Abstract
Findings from fear-conditioning studies in rats and functional neuroimaging with human volunteers have led to the suggestion that the amygdala is involved in the preattentive detection of threat-related stimuli. However, some neuroimaging findings point to attentional modulation of the amygdala response. The clinical-cognitive literature suggests that the extent to which the processing of threat-related stimuli is modulated by attention is crucially dependent on participants' anxiety levels. Here, we conducted a functional magnetic resonance imaging study with 27 healthy volunteers to examine whether amygdala responsivity to unattended threat-related stimuli varies with individual differences in state anxiety. Pairs of houses and faces (both fearful or neutral in expression) were presented, and participants attended to either the faces or the houses and matched these stimuli on identity. “Low-anxious” participants showed a reduced amygdala response to unattended versus attended fearful faces, but “high-anxious” participants showed no such reduction, having an increased amygdala response to fearful versus neutral faces regardless of attentional focus. These findings suggest that anxiety may interact with attentional focus to determine the magnitude of the amygdala response to threat-related stimuli.
Keywords: attention, emotion, fMRI, amygdala, anxiety, fear
Introduction
The amygdala is a key structure in the processing of threat-related stimuli. Fear-conditioning studies suggest that information about auditory or visual threat-related stimuli can reach the amygdala by a fast subcortical thalamoamygdala route as well as by a slower thalamocortical-amygdala pathway (Romanski and Le Doux, 1992; Shi and Davis, 2001). This has led to the proposal that the amygdala is involved in the rapid preattentive detection of threat-related stimuli as well as more elaborate processing of emotional stimuli (Armony and Le Doux, 2000; Le Doux, 2000; Anderson et al., 2003; Dolan and Vuilleumier, 2003).
Several neuroimaging studies have provided support for this position. These have reported amygdala activation to unattended threat-related stimuli (Vuilleumier et al., 2001; Anderson et al., 2003), to briefly presented backward-masked threat-related stimuli (Morris et al., 1998; Whalen et al., 1998), and to perceptually suppressed (via binocular rivalry) threat-related stimuli (Williams et al., 2004). Increased amygdala activity has also been shown to accompany the presentation of threat-related stimuli in the extinguished hemifield in a patient with extinction after parietal damage (Vuilleumier et al., 2002) and in the blind field in a patient with blindsight (Morris et al., 2001). Such findings have been interpreted as indicating that amygdala activity to threat-related stimuli can occur without both attention and conscious awareness (Whalen et al., 1998; Anderson et al., 2003; Dolan and Vuilleumier, 2003).
However, a number of studies have failed to observe amygdala activation under conditions of reduced awareness or attention. Phillips et al. (2004) found amygdala activation to backward-masked fearful faces presented for 170 msec but not to backward-masked fearful faces presented for 30 msec. Pessoa et al. (2002a) also reported that when participants were asked to judge the orientation of bars presented in the periphery, no differential amygdala response was observed for fearful versus neutral faces presented at fixation.
Our current study brings an individual-differences approach to this area of debate and specifically to the question of whether amygdala activation is observed when threat-related stimuli are presented outside the focus of spatial attention. Pessoa et al. (2002b) proposed that previous findings of amygdala activation to unattended threat-related stimuli could result from the primary task having an insufficiently high processing load to fully engage attentional resources. Here, we focus on an alternative important source of variance that could contribute to the discrepancy in findings and that has been overlooked previously, namely, individual variation in anxiety levels. Heightened anxiety is associated with an increased tendency to orient attention toward threat-related stimuli (Mogg and Bradley, 1998, 1999), this having led to the suggestion that anxiety alters the strength of output from a preattentive threat evaluation system, increasing the likelihood that threat-related stimuli will capture attention (Mathews and Mackintosh, 1998; Mogg and Bradley, 1998). Given this, and the proposed role of the amygdala in the early detection of threat-related stimuli (Le Doux, 2000; Dolan and Vuilleumier, 2003), we tested the proposition that individual differences in anxiety may influence the amygdala response to threat-related stimuli presented outside the current focus of spatial attention.
Materials and Methods
Participants. Twenty-seven participants (20 female, 7 male, all right-handed, 18-38 years of age) performed an adapted version of the matching task used by Vuilleumier et al. (2001) while functional magnetic resonance imaging (fMRI) data were collected. The study was approved by the Cambridgeshire Local Research Ethics Committee and performed in compliance with their guidelines. Individuals with a history of inpatient psychiatric care, neurological disease, or head injury were excluded, as were individuals on medication for anxiety or depression. Participants completed the Spielberger State-Trait Anxiety Inventory (STAI) (Spielberger, 1983) before the fMRI session. Participants' state anxiety scores ranged from 20 to 51 (mean, 32; SD, 8), and their trait anxiety scores ranged from 23 to 51 (mean, 35; SD, 8). These scores are similar to the published norms for this age group (state: mean, 36; SD, 10; trait: mean, 36; SD, 10) (Spielberger, 1983).
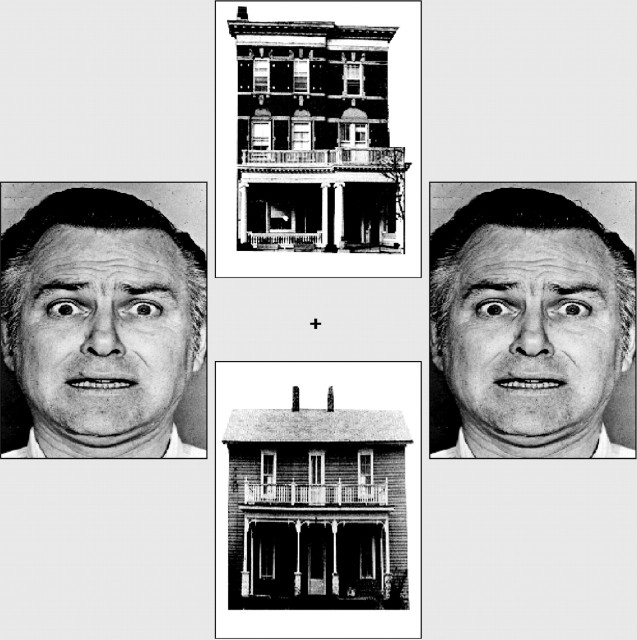
Task. Visual stimuli were back-projected onto a translucent screen positioned in the bore of the magnet, visible via an angled mirror placed above the participant's head. On each trial, two faces and two houses were presented in vertical and horizontal pairs around a central fixation cross (Fig. 1). The face stimuli used comprised 10 different individuals with fearful and neutral expressions taken from the Ekman series (Ekman and Friesen, 1976). Fearful facial expressions of conspecifics act as cues to potential danger and have been shown to share some of the functional properties of “prepared” (intrinsically threat-related) fear stimuli (Lanzetta and Orr, 1986). On each trial, the faces presented could vary in identity, but both were either fearful or neutral in expression. There were four imaging acquisition runs, each comprised of four blocks of 20 trials. At the start of each block, a cue indicated whether participants should attend to the vertical or horizontal pair of pictures. In one-half of the blocks, faces were presented in the attended locations, and in the other half, houses were presented in the attended locations. The task was to decide whether the attended pictures were identical or not. Within blocks, the interstimulus interval was randomly jittered using an exponential function with a mean of 6 sec and a minimum of 5 sec. A mixed model design was used, with the stimulus class attended to (houses or faces) being varied across blocks and the expression of the faces (fearful or neutral) being varied within blocks on a trial-by-trial basis. These two factors resulted in four conditions of interest: attended fearful faces, attended neutral faces, unattended fearful faces, and unattended neutral faces. The analyses reported below examined how amygdala activation varied as a function of attentional focus, face expression, and participant anxiety (as measured by the state subscale of the Spielberger State-Trait Anxiety Inventory) (Spielberger, 1983). The influence of anxiety on cortical “control” mechanisms during the processing of unattended fearful versus neutral faces and the role of distractor expectancy are dealt with by Bishop et al. (2004).
Figure 1.
Example stimuli. On each trial, two faces and two houses were presented in vertical and horizontal pairs around a central fixation cross. Participants matched either faces or houses, as cued by spatial location. Faces could differ in identity, but both were always either neutral or fearful in expression. Face stimuli copyright Paul Ekman [Ekman and Friesen (1976), reprinted with permission].
Image acquisition. Blood oxygenation level-dependent contrast functional images were acquired with echo-planar T2⋆-weighted (EPI) imaging using a MedSpec (Bruker, Ettlingen, Germany) 3 tesla magnetic resonance system with a head coil gradient set. Each image volume consisted of 21 interleaved 4-mm-thick slices (interslice gap, 1 mm; field of view, 25 × 25 cm; matrix size, 64 × 64; flip angle, 90°; echo time, 27 msec; voxel bandwidth, 100 kHz; acquisition time, 2.3 sec; repetition time, 3.02 sec). Slice acquisition was transverse oblique, angled to avoid the eyeballs, and covered the whole brain. Data were acquired in four scanning runs of ∼8 min. The first six volumes of each run were discarded to allow for T1 equilibration effects.
Image analysis. Data were analyzed using SPM99 software (Wellcome Department of Imaging Neuroscience, London, UK). Standard preprocessing was conducted comprising slice timing correction, realignment, and masked normalization of each participant's EPI data to the Montreal Neurological Institute (MNI)-International Consortium for Brain Mapping template. Images were resampled into this space with 2 mm isotropic voxels and smoothed with a Gaussian kernel of 8 mm full-width at half-maximum. Trials were modeled with step functions of 0.25 sec duration, convolved with the canonical hemodynamic response function to form regressors. Temporal derivatives of these regressors were also included, as were realignment parameters for each session, the latter to account for residual movement-related variance. A high-pass filter of 160 sec was used to remove low-frequency noise. A random effects analysis was used to analyze data at a group level. Modulations by anxiety were assessed by simple regression against state anxiety scores from the STAI.
Cluster-based regions of interest (ROIs) were used for left and right amygdala, left and right fusiform face area (FFA) (see Results), and left and right parahippocampal place area (PPA) (see Results). These ROIs were derived using data from a localizer task obtained from the same participants at the end of their fMRI session. In this task, participants were asked to passively view fearful and neutral faces, houses, and objects. The contrast for faces versus houses, using a random effects group analysis, produced bilateral activation in both the amygdala and FFA. The contrast for houses versus faces produced bilateral activation in the PPA. These clusters were extracted using the MarsBaR ROI toolbox for SPM99 (http://marsbar.sourceforge.net/). Significance thresholds were selected to give an approximately equivalent number of voxels in each ROI, with the criteria that all voxels selected should show activation associated with the relevant contrast at p < 0.001 uncorrected (activations in the PPA ROIs and right amygdala and right FFA ROIs were considerably stronger). The resulting left and right amygdala ROIs each comprised 46 voxels, and the left and right FFA and PPA ROIs each comprised 51-53 voxels. For the random effects analysis of data from the main experiment, voxelwise comparisons were conducted and small volume corrections applied for activations within each ROI (Worsley et al., 1996). All activations are reported using MNI coordinates.
Results
Effect of attention
Faces have been shown previously to produce a strong bilateral response in a region of fusiform cortex now commonly known as the fusiform face area (Kanwisher et al., 1997). Pictures of houses, meanwhile, have been shown to produce a strong bilateral response in a region of parahippocampal cortex now known as the parahippocampal place area (Epstein and Kanwisher, 1998). We first sought to establish whether we could replicate previous findings that FFA activation to faces and PPA activation to houses are modulated by attentional focus (Wojciulik et al., 1998; Vuilleumier et al., 2001). We used data from the localizer task (passive viewing of houses and faces) to create cluster-based ROIs for left and right FFA and PPA (see Materials and Methods). Activation in both FFA ROIs was significantly greater in the attend-face than in the attend-house conditions (x, y, z = 42, -52, -20, Z = 3.47, p corrected <0.005; x, y, z = -40, -50, -18, Z = 2.44, p corrected <0.05), whereas activation in both PPA ROIs was significantly greater in the attend-house than in the attend-face conditions (x, y, z = 28, -46, -12, Z = 5.22, p corrected <0.0001; x, y, z = -26, -50, -12, Z = 5.70, p corrected <0.0001). This suggests that the attentional manipulation was broadly successful, with participants focusing more on the faces in the attend-face conditions and more on the houses in the attend-house conditions.
Effect of expression
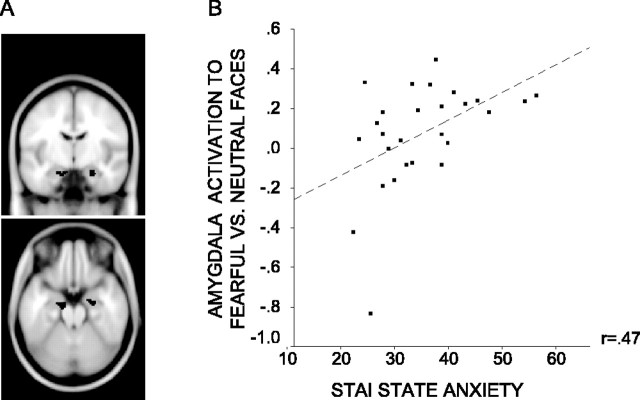
We investigated the main effect of emotional expression on the amygdala response. The localizer task described above was used to create cluster-based ROIs for the left and right amygdala (Fig. 2A) (see Materials and Methods). We examined how activation within both ROIs varied according to face expression (fearful vs neutral) averaging across both attended and unattended face trials. There was a significantly greater right amygdala response to trials with fearful versus neutral faces (x, y, z = 20, -8, -22, Z = 2.50, p corrected <0.05). Across participants, activation in the left amygdala ROI associated with this contrast did not reach significance (p corrected >0.1). However, there was a significant interaction between expression and anxiety level: left amygdala activation to fearful versus neutral faces showed a significant positive relationship with state anxiety (x, y, z = -14, -8, -22; Z = 2.48; p corrected <0.05) (Fig. 2B). No equivalent relationship was observed within the right amygdala ROI (p corrected >0.1)
Figure 2.
Amygdala activity to fearful versus neutral faces against participant state anxiety. A, Left and right amygdala ROIs (volume of each, 46 voxels, 368 mm3). Clusters extracted from analysis of localizer task data. B, Within the left amygdala ROI, the mean signal change (percentage of whole brain signal intensity) associated with the fearful versus neutral face contrast showed a positive relationship with participant state anxiety. This relationship is plotted for the peak voxel within the ROI (x, y, z =-14, -8, -22; Z = 2.48; p corrected <0.05).
Interaction of expression and attention
The extent to which the amygdala response to fearful versus neutral faces is modulated by attention is the key contrast of interest. Across participants, there was no significant interaction of expression by attention in either the left or right amygdala ROI (p corrected >0.1). In the left amygdala, however, there was a significant interaction of expression (fearful vs neutral) by attention (faces attended vs unattended) by state anxiety (x, y, z = -18, -10, -20; Z = 2.80; p corrected <0.02). Participants with higher anxiety levels showed less attentional modulation of the amygdala response to fearful versus neutral faces (Fig. 3A). There was a similar but nonsignificant pattern of activation in the right amygdala ROI (x, y, z = 26, -12, -18; Z = 2.05; p corrected = 0.10). For illustrative purposes, a median split was used to divide participants into low-anxious and high-anxious groups. Figure 3B shows the peak mean percentage signal change in the left amygdala to fearful versus neutral faces by attentional condition and by group. This indicates that whereas both low- and high-anxious participants showed an increased amygdala response to fearful versus neutral faces when they were attended, high-anxious participants additionally showed a selective amygdala response to unattended fearful faces.
Figure 3.
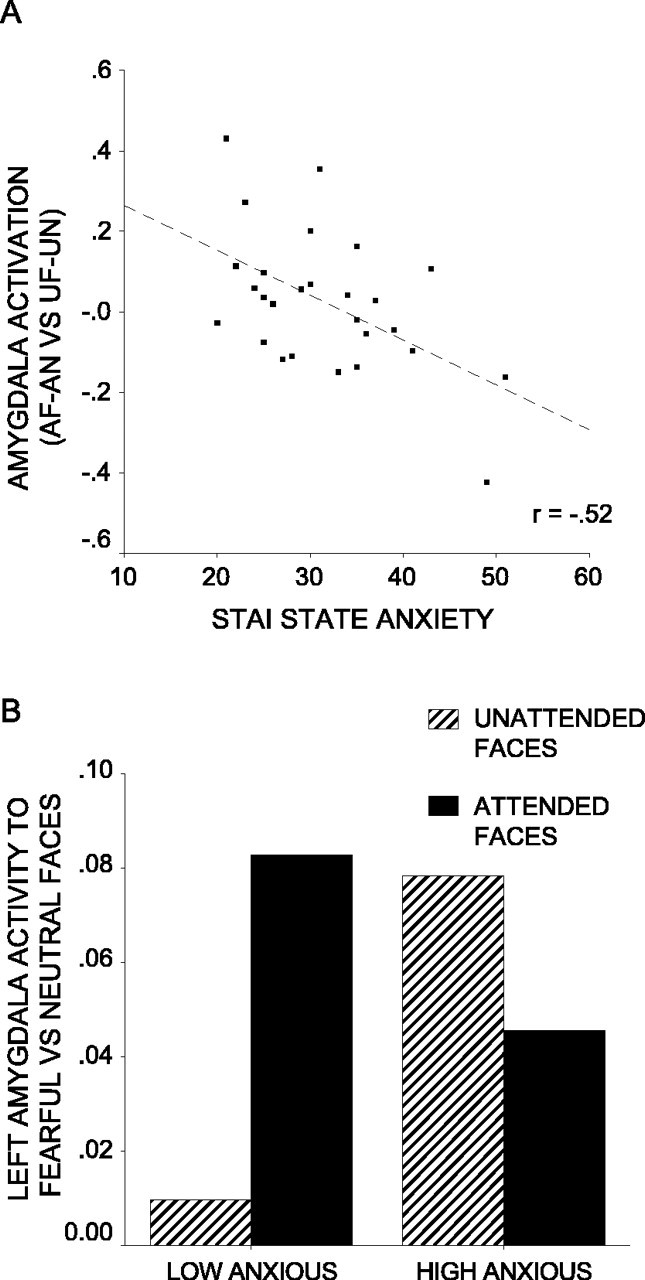
Anxiety influences attentional modulation of amygdala activity to fearful versus neutral faces. A, Amygdala activity to attended fearful (AF) faces versus attended neutral (AN) faces relative to unattended fearful (UF) faces versus unattended neutral (UN) faces against STAI state anxiety. Activation plotted is mean percentage signal change associated with this contrast for the peak voxel from the left amygdala ROI (x,y,z = -18, -10, -20; Z = 2.80; p corrected <0.02). A trend toward a similar relationship was observed with in the right amygdala ROI (x, y, z = 26, -12, -18; Z = 2.05; p corrected = 0.10). B, Amygdala activity to fearful versus neutral faces by attentional condition and anxiety level. Participants were divided into low- and high-anxious groups using a median split on STAI state anxiety scores. Amygdala activity is mean percentage signal change for the peak voxel from A.
Behavioral data
Mean reaction times for the four conditions of interest were as follows: attended fearful faces, mean, 849 msec, SD, 117 msec; attended neutral faces, mean, 836 msec, SD, 121 msec; unattended fearful faces, mean, 748 msec, SD, 100 msec; unattended neutral faces, mean, 746 msec, SD, 104 msec. In line with the findings by Vuilleumier et al. (2001), participants were generally slower at matching faces than houses (F(1,25) = 120.7; p < 0.001) and were also slower when the display contained threat-related stimuli (F(1,25) = 4.8; p < 0.05). There was no significant interaction of attentional condition by facial expression (F(1,25) = 1.3; p > 0.1), nor were there any significant interactions with anxiety (p > 0.1). Error rates were low and did not vary as a function of attentional condition, expression, or anxiety. It should be noted that speeded responding was not emphasized in the task instructions.
Discussion
Our data indicate that the amygdala response to threat-related stimuli located outside the current focus of spatial attention varies with individuals' levels of anxiety. High-anxious participants showed an increased amygdala response to fearful versus neutral faces both when faces were attended to and when they occurred outside the current focus of spatial attention. In contrast, low-anxious individuals showed only an increased amygdala response to attended fearful faces. This difference in amygdala responsivity between low- and high-anxious participants may have contributed to apparent discrepancies within the existing emotion-attention neuroimaging literature. To illustrate this, if one considers the current data from the high-anxious participants alone, there is no significant evidence for attentional modulation of the amygdala response to fearful faces; if anything, the response to unattended fearful versus neutral faces is stronger than that to attended fearful versus neutral faces. This falls in line with the findings by Vuilleumier et al. (2001). In contrast, if one considers the data from the low-anxious participants alone, there is evidence for attentional modulation of the amygdala response to fearful faces, which is in line with the findings by Pessoa et al. (2002a). The small sample sizes used in most neuroimaging studies increase the likelihood that random variations in participant anxiety levels could lead to differences in results. We suggest that routine consideration of anxiety as a covariate of interest is likely to make a useful contribution to this area of research.
As highlighted earlier, there has been much debate over whether the amygdala response to threat-related stimuli, such as fearful faces, requires attentional resources. According to one position, the amygdala shows a response to threat-related stimuli at an early preattentive processing stage, possibly exerting a bottom-up influence on the allocation of attention (Dolan and Vuilleumier, 2003). According to another, the amygdala response to threat-related stimuli occurs at a later postattentive stage such that “if attentional resources are depleted... face stimuli, regardless of valence, will fail to reach the amygdala and will fail to be tagged with emotional expression” (Pessoa et al., 2002b). In relation to our findings, supporters of the former position could argue that increased sensitivity of the proposed fast thalamoamygdala route in anxious participants may lead to a more robust amygdala response to mildly threatening stimuli presented outside the current focus of attention. This would fall in line with cognitive models of anxiety suggesting that anxiety acts to influence a preattentive threat evaluation system (Mathews and Mackintosh, 1998; Mogg and Bradley, 1998). Sensitization of the amygdala response to threat-related stimuli has indeed been held to play a role in anxiety (Rosen and Schulkin, 1998). Alternatively, advocates of a postattentive role for the amygdala might suggest that low-anxious participants are better able to maintain their task focus, hence, showing greater modulation of the amygdala response to threat by attention than high-anxious participants. Our previously reported findings that high-anxious participants show altered activity in the cortical circuitry implicated in controlling attention (a general reduction in rostral ACC activity and weaker recruitment of lateral PFC as expectancy of unattended threat-related stimuli increases) are arguably consistent with this (Bishop et al., 2004). However, these results do not provide any direct evidence that top-down control processes can modulate the amygdala response, an alternate possibility being that anxiety may have relatively independent additive effects on the top-down and bottom-up (salience-driven) mechanisms determining the allocation of attention to threat-related stimuli.
Our current findings do place a number of constraints on existing preattentive and postattentive accounts of amygdala function. They indicate that the amygdala response to threat-related stimuli cannot be entirely independent of attentional resources, because the low-anxious participants show a reduced amygdala response to unattended fearful faces. This finding has two main implications. The first is that a given threat-related stimulus does not lead to an automatic amygdala response of equal magnitude across participants. Instead, the magnitude of any preattentive amygdala response to threat-related stimuli is likely to be determined both by the nature of the given stimulus and by the sensitivity to threat-related stimuli of the individual. This falls in line with cognitive models of anxiety (Mathews and Mackintosh, 1998; Mogg and Bradley, 1998) and with findings of increased physiological reactivity to masked phobic stimuli in snake and spider fearful individuals (Öhman and Soares, 1994). Second, the increased amygdala response to attended relative to unattended fearful faces in low-anxious volunteers requires some mechanism for postattentional recruitment of the amygdala, at the very least a strengthening of subthreshold preattentive activity.
High-anxious participants, meanwhile, showed no reduction in the amygdala response to fearful faces presented outside the current focus of spatial attention, nor did they show particularly poor performance on the house-matching task in this condition: the interaction of attention by expression by anxiety failing to significantly influence reaction time scores. This indicates that in anxious participants, amygdala activity to threat-related stimuli cannot require sufficient attentional resources to significantly impede performance on the matching task, nor does direction of full attentional resources to threat-related stimuli lead to augmentation of the amygdala response. This suggests that there may be variation across individuals in the extent to which preattentive versus postattentive components contribute to the amygdala response to threat-related stimuli.
To conclude, the findings reported here indicate that the amygdala response to unattended versus attended threat-related stimuli is modulated by anxiety, with low-anxious participants showing greater modulation of the amygdala response to fearful faces by attentional focus than high-anxious participants. This may account for some of the discrepancies in results from previous studies examining the influence of attention on the amygdala response to threat-related stimuli and places certain constraints on both preattentive and postattentive models of amygdala function.
Footnotes
This work was conducted at the Cambridge University Wolfson Brain Imaging Centre (WBIC) and was supported by the United Kingdom Medical Research Council and a Betty Behrens Research Fellowship (Clare Hall, Cambridge University) awarded to S.J.B. We thank M. Brett and A. Andrade for statistical assistance, B. Cox for graphical assistance, and the radiographers at the WBIC who were involved with this project: R. Bisbrown-Chippendale, V. Lupson, and T. Donovan.
Correspondence should be addressed to Sonia Bishop, Medical Research Council Cognition and Brain Sciences Unit, 15 Chaucer Road, Cambridge CB2 2EF, UK. E-mail: sonia.bishop@mrc-cbu.cam.ac.uk.
Copyright © 2004 Society for Neuroscience 0270-6474/04/2410364-05$15.00/0
References
- Anderson AK, Christoff K, Panitz D, De Rosa E, Gabrieli JD (2003) Neural correlates of the automatic processing of threat facial signals. J Neurosci 23: 5627-5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armony JL, Le Doux JE (2000) How danger is encoded: toward a systems, cellular, and computational understanding of cognitive-emotional interactions in fear. In: The new cognitive neurosciences (Gazzaniga MS, ed), pp 1067-1080. Cambridge, MA: MIT.
- Bishop S, Duncan J, Brett M, Lawrence AD (2004) Prefrontal cortical function and anxiety: controlling attention to threat-related stimuli. Nat Neurosci 7: 184-188. [DOI] [PubMed] [Google Scholar]
- Dolan RJ, Vuilleumier P (2003) Amygdala automaticity in emotional processing. Ann NY Acad Sci 985: 348-355. [DOI] [PubMed] [Google Scholar]
- Ekman P, Friesen WV (1976) Pictures of facial affect. Palo Alto, CA: Consulting Psychologists.
- Epstein R, Kanwisher N (1998) A cortical representation of the local visual environment. Nature 392: 598-601. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM (1997) The fusiform face area: a module in human extrastriate cortex specialized for face perception. J Neurosci 17: 4302-4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzetta JT, Orr SP (1986) Excitatory strength of expressive faces: effects of happy and fear expressions and context on the extinction of a conditioned fear response. J Pers Soc Psychol 50: 190-194. [DOI] [PubMed] [Google Scholar]
- Le Doux JE (2000) Cognitive-emotional interactions: listen to the brain. In: Cognitive neuroscience of emotion (Lane RD, ed), pp 129-155. Oxford: Oxford UP.
- Mathews A, Mackintosh B (1998) A cognitive model of selective processing in anxiety. Cognit Ther Res 22: 539-560. [Google Scholar]
- Mogg K, Bradley BP (1998) A cognitive-motivational analysis of anxiety. Behav Res Ther 36: 809-848. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley BP (1999) Orienting of attention to threatening facial expressions presented under conditions of restricted awareness. Cognition Emotion 13: 713-740. [Google Scholar]
- Morris JS, Öhman A, Dolan RJ (1998) Conscious and unconscious emotional learning in the human amygdala. Nature 393: 467-470. [DOI] [PubMed] [Google Scholar]
- Morris JS, DeGelder B, Weiskrantz L, Dolan RJ (2001) Differential extra-geniculostriate and amygdala responses to presentation of emotional faces in a cortically blind field. Brain 124: 1241-1252. [DOI] [PubMed] [Google Scholar]
- Öhman A, Soares JJ (1994) “Unconscious anxiety”: phobic responses to masked stimuli. J Abnorm Psychol 103: 231-240. [DOI] [PubMed] [Google Scholar]
- Pessoa L, McKenna M, Gutierrez E, Ungerleider LG (2002a) Neural processing of emotional faces requires attention. Proc Natl Acad Sci USA 99: 11458-11463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L, Kastner S, Ungerleider LG (2002b) Attentional control of the processing of neutral and emotional stimuli. Brain Res Cogn Brain Res 15: 31-45. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Williams LW, Heining M, Herba CM, Russell T, Andrew C, Bullmore ET, Brammer MJ, Williams SC, Morgan M, Young AW, Gray JA (2004) Differential neural responses to overt and covert presentations of facial expressions of fear and disgust. NeuroImage 21: 1484-1496. [DOI] [PubMed] [Google Scholar]
- Romanski LM, Le Doux JE (1992) Equipotentiality of thalamo-amygdala and thalamo-cortico-amygdala circuits in auditory fear conditioning. J Neurosci 12: 4501-4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen JB, Schulkin J (1998) From normal fear to pathological anxiety. Psychol Rev 105: 325-350. [DOI] [PubMed] [Google Scholar]
- Shi C, Davis M (2001) Visual pathways involved in fear conditioning measured with fear-potentiated startle: behavioral and anatomic studies. J Neurosci 21: 9844-9855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD (1983) Manual for the state-trait anxiety inventory. Palo Alto, CA: Consulting Psychologists.
- Vuilleumier P, Armony JL, Driver J, Dolan RJ (2001) Effects of attention and emotion on face processing in the human brain: an event-related fMRI study. Neuron 30: 829-841. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Armony JL, Clarke K, Husain M, Driver J, Dolan RJ (2002) Neural response to emotional faces with and without awareness: event-related fMRI in a parietal patient with visual extinction and spatial neglect. Neuropsychologia 40: 2156-2166. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, Jenike MA (1998) Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. J Neurosci 18: 411-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MA, Morris AP, McGlone F, Abbott DF, Mattingley JB (2004) Amygdala responses to fearful and happy facial expressions under conditions of binocular suppression. J Neurosci 24: 2898-2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojciulik E, Kanwisher N, Driver J (1998) Covert visual attention modulates face-specific activity in the human fusiform gyrus: fMRI study. J Neurophysiol 79: 1574-1578. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC (1996) A unified statistical approach for determining significant signals in images of cerebral activation. Hum Brain Mapp 4: 58-73. [DOI] [PubMed] [Google Scholar]