Abstract
Innate immunity is a specific and organized immunological program engaged by peripheral organs and the CNS to maintain homeostasis after stress and injury. In neurodegenerative disorders, its putative deregulation, featured by inflammation and activation of glial cells resulting from inherited mutations or viral/bacterial infections, likely contributes to neuronal death. However, it remains unclear to what extent environmental factors and innate immunity cooperate to modulate the interactions between the neuronal and non-neuronal elements in the perturbed CNS. In the present study, we addressed the effects of acute and chronic administration of lipopolysaccharide (LPS), a Gram-negative bacterial wall component, in a genetic model of neurodegeneration. Transgenic mice expressing a mutant form of the superoxide dismutase 1 (SOD1G37R) linked to familial amyotrophic lateral sclerosis were challenged intraperitoneally with a single nontoxic or repeated injections of LPS (1 mg/kg). At different ages, SOD1G37R mice responded normally to acute endotoxemia. Remarkably, only a chronic challenge with LPS in presymptomatic 6-month-old SOD1G37R mice exacerbated disease progression by 3 weeks and motor axon degeneration. Closely associated with the severity of disease is the stronger and restricted upregulation of the receptor of innate immunity Toll-like receptor 2 and proinflammatory cytokines in degenerating regions of the ventral spinal cord and efferent fiber tracts of the brain from the LPS-treated SOD1G37R mice. This robust immune response was not accompanied by the establishment of acquired immunity. Our results provide solid evidence that environmental factors and innate immunity can cooperate to influence the course of disease of an inherited neuropathology.
Keywords: innate immunity, neurodegeneration, lipopolysaccharide, microglia, amyotrophic lateral sclerosis, superoxide dismutase 1, proinflammatory cytokines, transgenic mice
Introduction
The innate immune response is a rapid and coordinated cascade of reactions by cells of the host to pathogens and insults (Akira et al., 2001; Nguyen et al., 2002). In the CNS, the accuracy of this system can protect neurons by favoring remyelination and trophic support afforded by glial cells. Conversely, its deregulation might be harmful for neuronal integrity and might trigger neurodegeneration (Nguyen et al., 2002; Wyss-Coray and Mucke, 2002). Actually, the dual nature of the innate immune response relies on the fine-tuned regulation of microglial cells, the resident macrophages of the CNS (Nguyen et al., 2002; Wyss-Coray and Mucke, 2002).
Interestingly, numerous proinflammatory genes are induced in the CNS of presymptomatic mice expressing a mutant form of superoxide dismutase 1 (SOD1G37R) linked to amyotrophic lateral sclerosis (ALS), the most common form of human motor neuron disease (Nguyen et al., 2001b, 2002). ALS is an adult-onset neurological disorder characterized by the selective degeneration of motor neurons, culminating in paralysis and death within 3-5 years (Cleveland and Rothstein, 2001; Julien, 2001). Missense mutations in the gene coding for the Cu-Zn SOD1, located on chromosome 21, account for ∼20% cases of familial ALS (Rosen et al., 1993; Cudkowicz et al., 1997). The SOD1 protein is a cytosolic metalloenzyme catalyzing the conversion of superoxide anions to hydrogen peroxide (Fridovich, 1986). Transgenic mice expressing mutant SOD1 develop motor neuron disease resembling ALS, through a gain of unidentified deleterious properties (Wong et al., 1995; Bruijn et al., 1997). Several mechanisms have been proposed to account for such toxicity including excitotoxicity (Bruijn et al., 1997), disruption of the calcium homeostasis (Morrison et al., 1996; Roy et al., 1998), cytoskeletal abnormalities (Wong et al., 1995), Fas ligand (FasL)-mediated death (Raoul et al., 2002), and deregulation of Cdk5 (cyclin-dependent kinase 5) (Nguyen et al., 2001a).
Despite these findings, the toxicity of SOD1 mutants linked to human ALS remains poorly understood. SOD1 is a ubiquitously expressed protein, and therefore, it is possible that cells other than motor neurons play a role in ALS (Clement et al., 2003). Indeed, a restricted expression of mutant SOD1G37R to neurons in transgenic mice was not sufficient to provoke motor neuron disease (Pramatarova et al., 2001; Lino et al., 2002). Neither did the selective expression of mutant SOD1 in astrocytes provoke pathology despite astrocytosis (Gong et al., 2000). Compatible with this view of non-cell autonomy are studies reporting dysfunction of astrocytes, inflammatory processes, and activation of microglia and other immune cells in ALS patients and mice (Bruijn et al., 1997; Nguyen et al., 2001b, 2002).
The use of antiinflammatory compounds has recently conferred protection in models of neurodegeneration, including ALS (Drachman and Rothstein, 2000; Kriz et al., 2002; Schenk, 2002). However, it remains unclear whether these compounds mediate their beneficial effects directly on neurons or through the modulation of microglial activity and innate immunity. In addition, the modulation of neurodegenerative processes by environmental factors remains unexplored. In the present study, we triggered the innate immunity of SOD1G37R mice with systemic administration of lipopolysaccharide (LPS), a potent activator of microglia. Our results demonstrate that chronic activation of innate immunity by systemic LPS is noxious to motor neurons bearing SOD1G37R linked to ALS. We advance a model linking environmental factors and innate immunity that, potentially, might be extrapolated to sporadic cases of neurodegeneration.
Materials and Methods
Generation of SOD1G37R mice and protocol for LPS injection. The inbred C57BL/6 SOD1G37R mice (line 29) used in this study have a life span of 10-12 months (Nguyen et al., 2000). C57BL/6 SOD1G37R mice (line 42) exhibit a life span of 5-6 months (Wong et al., 1995). The mouse genotypes were determined by Southern blotting of tail DNA. The SOD1G37R mice were housed at room temperature (21°C) and in a light-controlled environment with ad libitum access to food and water. To avoid any potential interference with the effects of LPS, mice were kept in a pathogen-free facility. To trigger an acute innate immune response in the CNS, presymptomatic 3-, 6-, and 9-month-old SOD1G37R mice received a single intraperitoneal injection of LPS (1 mg/kg of body weight; from Escherichia coli; serotype 055:B5; Sigma, Saint Louis, MO) diluted in 100 μl of vehicle (Veh) solution (sterile pyrogen-free saline) or vehicle solution. At different times after the systemic injections (from 30 min to 24 hr), animals were deeply anesthetized via an intraperitoneal injection of a mixture of ketamine hydrochloride (91 mg/ml) and xylazine (9 mg/ml) and then rapidly perfused transcardially with 0.9% saline, followed by 4% paraformaldehyde in 0.1 m borax buffer, pH 9.5, at 4°C. Brains and spinal cords were rapidly removed from the animals, postfixed for 2-8 d, and then placed in a solution containing 10% sucrose diluted in 4% paraformaldehyde-borax buffer overnight at 4°C. The frozen tissues were mounted on a microtome (Reichert-Jung; Cambridge Instruments, Deerfield, IL) and cut into 20 μm coronal or longitudinal (spinal cord) sections from the olfactory bulb to the end of the spinal cord. The slices were collected in a cold cryoprotectant solution (0.05 m sodium phosphate buffer, pH 7.3, 30% ethylene glycol, 20% glycerol) and stored at -20°C.
Another group of presymptomatic 6-month-old SOD1G37R mice received intraperitoneal LPS or vehicle injections once every 2 weeks for a duration of 3 months. Around month 9 (42-43 weeks of age), the chronically LPS-treated SOD1G37R mice exhibited the first signs of paralysis, and the injections were stopped. The end stage of disease was observed 3 weeks later (45-46 weeks; 10 months of age), which corresponds to the life span of these mice. At this stage, in situ hybridization, staining, and axonal counts were performed. At the same time, Veh-SOD1 or SOD1 mice that did not show signs of paralysis were killed to have a matched control group for the equivalent analysis. Some of the Veh-SOD1 or SOD1 were left until they exhibited the paralytic phenotype (3-5 weeks after the LPS-SOD1), which permitted the calculation of their life span. Thus, the analysis of the Veh-SOD1 and SOD1 mice was performed strictly before the onset and not at the end stage of disease.
In situ hybridization and histological preparations. The riboprobes used in this study are listed in Table 1. In situ hybridization using 35S-labeled cRNA probes and quantification of the signals were accomplished as described previously (Laflamme et al., 1999; Nadeau and Rivest, 2000). Double-labeling procedures were similar to those described in other studies published by our group (Laflamme et al., 1999; Nadeau and Rivest, 2000, 2002). Neuronal death was detected by the Fluoro-Jade B (FJB) method (see below). Briefly, every sixth section of the entire rostrocaudal extent of each brain and spinal cord was mounted onto poly-l-lysine-coated slides, dried under vacuum for 2 hr, dehydrated through graded concentrations of alcohol (50, 70, and 100%; 1 min), rehydrated through graded concentrations of alcohol (100, 70, and 50%; 1 min each) and 1 min in distillated water. They were then dipped and shacked into potassium permanganate (0.06%) for 10 min, rinsed 1 min in distilled water, and dipped and shacked in a solution containing Fluoro-Jade B [Fluoro-Jade B (0.0004%; Histochem, Jefferson, AR) plus acetic acid (0.1%; catalog #A-6404; Sigma) plus 4′,6′-diamidino-2-phenylindole (DAPI) (0.0002%; catalog #D-1306; Molecular Probes, Eugene, OR)] for 20 min. The slides were thereafter rinsed three times in distilled water (1 min each), dried, dipped in xylene three times (2 min each), and coverslipped with distrene plasticizer xylene. FJB is novel fluorescent dye that has high affinity for dying neurons and does not discriminate between apoptosis and necrosis (Schmued et al., 1997). This explains our choice to use this dye, because it is still unclear whether unusual apoptosis or necrosis is the dominant form of cell death in ALS (Migheli et al., 1999; Pasinelli et al., 2000; Vukosavic et al., 2000).
Table 1.
Plasmids and enzymes used for the synthesis of the cRNA probes
|
Plasmid |
Vector |
Insert |
Antisense probe |
Sense probe |
Source |
|---|---|---|---|---|---|
| Mouse TLR2 | PCR-blunt II topo | 2.278 bp | EcoRV/SP6 | SpeI/T7 | PCR amplificationa |
| Mouse TNF-α | Bluescript SK+ + | 1.3 kb | PstI/T3 | BamHI/T7 | Dr. D. Radzioch, McGill University, Montreal, Canada |
| Mouse IFN-γ | pGEMEX | 550 bp | HindIII/T3 | EcoRI/SP6 | Dr. I. Campbell, The Scripps Research Institute, LaJolla, CA |
| Mouse IL-12p40 | pCL-Neo | 1.05 kb | XhoI/T3 | NotI/T7 | Dr. K. Pahan, University of Nebraska, Lincoln, NE |
| Mouse IκBα |
Bluescript SK II+ |
1.114 kb |
BamHI/T7 |
HindIII/T3 |
Dr. A. Israel, Institut Pasteur, Paris, France |
The DNA fragment of 2.278 kb corresponding to the almost complete coding sequence (2.355 kb) of the reported mouse TLR2mRNA (nucleotides 307-2661, GenBank accession no.AF185284) was amplified by PCR from a cDNA macrophage B10R cell line library using a pair of 23 bp oligonucleotide primers complementary to nucleotides 323-345 (5′-GGCTCTTCTGGATCTTGGTGGCC-3′) and 2579-2601 (5′-GGGCCACTCCAGGTAGGTCTTGG-3′).
Morphological and morphometric analyses. Mice were killed by overdose of chloral hydrate, perfused with 0.9% NaCl and then with fixative (3% v/v glutaraldehyde in PBS buffer, pH 7.4). Tissue samples were immersed in fixative overnight, rinsed in phosphate buffer, and then postfixed in 1% phosphate-buffered osmium tetroxide. After three washes with phosphate buffer, each sample was dehydrated in a graded series of ethanol and embedded in Epon. The thin sections of spinal cord and L5 ventral root were stained with toluidine blue and examined under a light microscope. The counting of axons in the L5 ventral root was performed with the Image-1 software from Universal Imaging Corporation (West Chester, PA).
Western blots. Mice were killed by intraperitoneal injection of chloral hydrate. Total protein extracts of spinal cord were obtained by homogenization in SDS-urea β-mercaptoethanol, 0.5% SDS, 8 m urea in phosphate buffer, pH 7.4, with a mixture of protease inhibitors (PMSF, leupeptin, pepstatin, and aprotinin). The protein concentration was estimated by the Bradford procedure (Bio-Rad, Hercules, CA). Proteins were fractionated on 12-15% SDS-PAGE and blotted on a nitrocellulose or polyvinylidene difluoride membrane for Western blot analysis. Membranes were incubated with antibodies against SOD1 (Biodesign; Santa Cruz Biotechnology, Santa Cruz, CA), α-tubulin (B512; Sigma), and actin (MAB 1501; Chemicon, Temecula, CA). The Western blots were revealed by Renaissance, a Western blot chemiluminescence kit from NEN (Boston, MA).
Statistical analysis. The statistical analyses for the different dependent variables were performed by a two-way ANOVA followed by a Bonferroni-Dunn test procedure as post hoc comparison. Please see the figures for more specific details.
Results
SOD1G37R mice exhibited a normal innate immune response after an acute systemic LPS injection
A single systemic LPS injection caused a robust increase in the expression of Toll-like receptor (TLR)2 mRNA across the brain and spinal cord of both normal [wild-type (WT)] and SOD1G37R mice (Fig. 1). The expression wave of this transcript was similar in the CNS of both mouse strains at all of the times evaluated (i.e., 30 min, 3, 6, and 24 hr). These small, scattered positive cells that were found across the brain, L5 segment, and cervical part of the spinal cord are microglial cells (see Fig. 3) (Laflamme et al., 2001). Indeed, an antibody directed against the ionized calcium-binding adapter molecule 1 (iba1) was used to stain microglia that were positive for TLR2 transcript in the CNS of LPS-treated mice (Laflamme et al., 2001). This was also the case for numerous other genes involved in the innate immune system, such as IκBα [inhibitory protein of nuclear factor-κB (NF-κB)] (index of NF-κB activity), tumor necrosis factor-α (TNF-α), and CD14 (data not shown). Microglia are therefore the main group of cells expressing most of the genes involved in the control of the innate immune response in the CNS after LPS administration.
Figure 1.
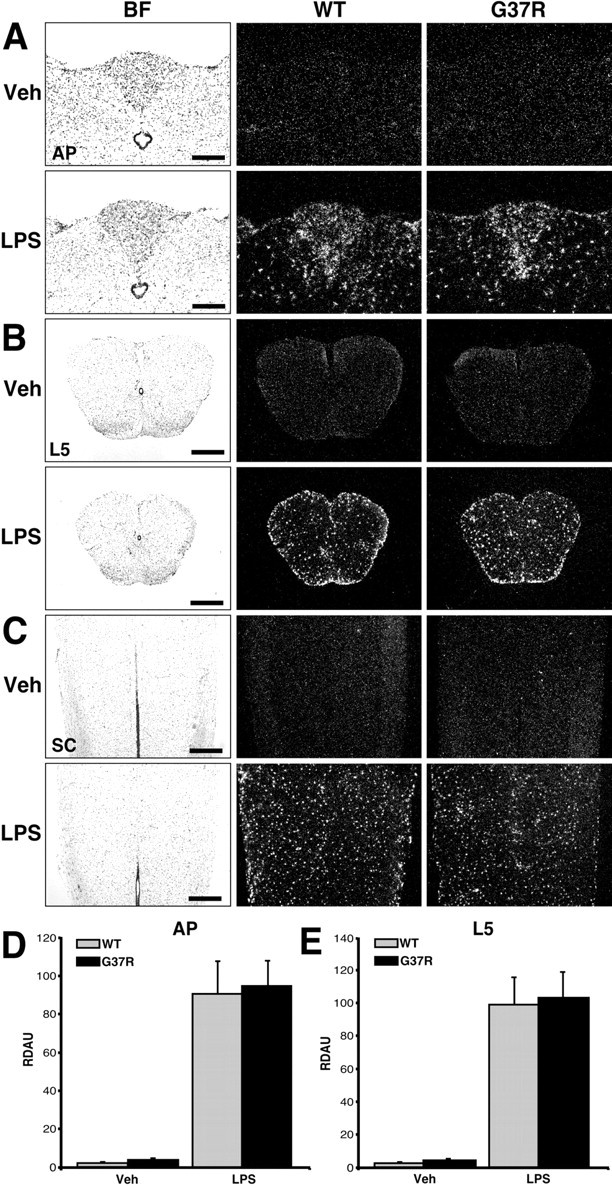
Effect of a single bolus of LPS on TLR2 gene expression in the brains and spinal cords of SOD1G37R mice and their WT littermates. These bright-field (BF) and dark-field photomicrographs depict the expression pattern of TLR2 mRNA 24 hr after a single intraperitoneal injection of vehicle (Veh) or LPS (1 mg/kg of body weight). Coronal and longitudinal sections were hybridized using a mouse TLR2 cRNA probe and dipped into NTB2 emulsion milk. A, Coronal sections at the level of the AP. B, Coronal sections within the L5 segment of the spinal cord (SC). C, Longitudinal slices of the SC. Note the strong and similar hybridization signal within the brain and spinal cord of both WT and SOD1G37R mice that received an intraperitoneal bolus of LPS. Semiquantitative analysis was performed in regions of the AP (D) and L5 segment (E). Data are means ± SEM. The expression levels were comparable in the CNS of both mouse strains after the acute endotoxemia. Statistical analysis was performed by a two-way ANOVA, which indicated a significant main effect (p < 0.0001) between the vehicle- and LPS-treated groups. Scale bars: A, 200 μm; B, C, 500 μm. RDAU, Refraction density in arbitrary units. (Means ± SEM).
Figure 3.

Chronic treatment with the endotoxin LPS increases the innate immune response and neurodegeneration in SOD1G37R mice. The bright-field (B.F.) and dark-field photomicrographs depict representative examples of the hybridization signal for TNF-α (B), IL-12 (C), and TLR2 (D) mRNA in the reticular formation just above the olivary complex. It is of interest to note that the hybridization signal for IL-12 and TLR2 overlaps with the fluorochrome FJB (C, D;vs E), used here as a marker of neuronal death. Degenerating axons were labeled by FJB staining in the reticular formation; DAPI-positive nuclei were essentially devoid of FJB signal in this structure (F). The bottom panels in G depict examples of microglial cells containing positive hybridization signal for TLR2 mRNA in the reticular formation. Cells of myeloid origin were labeled by immunoperoxidase using antisera directed against iba1 (brown immunoreactive cells). TLR2 mRNA was thereafter hybridized on the same sections by means of a radioactive in situ hybridization technique (silver grains). Note the presence of the mRNA encoding TLR2 within parenchymal microglia (agglomeration of silver grains within the cell cytoplasm) (black arrowheads). Scale bars: A-E, 500 μm; F, G, 50 μm. See the legend to Figure 2 for definitions of abbreviations.
Semiquantitative analyses revealed similar increases in expression levels of TLR2 mRNA in different regions of the CNS in both WT and SOD1G37R groups of mice challenged acutely with LPS (Fig. 1D,E). The area postrema (AP) and spinal L5 segment of LPS-treated SOD1G37R mice did not exhibit a different hybridization signal when compared with WT animals. These results indicate that SOD1G37R are not more sensitive to the endotoxin and, therefore, exhibit a normal cerebral innate immune response after acute endotoxemia.
Chronic systemic injections of LPS exacerbate disease progression and motor axon degeneration in SOD1G37R mice
Remarkably, a chronic systemic injection of an equal dose of LPS in presymptomatic 6-month-old SOD1G37R mice had a significant effect on their life span. Figure 2A shows the survival curve of SOD1G37R mice (line 29) treated chronically with Veh or LPS. The SOD1G37R mice treated with vehicle (n = 16) used as control exhibited an average life span of 49.4 ± 2.7 weeks and a median life probability of 49.0 weeks, which is not different from the life span of the nontreated SOD1G37R mice (Nguyen et al., 2000, 2001a). The median life probability is defined as the age at which the probability of survival is 50%. SOD1G37R mice treated chronically with LPS (n = 13) exhibited an average life span of 45.5 ± 2.2 weeks and a median life probability of 46.0 weeks. These results demonstrate that repeated intraperitoneal injections of a nontoxic dose of LPS in presymptomatic 6-month-old SOD1G37R mice exacerbated disease progression by 3 weeks.
Figure 2.
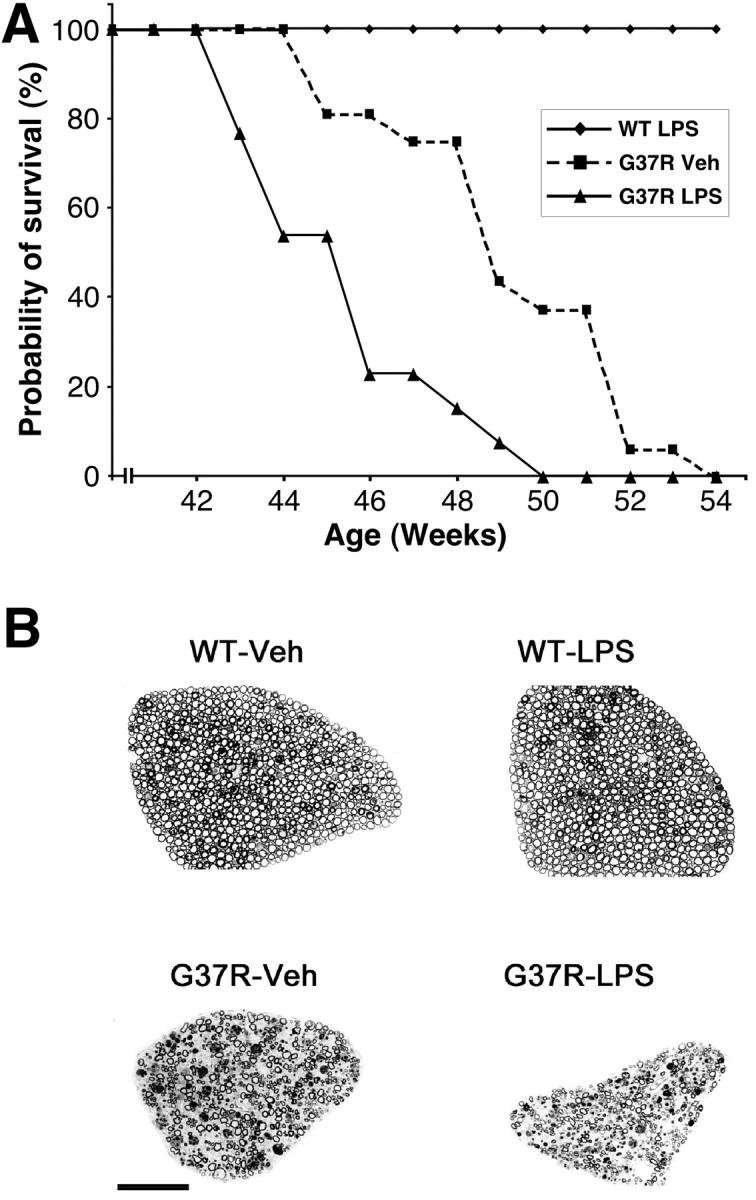
Exacerbation of motor axon degeneration in chronically LPS-treated SOD1G37R mice accelerates disease progression. A, Survival curves of transgenic mice expressing SOD1G37R challenged systemically with LPS or vehicle every 2 weeks. Disease progression of chronically LPS-treated mice is exacerbated by ∼3 weeks. Note that the life span of wild-type mice is unaffected by the same dose of LPS (see Materials and Methods). The survival probability of transgenic mice is plotted as a function of their age in weeks. B, Transverse sections of L5 ventral root from normal mice treated chronically with vehicle (WT-Veh) or LPS (WT-LPS) and from SOD1G37R mice challenged chronically with vehicle (G37R-Veh) or LPS (G37R-LPS). Massive degeneration is observed in the L5 ventral root of G37R-Veh. A more severe loss of motor axons is found in the L5 ventral root of G37R-LPS. It is noteworthy that WT-LPS mice do not show any sign of neurodegeneration. Scale bar, 100 μm.
To verify whether this acceleration in disease progression is caused by a more severe loss of motor axons, we examined at light microscopy and counted the number of axons in L5 ventral roots of SOD1G37R mice treated chronically with LPS or vehicle and killed at 10 months of age. At this age, the L5 ventral roots of LPS-treated SOD1G37R mice were smaller when compared with those dissected from Veh-SOD1G37R mice (Fig. 2B). Furthermore, LPS-treated SOD1G37R mice (n = 5) had 358 ± 48 axons, whereas Veh-treated SOD1G37R mice (n = 4) had 478 ± 40 (Table 2). Thus, at this age, there was a more severe loss of motor axons in SOD1G37R mice that received repeated injections with the endotoxin. These data indicate that exacerbation of disease progression is caused by accelerated degeneration of motor axons. It is noteworthy that the chronic injection of LPS in WT mice did not cause neurodegeneration (1033 ± 46; n = 4) as confirmed by axonal counts (Table 2).
Table 2.
Axonal counts of L5 ventral root
|
Genotype |
Number of axons at 10 months of age (SD) |
|---|---|
| Veh-WT | 1031 (57) (n = 6) |
| LPS-WT | 1033 (46) (n = 4) |
| Veh-SOD1G37R | 478 (40)* (n = 4) |
| LPS-SOD1G37R
|
358 (48)** (n = 5) |
Statistical analysis was performed by a two-way ANOVA, followed by a Bonferroni-Dunn test procedure as post hoc comparisons by means of the Statview program (version 4.01, Macintosh). *Significantly different (p<0.05) from WT groups of mice. **Significantly different (p<0.05) from all of the other groups.
Inflammatory response in chronically treated mice
We next assessed the transcriptional activation of the receptor of innate immunity TLR2 and the proapoptotic cytokine TNF-α, in the SOD1G37R mice challenged chronically with LPS. TLR2 is induced during disease progression of SOD1G37R mice and especially, in late stages, where massive neurodegeneration occurs (Gurney et al., 1994; Nguyen et al., 2001a,b). The physiological role for such induction remained unexplained. During endotoxemia, TLR expression has been suggested to be neuroprotective by favoring the elimination of pathogens (Akira et al., 2001). Similarly, a chronic expression of TLR2 might also trigger microglial apoptosis and thereby prevent the detrimental effects of subsequent inflammation (Aliprantis et al., 1999, 2000; Laflamme et al., 2001).
The innate immune response was much more pronounced in chronically LPS-treated SOD1G37R mice than in Veh-treated SOD1G37R mice (Figs. 3, 4, 5). The robust expression of TLR2 mRNA (Fig. 3D,G) is associated with strong hybridization signals for the genes encoding the proapoptotic cytokines TNF-α andinterleukin-12 (IL-12) in degenerating efferent fiber tracts of the brain (Fig. 3B,C) and in degenerating ventral spinal horns (Fig. 4A, rows 2-4). Degenerating neurons were labeled via FJB staining that clearly overlapped with the hybridization signal (Figs. 3E,F, 4, row 5). Indeed, positive FJB fibers were found in the reticular formation just above the olivary complex (Fig. 3E) and numerous other regions receiving projections from the spinal cord. The immune response and microglial activation were therefore highly associated with the degenerative groups of neurons, a phenomenon that was clearly exacerbated by chronic treatment with the bacterial cell wall component (Fig. 5).
Figure 4.
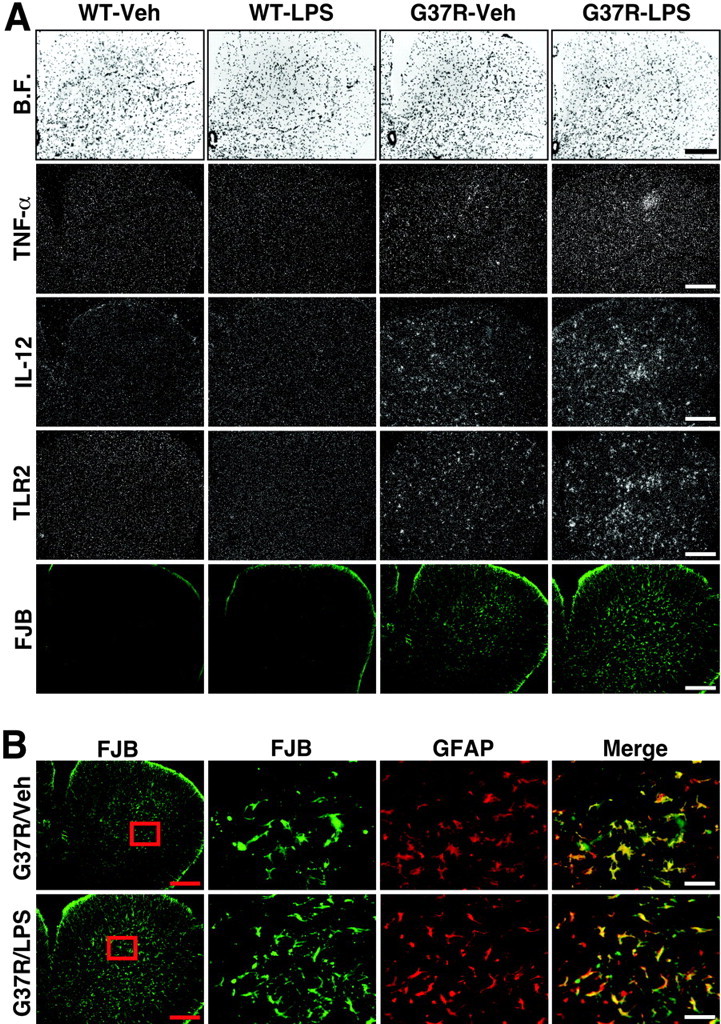
Robust inflammatory response in ventral spinal horn of chronically LPS-treated SOD1G37R mice associated with massive degeneration of astrocytes. The bright-field (B.F.) and dark-field photomicrographs depict representative examples of the hybridization signal for TNF-α, IL-12, and TLR2 mRNA in the L5 segment of the spinal cord (A). Here also the hybridization signal for IL-12 and TLR2 overlaps with the fluorochrome FJB. Neuronal cell bodies and axons contained FJB staining in this region. Please also note the robust FJB signal over astrocytes, a phenomenon that was specific to the L5 region (B). These data suggest an intimate link between degeneration of neurons and astrocytes in this region of spinal cord from SOD1G37R mice. Age of mice at time of analysis, 45-46 weeks. Scale bars: A and B (left panels), 500μm; B (high magnifications, merge), 50μm. See the legend to Figure 2 for definitions of abbreviations.
Figure 5.
Relative expression levels of TLR2 hybridization signal in the brains of SOD1G37R mice and their wild-type littermates that received chronic systemic injections of the endotoxin LPS or sterile saline solution (Veh). The selected structures were the areas adjacent to the mesencephalic nucleus of the trigeminal (A), the facial nucleus (B), and the reticular formation just above the olivary complex (C). These regions were chosen to facilitate the analysis among animals, although the hybridization signal was not limited to these specific nuclei (see Results). The signals revealed on dipped NTB2 nuclear emulsion slides were analyzed and quantified (relative levels) with an Olympus Optical System (BX-50; BMax) coupled to a Macintosh computer (PowerPC 7100/66) and Image software [version 1.59; non-FPU; W. Rasband (National Institutes of Health, Bethesda, MD)]. The refraction density in arbitrary units (RDAU) of the hybridization signal was measured under dark-field illumination at a magnification of 10×. Sections from experimental and control animals were digitized and subjected to densitometric analysis, yielding measurements of RDAU. The RDAU of each region was then corrected for the average background signal, which was determined by sampling cells immediately outside the cell group of interest. Data are reported as mean values (±SEM) for vehicle- and LPS-treated animals of both mouse stains. Statistical analysis was performed by a two-way ANOVA, followed by a Bonferroni-Dunn test procedure as post hoc comparisons by means of the Statview program (version 4.01; Macintosh). *Significantly different (p < 0.05) from WT groups of mice. **Significantly different (p < 0.05) from all of the other groups. Age of mice at time of analysis, 45-46 weeks.
In addition, a higher number of FJB-positive astrocytes were found in the spinal cord of chronically LPS-treated mutant SOD1 mice (Fig. 4B). Disturbance in functions of astrocytes is one of the first pathological changes observed in patients and rodent models with ALS (Rothstein et al., 1995; Bristol and Rothstein, 1996; Bruijn et al., 1997) that can lead to excitotoxicity-evoked motor neuron death (for review, see Cleveland and Rothstein, 2001; Julien, 2001).
Absence of adaptive immunity in the CNS of LPS-treated SOD1G37R mice
To determine whether upregulated innate immunity transfers to the adaptive form, we performed in situ hybridization on spinal cord and brain tissues using highly sensitive probes for interferon-γ (IFN-γ) and IL-12, two cytokines essential for the transfer from the innate to the acquired immunity. Figures 3C and 4A, row 3, show an upregulation of IL-12 in degenerating CNS regions of the chronically LPS-treated SOD1G37R mice when compared with chronically Veh-treated SOD1G37R mice. However, in situ hybridization failed to detect positive signal for IFN-γ transcript in the brain and spinal cord of SOD1G37R mice treated acutely or chronically with the endotoxin. Immunohistochemistry using antibodies directed against CD4+ or CD8+ also failed to provide anatomical evidence of infiltrating T cells in the CNS of both vehicle- and LPS-treated SOD1G37R mice (data not shown). The robust innate immune reaction is therefore not associated with an adaptive immune response.
Accelerated neurodegeneration in chronically LPS-treated SOD1G37R mice is not attributable to an upregulation in levels of superoxide dismutases 1
Emerging evidence indicates that LPS can induce SOD1, which subsequently might play an important role in mediating the immune response (Marikovsky et al., 2003). Central to the understanding of mechanisms causing accelerated neurodegeneration in chronically LPS-treated SOD1G37R mice is whether LPS upregulates expression of the transgene SOD1G37R. Indeed, transgenic mice having higher levels of SOD1G37R (line 42) exhibit a more aggressive pathology with previous onset (Wong et al., 1995). To address this question, protein levels for both endogenous mouse SOD1 (mSOD1) and human mutant SOD1G37R (hSOD1) were determined in SOD1G37R mice in response to acute or chronic injection of LPS. As shown in Figure 6, mSOD1 and hSOD1 levels in spinal cord and spleen were unchanged 24 hr after acute injection of LPS when compared with animals treated with vehicle solution (Veh). Similar effects were found 48 hr postinjection (data not shown). In addition, SOD1G37R mice chronically challenged with LPS did not display increased levels of both enzymes. These results clearly indicate that toxicity caused by chronic LPS treatment is not caused by enhanced SOD1 expression.
Figure 6.

Acute and chronic administration of LPS in SOD1G37R failed to alter expression of endogenous and transgene SOD1 Six-month-old WT and transgenic SOD1G37R littermates were analyzed for SOD1 levels 24 hr after acute injection of LPS (1 mg/kg of body weight). Expression of both endogenous mSOD1 and hSOD1 remained unaffected in spinal cord (A, lanes 5-8) and spleen (B, lanes 5-8) of SOD1G37R animals in response to saline (Veh) or LPS injection as detected by means of an antibody recognizing both SOD1 proteins. The endotoxin also failed to significantly upregulate mSOD1 expression in WT animals (A, B, lanes 1-4). Similar levels of both SOD1s were found in WT and SOD1G37R mice 48 hr after LPS or Veh administration (data not shown). In SOD1G37R mice that were chronically treated with LPS, expression of mutant SOD1 (detected with an antibody directed against the human transgene) remained stable when compared with littermates treated with Veh (C). Lysates from SOD1G37R line 42 (L42) overexpressing 2- to 2.5-fold the levels of line 29 (L29) were used as comparative control for expression levels. Thus, neither acute nor chronic administration of LPS in SOD1G37R mice affected expression of endogenous and transgene SOD1. Actin and α-tubulin were used as controls for loadings. Quantifications were corrected with levels of actin or tubulin, and performed with the Labscan program. Two to 4 animals were used for each condition. Experiments were repeated from three to eight times. Results represent means ± SD for all of the experiments and animals. See the legend to Figure 2 for definitions of abbreviations.
Discussion
Activation of innate immunity featured by the expression of TLR2 and several other inflammatory genes takes place in transgenic mice expressing SOD1G37R linked to ALS (Nguyen et al., 2001b). The results presented here demonstrate that repeated LPS injections exacerbated the pathogenesis and neuronal death processes of ALS caused by SOD1 mutation. Although SOD1G37R mice responded normally to an acute systemic injection of LPS, chronic stimulation of innate immune response with the bacterial cell wall component led to sustained induction of TLR2 receptor strictly within parenchymal microglial cells. This upregulation of TLR2 was not accompanied by enhanced expression of both endogenous and transgene SOD1 (Fig. 6). Most importantly, the degree of TLR2 induction correlated perfectly with degenerating motor neurons and motor axons, as demonstrated by the overlap between FJB, the in situ hybridization signals for the different immune transcripts, and axonal counts. As a consequence, the chronically LPS-treated SOD1G37R mice exhibited accelerated disease progression, motor axon degeneration, and a life span shortened by 3 weeks. Interestingly, chronically LPS-treated SOD1G37R mice exhibited a more important loss of astrocytes than Veh-treated or nontreated SOD1G37R mice. Thus, the profound innate immune reaction by microglial cells in the degenerating area is clearly detrimental for the cerebral tissue. These results are compatible with a non-cell-autonomous mechanism of motor neuron death in ALS mice (Clement et al., 2003).
The endotoxin LPS is able to activate microglia, because these cells are of myeloid lineage and express CD14 and TLR4 receptors (Lacroix et al., 1998; Laflamme and Rivest, 2001; Lehnardt et al., 2002). These receptors and proinflammatory cytokines are involved in controlling the innate immune response and, potentially, in orchestrating the transfer to an adaptive immune response (Nguyen et al., 2002). In the periphery, cytokines acting directly on macrophages are immediately produced on TLR activation, whereas those that mediate the transition from innate to adaptive immune response appear after a few hours. Binding of pathogen-associated molecular patterns to their respective TLRs leads to the release of IL-12, a cytokine that is involved in the transition from innate to the adaptive immunity. Indeed, macrophage-derived IL-12 stimulates the differentiation of a subset of T lymphocytes (CD4+) into T helper 1 cells that produce IFN-γ (Moser and Murphy, 2000; Nguyen et al., 2002). One of the mechanism by which the deregulated innate immune response may induce the selective killing of motor neurons is by establishing and orchestrating an adaptive immune response (Nguyen et al., 2002; Wyss-Coray and Mucke, 2002). This latter assumption is supported by studies on human patients with neurodegenerative disorders including ALS, reporting the expression of molecules of adaptive immunity, such as major histocompatibility complex (MHC) class I, MHC class II, and human histocompatibility leukocyte antigen in brains, spinal cords, CSF, and sera (Kriz et al., 2002; Nguyen et al., 2002; Schenk, 2002; Wyss-Coray and Mucke, 2002).
Although a robust signal for the gene encoding IL-12 was found in the CNS of LPS treated-SOD1G37R mice, the cerebral tissue of these animals did not exhibit positive signal for IFN-γ transcript. Immunohistochemistry also failed to detect infiltrating CD4+ and CD8+ cells in the brain and spinal cord of SOD1G37R mice treated chronically with vehicle or LPS. These results do not support the concept that deregulation of innate immunity is associated with a specific transfer to an adaptive immune response in ALS mice. Alternatively, defects in the fine interplay between innate and acquired immunity or in the transfer from innate to adaptive response may be toxic for the CNS of the LPS-treated SOD1G37R and SOD1G37R mice. For instance, upregulation of the local adaptive immune response in SOD1G93A mice with Copaxone (glatiramer acetate) vaccination eliminates destructive self-compounds associated with motor neuron death resulting in protection of motor neurons and extension of the life span of the animals. In addition, numerous studies have reported a protective role of the fine-tuned adaptive immune response (Warrington et al., 2000; Bieber et al., 2001; Kriz et al., 2002; Mitsunaga et al., 2002; Nguyen et al., 2002; Schenk, 2002; Wyss-Coray and Mucke, 2002).
The proapoptotic cytokine TNF-α is likely to play a determinant role in this model. The endotoxin LPS is able to trigger transcriptional activation of the gene encoding TNF-α in microglial cells across the CNS, and TNF-α gene expression progressively increased in the spinal cord of SOD1G37R mice (Nadeau and Rivest, 2000; Nguyen et al., 2001b). High TNF-α levels are also found in the CSF of ALS patients (Poloni et al., 2000). This cytokine shares with TLR2 common downstream effector kinases of the NF-κB pathway, which is critical for activating most genes involved in the innate immune response (Nguyen et al., 2002). Recent data also provided evidence supporting the participation of FasL-Fas receptor (FasR) in the selective killing of embryonic motor neurons derived from mutant SOD1 mice (Raoul et al., 2002). The FasR belongs to the superfamily of TNF receptors (TNFRs) that include TNFRI (p55) and TNFRII (p75NGFr) by which the cytokine stimulates signaling events (Locksley et al., 2001). The generation of SOD1 mice lacking TLR2, TNF-α, TNFRI, TNFRII, FasL, and FasR will be essential to clarify the roles of these molecules in the control of the innate immune response in this model of neurodegeneration. In addition, determining the endogenous ligand of TLR2 would help in deciphering the mechanistic details of its actions within activated microglia.
For a long time, the CNS was considered to be a privileged organ from an immunological point of view because of its inability to mount an immune response and process antigens. An accumulating body of evidence indicates that the CNS shows a well organized innate immune reaction in response to systemic bacterial infection and cerebral injury. This response rejuvenates the idea that environmental/immunological challenges might be an etiological factor in sporadic cases of neurodegeneration. It also indicates that primary causes of such neurodegeneration could originate outside the CNS. Indeed, the mechanisms that underlie 90% of ALS cases, sporadic Parkinson's disease, and Alzheimer's disease remain elusive (Nguyen et al., 2002; Wyss-Coray and Mucke, 2002).
Actually, sporadic ALS may result from gene-environment interactions. Factors such as insecticide, heavy metals, and dietary glutamate-fat intake have been proposed as etiological causes for ALS. In addition to lifestyle, viral and bacterial infections may induce motor neuron disease. This assumption is supported by cases of motor dysfunction observed in individuals afflicted by infections. In the same line, viral and bacterial components associated with infiltration of peripheral immune cells in the CNS of sporadic ALS cases have been reported (Nguyen et al., 2002). Nevertheless, because of the low number of cases, epidemiological studies have failed to consistently incriminate any specific environmental factors in this disease. Beyond the toxicity of chronic administration of LPS in the mutant SOD1 mice, our study constitutes a simple example of genetic modulation in mammals by a peripheral bacterial challenge during neurodegeneration. In light of studies reporting viral and bacterial infections in a wide diversity of neurodegenerative disorder including ALS, we provide evidence here that environmental factors and innate immunity can cooperate to influence the course of disease of an inherited neuropathology. Chronic activation of microglial cells and deregulated innate immunity have profound and detrimental effects on neuronal survival. The study of components of innate immune response and environmental factors such as infections therefore may require more attention and revision, especially in sporadic cases of neurodegeneration evolving over months and years.
Footnotes
This work was supported by the Canadian Institutes of Health Research (CIHR) and the Robert Packard Center for ALS Research at Johns Hopkins. M.D.N. was a recipient of a K. M. Hunter-CIHR scholarship and holds a long-term fellowship from the Human Frontier Science Program Organization. J.-P.J. holds CIHR Senior Investigator awards and a chair in neurodegenerative diseases. S.R. is a CIHR scientist and holds a chair in neuroimmunology. The technical help of Pascale Hince, Nathalie Laflamme, and Amélie Lapointe is gratefully acknowledged. We are grateful to Drs. D. L. Price (John Hopkins University, Baltimore, MD) and D. W. Cleveland (University of California, San Diego, La Jolla, CA) for the kind gift of SOD1G37R mice (line 29), Dr. Y. Imai (National Institute of Neuroscience, Kodaira, Tokyo, Japan) for the gift of iba1 antisera, Dr. A. Israel (Institut Pasteur, Paris, France) for the mouse IκBα cDNA, Dr. D. Radzioch (McGill University, Montréal, Québec, Canada) for the plasmid containing the mouse TNF-α cDNA, Dr. I. Campbell (The Scripps Research Institute, La Jolla, CA) for the mouse IFN-γ cDNA, Dr. K. Pahan (University of Nebraska, Lincoln, NE) for the mouse IL-12p40 cDNA, and Dr. Li-Huei Tsai for hosting the last steps of this study at Harvard Medical School.
Correspondence should be addressed to Dr. Serge Rivest, Laboratory of Molecular Endocrinology, Laval University Medical Center Research Center and Department of Anatomy and Physiology, Laval University, 2705 Boulevard Laurier, Sainte-Foy, Québec, G1V 4G2 Canada. E-mail: serge.rivest@crchul.ulaval.ca.
M. D. Nguyen's present address: Harvard Medical School, Department of Pathology, Howard Hughes Medical Institute, Harvard University, Boston, MA 02115.
Copyright © 2004 Society for Neuroscience 0270-6474/04/241340-10$15.00/0
References
- Akira S, Takeda K, Kaisho T (2001) Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol 2: 675-680. [DOI] [PubMed] [Google Scholar]
- Aliprantis AO, Yang RB, Mark MR, Suggett S, Devaux B, Radolf JD, Klimpel GR, Godowski P, Zychlinsky A (1999) Cell activation and apoptosis by bacterial lipoproteins through Toll-like receptor-2. Science 285: 736-739. [DOI] [PubMed] [Google Scholar]
- Aliprantis AO, Yang RB, Weiss DS, Godowski P, Zychlinsky A (2000) The apoptotic signaling pathway activated by Toll-like receptor-2. EMBO J 19: 3325-3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieber AJ, Warrington A, Pease LR, Rodriguez M (2001) Humoral autoimmunity as a mediator of CNS repair. Trends Neurosci 24: S39-S44. [DOI] [PubMed] [Google Scholar]
- Bristol LA, Rothstein JD (1996) Glutamate transporter gene expression in amyotrophic lateral sclerosis motor cortex. Ann Neurol 39: 676-679. [DOI] [PubMed] [Google Scholar]
- Bruijn LI, Becher MW, Lee MK, Anderson KL, Jenkins NA, Copeland NG, Sisodia SS, Rothstein JD, Borchelt DR, Price DL, Cleveland DW (1997) ALS-linked SOD1 mutant G85R mediates damage to astrocytes and promotes rapidly progressive disease with SOD1-containing inclusions. Neuron 18: 327-338. [DOI] [PubMed] [Google Scholar]
- Clement AM, Nguyen MD, Roberts EA, Garcia ML, Boillee S, Rule M, McMahon AP, Doucette W, Siwek D, Ferrante RJ, Brown Jr RH, Julien JP, Goldstein LS, Cleveland DW (2003) Wild-type nonneuronal cells extend survival of SOD1 mutant motor neurons in ALS mice. Science 302: 113-117. [DOI] [PubMed] [Google Scholar]
- Cleveland DW, Rothstein JD (2001) From Charcot to Lou Gehrig: deciphering selective motor neuron death in ALS. Nat Rev Neurosci 2: 806-819. [DOI] [PubMed] [Google Scholar]
- Cudkowicz ME, McKenna-Yasek D, Sapp PE, Chin W, Geller B, Hayden DL, Schoenfeld DA, Hosler BA, Horvitz HR, Brown RH (1997) Epidemiology of mutations in superoxide dismutase in amyotrophic lateral sclerosis. Ann Neurol 41: 210-221. [DOI] [PubMed] [Google Scholar]
- Drachman DB, Rothstein JD (2000) Inhibition of cyclooxygenase-2 protects motor neurons in an organotypic model of amyotrophic lateral sclerosis. Ann Neurol 48: 792-795. [PubMed] [Google Scholar]
- Fridovich I (1986) Superoxide dismutases. Adv Enzymol Relat Areas Mol Biol 58: 61-97. [DOI] [PubMed] [Google Scholar]
- Gong YH, Parsadanian AS, Andreeva A, Snider WD, Elliott JL (2000) Restricted expression of G86R Cu/Zn superoxide dismutase in astrocytes results in astrocytosis but does not cause motoneuron degeneration. J Neurosci 20: 660-665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney ME, Pu H, Chiu AY, Dal Canto MC, Polchow CY, Alexander DD, Caliendo J, Hentati A, Kwon YW, Deng HX, Chen W, Zhai P, Sufit RL, Siddique T (1994) Motor neuron degeneration in mice that express a human Cu, Zn superoxide dismutase mutation. Science 264: 1772-1775. [DOI] [PubMed] [Google Scholar]
- Julien JP (2001) Amyotrophic lateral sclerosis: unfolding the toxicity of the misfolded. Cell 104: 581-591. [DOI] [PubMed] [Google Scholar]
- Kriz J, Nguyen MD, Julien JP (2002) Minocycline slows disease progression in a mouse model of amyotrophic lateral sclerosis. Neurobiol Dis 10: 268-278. [DOI] [PubMed] [Google Scholar]
- Lacroix S, Feinstein D, Rivest S (1998) The bacterial endotoxin lipopolysaccharide has the ability to target the brain in upregulating its membrane CD14 receptor within specific cellular populations. Brain Pathol 8: 625-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laflamme N, Rivest S (2001) Toll-like receptor 4: the missing link of the cerebral innate immune response triggered by circulating Gram-negative bacterial cell wall components. FASEB J 15: 155-163. [DOI] [PubMed] [Google Scholar]
- Laflamme N, Lacroix S, Rivest S (1999) An essential role of interleukin-1β in mediating NF-κB activity and COX-2 transcription in cells of the blood-brain barrier in response to systemic and localized inflammation, but not during endotoxemia. J Neurosci 19: 10923-10930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laflamme N, Soucy G, Rivest S (2001) Circulating cell wall components derived from Gram-negative and not Gram-positive bacteria cause a profound transcriptional activation of the gene encoding Toll-like receptor 2 in the CNS. J Neurochem 70: 648-657. [DOI] [PubMed] [Google Scholar]
- Lehnardt S, Lachance C, Patrizi S, Lefebvre S, Follett PL, Jensen FE, Rosenberg PA, Volpe JJ, Vartanian T (2002) The Toll-like receptor TLR4 is necessary for lipopolysaccharide-induced oligodendrocyte injury in the CNS. J Neurosci 22: 2478-2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lino MM, Schneider C, Caroni P (2002) Accumulation of SOD1 mutants in postnatal motoneurons does not cause motoneuron pathology or motoneuron disease. J Neurosci 22: 4825-4832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locksley RM, Killeen N, Lenardo MJ (2001) The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell 104: 487-501. [DOI] [PubMed] [Google Scholar]
- Marikovsky M, Ziv V, Nevo N, Harris-Cerruti C, Mahler O (2003) Cu/Zn superoxide dismutase plays important role in immune response. J Immunol 170: 2993-3001. [DOI] [PubMed] [Google Scholar]
- Migheli A, Atzori C, Piva R, Tortarolo M, Girelli M, Schiffer D, Bendotti C (1999) Lack of apoptosis in mice with ALS. Nat Med 5: 966-967. [DOI] [PubMed] [Google Scholar]
- Mitsunaga Y, Ciric B, Van Keulen V, Warrington AE, Paz Soldan M, Bieber AJ, Rodriguez M, Pease LR (2002) Direct evidence that a human antibody derived from patient serum can promote myelin repair in a mouse model of chronic-progressive demyelinating disease. FASEB J 16: 1325-1327. [DOI] [PubMed] [Google Scholar]
- Morrison BM, Gordon JW, Ripps ME, Morrison JH (1996) Quantitative immunocytochemical analysis of the spinal cord in G86R superoxide dismutase transgenic mice: neurochemical correlates of selective vulnerability. J Comp Neurol 373: 619-631. [DOI] [PubMed] [Google Scholar]
- Moser M, Murphy KM (2000) Dendritic cell regulation of TH1-TH2 development. Nat Immunol 1: 199-205. [DOI] [PubMed] [Google Scholar]
- Nadeau S, Rivest S (2000) Role of microglial-derived tumor necrosis factor in mediating CD14 transcription and NF-κB activity in the brain during endotoxemia. J Neurosci 20: 3456-3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeau S, Rivest S (2002) Endotoxemia prevents the cerebral inflammatory wave induced by intraparenchymal lipopolysaccharide injection: role of glucocorticoids and CD14. J Immunol 169: 3370-3381. [DOI] [PubMed] [Google Scholar]
- Nguyen MD, Lariviere RC, Julien JP (2000) Reduction of axonal caliber does not alleviate motor neuron disease caused by mutant superoxide dismutase 1. Proc Natl Acad Sci USA 97: 12306-12311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen MD, Lariviere RC, Julien JP (2001a) Deregulation of Cdk5 in a mouse model of ALS: toxicity alleviated by perikaryal neurofilament inclusions. Neuron 30: 135-147. [DOI] [PubMed] [Google Scholar]
- Nguyen MD, Julien JP, Rivest S (2001b) Induction of proinflammatory molecules in mice with amyotrophic lateral sclerosis: no requirement for proapoptotic interleukin-1β in neurodegeneration. Ann Neurol 50: 630-639. [DOI] [PubMed] [Google Scholar]
- Nguyen MD, Julien JP, Rivest S (2002) Innate immunity: the missing link in neuroprotection and neurodegeneration? Nature Rev Neurosci 3: 216-227. [DOI] [PubMed] [Google Scholar]
- Pasinelli P, Houseweart MK, Brown RH, Cleveland DW (2000) Caspase-1 and -3 are sequentially activated in motor neuron death in Cu, Zn superoxide dismutase-mediated familial amyotrophic lateral sclerosis. Proc Natl Acad Sci USA 97: 13901-13906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poloni M, Facchetti D, Mai R, Micheli A, Agnoletti L, Francolini G, Mora G, Camana C, Mazzini L, Bachetti T (2000) Circulating levels of tumour necrosis factor-α and its soluble receptors are increased in the blood of patients with amyotrophic lateral sclerosis. Neurosci Lett 287: 211-214. [DOI] [PubMed] [Google Scholar]
- Pramatarova A, Laganiere J, Roussel J, Brisebois K, Rouleau GA (2001) Neuron-specific expression of mutant superoxide dismutase 1 in transgenic mice does not lead to motor impairment. J Neurosci 21: 3369-3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raoul C, Estevez AG, Nishimune H, Cleveland DW, deLapeyriere O, Henderson CE, Haase G, Pettmann B (2002) Motoneuron death triggered by a specific pathway downstream of Fas: potentiation by ALS-linked SOD1 mutations. Neuron 35: 1067-1083. [DOI] [PubMed] [Google Scholar]
- Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, Donaldson D, Goto J, O'Regan JP, Deng HX, Rahmani Z, Krizus A, McKenna-Yasek D, Cayabyab A, Gaston SM, Berger R, Tanzi RE, Halperin JJ, Herzfeldt B, Van den Bergh R, et al. (1993) Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 362: 59-62. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Van Kammen M, Levey AI, Martin LJ, Kuncl RW (1995) Selective loss of glial glutamate transporter GLT-1 in amyotrophic lateral sclerosis. Ann Neurol 38: 73-84. [DOI] [PubMed] [Google Scholar]
- Roy J, Minotti S, Dong L, Figlewicz DA, Durham HD (1998) Glutamate potentiates the toxicity of mutant Cu/Zn-superoxide dismutase in motor neurons by postsynaptic calcium-dependent mechanisms. J Neurosci 18: 9673-9684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk D (2002) Amyloid-β immunotherapy for Alzheimer's disease: the end of the beginning. Nat Rev Neurosci 3: 824-828. [DOI] [PubMed] [Google Scholar]
- Schmued LC, Albertson C, Slikker Jr W (1997) Fluoro-Jade: a novel fluorochrome for the sensitive and reliable histochemical localization of neuronal degeneration. Brain Res 751: 37-46. [DOI] [PubMed] [Google Scholar]
- Vukosavic S, Stefais L, Jackson-Lewis V, Guegan C, Romero N, Chen C, Dubois-Dauphin M, Przedborski S (2000) Delaying caspase activation by Bcl-2: a clue to disease retardation in a transgenic mouse model of amyotrophic lateral sclerosis. J Neurosci 20: 9119-9125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrington AE, Asakura K, Bieber AJ, Ciric B, Van Keulen V, Kaveri SV, Kyle RA, Pease LR, Rodriguez M (2000) Human monoclonal antibodies reactive to oligodendrocytes promote remyelination in a model of multiple sclerosis. Proc Natl Acad Sci USA 97: 6820-6825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong PC, Pardo CA, Borchelt DR, Lee MK, Copeland NG, Jenkins NA, Sisodia SS, Cleveland DW, Price DL (1995) An adverse property of a familial ALS-linked SOD1 mutation causes motor neuron disease characterized by vacuolar degeneration of mitochondria. Neuron 14: 1105-1116. [DOI] [PubMed] [Google Scholar]
- Wyss-Coray T, Mucke L (2002) Inflammation in neurodegenerative disease—a double-edged sword. Neuron 35: 419-432. [DOI] [PubMed] [Google Scholar]



