Abstract
The present study was designed to elucidate the inflammatory and apoptotic mechanisms of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced neurotoxicity in a model of Parkinson's disease. Our results showed that mutant mice lacking the caspase-11 gene were significantly more resistant to the effects of acute treatment with MPTP than their wild-type mice. Thus, the neurotoxicity of MPTP seems to be mediated by the induction of both mitochondrial dysfunction and free radical generation. Previously, we showed that overexpression of the Apaf-1 dominant-negative inhibitor inhibited the mitochondrial apoptotic cascade in chronic MPTP treatment but not in acute MPTP treatment. The present results indicate that MPTP neurotoxicity may be mediated via activation of the caspase-11 cascade and inflammatory cascade, as well as the mitochondrial apoptotic cascade.
Keywords: MPTP, Parkinson's disease, caspase-11, caspase-1, inflammation, apoptosis
Introduction
Parkinson's disease (PD) is a common neurodegenerative disease, the clinical manifestations of which include resting tremor, rigidity, slowness of movement, and postural instability (Fahn and Przedborski, 2000). Although the mechanism by which these neurons degenerate is still unknown, it is known that inflammation has an important role in the pathogenesis of PD (McGeer et al., 1988; Hunot and Hirsch, 2003). In PD, interleukin (IL)-1β expression is increased in the striatum (Mogi et al., 1994), CSF (Mogi et al., 1996), and mononuclear cells in the peripheral blood (Bessler et al., 1999). Elevation of IL-1β expression has been used as a sensitive and specific marker of caspase-1 activation, because caspase-1 is the primary activator of pro-IL-1β. Neurons of transgenic mice that express the dominant-negative (DN) form of caspase-1 are also shown to be resistant to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) toxicity and cell death induced by middle cerebral artery occlusion (Hara et al., 1997; Klevenyi et al., 1999). The DN form of caspase-1 has also been reported to extend the life span of neurons in murine models of Huntington's disease (Ona et al., 1999) and amyotrophic lateral sclerosis (Friedlander et al., 1997; Li et al., 2000). These findings indicate that caspase-1 may be an important common factor involved as an inflammatory component in several neurodegenerative disorders.
Caspase-11 is induced by stimulation with lipopolysaccharide (LPS) and mediates the activation of caspase-1 by physical interaction (Wang et al., 1998). Recent findings have shown that the substrate specificity of caspase-11 is similar to that of caspase-9 and that caspase-11 can promote the processing of caspase-3, suggesting that caspase-11 acts as the apical caspase in the cascade that activates downstream executioner caspases under pathological conditions (Kang et al., 2000). Therefore, caspase-11 is thought to be crucial in both inflammation and apoptosis under certain pathological conditions (Kang et al., 2002).
The results of our previous study indicated that chronic MPTP treatment induced apoptosis of dopaminergic neurons through activation of the mitochondrial apoptotic cascade (Mochizuki et al., 2001). The present study is an extension of our previous work and was designed to elucidate the inflammatory and apoptotic mechanisms of MPTP neurotoxicity in our model of PD. Specifically, we examined the role of caspase-11 in inflammation and apoptosis immediately after MPTP administration in caspase-11 knock-out -/- mice and in their wild-type mice. The results indicated that MPTP neurotoxicity is mediated through activation of the caspase-11 cascade and inflammatory cascade, as well as the mitochondrial apoptotic cascade. Identification of the relationship between inflammation and apoptosis in MPTP neurotoxicity should allow the design of novel treatments for PD.
Materials and Methods
Animals. C57/Black mice (8-12 weeks old; 19-22 gm; Charles River Laboratories, Tokyo, Japan) were used. Caspase-11-/- mice were generated by gene-targeting techniques and back-crossed for at least five generations (usually eight generations) into the C57/Black background (a gift from Prof. Junying Yuan, Harvard Medical School, Boston, MA) (Wang et al., 1998; Hisahara et al., 2001). Animals were housed five per cage in a temperature-controlled room under a 12 hr light/dark cycle with ad libitum access to food and water. All surgical operations were performed according to rules set forth by the Ethics Committee for the Use of Laboratory Animals at Juntendo University.
MPTP administration. The mice were divided into two groups that received either acute or chronic exposure to MPTP. In the acute exposure group (acute MPTP treatment), the mice received four intraperitoneal injections of MPTP-HCl (20 mg/kg; Sigma, St. Louis, MO) in saline at 2 hr intervals on the same day. In the chronic exposure group (chronic MPTP treatment), the mice received one intraperitoneal injection of MPTP-HCl (30 mg/kg) in saline daily for 5 consecutive days. Control mice received saline according to the same time and dosage schedules. MPTP was handled in accordance with the guidelines reported by Przedborski et al. (2001).
Immunohistochemistry. After anesthetization with pentobarbital, the control and MPTP-treated mice were perfused by the intracardiac route with PBS, followed by 4% paraformaldehyde in PBS. The mice were then decapitated, and their brains were removed and immersed for 48 hr in 4% paraformaldehyde for fixation. Nigral coronal sections (25 μm thick) were then prepared with a cryostat. For all immunostaining, the sections were first rinsed with PBS containing 0.1% Triton-X (PBS-T). They were then immersed in a solution of 0.5% H2O2 for 30 min, followed by incubation with one of the following primary antibodies in PBS-T containing 10% normal serum, overnight at 4°C with continuous shaking: rabbit polyclonal anti-ionized calcium-binding adapter molecule 1 (Iba-1; a gift from Drs. Shinichi Kohsaka and Yoshinori Imai, National Institute of Neuroscience, Tokyo, Japan) (Ito et al., 1998) for microglia, rat monoclonal anti-caspase-11 as Ich-3 (a gift from Prof. J. Yuan) (Wang et al., 1998), rabbit polyclonal anti-tyrosine hydroxylase (TH) (a gift from Prof. Ikuko Nagatsu, Fujita Health University, Aichi, Japan) for dopaminergic neurons, and mouse monoclonal anti-inducible nitric oxide synthase (iNOS) (BD Transduction Laboratories, Lexington, KY). The sections were then washed three times in PBS-T and incubated with a biotinylated secondary antibody (Vector Laboratories, Burlingame, CA) in PBS-T for 2 hr at room temperature. Then, sections were incubated in avidin-biotin peroxidase complex (Vector Laboratories) for 1 hr. A final incubation in DAB was performed for visualization. All the sections were then washed in PBS, mounted on amino propyltriethoxy silan-coated slides, dried, dehydrated in a graded series of ethanol, cleared in xylene, and coverslipped.
For immunofluorescence, rabbit polyclonal anti-Iba-1 for microglia, rat monoclonal anti-caspase-11 (a gift from Prof. J. Yuan), rabbit polyclonal anti-TH for dopaminergic neurons, rabbit polyclonal anti-GFAP for astrocytes (a gift from Dr. Haruhiko Akiyama, Psychiatric Research Institute of Tokyo, Tokyo, Japan), mouse monoclonal anti-O4 for oligodendrocytes (Chemicon, Temecula, CA), and mouse monoclonal anti-iNOS (BD Transduction Laboratories) were used as primary antibodies. FITC- or rhodamine-labeled anti-rabbit, anti-rat, or anti-mouse antibodies were used as secondary antibodies (Vector Laboratories). For caspase-11 immunofluorescence, biotin-conjugated anti-rat antibody (Vector Laboratories) was used as the secondary antibody, followed by streptavidin-FITC (Vector Laboratories). After immunostaining, mounted sections were observed under a confocal laser-scanning microscope (LSM-510; Zeiss, Oberkochen, Germany). For mouse monoclonal anti-iNOS immunostaining, we used Vector M.O.M. (mouse on mouse) kits (Vector Laboratories) to reduce undesired binding of the secondary antibody to significantly endogenous tissue immunoglobulin according to the instructions provided by the manufacturer.
Immunoblotting. The ventral midbrain tissues were lysed in CelLytic Lysis reagent (Sigma) with protease inhibitor (Calbiochem, La Jolla, CA). The protein concentration in the tissue lysates was determined using a BCA protein assay kit (Pierce, Rockford, IL). An equal amount of protein for each sample was separated by SDS-PAGE (15 or 10% gels). After electrophoresis, the proteins were transferred onto polyvinylidene difluoride (PVDF) membranes (Bio-Rad, Hercules, CA). The membranes were blocked with Block-Ace (Yukizirushi, Sapporo, Japan) and sequentially incubated with rabbit polyclonal anti-Iba-1 (a gift from Prof. S. Kohsaka) (Ito et al., 1998), rat monoclonal anti-caspase-11 (a gift from Prof. J. Yuan), and mouse monoclonal anti-α-tubulin antibody (Santa Cruz Biotechnology, Santa Cruz, CA), and then with HRP-conjugated anti-rabbit or anti-mouse antibody (Amersham Biosciences, Piscataway, NJ), followed by ECL detection (Amersham Biosciences). For caspase-11 immunoblotting, biotin-conjugated anti-rat antibody (Vector Laboratories) was used, followed by streptavidin-HRP (Dako, Carpinteria, CA) and ECL detection (Amersham Biosciences).
Total RNA extraction and reverse transcription-PCR. Total RNAs from the ventral midbrain of saline or MPTP-injected C57/Black and caspase-11-/- mice were prepared using an RNeasy kit (Qiagen, Hilden, Germany). The concentration and purity of the RNA preparations were determined by measuring the absorbance at 260 and 280 nm in a spectrophotometer. First-strand cDNA was synthesized from total RNA using a first-strand cDNA synthesis kit (Amersham Biosciences) according to the instructions provided by the manufacturer. The cDNA template was then amplified by PCR using Ex-Taq (TaKaRa, Kyoto, Japan). The nucleotide sequences of the primers were based on published cDNA sequences of IL-1β and iNOS (IL-1β forward, CTGTGTCTTTCCCGTGGACC; IL-1β reverse, CAGCTCATATGGGTCCGACA; iNOS forward, TCACTGGGACAGCACAGAAT; iNOS reverse, TGTGTCTGCAGATGTGCTGA). As an internal control, β-actin cDNA was coamplified using the primers ATCCTGAAAGACCTCTATGC and AACGCAGCTCAGTAACAGTC. After amplification, the products were separated on 1.5% agarose gels containing ethidium bromide. Bands were then visualized under UV transillumination. For IL-1β, iNOS, and β-actin, after electrophoresis the gels were exposed to phosphorimager screens (Fuji Film, Tokyo, Japan).
Measurement of striatal dopamine and DOPAC. HPLC with coulometric electrochemical detection was used to measure striatal levels of dopamine and DOPAC. MPTP- and saline-treated mice were killed 7 d after the last acute MPTP injection. For each mouse, the striatum was dissected, immediately frozen in dry ice, and then stored at -80°C for measurement of dopamine and its metabolites. Dissected striatal tissues were sonicated and centrifuged in chilled 0.1 m perchloric acid (PCA). The supernatants were analyzed for levels of dopamine and DOPAC using a reverse-phase C18 column (150 × 4.6 mm; Tosoh, Tokyo, Japan) and an eight-electrode electrochemical detection system. Concentrations of dopamine and DOPAC are expressed as picomoles per milligram of protein.
Measurement of 1-methyl-4-phenylpyridiniumion levels in the striatum. Caspase-11-/- and C57/Black mice (five mice per time point) were killed at 60 and 120 min after the fourth intraperitoneal injection of MPTP (20 mg/kg) in saline. Tissue samples were sonicated in 10 volumes of 0.1N PCA and 0.1 mm EDTA containing 10 μm 4-phenylpyridine (Aldrich, Milwaukee, WI) as an internal standard. After centrifugation, aliquots of 50 μl of supernatant were injected onto an ODS column (4.6 × 150 mm; Tosoh). The mobile phase was delivered at a flow rate of 1.0 ml/min and consisted of 0.02 m NaH2PO4, 3 mm tetra-n-butylammonium hydrogen sulfate, 0.5 mm 1-heptansulfonic acid sodium salt, and 10% isopropanol and was adjusted to pH 2.5 with H3PO4. AKTA Explore UV-900 (wavelength, 293 nm; Amersham Biosciences) was used for UV detection (Rollema et al., 1985; Mandir et al., 1999).
TH cell counting. To assay changes in the number of dopaminergic neurons in the substantia nigra pars compacta (SNpc), the total numbers of TH- and Nissl-stained SNpc neurons were counted in four mice per group. Immunohistochemistry was performed as described above, but using nigral coronal sections 20 μm in thickness. TH- and Nissl-double-positive neurons were counted in the right and left SNpc of every fourth section throughout the entire extent of the SNpc. For dopaminergic cell counting, sections covering the entire rostrocaudal axis of the mesencephalon were analyzed, which corresponded to five representative mesencephalic planes (plane numbers 321, 335, 343, 351, and 361) (Sidman et al., 1971). Each midbrain section was viewed at low power (100×), and the SNpc was outlined using the following anatomical landmarks (Jackson-Lewis et al., 1995; Przedborski et al., 1996). As the SNpc merges with the ventral tegmental area (VTA) at its medial border in planes 321, 351, and 361 (Sidman et al., 1971), we defined the boundary between the two structures in these planes as a line extending dorsally from the most medial boundary of the cerebral peduncle. In planes 335 and 343 (Sidman et al., 1971), the SNpc and the VTA are separated by the medial lemniscus and the tractus opticus basalis. Then, at random start points, the numbers of TH- and Nissl-double-positive cells were counted manually by researchers blinded to the treatment schedule. To avoid double counting of neurons with unusual shapes, TH- and Nissl-double-positive cells were counted only when their nuclei were optimally visualized. In addition, neurons were distinguished from non-neuronal cells, including glia, on Nissl staining by the exclusion of cells that did not have clearly defined nuclei, cytoplasm, and prominent nucleoli. Although some small neurons were probably excluded by this strategy, these criteria reliably exclude all non-neuronal cells (Mandir et al., 1999).
Statistical analysis. Data are expressed as means ± SEM. Differences between groups were examined for statistical significance using one-way ANOVA, followed by Scheffé's post hoc and Fisher's PLSD tests. The data were analyzed with a computer software system (StatView 5.0; Abacus Concepts, Berkeley, CA). A p value <0.01 denoted a statistically significant difference.
Results
MPTP-induced microglial activation in acute MPTP treatment but not in chronic MPTP treatment
To determine whether MPTP injection was associated with microglial activation, we examined brain sections of wild-type mice injected with acute or chronic MPTP and controls that were injected with saline using anti-Iba-1 antibody, a specific marker for microglia. Immunostaining revealed only a few faintly immunoreactive resting microglial cells in the SN of control mice (Fig. 1D,E). In acute MPTP treatment, numerous strongly immunoreactive Iba-1-positive activated microglial cells were observed 48 hr after the last injection of MPTP (Fig. 1F,G). However, the ventral midbrain expression of Iba-1 in chronic MPTP-treated mice was not activated (Fig. 1H,I), similar to control mice, although TH-positive cells in chronic MPTP treatment were reduced similar to the effect in acute MPTP treatment (Fig. 1 A-C).
Figure 1.

Microglial activation in the SN and striatum. TH immunohistochemistry in the SN of mice after saline injection (A), acute MPTP treatment (B), and chronic MPTP treatment (C) 48 hr after the last injection. Iba-1 immunohistochemistry in the SN (D-I) and striatum (J-O) after saline injection (D, E, J, K), acute MPTP treatment (F, G, L, M), and chronic MPTP treatment (H, I, N, O) 48 hr after the last injection in wild-type mice. Both acute and chronic MPTP injection induced TH-positive dopaminergic neuronal cell death in the SN. However, microglial activation in the SN and striatum was seen in the acute, but not chronic, MPTP treatment groups. Scale bars, 50 μm.
In the striatum, as in the SN, numerous strongly immunoreactive Iba-1-positive activated microglial cells were observed 48 hr after the last acute MPTP injection (Fig. 1L,M). Conversely, in chronic MPTP-injected mice, striatal Iba-1 immunostaining was similar to that of saline-injected wild-type controls (Fig. 1N,O).
Upregulation of IL-1β and caspase-11 in the SN in acute, but not chronic, MPTP treatment
We characterized MPTP-induced microglial activation by measuring the levels of IL-1β, a well documented marker of microglial activation. We confirmed the upregulation of IL-1β in only the SN of acute MPTP-treated, but not chronic MPTP-treated, mice (Fig. 2A). To investigate the expression of caspase-11, which acts upstream of caspase-1, we examined caspase-11 immunoblotting and immunohistochemistry in the SN. Immunoblotting revealed that the level of caspase-11 expression in the ventral midbrain was higher in acute MPTP-injected wild-type mice 12 hr after the last MPTP injection (Fig. 2B). This upregulation of caspase-11 was seen at a very early stage of microglial activation. Next, we investigated the location of caspase-11 expression in neuronal and glial cells after acute MPTP treatment using immunohistochemistry. As shown in Figure 3A, caspase-11 was expressed in very few cells in the SN of saline-injected mice, but numerous glial cells and a few neuronal cells were caspase-11 positive in the SN of acute MPTP-injected mice. Strong immunohistochemical reactivity for caspase-11 was found in the SNpc in which MPTP-induced dopaminergic neuronal cell death occurred (Fig. 3A-C). To identify the cells positive for caspase-11 after acute MPTP injection, the sections were stained with antibodies to neurons and glial cells. This revealed colocalization of caspase-11/Iba-1 and caspase-11/TH, indicating that the cells were microglia and dopaminergic neurons (Fig. 3D-I). However, we did not detect colocalization of GFAP (astrocytes) and O4 (oligodendrocytes) (data not shown).
Figure 2.
Upregulation of IL-1β and caspase-11 in the SN in acute MPTP treatment. A, Expression of IL-1β mRNA in the ventral midbrain 24 hr after the last MPTP injection. B, Immunoblot of caspase-11 and Iba-1 protein expression in the ventral midbrain of acute MPTP-treated wild-type mice at 0, 12, 24, and 48 hr after the last MPTP injection.
Figure 3.

Upregulation of caspase-11 in microglia and dopaminergic neurons in acute MPTP treatment. Caspase-11 immunohistochemistry in the SN of mice 12 hr after the last acute MPTP injection (A). B, C, High magnification of A. D-I, Expression of caspase-11 in microglial cells and dopaminergic neurons at 12 hr after the last acute MPTP injection. D, G, Laser confocal images of immunostaining for caspase-11 in the SNpc. E, Immunostaining for Iba-1 as a marker of microglial cells. H, Immunostaining for TH as a marker of dopaminergic neurons. F, I, Superimposed images. Scale bar, 50 μm.
Caspase-11-/- mice are resistant to acute MPTP neurotoxicity
To further explore the role of caspase-11 in MPTP-induced dopaminergic cell death, we studied the susceptibility of caspase-11-/- mice to MPTP neurotoxicity. Immunohistochemical analysis revealed that TH-positive neurons were still preserved in caspase-11-/- mice 7 d after the last injection in the acute MPTP treatment group (Fig. 4D,H). TH cell counts of SN dopaminergic neurons, defined by TH and Nissl staining, were not significantly different between saline-injected caspase-11-/- mice and their saline-injected wild-type littermates (Fig. 4A,B; Table 1). In wild-type mice, only ∼65% of the TH-positive neurons in the SN survived after MPTP injection. Thus, there was evidence of neuroprotection in the caspase-11-/- mice in acute MPTP treatment (Fig. 4A-D,G,H; Table 1). We also assessed the effects of acute administration of MPTP on striatal dopamine and DOPAC levels in wild-type mice and caspase-11-/- mice. No significant differences were noted in striatal dopamine or DOPAC concentrations after administration of saline in wild-type or caspase-11-/- mice. However, MPTP administration resulted in marked reductions in the concentrations of dopamine and DOPAC in the wild-type mice. In caspase-11-/- mice, these dopamine and DOPAC reductions were significantly attenuated (Fig. 4I) (p < 0.
Figure 4.
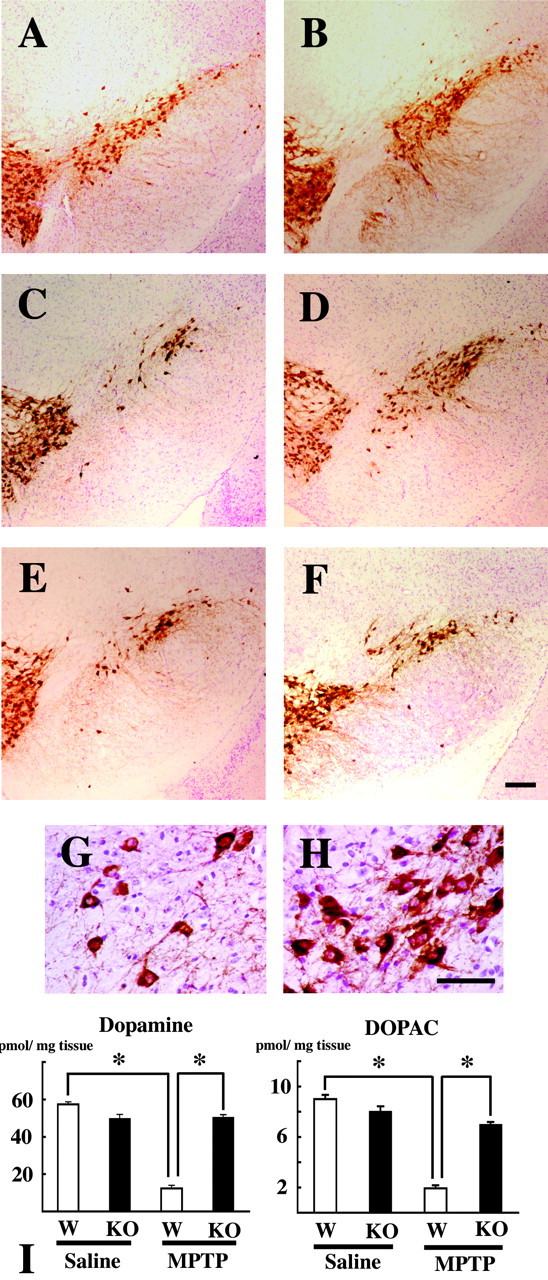
Effects of caspase-11 ablation on MPTP-induced neuronal cell death. A-H, TH and Nissl double staining in the SN 7 d after the last saline injection in wild-type (A) and caspase-11-/- (B) mice, 7 d after the last acute MPTP injection in wild-type (C, G) and caspase-11-/- (D, H) mice, and 21 d after the last chronic MPTP injection in wild-type (E) and caspase-11-/- (F) mice. G, H, Higher magnification of C and D, respectively. Scale bars, 100 μm. I, For all animals, the striatum was dissected for measurement of dopamine and DOPAC 7 d after the last administration of acute MPTP treatment between wild-type (□) and caspase-11-/- (▪) mice. Data are means ± SEM of five mice per group. *p < 0.01; Scheffé's test.
Table 1.
Numbers of TH- and Nissl-double-positive neurons in the SNpc of wild-type and caspase-11-/- mice
|
|
Wild type |
Caspase-11-/- |
||||
|---|---|---|---|---|---|---|
|
|
Saline |
MPTP |
Saline |
MPTP |
||
| Acute MPTP | 8866 ± 148 | 5714 ± 196*# | 9134 ± 314 | 9316 ± 209 | ||
| Chronic MPTP |
9165 ± 401 |
6056 ± 211*
|
9148 ± 316 |
6908 ± 613**
|
||
Data are the means ± SEM of four mice per group (Scheffée's test). The number of dopaminergic neurons represents the total number of TH- and Nissl-double-positive neurons in the SNpc per animal.
p < 0.01 compared with the other groups of saline-treated mice (n = 4 each).
p < 0.05 compared with the other groups of saline-treated mice (n = 4 each).
p < 0.01 compared with acute MPTP-injected caspase-11-/- mice (n = 4 each).
In contrast, in chronic MPTP treatment, TH cell counts of SNpc dopaminergic neurons were not significantly different between caspase-11-/- mice and wild-type mice 21 d after the last injection (p = 0.5644). The counts of dopaminergic neurons in chronic MPTP-injected caspase-11-/- mice were also less than those of saline-injected caspase-11-/- controls (p = 0.0018) (Fig. 4E,F; Table 1).
To confirm that the resistance of caspase-11-/- mice was attributable to the absence of the caspase-11 gene rather than to alternations in the glial production of 1-methyl-4-phenylpyridiniumion (MPP+), we measured striatal MPP+ content at different time intervals after MPTP injection. At no time did the striatal MPP+ content of caspase-11-/- mice differ significantly from that of their wild-type mice (Table 2).
Table 2.
Striatal MPP+ levels in wild-type and caspase-11-/- mice
|
MPP+ levels (nmol/gm striatum) |
|
|
|---|---|---|
|
|
60 min |
120 min |
| Wild-type | 25.08 ± 4.51 | 22.77 ± 1.76 |
| Caspase-11-/-
|
29.48 ± 2.2 |
28.93 ± 3.63 |
Data are the means ± SEM of five mice per group. Striatal MPP+ levels in wild-type and caspase-11-/- mice at 60 and 120 min after the fourth intraperitoneal injection of 20 mg/kg MPTP in saline are shown. There was no significant difference in the MPP+ level between caspase-11-/- and wild-type mice. p > 0.05; t test.
Blockade of caspase-11 attenuated MPTP-induced microglial activation in the acute MPTP treatment group
To determine whether neuroprotection in the caspase-11-/- mice was associated with inhibition of MPTP-induced glial activation, we examined brain sections for expression of Iba-1, a specific marker for microglia. Immunostaining revealed only a few faintly immunoreactive resting microglial cells in the SN of saline-injected caspase-11-/- mice (Fig. 5A). In the wild-type acute MPTP-treated mice, numerous strongly immunoreactive Iba-1-positive activated microglial cells were observed 48 hr after the last injection of MPTP (Fig. 1F,G). Immunoblotting revealed that the level of expression of Iba-1 in the ventral midbrain was significantly higher in acute MPTP-induced mice than in the saline-injected controls (Fig. 5C). Conversely, in caspase-11-/- acute MPTP-treated mice, Iba-1 immunostaining in the SN and ventral midbrain Iba-1 protein expression were similar to those seen in saline-injected controls (Fig. 5B,C).
Figure 5.
Caspase-11 ablation attenuated MPTP-induced microglial activation. Iba-1 immunohistochemistry in the SN of caspase-11-/- mice after saline injection (A) and acute MPTP treatment (B) 48 hr after the last MPTP injection. Scale bars, 50 μm. C, Immunoblotting analysis to detect Iba-1 protein expression in the ventral midbrain of saline- or MPTP-treated wild-type and caspase-11-/- mice. D, Expression of IL-1β mRNA in the ventral midbrain 24 hr after the last MPTP injection in caspase-11-/- mice.
Knock-out of caspase-11 prevents the production of microglia-deleterious mediators
Given the effect of caspase-11 on acute MPTP-induced microglial activation, we examined whether the production of known microglial noxious mediators, such as IL-1β, was also inhibited by knock-out of caspase-11. In saline-injected mice, ventral midbrain expression of IL-1β mRNA was minimal. In comparison, the expression was significantly higher in the acute MPTP-treated wild-type mice (Fig. 2A). However, in caspase-11-/- acute MPTP-treated mice, the ventral midbrain expression of IL-1β mRNA was similar to that seen in saline-injected controls (Fig. 5D).
Blockade of caspase-11 attenuated MPTP-induced upregulation of iNOS in acute MPTP treatment
Previous studies demonstrated that iNOS ablation attenuated MPTP neurotoxicity (Liberatore et al., 1999; Dehmer et al., 2000). To demonstrate whether knock-out of caspase-11 inhibits microglial iNOS production, we compared the expression of iNOS between acute MPTP-treated wild-type and caspase-11-/- mice. As shown in Figure 6, C and H, the expression of iNOS was not induced in acute MPTP-treated caspase-11-/- mice. In wild-type mice, iNOS expression was observed in microglia in the SNpc (Fig. 6B,D). iNOS-positive cells were colocalized with Iba-1-positive cells, indicating that iNOS-expressing cells were microglia (Fig. 6E-G). Acute MPTP treatment produced significant increases in iNOS levels in the ventral midbrain of MPTP-injected wild-type mice (Fig. 6H).
Figure 6.

Accumulation of MPTP-induced iNOS-positive cells in the SN. iNOS immunohistochemistry in the SN of wild-type (A, B) and caspase-11-/- (C) mice after saline injection (A) and acute MPTP treatment (B, C) 48 hr after the last MPTP injection. D, Higher magnification of B. Laser confocal images of iNOS (red; E) and Iba-1 as a marker of microglial cells (green; F) in the SNpc. G, Superimposed image. Scale bar, 50 μm. H, Expression of iNOS mRNA in the ventral midbrain 48 hr after saline injection and after the last acute MPTP injection in wild-type and caspase-11-/- mice.
Discussion
In the present study, we confirmed microglial activation in the SN from 12 hr after acute MPTP treatment in association with inflammatory events. Because these inflammatory events appeared in the SN before the reduction in the number of TH-positive neurons, these events apparently preceded dopaminergic cell death in the SN. Moreover, we also documented the roles of caspases-11 in the inflammatory events in the SN of acute MPTP-injected mice.
Caspase-11 is a member of the family of cysteine proteases initially identified as the mammalian homologs of the Caenorhabditis elegans death gene family (Wang et al., 1996). These proteases act as effector molecules that, when activated, can induce programmed cell death. Several recent studies have demonstrated that the role of caspase-11 is mediated in two ways (Hisahara et al., 2001). One is via the noncell-autonomous effects in which caspase-11 is expressed by infiltrating cells and regulates the production of cytotoxic cytokines in an experimental autoimmune encephalomyelitis model. Caspase-11 is also upregulated through LPS stimulation, a potent inducer of inflammation (Wang et al., 1998). Several previous studies have shown that injection of LPS into the SN of rats results in PD-like changes characterized by microglial activation and dopaminergic cell death (Castano et al., 1998; Gao et al., 2002). The other role of caspase-11 is the cell-autonomous effect, in which caspase-11 in neurons activate caspase-3 and results in cell death via a specific cell death pathway. In our previous experiments, the DN form of caspase-1 overexpresion only in dopaminergic neurons did not inhibit the MPTP neurotoxicity, whereas broad expression of DN caspase-1 in the brain inhibited MPTP toxicity in the SN (Klevenyi et al., 1999; Mochizuki et al., 2001). Therefore, high-level expression of caspase-11 in dopaminergic neurons and the cell-autonomous effect (i.e., the caspase-11 to caspase-3 cascade) may be the predominant cascade involved in the MPTP model. However, we detected only a few caspase-11-positive dopaminergic cells in the SN, and almost all caspase-11-positive cells were located in the microglia in the SN. Therefore, we concluded that the noncell-autonomous effect in which caspase-11 is expressed on microglia is the major effector of dopaminergic neuronal death in the acute MPTP model. Notwithstanding these findings, several studies have shown that acute MPTP treatment results in activation of caspase-3 in dopaminergic neurons (Viswanath et al., 2001). Therefore, we assume that a few caspase-11-positive dopaminergic neurons may die through the apoptotic cascade, via the cell-autonomous effect.
To confirm that the caspase-11 cascade is the major cascade involved in dopaminergic neuronal death in MPTP toxicity, we analyzed caspase-11-/- mice treated with acute or chronic MPTP. In these experiments, we demonstrated that caspase-11-/- mice were resistant to acute MPTP treatment. Immunohistochemical studies showed significant protection against the loss of TH-positive neurons in the SN of acute MPTP-treated caspase-11-/- mice. After acute MPTP treatment, there were significant reductions in the levels of dopamine and DOPAC in the striatum in the control mice, whereas this effect was significantly inhibited in caspase-11-/- mice. At 21 d after the last injection in the chronic MPTP treatment group, the TH-positive neuron count of caspase-11-/- mice was not significantly different from that of wild-type littermates (p = 0.5644). Thus, TH-positive neurons in the SNpc were more resistant to acute MPTP treatment than chronic MPTP treatment in caspase-11-/- mice. These results indicated that caspase-11 acts upstream of the mechanism of dopaminergic neuronal death in acute MPTP-treated mice.
These observations raise the question of the nature of the direct role of the caspase-11 cascade in dopaminergic cell death. The direct apoptotic cascade to caspase-3 from caspase-11 is a candidate for the cell death cascade described above. However, few neuronal cells express caspase-11, indicating that caspase-3 activation from caspase-11 is not a major cascade in our model. In our experiment, caspase-11-/- mice also showed downregulation of iNOS expression after MPTP treatment in the absence of microglial activation. Liberatore et al. (1999) reported that mutant mice lacking the iNOS gene were significantly more resistant to acute MPTP treatment than their wild-type littermates, in an experiment conducted to investigate the mechanisms of protection against inflammation. This is another explanation for protection against dopaminergic cell death in the MPTP model. However, Lee et al. (2001) recently reported that the caspase-11 cascade induced microglial cell death independent of the iNOS cascade. Therefore, iNOS regulation may be a secondary phenomenon mediated through the inhibition of microglial activation.
Tatton and Kish (1997) reported that chronic MPTP treatment induced apoptotic cell death in mice, which is the main apoptotic cascade involved in the associated neuronal injury. We also used an adeno-associated virus (AAV)-derived Apaf-1 DN inhibitor to demonstrate that inhibition of the mitochondrial apoptotic pathway prevented chronic MPTP toxicity. However, the AAV-derived Apaf-1 DN inhibitor did not inhibit dopaminergic neuronal death in acute MPTP toxicity (data not shown). These results indicated that chronic MPTP treatment induced injury of dopaminergic neurons through the mitochondrial apoptotic cascade. Therefore, the primary cascade activated by MPTP neurotoxicity might be different between mice that received acute and chronic MPTP treatment.
In PD, two different pathways of cell death must be considered (i.e., the inflammatory pathway and the apoptotic pathway), because the pathological findings differ between the two; some patients exhibit apoptotic cell death (Mochizuki et al., 1996; Tompkins et al., 1997), whereas others show glial accumulation in the SN (McGeer et al., 1988; Banati et al., 1998). Therefore, successful treatment of PD requires consideration and attention to inhibition of both of these pathways.
Footnotes
This work was supported in part by a High Technology Research Center grant and a grant-in-aid for Scientific Research (B) from the Ministry of Education, Culture, Sports, Science, and Technology, Japan. We are grateful to Prof. Junying Yuan (Harvard Medical School, Boston, MA) for providing caspase-11 knock-out mice and anti-caspase-11 antibody, Drs. Yoshinori Imai and Shinichi Kohsaka (National Institute of Neuroscience, Tokyo, Japan) for providing anti-Iba-1 antibody, Prof. Ikuko Nagatsu (Fujita Health University, Aichi, Japan) for providing anti-tyrosine hydroxylase antibody, Dr. Haruhiko Akiyama (Psychiatric Research Institute of Tokyo, Tokyo, Japan) for providing anti-GFAP antibody, and Prof. Tomoyoshi Kondo (Wakayama Medical University, Wakayama, Japan) for technical advice regarding HPLC.
Correspondence should be addressed to Dr. Hideki Mochizuki, Department of Neurology, Juntendo University School of Medicine, 2-1-1 Hongo, Bunkyo, Tokyo 113-8421, Japan. E-mail: hideki@med.juntendo.ac.jp.
Copyright © 2004 Society for Neuroscience 0270-6474/04/241865-08$15.00/0
References
- Banati RB, Daniel SE, Blunt SB (1998) Glial pathology but absence of apoptotic nigral neurons in long-standing Parkinson's disease. Mov Disord 13: 221-227. [DOI] [PubMed] [Google Scholar]
- Bessler H, Djaldetti R, Salman H, Bergman M, Djaldetti M (1999) IL1 beta, IL-2, IL-6 and TNF-alpha production by peripheral blood mononuclear cells from patients with Parkinson's disease. Biomed Pharmacother 53: 141-145. [DOI] [PubMed] [Google Scholar]
- Castano A, Herrera AJ, Cano J, Machado A (1998) Lipopolysaccharide intranigral injection induces inflammatory reaction and damage in nigrostriatal dopaminergic system. J Neurochem 70: 1584-1592. [DOI] [PubMed] [Google Scholar]
- Dehmer T, Lindenau J, Haid S, Dichgans J, Schulz JB (2000) Deficiency of inducible nitric synthase protects against MPTP toxicity in vivo. J Neurochem 74: 2213-2216. [DOI] [PubMed] [Google Scholar]
- Fahn S, Przedborski S (2000) Parkinsonism. In: Merritt's neurology (Rowland LP, ed), pp 679-693. New York: Lippincott Williams and Wilkins.
- Friedlander RM, Brown RH, Gagliardini V, Wang J, Yuan J (1997) Inhibition of ICE slows ALS in mice. Nature 388: 31. [DOI] [PubMed] [Google Scholar]
- Gao HM, Jiang J, Wilson B, Zhang W, Hong JS, Liu B (2002) Microglial activation-mediated delayed and progressive degeneration of rat nigral dopaminergic neurons: relevance to Parkinson's disease. J Neurochem 81: 1285-1297. [DOI] [PubMed] [Google Scholar]
- Hara H, Fink K, Endres M, Friedlander RM, Gagliardini V, Yuan J, Moskowitz MA (1997) Attenuation of transient focal cerebral ischemic injury in transgenic mice expressing a mutant ICE inhibitory protein. J Cereb Blood Flow Metab 17: 370-375. [DOI] [PubMed] [Google Scholar]
- Hisahara S, Yuan J, Momoi T, Okano H, Miura M (2001) Caspase-11 mediates oligodendrocyte cell death and pathogenesis of autoimmune-mediated demyelination. J Exp Med 193: 111-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunot S, Hirsch EC (2003) Neuroinflammatory processes in Parkinson's disease. Ann Neurol 53: S49-S60. [DOI] [PubMed] [Google Scholar]
- Ito D, Imai Y, Ohsawa K, Nakajima K, Fukuuchi Y, Kohsaka S (1998) Microglia-specific location of a novel calcium binding protein, Iba1. Mol Brain Res 57: 1-9. [DOI] [PubMed] [Google Scholar]
- Jackson-Lewis V, Jakowec M, Burke RE, Przedborski S (1995) Time course and morphology of dopaminergic neuronal death caused by the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Neurodegeneration 4: 257-269. [DOI] [PubMed] [Google Scholar]
- Kang SJ, Wang S, Hara H, Peterson EP, Namura S, Amin-Hanjani S, Huang Z, Srinivasan A, Tomaselli KJ, Thornberry NA, Moskowitz MA, Yuan J (2000) Dual role of caspase-11 in mediating activation of caspase-1 and caspase-3 under pathological conditions. J Cell Biol 149: 613-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SJ, Wang S, Kuida K, Yuan J (2002) Distinct downstream pathways of caspase-11 in regulating apoptosis and cytokine maturation during septic shock. Cell Death Differ 9: 1115-1125. [DOI] [PubMed] [Google Scholar]
- Klevenyi P, Andreassen O, Ferrante RJ, Scheicher Jr JR, Friedlander RM, Beal MF (1999) Transgenic mice expressing a dominant negative mutant interleukin-1beta converting enzyme show resistance to MPTP neurotoxicity. NeuroReport 10: 635-638. [DOI] [PubMed] [Google Scholar]
- Lee J, Hur J, Lee P, Kim JY, Cho N, Kim SY, Kim H, Lee MS, Suk K (2001) Dual role of inflammatory stimuli in activation-induced cell death of mouse microglial cells. Initiation of two separate apoptotic pathways via induction of interferon regulatory factor-1 and caspase-11. J Biol Chem 276: 32956-32965. [DOI] [PubMed] [Google Scholar]
- Li M, Ona VO, Gueogan C, Chen M, Jackson-Lewis V, Andrews LJ, Olszewski AJ, Stieg PE, Lee J, Przedborski S, Friedlander RM (2000) Functional role of caspase-1 and caspase-3 in an ALS transgenic mouse model. Science 288: 335-339. [DOI] [PubMed] [Google Scholar]
- Liberatore GT, Jackson-Lewis V, Vukosavic S, Mandir A, Vila M, Geoffrey-McAuliffe W, Dawson VL, Dawson TD, Prezedborski S (1999) Inducible nitric oxide synthase stimulates dopaminergic neurodegeneration in the MPTP model of Parkinson's disease. Nat Med 5: 1403-1409. [DOI] [PubMed] [Google Scholar]
- Mandir AS, Przedborski S, Jackson-Lewis V, Wang ZQ, Simbulan-Rosental CM, Smulson ME, Hoffman BE, Guastella DB, Dawson VL (1999) Poly (ADP-ribose) polymerase activation mediates 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced parkinsonism. Proc Natl Acad Sci USA 96: 5774-5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeer PL, Itagaki S, Boyes BE, McGeer EG (1988) Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson's and Alzheimer's disease brains. Neurology 38: 1285-1291. [DOI] [PubMed] [Google Scholar]
- Mochizuki H, Goto K, Mori H, Mizuno Y (1996) Histochemical detection of apoptosis in Parkinson's disease. J Neurol Sci 137: 120-123. [DOI] [PubMed] [Google Scholar]
- Mochizuki H, Hayakawa H, Migita M, Shibata M, Tanaka R, Suzuki A, Shimo-Nakanishi Y, Urabe T, Yamada M, Tamayose K, Shimada T, Miura M, Mizuno Y (2001) An AAV-derived Apaf-1 dominant negative inhibitor prevents MPTP toxicity as antiapoptotic gene therapy for Parkinson's disease. Proc Natl Acad Sci USA 98: 10918-10923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogi M, Harada M, Kondo T, Riederer P, Inagaki H, Minami M, Nagatsu T (1994) Interleukin-1 beta, interleukin-6, epidermal growth factor and transforming growth factor-alpha are elevated in the brain from parkinsonian patients. Neurosci Lett 180: 147-150. [DOI] [PubMed] [Google Scholar]
- Mogi M, Harada M, Narabayashi H, Inagaki H, Minami M, Nagatsu T (1996) Interleukin (IL)-1 beta, IL-2, IL-4, IL-6 and transforming growth factor-alpha levels are elevated in ventricular cerebrospinal fluid in juvenile parkinsonism and Parkinson's disease. Neurosci Lett 211: 13-16. [DOI] [PubMed] [Google Scholar]
- Ona VO, Li M, Vonsattel JP, Andrews LJ, Khan SQ, Chung WM, Frey AS, Menon AS, Li XJ, Stieg PE, Yuan J, Penney JB, Young AB, Cha JH, Friedlander RM (1999) Inhibition of caspase-1 slows disease progression in a mouse model of Huntington's disease. Nature 399: 263-267. [DOI] [PubMed] [Google Scholar]
- Przedborski S, Jackson-Lewis V, Yokoyama R, Shibata T, Dawson VL, Dawson TM (1996) Role of neuronal nitric oxide in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced dopaminergic neurotoxicity. Proc Natl Acad Sci USA 93: 4565-4571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przedborski S, Jackson-Lewis V, Naini A, Jakowec M, Petzinger G, Miller R, Akram M (2001) The parkinsonian toxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP): a technical review of its utility and safety. J Neurochem 76: 1265-1274. [DOI] [PubMed] [Google Scholar]
- Rollema H, Fries DS, Vries JB, Mastebroek D, Horn AS (1985) HPLC-assay with electrochemical detection for the neurotoxin MPTP, its metabolite MPP+ and MPTP-analogues in biological samples after purification over sephadex G10. Life Sci 37: 1633-1640. [DOI] [PubMed] [Google Scholar]
- Sidman RL, Angevine Jr JB, Pierce ET (1971) Atlas of the mouse brain and spinal cord. Cambridge, MA: Harvard UP.
- Tatton NA, Kish SJ (1997) In situ detection of apoptotic nuclei in the substantia nigra compacta of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated mice using terminal deoxynuceotidyl transferase labeling and acridine orange staining. Neuroscience 77: 1037-1048. [DOI] [PubMed] [Google Scholar]
- Tompkins MM, Basgall EJ, Zamrini E, William DH (1997) Apoptotic-like changes in Lewy-body-associated disorder and normal aging in substantia nigral neurons. Am J Pathol 150: 119-131. [PMC free article] [PubMed] [Google Scholar]
- Viswanath V, Wu Y, Boonplueang R, Chen S, Stevenson FF, Yantiri F, Yang L, Beal MF, Anderson JK (2001) Caspase-9 activation results in downstream caspase-8 activation and bid cleavage in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine induced Parkinson's disease. J Neurosci 21: 9519-9528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Miura M, Jung Y, Zhu H, Gagliardini V, Shi L, Greenberg AH, Yuan J (1996) Identification and characterization of Ich-3, a member of the interleukin-1beta converting enzyme (ICE)/Ced-3 family and an upstream regulator of ICE. J Biol Chem 271: 20580-20587. [DOI] [PubMed] [Google Scholar]
- Wang S, Miura M, Jung YK, Zhu H, Li E, Yuan J (1998) Murine caspase-11, an ICE-interacting protease, is essential for the activation of ICE. Cell 92: 501-509. [DOI] [PubMed] [Google Scholar]




