Abstract
Human hereditary hyperekplexia (“startle disease”) is a neurological disorder characterized by exaggerated, convulsive movements in response to unexpected stimuli. Molecular genetic studies have shown that this disease is often caused by amino acid substitutions at arginine 271 to glutamine or leucine of the α1 subunit of the inhibitory glycine receptor (GlyR). When exogenously expressed in Xenopus oocytes, agonist responses of mutant α1(R271Q) and α1(R271L) GlyRs show higher EC50 values and lower maximal inducible responses (relative efficacies) compared with oocytes expressing wild-type α1 GlyR subunits. Here, we report that the maximal glycine-induced currents (Imax) of mutant α1(R271Q) and α1(R271L) GlyRs were dramatically potentiated in the presence of the anesthetic propofol (PRO), whereas the Imax of wild-type α1 receptors was not affected. Quantitative analysis of the agonist responses of the isofunctionally substituted α1(R271K) mutant GlyR revealed that saturating concentrations of PRO decreased the EC50 values of both glycine and the partial agonist β-alanine by >10-fold, with relative efficacies increasing by 4- and 16-fold, respectively. Transgenic (tg) mice carrying the α1(R271Q) mutation (tg271Q-300) have both spontaneous and induced tremor episodes that closely resemble the movements of startled hyperekplexic patients. After treatment with subanesthetic doses of PRO, the tg271Q-300 mutant mice showed temporary reflexive and locomotor improvements that made them indistinguishable from wild-type mice. Together, these results demonstrate that the functional and behavioral effects of hyperekplexia mutations can be effectively reversed by drugs that potentiate GlyR responses.
Keywords: anesthesia, glycine, inhibition, IPSP (inhibitory potential), receptor, startle
Introduction
Patients affected by hyperekplexia, a neurological disorder commonly known as “startle disease,” reflexively jump, yell, or extend their arms in response to an unexpected touch or sound (Andermann et al., 1980). In severe cases, a mild stimulus produces stereotypic, convulsive jerks that resemble the effects of strychnine poisoning (Floeter and Hallet, 1993). In infants, the condition is often termed “stiff baby syndrome,” because a light touch to the tip of the nose can cause a muscle rigidity that temporarily halts breathing and may even lead to death caused by apnea (Stewart et al., 2002). Hyperekplexia is hereditary, and genetic linkage studies of affected patients have traced the disease to at least seven naturally occurring point mutations in glycine receptor (GlyR) α1 subunits (Schofield, 2002).
The most commonly observed hyperekplexia mutations are R271L and R271Q, found in the extracellular M2-M3 linker region of the α1 GlyR subunit (Shiang et al., 1993). When heterologously expressed, homo-oligomeric GlyRs containing α1(R271) substitutions show enormous reductions in agonist sensitivity and maximal inducible currents (Langosch et al., 1994). The isofunctional substitution R271K also increases the glycine EC50 value, but to a lesser extent than the natural occurring mutants (Langosch et al., 1994). Similarly, most α1(R271) substitutions increase the EC50 values and reduce the maximal responses of the partial agonists β-alanine and taurine (Laube et al., 1995).
Based on these findings, we recently developed an animal model for hyperekplexia by expressing human α1(R271Q) GlyR subunits in a transgenic (tg) mouse line (Becker et al., 2002). In these tg271Q-300 mice, mutant α1 subunits co-assemble with wild-type (wt) α1 subunits to reduce GlyR agonist sensitivity without decreasing GlyR surface expression (Becker et al., 2002). Behaviorally, tg271Q-300 mice have uncoordinated, spastic movements that lead to significantly slower righting times (>10 sec) than seen with wt mice (<1 sec). In response to a sudden noise or touch, mutant animals show uncontrolled jumps and jerks that closely resemble the startle reflexes of hyperekplexia patients. On more vigorous handling, the mice display tremor episodes that are especially pronounced in the limbs. Because the amplitudes of glycinergic IPSCs recorded from spinal cords of tg271Q-300 mice are threefold smaller than the IPSCs of wt mice, there is now direct evidence that hereditary hyperekplexia is caused by a reduction in GlyR function.
Here, we report that the intravenous anesthetic propofol (PRO) can compensate functionally for reduced GlyR response amplitudes in two model systems for hyperekplexia. In oocytes, PRO increases the maximal responses to agonists in α1(R271Q), α1(R271L), and α1(R271K) GlyRs expressed in oocytes. In tg271Q-300 mice, injections of PRO (15 mg/kg, i.p.) eliminates startle-induced tremor episodes without producing sedation. Despite the clear connection between hyperekplexia and specific GlyR mutations, patients are commonly treated with benzodiazepines (Stewart et al., 2002; Zhou et al., 2002) that indirectly target the disease by enhancing GABA type A receptor (GABAAR) activity. The results presented here suggest that strategies that enhance mutant GlyR responses may provide alternative, rational treatments for hyperekplexic patients.
Materials and Methods
Materials. β-Alanine was obtained from Merck (Darmstadt, Germany); all other chemicals were obtained from Sigma (Deisenhofen, Germany). For oocyte experiments, reagent-grade PRO (Tocris/Biotrend, Cologne, Germany) was dissolved in dimethylsulfoxide to form a stock solution of 1 m PRO before being added to buffer. The final dimethylsulfoxide concentration was <0.05% (v/v), and had no significant effect on glycine-induced currents. For animal experiments, a clinical formulation of 1% (w/v) PRO in soybean emulsion (Fresenius AG; Bad Homburg, Germany) was used.
Expression of mutant cDNAs in oocytes and mice. Human wt α1, α1(R271Q), α1(R271L), and α1(R271K) GlyR subunit cDNAs subcloned into the pRc/cytomegalovirus expression vector (Langosch et al., 1994) were used as templates for in vitro transcription of wt and mutant GlyR subunit cRNAs (mCAP kit; Invitrogen, Groningen, The Netherlands). Xenopus oocytes were prepared and injected with GlyR subunit cRNAs using established techniques (Langosch et al., 1994). For in vivo experiments, the heterozygous tg271Q-300 transgenic mouse line was bred and handled as described previously (Becker et al., 2002).
Electrophysiological recordings. Thirty-six hours after injection, two-electrode voltage clamp recordings were performed on oocytes held at a membrane potential of -70 mV. While determining concentration-effect relationships, the oocyte was continuously perfused with either frog Ringer's solution or frog Ringer's plus PRO. Frog Ringer's solution contained the following (in mm): 115 NaCl, 1 KCl, 1.8 CaCl2, and 10 HEPES, pH 7.2. Agonists (±PRO) were hand-applied to the oocyte chamber with a pipette. Osmotic controls with up to 500 mm sucrose produced no currents. Parameter values for agonist EC50, nH (the Hill coefficient), and ϵ (relative efficacy: Imax,test condition/Imax,Gly, where the test condition is either a partial agonist or an agonist + PRO) were obtained from fitting individual experiments with the following equation: I/Imax,Gly = ϵ*(DnH)/[(EC50nH) + (DnH)], where D represents the concentration of agonist, and Imax,Gly corresponds to the maximal response obtained with glycine in the absence of PRO.
Behavioral analysis of transgenic mice. Drug-naive animals were injected intraperitoneally with 10 or 15 mg/kg doses of a 1% (w/v) emulsion of PRO (Fresenius AG, Bad Homburg, Germany), and righting reflexes and pain sensitivities were monitored at 0, 3, 8, and 30 min after injection as described previously (Becker et al., 2000, 2002). At each time point, righting times were measured three times per side, and the data were pooled with the experimental results from 11 animals. Tremor was recorded as described previously (Becker et al., 2002). Pain sensitivity was determined by a hot-plate assay: animals were placed on a 52°C surface, and the time was measured until a paw lift or paw lick. All procedures were approved by the animal care committee and were in accordance with the German law on animal experimentation.
For all analyses, values are given as means ± SEM. Statistical significance was determined at the p < 0.05 and p < 0.01 levels using Student's t test.
Results
PRO increases the agonist affinities of recombinant wt α1 GlyRs
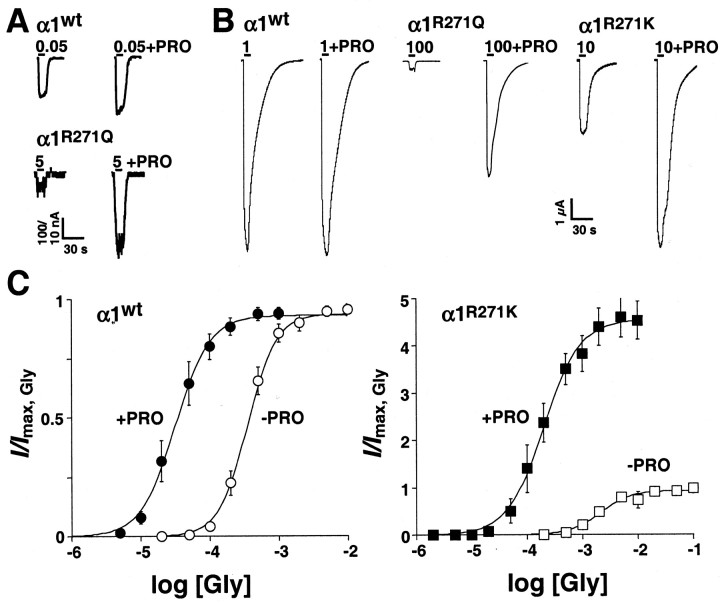
In oocytes expressing wt α1 GlyR subunits, 3-5 sec applications of 1 mm glycine produced maximal peak responses (Imax,Gly) that returned to baseline during agonist washout. Oocytes were allowed to recover for at least 30 sec between drug applications, and were periodically challenged with submaximal test concentrations of glycine to ensure that no response rundown had occurred. After establishing the Imax,Gly for each oocyte, PRO was added to the perfusion medium, and the oocytes were preincubated in the anesthetic for 2 min. This preincubation period with PRO alone caused no change in the holding current (e.g., no GlyR activation), but when co-applied with a submaximal concentration of glycine (i.e., 50 μm), PRO enhanced the response amplitude in a dose-dependent manner. PRO concentrations as low as 1 μm already produced potentiation (Fig. 1A), with maximal potentiation being seen at 0.5 mm PRO (data not shown). In contrast, PRO had no effect on α1 GlyR response amplitudes at saturating concentrations of glycine (Fig. 1B).
Figure 1.
PRO enhances the maximal responses of hyperekplexic mutant GlyRs to glycine. A, Agonist-induced responses from oocytes expressing, wt α1 or α1(R271Q) GlyRs in the absence (left traces) or presence (right traces) of bath-applied PRO (1 μm) in the presence of submaximal glycine concentrations. Bars indicate the duration of glycine application; glycine concentrations are given in mm. B, Agonist-induced responses from oocytes expressing, from left, wt α1, α1(R271Q), or α1(R271K) GlyRs in the absence (left traces) or presence (right traces) of bath-applied PRO (0.5 mm) at saturating glycine concentrations. Bars indicate the duration of glycine application; glycine concentrations are given in mm. C, Dose-response curves for glycine in the absence (open circles) and presence (filled circles) of PRO (0.5 mm) for wt α1 and α1(R271K) GlyRs. Data points represent means from six experiments, normalized to Imax,Gly, obtained with either 1 or 100 mm glycine. Error bars (shown when larger than symbols) indicate SEM.
Figure 1C shows pooled α1 GlyR concentration-effect data for multiple glycine concentrations in the presence and absence of 0.5 mm PRO. When normalized versus the Imax,Gly for each oocyte, PRO potentiation appears as a parallel, leftward shift of the glycine concentration-effect curve. Parameter values from curve fits of the data (Table 1) indicate that 0.5 mm PRO decreased the glycine EC50 value by 10-fold at α1 GlyRs, but had no effect on the maximal current elicited by glycine (relative efficacy ϵ) or Hill slope (nH) value (data not shown).
Table 1.
Agonist response parameters obtained in two-electrode voltage-clamp experiments.
|
Fitted parameter |
α1 GlyRs |
α1(R271K) GlyRs |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glycine (n = 8) |
β-Alanine (n = 5) |
Taurine (n = 3) |
Glycine (n = 5) |
β-Alanine (n = 5) |
||||||||||||||
|
|
−PRO |
+PRO |
−PRO |
+PRO |
−PRO |
+PRO |
−PRO |
+PRO |
−PRO |
+PRO |
||||||||
| EC50 (mm) | 0.39 ± 0.05 | 0.037 ± 0.008* | 2.02 ± 0.21 | 0.09 ± 0.02* | 4.51 ± 1.96 | 0.14 ± 0.01 | 2.05 ± 0.07 | 0.18 ± 0.05* | 5.77 ± 1.38 | 0.57 ± 0.16* | ||||||||
| EC50 ratio | 10.5 | 22.5 | 32.0 | 11.6 | 10.2 | |||||||||||||
| ϵ | 0.95 ± 0.01 | 0.93 ± 0.02 | 0.67 ± 0.05 | 0.92 ± 0.02* | 0.23 ± 0.04 | 0.90 ± 0.04* | 0.96 ± 0.01 | 4.1 ± 0.46* | 0.12 ± 0.05 | 1.9 ± 0.47* | ||||||||
| ϵ ratio |
1.0 |
|
1.4 |
|
3.9 |
|
4.3 |
|
15.8 |
|
||||||||
EC50 and ϵ values were averaged from curve fits of individual experiments and are expressed as means ± SEM. *Different by p < 0.05 from the agonist-alone condition. +PRO, Responses recorded after 2 min of preincubation with 0.5 mm PRO.
PRO restores glycine relative efficacy at hyperekplexic GlyRs
Previously, we have shown that glycine has increased EC50 values and smaller maximal responses at GlyRs carrying α1(R271) substitutions (Langosch et al., 1994). In contrast with wt α1 GlyRs, high concentrations of glycine (≥100 mm) are required to achieve the Imax,Gly at α1(R271Q) and α1(R271L) GlyRs (Langosch et al., 1994). Similar to wt α1 GlyRs, low concentrations of PRO (1 μm) were able to potentiate glycine-induced responses at α1(R271Q) GlyRs at low receptor occupancy (<EC10) (Fig. 1A). However, in contrast with α1 wt GlyRs, on addition of 0.5 mm PRO to the perfusion medium 100 mm glycine produced responses at α1(R271Q) and α1(R271L) GlyRs that were 26- and 19-fold larger than the Imax,Gly, respectively (Fig. 1B) (data not shown). Figure 1B also shows that in the absence of PRO, 100 mm glycine elicited only low-amplitude responses in α1(R271Q) GlyRs, a fact that impedes precise dose-response analysis. Therefore, to analyze in more detail the effect of PRO at GlyRs carrying a mutation at position 271, we chose the isofunctional mutation α1(R271K), which displays less-pronounced effects on agonist affinity and maximal inducible currents (Langosch et al., 1994). After the addition of 0.5 mm PRO to the perfusion medium, 10 mm glycine produced maximal responses at α1(R271K) GlyRs that also were significantly larger than the Imax,Gly (Fig. 1B), indicating that PRO has qualitatively similar effects at all α1(R271) GlyR mutants. In different concentration-effect experiments (Fig. 1C), PRO was found to potentiate the responses of α1(R271K) GlyRs to both submaximal and maximal concentrations of glycine. Fitted parameter values (Table 1) indicate that PRO decreased the glycine EC50 value by 12-fold, increased glycine ϵ values by 4-fold, and had no significant effect on glycine nH values at α1(R271K) GlyRs. Together, these results indicate that hyperekplexic mutant GlyRs have small maximal responses, but are potently potentiated by PRO at saturating concentrations of glycine.
PRO improves partial agonist relative efficacies at wt and hyperekplexic GlyRs
At GABAARs, PRO has been demonstrated to increase the ϵ value of a partial agonist (O'Shea et al., 2000). We therefore tested whether PRO might also improve the ϵ values of partial agonists at GlyRs. Neither β-alanine nor taurine produces detectable currents at α1(R271L) or α1(R271Q) GlyRs (Laube et al., 1995). At α1 (R271K) GlyRs, saturating concentrations of β-alanine generated 12 ± 5% of the Imax,Gly (Table 1). At these receptors, the addition of 0.5 mm PRO resulted in responses to 10 mm β-alanine that were larger than the Imax,Gly without PRO and comparable with the maximal inducible glycine currents in the presence of PRO (Fig. 2A). On average, β-alanine plus 0.5 mm PRO elicited responses that were roughly twofold larger than the Imax,Gly (Fig. 2B). This marked enhancement was reflected by a 16-fold increase in the ϵ value for β-alanine and a 10-fold decrease in the EC50 (Table 1). At wt GlyRs, saturating concentrations of β-alanine and taurine produced responses that typically reached 67 ± 5 and 23 ± 4%, respectively, of the Imax,Gly (Table 1) (Laube et al., 1995). PRO (0.5 mm) potentiated the maximal responses of both partial agonists to nearly the Imax,Gly. When comparing the effects of PRO on a range of β-alanine and taurine concentrations, PRO produced nonparallel, leftward shifts of both concentration effect curves (Fig. 2B). Table 1 summarizes these results: PRO decreased the EC50 values of β-alanine (23-fold) and taurine (32-fold), increased the ϵ values (1.4- and 4-fold, respectively), and significantly increased the nH values of both agonists at α1 GlyRs (data not shown). Therefore, assuming that the small responses seen with glycine, β-alanine, and taurine at R271-substituted GlyRs (Table 1) contribute to the small glycinergic IPSC amplitudes observed in spinal cord slices from tg271Q-300 mice (Becker et al., 2002), we wondered whether PRO could be used to improve the neuromotor deficits of this animal hyperekplexia model.
Figure 2.
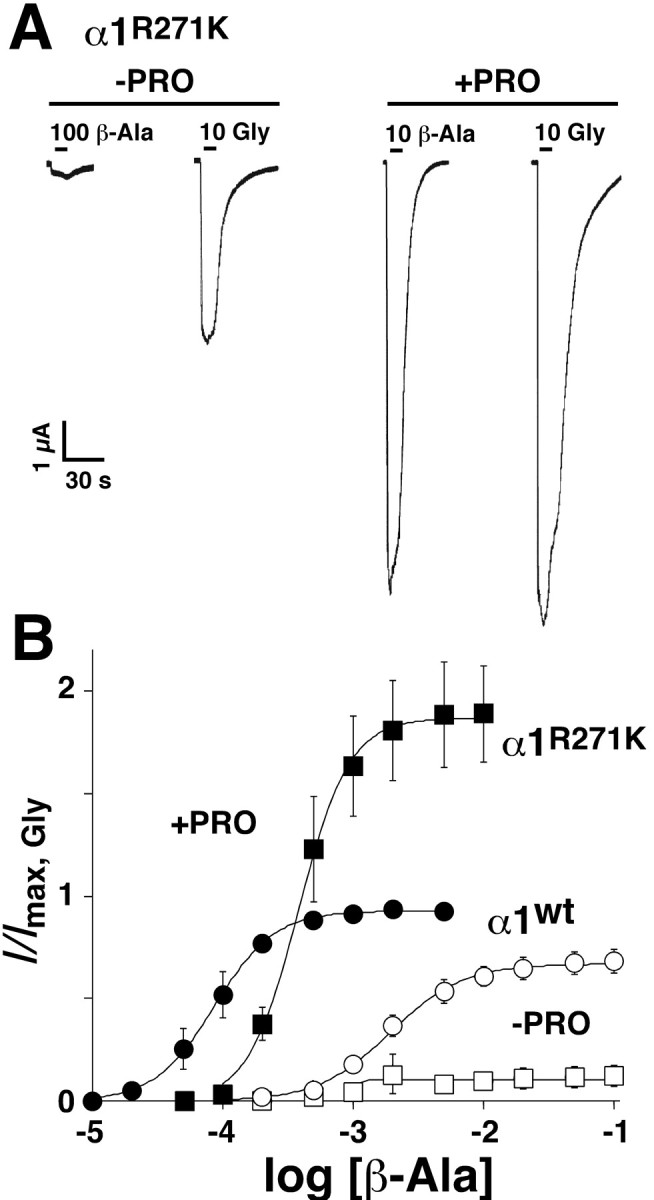
PRO enhances the maximal responses of wt and hyperekplexic α1 GlyRs to partial agonists. A, β-Alanine and glycine-induced responses recorded from oocytes expressing α1(R271K) GlyRs in the absence (-PRO) or presence (+PRO) of bath-applied PRO (0.5 mm). Bars indicate the duration of agonist application; agonist concentrations are given in mm. B, Pooled dose-response curves for β-alanine for α1 wt (circles) and α1(R271K) (squares) GlyRs in the absence (open symbols) and presence (filled symbols) of 0.5 mm PRO, respectively. Data are normalized to Imax,Gly obtained with saturating glycine concentrations (n = 5).
PRO reduces the startle responses and tremor episodes of tg271Q-300 mice
Compared with wt mice, transgenic hyperekplexic mice expressing human α1(R271Q) GlyRs show exaggerated jerks and jumps after a sudden noise or touch (Becker et al., 2002). Although a high inter-animal variability of these startle responses makes the behavior difficult to standardize and quantify, the differences in neuromotor performance between the two mouse lines are obvious to a trained observer. Notably, several minutes after an injection of 1% PRO (15 mg/kg, i.p.), the abnormal startle reflex of R271Q mice disappeared, and responses became indistinguishable from those of wt mice. This effect was transient, and the exaggerated startle response returned within 30 min after injection.
To further quantify the “anti-hyperekplexic” properties of PRO, we also measured its effect on handling-induced convulsive episodes. Tg271Q-300 animals show both spontaneous and handling-induced muscle tremors that occur with the same periodic frequency (∼25-30 Hz) as in startled hyperekplexic patients (Stayer and Meinck, 1998; Becker et al., 2002). Here, we conducted similar studies on tg271Q-300 mice both before and after an injection of PRO (15 mg/kg, i.p.). Figure 3A shows segments from a typical experiment, obtained by suspending a mouse from an electromechanical transducer by the tail. After an unexpected stimulus or handling, untreated tg271Q-300 mice often experienced prolonged tremor episodes (Fig. 3A). In mutants treated with PRO, the same stimulus produced no such tremors (Fig. 3A). Between tremor episodes, both untreated and PRO-treated transgenic mice resembled wt mice whose muscle tone was not changed on PRO injection (Fig. 3A). Like relief from the exaggerated startle response, the effect of PRO on tg271Q-300 mice was temporary, and the hyperekplexic phenotype returned within 30 min after injection.
Figure 3.
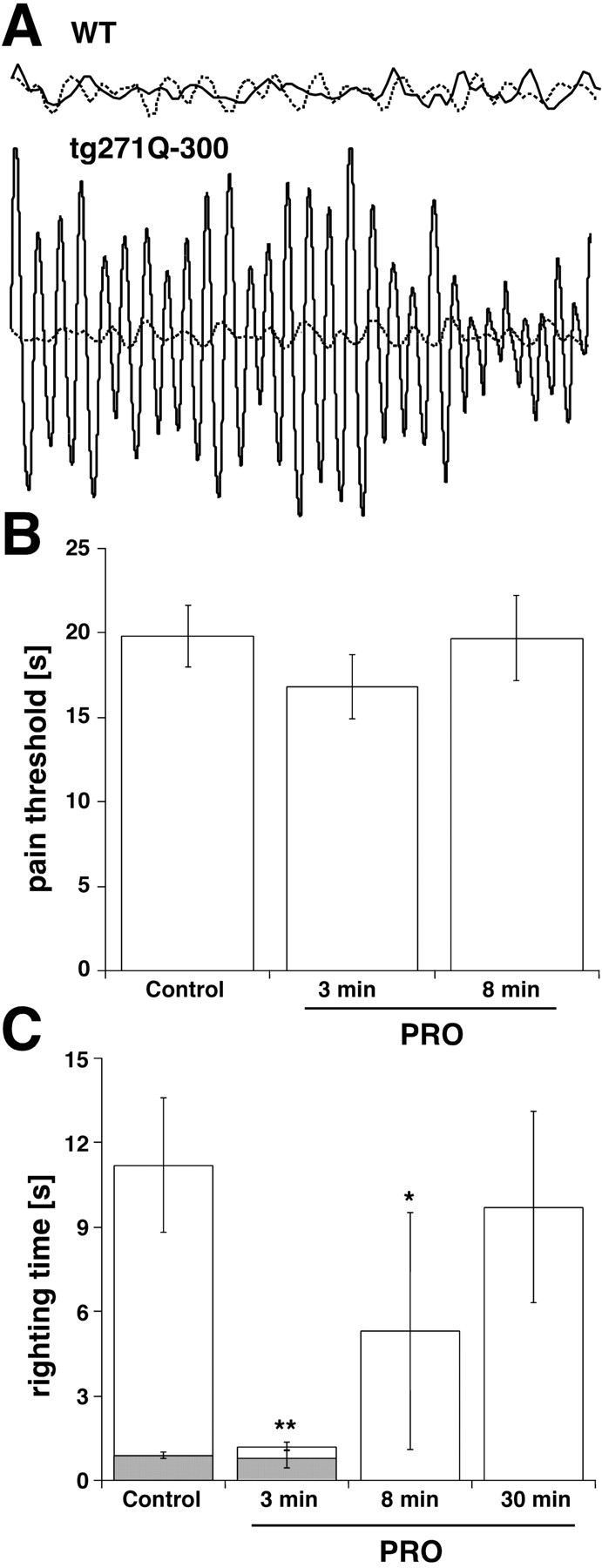
Behavioral effects of PRO in tg271Q-300 mice. A, PRO quiets tremor in hyperekplexic mice. Shown are typical 1 sec recordings obtained from wt and tg271Q-300 mice suspended by the tail from a mechanical transducer. In the upper traces, wt mice show no tremor activity before (solid line) and 3 min after (dotted line) a 15 mg/kg, i.p., injection of PRO. In the lower traces, untreated tg271Q-300 mice show high-frequency (25-30 Hz) tremors (solid line) that disappear 3 min after treatment with PRO. B, Acute pain responses to thermal stimuli in the hot-plate test. No significant differences in the latencies of withdrawal from thermal stimuli were observed between PRO-treated and control mice 3 and 8 min, after the intraperitoneal injection of 15 mg/kg PRO when using the hot-plate test (n = 11). C, PRO shortens the righting times of tg271-300 mice (white columns) without affecting those of wt mice (gray columns). Righting time latencies represent the time required for achieving a standing position either before (Control), or 3, 8, or 30 min after 15 mg/kg, i.p., injections of PRO. *p < 0.05 versus controls; **p < 0.01 versus controls (n = 11).
To exclude the possibility that the beneficial effects of PRO were attributable to oversedation, we tested tg271Q-300 mice using two classic behavioral assays for anesthesia. We checked for differences in pain sensitivity by placing the animal's foot on a hot plate and measuring the time required for a paw lift or paw lick. Before injection, mice showed 20 sec paw-lift latencies (19.6 ± 5.2 sec) that remained unchanged 3 min (16.3 ± 4.9 sec) and 8 min (19.5 ± 9.4 sec) after a 15 mg/kg dose of PRO (Fig. 3B). Second, the time required for the mice to right themselves after being rolled onto their backs was measured before and after the injection of PRO. As outlined below (Fig. 3C), instead of producing an increase in righting time associated with sedation, PRO significantly decreased the righting time of tg271Q-300 mice. In summary, the doses of PRO used here to treat hyperekplexia were too low to induce anesthesia, and produced no observable sedation in tg271Q-300 mice.
PRO restores normal locomotor coordination to tg271Q-300 mice
In addition to the exaggerated startle responses and muscle rigidity that are diagnostic features of hyperekplexia in humans, tg271Q-300 mice display two motor defects that distinguish them from wt mice: poorly coordinated movements and significantly slower righting times. The first is easily monitored as an uncoordinated walking behavior, and a clasping of the hindfeet when mutant animals are picked up by their tails (Becker et al., 2002). Surprisingly, tail-suspended mutants treated with PRO balanced their position by spreading out the legs and showed wt-like walking behavior (data not shown). Furthermore, compared with wt mice that normally achieve a standing position within less than a second after being rolled onto their backs, untreated tg271Q-300 mice showed mean righting times of ∼10 sec (Fig. 3C). Notably, 3 min after a 15 mg/kg injection of PRO, mutant mice showed a mean righting time of ≈1 sec, similar to those found for wt mice (Fig. 3C). After reaching an upright position, PRO-treated mice walked and moved normally. These improvements were temporary: 8 min after injection, the righting times had increased to 5 sec, and by 30 min the slow righting behavior had returned. To summarize, hyperekplexic mice treated acutely with PRO showed locomotor abilities that closely resembled those of untreated wt animals.
Discussion
Previous studies of GlyR allosteric modulation typically have focused on the enhancement of responses to submaximal concentrations of glycine (for review, see Laube et al., 2002). However, different reports suggest that saturating concentrations of agonist may produce better approximations of electrically evoked IPSCs in spinal cord and brainstem (Clements, 1996; Laube, 2002). Therefore, the ability of PRO to enhance the maximal responses of GlyRs carrying α1(R271) hyperekplexia mutations has relevance for glycinergic inhibition in hyperekplexic animals and patients.
As summarized in Table 1, PRO produced a fourfold increase in the ϵ value of glycine at α1(R271K) GlyRs, an improvement that should fully restore the threefold reductions observed in the glycinergic IPSC amplitudes of tg271Q-300 mice (Becker et al., 2002). Of the agonist-receptor combinations tested, PRO produced the largest ϵ ratio (16-fold) for β-alanine at α1(R271K) GlyRs, but PRO may produce even larger enhancements of glycine responses at α1(R271L) and α1(R271Q) GlyRs that could not be analyzed precisely because of the rather small responses seen in the absence of PRO. The ability of PRO to increase agonist ϵ values was not limited to mutants, because PRO converted β-alanine and taurine into full agonists at wt α1 GlyRs. The published concentrations of PRO producing GlyR potentiation show some variability, but modulating effects have consistently been seen at ≥1 μm (Belelli et al., 1999; Hadipour-Jahromy and Daniels, 2003).
PRO also increases the apparent agonist affinities of all GlyRs tested. For example, PRO decreased the EC50 value of glycine at α1(R271K) GlyRs by 12-fold, producing mutant GlyRs with EC50 values lower than of unmodulated wt α1 GlyRs (Table 1). PRO had similar effects on β-alanine elicited currents, and increased its EC50 value by 10-fold. Given that PRO had the largest effects on the ϵ value of β-alanine at α1(R271K) GlyRs, we expected to see a larger increase in the EC50 of β-alanine. In fact, there appeared to be a loose correlation between the EC50 values and ϵ ratios for most agonist-receptor combinations in the presence of PRO (Table 1). Our relatively slow agonist application speed and the decision to normalize all responses to Imax,Gly clearly influenced this relationship, but it is tempting to propose a mechanistic link between these two parameters (Colquhoun, 1998). We are currently investigating the kinetic properties of α1(R271K) GlyRs to determine if PRO influences agonist binding and/or channel gating.
In agreement with previous reports on wt GlyRs (Belelli et al., 1999; Hadipour-Jahromy and Daniels, 2003), low micromolar concentrations of PRO were effective in increasing the maximal response of the α1(R271Q) GlyR to glycine in oocytes (Fig. 1A). We therefore reasoned that PRO might also increase the amplitudes of IPSCs in mice expressing a human α1(R271Q) GlyR transgene. Tg271Q-300 mice show a number of behavioral phenotypes that make them an excellent model for hereditary hyperekplexia in humans (Becker et al., 2002). In response to sudden stimuli, mutant mice show exaggerated startle responses, muscle rigidity, and tremor episodes that mimic the behavior seen with affected patients (Stayer and Meinck, 1998). In addition to these reflexive deficits, mutant mice also show a reduction in muscle coordination associated with human hyperekplexia.
Shortly after an injection of PRO (15 mg/kg, i.p.), tg271Q-300 mice showed temporary abatements in tremor episodes and significantly improved righting responses that were consistent with a reduction in the hyperekplexia phenotype. More subjectively, the startle responses and locomotor coordination of PRO-treated mutant mice were indistinguishable from the behavior of wt mice. Presumably because of the improvement in muscle coordination, PRO-treated mice also showed normalization of righting times. Despite the well-known ability of PRO to enhance GABAAR activity (Belelli et al., 1999; Jurd et al., 2003), causing anesthesia on higher-dose intraperitoneal injection (e.g., 140 mg/kg; Irifune et al., 2003), the low doses of PRO used in this study did not appear to have anesthetic or sedative effects. Because a large reduction for GABAAR-mediated inhibitory transmission was also found in the tg271Q-300 mice (Becker et al., 2002) and mice carrying a point mutation in the β3 subunit of the GABAAR display a reduction in the loss of the righting reflex after PRO treatment (Jurd et al., 2003), a pleiotropic effect of subanesthetic PRO concentrations at both GABAARs and GlyRs cannot be excluded. However, because PRO concentrations as low as 1 μm potentiated GlyR responses in oocytes, PRO clearly can act as an in vivo potentiator of mutant GlyRs. Future behavioral tests will be required to determine the effects of PRO on higher cognitive functions (e.g., learning and memory, attention) and brain metabolism in mice, but PRO appears to be a promising lead compound for the rational design of drugs that target human hyperekplexia.
Footnotes
This work was supported by a fellowship from the Max-Planck-Gesellschaft (S.M.O.), Bundesministerium für Bildung und Forschung (H.W., H.B.), Deutsche Forschungsgemeinschaft Grant LA 1086/2-2 (B.L.), Fritz Thyssen Stiftung (H.W.), and Fonds der Chemischen Industrie (H.B.).
Correspondence should be addressed to Dr. Bodo Laube, Max-Planck-Institut für Hirnforschung, Abteilung Neurochemie, Deutschordentrasse 46, 60528 Frankfurt am Main, Germany. E-mail: laube@mpih-frankfurt.mpg.de.
S. M. O'Shea's present address: Auerbach Laboratory, Department of Physiology and Biophysics, 309 Cary Hall, State University of New York at Buffalo, Buffalo, NY 14214.
Copyright © 2004 Society for Neuroscience 0270-6474/04/242322-06$15.00/0
References
- Andermann F, Keene DL, Andermann E, Quesney LF (1980) Startle disease or hyperekplexia: future delineation of the syndrome. Brain 103: 985-997. [DOI] [PubMed] [Google Scholar]
- Becker L, Hartenstein B, Schenkel J, Kuhse J, Betz H, Weiher H (2000) Transient neuromotor phenotype in transgenic spastic mice expressing low levels of glycine receptor beta-subunit: an animal model of startle disease. Eur J Neurosci 12: 27-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker L, von Wegerer J, Schenkel J, Zeilhofer HU, Swandulla D, Weiher H (2002) Disease-specific human glycine receptor α1 subunit causes hyperekplexia phenotype and impaired glycine- and GABAA-receptor transmission in transgenic mice. J Neurosci 22: 2505-2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belelli D, Pistis M, Peters JA, Lambert JJ (1999) The interaction of general anaesthetics and neurosteroids with GABAA and glycine receptors. Neurochem Int 34: 447-452. [DOI] [PubMed] [Google Scholar]
- Clements JD (1996) Transmitter timecourse in the synaptic cleft: its role in central synaptic function. Trends Neurosci 19: 163-171. [DOI] [PubMed] [Google Scholar]
- Colquhoun D (1998) Binding, gating, affinity and efficacy: the interpretation of structure-activity relationships for agonists and of the effects of mutating receptors. Br J Pharmacol 125: 924-947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floeter MK, Hallet M (1993) Glycine receptors: a startling connection. Nat Genet 5: 319-320. [DOI] [PubMed] [Google Scholar]
- Hadipour-Jahromy M, Daniels S (2003) Binary combinations of propofol and barbiturates on human α1 glycine receptors expressed in Xenopus oocytes. Eur J Pharmacol 477: 81-86. [DOI] [PubMed] [Google Scholar]
- Irifune M, Takarada T, Shimizu Y, Endo C, Katayama S, Dohi T, Kawahara M (2003) Propofol-induced anaesthesia in mice is mediated by gamma-aminobutyric acid-A and excitatory amino acid receptors. Anaesth Analg 97: 424-429. [DOI] [PubMed] [Google Scholar]
- Jurd R, Arras M, Lambert S, Drexler B, Siegwart R, Crestani F, Zaugg M, Vogt KE, Ledermann B, Antkowiak B, Rudolph U (2003) General anesthetic actions in vivo strongly attenuated by a point mutation in the GABA(A) receptor beta3 subunit. FASEB J 17: 250-252. [DOI] [PubMed] [Google Scholar]
- Langosch D, Laube B, Rundstrom N, Schmieden V, Bormann J, Betz H (1994) Decreased agonist affinity and chloride conductance of mutant glycine receptors associated with human hereditary hyperekplexia. EMBO J 13: 4223-4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laube B (2002) Potentiation of inhibitory glycinergic neurotransmission by Zn2+: a synergistic interplay between presynaptic P2X2 and postsynaptic glycine receptors. Eur J Neurosci 16: 1025-1036. [DOI] [PubMed] [Google Scholar]
- Laube B, Langosch D, Betz H, Schmieden V (1995) Hyperekplexia mutations of the glycine receptor unmask the inhibitory subsite for beta-amino-acids. NeuroReport 6: 897-900. [DOI] [PubMed] [Google Scholar]
- Laube B, Maksay G, Schemm R, Betz H (2002) Modulation of glycine receptor function: a novel approach for therapeutic intervention at inhibitory synapses? Trends Pharmacol Sci 23: 519-527. [DOI] [PubMed] [Google Scholar]
- O'Shea SM, Wong LC, Harrison NL (2000) Propofol increases agonist efficacy at the GABA(A) receptor. Brain Res 852: 344-348. [DOI] [PubMed] [Google Scholar]
- Schofield PR (2002) The role of glycine and glycine receptors in myoclonus and startle syndromes. Adv Neurol 89: 263-274. [PubMed] [Google Scholar]
- Shiang R, Ryan SG, Zhu YZ, Hahn AF, O'Connell P, Wasmuth JJ (1993) Mutations in the alpha1 subunit of the inhibitory glycine receptor cause the dominant neurologic disorder, hyperekplexia. Nat Genet 5: 351-358. [DOI] [PubMed] [Google Scholar]
- Stayer C, Meinck HM (1998) Stiff-man syndrome: an overview. Neurologia 13: 83-88. [PubMed] [Google Scholar]
- Stewart WA, Wood EP, Gordon KE, Camfield PR (2002) Successful treatment of severe infantile hyperekplexia with low-dose clobazam. J Child Neurol 17: 154-156. [DOI] [PubMed] [Google Scholar]
- Zhou L, Chillag KL, Nigro MA (2002) Hyperekplexia: a treatable neurogenetic disease. Brain Dev 24: 669-674. [DOI] [PubMed] [Google Scholar]