Abstract
Neurogenesis from endogenous progenitor cells in the adult forebrain ventricular wall may be induced by the local viral overexpression of cognate neuronal differentiation agents, in particular BDNF. Here, we show that the overexpression of noggin, by acting to inhibit glial differentiation by subependymal progenitor cells, can potentiate adenoviral BDNF-mediated recruitment of new neurons to the adult rat neostriatum. The new neurons survive at least 2 months after their genesis in the subependymal zone and are recruited primarily as GABAergic DARPP-32+ medium spiny neurons in the caudate-putamen. The new medium spiny neurons successfully project to the globus pallidus, their usual developmental target, extending processes over several millimeters of the normal adult striatum. Thus, concurrent suppression of subependymal glial differentiation and promotion of neuronal differentiation can mobilize endogenous subependymal progenitor cells to achieve substantial neuronal addition to otherwise non-neurogenic regions of the adult brain.
Keywords: Huntington's disease, neurogenesis, subependymal zone, stem cells, gene therapy, regeneration
Introduction
Neural progenitor cells persist throughout the subependymal zone (SZ) of the adult mammalian brain (Smart, 1961; Boulder Committee, 1970; Doetsch et al., 1997, 1999; Goldman, 1998; Chiasson et al., 1999). Induction of division or directed differentiation of these progenitor cells via exogenous factor application can serve as a mechanism by which to repopulate the damaged brain. For example, SZ progenitor cells proliferate in response to epidermal growth factor (EGF) and FGF, both in vitro and in vivo (Vescovi et al., 1993; Palmer et al., 1995; Craig et al., 1996; Kuhn et al., 1997). In addition, these progenitor cells respond to BDNF with an increase in neuronal maturation and survival in vitro (Ahmed et al., 1995; Kirschenbaum and Goldman, 1995; Pincus et al., 1998). In vivo, BDNF delivery to SZ progenitors increases neuronal recruitment to the olfactory bulb, a typically neurogenic site in the adult rodent brain (Zigova et al., 1998). Importantly, intraventricular BDNF also elicits ectopic neuronal addition to the neostriatum, in which most of the newly added neurons develop as GABAergic, cabindin+/DARPP-32+ medium spiny neurons (Benraiss et al., 2001).
BDNF promotes the neuronal differentiation and survival of newly generated SZ daughter cells (Ahmed et al., 1995; Kirschenbaum and Goldman, 1995; Lindholm et al., 1996; Leventhal et al., 1999; Louissaint et al., 2002). In the absence of BDNF, these same daughter cells might otherwise generate glia or, alternatively, may undergo apoptotic death (Morshead and van der Kooy, 1992). Together, these observations raised the possibility that SZ progenitors might be driven to neuronal phenotype not only by promoting neuronal differentiation but also by suppressing glial differentiation. This possibility was testable, in that a number of humoral glial differentiation agents and their inhibitors have been identified. In particular, the bone morphogenetic proteins (BMPs) drive neural progenitors to glial fate in the adult and late fetal rodent brain (Gross et al., 1996; Lim et al., 2000). In the adult forebrain SZ, both the BMPs and their receptors are abundant (Mehler et al., 1995; Gross et al., 1996), in accordance with the gliogenic bias of most of the adult subependyma. Therefore, we reasoned that overexpression of noggin, a soluble BMP inhibitor (Zimmerman et al., 1996), might suppress astroglial differentiation of SZ cells and thereby promote their neuronal differentiation. Indeed, noggin expression has been shown to persist in some regions of ongoing neurogenesis in the adult rodent brain (Lim et al., 2000). Furthermore, we postulated that by suppressing glial differentiation, noggin might make more SZ daughter cells responsive to neuronal instruction by BDNF, thereby providing a concurrently permissive and instructive environment for neurogenesis.
To this end, we overexpressed noggin in the ventricular wall of adult rats and found that noggin substantially suppressed gliogenesis throughout the lateral ventricular subependyma. More remarkably, coinjection of AdBDNF and AdNoggin resulted in a dramatic increase in neuronal addition to the striatum, a typically non-neurogenic region, and did so to a much greater extent than did AdBDNF injection alone (Benraiss et al., 2001). The newly generated striatal neurons expressed the antigenic phenotype of medium spiny neurons of the caudate-putamen. Over the 2 month period following their genesis, FluoroGold (FG) backfills of AdBDNF-treated animals revealed that these cells extended fibers to their usual target, the globus pallidus. These new pallidal projection neurons survived and integrated, indicating that induced neurogenesis from resident progenitor cells might achieve the growth or regrowth of multinuclear circuits in the adult forebrain. These data indicate that noggin and BDNF cooperate to induce medium spiny neuronal recruitment from resident progenitor cells in the adult forebrain.
Materials and Methods
Adenovirus construction. Replication-incompetent AdBDNF (AdCMV:BDNF:IRES:GFP) and AdNull (AdCMV:GFP) were constructed and raised as described previously (Benraiss et al., 2001). Using the same previously described techniques (Graham and Prevec, 1991; Bajocchi, 1993), a ΔE1 type 5 adenovirus was made to encode, under cytomegalovirus (CMV) control, human noggin, from which the B2 heparin-binding domain had been deleted (Economides et al., 2000; Paine-Saunders et al., 2002).
Experimental design and stereotaxic injection. Twenty adult (12-13 weeks old; 255-270 gm) Sprague Dawley rats received bilateral 3 μl intraventricular injections of saline (n = 4), AdNoggin (n = 4), AdNull (n = 4), AdBDNF (n = 4), or AdBDNF/AdNoggin together (n = 4) (Paxinos and Watson, 1986; Benraiss et al., 2001). Another group of nine rats received bilateral 1 μl intrastriatal injections of AdNull (n = 3), AdBDNF (n = 3), or AdBDNF/AdNoggin together (n = 3) [anterior posterior (AP), +1.2; mediolateral (ML), ±2.1; dorsoventral (DV), -4.4] (Paxinos and Watson, 1986). Viruses were tittered to 2.5 × 1010 pfu/ml. All rats were then given 18 daily intraperitoneal injections of bromodeoxyuridine (BrdU; 100 mg/kg). On day 20 or 56, animals were killed, CSF was withdrawn, and brains were processed as described previously (Benraiss et al., 2001). Additionally, a cohort of animals that received intraventricular AdBDNF or AdNull (n = 3/group), followed by 18 daily injections of BrdU, were injected secondarily on day 42 with 1 μl of 2% FG (Biotium, Hayward, CA), injected bilaterally into the globus pallidus (from bregma: AP, -2.3 mm; ML, 4 mm; from dura: DV, -6.8) (Paxinos and Watson, 1986). The injected animals were killed 1 week later and perfused with 2% paraformaldehyde, and their brains were processed for BrdU immunolabeling, followed by confocal identification of BrdU+/FG-tagged striatal cells. Although FG did not diffuse significantly from its injection site, as evidenced by the persistence of a discrete focus of FG+ cells in the ventral portion of the globus pallidus, we ensured that the distance between the pallidal injection site and the region of BrdU+/FG+ cell identification within the neostriatum was at least 2 mm.
Another cohort of animals received bilateral intraventricular injections of 3 μl of saline (n = 9) or AdBDNF (n = 9). All rats were given seven daily intraperitoneal injections of BrdU and were killed on day 9, 16, or 23 after viral injection (n = 3 rats/time point/treatment). Their brains were then cut and stained for BrdU and doublecortin (Dcx) (see below). Distances of Dcx+/BrdU+ cells from the ventricular wall were determined using Bioquant (Nashville, TN) software.
ELISA. Noggin levels in the CSF were determined with a two-site ELISA using two rat-derived anti-human noggin monoclonal antibodies. Coat antibody RP57-16, which binds at the N-terminal half of noggin, was coated at 2 μg/ml in PBS, followed by an incubation with 10 mg/ml BSA solution in PBS for 2 hr to block any free protein-binding sites. On the ELISA plate, serial twofold dilutions of a known concentration of noggin protein were performed in triplicate, and CSF samples were added. One hundred microliters of a 1 μg/ml solution of the anti-noggin biotinylated monoclonal RP57-21-biotin, which binds to the cysteine-rich domain of noggin, were added to each well. To detect RP57-21-biotin, the plates were then incubated with a streptavidin-HRP conjugate (Invitrogen, Gaithersburg, MD) at a 1:5000 dilution for 1 hr.
In situ hybridization. Noggin RNA probes were made from pBlue.mNOG. This DNA plasmid was linearized with BamH1 for the sense control probe or with NotI for the antisense probe and in vitro transcribed with T3 RNA polymerase for the sense probe and T7 RNA polymerase for the antisense probe. The probes were radioactively labeled with S35-UTP. In situ hybridization was then performed on 15 μm sagittal brain sections of AdNoggin (n = 3) or AdNull (n = 3)-injected animals, as described (Valenzuela et al., 1993).
Immunochemistry and quantification. Sagittal 15 μm sections were stained for BrdU and neuronal/glial markers using double-immunofluorescence, as described previously (Benraiss et al., 2001; Nunes et al., 2003). Briefly, sections were denatured in 2N HCl at 37°C and stained for BrdU using a anti-BrdU rat IgG antibody (1:200; Serotec, Raleigh, NC), followed by a goat anti-rat Alexa-488 2° antibody (Molecular Probes, Eugene, OR). The sections were then washed and stained for one of the following: βIII-tubulin (monoclonal antibody TuJ1 at 1:400; a gift from Dr. A. Frankfurter, University of Virginia, Charlottesville, VA) (Lee et al., 1990), MAP2 (clone AP-20; 1:50; Sigma, St. Louis, MO), NeuN (mouse anti-NeuN; 1:400; Chemicon, Temecula, CA), DARPP-32 (1:5000; a gift from Dr. H. Hemmings, Cornell University, New York, NY) (Ivkovic and Ehrlich, 1999), GAD67 (rabbit anti-GAD67; 1:100; Chemicon), Dcx (rabbit antisera; 1:100; a gift from Dr. Chris Walsh, Harvard, Boston, MA), GFAP (rabbit anti-GFAP; 1:400; Sigma), and S100β (clone SH-B1; 1:400; Sigma). Immunostaining for green fluorescent protein (GFP) was performed using mouse anti-GFP (1:400; BD Biosciences-Clontech, Palo Alto, CA). All secondary antibodies (Molecular Probes) were preabsorbed to avoid nonspecific staining.
Striatal BrdU+ cells counts were done on six 15 μm sagittal sections per animal; every 16th section was analyzed at 240 μm intervals. The striatal region sampled began with the first appearance of striatal fascicles and proceeded 1.2 mm laterally. Section volumes were measured using Bioquant software. The number of striatal BrdU+/βIII-tubulin+ cells/mm3 in a given section was determined by multiplying the percentage of BrdU+ cells determined by confocal microscopy to express βIII-tubulin (see below) by the mean number of BrdU+ cells/mm3. Comparisons of the numbers of βIII-tubulin/BrdU+ cells/mm3 in saline-, AdNull-, AdNoggin-, AdBDNF-, and AdNoggin/AdBDNF-injected animals were performed using ANOVA, followed by post hoc Boneferroni t tests. Otherwise, pairwise comparisons were performed using two-sample Student's t test. All statistical analyses were performed using GB-Stat (Dynamic Microsystems, Silver Spring, MD).
Confocal imaging. In sections double-stained for BrdU and βIII-tubulin, Dcx, NeuN, MAP2, DARPP-32, GAD67, S100β, or GFAP, or in FG-injected animals immunostained for BrdU alone, single striatal BrdU+ cells were imaged using a Fluoview confocal microscope (Olympus, Lake Success, NY), images were acquired using an argon-krypton laser and were analyzed as described previously (Benraiss et al., 2001).
Results
Intraventricular AdNoggin yielded high-level noggin expression by the ventricular wall
Noggin overexpression was achieved via transduction of the ventricular wall with a recombinant adenovirus encoding human noggin. To ensure widespread local availability of the vector-encoded noggin, we used a noggin mutein, from which the heparin binding site was deleted to permit sustained solubility of secreted noggin (Paine-Saunders et al., 2002). To assess the production of noggin by this vector, we first used in situ hybridization to visualize noggin expression in both normal controls and in rats treated with adenoviral noggin. To this end, six adult rats were given injections of either AdNoggin or AdNull and killed 3 weeks later. Their brains were sectioned sagitally at 15 μm and subjected to in situ hybridization for noggin mRNA, using S35-UTP-labeled probes for mouse noggin. Endogenous noggin expression was seen in the septum and olfactory bulb as well as the dentate gyrus and CA1-3 of the hippocampus in both the AdNoggin- and AdNull-injected animals. Significant periventricular noggin, however, was only seen in animals that received AdNoggin (Fig. 1).
Figure 1.
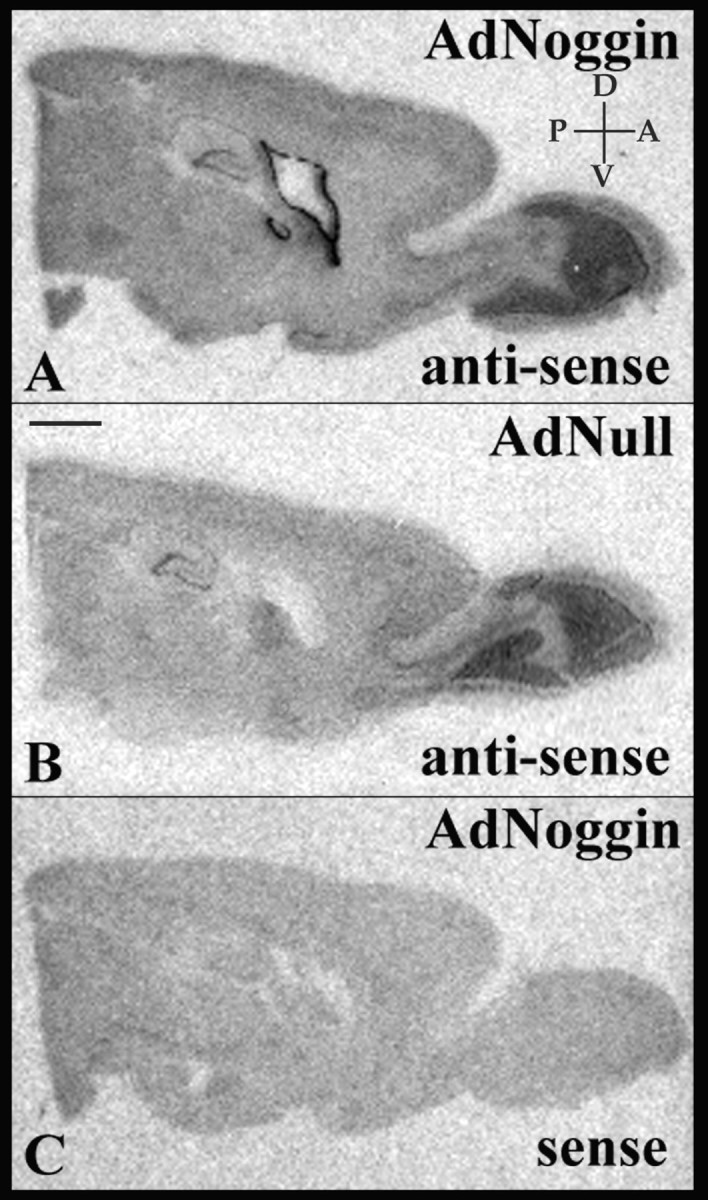
Intraventricular AdNoggin targets viral transgene overexpression to the ventricular wall. A-C, Sagittal sections of AdNoggin-injected (A, C) or AdNull-injected (B) rat brains were treated with antisense (A, B) or sense (C) probes for mouse noggin. Expression of the viral transgene is limited to the wall of the lateral ventricle. D, Dorsal; V, ventral; A, anterior; P, posterior. Scale bar, 2 mm.
We next asked whether AdNoggin injection raised noggin protein levels in the CSF. To this end, ventricular CSF was withdrawn by cisterna magna puncture 3 weeks after virus injection. In the AdNoggin-injected animals (n = 3), ELISA revealed that CSF noggin averaged 52.0 ± 4.5 ng/ml or 42.9 ± 7.2 μg/g protein (mean ± SE). In contrast, noggin was undetectable in the CSF of AdNull-injected animals (n = 3; p < 0.0001; two-sample Student's t test).
Noggin overexpression suppressed gliogenesis by the adult SZ
We had previously noted that AdBDNF induced ectopic neuronal addition to the neostriatum (Benraiss et al., 2001). Unlike the anterior subventricular zone and rostral migratory stream, the precursor cells of which are mostly committed to neuronal phenotype (Menezes et al., 1995), the striatal subependyma includes a mixed population of multipotential stem cells and committed progenitors and, hence, generates both neuronal and glial daughter cells (Doetsch et al., 1997; Goldman and Luskin, 1998). On this basis, we postulated that if noggin could suppress glial differentiation of striatal SZ daughter cells, then AdNoggin infection might be associated with a lower incidence of BrdU-labeled subependymal astrocytes, as defined by their coexpression of GFAP+ and S100β+. To this end, we scored the incidence of both BrdU+, GFAP+/BrdU+, and S100β+/BrdU+ cells in the subependyma (Fig. 2A,B) of AdNull- and AdNoggin-treated rats, at their respective 3 week survival points. We used both of these markers because whereas GFAP is an established marker of differentiated astrocytes, it is frequently lacking from immature or reactive astroglia. In contrast, S100β is more ubiquitously expressed by astrocytes, whether reactive or quiescent (Zhang and McKanna, 1997).
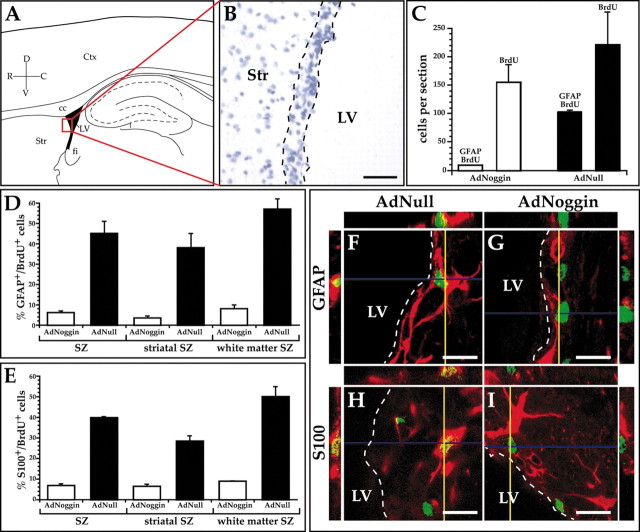
Figure 2.
AdNoggin suppresses glial production by the adult SZ. A, Schematic of sagittal rat brain section indicating the SZ scored for the incidence of BrdU+/GFAP+-S100β+ cells. The area in the red box is shown in B as a cresyl violet-stained section, with the SZ border indicated by the dashed lines. Scale bar, 64 μm. C, Despite a stable incidence of total SZ BrdU labeling between AdNoggin- and AdNull-injected animals, AdNoggin-injected rats exhibited substantial lower frequencies of BrdU+/GFAP+ subependymal astrocytes. D, E, In these graphs, the subependyma,which has a roughly triangular profile in the sagittal section, was divided into the striatal and callosal/fimbrial white matter segments, each of which was scored independently for GFAP+/BrdU+ (D) and S100β+/BrdU+ (E) cells. In all regions of the lateral ventricular lining, AdNoggin-injected rats exhibited substantial lower frequencies of BrdU+/GFAP+ subependymal astrocytes. F-I, Orthogonal views of subependymal BrdU+ cells (green), as viewed in the xz and yz planes, verify colabeling with GFAP (F, G) and S100β(H, I) (red). Fewer BrdU+ cells were colabeled with the astrocytic markers in AdNoggin-injected rats (G, I) than in AdNull-injected animals (F, H). Scale bars, 16 μm. D, dorsal; V, ventral; R, rostral; C, caudal; Ctx, cortex; cc, corpus callosum; LV, lateral ventricle; fi, fimbria; Str, striatum; SZ, subependymal zone.
We found that in the forebrain, the AdNoggin-injected rats exhibited substantial suppression of subependymal glial differentiation, as manifested by 80-90% reductions in both GFAP+/BrdU+ and S100β+/BrdU+ subependymal astrocytes. Specifically, whereas 46.8 ± 5.8% of BrdU+ SZ cells in AdNull-injected animals expressed GFAP, only 5.7 ± 1.1% of BrdU+ SZ cells were GFAP+ in AdNoggin-injected animals (Fig. 2D,F,G). Furthermore, whereas 39.9 ± 0.5% of BrdU+ SZ cells in AdNull-injected animals expressed glial S100β, only 6.8 ± 0.9% of BrdU+ SZ cells were S100β+ in AdNoggin-injected animals (Fig. 2E,H,I) (p < 0.001 for each marker; two-sample t test). The marked reduction in the proportion of GFAP+ and S100β+ cells among BrdU-incorporating SZ cells was noted although the incidence of SZ BrdU labeling was not significantly different between AdNoggin and AdNull-injected rats (Fig. 2C). These data indicated that noggin overexpression reduced the production of subependymal astrocytes.
We next asked whether progenitors of the striatal SZ, which overlies subcortical gray matter, and those of the fimbrial and callosal SZ, which appose white matter, respond analogously to AdNoggin. For this purpose, sagittal sections were taken that spanned the mediolateral extent of the striatum (L1.9-L3.9); these were divided into rostral (striatal) and dorsal/caudal (white matter) segments (Paxinos and Watson, 1986). In the white matter SZ of AdNull-injected animals, 64.9 ± 8.1% of all BrdU+ cells were GFAP+, and 49.9 ± 4.9% expressed S100β. In contrast, in AdNoggin-injected animals, only 7.6 ± 0.4% of BrdU+ cells were GFAP+ and 8.8 ± 0.3% S100β+ (p < 0.001 for each marker; two-sample t test) (Figs. 2D,E). Similarly, in the AdNull striatal SZ, 38.0 ± 4.7% of BrdU+ cells were GFAP+ and 28.3 ± 2.6% S100β+ in AdNull-injected animals, whereas in their AdNoggin-injected counterparts only 3.1 ± 1.9% were GFAP+ and 6.4 ± 1.1% S100β+ (p < 0.001) (Fig. 2D,E). These data confirmed that AdNoggin significantly decreased the proportion of GFAP+ and S100β+ astrocytes arising from SZ progenitor cells and did so in the subependymal layers adjacent to both gray and white matter.
AdNoggin and AdBDNF collaborated to increase neuronal addition to the neostriatum
We next postulated that by virtue of its suppression of gliogenesis, noggin overexpression might make more subependymal progenitor cells responsive to neuronal instruction by BDNF. As such, noggin and BDNF co-overexpression might be expected to provide a concurrently permissive and instructive environment for striatal neurogenesis. We tested this postulate in 250 gm Sprague Dawley rats that were given injections once intraventricularly with both AdBDNF and AdNoggin, then given injections daily for 3 weeks thereafter with BrdU. The animals were then killed, their brains were cut sagitally at 15 μm, and the sections were double-immunostained for βIII-tubulin and BrdU and scored as described in Materials and Methods (Benraiss et al., 2001). The neuronal phenotype was confirmed by coimunolabeling for the neuronal markers Dcx, NeuN, and MAP-2 (Fig. 3D,F-H).
Figure 3.
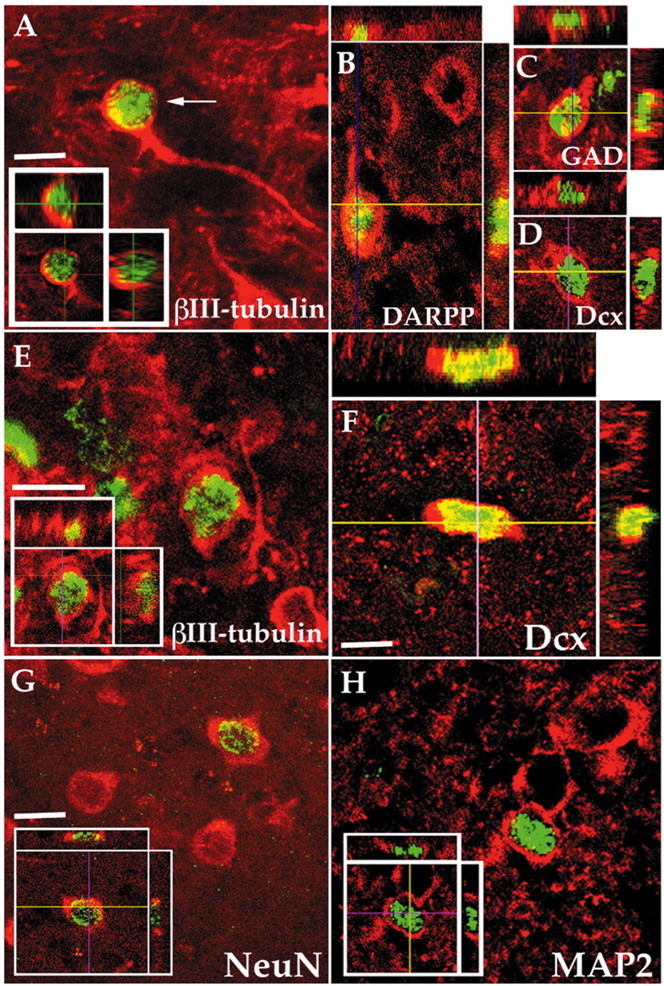
AdNoggin/AdBDNF-induced striatal neurons developed medium spiny phenotype. A, E, Composite images of newly generated neurons (arrows), double-immunostained for β-III tubulin+ (red) and BrdU+ (green), in the striata of rats injected either 20 d (A) or 56 d (E) before with AdBDNF and AdNoggin. The insets show orthogonal views. B-D, AdBDNF/AdNoggin-induced striatal cells double-immunostained for DARPP-32 (B), GAD67(C), or Dcx (D) (red) and BrdU (green), all shown with corresponding orthogonal views. F-H, AdBDNF/AdNoggin-induced striatal neurons (arrows), double-immunostained for BrdU (green) and Dcx (F), NeuN (G), or MAP-2 (H) (red). Scale bar, 12 μm.
We found that AdNoggin-treated animals had an average of 1436 ± 43 BrdU+ cells/mm3 in their striata, no different from the AdNull-treated animals that exhibited an average of 1455 ± 25 BrdU+ striatal cells/mm3. Among a randomly chosen sample of 750 BrdU+ striatal cells located in sections (n = 24) selected at random from four AdNoggin-treated brains, 18 cells were confirmed as labeled for both BrdU and βIII-tubulin by confocal imaging. By this criterion, 2.4 ± 0.1% (18/750 × 100) of the BrdU+ cells in the AdNoggin-treated rat striata, or 34 cells/mm3, were neurons. This was not significantly different from AdNull-injected animals, in which 2.1 ± 0.2% of the BrdU+ cells expressed βIII-tubulin. Thus, noggin alone had no significant effect on striatal neuronal addition (Table 1; Fig. 4).
Table 1.
Total scored BrdU+ cells/mm3 and percentage of β-III tubulin+/BrdU+ cells as a function of treatment
|
Treatment |
A. BrdU+ cells/mm3 |
B. β-III tubulin+/BrdU+ (%) |
C. BrdU+/β-III tubulin+ cells/mm3 |
|---|---|---|---|
| Saline | 1198 ± 45 | 0 | 0 |
| AdNull | 1455 ± 25 | 2.1 ± 0.2 | 31 ± 3 |
| AdNoggin | 1436 ± 43 | 2.4 ± 0.1 | 34 ± 2 |
| AdBDNF | 1573 ± 23 | 8.3 ± 0.2 | 131 ± 2 |
| AdBDNF/noggin |
2036 ± 374 |
13.4 ± 0.3 |
273 ± 46 |
The counts (column A) and their derived percentages (column B) were obtained from six striatal sections derived from each of four animals per treatment group. The data were then used to predict the number of BrdU+/β-III tubulin cells/mm3 for each animal; the per animal values were averaged to give the numbers reported in column C.
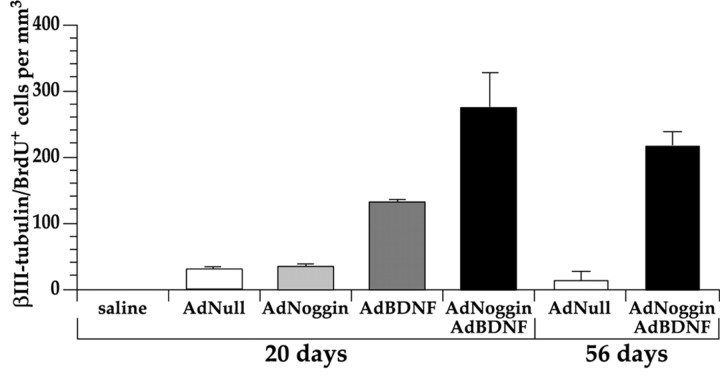
Figure 4.
AdNoggin significantly increased AdBDNF-induced neuronal addition to the striatum. The mean density of β-III tubulin+/BrdU+ cells in the neostriata of saline-, AdNull-, AdNoggin-, AdBDNF-, and AdNoggin/AdBDNF-injected rats compared with animals killed on days 20 (left) and 56 (right).
In contrast, the concurrent use of AdBDNF and AdNoggin greatly enhanced neuronal addition to the striatum. Among 709 BrdU+ striatal cells sampled randomly from four AdNoggin/AdBDNF brains, 95 (13.4 ± 0.3%) were found on confocal imaging to be double-labeled for BrdU and βIII-tubulin (Fig. 3A). Thus, ∼273 new neurons/mm3 (13.4% × 2036 BrdU+ cells/mm3) were added to the rat striatum within 3 weeks of AdNoggin/AdBDNF infection. This was substantially more neuronal addition than that which we observed in response to AdBDNF alone (8.3% × 1573 BrdU+ cells/mm3, or 131 new neurons/mm3; p < 0.01) and greatly exceeded the rare striatal neuronal addition noted in AdNull-injected rats, in which only 2.1 ± 0.2% of BrdU+ cells, or 31/mm3, expressed βIII-tubulin (p < 0.01) (Tables 1, 2; Fig. 4).
Table 2.
Post hoc pairwise comparisons of β-III tubulin+/BrdU+ cells/mm3 data (see Table 1) after one-way ANOVA
|
Treatment |
Saline |
AdNull |
AdNoggin |
AdBDNF |
AdBDNF/noggin |
|---|---|---|---|---|---|
| Saline | 0 | 6.32 | 9.82 | 141.48* | 596.19* |
| AdNull | 6.32 | 0 | 0.38 | 88.00* | 479.75* |
| AdNoggin | 9.82 | 0.38 | 0 | 76.75* | 452.96* |
| AdBDNF | 141.48* | 88.00* | 76.75* | 0 | 156.81* |
| AdBDNF/noggin |
596.19*
|
479.75*
|
452.96*
|
156.81*
|
0 |
Numbers indicate the post hoc Boneferroni t value for each comparison. *Significant to p<0.01.
The rise in striatal neuronal addition in AdBDNF/AdNoggin coinjected animals was accompanied by a fall in subpendymal gliogenesis in these same striata, as had been noted in animals treated with AdNoggin alone (Fig. 2). Thus, whereas 38.0 ± 4.7% of BrdU+ striatal subependymal cells in AdNull-injected animals expressed GFAP, only 4.8 ± 0.8% of BrdU+ striatal subependymal cells were GFAP+ in the AdBDNF/AdNoggin-injected animals. This 87% decline in the incidence of GFAP+/BrdU+ cells in the AdBDNF/AdNoggin-treated SZ was accompanied by the aforementioned increase in βIII-tubulin+/BrdU+ striatal neurons in AdBDNF/AdNoggin animals. The incidence of striatal neuronal recruitment, therefore, increased in the same AdBDNF/AdNoggin-treated striata in which the incidence of subependymal gliogenesis fell by 87%. At the same time, the AdBDNF/AdNoggin-triggered onset of striatal neuronal addition was associated with an increment in the incidence of striatal BrdU+ cells, to 2036 ± 374/mm3 (p < 0.01; two-sample t test). Together, these data indicated that noggin overexpression significantly potentiated BDNF-induced neuronal addition to the adult striatum, pare passu with its suppression of subependymal gliogenesis.
AdBDNF/AdNoggin-induced neurons survived striatal integration
By 8 weeks after viral infection and 5 weeks after the cessation of BrdU injection, the AdNoggin/AdBDNF-treated animals retained an average of 1986 ± 185 BrdU+ striatal cells/mm3, compared with 1351 ± 65 BrdU+ striatal cells/mm3 noted in AdNull-injected controls (p < 0.02; two-sample t test). Among 180 BrdU+ striatal cells selected at random from three AdNoggin/AdBDNF-treated brains, 20 cells (11.1 ± 1.7%) were identified by confocal analysis as BrdU+/βIII-tubulin+. Even at this late time point, an average of 220 new striatal neurons/mm3 remained in AdNoggin/AdBDNF coinjected animals, significantly more (p < 0.05) than AdNull-injected animals, in whom only 0.6 ± 0.6% of BrdU+ cells, or 8 cells/mm3, fulfilled our antigenic criteria for neuronal designation (Fig. 3E). Taken together, these data indicate that newly generated, AdNoggin/AdBDNF-induced neurons survive in the adult neostriatum, long after their mitotic generation and initial parenchymal recruitment.
AdBDNF/AdNoggin-induced striatal neurons differentiated into medium spiny cells
We had previously noted that AdBDNF-induced striatal neurons express markers characteristic of medium spiny neurons, including calbindin, glutamic acid decarboxylase (GAD67), an enzyme involved in the synthesis of GABA from glutamate, and DARPP-32, a dopamine-regulated phosphoprotein specifically expressed in the neostriatum by medium spiny neurons (Ivkovic and Ehrlich, 1999; Benraiss et al., 2001). To determine whether AdBDNF/AdNoggin-induced cells likewise differentiated as medium spiny neurons, we immunolabeled sections taken from AdBDNF/AdNoggin-injected animals killed at 3 weeks for BrdU and either GAD67 or DARPP-32 (Fig. 3B,C). We found that 8.5% of the imaged BrdU+ cells (11 of 130) coexpressed GAD67+, whereas 7.9% (15 of 189) coexpressed DARPP-32. This compared with the 13.4 ± 0.3% of striatal BrdU+ cells that were defined antigenically as neurons by their coexpression of βIII-tubulin. The high proportion of both GAD67+/BrdU+ and DARPP32+/BrdU+ cells to total βIII-tubulin+/BrdU+ cells in these animals, 63.4 and 59.0%, respectively, suggests that most AdBDNF/AdNoggin-induced striatal cells indeed matured as GABAergic medium spiny neurons.
Newly generated striatal neurons developed projections to the globus pallidus
We next asked whether the new neurons of the rat caudate-putamen extended processes to their normal developmental target, the globus pallidus. To address this question, we injected the retrograde tracer FG into the globus pallidus of rats injected with AdBDNF 6 weeks earlier, who had been given daily BrdU injections for the first 18 d after viral injection. One week after FG delivery, the rats were killed, and their striata were assessed for the incidence of BrdU+/FG+ cells, which we thereby defined as newly generated pallidal projection neurons (see Materials and Methods). We found that 2.3 ± 1.1% of striatal BrdU+ cells in AdBDNF-injected animals, or 17.1 ± 1.9 cells/mm3, projected to the globus pallidus (Fig. 5). In these same sections, 3.1 ± 0.2% of the striatal BrdU+ cells coexpressed NeuN of the 770 ± 174 total BrdU+ cells/mm3. Thus, these striata harbored an average of 24.6 NeuN+/BrdU+ cells/mm3. (Of note, NeuN rather than βIII-tubulin was used to identify neurons in FG-labeled sections, because the light fixation required for FG visualization diminished βIII-tubulin immunolabeling but did not noticeably affect NeuN.) Because 17.1 ± 1.9 cells/mm3 BrdU+ cells in the same striata incorporated FG, one can extrapolate that ∼70% (17.1/24.6 × 100) of newly generated striatal neurons extended processed to the globus pallidus within the observed time frame, which spanned the 7 weeks after AdBDNF injection.
Figure 5.
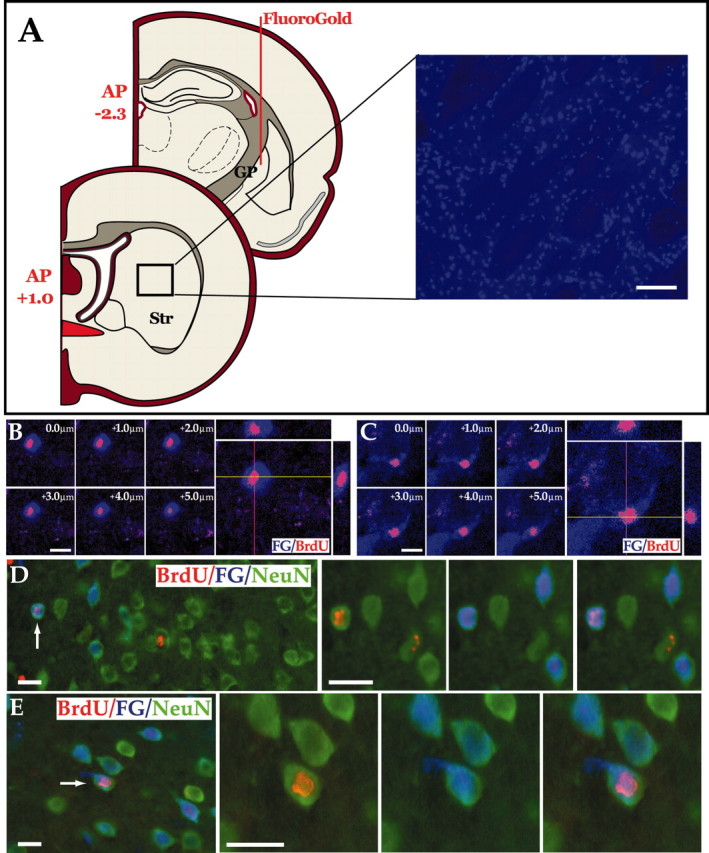
Newly generated striatal neurons project to the globus pallidus. A, FG was injected into the ventral portion of the globus pallidus of rats that had received AdBDNF 6 weeks earlier. The rats were then killed 1 week later, and FG uptake by striatopallidal projection neurons was assessed. Diffusion of the retrograde tracer averaged 0.7 mm from the injection site at P -2.3, so that the most rostral extent of diffusion was typically to P -1.6 mm; no evidence of any diffusion of FG to the striatum was ever noted. Striatal sections were, thus, scored rostrally from AP +1.0, to avoid any possible diffusion artifact. Scale bar, 100 μm. B, C, Confocal images of FG+ (blue)/BrdU+ (red) double-immunolabeled cells in the striata of AdBDNF-injected rats (7 week survival), shown as both single optical sections and orthogonal views in the xz and yz planes, to confirm that scored BrdU+ cells were FG+. D, E, Two examples of BrdU+ (pink)/NeuN+ (green) striatal neurons identified in an AdBDNF-treated rat, 7 weeks after viral injection. These cells (arrows) incorporated FG (blue) injected into the globus pallidus. The triple-labeled cells represent newly generated striatal neurons that have extended fibers to the pallidal targets. Scale bar, 16 μm.
Because the globus pallidus is the major target of caudate-putaminal medium spiny axons, these results suggest that newly generated medium spiny neurons can project axons to appropriate postsynaptic targets. Most strikingly, these observations argue that induction of endogenous progenitor cells may be sufficient to initiate this process in the adult brain.
Parenchymal progenitors did not contribute to AdBDNF/AdNoggin-induced neurogenesis
We next considered whether those new striatal neurons added in response to AdBDNF, with or without AdNoggin treatment, were derived from the ventricular subependyma or from the parenchymal progenitor pools of the striatum or striatal white matter (Palmer et al., 1995, 1999; Nunes et al., 2003). To address this issue, we first asked whether direct injection of AdBDNF and AdNoggin into the striatal parenchyma, in the absence of periventricular viral transduction, was sufficient to stimulate striatal neuronal addition. To wit, either AdNull, AdBDNF alone, or AdBDNF and AdNoggin together were administered to the striatal parenchyma of nine adult rats (n = 3/group), at sites at least 2 mm distant from the ventricular wall. Beginning 2 d later, the injected animals were given daily injections of BrdU for 18 d and then killed 1 day later and assessed histologically for the persistent expression of the adenoviral transgenes. To assess the distribution and persistence of adenoviral transgene expression, we then immunostained sections for GFP over a 2 mm mediolateral span centered on the injection site. We were able to use GFP as our reporter here because both AdBDNF and AdNull were set up as bicistronic vectors that included GFP, placed under internal ribosomal entry site or CMV transcriptional control, respectively. We found that GFP+ cells populated a striatal volume with an average radius of 0.31 ± 0.04 mm, suggesting effective viral transgene delivery throughout at least that volume of tissue (Figs. 6C,D).
Figure 6.
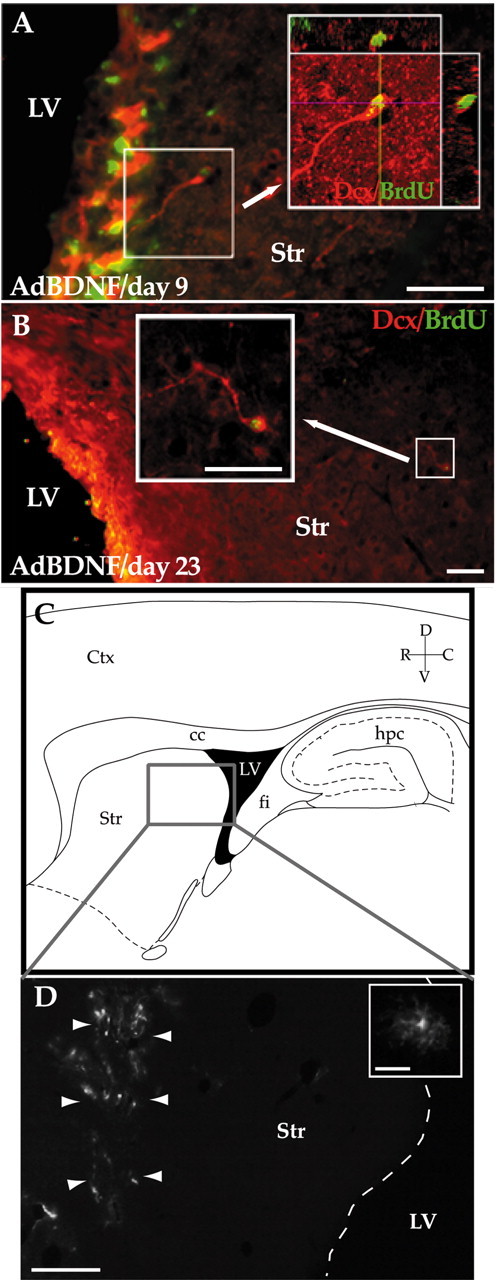
AdBDNF-induced neurons arose from the SZ. A shows the neostriatal wall of a rat killed 9 d after AdBDNF that had been treated daily with BrdU for the week before sacrifice. Many Dcx+ (red)/BrdU+ (green) cells, likely neuronal migrants, were noted arising from the ventricular wall. No BrdU+/Dcx+ cells were seen beyond 100 μm from the ventricular wall at this time point. B, Low-power image of the striatum of a rat killed 23 d after AdBDNF that had been given BrdU for only the first week after viral injection. BrdU-tagged Dcx+ cells (arrow) were identified throughout much of the neostriatum by this time. C, D, The striatal parenchyma is not a source of new striatal neurons. C, Schematic of sagittal rat brain section. The enclosed area is as imaged in D, which shows the striatum of a rat killed 20 d after an intrastriatal, rather than intraventricular, injection of AdBDNF. The volume of distribution of the adenoviral vector is delimited by infected cells expressing its GFP reporter (arrowheads; inset). Nonetheless, virtually no new neurons were observed in these striata after 3 weeks of daily BrdU injections. The dashed line in D delineates the border between the lateral ventricle and the striatum. Scale bars: A, B, 50 μm; D, 640 μm; D (inset), 32 μm. LV, Lateral ventricle; Str, striatum; hpc, hippocampus; fi, fimbria; cc, corpus callosum; D, dorsal; V, ventral; R, rostral; C, caudal.
We next looked for the presence of any BrdU-incorporating neurons in the striatal parenchyma of these animals, as defined by βIII-tubulin or NeuN immunolabeling. All brains were examined to ensure the absence of any inadvertent needle passage through the ventricular lumen that might have served to introduce virus to the ventricular progenitor population. In the AdNull-injected animals, no BrdU-incorporating βIII-tubulin-defined neurons were noted within a 1.5 mm radius of the injection site in any of the stereologically sampled sections, among a total of 868 BrdU+ cells scored in 15 sections. Similarly, in the AdBDNF and AdBDNF/AdNoggin groups, no parenchymal cells were noted to coexpress BrdU and βIII-tubulin within 1.5 mm of the viral injection sites. Thus, in no instances were new neurons identified as such at the sites of parenchymal viral injection. However, rare BrdU+/βIII-tubulin+ neurons (<4/mm3) did appear within 1 mm of the ventricular wall in both AdBDNF and AdBDNF/AdNoggin-injected rats. Given their periventricular locales, these few new neurons induced after parenchymal viral delivery appeared to arise from periventricular progenitors activated by transgene-encoded protein that gained access to the SZ, rather than from parenchymal progenitors induced to neurogenesis. Together, these observations indicated that those new neurons recruited to the striatum in response to AdBDNF and AdBDNF/AdNoggin did not arise from latent progenitors within the striatal parenchyma itself.
AdBDNF-induced neurons arose from the SZ
On the basis of these data, we concluded that the ventricular subependyma was likely the sole source of neurons added to the striatum in response to both periventricular AdBDNF and AdBDNF/AdNoggin overexpression. To positively demonstrate this point, we tracked the departure of new neurons from the ventricular wall, by observing the fate of BrdU-tagged neurons in rats sampled over a range of time points after intraventricular AdBDNF injection. Each group of rats received injections once daily of BrdU for 7 d after either AdBDNF or saline injection, then were killed on either day 9, 16, or 23 after viral injection (n = 3 rats/time point/treatment). Their brains were then cut and stained for BrdU and Dcx, a migration-associated protein (Gleeson et al., 1999). By this means, we tracked the migration of new neurons recruited to the striata of these animals.
No striatal Dcx+/BrdU+ cells were found in saline-injected control animals at any time point. In contrast, in AdBDNF-injected animals, Dcx+/BrdU+ neurons could be identified as early as 9 d after AdBDNF injection, although these cells were closely apposed to the striatal wall of the lateral ventricle (Fig. 6A). By 16 d, many more Dcx+/BrdU+ cells were noted in the striatal parenchyma, the average closest distance to the ventricular wall of which had increased substantially. By 23 d, extensive dispersal of new neurons from the ventricular wall had been achieved; the migrants were noted to have infiltrated over one-third of the striatal volume within that span (Fig. 6B). As a result, Dcx+/BrdU+ cells were found at mean distances from the ventricular wall of 79 ± 6.7 μm at 9 d, 188 ± 28 μm at 16 d, and 388 ± 89 μm at 23 d [p = 0.007; F = 12.5 (2.8 df) by one-way ANOVA]. Together, these data indicate that ependymal BDNF overexpression induces neuronal migration from the ventricular wall into the neostriatal parenchyma.
Discussion
These results indicate that the concurrent overexpression of noggin and BDNF may be used to stimulate and direct neuronal production from endogenous progenitor cells in the adult mammalian neostriatum. This strategy of using noggin to suppress glial lineage, while simultaneously using BDNF to direct the neuronal differentiation of SZ progenitors, yielded a marked accentuation of both neostriatal neurogenesis and parenchymal recruitment. By inhibiting glial differentiation, noggin may have increased the pool of progenitor cells potentially responsive to BDNF; together, the two cooperated in effecting neuronal recruitment to the striatum.
We found here that AdNoggin/AdBDNF-treated rats exhibited 273 βIII-tubulin+/BrdU+ striatal neurons/mm3, whereas AdBDNF alone yielded 131/mm3 and AdNoggin alone yielded only 34/mm3. Because AdNoggin alone yielded little striatal neuronal addition, noggin appeared instead to potentiate BDNF-induced striatal neurogenesis. Indeed, we found that the BDNF/Noggin-treated animals exhibited significantly greater numbers of striatal neurons than did rats treated with AdBDNF alone (p < 0.01 by one-way ANOVA with post hoc Boneferroni comparisons) (Table 2). Importantly in this regard, although noggin overexpression substantially suppressed astrocyte neogenesis within the SZ, its use alone was not associated with any decrement in the total number of striatal BrdU+ cells/mm3 (Table 1). Together, these observations suggest a model whereby noggin treatment may permit the maintenance of an undifferentiated progenitor pool otherwise destined to generate a fraction of differentiated astrocytic daughters. In the presence of noggin alone, astrocytic numbers are sharply reduced in the SZ, whereas fewer dividing progenitors progress to terminal differentiation. More of those cells are then amenable to neuronal differentiation in the presence of BDNF, allowing positive interaction of the two.
Interestingly, whereas glial differentiation was suppressed in AdNoggin-injected animals throughout the entire lateral ventricular wall, the combination of AdBDNF and AdNoggin resulted in ectopic neuronal addition only to the striatum and not to the neocortex or septum (data not shown). The regionally restricted nature of noggin-accentuated neurogenesis argues that glial suppression may be necessary, but not sufficient, for inducing neurogenesis in otherwise non-neurogenic regions of the adult CNS. In addition, endogenous noggin is expressed not only in neurogenic areas like the olfactory bulb and dentate gyrus but also in the non-neurogenic septum (Valenzuela et al., 1995) (Fig. 1), again suggesting that noggin expression alone is not sufficient for neuronal production and recruitment in vivo. Rather, noggin seems to be an important contributor, the relative importance of which in a given brain region is likely a function not only of the levels and species of endogenous pro-gliogenic BMPs but also of the responsiveness of the resident pro-genitor population. Together, these findings argue that locally active and regionally restricted factors delimit neuronal production and recruitment in the adult forebrain.
We also asked whether parenchymal progenitor pools might contribute to striatal neuronal addition. Our experiments revealed no such contribution from parenchymal progenitors, because we observed only rare instances of induced neurogenesis in response to intrastriatal AdBDNF or AdBDNF/AdNoggin injection, and even these few new neurons were invariably found in the periventricular regions, rather than near the parenchymal injection sites. In contrast, timed sacrifices suggested the dispersal of newly generated neurons from the striatal ventricular wall, spanning the 3 week period after AdBDNF injection.
The generation and migration of new striatal neurons was followed by their parenchymal integration and survival. The cells scored at 3 weeks mostly remained at 8 weeks, such that no appreciable loss of the newly generated striatal cohort was noted during this period. Remarkably, FG backfills revealed that by 7 weeks after viral injection, a large proportion of newly generated, AdBDNF-induced BrdU+ striatal neurons had extended fibers to their usual target, the globus pallidus. These fibers traversed a distance of at least 2 mm, typical for the striatopallidal projection in rats. The long distance extension of fibers from new projection neurons to distant nuclear targets has rarely been demonstrated in the adult CNS, and never before in uninjured mammals. A testosterone-mediated process of fiber extension from the principal vocal control nucleus to its motor target nucleus has been demonstrated in normal adult songbirds, and our current findings would seem conceptually analogous to this process, in that in each of these instances, adult-derived axogenic neurons have been newly generated (Alvarez-Buylla and Kirn, 1997). A similar instance of long distance fiber extension has also been reported by new neurons in the injured rat neocortex, after local compensatory neurogenesis (Magavi et al., 2000). These disparate examples of axogenesis by newly generated neurons in the adult CNS indicate not only that newly generated striatal neurons can generate projection fibers, but also that the regional environment may retain the developmental cues that direct axonal extension to appropriate targets. Together, these data argue that neurons induced from resident progenitor cells might be capable of at least limited tract regeneration in the adult forebrain.
These results indicate that the concurrent suppression of glial differentiation by noggin and promotion of both neuronal differentiation and survival by BDNF is an effective strategy for mobilizing endogenous progenitor cells in the adult forebrain. These cells may be thereby stimulated to achieve the quantitatively significant addition of new neurons to the adult neostriatum, a region that otherwise does not recruit new neurons in postnatal or adult animals. The differentiation of these new striatal cells as GABAergic DARPP-32+ neurons, the long distance projection of these new neurons to the globus pallidus, and the survival of these cells for at least 2 months after their genesis all suggest their ability to mature, function, and survive in the adult forebrain. The induced production of these cells by a strategy of concurrent glial suppression and neuronal induction thereby suggests a means by which phenotypically appropriate neurons might be regenerated from endogenous progenitor cells, in sufficient numbers to restore those medium spiny cells lost in striatal degenerations such as Huntington's disease.
Footnotes
This work was supported by the Cure HD Initiative of the Hereditary Disease Foundation and the National Institutes of Health-National Institute of Neurological Disorders and Stroke. We are grateful to Regeneron Pharmaceuticals and to Drs. Frederick Kaplan and Eileen Shore of the University of Pennsylvania Medical School (Philadelphia, PA) for providing AdNoggin. We are also grateful to Drs. Chris Walsh (Harvard, Boston, MA), Tony Frankfurter (University of Virginia, Charlottesville, VA), and Hugh Hemmings (Cornell University, New York, NY) for their gifts of antibodies, and to Dr. Susan Croll (Regeneron) for advice.
Correspondence should be addressed to Dr. Steven A. Goldman, Department of Neurology, University of Rochester, 601 Elmwood Avenue, Box 645, Rochester, NY 14642. E-mail: Steven_Goldman@urmc.rochester.edu.
Copyright © 2004 Society for Neuroscience 0270-6474/04/242133-10$15.00/0
References
- Ahmed S, Reynolds BA, Weiss S (1995) BDNF enhances the differentiation but not the survival of CNS stem cell- derived neuronal precursors. J Neurosci 15: 5765-5778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Kirn J (1997) Birth, migration, incorporation and death of vocal control neurons in adult songbirds. J Neurosci 33: 585-601. [PubMed] [Google Scholar]
- Bajocchi G, Feldman S, Crystal R, Mastrangeli A (1993) Direct in vivo gene transfer to ependymal cells in the central nervous system using recombinant adenovirus vectors. Nat Genet 3: 229-234. [DOI] [PubMed] [Google Scholar]
- Benraiss A, Chmielnicki E, Lerner K, Roh D, Goldman SA (2001) Adenoviral brain-derived neurotrophic factor induces both neostriatal and olfactory neuronal recruitment from endogenous progenitor cells in the adult forebrain. J Neurosci 21: 6718-6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulder Committee (1970) Embryonic vertebrate central nervous system: revised terminology. Anat Rec 166: 257-261. [DOI] [PubMed] [Google Scholar]
- Chiasson B, Tropepe V, Morshead C, van der Kooy D (1999) Adult mammalian forebrain ependymal and subependymal cells demonstrate proliferative potential, but only subependymal cells have neural stem cell characteristics. J Neurosci 19: 4462-4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig CG, Tropepe V, Morshead CM, Reynolds BA, Weiss S, van der Kooy D (1996) In vivo growth factor expansion of endogenous subependymal neural precursor cell populations in the adult mouse brain. J Neurosci 16: 2649-2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A (1997) Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci 17: 5046-5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A (1999) Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell 97: 703-716. [DOI] [PubMed] [Google Scholar]
- Economides AN, Stahl NE, Harlan RM (2000) Modified noggin polypeptide and compositions. U.S. Patent 6,075,007 (June 13, 2000).
- Gleeson J, Lin P, Flanagan L, Walsh C (1999) Doublecortin is a microtubule-associated protein and is expressed widely by migrating neurons. Neuron 23: 257-271. [DOI] [PubMed] [Google Scholar]
- Goldman SA (1998) Adult neurogenesis: from canaries to the clinic. J Neurobiol 36: 267-286. [PubMed] [Google Scholar]
- Goldman SA, Luskin MB (1998) Strategies utilized by migrating neurons of the postnatal vertebrate forebrain. Trends Neurosci 21: 107-114. [DOI] [PubMed] [Google Scholar]
- Graham F, Prevec L (1991) Manipulation of adenovirus vectors. In: Methods in molecular biology (Murray E, ed), pp 109-128. Totowa, NJ: Humana. [DOI] [PubMed]
- Gross R, Mehler M, Mabie P, Zang Z, Santschi L, Kessler J (1996) Bone morphogenetic proteins promote astroglial lineage commitment by mammalian subventricular zone progenitor cells. Neuron 17: 595-606. [DOI] [PubMed] [Google Scholar]
- Ivkovic S, Ehrlich M (1999) Expression of the striatal DARPP-32/ARPP-21 phenotype in GABAergic neurons requires neurotrophins in vivo and in vitro. J Neurosci 19: 5409-5419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschenbaum B, Goldman SA (1995) Brain-derived neurotrophic factor promotes the survival of neurons arising from the adult rat forebrain subependymal zone. Proc Natl Acad Sci USA 92: 210-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn HG, Winkler J, Kempermann G, Thal L, Gage F (1997) Epidermal growth factor and fibroblast growth factor-2 have different effects on neural progenitors in the adult rat brain. J Neurosci 17: 5820-5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MK, Rebhun LI, Frankfurter A (1990) Posttranslational modification of class III beta-tubulin. Proc Natl Acad Sci USA 87: 7195-7199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal C, Rafii S, Rafii D, Shahar A, Goldman SA (1999) Endothelial trophic support of neuronal production and recruitment from the adult mammalian subependyma. Mol Cell Neurosci 13: 450-464. [DOI] [PubMed] [Google Scholar]
- Lim D, Tramontin A, Trevejo J, Herrera D, Garcia-Verdugo J, Alvarez-Buylla A (2000) Noggin antagonizes BMP signaling to create a niche for adult neurogenesis. Neuron 28: 713-726. [DOI] [PubMed] [Google Scholar]
- Lindholm D, Carroll P, Tzimagiogis G, Thoenen H (1996) Autocrine-paracrine regulation of hippocampal neuron survival by IGF-1 and the neurotrophins BDNF, NT-3 and NT-4. Eur J Neurosci 8: 1452-1460. [DOI] [PubMed] [Google Scholar]
- Louissaint A, Rao S, Leventhal C, Goldman SA (2002) Coordinated interaction of angiogenesis and nurogenesis in the adult songbird brain. Neuron 34: 945-960. [DOI] [PubMed] [Google Scholar]
- Magavi S, Leavitt B, Macklis J (2000) Induction of neurogenesis in the neocortex of adult mice. Nature 405: 951-955. [DOI] [PubMed] [Google Scholar]
- Mehler MF, Marmur R, Gross R, Mabie PC, Zang Z, Papavasiliou A, Kessler JA (1995) Cytokines regulate the cellular phenotype of developing neural lineage species. Int J Dev Neurosci 13: 213-240. [DOI] [PubMed] [Google Scholar]
- Menezes JR, Smith CM, Nelson KC, Luskin MB (1995) The division of neuronal progenitor cells during migration in the neonatal mammalian forebrain. Mol Cell Neurosci 6: 496-508. [DOI] [PubMed] [Google Scholar]
- Morshead C, van der Kooy D (1992) Postmitotic death is the fate of constitutively proliferating cells in the subependymal layer of the adult mouse brain. J Neurosci 12: 249-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes M, Roy NS, Keyoung HM, Goodman R, McKhann G, Kang J, Jiang L, Nedergaard M, Goldman S (2003) Identification and isolation of multipotential neural progenitor cells from the subcortical white matter of the adult human brain. Nat Med 9: 439-447. [DOI] [PubMed] [Google Scholar]
- Paine-Saunders S, Viviano B, Economides AN, Saunders S (2002) Heparan sulfate proteoglycans retain noggin at the cell surface: a potential mechanism for shaping bone morphogenetic protein gradients. J Biol Chem 277: 2089-2096. [DOI] [PubMed] [Google Scholar]
- Palmer TD, Ray J, Gage FH (1995) FGF-2-responsive neuronal progenitors reside in proliferative and quiescent regions of the adult rodent brain. Mol Cell Neurosci 6: 474-486. [DOI] [PubMed] [Google Scholar]
- Palmer TD, Markakis EA, Willhoite AR, Safar F, Gage FH (1999) Fibroblast growth factor-2 activates a latent neurogenic program in neural stem cells from diverse regions of the adult CNS. J Neurosci 19: 8487-8497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C (1986) The rat brain in stereotaxic coordinates, Ed 2. Orlando, FL: Academic.
- Pincus DW, Harrison C, Goodman RR, Edgar M, Keyoung H, Fraser RA, Sakakibara S, Okano H, Nedergaard M, Goldman SA (1998) FGF2/BDNF- associated maturation of new neurons generated from adult human subependymal cells. Ann Neurol 43: 576-585. [DOI] [PubMed] [Google Scholar]
- Smart I (1961) The subependymal layer of the mouse brain and its cell production as shown by radioautography after thymidine injection. J Comp Neurol 116: 325-347. [Google Scholar]
- Valenzuela DM, Maisonpierre PC, Glass DJ, Rojas E, Nunez L, Kong Y, Gies DR, Stitt TN, Ip NY, Yancopoulos GD (1993) Alternate forms of rat trkC with different functional capabilities. Neuron 10: 963-974. [DOI] [PubMed] [Google Scholar]
- Valenzuela DM, Economides AN, Rojas E, Lamb TM, Nunez L, Jones P, Lp NY, Espinosa III R, Brannan CI, Gilbert DJ, Copeland N, Jenkins N, Le Beau M, Harland R, Yancopoulos G. (1995) Identification of mammalian noggin and its expression in the adult nervous system. J Neurosci 15: 6077-6084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vescovi AL, Reynolds BA, Fraser DD, Weiss S (1993) bFGF regulates the proliferative fate of unipotent (neuronal) and bipotent (neuronal/astroglial) EGF-generated CNS progenitor cells. Neuron 11: 951-966. [DOI] [PubMed] [Google Scholar]
- Zhang M, McKanna J (1997) Gliogenesis in postnatal rat optic nerve: LC1+ microglia and S100b+ astrocytes. Dev Brain Res 101: 27-36. [DOI] [PubMed] [Google Scholar]
- Zigova T, Pencea V, Wiegand SJ, Luskin MB (1998) Intraventricular administration of BDNF increases the number of newly generated neurons in the adult olfactory bulb. Mol Cell Neurosci 11: 234-245. [DOI] [PubMed] [Google Scholar]
- Zimmerman L, Jesus-Escobar J, Harlan R (1996) The Spemann organizer signal Noggin binds and inactivates bone morphogenetic protein 4. Cell 86: 599-606. [DOI] [PubMed] [Google Scholar]