Abstract
The hippocampus and the amygdala are involved in avoidance learning in mammals. The medial and lateral pallia of actinopterygian fish have been proposed as homologous to the mammalian pallial amygdala and hippocampus, respectively, on the basis of neuroanatomical findings. This work was aimed at studying the effects of ablation of the medial telencephalic pallia (MP) and lateral telencephalic pallia (LP) in goldfish on the retention of a conditioned avoidance response previously acquired in two experimental conditions. In the first experiment, fish were trained in nontrace avoidance conditioning. In the second experiment, fish were trained in trace avoidance conditioning in which temporal cues were crucial for the learning process. An MP lesion affected the retention of the avoidance response in both procedures; in contrast, an LP lesion impaired the retention only in the trace-conditioning procedure. These data support the presence of two different systems of memory in fish, based on discrete telencephalic areas: the MP, involved in an emotional memory system; and the LP, involved in a spatial, relational, or temporal memory system. Moreover, these differential effects were similar to those produced by amygdalar and hippocampal lesions in mammals. We conclude that these specialized systems of memory could have appeared early during phylogenesis and could have been conserved throughout vertebrate evolution.
Keywords: amygdala, hippocampus, avoidance learning, memory systems, telencephalon, brain evolution, teleost fish
Introduction
In mammals, the amygdalar and hippocampal systems accomplish an important role in the acquisition and retention of conditioned avoidance response (McIntyre and Stein, 1973; Grossman et al., 1975; Sánchez-Riolobos, 1986; Rawlins et al., 1993; Winocur, 1997). The amygdala is involved in emotional behaviors and emotional memory (LeDoux, 1995). It is also involved in avoidance learning because lesions of amygdala nuclei abolish the acquired conditioned response (Sánchez-Riolobos, 1986; Ambrogi-Lorenzini et al., 1991). It is known that hippocampal and septal lesions facilitate avoidance learning in mammals in some circumstances (O'Keefe and Nadel, 1978; Sara and David-Remacle, 1981; Gray and McNaughton, 1983; Weiner et al., 1998). However, these lesions produce harmful effects when the contextual or time cues are significant for the conditioning process, as in the case of avoidance learning or trace emotional conditioning procedures (Woodruff and Kantor, 1983; Moyer et al., 1990; Phillips and LeDoux, 1992). Thus, the hippocampus is involved in spatial learning (O'Keefe and Nadel, 1978), in relational memory (Eichenbaum et al., 1992; Squire, 1992), and in the processing of temporal attributes of events and situations (Kesner and DiMattia, 1987).
In actinopterygian fish, the lateral telencephalic pallium has been proposed as homologous to the hippocampus on the basis of both neuroanatomical evidence (Nieuwenhuys and Meek, 1990; Braford, 1995; Northcutt, 1995; Butler, 2000) and the involvement of this area in place learning by means of allocentric frames of reference (Rodríguez et al., 2002). The medial pallium has been proposed as homologous to pallial amygdala (Marino-Neto and Sabbatini, 1983; Nieuwenhuys and Meek, 1990; Braford, 1995; Northcutt, 1995; Butler, 2000).
The behavioral nature of the conditioned avoidance response in tetrapods has been explained by means of the two-process theory of Mowrer (1960). Interestingly, complete telencephalic ablation in fish produces devastating effects on the acquisition and maintenance of a two-way conditioned avoidance response (Overmier and Papini, 1985, 1986; Papini, 1985; Overmier and Hollis, 1990). Flood et al. (1976) proposed, on the basis of Mowrer's theory, that the function of the fish telencephalon is to use the emotional states as conditioned reinforcers to produce instrumental responses. Ablation of the telencephalon therefore disrupts avoidance conditioning because it prevents engagement between two processes of learning (Pavlovian and instrumental). Furthermore, it is probable that restricted telencephalic areas will be directly involved in this function as a part of various specialized systems of memory, as has been proposed in land vertebrates (Schacter and Tulving, 1994).
The aims of this work were to determine whether medial and lateral pallium lesions in teleost fish produce differential effects on the retention of the avoidance response previously acquired in two different situations (two-way active avoidance conditioning and trace two-way active avoidance conditioning), to test whether these separate pallial areas share functional similarities with their mammalian brain homologues, and to analyze the possible presence of multiple telencephalon-based memory systems in fish.
Materials and Methods
Experiment 1: two-way active avoidance conditioning
As described above, the fish medial and lateral pallia have been proposed as homologous to the mammalian pallial amygdala and hippocampus, respectively (Nieuwenhuys and Meek, 1990; Braford, 1995; Northcutt, 1995; Butler, 2000). Various experiments have shown that the lateral pallium has functional implications in spatial learning, similarly to the hippocampus (Vargas et al., 2000; Rodríguez et al., 2002), and others suggest the presence of a telencephalon-based emotional learning system in fish (Portavella et al., 2003). The aim of this experiment was to determine whether specific damage to the medial or lateral pallium could produce differential deficits in two-way avoidance conditioning in goldfish, similar to those caused by damage to pallial amygdala and hippocampus, respectively. An experiment of two-way avoidance conditioning was performed using a previous design of a fish shuttle box (Horner et al., 1961). The presentation of shock (used as an aversive stimulus) was completely overlapped with a previous green light presentation (used as a discriminative stimulus) to minimize the interstimulus temporal factor.
Subjects
Forty experimentally naive goldfish, purchased from a local supplier (in Seville), served as subjects in this experiment. The animals were between 9 and 11 cm in body length and were housed in small groups in glass aquaria (200 l) with aerated and filtered water at a constant temperature of 20°C. The aquarium room was subject to a 14/10 hr light/dark cycle (lights on from 7 A.M. to 9 P.M.). Pellets of dry food (Tetra-Pond; Ulrich Baemsch GmbH, Melle, Germany) were provided daily ad libitum during the experimental period. The experimental manipulations described in this article were conducted in accordance with Directive 86/609/CEE of the European Community Council and Spanish Real Decreto 223/1988.
Apparatus
Four similar shuttle boxes were used, following a previously described design (Horner et al., 1961) (Fig. 1). Each shuttle box consisted of a water-filled glass tank (50 × 25 × 14 cm). Black polyvinyl chloride (PVC) covered each long side; the floor was covered with white PVC; and the two box ends were clear and translucent to permit the green light presentation as a discriminative stimulus (10 W, 220 V AC, 50 Hz). On each long side, two stainless steel bars attached to metal plates were used as electrodes to deliver a uniform, mild electric shock as an aversive stimulus (0.39 V/cm, 50 Hz, pulsed 200 msec on and 800 msec off). A trapezoidal barrier (7.5 cm high, 10 cm wide at the top, and 18 cm wide at the bottom) divided the shuttle box into two compartments. On the barrier, two pairs of photoemitters (red lights, 24 V DC, 0.3 W) and photoreceptors (photoresistors, 3 V cc) detected the fish shuttle responses across it. The water level over the barrier was kept constant at 2 cm, giving a water level of 9.5 cm in each of the compartments. The water was aerated continuously. The shuttle boxes were controlled by a computerized system (Letica SL) for rat conditioning, adapted and modified in our laboratory. The software driving the shuttle boxes (Skinner; Cibertec SA) was also adapted to deliver the stimuli and to record the fish responses.
Figure 1.
Schematic representation of the shuttle box adapted to fish conditioning. A, Top view. B, Side view. The system for detecting the fish's passing across the barrier consisted of two red lights (24 V cc, 0.3 W) used as photoemitters, and a pair of photoresistors (3 V cc) used as photoreceptors, all on the barrier. The barrier was made of gray PVC. To deliver the electric shock (ES) as an unconditioned stimulus (0.39 V/cm, 50 Hz), two stainless steel bars in contact with four metal plates were used as electrodes. Two green lights [discriminative stimulus (DS)] were used as a conditioned stimulus (10 W, 220 V AC, 50 Hz), which was presented through the translucent end walls of the box.
Procedure
Preexposure. Before training sessions, fish were preexposed individually to the experimental apparatus on 3 consecutive days. On the first day, the animals were placed in the shuttle box with the water level set at 6 cm above the barrier and were allowed to swim freely through the apparatus for 30 min without any stimulus presentation. For the following two sessions, each of 5 min, the water level was dropped to 2 cm above the barrier; the animals did not receive conditioning stimuli.
Acquisition. All animals were trained using the same procedure. This consisted of daily sessions of 10 trials separated by an intertrial interval (ITI) of varying duration, ranging between 1 and 2 min. At the end of the ITI, the trial began. The discriminative stimulus was turned on for a maximum duration of 15 sec in the compartment where the fish was located. If the fish did not respond (swimming across the barrier) within 10 sec of green light onset, the electric shock was turned on for a maximum of 5 sec. Thus, the temporal separation between cue onset and shock onset was 10 sec. A response during the first 10 sec finished the warning stimulus (green light), and the shock was not delivered. A response during the 10-15 sec period canceled both the warning stimulus and the shock. Before starting and after finishing the daily training session, the subject rested in the shuttle box for 5 min without any stimulus presentation. The animals were trained until they reached the learning criterion, to a maximum of 20 sessions. If an animal had not reached the criterion in this period, it was rejected from the experiment. A learning criterion was established of at least 70% of avoidance responses in five of six consecutive training sessions (Overmier and Papini, 1986). All subjects reached the criterion between sessions 8 and 15 and were randomly assigned to one of five groups: medial pallium (MP) ablation (n = 8), lateral pallium (LP) ablation (n = 8), telencephalon (TEL) ablation (n = 8), sham-operated (n = 8), and control (n = 8). The variables recorded for the criterion sessions were latency of responses and percentage of avoidance (shuttle responses occurring before shock onset; the latency was <10 sec) and escape (shuttle responses occurring during the shock; the latency was between 10 and 15 sec). ANOVA with repeated measurements was used for statistical analysis.
Surgery. After the acquisition phase, fish were anesthetized by immersion in a 1:20,000 solution of tricaine methane sulfonate (Sigma, St. Louis, MO) with constant flow of aerated water through the gills. The animal was then placed in the surgical chamber, where it remained fixed in place by lateral holders and partially bathed in water. An adjustable tube was inserted into the animal's mouth and connected to a pump that provided a constant flow of water with a constant concentration of anesthetic during surgery.
The dorsal skin and skull were carefully removed, and the underlying fatty tissue was removed by aspiration. The telencephalic tissue was aspirated carefully with a micropipette connected to a manual vacuum system. Surgery was performed under visual inspection by means of a binocular microscope. The sulcus ypsiliformis, sulcus lateralis, sulcus limitans telencephali, and anterior commissure were used as anatomical references to determine the location and extent of the neural tissue to be removed. After the ablation, the piece of skull was replaced in its original position and fixed with cyanocrylate glue. The fish was returned to its home tank for a recovery period of 5 d. Sham operations were performed exactly as described, except that the nervous tissue was not injured. The control group did not receive any surgical intervention.
At the end of the experiment, the fish with telencephalic lesions and sham operations were deeply anesthetized (1:5000) and perfused with 50 mL of 0.9% saline solution, followed by 125 mL of fixative solution (10% formalin in phosphate buffer, 0.1 m, pH 7.4). The brain was removed from the skull, inspected for a preliminary evaluation of the ablation, and cut in transversal sections (50 μm thick) for histological analysis.
Retention. After the recovery period, the animals were placed in the shuttle box, following the same procedure, for six additional sessions. Latency and avoidance and escape responses were recorded. The performance during the sessions in the postsurgical period was analyzed and compared with the postcriterion sessions before surgery for each group. The recorded variables were the same as in the presurgical period. ANOVA with repeated measurements was used for statistical analysis. Statistical post hoc analysis was performed [honestly significant difference (HSD)-Tukey test]. Student's t test was used to compare presurgical and postsurgical intragroup differences.
Experiment 2: trace two-way active avoidance conditioning
Damage to the hippocampus in mammals produces a deficit in trace conditioning (Thompson et al., 1987; Moyer et al., 1990). The aim of this experiment was to determine whether a lesion of the lateral pallium of the fish telencephalon could produce a deficit in trace avoidance conditioning comparable with that produced by a hippocampal lesion in mammals. To this end, the duration and temporal relation between the discriminative stimulus (green light) and aversive stimulus (electric shock) were modified. The stimuli were not overlapped; a gap of 5 sec between green light off and electric shock on was introduced. The method performed here had been successfully used in previous studies in fish. Thus, conditioning of the emotional response in telencephalon-ablated fish was obtained with the same temporal interstimulus interval (Overmier and Savage, 1974); and the cue-shock onset interval (15 sec) allows two-way avoidance conditioning in fish (Davis, 1968; Savage, 1969; Zerbolio and Royalty, 1983; Portavella et al., 2003).
Subjects
Fifty experimentally naive goldfish, from the same source as and with characteristics similar to those of the animals of the previous experiment, served as subjects. The animals were kept under the same conditions of temperature, light/dark cycle, and food described for Experiment 1.
Apparatus
The experimental apparatus was the same as described for experiment 1.
Procedure
Preexposure. The preexposure procedure was the same as that used in the previous experiment.
Acquisition. All animals were trained using the same procedure. This consisted of a daily session of 10 trials separated by an ITI of varying duration, ranging between 1 and 2 min. At the end of the ITI, the trial began. The discriminative stimulus was turned on for a maximum duration of 10 sec in the compartment where the fish was located, followed by a gap period of 5 sec after termination of the discriminative stimulus. Thus, the temporal separation between cue onset and shock onset was 15 sec. If the fish did not respond (swimming across the barrier) within 15 sec, the electric shock was turned on for a maximum of 5 sec. A response during the first 15 sec finished the warning stimulus (green light), and the shock was not delivered. A response during the 15-20 sec period canceled both the warning stimulus and the shock. Before starting and after finishing the daily training session, the subject rested in the shuttle box for 5 min without any stimulus presentation. The animals were trained until they reached the learning criterion, to a maximum of 30 sessions. The learning criterion was the same as described above. The animals reached the criterion between sessions 12 and 18 and were randomly assigned to one of five groups: MP ablation (n = 8), LP ablation (n = 8), TEL ablation (n = 8), sham-operated (n = 8), and control (n = 8). The recorded variables were latency of responses and percentage of avoidance (shuttle responses occurring before shock onset, when the latency was <15 sec) and escape (shuttle responses occurring during shock, when the latency was between 15 and 20 sec). ANOVA with repeated measurements was used for statistical analysis.
Surgery. The surgical procedure was the same as for experiment 1.
Retention. After the recovery period, the animals were placed in the shuttle box, following the same procedure, for six additional acquisition sessions. Latency and avoidance and escape responses were recorded. The performance during the sessions in the postsurgical period was analyzed and compared with that for the postcriterion sessions before surgery for each group. The recorded variables were the same as in the presurgical period. ANOVA with repeated measurements was used for statistical analysis. Statistical post hoc analysis was performed (HSD-Tukey test). Student's t test was used to compare presurgical and postsurgical intragroup differences.
Results
Experiment 1: two-way active avoidance conditioning
Histological analysis
After visual and histological inspection of the brain, one animal from each experimental group showed intracranial hemorrhage, and their data were excluded from the analysis. Thus, the groups were control (n = 8), sham-operated (n = 8), TEL ablation (n = 7), MP lesion (n = 7), and LP lesion (n = 7).
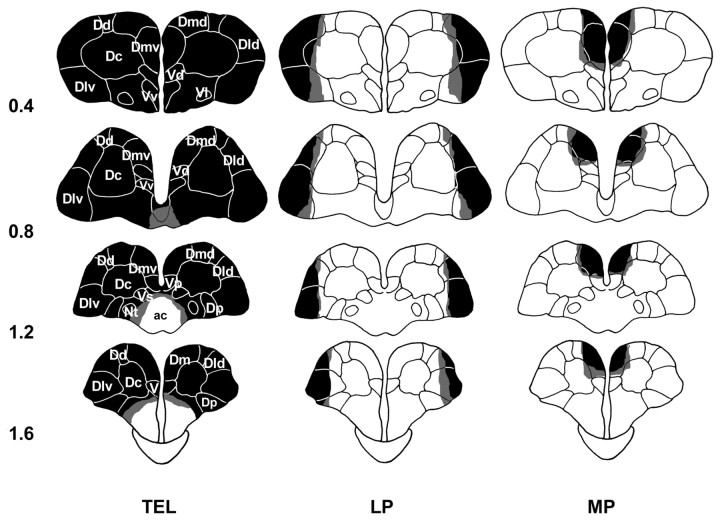
Figure 2 shows the largest (pale shading) and the smallest (dark shading) lesions for each experimental group. The borders of each telencephalic area follow the nomenclature used elsewhere (Peter and Gill, 1975; Nieuwenhuys and Meek, 1990; Rodríguez et al., 2002). The medial pallium lesions affected the area dorsalis telencephali pars medialis ventralis (Dmv) completely and part of the area dorsalis telencephali pars medialis dorsalis (Dmd). Collaterally, in the more extensive lesions, parts of the area telencephali pars dorsalis centralis (Dc), area telencephali ventralis pars dorsalis (Vd), and area telencephali ventralis pars post-commissuralis (Vp) were also affected. Lateral pallium lesions extended to the area dorsalis telencephali pars lateralis ventralis (Dlv) and the area dorsalis telencephali pars lateralis dorsalis (Dld). Major extension included small parts of the Dc and area dorsalis telencephali pars dorsalis (Dd). The telencephalon-ablated animals showed complete ablation of the telencephalon, and the histological analysis showed no damage to the preoptic area and optic tracts. The optic tectum was also spared. None of the sham-operated animals exhibited any evidence of damage to the telencephalon or optic tectum.
Figure 2.
Schematic representation of the location and extent of the lesions in experiment 1: whole TEL, LP, and MP lesions. Light shading represents the largest extension, and dark shading represents the smallest. ac, Anterior commissure; Nt, nucleus taenia. Pallium: Dd, Area dorsalis telencephali pars dorsalis. Subpallium: V, Ventral pallium; Vl, area ventralis telencephali pars lateralis; Vv, area ventralis telencephali pars ventralis. For other abbreviations, see Results. The numbers indicate the distance (millimeters) from the rostral pole of the telencephalon.
Acquisition of conditioned avoidance response
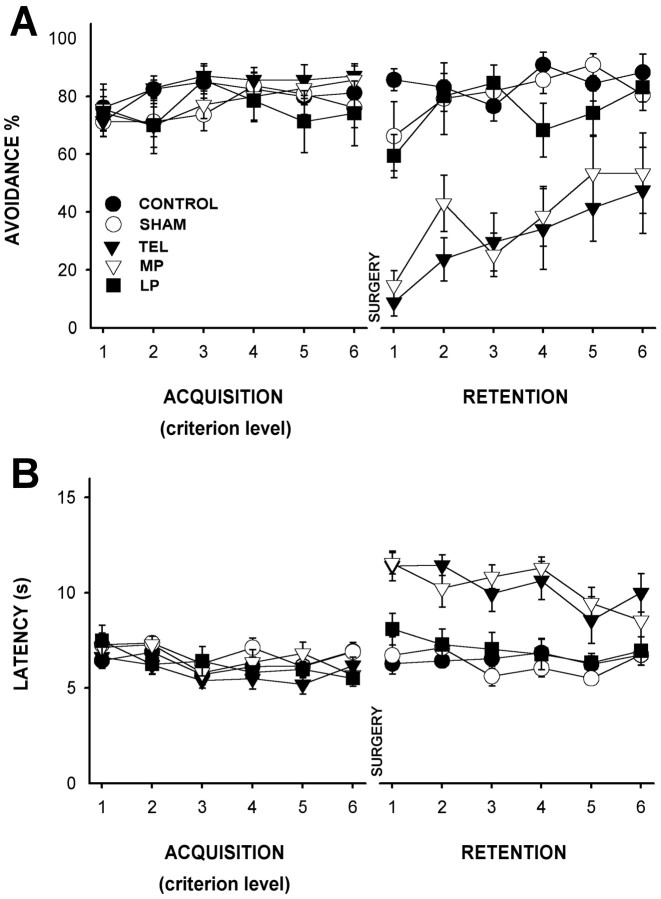
Once the animals reached the criterion of learning, the training and acquisition period finished. At this time, the latency and percentage of avoidance for the six sessions of criterion level were analyzed and compared between the experimental groups. An ANOVA with repeated measurements was used for statistical analysis. There were no significant differences between the five groups in either latency (F(4,32) = 1.975; p > 0.19) or avoidance (F(4,32) = 1.256; p > 0.3). Before surgery, the avoidance and latency values were similar among all groups in this phase (Fig. 3).
Figure 3.
Nontrace avoidance conditioning performance of the different groups during the acquisition and retention phases in experiment 1. A, Mean ± SEM of percentage of avoidance response during acquisition (6 sessions after reaching learning criterion before surgery) and retention (6 sessions after surgery) phases. B, Mean ± SEM of response latency (seconds) during acquisition and retention periods.
Retention of conditioned avoidance response
The results for the retention period, after surgery, showed statistically significant intergroup differences in latency (ANOVA with repeated measurements, F(4,32) = 34.237; p < 0.001) and avoidance response (F(4,32) = 18.894; p < 0.001; Fig. 3A,B). Post hoc analyses presented differences between the telencephalon-ablated group and the control, sham-operated, and LP groups in avoidance (HSD-Tukey, α = 0.05; p < 0.001) and latency (p < 0.001). The MP group showed similar differences with the three named groups (control, sham-operated, and LP) in avoidance and latency (p < 0.001; Fig. 3A,B). In contrast, the analyses from the telencephalon-ablated and MP groups did not indicate any difference in latency (p = 1.0) or avoidance response (p > 0.91; Fig. 3A,B). There was no significant difference among control, sham-operated, and LP groups in either avoidance response (p > 0.74) or latency (p > 0.51; Fig. 3A,B).
The comparison between presurgical (acquisition) and postsurgical (retention) phases indicated considerable impairment in the retention of the previously acquired avoidance response, produced by whole telencephalon and medial ablations (Fig. 3A). The two-tailed Student's t test (last session of acquisition vs first session of retention) revealed statistical differences in avoidance in the telencephalon-ablated group (t(6) > 19.4; p < 0.001) and MP group (t(6) > 17.6; p < 0.001). The other groups did not show any statistically significant difference in avoidance (control, t(7) < 0.32; p > 0.76; sham-operated, t(7) < 1.35; p > 0.21; and LP, t(6) < 1.018; p > 0.34; Fig. 3A). The latency of sham-operated and control groups did not present any significant difference (t(7) < 1.82; p > 0.18; t(7) < 1.737; p > 0.12, respectively); the LP group presented significant differences with the first session of the retention phase (t(6) = 3.992; p < 0.007) but maintained a high level of avoidance response in the following sessions; the telencephalon-ablated and MP groups showed significant differences (t(6) > 6.59; p < 0.001; t(6) > 7.64; p < 0.001; Fig. 3B). These results clearly show that the previously acquired conditioned avoidance response was impaired by medial pallium and whole telencephalon ablations (Fig. 3A,B). Although the data for latency showed a progressive decrease of the latency values in all groups (F(5, 160) = 11.716; p < 0.012) along the postsurgical retention period (Fig. 3B), the latency values in the LP, sham-operated, and control groups remained lower than 10 sec (avoidance latency). In contrast, latency in the telencephalon-ablated and MP groups latency exceeded 10 sec (escape latencies; Fig. 3B).
Experiment 2: trace two-way active avoidance conditioning
Histological analysis
No vascular accidents were detected by visual and histological inspection in any of the animals; thus, the experimental groups were control (n = 8), sham-operated (n = 8), TEL ablation (n = 8), MP lesion (n = 8), and LP lesion (n = 8).
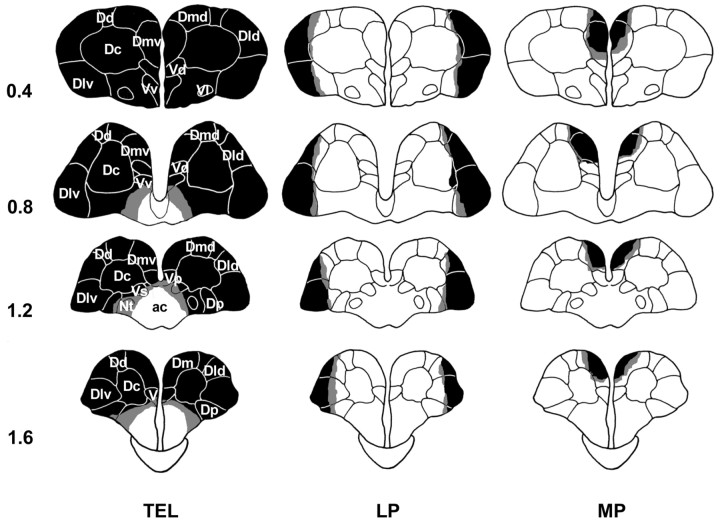
Figure 4 shows the extent of the lesions in the experimental groups. The largest (pale shading) and smallest (dark shading) medial and lateral pallium lesions were reconstructed on standard coronal sections of the same goldfish atlas. The medial pallium lesions affected the Dmv area almost completely and the Dmd area partially, with minor damage to adjacent areas. Collaterally, in the more extensive lesions, portions of the Dc, Vd, Vp, and area ventralis telencephali pars supracommissuralis (Vs) were also affected. Lateral pallium lesions included the Dlv and Dld, and the major extension lesions included small parts of the Dc and Dd. The telencephalon ablations were complete and did not affect the preoptic area, optic tracts, or optic tectum. The sham-operated animals did not present any damage in the telencephalon or optic tectum.
Figure 4.
Schematic representation of the location and extent of the lesions in experiment 2: whole TEL, LP, and MP lesions. Light shading represents the largest extension, and dark shading represents the smallest. For abbreviations, see Results and Figure 2 legend.
Acquisition of conditioned avoidance response
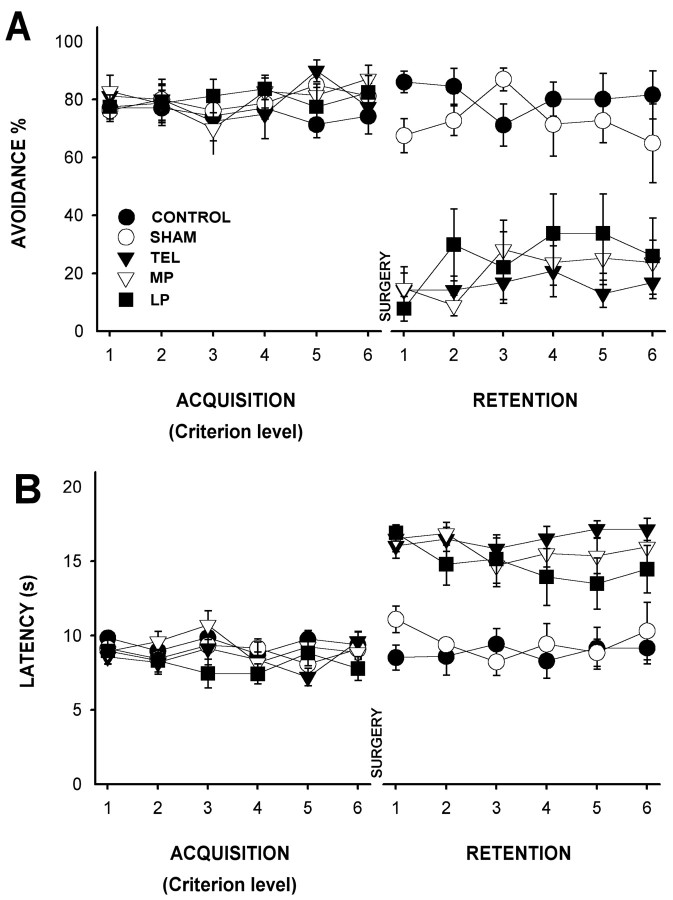
As in the previous experiment, the training and the acquisition period finished when the animals reached the criterion of learning. The latency and avoidance of the acquisition criterion level sessions were compared and analyzed between the experimental groups. The ANOVA analysis with repeated measurements showed no significant differences between the five groups in either latency (F(4, 33) = 2.3; p > 0.08) or avoidance (F(4, 33) = 0.833; p > 0.51; Fig. 3A,B). Before surgery, the avoidance response and the latency values were similar in all groups (Fig. 5).
Figure 5.
Trace avoidance conditioning performance of the different groups during acquisition and retention phases in experiment 2. A, Mean ± SEM of percentage of avoidance response in acquisition (6 sessions after reaching the learning criterion before surgery) and retention (6 sessions after surgery) phases. B, Mean ± SEM of response latency (seconds) in acquisition and retention periods.
Retention of conditioned avoidance response
In this phase, statistically significant differences between the experimental groups in avoidance response (ANOVA with repeated measurements; F(4, 33) = 21.742; p < 0.001) and latency (F(4, 33) = 16.58; p < 0.001) were found (Fig. 4A,B). Post hoc analyses indicated significant differences between the telencephalon-ablated, sham-operated, and control groups in avoidance (HSD-Tukey, α = 0.05; p < 0.001) and latency (p < 0.001; Fig. 5A,B). The MP group showed significant differences with the sham-operated and control groups in avoidance and latency (p < 0.001; Fig. 5A,B). Interestingly, in this experiment, the LP group also presented statistically significant differences with the sham-operated and control groups in avoidance (p < 0.001) and latency (p < 0.001; Fig. 5A,B). The sham-operated and control groups did not present significant differences in either avoidance (p > 0.95) or latency (p > 0.98). Finally, the performance of the three brain-injured experimental groups was similar in avoidance (p > 0.83) and latency (p > 0.61; Fig. 5).
The acquisition and the retention phase were compared by means of a two-tailed Student's t test (last session of acquisition vs first session of retention). Results indicated significant differences for the telencephalon-ablated, MP, and LP groups in avoidance (t(7) > 7.04; p < 0.001; t(6) > 11.310; p < 0.001; t(7) > 10.247; p < 0.001, respectively) and latency (t(7) > 4.959; p < 0.002; t(6) > 6.55; p < 0.001; t(7) > 13.69; p < 0.001, respectively; Fig. 5A,B). The sham-operated and control groups did not present any differences in either avoidance (t(7) < 2.03; p > 0.82; t(6) < 1.68; p > 0.14, respectively) or latency (t(7) < 1.452; p > 0.19; t(6) < 1.733; p > 0.13, respectively; Fig. 5A,B). Thus, the results of experiment 2 showed that when the experimental procedure emphasized the temporal factor, the LP lesion produced devastating deficits in conditioned avoidance learning (Figs. 3, 5).
Discussion
Involvement of the goldfish medial pallium in avoidance conditioning
The present results show, for the first time, that damage to the teleost fish medial pallium produces a deficit in the retention of conditioned avoidance as severe as that after ablation of the whole telencephalon (Savage, 1969; Overmier and Papini, 1985, 1986; Papini, 1985; Overmier and Hollis, 1990). In contrast, the lateral pallium lesion had no significant effects on the retention of avoidance in the nontrace procedure. Therefore, the present results suggest that the medial pallium could be the main telencephalic area involved in this kind of learning.
Although the latency values decreased progressively in the telencephalon-ablated and MP-lesioned groups along the postsurgical training sessions in the nontrace procedure (experiment 1), latency was consistently >10 sec (escape latency) without reaching avoidance values during the retention period (Fig. 3). These data indicate that the telencephalon-ablated and MP-lesioned animals were able to improve escape responses along the postsurgical sessions but not to produce avoidance responses. In a previous study (Savage, 1969), forebrain-less fish reached, after surgery, avoidance response levels similar to those of the MP group in the present experiment (∼50%), but they did not exceed this percentage along 26 sessions.
There is good evidence that avoidance learning is based on the acquisition of a mediational state of fear in goldfish (Gallon, 1972; Overmier and Starkman, 1974; Portavella et al., 2003), which, in turn, contributes to the development of the instrumental avoidance stimulus-response association because of a reduction in fear after the shuttle response (Mowrer, 1960; Flood et al., 1976; Overmier and Hollis, 1990; Zhuikov et al., 1994; Portavella et al., 2003). The deficit caused by the MP lesion in goldfish could be attributable to a deficit in the retrieval of the anticipatory fear response to the warning stimulus or could be caused by interfering with the ability of an internal state of fear to induce an avoidance response (Mowrer, 1960; Flood et al., 1976). Thus, the present results indicate that the fish telencephalon contains an emotional system that is critical for fear conditioning, and the MP is an essential element in this specialized system. The MP of teleost fish has been proposed as anatomically homologous to the pallial amygdala of mammals (Nieuwenhuys and Meek, 1990; Braford, 1995; Northcutt, 1995; Butler 2000). In consonance with this proposal, the present results demonstrate a striking functional similarity between the teleost MP and the pallial amygdala (Aggleton, 1992, 2001; LeDoux, 1995). In contrast, an MP lesion does not impair learned motor responses (as indicated by the low escape latencies of the animals in the MP-lesioned group), spatial memory (Rodríguez et al., 2002), or trace avoidance learning (present results, experiment 2). Thus, the present results do not support the proposal of homology between the teleost fish MP (Dmv) and the land vertebrate basal ganglia or the hippocampus (Echteler and Saidel, 1981; Murakami et al., 1983; Ito et al., 1986; Parent, 1986) on the basis of functional features.
Involvement of the goldfish lateral pallium in trace avoidance conditioning
Unlike in experiment 1, the LP lesion impaired performance in the trace-conditioning procedure. The main difference between the behavioral procedures used in experiments 1 and 2 was the presence of an interstimulus temporal gap of 5 sec (trace avoidance conditioning). Consequently, our results show that the LP of actinopterygian fish plays a major role in the retention of conditioned avoidance in a trace procedure. The lateral pallium of teleost fish, like the hippocampus of mammals, is involved in the analysis of temporal attributes of the task, the maintenance of the trace of the warning stimulus for conditioning, or both. The mammalian hippocampus seems to be involved in the acquisition and retention of conditioned avoidance, conditioned fear, and trace conditioning when contextual, spatial, and temporal factors are relevant to establishing associations between stimuli and responses (O'Keefe and Nadel, 1978; Woodruff and Kantor, 1983; Meck et al., 1984; Olton, 1986; Kesner and DiMattia, 1987; Thompson et al., 1987; Moyer et al., 1990; Phillips and LeDoux, 1992; Rawlins et al., 1993; Yee and Rawlins, 1994; Cassaday and Rawlins, 1995; Corodimas and LeDoux, 1995; Winocur, 1997). More specifically, it has been proposed that the mammalian hippocampus is directly involved in the processing of the duration, sequence, and temporal order of stimuli (Meck et al., 1984; Olton, 1986; Kesner and DiMattia, 1987). The present results thus demonstrate a striking functional similarity between the mammalian hippocampal pallium and the LP of teleost fish and support the hypothesis of homology between the fish LP and the mammalian hippocampus previously proposed on the basis of morphological features (Nieuwenhuys and Meek, 1990; Braford, 1995; Northcutt, 1995; Butler, 2000) and functional data (Rodríguez et al., 2002).
However, the hippocampus has not been related to temporal processing functions in nonmammalian vertebrates (Macphail, 1987, 1996; Bingman, 1990; Hampton and Shettleworth, 1996; Macphail, 1987, 1996). Furthermore, some authors have proposed that the nonspatial functions of the mammalian hippocampus may be related to unsought damage to cortical areas adjacent to the hippocampus (e.g., entorhinal cortex) during the surgical procedure (O'Keefe, 1993; Nadel, 1995; Guillazo-Blanch et al., 2002). Thus, these cortical areas (close to the hippocampus) may be involved in nonspatial functions (i.e., temporal attribute processing). In our case, the lesions of the LP extended to the Dld, which has been proposed as homologous to the isocortex of tetrapods (Braford and Northcutt, 1974; Northcutt and Braford, 1980; Northcutt and Davis, 1983; Butler, 1994). Although further studies on the functional implications of the fish lateral pallium are necessary, our results present a thought-provoking scene in which the functional involvement of the teleost fish LP is discussed in functional and theoretical terms similar to those for hippocampal formation.
Teleost fish medial and lateral telencephalic pallia maintain functional parallelism with mammalian hippocampus and amygdala: implications for vertebrate forebrain evolution
The present results demonstrate a striking functional similarity between the MP and LP of teleost fish and the pallial amygdala and hippocampal pallium of mammals, respectively. On the basis of anatomical and developmental evidence, the MP of teleost fish is considered homologous to the pallial amygdala of mammals, whereas the LP corresponds to the hippocampal pallium (Northcutt and Braford, 1980; Nieuwenhuys and Meek, 1990; Braford, 1995; Butler, 2000). The forebrain of ray-finned fishes develops by a process of eversion or outward bending of the prosencephalic walls of the prosencephalic vesicle (Nieuwenhuys, 1963; Northcutt and Braford, 1980). This eversion process reverses the pallial to medial-to-lateral topography observed in mammals. Thus, the developmentally lateral (amygdalar) pallium is predicted as lying medially in ray-finned fishes, whereas the developmentally lateral (hippocampal) pallium is predicted as occupying a medial position. The present functional data provide additional support for the eversion hypothesis of teleost telencephalon development, with considerable preservation of the original topology, and could contribute significantly to clarifying the identity of the pallial areas in ray-finned fishes.
Furthermore, the close similarity between the function of homologous telencephalic pallial areas suggests that the forebrain of vertebrates contains a common, conserved pattern of basic organization. Ray-finned fishes and land vertebrates share a common ancestor that lived some 400 million years ago (Carroll, 1988). Following a parsimony principle, the present results suggest that a system of emotional memory (medial pallium based) and another (or others) of spatial, relational, or temporal memory (lateral pallium based) could have appeared early during phylogenesis and could have been conserved throughout vertebrate evolution.
Footnotes
This research was supported by Spanish Ministerio de Ciencia y Tecnología Grants BF I2001-3178, I2000-0315, I2003-0029, PB 96-1334) and Junta de Andalucía CVI-242. We thank M. T. Gutiérrez, G. Labrador, and E. Cuetos for technical assistance.
Correspondence should be addressed to Manuel Portavella, Laboratorio de Psicobiología, Departamento de Psicología Experimental, Facultad de Psicología, Universidad de Sevilla, C/Camilo José Cela s/n, 41018 Seville, Spain. E-mail: portavel@us.es.
Copyright © 2004 Society for Neuroscience 0270-6474/04/242335-08$15.00/0
References
- Aggleton JP (1992) The amygdala: neurobiological aspects of emotion, memory, and mental dysfunction. New York: Wiley-Liss.
- Aggleton JP (2001) The amygdala: a functional analysis. Oxford: Oxford UP.
- Ambrogi-Lorenzini C, Bucherelli C, Giachetti A, Mugnai L, Tassoni G (1991) Effects of nucleus basolateralis amygdalae neurotoxic lesions on aversive conditioning in the rat. Physiol Behav 49: 765-770. [DOI] [PubMed] [Google Scholar]
- Bingman VP (1990) Spatial navigation in birds. In: Neurobiology of comparative cognition (Kesner R, Olton DS, eds), pp 423-447. Hillsdale, NJ: Erlbaum.
- Braford MR (1995) Comparative aspects of forebrain organization in the ray-finned fishes: touchstones or not? Brain Behav Evol 46: 259-274. [DOI] [PubMed] [Google Scholar]
- Braford MR, Northcutt GR (1974) Olfactory bulb projections in the bichir, Polypterus J Comp Neurol 156: 165-178. [DOI] [PubMed] [Google Scholar]
- Butler AB (1994) The evolution of the dorsal pallium in the telencephalon of amniotes: cladistic analysis and a new hypothesis. Brain Res Rev 19: 66-101. [DOI] [PubMed] [Google Scholar]
- Butler AB (2000) Topography and topology of the teleost telencephalon: a paradox resolved. Neurosci Lett 293: 95-98. [DOI] [PubMed] [Google Scholar]
- Carroll RL (1988) Vertebrate paleontology and evolution. New York: Freeman.
- Cassaday HJ, Rawlins JNP (1995) Fornix-fimbria section and working memory deficits in rats: stimulus complexity and stimulus size. Behav Neurosci 109: 594-606. [DOI] [PubMed] [Google Scholar]
- Corodimas KP, LeDoux JE (1995) Disruptive effects of posttraining perirhinal cortex lesions on conditioned fear: contributions of contextual cues. Behav Neurosci 109: 613-619. [DOI] [PubMed] [Google Scholar]
- Davis RE (1968) Environmental control of memory fixation in goldfish. J Comp Physiol Psychol 65: 72-78. [DOI] [PubMed] [Google Scholar]
- Echteler SM, Saidel WM (1981) Forebrain projections in the goldfish support telencephalic homologies with land vertebrates. Science 212: 683-685. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Otto T, Cohen NJ (1992) The hippocampus: what does it do? Behav Neural Biol 57: 2-36. [DOI] [PubMed] [Google Scholar]
- Flood NB, Overmier JB, Savage GE (1976) The teleost telencephalon and learning: an interpretative review of data and hypotheses. Physiol Behav 16: 783-798. [DOI] [PubMed] [Google Scholar]
- Gallon RL (1972) Effects of pre-training with fear and escape conditioning on shuttle-box avoidance acquisition by goldfish. Psychol Rep 31: 919-924. [DOI] [PubMed] [Google Scholar]
- Gray JA, McNaughton N (1983) Comparison between the behavioural effects of septal and hippocampal lesions: a review. Neurosci Biobehav Rev 7: 119-188. [DOI] [PubMed] [Google Scholar]
- Grossman SP, Grossman L, Walsh L (1975) Functional organization of the rat amygdala with respect to avoidance behavior. J Comp Physiol Psychol 88: 829-850. [DOI] [PubMed] [Google Scholar]
- Guillazo-Blanch G, Nadal R, Vale-Martinez A, Martí-Nicolovius M, Arévalo R, Morgado-Bernal I (2002) Effects of fimbria lesions on trace two-way active avoidance acquisition and retention in rats. Neurobiol Learn Mem 78: 406-425. [DOI] [PubMed] [Google Scholar]
- Hampton RR, Shettleworth S (1996) Hippocampal lesions impair memory for location but not color in passerine birds. Behav Neurosci 110: 831-835. [DOI] [PubMed] [Google Scholar]
- Horner JL, Longo N, Bitterman ME (1961) A shuttle box for fish and a control circuit of general applicability. Am J Psychol 74: 114-120. [PubMed] [Google Scholar]
- Ito H, Murakami T, Fukuoka T, Kishida R (1986) Thalamic fiber connections in a teleost (Sebasticus marmoratus): visual, somatosensory, octavolateral, and cerebellar relay region to the telencephalon. J Comp Neurol 250: 215-227. [DOI] [PubMed] [Google Scholar]
- Kesner RP, DiMattia BV (1987) Neurobiology of an attribute model of memory. Prog Psychobiol Physiol Psychol 12: 207-277. [Google Scholar]
- LeDoux JE (1995) Emotions: clues from the brain. Annu Rev Psychol 46: 209-235. [DOI] [PubMed] [Google Scholar]
- Macphail EM (1987) Brain and intelligence in vertebrates. Oxford: Clarendon.
- Macphail EM (1996) Cognitive function in mammals: the evolutionary perspective. Cognit Brain Res 3: 279-290. [DOI] [PubMed] [Google Scholar]
- Marino-Neto J, Sabbatini RM (1983) Discrete telencephalic lesions accelerate the habituation rate of behavioral arousal responses in Siamese fighting fish (Betta splendens). Braz J Med Biol Res 16: 271-278. [PubMed] [Google Scholar]
- McIntyre M, Stein DG (1973) Differential effects of one- versus two-stage amygdaloid lesions on activity, exploration, and avoidance behavior in the albino rat. Behav Biol 9: 451-465. [DOI] [PubMed] [Google Scholar]
- Meck WH, Church RM, Olton DS (1984) Hippocampus, time and memory. Behav Neurosci 98: 3-22. [DOI] [PubMed] [Google Scholar]
- Mowrer OH (1960) Learning theory and behavior. New York, Wiley.
- Moyer Jr JR, Deyo RA, Disterhoff JE (1990) Hippocampectomy disrupts trace eye-blink conditioning in rabbits. Behav Neurosci 104: 243-252. [DOI] [PubMed] [Google Scholar]
- Murakami T, Morita Y, Ito H (1983) Extrinsic and intrinsic fiber connections of the telencephalon in a teleost, Sebasticus marmoratus J Comp Neurol 216: 115-131. [DOI] [PubMed] [Google Scholar]
- Nadel L (1995) The role of the hippocampus in declarative memory: a comment on Zola-Morgan, Squire, and Ramus (1994). Hippocampus 5: 232-239. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuys R (1963) The comparative anatomy of the actinopterigian forebrain. J Hirnforsch 6: 171-200. [PubMed] [Google Scholar]
- Nieuwenhuys R, Meek J (1990) The telencephalon of actinopterygian fishes. In: Comparative structure and evolution of the cerebral cortex (Jones EG, Peters A, eds), pp 31-73 New York: Plenum.
- Northcutt RG (1995) The forebrain of gnathostomes: in search of a morphotype. Brain Behav Evol 46: 275-318. [DOI] [PubMed] [Google Scholar]
- Northcutt RG, Braford MR (1980) New observations on the organization and evolution of the telencephalon of actinopterygian fishes. In: Comparative neurology of the telencephalon (Ebbesson SOE, ed), pp 41-98. New York: Plenum.
- Northcutt RG, Davis RE (1983) Telencephalic organization in ray-finned fishes. In: Fish neurobiology, Vol 2 (Northcutt RG, Davis RE, eds), pp 203-236. Ann Arbor: University of Michigan. [Google Scholar]
- O'Keefe J (1993) Hippocampus, theta, and spatial memory. Curr Opin Neurobiol 3: 917-924. [DOI] [PubMed] [Google Scholar]
- O'Keefe J, Nadel L (1978) The hippocampus as a cognitive map. Oxford: Clarendon.
- Olton DS (1986) Hippocampal function and memory for temporal context. In: The hippocampus, Vol 3 (Isaacson RL, Pribram KH, eds), pp 281-298. New York: Plenum. [Google Scholar]
- Overmier JB, Hollis KL (1990) Fish in the think tank: learning, memory and integrated behavior. In: Neurobiology of comparative cognition (Kesner RP, Olton DS, eds), pp 204-236. Hillsdale, NJ: Erlbaum.
- Overmier JB, Papini MR (1985) Serial ablations of the telencephalon and avoidance learning by goldfish (Carassius auratus). Behav Neurosci 99: 509-520. [DOI] [PubMed] [Google Scholar]
- Overmier JB, Papini MR (1986) Factors modulating the effects of teleost telencephalon ablation on retention, relearning, and extinction of instrumental avoidance behavior. Behav Neurosci 100: 190-199. [DOI] [PubMed] [Google Scholar]
- Overmier JB, Savage GE (1974) Effects of telencephalic ablation on trace classical conditioning of heart rate in goldfish. Exp Neurol 42: 339-346. [DOI] [PubMed] [Google Scholar]
- Overmier JB, Starkman N (1974) Transfer of control of avoidance behavior in normal and telencephalon ablated goldfish (Carassius auratus). Physiol Behav 12: 605-608. [DOI] [PubMed] [Google Scholar]
- Papini MR (1985) Avoidance learning after simultaneous versus serial telencephalic ablations in the goldfish. Bull Psychon Soc 23: 160-163. [Google Scholar]
- Parent A (1986) Comparative neurobiology of the basal ganglia. New York: Wiley.
- Peter RE, Gill VE (1975) A stereotaxic atlas and technique for forebrain nuclei of the goldfish, Carassius auratus. J Comp Neurol 159: 69-102. [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE (1992) Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci 106: 274-285. [DOI] [PubMed] [Google Scholar]
- Portavella M, Vargas JP, Salas C, Papini MR (2003) Involvement of the telencephalon in spaced-trial avoidance learning in the goldfish (Carassius auratus). Physiol Behav 80: 49-56. [DOI] [PubMed] [Google Scholar]
- Rawlins JNP, Lyford GL, Seferiades A, Deacon RMJ, Cassaday HJ (1993) Critical determinants of non-spatial working memory deficits in rats with conventional lesions of the hippocampus or fornix. Behav Neurosci 107: 236-249. [DOI] [PubMed] [Google Scholar]
- Rodríguez R, López JC, Vargas JP, Gómez Y, Broglio C, Salas C (2002) Conservation of spatial memory function in the pallial forebrain of reptiles and ray-finned fishes. J Neurosci 22: 2894-2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Riolobos A (1986) Differential effect of chemical lesion and electrocoagulation of the central amygdaloid nucleus on active avoidance responses. Physiol Behav 36: 441-444. [DOI] [PubMed] [Google Scholar]
- Sara SJ, David-Remacle M (1981) Discriminative avoidance learning in hippocampal and cortical rats: acquisition rate, behavioral strategies, and long term retention. Physiol Psychol 9: 37-48. [Google Scholar]
- Savage GE (1969) Telencephalic lesions and avoidance behavior in the goldfish (Carassius auratus). Anim Behav 17: 362-373. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Tulving E (1994) Memory system. Cambridge, MA: MIT.
- Squire LR (1992) Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol Rev 99: 195-231. [DOI] [PubMed] [Google Scholar]
- Thompson RF, Donegan NH, Nelson H, Clark GA, Lavond DG, David G, Lincoln JS, Madden IV J, Manounas LA, Laura A, Mauk MD, McCormick DA, David A (1987) Classical conditioning, Chap 14 (Gormezano I, Prokasy WF, eds), pp 371-399. Hillsdale, NJ: Erlbaum.
- Vargas JP, Rodríguez F, López JC, Arias JL, Salas C (2000) Spatial learning-induced increase in the argyrophilic nucleolar organizer region of dorsolateral telencephalic neurons in goldfish. Brain Res 865: 77-84. [DOI] [PubMed] [Google Scholar]
- Weiner I, Feldon J, Tarrasch R, Hairston I, Joel D (1998) Fimbria-fornix cut affects spontaneous activity, two-way avoidance and delayed non matching to sample, but not latent inhibition. Behav Brain Res 96: 59-70. [DOI] [PubMed] [Google Scholar]
- Winocur G (1997) Hippocampal lesions alter conditioning to conditional and contextual stimuli. Behav Brain Res 88: 219-229. [DOI] [PubMed] [Google Scholar]
- Woodruff ML, Kantor H (1983) Fornix lesions, plasma ACTH levels, and shuttle box avoidance in rats. Behav Neurosci 97: 897-907. [DOI] [PubMed] [Google Scholar]
- Yee BK, Rawlins JNP (1994) The effects of hippocampal formation ablation or fimbria-fornix section on performance of a non-spatial radial arm maze task by rats. J Neurosci 14: 3766-3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerbolio DJ, Royalty JL (1983) Matching and oddity conditional discrimination in the goldfish as avoidance responses: evidence for conceptual avoidance learning. Anim Learn Behav 11: 341-348. [Google Scholar]
- Zhuikov AY, Couvillon PA, Bitterman ME (1994) Quantitative two-process analysis of avoidance conditioning in goldfish. J Exp Psychol Anim Behav Process 20: 32-43. [PubMed] [Google Scholar]