Abstract
Huntington's disease (HD) is a devastating neurodegenerative disorder caused by a CAG repeat expansion encoding an extended polyglutamine tract in the huntingtin protein. Transgenic mice expressing a human huntingtin transgene containing an expanded CAG repeat (R6/1 model) develop a neurodegenerative disorder closely resembling human HD. Previous work demonstrated that environmental enrichment delays the onset of motor symptoms in this mouse model. We confirmed that at 5 months of age, enrichment ameliorates motor symptoms (assessed using the rotarod test) and prevents loss of body weight induced by the HD transgene. We further examined molecular consequences of enrichment by determining changes in protein levels in the neostriatum, hippocampus, and anterior cortex using quantitative Western blot analysis. Non-enriched HD mice have severe reductions in BDNF in the hippocampus and striatum at 5 months, which are entirely rescued by enrichment. BDNF levels are unaltered by HD in the anterior cortex, suggesting that enrichment might prevent HD-induced impairment of anterograde transport of this neurotrophin to the striatum. NGF is unaffected by HD. Non-enriched HD mice also exhibit deficits in dopamine and cAMP-regulated phosphoprotein (32 kDa) in striatum and anterior cortex. Environmental enrichment rescues the cortical but not the striatal deficit at 5 months. These results suggest that environmental enrichment benefits animals at early stages of the disease by rescuing protein deficits, possibly through rescuing transcription or protein transport problems.
Keywords: Huntington's disease, environmental enrichment, BDNF, DARPP-32, transgenic mouse model, R6/1, NGF
Introduction
Huntington's disease (HD) is a fatal neurodegenerative disorder characterized by progressive cognitive, psychological, and motor symptoms. It is a trinucleotide repeat disease (Harper, 1996) caused by an expanded CAG repeat in exon 1 of the huntingtin gene, which translates into an abnormally long polyglutamine tract in the huntingtin protein (Huntington's Disease Collaborative Research Group, 1993). Neuropathology of HD includes neuronal death in the corpus striatum and neocortex, preceded by neuronal dysfunction (for review, see Davies and Ramsden, 2001). The mechanisms leading from the expanded polyglutamine tract to cell dysfunction and death are not yet fully understood.
Several transgenic mouse models have been developed to study HD, of which the R6 lines are probably the best characterized. These mice express exon 1 of the huntingtin gene containing an expanded CAG repeat. The R6/1 model used in these experiments, with some 112-120 repeats, closely resembles human HD in symptomatology and neuropathology (Mangiarini et al., 1996; Sathasivam et al., 1999).
Environmental enrichment has been shown to delay disease progress in the R6/1 mouse model of HD (van Dellen et al., 2000a) and in the related R6/2 model with a longer expansion (Hockly et al., 2002). Interestingly, remotivation therapy improves functioning in HD patients (Sullivan et al., 2001), indicating that environmental stimulation may also benefit human HD sufferers. However, the mechanisms underlying the beneficial effects of enrichment remain unknown.
HD is known to cause transcriptional dysregulation of several molecular systems, including neurotransmitter receptors and intracellular signaling mechanisms (Cha, 2000; Luthi Carter et al., 2000), and environmental enrichment increases transcription of some proteins, including neurotrophic factors, in the hippocampus (Pham et al., 1999a,b; Young et al., 1999). Neurotrophins contribute to the regulation of neuronal development, plasticity, and survival, making them important in mediating the progression of neurodegenerative diseases. BDNF expression has specifically been shown to be downregulated in human HD sufferers (Ferrer et al., 2000a; Zuccato et al., 2001) and in R6/2 HD mice (Luthi Carter et al., 2002), and wild-type huntingtin protein upregulates BDNF transcription (Zucatto et al., 2001).
Expression of the HD mutation in transgenic mice also causes downregulation of dopamine and cAMP-regulated phosphoprotein, 32 kDa (DARPP-32), a pivotal regulator of dopamine and serotonin signaling (Bibb et al., 2000; van Dellen et al., 2000b), and of dopamine receptors D1 and D2 (Cha et al., 1998). DARPP-32 is enriched in prefrontal cortex and striatum (Ouimet et al., 1984, 1992; Perez and Lewis, 1992), where it mediates dopamine and serotonin signaling (Svenningsson et al., 2002).
Here, we test whether environmental enrichment could be ameliorating HD symptoms by rescuing protein deficits caused by transcriptional dysregulation or disrupted protein trafficking. In particular, we examine BDNF, NGF, and DARPP-32 protein in the hippocampus, striatum, and the anteromedial cortex, which projects densely to the striatum. Our results not only suggest a molecular basis for the beneficial effects of enrichment but also point to a possible mechanism of the disease process.
Materials and Methods
Animals. R6/1 mice transgenic for the promoter and exon 1 of the expanded human huntingtin gene and littermate wild-type controls were bred in-house from a colony that originated from crossing male R6/1 mice (Mangiarini et al., 1996) (The Jackson Laboratory, Bar Harbor, ME) with female CBA mice. The R6/1 male offspring were crossed again with CBA females for several generations (past F10) to attain an almost pure CBA background. A single background strain is important to ensure consistency in behavioral testing. Groups of four to six mice were housed in large-sized rodent cages (dimensions, 44 × 28 × 12.5 cm) with ad libitum access to food and water. Half of the mice (selected randomly) were given environmental enrichment in the home cage from the age of 4 weeks, whereas the other half received only normal bedding. At 4 weeks of age, tail tissue was taken from experimental animals for PCR genotyping, and a microchip (Labtrac, Uckfield, UK) was inserted subcutaneously for identification under general anesthesia induced with hypnorm (fentanyl citrate; Janssen Pharmaceutica, Berse, Belgium) and hypnovel (midazolam; Roche, Hertforshire, UK) in distilled water (1:1:2; 2.7 ml/kg). Approximately half of the animals carried the HD transgene. Both male and female mice were used in these experiments in equal numbers. In previous studies, BDNF immunoreactivity had been indistinguishable between the sexes (Conner et al., 1997). All animal work conformed to United Kingdom regulations and was performed under a Home Office project license.
Environmental enrichment. Enriched mice were exposed to nontoxic objects placed in the home cage that were changed every 2 d (van Dellen et al., 2000a). The objects consisted of small cardboard boxes, small open wooden boxes, cylindrical cardboard tunnels ∼3 cm in diameter, and folded sheets of paper ∼10 × 10 cm.
Behavioral testing. A cohort of mice at 5 months of age consisting of 80 mice [15 wild-type non-enriched (WTNE), 24 HD non-enriched (HDNE), 18 wild-type enriched (WTE), and 23 HD enriched (HDE)] was tested for motor function.
A rotarod (Ugo Basile model 7650; Sandown Scientific, Hampton, UK) was used as a measure of motor symptoms. For this test, mice were placed on a 6 cm section of the central cylinder of the apparatus, which was 3 cm in diameter and had 2 mm longitudinal ridges. The cylinder rotated at an initial rate of 3.5 rpm and accelerated gradually at 20 rpm/min to a maximum of 40 rpm. When a mouse could no longer stay on the rod, it fell a few centimeters onto a sprung platform, and the amount of time that the mouse remained on the accelerating rod was recorded.
Tissue preparation. Tissue samples were obtained from mice aged 5 months (10 WTNE, 7 HDNE, 7 WTE, and 6 HDE). Mice were weighed (N2B110; Ohaus, Pine Brook, NJ) then deeply anesthetized with pentobarbitone (Euthetal; 200 mg/kg, i.p.; Rhone Merieux, Harlow, UK) and decapitated. Brains were removed quickly and weighed (Precisa 125A; Precisa, Milton Keynes, UK), and the striatum, anteromedial quarter of the cortical sheet, and hippocampus were dissected on ice. Dissected tissue samples were weighed and homogenized (homogenizer from Fischer Scientific, Pittsburgh, PA) in 0.1 m PBS (10× v/w; Sigma, St Louis, MO) containing protease inhibitor mixture (1:25 v/v; P8340; Sigma). Protein concentration in homogenized samples was determined using the Bradford method (Bio-Rad DC protein assay; Bio-Rad, Hercules, CA), and samples were stored at -80°C. Each sample was coded so that experiments and analyses were performed blind to genotype and housing conditions.
Immunoblotting. Equal amounts of protein from each sample were boiled for 5 min in sample buffer containing 5% mercaptoethanol, 2% SDS, and 10% glycerol (Sigma). Proteins were separated on 10% polyacrylamide gels by SDS-PAGE at 50 mA (Bio-Rad mini-protean system), then transferred overnight at ∼30 V onto 0.45 μm nitrocellulose (Bio-Rad). To confirm that equal amounts of protein were transferred from each sample, membranes were stained with 0.1% amido dye (Sigma) in distilled water then digitally scanned and analyzed using Image software (Scion, Fredrick, MD). The total amount of protein on the tested membranes never varied >5% between lanes. After amido staining, membranes were rinsed, then probed for BDNF, NGF, or DARPP-32 immunoreactivity using ECL (Amersham, Little Chalfont, UK) according to the manufacturer's instructions. Briefly, membranes were blocked for 1 hr in 5% powdered milk in PBS buffer containing 0.1% Triton detergent (PBST; Sigma) at pH 7.4, followed by a 2 hr incubation in primary antibodies to BDNF (Santa Cruz 546; Santa Cruz Biotechnology, Santa Cruz, CA), NGF (Santa Cruz Biotechnology), or DARPP-32 (Chemicon, Temecula, CA) diluted in PBST to final concentrations of 1:1000 BDNF, 1:1000 NGF, or 1:10,000 DARPP-32. After washing, a solution of secondary HRP-conjugated anti-rabbit antibody (Amersham) at a dilution of 1:1000 in PBST was applied to the membrane and incubated for 1 hr. Bands were visualized with ECL developing solution (Amersham). For each condition, more than one immunoblot was performed to ensure reproducibility. One of the blots from each condition was also stripped in buffer containing 0.2 m glycine (Sigma), pH 2.5, for 20 min at 80°C, then reprobed for β-tubulin (Covance Research Products, Berkeley, CA) at a concentration of 1:5000 as an additional control of equal protein loading.
Analysis. Averages (and SEM) were calculated for rotarod performance, body weight, and brain weight for each experimental group. The effects of the R6/1 genotype and enriched versus non-enriched housing conditions were assessed by two-way ANOVA using SPSS software (SPSS, Chicago, IL). The means of individual groups were compared using post hoc two-tailed t tests for independent variables with equal variance not assumed (SPSS).
Relative protein concentrations were quantified by comparing optical densities of protein bands using Image software (Scion). The optical density of each sample on the blot was normalized to the average optical density of all WTNE samples, giving a value of protein level as a percentage of WTNE. Averages for each group and SEM were calculated. The effects of the HD transgene and housing condition were analyzed using two-way ANOVA, and the means of individual groups were again compared using t tests (SPSS).
Results
Environmental enrichment ameliorates motor symptoms of HD
In non-enriched R6/1 mice, the onset of motor symptoms occurs at around 2 months of age, and by 5 months, all HDNE mice exhibit motor deficits (Mangiarini et al., 1996; van Dellen et al., 2000a). Environmental enrichment delays disease onset, and at 5 months, less than half of HDE mice exhibit motor symptoms (van Dellen et al., 2000a).
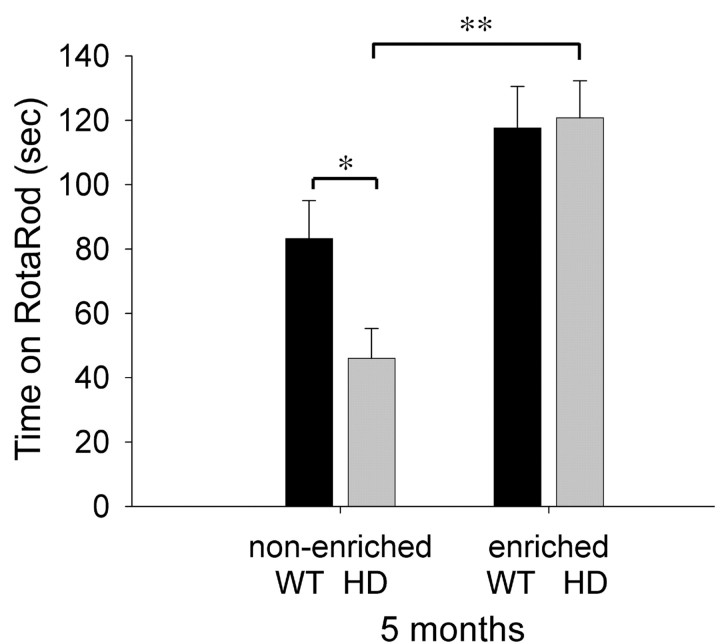
Using the accelerating rotarod test as an indicator of motor symptoms, we confirmed that prior environmental enrichment improves motor performance at 5 months (Fig. 1) (two-way ANOVA; F(1,76) = 22.452; p < 0.0001). HDNE mice performed much worse than WTNE animals (t test; p = 0.019), and enrichment increases the time that HD animals spend on the rod compared with WTNE animals (t test; p = 0.029) and HDNE animals (t test; p < 0.001). Thus, environmental enrichment completely alleviates the HD-induced deficit in rotarod performance at this age.
Figure 1.
Environmental enrichment enhances rotarod performance in HD and wild-type mice. At 5 months, environmental enrichment rescues an HD-induced deficit in performance on the accelerating rotarod. Bars represent post hoc t tests: *p < 0.05; **p ≤ 0.001.
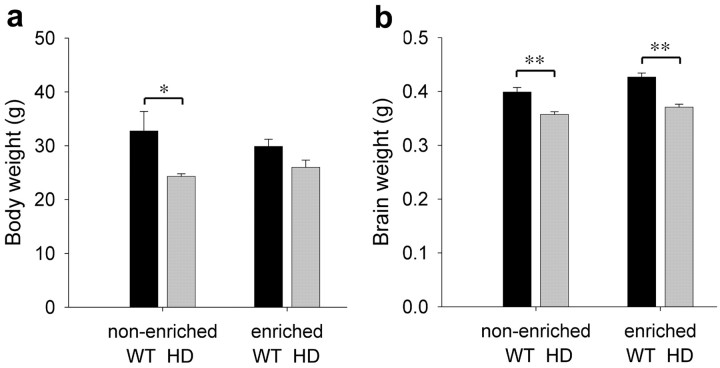
Body weight is significantly decreased by the HD transgene (Fig. 2) (two-way ANOVA; F(1,27) = 5.690; p = 0.024). Environmental enrichment partially ameliorates this HD-induced loss. Post hoc t tests confirmed that HDNE animals weigh less than WTNE mice (t test; p = 0.045). Enriched animals carrying the transgene are not significantly lighter than WTNE or WTE animals, indicating that enrichment prevents some of the weight loss associated with HD.
Figure 2.
HD causes a decrease in body and brain weight. a, The HD transgene causes a dramatic decrease in body weight of R6/1 mice at 5 months of age, and enrichment compensates for this loss. b, Brain weight is also reduced by the HD transgene, but enrichment increases brain weight in wild-type animals at 5 months. The enrichment-induced increase is not enough to compensate for the weight loss in HD. Bars represent post hoc t tests: *p < 0.05; **p ≤ 0.001.
The HD transgene also causes a decrease in brain weight (Fig. 2b) (two-way ANOVA; F(1,27) = 42.118; p < 0.001), and enrichment increases brain weight (two-way ANOVA; F(1,27) = 7.597; p = 0.010). Environmental enrichment slightly, but significantly, increases brain weight in wild-type animals (t test; p = 0.027). However, the HD-induced decrease in brain weight of non-enriched animals is not rescued. HDE animals have lower brain weight than either WTNE (t test; p = 0.013) or WTE (t test; p < 0.001) animals. Enrichment does not significantly increase brain weight in HD animals at this age, although there is a trend toward heavier brains in HDE animals compared with HDNE animals (p = 0.083).
Environmental enrichment rescues specific striatal and hippocampal BDNF deficits
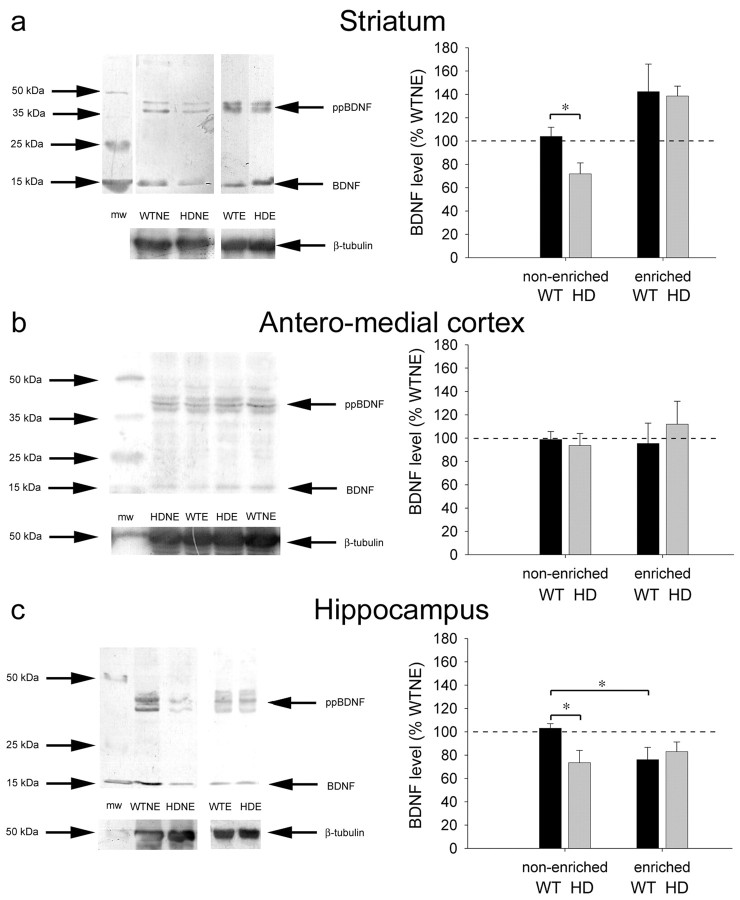
ECL for BDNF (Fig. 3) labeled a single band at 14 kDa corresponding to the dimer of mature BDNF. A doublet (or triplet) averaging 42 kDa was also labeled in all three brain areas examined, which may correspond to a precursor form of the protein prepro BDNF (ppBDNF). The Santa Cruz BDNF antibody used here has been used previously to quantify BDNF protein levels in human (Ferrer et al., 2000a,b; Dwivedi et al., 2003) and rodent (Fawcett et al., 2000).
Figure 3.
An HD-induced striatal BDNF deficit is rescued by environmental enrichment. ECL using an anti-BDNF antibody on tissue from the striatum, anteromedial cortex, and hippocampus shows the mature form of BDNF protein at 14 kDa and a doublet (or triplet) at 42 kDa, which corresponds to the prepro form of the protein (ppBDNF). a, Histograms of average BDNF levels (expressed as a percentage of WTNE levels ± SEM), show that HD causes a decrease of 30% in BDNF protein in the striatum at 5 months that is rescued by enrichment. Environmental enrichment rescues the deficit by increasing BDNF levels ∼40% over WTNE levels (two-way ANOVA; F(1,25) = 6.387; p < 0.05). b, In the anteromedial cortex, there is no effect of HD or enrichment on BDNF levels, suggesting that enrichment may compensate for a deficit in corticostriatal anterograde transport of BDNF. c, In the hippocampus, there is an HD-induced BDNF deficit in non-enriched animals that is rescued by environmental enrichment. In wild-type animals, enrichment actually decreases the level of BDNF in the hippocampus. After probing for BDNF, blots were stripped and reprobed for neuronal β-tubulin as a control of equal protein loading. Control bands are shown below BDNF bands in all conditions. Bars represent post hoc t tests: *p < 0.05.
We found that BDNF is dramatically reduced in the striatum of non-enriched symptomatic HD mice, as in human HD (Ferrer et al., 2000a). Even as early as 5 months of age, BDNF protein levels are 30% lower in the striatum of HDNE mice than in WTNE animals (t test; p = 0.021) (Fig. 3a). Encouragingly, enrichment, which clearly reduces symptoms at this early stage in the disease (Fig. 1) (van Dellen et al., 2000a), increases striatal BDNF levels (two-way ANOVA; F(1,25) = 16.055; p < 0.001). In the striatum of HDE mice, this increase more than compensates for the reduction of BDNF seen in HDNE animals, so that no deficit in protein level remains (Fig. 3a). Post hoc t tests reveal an increase in BDNF with enrichment in HD mice when compared with WTNE (p = 0.010) and HDNE (p < 0.001) animals. WTE animals also have higher levels of BDNF than HDNE mice (p = 0.015).
BDNF is not manufactured in the striatum but is anterogradely transported to it, especially from the cerebral cortex (Altar et al., 1997; Conner et al., 1997). In the anteromedial cortex of R6/1 HD mice, there is no obvious effect of HD on BDNF expression, suggesting that the HD mutation perturbs transport of BDNF from the cortex to striatum, and this effect is rectified by enrichment (Fig. 3b).
In the hippocampus, there is also an HD-induced BDNF deficit in non-enriched mice (Fig. 3c) but no difference in BDNF levels between HDE mice and WTE mice (t test; p = 0.621) or WTNE mice (trend toward decrease: t test; p = 0.064). This suggests that enrichment rescues the BDNF levels in hippocampus as in the striatum. Interestingly, although it prevents the decrease of BDNF levels associated with HD, environmental enrichment causes a decrease in BDNF levels in WT mice. BDNF mRNA is found in the hippocampus (Conner et al., 1997), indicating that the deficit in this area may be attributable to transcriptional dysregulation or altered catabolism of the protein and not necessarily disrupted protein trafficking, as we suggest for the striatum. However, we cannot rule out the possibility that areas projecting to the hippocampus (such as the entorhinal cortex) send BDNF to this area and that HD disrupts transport.
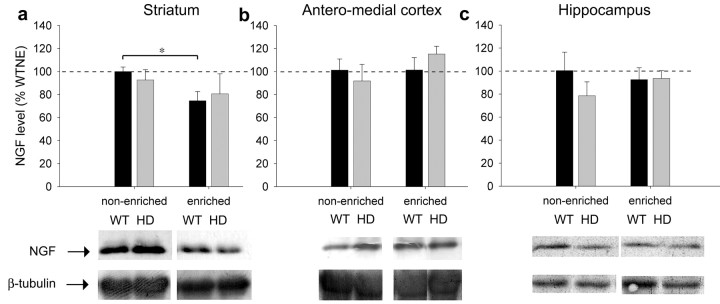
NGF, another important neurotrophin, is not affected by the HD transgene in the striatum, anterior cortex, or hippocampus (Fig. 4), indicating that the deficit in BDNF and the ameliorating effect of enrichment are specific to BDNF and that enrichment is not acting via blanket upregulation of neurotrophins. In fact, enrichment slightly decreases NGF levels in the striatum of wild-type mice (Fig. 4a).
Figure 4.
HD does not affect NGF levels. NGF protein levels are not affected by HD at 5 months in the striatum (a), anteromedial cortex (b), or hippocampus (c). Enrichment slightly decreases the level of NGF in the striatum but does not affect levels in the other areas (t test; * p < 0.05).
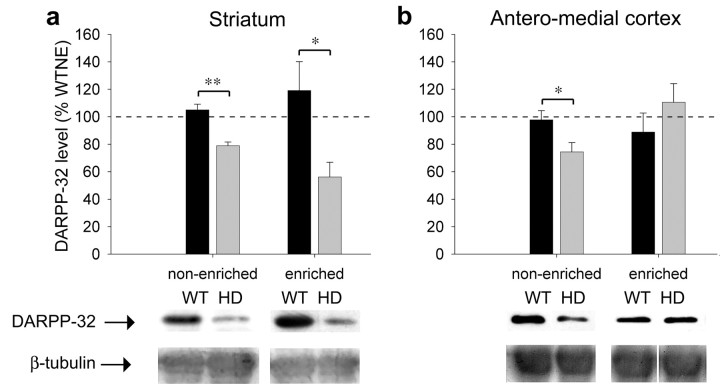
Environmental enrichment rescues a cortical DARPP-32 deficit
DARPP-32, a pivotal regulator of dopamine signaling, is downregulated in the striatum of R6/2 HD mice (Bibb et al., 2000; Luthi Carter et al., 2000), and reductions in DARPP-32 have been shown by qualitative observation of immunostaining in R6/1 mice (Van Dellen et al., 2000b). Here, we quantitatively confirm the DARPP-32 deficit in R6/1 symptomatic mice using Western blotting and ECL and explore the effects of environmental enrichment on DARPP-32 expression.
In the striatum at 5 months, DARPP-32 levels are significantly reduced, by ∼25%, in the striatum of HDNE mice (Fig. 5a) (t test; p < 0.001). However, this deficit is not rescued by enrichment: striatal DARPP-32 levels are decreased in HDE mice when compared with WTNE animals (t test; p = 0.004) and WTE animals (t test; p = 0.026). At the same age in the anterior cortex, the HD transgene also causes a decrease in DARPP-32 protein levels of ∼25% in non-enriched mice (Fig. 5b) (t test; p = 0.031). However in this area, environmental enrichment rescues the HD-induced deficit. DARPP-32 levels in the anterior cortex of HDE mice do not differ from WTNE (p = 0.433) or WTE (p = 0.290) mice. The increase in DARPP-32 with enrichment in HD mice, compared with HDNE mice, almost reached significance (p = 0.056).
Figure 5.
Enrichment corrects cortical DARPP-32 deficits. a, DARPP-32 levels are reduced, by ∼25%, in the striatum of HD mice at 5 months; however, this deficit is not rescued by enrichment at this age. In the anteromedial cortex of non-enriched mice (b), DARPP-32 levels are significantly lower in HD as opposed to wild-type (WT) mice. Environmental enrichment entirely rescues the HD-induced DARPP-32 deficit in this area. Bars represent post hoc t tests: *p < 0.05; **p < 0.01.
DARPP-32 mRNA is produced in both the striatum and cortex (Perez and Lewis, 1992), and striatal mRNA levels are known to be decreased by HD in another mouse model (Bibb et al., 2000; Luthi Carter et al., 2000). Thus, the decreases in the cortex and striatum of DARPP-32 protein observed here are probably attributable to transcriptional downregulation that is rectified by enrichment in the cortex.
Discussion
We have demonstrated that environmental enrichment ameliorates motor symptoms and rescues specific protein deficits in R6/1 mice. This indicates a possible molecular basis for the observed behavioral benefits of increased environmental stimulation, in both mice and human HD sufferers (van Dellen et al., 2000a; Sullivan et al., 2001; Hockly et al., 2002), and it points to a possible pathogenic mechanism of the disease process itself.
We show that environmental enrichment ameliorates motor symptoms at 5 months measured using the accelerating rotarod test, as would be expected from previous studies showing alleviation of symptoms at this age (van Dellen et al., 2000a). Enrichment also rescues the HD-induced decrease in body weight at 5 months. Although we find that enrichment increases total brain weight in wild-type animals as has been seen previously (Bennett et al., 1964; Rosenzweig and Bennett, 1996), the increase does not compensate for the HD-induced reduction. However, at the same age, enrichment of HD mice does rescue the specific decrease in volume of the area of forebrain surrounding the neostriatum (van Dellen et al., 2000a).
We investigate further the underlying molecular mechanisms of enrichment by examining protein levels in the striatum, anterior cortex, and hippocampus. Environmental enrichment rescues cortical, but not striatal, DARPP-32 levels in R6/1 HD mice at 5 months. Because DARPP-32 mRNA is present in both the cortex and striatum, we propose that the reduction of this protein with HD is attributable to transcriptional downregulation, as has been reported previously (Bibb et al., 2000; Luthi Carter et al., 2002). In the case of cortical DARPP-32, enrichment may then act by rescuing transcriptional abnormalities.
Downregulation of DARPP-32 (Bibb et al., 2000; van Dellen et al., 2000b) and of D1 and D2 dopamine receptors (Cha, 2000) disrupts dopamine signaling in the striatum and may contribute to neuronal dysfunction. The serotonergic and dopaminergic neurotransmitter systems, which signal through DARPP-32, contribute to cognition, emotion, mood, and reward. Deficits in these pathways caused by HD could contribute to psychiatric and cognitive symptoms of the disease. In the DARPP-32 knock-out mouse, both dopamine and serotonin signaling are disrupted (Fienberg and Greengard, 2000; Svenningsson et al., 2002), and reversal learning is impaired (Heyser et al., 2000). Hence, the rescue of cortical DARPP-32 deficits in R6/1 HD mice may contribute to observed behavioral benefits of enrichment by ameliorating aspects of neuronal dysfunction caused by disruption of dopamine and serotonin signaling.
Our most compelling finding is that environmental enrichment prevents striatal and hippocampal deficits in BDNF at a stage of the disease when HDE mice show far less severe motor symptoms than their HDNE littermates, as demonstrated in previous studies (van Dellen et al., 2000a) and by the rotarod experiment reported here (Fig. 1). In the striatum, enrichment causes an upregulation of BDNF that could explain the enhanced performance of both WTE and HDE mice on the rotarod. Neurotrophic factors facilitate neuronal development, plasticity, and survival, so their reduction could potentially be important in the pathological progression of neurodegenerative diseases. BDNF has specifically been shown to induce neurogenesis in the striatum, with new neurons expressing markers specific for medium spiny neurons, including DARPP-32 (Benraiss et al., 2001).
The upregulation of BDNF in the striatum with enrichment is specific to this neurotrophin because NGF levels are not raised by enrichment in this area. On the contrary, NGF levels in striatum are decreased by enrichment in wild-type mice. Differential response of neurotrophins to striatal distress has been observed previously (Canals et al., 1998).
We suggest that the decrease in BDNF protein in R6/1 striatum could be attributable to a disruption of corticostriatal anterograde transport of BDNF because levels of this neurotrophin are unaffected by the transgene in the cortex, which transports it to the striatum. However, the unchanged cortical levels of BDNF do not necessarily mean that the transgene is not affecting transcription. Transcription could be decreased by the transgene, but impaired anterograde trafficking could result in BDNF staying in the cortex instead of being shipped to the striatum. If the decrease of BDNF in the striatum is indeed attributable to disrupted corticostriatal transport, we would not expect to see an effect on NGF acting through the same mechanism, because NGF is not transported in the same manner. NGF is typically transported retrogradely, as in the case of basal forebrain cholinergic neurons (for review, see Sofroniew et al., 2001). Furthermore, it is endogenously expressed in the striatum by a subpopulation of interneurons (Bizon et al., 1999).
Rescuing BDNF levels in the striatum might promote cell survival and upregulate genes that are normally transcriptionally disrupted in HD, thus protecting against neuronal dysfunction. Indeed, BDNF specifically promotes survival of striatal projection neurons in an excitotoxic model of striatal neurodegeneration (Alberch et al., 2002). Furthermore, excitotoxic lesioning of the striatum in normal mice induces upregulation of BDNF in the cortical neurons responsible for supplying BDNF to the striatum via anterograde transport (Canals et al., 2001).
Normal huntingtin protein promotes BDNF transcription (Zuccato et al., 2001) and is involved in vesicular trafficking (DiFiglia et al., 1995). If environmental enrichment does indeed mitigate a deficit in corticostriatal anterograde transport of BDNF resulting from aberrant huntingtin, it might then partially compensate for this basic genetic failure. Our results lead us, then, to speculate that defects in corticostriatal transport might be a basic early pathogenic mechanism in HD.
We observe that environmental enrichment rescues an HD-induced BDNF deficit in the hippocampus as well as the striatum, but unlike in the striatum, enrichment causes a decrease in hippocampal BDNF level of wild-type animals. Although enrichment is generally seen to upregulate neurotrophins (Pham et al., 1999b; Young et al., 1999), inhibitory effects of enrichment on protein expression have also been observed. For example, in rats with focal strokes induced by cerebral ischemia, environmental enrichment prevents the increase in BDNF levels in hippocampus and cortex seen in non-enriched animals (Zhao et al., 2001). Functionally, the decrease in BDNF levels in the hippocampus may lead to decreased excitation, which would prevent excitotoxicity, because BDNF enhances spontaneous activity (Lessmann et al., 1994) and inhibits GABAergic transmission in this brain area (Tanaka et al., 1997).
The experiments presented here do not address which aspects of enrichment (cognitive stimulation, exercise, or feeding patterns) contribute to its beneficial effects. Cognitive stimulation in humans is important in preventing some aspects of neurodegenerative disease. Participating in cognitively stimulating activities has been associated with a reduced risk of Alzheimer's disease (Wilson et al., 2002), and lack of education increases the risk of dementia in Parkinson's disease (Glatt et al., 1996). Recent studies showed that exercise can increase BDNF levels in rats (Russo Neustadt et al., 2001; Berchtold et al., 2002). Enriched animals also eat less than non-enriched animals (Fiala et al., 1977), and a recent study has shown that dietary restriction can rescue BDNF levels and enhance motor performance in an HD model (Duan et al., 2003).
The involvement of corticostriatal BDNF transport in HD pathology and the rescue of DARPP-32 levels in the cortex highlight the importance of the cortex, and specifically the anterior cortex in this disease. In R6 transgenic mouse models, neurodegeneration, in the form of dark degenerating neurons, is most pronounced in the dorsal striatum and anterior cingulate cortex (Davies et al., 1999; Turmaine et al., 2000). The anteromedial area of cortex examined in our experiments includes anterior cingulate cortex, which has dense projections to the striatal medium spiny neurons that selectively degenerate in HD. Anterior cingulate cortex is involved in emotional responses and in sensory, motor, and cognitive processing (Vogt et al., 1992). Positron emission tomographic studies in patients with HD have revealed impairment of activity in the anterior cingulate cortex as well as the striatum (Mayberg et al., 1992). In R6/1 mice, transplantation of wild-type donor cortex to replace diseased anterior cingulate cortex delays the onset of a specific motor symptom of HD (van Dellen et al., 2001).
Taken together, these results imply that enrichment might act by reducing specific striatal, cortical, and hippocampal protein deficits. Specifically, the increase in neuronal activity in somatosensory, motor, and frontal cortices associated with enrichment may compensate for corticostriatal BDNF trafficking deficits. Indeed, BDNF transported anterogradely along axons has been shown to transfer to postsynaptic cells in an activity-dependent manner (Kohara et al., 2001). Reductions in BDNF expression in the hippocampus and DARPP-32 in the anterior cortex and striatum may also be counteracted by increased activity causing transcriptional upregulation (as seen in enriched rats) (Pham et al., 1999b). Our results show that environmental enrichment strongly influences the cortex and support the idea that early changes in the cortex contribute to HD pathogenesis. Finally, we suggest that BDNF might be a promising target for new therapeutic approaches to this devastating disease.
Footnotes
This work was supported by the Medical Research Council, Wellcome Trust, National Science Foundation, and Marshall Aid Commemoration Commission. We thank Andrew Ladwiniec and Denise Jelfs for excellent technical assistance.
Correspondence should be addressed to Dr. Tara Spires, Massachusetts General Hospital, Department of Neurology-Alzheimer's Disease Research Laboratory, 114 16th Street, Charlestown, MA 02129. E-mail: tara.spires@physiol.ox.ac.uk.
A. J. Hannan's present address: Howard Florey Institute, University of Melbourne, Melbourne, Australia VIC 2010.
Copyright © 2004 Society for Neuroscience 0270-6474/04/242270-07$15.00/0
References
- Alberch J, Perez Navarro E, Canals JM (2002) Neuroprotection by neurotrophins and GDNF family members in the excitotoxic model of Huntington's disease. Brain Res Bull 57: 817-822. [DOI] [PubMed] [Google Scholar]
- Altar CA, Cai N, Bliven T, Juhasz M, Conner JM, Acheson AL, Lindsay RM, Wiegand SJ (1997) Anterograde transport of brain-derived neurotrophic factor and its role in the brain. Nature 389: 856-860. [DOI] [PubMed] [Google Scholar]
- Bennett DAD, Diamond MC, Krech D, Rosenzweig MR (1964) Chemical and anatomical plasticity of brain. Science 146: 610-619. [DOI] [PubMed] [Google Scholar]
- Benraiss A, Chmielnicki E, Lerner K, Roh D, Goldman SA (2001) Adenoviral brain-derived neurotrophic factor induces both neostriatal and olfactory neuronal recruitment from endogenous progenitor cells in the adult forebrain. J Neurosci 21: 6718-6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berchtold NC, Kesslak JP, Cotman CW (2002) Hippocampal brain-derived neurotrophic factor gene regulation by exercise and the medial septum. J Neurosci Res 68: 511-521. [DOI] [PubMed] [Google Scholar]
- Bibb JA, Yan Z, Svenningsson P, Snyder GL, Pieribone VA, Horiuchi A, Nairn AC, Messer A, Greengard P (2000) Severe deficiencies in dopamine signaling in presymptomatic Huntington's disease mice. Proc Natl Acad Sci USA 97: 6809-6814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizon JL, Lauterborn JC, Gall CM (1999) Subpopulations of striatal interneurons can be distinguished on the basis of neurotrophic factor expression. J Comp Neurol 408: 283-298. [PubMed] [Google Scholar]
- Canals JM, Marco S, Checa N, Michels A, Perez Navarro E, Arenas E, Alberch J (1998) Differential regulation of the expression of nerve growth factor, brain-derived neurotrophic factor, and neurotrophin-3 after excitotoxicity in a rat model of Huntington's disease. Neurobiol Dis 5: 357-364. [DOI] [PubMed] [Google Scholar]
- Canals JM, Checa N, Marco S, Akerud P, Michels A, Perez Navarro E, Tolosa E, Arenas E, Alberch J (2001) Expression of brain-derived neurotrophic factor in cortical neurons is regulated by striatal target area. J Neurosci 21: 117-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha JH (2000) Transcriptional dysregulation in Huntington's disease. Trends Neurosci 23: 387-392. [DOI] [PubMed] [Google Scholar]
- Cha J-HJ, Kosinski CM, Kerner JA, Alsdorf SA, Mangiarini L, Davies SW, Penney JB, Bates GP, Young AB (1998) Altered brain neurotransmitter receptors in transgenic mice expressing a portion of an abnormal human Huntington disease gene. Proc Natl Acad Sci USA 95: 6480-6485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner JM, Lauterborn JC, Yan Q, Gall CM, Varon S (1997) Distribution of brain-derived neurotrophic factor (BDNF) protein and mRNA in the normal adult rat CNS: evidence for anterograde axonal transport. J Neurosci 17: 2295-2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies S, Ramsden DB (2001) Huntington's disease. Mol Pathol 54: 409-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies SW, Turmaine M, Cozens BA, Raza AS, Mahal A, Mangiarini L, Bates GP (1999) From neuronal inclusions to neurodegeneration: neuropathological investigation of a transgenic mouse model of Huntington's disease. Philos Trans R Soc Lond B Biol Sci 354: 971-979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFiglia M, Sapp E, Chase K, Schwarz C, Meloni A, Young C, Martin E, Vonsattel JP, Carraway R, Boyce FM, Aronin N (1995) Huntingtin is a cytoplasmic protein associated with vesicles in human and rat brain neurons. Neuron 14: 1075-1081. [DOI] [PubMed] [Google Scholar]
- Duan W, Guo Z, Jiang H, Ware M, Li XJ, Mattson MP (2003) Dietary restriction normalizes glucose metabolism and BDNF levels, slows disease progression, and increases survival in huntingtin mutant mice. Proc Natl Acad Sci USA 100: 2911-2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwiveldi Y, Rizavi HS, Conley RR, Roberts RC, Tamminga CA, Pandey GN (2003) Altered gene expression of brain-derived neurotrophic factor and receptor tyrosine kinase B in postmortem brain of suicide subjects. Arch Gen Psychiatry 60: 804-815. [DOI] [PubMed] [Google Scholar]
- Fawcett JP, Alonso-Vanegas MA, Morris SJ, Miller FD, Sadikot AF, Murphy RA (2000) Evidence that brain-derived neurotrophic factor from presynaptic nerve terminals regulates the phenotype of calbindin-containing neurons in the lateral septum. J Neurosci 20: 274-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer I, Goutan E, Marin C, Rey MJ, Ribalta T (2000a) Brain-derived neurotrophic factor in Huntington disease. Brain Res 866: 257-261. [DOI] [PubMed] [Google Scholar]
- Ferrer I, Marin C, Rey MJ, Ribalta T (2000b) Brain-derived neurotrophic factor in patients with frontotemproal dementia. Neurosci Lett 279: 33-36. [DOI] [PubMed] [Google Scholar]
- Fiala B, Snow FM, Coreenough WT (1977) “Impoverished” rats weigh more than “enriched” rats because they eat more. Dev Psychobiol 10: 537-541. [DOI] [PubMed] [Google Scholar]
- Fienberg AA, Greengard P (2000) The DARPP-32 knockout mouse. Brain Res Brain Res Rev 31: 313-319. [DOI] [PubMed] [Google Scholar]
- Glatt SL, Hubble JP, Lyons K, Paolo A, Troster AI, Hassanein RE, Koller WC (1996) Risk factors for dementia in Parkinson's disease: effect of education. Neuroepidemiology 15: 20-25. [DOI] [PubMed] [Google Scholar]
- Huntington's Disease Collaborative Research Group (1993) A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. Cell 72: 971-983. [DOI] [PubMed] [Google Scholar]
- Harper PS (1996) Huntington's disease, Ed 2. London: Saunders.
- Heyser CJ, Fienberg AA, Greengard P, Gold LH (2000) DARPP-32 knockout mice exhibit impaired reversal learning in a discriminated operant task. Brain Res 867: 122-130. [DOI] [PubMed] [Google Scholar]
- Hockly E, Cordery P-M, Woodman B, Mahal A, Van Dellen A, Blakemore C, Lewis C-M, Hannan A-J, Bates G-P (2002) Environmental enrichment slows disease progression in R6/2 Huntington's disease mice. Ann Neurol 51: 235-242. [DOI] [PubMed] [Google Scholar]
- Kohara K, Kitamura A, Morishima M, Tsumoto T (2001) Activity-dependent transfer of brain-derived neurotrophic factor to postsynaptic neurons. Science 291: 2419-2423. [DOI] [PubMed] [Google Scholar]
- Lessmann V, Gottmann K, Heumann R (1994) BDNF and NT-4/5 enhance glutamatergic synaptic transmission in cultured hippocampal neurones. NeuroReport 6: 21-25. [DOI] [PubMed] [Google Scholar]
- Luthi Carter R, Strand A, Peters NL, Solano SM, Hollingsworth ZR, Menon AS, Frey AS, Spektor BS, Penney EB, Schilling G, Ross CA, Borchelt DR, Tapscott SJ, Young AB, Cha JH, Olson JM (2000) Decreased expression of striatal signaling genes in a mouse model of Huntington's disease. Hum Mol Genet 9: 1259-1271. [DOI] [PubMed] [Google Scholar]
- Luthi Carter R, Hanson SA, Strand AD, Bergstrom DA, Chun W, Peters NL, Woods AM, Chan EY, Kooperberg C, Krainc D, Young AB, Tapscott SJ, Olson JM (2002) Dysregulation of gene expression in the R6/2 model of polyglutamine disease: parallel changes in muscle and brain. Hum Mol Genet 11: 1911-1926. [DOI] [PubMed] [Google Scholar]
- Mangiarini L, Sathasivam K, Seller M, Cozens B, Harper A, Hetherington C, Lawton M, Trottier Y, Lehrach H, Davies SW, Bates GP (1996) Exon 1 of the HD gene with an expanded CAG repeat is sufficient to cause a progressive neurological phenotype in transgenic mice. Cell 87: 493-506. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Starkstein SE, Peyser CE, Brandt J, Dannals RF, Folstein SE (1992) Paralimbic frontal lobe hypometabolism in depression associated with Huntington's disease. Neurology 42: 1791-1797. [DOI] [PubMed] [Google Scholar]
- Ouimet CC, Miller PE, Hemmings Jr HC, Walaas SI, Greengard P (1984) DARPP-32, a dopamine- and adenosine 3′:5′-monophosphate-regulated phosphoprotein enriched in dopamine-innervated brain regions. III. Immunocytochemical localization. J Neurosci 4: 111-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouimet CC, LaMantia AS, Goldman Rakic P, Rakic P, Greengard P (1992) Immunocytochemical localization of DARPP-32, a dopamine and cyclic-AMP-regulated phosphoprotein, in the primate brain. J Comp Neurol 323: 209-218. [DOI] [PubMed] [Google Scholar]
- Perez RG, Lewis RM (1992) Regional distribution of DARPP-32 (dopamine- and adenosine 3′,5′-monophosphate-regulated phosphoprotein of Mr = 32,000) mRNA in mouse brain. J Comp Neurol 318: 304-315. [DOI] [PubMed] [Google Scholar]
- Pham TM, Soderstrom S, Winblad B, Mohammed AH (1999a) Effects of environmental enrichment on cognitive function and hippocampal NGF in the non-handled rats. Behav Brain Res 103: 63-70. [DOI] [PubMed] [Google Scholar]
- Pham TM, Ickes B, Albeck D, Soderstrom S, Granholm AC, Mohammed AH (1999b) Changes in brain nerve growth factor levels and nerve growth factor receptors in rats exposed to environmental enrichment for one year. Neuroscience 94: 279-286. [DOI] [PubMed] [Google Scholar]
- Rosenzweig MR, Bennett EL (1996) Psychobiology of plasticity: effects of training and experience on brain and behaviour. Behav Brain Res 78: 57-65. [DOI] [PubMed] [Google Scholar]
- Russo Neustadt A, Ha T, Ramirez R, Kesslak JP (2001) Physical activity-antidepressant treatment combination: impact on brain-derived neurotrophic factor and behavior in an animal model. Behav Brain Res 120: 87-95. [DOI] [PubMed] [Google Scholar]
- Sathasivam K, Hobbs C, Mangiarini L, Mahal A, Turmaine M, Doherty P, Davies SW, Bates GP (1999) Transgenic models of Huntington's disease. Philos Trans R Soc Lond B Biol Sci 354: 963-969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew MV, Howe CL, Mobley WC (2001) Nerve growth factor signaling, neuroprotection, and neural repair. Annu Rev Neurosci 24: 1217-1281. [DOI] [PubMed] [Google Scholar]
- Sullivan FR, Bird ED, Alpay M, Cha JH (2001) Remotivation therapy and Huntington's disease. J Neurosci Nurs 33: 136-142. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Tzavara ET, Liu F, Fienberg AA, Nomikos GG, Greengard P (2002) DARPP-32 mediates serotonergic neurotransmission in the forebrain. Proc Natl Acad Sci USA 99: 3188-3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Saito H, Matsuki N (1997) Inhibition of GABAA synaptic responses by brain-derived neurotrophic factor (BDNF) in rat hippocampus. J Neurosci 17: 2959-2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turmaine M, Raza A, Mahal A, Mangiarini L, Bates GP, Davies SW (2000) Nonapoptotic neurodegeneration in a transgenic mouse model of Huntington's disease. Proc Natl Acad Sci USA 97: 8093-8097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dellen A, Blakemore C, Deacon R, York D, Hannan AJ (2000a) Delaying the onset of Huntington's in mice. Nature 404: 721-722. [DOI] [PubMed] [Google Scholar]
- van Dellen A, Welch J, Dixon RM, Cordery P, York D, Styles P, Blakemore C, Hannan AJ (2000b) N-acetylaspartate and DARPP-32 levels decrease in the corpus striatum of Huntington's disease mice. NeuroReport 11: 3751-3757. [DOI] [PubMed] [Google Scholar]
- van Dellen A, Deacon R, York D, Blakemore C, Hannan AJ (2001) Anterior cingulate cortical transplantation in transgenic Huntington's disease mice. Brain Res Bull 56: 313-318. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Finch DM, Olson CR (1992) Functional heterogeneity in cingulate cortex: the anterior executive and posterior evaluative regions. Cereb Cortex 2: 435-443. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Mendes De Leon CF, Barnes LL, Schneider JA, Bienias JL, Evans DA, Bennett DA (2002) Participation in cognitively stimulating activities and risk of incident Alzheimer disease. JAMA 287: 742-748. [DOI] [PubMed] [Google Scholar]
- Young DH, Lawlor PA, Leone P, Dragunow M, During MJ (1999) Environmental enrichment inhibits spontaneous apoptosis, prevents seizures and is neuroprotective. Nat Med 5: 448-453. [DOI] [PubMed] [Google Scholar]
- Zhao LR, Risedal A, Wojcik A, Hejzlar J, Johansson BB, Kokaia Z (2001) Enriched environment influences brain-derived neurotrophic factor levels in rat forebrain after focal stroke. Neurosci Lett 305: 169-172. [DOI] [PubMed] [Google Scholar]
- Zuccato C, Ciammola A, Rigamonti D, Leavitt BR, Goffredo D, Conti L, MacDonald ME, Friedlander RM, Silani V, Hayden MR, Timmusk T, Sipione S, Cattaneo E (2001) Loss of huntingtin-mediated BDNF gene transcription in Huntington's disease. Science 293: 493-498. [DOI] [PubMed] [Google Scholar]