Abstract
Inhibition of primary afferent neurons contributes to the antihyperalgesic effects of opioid and CB1 receptor agonists. Two bioassays were used to compare the effects of the CB1 receptor agonist CP 55,940 and morphine on dissociated adult rat DRG neurons. Both agonists inhibited the increase in free intracellular Ca2+ concentration evoked by depolarization; however, effects of CP 55,940 occurred primarily in large neurons (cell area, >800 μm2), whereas morphine inhibited the response in smaller neurons. Cotreatment with selective blockers of L-, N-, and P/Q-type voltage-dependent Ca2+ channels indicated that CB1 receptors on DRG neurons couple solely with N-type channels but opioid receptors couple with multiple subtypes. Experiments with selective agonists and antagonists of opioid receptors indicated that μ and δ, but not κ, receptors contributed to the inhibitory effect of morphine on voltage-dependent Ca2+ influx. Because Ca2+ channels underlie release of transmitters from neurons, the effects of opioid agonists and CP 55,940 on depolarization-evoked release of calcitonin gene-related peptide (CGRP) were compared. Morphine inhibited release through δ receptors but CP 55,940 had no effect. Colocalization of CGRP with δ-opioid but not μ-opioid or CB1 receptor immunoreactivity in superficial laminae of the dorsal horn of the spinal cord was consistent with the data for agonist inhibition of peptide release. Therefore, CB1 and opioid agonists couple with different voltage-dependent Ca2+ channels in different populations of DRG neurons. Furthermore, differences occur in the distribution of receptors between the cell body and terminals of DRG neurons. The complementary action of CB1 and opioid receptor agonists on populations of DRG neurons provides a rationale for their combined use in modulation of somatosensory input to the spinal cord.
Keywords: opioid agonist, CB1 receptor, dorsal root ganglion, neuron, VDCC, CGRP
Introduction
Cannabinoids are antihyperalgesic when administered peripherally at the site of tissue injury as well as when administered to the spinal cord (Calignano et al., 1998; Ko and Woods, 1999; Martin et al., 1999; Fox et al., 2001; Johanek et al., 2001; Malan et al., 2001). CB1 receptors on primary afferent neurons (Hohmann and Herkenham, 1999; Ahluwalia et al., 2000) and CB2 receptors on cells that generate mediators of inflammation (Galiegue et al., 1995; Mazzari et al., 1996; Malan et al., 2001, 2002) underlie the antihyperalgesic effects of cannabinoids in the periphery. Within adult rat dorsal root ganglia, CB1 receptor mRNA occurs predominately in intermediate to large neurons (Hohmann and Herkenham, 1999; Bridges et al., 2003), whereas CB1 receptor-immunoreactivity (-ir) has been localized to small (Ahluwalia et al., 2000) as well as large neurons (Khasabova et al., 2002).
Most of what we know about cellular actions of cannabinoids mediated by CB1 receptors has been generated on cells maintained in culture (Howlett and Fleming, 1984; Mackie and Hille, 1992; Mackie et al., 1995; Pan et al., 1996), including neonatal neurons (Twitchell et al., 1997; Hampson et al., 2000; Ross et al., 2001), and slices of brain or spinal cord obtained from young rats (Gerdeman and Lovinger, 2001; Morisset and Urban, 2001; Robbe et al., 2001). These studies show that CB1 receptors couple with G-proteins to inhibit adenylyl cyclase, decrease calcium conductance through voltage-gated calcium channels (VDCCs), and differentially modulate potassium channels. These models have use as experimental systems, but neuronal phenotypes are modified by culture conditions (Aguayo and White, 1992; Petersen et al., 1998). Furthermore, expression of receptors (Molliver et al., 1997; Beland and Fitzgerald, 2001) and VDCCs (Iwasaki et al., 2000) changes between birth and maturation of neuronal circuits. Therefore, data on effects of cannabinoids on adult DRG neurons are important to understand the role of these receptors in mature somatosensory systems. We recently reported that activation of CB1 receptors on dissociated, adult DRG neurons inhibited the influx of Ca2+ evoked by depolarization with 50 mm KCl (Khasabova et al., 2002). Interestingly, the effect of CB1 agonists on VDCCs occurred predominately on neurons with somal areas >800 μm2.
One goal of the present study was to extend our studies of adult DRG neurons by determining the subtype(s) of VDCCs that is modulated by the CB1 agonist CP 55,940. Because VDCCs are involved in transmitter release and cannabinoids have been shown to decrease release of transmitters from terminals of DRG neurons in spinal cord and skin (Richardson et al., 1998a,b; Morisset and Urban, 2001), we also investigated whether CP 55,940 modulated depolarization-evoked release of calcitonin gene-related peptide (CGRP) from isolated DRG neurons. Opioid receptors also couple negatively with VDCCs in DRG neurons (Moises et al., 1994; Wiley et al., 1997; Acosta and Lopez, 1999), and morphine is analgesic in the same models as cannabinoid agonists (Yaksh and Stevens, 1988; Sawynok, 2003). Therefore, direct comparisons were made between effects of opioid agonists and CP 55,940 on two populations of DRG neurons defined by size. Finally, the codistribution of CGRP-ir with μ-opioid receptor (MOR)-ir and δ-opioid receptor (DOR)-ir as well as CB1 receptor-ir in the spinal cord was examined using confocal microscopy. The results have implications for the complementary use of opioids and cannabinoids in alleviating pain.
Materials and Methods
Preparation of dissociated cells. Adult male Sprague Dawley rats (200-225 gm) were used in these studies. Procedures were approved by the University of Minnesota Institutional Animal Care and Use Committee. After euthanasia, dorsal root ganglia were dissected and dissociated as described previously (Khasabova et al., 2002). The final cell suspension was plated at a density of 10,000 cells/25 mm cover glass (Fisher Scientific, Pittsburgh, PA) or 30,000 cells/17 mm well of a tissue culture plate (Corning, Corning, NY) on laminin-coated surfaces. Cells were incubated in Ham's F12 medium (Invitrogen, Grand Island, NY) supplemented with l-glutamine (2 mm), penicillin (100 U/ml), streptomycin (100 μg/ml), and DNAase I (0.15 mg/ml; Sigma, St. Louis, MO). Cells were maintained in a humidified atmosphere of 5% CO2 at 37°C for 20-28 hr before use for neurons to adhere to cover glasses or wells.
Measurement of free intracellular calcium concentration ([Ca2+]i). Cells were incubated with the Ca2+-sensitive fluorescent indicator indo-1 acetoxymethyl ester (3 μm; Molecular Probes, Eugene, OR) in HEPES-buffered HBSS (Khasabova et al., 2002) containing 2% BSA for 45-60 min at 37°C before recordings. For microfluorimetry, a cover glass was mounted in a superfusion chamber and placed on an inverted microscope. Cells were superfused with HEPES buffer (1.8 ml/min) (Khasabova et al., 2002) at room temperature. The maximum and minimum diameters of a neuron were estimated using a grid mounted in the eyepiece of the microscope, and the average was used to calculate somal area. Measurements of [Ca2+]i were made in soma of single neurons using a dual-emission microfluorimeter (Photoscan; Photon Technology International, Princeton, NJ) to monitor fluorescence of indo-1 as described previously in our laboratory (Stucky et al., 1996; Khasabova et al., 2002). Counts from the photomultiplier tubes were recorded at one point/0.5 sec via computer with Fluorescence System hardware and Felix software (Photon Technology International). Values for [Ca2+]i were calculated from the equation [Ca2+]i = KDb(R - Rmin)/(Rmax - R), where r = 405 nm/485 nm fluorescence emission ratio corrected for background fluorescence. The dissociation constant (KD) for indo-1 was 250 nm (Grynkiewicz et al., 1985), and β was the ratio of fluorescence at 485 nm in the absence and presence of a saturating concentration of Ca2+. Other values, empirically determined in adult DRG neurons, were: Rmin = 0.38; Rmax = 3.28; β = 3.9.
The basic protocol used to explore the effect of drugs on responses of DRG neurons was three applications of KCl (50 mm; 10 sec) separated by superfusion for 5 min with HEPES buffer. Data for the control group (KCl alone) as well as morphine or CP 55,940 plus KCl were collected each day of an experiment to control for variation among preparations of neurons. Drugs were included in the superfusate after the first application of KCl and through the two following applications of KCl.
Stock solutions of opioid receptor ligands morphine (10 mm; Sigma), DAMGO (D-Ala 2, N-Me-Phe 4, Gly 5-ol-Enkephalin; 10 mm; Bachem, Torrence, CA), D-Ala 2-Deltorphin II (10 mm; Bachem), CTAP (D-Phe-Cys-Tyr-D-Trp-Arg-Thr-Pen-Thr-NH2; 1 mm; Sigma), and naltrindole (3 mm; Sigma) were prepared in dH2O; nor-binaltorphimine (nor-BNI; 10 mm) was dissolved in DMSO. Stock solutions of the cannabinoid receptor agonist CP 55,940 (10 mm; Tocris, Bristol, UK) and the L-type VDCC blocker nimodipine (20 mm; Sigma) were prepared in ethanol. All drugs were diluted in the HEPES buffer to the final concentrations indicated for superfusion. Solutions of ω-conotoxin GVIA and ω-agatoxin TK (Alomone Labs, Jerusalem, Israel) were prepared at their final concentration in HEPES buffer on the day of use. Based on the literature, the lowest concentration tested for each opioid antagonist was selective for one receptor subtype (Pelton et al., 1986; Portoghese et al., 1990; Bonner et al., 1997; Ananthan et al., 1998). The calcium channel blockers were used at concentrations reported to be effective and selective in blocking VDCCs in DRG neurons in vitro (Cardenas et al., 1997; Endoh and Suzuki, 1998; Formenti et al., 1998). The amplitude of the change in [Ca2+]i in response to treatments was calculated as the difference between baseline (average of values for 2 min before KCl) and the peak response evoked by KCl. To minimize variability within treatment groups, responses to the second and third treatments with KCl in the presence of drug were expressed as a percentage of the first response. Data for individual neurons were sorted by size of the neuron, with small neurons defined as <800 μm 2 in area and intermediate-size neurons as 800-1500 μm 2. These ranges in size were based on differential responses to CB1 agonists observed in a previous study (Khasabova et al., 2002).
Measurement of CGRP release. Neurons adherent to wells in tissue culture plates were used in studies of cannabinoid and opioid modulation of CGRP release. Experiments were conducted at room temperature. After a 10 min rinse in HEPES buffer (same buffer as that used for superfusion), cells were preincubated with CP 55,940 (100 nm; 10 min) or morphine (1 μm; 5 min) in fresh HEPES buffer and then stimulated with KCl (50 mm; 75 sec). To define receptor-mediated effects, cannabinoid and opioid receptor antagonists were included in the preincubation period. After the stimulation period, the buffer from each well was transferred to glass test tubes and frozen at -80°C until the time of assay. Samples of buffer containing released peptides were assayed without dilution. Rabbit anti-CGRP antibody (100 μl; 1:1,000,000 final dilution; provided by M. Iadarola, National Institutes of Health, Bethesda, MD) was added to each sample (400 μl), and tubes were mixed and incubated at 4°C for 24 hr. [125I]-Tyr 0CGRP (100 μl; 20,000-25,000 cpm) and goat anti-rabbit antiserum coupled with ferric beads (50 μl; 1 mg/ml; PerSeptive Biosystems, Framingham, MA) were then added to each tube. The reactants were mixed and incubated for 24 hr longer. The assay was stopped by immunomagnetic separation. The liquid was aspirated from each tube, and the immunoprecipitated product was counted on a gamma counter (Wallac, Gaithersburg, MD). Standard curves were generated in HEPES buffer. Levels of CGRP-ir in the samples were determined using logit-log analysis. The minimum detection limit for this assay is 2-3 fmol/tube with 50% displacement at ∼15 fmol/tube.
Immunochemical studies. Animals were anesthetized deeply with sodium pentobarbital (50 mg/kg, i.p.) and perfused intracardially with PBS, pH 7.35, followed by 4% (w/v) paraformaldehyde and 14% saturated picric acid in 160 mm phosphate buffer, pH 6.9. Lumbar segments of spinal cord were removed, postfixed for 2 hr, and incubated overnight in PBS with 30% sucrose. Serial transverse or horizontal sections (50 μm) of the spinal cord were cut on a sliding microtome, collected in PBS, and processed as free-floating sections. Sections were preincubated in a blocking solution of 5% normal donkey serum (Jackson ImmunoResearch, West Grove, PA) with 0.3% Triton X-100 and 0.1% sodium azide for 1 hr at room temperature. Each section was immunostained for two antigens: goat anti-CGRP serum (1:500; a gift from H. Heath, Mayo Clinic, Rochester, MN) (Carter et al., 1991) plus rabbit anti-CB1 receptor (1:500; a gift from K. Mackie, University of Washington, Seattle, WA), rabbit anti-MOR (serum 551; 1:1000; a gift from R. Elde, University of Minnesota, Minneapolis, MN) (Arvidsson et al., 1995b), or rabbit anti-DOR (serum 442; 1:1000 final dilution; a gift from R. Elde) (Arvidsson et al., 1995a) antibodies. Sections were incubated with primary antibodies overnight at room temperature. After rinses with PBS, tissue sections were incubated for 1 hr with a combination of Cy3-donkey anti-rabbit (1:400) and FITC-donkey anti-goat (1:100; Jackson ImmunoResearch) antibodies. Finally, the sections were rinsed in PBS, mounted on gelatin-coated slides, air dried, dehydrated in a series of graded ethanol (70, 90, and 100%), cleared in xylene, and coverslipped. Immunoreactivity was visualized with a CARV nonlaser Confocal Microscope System (ATTO Instruments, Rockville, MD). Images were collected in successive frames of 2 or 1 μm optical sections (Z-series) through the thickness of the sections using a 20× or 40× oil plan apochromat objective (Zeiss, Oberkochen, Germany), respectively.
The specificity of the MOR and DOR antibodies in immunohistochemistry has been described (Arvidsson et al., 1995a,b). The CB1 antibody was raised in rabbits against a glutathione S-transferase (GST) fusion protein that included a 15 amino acid sequence from the C-terminal tail of the CB1 receptor (GIP-KVTMSVSTDTSAEAL). The rabbit antiserum was affinity purified against GST and the full-length fusion protein. The affinity-purified antibody immunostained AtT20 cells transfected with the CB1 receptor but not untransfected cells. Similarly, in Western blots, the antibody recognized the appropriate band in extracts from AtT20 cells transfected with the CB1 receptor but did not cross-react with extracts from untransfected cells (K. Mackie, personal communication). Incubation of the diluted antibody with the immunizing peptide (1 μm) blocked detection of immunoreactivity in spinal cord. The cross-reactivity of the goat anti-CGRP serum with other peptides was characterized using the model system of Larsson (1981). The antiserum bound to CGRP but did not bind to 15 other neurosecretory peptides that are prominent in the superficial laminae of the dorsal horn of the spinal cord (100 pmol to 10 nmol). Incubation of the diluted CGRP antiserum with CGRP (1 μm) blocked detection of immunoreactivity in the spinal cord. No immunofluorescence above background was observed when primary antibodies were omitted from the staining protocol. Based on these data, all antibodies were judged to be selective for their respective antigens.
Statistical analyses. Data are presented as the mean ± SEM for the group unless stated otherwise. To minimize variability, responses to the second and third treatments with KCl were expressed as a percentage of the first response. Analysis of data for effects of blockers of VDCCs was a repeated measures ANOVA. Effects of remaining treatments on responses to KCl and release of CGRP were compared using one- and two-way ANOVAs as appropriate. Differences between groups were identified using multiple comparisons tests.
Results
K+-evoked increases in [Ca2+]i in two populations of adult DRG neurons defined by size
The basal level of [Ca2+]i in adult DRG neurons was 97.8 ± 2.5 nm (n = 201) among all neurons assayed, and this level did not differ between small (99.5 ± 3.5 nm; n = 98) and intermediate-size (93.3 ± 2.8 nm; n = 103) neurons. Application of KCl (50 mm) evoked more than a threefold increase in [Ca2+]i over basal levels in both populations of DRG neurons (Fig. 1A,C). A difference in the peak amplitude of the evoked increase in [Ca2+]i was noted between small and intermediate-size DRG neurons (small, 373 ± 22 nm; intermediate, 290 ± 15 nm; two-way ANOVA; p < 0.01). Previous studies in our laboratory established that the amplitude of the KCl-evoked increase in [Ca2+]i was constant among the three stimulations with KCl under control conditions and was attributable to an influx of extracellular Ca2+ (Khasabova et al., 2002).
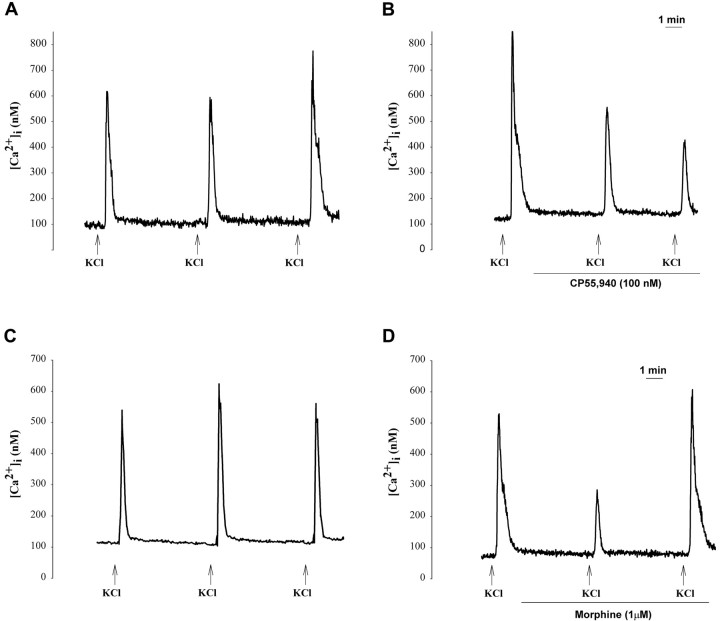
Figure 1.
Transient changes in [Ca2+]i in adult DRG neurons induced by depolarization with a high concentration of KCl were modulated by CP 55,940 and morphine. Shown are representative examples of responses of intermediate-size (A; 800-1200 μm2) and small (C; 240-780 μm2) neurons to repeated application of KCl (50 mm; 10 sec). The magnitude of increase in [Ca2+]i did not change across three applications of KCl. B, Representative example of inhibition of the response of an intermediate-size DRG neuron to KCl during superfusion with CP 55,940 (100 nm). D, Representative example of inhibition of the response of a small DRG neuron to KCl during superfusion with morphine (1 μm). Arrows below the tracings indicate the time of superfusion with KCl; lines indicate the duration of superfusion with CP 55,940 or morphine.
Effects of CP 55,940 and morphine on the K+-evoked increase in [Ca2+]i
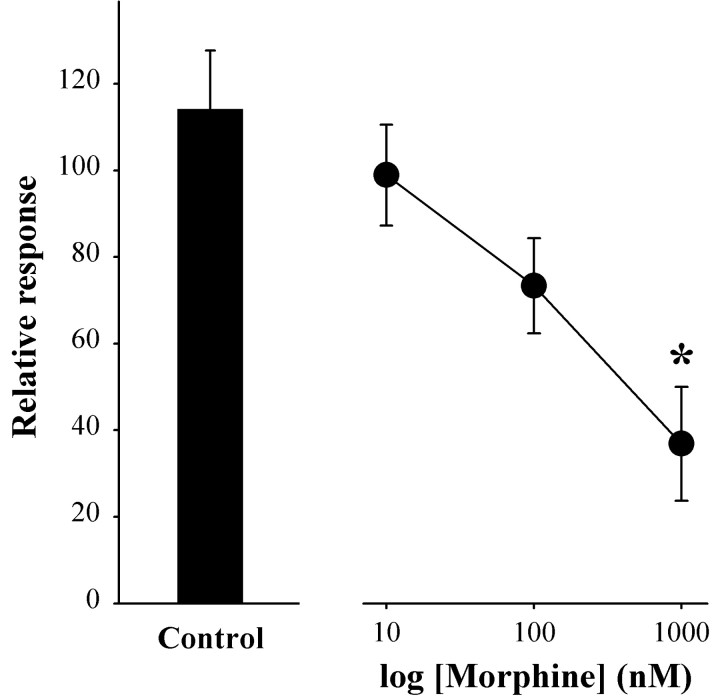
Data generated in the present study confirmed our previous results that the cannabinoid agonist CP 55,940 (100 nm) attenuated the K+-evoked increase in [Ca2+]i in intermediate-size neurons by 40% (Table 1). The inhibition was maximal after treatment for 10 min (Fig. 1B). No effect was observed on small neurons. In contrast, morphine attenuated the K+-evoked increase in [Ca2+]i in small DRG neurons by 50% (Fig. 1D) but had no effect on intermediate-size neurons (Table 1) The lowest concentration of morphine that produced inhibition was 1 μm (Fig. 2). The inhibitory effect of morphine was apparent at 5 min but not at 10 min, despite continuous superfusion (Fig. 1D).
Table 1.
CP 55,940 and morphine differentially modulated the K+ -evoked increase in [Ca2+]i in small and intermediate-size DRG neurons
|
Population |
Control |
CP 55,940 |
Morphine |
||||
|---|---|---|---|---|---|---|---|
|
|
Change in [Ca2+]i
|
Change in [Ca2+]ia
|
% Neurons respondingb
|
Change in [Ca2+]i
|
% Neurons responding |
||
| Small | 112 ± 10 (13) | 108 ± 16 (7) | 30 | 60 ± 7†(21) | 62** | ||
| Intermediate |
119 ± 6(20) |
71 ± 5†(12) |
65*
|
121 ± 17(15) |
6 |
||
*p<0.05, significantly different from control and from small neurons; **p<0.005, significantly different from control and from intermediate-size neurons; Fisher's exact test. †p<0.001 compared with nontreated control within the same population of neurons; two-way ANOVA with Tukey's multiple comparisons test.
The change in [Ca2+]i was defined as the amplitude of the response to 50 mm KCl at 5 or 10 min after incubation with morphine (1 μm) or CP 55,940 (100 nm), respectively, divided by the amplitude of the response to the first application of KCl (absence of agonist) and multiplied by 100. The mean somal area of small neurons tested was 499 μm2 (range, 340-642 μm2) and was 1030 μm2 (range, 829-1445 μm2) for intermediate-size neurons. Values are the means ± SEM for the treatment group; the number in parentheses is the number of neurons tested.
To be scored as responsive to agonist, the relative response of neurons to KCl in the presence of agonist had to be <78 (mean of the control population minus 1 SD). Applying this criterion to the control population indicated that 18% of the small neurons and 11% of the intermediate-size neurons had a nonspecific response. Previously published data for CP 55,940 (Khasabova et al., 2002) were included in this analysis.
Figure 2.
Morphine inhibition of the K+-evoked increase in [Ca2+]i in small neurons was concentration dependent. The relative response was defined as the amplitude of the response to KCl at 5 min after superfusion with morphine, divided by the amplitude of the response to the first application of KCl (absence of drug) and multiplied by 100. The mean somal area of neurons tested was 499 μm2 (range, 290-764 μm2). Values are the means ± SEM for the treatment group (n = 5-7 neurons/group). *p < 0.001 compared with nontreated control; one-way ANOVA with Dunnett's test for multiple comparisons with control.
The effects of cannabinoid and opioid agonists were apparent only as attenuation of the K+-evoked elevation in [Ca2+]i. No changes occurred in basal [Ca2+]i during the 10 min superfusion with either agonist between the first and third application of KCl (Fig. 1B,D). No change in basal [Ca2+]i was observed in small neurons during superfusion of morphine alone (within culture preparation control, 83.2 ± 10.0 nm [Ca2+]i; morphine, 82.4 ± 11 [Ca2+]i; n = 9 for each group). Similar data were reported previously for CP 55,940 (Khasabova et al., 2002). Furthermore, effects of CP 55,940 and morphine were pertussis toxin sensitive. Incubation of DRG neurons with pertussis toxin (500 ng/ml) overnight abolished the effects of cannabinoid and opioid agonists (Table 2), indicating the inhibitory effects of the agonists in adult rat DRG neurons were mediated by Gi/o proteins. Pertussis toxin had no effect on the response to KCl within either population of neurons (p = 0.895 for small neurons; p = 0.712 for intermediate-size neurons; Student's t test; n = 5-6 neurons/group).
Table 2.
Pertussis toxin (PT) blocked the effect of CP 55,940 and morphine on the K+ -evoked increase in [Ca2+]i in small and intermediate-size DRG neurons
|
|
Relative response to KCla |
|
|
|---|---|---|---|
| Population | PT | PT + CP 55,940 | PT + morphine |
| Small | 140 ± 21 (5) | n.d. | 154 ± 36 (5) |
| Intermediate |
95 ± 12 (5) |
92 ± 11 (6) |
n.d. |
Relative response was defined as the amplitude of the response to KCl at 5 or 10 min after incubation with morphine or CP 55,940, respectively, divided by the amplitude of the response to the first application (absence of drug) and multiplied by 100. CP 55,940 was used at 100 nm and morphine at 1 μm. The mean somal area of small neurons tested was 468 μm2 (range, 340-585 μm2) and was 1005 μm2 (range, 897-1116 μm2) for intermediate-size neurons. Values are the means ± SEM for the treatment group; the number in parentheses is the number of neurons tested. n.d., No determination.
Contribution of VDCCs to the K+-evoked increase in [Ca2+]i in two populations of DRG neurons
The differential effects of morphine and cannabinoids on small and intermediate-size DRG neurons could be attributable to a differential distribution of receptors for the two ligands between the two populations of neurons or a differential distribution of one or more VDCCs to which the receptors selectively couple. Treatments with blockers for L-, N-, and P/Q- type VDCCs were used to determine the contribution of subtypes of VDCCs to the K+-evoked increases in [Ca2+]i in small and intermediate-size neurons. Data were analyzed in a repeated measures ANOVA in which the dependent variable was response; the “subject” was cell; the between-subject fixed effects were drug, cell size, and their interaction; and the within-subject fixed effects were time (5 min vs 10 min) and its interaction with drug and cell size. The blockers of VDCCs had a significant effect (p < 0.0001), but there was no interaction between drug and cell size (p = 0.1444). For clarity in presentation (Fig. 3), data collected 5 min after administration of blockers is presented for small neurons because this was the time used to describe the effect of morphine; data collected 10 min after drug administration are presented for intermediate-size neurons because this was the time used to describe the effect of CP 55,940. There were no differences in responses of intermediate-size neurons after treatment with blockers of VDCCs for 5 or 10 min. Each VDCC blocker inhibited the depolarization-evoked increase in [Ca2+]i by 25-35%. Coapplication of all three channel blockers inhibited the depolarization-evoked increase in [Ca2+]i by 70-75% (small neurons: 23.0 ± 4.9% of control, n = 4; intermediate-size neurons: 32.4 ± 11.0%, n = 5). The residual increase in [Ca2+]i is most likely mediated by R-type VDCCs (Scamps et al., 1998). Our conclusion from these data is that L-, N-, P/Q-, and R-type VDCCs did not differ singly, or together, in their contribution to the K+-evoked increase in [Ca2+]i in the two populations of DRG neurons.
Figure 3.
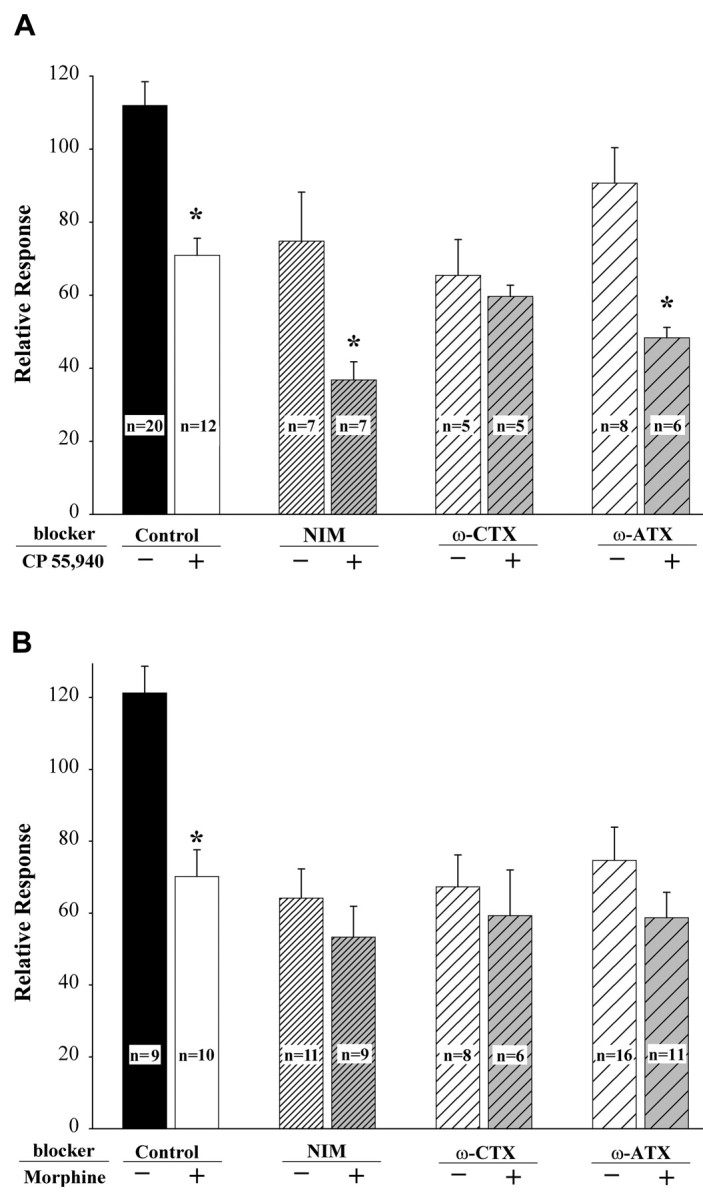
Modulation of the inhibitory effects of CP 55,940 (A) and morphine (B) by blockers of voltage-dependent Ca2+ channels: nimodipine (NIM), ω-conotoxin (ω-CTX), and ω-agatoxin (ω-ATX). The relative response was defined as the amplitude of the response to KCl at 10 min (A) or 5 min (B) after superfusion with an agonist plus a VDCC blocker, divided by the amplitude of the response to the first application (absence of drug) and multiplied by 100. The mean somal area of small neurons tested was 505 μm2 (range, 298-764 μm2) and was 1062 μm2 (range, 856-1275 μm2) for intermediate-size neurons. Values are the means ± SEM for the treatment group; the number in each bar is the number of neurons tested. *p < 0.01 compared with treatment with the respective VDCC blocker alone; two-way ANOVA within each population of neurons with Tukey's multiple comparisons test.
Identification of VDCCs modulated by CP 55,940 and morphine
To test the possibility that cannabinoid and opioid receptors couple negatively with specific types of VDCCs, we examined the ability of Ca2+ channel blockers to attenuate the effects of CP 55,940 and morphine on the K+-evoked increase in [Ca2+]i. The time courses of treatments were 10 min for CP 55,940 with intermediate-size neurons and 5 min for morphine with small neurons. These times are consistent with maximal effects of agonists and blockers for each population of neurons. Whereas superfusion with nimodipine or ω-agatoxin for 10 min did not block the ability of CP 55,940 to inhibit the depolarization-evoked increase in [Ca2+]i in intermediate-size neurons, the coapplication of the cannabinoid with ω-conotoxin GVIA blocked the effect of CP 55,940 (Fig. 3A). These data indicate that the inhibitory effect of CP 55,940 on the K+-evoked increase in [Ca2+]i in intermediate-size DRG neurons was mediated by N-type VDCCs.
In contrast to the selective relationship of the cannabinoid agonist to one type of VDCC, the inhibitory effect of morphine was mediated by multiple types of VDCCs. In the presence of nimodipine, ω-conotoxin, or ω-agatoxin, morphine no longer inhibited the K+-evoked increase in [Ca2+]i (Fig. 3B). Taken together, these data indicate that L-, N-, and P/Q-type VDCCs contribute to the inhibitory effect of morphine on the K+-evoked elevation in [Ca2+]i in small DRG neurons.
Effects of μ-, δ-, and κ-opioid ligands
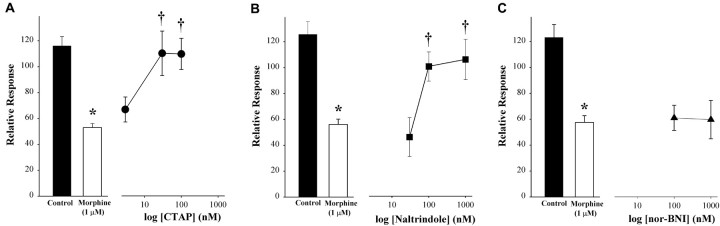
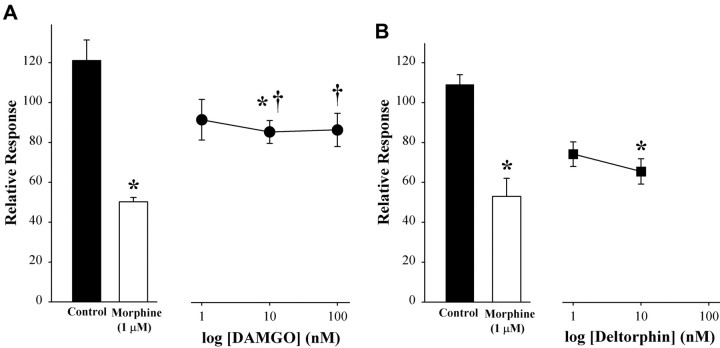
Although morphine exhibits a preference for MORs (Emmerson et al., 1994; Guirimand et al., 1994), it can also bind to DORs and κ-opioid receptors (Corbett et al., 1993). To determine which subtype(s) of the opioid receptor contributed to the effect of morphine on the K+-evoked increase in [Ca2+]i, selective opioid receptor antagonists were coapplied with morphine. The inhibitory effect of morphine was abolished by CTAP (30 nm; p < 0.05) (Fig. 4A), an antagonist of the MOR. A lower concentration of CTAP had no effect. The DOR antagonist naltrindole blocked the effect of morphine at a higher concentration (100 nm) (Fig. 4B). nor-BNI (100 nm to 1 μm), a κ-opioid receptor antagonist, had no effect (Fig. 4C). The vehicle for nor-BNI (0.01% DMSO; maximum final concentration) had no effect on basal or K+-evoked levels of [Ca2+]i (p = 0.90 for basal and p = 0.32 for depolarization-evoked response compared with control; Student's t test), so data for the vehicle alone were combined in the control group. Conversely, the effectiveness of opioid peptides in mimicking the effect of morphine was tested. Both DAMGO (10 nm) and deltorphin II (10 nm) inhibited the K+-evoked increase in [Ca2+]i, but only deltorphin II produced a level of inhibition that was comparable with that of morphine (Fig. 5). Therefore, MORs and DORs, but not κ-opioid receptors, mediate the effects of morphine on the K+-evoked increase in [Ca2+]i.
Figure 4.
CTAP (A) and naltrindole (B), but not nor-BNI (C), attenuated the inhibition of the K+-evoked increase in [Ca2+]i by morphine in small DRG neurons. Opioid receptor antagonists were included in the superfusion with morphine. The relative response was defined as the amplitude of the response to KCl at 5 min after superfusion with an agonist plus a receptor antagonist divided by the amplitude of the response to the first application (absence of drug) and multiplied by 100. The mean somal area of neurons tested was 491 μm2 (range, 242-642 μm2). Values are the means ± SEM for the treatment group (5-12 neurons/group). *p < 0.001 compared with control; †p < 0.05 compared with morphine; one-way ANOVA with multiple comparisons using Bonferonni's t test.
Figure 5.
DAMGO and deltorphin II mimicked the effect of morphine on small neurons. DAMGO (10 nm) and deltorphin II (10 nm) inhibited K+-evoked increase in [Ca2+]i, but the maximal effect of DAMGO was significantly different from morphine alone. The mean somal area of neurons tested was 487 μm2 (range, 298-585 μm2). Values are the means ± SEM for each treatment group (n = 6-18). *p < 0.05 compared with control; †p < 0.05 compared with morphine; one-way ANOVA with Tukey's multiple comparisons test.
Differential modulation of evoked CGRP release by CP 55,940 and morphine
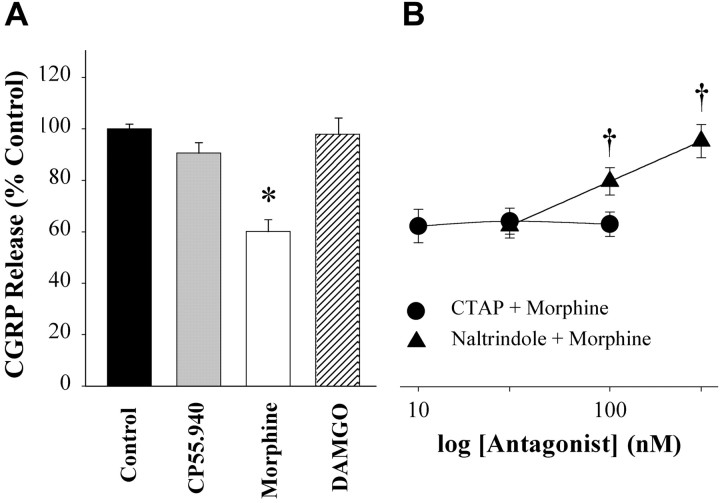
Because VDCCs are involved in transmitter release and the channels appeared to be modulated by cannabinoid and opioid agonists, we examined the effects of CP 55,940 and morphine on release of CGRP from adult DRG neurons in response to 50 mm KCl in vitro. The duration of pretreatment with agonists was based on the maximum effect of each agonist on the K+-evoked increase in [Ca2+]i. The basal release of CGRP was 4.23 ± 0.48 fmol/min in this preparation. Neither CP 55,940 nor morphine alone altered the level of basal CGRP release. Stimulation with KCl (50 mm) evoked a fivefold increase in peptide release (22.74 ± 4.20 fmol/min; p < 0.001). Pretreatment with CP 55,940 (100 nm; 10 min) did not change the amount of CGRP released in response to depolarization (Fig. 6A). However, pretreatment with morphine (1 μm; 5 min) attenuated the K+-evoked release of CGRP by 40%. Therefore morphine, but not a cannabinoid, inhibited depolarization-evoked release of CGRP from DRG neurons.
Figure 6.
Morphine, but not DAMGO or CP 55,940, inhibited K+-evoked release of CGRP-ir from adult DRG neurons, and the effect was mediated by DORs. A, Cultures of adult DRG neurons were incubated in the presence of CP 55,940 (100 nm; 10 min), morphine (1 μm; 5 min), or DAMGO (100 nm; 5 min) before stimulation with KCl. B, CTAP and naltrindole, a MOR and DOR antagonist, respectively, were coincubated with morphine before stimulation with KCl. The y-axis in B is the same as in A. Values are the means ± SEM for each treatment group (n = 9 wells from 3 different experiments). *p < 0.001 compared with control; †p < 0.005 compared with morphine; one-way ANOVA with Tukey's multiple comparisons test.
Because MOR and DOR agonists and antagonists mediated opioid effects on the depolarization-induced increase in [Ca2+]i, antagonists of these receptors were tested for effects on morphine inhibition of CGRP release. Neither antagonist alone modulated K+-evoked release of CGRP [CTAP (100 nm): 90.2 ± 3.6% of control, n = 9 wells; naltrindole (300 nm): 112 ± 16%, n = 9 wells]. Coadministration of the DOR antagonist naltrindole with morphine blocked the inhibitory effect of morphine in a concentration-dependent manner (Fig. 6B), but the MOR antagonist CTAP had no effect. Naltrindole, CTAP, or vehicle alone did not alter basal peptide release; vehicles did not alter K+-evoked CGRP release. Conversely, pretreatment with DAMGO (100 nm) had no effect on CGRP release (97.9 ± 6.3% of control; n = 9 wells). These data demonstrate that the inhibitory effect of morphine on CGRP release in DRG neurons was mediated by DORs.
Immunohistochemistry
Peptide release from dissociated DRG neurons could occur from the cell bodies (Huang and Neher, 1996; Harding et al., 1999) or terminals. We used immunohistochemistry and confocal microscopy to visualize the relationship between CB1 and opioid receptors with CGRP-immunoreactive axons and varicosities in which CGRP is released centrally: the superficial laminae of the dorsal horn of the spinal cord. Using two fluorophores and combinations of two primary antibodies raised in different species, we visualized CGRP-ir (FITC; green) with CB1-, MOR-, or DOR-ir (Cy3; red) within the same tissue sections (Fig. 7). Whereas CGRP-, MOR-, and DOR-ir were localized predominately within the marginal zone and the outer region of substantia gelatinosa, CB1-ir occurred in the marginal zone and inner substantia gelatinosa. Furthermore, the majority of DOR-immunoreative axons were also immunoreactive for CGRP (i.e., yellow) (Fig. 7E,H), but coexistence of CGRP-ir with CB1- and MOR-ir was rare (Fig. 7D,F,G,I). Therefore, immunohistochemical data are consistent with pharmacological data on cannabinoid and opioid receptor-mediated inhibition of CGRP release from dissociated DRG neurons.
Figure 7.
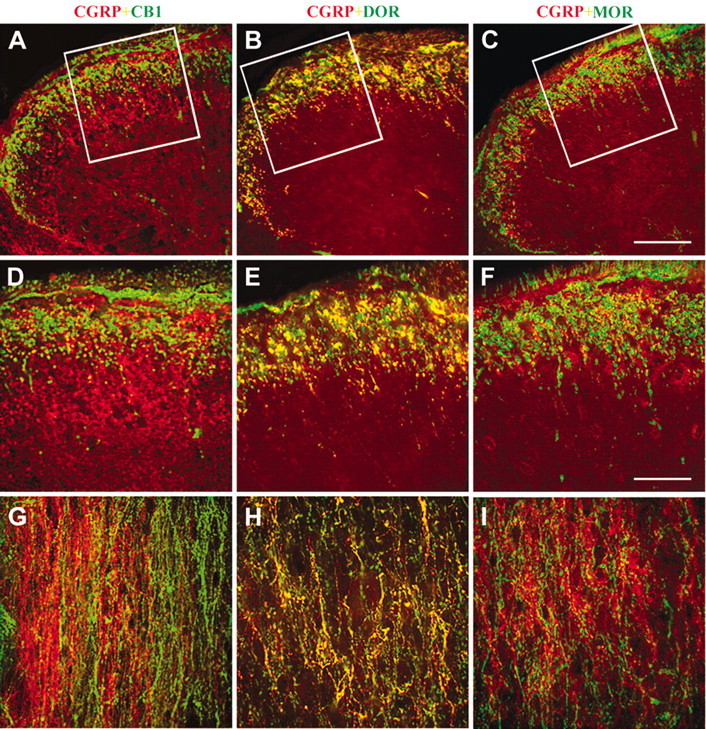
Digital confocal images of sections of the lumbar spinal cord immunostained for the dual localization of CGRP-ir with MOR-ir, DOR-ir, or CB1 receptor-ir. Green, CGRP-ir; red, CB1-ir (A,D,G), DOR-ir (B,E,H), or MOR-ir (C,F,I) in transverse (A-F) and horizontal (G-H) sections of the spinal cord. The boxes in A-C indicate regions enlarged in D-F, respectively. Bars: C, 100 μm; F, 50 μm. D-I are the same magnification. Images were digitally altered to enhance contrast.
Discussion
These results provide evidence that cannabinoid and opioid agonists have direct effects on adult DRG neurons that vary with neuronal size and bioassay. Both CP 55,940 and morphine modulated the depolarization-evoked increase in [Ca2+]i, but they differed in their inhibition of specific VDCCs and CGRP release. The localization of CGRP with CB1 or opioid receptors in adult spinal cord was consistent with the evidence for differential effects of the two classes of drugs on CGRP release.
Cannabinoids inhibit the depolarization-evoked increase in [Ca2+]i predominately in DRG neurons of at least intermediate size (>800 μm2) (Khasabova et al., 2002). Neurons in this population are generally insensitive to capsaicin and are likely to give rise to myelinated axons. The present results show that this population of adult DRG neurons is not sensitive to morphine. In contrast, morphine reduced the K+-evoked increase in [Ca2+]i in adult DRG neurons with somal areas <800 μm2. Adult rat DRG neurons of this smaller size are predominately capsaicin sensitive (Kirschstein et al., 1999; Khasabova et al., 2002). The selective effect of morphine on small DRG neurons is consistent with morphological data that MORs and DORs are downregulated postnatally in large DRG neurons (Beland and Fitzgerald, 2001) but expression continues in small neurons (Ji et al., 1995). Similarly, inhibitory effects of opiates on whole-cell currents have been described in small, but not large, DRG neurons from adult rodents (Taddese et al., 1995; Silbert et al., 2003). Thus, adult DRG neurons of different sizes, which generally process different sensory modalities (Willis and Coggeshall, 1991), respond differently to cannabinoid and opioid agonists in a measure of K+-evoked influx of Ca2+.
CP 55,940 and morphine did not modulate basal [Ca2+]i. Increases in [Ca2+]i in response to high concentrations of opioid agonists have been described in cultured embryonic mouse DRG neurons (Tang et al., 1996) and cells transfected with a cloned MOR (Quillan et al., 2002). It is not likely that our protocol masked an effect of opioids on basal [Ca2+]i. In some experiments, small neurons were treated with morphine alone (1 μm), and no changes in [Ca2+]i occurred. The desensitization to the inhibitory effect of morphine within 10 min of treatment is consistent with previous reports (Nomura et al., 1994; Morikawa et al., 1998).
A major conclusion of this study is that opioid receptors inhibited all pharmacological components of VDCCs (L, N, and P/Q) in small neurons, whereas CB1 receptors modulated only N-type VDCCs in intermediate-size adult DRG neurons. The absence of a selective relationship of opioid receptors with one type of VDCC is consistent with studies of subtypes of opioid receptors on DRG neurons cultured from neonatal or young rats (Moises et al., 1994; Wiley et al., 1997; Acosta and Lopez, 1999). Studies of CB1 agonists on neonatal hippocampal neurons indicate the receptor inhibits N-, P-, and Q-type VDCCs (Twitchell et al., 1997; Sullivan, 1999). These observations most likely reflect differences between hippocampal and primary afferent neurons and not a change in phenotype with development because ω-conotoxin also blocked the effect of a CB1 agonist in neonatal neurons (Ross et al., 2001). Modulation of N-type channels in soma of DRG neurons does not exclude other actions of CB1 receptors in primary afferent neurons. For example, CB1 agonists increase K+ currents in presynaptic terminals in nucleus accumbens (Robbe et al., 2001). Similarly, we observed a decrease in the excitability of dissociated adult DRG neurons after treatment with cannabinoids (L. Johanek, D. Simone, and J.-M. Zhang, unpublished observations).
In contrast to a previous report (Scroggs and Fox, 1992), there were no differences between small and intermediate-size DRG neurons in the relative contribution of individual VDCCs to the depolarization-induced increase in [Ca2+]i. Other studies of calcium currents in small DRG neurons noted a greater contribution of N-type VDCCs to the total calcium current (Rusin and Moises, 1995; Wiley et al., 1997; Acosta and Lopez, 1999). The variability in results is most likely attributable to technical factors in that currents through VDCCs in previous studies were isolated by suppressing other cation currents and were evoked with brief (30-100 msec) changes in voltage. Depolarization with 50 mm K+ also recruits multiple inputs to change [Ca2+]i, including activation of plasmalemmal VDCCs and Ca2+-activated Ca2+ release from intracellular stores (Garaschuk et al., 1997; Scamps et al., 1998). Measurement of somal [Ca2+]i with microfluorimetry may mask the contribution of individual elements but it provides an index of total cytoplasmic [Ca2+] within the cell body.
Opioid receptor pharmacology
The high concentration of morphine (1 μm) that was required to inhibit the K+-evoked increase in [Ca2+]i suggests the effect of morphine was mediated by multiple subtypes of opioid receptors. Evidence for involvement of MORs includes blockade of the effect of morphine by CTAP at a concentration that selectively inhibits MORs (Bonner et al., 1997) and blockade by naltrindole at a concentration that blocks MORs and DORs (Portoghese et al., 1991; Raynor et al., 1994). Furthermore, DAMGO mimicked the effect of morphine at a concentration that selectively binds μ receptors (Erspamer et al., 1989). However, DAMGO alone did not achieve the level of inhibition produced by morphine. A contribution of the DORs is based on evidence that deltorphin II was equally effective as DAMGO in inhibiting [Ca2+]i. The lack of effect of nor-BNI, a potent and highly selective κ-opioid receptor antagonist, suggested κ receptors are not involved. Together, these data suggest that MORs and possibly DORs, but not κ-opioid receptors, couple with VDCCs in the soma of small DRG neurons. This conclusion is consistent with effects of opioid receptor agonists on isolated adult DRG neurons (Taddese et al., 1995) but challenges the absence of cellular localization of DOR-ir to the plasmalemma in DRG of adult rats (Ji et al., 1995).
Modulation of CGRP release
The absence of an effect of CP 55,940 on evoked CGRP release was unexpected for several reasons. First, 70% of CGRP-immunoreactive adult DRG neurons in this preparation are CB1 receptor immunoreactive, indicating codistribution of the relevant elements (Khasabova et al., 2002). In addition, CB1 receptors couple with N-type VDCCs, and these channels contribute to release of peptide from primary afferent neurons (Harding et al., 1999; Kress et al., 2001). Furthermore, low concentrations of anandamide and CP 55,940 inhibit capsaicin-evoked release of immunoreactive CGRP release from skin and spinal cord in vitro through CB1 receptors (Richardson et al., 1998a,b; Ellington et al., 2002). The absence of an effect of CP 55,940 on CGRP release from dissociated adult DRG neurons suggests that CB1 receptors are not associated with sites for peptide release on terminals of DRG neurons and that effects of cannabinoids on peptide release in tissue preparations are mediated by other cells in the preparation. This interpretation is supported by evidence that neonatal capsaicin causes only a modest decrease in 3H-CP 55,940 binding in superficial dorsal horn (Hohmann and Herkenham, 1998). Furthermore, the selective coupling of CB1 receptors to N-type VDCCs may not be sufficient to block the release of peptide from primary afferent neurons. Peptide release from primary afferent neurons in response to high K+ is mediated by L, N, and P/Q types of Ca2+ channels (Harding et al., 1999; Asakura et al., 2000; Kress et al., 2001). Thus, L- and P/Q-type channels may be sufficient to overcome the selective inhibition of N-type channels. We cannot exclude the possibility that the preparation of dissociated neurons and growth of processes that provide adherence of neurons to the substrate altered the distribution of cannabinoid receptors. However, this is unlikely because morphine inhibited CGRP release in the same model.
Both MOR and DOR agonists are effective in inhibiting K+-evoked release of CGRP from terminals of primary afferent neurons within slices of rat spinal cord in vitro (Pohl et al., 1989). However, the present study demonstrated that only naltrindole-sensitive opioid receptors mediated the effect of morphine on CGRP release from isolated DRG neurons. The differential effects of naltrindole and CTAP on release contrast with their effects on VDCCs at the soma and suggest differences in the distribution of MORs and DORs on soma and terminals of DRG neurons. In contrast to the CB1 agonist, we speculate that the modulation of morphine on CGRP release was optimized because of the interaction of opioid receptors with multiple subtypes of VDCCs that contribute to the increase in [Ca2+]i, which in turn mobilizes release of peptides from terminals of primary afferent neurons (see above). The comparable inhibitory effect of morphine on the voltage-dependent increase in [Ca2+]i and CGRP release support this interpretation.
Interpretations of data on peptide release are supported by immunohistochemical data. Although CB1-ir and MOR-ir were present within the same laminae as CGRP-ir, there was limited colocalization in axons. The absence of codistribution of CGRP with CB1 receptors within fibers is consistent with observations made with an antibody directed against the C terminus of the receptor (Farquhar-Smith et al., 2000). Whereas immunoreactivity in lamina I is likely to be associated with axons, CB1 receptor-ir in deeper layers is most likely associated with dendrites and cell bodies of spinal interneurons (Farquhar-Smith et al., 2000; Salio et al., 2002). Only DOR-ir demonstrated a high colocalization with CGRP-ir. This result is consistent with observations of the same markers in DRG neurons as well as central (Dado et al., 1993) and peripheral terminals (Wenk and Honda, 1999). The absence of coexistence of CGRP-ir and MOR-ir supports conclusions based on distribution of the two markers in adjacent tissue sections after unilateral dorsal rhizotomy (Abbadie et al., 2002).
In summary, our results indicate that VDCCs in DRG neurons are under direct opioid and cannabinoid control, but the effects of agonists at these receptors on VDCCs are different, and the effects differ by cell size. Furthermore, differential coupling between receptors and VDCCs in soma and terminals may contribute to differences in the effects of the ligands on depolarization-evoked release of CGRP. The complementary action of CB1 and opioid receptor agonists on populations of DRG neurons provides a rationale for their combined use in modulation of somatosensory input to the spinal cord.
Footnotes
This work was supported by Grant DA11471 from the National Institute of Drug Abuse (NIDA) to D.A.S. Generation of the CB1 antibody was funded by NIDA Grant DA11322 to K. Mackie. We are grateful to W. R. Kennedy and members of his laboratory for instruction and use of the confocal microscope and to J. Hodges for some of the statistical analyses. The gifts of the CB1, opioid receptor, and CGRP antibodies from K. Mackie, R. P. Elde, and H. Heath, respectively, were appreciated.
Correspondence should be addressed to Dr. Virginia S. Seybold, Department of Neuroscience, University of Minnesota, 6-145 Jackson Hall, 321 Church Street Southeast, Minneapolis, MN 55455. E-mail: ginger@med.umn.edu.
Copyright © 2004 Society for Neuroscience 0270-6474/04/241744-10$15.00/0
References
- Abbadie C, Lombard MC, Besson JM, Trafton JA, Basbaum AI (2002) Mu and delta opioid receptor-like immunoreactivity in the cervical spinal cord of the rat after dorsal rhizotomy or neonatal capsaicin: an analysis of pre- and postsynaptic receptor distributions. Brain Res 930: 150-162. [DOI] [PubMed] [Google Scholar]
- Acosta CG, Lopez HS (1999) Delta opioid receptor modulation of several voltage-dependent Ca(2+) currents in rat sensory neurons. J Neurosci 19: 8337-8348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguayo LG, White G (1992) Effects of nerve growth factor on TTX- and capsaicin-sensitivity in adult rat sensory neurons. Brain Res 570: 61-67. [DOI] [PubMed] [Google Scholar]
- Ahluwalia J, Urban L, Capogna M, Bevan S, Nagy I (2000) Cannabinoid 1 receptors are expressed in nociceptive primary sensory neurons. Neuroscience 100: 685-688. [DOI] [PubMed] [Google Scholar]
- Ananthan S, Johnson CA, Carter RL, Clayton SD, Rice KC, Xu H, Davis P, Porreca F, Rothman RB (1998) Synthesis, opioid receptor binding, and bioassay of naltrindole analogues substituted in the indolic benzene moiety. J Med Chem 41: 2872-2881. [DOI] [PubMed] [Google Scholar]
- Asakura K, Kanemasa T, Minagawa K, Kagawa K, Yagami T, Nakajima M, Ninomiya M (2000) Alpha-eudesmol, a P/Q-type Ca(2+) channel blocker, inhibits neurogenic vasodilation and extravasation following electrical stimulation of trigeminal ganglion. Brain Res 873: 94-101. [DOI] [PubMed] [Google Scholar]
- Arvidsson U, Dado RJ, Riedl M, Lee JH, Law PY, Loh HH, Elde R, Wessendorf MW (1995a) delta-Opioid receptor immunoreactivity: distribution in brainstem and spinal cord, and relationship to biogenic amines and enkephalin. J Neurosci 15: 1215-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvidsson U, Riedl M, Chakrabarti S, Lee JH, Nakano AH, Dado RJ, Loh HH, Law PY, Wessendorf MW, Elde R (1995b) Distribution and targeting of a mu-opioid receptor (MOR1) in brain and spinal cord. J Neurosci 15: 3328-3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beland B, Fitzerald M (2001) Mu- and delta-opioid receptors are downregulated in the largest diameter primary sensory neurons during postnatal development in rats. Pain 90: 143-150. [DOI] [PubMed] [Google Scholar]
- Bonner GG, Davis P, Stropova D, Ferguson R, Yamamura HI, Porreca F, Hruby VJ (1997) Opioid peptides: simultaneous delta agonism and mu antagonism in somatostatin analogues. Peptides 18: 93-100. [DOI] [PubMed] [Google Scholar]
- Bridges D, Rice AS, Egertova M, Elphick MR, Winter J, Michael GJ (2003) Localisation of cannabinoid receptor 1 in rat dorsal root ganglion using in situ hybridisation and immunohistochemistry. Neuroscience 119: 803-812. [DOI] [PubMed] [Google Scholar]
- Calignano A, La Rana G, Giuffrida A, Piomelli D (1998) Control of pain initiation by endogenous cannabinoids. Nature 394: 277-281. [DOI] [PubMed] [Google Scholar]
- Cardenas CG, Del Mar LP, Scroggs RS (1997) Two parallel signaling pathways couple 5HT1A receptors to N- and L-type calcium channels in C-like rat dorsal root ganglion cells. J Neurophysiol 77: 3284-3296. [DOI] [PubMed] [Google Scholar]
- Carter WB, Taylor RL, Kao PC, Heath 3rd H (1991) Determination of plasma calcitonin gene-related peptide concentrations by a new immunochemiluminometric assay in normal persons and patients with medullary thyroid carcinoma and other neuroendocrine tumors. J Clin Endocrinol Metab 72: 327-335. [DOI] [PubMed] [Google Scholar]
- Corbett AD, Peterson SJ, Kosterlitz HW (1993) Selectivity of ligands for opioid receptors. In: Handbook of experimental pharmacology: opioids I (Herz A, ed), pp 645-673. Berlin: Springer.
- Dado RJ, Law PY, Loh HH, Elde R (1993) Immunofluorescent indentification of a δ-opioid receptor on primary afferent nerve terminals. NeuroReport 5: 341-344. [DOI] [PubMed] [Google Scholar]
- Ellington HC, Cotter MA, Cameron NE, Ross RA (2002) The effect of cannabinoids on capsaicin-evoked calcitonin gene-related peptide (CGRP) release from the isolated paw skin of diabetic and non-diabetic rats. Neuropharmacology 42: 966-975. [DOI] [PubMed] [Google Scholar]
- Emmerson PJ, Liu MR, Woods JH, Medzihradsky F (1994) Binding affinity and selectivity of opioids at mu, delta and kappa receptors in monkey brain membranes. J Pharmacol Exp Ther 271: 1630-1637. [PubMed] [Google Scholar]
- Endoh T, Suzuki T (1998) The regulating manner of opioid receptors on distinct types of calcium channels in hamster submandibular ganglion cells. Arch Oral Biol 43: 221-233. [DOI] [PubMed] [Google Scholar]
- Erspamer V, Melchiorri P, Falconieri-Erspamer G, Negri L, Corsi R, Severini C, Barra D, Simmaco M, Kreil G (1989) Deltorphins: a family of naturally occurring peptides with high affinity and selectivity for delta opioid binding sites. Proc Natl Acad Sci USA 86: 5188-5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar-Smith WP, Egertova M, Bradbury EJ, McMahon SB, Rice AS, Elphick MR (2000) Cannabinoid CB(1) receptor expression in rat spinal cord. Mol Cell Neurosci 15: 510-521. [DOI] [PubMed] [Google Scholar]
- Formenti A, Martina M, Plebani A, Mancia M (1998) Multiple modulatory effects of dopamine on calcium channel kinetics in adult rat sensory neurons. J Physiol (Lond) 509: 395-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox A, Kesingland A, Gentry C, McNair K, Patel S, Urban L, James I (2001) The role of central and peripheral cannabinoid1 receptors in the antihyperalgesic activity of cannabinoids in a model of neuropathic pain. Pain 92: 91-100. [DOI] [PubMed] [Google Scholar]
- Galiegue S, Mary S, Marchand J, Dussossoy D, Carriere D, Carayon P, Bouaboula M, Shire D, Le Fur G, Casellas P (1995) Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur J Biochem 232: 54-61. [DOI] [PubMed] [Google Scholar]
- Garaschuk O, Yaari Y, Konnerth A (1997) Release and sequestration of calcium by ryanodine-sensitive stores in rat hippocampal neurones. J Physiol (Lond) 502: 13-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdeman G, Lovinger DM (2001) CB1 cannabinoid receptor inhibits synaptic release of glutamate in rat dorsalateral striatum. J Neurophysiol 85: 468-471. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY (1985) A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260: 3440-3450. [PubMed] [Google Scholar]
- Guirimand F, Strimbu-Gozariu M, Willer JC, Le Bars D (1994) Effects of mu, delta and kappa opioid antagonists on the depression of a C-fiber reflex by intrathecal morphine and DAGO in the rat. J Pharmacol Exp Ther 269: 1007-1020. [PubMed] [Google Scholar]
- Hampson RE, Mu J, Deadwyler SA (2000) Cannabinoid and kappa opioid receptors reduce potassium K current via activation of G(s) proteins in cultured hippocampal neurons. J Neurophysiol 84: 2356-2364. [DOI] [PubMed] [Google Scholar]
- Harding LM, Beadle DJ, Bermudez I (1999) Voltage-dependent calcium channel subtypes controlling somatic substance P release in the peripheral nervous system. Prog Neuropsychopharmacol Biol Psychiatry 23: 1103-1112. [DOI] [PubMed] [Google Scholar]
- Hohmann AG, Herkenham M (1998) Regulation of cannabinoid and mu opioid receptors in rat lumbar spinal cord following neonatal capsaicin treatment. Neurosci Lett 252: 13-16. [DOI] [PubMed] [Google Scholar]
- Hohmann AG, Herkenham M (1999) Localization of central cannabinoid CB1 receptor messenger RNA in neuronal subpopulations of rat dorsal root ganglia: a double-label in situ hybridization study. Neuroscience 90: 923-931. [DOI] [PubMed] [Google Scholar]
- Howlett AC, Fleming RM (1984) Cannabinoid inhibition of adenylate cyclase. Pharmacology of the response in neuroblastoma cell membranes. Mol Pharmacol 26: 532-538. [PubMed] [Google Scholar]
- Huang LY, Neher E (1996) Ca2+-dependent exocytosis in the somata of the dorsal root ganglion neurons. Neuron 17: 135-145. [DOI] [PubMed] [Google Scholar]
- Iwasaki S, Momiyama A, Uchitel OD, Takahashi T (2000) Developmental changes in calcium channel types mediating central synaptic transmission. J Neurosci 20: 59-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji RR, Zhang Q, Law PY, Low HH, Elde R, Hokfelt T (1995) Expression of mu-, delta-, and kappa-opioid receptor-like immunoreactivities in rat dorsal root ganglia after carrageenan-induced inflammation. J Neurosci 15: 8156-8166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanek LM, Heitmiller DR, Turner M, Nader N, Hodges J, Simone DA (2001) Cannabinoids attenuate capsaicin-evoked hyperalgesia through spinal and peripheral mechanisms. Pain 93: 303-315. [DOI] [PubMed] [Google Scholar]
- Khasabova IA, Simone DA, Seybold VS (2002) Cannabinoids attenuate depolarization-dependent Ca2+ influx in intermediate-size primary afferent neurons of adult rats. Neuroscience 115: 613-625. [DOI] [PubMed] [Google Scholar]
- Kirschstein T, Greffrath W, Busselberg D, Treede RD (1999) Inhibition of rapid heat responses in nociceptive primary sensory neurons of rats by vanilloid receptor antagonists. J Neurophysiol 82: 2853-2860. [DOI] [PubMed] [Google Scholar]
- Kress M, Izydorczyk I, Kuhn A (2001) N- and L- but not P/Q-type calcium channels contribute to neuropeptide release from rat skin in vitro. NeuroReport 12: 867-870. [DOI] [PubMed] [Google Scholar]
- Ko M, Woods JH (1999) Local administration of Δ 9-tetrahydrocannabinol attenuates capsaicin-induced thermal nociception in rhesus monkeys: a peripheral cannabinoid action. Psychopharmacology 143: 322-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson LI (1981) A novel immunocytochemical model system for specificity and sensitivity screening of antisera against multiple antigens. J Histochem Cytochem 29: 408-410. [DOI] [PubMed] [Google Scholar]
- Mackie K, Hille B (1992) Cannabinoids inhibit N-type calcium current in neuroblastoma-glioma cells. Proc Natl Acad Sci USA 89: 3825-3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie K, Lai Y, Westenbroek R, Mitchell R (1995) Cannabinoids activate an inwardly rectifying potassium conductance and inhibit Q-type calcium currents in AtT20 cells transfected with rat brain cannabinoid receptor. J Neurosci 15: 6552-6561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malan Jr TP, Ibrahim MM, Deng H, Liu Q, Mata HP, Vanderah T, Porreca F, Makriyannis A (2001) CB2 cannabinoid receptor-mediated peripheral antinociception. Pain 93: 239-245. [DOI] [PubMed] [Google Scholar]
- Malan TP, Ibrahim MM, Vanderah TW, Makriyannis A, Porreca F (2002) Inhibition of pain responses by activation of CB(2) cannabinoid receptors. Chem Phys Lipids 121: 191-200. [DOI] [PubMed] [Google Scholar]
- Martin WJ, Loo CM, Basbaum AI (1999) Spinal cannabinoids are anti-allodynic in rats with persistent inflammation. Pain 82: 199-205. [DOI] [PubMed] [Google Scholar]
- Mazzari S, Canella R, Petrelli L, Marcolongo G, Leon A (1996) N-(2-hydroxyethyl)hexadecanamide is orally active in reducing edema formation and inflammatory hyperalgesia by down-modulating mast cell activation. Eur J Pharmacol 300: 227-236. [DOI] [PubMed] [Google Scholar]
- Moises HC, Rusin MI, Macdonald RL (1994) Mu- and kappa-opioid receptors selectively reduce the same transient components of high-threshold calcium current in rat dorsal root ganglion sensory neurons. J Neurosci 14: 5903-5916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molliver DC, Wright DE, Leitner ML, Parsadanian AS, Doster K, Wen D, Yan Q, Snider WD (1997) IB4-binding DRG neurons switch from NGF to GDNF dependence in early postnatal life. Neuron 19: 849-861. [DOI] [PubMed] [Google Scholar]
- Morikawa H, Fukuda K, Mima H, Shoda T, Kato S, Mori K (1998) Desensitization and resensitization of delta-opioid receptor-mediated Ca2+ channel inhibition in NG108-15 cells. Br J Pharmacol 123: 1111-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morisset V, Urban L (2001) Cannabinoid-induced presynaptic inhibition of glutamatergic EPSCs in substantia gelatinosa neurons of the rat spinal cord. J Neurophysiol 86: 40-48. [DOI] [PubMed] [Google Scholar]
- Nomura K, Reuveny E, Narahashi T (1994) Opioid inhibition and desensitization of calcium channel currents in rat dorsal root ganglion neurons. J Pharmacol Exp Ther 270: 466-474. [PubMed] [Google Scholar]
- Pan XH, Ikeda SR, Lewis DL (1996) Rat brain cannabinoid receptor modulates N-type Ca2+ channels in a neuronal expression system. Mol Pharmacol 49: 707-714. [PubMed] [Google Scholar]
- Pelton JT, Kazmierski W, Gulya K, Yamamura HI, Hruby VJ (1986) Design and synthesis of conformationally constrained somatostatin analogues with high potency and specificity for mu opioid receptors. J Med Chem 29: 2370-2375. [DOI] [PubMed] [Google Scholar]
- Petersen M, Klusch A, Eckert A (1998) The proportion of isolated rat dorsal root ganglion neurones responding to bradykinin increases with time in culture. Neurosci Lett 252: 143-146. [DOI] [PubMed] [Google Scholar]
- Pohl M, Lombard MC, Bourgoin S, Carayon A, Benoliel JJ, Mauborgne A, Besson JM, Hamon M, Cesselin F (1989) Opioid control of the in vitro release of calcitonin gene-related peptide from primary afferent fibres projecting in the rat cervical cord. Neuropeptides 14: 151-159. [DOI] [PubMed] [Google Scholar]
- Portoghese PS, Sultana M, Takemori AE (1990) Design of peptidomimetic delta opioid receptor antagonists using the message-address concept. J Med Chem 33: 1714-1720. [DOI] [PubMed] [Google Scholar]
- Portoghese PS, Nagase H, MaloneyHuss KE, Lin CE, Takemori AE (1991) Role of spacer and address components in peptidomimetic delta opioid receptor antagonists related to naltrindole. J Med Chem 34: 1715-1720. [DOI] [PubMed] [Google Scholar]
- Quillan JM, Carlson KW, Song C, Wang D, Sadee W (2002) Differential effects of mu-opioid receptor ligands on Ca(2+) signaling. J Pharmacol Exp Ther 302: 1002-1012. [DOI] [PubMed] [Google Scholar]
- Raynor K, Kong H, Chen Y, Yasuda K, Yu L, Bell GI, Reisine T (1994) Pharmacological characterization of the cloned kappa-, delta-, and mu-opioid receptors. Mol Pharmacol 45: 330-334. [PubMed] [Google Scholar]
- Richardson JD, Aanonsen L, Hargreaves KM (1998a) Antihyperalgesic effects of spinal cannabinoids. Eur J Pharmacol 345: 145-153. [DOI] [PubMed] [Google Scholar]
- Richardson JD, Kilo S, Hargreaves KM (1998b) Cannabinoids reduce hyperalgesia and inflammation via interaction with peripheral CB1 receptors. Pain 75: 111-119. [DOI] [PubMed] [Google Scholar]
- Robbe D, Alonso G, Duchamp F, Bockaert J, Manzoni OJ (2001) Localization and mechanisms of action of cannabinoid receptors at the glutamatergic synapses of the mouse nucleus accumbens. J Neurosci 21: 109-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross RA, Coutts AA, McFarlane SM, Anavi-Goffer S, Irving AJ, Pertwee RG, MacEwan DJ, Scott RH (2001) Actions of cannabinoid receptor ligands on rat cultured sensory neurones: implications for antinociception. Neuropharmacology 40: 221-232. [DOI] [PubMed] [Google Scholar]
- Rusin KI, Moises HC (1995) Mu-opioid receptor activation reduces multiple components of high-threshold calcium current in rat sensory neurons. J Neurosci 15: 4315-4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salio C, Fischer J, Franzoni MF, Conrath M (2002) Pre- and postsynaptic localizations of the CB1 cannabinoid receptor in the dorsal horn of the rat spinal cord. Neuroscience 110: 755-764. [DOI] [PubMed] [Google Scholar]
- Sawynok J (2003) Topical and Peripherally acting analgesics. Pharmacol Rev 55: 1-20. [DOI] [PubMed] [Google Scholar]
- Scamps F, Valentin S, Dayanithi G, Valmier J (1998) Calcium channel subtypes responsible for voltage-gated intracellular calcium elevations in embryonic rat motoneurons. Neuroscience 87: 719-730. [DOI] [PubMed] [Google Scholar]
- Scroggs RS, Fox AP (1992) Calcium current variation between acutely isolated adult rat dorsal root ganglion neurons of different size. J Physiol (Lond) 445: 639-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silbert SC, Beacham DW, McCleskey EW (2003) Quantitative single-cell differences in mu-opioid receptor mRNA distinguish myelinated and unmyelinated nociceptors. J Neurosci 23: 34-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stucky CL, Thayer SA, Seybold VS (1996) Prostaglandin E2 increases the proportion of neonatal rat dorsal root ganglion neurons that respond to bradykinin. Neuroscience 74: 1111-1123. [DOI] [PubMed] [Google Scholar]
- Sullivan JM (1999) Mechanisms of cannabinoid-receptor-mediated inhibition of synaptic transmission in cultured hippocampal pyramidal neurons. J Neurophysiol 82: 1286-1294. [DOI] [PubMed] [Google Scholar]
- Taddese A, Nah SY, McCleskey EW (1995) Selective opioid inhibition of small nociceptive neurons. Science 270: 1366-1369. [DOI] [PubMed] [Google Scholar]
- Tang T, Stevens BA, Cox BM (1996) Opioid regulation of intracellular free calcium in cultured mouse dorsal root ganglion neurons. J Neurosci Res 44: 338-343. [DOI] [PubMed] [Google Scholar]
- Twitchell W, Brown S, Mackie K (1997) Cannabinoids inhibit N- and P/Q-type calcium channels in cultured rat hippocampal neurons. J Neurophysiol 78: 43-50. [DOI] [PubMed] [Google Scholar]
- Wenk HN, Honda CN (1999) Immunohistochemical localization of delta opioid receptors in peripheral tissues. J Comp Neurol 408: 567-579. [DOI] [PubMed] [Google Scholar]
- Wiley JW, Moises HC, Gross RA, MacDonald RL (1997) Dynorphin A-mediated reduction in multiple calcium currents involves a G(o) alpha-subtype G protein in rat primary afferent neurons. J Neurophysiol 77: 1338-1348. [DOI] [PubMed] [Google Scholar]
- Willis WD, Coggeshall RE (1991) Dorsal root ganglion cells and their processes. In: Sensory mechanism of the spinal cord, Ed 2, pp 47-78. New York: Plenum.
- Yaksh TL, Stevens CW (1988) Properties of the modulation by receptor-selective agents in spinal nociceptive processing. In: Proceedings of the Vth World Congress on Pain, Vol 3 (Dubner R, Gebhart GF, Bond MR, eds), pp 417-435. Amsterdam: Elsevier. [Google Scholar]