Abstract
Mature retinal ganglion cells (RGCs), like other CNS neurons, cannot regrow injured axons into a myelin-rich environment. If stimulated by macrophage-derived factors, however, RGCs can regenerate their axons for considerable distances through the distal optic nerve. Using this “sensitized background,” we investigated the effects of either increasing the expression or suppressing the activity of the Nogo receptor (NgR). NgR mediates the growth-inhibiting effects of three myelin proteins, Nogo, OMgp (oligodendrocyte-myelin glycoprotein), and MAG (myelin-associated glycoprotein). Transfecting growth-sensitized RGCs with adeno-associated viruses expressing a dominant-negative form of NgR (NgRDN) increased axon regeneration several-fold; however, when the growth program of RGCs was not activated, NgRDN expression had no beneficial effects. Overexpression of wild-type NgR blocked almost all regeneration from growth-sensitized RGCs and caused axons proximal to the lesion site to retract. We conclude that gene therapy is an effective approach to enhancing axon regeneration in the CNS and that inactivation of NgR functioning greatly enhances axon regeneration provided the intrinsic growth program of neurons is activated.
Keywords: axon, macrophage, myelin, regeneration, retinal ganglion cell, virus, Nogo receptor, gene therapy, CNS, Nogo, MAG, OMgp
Introduction
The inability of CNS neurons to regenerate damaged axons severely limits functional recovery after traumatic injury, stroke, or certain neurodegenerative diseases. Regenerative failure has been attributed in part to proteins associated with CNS myelin and the scar that forms at an injury site. Several myelin inhibitors of axon growth, including the C-terminal of NogoA (Nogo66) (Chen et al., 2000; GrandPre et al., 2000), myelin-associated glycoprotein (MAG) (McKerracher et al., 1994; Mukhopadhyay et al., 1994), and oligodendrocyte-myelin glycoprotein (OMgp) (Wang et al., 2002a), exert their effects via the Nogo receptor (NgR) and p75NTR or another co-receptor (Fournier et al., 2001; Domeniconi et al., 2002; Liu et al., 2002; Wang et al., 2002a,b). In culture, ectopic NgR expression causes growth cones of embryonic chick retinal ganglion cells (RGCs) to collapse after contacting Nogo66 (Fournier et al., 2001) and inhibits cerebellar granule cells from extending neurites on MAG, OMgp, or myelin (Wang et al., 2002a,b). Conversely, transfection with a dominant-negative form of NgR (NgRDN) enables cerebellar granule cells in culture to overcome the inhibitory effects of myelin, Nogo66, OMgp, and MAG (Domeniconi et al., 2002; Wang et al., 2002a,b). Antibodies to NogoA or a small peptide inhibitor of NgR increase corticospinal tract (CST) regeneration to some extent in rats (Schnell et al., 1994; Bregman et al., 1995; GrandPre et al., 2002; Sicotte et al., 2003), whereas genetic deletion of the NogoA gene in mice results in either modest CST regeneration (Kim et al., 2003b; Simonen et al., 2003) or none (Zheng et al., 2003). These findings have raised the question of whether overcoming specific myelin inhibitors, or suppression of signaling through NgR, is sufficient to promote extensive CNS regeneration in vivo (Steward et al., 2003; Woolf, 2003; Zheng et al., 2003).
The optic nerve is a classic model for understanding regenerative failure or success in the mature mammalian CNS (Aguayo et al., 1991; Ramon y Cajal, 1991). Axons that are injured in the mature rat optic nerve cannot grow back into the myelin-rich environment distal to the injury site. In addition, if axonal damage occurs close to the eye, RGCs undergo apoptosis after several days (Berkelaar et al., 1994). Several intraocular manipulations, including injuring the lens (Fischer et al., 2000, 2001; Leon et al., 2000), injecting the proinflammatory agent zymosan (Yin et al., 2003), or inserting a peripheral nerve fragment (Berry et al., 1996), partially reverse this situation and allow many RGCs to survive injury and regenerate lengthy axons into the optic nerve; these effects appear to be mediated via macrophage-derived factors (Yin et al., 2003) acting in concert with a carbohydrate that is constitutively present in the eye (Li et al., 2003). The partial regeneration that occurs under these conditions provides a sensitized background on which to investigate the significance of NgR in CNS regeneration. This was done here by transfecting RGCs with adeno-associated viruses (AAVs) carrying a gene for either wild-type NgR or a dominant-negative NgR.
Materials and Methods
Viral transfections. cDNAs encoding either wild-type NgR (Fournier et al., 2001) or a C-terminal truncated, dominant-negative variant of NgR that retains the ligand binding domain but does not associate with its co-receptor (Domeniconi et al., 2002; Wang et al., 2002a) were inserted into the AAV-MCS2 plasmid, described on the website of the Harvard Gene Therapy Initiative (http://hgti.med.harvard.edu/pp/AAV-MCS2.png). Gene expression was driven by a cytomegalovirus (CMV) promoter. Constructs expressed enhanced green fluorescent protein (GFP) from an internal ribosome entry site. NgR constructs contained a hemagglutinin antigen (HA) epitope tag, as described (Wang et al., 2002b). Controls were transfected with viruses expressing GFP alone. Virus production was performed at the Harvard Gene Therapy Initiative Core Facility. To transfect RGCs, female Sprague Dawley rats (160-180 gm) were anesthetized with ketamine-xylazine, and the back of the eye was exposed intraorbitally. After withdrawing 10 μl of fluid from the eye, ∼1010 AAV particles in 10 μl PBS were injected into the vitreous body using a micropipette, with care taken to avoid injuring the lens (Fischer et al., 2000). Injections were done 3 weeks before optic nerve surgery to maximize levels of transgene expression at the onset of axon regeneration (Cheng et al., 2002). All procedures were performed under protocols approved by the Children's Hospital Institutional Animal Care and Use Committee.
Optic nerve surgery and lens injury. Animals were anesthetized using ketamine-xylazine, immobilized in a stereotaxic apparatus, and the left optic nerve was surgically exposed intraorbitally. After the meninges was opened longitudinally, the optic nerve was crushed 2 mm from the orbit by applying pressure with jewelers' forceps under a dissecting microscope for 10 sec. Lens injury was accomplished by puncturing the lens capsule with a microcapillary through a posterior approach (Fischer et al., 2000). Lens injury leads to macrophage activation, and factors secreted from activated macrophages stimulate RGCs to regenerate their axons (Yin et al., 2003). Controls sustained nerve injury but no lens damage. Nerve injury was verified by the appearance of a clearing at the crush site; the vascular integrity of the retina was verified by fundoscopic examination.
Retinal explants. Explants of viral-transfected retinas were prepared 4 d after crushing the optic nerve and either injuring the lens or performing sham surgery. Animals were killed, and their retinas were dissected out, cut into eight radial pieces, and cultured in DMEM-B27 (Invitrogen) on a poly-d-lysine-laminin (PLL) substrate (Bahr et al., 1988) with or without myelin, prepared as described (Wang et al., 2002a). Two days later, the number of axons growing ≥50 μm beyond the margin of each explant was counted with the aid of an inverted phase-contrast microscope (Axiovert, Zeiss) and a calibrated ocular micrometer at a magnification of 200×. In cases with strong regeneration, some fiber fasciculation was observed, and these were counted as single axons. Results from individual explants were averaged within each treatment group, and between-group differences were evaluated with Student's t test. To evaluate growth on myelin, we calculated the ratio of axons growing >500 μm to total axons ≥50 μm in explants immunostained for βIII tubulin. This was done to account for the variability in adhesion and outgrowth of explants grown on the mixed myelin-laminin substrate and to visualize axons against a particulate background. Results were averaged from six explants per retina and four to five retinas per condition.
Histology: retinal explants. After 2 d in culture, retinas were fixed in 4% paraformaldehyde in PBS, treated with methanol for 10 min and blocking solution containing 10% serum from the same species as the secondary antibody for 1 hr (room temperature), and then incubated overnight (4°C) with antibodies against GFP (prepared in rabbit; Molecular Probes, Eugene, OR, 1:1000), βIII tubulin (mouse monoclonal antibody TUJ1; Babco, Richmond, CA; 1:500), or the HA epitope tag (mouse monoclonal antibody; Molecular Probes, 1:100) fused to NgR. Primary antibodies were prepared in Tris-buffered saline (TBS) containing 2× physiological saline, 5% serum, 2% BSA, and 0.1% Tween 20. After three rinses in TBS, sections were incubated with fluorescent-tagged secondary antibodies, i.e., AlexaFluor 488-conjugated goat antibody to rabbit IgG or AlexaFluor 594-conjugated goat antibody to mouse IgG (1:500; 2 hr; room temperature), rinsed, and covered.
Optic nerve and retinal cross sections. Two weeks after nerve surgery, animals were killed with an overdose of anesthesia and perfused with PBS followed by 4% paraformaldehyde in PBS. Optic nerves with retinas attached were dissected and prepared for longitudinal sectioning as described (Yin et al., 2003). Sections were stained to visualize either GAP-43 [primary antibody prepared in sheep (Benowitz et al., 1988); 1:1000, followed by a fluorescent-tagged donkey anti-sheep IgG] or GFP, as above. Retinal cross sections were stained to visualize either GFP or βIII tubulin (as above) or NgR. The latter was visualized using a primary antibody made in goat to the N terminus of NgR (1:10; Santa Cruz Biotechnology), followed by a fluorescent secondary antibody to goat IgG made in donkey (1:500).
Axon regeneration: quantitation. Regeneration was quantified as described (Leon et al., 2000; Yin et al., 2003). In four longitudinal sections per case, under 400× magnification, we counted the number of GAP-43-positive axons extending ≥500 μm and ≥1 mm from the injury site and divided these numbers by the cross-sectional width of the nerve at the point where axons were counted. The number of axons per unit width of optic nerve was averaged across the four sections and used to calculate the total number of axons regenerating in the nerve (Leon et al., 2000; Yin et al., 2003). The significance of intergroup differences was evaluated by Student's t tests.
Cell survival. Cross sections through the center of the retina were double-stained with antibodies to GFP and βIII tubulin as described above. The numbers of βIII tubulin-positive cells per section were counted in four to six sections per case, averaged for each case, and then averaged across all similarly treated animals to obtain group means and SEs.
Results
To investigate the role of NgR in vivo, we injected mature rats intravitreally with AAV (serotype 2) carrying a plasmid expressing either the wild-type Nogo receptor (NgRWT) (Fournier et al., 2001) or a truncated, dominant-negative variant of NgR (Domeniconi et al., 2002; Wang et al., 2002a) from a CMV promoter, along with enhanced GFP from an internal ribosome entry site (AAV-NgRWT-IGFP and AAV-NgRDN-IGFP, respectively). Controls were transfected with viruses expressing GFP alone (AAV-GFP). When examined 3 weeks later, the GFP reporter was detected in >75% of all RGCs (Fig. 1A-C), in agreement with previous studies using a similar virus (Cheng et al., 2002; Martin et al., 2002). GFP-labeled cells were localized almost exclusively within the ganglion cell layer (Fig. 1D) in cells that are immunopositive for βIII tubulin (Fig. 1E,F). Within the retina, this tubulin isoform is expressed only in RGCs (Cui et al., 2003; Yin et al., 2003), which we verified by showing a complete overlap of βIII tubulin immunostaining with Fluorogold labeling in RGCs after injecting the latter into the superior colliculus (data not shown). The specificity of transfection to RGCs presumably reflects a combination of the neural selectivity of AAV2 (Bartlett et al., 1998) and the ready access of intravitreal viral particles to RGC axons and somata.
Figure 1.
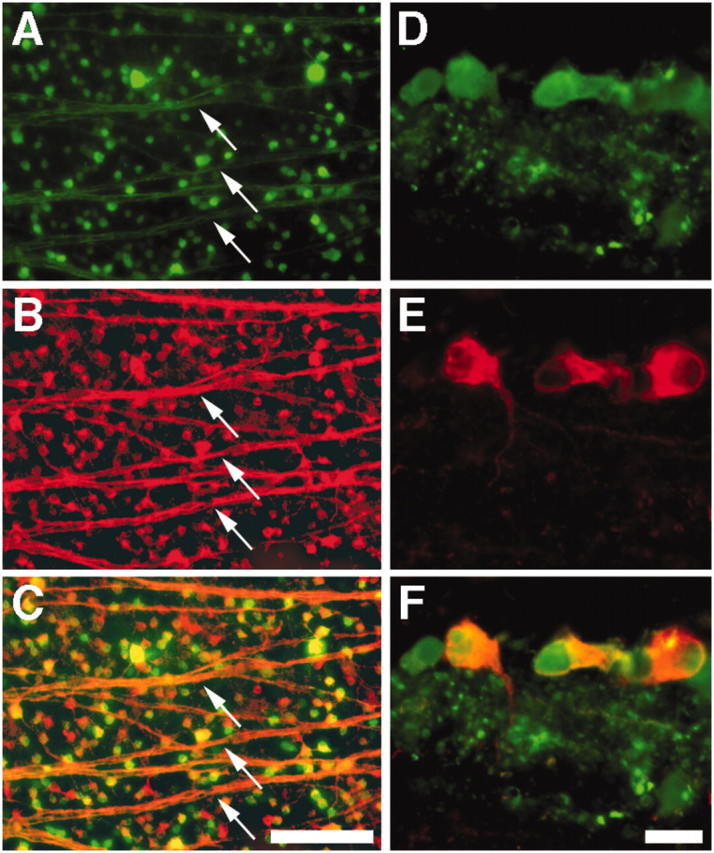
AAV-mediated transfection of RGCs. A-C, Flat-mounted rat retina 3 weeks after intravitreal injection with AAV-NgRWT-IGFP, double-stained for GFP to detect transfected cells (A) and βIII tubulin, a selective marker for RGCs in the retina (B). Note staining of cell bodies and axon fascicles (arrows) throughout the retina. C, Merged image. D-F, Retinal cross section, double labeled as above. GFP-expressing cells (D) are located within the innermost retina and show positive staining for βIII tubulin (E). F, Merged image. Scale bars: A-C, 100 μm; D-F, 20 μm.
NgR immunostaining was modest or weak in controls transfected with AAV-GFP (Fig. 2A-C) but was strong in retinas transfected with AAV-NgRWT-IGFP (Fig. 2D-F). Thus, in transfected cells, levels of transgene expression exceed those of the endogenous protein. Three weeks after transfections, animals were anesthetized, and the left optic nerve was crushed 2 mm from the back of the eye. In half of these animals, the lens was damaged to activate macrophages and promote regeneration (Fischer et al., 2000; Leon et al., 2000; Yin et al., 2003); the remaining animals received no further surgery.
Figure 2.
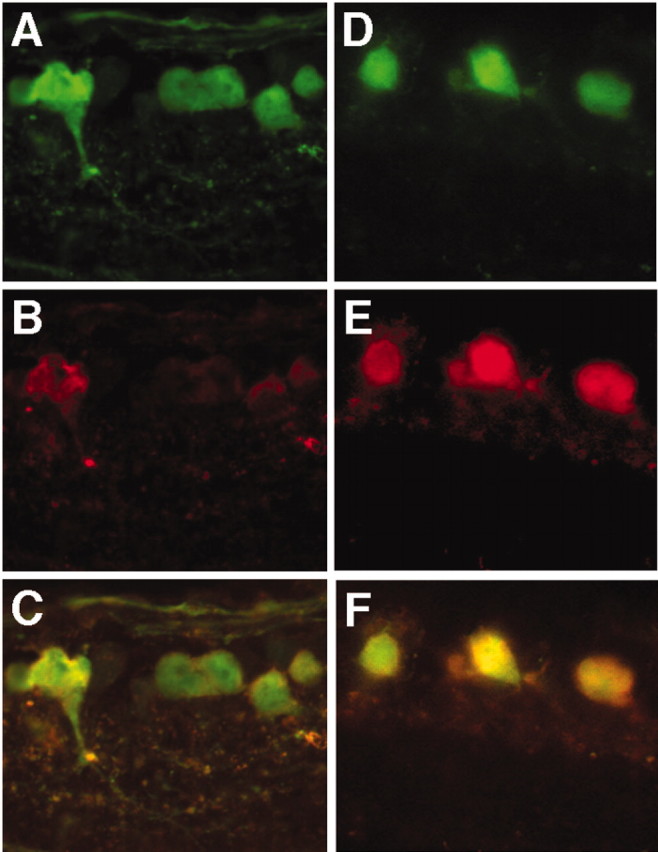
Overexpression of NgR in transfected RGCs. A-C, Controls transfected with AAV-GFP and immunostained for GFP (A) and NgR (B). C, Merged image. D-F, Animals transfected with AAV-NgRWT-IGFP and immunostained for GFP (D) and NgR (E). F, Merged image. Scale is the same as in Figure 1D-F.
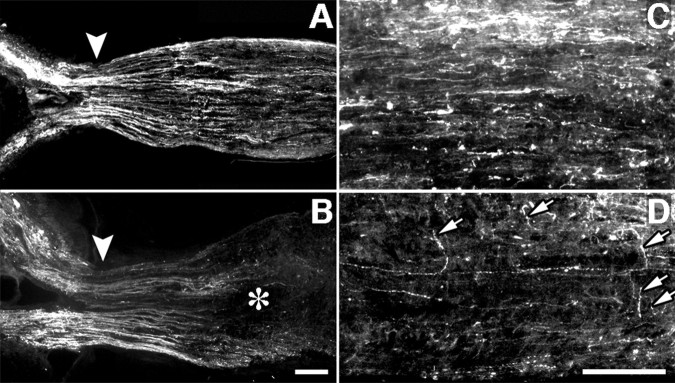
Regeneration was investigated 2 weeks after optic nerve injury; previous work has shown that damaged axons have begun to grow back into the distal optic nerve by this time, provided macrophages have been activated intravitreally (Leon et al., 2000). Regenerating axons are readily distinguished by staining with antibodies to GAP-43. GAP-43 is normally undetectable in the mature optic nerve but is strongly upregulated in RGC axons undergoing regeneration (Schaden et al., 1994; Berry et al., 1996; Leon et al., 2000). The origin of the GAP-43-positive axons in RGCs has been shown previously by anterograde labeling and double immunostaining (Leon et al., 2000). Controls with lens injury transfected with AAV-GFP (n = 8) showed a moderate amount of regeneration distal to the injury site (Fig. 3A), comparable with that reported in similarly treated animals without viral transfections (Fig. 4A) (Leon et al., 2000).
Figure 3.
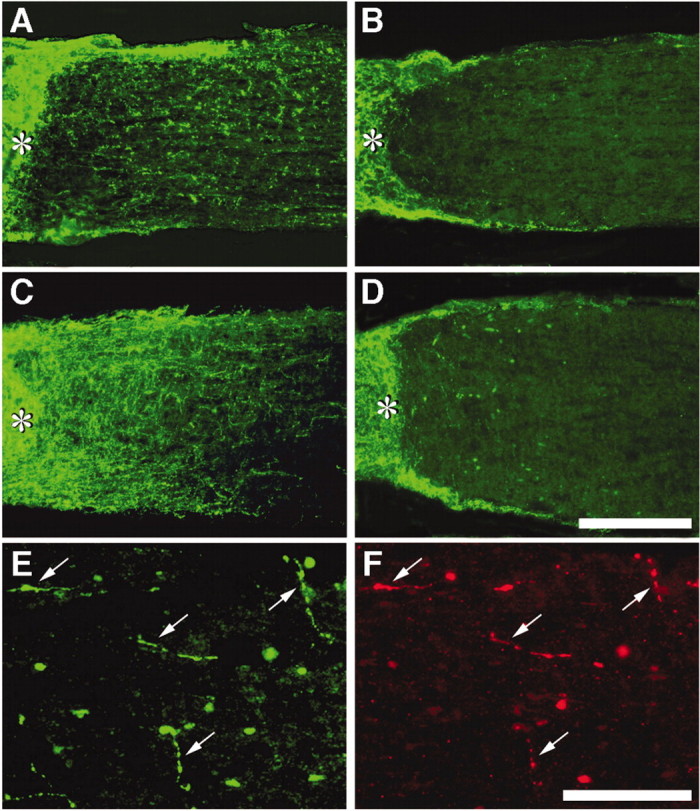
Role of the Nogo receptor in axon regeneration. A-F, Longitudinal sections through the optic nerve showing GAP-43-positive axons distal to the injury site (asterisk) 2 weeks after surgery with or without lens injury. A-C, Axon regeneration after transfection of growth-activated RGCs with AAV expressing GFP alone (A), NgRWT (B), or NgRDN (C). D, NgRDN expression fails to increase regeneration when RGCs have not been sensitized to grow. E, F, Distal optic nerve from the NgRDN-expressing, growth-sensitized case shown in C. Many of the longest axons coexpress GAP-43 (E) and GFP (F) (arrows). Scale bars: A-D, 200 μm; E, F, 100 μm.
Figure 4.
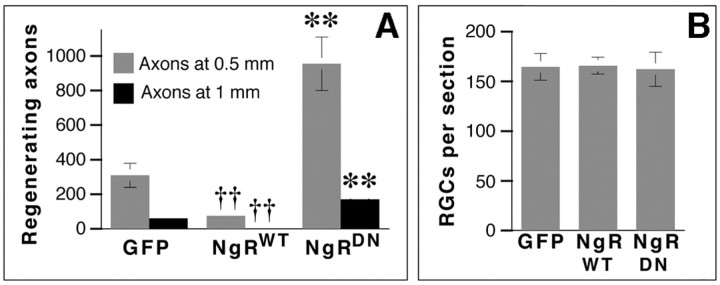
Quantitation of axon regeneration and RGC survival. A, Total number of axons at 0.5 mm (light bars) and 1 mm (dark bars) distal to the injury site. B, Cell survival (βIII tubulin-positive RGCs per section). ††p < 0.01, decrease relative to GFP-transfected controls; **p < 0.01, increase relative to GFP-transfected controls.
Two weeks after nerve crush and lens injury, animals overexpressing NgRWT showed 76% fewer axons regenerating ≥0.5 mm from the injury site than controls (Figs. 3B, 4A) (n = 9; p < 0.01), and 96% fewer axons extending ≥1 mm (p < 0.01). Many NgRWT-containing axons retracted from the lesion site toward the optic nerve head (Fig. 5B,D), reflecting the sensitivity of these axons to myelin; this phenomenon was never observed in animals expressing GFP alone (Fig. 5A,C) or NgRDN (data not shown).
Figure 5.
Overexpression of NgRWT causes axon retraction. GFP-labeled axons in the optic nerve proximal to the injury site 2 weeks after nerve surgery and lens injury. A, C, Growth-sensitized control transfected with AAV-GFP. B, D, Growth-sensitized case transfected with AAV-NgRWT-IGFP. Note reduced number of axons proximal to the injury site (B, asterisk) and axons turning away from the long axis of the nerve (D, arrows). A, B, Arrowheads show the head of the myelinated portion of the nerve. In all cases, the lesion site (not shown) is to the right of the area depicted. Scale bar, 100 μm.
In striking contrast, expression of NgRDN greatly enhanced the number of axons regenerating into the optic nerve (Fig. 3C). Two weeks after nerve crush and lens injury, animals expressing NgRDN (n = 5) extended approximately 3 times more axons ≥1 mm beyond the injury site than growth-sensitized controls expressing GFP alone, and 75 times more axons than growth-sensitized cases expressing NRWT (Fig. 4A). In general, although GFP could be visualized in many axons proximal to the injury site, fewer than half of the axons that extended beyond this point exhibited GFP immunofluorescence, presumably because of decreasing concentrations of the cytoplasmic reporter protein far from RGC somata; however, the longest regenerating axons frequently exhibited GFP staining (Fig. 3E,F), which suggests that they may have arisen from RGCs that express high levels of NgRDN. This colocalization further confirms the origin of GAP-43-immunopositive axons in RGCs. Two weeks after nerve crush and lens injury, the longest axons in NgRDN-expressing cases extended ∼3 mm from the injury site.
On average, the number of fibers that grew ≥0.5 mm distal to the injury site in growth-activated cases expressing NgRDN represents growth from ∼1% of all RGCs. This number is likely to increase with time, as has been observed after macrophage activation alone (Leon et al., 2000). Several factors, however, limit the amount of regeneration that can ultimately occur under these experimental conditions, including the rapid decline of the macrophage response to lens injury and hence the availability of appropriate trophic factors (Leon et al., 2000); the continued death of RGCs (Fischer et al., 2000; Leon et al., 2000) and the likelihood that many of the surviving ones are in a metabolically compromised state; diminished transgene expression shortly after optic nerve surgery (Cheng et al., 2002); and the strong inhibitory effects of the glial scar (McKeon et al., 1995), which is apparent in comparing the number of GAP-43-positive axons proximal versus distal to the injury site (Leon et al., 2000). Further research will be required to determine whether overcoming these problems will enable growth-activated, NgRDN-expressing RGCs to extend axons back to their central targets.
In the absence of lens injury, NgRDN expression did not enable RGCs to regenerate their axons into the optic nerve (Fig. 3D). Quantitatively, no axons were counted ≥0.5 mm distal to the injury site in any animal without lens injury, regardless of which transgene was expressed (data not shown).
To investigate whether the effects of the three transgenes on axon regeneration might reflect differences in cell survival, we counted TUJ1-positive cells in retinal cross sections 2 weeks after nerve crush and lens injury. Transgene expression had no measurable effect on cell survival (Fig. 4B).
To investigate whether altering NgR levels or function might affect the intrinsic ability of RGCs to extend axons, we investigated outgrowth on a more permissive substrate. As before, we transfected RGCs in vivo with either AAV-NgRWT-IGFP or AAV-NgRDN-IGFP and then performed optic nerve surgery combined with lens injury or sham intraocular surgery 3 weeks later.After 4 d, a time at which axotomized RGCs stimulated bymacrophage-derived factors go into a growth state (Fischer et al., 2000), we explanted wedges of retinas onto a PLL substrate. Little outgrowth was seen in explants not exposed to growth factors in vivo (i.e., no lens injury) (Fig. 6A) regardless of transgene expression (Fig. 6F). It should be noted that axotomized RGCs do not show signs of apoptosis at this time point (Berkelaar et al., 1994). Retinas primed to grow as a result of lens injury in vivo showed strong outgrowth regardless of which transgene was expressed (Fig. 6B-F). Figure 6B-D illustrates strong outgrowth from RGCs expressing NgRWT, whereas Figure 6E illustrates outgrowth from a growth-activated retina expressing NgRDN.
Figure 6.
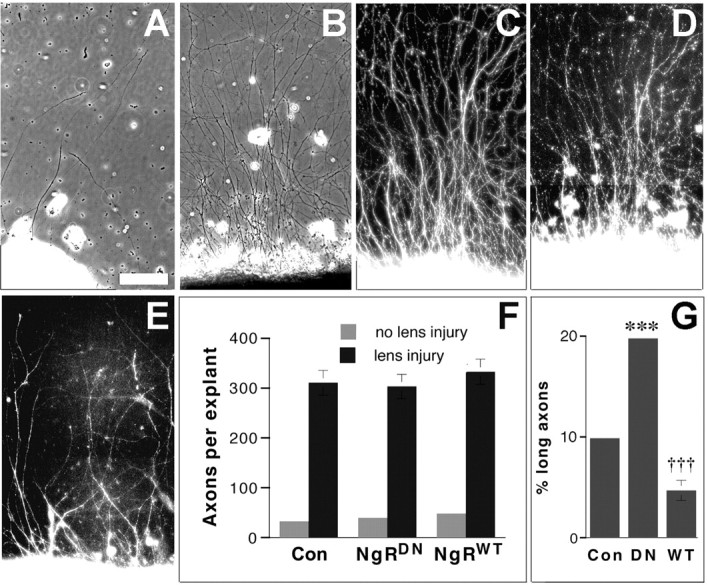
Axon regeneration on permissive and nonpermissive substrates. A-F, Retinal explants grown on a permissive PLL substrate. A, Control retina not exposed to macrophage-derived factors in vivo (i.e., no lens injury). B-D, Retinas transfected with AAV-NgRWT-IGFP and exposed to macrophage-derived factors in vivo. Axons (visualized by phase-contrast microscopy in B) arise from transfected RGCs, as shown by positive immunostaining for βIII tubulin (C) and GFP (D). E, Axons arising from growth-activated retina transfected with AAV-NgRDN-IGFP show positive immunostaining for the epitope tag expressed on the NgR-HA fusion protein. F, Quantitation of axon growth. G, Growth of transfected retinal explants (exposed to macrophage-derived factors in vivo) on myelin (percentage of axons arising from explants that extend ≥500 μm). †††p < 0.001, decrease relative to controls; ***p < 0.001, increase relative to controls. Scale bar, 100 μm.
As expected, the effects of transgene expression became apparent when explants were plated on a substrate containing myelin (Fig. 6G). NgRWT overexpression decreased the percentage of axons growing ≥0.5 mm on a mixed myelin-laminin substrate by ∼50% relative to controls, whereas expression of NgRDN doubled the percentage of long axons (p < 0.001 in both cases).
Discussion
The results of this study show that NgR plays a major role in limiting axon regeneration in the mature optic nerve; however, extensive regeneration requires that, in addition to suppressing NgR activity, the intrinsic growth state of neurons must be activated. Our results show further that AAV-mediated transfection provides a highly effective means of altering either the levels or functioning of gene products important for axon regeneration in CNS neurons.
The critical role of NgR for optic nerve regeneration is evident from the dramatic enhancement of axon growth that occurs when growth-sensitized RGCs express a dominant-negative form of NgR, and conversely, from the near-complete failure of growth-sensitized RGCs to regenerate their axons when overexpressing wild-type NgR. In mature mice, a null mutation of the NgR gene does not enhance regeneration of the CST but does increase sprouting of essential descending serotonergic projections after spinal cord injury (Kim et al., 2003a). On the basis of the present study, we would propose that the contrasting results seen in CST versus serotonergic axons after NgR deletion may reflect intrinsic differences in the growth state of cortical pyramidal cells versus raphe neurons, and that activation of the former with appropriate trophic factors could lead to better CST regeneration.
Alterations of NgR functioning (or levels) and activation of the axonal growth program appear to be mostly independent of one another. As shown in the explant studies, altering NgR functioning or levels did not affect the ability of neurons to extend axons on a permissive substrate, and activating the intrinsic growth state of RGCs still left axons partially responsive to the effects of myelin proteins. Activation of the growth program of RGCs by macrophage-derived factors greatly increases the expression of GAP-43 (Yin et al., 2003) and other regeneration-associated genes, but does not appreciably alter mRNA levels for NgR or p75, a NgR co-receptor (D. Fischer and L. Benowitz, unpublished gene profiling results). Inhibition of RhoA, an essential downstream mediator of NgR functioning, allows for limited axon regeneration when an ADP ribosyl transferase is delivered at the site of optic nerve injury in the absence of intravitreal macrophage activation (Lehmann et al., 1999). The presence of intense GAP-43 immunoreactivity in axons extending beyond the injury site provides strong evidence that these are regenerating rather than spared axons, because the latter do not show detectable levels of GAP-43 (Schaden et al., 1994; Berry et al., 1996; Leon et al., 2000). The fact that the GAP-43-positive axons do not extend the length of the optic nerve further suggests that they are regenerating, because spared axons would extend the full length of the nerve.
AAV-mediated transfection of growth-sensitized RGCs represents a general approach for investigating the role of various gene products in axon regeneration. By this method, one can readily obtain precise temporal and spatial control of gene expression without the expense, time delays, and possible developmental problems inherent in transgenic technology. The specificity and efficiency of RGC transfection by AAV found here has also been demonstrated in other studies (Cheng et al., 2002; Martin et al., 2002).
The clinical implications of this work are clear: extensive axon regeneration may not be attainable in the mature CNS by overcoming inhibitory signals alone but may require that the intrinsic growth state of neurons be activated at the same time (Schnell et al., 1994; Cheng et al., 1996; Guest et al., 1997). Even when these two approaches are combined, additional barriers to growth remain to be breached, e.g., chondroitin sulfate proteoglycans and other proteins associated with the glial scar (McKeon et al., 1995; Moon et al., 2001), amino-NogoA (Spillmann et al., 1998; Fournier et al., 2001; Niederost et al., 2002; Oertle et al., 2003), diffusible axon-repellant signals (Oster et al., 2003), and a possible developmental loss of appropriate guidance cues. Nonetheless, the strong regeneration that results from simultaneously overcoming inhibitory signaling and activating the growth program of neurons lends encouragement to the possibility that clinically significant axon regeneration can be achieved in the injured human CNS.
Footnotes
This work was supported by National Eye Institute (National Institutes of Health Grant R01 EY05690) (L.B.), Boston Life Sciences, Inc., the German Research Foundation (D.F.), and the Paralyzed Veterans of America. We thank Fan Jiang for assistance with histology, David Goldberg for help with figures, Jeng-shin Lee of the Harvard Gene Therapy Initiative for assistance with virus constructions, Joshua Gooley (Harvard Medical School) for help in preliminary AAV studies, and Clifford Woolf and Gabriel Corfas for helpful comments on this manuscript.
Correspondence should be addressed to Dr. Larry Benowitz, Laboratories for Neuroscience Research in Neurosurgery, Children's Hospital, 300 Longwood Avenue, Boston, MA 02115. E-mail: larry.benowitz@tch.harvard.edu.
Copyright © 2004 Society for Neuroscience 0270-6474/04/241646-06$15.00/0
References
- Aguayo AJ, Rasminsky M, Bray GM, Carbonetto S, McKerracher L, Villegas-Perez MP, Vidal-Sanz M, Carter DA (1991) Degenerative and regenerative responses of injured neurons in the central nervous system of adult mammals. Philos Trans R Soc Lond B Biol Sci 331: 337-343. [DOI] [PubMed] [Google Scholar]
- Bahr M, Vanselow J, Thanos S (1988) In vitro regeneration of adult rat ganglion cell axons from retinal explants. Exp Brain Res 73: 393-401. [DOI] [PubMed] [Google Scholar]
- Bartlett JS, Samulski RJ, McCown TJ (1998) Selective and rapid uptake of adeno-associated virus type 2 in brain. Hum Gene Ther 9: 1181-1186. [DOI] [PubMed] [Google Scholar]
- Benowitz LI, Apostolides PJ, Perrone-Bizzozero N, Finklestein SP, Zwiers H (1988) Anatomical distribution of the growth-associated protein GAP-43/B-50 in the adult rat brain. J Neurosci 8: 339-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkelaar M, Clarke DB, Wang YC, Bray GM, Aguayo AJ (1994) Axotomy results in delayed death and apoptosis of retinal ganglion cells in adult rats. J Neurosci 14: 4368-4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry M, Carlile J, Hunter A (1996) Peripheral nerve explants grafted into the vitreous body of the eye promote the regeneration of retinal ganglion cell axons severed in the optic nerve. J Neurocytol 25: 147-170. [DOI] [PubMed] [Google Scholar]
- Bregman BS, Kunkel-Bagden E, Schnell L, Dai HN, Gao D, Schwab ME (1995) Recovery from spinal cord injury mediated by antibodies to neurite growth inhibitors. Nature 378: 498-501. [DOI] [PubMed] [Google Scholar]
- Chen MS, Huber AB, van der Haar ME, Frank M, Schnell L, Spillmann AA, Christ F, Schwab ME (2000) Nogo-A is a myelin-associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1. Nature 403: 434-439. [DOI] [PubMed] [Google Scholar]
- Cheng H, Cao Y, Olson L (1996) Spinal cord repair in adult paraplegic rats: partial restoration of hind limb function. Science 273: 510-513. [DOI] [PubMed] [Google Scholar]
- Cheng L, Sapieha P, Kittlerova P, Hauswirth WW, Di Polo A (2002) TrkB gene transfer protects retinal ganglion cells from axotomy-induced death in vivo J Neurosci 22: 3977-3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Q, Yip HK, Zhao RC, So KF, Harvey AR (2003) Intraocular elevation of cyclic AMP potentiates ciliary neurotrophic factor-induced regeneration of adult rat retinal ganglion cell axons. Mol Cell Neurosci 22: 49-61. [DOI] [PubMed] [Google Scholar]
- Domeniconi M, Cao Z, Spencer T, Sivasankaran R, Wang K, Nikulina E, Kimura N, Cai H, Deng K, Gao Y, He Z, Filbin M (2002) Myelin-associated glycoprotein interacts with the nogo66 receptor to inhibit neurite outgrowth. Neuron 35: 283-290. [DOI] [PubMed] [Google Scholar]
- Fischer D, Pavlidis M, Thanos S (2000) Cataractogenic lens injury prevents traumatic ganglion cell death and promotes axonal regeneration both in vivo and in culture. Invest Ophthalmol Vis Sci 41: 3943-3954. [PubMed] [Google Scholar]
- Fischer D, Heiduschka P, Thanos S (2001) Lens-injury-stimulated axonal regeneration throughout the optic pathway of adult rats. Exp Neurol 172: 257-272. [DOI] [PubMed] [Google Scholar]
- Fournier AE, GrandPre T, Strittmatter SM (2001) Identification of a receptor mediating Nogo-66 inhibition of axonal regeneration. Nature 409: 341-346. [DOI] [PubMed] [Google Scholar]
- GrandPre T, Nakamura F, Vartanian T, Strittmatter SM (2000) Identification of the Nogo inhibitor of axon regeneration as a Reticulon protein. Nature 403: 439-444. [DOI] [PubMed] [Google Scholar]
- GrandPre T, Li S, Strittmatter SM (2002) Nogo-66 receptor antagonist peptide promotes axonal regeneration. Nature 417: 547-551. [DOI] [PubMed] [Google Scholar]
- Guest JD, Hesse D, Schnell L, Schwab ME, Bunge MB, Bunge RP (1997) Influence of IN-1 antibody and acidic FGF-fibrin glue on the response of injured corticospinal tract axons to human Schwann cell grafts. J Neurosci Res 50: 888-905. [DOI] [PubMed] [Google Scholar]
- Kim JE, Liu BP, Yang X, Strittmatter SM (2003a) Recovery from spinal cord injury in mice lacking the Nogo-66 receptor. Soc Neurosci Abstr 29: 415.11. [Google Scholar]
- Kim JE, Li S, GrandPre T, Qiu D, Strittmatter SM (2003b) Axon regeneration in young adult mice lacking nogo-a/b. Neuron 38: 187-199. [DOI] [PubMed] [Google Scholar]
- Lehmann M, Fournier A, Selles-Navarro I, Dergham P, Sebok A, Leclerc N, Tigyi G, McKerracher L (1999) Inactivation of Rho signaling pathway promotes CNS axon regeneration. J Neurosci 19: 7537-7547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon S, Yin Y, Nguyen J, Irwin N, Benowitz LI (2000) Lens injury stimulates axon regeneration in the mature rat optic nerve. J Neurosci 20: 4615-4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Irwin N, Yin Y, Lanser M, Benowitz LI (2003) Axon regeneration in goldfish and rat retinal ganglion cells: differential responsiveness to carbohydrates and cAMP. J Neurosci 23: 7830-7838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu BP, Fournier A, GrandPre T, Strittmatter SM (2002) Myelin-associated glycoprotein as a functional ligand for the Nogo-66 receptor. Science 27: 1190-1193. [DOI] [PubMed] [Google Scholar]
- Martin KR, Klein RL, Quigley HA (2002) Gene delivery to the eye using adeno-associated viral vectors. Methods 28: 267-275. [DOI] [PubMed] [Google Scholar]
- McKeon RJ, Hoke A, Silver J (1995) Injury-induced proteoglycans inhibit the potential for laminin-mediated axon growth on astrocytic scars. Exp Neurol 136: 32-43. [DOI] [PubMed] [Google Scholar]
- McKerracher L, David S, Jackson DL, Kottis V, Dunn RJ, Braun PE (1994) Identification of myelin-associated glycoprotein as a major myelin-derived inhibitor of neurite growth. Neuron 13: 805-811. [DOI] [PubMed] [Google Scholar]
- Moon LD, Asher RA, Rhodes KE, Fawcett JW (2001) Regeneration of CNS axons back to their target following treatment of adult rat brain with chondroitinase ABC. Nat Neurosci 4: 465-466. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay G, Doherty P, Walsh FS, Crocker PR, Filbin MT (1994) A novel role for myelin-associated glycoprotein as an inhibitor of axonal regeneration. Neuron 13: 757-767. [DOI] [PubMed] [Google Scholar]
- Niederost B, Oertle T, Fritsche J, McKinney RA, Bandtlow CE (2002) Nogo-A and myelin-associated glycoprotein mediate neurite growth inhibition by antagonistic regulation of RhoA and Rac1. J Neurosci 22: 10368-10376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oertle T, van der Haar ME, Bandtlow CE, Robeva A, Burfeind P, Buss A, Huber AB, Simonen M, Schnell L, Brosamle C, Kaupmann K, Vallon R, Schwab ME (2003) Nogo-A inhibits neurite outgrowth and cell spreading with three discrete regions. J Neurosci 23: 5393-5406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oster SF, Bodeker MO, He F, Sretavan DW (2003) Invariant Sema5A inhibition serves an ensheathing function during optic nerve development. Development 130: 775-784. [DOI] [PubMed] [Google Scholar]
- Ramon y Cajal S (1991) Degeneration and regeneration of the nervous system. New York: Oxford UP.
- Schaden H, Stuermer CA, Bahr M (1994) GAP-43 immunoreactivity and axon regeneration in retinal ganglion cells of the rat. J Neurobiol 25: 1570-1578. [DOI] [PubMed] [Google Scholar]
- Schnell L, Schneider R, Kolbeck R, Barde YA, Schwab ME (1994) Neurotrophin-3 enhances sprouting of corticospinal tract during development and after adult spinal cord lesion. Nature 367: 170-173. [DOI] [PubMed] [Google Scholar]
- Sicotte M, Tsatas O, Jeong SY, Cai CQ, He Z, David S (2003) Immunization with myelin or recombinant Nogo-66/MAG in alum promotes axon regeneration and sprouting after corticospinal tract lesions in the spinal cord. Mol Cell Neurosci 23: 251-263. [DOI] [PubMed] [Google Scholar]
- Simonen M, Pedersen V, Weinmann O, Schnell L, Buss A, Ledermann B, Christ F, Sansig G, van der Putten H, Schwab ME (2003) Systemic deletion of the myelin-associated outgrowth inhibitor nogo-a improves regenerative and plastic responses after spinal cord injury. Neuron 38: 201-211. [DOI] [PubMed] [Google Scholar]
- Spillmann AA, Bandtlow CE, Lottspeich F, Keller F, Schwab ME (1998) Identification and characterization of a bovine neurite growth inhibitor (bNI-220). J Biol Chem 273: 19283-19293. [DOI] [PubMed] [Google Scholar]
- Steward O, Zheng B, Tessier-Lavigne M (2003) False resurrections: distinguishing regenerated from spared axons in the injured central nervous system. J Comp Neurol 459: 1-8. [DOI] [PubMed] [Google Scholar]
- Wang KC, Koprivica V, Kim JA, Sivasankaran R, Guo Y, Neve RL, He Z (2002a) Oligodendrocyte-myelin glycoprotein is a Nogo receptor ligand that inhibits neurite outgrowth. Nature 417: 941-944. [DOI] [PubMed] [Google Scholar]
- Wang KC, Kim JA, Sivasankaran R, Segal R, He Z (2002b) p75 interacts with the Nogo receptor as a co-receptor for Nogo, MAG and OMgp. Nature 420: 74-78. [DOI] [PubMed] [Google Scholar]
- Woolf CJ (2003) No Nogo: now where to go? Neuron 38: 153-156. [DOI] [PubMed] [Google Scholar]
- Yin Y, Cui Q, Li Y, Irwin N, Fischer D, Harvey AR, Benowitz LI (2003) Macrophage-derived factors stimulate optic nerve regeneration. J Neurosci 23: 2284-2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng B, Ho C, Li S, Keirstead H, Steward O, Tessier-Lavigne M (2003) Lack of enhanced spinal regeneration in nogo-deficient mice. Neuron 38: 213-224. [DOI] [PubMed] [Google Scholar]