Health care-associated infections (HAIs) are a global problem associated with significant morbidity and mortality. Controlling the spread of antimicrobial-resistant bacteria is a major public health challenge, and antimicrobial resistance has become one of the most important global problems in current times.
KEYWORDS: health care-associated infections, antibiotic resistance, antimicrobial activity, antimicrobial agents, copper, gynecology, microorganisms, obstetrics, wound healing
SUMMARY
Health care-associated infections (HAIs) are a global problem associated with significant morbidity and mortality. Controlling the spread of antimicrobial-resistant bacteria is a major public health challenge, and antimicrobial resistance has become one of the most important global problems in current times. The antimicrobial effect of copper has been known for centuries, and ongoing research is being conducted on the use of copper-coated hard and soft surfaces for reduction of microbial contamination and, subsequently, reduction of HAIs. This review provides an overview of the historical and current evidence of the antimicrobial and wound-healing properties of copper and explores its possible utility in obstetrics and gynecology.
INTRODUCTION
Health care-associated infections (HAIs) are a global problem associated with significant morbidity and mortality. With an overall prevalence rate of 7.1%, over 4.1 million patients are affected in Europe (1). In the emergency department, more than half of the patients suffer from an HAI, leading to 16 million extra days of hospitalization, with a cost estimate of approximately 7 billion euros (1). In Europe, 37,000 deaths can be attributed directly to HAIs, and in an additional 110,000 deaths, HAI is a contributory factor (1). The management of HAIs with antibiotic-resistant bacteria such as methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus (VRE) is becoming increasingly problematic (2). Although the prevalence of MRSA in Europe is decreasing, an incidence rate of up to 65% of all isolates in 2013 has been reported (3). The most concerning trends in Europe over recent years were related to the occurrence of antibiotic-resistant Gram-negative bacteria (Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Acinetobacter species). The incidence of antimicrobial-resistant isolates is currently one of the most important benchmarks of global health care. Attempts to reduce the distribution of antimicrobial-resistant pathogens have become a major priority of health services. In the United Kingdom, antibiotic consumption during 2011 to 2014 (4) increased by 6.5%, and in the United States, 68% of the courses of antibiotics were inappropriately prescribed (5). Even more concerning is that it has been 30 years since the last antibiotic agent has been developed, and only 3 out of the 41 newly developing antibiotics are sensitive against the majority of resistant bacteria (6). Developing a new generation of antibiotics is going to be a challenge and would provide only a temporary option before resistance develops. Furthermore, these antibiotics may not be easily available in low-resource countries. It is therefore crucial that investment is made into research to identify other antimicrobial agents that can prevent and treat hospital-acquired infections.
Recent research in microbiology has begun to focus on copper. The biocidal effect of copper on a wide range of pathogens, such as bacteria, fungi, and viruses, has been demonstrated by many laboratory studies (7, 8). The effectiveness of copper extends to MRSA (9) and VRE (1). Apart from being an effective antimicrobial agent, exposure to copper has been correlated with enhanced wound healing (10). The substitution of copper alloys on surfaces within a clinical setting has been demonstrated to significantly reduce bacterial colonization (11, 12), and more recently, copper-infused fabrics have also been studied (13).
In the field of obstetrics and gynecology, infection prevention practices are challenging. The “normal” microflora of the vagina and cervix can, under certain circumstances, lead to infection of the female genital tract, and these infections are usually caused by more than one microbe, both aerobic and anaerobic (14, 15). From a global perspective, maternal sepsis is the third major cause of death, representing about 11% of all maternal deaths (16). Sepsis is the leading direct cause of maternal mortality in the United Kingdom (17).
In this review, our aim is primarily to explore the current available evidence with regard to the antimicrobial effects of copper-based materials. Given the high morbidity and mortality associated with maternal sepsis (16), we also evaluate the potential benefits and clinical applications of copper within obstetrics and gynecology.
HISTORICAL PERSPECTIVE
The Egyptians were the first to mention the antimicrobial effects of copper in 2600 BC. They used copper vessels to sanitize drinking water and also to treat chest wounds. In papyrus circa 1500 BC, it was stated that various adaptations of copper were used to treat infections, scalds, and itching (18). In Cyprus, a Greek island, copper was found in large quantities, and therefore, it was used frequently by the ancient Greeks. This is also the basis from which the Latin name for copper, cuprus, was derived. In the Hippocratic Collection, which was partially written by the great Greek physician Hippocrates (460 to 377 BC), it was stated that copper-containing wound dressings were recommended to treat leg ulcers, and infection of fresh wounds was prevented by sprinkling dry copper powder on the wound. In another important first-century medical writing, De Medicina, written by the Roman Aulus Cornelius Celsus, copper is mentioned as an important drug for practicing physicians, namely, to treat venereal diseases and nonhealing chronic ulcers. During the two great European cholera epidemics in the 19th century, copper suppressed a cholera outbreak among workers in the copper industry (19).
Despite the demonstrated benefits, interest in copper diminished due to the limited understanding of the mechanisms of antimicrobial action and basis of promotion of wound healing, but more importantly, it was superseded by the discovery of antibiotics.
IN VITRO ANTIMICROBIAL ACTIVITY OF COPPER
Considerable research has been conducted globally to establish the antimicrobial spectrum of copper. Copper been demonstrated not only to inhibit growth but also to effectively eradicate microorganisms, including those causing HAIs.
Bacteria
In 1975, Majno was the first to reproduce and experimentally demonstrate the antibacterial abilities of ancient copper compounds (20). Recreating the copper compounds mentioned in the Smith Papyrus, he ground malachite and chrysocolla to a fine powder and made verdigris, using the oldest method known, by converting copper to vapor from vinegar. He then inoculated the three different compounds with three bacteria isolated from a wound and discovered that no bacteria grew in the proximity of the three compounds, thereby providing evidence of their antiseptic abilities. In the 1990s, Bill Keevil and his team conducted considerable research at the University of Southampton, United Kingdom. His interest was piqued when he observed lower levels of contamination with Legionella on plumbing systems made with copper than on those made with stainless steel or plastic (21). Through a series of laboratory tests, including a broad spectrum of microbes and testing under different conditions (temperature and humidity), he confirmed the antimicrobial ability of copper and copper alloys (22–25). His team tested the survival of high concentrations of Escherichia coli O157 bacteria on different metal surfaces such as stainless steel, copper, and copper alloys and showed survival for more than 28 days on stainless steel. In contrast, no bacteria were found after 90 to 270 min at 20°C and 4°C, respectively, on a copper surface. Copper alloys also demonstrated the antibacterial ability, whereby the effect increased with increasing copper concentrations (22). They continued their research by looking at the survival time of Listeria monocytogenes on 25 different metal alloys and found a great reduction of survival times on copper-based alloys compared to stainless steel. On stainless steel, viable bacteria could still be retrieved after 24 h, whereas on copper alloys, no viable bacteria could be retrieved after 60 min (23).
Copper has also been shown to be effective against antibiotic-resistant bacteria such as MRSA (9, 26) or VRE (27). The first study was published in 2006 whereby copper and stainless steel coupons were inoculated with three MRSA strains (MRSA, EMRSA-1, and EMRSA-16) and incubated at 22°C and 4°C. The results demonstrated a complete kill of MRSA in 45 to 90 min on pure copper at room temperature. A complete kill was achieved within 6 h for all three strains at 4°C. In contrast, MRSA was still viable after 72 h on stainless steel (9).
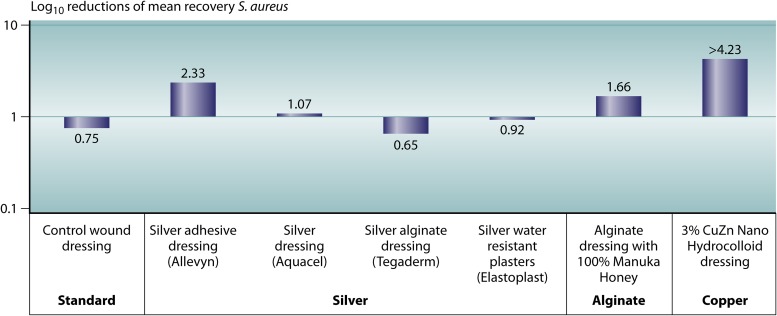
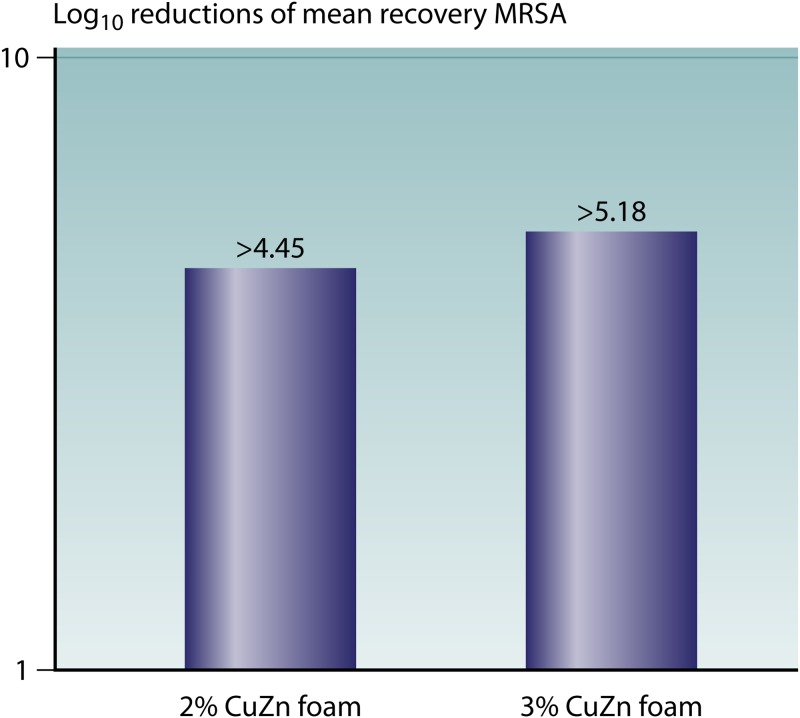
An independent laboratory in the United Kingdom has done several in vitro tests comparing the antibacterial abilities of several bioactive wound dressings (Fig. 1 to 3). The different wound dressing coupons were inoculated with approximately 105 to 106 CFU of S. aureus, K. pneumoniae, and MRSA and incubated for 24 h at 24°C at >95% humidity. Their results showed a significant >1-log reduction of S. aureus on two of the four silver dressings, and there was no significant reduction with the other two silver dressings. A significant reduction of K. pneumoniae was observed on all four silver dressings, ranging between a 2-log and a >4-log reduction. On the alginate-honey dressing, a significant >1-log reduction was observed for both bacteria. On the 3% copper–zinc dressing, a >4-log reduction of both S. aureus and K. pneumoniae was observed. Dressings with two different copper concentrations (2% and 3%) were also tested against MRSA, which showed a greater reduction of MRSA with increased copper concentrations (Fig. 3). In summary, copper-impregnated dressings showed a greater reduction of S. aureus and a similar reduction of K. pneumoniae. The alginate-honey dressing showed a similar reduction of S. aureus compared to the silver dressings and a lower reduction of K. pneumoniae than the silver and copper dressings. A higher copper concentration resulted in a greater log reduction of MRSA. Borkow et al. conducted in vitro and animal studies on a copper oxide-impregnated wound dressing (28). The in vitro studies demonstrated potent broad-spectrum antibacterial and antifungal activities of the wound dressings, with a reduction of the bacterial burden associated with the tissues of >99.9%. Tests comparing copper oxide-impregnated dressings to those impregnated with silver leachates found that the copper dressings were superior in providing a significantly greater reduction of the bacterial load.
FIG 1.
Antibacterial activities of different wound dressings against S. aureus. Staphylococcus aureus was prepared to approximately 106 CFU/ml in 0.85% NaCl. For each sample of wound dressing, five replicate test pieces were inoculated on the wound-facing surface with an appropriate volume of S. aureus. The inoculates were held for 24 h at 24°C ± 1°C at >95% humidity. A significant log reduction in the numbers of S. aureus bacteria was observed for two of the four silver dressings, the alginate-honey dressing, and the 3% CuZn dressing, with the highest log reduction observed for the copper dressing.
FIG 2.
Antibacterial activities of different wound dressings against K. pneumoniae. Klebsiella pneumoniae was prepared to approximately 106 CFU/ml in 0.85% NaCl. For each sample of wound dressing, five replicate test pieces were inoculated on the wound-facing surface with an appropriate volume of K. pneumoniae. The inoculates were held for 24 h at 24°C ± 1°C at >95% humidity. A significant log reduction in the numbers of K. pneumoniae bacteria was observed for all the silver dressings, the alginate-honey dressing, and the 3% CuZn dressing.
FIG 3.
Antibacterial activities of two wound dressings with different copper concentrations against MRSA. Methicillin-resistant Staphylococcus aureus (MRSA) (NC 11939-09) was prepared to approximately 106 CFU/ml in 0.85% NaCl. For each sample of wound dressing, five replicate test pieces were inoculated on the wound-facing surface with an appropriate volume of MRSA. The inoculates were held for 24 h at 24°C ± 1°C at >95% humidity. A significant log reduction was observed for both copper dressings, and an increased log reduction was observed with an increased copper concentration.
See Table 1 for a complete overview of the antimicrobial spectrum of copper.
TABLE 1.
Antimicrobial efficacy of copper
| Organism | Reference(s) |
|---|---|
| Bacteria | |
| Gram positive | |
| Bacillus spp. | 38, 112, 256–269 |
| Brachybacterium conglomeratum | 270 |
| Clostridium difficile | 25, 101, 271, 272 |
| Clostridium tyrobutyricum | 273 |
| Deinococcus radiodurans | 38 |
| Enterococcus spp.a | 12, 13, 28, 36, 45, 46, 55, 83, 101, 103, 104, 107, 258, 271, 274–280 |
| Kocuria spp. | 270 |
| Listeria monocytogenes | 23, 55, 274, 281, 282 |
| Micrococcus luteus | 45, 46, 260, 270 |
| Mycobacterium tuberculosisa | 35 |
| Sarcina lutea | 256 |
| Staphylococcus spp.a | 9, 12, 13, 26, 28, 35, 44–46, 55–57, 68, 101, 103, 104, 107, 112, 250, 251, 256–259, 262, 263, 266–268, 270, 271, 275, 276, 281–298 |
| Streptococcus spp. | 107, 257–260, 297, 299 |
| Gram negative | |
| Acinetobacter spp.a | 35, 112, 171, 257, 270, 271, 275, 276, 285, 300, 301 |
| Campylobacter jejuni | 173 |
| Citrobacter freundii | 257, 302 |
| Cronobacter sakazakii | 42, 344 |
| Desulfovibrio desulfuricans | 303 |
| Edwardsiella tarda | 304 |
| Enterobacter spp.a | 45, 46, 112, 258, 259, 274, 276, 286, 301 |
| Escherichia colia | 13, 22, 28, 34, 38, 40, 41, 45, 46, 55–57, 64, 65, 83, 112, 257, 258, 260–263, 266–268, 270, 276, 278, 286–290, 297, 301, 305–313 |
| Klebsiella pneumoniaea | 28, 35, 112, 257, 260, 275, 276, 284, 287, 301, 305, 306, 314 |
| Legionella pneumophila | 23, 271, 315–318 |
| Methylobacterium spp. | 261 |
| Morganella morganii | 302 |
| Pantoea stewartii | 270 |
| Photobacterium leiognathi | 28 |
| Proteus spp. | 258, 314 |
| Pseudomonas spp.a | 28, 35, 45, 46, 112, 171, 256, 258–260, 262, 263, 267, 269, 270, 275, 276, 281, 286, 301, 314, 319–321 |
| Salmonella spp.a | 43, 55, 64, 173, 257, 268, 281, 302, 307, 309, 314, 319, 321–324 |
| Serratia marcescens | 267 |
| Shewanella putrefaciens | 281 |
| Shigella spp. | 257, 268, 302, 314, 322 |
| Sphingomonas panni | 270 |
| Sphingomonas sp. | 261 |
| Stenotrophomonas maltophilia | 171 |
| Vibrio choleraea | 307, 323, 325 |
| Xanthomonas campestris | 326 |
| Yersinia pseudotuberculosis | 274 |
| Viruses | |
| Enveloped | |
| Avian influenza virus | 61, 283 |
| Bacteriophages | 327–331 |
| Cytomegalovirus | 332 |
| Herpes simplex virus | 327, 328 |
| Human immunodeficiency virus | 13, 332–334 |
| Infectious bronchitis virus | 335 |
| Influenza A virus | 24, 61, 332, 336 |
| Junin virus | 328 |
| Measles virus | 332 |
| Parainfluenza virus 3 | 332 |
| Pichinde virus | 332 |
| Punta Toro virus | 332 |
| Respiratory syncytial virus | 332 |
| Vaccinia virus | 332 |
| West Nile virus | 13 |
| Yellow fever virus | 332 |
| Nonenveloped | |
| Adenovirus type 1 | 332 |
| Bacteriophages | 327–331 |
| Murine norovirus | 30 |
| Poliovirus | 331, 337 |
| Rhinovirus 2 | 332 |
| Fungi/yeast | |
| Alternaria brassicae | 326 |
| Aspergillus spp. | 31, 260, 262, 319, 322, 326, 338–342 |
| Candida spp. | 13, 28, 31, 32, 35, 55, 56, 108, 112, 258, 260, 263, 264, 274–276, 305, 314, 322, 338, 340, 342, 343 |
| Cryptococcus neoformans | 340 |
| Curvularia lunata | 284 |
| Epidermophyton floccosum | 340 |
| Fusarium spp. | 31, 284, 322, 326 |
| Microsporum canis | 322, 340 |
| Myrothecium verrucaria | 341 |
| Penicillium chrysogenum | 31 |
| Pleurotus ostreatus | 308 |
| Pycnoporus cinnabarinus | 308 |
| Rhizoctonia spp. | 284, 319 |
| Saccharomyces cerevisiae | 32, 62, 263, 345 |
| Torulopsis pintolopesii | 343 |
| Trichoderma viride | 341 |
| Trichophyton spp. | 314, 322, 338, 340, 341 |
Including antibiotic-resistant strains.
Viruses
As early as 1958, experiments demonstrated the possible antiviral abilities of copper and copper compounds (29). In 2007, Keevil’s team demonstrated the effectiveness of copper against influenza A virus (24). They inoculated coupons of either copper or stainless steel with 2 × 106 virus particles and incubated the coupons at room temperature for 1 to 24 h. After 24 h, 5 × 105 infectious virus particles were observed, in contrast to 5 × 102 infectious virus particles on copper (24). In a similar study, they looked at the survival of norovirus on stainless steel and compared it to that on copper alloys; they demonstrated a significant reduction of active norovirus after 2 h on copper alloys. Furthermore, they found that inactivation of the virus was dependent on humidity and temperature; there was a lower rate of inactivation when the inoculum was suspended in a larger volume, such that evaporation was slower at the lower temperature (30).
See Table 1 for a complete overview of the antiviral spectrum of copper.
Fungi
In contrast to the antibacterial effect, the antifungal ability of copper has not been studied extensively. Weaver et al. studied the effectiveness of copper against various fungi (Aspergillus spp., Fusarium spp., Penicillium chrysogenum, and Candida albicans) (31). Copper coupons were inoculated with 20 μl of a suspension of fungal spores for exposure times varying from 0 to 576 h at room temperature and humidity. Surviving spores were visualized using the fluorochrome FUN-1. They found that copper was able to kill all the fungi except Aspergillus niger. Moreover, copper was able to prevent the germination of fungal spores. The mechanism by which fungi are killed on copper was found to be similar to the mechanism by which bacteria are killed. Quaranta et al. found that the antifungal effect starts with extensive fungal membrane damage, which was followed by enlargement and disappearance of vacuoles and oxidative stress (32).
See Table 1 for a complete overview of the antifungal spectrum of copper.
Effect of Copper Surfaces
Copper and copper alloys have been studied in experiments simulating a clinical setting by using moist and dry inoculation techniques (33). Many in vitro studies have demonstrated the antimicrobial effect of copper on a wide spectrum of Gram-positive bacteria (i.e., Enterococcus spp. and Staphylococcus spp.), Gram-negative bacteria (i.e., Acinetobacter spp., Campylobacter jejuni, E. coli, and Pseudomonas spp.), viruses (i.e., influenza A virus, herpes simplex virus, and human immunodeficiency virus [HIV]), and fungi and yeasts (i.e., Candida spp.) (Table 1). The biocidal efficacy is dependent on the copper concentration, length of exposure, humidity, and temperature. Various studies have experimented with the copper concentration (9, 22, 24, 25, 32, 34–38) and different lengths of exposure (9, 22, 23, 39) to achieve the most efficient biocidal effect, with the majority of the research having been conducted by the research team of Keevil in Southampton, United Kingdom (9, 22, 24–26, 30, 34, 36). They placed a higher concentration of bacteria (40) onto coupons with different concentrations of copper ranging between 0% (100% stainless steel or 100% metallic zinc) and 100% but varying the time of surface exposure of the microbes to the metal from several minutes to up to 24 h. Viable microbes were counted after staining for visualization by epifluorescence microscopy. They demonstrated that the biocidal efficacy increases with higher copper concentrations. A significant biocidal effect was reached using metals with a copper concentration of between 55% and 100%; there was a complete eradication of microbes within 90 min on pure copper and 120 min on copper nickel with a 75% copper concentration (22). Copper continuously reduced microbial contamination, with a 99.9% reduction within 2 h of exposure. Initial studies were done using a wet inoculum at high relative humidity (>90%) and incubated at high temperatures (35°C). However, as this would not reflect a clinical setting, different laboratory techniques have been used. One difference is the use of either a dry inoculum (reflecting touch contamination) or a wet inoculum (reflecting wet contamination such as a sneeze). It was found that bacterial killing occurred more rapidly under dry conditions than under moist ones (38, 41–43). Using a wet inoculum, bacteria such as Enterococcus faecalis or Enterococcus faecium were killed in 1 h, compared to 10 min when presenting the inocula to the metal coupons in lower volumes (20 μl) (27). Also, the effects of temperature (26, 34, 44) and relative humidity (26, 44–46) were explored.
Michels et al. conducted laboratory tests studying the effects of temperature and humidity on the biocidal efficacy of copper- and silver-containing materials on MRSA (26). They used the standard methodology of that time, JIS Z 2801, originally the Japanese Industrial Standard (JIS Z 2801), which utilizes temperatures approximating that of human skin (35°C) and high humidity (>90%) and compared it to situations with lower temperatures (20°C) and lower humidity (20%). Under all test conditions, a >5-log reduction of CFU after 24 h was achieved on copper, although slight differences were seen when changing the temperature and humidity. Their results were a >6.4-log reduction of CFU at 35°C and >90% humidity on copper, which dropped slightly to a >6.1-log reduction when lowering the temperature to 20°C. Testing under lower humidity showed a larger difference: at 20% humidity and 35°C, there was a slightly lower reduction than that at >90% humidity (>5.5-log versus >6.4-log reduction, respectively). Interestingly, silver also demonstrated >6.4- and 5.5-log reductions at high humidity at 35°C and 20°C, respectively; however, this was quickly reduced to a <0.3-log reduction at 24% humidity and 20°C but no log reduction at 20% humidity and 35°C.
As a result of the substantial amount of evidence, copper is the only hard surface material that has been approved by the U.S. Environmental Protection Agency (EPA) to make public health claims under the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA), a federal law of the United States (47) that is responsible for the governance of pesticides to protect applicators, consumers, and the environment (48). Registered public health claims are as follows: antimicrobial copper alloys continuously reduce bacterial contamination, antimicrobial copper is able to kill >99.9% of bacteria within 2 h of contact, antimicrobial copper remains effective in killing >99.9% of bacteria after repeated recolonization, and antimicrobial copper inhibits bacterial growth between routine cleaning activities.
Apart from the horizontal transmission of pathogens through hard surfaces, studies are suggesting the potential hazard of soft surfaces in health care environments (white coats, privacy curtains, and bed linen, etc.) for cross-contamination (49–52). To address this issue, the introduction of copper into textiles and liquids was explored. Due to recent developments in nanotechnology, it has been made possible to impregnate textiles, latex, and other polymer products with copper oxide (13). Metal nanoparticles are particularly interesting because of their enhanced antimicrobial ability compared to hard surfaces and the possibility of embedding them into polymer structures. Their enhanced antimicrobial ability is explained by their high surface area compared to their volume; therefore, cell membranes are more rapidly penetrated (53, 54). Laboratory studies have provided solid evidence of the broad-spectrum antimicrobial properties of copper-impregnated fibers of which socks, face masks, filters, bed linen, and scrubs, etc. can be made (8, 13, 28, 55–61) (Table 1). A research team in Israel was the first to report on copper-impregnated products and their antimicrobial properties (13). Cotton fibers and latex were impregnated with copper ions, and their effectiveness against bacteria (E. coli, S. aureus, MRSA, and VRE), fungi (Candida albicans), and viruses (HIV-1IIIB and West Nile virus) was compared to that of a noncopper equivalent. A >2-log reduction of all four bacteria was demonstrated within 2 h of exposure to copper fabrics. The copper fabric was also able to completely inactivate fungi in 60 min of exposure, and copper-impregnated latex gloves were able to reduce the infectivity of both viruses. The same team also conducted animal studies and demonstrated that copper-impregnated fabrics did not possess skin-sensitizing properties. Since then, more studies have been conducted, especially on copper impregnated fabrics. In 2006, the same research team reported results of biocidal studies of different copper oxide-impregnated fabrics (cotton, polyester, polypropylene, and nylon) against a wide variety of bacteria, fungi, and viruses (55), again showing that copper fabrics reduced the numbers of viable bacteria, fungi, and viruses by >98.7% within 20 min to 4 h of exposure.
Mechanism of Action
The mechanism by which copper kills microorganisms is multifaceted and not yet fully understood. Literature suggests that copper interacts with microorganisms on different cellular levels, all resulting in cell death, including cell membrane permeabilization, membrane lipid peroxidation, protein alteration, and denaturation of nucleic acids.
Copper ions released from the surface are transported through the cell membrane via an energy-dependent transport system. A decreased barrier function of the plasma membrane occurs upon reaching a crucial intracellular concentration of copper ions. The membrane becomes increasingly permeable, leading to leakage of vital cell content such as nucleotides, amino acids, and potassium (62, 63); Warnes et al. (64) demonstrated rapid membrane depolarization and loss of membrane integrity upon exposure to metallic copper surfaces, leading to cytoplasmic accumulation of free radicals.
Another mechanism by which copper damages the cell membrane is via the formation of reactive oxygen species (ROS). Copper ions are able to donate and accept electrons and thereby can change between Cu+ and Cu2+ via oxidation. This catalyzes the formation of ROS such as hydroxyl radicals (HO) and superoxide anions (O2−); causes oxidative stress to membrane lipids (lipid peroxidation), amino acids in proteins, and nucleic acids; and causes oxidative deactivation of enzymes (27, 63–69).
Essential metals are required for proper protein function through a process called coordination chemistry (70). They are needed as cofactors and to catalyze cytotoxic reactions. Proteins controlling the activity of intracellular metal ions include membrane transporters, metalloregulating proteins, and metallochaperoning proteins. Metal ions are able to bind to certain atoms of protein complexes, such as oxygen (O), nitrogen (N), and sulfur (S) (71). Proteins exert preferences for binding specific metal ions because of their atomic structure (72). However, when metal homeostasis is distorted, metal ions such as Cu2+ are able to compete with other essential metals, for instance, iron ions (Fe2+), and prevent proteins from binding with the correct cofactor and consequently inhibiting protein function (63). For instance, the protease of HIV-1 is inhibited by the binding of copper ions to the amino acid cysteine (73). Another example is the inactivation of protein tyrosine phosphatases through the oxidation of cysteine residues on the phosphatases by copper ions (74). Furthermore, the enzyme isopropylmalate dehydratase in E. coli is inactivated by displacing iron atoms by copper ions from the iron-sulfur clusters within the enzyme, a process called mismetallation (75).
Antimicrobial Resistance
Bacterial resistance to antibiotics, which has emerged in less than 50 years since the widespread use of antibiotics, is a major concern. However, only very recently has evidence of copper resistance been demonstrated despite its use over thousands of years (76). More frequently, a reduced sensitivity to copper has been observed but not at the exposure levels present in products used to control the concentration of microbes in the built environment. Bacteria which have been exposed to low concentrations of copper for a long period of time demonstrate increased tolerance to copper through the development of efflux pumps for active excretion of excess copper (77), impermeability to copper by membrane alterations (78), intra- and extracellular binding of copper (78–81), and detoxification of copper by enzymes (82). Despite these mechanisms, continuous exposure to high concentrations of copper is fatal for copper-tolerant microorganisms (83, 84). The rapid and multifactorial mechanism of action, which includes denaturation of DNA, makes it highly unlikely that microorganisms will develop resistance to copper. However, exposure to copper may upregulate nonspecific antibiotic resistance mechanisms, such as reduced membrane permeability, antibiotic alteration, efflux pumps for antibiotics, alteration of cellular targets, and antibiotic sequestration, which eventually could lead to increased antibiotic resistance (85).
ANTIMICROBIAL ACTIVITY OF COPPER IN A CLINICAL SETTING
There is ample evidence to support an important role of the surface contamination of the environment in the spread of HAIs (86). Research into the survival of nosocomial pathogens on surfaces has demonstrated that many pathogens, such as MRSA, VRE, Acinetobacter spp., Pseudomonas spp., and norovirus, are able to survive days to weeks on environmental surfaces, with a survival of months for Clostridium difficile (87, 88). Surface contamination with MRSA or VRE has been documented for up to 60 to 70% of hospital surfaces (89, 90). Hand contamination is a well-known vector of spreading pathogens, particularly through health workers (91). The bacterial burden, which can be expressed by positive environmental surface cultures, is the most important risk factor for hand contamination (92). Clinical research has shown that fewer than half of the hospital touch surfaces are clean due to inadequate disinfection (93, 94). Apart from techniques such as cleaning and disinfection to decrease the transmission of pathogens (95, 96), self-disinfecting touch surfaces have been developed (97).
Copper as an Antimicrobial Hard Surface
Copper was first clinically investigated as a biocidal surface material. The first clinical article was written in 1983 by an American physician from Philadelphia, PA, Phyllis J. Kuhn (98). She involved domestic staff from the local hospital in a small study to increase their awareness of modes of infection. They were given culture plates with instructions to return them with cultures from different sites in the hospital. The test showed that a brass doorknob (67% copper and 33% zinc) had very few bacterial colonies, in contrast to stainless steel doorknobs, with heavy growths of both Gram-positive and Gram-negative bacteria. Repeated experiments using a high inoculum of E. coli, S. aureus, group D Streptococcus, and Pseudomonas sp. showed rapid disinfection on brass and copper, compared to heavy growths on stainless steel and aluminum.
Since then, a number of clinical studies have been carried out in different parts of the world, serving to lend clinical relevance to incorporating copper surfaces into the built environment in order to reduce the resident microbial burden and thereby reduce the risk of infection (the United Kingdom [99–101], Germany [102], the United States [12, 103–105], Chile [106], and South Africa [40]). Work done in a university hospital in Birmingham, United Kingdom, replaced three items in an acute medical ward using copper facsimiles for the toilet seat, tap handles, and door push plates for a period of 10 weeks. Here it was observed that when the ward was sampled for the presence of microorganisms, the copper facsimiles harbored 90% fewer microbes (median values) than the original objects to no microbes at all that could be retrieved (99). This study was further extended by replacing 14 frequently touched items (trolleys, light pulls, flush handles, overbed tables, dressing trolleys, and commode chairs) for copper-containing items on the same medical ward. Again, these items were compared to noncopper equivalents over a period of 24 weeks. This study showed that all 14 items had a reduced bacterial load and that 8 of the 14 items demonstrated a significant reduction compared to noncopper items. Furthermore, antibiotic-resistant bacteria were significantly less frequently cultured on copper items (101).
Research conducted in other parts of the world showed similar results. For example, a study in a South African primary health care clinic showed a 71% reduction of CFU on copper surfaces (40). A study on an oncology/pneumatology and geriatric ward in Germany showed a CFU reduction of 30% on copper and a significantly delayed repopulation compared to standard items (102). In Chile, copper surfaces were installed in 3 rooms on an intensive care unit (ICU) and compared with 3 control rooms. The mean microbial burden was significantly reduced by 49 to 93% in the copper-containing rooms (106).
The U.S. army funded a large-scale, multicenter, randomized, controlled trial with the aim to assess the antimicrobial properties of copper surfaces in ICUs. Eight ICU rooms in three different hospitals had the most contaminated surfaces replaced with copper and were matched with control rooms. The average microbial burden was 83% lower on copper surfaces than on noncopper surfaces (103). In 2011, Casey et al. studied the contamination of copper and stainless steel pens used in the hospital (100). Fifty copper pens (85% copper and 15% zinc) and 50 stainless steel pens (100% stainless steel) were disinfected and distributed to nurses on two critical care units. After being used for one 12.5-h shift, half of the pens (25 copper and 25 stainless steel pens) were immediately sent to the laboratory for microbial sampling, and the remaining 50 pens were stored for 11 h at room temperature before being sent to the laboratory. They found that upon immediate sampling, there was a nonsignificantly lower number of contaminated copper pens than stainless steel pens (48% and 68%, respectively). After storing the pens for 11 h, a significantly lower number of contaminated copper pens was found (20% versus 72%; P = 0.0005). Likewise, a significantly lower number of CFU was found on copper pens than on stainless steel pens upon immediate testing (median [lower to upper quartiles] of 0 [0 to 4] versus 12 [0 to 20]; P = 0.04) and after 11 h of storage (median [lower to upper quartiles] of 0 [0 to 0] versus 8 [0 to 80]; P = 0.0002). More recently, the microbial burden of a standard stethoscope was compared to that of a similar one with 4 parts replaced with copper-containing parts (diaphragm, binaural tube, and the two ear tubes) (107). Different health care professionals (fellow and resident physicians and pediatric nurse practitioners) alternately used the control and copper stethoscopes for 1 week on four separate occasions. They found significantly lower concentrations of bacteria on copper stethoscopes than on the control ones (11.7 versus 127.1 CFU; P < 0.00001). Salgado et al. (12) were the first to report a study on the effect of copper surfaces on the incidence of HAIs. During the study period of almost 1 year, patients were randomly placed into rooms with or without copper touch surfaces. The incidence of HAI and/or MRSA or VRE colonization in ICU rooms with copper showed a significant reduction of 42%, and the incidence of HAI alone was 58% lower in copper rooms than in the control rooms (12).
All the studies mentioned above provide substantial evidence of the antimicrobial and infection prevention abilities of copper surfaces in clinical settings.
Antimicrobial Copper-Impregnated Soft Surfaces
Studies in a clinical setting have been performed only more recently. Gabbay et al. reported the first study using copper oxide-impregnated socks and sheets (55). The copper-impregnated sheets were tested in a clinical setting on a general internal medicine hospital ward, where 30 patients slept overnight on regular sheets and copper-impregnated sheets were used the following night. They reported that bacterial colonization was significantly lower on copper sheets than on regular sheets. A study population of 100 individuals with tinea pedis were asked to wear copper oxide-impregnated socks on a daily basis and to stop antifungal treatment. All 100 individuals reported a complete relief of symptoms of burning and itching within 1 to 3 days of wearing the socks. The skin affected by tinea pedis significantly improved within a week. Subsequently, the same team in Israel conducted another study on copper oxide-impregnated socks (108). Fifty-six patients with severe tinea pedis were asked to wear normal socks for the first week and continue wearing copper socks from the second week onwards in the absence of any other treatment. Follow-up results after averages of 9 days and 40 days showed either improvement or resolution of symptoms of tinea pedis (erythema, fissuring, vesicular eruptions, scaling, and burning and itching) for the whole cohort. No adverse reactions to the copper-impregnated socks were identified (108). These results were then confirmed in a study using photographs taken in the previous study and quantitatively measuring them with a computer program (109). An unplanned study was carried out in 2010 when 33 miners were trapped for 69 days in a mine in Chile (110). The miners suffered from severe fungal skin problems, mainly of their feet. An antifungal cream was delivered to the trapped miners, but the cream application was unsuccessful. Therefore, after more than a month, copper oxide-impregnated socks were delivered, and this proved to be effective. After rescue, it was noted that the miners’ skin condition was exceptionally good given the circumstances in which they were trapped. In 2013, a study on the use of copper oxide-impregnated socks for the protection of soldiers’ feet was published (111). Fifty-three soldiers filled out a questionnaire after wearing the socks for 3 weeks during intensive basic training. The majority reported a significant reduction of skin irritation, itching, and dryness of their feet and reduction of foot and sock odor.
More recently, the effect of copper-impregnated textiles on HAIs has been studied in two clinical trials (112, 113). The first trial compared the HAI rates on a specialized ward for patients with head injuries over 1 year, separated into two time periods: one period prior to the replacement of bed linens and uniforms worn by staff with copper-impregnated textiles and the second period after substitution of the hospital textiles (112). Replacing regular textiles with copper-impregnated textiles resulted in a significant reduction of 24% in the HAI rate. Furthermore, significant reductions were observed in the number of fever days (reduction of 47%) and in the total number of days of antibiotic treatment (32.8% reduction), and a savings of 27% in hospital costs was accomplished. A second study conducted on two ventilator-dependent wards showed similar results for HAI indicators in a crossover, double-blind, controlled trial (113). Copper fabrics (bed linen, clothing, and towels for patients) were given to one ward, and nonimpregnated textiles were given to the other ward, for a period of 3 months. After a 1-month washout period with the hospital’s usual textiles, copper and control textiles were exchanged for another 3 months. All staff were blind to the nature of the fabrics. Significant reductions were observed in all four indicators for infection, namely, the need for antibiotic treatment (29.3% reduction), number of days with a fever (55.5% reduction), duration of antibiotic therapy (23.0% reduction), and daily dose of antibiotics used (27.5% reduction). Adverse events or skin irritation was not reported.
These trials suggest a positive effect of the use of copper-impregnated textiles to augment infection control practices in a health care environment. Additional and more robust trials (e.g., randomized, blind trials) are required to learn the complete benefit that copper textiles offer in lowering the risk of HAI.
WOUND HEALING
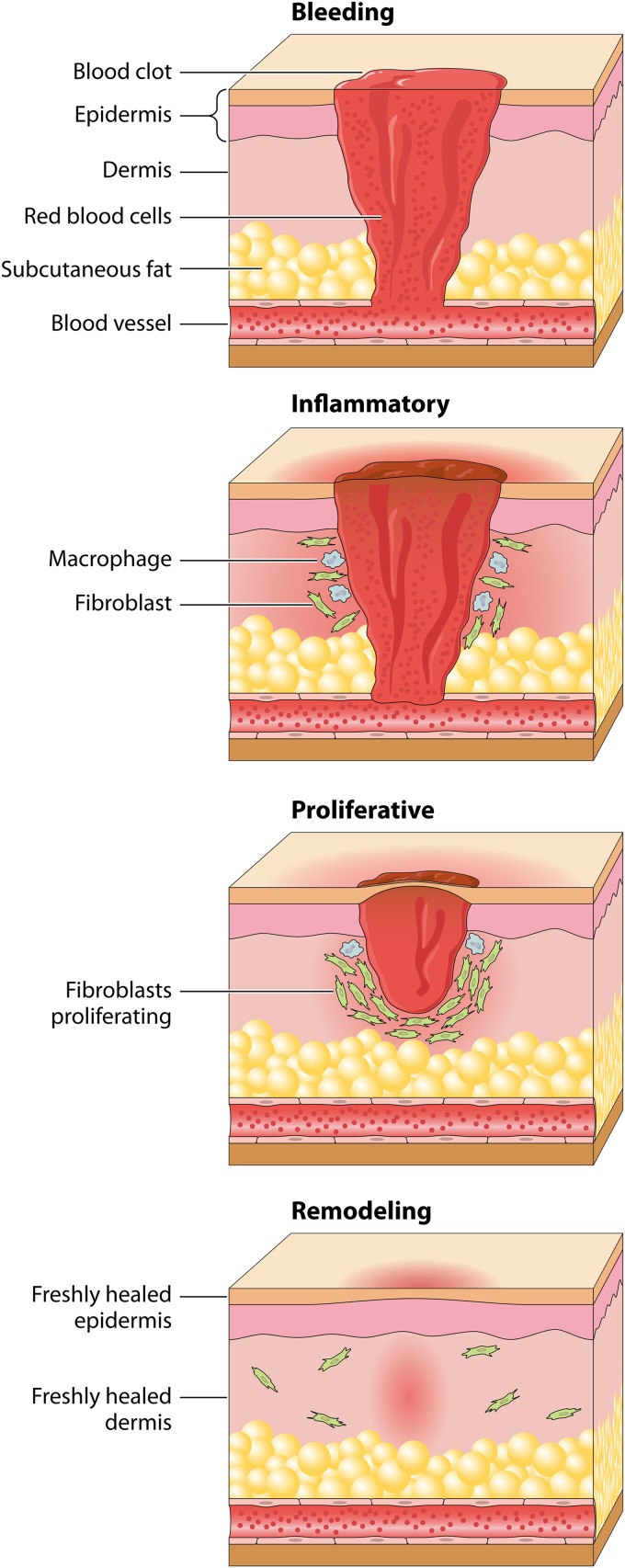
Wound healing is a complex cascade of events on a cellular level which comprises four stages: hemostasis, inflammation, proliferation, and remodeling (114) (Fig. 4). Hemostasis is dominated by platelets, which release two main factors: platelet-derived growth factor (PDGF) and transforming growth factor beta (TGF-β). Inflammatory cells such as neutrophils, mast cells, macrophages, and T lymphocytes clean the wound from injured and contaminated tissue in the inflammatory phase. Characteristic features of the proliferation phase epithelialization, fibroplasia, and angiogenesis. Epithelialization is stimulated by epidermal growth factor (EGF) and TGF-α, which are produced by macrophages. Angiogenesis is initiated by vascular endothelial cell growth factor (VEGF), basic fibroblast growth factor (bFGF), and TGF-β. During remodeling, matrix proteins such as collagen, proteoglycans, and fibronectin are produced and form tight connections to increase the strength of the scar.
FIG 4.
Mechanism of wound healing. Wound healing is a complex cascade characterized by four overlapping phases: bleeding and hemostasis, inflammation, proliferation, and remodeling. At the time of injury, the tissue is disrupted, and the platelets stick together to seal damage to the blood vessel. After this, fibrin strands begin to adhere. Following hemostasis, the neutrophils enter the wound site and begin removing foreign materials, bacteria, and damaged tissue. The inflammatory phase continues with highly phagocytic macrophages that are responsible for removing nonfunctional host cells, bacterium-filled neutrophils, damaged matrix, foreign debris, and any remaining bacteria. Once the wound site is cleaned out, the migrated fibroblasts begin the proliferation phase. The objective of the proliferation phase is for the wound to be “rebuilt” with new granulation tissue, which is comprised of collagen and extracellular matrix and into which a new network of blood vessels develops, a process known as angiogenesis. Epithelial cells finally resurface the wound, a process known as epithelization. During the final remodeling phase, the collagen forms well-organized stable cross-links.
Dysregulation of this complex mechanism will result in fibrosis and chronic ulceration and is associated with increased morbidity and mortality (115–118). According to the literature, the function of many factors involved in wound healing is dependent on and modulated by copper ions, and thus, copper is involved in all four stages of wound healing (74, 119–132). It is hypothesized that delayed wound healing could partially be explained by depleted copper levels (10), which in turn reinforces a role for this element as an essential micro-trace nutrient required from diet.
Effect of Copper on Hemostasis and the Inflammatory Response
PDGF plays a significant role not only during hemostasis but also during the several stages of wound repair. It stimulates not only the proliferation and migration of fibroblasts and smooth muscle cells but also the migration of inflammatory cells such as neutrophils and macrophages (133). In vitro studies have provided insight that the cell migration stimulated by PDGF is copper dependent (130) and that copper plays an important role in the first two stages of wound healing. Copper accumulates during the inflammation phase, where it will be used by inflammatory cells such as macrophages (134, 135).
Effect of Copper on Proliferation
Copper’s role in facilitating angiogenesis has been known for some time (136–138), but only more recent studies have shed light on the specific copper targets. VEGF is an important growth factor which stimulates angiogenesis, a key part of the proliferation phase. Copper is able to induce VEGF expression in keratinocytes, which is confirmed by enhanced VEGF expression in copper-treated wounds (125). Furthermore, the release of two polypeptides also involved in angiogenesis, fibroblast growth factor 1 (FGF-1) and interleukin 1α (IL-1α), is upregulated by copper (139).
Effect of Copper on Remodeling
During remodeling, fibronectin mats are formed to strengthen the scar. Fibronectin stabilization and cell ingrowth are stimulated by low concentrations of copper (140). Copper also forms complexes with the tripeptide glycyl-l-histidyl-l-lysine (GHK), which upregulate important remodeling proteins such as collagen, elastin, metalloproteinases, VEGF, fibroblast growth factor, and nerve growth factor (141, 142). Furthermore, cross-linking of collagen and elastin is catalyzed by the copper-dependent enzyme lysyl oxidase (LOX) (143).
Copper-Impregnated Products for Antiaging Skin Therapy and Improvement of Skin Pathologies
The positive effect of copper oxide-impregnated products on the skin was also demonstrated in two double-blind randomized trials using copper-impregnated pillowcases. Borkow et al. reported a double-blind, placebo-controlled, randomized study (144). Fifty-seven volunteers aged 40 to 60 years slept on either a copper oxide-impregnated or a control pillowcase during the study period of 4 weeks. Facial photographs at baseline and after 2 and 4 weeks were evaluated by a dermatologist and a cosmetologist. Sleeping on a copper oxide-impregnated pillowcase resulted in a significant reduction of wrinkles and crow’s-feet and resulted in an overall improved facial appearance at both 2 and 4 weeks compared to sleeping on a normal pillowcase. A similar study was conducted by Baek et al. (145), although a longer study period was chosen (8 weeks), and facial photographs were also evaluated by GFM PRIMOS three-dimensional (3D) image analysis at baseline and at 4 and 8 weeks. Results from 61 volunteers using either a copper oxide-impregnated or control pillowcase showed a significant decrease of crow’s-feet in the copper group and no decrease in the control group. The 3D analysis revealed a decrease of facial wrinkles in the copper group but failed to detect changes in the control group. These results were substantiated by Dykes (146), who studied the effect of copper oxide-impregnated socks on skin elasticity. In a double-blind, placebo-controlled study involving 60 healthy volunteers in which about half of the group wore copper oxide-impregnated socks and the other half wore identical socks without copper for 4 weeks, skin elasticity on the side of the ankle was measured at baseline and after 2 and 4 weeks using a cutometer. The results showed an increase of mean net skin elasticity of 6.4% at 2 weeks and a significant increase of 31.4% at 4 weeks in the group using copper socks but no increase in elasticity in the control group. In a preliminary clinical study regarding the safety of adult diapers impregnated with copper oxide in elderly patients, an improvement of skin appearance was found (147). Sixteen patients used the copper diapers daily for a period of half a year. During the study period, no skin irritation or adverse reactions related to the diapers were observed. After 1 month of usage, an improved skin appearance in the diaper area was observed in all participants. Arendsen et al. studied the effect of copper-impregnated compression stockings on the skin condition lipodermatosclerosis secondary to chronic venous insufficiency (148). In a double-blind, randomized, controlled study, they included patients with bilateral lipodermatosclerosis. The participants were given pairs of study compression stockings, of which one of the pairs was impregnated with copper and the other was a control stocking. This study demonstrated a significant reduction of lipodermatosclerosis using copper-impregnated stockings.
SAFETY OF COPPER
Copper is one of a few metallic minerals which are essential for normal functions of several processes in the human body. It is estimated that humans digest about 1 mg of copper per kg of body weight daily (149). Depending on the copper content and form of copper, 15 to 97% of the copper is absorbed in the stomach and small intestine (150–153). Various copper transporters move the ingested copper through cell membranes (154, 155), after which copper binds to albumin, glutathione, and amino acids (156, 157). The primary site of copper uptake is the liver, where it is incorporated into copper-dependent proteins. The liver controls copper release and thereby maintains copper homeostasis (158). Copper is excreted through feces, and the majority of fecal copper comes from biliary excretion (159–161). The human body utilizes copper for the innate immune response by increasing the phagocytic and bactericidal activities of neutrophils and the antimicrobial function of macrophages (162). Signs and symptoms of copper deficiency include anemia, neutropenia, myelopathy, and peripheral and optic neuropathy (163–165). Copper toxicity due to excess ingestion of copper is rare; however, it can lead to nausea, vomiting, hematemesis, hypotension, melena, jaundice, and diarrhea (166). Chronic copper excess can damage the liver and kidneys (167). High internal copper concentrations can result from a genetic disorder, Wilson’s disease is a rare autosomal recessive disease affecting between 1 in 30,000 and 1 in 100,000 of the population. Wilson’s disease affects the proper metabolism of copper and is associated with a reduced rate of copper excretion through bile, resulting in a toxic accumulation of the element within the liver (168). Clinical manifestations include hepatic (elevated serum aminotransferase levels, chronic hepatitis, cirrhosis, and hepatic failure), neurological (tremor, choreiform movements, Parkinsonism, gait disturbances, dysarthria, seizures, and migraine), ophthalmic (K-F rings and sunflower cataracts), psychiatric (depression, neuroses, and psychosis), and renal abnormalities.
Even though a high internal concentration of copper is toxic, the exogenous application of copper is considered safe to humans. Such a claim is supported by the fact that, for almost 50 years, copper intrauterine devices (IUDs) have been safely used for up to 10 years of single placements, with no reported adverse effects (169). Globally, copper pipes are widely used for the distribution of water because of their ability to resist corrosion. Copper is also safely used in health care for the control of Legionella (170) and other waterborne bacteria (171) through a copper-silver ionization water filter, in dentistry for the reduction of carries (172) by using high copper concentrations in dental amalgam alloys, and for the prevention of foodborne diseases (23, 34, 173) on copper alloy surfaces. Furthermore, the use of copper as a biocide has become indispensable and is frequently used in antifouling paints (174, 175); for wood preservation (176); for the control of green algae in lakes, rice fields, canals, rivers, and swimming pools (90); and for the prevention of the fungal disease of grapes (downy mildew) (177).
The safety of copper-impregnated textiles is studied through extensive animal studies, which all demonstrate no skin irritation or sensitization or any other adverse reaction to copper oxide-impregnated textiles (13, 28, 55, 61). Subsequently, its safety in humans was demonstrated in various clinical trials (108, 110, 144, 145, 178). Contrary to microbes, human skin cells are able to metabolize and utilize copper, and consequently, the risk of adverse reactions is negligible (179). In contrast to human cells, microorganisms are exceedingly sensitive to excess concentrations of copper, as they cannot control their extracellular environment to allow for excretion (75). Other metals commonly used in health care, such as aluminum, nickel, and silver, cause harm to the environment, and even though human cells are also able to utilize aluminum and other trace metals, significant health issues have been documented. Apart from the obvious adverse effects on the respiratory tract for aluminum industry employees, aluminum drinking levels in potable water are also positively associated with the risk of cognitive impairment, dementia, or Alzheimer’s disease (180). The use of nickel-containing products is increasing, which led to an increased environmental burden of nickel compounds, a serious health risk for humans, as nickel is not an essential element for humans. Nickel can result in skin sensitization, lung fibrosis, poisoning of the kidneys and the cardiovascular system, and tumor formation (181).
Silver is applied in hygiene, personal care, and health care as silver coatings on hard surfaces, spray for prevention of sore throats, and silver-impregnated dressings for treatment of chronic wounds (182). The production and usage of silver nanoparticles pose a threat for the environment and humans. Environmental toxicity is caused by soluble free silver ions that are released from industrial wastes. Adverse effects on humans include permanent discoloration of the skin (argyria) or the eyes (argyrosis); liver and kidney damage; irritations of the eyes, skin, respiratory system, and intestinal tract; and strong toxic effects on the proliferation of and cytokine expression by peripheral blood mononuclear cells (183). As silver ions are soluble, silver-impregnated products will leach silver ions during washing or recycling. In contrast, copper oxide is nonsoluble, and copper oxide-impregnated products will therefore not pollute the environment and, as such, will have permanent antimicrobial properties.
CLINICAL APPLICATION IN OBSTETRICS AND GYNECOLOGY
To date, there is little to no research on copper specifically in the field of obstetrics and gynecology, although the clinical application of copper can be extrapolated from current evidence. However, infection prevention practices can be very challenging. The “normal” microflora of the vagina and cervix can, under certain circumstances, lead to infection of the female genital tract, and these infections are usually polymicrobial (14). Particularly in obstetric practices, there is a higher risk of transmission of infectious diseases due to frequent contact with blood and other bodily fluids. During the 19th century, Ignaz Semmelweis discovered that puerperal fever was transmitted from the morgue to laboring women via the hands of medical students. Due to his introduction of antiseptic procedures, the mortality rate from puerperal fever dropped to below 1% (184). HAIs are a global problem associated with a significant impact on morbidity and mortality. Plowman et al. reported an overall incidence of HAI of 7.8%, which varied considerably with specialty, with the highest incidence of 13.1% in gynecology and the third highest incidence of 10.1% in obstetrics (185). Johnson et al. have shown that 10% of women undergoing vaginal delivery who sustained perineal trauma that required suturing developed a wound infection (186).
Surgical site infections (SSIs) are one of the most prevalent reported nosocomial infections. They account for 14 to 16% of all hospital-acquired infections. With cesarean section (CS) rates rising (187–190), the risk of surgical site infections is also subject to an increased frequency despite the use of prophylactic antibiotics, with the rate ranging between 2% and 15% (191–197). MRSA has caused a larger number of severe postpartum infections, especially after CS (198–200). Approximately 10% of asymptomatic pregnant women are colonized with MRSA (201, 202). Reduction of the microbial burden in the vicinity of a surgical wound may limit surgical site infections or at least simply reduce the incident risk following not only CS but also other gynecological (abdominal) surgeries. Recently, the results of the first clinical trial studying the effect of copper-impregnated wound dressings on SSI rates following CS were presented by Arendsen et al. at the European Congress of the European Board and College of Obstetrics and Gynaecology (203). This comprehensive, single-center, double-blind, randomized, controlled trial included 324 participants, of whom 156 were randomized to the copper-impregnated dressing group (study group) and 159 were randomized to the noncopper wound dressing group (control group). All women following cesarean section, both planned and emergency, were eligible for study enrollment. The primary study outcome was the incidence of SSI within a 30-day period following CS. The Health Protection Agency’s Post Discharge Questionnaire for national (United Kingdom) SSI surveillance was used for the assessment of SSI (204), which is in keeping with the worldwide-used definition of SSI by the Centers for Disease Control and Prevention (205). With a follow-up rate of 100%, there was a significant 38% lower overall SSI rate in the copper intervention arm than in the control group (adjusted odds ratio [OR], 0.44 [95% confidence interval {CI}, 0.25 to 0.77]; P = 0.004).
Vaginal trauma from vaginal surgery or delivery can also lead to wound infection. The prevalence of vaginal lacerations following vaginal delivery is as high as 85%, and up to 70% will need suturing (206). Because of factors supporting the development of inflammation, such as higher local humidity and the presence of various microbes, obstetric trauma has a high risk of wound infections of 11% (186). Wound infections can cause wound breakdown associated with pain, resulting in a delay in healing, and this can have a negative impact on motherhood (207). Furthermore, antibiotics are able to pass into breast milk and can disrupt the microbiome of the developing infant gut. One of the leading causes of maternal mortality worldwide is bacterial infections resulting from delivery and during the postpartum period. These infections account for 11% of the global burden of maternal deaths (16, 208). The use of copper-impregnated sanitary pads may help limit (wound) infections after vaginal surgery or vaginal delivery. As endometritis is often caused by an ascending infection from the lower genital tract, it was hypothesized that using sanitary pads impregnated with copper in the time immediately postpartum may be able to limit this serious maternal complication. The results of the first trial studying copper-impregnated sanitary towels was recently presented by Arendsen et al. at the European Congress of the European Board and College of Obstetrics and Gynaecology (209). This double-blind, randomized, controlled trial included 450 women who sustained a secondary tear or episiotomy during a vaginal delivery. Half of the study population used copper-impregnated maternity pads. The study demonstrated a significant reduction of the overall infection rate in the copper group by 78%.
Copper-impregnated sanitary pads or tampons could also be effective for the treatment of vaginitis. Vaginal microbiota dominated by the anaerobic Lactobacillus are a well-known marker of healthy vaginal flora (210). Lactobacillus is able to inhibit the growth of pathogenic microorganisms such as Bacteroides fragilis, E. coli, Gardnerella vaginalis, Mobiluncus spp., Neisseria gonorrhoeae, Peptostreptococcus anaerobius, Prevotella bivia, and S. aureus (211–214). Lactobacillus spp. generate a low pH, resulting in a vaginal pH of less than 4.5 (215). Additionally, Lactobacillus spp. generate hydrogen peroxide, a significant inhibitor of anaerobes and facultative anaerobic bacteria lacking or possessing reduced levels of catalase (216, 217). Studies suggest that the biocidal ability of copper is enhanced by the conversion of hydrogen peroxide to hydroxyl radicals (ROS) catalyzed by copper, and copper is a more efficient catalyst (∼100-fold) than Fe2+ in catalyzing the Fenton reaction, which generates significant hydrogen peroxide (218). A distorted balance of the vaginal microbiota can lead to an overgrowth of anaerobic microbes (bacterial vaginosis); facultative anaerobic species such as E. coli, enterococci, and group B streptococci (aerobic vaginitis); or both. A healthy, acidic vaginal microflora is especially important during pregnancy, as studies have suggested that vaginal infection is an important risk factor for complications of pregnancy. For instance, premature births and spontaneous abortions have been associated with bacterial vaginosis, which occurs in up to 20% of pregnant women (219). No research has been done to establish the effect of copper on Lactobacillus spp.; however, given the fact that the generation of ROS is part of the antimicrobial efficacy of copper as well as Lactobacillus spp., it can be hypothesized that these bacteria could be less sensitive to copper. It is expected that during continued exposure to copper, members of the healthy vaginal microflora such as Lactobacillus would eventually be harmed. In contrast to antibiotics, which will kill only a specific spectrum of bacteria, leaving other bacteria or microbes to flourish, copper is able to kill all microbes, preventing an overgrowth of a particular microorganism. Local application of copper-impregnated products such as underpants, sanitary/maternity pads, and tampons could help in the treatment of vaginitis and might also have the ability to prevent complications related to vaginitis in pregnancy.
Copper IUDs have been safely used for almost 50 years, with up to 10-year single placements without any adverse effects (169). A common concern among health care providers is whether the insertion of an IUD increases the risk of lower and upper genital tract infections, which can lead to pelvic inflammatory disease (PID), with its associated risk of infertility. This is particularly important since the majority of IUD users are young women who are at a higher risk of sexually transmitted infections (STIs) (220). However, extensive research shows that the risk of PID was not increased with the use of an IUD (both copper- and levonorgestrel-containing IUDs) compared to other contraceptive methods, and there was no association with infertility (221–226). A case-control study assessing the association between the use of a copper-containing IUD and the level of antichlamydial antibodies even found evidence of a possible protective effect against chlamydia infection (227). Compared to women with an inert IUD (not containing a bioactive component), women with a copper-containing IUD had significantly lower levels of antichlamydial antibodies.
In recent years, more attention has been given to the prevention of human papillomavirus (HPV) to reduce the mortality and morbidity of cervical cancer (228). Lekovich et al. studied the differences in clearance of HPV in women using copper-containing and levonorgestrel-containing IUDs (229). In this retrospective study, the cervical cytology of 150 women using a copper IUD was compared with the cervical cytology of 152 women using the levonorgestrel IUD, both before and after placement of the IUD. The results showed that a significantly lower proportion of the women using the levonorgestrel IUD cleared HPV infection than women using the copper IUD (42% versus 70%; P = 0.04). Furthermore, women using the levonorgestrel IUD had a 4-times-higher risk of acquiring a new HPV infection. It is still debatable whether this study shows a positive effect of the copper IUD on HPV clearance, a negative effect of the levonorgestrel IUD, or maybe a combination of the two. Spontaneous clearance of HPV has been documented in the literature to occur in 61% of women within 1 year (230). A higher rate of clearance was found in this study, although this was not statistically significant. The HPV clearance rate in women with a levonorgestrel IUD was found to be significantly lower than the spontaneous clearance rate (P = 0.03). Another study evaluated the association between recent IUD use and cervical intraepithelial neoplasia grade 2 (CIN2) and CIN3, adenocarcinoma, or cancer and found an increased risk of CIN grade 2 and higher (relative risk [RR], 1.18 [95% CI, 1.08 to 1.30]; P < 0.001) associated with the use of a levonorgestrel-containing IUD (231). Copper IUDs showed no association with precancer or cancer; a possible trend of a lower risk of CIN3+ (RR, 0.81 [95% CI, 0.64 to 1.02]; P = 0.07) was found with the use of a copper IUD. Unfortunately, the group of women with a copper IUD was almost four times smaller than the levonorgestrel-containing IUD group, which could explain why statistical significance could not have been reached.
Twenty-five percent of all hospitalized patients have a urinary catheter (232), and nosocomial urinary tract infection (UTI) associated with urinary catheters accounts for 80% of all nosocomial infections (233). Symptoms of urinary incontinence are highly prevalent in both sexes; however, they are much more frequent in women. In the United Kingdom, as many as 50% of women suffer from urinary incontinence (234); therefore, urinary incontinence is a common problem seen in gynecological outpatient departments. Women suffering from severe urinary incontinence often end up with long-term catheterization (235). For this reason, research has been undertaken to investigate antimicrobial coatings for urinary catheters (236). Currently, silver is one of the few ingredients for medical products, such as coatings for urinary catheters, that is approved by the FDA. Extensive research has been carried out suggesting the antimicrobial ability of silver cations (237–243). The data concerning the overall effectiveness of silver cation-releasing catheters for their ability to prevent catheter-mediated UTI are limited (244–249). No studies to date have been done looking at the possibility of the use of copper-impregnated urinary catheters; however, there has been research done with copper-impregnated silicone in a different field of medicine. Gosau et al. investigated the antibacterial ability of copper-impregnated silicone for use in breast implants (250, 251). Their research showed reduced adherence of bacteria and bactericidal abilities of the copper-impregnated silicone.
Another possibility to reduce UTIs, especially in the elderly, is copper oxide-impregnated incontinence pads. The reported association of urinary incontinence and UTIs in the literature has been suggested to be caused by the transmission of bacteria during daily incontinence care (252, 253) and through the use of incontinence pads (254). Omli et al. demonstrated an almost 4-times-increased risk of UTI with the use of incontinence pads (255). Weinberg et al. studied copper oxide-impregnated incontinence diapers for chronic care patients (147). The frequencies of infections that occurred over the study period were similar to, if not slightly lower than, those for the same period a year before. Unfortunately, this study was not designed to detect a significant difference in infection rates.
CONCLUSION
Although there is considerable in vitro research supporting the antimicrobial properties of copper, there is still a paucity of in vivo studies. With the advancement of research toward clinical studies, the antimicrobial application of copper in health care is becoming more evident. The continuous and rapid broad-spectrum biocidal properties of both copper-containing hard surfaces and soft surfaces such as textiles and polymers may offer a novel and innovative advancement in the battle against health care-associated infections. Although traditional infection prevention practices should not be abandoned, copper-coated surfaces and textiles could increase their effectiveness. This review has provided an overview of the current evidence of the antimicrobial and wound-healing properties of copper and explored its potential uses in obstetrics and gynecology. Although some studies provide evidence for clinical applications of copper in a health care setting, more robust clinical, randomized, controlled research is needed to further confirm the use of various copper-impregnated (medical) products for the prevention of infection. Within obstetrics and gynecology, copper-impregnated products may be useful for infection prevention, e.g., copper-impregnated wound dressings for the prevention of surgical site infection following cesarean section and other abdominal gynecological surgeries; copper-impregnated incontinence pads for the prevention of wound infection after perineal suturing (following vaginal delivery or vaginal surgery), for the prevention of UTIs associated with the use of incontinence pads, and for the treatment of vaginitis; copper-containing IUDs, which may have a protective ability against chlamydia and HPV infection; and copper-impregnated urinary catheters for the prevention of UTIs associated with long-term catheterization. As sepsis is one of the major causes of maternal mortality, the introduction of copper-impregnated materials in the health care setting could have a dramatic impact on the reduction of infections. Given the emerging and constant resistance to antibiotics, copper provides a nonpharmacological alternative in terms of both prevention and treatment of infections. The impact of these benefits is likely to be greater in low-resource countries where access to medicine, especially antibiotics, is limited.
ACKNOWLEDGMENTS
We thank The Mayday Childbirth Trust, Croydon, for providing a grant to cover the salary of L.P.A. as a research fellow for 3 years.
We are grateful to Copper Clothing Ltd. for providing the figures for the manuscript.
R.T. and A.H.S. conceived the study. L.P.A. was responsible for writing the first draft of the manuscript. All authors reviewed and edited the manuscript and approved the final version of the manuscript.
Biographies

Linda P. Arendsen obtained her medical degree from the Radboud University of Nijmegen, The Netherlands. In the Radboud University of Nijmegen, she carried out multicenter research on obstetric anal sphincter injuries (OASIs) and endoanal ultrasound, which was published in 2016. From 2015, she started as a Research Fellow at Croydon University Hospital, London, United Kingdom. Under the supervision of A. H. Sultan and R. Thakar, she conducted extensive research on the effect of copper-impregnated products on the infection rate in the Obstetrics and Gynecology Department. She also carried out research on the effect of copper on lipodermatosclerosis secondary to chronic venous insufficiency. Furthermore, she gained clinical experience in urogynecology working at the highly specialized Pelvic Floor and Reconstruction Centre at Croydon University Hospital.

Ranee Thakar is a Subspecialist in Urogynecology and Consultant Obstetrician and Gynecologist at Croydon University Hospital as well as an honorary senior lecturer at St George’s University of London. Her research evaluating pelvic floor dysfunction following hysterectomy led to a landmark paper which was published in the New England Journal of Medicine and to a doctorate (M.D.) from the University of London. She is a consultant in a busy tertiary referral urogynecology department at Croydon University Hospital, with a large clinical workload, dealing with complex urogynecological problems, teaching medical students, training junior doctors, and undertaking clinical research. Her publications include many original papers in peer-reviewed journals and chapters in books. She has edited four textbooks. Along with Abdul H. Sultan, she runs the popular hands-on perineal repair and pelvic floor ultrasound courses at Croydon University Hospital, United Kingdom.

Abdul H. Sultan is a Consultant Obstetrician and Urogynecologist at Croydon University Hospital and is an Honorary Reader at St George’s University of London. He completed his postgraduate training at St George’s Hospital. His research involved studying the effects of childbirth on anal sphincters. This led to a landmark paper published in the New England Journal of Medicine and to a doctor of Medicine. His pioneering work highlighted the deficiencies in training of doctors and midwives in detecting and repairing anal sphincter injuries. He is the codirector of a tertiary referral urogynecology unit with subspecialty training and has over 200 publications. He is the codirector of the popular international Perineal Trauma and Pelvic Floor Ultrasound courses (www.perineum.net). He has been awarded the prestigious overall “UK Hospital Doctor of the Year” award and is the past President of the Section of Obstetrics and Gynecology at the Royal Society of Medicine.
REFERENCES
- 1.World Health Organization. 2010. The burden of health care-associated infection worldwide: a summary. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.European Centre for Disease Prevention and Control, European Medicines Agency. 2009. The bacterial challenge, time to react: a call to narrow the gap between multidrug-resistant bacteria in the EU and the development of new antibacterial agents. European Centre for Disease Prevention and Control, European Medicines Agency, Stockholm, Sweden. [Google Scholar]
- 3.European Centre for Disease Prevention and Control. 2014. Antimicrobial resistance surveillance in Europe: annual report of the European Antimicrobial Resistance Surveillance Network (EARS-Net) 2013. European Centre for Disease Prevention and Control, Luxembourg, Luxembourg. [Google Scholar]
- 4.Public Health England. 2015. English Surveillance Programme for Antimicrobial Utilisation and Resistance (ESPAUR) report. Public Health England, London, United Kingdom. [Google Scholar]
- 5.Shapiro DJ, Hicks LA, Pavia AT, Hersh AL. 2014. Antibiotic prescribing for adults in ambulatory care in the USA, 2007-09. J Antimicrob Chemother 69:234–240. doi: 10.1093/jac/dkt301. [DOI] [PubMed] [Google Scholar]
- 6.Review on Antimicrobial Resistance. 2015. Securing new drugs for future generations: the pipeline of antibiotics. Review on Antimicrobial Resistance, London, United Kingdom. [Google Scholar]
- 7.Borkow G, Gabbay J. 2005. Copper as a biocidal tool. Curr Med Chem 12:2163–2175. doi: 10.2174/0929867054637617. [DOI] [PubMed] [Google Scholar]
- 8.Borkow G, Gabbay J. 2009. Copper, an ancient remedy returning to fight microbial, fungal and viral infections. Curr Chem Biol 3:272–278. doi: 10.2174/2212796810903030272. [DOI] [Google Scholar]
- 9.Noyce JO, Michels H, Keevil CW. 2006. Potential use of copper surfaces to reduce survival of epidemic meticillin-resistant Staphylococcus aureus in the healthcare environment. J Hosp Infect 63:289–297. doi: 10.1016/j.jhin.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 10.Borkow G, Gabbay J, Zatcoff RC. 2008. Could chronic wounds not heal due to too low local copper levels? Med Hypotheses 70:610–613. doi: 10.1016/j.mehy.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 11.O’Gorman J, Humphreys H. 2012. Application of copper to prevent and control infection. Where are we now? J Hosp Infect 81:217–223. doi: 10.1016/j.jhin.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 12.Salgado CD, Sepkowitz KA, John JF, Cantey JR, Attaway HH, Freeman KD, Sharpe PA, Michels HT, Schmidt MG. 2013. Copper surfaces reduce the rate of healthcare-acquired infections in the intensive care unit. Infect Control Hosp Epidemiol 34:479–486. doi: 10.1086/670207. [DOI] [PubMed] [Google Scholar]
- 13.Borkow G, Gabbay J. 2004. Putting copper into action: copper-impregnated products with potent biocidal activities. FASEB J 18:1728–1730. doi: 10.1096/fj.04-2029fje. [DOI] [PubMed] [Google Scholar]
- 14.Garber GE, Chow AW. 1989. Female genital tract infections, p 429–453. In Finegold SM, George WL (ed), Anaerobic infections in humans, 1st ed Academic Press, Inc, San Diego, CA. [Google Scholar]
- 15.van Dillen J, Zwart J, Schutte J, van Roosmalen J. 2010. Maternal sepsis: epidemiology, etiology and outcome. Curr Opin Infect Dis 23:249–254. doi: 10.1097/QCO.0b013e328339257c. [DOI] [PubMed] [Google Scholar]
- 16.Say L, Chou D, Gemmill A, Tunçalp Ö, Moller A-B, Daniels J, Gülmezoglu AM, Temmerman M, Alkema L. 2014. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health 2:e323–e333. doi: 10.1016/S2214-109X(14)70227-X. [DOI] [PubMed] [Google Scholar]
- 17.Knight M, Kenyon S, Brocklehurst P, Neilson J, Shakespeare J, Kurinczuk JJE (ed). 2014. Saving lives, improving mothers’ care: lessons learned to inform future maternity care from the UK and Ireland Confidential Enquiries into Maternal Deaths and Morbidity 2009-2012. University of Oxford, Oxford, United Kingdom. [Google Scholar]
- 18.Dollwet HH. 1985. Historic uses of copper compounds in medicine. Trace Elem Med 2:80–87. [Google Scholar]
- 19.Walusinski O. 2018. The scientific illusion of Victor Burq (1822–1884). Eur Neurol 79:135–149. doi: 10.1159/000487667. [DOI] [PubMed] [Google Scholar]
- 20.Majno G. 1991. The healing hand: man and wound in the ancient world. Harvard University Press, Cambridge, MA. doi: 10.1086/ahr/82.1.66-a. [DOI] [Google Scholar]
- 21.Rogers J, Dowsett AB, Dennis PJ, Lee JV, Keevil CW. 1994. Influence of temperature and plumbing material selection on biofilm formation and growth of Legionella pneumophila in a model potable water system containing complex microbial flora. Appl Environ Microbiol 60:1585–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilks SA, Michels H, Keevil CW. 2005. The survival of Escherichia coli O157 on a range of metal surfaces. Int J Food Microbiol 105:445–454. doi: 10.1016/j.ijfoodmicro.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 23.Wilks SA, Michels HT, Keevil CW. 2006. Survival of Listeria monocytogenes Scott A on metal surfaces: implications for cross-contamination. Int J Food Microbiol 111:93–98. doi: 10.1016/j.ijfoodmicro.2006.04.037. [DOI] [PubMed] [Google Scholar]
- 24.Noyce JO, Michels H, Keevil CW. 2007. Inactivation of influenza A virus on copper versus stainless steel surfaces. Appl Environ Microbiol 73:2748–2750. doi: 10.1128/AEM.01139-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weaver L, Michels HT, Keevil CW. 2008. Survival of Clostridium difficile on copper and steel: futuristic options for hospital hygiene. J Hosp Infect 68:145–151. doi: 10.1016/j.jhin.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 26.Michels HT, Noyce JO, Keevil CW. 2009. Effects of temperature and humidity on the efficacy of methicillin‐resistant Staphylococcus aureus challenged antimicrobial materials containing silver and copper. Lett Appl Microbiol 49:191–195. doi: 10.1111/j.1472-765X.2009.02637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Warnes SL, Keevil CW. 2011. Mechanism of copper surface toxicity in vancomycin-resistant enterococci following wet or dry contact. Appl Environ Microbiol 77:6049–6059. doi: 10.1128/AEM.00597-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Borkow G, Okon-Levy N, Gabbay J. 2010. Copper oxide impregnated wound dressing: biocidal and safety studies. Wounds 22:301–310. [PubMed] [Google Scholar]
- 29.Bauer DJ. 1958. The chemotherapeutic activity of compounds of copper, rhodium and certain other metals in mice infected with neurovaccinia and ectromelia viruses. Br J Exp Pathol 39:480–489. [PMC free article] [PubMed] [Google Scholar]
- 30.Warnes SL, Keevil CW. 2013. Inactivation of norovirus on dry copper alloy surfaces. PLoS One 8:e75017. doi: 10.1371/journal.pone.0075017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weaver L, Michels HT, Keevil CW. 2010. Potential for preventing spread of fungi in air-conditioning systems constructed using copper instead of aluminium. Lett Appl Microbiol 50:18–23. doi: 10.1111/j.1472-765X.2009.02753.x. [DOI] [PubMed] [Google Scholar]
- 32.Quaranta D, Krans T, Santo CE, Elowsky CG, Domaille DW, Chang CJ, Grass G. 2011. Mechanisms of contact-mediated killing of yeast cells on dry metallic copper surfaces. Appl Environ Microbiol 77:416–426. doi: 10.1128/AEM.01704-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dancer SJ. 2004. How do we assess hospital cleaning? A proposal for microbiological standards for surface hygiene in hospitals. J Hosp Infect 56:10–15. doi: 10.1016/j.jhin.2003.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Noyce JO, Michels H, Keevil CW. 2006. Use of copper cast alloys to control Escherichia coli O157 cross-contamination during food processing. Appl Environ Microbiol 72:4239–4244. doi: 10.1128/AEM.02532-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mehtar S, Wiid I, Todorov SD. 2008. The antimicrobial activity of copper and copper alloys against nosocomial pathogens and Mycobacterium tuberculosis isolated from healthcare facilities in the Western Cape: an in-vitro study. J Hosp Infect 68:45–51. doi: 10.1016/j.jhin.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 36.Warnes SL, Green SM, Michels HT, Keevil CW. 2010. Biocidal efficacy of copper alloys against pathogenic enterococci involves degradation of genomic and plasmid DNAs. Appl Environ Microbiol 76:5390–5401. doi: 10.1128/AEM.03050-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grass G, Rensing C, Solioz M. 2011. Metallic copper as an antimicrobial surface. Appl Environ Microbiol 77:1541–1547. doi: 10.1128/AEM.02766-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Santo CE, Lam EW, Elowsky CG, Quaranta D, Domaille DW, Chang CJ, Grass G. 2011. Bacterial killing by dry metallic copper surfaces. Appl Environ Microbiol 77:794–802. doi: 10.1128/AEM.01599-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Michels HT, Anderson DG. 2008. Antimicrobial regulatory efficacy testing of solid copper alloy surfaces in the USA. Met Ions Biol Med 10:185–190. [Google Scholar]
- 40.Marais F, Mehtar S, Chalkley L. 2010. Antimicrobial efficacy of copper touch surfaces in reducing environmental bioburden in a South African community healthcare facility. J Hosp Infect 74:80–82. doi: 10.1016/j.jhin.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 41.Santo CE, Taudte N, Nies DH, Grass G. 2008. Contribution of copper ion resistance to survival of Escherichia coli on metallic copper surfaces. Appl Environ Microbiol 74:977–986. doi: 10.1128/AEM.01938-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Elguindi J, Alwathnani HA, Rensing C. 2012. Rapid inactivation of Cronobacter sakazakii on copper alloys following periods of desiccation stress. World J Microbiol Biotechnol 28:1837–1841. doi: 10.1007/s11274-011-0972-3. [DOI] [PubMed] [Google Scholar]
- 43.Zhu L, Elguindi J, Rensing C, Ravishankar S. 2012. Antimicrobial activity of different copper alloy surfaces against copper resistant and sensitive Salmonella enterica. Food Microbiol 30:303–310. doi: 10.1016/j.fm.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 44.Ojeil M, Jermann C, Holah J, Denyer SP, Maillard J-Y. 2013. Evaluation of new in vitro efficacy test for antimicrobial surface activity reflecting UK hospital conditions. J Hosp Infect 85:274–281. doi: 10.1016/j.jhin.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 45.De Veer I, Wilke K, Rüden H. 1993. Bacterial reducing qualities of copper-containing and non-copper-containing materials. I. Contamination and sedimentation in humid and dry conditions. Zentralbl Hyg Umweltmed 195:66–87. [PubMed] [Google Scholar]
- 46.De Veer I, Wilke K, Rüden H. 1994. Bacteria-reducing properties of copper-containing and non-copper-containing materials. II. Relationship between microbiocide effect of copper-containing materials and copper ion concentration after contamination with moist and dry hands. Zentralbl Hyg Umweltmed 195:516–528. [PubMed] [Google Scholar]
- 47.US Congress. 2006. 7 United States Code Chapter 6—insecticides and environmental pesticide control.
- 48.US Environmental Protection Agency. 2006. Reregistration eligibility decision (RED) for coppers. EPA 738-R-06-020 US Environmental Protection Agency, Washington, DC. [Google Scholar]
- 49.Burden M, Cervantes L, Weed D, Keniston A, Price CS, Albert RK. 2011. Newly cleaned physician uniforms and infrequently washed white coats have similar rates of bacterial contamination after an 8‐hour workday: a randomized controlled trial. J Hosp Med 6:177–182. doi: 10.1002/jhm.864. [DOI] [PubMed] [Google Scholar]
- 50.Munoz-Price LS, Arheart KL, Mills JP, Cleary T, DePascale D, Jimenez A, Fajardo-Aquino Y, Coro G, Birnbach DJ, Lubarsky DA. 2012. Associations between bacterial contamination of health care workers’ hands and contamination of white coats and scrubs. Am J Infect Control 40:e245–e248. doi: 10.1016/j.ajic.2012.03.032. [DOI] [PubMed] [Google Scholar]
- 51.Neely AN, Maley MP. 2000. Survival of enterococci and staphylococci on hospital fabrics and plastic. J Clin Microbiol 38:724–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ohl M, Schweizer M, Graham M, Heilmann K, Boyken L, Diekema D. 2012. Hospital privacy curtains are frequently and rapidly contaminated with potentially pathogenic bacteria. Am J Infect Control 40:904–906. doi: 10.1016/j.ajic.2011.12.017. [DOI] [PubMed] [Google Scholar]
- 53.De M, Ghosh PS, Rotello VM. 2008. Applications of nanoparticles in biology. Adv Mater 20:4225–4241. doi: 10.1002/adma.200703183. [DOI] [Google Scholar]
- 54.Biener J, Wittstock A, Baumann TF, Weissmüller J, Bäumer M, Hamza AV. 2009. Surface chemistry in nanoscale materials. Materials 2:2404–2428. doi: 10.3390/ma2042404. [DOI] [Google Scholar]
- 55.Gabbay J, Borkow G, Mishal J, Magen E, Zatcoff R, Shemer-Avni Y. 2006. Copper oxide impregnated textiles with potent biocidal activities. J Ind Text 35:323–335. doi: 10.1177/1528083706060785. [DOI] [Google Scholar]
- 56.Borkow G, Gabbay J. 2006. Endowing textiles with permanent potent biocidal properties by impregnating them with copper oxide. J Text Apparel Technol Manag 5:1–3. [Google Scholar]
- 57.Sun C, Li Y, Li Z, Su Q, Wang Y, Liu X. 2018. Durable and washable antibacterial copper nanoparticles bridged by surface grafting polymer brushes on cotton and polymeric materials. J Nanomater 2018:6546193. doi: 10.1155/2018/6546193. [DOI] [Google Scholar]
- 58.Anita S, Ramachandran T, Rajendran R, Koushik CV, Mahalakshmi M. 2011. A study of the antimicrobial property of encapsulated copper oxide nanoparticles on cotton fabric. Text Res J 81:1081–1088. doi: 10.1177/0040517510397577. [DOI] [Google Scholar]
- 59.Chattopadhyay DP, Patel BH. 2010. Effect of nanosized colloidal copper on cotton fabric. J Eng Fiber Fabr 5:1–6. [Google Scholar]
- 60.Berendjchi A, Khajavi R, Yazdanshenas ME. 2011. Fabrication of superhydrophobic and antibacterial surface on cotton fabric by doped silica-based sols with nanoparticles of copper. Nanoscale Res Lett 6:594. doi: 10.1186/1556-276X-6-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Borkow G, Zhou SS, Page T, Gabbay J. 2010. A novel anti-influenza copper oxide containing respiratory face mask. PLoS One 5:e11295. doi: 10.1371/journal.pone.0011295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ohsumi Y, Kitamoto K, Anraku Y. 1988. Changes induced in the permeability barrier of the yeast plasma membrane by cupric ion. J Bacteriol 170:2676–2682. doi: 10.1128/JB.170.6.2676-2682.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lemire JA, Harrison JJ, Turner RJ. 2013. Antimicrobial activity of metals: mechanisms, molecular targets and applications. Nat Rev Microbiol 11:371–384. doi: 10.1038/nrmicro3028. [DOI] [PubMed] [Google Scholar]
- 64.Warnes SL, Caves V, Keevil CW. 2012. Mechanism of copper surface toxicity in Escherichia coli O157:H7 and Salmonella involves immediate membrane depolarization followed by slower rate of DNA destruction which differs from that observed for Gram-positive bacteria. Environ Microbiol 14:1730–1743. doi: 10.1111/j.1462-2920.2011.02677.x. [DOI] [PubMed] [Google Scholar]
- 65.Nan L, Liu Y, Lü M, Yang K. 2008. Study on antibacterial mechanism of copper-bearing austenitic antibacterial stainless steel by atomic force microscopy. J Mater Sci Mater Med 19:3057–3062. doi: 10.1007/s10856-008-3444-z. [DOI] [PubMed] [Google Scholar]
- 66.Avery SV, Howlett NG, Radice S. 1996. Copper toxicity towards Saccharomyces cerevisiae: dependence on plasma membrane fatty acid composition. Appl Environ Microbiol 62:3960–3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hazel JR, Williams EE. 1990. The role of alterations in membrane lipid composition in enabling physiological adaptation of organisms to their physical environment. Prog Lipid Res 29:167–227. doi: 10.1016/0163-7827(90)90002-3. [DOI] [PubMed] [Google Scholar]
- 68.Weaver L, Noyce JO, Michels HT, Keevil CW. 2010. Potential action of copper surfaces on meticillin‐resistant Staphylococcus aureus. J Appl Microbiol 109:2200–2205. doi: 10.1111/j.1365-2672.2010.04852.x. [DOI] [PubMed] [Google Scholar]
- 69.Rifkind JM, Shin YA, Heim JM, Eichhorn GL. 1976. Cooperative disordering of single‐stranded polynucleotides through copper crosslinking. Biopolym Orig Res Biomol 15:1879–1902. doi: 10.1002/bip.1976.360151002. [DOI] [PubMed] [Google Scholar]
- 70.Finney LA, O’Halloran TV. 2003. Transition metal speciation in the cell: insights from the chemistry of metal ion receptors. Science 300:931–936. doi: 10.1126/science.1085049. [DOI] [PubMed] [Google Scholar]
- 71.Ma Z, Jacobsen FE, Giedroc DP. 2009. Coordination chemistry of bacterial metal transport and sensing. Chem Rev 109:4644–4681. doi: 10.1021/cr900077w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Waldron KJ, Robinson NJ. 2009. How do bacterial cells ensure that metalloproteins get the correct metal? Nat Rev Microbiol 7:25–35. doi: 10.1038/nrmicro2057. [DOI] [PubMed] [Google Scholar]
- 73.Karlstrom AR, Shames BD, Levine RL. 1993. Reactivity of cysteine residues in the protease from human immunodeficiency virus: identification of a surface-exposed region which affects enzyme function. Arch Biochem Biophys 304:163–169. doi: 10.1006/abbi.1993.1334. [DOI] [PubMed] [Google Scholar]
- 74.Kim J-H, Cho H, Ryu S-E, Choi M-U. 2000. Effects of metal ions on the activity of protein tyrosine phosphatase VHR: highly potent and reversible oxidative inactivation by Cu2+ ion. Arch Biochem Biophys 382:72–80. doi: 10.1006/abbi.2000.1996. [DOI] [PubMed] [Google Scholar]
- 75.Macomber L, Imlay JA. 2009. The iron-sulfur clusters of dehydratases are primary intracellular targets of copper toxicity. Proc Natl Acad Sci U S A 106:8344–8349. doi: 10.1073/pnas.0812808106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Purves J, Thomas J, Riboldi GP, Zapotoczna M, Tarrant E, Andrew PW, Londoño A, Planet PJ, Geoghegan JA, Waldron KJ, Morrissey JA. 2018. A horizontally gene transferred copper resistance locus confers hyper-resistance to antibacterial copper toxicity and enables survival of community acquired methicillin resistant Staphylococcus aureus USA300 in macrophages. Environ Microbiol 20:1576–1589. doi: 10.1111/1462-2920.14088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hasman H, Kempf I, Chidaine B, Cariolet R, Ersbøll AK, Houe H, Hansen HCB, Aarestrup FM. 2006. Copper resistance in Enterococcus faecium, mediated by the tcrB gene, is selected by supplementation of pig feed with copper sulfate. Appl Environ Microbiol 72:5784–5789. doi: 10.1128/AEM.02979-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rouch DA, Lee BT, Morby AP. 1995. Understanding cellular responses to toxic agents: a model for mechanism-choice in bacterial metal resistance. J Ind Microbiol 14:132–141. doi: 10.1007/BF01569895. [DOI] [PubMed] [Google Scholar]
- 79.Silver S, Phung LT. 1996. Bacterial heavy metal resistance: new surprises. Annu Rev Microbiol 50:753–789. doi: 10.1146/annurev.micro.50.1.753. [DOI] [PubMed] [Google Scholar]
- 80.Joho M, Inouhe M, Tohoyama H, Murayama T. 1995. Nickel resistance mechanisms in yeasts and other fungi. J Ind Microbiol 14:164–168. doi: 10.1007/BF01569899. [DOI] [PubMed] [Google Scholar]
- 81.Teitzel GM, Parsek MR. 2003. Heavy metal resistance of biofilm and planktonic Pseudomonas aeruginosa. Appl Environ Microbiol 69:2313–2320. doi: 10.1128/AEM.69.4.2313-2320.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bruins MR, Kapil S, Oehme FW. 2000. Microbial resistance to metals in the environment. Ecotoxicol Environ Saf 45:198–207. doi: 10.1006/eesa.1999.1860. [DOI] [PubMed] [Google Scholar]
- 83.Elguindi J, Moffitt S, Hasman H, Andrade C, Raghavan S, Rensing C. 2011. Metallic copper corrosion rates, moisture content, and growth medium influence survival of copper ion-resistant bacteria. Appl Microbiol Biotechnol 89:1963–1970. doi: 10.1007/s00253-010-2980-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Andersen GL, Menkissoglou O, Lindow SE. 1991. Occurrence and properties of copper-tolerant strains of Pseudomonas syringae isolated from fruit trees in California. Phytopathology 81:648–656. doi: 10.1094/Phyto-81-648. [DOI] [Google Scholar]
- 85.Singer AC, Shaw H, Rhodes V, Hart A. 2016. Review of antimicrobial resistance in the environment and its relevance to environmental regulators. Front Microbiol 7:1728. doi: 10.3389/fmicb.2016.01728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Weber DJ, Anderson D, Rutala WA. 2013. The role of the surface environment in healthcare-associated infections. Curr Opin Infect Dis 26:338–344. doi: 10.1097/QCO.0b013e3283630f04. [DOI] [PubMed] [Google Scholar]
- 87.Hota B. 2004. Contamination, disinfection, and cross-colonization: are hospital surfaces reservoirs for nosocomial infection? Clin Infect Dis 39:1182–1189. doi: 10.1086/424667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kramer A, Schwebke I, Kampf G. 2006. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect Dis 6:130. doi: 10.1186/1471-2334-6-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Boyce JM. 2007. Environmental contamination makes an important contribution to hospital infection. J Hosp Infect 65(Suppl 2):50–54. doi: 10.1016/S0195-6701(07)60015-2. [DOI] [PubMed] [Google Scholar]
- 90.Weber DJ, Rutala WA. 1997. Role of environmental contamination in the transmission of vancomycin-resistant enterococci. Infect Control Hosp Epidemiol 18:306–309. doi: 10.2307/30141222. [DOI] [PubMed] [Google Scholar]
- 91.Huslage K, Rutala WA, Sickbert-Bennett E, Weber DJ. 2010. A quantitative approach to defining “high-touch” surfaces in hospitals. Infect Control Hosp Epidemiol 31:850–853. doi: 10.1086/655016. [DOI] [PubMed] [Google Scholar]
- 92.Morgan DJ, Rogawski E, Thom KA, Johnson JK, Perencevich EN, Shardell M, Leekha S, Harris AD. 2012. Transfer of multidrug-resistant bacteria to healthcare workers’ gloves and gowns after patient contact increases with environmental contamination. Crit Care Med 40:1045–1051. doi: 10.1097/CCM.0b013e31823bc7c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Blue J, O’Neill C, Speziale P, Revill J, Ramage L, Ballantyne L. 2008. Use of a fluorescent chemical as a quality indicator for a hospital cleaning program. Can J Infect Control 23:216–219. [PubMed] [Google Scholar]
- 94.Carling PC, Parry MF, Bruno-Murtha LA, Dick B. 2010. Improving environmental hygiene in 27 intensive care units to decrease multidrug-resistant bacterial transmission. Crit Care Med 38:1054–1059. doi: 10.1097/CCM.0b013e3181cdf705. [DOI] [PubMed] [Google Scholar]
- 95.Carling PC, Bartley JM. 2010. Evaluating hygienic cleaning in health care settings: what you do not know can harm your patients. Am J Infect Control 38:S41–S50. doi: 10.1016/j.ajic.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 96.Dancer SJ. 2011. Hospital cleaning in the 21st century. Eur J Clin Microbiol Infect Dis 30:1473–1481. doi: 10.1007/s10096-011-1250-x. [DOI] [PubMed] [Google Scholar]
- 97.Weber DJ, Rutala WA. 2013. Self-disinfecting surfaces: review of current methodologies and future prospects. Am J Infect Control 41:S31–S35. doi: 10.1016/j.ajic.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 98.Kuhn PJ. 1983. Doorknobs: a source of nosocomial infection. Diagn Med 6:62–63. [Google Scholar]
- 99.Casey AL, Adams D, Karpanen TJ, Lambert PA, Cookson BD, Nightingale P, Miruszenko L, Shillam R, Christian P, Elliott TSJ. 2010. Role of copper in reducing hospital environment contamination. J Hosp Infect 74:72–77. doi: 10.1016/j.jhin.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 100.Casey AL, Karpanen TJ, Adams D, Lambert PA, Nightingale P, Miruszenko L, Elliott TS. 2011. A comparative study to evaluate surface microbial contamination associated with copper-containing and stainless steel pens used by nurses in the critical care unit. Am J Infect Control 39:e52–e54. doi: 10.1016/j.ajic.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 101.Karpanen TJ, Casey AL, Lambert PA, Cookson BD, Nightingale P, Miruszenko L, Elliott TS. 2012. The antimicrobial efficacy of copper alloy furnishing in the clinical environment: a crossover study. Infect Control Hosp Epidemiol 33:3–9. doi: 10.1086/663644. [DOI] [PubMed] [Google Scholar]
- 102.Mikolay A, Huggett S, Tikana L, Grass G, Braun J, Nies DH. 2010. Survival of bacteria on metallic copper surfaces in a hospital trial. Appl Microbiol Biotechnol 87:1875–1879. doi: 10.1007/s00253-010-2640-1. [DOI] [PubMed] [Google Scholar]
- 103.Rai S, Hirsch BE, Attaway HH, Nadan R, Fairey S, Hardy J, Miller G, Armellino D, Moran WR, Sharpe P, Estelle A, Michel JH, Michels HT, Schmidt MG. 2012. Evaluation of the antimicrobial properties of copper surfaces in an outpatient infectious disease practice. Infect Control Hosp Epidemiol 33:200–201. doi: 10.1086/663701. [DOI] [PubMed] [Google Scholar]
- 104.Schmidt MG, Attaway HH, Sharpe PA, John J, Sepkowitz KA, Morgan A, Fairey SE, Singh S, Steed LL, Cantey JR, Freeman KD, Michels HT, Salgado CD. 2012. Sustained reduction of microbial burden on common hospital surfaces through the introduction of copper. J Clin Microbiol 50:2217–2223. doi: 10.1128/JCM.01032-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Schmidt MG, Attaway HH, Fairey SE, Steed LL, Michels HT, Salgado CD. 2013. Copper continuously limits the concentration of bacteria resident on bed rails within the intensive care unit. Infect Control Hosp Epidemiol 34:530–533. doi: 10.1086/670224. [DOI] [PubMed] [Google Scholar]
- 106.Prado VJ, Vidal RA, Durán CT. 2012. Application of copper bactericidal properties in medical practice. Rev Med Chil 140:1325–1332. doi: 10.4067/S0034-98872012001000014. [DOI] [PubMed] [Google Scholar]
- 107.Schmidt MG, Tuuri RE, Dharsee A, Attaway HH, Fairey SE, Borg KT, Salgado CD, Hirsch BE. 2017. Antimicrobial copper alloys decreased bacteria on stethoscope surfaces. Am J Infect Control 45:642–647. doi: 10.1016/j.ajic.2017.01.030. [DOI] [PubMed] [Google Scholar]
- 108.Zatcoff RC, Smith MS, Borkow G. 2008. Treatment of tinea pedis with socks containing copper-oxide impregnated fibers. Foot (Edinb) 18:136–141. doi: 10.1016/j.foot.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 109.Gargiulo ME, del Carmen Elías A, Borkow G. 2012. Analysis of the effect of wearing copper oxide impregnated socks on tinea pedis based on “before and after” pictures—a statistical follow-up tool. Open Biol J 5:17–22. doi: 10.2174/1874196701205010017. [DOI] [Google Scholar]
- 110.Borkow G, Mellibovsky JC. 2012. Resolution of skin maladies of the trapped Chilean miners: the unplanned underground copper-impregnated antifungal socks “trial.” Arch Dermatol 148:134–136. doi: 10.1001/archdermatol.2011.1280. [DOI] [PubMed] [Google Scholar]
- 111.Borkow G. 2013. Protection of soldiers’ feet by copper oxide impregnated socks. Adv Mil Technol 8:101–108. [Google Scholar]
- 112.Lazary A, Weinberg I, Vatine J-J, Jefidoff A, Bardenstein R, Borkow G, Ohana N. 2014. Reduction of healthcare-associated infections in a long-term care brain injury ward by replacing regular linens with biocidal copper oxide impregnated linens. Int J Infect Dis 24:23–29. doi: 10.1016/j.ijid.2014.01.022. [DOI] [PubMed] [Google Scholar]
- 113.Marcus E-L, Yosef H, Borkow G, Caine Y, Sasson A, Moses AE. 2017. Reduction of health care-associated infection indicators by copper oxide-impregnated textiles: crossover, double-blind controlled study in chronic ventilator-dependent patients. Am J Infect Control 45:401–403. doi: 10.1016/j.ajic.2016.11.022. [DOI] [PubMed] [Google Scholar]
- 114.Richardson M. 2004. Acute wounds: an overview of the physiological healing process. Nurs Times 100:50–53. [PubMed] [Google Scholar]
- 115.Clark RA. 1993. Biology of dermal wound repair. Dermatol Clin 11:647–666. doi: 10.1016/S0733-8635(18)30218-3. [DOI] [PubMed] [Google Scholar]
- 116.Broughton G II, Janis JE, Attinger CE. 2006. The basic science of wound healing. Plast Reconstr Surg 117:12S–34S. doi: 10.1097/01.prs.0000225430.42531.c2. [DOI] [PubMed] [Google Scholar]
- 117.Singer AJ, Clark RA. 1999. Cutaneous wound healing. N Engl J Med 341:738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 118.Friedman A. 2011. Wound healing: from basic science to clinical practice and beyond. J Drugs Dermatol 10:427–433. [PubMed] [Google Scholar]
- 119.Maquart F-X, Pickart L, Laurent M, Gillery P, Monboisse J-C, Borel J-P. 1988. Stimulation of collagen synthesis in fibroblast cultures by the tripeptide‐copper complex glycyl‐L‐histidyl‐L‐lysine‐Cu2+. FEBS Lett 238:343–346. doi: 10.1016/0014-5793(88)80509-X. [DOI] [PubMed] [Google Scholar]
- 120.Soncin F, Guitton J-D, Cartwright T, Badet J. 1997. Interaction of human angiogenin with copper modulates angiogenin binding to endothelial cells. Biochem Biophys Res Commun 236:604–610. doi: 10.1006/bbrc.1997.7018. [DOI] [PubMed] [Google Scholar]
- 121.Hu G. 1998. Copper stimulates proliferation of human endothelial cells under culture. J Cell Biochem 69:326–335. doi:. [DOI] [PubMed] [Google Scholar]
- 122.Tenaud I, Sainte-Marie I, Jumbou O, Litoux P, Dréno B. 1999. In vitro modulation of keratinocyte wound healing integrins by zinc, copper and manganese. Br J Dermatol 140:26–34. doi: 10.1046/j.1365-2133.1999.02603.x. [DOI] [PubMed] [Google Scholar]
- 123.Ross GM, Shamovsky IL, Woo SB, Post JI, Vrkljan PN, Lawrance G, Solc M, Dostaler SM, Neet KE, Riopelle RJ. 2001. The binding of zinc and copper ions to nerve growth factor is differentially affected by pH: implications for cerebral acidosis. J Neurochem 78:515–523. doi: 10.1046/j.1471-4159.2001.00427.x. [DOI] [PubMed] [Google Scholar]
- 124.Siméon A, Monier F, Emonard H, Gillery P, Hornebeck W, Maquart F-X, Birembaut P. 1999. Expression and activation of matrix metalloproteinases in wounds: modulation by the tripeptide-copper complex glycyl-L-histidyl-L-lysine-Cu2+. J Invest Dermatol 112:957–964. doi: 10.1046/j.1523-1747.1999.00606.x. [DOI] [PubMed] [Google Scholar]
- 125.Sen CK, Khanna S, Venojarvi M, Trikha P, Ellison EC, Hunt TK, Roy S. 2002. Copper-induced vascular endothelial growth factor expression and wound healing. Am J Physiol Heart Circ Physiol 282:H1821–H1827. doi: 10.1152/ajpheart.01015.2001. [DOI] [PubMed] [Google Scholar]
- 126.Mandinov L, Mandinova A, Kyurkchiev S, Kyurkchiev D, Kehayov I, Kolev V, Soldi R, Bagala C, de Muinck ED, Lindner V, Post MJ, Simons M, Bellum S, Prudovsky I, Maciag T. 2003. Copper chelation represses the vascular response to injury. Proc Natl Acad Sci U S A 100:6700–6705. doi: 10.1073/pnas.1231994100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Xie H, Kang YJ. 2009. Role of copper in angiogenesis and its medicinal implications. Curr Med Chem 16:1304–1314. doi: 10.2174/092986709787846622. [DOI] [PubMed] [Google Scholar]
- 128.Feng W, Ye F, Xue W, Zhou Z, Kang YJ. 2009. Copper regulation of hypoxia-inducible factor-1 activity. Mol Pharmacol 75:174–182. doi: 10.1124/mol.108.051516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Philips N, Hwang H, Chauhan S, Leonardi D, Gonzalez S. 2010. Stimulation of cell proliferation and expression of matrixmetalloproteinase-1 and interluekin-8 genes in dermal fibroblasts by copper. Connect Tissue Res 51:224–229. doi: 10.3109/03008200903288431. [DOI] [PubMed] [Google Scholar]
- 130.Ashino T, Sudhahar V, Urao N, Oshikawa J, Chen G-F, Wang H, Huo Y, Finney L, Vogt S, McKinney RD, Maryon EB, Kaplan JH, Ushio-Fukai M, Fukai T. 2010. Unexpected role of the copper transporter ATP7A in PDGF-induced vascular smooth muscle cell migration: novelty and significance. Circ Res 107:787–799. doi: 10.1161/CIRCRESAHA.110.225334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tsai C-Y, Finley JC, Ali SS, Patel HH, Howell SB. 2012. Copper influx transporter 1 is required for FGF, PDGF and EGF-induced MAPK signaling. Biochem Pharmacol 84:1007–1013. doi: 10.1016/j.bcp.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.La Mendola D, Farkas D, Bellia F, Magrì A, Travaglia A, Hansson Ö, Rizzarelli E. 2012. Probing the copper(II) binding features of angiogenin. Similarities and differences between a N-terminus peptide fragment and the recombinant human protein. Inorg Chem 51:128–141. doi: 10.1021/ic201300e. [DOI] [PubMed] [Google Scholar]
- 133.Heldin C-H, Westermark B. 1999. Mechanism of action and in vivo role of platelet-derived growth factor. Physiol Rev 79:1283–1316. doi: 10.1152/physrev.1999.79.4.1283. [DOI] [PubMed] [Google Scholar]
- 134.White C, Lee J, Kambe T, Fritsche K, Petris MJ. 2009. A role for the ATP7A copper-transporting ATPase in macrophage bactericidal activity. J Biol Chem 284:33949–33956. doi: 10.1074/jbc.M109.070201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Johnson MDL, Kehl-Fie TE, Klein R, Kelly J, Burnham C, Mann B, Rosch JW. 2015. Role of copper efflux in pneumococcal pathogenesis and resistance to macrophage-mediated immune clearance. Infect Immun 83:1684–1694. doi: 10.1128/IAI.03015-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Raju KS, Alessandri G, Ziche M, Gullino PM. 1982. Ceruloplasmin, copper ions, and angiogenesis. J Natl Cancer Inst 69:1183–1188. [PubMed] [Google Scholar]
- 137.Alessandri G, Raju K, Gullino PM. 1984. Angiogenesis in vivo and selective mobilization of capillary endothelium in vitro by heparin-copper complex. Microcirc Endothelium Lymphatics 1:329–346. [PubMed] [Google Scholar]
- 138.Alessandri G, Raju K, Gullino PM. 1983. Mobilization of capillary endothelium in vitro induced by effectors of angiogenesis in vivo. Cancer Res 43:1790–1797. [PubMed] [Google Scholar]
- 139.Prudovsky I, Mandinova A, Soldi R, Bagala C, Graziani I, Landriscina M, Tarantini F, Duarte M, Bellum S, Doherty H, Maciag T. 2003. The non-classical export routes: FGF1 and IL-1α point the way. J Cell Sci 116:4871–4881. doi: 10.1242/jcs.00872. [DOI] [PubMed] [Google Scholar]
- 140.Ahmed Z, Briden A, Hall S, Brown RA. 2004. Stabilisation of cables of fibronectin with micromolar concentrations of copper: in vitro cell substrate properties. Biomaterials 25:803–812. doi: 10.1016/S0142-9612(03)00596-9. [DOI] [PubMed] [Google Scholar]
- 141.Pollard JD, Quan S, Kang T, Koch RJ. 2005. Effects of copper tripeptide on the growth and expression of growth factors by normal and irradiated fibroblasts. Arch Facial Plast Surg 7:27–31. doi: 10.1001/archfaci.7.1.27. [DOI] [PubMed] [Google Scholar]
- 142.Pickart L, Vasquez-Soltero JM, Margolina A. 2012. The human tripeptide GHK-Cu in prevention of oxidative stress and degenerative conditions of aging: implications for cognitive health. Oxid Med Cell Longev 2012:324832. doi: 10.1155/2012/324832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Olczyk P, Mencner Ł, Komosinska-Vassev K. 2014. The role of the extracellular matrix components in cutaneous wound healing. Biomed Res Int 2014:747584. doi: 10.1155/2014/747584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Borkow G, Gabbay J, Lyakhovitsky A, Huszar M. 2009. Improvement of facial skin characteristics using copper oxide containing pillowcases: a double‐blind, placebo‐controlled, parallel, randomized study. Int J Cosmet Sci 31:437–443. doi: 10.1111/j.1468-2494.2009.00515.x. [DOI] [PubMed] [Google Scholar]
- 145.Baek JH, Yoo MA, Koh JS, Borkow G. 2012. Reduction of facial wrinkles depth by sleeping on copper oxide‐containing pillowcases: a double blind, placebo controlled, parallel, randomized clinical study. J Cosmet Dermatol 11:193–200. doi: 10.1111/j.1473-2165.2012.00624.x. [DOI] [PubMed] [Google Scholar]
- 146.Dykes P. 2015. Increase in skin surface elasticity in normal volunteer subjects following the use of copper oxide impregnated socks. Skin Res Technol 21:272–277. doi: 10.1111/srt.12187. [DOI] [PubMed] [Google Scholar]
- 147.Weinberg I, Lazary A, Jefidoff A, Vatine J-J, Borkow G, Ohana N. 2013. Safety of using diapers containing copper oxide in chronic care elderly patients. Open Biol J 6:1–7. [Google Scholar]
- 148.Arendsen LP, Vig S, Thakar R, Sultan AH. 2019. Impact of copper compression stockings on venous insufficiency and lipodermatosclerosis: a randomised controlled trial. Phlebology 34:224–230. doi: 10.1177/0268355518795329. [DOI] [PubMed] [Google Scholar]
- 149.Agency for Toxic Substances and Disease Registry. 2004. Toxicological profile for copper. Agency for Toxic Substances and Disease Registry, Atlanta, GA. [Google Scholar]
- 150.Strickland GT, Beckner WM, Leu ML. 1972. Absorption of copper in homozygotes and heterozygotes for Wilson’s disease and controls: isotope tracer studies with 67 Cu and 64 Cu. Clin Sci 43:617–625. doi: 10.1042/cs0430617. [DOI] [PubMed] [Google Scholar]
- 151.Strickland GT, Beckner WM, Leu M-L, O’Reilly S. 1972. Turnover studies of copper in homozygotes and heterozygotes for Wilson’s disease and controls: isotope tracer studies with 67 Cu. Clin Sci 43:605–615. doi: 10.1042/cs0430605. [DOI] [PubMed] [Google Scholar]
- 152.Turnlund JR, Keyes WR, Anderson HL, Acord LL. 1989. Copper absorption and retention in young men at three levels of dietary copper by use of the stable isotope 65Cu. Am J Clin Nutr 49:870–878. doi: 10.1093/ajcn/49.5.870. [DOI] [PubMed] [Google Scholar]
- 153.Ehrenkranz RA, Gettner PA, Nelli CM. 1989. Nutrient balance studies in premature infants fed premature formula or fortified preterm human milk. J Pediatr Gastroenterol Nutr 8:58–67. doi: 10.1097/00005176-198901000-00012. [DOI] [PubMed] [Google Scholar]
- 154.Lutsenko S, Kaplan JH. 1995. Organization of P-type ATPases: significance of structural diversity. Biochemistry 34:15607–15613. doi: 10.1021/bi00048a001. [DOI] [PubMed] [Google Scholar]
- 155.Solioz M, Vulpe C. 1996. CPx-type ATPases: a class of P-type ATPases that pump heavy metals. Trends Biochem Sci 21:237–241. doi: 10.1016/S0968-0004(96)20016-7. [DOI] [PubMed] [Google Scholar]
- 156.Marceau N, Aspin N, Sass-Kortsak A. 1970. Absorption of copper 64 from gastrointestinal tract of the rat. Am J Physiol 218:377–383. doi: 10.1152/ajplegacy.1970.218.2.377. [DOI] [PubMed] [Google Scholar]
- 157.Bligh SW, Boyle HA, McEwen AB, Sadler PJ, Woodham RH. 1992. 1H NMR studies of reactions of copper complexes with human blood plasma and urine. Biochem Pharmacol 43:137–145. doi: 10.1016/0006-2952(92)90270-S. [DOI] [PubMed] [Google Scholar]
- 158.Harris ED. 2000. Cellular copper transport and metabolism. Annu Rev Nutr 20:291–310. doi: 10.1146/annurev.nutr.20.1.291. [DOI] [PubMed] [Google Scholar]
- 159.Cousins RJ. 1985. Absorption, transport, and hepatic metabolism of copper and zinc: special reference to metallothionein and ceruloplasmin. Physiol Rev 65:238–309. doi: 10.1152/physrev.1985.65.2.238. [DOI] [PubMed] [Google Scholar]
- 160.Winge DR, Mehra RK. 1990. Host defenses against copper toxicity. Int Rev Exp Pathol 31:47–83. doi: 10.1016/B978-0-12-364931-7.50007-0. [DOI] [PubMed] [Google Scholar]
- 161.Turnlund JR. 1998. Human whole-body copper metabolism. Am J Clin Nutr 67:960S–964S. doi: 10.1093/ajcn/67.5.960S. [DOI] [PubMed] [Google Scholar]
- 162.Djoko KY, Ong CY, Walker MJ, McEwan AG. 2015. The role of copper and zinc toxicity in innate immune defense against bacterial pathogens. J Biol Chem 290:18954–18961. doi: 10.1074/jbc.R115.647099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Klevay LM. 2006. “Myelodysplasia,” myeloneuropathy, and copper deficiency. Mayo Clin Proc 81:132. doi: 10.4065/81.1.132 (Reply, 81:132.) [DOI] [PubMed] [Google Scholar]
- 164.Jaiser SR, Winston GP. 2010. Copper deficiency myelopathy. J Neurol 257:869–881. doi: 10.1007/s00415-010-5511-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kumar N. 2006. Copper deficiency myelopathy (human swayback). Mayo Clin Proc 81:1371–1384. doi: 10.4065/81.10.1371. [DOI] [PubMed] [Google Scholar]
- 166.Klaassen CD, Watkins JB. 1996. Casarett and Doull’s toxicology: the basic science of poisons. McGraw-Hill, New York, NY. [Google Scholar]
- 167.de Romaña DL, Olivares M, Uauy R, Araya M. 2011. Risks and benefits of copper in light of new insights of copper homeostasis. J Trace Elem Med Biol 25:3–13. doi: 10.1016/j.jtemb.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 168.Ala A, Walker AP, Ashkan K, Dooley JS, Schilsky ML. 2007. Wilson’s disease. Lancet 369:397–408. doi: 10.1016/S0140-6736(07)60196-2. [DOI] [PubMed] [Google Scholar]
- 169.Goodwin TM, Montoro MN, Muderspach L, Paulson R, Roy S (ed). 2010. Management of common problems in obstetrics and gynecology, 5th ed Wiley-Blackwell, Chichester, United Kingdom. [Google Scholar]
- 170.Chen YS, Lin YE, Liu Y-C, Huang WK, Shih HY, Wann SR, Lee SS, Tsai HC, Li CH, Chao HL, Ke CM, Lu HH, Chang CL. 2008. Efficacy of point-of-entry copper-silver ionisation system in eradicating Legionella pneumophila in a tropical tertiary care hospital: implications for hospitals contaminated with Legionella in both hot and cold water. J Hosp Infect 68:152–158. doi: 10.1016/j.jhin.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 171.Huang H-I, Shih H-Y, Lee C-M, Yang TC, Lay J-J, Lin YE. 2008. In vitro efficacy of copper and silver ions in eradicating Pseudomonas aeruginosa, Stenotrophomonas maltophilia and Acinetobacter baumannii: implications for on-site disinfection for hospital infection control. Water Res 42:73–80. doi: 10.1016/j.watres.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 172.Mahler DB. 1997. The high-copper dental amalgam alloys. J Dent Res 76:537–541. doi: 10.1177/00220345970760010301. [DOI] [PubMed] [Google Scholar]
- 173.Faúndez G, Troncoso M, Navarrete P, Figueroa G. 2004. Antimicrobial activity of copper surfaces against suspensions of Salmonella enterica and Campylobacter jejuni. BMC Microbiol 4:19. doi: 10.1186/1471-2180-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Cooney TE. 1995. Bactericidal activity of copper and noncopper paints. Infect Control Hosp Epidemiol 16:444–450. doi: 10.2307/30141081. [DOI] [PubMed] [Google Scholar]
- 175.Cooney JJ, Tang RJ. 1999. Quantifying effects of antifouling paints on microbial biofilm formation. Methods Enzymol 310:637–644. doi: 10.1016/S0076-6879(99)10049-1. [DOI] [PubMed] [Google Scholar]
- 176.Schultz TP, Nicholas DD, Preston AF. 2007. A brief review of the past, present and future of wood preservation. Pest Manag Sci 63:784–788. doi: 10.1002/ps.1386. [DOI] [PubMed] [Google Scholar]
- 177.La Torre A, Talocci S, Spera G, Valori R. 2008. Control of downy mildew on grapes in organic viticulture. Commun Agric Appl Biol Sci 73:169–178. [PubMed] [Google Scholar]
- 178.Borkow G. 2012. Safety of using copper oxide in medical devices and consumer products. Curr Chem Biol 6:86–92. doi: 10.2174/187231312799984349. [DOI] [Google Scholar]
- 179.Hostynek JJ, Dreher F, Maibach HI. 2011. Human skin penetration of a copper tripeptide in vitro as a function of skin layer. Inflamm Res 60:79–86. doi: 10.1007/s00011-010-0238-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Krewski D, Yokel RA, Nieboer E, Borchelt D, Cohen J, Harry J, Kacew S, Lindsay J, Mahfouz AM, Rondeau V. 2007. Human health risk assessment for aluminium, aluminium oxide, and aluminium hydroxide. J Toxicol Environ Health B Crit Rev 10(Suppl 1):1–269. doi: 10.1080/10937400701597766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Denkhaus E, Salnikow K. 2002. Nickel essentiality, toxicity, and carcinogenicity. Crit Rev Oncol Hematol 42:35–56. doi: 10.1016/S1040-8428(01)00214-1. [DOI] [PubMed] [Google Scholar]
- 182.Edwards-Jones V. 2009. The benefits of silver in hygiene, personal care and healthcare. Lett Appl Microbiol 49:147–152. doi: 10.1111/j.1472-765X.2009.02648.x. [DOI] [PubMed] [Google Scholar]
- 183.Prabhu S, Poulose EK. 2012. Silver nanoparticles: mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int Nano Lett 2:32. doi: 10.1186/2228-5326-2-32. [DOI] [Google Scholar]
- 184.Wyklicky H, Skopec M. 1983. Ignaz Philipp Semmelweis, the prophet of bacteriology. Infect Control 4:367–370. doi: 10.1017/S0195941700059762. [DOI] [PubMed] [Google Scholar]
- 185.Plowman R, Graves N, Griffin MAS, Roberts JA, Swan AV, Cookson B, Taylor L. 2001. The rate and cost of hospital-acquired infections occurring in patients admitted to selected specialties of a district general hospital in England and the national burden imposed. J Hosp Infect 47:198–209. doi: 10.1053/jhin.2000.0881. [DOI] [PubMed] [Google Scholar]
- 186.Johnson A, Thakar R, Sultan AH. 2012. Obstetric perineal wound infection: is there underreporting? Br J Nurs 21:S28–S35. doi: 10.12968/bjon.2012.21.Sup3.S28. [DOI] [PubMed] [Google Scholar]
- 187.Thomas J, Callwood A, Brocklehurst P, Walker J. 2000. The national sentinel caesarean section audit. BJOG 107:579–580. doi: 10.1111/j.1471-0528.2000.tb13296.x. [DOI] [PubMed] [Google Scholar]
- 188.Betrán AP, Ye J, Moller A-B, Zhang J, Gülmezoglu AM, Torloni MR. 2016. The increasing trend in caesarean section rates: global, regional and national estimates: 1990-2014. PLoS One 11:e0148343. doi: 10.1371/journal.pone.0148343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Boerma T, Ronsmans C, Melesse DY, Barros AJD, Barros FC, Juan L, Moller A-B, Say L, Hosseinpoor AR, Yi M, de Lyra Rabello Neto D, Temmerman M. 2018. Global epidemiology of use of and disparities in caesarean sections. Lancet 392:1341–1348. doi: 10.1016/S0140-6736(18)31928-7. [DOI] [PubMed] [Google Scholar]
- 190.Kirchengast S, Hartmann B. 2018. Recent lifestyle parameters are associated with increasing caesarean section rates among singleton term births in Austria. Int J Environ Res Public Health 16:E14. doi: 10.3390/ijerph16010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ward VP, Charlett A, Fagan J, Crawshaw SC. 2008. Enhanced surgical site infection surveillance following caesarean section: experience of a multicentre collaborative post-discharge system. J Hosp Infect 70:166–173. doi: 10.1016/j.jhin.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 192.Wloch C, Wilson J, Lamagni T, Harrington P, Charlett A, Sheridan E. 2012. Risk factors for surgical site infection following caesarean section in England: results from a multicentre cohort study. BJOG 119:1324–1333. doi: 10.1111/j.1471-0528.2012.03452.x. [DOI] [PubMed] [Google Scholar]
- 193.Wilson J, Wloch C, Saei A, McDougall C, Harrington P, Charlett A, Lamagni T, Elgohari S, Sheridan E. 2013. Inter-hospital comparison of rates of surgical site infection following caesarean section delivery: evaluation of a multicentre surveillance study. J Hosp Infect 84:44–51. doi: 10.1016/j.jhin.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 194.Carter EB, Temming LA, Fowler S, Eppes C, Gross G, Srinivas SK, Macones GA, Colditz GA, Tuuli MG. 2017. Evidence-based bundles and cesarean delivery surgical site infections: a systematic review and meta-analysis. Obstet Gynecol 130:735–746. doi: 10.1097/AOG.0000000000002249. [DOI] [PubMed] [Google Scholar]
- 195.Ahmadzia HK, Patel EM, Joshi D, Liao C, Witter F, Heine RP, Coleman JS. 2015. Obstetric surgical site infections: 2 grams compared with 3 grams of cefazolin in morbidly obese women. Obstet Gynecol 126:708–715. doi: 10.1097/AOG.0000000000001064. [DOI] [PubMed] [Google Scholar]
- 196.McKibben RA, Pitts SI, Suarez-Cuervo C, Perl TM, Bass EB. 2015. Practices to reduce surgical site infections among women undergoing cesarean section: a review. Infect Control Hosp Epidemiol 36:915–921. doi: 10.1017/ice.2015.116. [DOI] [PubMed] [Google Scholar]
- 197.Brown J, Thompson M, Sinnya S, Jeffery A, de Costa C, Woods C, Howat P, Raulli A. 2013. Pre-incision antibiotic prophylaxis reduces the incidence of post-caesarean surgical site infection. J Hosp Infect 83:68–70. doi: 10.1016/j.jhin.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 198.Stumpf PG, Flores M, Murillo J. 2008. Serious postpartum infection due to MRSA in an asymptomatic carrier: case report and review. Am J Perinatol 25:413–415. doi: 10.1055/s-2008-1075032. [DOI] [PubMed] [Google Scholar]
- 199.Thurman AR, Anca Y, White CA, Soper DE. 2010. Post-cesarean delivery infectious morbidity: focus on preoperative antibiotics and methicillin-resistant Staphylococcus aureus. Am J Infect Control 38:612–616. doi: 10.1016/j.ajic.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 200.American College of Obstetricians and Gynecologists. 2011. ACOG practice bulletin no. 120: use of prophylactic antibiotics in labor and delivery. Obstet Gynecol 117:1472–1483. doi: 10.1097/AOG.0b013e3182238c31. [DOI] [PubMed] [Google Scholar]
- 201.Creech CB, Litzner B, Talbot TR, Schaffner W. 2010. Frequency of detection of methicillin-resistant Staphylococcus aureus from rectovaginal swabs in pregnant women. Am J Infect Control 38:72–74. doi: 10.1016/j.ajic.2009.06.015. [DOI] [PubMed] [Google Scholar]
- 202.Beigi R, Hanrahan J. 2007. Staphylococcus aureus and MRSA colonization rates among gravidas admitted to labor and delivery: a pilot study. Infect Dis Obstet Gynecol 2007:70876. doi: 10.1155/2007/70876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Arendsen L, Thakar R, Sultan A. 2019. The impact of copper impregnated wound dressings on surgical site infection following caesarean section: a double blind randomised controlled study. Eur J Obstet Gynecol Reprod Biol 234:e171. doi: 10.1016/j.ejogrb.2018.08.534. [DOI] [PubMed] [Google Scholar]
- 204.Public Health England. 2013. Surgical site infection surveillance service: protocol and guides. Public Health England, London, United Kingdom. [Google Scholar]
- 205.Horan TC, Andrus M, Dudeck MA. 2008. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control 36:309–332. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 206.McCandlish R, Bowler U, van Asten H, Berridge G, Winter C, Sames L, Garcia J, Renfrew M, Elbourne D. 1998. A randomised controlled trial of care of the perineum during second stage of normal labour. Br J Obstet Gynecol 105:1262–1272. doi: 10.1111/j.1471-0528.1998.tb10004.x. [DOI] [PubMed] [Google Scholar]
- 207.American College of Obstetrics and Gynecology. 2001. Operative vaginal delivery. Clinical management guidelines for obstetrician-gynecologists. American College of Obstetrics and Gynecology. Int J Gynaecol Obstet 74:69–76. doi: 10.1016/S0020-7292(01)00434-9. [DOI] [PubMed] [Google Scholar]
- 208.Khan KS, Wojdyla D, Say L, Gülmezoglu AM, Van Look PF. 2006. WHO analysis of causes of maternal death: a systematic review. Lancet 367:1066–1074. doi: 10.1016/S0140-6736(06)68397-9. [DOI] [PubMed] [Google Scholar]
- 209.Arendsen L, Thakar R, Sultan A. 2019. Can perineal wound infection following vagina delivery be reduced? A double blind randomised controlled trial using copper impregnated maternity sanitary towels. Eur J Obstet Gynecol Reprod Biol 234:e180. doi: 10.1016/j.ejogrb.2018.08.554. [DOI] [Google Scholar]
- 210.Witkin SS, Linhares IM, Giraldo P. 2007. Bacterial flora of the female genital tract: function and immune regulation. Best Pract Res Clin Obstet Gynaecol 21:347–354. doi: 10.1016/j.bpobgyn.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 211.Skarin A, Sylwan J. 1986. Vaginal lactobacilli inhibiting growth of Gardnerella vaginalis, Mobiluncus and other bacterial species cultured from vaginal content of women with bacterial vaginosis. Acta Pathol Microbiol Immunol Scand B 94:399–403. [DOI] [PubMed] [Google Scholar]
- 212.Strus M, Malinowska M, Heczko PB. 2002. In vitro antagonistic effect of Lactobacillus on organisms associated with bacterial vaginosis. J Reprod Med 47:41–46. [PubMed] [Google Scholar]
- 213.Matu MN, Orinda GO, Njagi ENM, Cohen CR, Bukusi EA. 2010. In vitro inhibitory activity of human vaginal lactobacilli against pathogenic bacteria associated with bacterial vaginosis in Kenyan women. Anaerobe 16:210–215. doi: 10.1016/j.anaerobe.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 214.Graver MA, Wade JJ. 2011. The role of acidification in the inhibition of Neisseria gonorrhoeae by vaginal lactobacilli during anaerobic growth. Ann Clin Microbiol Antimicrob 10:8. doi: 10.1186/1476-0711-10-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Tachedjian G, Aldunate M, Bradshaw CS, Cone RA. 2017. The role of lactic acid production by probiotic Lactobacillus species in vaginal health. Res Microbiol 168:782–792. doi: 10.1016/j.resmic.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 216.Wang ZK, Yang YS, Stefka AT, Sun G, Peng LH. 2014. Review article: fungal microbiota and digestive diseases. Aliment Pharmacol Ther 39:751–766. doi: 10.1111/apt.12665. [DOI] [PubMed] [Google Scholar]
- 217.Erdogan A, Rao SSC. 2015. Small intestinal fungal overgrowth. Curr Gastroenterol Rep 17:16. doi: 10.1007/s11894-015-0436-2. [DOI] [PubMed] [Google Scholar]
- 218.Gunther MR, Hanna PM, Mason RP, Cohen MS. 1995. Hydroxyl radical formation from cuprous ion and hydrogen peroxide: a spin-trapping study. Arch Biochem Biophys 316:515–522. doi: 10.1006/abbi.1995.1068. [DOI] [PubMed] [Google Scholar]
- 219.Waites KB, Katz B, Schelonka RL. 2005. Mycoplasmas and ureaplasmas as neonatal pathogens. Clin Microbiol Rev 18:757–789. doi: 10.1128/CMR.18.4.757-789.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Workowski KA, Bolan GA. 2015. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recommend Rep 64:1–137. [PMC free article] [PubMed] [Google Scholar]
- 221.Grimes DA. 2000. Intrauterine device and upper-genital-tract infection. Lancet 356:1013–1019. doi: 10.1016/S0140-6736(00)02699-4. [DOI] [PubMed] [Google Scholar]
- 222.Hubacher D, Lara-Ricalde R, Taylor DJ, Guerra-Infante F, Guzmán-Rodríguez R. 2001. Use of copper intrauterine devices and the risk of tubal infertility among nulligravid women. N Engl J Med 345:561–567. doi: 10.1056/NEJMoa010438. [DOI] [PubMed] [Google Scholar]
- 223.Mohllajee AP, Curtis KM, Peterson HB. 2006. Does insertion and use of an intrauterine device increase the risk of pelvic inflammatory disease among women with sexually transmitted infection? A systematic review. Contraception 73:145–153. doi: 10.1016/j.contraception.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 224.Jatlaoui TC, Riley HEM, Curtis KM. 2017. The safety of intrauterine devices among young women: a systematic review. Contraception 95:17–39. doi: 10.1016/j.contraception.2016.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Lohr PA, Lyus R, Prager S. 2017. Use of intrauterine devices in nulliparous women. Contraception 95:529–537. doi: 10.1016/j.contraception.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 226.Usinger KM, Gola SB, Weis M, Smaldone A. 2016. Intrauterine contraception continuation in adolescents and young women: a systematic review. J Pediatr Adolesc Gynecol 29:659–667. doi: 10.1016/j.jpag.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 227.Mehanna MT, Rizk MA, Ramadan M, Schachter J. 1994. Chlamydial serologic characteristics among intrauterine contraceptive device users: does copper inhibit chlamydial infection in the female genital tract? Am J Obstet Gynecol 171:691–693. doi: 10.1016/0002-9378(94)90083-3. [DOI] [PubMed] [Google Scholar]
- 228.Deligeoroglou E, Giannouli A, Athanasopoulos N, Karountzos V, Vatopoulou A, Dimopoulos K, Creatsas G. 2013. HPV infection: immunological aspects and their utility in future therapy. Infect Dis Obstet Gynecol 2013:540850. doi: 10.1155/2013/540850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Lekovich JP, Amrane S, Pangasa M, Pereira N, Frey MK, Varrey A, Holcomb K. 2015. Comparison of human papillomavirus infection and cervical cytology in women using copper-containing and levonorgestrel-containing intrauterine devices. Obstet Gynecol 125:1101–1105. doi: 10.1097/AOG.0000000000000760. [DOI] [PubMed] [Google Scholar]
- 230.Schmeink CE, Massuger LFAG, Lenselink CH, Quint WGV, Witte BI, Berkhof J, Melchers WJG, Bekkers RLM. 2013. Prospective follow-up of 2,065 young unscreened women to study human papillomavirus incidence and clearance: HPV incidence and clearance in young women. Int J Cancer 133:172–181. doi: 10.1002/ijc.27986. [DOI] [PubMed] [Google Scholar]
- 231.Averbach S, Silverberg MJ, Leyden W, Smith-McCune K, Raine-Bennett T, Sawaya GF. 2018. Recent intrauterine device use and the risk of precancerous cervical lesions and cervical cancer. Contraception 98:130–134. doi: 10.1016/j.contraception.2018.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Haley RW, Hooton TM, Culver DH, Stanley RC, Emori TG, Hardison CD, Quade D, Shachtman RH, Schaberg DR, Shah BV, Schatz GD. 1981. Nosocomial infections in U.S. hospitals, 1975-1976: estimated frequency by selected characteristics of patients. Am J Med 70:947–959. doi: 10.1016/0002-9343(81)90561-1. [DOI] [PubMed] [Google Scholar]
- 233.Lo J, Lange D, Chew BH. 2014. Ureteral stents and Foley catheters-associated urinary tract infections: the role of coatings and materials in infection prevention. Antibiotics (Basel) 3:87–97. doi: 10.3390/antibiotics3010087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Shaw C, Gupta RD, Bushnell DM, Assassa RP, Abrams P, Wagg A, Mayne C, Hardwick C, Martin M. 2006. The extent and severity of urinary incontinence amongst women in UK GP waiting rooms. Fam Pract 23:497–506. doi: 10.1093/fampra/cml033. [DOI] [PubMed] [Google Scholar]
- 235.Lawrence EL, Turner IG. 2005. Materials for urinary catheters: a review of their history and development in the UK. Med Eng Phys 27:443–453. doi: 10.1016/j.medengphy.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 236.Singha P, Locklin J, Handa H. 2017. A review of the recent advances in antimicrobial coatings for urinary catheters. Acta Biomater 50:20–40. doi: 10.1016/j.actbio.2016.11.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Heidari Zare H, Juhart V, Vass A, Franz G, Jocham D. 2017. Efficacy of silver/hydrophilic poly(p-xylylene) on preventing bacterial growth and biofilm formation in urinary catheters. Biointerphases 12:011001. doi: 10.1116/1.4974197. [DOI] [PubMed] [Google Scholar]
- 238.Kart D, Kustimur AS, Sağıroğlu M, Kalkancı A. 2017. Evaluation of antimicrobial durability and anti-biofilm effects in urinary catheters against Enterococcus faecalis clinical isolates and reference strains. Balkan Med J 34:546–552. doi: 10.4274/balkanmedj.2016.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Ogilvie AT, Brisson BA, Singh A, Weese JS. 2015. In vitro evaluation of the impact of silver coating on Escherichia coli adherence to urinary catheters. Can Vet J 56:490–494. [PMC free article] [PubMed] [Google Scholar]
- 240.Johnson JR, Johnston B, Kuskowski MA. 2012. In vitro comparison of nitrofurazone- and silver alloy-coated Foley catheters for contact-dependent and diffusible inhibition of urinary tract infection-associated microorganisms. Antimicrob Agents Chemother 56:4969–4972. doi: 10.1128/AAC.00733-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Samuel U, Guggenbichler JP. 2004. Prevention of catheter-related infections: the potential of a new nano-silver impregnated catheter. Int J Antimicrob Agents 23:75–78. doi: 10.1016/j.ijantimicag.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 242.Roe D, Karandikar B, Bonn-Savage N, Gibbins B, Roullet J-B. 2008. Antimicrobial surface functionalization of plastic catheters by silver nanoparticles. J Antimicrob Chemother 61:869–876. doi: 10.1093/jac/dkn034. [DOI] [PubMed] [Google Scholar]
- 243.Thokala N, Kealey DC, Kennedy DJ, Brady DDB, Farrell DJ. 2018. Comparative activity of silver-based antimicrobial composites for urinary catheters. Int J Antimicrob Agents 52:166–171. doi: 10.1016/j.ijantimicag.2018.03.015. [DOI] [PubMed] [Google Scholar]
- 244.Riley DK, Classen DC, Stevens LE, Burke JP. 1995. A large randomized clinical trial of a silver-impregnated urinary catheter: lack of efficacy and staphylococcal superinfection. Am J Med 98:349–356. doi: 10.1016/S0002-9343(99)80313-1. [DOI] [PubMed] [Google Scholar]
- 245.Pickard R, Lam T, MacLennan G, Starr K, Kilonzo M, McPherson G, Gillies K, McDonald A, Walton K, Buckley B, Glazener C, Boachie C, Burr J, Norrie J, Vale L, Grant A, N’Dow J. 2012. Antimicrobial catheters for reduction of symptomatic urinary tract infection in adults requiring short-term catheterisation in hospital: a multicentre randomised controlled trial. Lancet 380:1927–1935. doi: 10.1016/S0140-6736(12)61380-4. [DOI] [PubMed] [Google Scholar]
- 246.Desai DG, Liao KS, Cevallos ME, Trautner BW. 2010. Silver or nitrofurazone impregnation of urinary catheters has a minimal effect on uropathogen adherence. J Urol 184:2565–2571. doi: 10.1016/j.juro.2010.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Karchmer TB, Giannetta ET, Muto CA, Strain BA, Farr BM. 2000. A randomized crossover study of silver-coated urinary catheters in hospitalized patients. Arch Intern Med 160:3294–3298. doi: 10.1001/archinte.160.21.3294. [DOI] [PubMed] [Google Scholar]
- 248.Lai KK, Fontecchio SA. 2002. Use of silver-hydrogel urinary catheters on the incidence of catheter-associated urinary tract infections in hospitalized patients. Am J Infect Control 30:221–225. doi: 10.1067/mic.2002.120128. [DOI] [PubMed] [Google Scholar]
- 249.Singh R, Hokenstad ED, Wiest SR, Kim-Fine S, Weaver AL, McGree ME, Klingele CJ, Trabuco EC, Gebhart JB. 2019. Randomized controlled trial of silver-alloy-impregnated suprapubic catheters versus standard suprapubic catheters in assessing urinary tract infection rates in urogynecology patients. Int Urogynecol J 30:779–787. doi: 10.1007/s00192-018-3726-z. [DOI] [PubMed] [Google Scholar]
- 250.Gosau M, Prantl L, Feldmann M, Kokott A, Hahnel S, Burgers R. 2010. The effects of copper additives on the quantity and cell viability of adherent Staphylococcus epidermidis in silicone implants. Biofouling 26:359–365. doi: 10.1080/08927011003629300. [DOI] [PubMed] [Google Scholar]
- 251.Gosau M, Bürgers R, Vollkommer T, Holzmann T, Prantl L. 2013. Effectiveness of antibacterial copper additives in silicone implants. J Biomater Appl 28:187–198. doi: 10.1177/0885328212441957. [DOI] [PubMed] [Google Scholar]
- 252.Loeb MB, Craven S, McGeer AJ, Simor AE, Bradley SF, Low DE, Armstrong-Evans M, Moss LA, Walter SD. 2003. Risk factors for resistance to antimicrobial agents among nursing home residents. Am J Epidemiol 157:40–47. doi: 10.1093/aje/kwf173. [DOI] [PubMed] [Google Scholar]
- 253.Thompson BL, Dwyer DM, Ussery XT, Denman S, Vacek P, Schwartz B. 1997. Handwashing and glove use in a long-term-care facility. Infect Control Hosp Epidemiol 18:97–103. doi: 10.2307/30142397. [DOI] [PubMed] [Google Scholar]
- 254.Aggazzotti G, Pesce F, Grassi D, Fantuzzi G, Righi E, De Vita D, Santacroce S, Artibani W. 2000. Prevalence of urinary incontinence among institutionalized patients: a cross-sectional epidemiologic study in a midsized city in northern Italy. Urology 56:245–249. doi: 10.1016/S0090-4295(00)00643-9. [DOI] [PubMed] [Google Scholar]
- 255.Omli R, Skotnes LH, Romild U, Bakke A, Mykletun A, Kuhry E. 2010. Pad per day usage, urinary incontinence and urinary tract infections in nursing home residents. Age Ageing 39:549–554. doi: 10.1093/ageing/afq082. [DOI] [PubMed] [Google Scholar]
- 256.Podunavac-Kuzmanovic S, Cvetkovic D. 2010. Antimicrobial investigations of copper(II) complexes with some 1-benzylbenzimidazole derivatives. Rev Roum Chim 55:363–367. [Google Scholar]
- 257.Cortés P, Atria AM, Contreras M, Garland MT, Peña O, Corsini G. 2006. Magnetic properties and antibacterial activity of tetranuclear copper complexes bridged by oxo group. J Chil Chem Soc 51:957–960. [Google Scholar]
- 258.Arslan H, Duran N, Borekci G, Koray Ozer C, Akbay C. 2009. Antimicrobial activity of some thiourea derivatives and their nickel and copper complexes. Molecules 14:519–527. doi: 10.3390/molecules14010519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Xie LJ, Chu W, Sun JH, Wu P, Tong DG. 2011. Synthesis of copper oxide vegetable sponges and their antibacterial, electrochemical and photocatalytic performance. J Mater Sci 46:2179–2184. doi: 10.1007/s10853-010-5055-6. [DOI] [Google Scholar]
- 260.Ramyadevi J, Jeyasubramanian K, Marikani A, Rajakumar G, Rahuman AA. 2012. Synthesis and antimicrobial activity of copper nanoparticles. Mater Lett 71:114–116. doi: 10.1016/j.matlet.2011.12.055. [DOI] [Google Scholar]
- 261.Schmidt MG, Attaway HH, Terzieva S, Marshall A, Steed LL, Salzberg D, Hamoodi HA, Khan JA, Feigley CE, Michels HT. 2012. Characterization and control of the microbial community affiliated with copper or aluminum heat exchangers of HVAC systems. Curr Microbiol 65:141–149. doi: 10.1007/s00284-012-0137-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Revanasiddappa HD, Vijaya B, Kumar S, Prasad S. 2010. Synthesis, characterization and antimicrobial activity of Cu(II), Co(II), Ni(II) and Mn(II) complexes with desipramine. World J Chem 5:18–25. [Google Scholar]
- 263.Sun L, Zhang C, Li P. 2011. Characterization, antimicrobial activity, and mechanism of a high-performance (−)-epigallocatechin-3-gallate (EGCG)-CuII/polyvinyl alcohol (PVA) nanofibrous membrane. J Agric Food Chem 59:5087–5092. doi: 10.1021/jf200580t. [DOI] [PubMed] [Google Scholar]
- 264.Suksrichavalit T, Prachayasittikul S, Nantasenamat C, Isarankura-Na-Ayudhya C, Prachayasittikul V. 2009. Copper complexes of pyridine derivatives with superoxide scavenging and antimicrobial activities. Eur J Med Chem 44:3259–3265. doi: 10.1016/j.ejmech.2009.03.033. [DOI] [PubMed] [Google Scholar]
- 265.Cross JB, Currier RP, Torraco DJ, Vanderberg LA, Wagner GL, Gladen PD. 2003. Killing of Bacillus spores by aqueous dissolved oxygen, ascorbic acid, and copper ions. Appl Environ Microbiol 69:2245–2252. doi: 10.1128/AEM.69.4.2245-2252.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Ruparelia JP, Chatterjee AK, Duttagupta SP, Mukherji S. 2008. Strain specificity in antimicrobial activity of silver and copper nanoparticles. Acta Biomater 4:707–716. doi: 10.1016/j.actbio.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 267.Patel MN, Dosi PA, Bhatt BS, Thakkar VR. 2011. Synthesis, characterization, antibacterial activity, SOD mimic and interaction with DNA of drug based copper(II) complexes. Spectrochim Acta A Mol Biomol Spectrosc 78:763–770. doi: 10.1016/j.saa.2010.11.056. [DOI] [PubMed] [Google Scholar]
- 268.Chohan ZH, Iqbal MS, Aftab SK, Rauf A. 2012. Antibacterial dimeric copper(II) complexes with chromone-derived compounds. J Enzyme Inhib Med Chem 27:223–231. doi: 10.3109/14756366.2011.585135. [DOI] [PubMed] [Google Scholar]
- 269.Chandra S, Raizada S, Tyagi M, Sharma PK. 2008. Spectroscopic and biological approach of Ni(II) and Cu(II) complexes of 2-pyridinecarboxaldehyde thiosemicarbazone. Spectrochim Acta A Mol Biomol Spectrosc 69:816–821. doi: 10.1016/j.saa.2007.05.033. [DOI] [PubMed] [Google Scholar]
- 270.Santo CE, Morais PV, Grass G. 2010. Isolation and characterization of bacteria resistant to metallic copper surfaces. Appl Environ Microbiol 76:1341–1348. doi: 10.1128/AEM.01952-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Gant VA, Wren MWD, Rollins MSM, Jeanes A, Hickok SS, Hall TJ. 2007. Three novel highly charged copper-based biocides: safety and efficacy against healthcare-associated organisms. J Antimicrob Chemother 60:294–299. doi: 10.1093/jac/dkm201. [DOI] [PubMed] [Google Scholar]
- 272.Wheeldon LJ, Worthington T, Lambert PA, Hilton AC, Lowden CJ, Elliott TSJ. 2008. Antimicrobial efficacy of copper surfaces against spores and vegetative cells of Clostridium difficile: the germination theory. J Antimicrob Chemother 62:522–525. doi: 10.1093/jac/dkn219. [DOI] [PubMed] [Google Scholar]
- 273.Mato Rodriguez L, Alatossava T. 2010. Effects of copper on germination, growth and sporulation of Clostridium tyrobutyricum. Food Microbiol 27:434–437. doi: 10.1016/j.fm.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 274.Colak A, Terzi Ü, Col M, Karaoglu ŞA, Karaböcek S, Küçükdumlu A, Ayaz FA. 2010. DNA binding, antioxidant and antimicrobial activities of homo- and heteronuclear copper(II) and nickel(II) complexes with new oxime-type ligands. Eur J Med Chem 45:5169–5175. doi: 10.1016/j.ejmech.2010.08.031. [DOI] [PubMed] [Google Scholar]
- 275.Irene G, Georgios P, Ioannis C, Anastasios T, Diamantis P, Marianthi C, Philippe W, Maria S. 2016. Copper-coated textiles: armor against MDR nosocomial pathogens. Diagn Microbiol Infect Dis 85:205–209. doi: 10.1016/j.diagmicrobio.2016.02.015. [DOI] [PubMed] [Google Scholar]
- 276.Cervantes HI, Alvarez JA, Muñoz JM, Arreguín V, Mosqueda JL, Macías AE. 2013. Antimicrobial activity of copper against organisms in aqueous solution: a case for copper-based water pipelines in hospitals? Am J Infect Control 41:e115–e118. doi: 10.1016/j.ajic.2013.03.309. [DOI] [PubMed] [Google Scholar]
- 277.Robine E, Boulangé-Petermann L, Derangère D. 2002. Assessing bactericidal properties of materials: the case of metallic surfaces in contact with air. J Microbiol Methods 49:225–234. doi: 10.1016/S0167-7012(01)00371-2. [DOI] [PubMed] [Google Scholar]
- 278.Nie Y, Kalapos C, Nie X, Murphy M, Hussein R, Zhang J. 2010. Superhydrophilicity and antibacterial property of a Cu-dotted oxide coating surface. Ann Clin Microbiol Antimicrob 9:25. doi: 10.1186/1476-0711-9-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Molteni C, Abicht HK, Solioz M. 2010. Killing of bacteria by copper surfaces involves dissolved copper. Appl Environ Microbiol 76:4099–4101. doi: 10.1128/AEM.00424-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Mathews S, Hans M, Mücklich F, Solioz M. 2013. Contact killing of bacteria on copper is suppressed if bacterial-metal contact is prevented and is induced on iron by copper ions. Appl Environ Microbiol 79:2605–2611. doi: 10.1128/AEM.03608-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Russell SM. 2008. The effect of an acidic, copper sulfate-based commercial sanitizer on indicator, pathogenic, and spoilage bacteria associated with broiler chicken carcasses when applied at various intervention points during poultry processing. Poult Sci 87:1435–1440. doi: 10.3382/ps.2007-00339. [DOI] [PubMed] [Google Scholar]
- 282.López-Carballo G, Hernández-Muñoz P, Gavara R, Ocio MJ. 2008. Photoactivated chlorophyllin-based gelatin films and coatings to prevent microbial contamination of food products. Int J Food Microbiol 126:65–70. doi: 10.1016/j.ijfoodmicro.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 283.Carson KC, Bartlett JG, Tan T-J, Riley TV. 2007. In vitro susceptibility of methicillin-resistant Staphylococcus aureus and methicillin-susceptible Staphylococcus aureus to a new antimicrobial, copper silicate. Antimicrob Agents Chemother 51:4505–4507. doi: 10.1128/AAC.00771-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Raman N, Sobha S. 2010. Synthesis, characterization, DNA interaction and antimicrobial screening of isatin-based polypyridyl mixed ligand Cu(II) and Zn(II) complexes. J Serbian Chem Soc 75:773–788. doi: 10.2298/JSC091020054R. [DOI] [Google Scholar]
- 285.Hall TJ, Wren MWD, Jeanes A, Gant VA. 2009. A comparison of the antibacterial efficacy and cytotoxicity to cultured human skin cells of 7 commercial hand rubs and Xgel, a new copper-based biocidal hand rub. Am J Infect Control 37:322–326. doi: 10.1016/j.ajic.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 286.Monk AB, Kanmukhla V, Trinder K, Borkow G. 2014. Potent bactericidal efficacy of copper oxide impregnated non-porous solid surfaces. BMC Microbiol 14:57. doi: 10.1186/1471-2180-14-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Steindl G, Heuberger S, Springer B. 2012. Antimicrobial effect of copper on multidrug-resistant bacteria. Wien Tierarztl Monatsschr 99:38–43. [Google Scholar]
- 288.Ren G, Hu D, Cheng EWC, Vargas-Reus MA, Reip P, Allaker RP. 2009. Characterisation of copper oxide nanoparticles for antimicrobial applications. Int J Antimicrob Agents 33:587–590. doi: 10.1016/j.ijantimicag.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 289.Yates HM, Brook LA, Ditta IB, Evans P, Foster HA, Sheel DW, Steele A. 2008. Photo-induced self-cleaning and biocidal behaviour of titania and copper oxide multilayers. J Photochem Photobiol Chem 197:197–205. doi: 10.1016/j.jphotochem.2007.12.023. [DOI] [Google Scholar]
- 290.Teli MD, Sheikh J. 2013. Modified bamboo rayon-copper nanoparticle composites as antibacterial textiles. Int J Biol Macromol 61:302–307. doi: 10.1016/j.ijbiomac.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 291.Luna VA, Hall TJ, King DS, Cannons AC. 2010. Susceptibility of 169 USA300 methicillin-resistant Staphylococcus aureus isolates to two copper-based biocides, CuAL42 and CuWB50. J Antimicrob Chemother 65:939–941. doi: 10.1093/jac/dkq092. [DOI] [PubMed] [Google Scholar]
- 292.Neel EAA, Ahmed I, Pratten J, Nazhat SN, Knowles JC. 2005. Characterisation of antibacterial copper releasing degradable phosphate glass fibres. Biomaterials 26:2247–2254. doi: 10.1016/j.biomaterials.2004.07.024. [DOI] [PubMed] [Google Scholar]
- 293.Rio L, Kusiak-Nejman E, Kiwi J, Bétrisey B, Pulgarin C, Trampuz A, Bizzini A. 2012. Comparison of methods for evaluation of the bactericidal activity of copper-sputtered surfaces against methicillin-resistant Staphylococcus aureus. Appl Environ Microbiol 78:8176–8182. doi: 10.1128/AEM.02266-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Santo CE, Quaranta D, Grass G. 2012. Antimicrobial metallic copper surfaces kill Staphylococcus haemolyticus via membrane damage. Microbiologyopen 1:46–52. doi: 10.1002/mbo3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Emam HE, Manian AP, Široká B, Duelli H, Merschak P, Redl B, Bechtold T. 2014. Copper(I)oxide surface modified cellulose fibers—synthesis, characterization and antimicrobial properties. Surf Coat Technol 254:344–351. doi: 10.1016/j.surfcoat.2014.06.036. [DOI] [Google Scholar]
- 296.Huang L, Fozo EM, Zhang T, Liaw PK, He W. 2014. Antimicrobial behavior of Cu-bearing Zr-based bulk metallic glasses. Mater Sci Eng C Mater Biol Appl 39:325–329. doi: 10.1016/j.msec.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 297.Klinkajon W, Supaphol P. 2014. Novel copper(II) alginate hydrogels and their potential for use as anti-bacterial wound dressings. Biomed Mater 9:045008. doi: 10.1088/1748-6041/9/4/045008. [DOI] [PubMed] [Google Scholar]
- 298.Warnes SL, Keevil CW. 2016. Lack of involvement of Fenton chemistry in death of methicillin-resistant and methicillin-sensitive strains of Staphylococcus aureus and destruction of their genomes on wet or dry copper alloy surfaces. Appl Environ Microbiol 82:2132–2136. doi: 10.1128/AEM.03861-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Mulligan AM, Wilson M, Knowles JC. 2003. The effect of increasing copper content in phosphate-based glasses on biofilms of Streptococcus sanguis. Biomaterials 24:1797–1807. doi: 10.1016/S0142-9612(02)00577-X. [DOI] [PubMed] [Google Scholar]
- 300.Moore G, Hall TJ, Wilson APR, Gant VA. 2008. The efficacy of the inorganic copper-based biocide CuWB50 is compromised by hard water. Lett Appl Microbiol 46:655–660. doi: 10.1111/j.1472-765X.2008.02369.x. [DOI] [PubMed] [Google Scholar]
- 301.Souli M, Galani I, Plachouras D, Panagea T, Armaganidis A, Petrikkos G, Giamarellou H. 2013. Antimicrobial activity of copper surfaces against carbapenemase-producing contemporary Gram-negative clinical isolates. J Antimicrob Chemother 68:852–857. doi: 10.1093/jac/dks473. [DOI] [PubMed] [Google Scholar]
- 302.Agwara MO, Ndifon PT, Ndosiri NB, Paboudam AG, Yufanyi DM, Mohamadou A. 2010. Synthesis, characterisation and antimicrobial activities of cobalt(II), copper(II) and zinc(II) mixed-ligand complexes containing 1,10-phenanthroline and 2,2′-bipyridine. Bull Chem Soc Ethiop 24:383–389. [Google Scholar]
- 303.Sani RK, Peyton BM, Brown LT. 2001. Copper-induced inhibition of growth of Desulfovibrio desulfuricans G20: assessment of its toxicity and correlation with those of zinc and lead. Appl Environ Microbiol 67:4765–4772. doi: 10.1128/AEM.67.10.4765-4772.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Hu Y-H, Dang W, Liu C-S, Sun L. 2010. Analysis of the effect of copper on the virulence of a pathogenic Edwardsiella tarda strain. Lett Appl Microbiol 50:97–103. doi: 10.1111/j.1472-765X.2009.02761.x. [DOI] [PubMed] [Google Scholar]
- 305.Rosu T, Pahontu E, Pasculescu S, Georgescu R, Stanica N, Curaj A, Popescu A, Leabu M. 2010. Synthesis, characterization antibacterial and antiproliferative activity of novel Cu(II) and Pd(II) complexes with 2-hydroxy-8-R-tricyclo[7.3.1.0.(2,7)]tridecane-13-one thiosemicarbazone. Eur J Med Chem 45:1627–1634. doi: 10.1016/j.ejmech.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 306.Warnes SL, Highmore CJ, Keevil CW. 2012. Horizontal transfer of antibiotic resistance genes on abiotic touch surfaces: implications for public health. mBio 3:e00489-12. doi: 10.1128/mBio.00489-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Sudha VBP, Singh KO, Prasad SR, Venkatasubramanian P. 2009. Killing of enteric bacteria in drinking water by a copper device for use in the home: laboratory evidence. Trans R Soc Trop Med Hyg 103:819–822. doi: 10.1016/j.trstmh.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 308.Malachová K, Praus P, Rybková Z, Kozák O. 2011. Antibacterial and antifungal activities of silver, copper and zinc montmorillonites. Appl Clay Sci 53:642–645. doi: 10.1016/j.clay.2011.05.016. [DOI] [Google Scholar]
- 309.Ibrahim SA, Yang H, Seo CW. 2008. Antimicrobial activity of lactic acid and copper on growth of Salmonella and Escherichia coli O157:H7 in laboratory medium and carrot juice. Food Chem 109:137–143. doi: 10.1016/j.foodchem.2007.12.035. [DOI] [PubMed] [Google Scholar]
- 310.Qiu J-H, Zhang Y-W, Zhang Y-T, Zhang H-Q, Liu J-D. 2011. Synthesis and antibacterial activity of copper-immobilized membrane comprising grafted poly(4-vinylpyridine) chains. J Colloid Interface Sci 354:152–159. doi: 10.1016/j.jcis.2010.09.090. [DOI] [PubMed] [Google Scholar]
- 311.Zhang W, Zhang Y, Ji J, Yan Q, Huang A, Chu PK. 2007. Antimicrobial polyethylene with controlled copper release. J Biomed Mater Res A 83:838–844. doi: 10.1002/jbm.a.31436. [DOI] [PubMed] [Google Scholar]
- 312.Grey B, Steck TR. 2001. Concentrations of copper thought to be toxic to Escherichia coli can induce the viable but nonculturable condition. Appl Environ Microbiol 67:5325–5327. doi: 10.1128/AEM.67.11.5325-5327.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Torres A, Ruales C, Pulgarin C, Aimable A, Bowen P, Sarria V, Kiwi J. 2010. Innovative high-surface-area CuO pretreated cotton effective in bacterial inactivation under visible light. ACS Appl Mater Interfaces 2:2547–2552. doi: 10.1021/am100370y. [DOI] [PubMed] [Google Scholar]
- 314.Chohan ZH, Arif M, Rashid A. 2008. Copper(II) and zinc(II) metal-based salicyl-, furanyl-, thienyl- and pyrrolyl-derived ONNO, NNNO, ONNS & NNNS donor asymmetrically mixed Schiff-bases with antibacterial and antifungal potentials. J Enzyme Inhib Med Chem 23:785–796. doi: 10.1080/14756360701450145. [DOI] [PubMed] [Google Scholar]
- 315.Stout JE, Yu VL. 2003. Experiences of the first 16 hospitals using copper-silver ionization for Legionella control: implications for the evaluation of other disinfection modalities. Infect Control Hosp Epidemiol 24:563–568. doi: 10.1086/502251. [DOI] [PubMed] [Google Scholar]
- 316.Casari E, Ferrario A, Montanelli A. 2007. Prolonged effect of two combined methods for Legionella disinfection in a hospital water system. Ann Ig 19:525–532. [PubMed] [Google Scholar]
- 317.Stout JE, Lin YS, Goetz AM, Muder RR. 1998. Controlling Legionella in hospital water systems: experience with the superheat-and-flush method and copper-silver ionization. Infect Control Hosp Epidemiol 19:911–914. doi: 10.1086/647762. [DOI] [PubMed] [Google Scholar]
- 318.Landeen LK, Yahya MT, Gerba CP. 1989. Efficacy of copper and silver ions and reduced levels of free chlorine in inactivation of Legionella pneumophila. Appl Environ Microbiol 55:3045–3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Raman N, Baskaran T, Selvan A, Jeyamurugan R. 2008. DNA interaction and antimicrobial studies of novel copper(II) complex having ternary Schiff base. J Iran Chem Res 1:129–139. [Google Scholar]
- 320.Elguindi J, Wagner J, Rensing C. 2009. Genes involved in copper resistance influence survival of Pseudomonas aeruginosa on copper surfaces. J Appl Microbiol 106:1448–1455. doi: 10.1111/j.1365-2672.2009.04148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Keyhani E, Abdi-Oskouei F, Attar F, Keyhani J. 2006. DNA strand breaks by metal-induced oxygen radicals in purified Salmonella Typhimurium DNA. Ann N Y Acad Sci 1091:52–64. doi: 10.1196/annals.1378.054. [DOI] [PubMed] [Google Scholar]
- 322.Chohan ZH, Shaikh AU, Supuran CT. 2006. In-vitro antibacterial, antifungal and cytotoxic activity of cobalt(II), copper(II), nickel(II) and zinc(II) complexes with furanylmethyl- and thienylmethyl-dithiolenes: [1, 3-dithiole-2-one and 1,3-dithiole-2-thione]. J Enzyme Inhib Med Chem 21:733–740. doi: 10.1080/14756360600810308. [DOI] [PubMed] [Google Scholar]
- 323.Sharan R, Chhibber S, Reed RH. 2011. Inactivation and sub-lethal injury of Salmonella Typhi, Salmonella Typhimurium and Vibrio cholerae in copper water storage vessels. BMC Infect Dis 11:204. doi: 10.1186/1471-2334-11-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Sharan R, Chhibber S, Reed RH. 2011. A murine model to study the antibacterial effect of copper on infectivity of Salmonella enterica serovar Typhimurium. Int J Environ Res Public Health 8:21–36. doi: 10.3390/ijerph8010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Bhattacharya K, Niyogi SK, Choudhuri SK. 2011. Role of a novel copper chelate in modulation of resistance by time and dose-dependent potential on the growth of tetracycline-resistant Vibrio cholerae O1. Int J Antimicrob Agents 38:182–183. doi: 10.1016/j.ijantimicag.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 326.Chandra S, Jain D, Sharma AK, Sharma P. 2009. Coordination modes of a Schiff base pentadentate derivative of 4-aminoantipyrine with cobalt(II), nickel(II) and copper(II) metal ions: synthesis, spectroscopic and antimicrobial studies. Molecules 14:174–190. doi: 10.3390/molecules14010174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Sagripanti JL. 1992. Metal-based formulations with high microbicidal activity. Appl Environ Microbiol 58:3157–3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Sagripanti JL, Routson LB, Lytle CD. 1993. Virus inactivation by copper or iron ions alone and in the presence of peroxide. Appl Environ Microbiol 59:4374–4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Wong K, Morgan AR, Paranchych W. 1974. Controlled cleavage of phage R17 RNA within the virion by treatment with ascorbate and copper(II). Can J Biochem 52:950–958. doi: 10.1139/o74-133. [DOI] [PubMed] [Google Scholar]
- 330.Yamamoto N, Hiatt CW, Haller W. 1964. Mechanism of inactivation of bacteriophages by metals. Biochim Biophys Acta 91:257–261. doi: 10.1016/0926-6550(64)90249-x. [DOI] [PubMed] [Google Scholar]
- 331.Yahya MT, Straub TM, Gerba CP, Margolin AB. 1991. Inactivation of poliovirus and bacteriophage MS-2 in copper, galvanised and plastic domestic water pipes. Int J Environ Health Res 1:76–86. doi: 10.1080/09603129109356707. [DOI] [Google Scholar]
- 332.Borkow G, Sidwell RW, Smee DF, Barnard DL, Morrey JD, Lara-Villegas HH, Shemer-Avni Y, Gabbay J. 2007. Neutralizing viruses in suspensions by copper oxide-based filters. Antimicrob Agents Chemother 51:2605–2607. doi: 10.1128/AAC.00125-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Borkow G, Lara HH, Covington CY, Nyamathi A, Gabbay J. 2008. Deactivation of human immunodeficiency virus type 1 in medium by copper oxide-containing filters. Antimicrob Agents Chemother 52:518–525. doi: 10.1128/AAC.00899-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Sagripanti JL, Lightfoote MM. 1996. Cupric and ferric ions inactivate HIV. AIDS Res Hum Retroviruses 12:333–337. doi: 10.1089/aid.1996.12.333. [DOI] [PubMed] [Google Scholar]
- 335.Jordan FT, Nassar TJ. 1971. The influence of copper on the survival of infectious bronchitis vaccine virus in water. Vet Rec 89:609–610. doi: 10.1136/vr.89.23.609. [DOI] [PubMed] [Google Scholar]
- 336.Fujimori Y, Sato T, Hayata T, Nagao T, Nakayama M, Nakayama T, Sugamata R, Suzuki K. 2012. Novel antiviral characteristics of nanosized copper(I) iodide particles showing inactivation activity against 2009 pandemic H1N1 influenza virus. Appl Environ Microbiol 78:951–955. doi: 10.1128/AEM.06284-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Totsuka A, Otaki K. 1974. The effects of amino acids and metals on the infectivity of poliovirus ribonucleic acid. Jpn J Microbiol 18:107–112. doi: 10.1111/j.1348-0421.1974.tb00797.x. [DOI] [PubMed] [Google Scholar]
- 338.Mumcuoglu KY, Gabbay J, Borkow G. 2008. Copper oxide-impregnated fabrics for the control of house dust mites. Int J Pest Manag 54:235–240. doi: 10.1080/09670870802010856. [DOI] [Google Scholar]
- 339.Bellí N, Marín S, Sanchis V, Ramos AJ. 2006. Impact of fungicides on Aspergillus carbonarius growth and ochratoxin A production on synthetic grape-like medium and on grapes. Food Addit Contam 23:1021–1029. doi: 10.1080/02652030600778702. [DOI] [PubMed] [Google Scholar]
- 340.Kumbhar AS, Padhye SB, Saraf AP, Mahajan HB, Chopade BA, West DX. 1991. Novel broad-spectrum metal-based antifungal agents. Correlations amongst the structural and biological properties of copper(II) 2-acetylpyridine N4-dialkylthiosemicarbazones. Biol Met 4:141–143. doi: 10.1007/BF01141304. [DOI] [PubMed] [Google Scholar]
- 341.Gershon H, Clarke DD, Gershon M. 1989. Synergistic antifungal action of 8-quinolinol and its bischelate with copper(II) and with mixed ligand chelates composed of copper(II), 8-quinolinol, and aromatic hydroxy acids. J Pharm Sci 78:975–978. doi: 10.1002/jps.2600781120. [DOI] [PubMed] [Google Scholar]
- 342.Tajudeen SS, Santhalakshmi S, Kannappan G. 2010. Studies on antimicrobial activity of some hydrazones and its copper complexes. J Pharm Res 3:2759–2760. [Google Scholar]
- 343.Lin MY, Huang KJ, Kleven SH. 1989. In vitro comparison of the activity of various antifungal drugs against new yeast isolates causing thrush in poultry. Avian Dis 33:416–421. doi: 10.2307/1591098. [DOI] [PubMed] [Google Scholar]
- 344.Al-Holy MA, Castro LF, Al-Qadiri HM. 2010. Inactivation of Cronobacter spp. (Enterobacter sakazakii) in infant formula using lactic acid, copper sulfate and monolaurin. Lett Appl Microbiol 50:246–251. doi: 10.1111/j.1472-765X.2009.02782.x. [DOI] [PubMed] [Google Scholar]
- 345.Vagabov VM, Ivanov AY, Kulakovskaya TV, Kulakovskaya EV, Petrov VV, Kulaev IS. 2008. Efflux of potassium ions from cells and spheroplasts of Saccharomyces cerevisiae yeast treated with silver and copper ions. Biochemistry (Mosc) 73:1224–1227. doi: 10.1134/S0006297908110084. [DOI] [PubMed] [Google Scholar]