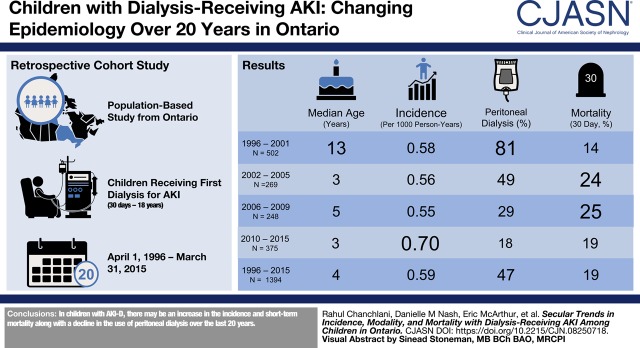
Visual Abstract
Keywords: acute renal failure; children; clinical epidemiology; dialysis; child; humans; renal dialysis; incidence; length of stay; Ontario; child, hospitalized; chronic kidney failure; peritoneal dialysis; acute kidney injury; metabolism, inborn errors; kidney failure, chronic
Abstract
Background and objectives
There is a limited appreciation of the epidemiology of dialysis-receiving AKI in children. The primary objective of the study was to evaluate changes in the incidence of dialysis-receiving AKI among children over a period of 20 years in Ontario, Canada. The secondary objectives were to assess temporal trends in the utilization of various dialysis modalities and 30-day mortality among children with dialysis-receiving AKI.
Design, setting, participants, & measurements
All children (29 days to 18 years) who received their first dialysis for AKI between 1996 and 2015 were identified from healthcare administrative databases. Those who received dialysis for ESKD, inborn errors of metabolism, and poisonings were excluded. The incidence rates of dialysis-receiving AKI were reported annually. The Cochran—Armitage test was used to assess trends in the incidence and short-term mortality after dialysis-receiving AKI.
Results
We identified 1394 children treated with dialysis for AKI during a hospital stay. There was a significant increase in the incidence of dialysis-receiving AKI among hospitalized children from 1996 (0.58 per 1000 person-years) to 2015 (0.65 per 1000 person-years) (P=0.01). The use of continuous kidney replacement therapy and intermittent hemodialysis increased whereas the relative use of peritoneal dialysis declined over time. Thirty-day mortality rates after dialysis-receiving AKI increased from 14% to 25% between 1996 and 2009 and reduced to 19% in the more recent years (P=0.03).
Conclusions
In Ontario, the incidence of dialysis-receiving AKI among children has increased between 1996 and 2015. The use of peritoneal dialysis for AKI has declined and the short-term mortality after dialysis-receiving AKI has increased.
Introduction
AKI has been increasingly recognized as an important complication in hospitalized children. AKI occurs in up to 60% of children after cardiac surgery (1–3) and 10%–80% of all children admitted to an intensive care unit (ICU) (4–6). AKI is strongly associated with hospital mortality in children (6–8), and among those with severe AKI receiving dialysis, hospital mortality is as high as 50%–70% (9,10). Similarly, among neonates, AKI occurs in around 18%–70% of patients undergoing cardiac surgery or those on extracorporeal membrane oxygenation (ECMO) (11–13), and has been shown to be associated with high mortality rates in the range of 13%–55% on the basis of data from various multicenter studies (12,14,15).
Recent data suggest that the incidence of dialysis-receiving AKI has increased among adults over the past two decades (16,17). However, trends in the incidence of AKI over time have not been well documented in children. Previous studies in children on AKI were from single centers or (18) cross-sectional in design (19), and there is no information on secular trends in AKI incidence.
We conducted a population-based cohort study to determine the epidemiology of dialysis-receiving AKI among children over a period of 20 years, using health administrative databases in Ontario. Our main objectives were to determine the trends in the incidence of dialysis-receiving AKI, relative utilization of various dialysis modalities, and risk of short-term mortality after dialysis-receiving AKI among children. We hypothesized that the incidence of dialysis-receiving AKI and the utilization of intermittent hemodialysis (HD) and continuous kidney replacement therapy (CKRT) have increased but the risk of mortality after AKI has decreased over the past two decades among hospitalized children.
Materials and Methods
Setting and Design
This was a population-based, retrospective cohort study of children who received their first dialysis treatment for AKI in any one of the four tertiary care pediatric hospitals in Ontario between April 1, 1996 and March 31, 2015. Ontario is Canada’s most populous province in which comprehensive publicly funded health care is available for its >13 million residents. The use of data in this project was authorized under section 45 of Ontario’s Personal Health Information Protection Act, which is exempt from review by a research ethics board.
Population
All children (29 days to 18 years) who were hospitalized between April 1, 1996 and March 31, 2015, were included in this study. To assess trends in dialysis-receiving AKI over time, separate cohorts were created for each fiscal year (April 1 to March 31), and only children alive at the beginning of the fiscal year were included. Study entry date was considered the start of each fiscal year. To calculate the incidence of dialysis-receiving AKI among children, we included not only the hospitalized population but also the total childhood population in Ontario between 1996 and 2015. We then followed them for 1 year during each fiscal year for evidence of dialysis-receiving AKI. Emigration from Ontario is very low (0.1% per year) and was the only reason for loss to follow-up.
We also analyzed the trends in dialysis-receiving AKI among hospitalized neonates (0–28 days) as an exploratory analysis. For neonates, date of birth was used as the study entry date and they were followed for 28 days from birth for evidence of dialysis-receiving AKI.
We excluded neonates and children who were not residents of Ontario at the time of entry into the study, and those who received any dialysis or a kidney transplant before the study entry date. The exclusion of kidney transplant did not apply to the index hospitalization.
Data Sources
The study utilized provincial health care administrative databases housed at ICES. The details of the databases used in this study are presented in Supplemental Table 1.
Outcomes
Dialysis-Receiving AKI.
The primary outcome of the study was the annualized incidence of dialysis-receiving AKI among hospitalized children in Ontario. We ascertained dialysis-receiving AKI by the presence of at least one acute dialysis code (intermittent HD, CKRT, or peritoneal dialysis [PD]) or access code (for HD or PD catheter) (Supplemental Table 2) billed between the admission and discharge dates during the index hospitalization. To exclude patients starting maintenance dialysis for ESKD, we did not consider children who received dialysis at 90 days beyond their initial acute dialysis date. This strategy of identifying those with dialysis dependence through the ICES databases has been published previously (20). Patients who received dialysis but who had a diagnostic code for inborn errors of metabolism (e.g., organic acidemia, maple syrup urine disease, and urea cycle defect) or poisonings (methanol, salicylates, and ethylene glycol) were not considered as dialysis-receiving AKI cases. For patients with multiple eligible hospitalizations during each fiscal year (i.e., hospitalization with dialysis-receiving AKI), the first hospitalization during the study year was chosen. Children with dialysis-receiving AKI during a particular fiscal year were included in the analysis again if they had a hospitalization for dialysis-receiving AKI during the subsequent years. For patients treated with multiple modalities of dialysis used during an episode of AKI, only the first modality was considered for all analyses.
To define dialysis-receiving AKI among hospitalized neonates, a slightly different strategy was used. This was because many hospitalized neonates originally identified as receiving acute dialysis had an access code (HD or PD) only without an associated acute dialysis code, especially those receiving cardiac surgery (Supplemental Table 3). Because of the nature of our health administrative databases, it was difficult to ascertain whether the neonates who had an access code only without an acute dialysis code did actually receive dialysis. Hence, we created two definitions for dialysis-receiving AKI among hospitalized neonates: (1) the presence of an acute dialysis code (HD, PD, or CKRT) (considered as main definition to reduce misclassification bias), and (2) the presence of either an acute dialysis code or an access code.
Dialysis Modalities.
The relative utilization of various dialysis modalities (HD, CKRT, and PD) for each fiscal year was determined using the acute dialysis codes.
Short-Term Mortality.
Secular trends in all-cause mortality within 30 days of dialysis-receiving AKI were also evaluated.
Demographics and Comorbidities
We considered the demographic and clinical characteristics of our cohorts by time periods: (1996–2001, 2002–2005, 2006–2009, and 2010–2015). The International Classification of Disease (ICD) Ninth Revision coding system was in use during the first time period and the ICD-10 was used in all subsequent periods. Variables of interest included age, sex, neighborhood income quintile, and rural residence.
We also considered various features of the index hospitalization (e.g., most responsible diagnosis, sepsis, Risk Adjustment in Congenital Heart Surgery-1 score [21], receipt of cardiac surgery, ECMO, and mechanical ventilation). We also described the presence of hypertension, CKD, liver disease, and malignancy in the preceding 5 years. Income quintile was assigned according to the average neighborhood household income in which the patient resided (22). Rurality was defined by residence in a municipality with a population of <10,000 persons (23).
Statistical Analyses
All analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC). As we expected all continuous baseline variables to be non-normally distributed, we presented all continuous variables using median (interquartile range [IQR]). Categorical and binary variables were reported as numbers and percentages. For each study year, neonates were followed for 28 days and children were followed for 365 days to identify the incidence rate of dialysis-receiving AKI. For both neonates and children, the incidence rate of dialysis-receiving AKI was reported annually. We used incidence rate ratios to compare differences in the incidence across the study years. To evaluate the incidence of dialysis-receiving AKI we divided the number of children with dialysis-receiving AKI by (1) the total number of hospitalized children in Ontario, and (2) the entire pediatric population of Ontario. Relative utilization of different dialysis modalities was calculated across the study period (24). We also calculated the proportion of childhood deaths within 30 days of dialysis-receiving AKI across four time periods (i.e., 1996–2001, 2002–2005, 2006–2009, and 2010–2015). The Cochran–Armitage test was used to assess trends in in the incidence and mortality after dialysis-receiving AKI over time. This test is sensitive to the linearity between response variable and experimental variables (25).
Sensitivity Analyses
We performed a number of sensitivity analyses to confirm the robustness of our findings. Similar to neonates, a proportion of children, especially those receiving cardiac surgery, had an access code (HD or PD) only without an associated acute dialysis code (Supplemental Table 3). Because it was difficult to ascertain the actual receipt of dialysis in those patients, we analyzed the trend in dialysis-receiving AKI by including only those children who had an acute dialysis code (HD, PD, or CKRT). For the same reason, we performed a subgroup analysis in which the trend in dialysis-receiving AKI (defined by presence of either an acute dialysis code or an access code) was analyzed among only hospitalized children who did not receive cardiac surgery.
We did not include AKI diagnosis codes in the main algorithm to define dialysis-receiving AKI as they are known to have low sensitivity (26). However, because of the high specificity of these codes, we performed an additional sensitivity analysis by defining dialysis-receiving AKI as those children who had an AKI diagnosis code in addition to the acute dialysis/access codes.
Any analyses with a cell count of five or fewer study participants was reported as “<6” in accordance with ICES data privacy policies. A two-sided P value <0.05 was considered statistically significant.
Results
During the study period, 1394 children experienced dialysis-receiving AKI during a hospitalization. Supplemental Figure 1 illustrates the cohort assembly for the last fiscal year (2014–2015). The identical approach was used to identify children with dialysis-receiving AKI during previous study years.
Baseline characteristics of children with dialysis-receiving AKI stratified by the designated time periods is described in Table 1. The median age at dialysis initiation among children consistently decreased over time and was 3 years (IQR, 0–13) during 2010–2015 compared with 13 years (IQR, 6–16) during 1996–2001. The relative proportion of children with cardiac surgery associated AKI and the receipt of mechanical ventilation and ECMO increased over time, whereas the proportion of children with concomitant sepsis has remained somewhat stable over the past decade. Supplemental Table 4 shows the reasons for the most responsible diagnosis leading to index hospitalization.
Table 1.
Baseline characteristics of the hospitalized children who received dialysis for AKI in Ontario between April 1, 1996 and March 31, 2015
| Baseline Characteristic | 1996–2001 | 2002–2005 | 2006–2009 | 2010–2015 | ||||
|---|---|---|---|---|---|---|---|---|
| Total | 502 | 269 | 248 | 375 | ||||
| Median age at first dialysis (IQR), years | 13 | (6–16) | 3 | (0–13) | 5 | (0–14) | 3 | (0–13) |
| Male, n (%) | 252 | 50% | 136 | 51% | 132 | 53% | 207 | 55% |
| Rural status, n (%) | 121 | 24% | 33 | 12% | 33 | 13% | 47 | 13% |
| Income quintile, n (%) | ||||||||
| 1 | 100 | 20% | 70 | 26% | 52 | 21% | 98 | 26% |
| 2 | 100 | 20% | 63 | 23% | 40 | 16% | 61 | 16% |
| 3 | 118 | 24% | 54 | 20% | 59 | 24% | 58 | 16% |
| 4 | 84 | 17% | 40 | 15% | 53 | 21% | 71 | 19% |
| 5 | 100 | 20% | 42 | 16% | 44 | 18% | 77 | 21% |
| Interventions during index hospitalization | ||||||||
| Need for mechanical ventilation, n (%) | 88 | 18% | 195 | 73% | 179 | 72% | 295 | 79% |
| Cardiac surgery, n (%) | 36 | 7% | 95 | 35% | 79 | 32% | 154 | 41% |
| Risk Assessment for Congenital Heart Surgery-1 score, n (%) | ||||||||
| 0 (not relevant) | 478 | 95% | 206 | 77% | 196 | 79% | 242 | 65% |
| 1 | 0 | 0% | 20 | 7% | 28 | 11% | 18 | 5% |
| 2 | 11 | 2% | 62 | 17% | ||||
| 3 | 13 | 3% | 23 | 9% | 16 | 7% | 36 | 10% |
| ≥4 | 20 | 7% | 8 | 3% | 17 | 5% | ||
| Sepsis, n (%) | 55 | 11% | 59 | 22% | 44 | 18% | 57 | 15% |
| Extracorporeal membrane oxygenation, n (%) | 13 | 3% | 9 | 3% | 54 | 22% | 57 | 15% |
| Index hospital | ||||||||
| Hospital for Sick Children, Toronto, n (%) | 106 | 21% | 165 | 61% | 138 | 56% | 154 | 41% |
| Children’s Hospital of Eastern Ontario, Ottawa, n (%) | 39 | 8% | 26 | 10% | 37 | 15% | 124 | 33% |
| London Health Sciences Center, London, n (%) | 41 | 8% | 23 | 9% | 26 | 11% | 26 | 7% |
| McMaster Children’s Hospital, Hamilton, n (%) | 39 | 8% | 27 | 10% | 19 | 8% | 30 | 8% |
| Other, n (%) | 267 | 53% | 25 | 9% | 26 | 11% | 36 | 10% |
| Median duration of index hospitalization among those who survived hospitalization, days (IQR) | 7 | (4–15) | 21.5 | (13–42) | 22 | (12–41) | 15 | (7–35) |
| Liver disease in past 5 yr, n (%) | 25 | 5% | 35 | 13% | 33 | 13% | 40 | 11% |
| Hypertension in past 5 yr, n (%) | 17 | 3% | 20 | 7% | 41 | 17% | 56 | 15% |
| CKD in past 5 yr, n (%) | 49 | 10% | 36 | 13% | 30 | 12% | 28 | 8% |
| Malignancy in past 5 yr, n (%) | 46 | 9% | 46 | 17% | 51 | 21% | 60 | 16% |
IQR, interquartile range.
Incidence of Dialysis-Receiving AKI Among Children
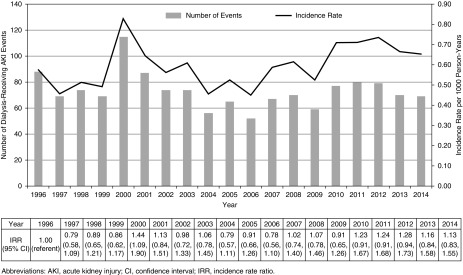
The incidence of AKI-D among 2,363,053 hospitalized children between 1996 and 2015 was 0.59 per 1000 person-years. There was a significant change in the incidence of dialysis-receiving AKI among children from 1996 (0.58 per 1000 person-years) to 2015 (0.65 per 1000 person-years) (Cochran–Armitage test for trend, P=0.01). The incidence of dialysis-receiving AKI peaked in the year 2000 (0.83 per 1000 person-years) and then stabilized (Figure 1). When examined as a proportion of the total Ontario pediatric population, there was a slight reduction in the incidence of dialysis-receiving AKI from 0.03 per 1000 person-years in 1996 to 0.02 per 1000 person-years in 2015 (Cochran–Armitage test for trend, P=0.02) (Supplemental Figure 2).
Figure 1.
Incidence of dialysis-receiving AKI among hospitalized children (n=1394) between April 1, 1996 and March 31, 2015 in Ontario. 95% CI, 95% confidence interval; IRR, incidence rate ratio.
Trends in the Use of Various Dialysis Modalities for AKI
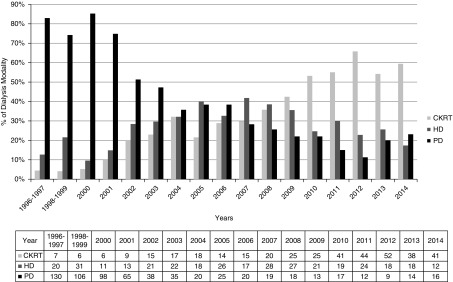
Among children, there was a relative decline in the use of PD over years. HD and CKRT were more frequently deployed as initial dialysis modalities for AKI in more recent years (Figure 2).
Figure 2.
Relative utilization of various dialysis modalities for the management of dialysis-receiving AKI among hospitalized children in Ontario between April 1, 1996 and March 31, 2015. CKRT, continuous kidney replacement therapy; HD, hemodialysis; PD, peritoneal dialysis.
Trends in the 30-Day Mortality after Dialysis-Receiving AKI
Among children with dialysis-receiving AKI, 30-day mortality increased in the early 2000s compared with 1996–2001, but has stabilized around 20% in the past 10 years (Cochran–Armitage test, P=0.03) (Table 2). The mortality rates differed on the basis of the dialysis modality. The relative proportion of deaths among children was higher among those on CKRT (146 out of 393, 37%) compared with those on HD or PD (Supplemental Table 5).
Table 2.
30-Day mortality after dialysis-receiving AKI among children in Ontario between April 1, 1996 and March 31, 2015
| Years | N | Deaths | |
|---|---|---|---|
| n | % (95% Confidence Interval) | ||
| 1996–2001 | 502 | 69 | 14 (11 to 17) |
| 2002–2005 | 269 | 64 | 24 (19 to 29) |
| 2006–2009 | 248 | 61 | 25 (20 to 30) |
| 2010–2015 | 375 | 71 | 19 (15 to 23) |
| Total | 1394 | 265 | 19 (17 to 21) |
N, number of children; n, number of deaths.
Exploratory Analysis on the Incidence of Dialysis-Receiving AKI among Neonates
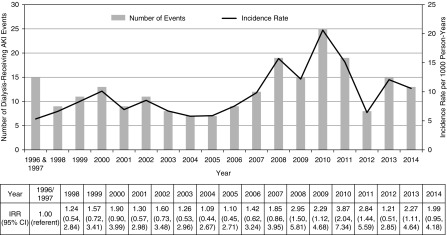
During the study period, 2,481,043 neonates were born, and among them, 293,583 were hospitalized. Of the hospitalized neonates, 225 experienced dialysis-receiving AKI as defined using only acute dialysis codes (incidence rate 9.38 per 1000 person-years). The incidence of dialysis-receiving AKI was relatively stable from 1996 to 2005. Thereafter, there was an increase in the number of cases that peaked in 2010 and declined thereafter (Figure 3) (Cochran–Armitage test for trend, P<0.001).
Figure 3.
Incidence of dialysis-receiving AKI among hospitalized neonates (n=225) between April 1, 1996 and March 31, 2015 in Ontario using only acute dialysis codes. 95% CI, 95% confidence interval; IRR, incidence rate ratio.
On defining dialysis-receiving AKI as those neonates who had either an access code or an acute dialysis code (n=531), the incidence rate of dialysis-receiving AKI was 22.13 per 1000 person-years. From the fiscal years 1996 and 1997, the incidence of dialysis-receiving AKI among neonates increased from 5.33 per 1000 person-years to 47.92 per 1000 person-years in 2005 and then declined and stabilized at 20.35 per 1000 person-years in the year 2015 (Cochran–Armitage test for trend, P<0.001) (Supplemental Figure 3).
Sensitivity Analyses
Among hospitalized children in whom dialysis-receiving AKI was defined only on the basis of the presence of acute dialysis codes (n=1249), the trend in dialysis-receiving AKI was not statistically significant (Cochran–Armitage test for trend, P=0.3) (Supplemental Figure 4). Similarly, on analyzing a subset of hospitalized children who did not have a cardiac surgery, there was a reduction in the incidence of dialysis-receiving AKI (n=1030; Cochran–Armitage test for trend, P<0.001) (Supplemental Figure 5).
Finally, when including AKI diagnosis codes in addition to the acute dialysis codes to define dialysis-receiving AKI among hospitalized children, there was a slight change in the trend compared with the main analysis (n=455; Cochran–Armitage test for trend, P<0.001) (Supplemental Figure 6).
Discussion
This comprehensive, population-wide study evaluated the epidemiology of pediatric dialysis-receiving AKI over a period spanning two decades. The incidence of dialysis-receiving AKI has increased significantly among hospitalized neonates and children from 1996 to 2015. Our study also shows the relative increase in the use of intermittent HD and CKRT as the initial modality of dialysis for AKI among children. Moreover, one fifth of children die within 30 days of initiating dialysis for AKI and the short-term mortality after dialysis-receiving AKI has increased significantly over time.
This is the only population-wide study examining the trends in dialysis-receiving AKI among children and represents the largest reported cohort of nonadults with dialysis-receiving AKI. Using the Nationwide Inpatient Sample database in the United States, Hsu et al. (16) showed that the incidence of dialysis-receiving AKI among children and adults increased by 10% per year (incidence rate ratio, 1.10; 95% confidence interval, 1.10 to 1.11 per year) from 2000 to 2009. Among children (0–19 years), the increase was 7% per year. The authors identified dialysis-receiving patients with AKI by using both AKI diagnosis and dialysis procedure codes through a national database. We also documented an increase in the incidence of dialysis-receiving AKI; however, we defined it using only the acute dialysis and access codes and not AKI diagnosis codes in our main analysis. Of note, in sensitivity analyses when we defined dialysis-receiving AKI using only acute dialysis codes, the trend in the incidence was no longer statistically significant. However, the result of this sensitivity analysis should be interpreted with caution because of the reduced sample size (Supplemental Figure 4), and may have occurred because of changes in coding practice after 2001. This is also reflected in the fact that there was a greater than three times increase in the proportion of children with PD access and no dialysis codes among those who had cardiac surgery between 1996–2001 and 2002–2005, which is unlikely to be a true increase in dialysis over that time period. Moreover, changes in the practice of inserting prophylactic dialysis catheters among cardiac surgery patients could also be an explanation of this finding. When we included AKI diagnosis codes in the algorithm (in addition to acute dialysis/access codes), the sample size was reduced, and as such the trend in dialysis-receiving AKI looked different from the main analysis. The increase in dialysis-receiving AKI cases after 2002 (Supplemental Figure 6) in this sensitivity analysis can also be potentially explained by changes in coding practice after 2002. On analyzing a subset of children who did not have cardiac surgery, the incidence in dialysis-receiving AKI appeared to be decreasing. This can be explained by improved quality of care, awareness of AKI, and prevention of fluid overload strategies in pediatric ICUs over time (27–29).
Intermittent HD and CKRT have become the predominant dialysis modalities in children with AKI, with the use of PD diminishing. This trend can be explained by significant advances in extracorporeal therapy technology tailored to the pediatric population. This includes the availability of smaller dialyzers permitting the use of smaller extracorporeal volumes, which has made the delivery of HD and CKRT feasible and safe even in extremely low-birth-weight babies (30). With the introduction of newer machines for CKRT, it is expected that CKRT utilization will continue to grow. The Newcastle infant dialysis and ultrafiltration system and CARPEDIEM (Cardio-renal pediatric dialysis emergency machine) have lower extracorporeal volumes for providing dialysis to infants <8 kg. There are emerging reports on the safety and efficacy of these and other machines, which will make CKRT a more accessible option in the future for critically ill infants and children (31,32).
Children with dialysis-receiving AKI are at a high risk of death. In a multicenter study of >2 million hospitalized children where AKI was defined using ICD-9 codes, those with dialysis-receiving AKI had a high risk of mortality that was substantially higher than those with AKI not receiving dialysis (27.1% versus 14.2%; P<0.001) (19). In a more recent study of 4683 hospitalized children in which AKI was defined by serum creatinine criteria (33), dialysis-receiving AKI (n=73) was associated with a three-fold risk of 28-day mortality (odds ratio, 3.38; 95% confidence interval, 1.74 to 6.54) compared with children with no AKI (4). In our study, there was initially an increase in the 30-day mortality from 14% to 25% until 2009, followed by a decline to around 20% in the more recent years despite an increasing burden of comorbid conditions such as cardiac surgery and mechanical ventilation. This may be due to various reasons, including significant advancement in clinical care of the underlying conditions, better availability of ICUs, and earlier initiation of dialysis. The overall mortality rate in our study among children (19.0%) was lower than those shown in the ppCKRT registry (>40%), which could be because children in the latter study were all critically ill and recipients of CKRT, which suggest a higher degree of acuity. It is notable that in our cohort, mortality was highest among children who commenced CKRT (37%). In summary, our work and those of others highlight the important association between dialysis-receiving AKI and short-term mortality among children.
In our study, there was an increase in the number and complexity of cardiac surgeries after 2001 during which a prophylactic PD catheter or a central venous line may have been inserted before cardiopulmonary bypass depending on the institutional practices. However, we recognize that placement of access for kidney replacement therapy may not have necessarily resulted in the administration of dialysis. In fact, a proportion of patients in our study, especially neonates with cardiac surgery, had an access code (PD or HD) but no acute dialysis code. Because of the nature of our databases, we could not ascertain whether those neonates and children with an access code actually received dialysis. Also, because this issue was more profound in neonates, we primarily defined dialysis-receiving AKI as only those neonates who had an acute dialysis code. Since fluid overload has been shown to be a major determinant of morbidity and mortality among infants after cardiac surgery, various interventions have been tried to prevent it. Pharmacologic management, such as use of diuretics and aminophylline, have shown mixed results. Because of these reasons, early kidney replacement therapy, especially PD, is being increasingly used to prevent fluid overload. However, there is no consensus on the use of prophylactic PD catheter insertion after cardiac surgery among infants and children and there is a significant variation in practice. The evidence to support prophylactic PD catheter insertion to prevent fluid overload secondary to AKI or hemodynamic stability is quite conflicting. Some studies have shown a higher risk of mortality in patients who received PD (34), whereas others have shown a significant improvement in the fluid overload compared with medical treatment (35).
Our study also demonstrates that the trends in dialysis-receiving AKI among children are more nuanced than those in adults. Most of the adult studies have shown a rapid and consistent increase in the incidence of dialysis-receiving AKI over time. In our study, there was a significant periodic variation in the incidence of dialysis-receiving AKI over the past two decades despite a consistent increase in the comorbid conditions and procedures. Some potential reasons could be administrative (changes in the coding strategy after 2001), changes in patient characteristics (case-mix as suggested by increase in the number of cardiac surgeries and ECMO), and changes in provider characteristics (improving awareness of AKI, practice patterns related to prophylactic dialysis catheter insertion, better availability of various dialysis modalities, and lower threshold of offering dialysis over years). These reasons potentially explain the dynamic variation seen in the trend of dialysis-receiving AKI among children in our study and provide explanation of the modest change in dialysis-receiving AKI compared with adults.
Our study has several strengths. We assembled the largest described cohort of neonates and children receiving dialysis for AKI that spanned two decades and encompassed the entire Ontario population of over 13 million residents. The universal access to publicly funded health care in Ontario gave us a unique opportunity to study the changing incidence and outcomes of severe AKI among the entire neonatal and pediatric population in our province.
Our study also has several important limitations. The nature of our datasets did not allow us to account for important factors, most notably the severity of acute illness, which may have confounded the secular trends in dialysis-receiving AKI over time. Second, administrative codes for dialysis-receiving AKI have not been validated in the pediatric population. The sensitivity of AKI diagnosis codes has been shown to be limited but because acute dialysis is associated with reimbursement, it is likely to be captured with greater accuracy (36). In addition, codes for identifying individuals with dialysis-receiving AKI have been validated in adults and have been shown to have a sensitivity of 90.3% and specificity of 93.8% compared with chart review (37). Third, we excluded children who received kidney transplant before index date but not those who received it on the index date, so our dialysis-receiving AKI cohort may have included a small proportion of children with kidney transplant. Fourth, we excluded neonates and children (n=79) if they were receiving dialysis on or after day 90 to exclude those in whom dialysis was presumably started in the context of ESKD. However, through this process we may have inadvertently excluded those who had true AKI that did not recover. Fifth, we performed all analyses on the basis of the first modality of dialysis used for AKI among children. Because patients may transition between modalities in the course of a hospitalization complicated by AKI, our data on the use of dialysis modalities may not reflect the actual usage of different therapies.
In conclusion, the incidence of dialysis-receiving AKI has increased significantly among children in Ontario between 1996 and 2015. The utilization of HD and CKRT have increased with a decline in the use PD over the past two decades. Dialysis-receiving AKI continues to be associated with an unacceptably high risk of short-term mortality. Future research should aim to test strategies that will improve outcomes in this vulnerable population.
Disclosures
The authors have nothing to disclose.
Funding
Dr. Chanchlani reports a grant from Hamilton Health Sciences (NIF17405). Dr. Wald reports grants from Baxter (IPR139081).
Supplementary Material
Acknowledgments
We gratefully acknowledge the New Investigator Fund received from the Hamilton Health Sciences for this project in 2017. We are extremely thankful to Ms. Nivethika Jeyakumar for her assistance in preparing the tables and figures for this manuscript.
Dr. Chanchlani, Dr. Nash, and Dr. Wald conceptualized and designed the study, drafted the initial manuscript, and reviewed and revised the manuscript. Dr. Kuwornu and Mr. McArthur carried out the analyses, and reviewed and revised the manuscript. Dr. Zappitelli, Dr. Garg, Dr. Greenberg, Ms. Archer, Dr. Goldstein, and Dr. Thabane supervised data collection, and critically reviewed and revised the manuscript for important intellectual content. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.08250718/-/DCSupplemental.
Supplemental Table 1. ICES databases used for cohort creation, outcomes, and baseline characteristics.
Supplemental Table 2. Administrative data codes used in the study to identify children receiving dialysis for AKI.
Supplemental Table 3. Proportion of neonates and children with dialysis-receiving AKI in various time-periods stratified by the cardiac surgery status.
Supplemental Table 4. Most prevalent diagnoses during a hospital stay for children who received dialysis for AKI in Ontario between April 1, 1996 and March 31, 2015.
Supplemental Table 5. Risk of 30-day mortality among hospitalized children after dialysis-receiving AKI stratified by dialysis modality.
Supplemental Figure 1. Cohort creation for children to assess incidence of dialysis-receiving AKI for the fiscal year April 1, 2014 to March 31, 2015.
Supplemental Figure 2. Incidence of dialysis-receiving AKI among total pediatric population between April 1, 1996 and March 31, 2015 in Ontario.
Supplemental Figure 3. Incidence of dialysis-receiving AKI (defined using acute dialysis or access codes) among hospitalized neonates between April 1, 1996 and March 31, 2015 in Ontario.
Supplemental Figure 4. Incidence of dialysis-receiving AKI (defined using only acute dialysis codes) among hospitalized children (n=1249) between April 1, 1996 and March 31, 2015 in Ontario.
Supplemental Figure 5. Incidence of dialysis-receiving AKI (defined using acute dialysis or access codes) among hospitalized children not receiving cardiac surgery (n=1030) between April 1, 1996 and March 31, 2015 in Ontario.
Supplemental Figure 6. Incidence of dialysis-receiving AKI (defined using AKI diagnosis combined with acute dialysis codes) among hospitalized children (n=455) between April 1, 1996 and March 31, 2015 in Ontario.
References
- 1.Brown JR, Hisey WM, Marshall EJ, Likosky DS, Nichols EL, Everett AD, Pasquali SK, Jacobs ML, Jacobs JP, Parikh CR: Acute kidney injury severity and long-term readmission and mortality after cardiac surgery. Ann Thorac Surg 102: 1482–1489, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vives M, Wijeysundera D, Marczin N, Monedero P, Rao V: Cardiac surgery-associated acute kidney injury. Interact Cardiovasc Thorac Surg 18: 637–645, 2014 [DOI] [PubMed] [Google Scholar]
- 3.Li S, Krawczeski CD, Zappitelli M, Devarajan P, Thiessen-Philbrook H, Coca SG, Kim RW, Parikh CR; TRIBE-AKI Consortium : Incidence, risk factors, and outcomes of acute kidney injury after pediatric cardiac surgery: A prospective multicenter study. Crit Care Med 39: 1493–1499, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaddourah A, Basu RK, Bagshaw SM, Goldstein SL; AWARE Investigators : Epidemiology of acute kidney injury in critically ill children and young adults. N Engl J Med 376: 11–20, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akcan-Arikan A, Zappitelli M, Loftis LL, Washburn KK, Jefferson LS, Goldstein SL: Modified RIFLE criteria in critically ill children with acute kidney injury. Kidney Int 71: 1028–1035, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Schneider J, Khemani R, Grushkin C, Bart R: Serum creatinine as stratified in the RIFLE score for acute kidney injury is associated with mortality and length of stay for children in the pediatric intensive care unit. Crit Care Med 38: 933–939, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Alkandari O, Eddington KA, Hyder A, Gauvin F, Ducruet T, Gottesman R, Phan V, Zappitelli M: Acute kidney injury is an independent risk factor for pediatric intensive care unit mortality, longer length of stay and prolonged mechanical ventilation in critically ill children: A two-center retrospective cohort study. Crit Care 15: R146, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greenberg JH, Zappitelli M, Devarajan P, Thiessen-Philbrook HR, Krawczeski C, Li S, Garg AX, Coca S, Parikh CR; TRIBE-AKI Consortium : Kidney outcomes 5 Years after pediatric cardiac surgery: The TRIBE-AKI study. JAMA Pediatr 170: 1071–1078, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayes LW, Oster RA, Tofil NM, Tolwani AJ: Outcomes of critically ill children requiring continuous renal replacement therapy. J Crit Care 24: 394–400, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Symons JM, Chua AN, Somers MJ, Baum MA, Bunchman TE, Benfield MR, Brophy PD, Blowey D, Fortenberry JD, Chand D, Flores FX, Hackbarth R, Alexander SR, Mahan J, McBryde KD, Goldstein SL: Demographic characteristics of pediatric continuous renal replacement therapy: A report of the prospective pediatric continuous renal replacement therapy registry. Clin J Am Soc Nephrol 2: 732–738, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Alabbas A, Campbell A, Skippen P, Human D, Matsell D, Mammen C: Epidemiology of cardiac surgery-associated acute kidney injury in neonates: A retrospective study. Pediatr Nephrol 28: 1127–1134, 2013 [DOI] [PubMed] [Google Scholar]
- 12.Askenazi DJ, Ambalavanan N, Hamilton K, Cutter G, Laney D, Kaslow R, Georgeson K, Barnhart DC, Dimmitt RA: Acute kidney injury and renal replacement therapy independently predict mortality in neonatal and pediatric noncardiac patients on extracorporeal membrane oxygenation. Pediatr Crit Care Med 12: e1–e6, 2011 [DOI] [PubMed] [Google Scholar]
- 13.Zwiers AJ, de Wildt SN, Hop WC, Dorresteijn EM, Gischler SJ, Tibboel D, Cransberg K: Acute kidney injury is a frequent complication in critically ill neonates receiving extracorporeal membrane oxygenation: A 14-year cohort study. Crit Care 17: R151, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jetton JG, Boohaker LJ, Sethi SK, Wazir S, Rohatgi S, Soranno DE, Chishti AS, Woroniecki R, Mammen C, Swanson JR, Sridhar S, Wong CS, Kupferman JC, Griffin RL, Askenazi DJ; Neonatal Kidney Collaborative (NKC) : Incidence and outcomes of neonatal acute kidney injury (AWAKEN): A multicentre, multinational, observational cohort study. Lancet Child Adolesc Health 1: 184–194, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Askenazi DJ, Goldstein SL, Koralkar R, Fortenberry J, Baum M, Hackbarth R, Blowey D, Bunchman TE, Brophy PD, Symons J, Chua A, Flores F, Somers MJ: Continuous renal replacement therapy for children ≤10 kg: A report from the prospective pediatric continuous renal replacement therapy registry. J Pediatr 162: 587–592.e3, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsu RK, McCulloch CE, Dudley RA, Lo LJ, Hsu CY: Temporal changes in incidence of dialysis-requiring AKI. J Am Soc Nephrol 24: 37–42, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wald R, McArthur E, Adhikari NK, Bagshaw SM, Burns KE, Garg AX, Harel Z, Kitchlu A, Mazer CD, Nash DM, Scales DC, Silver SA, Ray JG, Friedrich JO: Changing incidence and outcomes following dialysis-requiring acute kidney injury among critically ill adults: A population-based cohort study. Am J Kidney Dis 65: 870–877, 2015 [DOI] [PubMed] [Google Scholar]
- 18.Vachvanichsanong P, Dissaneewate P, Lim A, McNeil E: Childhood acute renal failure: 22-year experience in a university hospital in southern Thailand. Pediatrics 118: e786–e791, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Sutherland SM, Ji J, Sheikhi FH, Widen E, Tian L, Alexander SR, Ling XB: AKI in hospitalized children: Epidemiology and clinical associations in a national cohort. Clin J Am Soc Nephrol 8: 1661–1669, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wald R, Quinn RR, Luo J, Li P, Scales DC, Mamdani MM, Ray JG; University of Toronto Acute Kidney Injury Research Group : Chronic dialysis and death among survivors of acute kidney injury requiring dialysis. JAMA 302: 1179–1185, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Jenkins KJ, Gauvreau K, Newburger JW, Spray TL, Moller JH, Iezzoni LI: Consensus-based method for risk adjustment for surgery for congenital heart disease. J Thorac Cardiovasc Surg 123: 110–118, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Statistics Canada: Analysis. Available at: http://www.statcan.gc.ca/pub/75-202-x/2010000/analysis-analyses-eng.htm. 2015. Accessed April 18, 2019
- 23.Statistics Canada: Rural and Small Town Canada Analysis Bulletin. Available at: https://www150.statcan.gc.ca/n1/en/pub/21-006-x/21-006-x2001003-eng.pdf?st=mQ_P6Yqr. 2001. Accessed April 18, 2019
- 24.Quinn RR, Laupacis A, Austin PC, Hux JE, Garg AX, Hemmelgarn BR, Oliver MJ: Using administrative datasets to study outcomes in dialysis patients: A validation study. Med Care 48: 745–750, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Armitage P: Tests for linear trends in proportions and frequencies. Biometrics 11: 375–386, 1955 [Google Scholar]
- 26.Grams ME, Waikar SS, MacMahon B, Whelton S, Ballew SH, Coresh J: Performance and limitations of administrative data in the identification of AKI. Clin J Am Soc Nephrol 9: 682–689, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goldstein SL, Currier H, Graf Cd, Cosio CC, Brewer ED, Sachdeva R: Outcome in children receiving continuous venovenous hemofiltration. Pediatrics 107: 1309–1312, 2001 [DOI] [PubMed] [Google Scholar]
- 28.Arikan AA, Zappitelli M, Goldstein SL, Naipaul A, Jefferson LS, Loftis LL: Fluid overload is associated with impaired oxygenation and morbidity in critically ill children. Pediatr Crit Care Med 13: 253–258, 2012 [DOI] [PubMed] [Google Scholar]
- 29.Sutherland SM, Zappitelli M, Alexander SR, Chua AN, Brophy PD, Bunchman TE, Hackbarth R, Somers MJ, Baum M, Symons JM, Flores FX, Benfield M, Askenazi D, Chand D, Fortenberry JD, Mahan JD, McBryde K, Blowey D, Goldstein SL: Fluid overload and mortality in children receiving continuous renal replacement therapy: The prospective pediatric continuous renal replacement therapy registry. Am J Kidney Dis 55: 316–325, 2010 [DOI] [PubMed] [Google Scholar]
- 30.Goldstein SL: Advances in pediatric renal replacement therapy for acute kidney injury. Semin Dial 24: 187–191, 2011 [DOI] [PubMed] [Google Scholar]
- 31.Ronco C, Garzotto F, Brendolan A, Zanella M, Bellettato M, Vedovato S, Chiarenza F, Ricci Z, Goldstein SL: Continuous renal replacement therapy in neonates and small infants: Development and first-in-human use of a miniaturised machine (CARPEDIEM). Lancet 383: 1807–1813, 2014 [DOI] [PubMed] [Google Scholar]
- 32.Askenazi D, Ingram D, White S, Cramer M, Borasino S, Coghill C, Dill L, Tenney F, Feig D, Fathallah-Shaykh S: Smaller circuits for smaller patients: Improving renal support therapy with Aquadex™. Pediatr Nephrol 31: 853–860, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kidney Disease Improving Global Outcomes (KDIGO): Clinical Practice Guideline for Acute Kidney Injury. Kidney Int Suppl 2: 1–138, 2012 [Google Scholar]
- 34.Madenci AL, Thiagarajan RR, Stoffan AP, Emani SM, Rajagopal SK, Weldon CB: Characterizing peritoneal dialysis catheter use in pediatric patients after cardiac surgery. J Thorac Cardiovasc Surg 146: 334–338, 2013 [DOI] [PubMed] [Google Scholar]
- 35.Kwiatkowski DM, Goldstein SL, Cooper DS, Nelson DP, Morales DL, Krawczeski CD: Peritoneal dialysis vs furosemide for prevention of fluid overload in infants after cardiac surgery: A randomized clinical trial. JAMA Pediatr 171: 357–364, 2017 [DOI] [PubMed] [Google Scholar]
- 36.Siew ED, Davenport A: The growth of acute kidney injury: A rising tide or just closer attention to detail? Kidney Int 87: 46–61, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Waikar SS, Wald R, Chertow GM, Curhan GC, Winkelmayer WC, Liangos O, Sosa MA, Jaber BL: Validity of International classification of diseases, ninth revision, clinical modification codes for acute renal failure. J Am Soc Nephrol 17: 1688–1694, 2006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.