Abstract
Extracorporeal therapies have been used to remove toxins from the body for over 50 years and have a greater role than ever before in the treatment of poisonings. Improvements in technology have resulted in increased efficacy of removing drugs and other toxins with hemodialysis, and newer extracorporeal therapy modalities have expanded the role of extracorporeal supportive care of poisoned patients. However, despite these changes, for at least the past three decades the most frequently dialyzed poisons remain salicylates, toxic alcohols, and lithium; in addition, the extracorporeal treatment of choice for therapeutic removal of nearly all poisonings remains intermittent hemodialysis. For the clinician, consideration of extracorporeal therapy in the treatment of a poisoning depends upon the characteristics of toxins amenable to extracorporeal removal (e.g., molecular mass, volume of distribution, protein binding), choice of extracorporeal treatment modality for a given poisoning, and when the benefit of the procedure justifies additive risk. Given the relative rarity of poisonings treated with extracorporeal therapies, the level of evidence for extracorporeal treatment of poisoning is not robust; however, extracorporeal treatment of a number of individual toxins have been systematically reviewed within the current decade by the Extracorporeal Treatment in Poisoning workgroup, which has published treatment recommendations with an improved evidence base. Some of these recommendations are discussed, as well as management of a small number of relevant poisonings where extracorporeal therapy use may be considered.
Keywords: toxicology; extracorporeal therapies; hemodialysis; poisoning; intoxication; acidosis; therapeutic plasma exchange; toxic alcohols; lithium; salicylates; Poisons; Toxins, Biological; hemoperfusion
Introduction
Since 1914, when the first in vivo hemodialysis (HD) was performed, extracorporeal therapies have been used for the removal of drugs and other toxins (1). Over the past 50 years, there has been increasing use of extracorporeal therapies for the treatment of poisoning, both for removal of toxins and supportive therapy; this review focuses primarily upon the former (2). Although the increasing importance of extracorporeal therapies in managing poisons is likely due in part to the greater number of available modalities, intermittent HD has become more efficacious at removing toxins over time as technology has progressed, and it is likely that the role of extracorporeal therapies for poisoning will continue to grow and evolve over time (3,4).
Principles of Toxin Removal by Extracorporeal Therapies
A number of parameters influence the ability of extracorporeal therapies to remove poisons; the ideal dialyzable substance is a small molecule, has a low volume of distribution, low protein binding, and rapidly distributes from tissue to plasma (3,5–7). Table 1 explores these alongside different extracorporeal modalities.
Table 1.
Utility of extracorporeal modalities in poisoning
| Modality | Toxin Molecular Mass (Da) | Toxin Volume of Distribution (L/kg) | Protein Binding of Toxin | Examples of Toxins Amenable to Therapy | Primary Limitations of Therapy |
|---|---|---|---|---|---|
| Hemodialysis | Up to 10,000–15,000 | ≤1.5–2 | ≤80% | Salicylates, toxic alcohols, lithium | Hemodynamic stability |
| HCO filter HD | Up to 50,000 | ≤1.5–2 | ≤80% | Small peptide therapeutics; any therapy amenable to HD | Limited availability |
| Limited role in poisoning | |||||
| CRRT | Up to 15,000–25,000 | ≤1.5–2 | ≤80% | Lithium | Slow toxin clearance (excepting toxins with slow redistribution) |
| Hemoperfusion | Unclear, but high | ≤1 L/kg | Any | Valproic acid, carbamazepine | Limited availability |
| Clotting | |||||
| Hypocalcemia | |||||
| Plasma exchange | No limit | ≤1 L/kg | Any | Monoclonal antibodies, arsine | Limited availability |
| Very slow clearance |
HCO, high-molecular-mass cutoff; HD, hemodialysis; CRRT, continuous renal replacement therapy.
To clear any substance from the body extracorporeally, it must be present in appreciable quantity in the intravascular space; thus, volume of distribution is typically the greatest determinant of extracorporeal removal of poisons. The volume of distribution, a theoretical volume which represents how much of a substance is present in plasma versus other spaces, is determined primarily by the lipophilicity of a substance; nonpolar, lipophilic toxins have high volumes of distribution and are not generally dialyzable, whereas hydrophilic toxins have lower volumes of distribution and are more easily dialyzable. Solutes with volumes of distribution >1–1.5 L/kg are poorly amenable to extracorporeal removal (see Table 2) (5,6).
Table 2.
Sample pharmacokinetic characteristics of toxins
| Toxin | Molecular Mass (Da) | Volume of Distribution (L/kg) | Protein Binding | Speed of Distribution from Plasma to Tissue | Dialyzability | Optimal Extracorporeal Modality for Removal |
|---|---|---|---|---|---|---|
| Amitriptyline | 277 | 19 | 95% | Fast | Not dialyzable | None |
| Colchicine | 399 | 5–8 | 40% | Fast | Not dialyzable | None |
| Ethylene glycol | 62 | 0.6–0.8 | Little to none | Fast | Dialyzable | HD |
| Lithium | 7 | 0.6–0.9 | Approximately 10% | Slower, may have rebound after HD | Dialyzable | HD; CRRT (nonemergent cases) |
| Metformin | 129 | 1–5 | None | Slower, may have rebound after HD | Moderately dialyzable | HD |
| Methotrexate | 454 | 0.8–2 | 35%–50% | Slower, may have rebound after HD | Moderately dialyzable | HD |
| Rituximab | 144,000 | <0.2 | None | Not applicable | Not dialyzable | Plasma exchange |
| Vancomycin | 1449 | <0.4–1 | <60% (varies) | Slower, may have rebound after HD | Moderately dialyzable | HD |
HD, hemodialysis; CRRT, continuous renal replacement therapy.
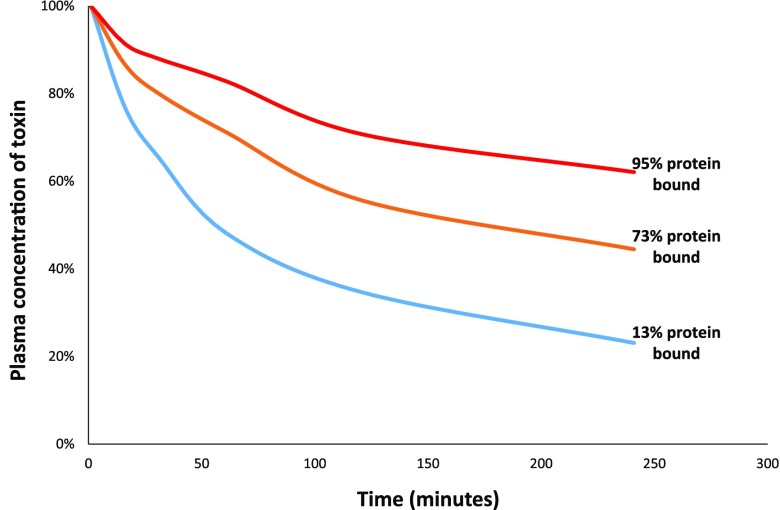
In plasma, almost all solutes are protein-bound to some degree (5). The plasma proteins that bind drugs are too large to be removed via extracorporeal therapies other than apheresis, and the fraction of toxins that are protein-bound are not removed via HD (6,7). Substances with <80% protein binding are typically amenable to extracorporeal removal via HD (6,8). The logarithmic nature of extracorporeal solute removal (Figure 1) results in little difference between the removal of a substance that is, for example, 20% protein-bound and one that is 70% protein-bound, but at >80% protein binding clearance drops sharply (8). Protein binding is saturable: certain drugs (salicylates, valproic acid, carbamazepine, and phenytoin, among others) have high protein binding at therapeutic concentrations, which limits dialyzability, but become amenable to removal at various toxic concentrations after binding sites are occupied and free drug levels rise (9–12).
Figure 1.
Removal of toxins via HD decreases with greater degrees of protein binding. Comparison of removal of three uremic toxins (p-cresyl glucuronide, 13% protein-bound; indole 3-acetic acid, 73% protein-bound; and p-cresyl sulfate, 95% protein-bound) during a single HD session averaged over ten patients. Blood flow rates were 300 ml/min, dialysate flow rates were 700 ml/min, and dialyzer urea clearances varied. Modified from reference 8, with permission.
Improvements in HD, which have been made to clear greater amounts of uremic toxins, particularly middle molecules, have increased the molecular mass of substances amenable to extracorporeal removal; for HD, conventional dialyzers may clear substances up to 15,000 Da, whereas high-cutoff hemofilters may clear substances closer to 50,000 Da in size (5,6,13,14). Plasmapheresis may clear substances of any size (15).
As toxins are removed from plasma via HD, they diffuse from tissue along their concentration gradient. Although most dialyzable substances move relatively quickly from tissue to plasma, certain toxins exhibit slower transit, leading to the phenomenon of “rebound,” where plasma levels of a given substance will increase hours after dialysis. Lithium is the best-known example, although others (metformin, methotrexate, vancomycin, dabigatran, etc.) may exhibit rebound after extracorporeal removal (16,17).
Optimization of extracorporeal removal of poisons depends primarily on increasing the volume of plasma filtered. Consider HD, where increasing blood flow rate, dialysate flow rate, dialyzer surface area, and time maximize toxin removal (18,19). This process is not linear: doubling therapy time does not result in removal of twice the amount of toxin. Utilizing a large overdose of methanol (serum level 400 mg/dl) as an example, in an 80 kg man, intermittent HD (2.5 m2 dialyzer, blood flow 400 ml/min, dialysate flow 800 ml/min) would take nearly 8 hours to lower serum concentrations to 20 mg/dl, whereas high-dose continuous venovenous HD (dialysate flow 6000 ml/h) would require almost 48 hours to achieve this (20).
A complicated issue not fully addressed in this review is when to consider the use of extracorporeal therapies for poisoning. There are relatively few drugs and other toxins amenable to extracorporeal removal, and some dialyzable poisonings rarely require this (e.g., acetaminophen). In addition, some toxins have greater endogenous clearance (kidney, hepatic, or otherwise) than extracorporeal elimination can provide, reducing the added value of the procedure; Table 3 displays suitability of extracorporeal removal for a variety of drug classes. The risks of the procedure need to be balanced against the risks of toxicity. Optimal use of extracorporeal therapies for poisoning generally involves performing the procedure emergently and maximizing the rate of toxin removal, particularly when a toxin produces life-threatening or irreversible toxicity. When needed, we would recommend to consider the involvement of a medical or clinical toxicologist, as not all nephrologists are comfortable managing difficult or rare forms of poisoning cases. Further, most nephrologists are rarely called to manage poisoning cases except when kidney replacement therapy is needed, and toxicologists may aid in managing aspects of poisoning other than extracorporeal therapy utilization; for example, glucarpidase (an enzyme which rapidly and effectively breaks down methotrexate) may be used instead of or in addition to HD in the treatment of methotrexate poisoning in patients with AKI (3).
Table 3.
Dialyzability of selected toxins by therapeutic class
| Therapeutic Class/Group of Poisons | Examples | General Pharmacokinetic Properties | Selected Agents Amenable to Extracorporeal Removal | Selected Agents Not Amenable to Extracorporeal Removal |
|---|---|---|---|---|
| Antiarrhythmics | Amiodarone, flecainide, lidocaine, sotalol | Lipophilic with high volume of distribution, protein binding | Sotalol (HD) | Amiodarone, flecainide, lidocaine |
| Anti-diabetic medications | Insulin, metformin, sulfonylureas | Varied | Metformin (HD) | Insulin, sulfonylureas |
| Antidepressants and antipsychotics | Amitriptyline, haloperidol, quetiapine, sertraline | Lipophilic with high volume of distribution, protein binding | Lithium (HD, CRRT) | SSRIs/SNRIs, TCAs, antipsychotics |
| Antiepileptics | Barbiturates, benzodiazepines, carbamazepine, phenytoin | Varied; generally at least moderately lipophilic | Barbiturates (HD); after massive ingestion, carbamazepine, phenytoin, valproic acid (HD) | Benzodiazepines, lamotrigine |
| Antimicrobials | Antifungals, antivirals, β-lactams, glycopeptides | Varied | Cefepime, vancomycin (HD) | Amphotericin |
| β-Adrenergic receptor blockers | Acebutolol, atenolol, carvedilol, labetalol, metoprolol | Varied | Acebutolol, atenolol, metoprolol (HD) | Most agents; carvedilol, propranolol, labetalol |
| Calcium channel blockers | Amlodipine, diltiazem, nifedipine, verapamil | Lipophilic with high volume of distribution, protein binding | None | Amlodipine, diltiazem, nifedipine, verapamil |
| Cardiac glycosides | Digoxin, plant toxins (e.g., oleandrin) | Lipophilic with high volume of distribution, protein binding | None | All |
| Chemotherapeutic agents | Cisplatin, methotrexate, rituximab, vincristine, doxorubicin | Varied | Methotrexate (HD), rituximab, cisplatin (plasma exchange) | Vincristine, doxorubicin |
| Methylxanthines | Caffeine, theophylline | Hydrophilic with low volume of distribution, low protein binding | All | None |
| Oral anticoagulants | Apixaban, dabigatran, rivaroxaban, warfarin | Most lipophilic with high volume of distribution, protein binding | Dabigatran (HD) | Apixaban, rivaroxaban, warfarin |
| Sympathomimetic agents | Cocaine, amphetamines, synthetic cathinones (“bath salts”) | Lipophilic with high volume of distribution, protein binding | None | Cocaine, amphetamines, synthetic cathinones |
HD, hemodialysis; CRRT, continuous renal replacement therapy; SSRIs, selective serotonin reuptake inhibitors; SNRIs, serotonin-norepinephrine reuptake inhibitors; TCAs, tricyclic antidepressants.
Modalities of Extracorporeal Therapy for Poisoning
Although HD is the preeminent extracorporeal modality utilized for poisoning, other modalities include continuous RRT (CRRT), hemoperfusion, therapeutic plasma exchange, exchange transfusion, and albumin dialysis (3). Comparison between these modalities is addressed in Table 1. As clearance of greater volumes of plasma will increase toxin removal, HD is the optimal choice for nearly all toxins amenable to extracorporeal removal. Peritoneal dialysis is not addressed in this review, primarily because its clearance of toxins is very slow; it should only be used if all other extracorporeal modalities are unavailable in resource-limited settings (3,4).
Although studies are scarce, CRRT use for poisoning has significantly increased; this is likely because of the ease of the procedure, especially in the context of shock (2). Unfortunately, because of slow blood and dialysate flow rates, CRRT has limited utility for the treatment of poisoning and should be foregone in most cases in favor of HD; lithium and other substances with slow movement from tissue to plasma, including some moderately lipophilic substances, are potential exceptions (21). Occasionally, CRRT may be used for convenience between HD sessions; although logistically more difficult to achieve, prolonged HD with more rapid toxin removal over time may be preferable for toxins (e.g., caffeine, salicylates) where rapidity of clearance may greatly affect the clinical course (3,12). Recent reports suggest that extracorporeal therapies are being used increasingly for supportive care of AKI in poisonings not amenable to extracorporeal removal. On the basis of current epidemiology, opioids and other sedative-hypnotic agents causing shock, sympathomimetics (e.g., cocaine) causing rhabdomyolysis and AKI, and severe acetaminophen poisonings (which may result in AKI and multiorgan failure) are some of the more common nondialyzable toxins receiving dialysis; it is likely that CRRT is often the therapy used in such cases (2,22).
Hemoperfusion, utilizing charcoal or resin-containing hemofilters, has traditionally been used primarily for poisoning; today, use has declined to roughly 1% of HD utilization in the United States (2). This likely reflects improvements in HD technique and availability, frequent complications of hemoperfusion (clotting, hypocalcemia, etc.), and cartridge cost (18,23). Other than for treatment of paraquat poisoning (largely in Asia, and often in combination with HD), hemoperfusion has little utility in treatment of poisonings nowadays (3,24).
Apheresis has utility in a relatively small number of poisonings, but has the advantage of removing toxins that may be impossible to remove via other methods (15). Poisons removed via apheresis optimally have very low volumes of distribution, as a lower volume of blood is processed during a single apheresis treatment than during a standard HD treatment. Exchange transfusion and therapeutic plasma exchange have been utilized for rare poisonings not otherwise amenable extracorporeal removal, such as snake envenomation, iatrogenic poisoning with monoclonal antibodies, arsine gas, and Amanita phalloides (a highly poisonous basidiomycete mushroom) poisoning, but it is unclear how much benefit is gained via these procedures (15,25,26).
Albumin dialysis refers to HD (typically CRRT) against a dialysate that contains circulating albumin, and is utilized primarily as support in liver failure (3,27). Although such systems can theoretically be useful to remove protein-bound toxins, and case reports of use in poisoning exist, albumin dialysis typically is inferior to HD at clearing poisons, with clearances of a number of protein-bound drugs (e.g., phenytoin, valproic acid) that are typically lower than that achievable by HD (3).
Extracorporeal Treatment for Individual Toxins
The use of extracorporeal therapies for poisoning has steadily grown over the past 50 years (2). In the United States, best-available data suggest that extracorporeal treatment is performed in 0.45% of poisoning exposures; detailed information (from the American Association of Poison Centers) is only available for roughly one fourth of these cases (2,28). Across different Western countries, ethylene glycol, lithium, and salicylates have consistently comprised roughly two thirds of poisonings treated via extracorporeal therapies (2).
Extracorporeal treatment of commonly dialyzable toxins is discussed below; the literature does not provide robust evidence for extracorporeal treatment of poisoning, and optimal treatment would involve a nephrologist and medical toxicologist working in concert for most dialyzable poisonings. The Extracorporeal Treatment in Poisoning (EXTRIP) workgroup, an international, multidisciplinary group that systematically reviewed the literature published concerning extracorporeal therapies for poisoning, has published the most evidence-based, thorough recommendations regarding extracorporeal treatment of poisoning; their work is available at www.extrip-workgroup.org (29).
Methanol
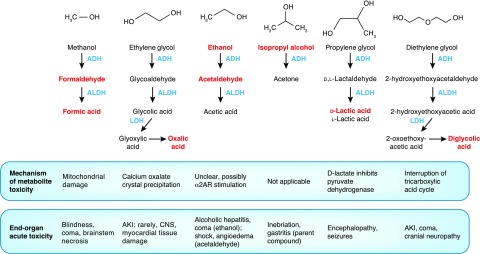
Methanol is widely used in a variety of products ranging from windshield wiper fluid, racing car fuel, to camping fuel, and in a number of industrial settings; outbreaks may also occur from tainted homemade ethanol (“moonshine”). Like other toxic alcohols, it is metabolized by alcohol dehydrogenase and aldehyde dehydrogenase into formaldehyde and formic acid, respectively (Figure 2), which both cause direct mitochondrial toxicity (30,31). The toxic effects of methanol metabolites are most prevalent in the central nervous system (CNS), where a variety of sequelae such as blindness, coma, and paresis may result; unfortunately, these are secondary to neural tissue death and are often poorly reversible (30,31). Although the alcohol dehydrogenase inhibitor fomepizole is commonly used to treat toxic alcohol poisonings, it does not remove these (readily dialyzable) toxic metabolites (31,32). After fomepizole administration, endogenous methanol clearance is exceedingly slow, with a t1/2 of roughly 50 hours; aggressive extracorporeal removal is often ideal given the high cost of fomepizole and prolonged hospitalization (32).
Figure 2.
Toxic alcohol metabolic pathways with mechanisms of toxicity of parent compounds and metabolites. Parent compounds and metabolites causing toxic effects (detailed at bottom of figure) are represented in red font. ADH, alcohol dehydrogenase; ALDH, aldehyde dehydrogenase; α2AR, α-2 adrenergic receptor; CNS, central nervous system; LDH, lactate dehydrogenase.
Similar to other toxic alcohols, methanol and metabolites are well removed via HD. HD clears methanol at roughly 200 ml/min, cutting the t1/2 to below 4 hours; importantly, the same is true of HD clearance of formic acid (31,32). The EXTRIP workgroup recommends extracorporeal treatment for severe methanol poisoning with acidemia, seizures, or vision deficits, or for serum concentrations >70 mg/dl after fomepizole; as most clinicians will not have access to real-time toxic alcohol levels, the osmolality gap (discussed below) may need to substitute for this (31). Because of the latent period between intoxication and onset of symptoms, as well as potential irreversibility of toxicity, early use of dialysis may need to be considered in concerning methanol ingestions.
Ethylene Glycol
Ethylene glycol is primarily used as antifreeze and in chemical manufacture. A small alcohol with two hydroxyl groups, it is metabolized via alcohol dehydrogenase, aldehyde dehydrogenase, and lactate dehydrogenase. Although oxalate is the metabolite primarily responsible for end-organ toxicity, glycolic acid is mostly responsible for metabolic acidosis (33,34). Clinical effects due to ethylene glycol poisoning include sedation and coma (due to parent compound), AKI, and occasionally CNS and myocardial damage. The latter effects are due to precipitation of calcium oxalate crystals, which directly damage tissue; although most commonly seen in kidney tubules where oxalate concentrations are highest, in massive ingestion these crystals may form in other tissues (33–35).
As access to real-time serum levels is uncommon, diagnosis of ethylene glycol poisoning commonly relies upon measuring an osmolality gap between calculated and measured serum osmolality (33). Notably, this method may miss small ingestions of ethylene glycol and serial chemistries observing for development of acidosis may be of use. Point-of-care blood gas analyzers, which commonly utilize the enzyme lactate oxidase, may misread chemically similar glycolic acid as lactate and return a falsely elevated serum lactate level; the “lactate gap” resulting from the difference between serum and whole blood lactate may be diagnostically useful if ethylene glycol intoxication is suspected (36).
Ethylene glycol and its metabolites are readily dialyzable. Clearance of ethylene glycol and metabolites via HD results in an elimination t1/2 under 3 hours (34). Although virtually all published recommendations suggest HD for severe acidemia or AKI after ethylene glycol poisoning, in recent years a number of cases have been treated with fomepizole alone given that endogenous kidney clearance results in a t1/2 of <18 hours (37). Recent evidence-based recommendations for when to perform extracorporeal therapy for ethylene glycol are not available; consideration should be given to massive ingestions without acidemia, particularly as ethylene glycol itself may cause coma.
Other Toxic Alcohols
Other than methanol and ethylene glycol, a number of short-chain alcohols may cause toxicity that may warrant consideration of extracorporeal removal. Short-chain alcohols share similar pharmacokinetic parameters with methanol and ethylene glycol, and as a rule are quite amenable to extracorporeal removal. Some of these alcohols are summarized briefly in Figure 2. Other than ethanol and isopropanol, HD and fomepizole are commonly used to treat these poisonings (38–40).
Salicylates
Readily dialyzable, salicylic acid was one of the first compounds removed via HD during early 20th century trials (1). Salicylates cause toxicity through multiple mechanisms, the primary one being uncoupling of mitochondrial oxidative phosphorylation (12,41,42). The clinical picture of salicylate poisoning is protean, with manifestations ranging from tinnitus, vomiting, metabolic acidosis (from lactate, ketones, and salicylate itself), primary respiratory alkalosis, agitated delirium, and/or somnolence; more severe presentations may include acute respiratory distress syndrome, AKI, hyperthermia, seizures, and shock (41,42). Fatalities are usually due to cerebral edema. Poisonings may be easily misdiagnosed (e.g., as sepsis), and events that lower blood pH (such as seizures or medications that slow the respiratory rate) may provoke rapid shifts of salicylate across the blood-brain barrier, worsening toxicity acutely (41,42). Early consideration of HD is critical in severe salicylate poisoning.
The EXTRIP workgroup reviewed extracorporeal treatment for salicylate poisoning; they recommend extracorporeal therapy for salicylate concentrations >100 mg/dl, as well as altered mental status and hypoxemia (12). However, perhaps the most compelling indication for extracorporeal therapy in salicylate poisoning is progressive toxicity despite appropriate medical treatment (e.g., intravenous sodium bicarbonate).
Acetaminophen (Paracetamol)
Although acetaminophen is readily dialyzable, most acetaminophen poisonings do not require extracorporeal removal because of the effectiveness of N-acetylcysteine (NAC) for early presentations (43,44). Classically, severe acetaminophen poisoning results in acute liver failure. However, in extremely large overdoses, acetaminophen may produce encephalopathy and lactic acidosis; this may be due to mitochondrial injury from acetaminophen itself, or locally elevated metabolite concentrations. Typically, this occurs at acetaminophen concentrations >750 mg/L (43). The effectiveness of NAC is not well established for these scenarios, and extracorporeal removal may have clinical benefit in preventing toxicity.
The EXTRIP workgroup reviewed 22 case reports and series, one observational trial, and one randomized, controlled trial comprising 127 patients; they concluded that there was sufficient evidence to suggest extracorporeal treatment of severe acetaminophen poisoning, with a recommendation to treat levels >900 mg/L in patients with altered mental status and lactic acidosis who are receiving NAC (43). It should be noted that NAC dosing may need alteration during dialysis (43).
Lithium
Despite more than a century of clinical use, lithium’s mechanism of toxicity remains poorly understood (16,45). Although lithium may cause comparatively mild gastrointestinal and cardiac toxicity, its major site of therapeutic and toxic action remains the CNS (16,45). Ataxia, myoclonus, and tremor are most commonly seen with lithium toxicity; severe symptoms include seizures, delirium, and coma, with fatalities stemming from severe neurotoxicity. Clinical presentation does not correlate well with lithium levels (16).
It is recommended practice that seizures, arrhythmias, or coma related to lithium toxicity should receive extracorporeal therapy. Although the EXTRIP workgroup recommends extracorporeal therapy for lithium poisoning at concentrations >4.0 mEq/L with reduced kidney function, and suggests this for levels >5.0 mEq/L, there is a relatively low level of evidence for optimal treatment of lithium even compared with other frequently dialyzed toxins, and clinical practice varies significantly (16).
Lithium has a low molecular mass, volume of distribution, and protein binding; it is readily removed from plasma by HD (3,4,16). Unlike most toxins, however, lithium travels from cells to plasma via sodium channels, and thus equilibrates more slowly than nearly all other dialyzable toxins, which directly diffuse across cell membranes; its extracorporeal removal is analogous to electrolytes such as phosphate (3,16). As a result, repeat HD sessions are commonly needed as rebound of plasma levels is seen several hours after therapy (3,4,16). CRRT may be used to avoid the issue of rebound, although caution is advised to consider HD initially in severe toxicity for rapid resolution of life-threatening symptoms.
One of the more severe complications of lithium toxicity is permanent neurotoxicity; it is unclear whether extracorporeal treatments affect this (45). Case series are conflicting on whether neurotoxicity is less common or more common after extracorporeal therapy, and it is uncertain if rapid removal of lithium avoids or increases the risk of long-lasting sequelae (45,46).
Metformin
Despite literature questioning the existence of metformin-associated lactic acidosis (MALA), numerous cases have been published in the literature (17,47). Although MALA is rare, with an estimated prevalence of <0.03 cases per 1000 patient-years, mortality of cases is high (17). Metformin inhibits glycerophosphate dehydrogenase and other enzymes that lead to decreased mitochondrial respiration (48). Metformin is solely removed through kidney excretion; almost all cases of MALA occur in the context of impaired kidney function. However, rarely, large acute metformin overdoses have provoked severe toxicity (17). Metformin toxicity results in lactic acidosis, shock, and multiorgan failure (likely due to both acidemia and reduced energetic substrates) (17,48).
Metformin is moderately dialyzable, with an average t1/2 of roughly 4 hours with HD and 16 hours with CRRT; endogenous clearance with normal kidney function is significantly faster (17). EXTRIP recommends HD for severe acidemia and lactate levels >20 mmol/L, and suggests consideration at lower levels with concomitant organ failure (17). Given the high mortality of MALA, consideration should be given to aggressive HD even in the presence of shock, to ensure adequate drug removal.
Opioids
In general, opioid poisoning is not best treated extracorporeally, antidotal and supportive therapies are generally sufficient. However, patients with ESKD may accumulate certain opioids and metabolites, and HD may be key to limiting this toxicity. Morphine-6-glucuronide, a major active morphine metabolite, may cause prolonged opioid effect in ESKD; this compound is moderately dialyzable (49). Similarly, hydromorphone-3-glucuronide, which may accumulate in patients with impaired kidney function receiving prolonged high-dose hydromorphone causing encephalopathy and seizures, may be removed via HD (50).
Conclusions
Extracorporeal therapies will undoubtedly continue as an increasingly important treatment option for poisoning, and nephrologists are central to their use in this context. Groups such as EXTRIP are expanding the relevant literature, and it is likely that extracorporeal therapy use will increase both for removal of toxins and supportive therapy after intoxication. Although not all extracorporeal modalities are available broadly, it behooves the practicing nephrologist to be familiar with the potential utility of extracorporeal therapy for poisoning even if they do not provide each therapy. However, given the rarity of extracorporeal therapy use in these settings, partnering with a medical toxicologist or a poison center for optimal care of these patients is advised.
Disclosures
Dr. Jaar reports receiving royalties from UpToDate for writing a chapter on “Lead-Related Nephrotoxicity” outside of the submitted work. Dr. Kern and Dr. King have nothing to disclose.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Abel JJ, Rowntree LG, Turner BB: On the removal of diffusible substances from the circulating blood of living animals by dialysis. J Pharmacol Exp Ther 53: 275–316, 1914 [Google Scholar]
- 2.Ghannoum M, Lavergne V, Gosselin S, Mowry JB, Hoegberg LC, Yarema M, Thompson M, Murphy N, Thompson J, Purssell R, Hoffman RS: Practice trends in the use of extracorporeal treatments for poisoning in four countries. Semin Dial 29: 71–80, 2016 [DOI] [PubMed] [Google Scholar]
- 3.Ouellet G, Bouchard J, Ghannoum M, Decker BS: Available extracorporeal treatments for poisoning: Overview and limitations. Semin Dial 27: 342–349, 2014 [DOI] [PubMed] [Google Scholar]
- 4.Garlich FM, Goldfarb DS: Have advances in extracorporeal removal techniques changed the indications for their use in poisonings? Adv Chronic Kidney Dis 18: 172–179, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Keller F, Wilms H, Schultze G, Offerman G, Molzahn M: Effect of plasma protein binding, volume of distribution and molecular weight on the fraction of drugs eliminated by hemodialysis. Clin Nephrol 19: 201–205, 1983 [PubMed] [Google Scholar]
- 6.Roberts DM, Buckley NA: Pharmacokinetic considerations in clinical toxicology: Clinical applications. Clin Pharmacokinet 46: 897–939, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Lam YW, Banerji S, Hatfield C, Talbert RL: Principles of drug administration in renal insufficiency. Clin Pharmacokinet 32: 30–57, 1997 [DOI] [PubMed] [Google Scholar]
- 8.Eloot S, Schneditz D, Cornelis T, Van Biesen W, Glorieux G, Dhondt A, Kooman J, Vanholder R: Protein-bound uremic toxin profiling as a tool to optimize hemodialysis. PLoS One 11: e0147159, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thanacoody RH: Extracorporeal elimination in acute valproic acid poisoning. Clin Toxicol (Phila) 47: 609–616, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Ghannoum M, Laliberté M, Nolin TD, MacTier R, Lavergne V, Hoffman RS, Gosselin S; EXTRIP Workgroup: Extracorporeal treatment for valproic acid poisoning: Systematic review and recommendations from the EXTRIP workgroup. Clin Toxicol (Phila) 53: 454–465, 2015 [DOI] [PubMed] [Google Scholar]
- 11.Ghannoum M, Yates C, Galvao TF, Sowinski KM, Vo TH, Coogan A, Gosselin S, Lavergne V, Nolin TD, Hoffman RS; EXTRIP Workgroup: Extracorporeal treatment for carbamazepine poisoning: Systematic review and recommendations from the EXTRIP workgroup. Clin Toxicol (Phila) 52: 993–1004, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Juurlink DN, Gosselin S, Kielstein JT, Ghannoum M, Lavergne V, Nolin TD, Hoffman RS; EXTRIP Workgroup: Extracorporeal treatment for salicylate poisoning: Systematic review and recommendations from the EXTRIP workgroup. Ann Emerg Med 66: 165–181, 2015 [DOI] [PubMed] [Google Scholar]
- 13.Kirsch AH, Lyko R, Nilsson LG, Beck W, Amdahl M, Lechner P, Schneider A, Wanner C, Rosenkranz AR, Krieter DH: Performance of hemodialysis with novel medium cut-off dialyzers. Nephrol Dial Transplant 32: 165–172, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gondouin B, Hutchison CA: High cut-off dialysis membranes: Current uses and future potential. Adv Chronic Kidney Dis 18: 180–187, 2011 [DOI] [PubMed] [Google Scholar]
- 15.Ibrahim RB, Liu C, Cronin SM, Murphy BC, Cha R, Swerdlow P, Edwards DJ: Drug removal by plasmapheresis: An evidence-based review. Pharmacotherapy 27: 1529–1549, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Decker BS, Goldfarb DS, Dargan PI, Friesen M, Gosselin S, Hoffman RS, Lavergne V, Nolin TD, Ghannoum M; EXTRIP Workgroup: Extracorporeal treatment for lithium poisoning: Systematic review and recommendations from the EXTRIP workgroup. Clin J Am Soc Nephrol 10: 875–887, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calello DP, Liu KD, Wiegand TJ, Roberts DM, Lavergne V, Gosselin S, Hoffman RS, Nolin TD, Ghannoum M; Extracorporeal Treatments in Poisoning Workgroup: Extracorporeal treatment for metformin poisoning: Systematic review and recommendations from the extracorporeal treatments in poisoning workgroup. Crit Care Med 43: 1716–1730, 2015 [DOI] [PubMed] [Google Scholar]
- 18.Bouchard J, Roberts DM, Roy L, Ouellet G, Decker BS, Mueller BA, Desmeules S, Ghannoum M: Principles and operational parameters to optimize poison removal with extracorporeal treatments. Semin Dial 27: 371–380, 2014 [DOI] [PubMed] [Google Scholar]
- 19.Leypoldt JK, Cheung AK, Agodoa LY, Daugirdas JT, Greene T, Keshaviah PR: Hemodialyzer mass transfer-area coefficients for urea increase at high dialysate flow rates. The Hemodialysis (HEMO) Study. Kidney Int 51: 2013–2017, 1997 [DOI] [PubMed] [Google Scholar]
- 20.Hirsch DJ, Jindal KK, Wong P, Fraser AD: A simple method to estimate the required dialysis time for cases of alcohol poisoning. Kidney Int 60: 2021–2024, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Kim Z, Goldfarb DS: Continuous renal replacement therapy does not have a clear role in the treatment of poisoning. Nephron Clin Pract 115: c1–c6, 2010 [DOI] [PubMed] [Google Scholar]
- 22.Gummin DD, Mowry JB, Spyker DA, Brooks DE, Fraser MO, Banner W: 2016 annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 34th annual report. Clin Toxicol (Phila) 55: 1072–1252, 2017 [DOI] [PubMed] [Google Scholar]
- 23.Ghannoum M, Bouchard J, Nolin TD, Ouellet G, Roberts DM: Hemoperfusion for the treatment of poisoning: Technology, determinants of poison clearance, and application in clinical practice. Semin Dial 27: 350–361, 2014 [DOI] [PubMed] [Google Scholar]
- 24.Gil HW, Kim SJ, Yang JO, Lee EY, Hong SY: Clinical outcome of hemoperfusion in poisoned patients. Blood Purif 30: 84–88, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Schwartz J, Padmanabhan A, Aqui N, Balogun RA, Connelly-Smith L, Delaney M, Dunbar NM, Witt V, Wu Y, Shaz BH: Guidelines on the use of therapeutic apheresis in clinical practice-evidence-based approach from the writing committee of the American Society for Apheresis: The seventh special issue. J Clin Apher 31: 149–162, 2016 [DOI] [PubMed] [Google Scholar]
- 26.Song Y, Wang D, Li H, Hao F, Ma J, Xia Y: Severe acute arsine poisoning treated by plasma exchange. Clin Toxicol (Phila) 45: 721–727, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Krisper P, Stadlbauer V, Stauber RE: Clearing of toxic substances: Are there differences between the available liver support devices? Liver Int 31[Suppl 3]: 5–8, 2011 [DOI] [PubMed] [Google Scholar]
- 28.Ghannoum M, Hoffman RS, Gosselin S, Nolin TD, Lavergne V, Roberts DM: Use of extracorporeal treatments in the management of poisonings. Kidney Int 94: 682–688, 2018 [DOI] [PubMed] [Google Scholar]
- 29.Lavergne V, Nolin TD, Hoffman RS, Roberts D, Gosselin S, Goldfarb DS, Kielstein JT, Mactier R, Maclaren R, Mowry JB, Bunchman TE, Juurlink D, Megarbane B, Anseeuw K, Winchester JF, Dargan PI, Liu KD, Hoegberg LC, Li Y, Calello DP, Burdmann EA, Yates C, Laliberté M, Decker BS, Mello-Da-Silva CA, Lavonas E, Ghannoum M: The EXTRIP (EXtracorporeal TReatments In Poisoning) workgroup: Guideline methodology. Clin Toxicol (Phila) 50: 403–413, 2012 [DOI] [PubMed] [Google Scholar]
- 30.Hovda KE, Hunderi OH, Tafjord AB, Dunlop O, Rudberg N, Jacobsen D: Methanol outbreak in Norway 2002-2004: Epidemiology, clinical features and prognostic signs. J Intern Med 258: 181–190, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Roberts DM, Yates C, Megarbane B, Winchester JF, Maclaren R, Gosselin S, Nolin TD, Lavergne V, Hoffman RS, Ghannoum M; EXTRIP Work Group: Recommendations for the role of extracorporeal treatments in the management of acute methanol poisoning: A systematic review and consensus statement. Crit Care Med 43: 461–472, 2015 [DOI] [PubMed] [Google Scholar]
- 32.Zakharov S, Pelclova D, Navratil T, Belacek J, Kurcova I, Komzak O, Salek T, Latta J, Turek R, Bocek R, Kucera C, Hubacek JA, Fenclova Z, Petrik V, Cermak M, Hovda KE: Intermittent hemodialysis is superior to continuous veno-venous hemodialysis/hemodiafiltration to eliminate methanol and formate during treatment for methanol poisoning. Kidney Int 86: 199–207, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barceloux DG, Krenzelok EP, Olson K, Watson W: American academy of clinical toxicology practice guidelines on the treatment of ethylene glycol poisoning. Ad hoc committee. J Toxicol Clin Toxicol 37: 537–560, 1999 [DOI] [PubMed] [Google Scholar]
- 34.Moreau CL, Kerns W 2nd, Tomaszewski CA, McMartin KE, Rose SR, Ford MD, Brent J: Glycolate kinetics and hemodialysis clearance in ethylene glycol poisoning. META study group. J Toxicol Clin Toxicol 36: 659–666, 1998 [DOI] [PubMed] [Google Scholar]
- 35.Armstrong EJ, Engelhart DA, Jenkins AJ, Balraj EK: Homicidal ethylene glycol intoxication: A report of a case. Am J Forensic Med Pathol 27: 151–155, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Brindley PG, Butler MS, Cembrowski G, Brindley DN: Falsely elevated point-of-care lactate measurement after ingestion of ethylene glycol. CMAJ 176: 1097–1099, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buchanan JA, Alhelail M, Cetaruk EW, Schaeffer TH, Palmer RB, Kulig K, Brent J: Massive ethylene glycol ingestion treated with fomepizole alone-a viable therapeutic option. J Med Toxicol 6: 131–134, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kraut JA, Kurtz I: Toxic alcohol ingestions: Clinical features, diagnosis, and management. Clin J Am Soc Nephrol 3: 208–225, 2008 [DOI] [PubMed] [Google Scholar]
- 39.Satoh Y, Ide Y, Sugano T, Koda K, Momose Y, Tagami M: Hypotensive and hypertensive effects of acetaldehyde on blood pressure in rats. Nihon Arukoru Yakubutsu Igakkai Zasshi 43: 188–193, 2008 [PubMed] [Google Scholar]
- 40.Landry GM, Dunning CL, Conrad T, Hitt MJ, McMartin KE: Diglycolic acid inhibits succinate dehydrogenase activity in human proximal tubule cells leading to mitochondrial dysfunction and cell death. Toxicol Lett 221: 176–184, 2013 [DOI] [PubMed] [Google Scholar]
- 41.Hill JB: Salicylate intoxication. N Engl J Med 288: 1110–1113, 1973 [DOI] [PubMed] [Google Scholar]
- 42.Chyka PA, Erdman AR, Christianson G, Wax PM, Booze LL, Manoguerra AS, Caravati EM, Nelson LS, Olson KR, Cobaugh DJ, Scharman EJ, Woolf AD, Troutman WG; Americal Association of Poison Control Centers; Healthcare Systems Bureau, Health Resources and Sevices Administration, Department of Health and Human Services: Salicylate poisoning: An evidence-based consensus guideline for out-of-hospital management. Clin Toxicol (Phila) 45: 95–131, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Gosselin S, Juurlink DN, Kielstein JT, Ghannoum M, Lavergne V, Nolin TD, Hoffman RS; Extrip Workgroup: Extracorporeal treatment for acetaminophen poisoning: Recommendations from the EXTRIP workgroup. Clin Toxicol (Phila) 52: 856–867, 2014 [DOI] [PubMed] [Google Scholar]
- 44.American College of Medical Toxicology: ACMT position statement: Duration of intravenous acetylcysteine therapy following acetaminophen overdose. J Med Toxicol 13: 126–127, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Adityanjee, Munshi KR, Thampy A: The syndrome of irreversible lithium-effectuated neurotoxicity. Clin Neuropharmacol 28: 38–49, 2005 [DOI] [PubMed] [Google Scholar]
- 46.Swartz CM, Jones P: Hyperlithemia correction and persistent delirium. J Clin Pharmacol 34: 865–870, 1994 [DOI] [PubMed] [Google Scholar]
- 47.Salpeter SR, Greyber E, Pasternak GA, Salpeter EE: Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus. Cochrane Database Syst Rev (1): CD002967, 2010 [DOI] [PubMed] [Google Scholar]
- 48.Madiraju AK, Erion DM, Rahimi Y, Zhang XM, Braddock DT, Albright RA, Prigaro BJ, Wood JL, Bhanot S, MacDonald MJ, Jurczak MJ, Camporez JP, Lee HY, Cline GW, Samuel VT, Kibbey RG, Shulman GI: Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature 510: 542–546, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bodd E, Jacobsen D, Lund E, Ripel A, Mørland J, Wiik-Larsen E: Morphine-6-glucuronide might mediate the prolonged opioid effect of morphine in acute renal failure. Hum Exp Toxicol 9: 317–321, 1990 [DOI] [PubMed] [Google Scholar]
- 50.Gagnon DJ, Jwo K: Tremors and agitation following low-dose intravenous hydromorphone administration in a patient with kidney dysfunction. Ann Pharmacother 47: e34, 2013 [DOI] [PubMed] [Google Scholar]