Abstract
Objective
To investigate the effect of gross saponins of Tribulus terrestris (GSTT) on erectile function in rats resulting from type 2 diabetes mellitus (T2DMED).
Methods
The T2DMED model was constructed by high-fat and high-sugar feeding and streptozotocin injection. After 4 weeks of GSTT intervention. Intracavernous pressure (ICP) and mean arterial pressure (MAP) were measured in each group. The level of nitric oxide (NO) in the cavernous tissue was detected using the nitrate reductase method. The production of reactive oxygen species (ROS) was detected using DHE fluorescent probe detection. Cyclic adenosine monophosphate (cGMP) level was detected by enzyme-linked immunosorbent assay, and endothelial nitric oxide synthase (eNOS) was detected using immunohistochemistry. Masson staining was used to detect the cavernosal smooth muscle/collagen ratio. Apoptosis in endothelial cells was measured using TUNEL. Western blotting method to detect the protein expression level of eNOS, TIMP-1, cleaved caspase 3, and cleaved caspase 9.
Results
After treatment, the ICP and ICP/MAP values of the GSTT were significantly higher than those of the T2DMED group (P<0.05). Unlike the T2DMED group, the GSTT group showed significantly increased NO levels (P<0.05) and decreased ROS levels (P<0.05). There was no significant difference between the GSTT group and the sildenafil group in increasing cGMP levels (P>0.05), and the mixed group had higher levels than these two groups (P<0.05). Immunohistochemistry and Western blotting showed that the expression of eNOS in the GSTT was significantly higher than that in the T2DMED groups (P<0.05). Masson staining showed that the smooth muscle/collagen ratio of the GSTT group was significantly higher than that of the T2DMED groups (P<0.05), the expression of TIMP-1 was lower than that of T2DMED group (P<0.05). TUNEL assay showed that the apoptotic index and cleaved caspase 3 and cleaved caspase 9 expression level of GSTT group were lower than that of the T2DMED group (P<0.05).
Conclusion
GSTT can protect T2DMED rats’ erectile function by improving penile endothelial function and inhibiting cavernosum fibrosis, inhibiting apoptosis, and is synergistic with sildenafil.
Keywords: erectile function, type 2 diabetes mellitus, endothelial cells, endothelial nitric oxide synthase, nitric oxide
Introduction
Erectile dysfunction (ED) is a common male disease, with glycolipid metabolism disorder and oxidative stress induced by type 2 diabetes mellitus (T2DM) as an important aspect of pathogenesis. Previous studies have confirmed that the incidence of ED in diabetes mellitus (DM) patients is as high as 32–90%,1,2 with an age of onset, is about 10 years earlier than non-DM patients. Although there are many treatments for ED, the efficacy is not always completely satisfactory, especially for ED related to DM. First-line therapy including sildenafil recommended by each guideline shows only a slight improvement.3–5 Gross saponins extracted form of Tribulus terrestris herbs (GSTT) constitute the main active ingredients used in traditional Chinese medicine for the treatment of impotence. This was first recorded in the “Shen Nong’s Herbal Classic” in China, and was also used to treat tract infections, inflammation, and other ailments. Previous studies have shown that T. terrestris improves glycolipid metabolism,6,7 anti-oxidative stress,8–10 and inhibits apoptosis. In this study, we aimed to explore the efficacy and mechanisms of GSTT in the treatment of erectile function in T2DMED rats.
Materials and methods
Experimental materials
Animals
Fifty adult male-specific pathogen-free (SPF) Sprague–Dawley rats 6–7 weeks old and weighing 180–220 g were provided by the Experimental Animal Center of Guangzhou University of Chinese Medicine (Laboratory Animal Certificate No. SCXK Yue 2013-0034) and raised in the SPF animal laboratory of the First Affiliated Hospital of Guangzhou University of Chinese Medicine under ethical approval (No. TCMF1-2018007) of this institute. All experiments were conducted in accordance with the approved guidelines (the National Standard GB/T35892-2018 of the People’s Republic of China). All efforts were made to minimize the suffering of rat and the minimum number of rats were used to meet the valid statistical evaluation based on the guidelines of the animal ethics committee of the institute.
Study design
Establishment of a T2DMED rat model
Rats were acclimated to the laboratory for 3 days and fed a normal diet, then weighed and given apomorphine (100 μg/kg; Sigma, USA) subcutaneously at the back of the neck and placed in a transparent experimental cage in a dark and quiet environment for 30 mins.12 Erectile function was recorded based on a standard of penis enlargement, foreskin retreat, glans exposure, and congestion. After removing rats with preexisting ED, six rats were randomly selected as a control group (n=6) provided with standard rat chow ad libitum. The remaining rats were fed with a high-fat and high-sugar diet containing 15% animal fat, 20% sucrose, and 5% cholesterol added to standard rat chow provided ad libitum for 8 weeks. An unlimited 50% glucose-water solution was also provided during this period. At the end of this duration, after fasting for 12 hrs, the control group received an intraperitoneal injection of sodium citrate buffer, while other rats were intraperitoneally injected with STZ (Sigma, USA) at 30 mg/kg in order to destroy islet β cells and induce T2DM. Blood glucose was measured using a tail-cut method on the third and seventh day after STZ injection, with random blood glucose >16.67 mmol/L criteria for T2DM development. Following this, blood glucose was measured once every 2 weeks. During this time, rats continued to receive a high-fat and high-sugar diet. At week 16, fasting blood glucose was measured, and APO was injected again to screen rats for erectile function. T2DMED rats with random blood glucose >16.67 mmol/L and no erectile function were divided into four groups of six rats apiece, including a T2DMED group receiving no treatment, a group treated with GSTT, a group treated with sildenafil, and a mixed group treated with GSTT and sildenafil. Including the non-diabetic control group, this yielded five groups in total.
Drug intervention
Treatments for all rats were administered via intragastric gavage daily for 4 weeks. The GSTT group was treated with GSTT obtained from Xi’an Wanfang Biotech Co., Ltd. dissolved in purified water at a dose of 40 mg/kg/d, and the sildenafil group was given sildenafil citrate (Pfizer Inc. NY, USA) suspension dissolved in purified water after grinding into powder at 5 mg/kg/d. The mixed group was given equal amounts of saponin solution at 40 mg/kg/d and sildenafil citrate suspension at 5 mg/kg/d. Rats in the untreated T2DMED group were given the same volume of purified water.
Evaluation of erectile function
After 4 weeks of treatment,13 an intraperitoneal injection of 10% chloral hydrate at a dose of 100 mg/kg was administered to each rat. A midline incision of the abdomen and neck was made to expose the bilateral cavernous body nerve and common carotid artery, respectively. A bipolar silver electrode was placed in the right cavernous nerve, and two 23-G infusion needles which filled with 250 U/mL heparin saline were inserted into the right penis root and the right common carotid artery and connected to a pressure transducer linked to record intracavernous pressure (ICP) and mean arterial pressure (MAP). Stimulation parameters were set to 5 V voltage and 20 Hz frequency for 30 s at intervals of 5 mins. ICP-value and the MAP-value were recorded using a Delphis electrophysiometer recorder (Laborie Medical Technologies, Ontario, Canada).
Drawing and slicing
After the erectile function evaluation test was completed, the penis of the rat was cut from the root, the fascia was separated, and the removed organ was divided into two parts. A part of the tissue was fixed with 4% paraformaldehyde and embedded in paraffin and another part of the tissue was frozen in liquid nitrogen and stored at ‒80°C for later use.
Determination of endothelial nitric oxide synthase (eNOS) expression level
Tissue samples were fixed, embedded in paraffin, and sectioned at a thickness of 5 μm. After dewaxing and rehydration, antigen was repaired and blocked, and incubated with anti-eNOS-antibody overnight at 4°C. The sections were then incubated with biotin-conjugated secondary antibody for 30 mins at 37°C, followed by incubation with avidin-4 horseradish peroxidase complex for 30 mins at 37°C, then the protoplast was processed to perform the reaction and visualize by DAB chromogen. Sections were counterstained with diluted hematoxylin and fixed with neutral glue. Five high power fields (200×) were randomly selected for each slice. The image of eNOS immunohistochemistry in each group of penis tissues was analyzed using Image-Pro Plus 6.0 image analysis software.
Determination of nitric oxide (NO) level
Penile tissue stored at ‒80°C was homogenized and in a cold phosphate buffer solution at 2–8°C. Each homogenized sample was centrifuged at 1500 rpm for 15 mins at 4°C, and the supernatant was recovered for detection of NO levels. The protein content per milligram of tissue was determined using a Coomassie Brilliant Blue Assay Kit (Nanjing Institute of Bioengineering).
Determination of cyclic adenosine monophosphate (cGMP) expression level
The obtained penile tissue was homogenized in cold sodium acetate, and cGMP expression level in the corpus cavernosum was detected based on a rat cGMP ELISA kit (R&D Systems, Minneapolis, USA). Protein levels were determined using a BCA Protein Assay Kit (Beyotime Biotechnology, Shanghai, China).
Determination of reactive oxygen species (ROS) levels
As per Davidson et al,14 the expression of ROS in each group was detected using a fluorescent probe with DHE superoxide anion level (Servicebio, Wuhan, China). Each section was photographed in five areas under a 200× optical microscope, using Image-Pro Plus 6.0 image analysis software (Media Cybernetics, Maryland, USA). The determination of tissue ROS content and tissue homogenate crude extract protein was carried out using Nanjing Jiancheng Bioengineering Research Institute kits per manufacturer’s instructions. ROS content was defined as the reduction of hydrogen peroxide concentration in the reaction system by 1 μmol per milligram of tissue protein per minute at 37°C as an anti-reactive oxygen unit (U/mg).
Masson staining
Tissue sections of the middle part of the penis were stained according to Masson kit instructions (Servicebio, Wuhan, China). The collagen tissues of the penile sponge (stained blue or green) and smooth muscle tissues (stained red) were observed under a light microscope. The cavernous smooth muscle/collagen ratio was analyzed using Image-Pro plus 6.0 software.
TUNEL staining
Apoptosis of the penile tissue of each group was detected using a TUNEL apoptosis detection kit following manufacturer’s instructions. The TUNEL picture (200×) of each group of penis tissue was analyzed using Image-pro plus 6.0. Each sample was randomly selected from four fields of view. Cells with brown staining were counted as apoptotic, and the ratio of apoptotic cells to the total number of cells in the field of view yielded the rate of apoptosis.
Western blotting
The sample protein concentration was quantified by BCA method, and each sample protein extract was adjusted to the same concentration level by PBS buffer. SDS-PAGE electrophoresis, transfection, 5% skim milk powder blocking, routine anti-eNOS (Servicebio, China), TIMP-1 (ZSGB-BIO, China), cleaved caspase 3 (Abcam, USA), cleaved caspase 9 (Abcam, USA), overnight incubation at 4°C, TBST wash membrane three times, 10 mins each time, the corresponding horseradish peroxidase IgG was incubated at room temperature for 120 mins, and TBST was washed three times for 10 mins each time, and exposed by ECL method. Gray value analysis was performed using Image-pro plus 6.0 software, and the results were expressed as the ratio of the gray value of the target protein to the GAPDH band of the internal reference protein.
Statistical analysis
Data processing was performed using SPSS 20.0 software. All data were expressed as mean ± standard error. One-way ANOVA was used to compare means and a Tukey test was performed to determine significant differences. P<0.05 was considered statistically significant.
Results
General conditions
Bodyweight and blood glucose levels of the rats in each time period are shown in Table 1. At the end of the experiment (20 weeks), all groups showed changes in body weight and blood glucose. Mean weight in the non-diabetic control group was significantly higher than that of the T2DMED group (P<0.05). Compared with the T2DMED group, the weight of the GSTT group and the mixed group increased slightly, but the difference between GSTT group and the mixed group was not statistically significant. By the end of the experiment, the blood glucose of each group was significantly higher than that of the non-diabetic control group (P<0.05). Blood glucose in the GSTT group and the mixed group was significantly different from the T2DMED group (P<0.05).
Table 1.
Rat body weight and blood glucose levels during the experiment (x±s)
| n | Body weight (g) | Blood glucose (mmol/L) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Initial | 8 weeks | 16 weeks | 20 weeks | Initial | 8 weeks | 16 weeks | 20 weeks | ||
| Control | 6 | 191±12.3 | 382.4±10.2 | 486.1±9.5 | 522.1±15.4 | 6.7±0.4 | 6.5±0.5 | 6.6±0.5 | 6.7±0.6 |
| DM | 6 | 201.3±3.3 | 407.9±17.6a | 402.3±15.3a | 408.1±16.1a | 6.1±0.2 | 7.2±0.5 | 26±1.2a | 23.8±1.6a |
| Sildenafil | 6 | 202.6±6 | 407.7±15a | 404.3±17.7a | 402.1±9.2a | 6.6±0.4 | 6.6±0.3 | 27.1±1.8a | 25.6±1.4* |
| GSTT | 6 | 197.6±6.6 | 405.9±14.7a | 393.1±7.6a | 419.4±20.6a | 6.5±0.5 | 7±0.9 | 24±0.9a | 20.1±1.3a,b |
| Mixed | 6 | 192.9±15 | 403±15.6a | 400.8±16a | 420.2±20.9a | 6.3±0.4 | 6.6±0.4 | 26.3±1.6a | 21.1±1a,b |
Notes: aCompared with the blank group, P<0.05; bCompared with the model group, P<0.05.
Abbreviations: DM, diabetes mellitus; GSTT, gross saponins of Tribulus terrestris.
The levels of blood lipids and insulin secretion are shown in Table 2. By the end of the experiment, Total Cholesterol, triglyceride, and low-density lipoprotein (LDL) were significantly increased in each group compared with the control group. Only LDL expression was observed decrease by GSTT intervention. In the 16th week, before the injection of STZ, the fasting insulin of the rats in each group showed a significant increase (P<0.05). At the 20th week, the insulin expression in the rats decreased with the intervention of GSTT (P<0.05). The insulin sensitivity index was significantly improved (P<0.05). This suggests that the rat model showing the characteristics of insulin resistance. Combined with hyperlipidemia, the model simulates the characteristics of patients with T2DM well.
Table 2.
Rat blood lipids and insulin level during the experiment (x±s)
| n | TC (mmol/L) | TG (mmol/L) | LDL (mmol/L) | FINS (ng/mL) | ISI | ||
|---|---|---|---|---|---|---|---|
| 20 weeks | 16 weeks | 20 weeks | 20 weeks | ||||
| Control | 6 | 1.31±0.08b | 0.68±0.04b | 0.73±0.05b | 0.51±0.02b | 0.52±0.02b | −4.57±0.11 |
| T2DMED | 6 | 2.36±0.15a | 0.83±0.08a | 1.60±0.08a | 0.75±0.05a | 0.79±0.04a | −5.16±0.13a |
| Sildenafil | 6 | 2.33±0.13a | 0.82±0.10a | 1.64±0.06a | 0.74±0.02a | 0. 80±0.03a | −5.21±0.09a |
| GSTT | 6 | 2.29±0.10a | 0.83±0.11a | 1.25±0.10a,b | 0.78±0.04a | 0. 67±0.04a | −5.07±0.10a,b |
| Mixed | 6 | 2.36±0.13a | 0.86±0.05a | 1.25±0.06a,b | 0.80±0.03a | 0.68±0.04a,b | −4.97±0.11a,b |
Notes: aCompared with the blank group, P<0.05; bCompared with the model group, P<0.05.
Abbreviations: TC, total cholesterol; TG, triglyceride; LDL, low-density lipoprotein; T2DMED, type 2 diabetes mellitus; GSTT, gross saponins of Tribulus terrestrise.
Evaluation of erectile function
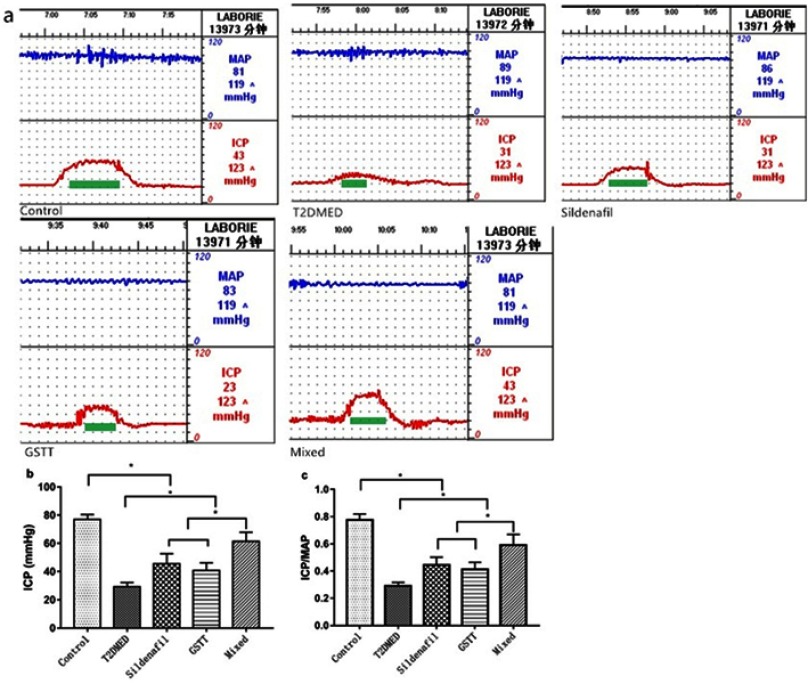
After the experiment, cavernous nerve was electrically stimulated to measure ICP and MAP. The results showed that the ICP (61±6.7) mmHg and ICP/MAP (0.59±0.08) in the mixed group were significantly greater than in the GSTT group (40±5.3 mmHg, 0.41±0.05), sildenafil group (45±7.3 mmHg, 0.45±0.06), and T2DMED group (29±3.1 mmHg, 0.77±0.04) (P<0.05), but still lower than the non-diabetic control group (77±3.4 mmHg, 0.74±0.05) (P<0.05). The corresponding values of the GSTT and sildenafil groups were significantly higher than those of T2DMED group (P<0.05), but there was no significant difference between the two groups (P>0.05) (Figure 1). This suggests that both GSTT and sildenafil can improve erectile function, with synergistic effects.
Figure 1.
Comparison of ICP and ICP/MAP after the end of experiment in each group of rats.
Note: (A): Representative rat ICP and MAP-values; (B): ICP comparison between groups, n=6; c: ICP comparison between groups, n=6; *P<0.05.
Abbreviations: T2DMED, type 2 diabetes mellitus; GSTT, gross saponins of Tribulus terrestris; ICP, intracavernous pressure; MAP, mean arterial pressure.
eNOS expression level
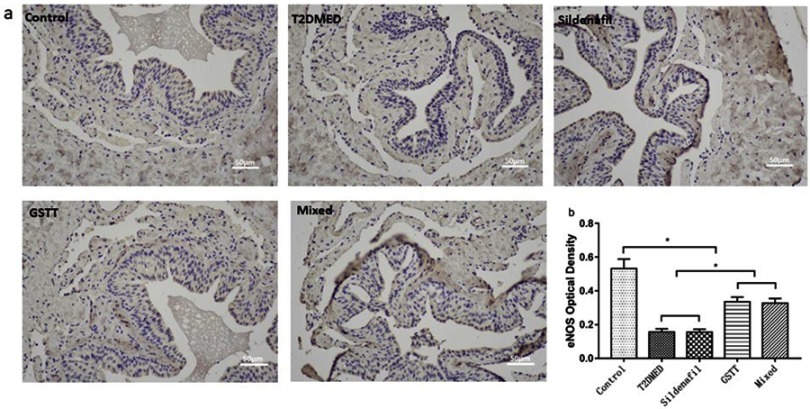
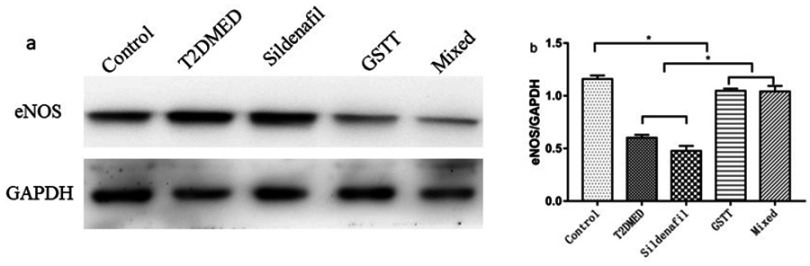
The expression of eNOS in rat corpus cavernosum was detected using immunohistochemistry. The results showed that the expression of eNOS in the penis of the control group was strongly positive, while expression in the GSTT and mixed groups was slightly but significantly weaker than in the control group (P<0.05), but significantly higher than in the T2DMED and sildenafil groups (Figure 2, P<0.05). And this trend was also verified in Western blotting (Figure 3).
Figure 2.
Shows the expression of eNOS in the corpus cavernosum of rats.
Note: (A): Representative rat penile corpus cavernosum eNOS immunohistochemistry picture (nucleus is blue, positive expression is brownish yellow); (B): Statistical cube of eNOS ratio in penile sponge of each group; *P<0.05.
Abbreviations: T2DMED, type 2 diabetes mellitus; GSTT, gross saponins of Tribulus terrestrise; NOS, endothelial nitric oxide synthase.
Figure 3.
Comparison of eNOS protein expression in the corpus cavernosum of each group.
Note: The representative rat corpus cavernosum eNOS protein band diagram and the statistical cube of eNOS protein expression and internal reference value in the penis of each group; *P<0.05.
Abbreviations: T2DMED, type 2 diabetes mellitus; GSTT, gross saponins of Tribulus terrestrise; eNOS, endothelial nitric oxide synthase.
NO expression level
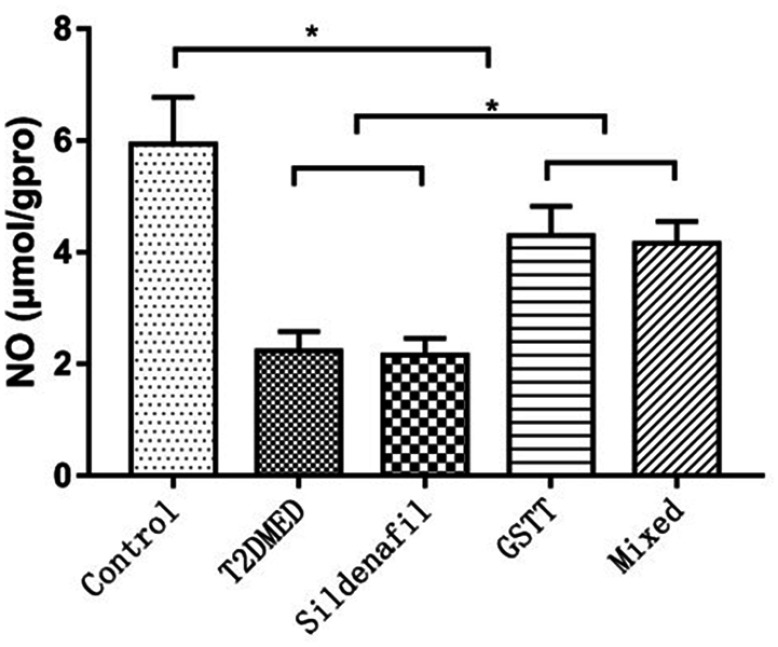
The NO levels in the penis of the non-diabetic control, T2DMED, sildenafil, GSTT, and mixed groups were 5.93±0.85, 2.23±0.35, 2.16±0.30, 4.30±0.52, and 4.16±0.39 μmol/gprot, respectively. The level was significantly higher in the GSTT and mixed groups than in the T2DMED group (P<0.05), and significantly lower than that in the non-diabetic control group (P<0.05) (Figure 4).
Figure 4.
Statistical cube diagram of NO ratio in penile sponge of rats in each group; *P<0.05.
Abbreviations: T2DMED, type 2 diabetes mellitus; GSTT, gross saponins of Tribulus terrestrise;NO, nitric oxide.
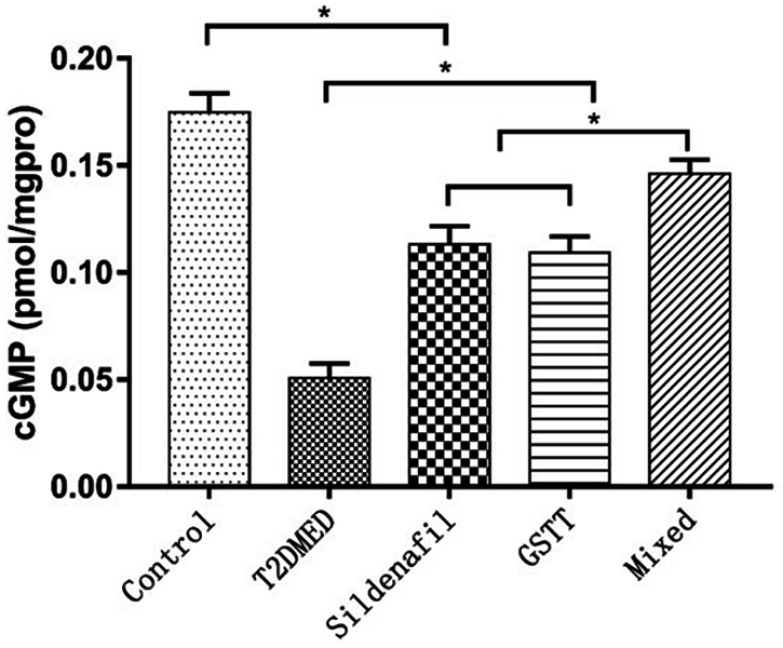
cGMP expression level
The expression levels of cGMP in the control, T2DMED, sildenafil, GSTT, and mixed groups were 0.175±0.009, 0.051±0.001, 0.113±0.008, 0.109±0.008, and 0.146±0.006 pmol/gprot, respectively. The cGMP level in the T2DMED group was significantly lower than in the non-diabetic control group (P<0.05). After treatment, cGMP expression in the sildenafil, GSTT, and mixed groups was significantly higher than that in the T2DMED group, and the difference was statistically significant (P<0.05). At the same time, the expression of cGMP in the mixed group was significantly higher than in the sildenafil and the GSTT groups (P<0.05), and significantly lower than in the non-diabetic control group (P<0.05) (Figure 5). This suggests that GSTT and sildenafil may have synergistic effects in increasing cGMP expression.
Figure 5.
Statistical cube diagram of cGMP ratio in the penis of each group of rats; *P<0.05.
Abbreviations: T2DMED, type 2 diabetes mellitus; GSTT, gross saponins of Tribulus terrestrise; cGMP, cyclic adenosine monophosphate.
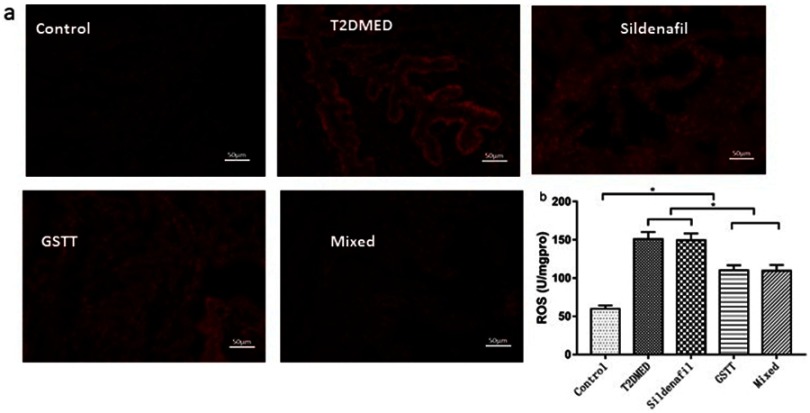
ROS levels in each group
The expression levels of ROS in the penis of the non-diabetic control, T2DMED, sildenafil, GSTT, and mixed groups were: 59.67±4.55, 151.01±0.099, 149.33±0.89194, 110.01±0.69328, and 109.33±0.84007 µmol/gprot. ROS expression level in the T2DMED group was significantly higher than in the non-diabetic control group (P<0.05). After GSTT treatment, the expression of ROS in the GSTT and mixed groups decreased below the level in the T2DMED group (P<0.05), but were still significantly higher than in the control group (P<0.05) (Figure 6).
Figure 6.
Differences in ROS expression between groups of rats.
Note: (A): Representative rat corpus cavernosum ROS fluorescence staining; (B): statistical cube of ROS ratio in the penis of each group; *P<0.05.
Abbreviations: T2DMED, type 2 diabetes mellitus; GSTT, gross saponins of Tribulus terrestrise; ROS, reactive oxygen species.
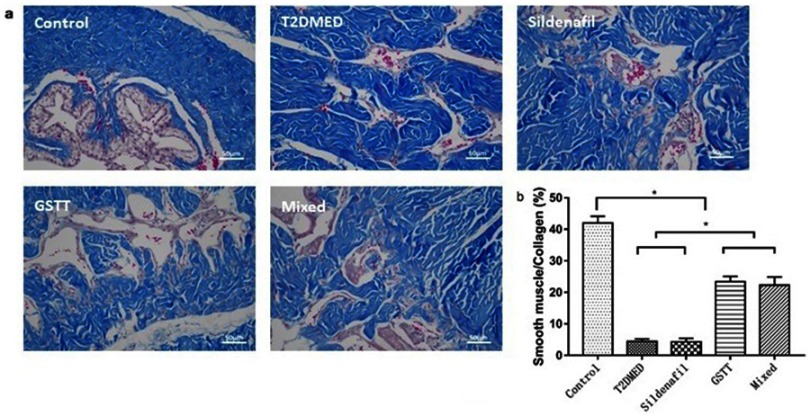
Assessment of smooth muscle fibrosis
Masson staining
The ratio of cavernosum smooth muscle/collagen in the non-diabetic control, T2DMED, sildenafil, GSTT, and mixed groups was 41.99±2.13, 4.44±0.78, 23.39±1.65, 4.29±1.10, and 22.28±2.63, respectively. The smooth muscle/collagen ratio of the GSTT and mixed groups was significantly higher than in the T2DMED and sildenafil groups (P<0.05); but significantly lower than in the control group (P<0.05) (Figure 7). This indicates that GSTT can improve cavernosum fibrosis in T2DMED rats.
Figure 7.
Comparison of the ratio of smooth muscle/fibrous collagen in the corpus cavernosum of each group.
Note: (A): Representative rat corpus cavernosum Masson staining map (red for smooth muscle, blue for collagen tissue); (B): statistical cube for ratio of smooth muscle/collagen fiber in penile sponge of each group; *P<0.05.
Abbreviations: T2DMED, type 2 diabetes mellitus; GSTT, gross saponins of Tribulus terrestrise.
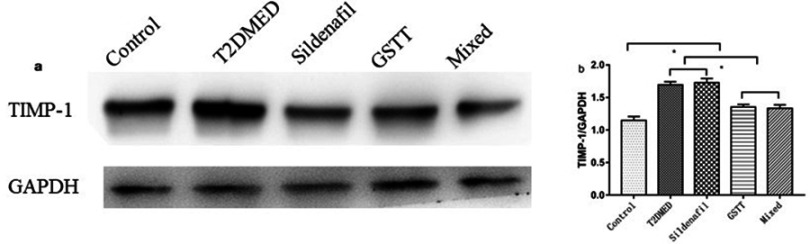
Expression of TIMP-1 in the corpus cavernosum of rats
The expression of TIMP-1 protein in rat corpus cavernosum was detected by Western blotting (Figure 8). The results showed that the expression of TIMP-1 in the penis of the control group was weak, and the expression of T2DMED group and Sildenafil group increased significantly. After intervention, GSTT group and mixed group express TIMP-1 protein significantly lower than the T2DMED group (P<0.05).
Figure 8.
Comparison of TIMP-1 protein expression in the corpus cavernosum of each group.
Note: (A): The representative rat corpus cavernosum TIMP-1 protein band diagram; (B): statistical cube of TIMP-1 protein expression and internal reference value in the penis of each group; *P<0.05.
Abbreviations: T2DMED, type 2 diabetes mellitus; GSTT, gross saponins of Tribulus terrestrise.
Assessment of apoptotic index
TUNEL staining
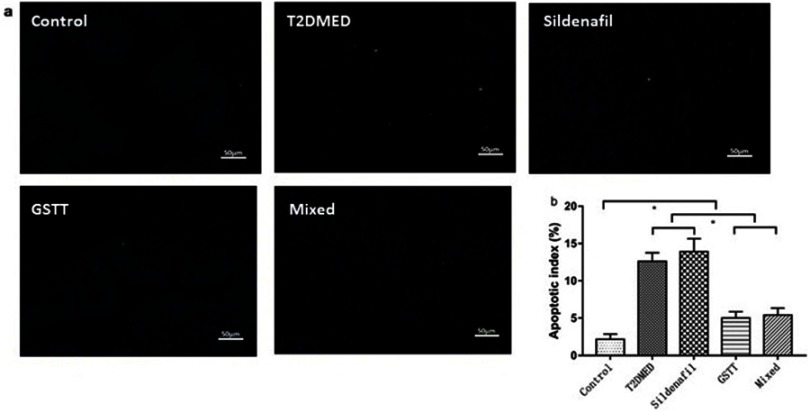
The apoptotic index (%) of the penis in the non-diabetic control, T2DMED, sildenafil, GSTT, and mixed groups were 2.19±0.68, 12.59±1.17, 5.03±0.87, 13.92±1.75, and 5.43±0.93, respectively. The apoptotic index of the GSTT and mixed groups were significantly lower than the T2DMED and sildenafil groups (P<0.05), but higher than the control group (P<0.05) (Figure 9). This indicates that GSTT can reduce the level of apoptosis in the corpus cavernosum of T2DMED rats.
Figure 9.
Comparison of apoptotic index in the corpus cavernosum of each group.
Note: (A): Representative TUNEL staining map, positive apoptotic cell nucleus is fluorescent green; (B): difference in apoptosis index between different treatment groups; *P<0.05.
Abbreviations: T2DMED, type 2 diabetes mellitus; GSTT, gross saponins of Tribulus terrestrise.
Expression of Cleaved caspase 3 and Cleaved caspase 9
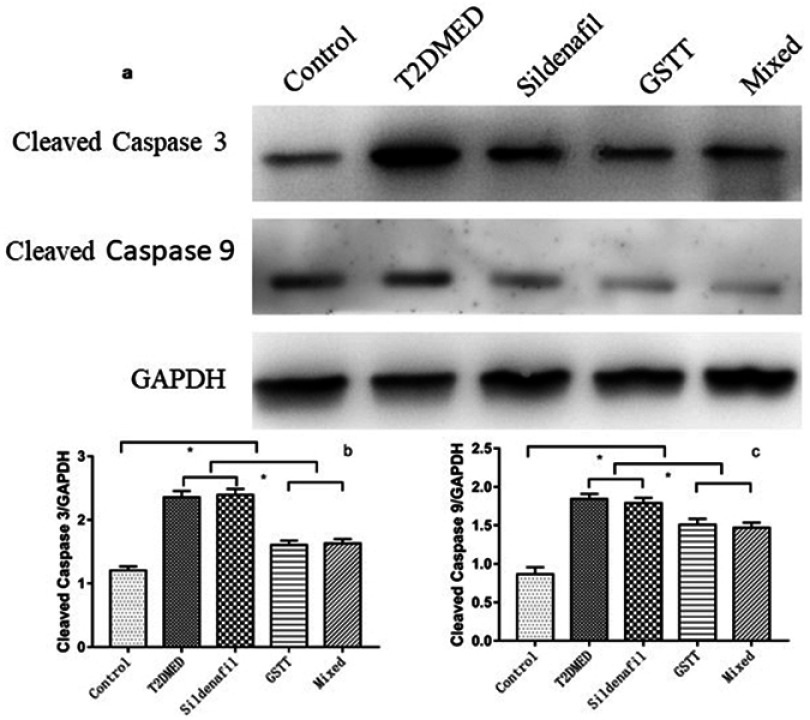
Western blotting was used to detect the expression of Cleaved caspase 3 and cleaved caspase 9 in rat corpus cavernosum. As shown in Figure 10, the expression of Cleaved caspase 3 and cleaved caspase 9 in the penis of control group was weak, and the expression of T2DMED group and Sildenafil group was significantly increased, while the GSTT group and the mixed group were significantly lower than the T2DMED group (P<0.05).
Figure 10.
Comparison of Cleaved caspase 3 and Cleaved caspase 9 protein expression in the corpus cavernosum of each group.
Note: (A): The representative corpus cavernosum Cleaved caspase 3 and Cleaved caspase 9 protein band diagram; (B, C): the statistical cube of Cleaved caspase 3 and Cleaved caspase 9 protein expression and internal reference value in the penis of each group; *P<0.05.
Abbreviations: T2DMED, type 2 diabetes mellitus; GSTT, gross saponins of Tribulus terrestrise.
Discussion
Complete endothelial function is an important physiological basis for erectile function. A long-term high glucose environment causes an accumulation of oxidative stress and damage in endothelial cells,15 which can lead to intravascular penile lumen stenosis and thrombosis followed by ischemia and hypoxia of the cavernous tissue, damage to smooth muscle, and fibrosis.16 eNOS plays a key role in NO production and relies on intact endothelial cell function. Abnormalities in eNOS expression and activity due to endothelial dysfunction and NO synthesis disorders are considered to be caused by various vascular diseases, including DM. This is an important pathological basis for diseases such as ED,17,18 and this change often continues after blood glucose is controlled.
Various methods have been investigated for treating T2DMED, including the use of stem cells to repair damaged tissues,19 or using islet kininase to improve oxidative stress.20 These treatments have had some effect, but are not yet sufficiently developed for clinical application. Therefore, the current first-line treatment plan is still oral administration of a Phosphodiesterase type 5 (PDE5) inhibitor such as sildenafil. This can promote erectile function by inhibiting the activity of PDE5 and reducing the degradation of cGMP in the NO/cGMP pathway. However, long-term clinical studies have found that patients with T2DMED do not have the desired outcome under PDE5 inhibitor treatment, and may show only a slight improvement.3–5 We speculate that this is because T2DMED is not due to excessive degradation of cGMP, but due to an insufficient source of cGMP. That is, a long-term high glucose environment leads to endothelial cell damage, fibrosis, and increased apoptosis, which reduces NO synthesis and eventually leads to insufficient cGMP,21,22 causing ED. Therefore, repairing the vascular endothelial cells, increasing NO expression, improving smooth muscle fibrosis, and inhibiting apoptosis are key in the treatment of T2DMED.
Tribulus terrestris is a commonly used traditional Chinese medicine, which functions to tonify the kidney and calm the liver, and is often used for the treatment of eyesight, diuretic, cough, skin itching, headache and dizziness, and blockage of the mammary duct. In India, its fruit is also used to treat diseases such as infertility, impotence, ED, and low libido.23 Modern research has shown that the main role of T. terrestris depends on the anti-inflammatory and anti-aging activities exhibited by steroidal saponins and steroids.
GSST is often used to improve sexual function, and has also been shown to improve insulin-dependent DM by inhibiting α-glucosidase.6,7 Clinical trials have demonstrated that GSTT can significantly improve blood glucose and anti-oxidative stress,8,10 and can inhibit apoptosis induced by oxidative stress,11 ROS is a product of the combination of AGEs (Advanced Glycation End Products) and RAGE (Receptor of Advanced Glycosylation End Products), and plays an important role in oxidative stress, which activates the redox-sensitive transcription factor nuclear factor-JB in vascular wall cells, promoting the expression of various atherosclerosis-associated genes and RAGE.24 In this study, we demonstrated that GSTT alone can reduce ROS content in the cavernous and reduce oxidative stress. We also found that GSTT can improve blood glucose levels and insulin secretion in rats, though not entirely normalize it. At the same time, ICP and ICP/MAP records of the rats in this study also showed that the erectile function of the mixed treatment group significantly improved compared with the T2DMED group, the GSTT-only group, or the sildenafil-only group. This confirms previous studies on GSTT in basic and clinical trials to improve erectile function.25–28 We further found that GSTT can improve the expression of eNOS in rat endothelial cells, directly increasing the concentration of NO, and ultimately improving the level of cGMP in each group. Moreover, the final concentration of cGMP in the mixed treatment group was significantly higher than in rats treated with only GSTT or only sildenafil, suggesting a synergistic effect. Finally, in terms of tissue structure, long-term DM causes smooth muscle/collagen ratio imbalance, which is an important factor in the occurrence of T2DMED.29 In our study, Masson staining showed a significant decrease in the smooth muscle/collagen ratio of the corpus cavernosum in the T2DMED group compared to the non-diabetic control group, and the expression of TIMP-1 suggesting that GSTT can improve penile fibrosis and protect the structure of the corpus cavernosum.
This study has some shortcomings, although the T2DM model in this study is better representative of human T2DM than the model used in a previous study30-32 and more economical. Our experiment try to simulate the normal pathogenesis of T2DM, i.e., via long-term high-sugar and high-fat diet causing insulin resistance, followed by small doses of STZ to destroy rat islet cells. And based on our data with regard to body weight and blood glucose and insulin, most of our model is closer to T2DM, but there are still some models (about 40%) are fail to build, suggest that the modeling method still needs improvement. In addition, although we have identified clear histological and molecular changes that played a role in improving erectile function, there may be other factors that may regulate this functional improvement which require further study.
Conclusion
This study demonstrates that gross saponin of T. terrestris improves erectile function of T2DMED rats by repairing endothelial cells, improving cavernosal smooth muscle fibrosis, and inhibiting smooth muscle cell apoptosis. In addition, there is a synergistic effect with sildenafil in the improvement of erectile function.
Acknowledgment
This project was funded by the Jiangxi Provincial Administration of Traditional Chinese Medicine, China (Project no: 2018B129) and we would like to thank Editage for English language editing.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Kamenov ZA. A comprehensive review of erectile dysfunction in men with diabetes. Exp Clin Endocrinol Diab. 2015;123(3):141–158. [DOI] [PubMed] [Google Scholar]
- 2.Giugliano F, Maiorino M, Bellastella G, et al. Determinants of erectile dysfunction in type 2 diabetes. Int J Impot Res. 2010;22(3):204. doi: 10.1038/ijir.2010.1 [DOI] [PubMed] [Google Scholar]
- 3.Hidalgo-Tamola J, Chitaley K. Type 2 diabetes mellitus and erectile dysfunction. J Sex Med. 2009;6(4):916–926. doi: 10.1111/j.1743-6109.2008.01116.x [DOI] [PubMed] [Google Scholar]
- 4.Ruan Y, Li M, Wang T, et al. Taurine supplementation improves erectile function in rats with streptozotocin-induced type 1 diabetes via amelioration of penile fibrosis and endothelial dysfunction. J Sex Med. 2016;13(5):778–785. doi: 10.1016/j.jsxm.2016.02.164 [DOI] [PubMed] [Google Scholar]
- 5.Defeudis G, Gianfrilli D, Di Emidio C, et al. Erectile dysfunction and its management in patients with diabetes mellitus. Rev Endocr Metab Dis. 2015;16(3):213–231. doi: 10.1007/s11154-015-9321-4 [DOI] [PubMed] [Google Scholar]
- 6.Ercan P, El SN. Inhibitory effects of chickpea and Tribulus terrestris on lipase, α-amylase and α-glucosidase. Food Chem. 2016;205:163–169. doi: 10.1016/j.foodchem.2016.03.012 [DOI] [PubMed] [Google Scholar]
- 7.Zhang SJ, Feng SC. Effects of saponins from Tribulus terrestris on postprandial blood glucose levels in normal and type 2 diabetic rats. Pract Pharm Clin Remed. 2012;15(1):1–3. [Google Scholar]
- 8.Hemalatha S, Hari R. Comparative antioxidant activities of crude ethanolic and saponin rich butanol extracts of Tribulus terrestris fruits. Int J Pharma Biol Sc. 2013;4(4):784–793. [Google Scholar]
- 9.Yogendra KG, Vimala Y, Yogendra KG. Antioxidant activity and RP-HPLC analysis of diosgenin from the callus of Tribulus terrestris Linn. Int J Res Ayurveda Pharm. 2014;5(3):343–346. doi: 10.7897/2277-4343.05371 [DOI] [Google Scholar]
- 10.Zhang S, Li H, Yang S. Tribulosin protects rat hearts from ischemia/reperfusion injury. Acta Pharmacol Sin. 2010;31(6):671. doi: 10.1038/aps.2010.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reshma PL, Lekshmi VS, Sankar V, et al. Tribulus terrestris (Linn.) attenuates cellular alterations induced by ischemia in H9c2 cells via antioxidant potential. Phytother Res. 2015;29(6):933–943. doi: 10.1002/ptr.5336 [DOI] [PubMed] [Google Scholar]
- 12.Heaton JPW, Varrin SJ, Morales A. The characterization of a bio-assay of erectile function in a rat model. J Urol. 1991;145(5):1099–1102. doi: 10.1016/s0022-5347(17)38543-9 [DOI] [PubMed] [Google Scholar]
- 13.Gou X, He WY, Xiao MZ, et al. Transplantation of endothelial progenitor cells transfected with VEGF165 to restore erectile function in diabetic rats. Asian J Androl. 2011;13(2):332. doi: 10.1038/aja.2010.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller FJ, Gutterman DD, Rios CD, et al. Superoxide production in vascular smooth muscle contributes to oxidative stress and impaired relaxation in atherosclerosis. Circ Res. 1998;82(12):1298–1305. doi: 10.1161/01.res.82.12.1298 [DOI] [PubMed] [Google Scholar]
- 15.Hosseini A, Abdollahi M. Diabetic neuropathy and oxidative stress: therapeutic perspectives. Oxid Med Cell Longev. 2013;2013. doi: 10.1155/2013/168039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malavige LS, Levy JC. Erectile dysfunction in diabetes mellitus. J Sex Med. 2009;6(5):1232–1247. doi: 10.1111/j.1743-6109.2008.01168.x [DOI] [PubMed] [Google Scholar]
- 17.Gur S, Kadowitz PJ, Gurkan L, et al. Chronic inhibition of nitric‐oxide synthase induces hypertension and erectile dysfunction in the rat that is not reversed by sildenafil. BJU Int. 2010;106(1):78–83. doi: 10.1111/j.1464-410X.2009.09104.x [DOI] [PubMed] [Google Scholar]
- 18.Scallan JP, Hill MA, Davis MJ. Lymphatic vascular integrity is disrupted in type 2 diabetes due to impaired nitric oxide signalling. Cardiovasc Res. 2015;107(1):89–97. doi: 10.1093/cvr/cvv117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ouyang B, Sun X, Han D, et al. Human urine-derived stem cells alone or genetically-modified with FGF2 Improve type 2 diabetic erectile dysfunction in a rat model. PLoS One. 2014;9(3):e92825. doi: 10.1371/journal.pone.0092825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen GT, Yang BB, Chen JH, et al. Pancreatic kininogenase improves erectile function in streptozotocin-induced type 2 diabetic rats with erectile dysfunction. Asian J Androl. 2018;20(5):448. doi: 10.4103/aja.aja_54_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gonzalez‐Cadavid NF. Mechanisms of penile fibrosis. J Sex Med. 2009;6(S3):353–362. doi: 10.1111/j.1743-6109.2008.01195.x [DOI] [PubMed] [Google Scholar]
- 22.Andersson KE. Mechanisms of penile erection and basis for pharmacological treatment of erectile dysfunction. Pharmacol Rev. 2011;63(4):811–859. doi: 10.1124/pr.111.004515 [DOI] [PubMed] [Google Scholar]
- 23.Khare CP. Indian Medicinal Plants: an Illustrated Dictionary. Berlin, Heidelberg: Springer Verlag; 2007:669–671. [Google Scholar]
- 24.Tanaka N, Yonekura H, Yamagishi S, et al. The receptor for advanced glycation endproducts is induced by the glycation products themselves and TNF-α through NF-κB, and by 17β-estradiol through Sp-1 in human vascular endothelial cells. J Biol Chem. 2000. doi: 10.1074/jbc.M001235200 [DOI] [PubMed] [Google Scholar]
- 25.Sahin K, Orhan C, Akdemir F, et al. Comparative evaluation of the sexual functions and NF-κB and Nrf2 pathways of some aphrodisiac herbal extracts in male rats. BMC Complement Altern Med. 2016;16(1):318. doi: 10.1186/s12906-016-1303-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kamenov Z, Fileva S, Kalinov K, et al. Evaluation of the efficacy and safety of Tribulus terrestris in male sexual dysfunction—a prospective, randomized, double-blind, placebo-controlled clinical trial. Maturitas. 2017;99:20–26. doi: 10.1016/j.maturitas.2017.01.011 [DOI] [PubMed] [Google Scholar]
- 27.Santos CA Jr, Reis LO, Destro-Saade R, et al. Tribulus terrestris versus placebo in the treatment of erectile dysfunction: a prospective, randomized, double-blind study. Actas Urológicas Españolas. 2014;38(4):244–248. doi: 10.1016/j.acuroe.2014.03.009 [DOI] [PubMed] [Google Scholar]
- 28.GamalEl Din SF, Abdel Salam MA, Mohamed MS, et al. Tribulus terrestris versus placebo in the treatment of erectile dysfunction and lower urinary tract symptoms in patients with late-onset hypogonadism: a placebo-controlled study. Urol J. 2018;86. doi: 10.1177/0391560318802160. [DOI] [PubMed] [Google Scholar]
- 29.Liu T, Peng Y, Jia C, et al. Hepatocyte growth factor-modified adipose tissue-derived stem cells improve erectile function in streptozotocin-induced diabetic rats. Growth Factors. 2015;33(4):282–289. doi: 10.3109/08977194.2015.1077825 [DOI] [PubMed] [Google Scholar]
- 30.Bell-Quint J, Forte T, Graham P. Synthesis of two forms of apolipoprotein B by cultured rat hepatocytes. Biochem Biophys Res Commun. 1981;99(2):700–706. doi: 10.1016/0006-291x(81)91800-3 [DOI] [PubMed] [Google Scholar]
- 31.Winzell MS, Ahrén B. The high-fat diet–fed mouse: a model for studying mechanisms and treatment of impaired glucose tolerance and type 2 diabetes. Diabetes. 2004;53(suppl 3):S215–S219. doi: 10.2337/diabetes.53.6.1467 [DOI] [PubMed] [Google Scholar]
- 32.Albersen M, Lin G, Fandel TM, et al. Functional, metabolic, and morphologic characteristics of a novel rat model of type 2 diabetes-associated erectile dysfunction. Urology. 2011;78(2):476. e1-476. e8. doi: 10.1016/j.urology.2011.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]