Abstract
Insulin-like growth factor-1 receptor (IGF1R) is a transmembrane tyrosine kinase receptor that plays a crucial role in cell proliferation, growth, differentiation, and apoptosis. IGF1R overexpression has been observed in several cancers, including invasive bladder carcinomas, as a potential prognostic factor. Given known biologic differences between upper and lower urinary tract urothelial carcinoma, we assessed the expression status and prognostic significance of IGF1R in upper tract urothelial carcinoma (UTUC). Two tissue microarrays (TMAs) were built from 99 Japanese patients with non-metastatic UTUC submitted to radical nephroureterectomy between 1997 and 2011. TMAs were constructed with triplicate tumor and paired benign urothelium. Membranous IGF1R staining was evaluated using immunohistochemistry. Two scoring methods were applied (Her2-score and H-score). The highest score was assigned to each tumor. IGF1R positivity was defined as Her2-score ≥ 1+. Association with clinicopathologic parameters and outcome was assessed using hazard ratios (HR) with 95% confidence intervals (CI) and adjusted P values. We found positive IGF1R expression in 70% of UTUC. Outcomes were as follows: tumor recurrence, 33%; tumor progression, 59%; overall mortality, 33%; and cancer-specific mortality, 30%. IGF1R was not associated with any clinicopathologic features. In addition, IGF1R expression was not associated with tumor recurrence (HR = 0.54, CI = 0.25–1.1, P =0.11), tumor progression (HR= 1.6, CI = 0.8–3.1, P =0.19), overall mortality (HR= 1.5, CI = 0.68–3.4, P = 0.31), or cancer-specific mortality (HR = 1.6, CI = 0.68–3.8, P = 0.27). Positive IGF1R expression was found in more than two thirds of UTUC. This finding provides a rationale to investigate IGF1R as a potential therapeutic target in UTUC. In contrast to bladder cancer, IGF1R expression in UTUC did not correlate with outcome, further pointing to biologic differences between UTUC and bladder cancer.
Keywords: Insulin-like growth factor-1 receptor, IGF1R, Urothelial carcinoma, Upper tract urothelial carcinoma
Introduction
Upper tract urothelial carcinoma (UTUC) accounts for 5 to 10% of all urothelial carcinomas [1]. Although these tumors share many similarities with urothelial carcinomas of the bladder, less is known about their pathogenesis, due in part to their lower prevalence. Important differences in clinical, pathologic, and biological characteristics exist between lower and upper tract urothelial neoplasms [2–5]. Further understanding of oncogenic mechanisms underlying the development of UTUC will certainly help identify biological markers for prognostication and potential targets of therapy.
Insulin-like growth factor-1 receptor (IGF1R) is a transmembrane tyrosine kinase receptor that plays a crucial role as an anti-apoptotic factor enhancing cell survival. Binding of the ligand insulin-like growth factor 1 (IGF1) activates downstream pathways, increasing cellular proliferation and inhibiting apoptosis [6, 7]. IGF1R overexpression has been documented in several human malignancies, including lung, pancreatic, breast, prostate, renal, and bladder carcinoma [8–13].
We have previously demonstrated IGF1R overexpression in invasive bladder carcinoma as a potential prognostic factor [11]. Given known biologic differences between upper and lower urinary tract urothelial carcinoma, in the current study, we assessed the expression status and prognostic significance of IGF1R in UTUC.
Materials and methods
Patient cohort and tissue microarray construction
The study was approved by the corresponding Institutional Review Boards. The cohort included 99 Japanese UTUC patients who underwent radical nephroureterectomy with curative intent between 1997 and 2011 at Osaka General Medical Center, Osaka, Japan. Written informed consent was obtained from all the patients. Forty-six patients underwent laparoscopic radical nephroureterectomy, 53 underwent open radical nephroureterectomy, and 96 underwent concomitant retroperitoneal lymphadenectomy. All sections were retrieved and reviewed by a urologic pathologist for confirmation of the original diagnosis, grade, and stage according to the World Health Organization (WHO) 2016 classification and the 8th edition of the AJCC cancer staging manual [14, 15], and selection of a representative paraffin block for tissue microarray (TMA) construction. Two TMAs were constructed with spotted triplicate tumor samples and paired benign urothelium from each UTUC, as previously described [16].
Clinicopathologic and follow-up data
Clinicopathologic data were obtained from medical records and included patient demographics and follow-up data. Tumor progression was defined as the development of recurrence at the radical nephroureterectomy site, lymph node metastasis, or visceral metastasis. Metachronous or synchronous lower tract recurrence (e.g., bladder) was not considered as tumor progression.
Patients were followed from their initial diagnosis to the appearance of an event of interest or the end of the study. Patients who did not show an event of interest by the end of the study were censored for time-to-event analyses. The primary end point of the study was cancer-specific mortality. The secondary end point was tumor progression, as defined above.
Immunohistochemistry
Membranous IGF1R expression was evaluated using standard immunohistochemistry. Staining was performed on 5-μm TMA sections using a rabbit monoclonal anti-IGF1R primary antibody (clone G11, Ventana Medical Systems, Tucson, AZ) and automated BenchMark XT slide stainer, according to the manufacturer’s protocol. Sections were deparaffinized, rehydrated, and incubated for 32 min. The reaction was developed using 3–3-O-diaminobenzidine chromogen and counterstained with hematoxylin. Appropriate negative and positive controls were used.
Scoring system
IGF1R expression was evaluated using two different approaches. The first approach consisted of a quantitative H-score system (ranging from 0 to 300) in which IGF1R expression was estimated as the sum of the products of the intensity (0 for negative, 1 for weakly positive, 2 for moderately positive, and 3 for strongly positive) multiplied by the extent of the staining (0 to 100%). Estimations of the H-score were made spot by spot. The highest score was adopted and used for statistical analyses. Cases with IGF1R H-score higher than the median were considered to show high IGF1R expression.
The second approach for evaluating IGF1R expression consisted of a semi-quantitative score system similar to the standardized scoring system used for evaluating HER2 immunohistochemical expression in breast carcinoma. TMA spots were classified by one of the following categories: 0, no reactivity or membranous reactivity in < 10% of tumor cells; 1+, faint or barely perceptible membranous partial reactivity in 10% or more of tumors cells; 2+, weak to moderate complete membranous reactivity in 10% or more of tumor cells; or 3+, strong complete membranous reactivity in 10% or more of tumor cells [17]. The highest score among the TMA spots was assigned as the case’s score and used for statistical analysis.
Adopting a similar cutoff to that used in our previous study on bladder cancer, tumors with IGF1R score ≥ 1+ in the HER2 scoring system were considered to be IGF1R positive.
Statistical analysis
The association between clinicopathologic features and IGF1R expression was evaluated using Kruskal-Wallis test for continuous variables and Pearson’s chi-squared test or Mann-Whitney U test for the sum of ranks for categorical variables.
The outcome analysis included regression modeling and time-to-event (survival) analysis. Outcome variables included tumor recurrence, tumor progression, overall mortality, and cancer-specific mortality. Hazard ratios were estimated using adjusted Cox’s proportional hazards regression models. Survival curves were built using the Kaplan-Meier estimator and compared using the log-rank test.
To prevent family-wise error rates due to multiple comparisons, all P values were adjusted using Bonferroni’s method. For hypothesis testing, statistical significance was established at Padjusted < 0.05 for two tails of distribution. Data was analyzed and plots were generated using R version 3.5.1 (2018–07-02) from the R Foundation for Statistical Computing (Vienna, Austria). All the data, code, and results are freely available as supplementary materials in a GitHub repository at https://alcideschaux.github.io/IGF1R-UTUC/. The repository includes the data analysis considering all the scoring systems used for evaluating IGF1R expression.
Results
Clinicopathologic features
Of the 99 patients, 60 were men and 39 were women. The median age at diagnosis was 71 years (range from 48 to 87 years). The distribution by pT stage was as follows: 19 pTa, 18 pT1, 8 pT2, 48 pT3, and 6 pT4. Considering the anatomical location of the primary tumor, 45 tumors were located in the renal pelvis, 50 tumors were located in the ureter, and 4 tumors invaded both the renal pelvis and ureter. Following the WHO grading system, 84 tumors were high grade and 15 tumors were low grade.
The median length of follow-up was 47 months (range from 3 to 173 months). Lymphovascular invasion was observed in 40 cases. Lymph node status was available for 96 patients. Of these patients, 12 patients had lymph node metastasis at the time of radical nephroureterectomy.
Metachronous or synchronous lower tract recurrence was observed in 32 patients. Tumor progression appeared in 49 patients. The distribution of patients by final outcome at the end of the follow-up period was as follows: 38 patients were alive with no evidence of disease, 5 patients were alive with evidence of disease, 30 patients died of disseminated cancer, 3 patients died of unrelated causes, and 23 patients were lost at follow-up. Thus, overall mortality was observed in 33 patients, while cancer-specific mortality was observed in 30 patients.
IGF1R expression and association analysis
Representative patterns of IGF1R immunohistochemical expression are shown in Fig. 1. IFG1R positivity was observed in 69 cases. Comparison of IGF1R expression and clinicopathologic features is shown in Table 1.
Fig. 1.
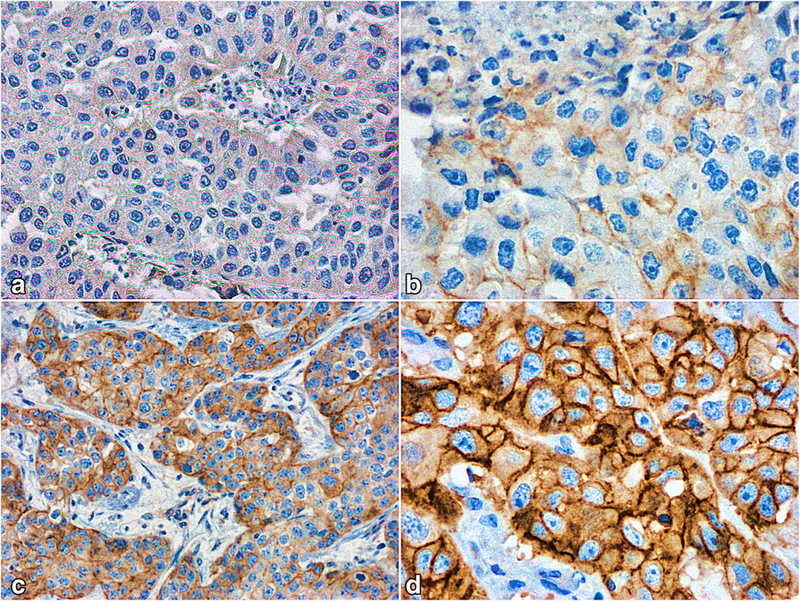
a–d Representative patterns of IGF1R immunohistochemical expression. (a) Score 0 ; (b) score 1+ ; (c) score 2+ ; (d) score 3+
Table 1.
Clinicopathologic and outcome features by IGF1R positivity
| IGF1R negative | IGF1R positive | P value | |
|---|---|---|---|
| Age, median (IQR) | 67 (15.25) | 72 (12) | 0.4 |
| Sex, male/female | 19/11 | 41/28 | 1.0 |
| Location, pelvis/ureter/both | 16/14/0 | 29/36/4 | 1.0 |
| High pT stage, pTa-pT2/pT3-pT4 | 12/18 | 33/36 | 1.0 |
| WHO grade, low grade/high grade | 4/26 | 11/58 | 1.0 |
| Lymphovascular invasion, absent/present | 20/10 | 39/30 | 1.0 |
| Lymph node metastasis, absent/present | 27/3 | 57/9 | 1.0 |
| Tumor recurrence, absent/present | 17/13 | 48/19 | 1.0 |
| Tumor progression, absent/present | 10/16 | 4/24 | 1.0 |
| Overall mortality, no/dead | 20/10 | 46/23 | 1.0 |
| Cancer-specific mortality, no/dead | 21/9 | 48/21 | 1.0 |
None of the evaluated clinicopathologic or outcome features was associated with IGF1R expression. In addition, there was no significant difference in IGF1R expression between paired tumor tissue and benign urothelium (P = 0.58). Similar results were observed with other scoring methods (see supplementary materials at the GitHub repository for further details).
IGF1R expression and outcome analysis
Figure 2 shows the survival curves for tumor recurrence, tumor progression, overall mortality, and cancer-specific mortality considering IGF1R positivity. As seen, IGF1R expression was not significantly associated with outcome.
Fig. 2.
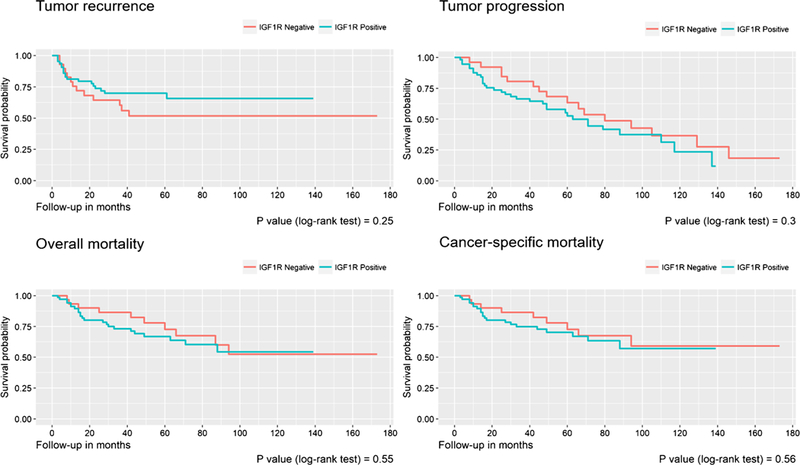
Survival curves for tumor recurrence, tumor progression, overall mortality, and cancer-specific mortality considering IGF1R positivity
Table 2 includes the adjusted hazard ratios for predicting tumor recurrence, tumor progression, overall mortality, and cancer-specific mortality using clinicopathologic features and IGF1R expression. Tumors located in both the renal pelvis and ureter were about 10 times more likely to develop tumor recurrence and 7 times more likely to develop tumor progression during follow-up. Tumors with high pT stage (>pT2) were also more likely to develop tumor progression. A tendency to develop tumor recurrence was observed in older patients, as well as a tendency to develop tumor progression in tumors with lymphovascular invasion.
Table 2.
Hazard ratios with 95% confidence intervals and P values for predicting tumor recurrence, tumor progression, overall mortality, and cancer-specific mortality using clinicopathologic features and IGF1R expression, estimated from adjusted proportional hazards regression models
| Tumor recurrence | Tumor progression | Overall mortality | Cancer-specific mortality | |||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| Age: Older vs younger patientsa | 2.0 (0.9–4.3) | 0.09 | 2.0 (1.0–4.1) | 0.04 | 1.5 (0.7–3.2) | 0.3 | 1.4 (0.6–3.3) | 0.4 |
| Sex: Female vs Male | 0.5 (0.2–1.2) | 0.1 | 1.0 (0.5–2.0) | 0.9 | 0.9 (0.4–2.2) | 0.9 | 0.7 (0.3–1.8) | 0.4 |
| Location: Ureter vs Pelvis | 1.3 (0.6–2.8) | 0.5 | 0.8 (0.4–1.6) | 0.6 | 1.0 (0.5–2.1) | 1 | 0.9 (0.4–1.9) | 0.7 |
| Location: Pelvis-ureter vs Pelvis | 9.8 (2.2–44.2) | 0.003 | 7.1 (1.5–34.5) | 0.01 | 1.3 (0.1–10.6) | 0.8 | 1.6 (0.2–13.8) | 0.7 |
| High pT stageb | 1.4 (0.6–3.2) | 0.4 | 2.8 (1.3–5.9 | 0.007 | 6.7 (2.1–21.5) | 0.001 | 11.9 (2.5–55.5) | 0.002 |
| Lymphovascular invasion | 0.5 (0.2–1.4) | 0.2 | 2.2 (0.9–5.2) | 0.07 | 2.0 (0.7–5.3) | 0.2 | 2.2 (0.8–6.4) | 0.1 |
| Lymph nodes metastasis | 1.1 (0.2–5.5) | 0.9 | 0.8 (0.3–2.0) | 0.6 | 0.9 (0.3–2.6) | 0.9 | 1.1 (0.4–3.1) | 0.9 |
| Positive IGF1R expression | 0.5 (0.3–1.1) | 0.1 | 1.6 (0.8–3.1) | 0.2 | 1.5 (0.7–3.4) | 0.3 | 1.6 (0.7–3.8) | 0.3 |
WHO grade was not included in the regression models due to the absence of low-grade cases with dismal prognosis.
Using the median age as the cutoff point.
Using pT2 as the cutoff point, i.e., tumors with pT0-pT2 stage are considered low pT stage, and tumors with pT3-pT4 stage are considered high pT stage
Regarding overall mortality and cancer-specific mortality, the only variable significantly associated with prognosis was high pT stage. Patients with high pT stage tumors were about 7 times more likely to have died of any cause and 12 times more likely to have died of disseminated cancer.
IGF1R positivity was not associated with any outcome. Similar results were obtained for all other scoring methods, i.e., HER2 scoring and H-scores (see supplementary materials at the GitHub repository for further details).
Discussion
The transmembrane tyrosine kinase receptor IGF1R is a promising candidate for the development of new targeted therapies. It is activated by IGF1 or IGF2. It triggers two downstream signaling pathways: Ras/mitogen-activated protein kinase and the phosphoinositide 3-kinase/protein kinase B (PI3K/AKT), which incites the inhibition of apoptosis and increases mitogenic activity and protein synthesis [2, 3]. Furthermore, IGF1R expression has been shown to be inhibited by phosphatase and tensin homolog (PTEN) [18] and wild-type p53 [19]. IGF1R activation has been suggested to promote tumor cell motility and invasion through proline-rich tyrosine kinase 2, which has also been implicated in the activation of mitogen-activated protein kinase and AKT pathways by IGF1 stimulation [20].
To date, clinical trials using monoclonal anti-IGF1R antibodies have spanned a diverse group of cancers including hematologic malignancies, sarcomas, and brain tumors [19, 21]. In addition to the direct targeting of the IGF1R, several members of the insulin-like growth factor (IGF) system have also been studied as possible therapeutic targets in different cancer types with variable success rates between tumor types [22]. IGF1R-targeted therapy has also been evaluated as a treatment option in benign tumors, such as in uterine leiomyomas where IGF1R expression has been found to play a role growth and development [23].
The overexpression of IGF1R has been associated with tumor progression and poor clinical outcome in several cancer types including breast, gastric, and colorectal cancers [21, 24]. Moreover, therapeutic strategies leading to the inhibition of IGF1R signaling have been described to play a role in improving the efficacy of response to radiotherapy and chemotherapy [24–26]. We have previously found IGF1R overexpression in 62% of bladder urothelial carcinoma. Furthermore, it is a significant independent predictor of overall mortality (hazard ratio =2.6, P =0.0002) and cancer-specific mortality (hazard ratio = 2.45, P = 0.01), suggesting its potential role as a prognosticator [27]. Our current study is the first to evaluate the immunohistochemical expression of IGF 1R in relation to clinicopathologic parameters and outcome in UTUC. We found IGF1R overexpression in 70% of UTUC. This finding can be seen as a rationale to investigate IGF1R as a potential target of therapy in UTUC. Unlike bladder cancer, IGF1R expression in UTUC lacked prognostic significance.
UTUCs are less common urological malignancies compared to their bladder counterpart; however, an increase in the overall incidence of UTUC has been observed over the past three decades [1]. Previous studies have shown differences in epidemiologic, molecular, and clinical features between upper and lower tract urothelial carcinomas [2–5], further emphasizing the need to assess biomarkers of prognostic or predictor significance in both groups of tumors. In our study, the difference in outcome prediction between bladder cancer and UTUC based on IGF1R expression is therefore not totally unexpected, but rather further supports the aforementioned observations. This finding is also in line with some studies which have demonstrated that the prognostic and clinical significance of IGF1R expression remains controversial, and that it differs across different cancer types [21]. Clinicopathologic factors which have been traditionally used to predict survival in the postoperative setting of UTUC, such as high pT stage, were also significant predictors of tumor progression and cancer-specific mortality in our cohort [28, 29].
IGF1R expression has been scored in several ways. In breast cancer, IGF1R expression is scored based on a modified HER2 scoring approach ranging from 0 to 3 in 0.5 increments, assessing membranous intensity [30]. The same score system was used in previous studies to assess IGF1R expression in bladder cancer, given that it is typically membranous in these tumors [11]. Also, previous studies have shown good inter-observer reproducibility of the HER2 scoring system [31, 32]. In our study, we applied the HER2 scoring approach as well as the H-score. Both approaches showed no association between IGFR1R expression and outcome.
The evaluation of immunohistochemical expression using TMAs instead of whole tissue sections could be a potential limitation in the present study due to intratumoral staining heterogeneity of some markers. However, several studies have proven the adequate representation of overall immunohistochemistry expression levels by using multiple TMA spots from the same tumor. Also, they provide an efficient way to evaluate a large number of cases under the same immunohistochemical conditions [33]. Nevertheless, our results should be reevaluated using whole sections to further confirm our findings. Furthermore, our study only included conventional urothelial carcinomas. Evaluations of IGF1R expression in variants of urothelial carcinoma could be of additional value. Another limitation of the current study is the retrospective nature of the cohort. Future prospective studies evaluating other members of the IGF system could help in assessing the significance of this signaling system in UTUC.
Conclusion
In summary, we found IGF1R overexpression in 70% of UTUC. The later provides a rationale to investigate IGF1R as a target of therapy in UTUC. Unlike urothelial carcinoma in the bladder, IGF1R expression of upper urinary tract carcinoma did not correlate with outcome.
Supplementary Material
Footnotes
Compliance with ethical standards
Informed consent Written informed consent was obtained from all the patients included in the study.
Conflict of interest The authors declare that they have no conflict of interest.
References
- 1.Raman JD, Messer J, Sielatycki JA, Hollenbeak CS (2011) Incidence and survival of patients with carcinoma of the ureter and renal pelvis in the USA, 1973–2005. BJU Int 107:1059–1064. 10.1111/j.1464-410X.2010.09675.x [DOI] [PubMed] [Google Scholar]
- 2.Colin P, Koenig P, Ouzzane A et al. (2009) Environmental factors involved in carcinogenesis of urothelial cell carcinomas of the upper urinary tract. BJU Int 104:1436–1440. 10.1111/j.1464-410X.2009.08838.x [DOI] [PubMed] [Google Scholar]
- 3.Rouprêt M, Fromont G, Azzouzi A-R et al. (2005) Microsatellite instability as predictor of survival in patients with invasive upper urinary tract transitional cell carcinoma. Urology 65:1233–1237. 10.1016/j.urology.2005.01.019 [DOI] [PubMed] [Google Scholar]
- 4.Green DA, Rink M, Xylinas E et al. (2013) Urothelial carcinoma of the bladder and the upper tract: disparate twins. J Urol 189:1214–1221. 10.1016/j.juro.2012.05.079 [DOI] [PubMed] [Google Scholar]
- 5.Moss TJ, Qi Y, Xi L et al. (2017) Comprehensive genomic characterization of upper tract urothelial carcinoma. Eur Urol 72:641–649. 10.1016/j.eururo.2017.05.048 [DOI] [PubMed] [Google Scholar]
- 6.Baserga R (2005) The insulin-like growth factor-I receptor as a target for cancer therapy. Expert Opin Ther Targets 9:753–768. 10.1517/14728222.9A753 [DOI] [PubMed] [Google Scholar]
- 7.Tao Y, Pinzi V, Bourhis J, Deutsch E (2007) Mechanisms of disease: signaling of the insulin-like growth factor 1 receptor pathway—therapeutic perspectives in cancer. Nat Clin Pract Oncol 4: 591–602. 10.1038/ncponc0934 [DOI] [PubMed] [Google Scholar]
- 8.Nakagawa M, Uramoto H, Oka S et al. (2012) Clinical significance of IGF1R expression in non-small-cell lung cancer. Clin Lung Cancer 13:136–142. 10.1016/j.cllc.2011.10.006 [DOI] [PubMed] [Google Scholar]
- 9.Law JH, Habibi G, Hu K et al. (2008) Phosphorylated insulin-like growth factor-i/insulin receptor is present in all breast cancer subtypes and is related to poor survival. Cancer Res 68:10238–10246. 10.1158/0008-5472.CAN-08-2755 [DOI] [PubMed] [Google Scholar]
- 10.Hellawell GO, Turner GDH, Davies DR et al. (2002) Expression of the type 1 insulin-like growth factor receptor is up-regulated in primary prostate cancer and commonly persists in metastatic disease. Cancer Res 62:2942–2950 [PubMed] [Google Scholar]
- 11.Gonzalez-Roibon N, Kim JJ, Faraj SF et al. (2014) Insulin-like growth factor-1 receptor overexpression is associated with outcome in invasive urothelial carcinoma of urinary bladder: a retrospective study of patients treated using radical cystectomy. Urology 83: 1444.e1–1444.e6. 10.1016/j.urology.2014.01.028 [DOI] [PubMed] [Google Scholar]
- 12.Ouban A, Muraca P, Yeatman T, Coppola D (2003) Expression and distribution of insulin-like growth factor-1 receptor in human carcinomas. Hum Pathol 34:803–808 [DOI] [PubMed] [Google Scholar]
- 13.Rochester MA, Patel N, Turney BW et al. (2007) The type 1 insulin-like growth factor receptor is over-expressed in bladder cancer. BJU Int 100:1396–1401. 10.1111/j.1464-410X.2007.06931.x [DOI] [PubMed] [Google Scholar]
- 14.Moch H, Humphrey PA, Ulbright TM, Reuter VE (2016) WHO classification of tumours of the urinary system and male genital organs, 4th ed International Agency for Research on Cancer (IARC), Lyon [Google Scholar]
- 15.Amin MB, Edge SB, Greene F et al. (2017) AJCC cancer staging manual, 8th edn Springer International Publishing, New York [Google Scholar]
- 16.Fedor HL, De Marzo AM (2005) Practical methods for tissue microarray construction. Methods Mol Med 103:89–101 [DOI] [PubMed] [Google Scholar]
- 17.Hammond ME, Hayes DF, Wolff AC (2011) Clinical notice for American Society of Clinical Oncology-College of American Pathologists guideline recommendations on ER/PgR and HER2 testing in breast cancer. J Clin Oncol 29:e458. 10.1200/JCO.2011.35.2245 [DOI] [PubMed] [Google Scholar]
- 18.Pantuck AJ, Seligson DB, Klatte T et al. (2007) Prognostic relevance of the mTOR pathway in renal cell carcinoma. Cancer 109:2257–2267. 10.1002/cncr.22677 [DOI] [PubMed] [Google Scholar]
- 19.Mansure JJ, Nassim R, Chevalier S et al. (2009) Inhibition of mammalian target of rapamycin as a therapeutic strategy in the management of bladder cancer. Cancer Biol Ther 8:2339–2347 [DOI] [PubMed] [Google Scholar]
- 20.Harris LD, De La Cerda J, Tuziak T et al. (2008) Analysis of the expression of biomarkers in urinary bladder cancer using a tissue microarray. Mol Carcinog 47:678–685. 10.1002/mc.20420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu J, Yu E (2014) Insulin-like growth factor receptor-1 (IGF-IR) as a target for prostate cancer therapy. Cancer Metastasis Rev 33:607–617. 10.1007/s10555-013-9482-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brahmkhatri VP, Prasanna C, Atreya HS (2015) Insulin-like growth factor system in cancer: novel targeted therapies. Biomed Res Int 2015:538019. 10.1155/2015/538019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gkioka E, Msaouel P, Philippou A et al. (2015) Review: the role of insulin-like growth factor-1 signaling pathways in uterine leiomyoma. In Vivo 29:637–649 [PubMed] [Google Scholar]
- 24.Zhang X, Yee D (2000) Tyrosine kinase signalling in breast cancer: insulin-like growth factors and their receptors in breast cancer. Breast Cancer Res 2:170–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goel HL, Sayeed A, Breen M et al. (2013) β1 integrins mediate resistance to ionizing radiation in vivo by inhibiting c-Jun amino terminal kinase 1. J Cell Physiol 228:1601–1609. 10.1002/jcp.24323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Casa AJ, Dearth RK, Litzenburger BC et al. (2008) The type I insulin-like growth factor receptor pathway: a key player in cancer therapeutic resistance. Front Biosci 13:3273–3287 [DOI] [PubMed] [Google Scholar]
- 27.Munari E, Fujita K, Faraj S et al. (2013) Dysregulation of mammalian target of rapamycin pathway in upper tract urothelial carcinoma. Hum Pathol 44:2668–2676. 10.1016/j.humpath.2013.07.008 [DOI] [PubMed] [Google Scholar]
- 28.Lughezzani G, Jeldres C, Isbarn H et al. (2010) A critical appraisal of the value of lymph node dissection at nephroureterectomy for upper tract urothelial carcinoma. Urology 75:118–124. 10.1016/j.urology.2009.07.1296 [DOI] [PubMed] [Google Scholar]
- 29.Novara G, Matsumoto K, Kassouf W et al. (2010) Prognostic role of lymphovascular invasion in patients with urothelial carcinoma of the upper urinary tract: an international validation study. Eur Urol 57:1064–1071. 10.1016/j.eururo.2009.12.029 [DOI] [PubMed] [Google Scholar]
- 30.Drury SC, Detre S, Leary A et al. (2011) Changes in breast cancer biomarkers in the IGF1R/PI3K pathway in recurrent breast cancer after tamoxifen treatment. Endocr Relat Cancer 18:565–577. 10.1530/ERC-10-0046 [DOI] [PubMed] [Google Scholar]
- 31.Turashvili G, Leung S, Turbin D et al. (2009) Inter-observer reproducibility of HER2 immunohistochemical assessment and concordance with fluorescent in situhybridization (FISH): pathologist assessment compared to quantitative image analysis. BMC Cancer 9: 165 10.1186/1471-2407-9-165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lacroix-Triki M, Mathoulin-Pelissier S, Ghnassia J-P et al. (2006) High inter-observer agreement in immunohistochemical evaluation of HER-2/neu expression in breast cancer: a multicentre GEFPICS study. Eur J Cancer 42:2946–2953. 10.1016/j.ejca.2006.06.020 [DOI] [PubMed] [Google Scholar]
- 33.Camp RL, Neumeister V, Rimm DL (2008) A decade of tissue microarrays: progress in the discovery and validation of cancer biomarkers. J Clin Oncol 26:5630–5637. 10.1200/JCO.2008.17.3567 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


