Abstract
Purpose:
To develop patient-derived Pediatric Eye Questionnaires (PedEyeQ) to separately assess eye-related quality of life (ER-QOL) and functional vision in children with eye conditions.
Design:
Questionnaire development study.
Methods:
444 children (0 to <18 years old), across 10 diagnostic categories, were enrolled at two sites, All parents (n=444) and 277 children (5 to <18 years old) completed master questionnaires, developed from patient-derived concerns. Factor analysis was performed to identify unidimensional domains (Eigenvalue >1.0) and Rasch analyses (differential item functioning, targeting, fit) to reduce items (separate analyses for 0–4, 5–11 and 12–17 year olds and for each factor).
Results:
The Child 5–11 year-old PedEyeQ consisted of four unidimensional domains/questionnaires: functional vision, bothered by eyes/vision, social, frustration/worry (10 items each). The Child 12–17 year-old PedEyeQ consisted of the same four domains (total 39 items). The Proxy 0–4 year PedEyeQ consisted of three questionnaires/domains: functional vision, bothered by eyes/vision, social (total 29 items). The Proxy 5–11 year-old consisted of five questionnaires/domains: functional vision, bothered by eyes/vision, social, frustration/worry, eye-care (total 39 items) as did the Proxy 12–17 year-old PedEyeQ (total 42 items). The Parent PedEyeQ, consisted of four questionnaires/domains: impact on parent/family, worry re child’s eye condition, worry re child’s self-perception and interactions, worry re child’s visual function (total 35 items). Rasch look-up tables were created for scoring.
Conclusions and Relevance:
By following a rigorous approach we have developed Pediatric Eye Questionnaires for separately assessing functional vision and ER-QOL domains in children of any age and with any eye condition.
INTRODUCTION
We previously reported the identification of specific functional vision and eye-related quality of life (ER-QOL) concerns of children with eye conditions, derived from individual interviews. Based on the complete interview data set for children with a broad spectrum of eye conditions, we created a large pool of potential questionnaire items.1 In the present study, we describe the second stage in the process, using these patient-derived items in a new cohort of patients, to develop new patient-reported outcome measures (in accordance with following Food and Drug Administration guidelines2), ensuring content validity. A subsequent third stage, evaluating construct validity, reliability and responsiveness of the derived questionnaires in a new patient population, will be described separately. The goal in developing these questionnaires was to meet the need for patient-derived, eye-specific instruments that can be used across the spectrum of childhood eye disorders, in children of any age, with child self-report, proxy-report, and parent components. Our specific aim was to develop short forms, representing individual, analysis-driven, unidimensional domains within the separate constructs of functional vision and ER-QOL, for administration in any clinical setting.
METHODS
Approval for this for this questionnaire development study was obtained from Institutional Review Boards at the Mayo Clinic, Rochester, Minnesota and the University of Texas Southwestern Medical Center, Dallas, Texas. All procedures and data collection were conducted in a manner compliant with the Health Insurance Portability and Accountability Act and all research procedures adhered to the tenets of the Declaration of Helsinki. Informed consent and informed assent were obtained as required by local IRBs. Both the child and one of their parents were enrolled.
Patients
As described previously,1, 3–6 patients were enrolled at two sites: Mayo Clinic, Rochester, Minnesota and Retina Foundation of the Southwest, Dallas, Texas. Eligible children were aged 0 to 17 years, with any eye condition.
The medical record was reviewed to establish the current primary diagnosis. Based on this diagnosis, each subject was then allocated to one of 10 broad diagnostic categories (esotropia, exotropia, hypertropia, nystagmus, amblyopia, refractive error, orbital and external conditions, anterior segment, retina and optic nerve, and central nervous system conditions affecting vision). Other diagnoses were listed as “additional.” In an attempt to adequately represent the concerns of children with less common as well as those with more common eye conditions we aimed to enroll equal numbers into each of the 10 diagnostic categories (480 overall, 48 into each of the 10 categories, 16 to each of three age bins [0–4, 5–11 and 12–17 years], at least 8 being from a racial/ethnic minority). We also aimed to cover a range of different disease severities. We attempted to enroll consecutive subjects and almost no invitees declined enrollment.
Phase 1: Creating Master Questionnaires
Item Selection
We previously described the process of identifying a large pool of 614 candidate child and proxy questionnaire items and 589 candidate parent items.1 We planned to add any additional items that could be identified in existing English-language, pediatric vision questionnaires, to be sure that our final, comprehensive list included all areas of concern. Review of the literature identified 14 previously-published vision-specific questionnaires addressing the child’s experience,7–20 and one that assessed the parent’s own experience15 with some parent-related items within proxy questionnaires.9, 11, 20 On review of these published questions we did not find any additional areas of concern that we had missed and therefore no additional questions derived from published questionnaires were added to our pool of candidate items.
Before commencing the process of item-reduction, we had determined to segregate items within the two distinct constructs of functional vision and ER-QOL. To reduce items within these constructs to smaller subsets, we used a previously-described process of winnowing.21, 22 All questions had been previously grouped by thematic content,1 and these bins of questions were reviewed, and questions removed if: 1) they were similar to another question; 2) the question’s content was too narrow, reducing applicability across socioeconomic and cultural groups; 3) the question was confusing or unclear.22 This process was repeated until the team agreed that the reduced number of questions formed a balance between adequate representation of concerns and reasonable burden of testing. Questions addressing the experience of the child themselves (expressed by the child or by the parent as proxy-reporter) were grouped either within functional vision or ER-QOL. For the current project, we chose to exclude questions that only addressed symptoms, and focused on the two specific constructs of functional vision and ER-QOL. Child and proxy-reported questions were collated separately from those addressing the experience of the parent themselves. While we collected concerns related to the treatment of pediatric eye conditions and formulated some candidate questions based on these concerns,1 only a fraction of the children in the present study were undergoing treatment, so the development of treatment-related questionnaires was deferred, pending a larger sample size.
Formatting Master Questionnaires
Questions were formatted to create three different questionnaires: Child (for completion by the child), Proxy (parallel to the Child questionnaire but phrased in the third person), and Parent (the parent’s own experience).
In a previous study,5 we established that a frequency rating scale performed better than difficulty or severity scales for both children and parents and therefore we utilized a 4-point frequency scale (“Never,” “Sometimes,” “Most of the time,” “All of the time”) for responses on each questionnaire. Questionnaires were also translated into Spanish and independently back-translated to ensure consistency of content.
Phase 2: Administering Master Questionnaires
Children aged 5–17 years completed Child questionnaires and one parent of each child aged 0–17 years completed Proxy and Parent questionnaires. No enrolled children or parents had been involved in previous phases of the study. Simple verbal and written instructions directed participants to select one response per question, and indicate if the question was confusing or unclear. Questions were read to 5- to 7-year-old children by a trained examiner; 8- to 17-year-olds were given the option of completing on their own.
Phase 3: Developing Pediatric Eye Questionnaire Modules
Our goal was to create short-form questionnaires, representing each of several analysis-driven unidimensional domains, with no more than 10 items per factor (to minimize testing burden). Item reduction was accomplished in a series of analytical steps (described below). Child, Proxy and Parent questionnaires were analyzed separately. For analysis, “Never” responses were scored as 3, “Sometimes” as 2, “Most of the time” as 1, and “All of the time” as 0.
1. Initial Item Reduction
Prior to performing factor analysis and Rasch analysis we excluded questionnaires with >10% missing responses, and questions ≥20% “not applicable” responses, or where >10% of respondents found it unclear or confusing (analyzed separately for 0–4, 5–11, and 12–17 year age groups). For questionnaires with <10% of responses missing we calculated Spearman rank correlations across questionnaires within the same age bin, and imputed the highest-correlated response, to allow use of all the non-imputed data for these children and parents (proportion of imputed responses: Child 8%, Proxy 4%, Parent 2%).
2. Factor Analysis
Factor analysis was performed to identify latent variables within each construct (Child and Proxy: functional vision and, ER-QOL separately; Parent: ER-QOL). Analyses were performed separately by age group for Child (5–11, 12–17 years) and Proxy (0–4, 5–11, 12–17 years) questionnaires. There were no separate analyses by child age for Parent questionnaires. Similar to previous analyses for other eye-related questionnaire instruments,23 Varimax rotation methods were used with a threshold Eigenvalue of 1.0 for identifying factors. Each factor analysis was initially performed unconstrained, and then constraining to fewer factors, based on review of thematic content, Eigenvalues, and the inflection point on the scree plot. Items that loaded on more than one factor were initially retained on the factor with the highest loading threshold but if an item cross-loaded (at a threshold of 0.3 or more) on another factor, we reviewed thematic content and if the thematic fit was better, we moved it to the other factor for subsequent analyses. No items were removed for low loading. Identified latent traits were assigned descriptive titles according to their thematic content.
3. Rasch Analysis
Separate Rasch analyses were performed for each latent trait and, for Child and Proxy questionnaires, also by age group. All Rasch analyses were performed using Winsteps software version 4.0.1, Winsteps Software Technologies, Seattle, WA utilizing the Masters Partial-Credit Model. All statistical analyses were performed using SAS version 9.4 (Cary, N.C). Response option category utilization was evaluated and categories combined for subsequent analyses if there was underutilization of a response option. Principal component analysis was performed to assess dimensionality of the individual components. If any of the components was found to be multidimensional, items were removed until it became unidimensional.
For each identified factor (by age group), we then evaluated differential item functioning, fit (outside a range of 0.7 to 1.3), targeting and local dependence (outside a range of −0.5 to 0.5). We prioritized removal of items due to differential item functioning (Child / Proxy questionnaires: child gender [male versus female]; child race/ethnicity [non-Hispanic white versus other]; Proxy / Parent questionnaires: person completing [mother versus not mother]; Parent questionnaires: parent race/ethnicity [non-Hispanic white versus other]). Items with a differential item functioning contrast of >1.0 logit were removed. We also iteratively reviewed the Rasch maps to decide which items to remove and which to retain, with the aim of achieving a good spread across item difficulty and to maintain optimal targeting. Item removal continued through iterative reevaluation of Rasch indices, and review of the Rasch maps until we arrived at 10 or fewer items per factor, optimizing person and item separation indices (aim ≥2.0) and person and item reliability indices (aim ≥0.8). If a factor had less than 10 items from the outset, we removed items for multidimensionality and differential item functioning, but accepted some degree of misfit and somewhat suboptimal targeting overall. Unidimensionality of each factor was re-confirmed once a final set of items had been derived.
RESULTS
Phase 1: Creating Master Questionnaires
The pre-determined criteria for eliminating questions were applied to the 462 functional vision and general ER-QOL questions in the Child and Proxy item pool1 (152 treatment-related questions not included in the current study). The pre-determined criteria were also applied to the 367 general ER-QOL questions in the Parent item pool1 (222 treatment-related questions not included in the current study). Child (and parallel Proxy) questions were reduced to 63 items for a master questionnaire, (399 eliminated) and Parent questionnaires were reduced to 47 items for a master questionnaire (320 eliminated).
Phase 2: Administering Master Questionnaires
Four hundred and forty-four children were enrolled, along with one of their parents, to complete master questionnaires. All parents completed Proxy and Parent master questionnaires (83% mothers, 16% fathers, 1% legal guardians), and 277 5- to 17-year-old children completed Child master questionnaires (22 [8%] of 5- to 17-year-old children were unable to complete the questionnaires due to cognitive impairment). Demographics of enrolled children and their parents are provided in Table 1: 220 (50%) of 444 children were male, and race/ethnicity was non-Hispanic white for 296 (67%). One hundred and sixty seven (38%) children were 0–4 years old, 156 (35%) were 5–11 years old, and 121 (27%) were 12–17 years old (Table 1). The number of children in each primary diagnostic category, along with associated diagnostic categories, are shown in Table 2.
Table 1:
Demographics of Children and Parents Completing Master Questionnaires
| N (%) | ||
|---|---|---|
| Sex of child | ||
| Female | 224 (50) | |
| Male | 220 (50) | |
| Age | ||
| 0–4 | 167 (38) | |
| 5–11 | 156 (35) | |
| 12–17 | 121 (27) | |
| Race | ||
| White (including Hispanic / Latino) | 339 (76) | |
| Asian | 35 (8) | |
| More than 1 race | 35 (8) | |
| Black/African American | 19 (4) | |
| American Indian / Alaskan Native | 5 (1) | |
| Native Hawaiian / Other Pacific Islander | 1 (<1) | |
| Other | 8 (2) | |
| Unknown/not reported | 2 (<1) | |
| Ethnicity | ||
| Not Hispanic / Latino and not Middle Eastern/North African and not Indian Subcontinent | 349 (79) | |
| Hispanic / Latino | 62 (14) | |
| Indian Subcontinental | 18 (4) | |
| Middle Eastern / North African | 7 (2) | |
| More than one | 4 (1) | |
| Unknown / Not Reported | 4 (1) | |
| Developmental delay | ||
| No delay | 365 (82%) | |
| Delay | 79 (18%) | |
| Parent / Legal Guardian Interviewed | ||
| Mother | 367 (83) | |
| Father | 71 (16) | |
| Legal guardian | 6 (1) | |
| Parent / Legal Guardian Age | ||
| Under 21 | 5 (1) | |
| 21 to 30 | 55 (12) | |
| 31 to 40 | 218 (49) | |
| 41 to 50 | 129 (29) | |
| 51 to 60 | 31 (7) | |
| Over 60 | 4 (1) | |
| Not Reported | 2 (<1) | |
| Parent / Legal Guardian Highest Level of Education | ||
| Attended high school | 28 (6) | |
| High school graduate | 29 (7) | |
| Attended college | 80 (18) | |
| College graduate | 175 (39) | |
| Post-graduate / professional degree | 126 (28) | |
| Not reported | 6 (1) | |
| Housing | ||
| Own | 366 (82) | |
| Rent | 65 (15) | |
| Other | 10 (2) | |
| Not reported | 3 (1) | |
| Number of Parents/Legal Guardians in Home | ||
| 1 | 45 (10) | |
| 2 | 398 (90) | |
| Not reported | 1 (<1) | |
| Care of Child | ||
| Parents /Legal Guardians only | 189 (43) | |
| Day care | 47 (11) | |
| Babysitter | 37 (8) | |
| Other relative | 76 (17) | |
| Other | 21 (5) | |
| After-school program | 19 (4) | |
| More than 1 source of assistance | 50 (11) | |
| Not reported | 5 (1) | |
| Visual acuity of worse eye | ||
| −0.1 to 0.54 LogMAR (20/16 to 20/70) | 287 (65%) | |
| <0.54 to 1.0 LogMAR (<20/70 to 20/200) | 77 (17%) | |
| <1.0 LogMAR (<20/200) | 28 (6%) | |
| Not recorded | 52 (12%) | |
| Visual acuity of better eye | ||
| −0.2 to 0.54 LogMAR (20/12 to 20/70) | 340 (77%) | |
| <0.54 to 1.0 LogMAR (<20/70 to 20/200)a | 52 (12%) | |
| <1.0 LogMAR (<20/200)b | 13 (3%) | |
| Not recorded | 39 (9%) | |
World Health Organization criteria for moderate visual impairment;
World Health Organization criteria for severe visual impairment or blindness (https://www.icd10monitor.com/looking-at-new-icd-10-cm-codes-for-blindness)
Table 2.
Diagnostic Categories of Children Enrolled for Completion of Master Questionnaires
| Secondary Diagnoses | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Current Primary Diagnosis | Esotropia | Exotropia | Hypertropia | Amblyopia | Nystagmus | Refractive Error | Orbital | Anterior Segment | Retina/Optic Nerve | Central Nervous System | |
| Esotropia | 56 | - | 1 | 6 | 16 | 0 | 45 | 1 | 0 | 3 | 0 |
| Exotropia | 52 | 10 | - | 11 | 7 | 1 | 35 | 0 | 2 | 5 | 1 |
| Hypertropia | 34 | 12 | 12 | - | 8 | 3 | 21 | 4 | 0 | 2 | 0 |
| Amblyopia | 44 | 13 | 3 | 2 | - | 2 | 42 | 2 | 1 | 2 | 0 |
| Nystagmus | 37 | 12 | 8 | 3 | 3 | - | 28 | 2 | 1 | 17 | 2 |
| Refractive Error | 50 | 2 | 2 | 0 | 3 | 0 | - | 1 | 0 | 1 | 0 |
| Orbital | 38 | 4 | 3 | 4 | 5 | 1 | 21 | - | 6 | 2 | 0 |
| Anterior segment | 51 | 9 | 6 | 7 | 11 | 6 | 40 | 4 | - | 4 | 0 |
| Retina / optic nerve | 55 | 9 | 11 | 5 | 10 | 10 | 38 | 5 | 9 | - | 0 |
| Central nervous system | 27 | 5 | 15 | 2 | 4 | 4 | 20 | 2 | 1 | 13 | - |
Phase 3: Developing Pediatric Eye Questionnaire Modules
1. Factor Analysis
For Child, Proxy and Parent master questionnaires, no items were removed in the master questionnaire item reduction phase (>10% missing, ≥20% questions “not applicable”, >10% found questions unclear or confusing).Factor analysis was then performed yielding, unidimensional domains described below for Rasch analysis.
2. Rasch Analysis
Child Questionnaires
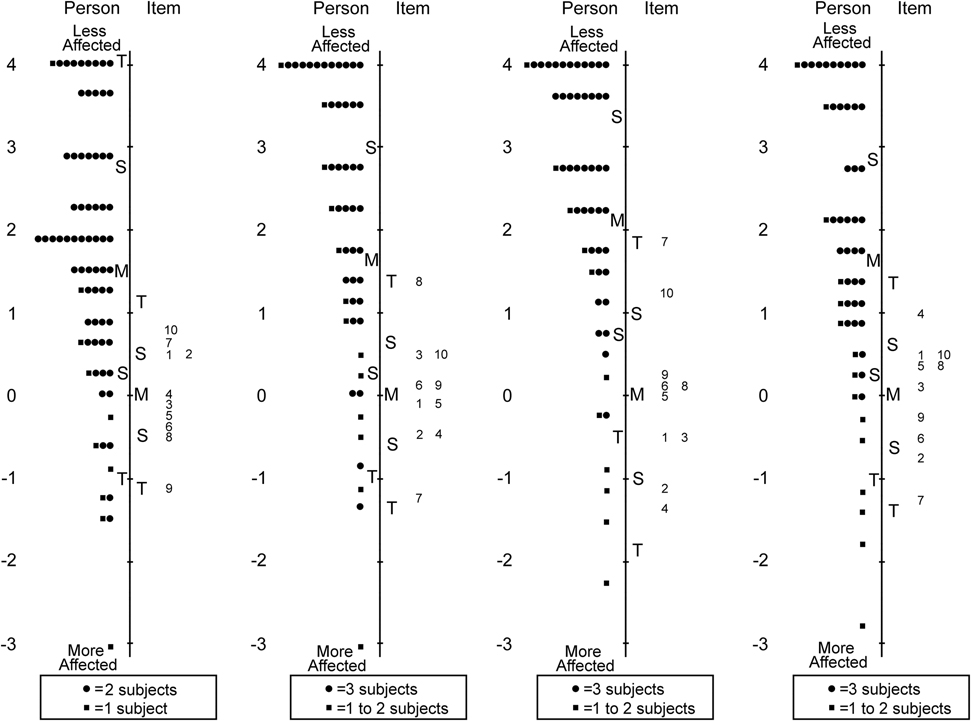
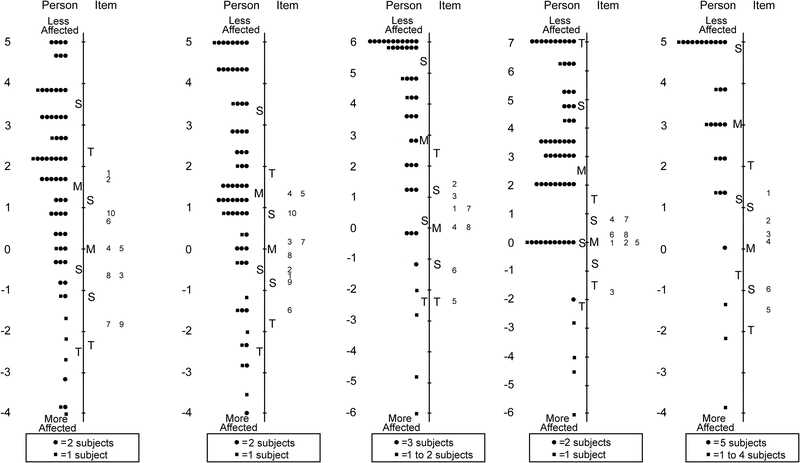
Analysis of both Child 5–11 and Child 12–17 year-old items showed one response category (“most of the time”) was consistently underutilized, and therefore the response scale was collapsed to three response options for subsequent analyses, combining “most of the time” with “sometimes.” After removing 23 of 63 items for either misfit, differential item functioning, and/or to improve targeting, the final Child 5–11 year old Pediatric Eye Questionnaires (PedEyeQ) had four unidimensional domains: 10 of 18 items in functional vision, 10 of 22 in bothered by eyes/vision, 10 of 12 in social, and 10 of 11 in frustration/worry (Table 3 and Figure 1).
Table 3:
Child 5–11 Year Pediatric Eye Questionnaires (PedEyeQ)
| PedEyeQ -Functional vision: Child 5–11 years |
| 1. Do your eyes make it hard to learn? |
| 2. Do you have a hard time seeing? |
| 3. Do you have to do things differently than other people because of your eyes? |
| 4. Do your eyes make it hard to concentrate? |
| 5. Do your eyes make it hard to do certain things? |
| 6. Do you have trouble reading close-up? |
| 7. Do you have to do certain things to help you see better? |
| 8. Is it hard to see the board at school? |
| 9. Do you run into things because of your eyes? |
| 10. Do your eyes get tired easily? |
| PedEyeQ -Bothered by eyes / vision: Child 5–11 years |
| 1. Does it bother you because your eyes make it hard to learn? |
| 2. Does it bother you because your eyes make it hard to play sports? |
| 3. Does it bother you because you have a hard time seeing? |
| 4. Does it bother you that you can’t do certain things because of your eyes? |
| 5. Does it bother you because your eyes make it hard to do certain things? |
| 6. Does it bother you because it’s hard to see the board at school? |
| 7. Does it bother you because it’s hard to see steps when you walk? |
| 8. Does it bother you that bright light makes it hard to do things outside? |
| 9. Is taking care of your eye condition hard for you? |
| 10. Does it bother you because your eyes hurt? |
| PedEyeQ -Social: Child 5–11 years |
| 1. Does it bother you that it’s hard to play/interact with others because of your eyes? |
| 2. Does your eye condition cause problems in your family? |
| 3. Are you shy because of your eyes? |
| 4. Do other people get frustrated with you because of your eyes? |
| 5. Do you get teased because of your eyes? |
| 6. Do you worry about getting hurt because of your eyes? |
| 7. Do you worry about your eyes getting worse? |
| 8. Do you worry about what other people think about you because of your eyes? |
| 9. Do you worry about getting teased because of your eyes? |
| 10. Do you worry about your eyes? |
| PedEyeQ -Frustration / worry: Child 5–11 years |
| 1. Are you bothered by the things you have to do to make your eyes better? |
| 2. Do your eyes make you feel unsure of yourself? |
| 3. Are you frustrated because your eyes aren’t getting better? |
| 4. Does it bother you when other people say things or ask questions about your eyes? |
| 5. Do you feel “different” because of your eyes? |
| 6. Does it bother you that you get extra attention because of your eyes? |
| 7. Do you feel left out because of your eyes? |
| 8. Do you worry that your eyes will make it hard to do things when you’re older? |
| 9. Do you worry about not being able to do things because of your eyes? |
| 10. Do you worry about what you might have to do to make your eyes better? |
Formatted questionnaires with response options, instructions, and Rasch scoring are freely available at www.pedig.net. Each domain can be administered separately or in any combination, and is scored separately.
Figure 1:
Person-item maps for the Child Age 5 to 11 Years Pediatric Eye Questionnaires (PedEyeQ): (first from left) 10 items in a functional vision domain, (second) 10 items in a bothered by eyes/vision domain, (third) 10 items in a social domain, and (fourth) 10 items in a frustration/worry domain (see Table 3 for items). Items higher on the scale are less frequently endorsed as “never.”
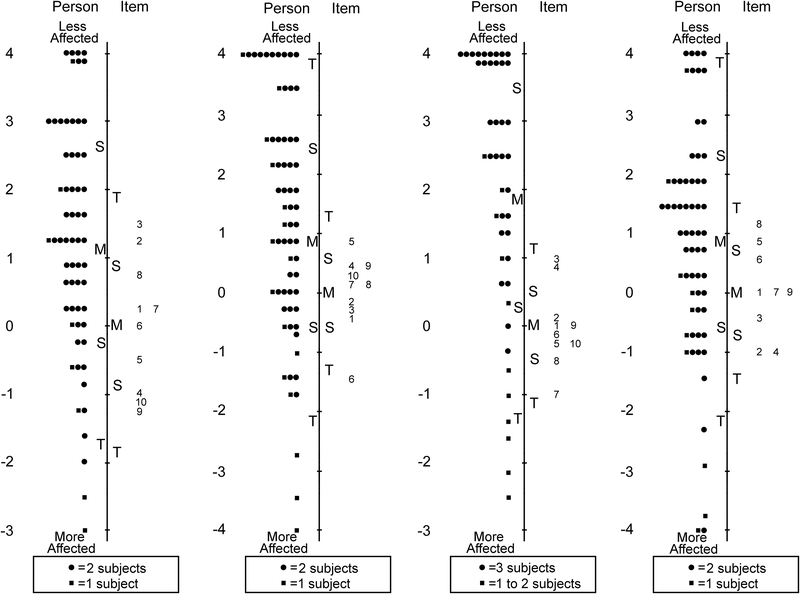
For the Child 12–17 year old PedEyeQ 24 of 63 items were removed across four unidimensional domains retaining: 10 of 18 items in functional vision, 10 of 21 in bothered by eyes/vision, 10 of 15 in social and 9 of 9 in frustration/worry (Table 4 and Figure 2). Separation and reliability indices for both Child 5–11 years and 12–17 years questionnaires are shown in Table 5. Formatted questionnaires and look-up tables with Rasch-scoring for Child 5–11 and 12–17 PedEyeQs are freely available at www.pedig.net.
Table 4:
Child 12–17 Year Pediatric Eye Questionnaires (PedEyeQ)
| PedEyeQ -Functional vision: Child 12–17 years |
| 1. Do your eyes make it hard to learn? |
| 2. Do your eyes make it hard to play sports? |
| 3. Do you have a hard time seeing? |
| 4. Do you need special help at school because of your eyes? |
| 5. Do you need help with certain things because of your eyes? |
| 6. Do you have to do things differently than other people because of your eyes? |
| 7. Do your eyes make it hard to concentrate? |
| 8. Is it hard to see the board at school? |
| 9. Is it hard to see steps when you walk? |
| 10. Do you run into things because of your eyes? |
| PedEyeQ -Bothered by eyes / vision: Child 12–17 years |
| 1. Does it bother you that you need help with certain things because of your eyes? |
| 2. Does it bother you to have to do things differently because of your eyes? |
| 3. Does it bother you because your eyes make it hard to learn? |
| 4. Does it bother you because your eyes make it hard to play sports? |
| 5. Does it bother you because you have a hard time seeing? |
| 6. Does it bother you to need special help at school because of your eyes? |
| 7. Does it bother you because your eyes make it hard to concentrate? |
| 8. Does it bother you because your eyes make it hard to do certain things? |
| 9. Does it bother you because it’s hard to see the board at school? |
| 10. Are you bothered by the things you have to do to make your eyes better? |
| PedEyeQ -Social: Child 12–17 years |
| 1. Are you bothered by how your eyes look? |
| 2. Do your eyes make you feel unsure of yourself? |
| 3. Does it bother you when other people say things or ask questions about your eyes? |
| 4. Do you feel “different” because of your eyes? |
| 5. Does it bother you that you get extra attention because of your eyes? |
| 6. Does it bother you when people look/stare at you because of your eyes? |
| 7. Are you shy because of your eyes? |
| 8. Do you get teased because of your eyes? |
| 9. Do you worry about what other people think about you because of your eyes? |
| 10. Do you worry about getting teased because of your eyes? |
| PedEyeQ -Frustration / worry: Child 12–17 years |
| 1. Do you hate going to the eye doctor? |
| 2. Is taking care of your eye condition hard for you? |
| 3. Are you frustrated because your eyes aren’t getting better? |
| 4. Do you worry about getting hurt because of your eyes? |
| 5. Do you worry about your eyes getting worse? |
| 6. Do you worry that your eyes will make it hard to do things when you’re older? |
| 7. Do you worry about not being able to do things because of your eyes? |
| 8. Do you worry about your eyes? |
| 9. Do you worry about what you might have to do to make your eyes better? |
Formatted questionnaires with response options, instructions, and Rasch scoring are freely available at www.pedig.net. Each domain can be administered separately or in any combination, and is scored separately.
Figure 2:
Person-item maps for the Child Age 12 to 17 Years Pediatric Eye Questionnaires (PedEyeQ): (first from left) 10 items in a functional vision domain, (second) 10 items in a bothered by eyes/vision domain, (third) 10 items in a social domain, and (fourth) 9 items in a frustration/worry domain (see Table 4 for items). Items higher on the scale are less frequently endorsed as “never.”
Table 5.
Rasch Indices for Each Unidimensional Domain Within Each Questionnaire, By Age Group
| Separation | Reliability | Mean person measure |
|||
|---|---|---|---|---|---|
| Person | Item | Person | Item | ||
| Child 5–11 years | |||||
| Functional vision | 1.55 | 2.86 | 0.71 | 0.89 | 1.47 |
| Bothered by eyes / vision | 1.33 | 3.07 | 0.64 | 0.90 | 1.67 |
| Social | 1.00 | 4.06 | 0.50 | 0.94 | 2.10 |
| Frustration / Worry | 1.27 | 3.19 | 0.62 | 0.91 | 1.56 |
| Child 12–17 years | |||||
| Functional vision | 1.91 | 4.40 | 0.78 | 0.95 | 1.18 |
| Bothered by eyes / vision | 1.81 | 2.83 | 0.77 | 0.89 | 0.88 |
| Social | 1.43 | 2.04 | 0.67 | 0.81 | 1.88 |
| Frustration / Worry | 1.99 | 3.43 | 0.80 | 0.92 | 0.87 |
| Proxy 0–4 years | |||||
| Functional vision | 2.11 | 4.30 | 0.82 | 0.95 | 1.73 |
| Bothered by eyes / vision | 1.81 | 3.92 | 0.77 | 0.94 | 3.08 |
| Social | 0.95 | 3.85 | 0.48 | 0.94 | 5.16 |
| Proxy 5–11 years | |||||
| Functional vision | 2.39 | 4.06 | 0.85 | 0.94 | 1.49 |
| Bothered by eyes / vision | 1.91 | 4.84 | 0.78 | 0.96 | 3.34 |
| Social | 1.35 | 2.22 | 0.65 | 0.83 | 2.59 |
| Frustration / Worry | 1.15 | 2.16 | 0.57 | 0.82 | 3.22 |
| Eye-care | 1.40 | 2.48 | 0.66 | 0.86 | 1.32 |
| Proxy 12–17 years | |||||
| Functional vision | 2.30 | 5.40 | 0.84 | 0.97 | 1.51 |
| Bothered by eyes / vision | 2.24 | 3.91 | 0.83 | 0.94 | 1.38 |
| Social | 1.97 | 3.78 | 0.79 | 0.93 | 2.84 |
| Frustration / Worry | 2.03 | 2.46 | 0.81 | 0.86 | 2.40 |
| Eye-care | 1.25 | 3.58 | 0.61 | 0.93 | 2.95 |
| Parent | |||||
| Impact on parent and family | 1.27 | 4.99 | 0.62 | 0.96 | 3.20 |
| Worry about child’s eye condition | 2.27 | 6.64 | 0.84 | 0.98 | 0.15 |
| Worry about self-perception / interactions | 1.96 | 7.17 | 0.79 | 0.98 | 0.86 |
| Worry about functional vision | 2.02 | 8.70 | 0.80 | 0.99 | 1.70 |
Proxy Questionnaires
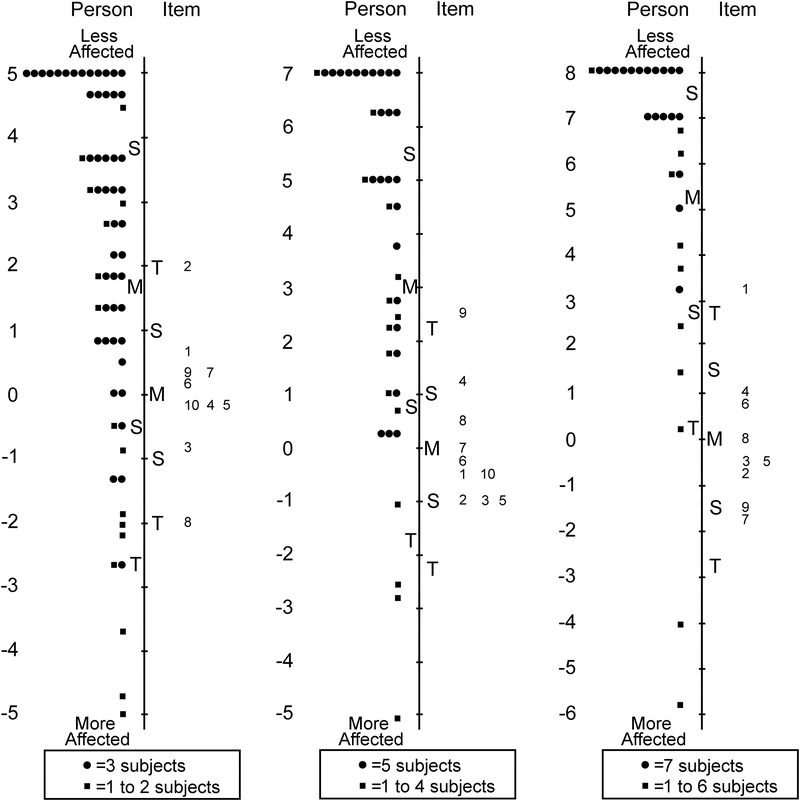
Across all Proxy questionnaires (0–4, 5–11 and 12–17 years) the response “most of the time” was consistently underutilized, and therefore the scale was collapsed to three response options, combining “most of the time” with “sometimes.” After further Rasch analysis of the Proxy 0–4 years questionnaire, 18 of 47 items were removed across three unidimensional domains retaining: 10 of 14 items in functional vision, 10 of 19 in bothered by eyes/vision, and 9 of 14 in social (Table 6 and Figure 3).
Table 6:
Proxy 0–4 Year Pediatric Eye Questionnaires (PedEyeQ)
| PedEyeQ -Functional vision: Proxy 0–4 years |
| 1. Do your child’s eyes make it hard for them to learn? |
| 2. Does your child have a hard time seeing? |
| 3. Are there certain things your child can’t do because of their eyes? |
| 4. Does your child need help with certain things because of their eyes? |
| 5. Does your child have to do things differently than other people because of their eyes? |
| 6. Do your child’s eyes make it hard for them to concentrate? |
| 7. Do your child’s eyes make it hard for them to do certain things? |
| 8. Is it hard for your child to play/interact with others because of their eyes? |
| 9. Is it hard for your child to see steps when they walk? |
| 10. Does your child run into things because of their eyes? |
| PedEyeQ -Bothered by eyes / vision: Proxy 0–4 years |
| 1. Does it bother your child that they need help with certain things because of their eyes? |
| 2. Does it bother your child because they have to do things differently than other people because of their eyes? |
| 3. Does it bother your child because their eyes make it hard to learn? |
| 4. Does it bother your child because they have a hard time seeing? |
| 5. Does it bother your child that they can’t do certain things because of their eyes? |
| 6. Does it bother your child because their eyes make it hard to concentrate? |
| 7. Does it bother your child because their eyes make it hard to do certain things? |
| 8. Does it bother your child to have to do certain things to help them see better? |
| 9. Is your child bothered by the things they have to do to make their eyes better? |
| 10. Does your child get upset because of their eyes? |
| PedEyeQ -Social: Proxy 0–4 years |
| 1. Does it bother your child that bright light makes it hard to do things outside? |
| 2. Is your child bothered by how their eyes look? |
| 3. Do your child’s eyes make them feel unsure of themself? |
| 4. Does your child feel “different” because of their eyes? |
| 5. Does it bother your child that they get extra attention because of their eyes? |
| 6. Does it bother your child when people look/stare at them because of their eyes? |
| 7. Is it hard for your child to make friends because of their eyes? |
| 8. Is your child shy because of their eyes? |
| 9. Does your child feel left out because of their eyes? |
Formatted questionnaires with response options, instructions, and Rasch scoring are freely available at www.pedig.net. Each domain can be administered separately or in any combination, and is scored separately.
Figure 3:
Person-item maps for the Proxy Age 0 to 4 Years Pediatric Eye Questionnaires (PedEyeQ): (first from left) 10 items in a functional vision domain, (second) 10 items in a bothered by eyes/vision domain, and (third) 9 items in a social domain (see Table 6 for items). Items higher on the scale are less frequently endorsed as “never.”
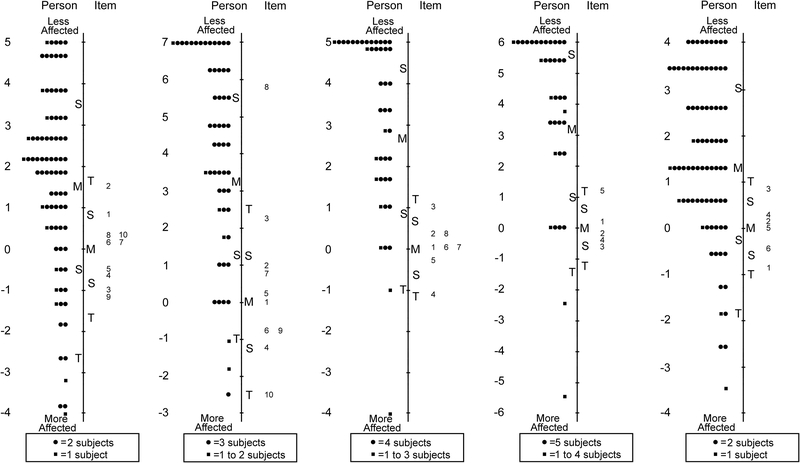
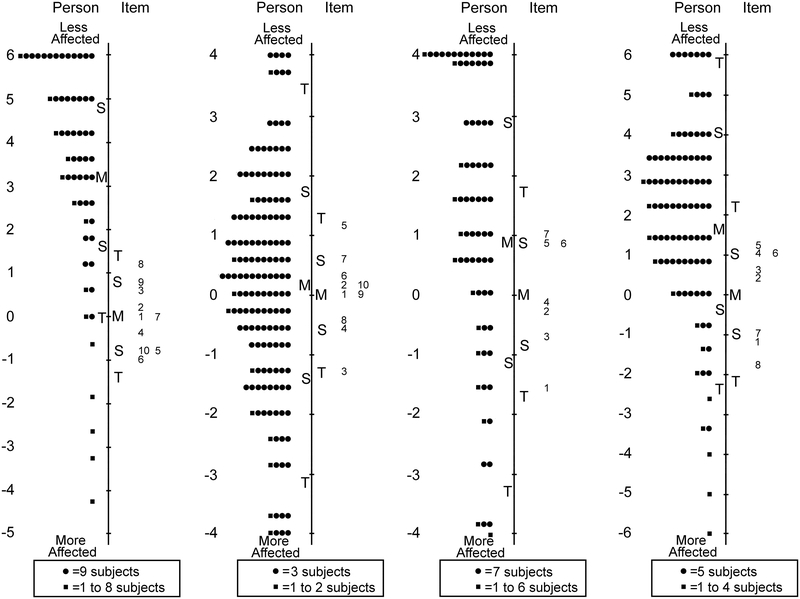
For Proxy 5–11 years 24 of 63 items were removed across five unidimensional domains retaining: 10 of 18 items in functional vision, 10 of 23 in bothered by eyes/vision, 8 of 11 in social, 5 of 5 in frustration/worry, and 6 of 6 in eye-care (Table 7 and Figure 4).
Table 7:
Proxy 5–11 Year Pediatric Eye Questionnaires (PedEyeQ)
| PedEyeQ -Functional vision: Proxy 5–11 years |
| 1. Do your child’s eyes make it hard for them to play sports? |
| 2. Does your child have a hard time seeing? |
| 3. Are there certain things your child can’t do because of their eyes? |
| 4. Does your child need help with certain things because of their eyes? |
| 5. Does your child have to do things differently than other people because of their eyes? |
| 6. Do your child’s eyes make it hard for them to concentrate? |
| 7. Do your child’s eyes make it hard to do certain things? |
| 8. Is it hard for your child to see the board at school? |
| 9. Does your child run into things because of their eyes? |
| 10. Do your child’s eyes get tired easily? |
| PedEyeQ -Bothered by eyes / vision: Proxy 5–11 years |
| 1. Does it bother your child because they have to do things differently than other people because of their eyes? |
| 2. Does it bother your child because their eyes make it hard to play sports? |
| 3. Does it bother your child because they have a hard time seeing? |
| 4. Does it bother your child to need special help at school because of their eyes? |
| 5. Does it bother your child that they can’t do certain things because of their eyes? |
| 6. Does it bother your child that they run into things because of their eyes? |
| 7. Does it bother your child because their eyes get tired easily? |
| 8. Does it bother your child because their eyes make it hard to do certain things? |
| 9. Does it bother your child that it’s hard to play/interact with others because of their eyes? |
| 10. Does your child feel left out because of their eyes? |
| PedEyeQ -Social: Proxy 5–11 years |
| 1. Do your child’s eyes make them feel unsure of themself? |
| 2. Does it bother your child when other people say things or ask questions about their eyes? |
| 3. Does your child feel “different” because of their eyes? |
| 4. Does it bother your child that they get extra attention because of their eyes? |
| 5. Does it bother your child when people look/stare at them because of their eyes? |
| 6. Does your child get teased because of their eyes? |
| 7. Does your child worry about what other people think about them because of their eyes? |
| 8. Does your child worry about getting teased because of their eyes? |
| PedEyeQ -Frustration / worry: Proxy 5–11 years |
| 1. Is your child frustrated because their eyes aren’t getting better? |
| 2. Does your child worry about their eyes getting worse? |
| 3. Does your child worry that their eyes will make it hard to do things when they’re older? |
| 4. Does your child worry about not being able to do things because of their eyes? |
| 5. Does your child worry about their eyes? |
| PedEyeQ -Eyecare: Proxy 5–11 years |
| 1. Does it bother your child to have to protect their eyes? |
| 2. Does it bother your child to have to do certain things to help them see better? |
| 3. Is your child bothered by the things they have to do to make their eyes better? |
| 4. Does your child hate going to the eye doctor? |
| 5. Is taking care of their eye condition hard for your child? |
| 6. Does your child worry about what they might have to do to make their eyes better? |
Formatted questionnaires with response options, instructions, and Rasch scoring are freely available at www.pedig.net. Each domain can be administered separately or in any combination, and is scored separately.
Figure 4:
Person-item maps for the Proxy Age 5 to 11 Years Pediatric Eye Questionnaires (PedEyeQ): (first from left) 10 items in a functional vision domain, (second) 10 items in a bothered by eyes/vision domain, (third) 8 items in a social domain, and (fourth) 5 items in a frustration/worry domain, and (fifth) 6 items in an eye-care domain (see Table 7 for items). Items higher on the scale are less frequently endorsed as “never.”
For Proxy 12–17 years 21 of 63 items were removed across five unidimensional domains, retaining: 10 of 18 items in functional vision, 10 of 19 in bothered by eyes/vision, 8 of 12 in social, 8 of 8 in frustration/worry, and 6 of 6 in eye-care (Table 8 and Figure 5). Separation and reliability indices for Proxy 0–4, 5–11 and 12–17 years questionnaires are shown in Table 5. Formatted questionnaires and look-up tables with Rasch-scoring for Proxy 0–4, 5–11 and 12–17 PedEyeQs are freely available at www.pedig.net.
Table 8:
Proxy 12–17 Pediatric Eye Questionnaires (PedEyeQ)
| PedEyeQ -Functional vision: Proxy 12–17 years |
| 1. Do your child’s eyes make it hard for them to play sports? |
| 2. Does your child have a hard time seeing? |
| 3. Does your child need special help at school because of their eyes? |
| 4. Does your child have to do things differently than other people because of their eyes? |
| 5. Do your child’s eyes make it hard for them to concentrate? |
| 6. Do your child’s eyes make it hard to do certain things? |
| 7. Is it hard for your child to play/interact with others because of their eyes? |
| 8. Is it hard for your child to see steps when they walk? |
| 9. Does your child run into things because of their eyes? |
| 10. Do your child’s eyes get tired easily? |
| PedEyeQ -Bothered by eyes / vision: Proxy 12–17 years |
| 1. Does it bother your child that they need help with certain things because of their eyes? |
| 2. Does it bother your child because they have to do things differently than other people because of their eyes? |
| 3. Does it bother your child because their eyes make it hard to learn? |
| 4. Does it bother your child because their eyes make it hard to play sports? |
| 5. Does it bother your child because they have a hard time seeing? |
| 6. Does it bother your child that they run into things because of their eyes? |
| 7. Does it bother your child because their eyes get tired easily? |
| 8. Does it bother your child because their eyes make it hard to concentrate? |
| 9. Does it bother your child because they have trouble reading close-up? |
| 10. Does it bother your child because it’s hard to see the board at school? |
| PedEyeQ -Social: Proxy 12–17 years |
| 1. Is your child bothered by how their eyes look? |
| 2. Do your child’s eyes make them feel unsure of themself? |
| 3. Does it bother your child when other people say things or ask questions about their eyes? |
| 4. Does it bother your child when people look/stare at them because of their eyes? |
| 5. Is it hard for your child to make friends because of their eyes? |
| 6. Is your child shy because of their eyes? |
| 7. Does your child worry about what other people think about them because of their eyes? |
| 8. Does your child worry about getting teased because of their eyes? |
| PedEyeQ -Frustration / worry: Proxy 12–17 years |
| 1. Does your child get upset because of their eyes? |
| 2. Is your child frustrated because their eyes aren’t getting better? |
| 3. Does your child worry about getting hurt because of their eyes? |
| 4. Does your child worry about their eyes getting worse? |
| 5. Does your child worry that their eyes will make it hard to do things when they’re older? |
| 6. Does your child worry about not being able to do things because of their eyes? |
| 7. Does your child worry about their eyes? |
| 8. Does your child worry about what they might have to do to make their eyes better? |
| PedEyeQ -Eyecare: Proxy 12–17 years |
| 1. Does your child hate going to the eye doctor? |
| 2. Is taking care of their eye condition hard for your child? |
| 3. Does it bother your child because their eyes hurt? |
| 4. Does it bother your child that they get extra attention because of their eyes? |
| 5. Does your child’s eye condition cause problems in your family? |
| 6. Do other people get frustrated with your child because of their eyes? |
Formatted questionnaires with response options, instructions, and Rasch scoring are freely available at www.pedig.net. Each domain can be administered separately or in any combination, and is scored separately
Figure 5:
Person-item maps for the Proxy Age 12 to 17 Years Pediatric Eye Questionnaires (PedEyeQ): (first from left) 10 items in a functional vision domain, (second) 10 items in a bothered by eyes/vision domain, (third) 8 items in a social domain, and (fourth) 8 items in a frustration/worry domain, and (fifth) 6 items in an eye-care domain (see Table 8 for items). Items higher on the scale are less frequently endorsed as “never.”
Parent Questionnaires
For Parent questionnaires the “most of the time” response option was once again underutilized, and therefore the response scale was collapsed to three response options, combining “most of the time” with “sometimes.” After further Rasch analysis of Parent questionnaire, 12 of 47 items were removed across four unidimensional domains retaining: 10 of 19 items in impact on parent and, 10 of 13 in worry about child’s eye condition, 7 of 7 in worry about child’s self-perception/interactions, and 8 of 8 in worry about child’s functional vision (Table 9 and Figure 6). Separation and reliability indices for Parent PedEyeQs are shown in Table 2. Formatted questionnaires and look-up tables with Rasch-scoring are freely available at www.pedig.net.
Table 9:
Parent Pediatric Eye Questionnaires (PedEyeQ)
| PedEyeQ -Impact on parent and family: Parent |
| 1. Do you feel different from other parents because of your child’s eye condition? |
| 2. Does taking care of your child’s eye condition cause stress on your family? |
| 3. Is it difficult to ensure that your child receives the help they need because of their eye condition? |
| 4. Is it hard because you need to be more involved in your child’s schooling because of their eye condition? |
| 5. Does it bother you to have to change how you do things because of your child’s eye condition? |
| 6. Does it bother you that you can’t do certain things because of your child’s eye condition? |
| 7. Is it hard because it takes extra time to do things because of your child’s eye condition? |
| 8. Is it hard work having to explain your child’s eye condition to others? |
| 9. Is it hard because you have to attend frequent eye exams for your child? |
| 10. Is it hard because your child needs more supervision because of their eye condition? |
| PedEyeQ -Worry about child’s eye condition: Parent |
| 1. Does it bother you that your child has an eye condition? |
| 2. Does it bother you that your child doesn’t see well out of one or both eyes? |
| 3. Does it bother you that your child’s eye condition causes physical discomfort? |
| 4. Do you worry about your child getting upset because of their eye(s)? |
| 5. Do you worry about your child’s eye condition getting worse? |
| 6. Do you worry about your child’s future because of their eye condition? |
| 7. Do you worry about protecting your child’s eye(s)? |
| 8. Do you worry about your child’s safety because of their eye condition? |
| 9. Do you worry about the treatment(s) your child may need for their eye condition? |
| 10. Do you worry that you don’t fully understand your child’s eye condition? |
| PedEyeQ -Worry about self-perception and interactions: Parent |
| 1. Are you bothered by your child’s appearance because of their eye condition? |
| 2. Does it bother you when others say things about your child’s eye condition? |
| 3. Does it bother you when others look/stare at your child because of their eye condition? |
| 4. Do you worry about your child being “different” because of their eye condition? |
| 5. Do you worry about your child getting teased because of their eye condition? |
| 6. Do you worry about your child’s eye condition affecting them socially? |
| 7. Do you worry about your child’s self-esteem because of their eye condition? |
| PedEyeQ -Worry about functional vision: Parent |
| 1. Does it bother you that your child can’t do certain things because of their eye condition? |
| 2. Do you worry about your child being unable to do certain things because of their eye condition? |
| 3. Do you worry about your child having a hard time reading because of their eye condition? |
| 4. Do you worry about your child’s eye condition affecting their development? |
| 5. Do you worry about your child’s eye condition affecting their learning? |
| 6. Do you worry about your child’s depth perception? |
| 7. Does it bother you when others aren’t patient with your child’s eye related needs? |
| 8. Is it hard because your child’s eye condition affects their behavior? |
Formatted questionnaires with response options, instructions, and Rasch scoring are freely available at www.pedig.net. Each domain can be administered separately or in any combination, and is scored separately.
Figure 6:
Person-item maps for the Parent Pediatric Eye Questionnaires (PedEyeQ): (first from left) 10 items in an impact on the parent and family domain, (second) 10 items in a worry about the child domain, (third) 7 items in a worry about the child’s self-perception/interactions domain, and (fourth) 8 items in a worry about the child’s functional vision domain (see Table 9 for items). Items higher on the scale are less frequently endorsed as “never.”
DISCUSSION
Using rigorous questionnaire development methods we have created new Pediatric Eye Questionnaires (PedEyeQ), with Child, Proxy and Parent components, for use across the entire spectrum of childhood eye conditions. The PedEyeQs have been designed to enable age-appropriate assessment of the impact of any eye condition on the functional vision and ER-QOL of the child themselves (self-report and proxy) as well as the impact on the parent, with individual, separately-scored domains that can be administered alone, or in any combination, depending on clinical and/or research needs. By making the PedEyeQ questions, short-forms and Rasch scoring system freely available to the community, we hope to improve assessment and reporting of how various eye conditions affect a child and their family in everyday life.
Existing pediatric questionnaires have been designed either as generic instruments (e.g., the PedsQL24, 25), or for specific eye conditions, (e.g., uveitis,7, 26 conjunctivitis,8 amblyopia10, 11, 17, 18, 27) or for low vision.12–14, 16, 20, 28–30 While we recognize a role for evaluating concerns peculiar to a certain disease, as well as a role for evaluating more general quality of life concerns, we identified a need for instruments that would allow evaluation of eye-related concerns, without being disease-specific, and therefore applicable across the entire spectrum of childhood eye conditions. By initially deriving potential questionnaire items from interviews with a large cohort of children with a diverse range of eye disorders,1, 3, 4, 6 and then using rigorous item-reduction methods, we believe the new PedEyeQs provide such a means of evaluating the functional vision and ER-QOL impact of any childhood eye disorder. In future studies the PedEyeQ domains will be evaluated for construct validity, reliability, and responsiveness, and compared with other instruments.
It is impractical to administer a large number of questionnaire items to children; many have a limited attention span making administration of more than 40–50 questions unrealistic, particularly in a clinical setting. Various strategies can be adopted to reduce a large number of items to a smaller representative sample. We elected to use Rasch analysis to identify which items to exclude to retain 10 items or fewer per unidimensional domain. We found that for older children and for parents, this approach to item-reduction typically resulted in a final set of questions that had the desired precision as assessed by Rasch parameters. Nevertheless, for younger children we had several examples of relatively poor targeting and relatively low precision even before any items were removed. Published target Rasch indices31, 32 are based on typical performance in adult populations, and it is likely that these thresholds are unrealistic for young children.
There are some limitations to our study. Although we recruited a large, diverse cohort of children and parents to complete master questionnaires, we may have under-represented certain socioeconomic and cultural groups, and final selection of items might have been different had we tested master questionnaires in other patient populations. Nevertheless, we included children and parents across a spectrum of socioeconomic and cultural groups. In addition, some children had coexisting systemic health conditions and the presence of such may have influenced responses. Nevertheless, we consistently used the qualifier “because of your eyes” and such responses are representative of children with eye conditions who have additional health concerns. We identified ER-QOL constructs using a data-driven approach, performing factor analysis and then labeling factors according to item content. However alternative approaches include determining constructs a priori, and we may have created different constructs had we used this approach. We provide Rasch-based scoring look-up tables for derived questionnaires, but these scores were derived from our specific study population and scoring may differ in other populations where a de novo Rasch analysis may be preferable.
By following a rigorous approach to questionnaire development and refinement, we have developed sets of Pediatric Eye Questionnaire short forms for separately assessing functional vision and individual ER-QOL domains in children of any age and with any eye condition. In future phases of this study these questionnaires will undergo reliability and validity testing in a new cohort of patients, and we will also separately report the development of treatment-related questionnaires.
ACKNOWLEDGMENTS:
a. Funding/Support: Financial assistance for this study came from National Institutes of Health Grants EY024333 (JMH, PI & EEB, Co-I), and EY022313 (EEB), and Mayo Foundation, Rochester, Minnesota.
b. Financial Disclosures: None of the authors have any financial disclosures
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Hatt SR, Leske DA, Castaneda YS, et al. Patient-derived questionnaire items for patient-reported outcome measures in pediatric eye conditions. J AAPOS 2018. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.U.S. Department of Health and Human Services, Food and Drug Admministration, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER), Center for Devices and Radiological Health (CDRH). Guidance for Industry. Patient-reported outcome measures: Use in medical product development to support labeling claims, 2009:http://www.fda.gov/downloads/Drugs/Guidances/UCM193282.pdf. [Google Scholar]
- 3.Liebermann L, Leske DA, Castañeda YS, et al. Childhood esotropia: child and parent concerns. J AAPOS 2016;20(4):295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castañeda YS, Cheng-Patel CS, Leske DA, et al. Quality of life and functional vision concerns of children with cataracts and their parents. Eye (Lond) 2016;30(9):1251–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hatt SR, Leske DA, Wernimont SM, Birch EE, Holmes JM. Comparison of rating scales in the development of patient-reported outcome measures for children with eye disorders. Strabismus 2017;25(1):33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liebermann L, Leske DA, Hatt SR, et al. Bilateral childhood visual impairment: child and parent concerns. J AAPOS 2017;21(3):183.e1–183.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Angeles-Han ST, Griffin KW, Harrison MJ, et al. Development of a vision-related quality of life instrument for children ages 8–18 years for use in juvenile idiopathic arthritis-associated uveitis. Arthritis Care Res (Hoboken) 2011;63(9):1254–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sacchetti M, Baiardini I, Lambiase A, et al. Development and testing of the quality of life in children with vernal keratoconjunctivitis questionnaire. Am J Ophthalmol 2007;144(4):557–563. [DOI] [PubMed] [Google Scholar]
- 9.Holmes JM, Leske DA, Cole SR, Chandler DL, Repka MX, Nasolacrimal Duct Obstruction Questionnaire Study Group. A symptom survey and quality of life questionnaire for nasolacrimal duct obstruction in children. Ophthalmology 2006;113(9):1675–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sabri K, Knapp CM, Thompson JR, Gottlob I. The VF-14 and psychological impact of amblyopia and strabismus. Invest Ophthalmol Vis Sci 2006;47(10):4386–4392. [DOI] [PubMed] [Google Scholar]
- 11.Hrisos S, Clarke MP, Wright CM. The emotional impact of amblyopia treatment in preschool children. Ophthamology 2004;1111550–1556. [DOI] [PubMed] [Google Scholar]
- 12.Gothwal VK, Lovie-Kitchin JE, Nutheti R. The development of the LV Prasad-Functional Vision Questionnaire: a measure of functional vision performance of visually impaired children. Invest Ophthalmol Vis Sci 2003;44(9):4131–4139. [DOI] [PubMed] [Google Scholar]
- 13.Khadka J, Ryan B, Margrain TH, Court H, Woodhouse JM. Development of the 25-item Cardiff Visual Ability Questionnaire for Children (CVAQC). Br J Ophthalmol 2010;94(6):730–735. [DOI] [PubMed] [Google Scholar]
- 14.Cochrane GM, Marella M, Keeffe JE, Lamoureux EL. The impact of vision impairment for children (IVI_C): validation of a vision-specific pediatric quality-of-life questionnaire using Rasch analysis. Invest Ophthalmol Vis Sci 2011;52(3):1632–1640. [DOI] [PubMed] [Google Scholar]
- 15.Hatt SR, Leske DA, Yamada T, Bradley EA, Cole SR, Holmes JM. Development and initial validation of quality of life questionnaires for intermittent exotropia. Ophthalmology 2010;117(1):163–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tadic V, Cooper A, Cumberland P, Lewando-Hundt G, Rahi JS. Development of the functional vision questionnaire for children and young people with visual impairment: the FVQ_CYP. Ophthalmology 2013;120(12):2725–2732. [DOI] [PubMed] [Google Scholar]
- 17.Carlton J Developing the draft descriptive system for the child amblyopia treatment questionnaire (CAT-Qol): a mixed methods study. Health Qual Life Outcomes 2013;11174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bokhary KA, Suttle C, Alotaibi AG, Stapleton F, Boon MY. Development and validation of the 21-item Children’s Vision for Living Scale (CVLS) by Rasch analysis. Clin Exp Optom 2013;96(6):566–576. [DOI] [PubMed] [Google Scholar]
- 19.Rah MJ, Walline JJ, Jones-Jordan LA, et al. Vision specific quality of life of pediatric contact lens wearers. Optom Vis Sci 2010;87(8):560–566. [DOI] [PubMed] [Google Scholar]
- 20.Felius J, Stager DR Sr, Berry PM, et al. Development of an instrument to assess vision-related quality of life in young children. Am J Ophthalmol 2004;138(3):362–372. [DOI] [PubMed] [Google Scholar]
- 21.DeWalt DA, Rothrock N, Yount S, Stone AA. Evaluation of item candidates: the PROMIS qualitative item review. Med Care 2007;45(5 Suppl 1):S12–S21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khadka J, McAlinden C, Craig JE, Fenwick EK, Lamoureux EL, Pesudovs K. Identifying content for the glaucoma-specific item bank to measure quality-of-life parameters. J Glaucoma 2013. [DOI] [PubMed] [Google Scholar]
- 23.van de Graaf ES, Felius J, van Kempen-du Saar H, Looman CWN, Passchier J, Kelderman HS, H.J. Construct validation of the Amblyopia and Strabismus Questionnaire (A&SQ) by factor analysis. Graefes Arch Clin Exp Ophthalmol 2009;247(9):1263–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Varni JW, Seid M, Rode CA. The PedsQL: measurement model for the pediatric quality of life inventory. Med Care 1999;37(2):126–139. [DOI] [PubMed] [Google Scholar]
- 25.Varni JW, Seid M, Kurtin PS. PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Med Care 2001;39(8):800–812. [DOI] [PubMed] [Google Scholar]
- 26.Angeles-Han ST, Yeh S, McCracken C, et al. Using the Effects of Youngsters’ Eyesight on Quality of Life Questionnaire to measure visual outcomes in children with uveitis. Arthritis Care Res (Hoboken) 2015;67(11):1513–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carlton J Identifying potential themes for the Child Amblyopia Treatment Questionnaire. Optom Vis Sci 2013;90(8):867–873. [DOI] [PubMed] [Google Scholar]
- 28.Gothwal VK, Wright TA, Lamoureux EL, Pesudovs K. Rasch analysis of the quality of life and vision function questionnaire. Optom Vis Sci 2009;86(7):E836–844. [DOI] [PubMed] [Google Scholar]
- 29.Cochrane G, Lamoureux E, Keeffe J. Defining the content for a new quality of life questionnaire for students with low vision (the Impact of Vision Impairment on Children: IVI_C). Ophthalmic Epidemiol 2008;15(2):114–120. [DOI] [PubMed] [Google Scholar]
- 30.Birch EE, Cheng CS, Felius J. Validity and reliability of the Children’s Visual Function Questionnaire (CVFQ). J AAPOS 2007;11(5):473–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khadka J, McAlinden C, Pesudovs K. Quality assessment of ophthalmic questionnaires: review and recommendations. Optom Vis Sci 2013;90(8):720–744. [DOI] [PubMed] [Google Scholar]
- 32.Pesudovs K, Burr JM, Harley C, Elliott DB. The development, assessment, and selection of questionnaires. Optom Vis Sci 2007;84(8):663–674. [DOI] [PubMed] [Google Scholar]