Abstract
Degenerative retinal diseases such as retinitis pigmentosa (RP) and Leber’s congenital amaurosis (LCA) may lead to blindness without effective treatment. With the rapid advancement of the CRISPR/Cas9 genome editing technology, in vivo application of CRISPR/Cas9 holds immense potential for treatment of these diseases. Adeno-associated virus (AAV) vectors are an ideal gene transfer tool for delivery of CRISPR components to the retina. Here, we describe a protocol for utilizing an AAV-based CRISPR/Cas9 system for in vivo genome editing in the retina.
Keywords: CRISPR/Cas9, AAV vector, Genome editing, Gene therapy, Retina, Retinal degeneration
1. Introduction
The rapidly advancing CRISPR/Cas9 genome editing technology has brought revolutionary changes in life sciences. In the CRISPR/Cas9 system, the Cas9 DNA endonuclease creates double-strand DNA breaks (DSBs) at desired locations in the genome, directed by a short guide RNA (sgRNA) comprised of 20 nucleotides that recognizes the target DNA via Watson-Crick base paring. Endogenous DNA repair mechanisms such as non-homologous end joining (NHEJ) or homology directed repair (HDR) are activated to repair the DSBs. NHEJ is the major DNA repair pathway in which insertion or deletion (indel) of random nucleotides usually occurs, and is used to achieve gene disruption or gene deletion for genome editing. In contrast, HDR leads to an error-free repair based on a template DNA with homologous sequences, though with much lower efficiency than NHEJ [1].
Numerous studies have shown the great potential of the CRISPR/Cas9 technique for disease treatment [2]. To develop therapies for various degenerative retinal diseases such as retinitis pigmentosa (RP), Leber’s congenital amaurosis (LCA), and age-related macular degeneration (AMD), the CRISPR components need to be efficiently delivered to the retina. To this end, three gene transfer methods have been reported, including electroporation of plasmids carrying CRISPR components [3–6], lipid-mediated transfection of CRISPR ribonucleoprotein (RNP) complexes [7], and viral vector (AAV and lentivirus) mediated CRISPR transfer [8–15]. Among them, the AAV vector approach appears to be most efficient for in vivo retinal gene delivery. Here we describe a protocol for the generation of AAV vectors carrying the CRISPR components and the delivery of these AAV vectors to the mouse retina.
2. Materials
2.1. Vector Construction and In Vitro sgRNA Selection
2.1.1. Design and Assembly of sgRNA
CRISPR design tool (http://crispr.mit.edu/).
Benchling (https://benchling.com/).
pV-Cas9 plasmid carrying the Cas9 expression cassette [14].
pV-sgRNA-tdTomato plasmid carrying the U6 promoter-driven sgRNA and a tdTomato expression cassette [14].
T4 polynucleotide kinase.
10× T4 PNK reaction buffer.
10 mM adenosine triphosphate (ATP).
UltraPure DNase/RNase-free distilled water.
Taq buffer.
Thermal cycler.
SapI restriction enzyme (New England Biolabs).
10× CutSmart buffer.
Alkaline phosphatase, calf intestinal (CIP).
Tris-acetate-EDTA (TAE) buffer.
Agarose gel.
Gel Extraction kit.
Quick Ligase (New England Biolabs).
Quick Ligase buffer.
DH10B competent cells (New England Biolabs).
SOC medium.
LB agar plates.
LB medium supplemented with 100 μg/mL ampicillin.
Plasmid miniprep kit.
Plasmid maxiprep kit.
2.1.2. In Vitro sgRNA Selection
HEK293 cells (ATCC® #CRL-1573).
HEK293-GFP cells (GenTarget #SC001).
Dulbecco’s modified Eagle’s medium (DMEM).
Fetal bovine serum (FBS).
Lipofectamine 2000.
Gel Extraction kit.
Opti-MEM® I reduced serum medium.
Phosphate buffered saline (PBS).
Trypsin-EDTA.
DNeasy Blood & Tissue Kit (QIAGEN).
- SURVEYOR assay kit (Integrated DNA Technologies):
- 0.15 M MgCl2 stock solution.
- SURVEYOR nuclease S.
- SURVEYOR enhancer S.
- Stop Solution.
High-fidelity DNA polymerase: PrimeSTAR polymerase (Clontech) or similar.
DNA polymerase buffer (2× PrimeSTAR GC buffer or similar).
25 mM Deoxy-ribonucleoside triphosphate (dNTP).
2.2. AAV Production
2.2.1. AAV Generation by Transient Transfection
Helper plasmid pHLP19-AAV8 carrying AAV2 Rep and AAV8 Cap genes.
Helper plasmid pLAdeno5 carrying adenovirus helper genes.
Roller bottles.
1 M HEPES buffer.
2× HBS buffer: 50 mM HEPES, 280 mM NaCl, and 1.5 mM Na2HPO4, pH 7.3.
0.3 M CaCl2 solution.
500 mL conical tubes.
TSM buffer: 50 mM TrisCl, 150 mM NaCl, and 2 mM MgCl2, pH 8.0.
Benchtop centrifuge.
2.2.2. AAV Isolation and Purification
Microfluidizer.
1 M CaCl2 solution.
Benzonase.
40% PEG8000/2.5 N NaCl solution.
HSSE-RNase A buffer: 50 mM HEPES, 150 mM NaCl, 1% Sarcosyl, 20 mM EDTA, and 10 μg/mL RNase A, pH 8.0.
1.5 g/mL CsCl: mix 60 g CsCl with approximately 80 mL of 25 mM Tris–HCl, pH 8.0. Determine density by weighing 1 mL solution.
1.4 g/mL CsCl: mix 60 g CsCl with approximately 100 mL of 25 mM Tris–HCl, pH 8.0.
1.3 g/mL CsCl: mix 60 g CsCl with approximately 125 mL of 25 mM Tris–HCl, pH 8.0.
Ultracentrifuge with SW32Ti and SW40Ti rotors (Beckman).
38.5 mL centrifuge tubes.
14 mL centrifuge tubes.
Halogen beam illuminator.
5 mL syringes.
18G needles.
Slide-A-Lyzer dialysis cassettes.
Tris-buffered saline: 10 mM Tris–HCl, 180 mM NaCl, pH 7.4.
2.2.3. AAV Titration by qPCR
Linearized AAV vector standard DNA.
Titration primers.
FAM-conjugated probes.
qPCR dilution buffer: 10 mM Tris–HCl pH 8.0, 1 mM EDTA, 10 μg/mL yeast tRNA, 0.01% Tween 80.
DNase buffer: 10 mM Tris–HCl, pH 8.0, and 10 mM MgCl2.
200 mM EDTA, pH 8.0.
2× PCR Master Mix.
LoBind microcentrifuge tubes.
96-well qPCR plate
qPCR optical adhesive film.
Real-Time PCR System (QuantStudio™ 3 or similar).
2.3. Delivery of AAV to the Retina by Subretinal Injection
100 mg/mL fluorescein (Alcon).
12.5 mg/mL ketamine.
2.5 mg/mL xylazine.
1% atropine eye drops.
0.5% tropicamide eye drops.
18G needles.
Ophthalmic surgical microscope.
Hamilton glass syringes.
34-gauge blunt-tip needles.
Antibiotic ointment.
2.4. Validation of In Vivo Genome Editing
2.4.1. Retina Whole Mount
Micro scissors.
Micro forceps.
4% paraformaldehyde (PFA).
PBS (prechilled or room temperature).
Microscope slides.
Micro cover glasses.
Mounting medium: Fluoromount-G or similar.
Fluorescence microscope or confocal scanning microscope.
2.4.2. Dissociation of Retinal Cells for FACS
StemPro™ accutase cell dissociation reagent (Thermo Fisher).
Thermal Shaker.
Test tubes with cell strainer snap cap and regular cap.
Hank’s balanced salt solution (HBSS).
Fluorescence-activated cell sorting (FACS) system (FACSAria or similar).
2.4.3. Targeted DNA High-Throughput Sequencing
2.5. Assessment After In Vivo Genome Editing
Espion E2 Electroretinography Console.
12.5 mg/mL ketamine.
2.5 mg/mL xylazine.
1% atropine eye drops.
0.5% tropicamide eye drops.
Ophthalmic gel (Gonak™ or similar).
OptoMotry VR system, version 1.4.0 (CerebralMechanics, Inc.).
10% sucrose in PBS.
20% sucrose in PBS.
PBST solution: 5% Donkey serum and 0.1% Triton X-100 in PBS.
Tissue-Tek™ OCT compound.
Cryostat microtome.
Primary antibodies and fluorochrome-conjugated secondary antibodies.
0.2 μg/mL 4,6-diamidino-2-phenylindole (DAPI) in PBST.
Mounting medium: Fluoromount-G or similar.
3. Methods
Carry out all procedures at room temperature unless otherwise specified.
3.1. Vector Construction and In Vitro sgRNA Selection
3.1.1. Design and Assembly of sgRNA
Input the DNA sequence of interest into the online programs CRISPR design tool and Benchling. Select five sgRNA target sequences based on the ranking of potential off-target and on-target activities.
Purchase synthesized oligonucleotides (oligos) of each sense and antisense target sequence with appropriate overhangs for assembly. For sgRNA assembly to pV-sgRNA-tdTomato, add 5′-ACCG-3′ to the 5′-end of the sense oligos, and add 5′-AAC-3′ to the 5′-end and a “C” to the 3′-end of the anti-sense oligos.
Phosphorylate the oligos in a 20 μL reaction containing: 1× T4 PNK reaction buffer, 200 μM oligonucleotide, 2 μL 10 mM ATP, and 10 U T4 PNK. Incubate at 37 °C for 30 min followed by 75 °C for 10 min. Dilute the reaction to 200 μL by adding 180 μL UltraPure water.
Anneal the sense and antisense oligos in a 20 μL reaction containing: 4 μM sense oligos, 4 μM antisense oligos, and 1× standard Taq buffer. Incubate at 95 °C for 5 min and ramp down to 25 °C at a rate of 1 °C per 20 s.
Digest 2 μg of pV-sgRNA-tdTomato in a 50 μL reaction containing 1× CutSmart buffer and 20 U SapI restriction enzyme. Incubate at 37 °C for 2 h. Add 10 U CIP to the reaction and incubate for 30 min to dephosphorylate the digested ends.
Separate the digested plasmid on a 1% agarose gel. Purify the digested plasmid using the Gel Extraction kit following manufacturer’s instructions.
Ligate the annealed oligos with the purified digested plasmid in a 20 μL reaction containing: 1× Quick Ligase buffer, 50 ng purified digested plasmid, 2 μL annealed oligos and 1 μL Quick Ligase. Incubate for 5 min.
Transform the ligation mixture into DH10B competent bacterial cells following manufacturer’s instructions. Briefly, add 4 μL of the ligation mixture to 100 μL competent cells and incubate on ice for 30 min, followed by incubation at 42 °C for 30 s. Immediately place the bacteria on ice for 2 min. Add 800 μL of SOC medium to the bacteria and shake at the speed of 220 rpm at 37 °C for 1 h. Centrifuge at 1500 × g for 3 min and discard the supernatant. Resuspend the bacteria in 100 μL of SOC medium and spread on LB agar plates with 100 μg/mL ampicillin. Incubate the plate at 37 °C overnight.
Pick several bacterial colonies for cultures in 3 mL of LB medium with 100 μg/mL ampicillin and incubate at 37 °C overnight with shaking at 220 rpm.
Isolate the plasmid DNA using a miniprep kit following manufacturer’s instructions. Elute plasmid DNA with 50 μL Tris–HCl solution, pH 8.0.
Validate the assembly of sgRNA sequences by redigestion using SapI restriction enzyme and/or by sanger sequencing.
3.1.2. In Vitro sgRNA Selection
Seed 4 × 105 HEK293-GFP cells in a 6-well plate filled with 2 mL of DMEM medium supplemented with 10% FBS. Culture cells in a 37 °C incubator supplemented with 10% CO2.
When the cells are approximately 80% confluent, transfect the cells in each well with 1 μg of pV-Cas9 plasmid and 1 μg of pV-sgRNA-tdTomato plasmid with verified insertion of the sgRNA target sequence, using Lipofectamine 2000 reagent and following manufacturer’s instructions.
Harvest the cells 48 h after transfection. Extract genomic DNA using the DNeasy Blood & Tissue Kit following manufacturer’s instructions.
Amplify fragments containing target sequences by PCR using high-fidelity DNA polymerase. Purify the PCR amplicons using a Gel Extraction kit following manufacturer’s instructions (see Note 1).
Detect and determine cleavage activity using the SURVEYOR assay, following manufacturer’s instructions and the previously published protocol [18]. Briefly, first set up a 20 μL reaction containing approximately 500 ng purified amplicon and 1× standard Taq buffer. Denature and reanneal the amplicons to allow heteroduplex formation by incubating at 95 °C for 5 min and ramping down to 25 °C at a rate of 5 °C per min. Add 2.5 μL of 0.15 M MgCl2 stock solution, 1 μL of SURVEYOR nuclease S, 1 μL of SURVEYOR enhancer S, and 0.5 μL of UltraPure water to the annealed heteroduplex solution to prepare a 25 μL reaction for SURVEYOR digestion. Incubate at 42 °C for 30 min, then add 2 μL of Stop Solution to terminate the reaction.
Visualize and image the DNA fragments on a 2% agarose gel.
-
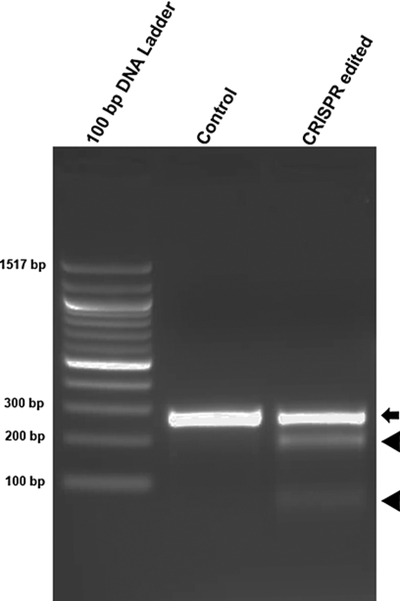
Estimate the integrated intensity of the PCR amplicon and cleaved bands using ImageJ or other software for gel quantification. Calculate the estimated SURVEYOR cleavage (fcut) using the following formula [18]:
where a is the integrated intensity of the undigested amplicons and b and c are the integrated intensities of each cleavage product (Fig. 1).
The sgRNA with higher value of fcut usually has higher on-target activity. Select one or two sgRNAs with the highest fcut for further studies.
Fig. 1.
Sample SURVEYOR assay result. The SURVEYOR nuclease recognizes the mismatch (if any) in the reannealed heteroduplex, which results from indels introduced into the predicted cleavage site in the CRISPR edited cells. Black arrow: undigested amplicons; black triangles: cleavage products of the SURVEYOR nuclease
3.2. AAV Vector Production
3.2.1. AAV Generation by Transient Transfection
Seed the HEK293 cells in 5 roller bottles at a density of 3 × 107 cells per roller bottle. Culture cells in 300 mL of DMEM medium with 10% FBS in 37 °C incubator supplemented with 10% CO2.
When the cells are approximately 80% confluent, add 5 mL of 1 M HEPES buffer to each roller bottle before transfection to stabilize the pH of the medium. To set up the transfection solution, for each roller bottle, mix 150 μg vector plasmid (either pV-Cas9 or pV-sgRNA-tdTomato containing the selected sgRNA sequence) with 150 μg pHLP19-AAV8 and 150 μg pLAdeno5 helper plasmids, and add the plasmid mixture to 15 mL of 0.3 M CaCl2. Add 15 mL of 2× HBS buffer and gently mix the solution by pipetting up and down five times. Immediately add the transfection solution to the roller bottle. Incubate the cells in a 37 °C incubator supplemented with 3% CO2 for 6 h to overnight and replace the medium with 100 mL of DMEM serum-free medium.
Incubate the cells in a 37 °C incubator supplemented with 5% CO2.
48 h after transfection, harvest cells from the roller bottles by vigorous swirling without excessive splashing or foaming.
Transfer the dislodged cells and medium from the 5 roller bottles to one 500 mL conical tube and centrifuge at 3000 × g for 30 min at 4 °C. Discard the supernatant and resuspend the pellets with 200 mL of TSM buffer. The harvested cells can be used for AAV purification or stored at −80 °C. If they are stored, thaw in a 37 °C water bath before proceeding to the next step.
3.2.2. AAV Isolation and Purification
Homogenize and break the cells using a microfluidizer (see Note 2) according to manufacturer’s instructions.
Centrifuge at 3000 × g for 30 min and pour supernatant into a new 500 mL centrifuge tube to eliminate cell debris.
Add 1 M CaCl2 to a final concentration of 25 mM. Mix thoroughly and incubate at 4 °C for 10 min. Centrifuge at 3000 × g for 1 h. Collect the supernatant and transfer it to a new 500 mL centrifuge tube.
To digest residual-free DNA, add Benzonase (100 U/mL) to the supernatant and incubate at 37 °C for 1 h.
To precipitate the AAV particles, add 40% PEG8000/2.5 N NaCl to a final concentration of 8% PEG, mix thoroughly, and incubate on ice for 2 h. Centrifuge at 3000 × g for 30 min at 4 °C. Discard the supernatant.
Resuspend the PEG pellet in 25 mL of HSSE-RNase A buffer. Incubate at 37 °C for 30 min.
Prepare the CsCl step gradient by adding 5 mL of 1.5 g/mL CsCl to the bottom of a new 38.5 mL centrifuge tube, followed by 8 mL of 1.3 g/mL CsCl as the middle layer. Add the viral suspension to the top. Carefully balance the tubes and centrifuge in a SW32Ti rotor at 28,000 rpm for 18 h.
After step gradient ultracentrifugation, carefully place the tube above a halogen beam illuminator, collect the viral bands using a 5 mL syringe and 18G needle, and transfer to a new 14 mL centrifuge tube. Add 1.4 g/mL CsCl to fill the tube and centrifuge in a SW40Ti rotor at 38,000 rpm for 72 h (see Note 3).
After linear gradient ultracentrifugation, carefully place the tube above a halogen beam illuminator, collect the viral band with 5 mL syringe and 18-gauge needle. Dialyze overnight using Slide-A-Lyzer cassettes in Tris-buffered saline. Store the virus solution at −80 °C (see Note 3).
3.2.3. AAV Titration by qPCR
-
Use LoBind tubes for all steps in this section. Prepare standard DNA for qPCR using 1 μg purified linearized AAV vector plasmid. Because 1 μg of a 1 kb plasmid contains 9.26 × 1011 plasmid molecules, 1 μg of the standard plasmid will contain a number of copies equal to:
where N is the length of the standard DNA plasmid in kb. Make serial fourfold dilutions of this digested DNA in qPCR dilution buffer, starting at 107 copies/μL and preparing a total of eight dilutions.
For each vector sample, set up two independent initial dilutions (100-fold and 300-fold) in qPCR dilution buffer.
To eliminate residual plasmid DNA and AAV replication intermediates from the vector samples, add 10 μL of each diluted vector sample to 40 μL of DNase buffer and 10 U DNase. 1 μg plasmid DNA with a volume of 10 μL was used as a control for DNase activity, and DNA negative sample was used as a control for contamination. Incubate all samples at 37 °C for 1 h. Add 50 μL of 200 mM EDTA (pH 8.0) and incubate at 95 °C for 30 min to terminate the reaction. This step further dilutes the samples by tenfold.
Dilute each sample 100-fold by adding 5 μL of sample to 495 μL qPCR dilution buffer and vortexing thoroughly. The vector samples are now diluted 105-fold or 3 × 105-fold.
For each sample prepare qPCR reactions in triplicate containing: 1× PCR Master Mix, 900 nM sense primer, 900 nM antisense primer, 250 nM FAM-conjugated probe, and 5 μL of diluted sample or standard DNA in a final volume of 25 μL qPCR dilution buffer. Load the reaction solution on a 96-well qPCR plate. Seal the plate with optical adhesive film and run the qPCR reaction in a real-time PCR machine following the program in Table 1.
-
Calculate the titer in vector genomes (vg) per mL using the formula:
where N1 and N2 are the average number of copies in the 5 μL sample from the 105-fold and 3 × 105-fold AAV vector dilution wells, respectively (see Note 4).
Table 1.
Program for real-time qPCR
| Step | Temperature (°C) | Time (s) | Fluorescent collection |
|---|---|---|---|
| 1 | 95 | 20 | No |
| 2 | 95 | 1 | No |
| 3 | 60 | 20 | Yes |
Repeat step 2 and 3 for an additional 39 cycles
3.3. Delivery of AAV to the Retina by Subretinal Injection
To prepare the working solution, dilute AAV vectors to the desired concentration using sterilized Tris-buffered saline containing 0.1% fluorescein.
Anesthetize the animal by intraperitoneal injection of a mixture of ketamine (80 mg/kg) and xylazine (8 mg/kg) and dilate the pupils with topical atropine (1%) and tropicamide (0.5%).
Position the animal on its side. Expose the eye that will be injected under an ophthalmic surgical microscope. Some fundus-vessels should be visible.
Gently make an incision through the cornea adjacent to the limbus at the nasal side using a sharp 18G needle.
Use a Hamilton syringe with a 0.5 in 34G blunt-tip needle to perform subretinal injection. Load 1 μL of vector working solution. Carefully insert the needle through the incision while avoiding the lens, and push through the retina. Depress the plunger to inject the solution into the subretinal space. The spread of the vector can be visualized by fluorescein.
After injection, apply antibiotic ointment to prevent potential infection.
3.4. Validation of In Vivo Genome Editing
3.4.1. Retina Whole Mount
Euthanize the mouse. Enucleate the eyeballs and incubate in chilled PBS for 15 min (see Note 5).
Gently squeeze the eyeballs several times to detach the retina.
Fix the eyeballs in 4% PFA for 1 h.
Remove the cornea using micro scissors. Gently pull out the lens using fine forceps.
Carefully separate the retina from the sclera and dissect it from the eye cup at the optic nerve head. Wash the retina with PBS.
Place the retina on a microscope slide with the vitreous side facing up. Make three or four cuts from the periphery half way to the center and flatten the retina using a fine brush.
The retina whole mount can be used for immunofluorescent staining or other treatments. To observe expression and knockdown of EGFP, coverslip the retina with mounting reagent and observe under a fluorescence microscope.
3.4.2. Dissociation of Retinal Cells for FACS
Euthanize the mouse and enucleate the eyes. Remove the cornea and lens and carefully separate the retina from other tissue.
Place the retina in 1 mL of accutase solution for dissociation. Incubate at 37 °C for 10 min with shaking at 1000 rpm in a thermal shaker.
Prepare test tubes with cell strainer caps. Rinse cell strainers with 0.5 mL of HBSS.
Pipette the retina-accutase solution up and down several times to completely dissociate the tissue.
Add the retina-accutase solution through the cell strainer cap.
Add 3 mL of HBSS through the cap to wash it. Replace the cell strainer cap with a regular cap.
Centrifuge at 450 × g for 4 min.
Discard the supernatant.
Resuspend the cells in 1 mL of HBSS. The suspension is ready for FACS.
Use FACS to analyze the number of EGFP-expressing cells. The AAV-transduced photoreceptor cells indicated by tdTomato expression can also be analyzed and sorted by FACS.
3.4.3. Targeted DNA High-Throughput Sequencing
Predicted on- and off-target sites are indicated by the online programs during sgRNA selection (see Subheading 3.1.1). Based on this information, design primers for amplifying DNA fragments containing each predicted on- and off-target site. The length of the amplicons should not exceed 600 bp.
Perform PCR amplification as described above in step 4 in Subheading 3.1.2. Gel purify the amplicons using a Gel Extraction Kit (see Note 1). Determine the quantity of each amplicon using a Nanodrop spectrophotometer.
For each treatment group, pool all amplicons containing each predicted on- and off-target site in equal ratio as one sample.
Run an aliquot of the pooled amplicons on an Illumina MiSeq device using MiSeq Reagent Nano kit v.2. Use 25 cycles followed by two index reads for quality control. Rebalance the pool based on the percentage of reads seen for each amplicon’s indexes.
Sequence the rebalanced pool on the MiSeq using MiSeq Reagent Nano Kit v.3.
Process the sequencing data using RTA 1.18.54 and CASAVA (v1.8.2.).
Convert the Aligned Binary Alignment/Map (BAM) files to FASTQ format using the Bedtools software package [16].
To obtain the mutation rate and pattern of the predicted on- and off-target sites, analyze the FASTQ data using the CRISPResso software package [17] or similar software.
3.5. Assessment After In Vivo Genome Editing
3.5.1. Electroretinography (ERG)
The ERG is used to measure retinal function. It records the electrical response of neuronal and nonneuronal cells in the retina to a light stimulus. The primary outcomes of the ERG test usually consist of the a-wave, b-wave, latency of response, etc. The a-wave is a negative deflection derived from rod (dark-adapted) and cone (light-adapted) photoreceptors. The b-wave is a positive deflection derived mainly from Müller and ON-bipolar cells.
Dark-adapt the mice overnight.
Conduct the test in a dark room with only dim red light.
Anesthetize the mouse by intraperitoneal injection of a mixture of ketamine (80 mg/kg) and xylazine (8 mg/kg). Dilate the pupils with topical atropine (1%) and tropicamide (0.5%).
Place the mouse on a warm pad. Gently place the reference electrode in its mouth and the recording electrodes on the cornea of each eye. Apply a drop of Gonak™ or similar contact gel on the electrodes or eyes to improve electrical contact and maintain corneal hydration before placing the recording electrodes.
Record the dark-adapted ERG at a light intensity of 10−4, 10−3, 10−2, 1, 10, 100, and 1000 cd.s/m2. The main ERG parameters of each step are listed in Table 2.
Light-adapt the mouse for 2 min.
Record the light-adapted ERG at a light intensity of 0.3, 1, 3, 10, 30, and 100 cd.s/m2. The main ERG parameters of each step are listed in Table 2.
Table 2.
Main parameters of ERG
| Light intensity (cd s/m2) | Total sweeps | Internal time (s) | ||
|---|---|---|---|---|
| Dark-adapted ERG | Step 1 | 10−4 | 5 | 5 |
| Step 2 | 10−3 | 3 | 10 | |
| Step 3 | 10−2 | 3 | 20 | |
| Step 4 | 1 | 3 | 30 | |
| Step 5 | 10 | 2 | 45 | |
| Step 6 | 102 | 2 | 60 | |
| Step 7 | 103 | 1 | 60 | |
| Light-adapted ERG | Step 1 | 0.3 | 20 | 0.2 |
| Step 2 | 1 | 20 | 0.2 | |
| Step 3 | 3 | 20 | 0.2 | |
| Step 4 | 10 | 20 | 0.2 | |
| Step 5 | 30 | 20 | 0.2 | |
| Step 6 | 100 | 20 | 0.2 | |
| Flicker | 100 | 20 | 0 |
3.5.2. Optomotor Test
The measurement of the optomotor response (compensatory head/body movement) is a well-established method to evaluate visual acuity in the context of impaired visual function in disease models [19, 20]. The animals do not need training for this visual behavior test.
Place the mouse on the platform surrounded with LCD screens. Close the lid of the instrument. The mouse should be allowed to move freely on the platform.
When starting the test, rotating images of virtual walls of cylinders with vertical sine wave grating will be displayed on the LCD screens at a spatial frequency controlled by the Opto-Motry VR software (v.1.4).
During the test, the display of the images can be reset by inputting the location of the mouse’s head on the video image using a crosshair superimposed on the computer display. The software will adjust the projection of images to center the rotation of the cylinder at the mouse’s viewing position.
The test starts at a spatial frequency of 0.042 cycles/degree. When you observe unambiguous mouse head movements tracking the cylinder rotation, accept the current spatial frequency and the software will proceed to the next test at an increased frequency. In contrast, if the mouse fails to track the cylinder rotation in 10 min, reject the current frequency and the software will proceed to the next test at a slightly decreased frequency. Visual behavior at serial frequency settings from 0.03 cycles/degree to 0.642 cycles/degree will be repeatedly tested to determine visual acuity.
Tracking clockwise rotation determines the visual acuity of the left eye and tracking counterclockwise rotation determines the visual acuity of the right eye [21].
3.5.3. Retinal Cryosection Preparation
Euthanize the mouse and enucleate the eyes after marking the orientation (see Note 5).
Fix the eyes in 4% PFA for 1 h.
Remove the cornea and lens. Place the eye cup in 10% sucrose and incubate at 4 °C for 30 min.
Place the eye cup in 20% sucrose and incubate at 4 °C for 30 min or overnight, until it completely sinks.
Embed the eye cup in OCT compound. Carefully adjust the position of the eye cup in the OCT, then perform rapid freezing using dry ice or liquid nitrogen. Store in −80 °C.
Cut retinal cryosections at 10 μm thickness using a cryostat microtome.
3.5.4. Immunofluorescent Staining
Completely air-dry the sections before beginning. Once the procedure starts, prevent sections from drying out.
Use PBS to rinse sections, and block with PBST containing 5% donkey serum for 1 h.
Incubate sections with primary antibodies diluted in 2% donkey serum in PBS for 2 h to overnight.
Wash sections with PBST three times for 5 min each.
Incubate sections with fluorochrome-conjugated secondary antibodies diluted in 2% donkey serum for 1 h.
Incubate the sections with 0.2 μg/mL 4,6-diamidino-2-phenylindole (DAPI) in PBST for 10 min.
Wash the sections with PBST four times for 10 min each.
Mount the sections in Fluoromount-G reagent. Keep away from light.
Once the mounting medium has dried, observe and take images using a fluorescence microscope.
4. Notes
The integrity and fidelity of the specific amplicon that contains the predicted CRISPR cleavage site is critical for obtaining accurate results in the SURVEYOR assay. Gel purification is necessary when nonspecific amplicons are present. To minimize potential DNA damage, UV exposure should be limited.
A microfluidizer is a high-pressure homogenizing device for large-scale homogenization. Alternatively, for small-scale production, sonication can be utilized for cell homogenization.
With halogen beam illumination, two bands can be visualized after step gradient ultracentrifugation. The lower/heavier band contains full AAV particles, while the upper/lighter band contains empty particles. Only the lower band is collected and subjected to linear gradient ultracentrifugation. After the second round of ultracentrifugation, only one band containing full AAV particles is visualized.
- Because a double-stranded DNA plasmid is used for the standard wells and a single-stranded AAV genome is measured in the unknown wells, this formula multiplies the average value of the unknown wells by 2. If a self-complementary (double-stranded) AAV genome is measured in the unknown wells, use the formula:
When enucleating the eyes, keep the nictitating membrane intact to indicate the orientation. Alternatively, mark the orientation with tissue-marking dye before enucleating the eyeball.
References
- 1.Mali P, Yang L, Esvelt KM, Aach J, Guell M, DiCarlo JE, Norville JE, Church GM (2013) RNA-guided human genome engineering via Cas9. Science 339(6121):823–826. 10.1126/science.1232033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lau CH, Suh Y (2017) In vivo genome editing in animals using AAV-CRISPR system: applications to translational research of human disease. F1000Res 6:2153 10.12688/f1000research.11243.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang S, Sengel C, Emerson MM, Cepko CL (2014) A gene regulatory network controls the binary fate decision of rod and bipolar cells in the vertebrate retina. Dev Cell 30(5):513–527. 10.1016/j.devcel.2014.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bakondi B, Lv W, Lu B, Jones MK, Tsai Y, Kim KJ, Levy R, Akhtar AA, Breunig JJ, Svendsen CN, Wang S (2016) In vivo CRISPR/Cas9 gene editing corrects retinal dystrophy in the S334ter-3 rat model of autosomal dominant retinitis pigmentosa. Mol Ther 24 (3):556–563. 10.1038/mt.2015.220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Latella MC, Di Salvo MT, Cocchiarella F, Benati D, Grisendi G, Comitato A, Marigo V, Recchia A (2016) In vivo editing of the human mutant rhodopsin gene by electroporation of plasmid-based CRISPR/Cas9 in the mouse retina. Mol Ther Nucleic Acids 5(11):e389 10.1038/mtna.2016.92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li P, Kleinstiver BP, Leon MY, Prew MS, Navarro-Gomez D, Greenwald SH, Pierce EA, Joung JK, Liu Q (2018) Allele-specific CRISPR/Cas9 genome editing of the single-base P23H mutation for rhodopsin associated dominant retinitis pigmentosa. bioRxiv. 10.1101/197962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim K, Park SW, Kim JH, Lee SH, Kim D, Koo T, Kim KE, Kim JH, Kim JS (2017) Genome surgery using Cas9 ribonucleoproteins for the treatment of age-related macular degeneration. Genome Res 27(3):419–426. 10.1101/gr.219089.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hung SS, Chrysostomou V, Li F, Lim JK, Wang JH, Powell JE, Tu L, Daniszewski M, Lo C, Wong RC, Crowston JG, Pebay A, King AE, Bui BV, Liu GS, Hewitt AW (2016) AAV-mediated CRISPR/Cas gene editing of retinal cells in vivo. Invest Ophthalmol Vis Sci 57(7):3470–3476. 10.1167/iovs.16-19316 [DOI] [PubMed] [Google Scholar]
- 9.Giannelli SG, Luoni M, Castoldi V, Massimino L, Cabassi T, Angeloni D, Demontis G, Leocani L, Andreazzoli M, Broccoli V (2018) Cas9/sgRNA selective targeting of the P23H Rhodopsin mutant allele for treating retinitis pigmentosa by intravitreal AAV9. PHP.B-based delivery. Hum Mol Genet 27 (5):761–779. 10.1093/hmg/ddx438 [DOI] [PubMed] [Google Scholar]
- 10.Kim E, Koo T, Park SW, Kim D, Kim K, Cho HY, Song DW, Lee KJ, Jung MH, Kim S, Kim JH, Kim JH, Kim JS (2017) In vivo genome editing with a small Cas9 orthologue derived from Campylobacter jejuni. Nat Commun 8:14500 10.1038/ncomms14500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang X, Zhou G, Wu W, Duan Y, Ma G, Song J, Xiao R, Vandenberghe L, Zhang F, D’Amore PA, Lei H (2017) Genome editing abrogates angiogenesis in vivo. Nat Commun 8 (1):112 10.1038/s41467-017-00140-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruan GX, Barry E, Yu D, Lukason M, Cheng SH, Scaria A (2017) CRISPR/Cas9-mediated genome editing as a therapeutic approach for leber congenital amaurosis 10. Mol Ther 25 (2):331–341. 10.1016/j.ymthe.2016.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suzuki K, Tsunekawa Y, Hernandez-Benitez R, Wu J, Zhu J, Kim EJ, Hatanaka F, Yamamoto M, Araoka T, Li Z, Kurita M, Hishida T, Li M, Aizawa E, Guo S, Chen S, Goebl A, Soligalla RD, Qu J, Jiang T, Fu X, Jafari M, Esteban CR, Berggren WT, Lajara J, Nunez-Delicado E, Guillen P, Campistol JM, Matsuzaki F, Liu GH, Magistretti P, Zhang K, Callaway EM, Zhang K, Belmonte JC (2016) In vivo genome editing via CRISPR/Cas9 mediated homology-independent targeted integration. Nature 540(7631):144–149. 10.1038/nature20565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu W, Mookherjee S, Chaitankar V, Hiriyanna S, Kim JW, Brooks M, Ataeijannati Y, Sun X, Dong L, Li T, Swaroop A, Wu Z (2017) Nrl knockdown by AAV-delivered CRISPR/Cas9 prevents retinal degeneration in mice. Nat Commun 8:14716 10.1038/ncomms14716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holmgaard A, Askou AL, Benckendorff JNE, Thomsen EA, Cai Y, Bek T, Mikkelsen JG, Corydon TJ (2017) In vivo knockout of the vegfa gene by lentiviral delivery of CRISPR/Cas9 in mouse retinal pigment epithelium cells. Mol Ther Nucleic Acids 9:89–99. 10.1016/j.omtn.2017.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quinlan AR, Hall IM (2010) BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics 26(6):841–842. 10.1093/bioinformatics/btq033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pinello L, Canver MC, Hoban MD, Orkin SH, Kohn DB, Bauer DE, Yuan GC (2016) Analyzing CRISPR genome-editing experiments with CRISPResso. Nat Biotechnol 34(7):695–697. 10.1038/nbt.3583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA, Zhang F (2013) Genome engineering using the CRISPR-Cas9 system. Nat Protoc 8 (11):2281–2308. 10.1038/nprot.2013.143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prusky GT, Alam NM, Beekman S, Douglas RM (2004) Rapid quantification of adult and developing mouse spatial vision using a virtual optomotor system. Invest Ophthalmol Vis Sci 45(12):4611–4616. 10.1167/iovs.04-0541 [DOI] [PubMed] [Google Scholar]
- 20.Kretschmer F, Sajgo S, Kretschmer V, Badea TC (2015) A system to measure the optokinetic and optomotor response in mice. J Neurosci Methods 256:91–105. 10.1016/j.jneumeth.2015.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Douglas RM, Alam NM, Silver BD, McGill TJ, Tschetter WW, Prusky GT (2005) Independent visual threshold measurements in the two eyes of freely moving rats and mice using a virtual-reality optokinetic system. Vis Neurosci 22(5):677–684. 10.1017/S0952523805225166 [DOI] [PubMed] [Google Scholar]