Abstract
Fresolimumab is an antibody capable of neutralizing all human isoforms of transforming growth factor beta (TGFβ) and has demonstrated anticancer activity in investigational studies. Inhibition of TGFβ by fresolimumab can potentially result in the development of cutaneous lesions. The aim of this study was to investigate the clinical, histological, and immunohistochemical characteristics of cutaneous neoplasms associated with fresolimumab. Skin biopsies (n = 24) were collected and analyzed from patients (n = 5) with treatment-emergent, cutaneous lesions arising during a phase 1 study of multiple doses of fresolimumab in patients (n = 29) with melanoma or renal cell carcinoma. Blinded, independent histological review and measurements of Ki-67, p53, and HPV integration were performed. Based on central review, four patients developed lesions with histological characteristics of keratoacanthomas, and of these patients, a single case of well-differentiated squamous cell carcinoma was also found. Expression of Ki-67, no evidence of p53 overexpression, and only focal positivity for human papillomavirus RNA by in situ hybridization in 4/18 cases were consistent with these findings. Following completion of fresolimumab, lesions spontaneously resolved. Therefore, benign, reversible keratoacanthomas were the most common cutaneous neoplasms observed, a finding of importance for adverse event monitoring, patient care, and optimization of therapies targeting TGFβ.
Keywords: Transforming growth factor β, GC1008, Fresolimumab, Keratoacanthoma, Squamous cell cancer
Introduction
Transforming growth factor beta (TGFβ) is a pleiotropic cytokine that is critical for normal cellular homeostasis [1, 2]. However, in advanced malignancies, TGFβ is typically overexpressed and is associated with higher pathological grade, increased frequency of metastases, and poorer survival in a variety of cancers. In these malignancies, TGFβ signaling is often altered, rendering TGFβ a tumor promoter by directly stimulating cell proliferation, migration, and invasion [2–4]. In addition, overproduction of TGFβ induces the development of a tumor growth-supportive microenvironment by increasing angiogenesis, activating stromal cells, increasing extracellular matrix production, and suppressing anticancer immunity [2–4]. For these reasons, targeting and neutralizing TGFβ represents a rational anticancer approach.
Fresolimumab, also known as GC1008, is a high affinity, human immunogobulin (IgG) 4 monoclonal antibody capable of binding and neutralizing all human isoforms of TGFβ (β1, β2, and β3). In a phase 1 study of fresolimumab in 29 patients with malignant melanoma (MM) or renal cell carcinoma (RCC), the development of keratoacanthomas (KAs) and squamous cell carcinoma (SCC) were reported in four MM patients [5, 6]. In skin, TGFβ inhibits normal keratinocyte proliferation and enhances differentiation [7]. TGFβ typically binds to cell surface receptors and induces the formation of a complex [TGFβ receptor R1, R2, and R3 (betaglycan)] with serine/threonine kinase activity [2, 3]. This causes the phosphorylation and activation of members of the Smad pathway that translocate to the nucleus and regulate gene expression. Although the Smad pathway plays a predominant role, TGFβ signaling is complex and a diverse number of pathways may be activated by cross talk between members of TGFβ family ligands, receptors, Smad components, and their transcription factors [8]. Non-Smad signaling through the activation of mitogen-activated (MAP) kinases, phosphatidylinositol-3′-kinase (PI3K), extracellular signal-regulated kinase (Erk), Jun N-terminal kinase (JNK), and protein kinase B (Akt) also occurs. In addition, TGFβ may signal through other receptors such as endoglin [2].
With the advent of novel targeted therapies, the development of KA and SCC have been reported following treatment with several different agents including the multikinase inhibitor sorafenib [9, 10], the TNF inhibitor infliximab [11], and the B-rapidly accelerated fibrosarcoma (RAF) inhibitors vemurafenib [12, 13] and dabrafenib [14, 15]. In a phase 1 trial, fresolimumab, an antibody that neutralizes TGFβ, was associated with the development of KAs. Herein, we report the clinical and histopathological characteristics of cutaneous neoplasms developing in patients receiving fresolimumab therapy.
Materials and methods
Patients
In the phase 1 study of fresolimumab [5, 6], MM and RCC patients were treated at 1 of 6 dose levels (0.1, 0.3, 1, 3, 10, or 15 mg/kg) in a 3 + 3 dose escalation design with 4 planned doses. Patients achieving at least stable disease were eligible to receive “extended treatment” consisting of 4 additional doses of fresolimumab every 2 weeks for up to two additional courses. For each extended treatment course, intrapatient dose escalation to the current fresolimumab dose determined to be safe was allowed. Twenty-two patients (21 MM, 1 RCC) were treated during the dose escalation portion of the study, including six patients at the highest dose 15 mg/kg. In an expansion cohort, seven additional MM patients were treated at 15 mg/kg to better evaluate safety and the incidence of skin events. The protocol for this study (clinicaltrials.gov: NCT00356460) was approved by the Institutional Review Board at each participating site, and written informed consent was obtained from each patient.
Skin biopsies and analyses
Patients developing new skin lesions while on study were evaluated at their local institution, and full thickness skin biopsies were recommended and obtained as appropriate. In general, 4-μm sections from formalin-fixed, paraffin-embedded biopsy samples of skin lesions were stained with hematoxylin and eosin and then evaluated by dermatopathologists at the treating institution. Stained and unstained slides were provided for independent central review. Hematoxylin- and eosin-stained slides were reviewed in a blinded fashion by a dermatopathologist (Joan Guitart), followed by an unblinded review with reports and history with the co-investigator (Mario E. Lacouture). In cases where a diagnosis was equivocal, the opinion of another dermatopathologist was to be sought; however, there were no discordances between investigators in the diagnoses.
Unstained slides and skin samples were further evaluated for the expression of a panel of immunohistochemical (IHC) markers. Unstained 4-μm sections of formalin-fixed, paraffin-embedded skin biopsies were analyzed for expression of kinesis (Ki)-67 (monoclonal MIB-1; Dako) and p53 protein (Thermo Scientific, Waltham, MA). Slides were deparaffinized and treated with an alcohol gradient to absolute ethanol. Endogenous peroxidase activity was blocked with the peroxidase-blocking solution (Dako; S2001). Antigen retrieval was achieved with target retrieval solution (Dako). Primary antibody was applied at 1:100 dilution and incubated at 4 °C for 1 h. Anti-mouse IgG with the Dako EnVision System (horseradish peroxidase) was employed according to the manufacturer’s instructions. Samples were also examined for human papillomavirus (HPV) using in situ hybridization to detect common sequences of low- and high-risk HPV genotypes (HPV type 6/11, 16/18, 31/33/35; Enzo Diagnostics, New York, NY).
Results
Overview of patients treated on study
As reported separately [5, 6], 29 patients (28 MM, 1 RCC) were treated on study with fresolimumab. Early evidence of clinical benefit, defined as stable disease or better, was observed in seven MM patients: one PR and six SD, including three patients with mixed tumor response. Skin toxicity was the most common adverse events/serious adverse events (AE/SAE) considered possibly related to fresolimumab. As determined by the local site, this included skin rash, the development of eruptive KAs and hyperkeratosis in two patients, cutaneous SCC in two patients, and basal cell carcinoma (BCC) in one patient with a history of multiple prior BCCs. Grade 1 or 2 skin rashes were reported in 10 patients that improved or resolved by end of study (various terms used to describe lesions; events were considered either related or unrelated), and six of these patients continued therapy without worsening of symptoms. Two SAEs were considered possibly related to fresolimumab, a well-differentiated SCC in a patient with a prior history of this cancer and a patient that developed herpes zoster. There were no drug-related grade 4 or 5 SAEs, and no AE led to study drug discontinuation.
Of the two cases of KAs reported by the treating institution, one occurred in a patient who initially received treatment at 1 mg/kg (4 doses) and then extended treatment at 3 mg/kg (4 doses) and 15 mg/kg (4 doses), and the second case occurred in a patient who received treatment at 15 mg/kg (4 doses). Of the two cases reported by a clinical site as representing SCC, one occurred in a patient who received treatment at 1 mg/kg (4 doses) and then extended treatment at 3 mg/kg (4 doses), and the second case occurred in a patient who received treatment at 15 mg/kg (4 doses). In all four patients, KA/SCC lesions were first definitively diagnosed by biopsy either during or following their final course of therapy (Table 1). However, in three of these four cases, mild non-specific rashes or skin lesions preceded the development of the KA/SCC lesions. Patients who developed skin lesions underwent biopsies and local treatment. Multiple KA/SCC lesions were present in all four of these patients, and following completion of fresolimumab, the remaining lesions were observed over time.
Table 1.
Initial observations of KA/SCC
| Patient | Fresolimumab treatment | Initial diagnosis of KA/SCC | Other lesions preceding KA/SCC diagnosis |
|---|---|---|---|
| 007 |
1 mg/kg × 4 3 mg/kg × 4 15 mg/kg × 4 |
After 2 doses fresolimumab at 15 mg/kg, biopsy-proven KA | Non-specific, transient papular rash noted during initial treatment at 1 mg/kg. After 4 doses of fresolimumab 3 mg/kg, keratosis pilaris-like papules on her face and shoulders, enlarged hair follicle with irregular keratinization and lichenoid keratosis, and generalized hyperkeratosis |
| 018 | 15 mg/kg × 4 | After fourth dose fresolimumab, biopsy-proven KA | Non-specific papular rash after first dose |
| 009 |
1 mg/kg × 4 3 mg/kg × 4 |
After fourth fresolimumab dose at 3 mg/kg, biopsy-proven KA | |
| 028 | 15 mg/kg × 4 | After fourth dose fresolimumab, biopsy-proven KA | After second dose at 15 mg/kg, few papules noted over forehead and a few bumps on the left lateral leg, minimally pruritic |
Histological analyses
Twenty-four skin biopsies were provided for independent central review. Unstained histopathology sections were available for 18 skin lesions from three subjects. IHC and in situ hybridization studies were performed in an attempt to distinguish KA from SCC. IHC was performed using antihuman p53 oncogene and anti-Ki-67 (MIB-1) proliferative marker to detect any specific patterns. Prior to testing the subject samples, a panel of six immunomarkers including beta-catenin, CD10, CD99, p63, bcl2, p53, and Ki-67 in control tissues were examined. These markers had been reported to provide diagnostic clues to distinguish KA from SCC [16–19]. The six markers were tested in prototypical cases of KA (2), verruca vulgaris (1), SCC well differentiated (1), and with keratoacanthomatous changes (2). The antibodies satisfactorily stained all control tissues. Based on this pilot sample, the markers that we found to be of best assistance at differentiating KA from SCC were p53 and Ki-67. Both of these markers showed distinct patterns in SCC and KA. Strong p53 staining has been reported in SCC, but this pattern is rare in KA. The proliferative marker Ki-67 is expressed diffusely in SCC, whereas in KA, Ki67 is expressed only during the proliferative phase and mostly at the periphery of the tumor.
Two slides from each case were examined by in situ hybridization against common sequences of low- and high-risk HPV genotypes (HPV type 6/11, 16/18, 31/33/35). Stained slides were evaluated and graded for nuclear expression.
Clinical and pathologic characterization of skin lesions
Of the skin lesions examined by independent central review, 16 KAs, 1 well-differentiated, microinvasive SCC, and 7 other dermatologic abnormalities (pilomatricoma; acantholytic acanthoma; actinic keratosis; seborrheic keratosis, inflamed; perivascular dermatitis with eosinophils; verruca vulgaris, inflamed; chronic spongiotic dermatitis with eosinophils) were noted in the 24 samples submitted (Table 2; representative examples are shown in Figs. 1, 2). Discordance of SCC/KA diagnoses between this independent central review and that of the local sites occurred in three lesions. In this central review, the two cases diagnosed at the treating institution as SCC were interpreted as representing KAs, and in another case, a lesion diagnosed as a squamous epithelial proliferation with KA-like features was interpreted as most consistent with SCC.
Table 2.
Clinical and histological characteristics of samples (N = 24) submitted for central review
| Patient | Location | Site review | Central review | Concordance site versus central |
|---|---|---|---|---|
| 007 | Skin, forearm | KA | KA, no atypia, or SCC | Yes |
| Skin, shoulder | Hair follicule showing disorderly keratosis | Evolving KA, no atypia, or SCC | No | |
| Skin, lateral eye | Pilomatricoma | Pilomatricoma, no atypia, or KA | Yes | |
| Skin, ear | KA, specimen represents portion of biopsy | Portion of a KA, no atypia, or SCC | Yes | |
| Skin, submandibular | KA | KA, no atypia, or SCC | Yes | |
| Skin, lateral neck | KA | KA, no atypia, or SCC | Yes | |
| Skin, ear | KA (superficial portion) | KA | Yes | |
| 018 | Skin, parascapular | Superficial portion of KA | Superficial portion of KA, no atypia, or SCC | Yes |
| Skin, anterior thigh | KA arising from hair follicle | KA, no SCC. Endophytic pattern noted which is consistent with an origin of the infindibulum of a hair follicle; however, this link cannot be established with certainty | Yes | |
| Skin, shoulder | KA | KA | Yes | |
| Skin, subscapular | KA and intradermal nevus | KA, no SCC. No evidence of nevus in the section examined | Yes | |
| Skin, preauricular | KA | KA, no atypia | Yes | |
| Skin, distal arm | KA | KA, no atypia | Yes | |
| Skin, clavicle | KA with squamous metaplasia-like changes in the sweat ducts | KA with focal atypia | Yes | |
| Skin, clavicle | KA | KA | Yes | |
| Skin, post-auricular | Atypical squamous epithelial proliferation with KA features | SCC. Lesion shows features of a KA; however, there is nuclear atypia, abnormal mitotic figures, and marked solar damage | No | |
| Skin, infrascapular region |
Grover’s disease There is suprabasilar acantholysis |
Acantholytic acanthoma, consistent with Grover’s disease. However, Grover’s diagnosis should be based on clinical presentation. No KA or SCC | Yes | |
| Skin, forearm | Hypertrophic actinic keratosis. No malignancy | Actinic keratosis, no KA, or SCC | Yes | |
| Skin, arm | Eosinophilic spongiosis | Seborrheic keratosis, inflamed. There is eosinophilic spongiosis; however, impression is that there was a preceding keratosis which became inflamed. No KA or SCC | Yes | |
| 026 | Skin, flank | Mild perivascular chronic inflammation; acute eczematous dermatitis with spongiosis | Perivascular dermatitis with eosinophils. No KA, atypia, or SCC | Yes |
| 009 | Skin, mid-back | SCC, well differentiated | KA with focal squamous atypia | No |
| 028 | Skin, nasal ala | Hypertrophic actinic keratosis | Consistent with verruca vulgaris, inflamed. No KA, atypia, or SCC | No |
| Skin, forehead | SCC, moderately differentiated (endophytic) | Consistent with KA, although biopsy is too superficial for a definitive diagnosis | No | |
| 029 | Skin, forearm | Interface and lichenoid dermatitis with eosinophils | Chronic spongiotic dermatitis with eosinophils. No KA, atypia, or SCC | Yes |
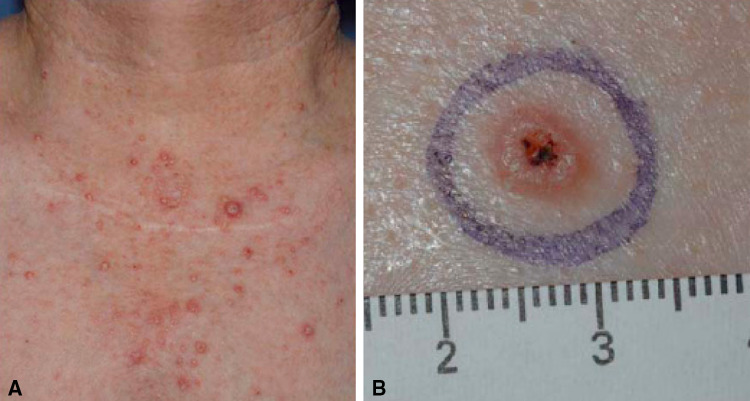
Fig. 1.
a Cutaneous neoplasms following fresolimumab treatment. Patient 007 was a 65-year-old woman with MM who received fresolimumab at 1 mg/kg ×4 doses followed by extended treatment at 3 and 15 mg/kg ×4 who achieved a PR. An asymptomatic, self-limited, papular rash developed after three doses of fresolimumab 1 mg/kg. At 3 mg/kg, a papular rash with a few keratosis pilaris-like papules developed, which on biopsy revealed an enlarged hair follicle with irregular keratinization and lichenoid keratosis. Between courses, rashes improved and tumor continued to regress. At 15 mg/kg, lesions with features of keratoacanthomas developed on her face, chest, and arms. These lesions completely regressed over a period of months following completion of therapy. b Characteristic appearance of a keratoacanthoma. Lesions were characterized by skin-colored, dome-shaped papules or nodules with a crateriform center and keratin plug
Fig. 2.
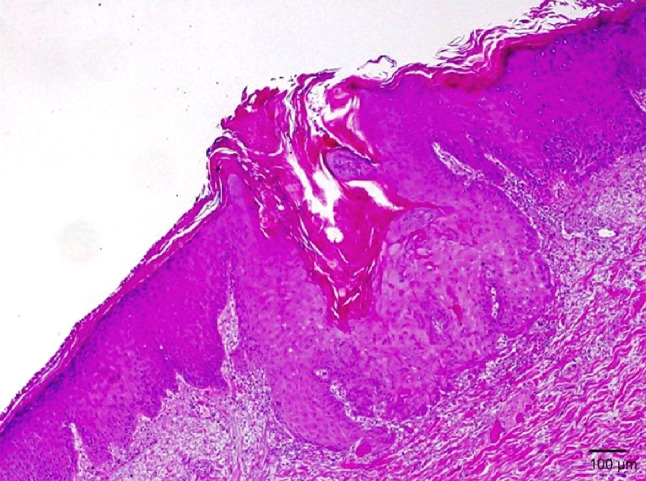
Histology of keratoacanthoma arising in a patient treated with fresolimumab. Hematoxylin- and eosin-stained skin biopsy section demonstrates a central keratinous plug present within an acanthotic epidermis with few atypical keratinocytes. A sparse inflammatory dermal infiltrate underlies this lesion
Mutant p53 expression was negative in most cases of KAs, consistent with prior reports. Focal and weak expression was observed in three cases of KA, but upon histology review, these focal areas showed no evidence of atypia. One case of squamous cell carcinoma and one case of hypertrophic actinic keratosis, both within a background of solar elastosis, were strongly positive (Table 3).
Table 3.
Immunohistochemical characteristics of fresolimumab-induced neoplasms
| Patient | Location | Ki-67 | P53 | HL | HH | Diagnosis | Comments |
|---|---|---|---|---|---|---|---|
| 007 | Skin, ear | + | – | – | – | KA | |
| Skin, ear | ++ | – | – | – | KA | ||
| Skin, submandibular | ++ | – | – | – | KA | ||
| Skin, neck | 0 | 0 | – | ++ | KA | ||
| Skin, forearm | 0 | 0 | + | – | KA | ||
| Skin, shoulder | + | – | – | – | KA | Probably early lesion | |
| Skin, lateral eye | ++ | – | – | – | pilomatricoma | ||
| 018 | Skin, forearm | ++ | ++ | – | – | Hypertrophic Actinic keratosis | |
| Skin, preauricular | 0 | 0 | – | – | KA | ||
| Skin, distal arm | + | + | – | – | KA | ||
| Skin, clavicle | ++ | – | – | – | KA (atypical) | ||
| Skin, clavicle | ++ | + | – | – | KA | ||
| Skin, post-auricular | ++ | + | – | – | SCC microinvasive | Some features of KA. Central review different from local site diagnosis of KA | |
| Skin, thigh | + | – | – | + | KA | ||
| Skin, shoulder | 0 | 0 | – | – | KA | ||
| Skin, subscapular | + | – | – | + | KA | ||
| Skin, parascapular | + | – | – | – | KA | ||
| 009 | Skin, mid-back | + | ++ | – | – | KA (atypical) | Central review different from local site diagnosis of SCC |
Ki67, MIB1 proliferative marker; +, low proliferation mostly at the periphery of the lesion; ++, high proliferation seen in growing phase of SCC or keratoacanthomas; P53, oncogene expressed only on mutant keratinocytes; HL, low-risk HPV mRNA by in situ hybridization; +, trace of uncertain significance; ++, positive focally; HH, high-risk HPV mRNA by in situ hybridization (HPV 16/18/33 are associated with certain SCC especially in mucosal surfaces), +, trace; ++, positive
Of the 15 lesions identified as KA, Ki-67 staining was 2+ in 20 % of lesions, compared to 66 % in SCC, p53 staining + in 13 % (compared to 100 % of SCC), high-risk HPV mRNA was + in 20 % of KA but none of the SCC. The proliferative marker Ki-67 reflected the growth phase of the lesion and showed a trend for increased expression in cases with morphological evidence of squamous atypia. The staining was primarily at the periphery or advancing border of the lesions. In situ hybridization for unrestricted low- and high-risk HPV were negative with the exception of one case that was focally positive for low-risk HPV and three cases also focally positive for high-risk HPV. The significance of focal positivity in these samples is unclear and could imply episomal viral products rather oncogenic viral integration.
Discussion
In this study, we report the development of KA lesions in melanoma patients following the blockade of TGFβ with a neutralizing antibody. KAs are characterized by rapidly growing, symmetrical, dome-shaped nodules that frequently undergo spontaneous resolution [20, 21]. The tumors are mostly endophytic with a verrucous exophytic component during the growing phase, but in late lesions, full keratinization occurs resulting in transepidermal extrusion of keratotic debris. KAs are often clinically indistinguishable from well-differentiated SCCs or other verrucous processes. While some authors have considered KAs to be low-grade cutaneous SCCs, the lack of frank atypia and the spontaneous resolution of the tumors indicate otherwise [22–24]. Known risk factors for cutaneous squamous cell proliferations are ultraviolet light exposure, especially in fair-skinned individuals, immunosuppression, and HPV infection. KAs most often arise on sun-exposed sites of light-skinned people of middle or older age and are more frequent in immunocompromised patients. The development of multiple KAs are typically rare. However, they can be seen as generalized eruptive lesions in the context of the Ferguson-Smith syndrome, the Muir–Torre syndrome, and the rare Grzybowski syndrome.
TGFβ is a pleiotropic cytokine with immune modulatory and other functions. It might be expected that blockade of TGFβ could be detrimental to normal homeostatic activities. In the extreme case, complete disruption of TGFβ1 in homozygous “knockout” (KO) mice can result in intrauterine death, and although more than a third of fetuses do develop to term, the resulting animals develop a fatal wasting syndrome characterized by inflammatory lesions in multiple organs including the lungs and heart, which results in death within a few weeks of birth [25, 26]. In these TGFβ1 knockout (KO) mice, no lesions were reported in the skin upon histological examination; however, the animals were only 10–21 days old at the time of analysis. With respect to the development of inflammatory multifocal disease, complete versus partial neutralization of TGFβ may have very different outcomes. Animals with partial heterozygous deletion of TGFβ have a normal phenotype [25]. In addition, preclinical models of chronic TGFβ neutralization have not replicated these multi-organ inflammatory phenotypes in mice [27] or non-human primates (Genzyme, unpublished observations), indicating that neutralizing antibodies to TGFβ reduce but cannot completely remove or KO TGFβ in vivo.
TGFβ is known to act as a tumor suppressor early in carcinogenesis, but then switches to a promoter of tumor growth and metastatic behavior in some late-stage cancers. In a skin chemical-induced carcinogenesis model, cutaneous TGFβ signaling appeared to promote the development of benign tumors but suppressed premalignant progression to cancerous lesions, and treatment with SB431542, a small molecule inhibitor of anaplastic lymphoma receptor tyrosine kinase (ALK)5 (aTGFβ type I receptor), was observed to increase the conversion of papillomas to SCCs [28]. However, the effect of TGFβ appears to be context dependent. Other investigators have examined the effect of overexpression of TGFβ in rat sarcoma (RAS)-transfected human malignant epidermal keratinocytes that represent the early stages of human SCC [29]. Cells that over-expressed TGFβ were more proliferative, grew into larger tumors, and were more invasive than controls, indicating a role of TGFβ in the growth and progression of these cancerous lesions. These data again indicate that TGFβ over-expression has a complex effect that is different for normal and premalignant cells compared to advanced cancer cells and likely reflect genetic changes that influence the signaling pathways in context-dependent manners. In the case of fresolimumab, the KAs and other lesions did not have an increased conversion to SCC.
In this study, we examined treatment-emergent skin lesions arising in MM or RCC patients participating in a clinical study of fresolimumab [5]. On central review, all lesions reported by the treating institution as SCC were interpreted as consistent with KA, and of all cases reported by the treating institution as KA, one case was interpreted as consistent with SCC. All institution-reported cases of SCCs came from a single collaborating site where the term “keratoacanthoma” was not used, and instead, all cases with features of KA were reported as SCC. In the single case where a lesion was interpreted as SCC by central review, the biopsy was read at the institution as an atypical squamous epithelial proliferation with keratoacanthoma features and arose in a skin cancer patient with a history of prior SCC lesions.
The diagnosis of KA is further supported by additional markers and the clinical behavior of the lesions. We used immunohistochemistry as an ancillary test, since morphological overlap by routine histology is a common problem in differentiating KA from SCC. Lack of significant p53 expression (<10 % of viable keratinocytes) is more consistent with KA, whereas high p53 expression is suggestive of SCC. Similarly, Ki67 (MIB-1) is not abundant in mature KA (<10 % of keratinocytes), whereas SCC tend to show persistent high expression (>50 % of viable cells).
In our study, increased expression of Ki-67 confined to the tumor periphery, decreased p53 expression, and lack of HPV integration were observed in most cases. These findings are consistent with that of KA and indicate that any potential role of HPV in the development of these lesions may be limited to a minority of cases. High-risk HPV strains are typically associated with mucosal SCC and with carcinomas of the skin in immunosuppressed patients. Clinically, these fresolimumab-treated patients developed multiple lesions that were noted to wax and wane over time. Lesions that remained following completion of treatment were observed, and over a period of weeks to months, they regressed, supporting a benign, reversible clinical behavior and the diagnosis of KA. Fresolimumab has a long half-life of approximately 3 weeks, and persistent antibody is likely a contributing factor to the slow regression of these lesions.
Four patients developed these KA/SCC lesions, and in each case, the patients were either treated with the highest dose level of fresolimumab, 15 mg/kg, or received extended treatment (Table 1). Although this may be a reflection of fresolimumab dose and exposure, other factors may be important for the development of these lesions. In preclinical studies with neutralizing anti-TGFβ antibodies in mice [27] and non-human primates (Genzyme, unpublished observations), animals did not develop skin lesions or KAs. However, some did develop hyperkeratosis in the oral cavity, notably adjacent to emerging teeth. Similar oral lesions were not observed in humans and may indicate either differences in species or differences in oral cavity tissues such as the state of cell growth and proliferation. In this clinical trial of fresolimumab, patients developing these KA/SCC lesions of the skin had MM and histories of solar damage.
In this study, 10 patients developed transient grade 1 or 2 skin lesions, which were described by various non-specific terms such as papular rash, maculopapular rash, or drug eruption and considered either related or unrelated. The majority of patients (80 %) with skin events reported developing these lesions after multiple doses of GC1008 or after single administration of the highest dose 15 mg/kg. It is interesting to speculate that some of these skin disorders may represent precursors of KAs and to note that dose intensity and the development of such lesions may be directly related. In the future, comparative gene profiling of these various lesions and KAs may be informative as to their etiology and development. Immunohistochemical stains revealed some differences between KA and SCC, namely, KA have a lower frequency of staining for Ki-67 when compared to SCC. Staining for high-risk HPV mRNA was low in KA and absent in SCC.
Recently, a clinical disease correlate has been described that could provide insights into the biology of KAs. In work by Goudie et al. [30], genomic sequencing was performed on families with Ferguson-Smith disease, an autosomal dominant skin condition characterized by the appearance of multiple KAs. In this study, a genotype–phenotype correlation between loss of function of TGFβ R1 (TGFβ receptor 1) and the development of the characteristic skin lesions were found. Interestingly, the lesions observed in patients receiving fresolimumab have similarities to those described with this genetic disorder. Therefore, effective neutralization of TGFβ with fresolimumab may mimic the loss of TGFβ signaling found in patients with Ferguson-Smith disease.
With the advent of novel, targeted therapies for the treatment of cancer, the development of skin lesions has emerged as an unexpected toxicity. The development of KAs and SCCs has been reported following treatment with a variety of agents that target different pathways. These include the multikinase inhibitor sorafenib [9, 10], the TNF inhibitor infliximab [11], the B-RAF inhibitors vemurafenib [12, 13] and dabrafenib [14, 15], and now fresolimumab, an anti-TGFβ monoclonal antibody.
The development of multiple KAs reported in patients treated with sorafenib, vemurafenib, and dabrafenib therapy show a similar clinical pattern and histological appearance to that observed in our study [31]. KAs and SCCs are frequent events observed with agents targeting the RAF pathway such as vemurafenib and dabrafenib and have been reported to have an incidence between 11 and 21 % in treated patients. The lesions occurring in these B-RAF inhibitor-treated patients typically harbor RAS mutations, most frequently HRAS [13]. Evidence suggests that RAF inhibitors induced BRAF–CRAF dimerization in BRAF wt keratinocytes, thereby activating the MAP kinase pathway with resultant keratinocyte proliferation. In a subset of sorafenib-induced neoplasms (n = 2), two lesions revealed somatic missense mutations in TGFBR1 in one KA (c.1120G > A; p.Gly374Arg) and in one SCC in situ (c.248C > T; Pro83Leu) [10]. Although fresolimumab and these other novel agents act on different signaling pathways, there may be significant cross talk between these affected pathways. However, a common etiology for the development of KA and SCC has not yet been identified. These data implicating an association of KAs and RAF, RAS, and TGFBR1 were not available at the time this trial was completed, and therefore, the collection of appropriate samples and assessment of these parameters were not planned or performed as part of this study.
In conclusion, neutralization of TGFβ by fresolimumab may induce the development of skin lesions consistent with eruptive KAs. Our data regarding the expression of Ki-67, p53, and HPV support this diagnosis, and the regression of lesions following completion of study drug demonstrates their reversible, non-malignant nature. However, close and frequent follow-up, as well as the standard management of KA/SCCs should be instituted in patients who develop these events, as there is a low, yet poorly defined risk of metastases [32]. Treatment-emergent KAs have been reported following other novel therapies such as sorafenib [9], vemurafenib [12], and infliximab [11] and may reflect cross talk between signaling pathways affected by these agents and TGFβ. Although not examined in this study of fresolimumab, data from studies of B-RAF inhibitors [13] and sorafenib [9, 10] have suggested a role for H-RAS mutations and the development of drug-associated KAs. Future research focusing on the common defects between these treatment-emergent skin lesions, the RAS pathway, and that of Ferguson-Smith disease may yield important insights into the development of these skin lesions.
Acknowledgments
This work was supported by Genzyme Corporation and in part by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute (NCI).
Conflict of interest
Frank J. Hsu was an employee of Genzyme. Mario E. Lacouture and Joan Guitart were consultants for Genzyme. Jay A. Berzofsky received research support from Genzyme. All other authors do not have any conflict of interest.
Abbreviations
- AE/SAE
Adverse events/serious adverse events
- Akt
Protein kinase B
- ALK
Anaplastic lymphoma receptor tyrosine kinase
- ERK
Extracellular signal-regulated kinase
- HPV
Human papillomavirus
- IHC
Immunohistochemical
- IgG
Immunoglobulin
- JNK
Jun N-terminal kinase
- KA
Keratoacanthoma
- Kg
Kilograms
- Ki
Kinesis
- KO
Knockout
- Mg
Milligrams
- MM
Malignant melanoma
- NCI
National Cancer Institute
- RAF
Rapidly accelerated fibrosarcoma
- RAS
Rat sarcoma
- RCC
Renal cell carcinoma
- SCC
Squamous cell carcinoma
- SMAD
Small body size mothers against decapentaplegic
- TGFβ
Transforming growth factor β
- TGFBR
Transforming growth factor β receptor
References
- 1.Roberts AB, Anzano MA, Lamb LC, Smith JM, Sporn MB. New class of transforming growth factors potentiated by epidermal growth factor: isolation from non-neoplastic tissues. Proc Natl Acad Sci USA. 1981;78(9):5339–5343. doi: 10.1073/pnas.78.9.5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Massague J. TGFbeta in cancer. Cell. 2008;134(2):215–230. doi: 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tan AR, Alexe G, Reiss M. Transforming growth factor-beta signaling: Emerging stem cell target in metastatic breast cancer? Breast Cancer Res Treat. 2009;115(3):453–495. doi: 10.1007/s10549-008-0184-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wrzesinski SH, Wan YY, Flavell RA. Transforming growth factor-beta and the immune response: implications for anticancer therapy. Clin Cancer Res. 2007;13(18 Pt 1):5262–5270. doi: 10.1158/1078-0432.CCR-07-1157. [DOI] [PubMed] [Google Scholar]
- 5.Morris JC, Shapiro GI, Tan AR, Lawrence DP, Olenki TE, Dezube BJ, Hsu FJ, Reiss M, Berzofsky JA (2008) Phase I/II Study of GC1008: a human anti-transforming growth factor-beta (TGFβ) monoclonal antibody (MAb) in patients with advanced malignant melanoma (MM) or renal cell carcinoma (RCC). J Clin Oncol. ASCO Annual Meeting Proceedings (Post-Meeting Edition). Vol 26, no 15S (May 20 Supplement): 9028
- 6.Morris JC, Tan AR, Olencki TE, Shapiro GI, Dezube BJ, Reiss M, Hsu FJ, Berzofsky JA, Lawrence DP. Phase I study of GC1008 (fresolimumab): a human anti-transforming growth factor-beta (TGFbeta) monoclonal antibody in patients with advanced malignant melanoma or renal cell carcinoma. PLoS One. 2014;9(3):e90353. doi: 10.1371/journal.pone.0090353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shirakata Y. Regulation of epidermal keratinocytes by growth factors. J Dermatol Sci. 2010;59(2):73–80. doi: 10.1016/j.jdermsci.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 8.Zhang YE. Non-smad pathways in TGF-beta signaling. Cell Res. 2009;19(1):128–139. doi: 10.1038/cr.2008.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arnault JP, Wechsler J, Escudier B, Spatz A, Tomasic G, Sibaud V, Aractingi S, Grange JD, Poirier-Colame V, Malka D, Soria JC, Mateus C, Robert C. Keratoacanthomas and squamous cell carcinomas in patients receiving sorafenib. J Clin Oncol. 2009;27(23):e59–e61. doi: 10.1200/JCO.2009.23.4823. [DOI] [PubMed] [Google Scholar]
- 10.Arnault JP, Mateus C, Escudier B, Tomasic G, Wechsler J, Hollville E, Soria JC, Malka D, Sarasin A, Larcher M, Andre J, Kamsu-Kom N, Boussemart L, Lacroix L, Spatz A, Eggermont AM, Druillennec S, Vagner S, Eychene A, Dumaz N, Robert C. Skin tumors induced by sorafenib; paradoxic RAS–RAF pathway activation and oncogenic mutations of HRAS, TP53, and TGFBR1. Clin Cancer Res. 2012;18(1):263–272. doi: 10.1158/1078-0432.CCR-11-1344. [DOI] [PubMed] [Google Scholar]
- 11.Esser AC, Abril A, Fayne S, Doyle JA. Acute development of multiple keratoacanthomas and squamous cell carcinomas after treatment with infliximab. J Am Acad Dermatol. 2004;50(5 Suppl):S75–S77. doi: 10.1016/j.jaad.2003.11.044. [DOI] [PubMed] [Google Scholar]
- 12.Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, Dummer R, Garbe C, Testori A, Maio M, Hogg D, Lorigan P, Lebbe C, Jouary T, Schadendorf D, Ribas A, O’Day SJ, Sosman JA, Kirkwood JM, Eggermont AM, Dreno B, Nolop K, Li J, Nelson B, Hou J, Lee RJ, Flaherty KT, McArthur GA. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364(26):2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Su F, Viros A, Milagre C, Trunzer K, Bollag G, Spleiss O, Reis-Filho JS, Kong X, Koya RC, Flaherty KT, Chapman PB, Kim MJ, Hayward R, Martin M, Yang H, Wang Q, Hilton H, Hang JS, Noe J, Lambros M, Geyer F, Dhomen N, Niculescu-Duvaz I, Zambon A, Niculescu-Duvaz D, Preece N, Robert L, Otte NJ, Mok S, Kee D, Ma Y, Zhang C, Habets G, Burton EA, Wong B, Nguyen H, Kockx M, Andries L, Lestini B, Nolop KB, Lee RJ, Joe AK, Troy JL, Gonzalez R, Hutson TE, Puzanov I, Chmielowski B, Springer CJ, McArthur GA, Sosman JA, Lo RS, Ribas A, Marais R. RAS mutations in cutaneous squamous-cell carcinomas in patients treated with BRAF inhibitors. N Engl J Med. 2012;366(3):207–215. doi: 10.1056/NEJMoa1105358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kefford R, Arkenau H, Brown MP, Millward M, Infante JR, Long GV, Ouellet D, Curtis M, Lebowitz PF, Falchook GS (2010) Phase I/II study of GSK2118436, a selective inhibitor of oncogenic mutant BRAF kinase, in patients with metastatic melanoma and other solid tumors. ASCO Annual Meeting, J Clin Oncol, 28:15s, (suppl; abstr 8503)
- 15.Anforth RM, Blumetti TC, Kefford RF, Sharma R, Scolyer RA, Kossard S, Long GV, Fernandez-Penas P. Cutaneous manifestations of dabrafenib (GSK2118436): a selective inhibitor of mutant BRAF in patients with metastatic melanoma. Br J Dermatol. 2012;167(5):1153–1160. doi: 10.1111/j.1365-2133.2012.11155.x. [DOI] [PubMed] [Google Scholar]
- 16.Suk JD, Park WS, Kim DK. Low rates of somatic p53 mutations in keratoacanthomas. J Dermatol Sci. 2009;53(1):72–73. doi: 10.1016/j.jdermsci.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 17.Batinac T, Zamolo G, Coklo M, Hadzisejdic I, Stemberger C, Zauhar G. Expression of cell cycle and apoptosis regulatory proteins in keratoacanthoma and squamous cell carcinoma. Pathol Res Pract. 2006;202(8):599–607. doi: 10.1016/j.prp.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 18.Sakiz D, Turkmenoglu TT, Kabukcuoglu F. The expression of p63 and p53 in keratoacanthoma and intraepidermal and invasive neoplasms of the skin. Pathol Res Pract. 2009;205(9):589–594. doi: 10.1016/j.prp.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 19.Kanner WA, Brill LB, 2nd, Patterson JW, Wick MR. CD10, p63 and CD99 expression in the differential diagnosis of atypical fibroxanthoma, spindle cell squamous cell carcinoma and desmoplastic melanoma. J Cutan Pathol. 2010;37(7):744–750. doi: 10.1111/j.1600-0560.2010.01534.x. [DOI] [PubMed] [Google Scholar]
- 20.Karaa A, Khachemoune A. Keratoacanthoma: a tumor in search of a classification. Int J Dermatol. 2007;46(7):671–678. doi: 10.1111/j.1365-4632.2007.03260.x. [DOI] [PubMed] [Google Scholar]
- 21.Putti TC, Teh M, Lee YS. Biological behavior of keratoacanthoma and squamous cell carcinoma: telomerase activity and COX-2 as potential markers. Mod Pathol. 2004;17(4):468–475. doi: 10.1038/modpathol.3800063. [DOI] [PubMed] [Google Scholar]
- 22.Ko CJ. Keratoacanthoma: facts and controversies. Clin Dermatol. 2010;28(3):254–261. doi: 10.1016/j.clindermatol.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 23.Schwartz RA. Keratoacanthoma. J Am Acad Dermatol. 1994;30(1):1–19. doi: 10.1016/S0190-9622(94)70001-X. [DOI] [PubMed] [Google Scholar]
- 24.Kossard S, Tan KB, Choy C. Keratoacanthoma and infundibulocystic squamous cell carcinoma. Am J Dermatopathol. 2008;30(2):127–134. doi: 10.1097/DAD.0b013e318161310c. [DOI] [PubMed] [Google Scholar]
- 25.Kulkarni AB, Huh CG, Becker D, Geiser A, Lyght M, Flanders KC, Roberts AB, Sporn MB, Ward JM, Karlsson S. Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci USA. 1993;90(2):770–774. doi: 10.1073/pnas.90.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yaswen L, Kulkarni AB, Fredrickson T, Mittleman B, Schiffman R, Payne S, Longenecker G, Mozes E, Karlsson S. Autoimmune manifestations in the transforming growth factor-beta 1 knockout mouse. Blood. 1996;87(4):1439–1445. [PubMed] [Google Scholar]
- 27.Vitsky A, Waire J, Pawliuk R, Bond A, Matthews D, Lacasse E, Hawes ML, Nelson C, Richards S, Piepenhagen PA, Garman RD, Andrews L, Thurberg BL, Lonning S, Ledbetter S, Ruzek MC. Homeostatic role of transforming growth factor-beta in the oral cavity and esophagus of mice and its expression by mast cells in these tissues. Am J Pathol. 2009;174(6):2137–2149. doi: 10.2353/ajpath.2009.080723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mordasky Markell L, Perez-Lorenzo R, Masiuk KE, Kennett MJ, Glick AB. Use of a TGFbeta type I receptor inhibitor in mouse skin carcinogenesis reveals a dual role for TGFbeta signaling in tumor promotion and progression. Carcinogenesis. 2010;31(12):2127–2135. doi: 10.1093/carcin/bgq191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davies M, Prime SS, Eveson JW, Price N, Ganapathy A, D’Mello A, Paterson IC. Transforming growth factor-beta enhances invasion and metastasis in RAS-transfected human malignant epidermal keratinocytes. Int J Exp Pathol. 2012;93(2):148–156. doi: 10.1111/j.1365-2613.2011.00806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goudie DR, D’Alessandro M, Merriman B, Lee H, Szeverenyi I, Avery S, O’Connor BD, Nelson SF, Coats SE, Stewart A, Christie L, Pichert G, Friedel J, Hayes I, Burrows N, Whittaker S, Gerdes AM, Broesby-Olsen S, Ferguson-Smith MA, Verma C, Lunny DP, Reversade B, Lane EB. Multiple self-healing squamous epithelioma is caused by a disease-specific spectrum of mutations in TGFBR1. Nat Genet. 2011;43(4):365–369. doi: 10.1038/ng.780. [DOI] [PubMed] [Google Scholar]
- 31.Oberholzer PA, Kee D, Dziunycz P, Sucker A, Kamsukom N, Jones R, Roden C, Chalk CJ, Ardlie K, Palescandolo E, Piris A, MacConaill LE, Robert C, Hofbauer GF, McArthur GA, Schadendorf D, Garraway LA. RAS mutations are associated with the development of cutaneous squamous cell tumors in patients treated with RAF inhibitors. J Clin Oncol. 2012;30(3):316–321. doi: 10.1200/JCO.2011.36.7680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hodak E, Jones RE, Ackerman AB. Solitary keratoacanthoma is a squamous-cell carcinoma: three examples with metastases. Am J Dermatopathol. 1993;15(4):332–342. doi: 10.1097/00000372-199308000-00007. [DOI] [PubMed] [Google Scholar]