Abstract
Purpose:
Triple-negative breast cancers (TNBCs) represent a heterogeneous group of tumors. The lack of targeted therapies combined with the inherently aggressive nature of TNBCs results in a higher relapse rate and poorer overall survival. We evaluated the heterogeneity of TNBC cell lines for TRPC channel expression and sensitivity to cation-disrupting drugs.
Methods:
The TRPC1/4/5 agonist englerin A was used to identify a group of TNBC cell lines sensitive to TRPC1/4/5 activation and intracellular cation disruption. Quantitative RT-PCR, the sulforhodamine B assay, pharmacological inhibition, and siRNA-mediated knockdown approaches were employed. Epifluorescence imaging was performed to measure intracellular Ca2+ and Na+ levels. Mitochondrial membrane potential changes were monitored by confocal imaging.
Results:
BT-549 and Hs578T cells express high levels of TRPC4 and TRPC1/4, respectively, and are exquisitely, 2000- and 430-fold, more sensitive to englerin A than other TNBC cell lines. While englerin A caused a slow Na+ and nominal Ca2+ accumulation in Hs578T cells, it elicited rapid increases in cytosolic Ca2+ levels that triggered mitochondrial depolarization in BT-549 cells. Interestingly, BT-549 and Hs578T cells were also more sensitive to digoxin as compared to other TNBC cell lines. Collectively, these data reveal TRPC1/4 channels as potential biomarkers of TNBC cell lines with dysfunctional mechanisms of cation homeostasis and therefore sensitivity to cardiac glycosides.
Conclusions:
The sensitivity of BT-549 and Hs578T cells to englerin A and digoxin suggests a subset of TNBCs are highly susceptible to cation disruption and encourages investigation of TRPC1 and TRPC4 as potential new biomarkers of sensitivity to cardiac glycosides.
Keywords: Triple-negative breast cancer, englerin A, TRPC1/4/5, Digoxin, cation disruption
Background
Triple negative breast cancers (TNBCs) represent 15–20% of all breast cancers and are defined by the lack of estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor (HER2) amplification. While targeted therapies have improved patient outcomes for receptor-positive breast cancers, there are no targeted therapies for TNBCs. These cancers are currently treated with cytotoxic chemotherapy and unfortunately, these therapies provide only 23–55% relapse-free 10-year survival, highlighting the need for new treatment options [1].
A major challenge with TNBC is tumor heterogeneity [2, 3]. Efforts have been undertaken to identify subtypes of TNBC and targeted therapies for these subtypes. Success in this approach includes ongoing clinical trials of androgen receptor antagonists bicalutamide or enzalutamide in androgen receptor expressing TNBCs [4]. Our goal is to identify new molecular liabilities of TNBC subtypes by evaluating natural products in a panel of diverse TNBC cell lines [5]. Compounds with selective cytotoxic activity against a subgroup of these cells are investigated further to identify molecular liabilities. Multiple compounds with selective activities against TNBC cell lines have been identified and their molecular targets defined [6–8].
We investigate natural products from higher plants and fungi because nature provides a rich source of bioactive compounds. Analyses show that 55% of small molecule anticancer agents approved by the FDA from 1981–2014 were natural product-derived [9]. Englerin A (EA) is a sesquiterpene isolated from the Tanzanian plant Phyllanthus engleri that demonstrated potent and selective effects on renal cell carcinoma cell lines in the NCI-60 panel [10]. Studies with EA implicated multiple mechanisms of action in renal carcinoma cells, including activation of canonical transient receptor potential cation channels (TRPC1/4/5) and PKCθ activation [11, 12]. Effects of TRPC1/4/5 activation by EA appear to be dictated by the homomeric (TRPC4/4) and heteromeric (TRPC1/4) channel composition, in that TRPC4 homomer activation causes primarily Ca2+ influx while TRPC1/4 heteromer activation initiates a greater Na+ influx [13–16]. EA has also been evaluated in Ewing sarcoma cell lines, where it increased intracellular Ca2+ and inhibited EWS-FLI1-driven transcription [17]. The NCI-60 data showed differential antiproliferative and cytotoxic activity across 6 breast cancer cell lines [10], but EA had not been evaluated extensively in TNBC cell lines. Based on the ability of EA to activate TRPC1/4/5 channels in renal and Ewing sarcoma cancer cells, we investigated TRPC1/4/5 expression in TNBC cells and their sensitivity to EA. The results show that BT-549 cells have high TRPC4 expression and that Hs578T cells have high TRPC1 and TRPC4 expression, and both cell lines are exquisitely sensitive to EA as compared to other TNBC cells. The underlying molecular liabilities that confer sensitivity to EA were determined to identify a new molecular subgroup of TNBC that could be highly susceptible to cation-disrupting drugs.
Materials and Methods
Chemicals and reagents
Englerin A was isolated from the plant Phyllanthus engleri [10]. Pico145 was generously provided by Dr. David Beech of the University of Leeds [18]. Digoxin was purchased from Sigma-Aldrich (St. Louis, MO, USA).
Cell culture
The TNBC cell lines, MDA-MB-453, Hs578T, MDA-MB-231, HCC1806, and HCC1937 were purchased from the American Type Culture Collection (Manassas, VA, USA). CAL-51 cells were purchased from Creative Bioarray (Shirley, NY, USA). BT-549 cells were obtained from the Lombardi Cancer Center of Georgetown University (Washington, DC, USA). A-498 renal cell carcinoma cells were provided by Dr. Brian Reeves (University of Texas Health Science Center at San Antonio). Cell line identity was confirmed by DNA short tandem repeat analyses by Genetica DNA Laboratories (Burlington, NC, USA). BT-549, CAL-51, HCC1937 and HCC1806 cells were grown in RPMI-1640 supplemented with 10% FBS and 50 μg/mL gentamicin. MDA-MB-231 and MDA-MB-453 cells were grown in IMEM supplemented with 10% FBS and 25 μg/mL gentamicin. Hs578T cells were grown in DMEM supplemented with 10% FBS and 50 μg/mL gentamicin. A-498 cells were grown in DMEM without sodium pyruvate and supplemented with 10% heat inactivated FBS and 50 μg/mL gentamicin. All cells were maintained at 37°C with 5% CO2 and used within 40 passages after revival from liquid nitrogen.
Sulforhodamine B Assay
The antiproliferative and cytotoxic effects of indicated compounds were evaluated after 24 or 48 hr treatments using the sulforhodamine B (SRB) assay [19, 20] as previously described [21]. The IC50 is defined as the concentration that causes a 50% reduction in absorbance as compared to vehicle (DMSO) control by nonlinear regression in Prism 6.01 (GraphPad Software, La Jolla, CA, USA).
RNA isolation, cDNA preparation, and qRT-PCR
RNA was isolated and concentration and purity assessed using a NanoDrop 2000 spectrophotometer (ThermoFisher Scientific, Waltham, MA, USA). A high capacity cDNA Reverse Transcription kit (Applied Biosystems, Foster City, CA, USA) was used to make cDNA and qRT-PCR completed with SYBR® Green PCR Master Mix (Applied Biosystems). Primer pair sequences for TRPC1, TRPC4, TRPC5[22] and GAPDH are provided (Supplementary Table S1).
Gene knockdown
Two siRNAs targeting each TRPC1 (SASI_Hs02_00335399 and SASI_Hs01_00060664) and TRPC4 (SASI_Hs01_00129366 and SASI_Hs01_00129367) (Sigma-Aldrich, St Louis, MO, USA) were transfected into BT-549 and Hs578T cells for 48 hr at a final concentration of 9.4 nM TRPC1 siRNA, 18.8 nM TRPC4 siRNA and 0.025% lipofectamine RNAi-MAX (ThermoFisher Scientific). Mock transfections contained only lipofectamine RNAi-MAX. Following transfection, cells were treated with EA for 24 hr, or RNA isolated to determine knockdown efficiency at the time of EA addition.
Intracellular cation imaging
BT-549, Hs578T, and HCC1806 cells were plated on glass coverslips, grown to 60–80% confluency and loaded with 2.5 μM Cal-520 AM (ATT Bioquest, Sunnyvale, CA, USA) or 5 μM CoroNA Green AM (ThermoFisher Scientific) for imaging of intracellular Ca2+ and Na+, respectively. Cell culture media was supplemented with 0.2% Pluronic F-127 (Biotium, Fremont, CA, USA) to facilitate dye loading. Cells were incubated with dye solution for 30 min at 37°C, washed with Hank’s Balanced Salt Solution (HBSS), and incubated at room temperature for 15 min in the dark. Dye-loaded cells were stored at 37 °C if imaged later than 1 hr after dye loading. Unless otherwise indicated, all cells were imaged in standard HBSS with 1.8 mM Ca2+ within 4 hr of dye loading. To investigate TRPC1/4/5 activity, cells were pretreated, after dye loading, for 5 min with 10 nM Pico145. Imaging was performed with a Nikon Eclipse TE2000-U inverted microscope with a 20x objective. Images were acquired with an Andor iXon camera and evaluated using Metamorph software. Fluorescent intensity was analyzed cell-by-cell using ImageJ software with personnel blinded to experimental conditions. Fluorescence intensity data are represented as corrected total cell fluorescence (CTCF) = Integrated density - (cell area X mean background fluorescence).
Simultaneous Measurements of Cytosolic Ca2+ and Mitochondrial Membrane Potential (ΔΨm)
BT-549 and HCC1806 cells were grown on collagen-coated glass coverslips. Cells were loaded with Fluo-4 AM and TMRE (tetramethylrhodamine, ethyl ester) for 20 min to measure intracellular Ca2+ and mitochondrial membrane potential, respectively. Time-lapse imaging was performed using a Leica SP8 DMi8 confocal microscope with a 63x objective in an environmental chamber. Cells were left untreated or treated with 200 nM EA after a 60 sec baseline recording. Cytosolic Ca2+ and ΔΨm changes were recorded and analyzed using Leica Application Suite X.
Statistical analysis
qRT-PCR data are reported as fold-difference compared to MDA-MB-231 cells and statistical significance was determined using a one-way ANOVA with Dunnett’s multiple comparisons test. For intracellular ion sensing in BT-549 cells with Cal-520 and CoroNa green, statistical significance was determined by two-way ANOVA with Dunnett’s or Bonferroni’s multiple comparisons test. For CoroNa green fluorescence in Hs578T cells, an unpaired t-test was sufficient to determine significance. An unpaired t-test was used to determine the significance of Ca2+ and ΔΨm changes between non-treated and EA-treated conditions for each cell line. A one-way ANOVA with Dunnett’s multiple comparisons evaluation was used to test for significant differences in sensitivity of BT-549 cells to digoxin as compared to other TNBC cell lines. All error bars represent the mean ± SEM. All statistical analyses were performed using GraphPad Prism 6.01.
Results
mRNA Expression of TRPC1/4/5 in TNBC cell lines.
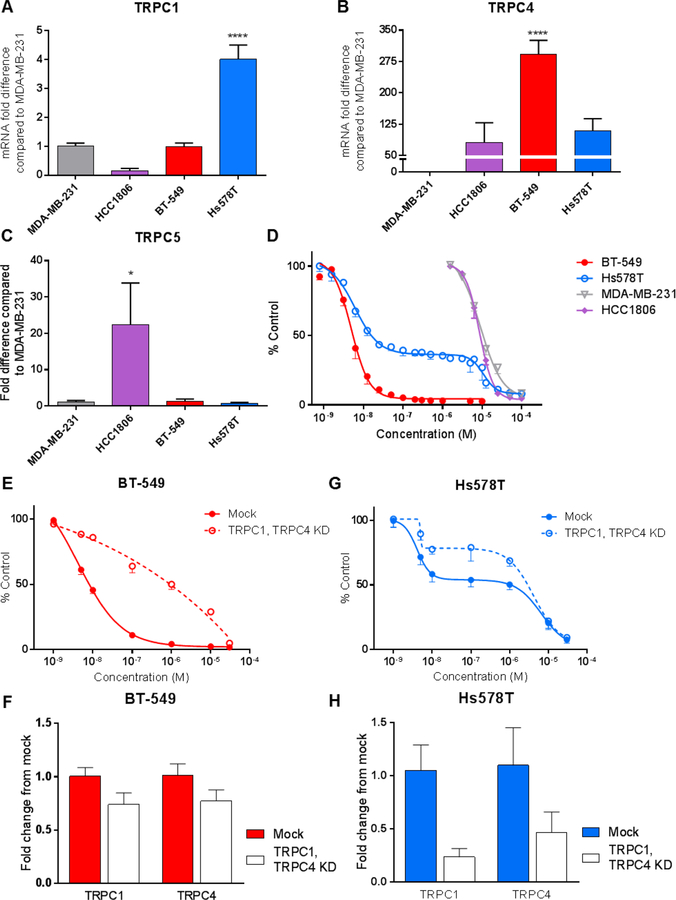
Because EA has demonstrated an ability to activate TRPC1/4/5 channels in some cell types [13–15, 23], the relative expression of TRPC1, TRPC4, and TRPC5 mRNA was evaluated in 4 TNBC cell lines: MDA-MB-231, HCC1806, BT-549, and Hs578T. The results show that Hs578T cells express 4 and 110-fold higher levels of TRPC1 and TRPC4, respectively, than MDA-MB-231 cells (Fig. 1a,b). BT-549 cells express 290-times more TRPC4 than MDA-MD-231 cells, but equivalent levels of TRPC1 (Fig. 1a,b). Expression levels of TRPC4 and TRPC5 in HCC1806 cells are 81 and 20-fold higher than in MDA-MB-231 cells (Fig. 1b,c). The sensitivity of these cells to EA-mediated cytotoxicity was then evaluated and the results show that BT-549 and Hs578T cells are exquisitely sensitive to EA, with IC50 values of 5.4 and 16 nM, respectively. MDA-MB-231 and HCC1806 cells are resistant to EA with IC50 values of 11 and 8.9 µM, respectively (Fig 1d). Evaluation of three additional TNBC cell lines show that BT-549 and Hs578T cells are 430 to 2000-fold more sensitive to EA-mediated cytotoxicity than the other TNBC cell lines (Table 1). Interestingly, the EA concentration response curve in Hs578T cells is biphasic with the first phase closely aligned with BT-549 cells, consistent with a shared mechanism of action. However, the distinct plateau and second phase of the EA concentration response curve in Hs578T cells that aligns with the resistant TNBC cell lines suggests mechanistic differences between these two sensitive cell lines.
Fig. 1.
TRPC1/4 expression correlates to TNBC cell line sensitivity to EA. a TRPC1 b TRPC4 and c TRPC5 expression was determined by qRT-PCR analysis of MDA-MB-231, HCC1806, BT-549, and Hs578T cells. Fold difference was analyzed as compared to MDA-MB-231 expression levels. Significance determined by one-way ANOVA with Dunnett’s multiple comparisons test *p = 0.02, ****p < 0.0001 d The effects of EA on BT-549, Hs578T, MDA-MB-231, and HCC1806 cell survival were measured by the SRB assay after 48 hr treatment (n=3–15). The effects of reduced TRPC1 and TRPC4 expression on EA mediated cytotoxicity were assessed by SRB assay following 48 hr siRNA-mediated knockdown of TRPC1 and TRPC4 and 24 hr EA treatment in e BT-549 and g Hs578T cells (n=3). The efficiency of TRPC1 and TRPC4 knockdown was determined by qRT-PCR analysis of f BT-549 and h Hs578T cells compared to the expression in mock transfected cells (n=3)
Table 1.
IC50 values of EA in TNBC cell lines
| Cell Line | IC50 (nM) ± SEM |
|---|---|
| BT-549 | 5.4 ± 1.3 |
| Hs578T | 16 ± 1.8 |
| CAL-51 | 6,900 ± 2000 |
| MDA-MB-231 | 11,000 ± 1300 |
| MDA-MB-453 | 8,600 ± 1500 |
| HCC1937 | 11,000 ± 1900 |
| HCC1806 | 8,900 ± 910 |
The effects of siRNA-mediated TRPC1/4 depletion on EA sensitivity in BT-549 and Hs578T cells were evaluated. Even a small reduction in TRPC1 and TRPC4 mRNA expression by 26% and 23%, respectively, in BT-549 cells decreased the potency of EA by 115-fold compared to mock controls, demonstrating the critical nature of TRPC1/4 channels to EA sensitivity in these cells (Fig. 1e,f). In Hs578T cells, an siRNA-mediated 76% decrease in TRPC1 and 53% decrease in TRPC4 expression caused a 2.6-fold decrease in sensitivity to EA (Fig. 1g,h). Taken together, these data suggest TRPC1/4 channel expression mediates cell line sensitivity to EA, albeit with differences between cell lines.
TRPC1/4/5 inhibition decreases the potency of EA in BT-549 and Hs578T cells.
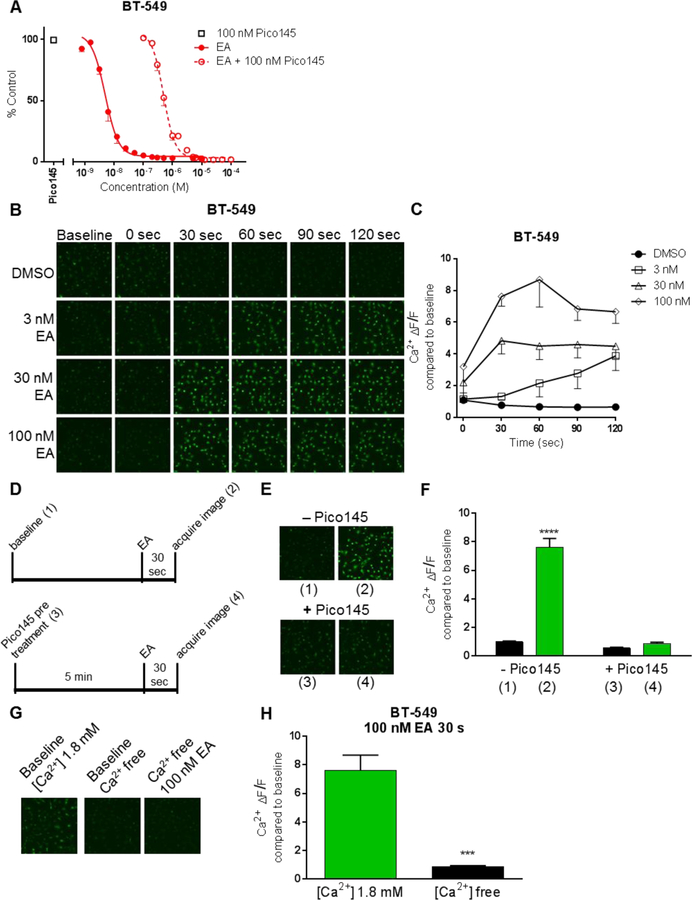
Pico145, a selective inhibitor of TRPC1/4/5 channels [18], was used to determine if the cytotoxic effects of EA are due to TRPC1/4/5 activation in sensitive TNBC cell lines. Pico145 reduced the potency of EA in BT-549 and Hs578T cell lines as evidenced by a 98- and 69-fold increase in the IC50, respectively (Fig. 2a, Supplementary Fig. S1a) demonstrating the importance of TRPC1/4/5 activity to the sensitivity of these cells to EA. In contrast, Pico145 did not decrease the potency of EA in MDA-MB-231 cells (Supplementary Fig. S1b).
Fig. 2.
Evidence that EA activity is mediated by TRPC1/4/5 channels. a Effects of Pico145 (100 nM) on EA concentration response in BT-549 cells were measured by SRB assay 48 hr after combined treatment. EA treatment alone data are also presented in Fig 1. 100 nM Pico145 and 100 nM Pico145 plus EA (n=4–8) b Representative images and c quantification of Ca2+ in BT-549 cells as a function of time and EA concentration measured by Cal-520 AM dye (n=2–4) Significance indicated in Supplementary TableS2. ΔF/F = CTCFtreatment CTCFbaseline d Schema of evaluation of Pico145 (10 nM) effects on EA induced Ca2+ influx. Parenthetical numbers correspond to representative images in e and quantification in f of Cal-520 fluorescence (n=3–13). Statistical significance determined by two-way ANOVA with Bonferroni’s multiple comparisons is indicated for baseline versus EA treatment ± 10 nM Pico145, ****p<0.0001 g Representative images and h quantification of 30 sec 100 nM EA exposure on Ca2+ in BT-549 cells in normal Ca2+ (1.8 mM) HBSS or Ca2+ free HBSS (n=3) significance determined by unpaired t-test, *** p=0.0004
TRPC channels are nonselective, tetrameric, cation channels composed of homomers or heteromers of 2 TRPCs [24–26]. The effects of EA on cytosolic levels of Na+ and Ca2+ were evaluated with fluorescent sensors selective for either Na+ or Ca2+. In BT-549 cells, over the course of 5 min, 100 nM EA caused a maximum increase in intracellular Na+ of 2.1-fold over baseline levels and did not increase further with higher EA concentrations (Supplementary Fig. S2a,b, Supplementary Table S2). A similar response was seen in Hs578T cells (Supplementary Fig. S2c,d). Pretreatment of BT-549 cells with Pico145 blocked the EA-mediated increase in intracellular Na+, suggesting that it is mediated by TRPC1/4/5 channel activation. In BT-549 cells, EA initiated a rapid increase in intracellular Ca2+ that was more robust than the increase in Na+. EA increased intracellular Ca2+ in a concentration (3–1000 nM) and time (0–120 sec)-dependent manner (Fig. 2b, Supplementary Table S3). With 3 nM EA treatment, a 3.9-fold increase in intracellular Ca2+ compared to baseline was detected within 120 sec, while 100 nM EA caused a 7.6-fold increase in Ca2+ within 30 sec that increased to 8.7-fold by 60 sec (Fig. 2c). Treatment with 1 µM EA, caused an immediate increase in intracellular Ca2+, 7.2 times higher than baseline (Supplementary Fig. S3a,b, Table S3). In BT-549 cells, pretreatment with Pico145 completely attenuated the EA-induced increase in intracellular Ca2+ (Fig. 2d,e,f), reinforcing that EA-induced cation influx is due to TRPC1/4/5 activation. Importantly, in Ca2+-free imaging solution EA caused no detectable change in intracellular Ca2+, demonstrating that the EA-induced cytosolic Ca2+ increase is primarily due to influx from the extracellular environment, and not released from intracellular stores (Fig. 2g,h). In stark contrast to BT-549 cells, EA had no effect on intracellular Ca2+ levels in Hs578T cells (Supplementary Fig. S4a,b). Of note, EA also had no effect on intracellular Ca2+ levels in EA-resistant HCC1806 cells at concentrations up to 250 µM (Supplementary Fig. S5a,b). These data suggest that EA activates TRPC1/4/5 channels and disrupts intracellular cation levels in the cells most sensitive to the cytotoxic effects of EA.
EA causes mitochondrial depolarization in BT-549 cells.
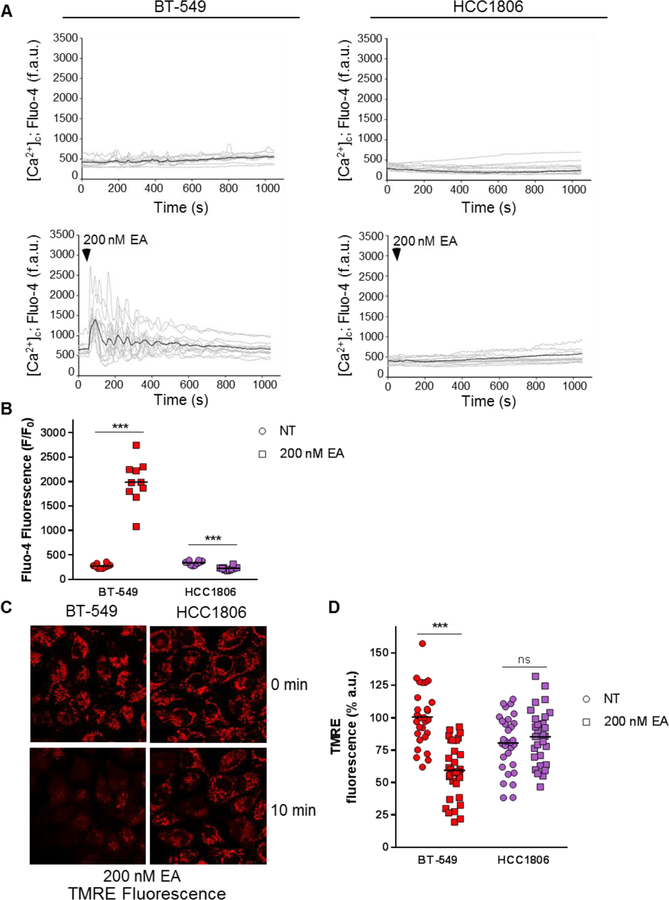
The effects of the EA-mediated increase in cytosolic Ca2+ on mitochondria were measured in BT-549 cells because dysregulated levels of Ca2+ can cause a loss of mitochondrial membrane potential (ΔΨm) and subsequent cell death. EA caused a rapid, within 60 sec, and robust 7.3-fold increase in intracellular Ca2+ compared to non-treated cells that was damped to a 5.7-fold increase when measured in Ca2+-free imaging media (Fig. 3a,b and Supplementary Figure S6a,b). The Ca2+ increase in BT-549 cells led to significant mitochondrial depolarization as measured by TMRE fluorescent intensity within 10 min after EA addition (Fig. 3c,d and Supplementary Figure S6c). Consistent with the aforementioned EA-mediated increases in intracellular Ca2+ in BT-549 cells, these data demonstrate that EA causes Ca2+ entry into the cytoplasm from the extracellular environment. Interestingly, BT-549 cells had baseline unsynchronized Ca2+ oscillations that were maintained following EA treatment with and without extracellular Ca2+ (Fig. 3a and Supplementary Figure S6a). In contrast, in the resistant HCC1806 cells, EA did not increase intracellular Ca2+ or cause mitochondrial depolarization (Fig. 3a,b and Supplementary Figure S6a,b,c). These data show that EA-induced increases in intracellular Ca2+ rapidly initiate mitochondrial depolarization and this likely contributes to EA cytotoxicity in BT-549 cells.
Fig. 3.
EA-induced Ca2+ influx corresponds with mitochondrial depolarization in the presence of extracellular Ca2+. a Ca2+ traces over time and b quantification of peak amplitude in BT-549 and HCC1806 cells in the presence of extracellular Ca2+ with or without 200 nM EA treatment at 60 sec. Intracellular Ca2+ is measured by Fluo-4 AM dye (n=4–8) Significance determined by unpaired t-test between non-treat and EA treatment conditions in each cell line, ***p<0.001 c Representative images and d quantification of TMRE fluorescence at 10 min following 200 nM EA treatment of BT-549 and HCC1806 cells (n=4–8) Significance determined by unpaired t-test between non-treat and EA treatment conditions in each cell line, ***p<0.001.
BT-549 and Hs578T cells are also sensitive to other cation-disrupting drugs.
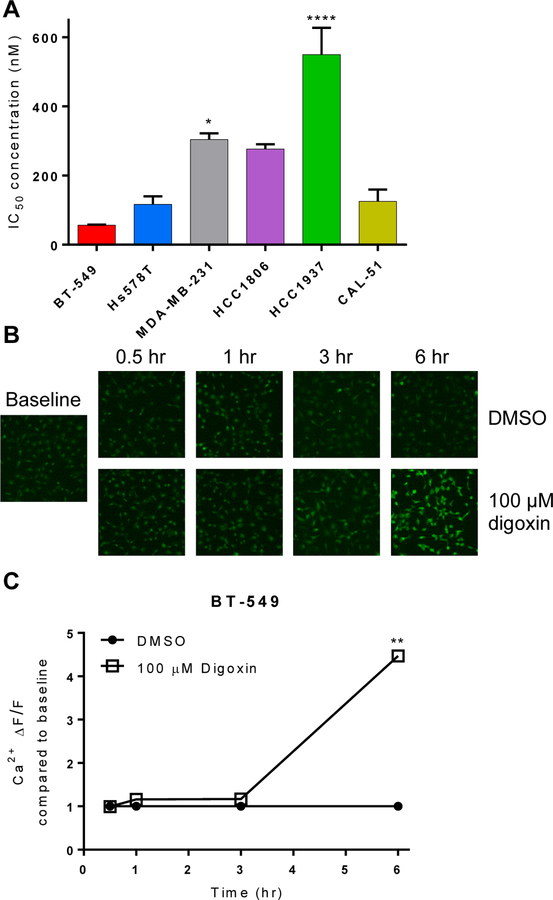
The overall goal of our work is to identify pharmacological liabilities of subgroups of TNBCs as a mechanism to develop new treatment options for TNBC. EA is not a clinical candidate because it causes cardiotoxicity [23] and reduction of locomotor activity mediated by TRPC4/5 [27]. Based on our findings that BT-549 and Hs578T cells are highly sensitive to EA-mediated cation disruption, we considered other FDA-approved drugs that alter intracellular cation concentrations. Cardiac glycosides improve cardiac contractility by increasing intracellular Ca2+ through inhibition of the Na+/K+ ATPase [28]. Interestingly, Ludlow et al [14] and Wu et al [11] both reported that EA synergizes with cardiac glycosides in renal cancer and Ewing sarcoma cells, consistent with the compounds acting through different mechanisms. Consistent with the effects of EA, BT-549 and Hs578T cells were the most sensitive TNBC cell lines to digoxin with IC50 values of 56 and 100 nM, respectively (Fig. 4a). MDA-MB-231 and HCC1937 cells were significantly less sensitive to digoxin with IC50 concentrations of 300 and 520 nM, respectively (Fig. 4a). The ability of digoxin to increase intracellular Ca2+ was confirmed in BT-549 cells where a 4.5-fold increase in the intracellular Ca2+ fluorescence occurred within 6 hr (Fig. 4b,c). These data suggest BT-549 and Hs578T cells represent a group of TNBCs that are particularly sensitive to multiple mechanisms of cation disruption.
Fig. 4.
Effects of digoxin on TNBC cells. a BT-549, Hs578T, MDA-MB-231, HCC1806, HCC1937, and CAL-51 TNBC cells were treated with digoxin for 48 hr, and IC50 determined by SRB assay. Significant difference of IC50 in each cell line compared to BT-549 IC50 determined by one-way ANOVA with Dunnett’s multiple comparisons test (*p<0.05, ***p<0.001, ****p<0.001 (n=3–5). b Representative images and c quantification of Ca2+ fluorescence in BT-549 cells treated with 100 μM digoxin measured by Cal-520 AM dye. Significance between DMSO and digoxin treatment at each time point determined by two-way ANOVA with Bonferroni’s multiple comparisons test (**p<0.0001) (n=111–990 cells)
DISCUSSION
Based on previous studies, we hypothesized that some TNBC cell lines would be sensitive to EA based on TRPC1/4/5 expression. Herein, we demonstrate that cell lines with high TRPC1/4 expression are highly sensitive to EA. While BT-549 and Hs578T cells are susceptible to EA-induced cytotoxicity because of TRPC1/4 expression, they have different TRPC1/4 expression levels, EA concentration response curves, and EA-mediated cation disruptions. While regarded as non-selective, the permeability of TRPC1/4/5 channels to Ca2+ and Na+ differs depending on the tetramer composition, for example, TRPC1/4 heteromers are less permeable to Ca2+ than TRPC4 homomers [16]. The differences between the effects of EA in BT-549 and Hs578T cells are likely due to the high levels of TRPC4 in BT-549 cells that form homomers that primarily conduct Ca2+ as compared to the high levels of TRPC1 and TRPC4 that might exist in heteromeric forms in Hs578T cells and conduct more Na+ [16]. There was no difference in Na+ influx between the 2 cell lines because each of the TRPC1/4 channel configurations are permeable to Na+. Interestingly, elevated levels of TRPC4/5 co-expression in HCC1806 cells did not lead to EA sensitivity, further suggesting that specific TRPC heteromer composition influences sensitivity to EA.
Our data suggest that Hs578T cells, like the renal carcinoma A-498 cells [14] and the synovial sarcoma SW982 cells [15], are sensitive to EA-mediated Na+ influx through the activation of TRPC1/4 heteromeric channels. When comparing the concentration response curves of EA in Hs578T and A-498 cells, both cell lines respond in a biphasic manner (Supplementary Fig. S7). Furthermore, Hs578T cells have more TRPC1 than the EA-resistant TNBC cell lines, and EA caused a marked increase in intracellular Na+, but not Ca2+. We postulate that Na+ influx through TRPC1/4 heteromeric channels drives the long-term accumulation of intracellular Ca2+. This is analogous to the mechanism of action of cardiac glycosides which inhibit the Na+/K+ ATPase pump, initially causing an increase in intracellular Na+ that is compensated for by inhibition or reversal of the Na+/Ca2+ exchanger, leading to a secondary increase in intracellular Ca2+ [29]. In contrast, our data suggest that BT-549 cells are sensitive to EA due to the acute activation of homomeric TRPC4 channels and rapid Ca2+ influx. Additionally, the unsynchronized, periodic oscillations in intracellular Ca2+ observed in BT-549, but not HCC1806 cells, suggests that BT-549 cells are continuously fine-tuning their intracellular Ca2+ levels. Our demonstration that even a slight decrease in TRPC1/4 expression can decrease the sensitivity to EA, further supports the delicate balance of Ca2+ regulation in BT-549 cells. EA-mediated rapid Ca2+ influx and subsequent mitochondrial depolarization demonstrates that these cells have no capacity to buffer the extensive EA-meditated Ca2+ influx. Additional evidence that BT-549 and Hs578T cells are different in how they modulate intracellular Ca2+ levels is their high expression of PMCA1 and PMCA4 Ca2+ pumps in comparison to other TNBC, luminal, and HER2 overexpressing breast cancer cell lines [30]. Notably, dysregulated Ca2+ signaling has been implicated in many of the “hallmarks of cancer”, including proliferation, migration, and metastasis [31].
This is the first study to identify a distinct group of TNBC cell lines that are exquisitely sensitive to EA and describe the mechanism underlying this sensitivity. While EA itself is not a clinical candidate, ongoing studies are aimed to identify new EA analogs with an improved therapeutic window [11]. In the meantime, we determined that EA-sensitive TNBC lines were also sensitive to the clinically approved cation-disrupting compound digoxin. For decades, it has been proposed that cardiac glycosides should be investigated for activity in breast cancer based on clinical observations. Breast cancer patients taking a cardiac glycoside for cardiac indications had more uniform and smaller breast tumors [32]. There lower breast cancer mortality among breast cancer patients using digitalis as compared to those that were not [33]. Preclinical studies show that cardiac glycosides can induce apoptosis [34, 35], inhibit topoisomerase II [36], initiate p21Cip1 expression [37], and cause immunogenic cell death [38] in cancer cells. While cardiac glycosides can also bind and activate the estrogen receptor, curbing further investigation in ER positive breast cancer [39–41], this is not a concern in TNBC. Consequently, digoxin use was associated with a high risk of relapse in the first year after diagnosis in patients with ER-positive breast cancer, but a low risk of relapse in patients with ER-negative breast cancers [40]. Two other studies found digoxin users had a lower risk of developing ER-negative disease than ER- positive disease [42, 43]. Although there is no evidence suggesting digoxin acts through the TRPC1/4/5 channels, our results demonstrating that TNBC cell lines with high TRPC1/4 expression are particularly sensitive to cardiac glycosides suggests that there is a subgroup of TNBC with defective Ca2+ handling abilities that could benefit from digoxin. Mining the METABRIC breast cancer data set [44] shows that 2.2% of patients (47/2173) have amplified copy number alterations in TRPC1 or TRPC4, and half of those were ER-negative [45, 46]. Thus, a population of breast cancer patients express these potential biomarkers of response to cardiac glycosides. Furthermore, it has been shown that TRPC1 expression is high in TNBCs of the mesenchymal phenotype, which is associated with poorer patient prognosis [47]. These studies, taken together with our findings, support future investigations of TRPC1 and TRPC4 as biomarkers of response to cardiac glycosides or other cation disruptors. This would allow for the selection of a more defined group of TNBC patients for this intervention, therefore increasing the chances of therapeutic benefit.
Supplementary Material
Acknowledgements
We thank Dr. Mark Shapiro (University of Texas Health Science Center at San Antonio) for providing instruments and space for intracellular ion imaging experiments. We also thank Dr. David J. Beech (University of Leeds) for providing us with Pico145.
Funding
This study was funded by grants to SLM and Robert Cichewicz from the National Cancer Institute (U01CA182740), and by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research (IZIA BC1146907).
Footnotes
Conflict of Interest
Corena V. Grant declares that she has no conflict of interest. Chase M. Carver declares that he has no conflict of interest. Shayne D. Hastings declares that she has no conflict of interest. Karthik Ramachandran declares that he has no conflict of interest. Madesh Muniswamy declares that he has no conflict of interest. April L. Risinger declares that she has no conflict of interest. John A. Beutler declares that he has no conflict of interest. Susan L. Mooberry has and continues to receive research funding, unrelated to this study, from Eisai, Inc.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors
Availability of data and material
Englerin A is available from Dr. John Beutler of the Molecular Targets Program, Center for Cancer Research, National Cancer Institute, Frederick, MD.
REFERENCES
- 1.Hatzis C, Symmans WF, Zhang Y, et al. (2016) Relationship between Complete Pathologic Response to Neoadjuvant Chemotherapy and Survival in Triple-Negative Breast Cancer. Clin Cancer Res 22:26–33 [DOI] [PubMed] [Google Scholar]
- 2.Bianchini G, Balko JM, Mayer IA, et al. (2016) Triple-negative breast cancer: challenges and opportunities of a heterogeneous disease. Nat Rev Clin Oncol 13:674–690. doi: 10.1038/nrclinonc.2016.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yam C, Mani SA, Moulder SL (2017) Targeting the Molecular Subtypes of Triple Negative Breast Cancer: Understanding the Diversity to Progress the Field. Oncologist 22:1086–1093. doi: 10.1634/theoncologist.2017-0095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. ClinicalTrials.gov. https://clinicaltrials.gov/
- 5.Lehmann BD, Bauer JA, Chen X, et al. (2011) Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest 121:2750–2767. doi: 10.1172/JCI45014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robles AJ, Du L, Cichewicz RH, Mooberry SL (2016) Maximiscin Induces DNA Damage, Activates DNA Damage Response Pathways, and Has Selective Cytotoxic Activity against a Subtype of Triple-Negative Breast Cancer. J Nat Prod 79:1822–1827. doi: 10.1021/acs.jnatprod.6b00290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robles AJ, Cai S, Cichewicz RH, Mooberry SL (2016) Selective activity of deguelin identifies therapeutic targets for androgen receptor-positive breast cancer. Breast Cancer Res Treat 157:475–488. doi: 10.1007/s10549-016-3841-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robles AJ, McCowen S, Cai S, et al. (2017) Structure–Activity Relationships of New Natural Product-Based Diaryloxazoles with Selective Activity against Androgen Receptor-Positive Breast Cancer Cells. J Med Chem 60:9275–9289. doi: 10.1021/acs.jmedchem.7b01228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Newman DJ, Cragg GM (2016) Natural Products as Sources of New Drugs from 1981 to 2014. J Nat Prod 79:629–661. doi: 10.1021/acs.jnatprod.5b01055 [DOI] [PubMed] [Google Scholar]
- 10.Ratnayake R, Covell D, Ransom TT, et al. (2009) Englerin A, a selective inhibitor of renal cancer cell growth, from Phyllanthus engleri. Org Lett 11:57–60. doi: 10.1021/ol802339w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu Z, Zhao S, Fash DM, et al. (2017) Englerins: A Comprehensive Review. J Nat Prod 80:771–781. doi: 10.1021/acs.jnatprod.6b01167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rubaiy HN (2019) Treasure Troves of Pharmacological Tools to Study TRPC1/4/5 Channels. Br J Pharmacol 10.1111/bph.14578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akbulut Y, Gaunt HJ, Muraki K, et al. (2015) (−)-Englerin A is a potent and selective activator of TRPC4 and TRPC5 calcium channels. Angew Chem Int Ed Engl 54:3787–3791. doi: 10.1002/anie.201411511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ludlow MJ, Gaunt HJ, Rubaiy HN, et al. (2016) (−)-Englerin A-evoked Cytotoxicity is Mediated by Na+ Influx and Counteracted by Na+/K+-ATPase. J Biol Chem 10.1074/jbc.M116.755678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muraki K, Ohnishi K, Takezawa A, et al. (2017) Na+ entry through heteromeric TRPC4/C1 channels mediates (−)Englerin A-induced cytotoxicity in synovial sarcoma cells. Sci Rep 7:16988. doi: 10.1038/s41598-017-17303-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Storch U, Forst A-L, Philipp M, et al. (2012) Transient receptor potential channel 1 (TRPC1) reduces calcium permeability in heteromeric channel complexes. J Biol Chem 287:3530–3540. doi: 10.1074/jbc.M111.283218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caropreso V, Darvishi E, Turbyville TJ, et al. (2016) Englerin A Inhibits EWS-FLI1 DNA Binding in Ewing Sarcoma Cells. J Biol Chem 291:10058–10066. doi: 10.1074/jbc.M115.701375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rubaiy HN, Ludlow MJ, Henrot M, et al. (2017) Picomolar, selective, and subtype-specific small-molecule inhibition of TRPC1/4/5 channels. J Biol Chem 292:8158–8173. doi: 10.1074/jbc.M116.773556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Skehan P, Storeng R, Scudiero D, et al. (1990) New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst 82:1107–1112 [DOI] [PubMed] [Google Scholar]
- 20.Vichai V, Kirtikara K (2006) Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat Protoc 1:1112–1116. doi: 10.1038/nprot.2006.179 [DOI] [PubMed] [Google Scholar]
- 21.Shaffer CV, Cai S, Peng J, et al. (2016) Texas Native Plants Yield Compounds with Cytotoxic Activities against Prostate Cancer Cells. J Nat Prod 79:. doi: 10.1021/acs.jnatprod.5b00908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wuensch T, Thilo F, Krueger K, et al. (2010) High glucose-induced oxidative stress increases transient receptor potential channel expression in human monocytes. Diabetes 59:844–849. doi: 10.2337/db09-1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carson C, Raman P, Tullai J, et al. (2015) Englerin A Agonizes the TRPC4/C5 Cation Channels to Inhibit Tumor Cell Line Proliferation. PLoS One 10:e0127498. doi: 10.1371/journal.pone.0127498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vazquez G, Wedel BJ, Aziz O, et al. (2004) The mammalian TRPC cation channels. Biochim Biophys Acta - Mol Cell Res 1742:21–36. doi: 10.1016/j.bbamcr.2004.08.015 [DOI] [PubMed] [Google Scholar]
- 25.Putney JW (2005) Physiological mechanisms of TRPC activation. Pflügers Arch - Eur J Physiol 451:29–34. doi: 10.1007/s00424-005-1416-4 [DOI] [PubMed] [Google Scholar]
- 26.Abramowitz J, Ahern G, Ambudkar I, et al. (2007) Handbook of Experimental Pharmacology [Google Scholar]
- 27.Cheung SY, Henrot M, Al-Saad M, et al. (2018) TRPC4/TRPC5 channels mediate adverse reaction to the cancer cell cytotoxic agent (−)-Englerin A. Oncotarget 9:29634–29643. doi: 10.18632/oncotarget.25659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fozzard HA, Sheets MF (1985) Cellular mechanism of action of cardiac glycosides. J Am Coll Cardiol 5:10A–15A. doi: 10.1016/S0735-1097(85)80458-7 [DOI] [PubMed] [Google Scholar]
- 29.Schoner W, Scheiner-Bobis G (2007) Endogenous and Exogenous Cardiac Glycosides and their Mechanisms of Action. Am J Cardiovasc Drugs 7:173–189. doi: 10.2165/00129784-200707030-00004 [DOI] [PubMed] [Google Scholar]
- 30.Varga K, Hollósi A, Pászty K, et al. (2018) Expression of calcium pumps is differentially regulated by histone deacetylase inhibitors and estrogen receptor alpha in breast cancer cells. BMC Cancer 18:1029. doi: 10.1186/s12885-018-4945-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stewart TA, Yapa KTDS, Monteith GR (2015) Altered calcium signaling in cancer cells. Biochim Biophys Acta - Biomembr 1848:2502–2511. doi: 10.1016/J.BBAMEM.2014.08.016 [DOI] [PubMed] [Google Scholar]
- 32.Stenkvist B, Bengtsson E, Eriksson O, et al. (1979) Cardiac glycosides and breast cancer. Lancet 1:563. [DOI] [PubMed] [Google Scholar]
- 33.Stenkvist B (1999) Is digitalis a therapy for breast carcinoma? Oncol Rep 6:493–496 [PubMed] [Google Scholar]
- 34.Zhao Y-T, Yan J-Y, Han X-C, et al. (2017) Anti-proliferative effect of digoxin on breast cancer cells via inducing apoptosis. Eur Rev Med Pharmacol Sci 21:5837–5842. doi: 10.26355/eurrev_201712_14032 [DOI] [PubMed] [Google Scholar]
- 35.Winnicka K, Bielawski K, Bielawska A, Surażyński A (2008) Antiproliferative Activity of Derivatives of Ouabain, Digoxin and Proscillaridin A in Human MCF-7 and MDA-MB-231 Breast Cancer Cells. Biol Pharm Bull 31:1131–1140. doi: 10.1248/bpb.31.1131 [DOI] [PubMed] [Google Scholar]
- 36.Bielawski K, Winnicka K, Bielawska A (2006) Inhibition of DNA Topoisomerases I and II, and Growth Inhibition of Breast Cancer MCF-7 Cells by Ouabain, Digoxin and Proscillaridin A. Biol Pharm Bull 29:1493–1497. doi: 10.1248/bpb.29.1493 [DOI] [PubMed] [Google Scholar]
- 37.Kometiani P, Liu L, Askari A (2005) Digitalis-induced signaling by Na+/K+-ATPase in human breast cancer cells. Mol Pharmacol 67:929–936. doi: 10.1124/mol.104.007302 [DOI] [PubMed] [Google Scholar]
- 38.Menger L, Vacchelli E, Adjemian S, et al. (2012) Cardiac glycosides exert anticancer effects by inducing immunogenic cell death. Sci Transl Med 4:143ra99. doi: 10.1126/scitranslmed.3003807 [DOI] [PubMed] [Google Scholar]
- 39.Biggar RJ (2012) Molecular Pathways: Digoxin Use and Estrogen-Sensitive Cancers--Risks and Possible Therapeutic Implications. Clin Cancer Res 18:2133–2137. doi: 10.1158/1078-0432.CCR-11-1389 [DOI] [PubMed] [Google Scholar]
- 40.Biggar RJ, Andersen EW, Kroman N, et al. (2013) Breast cancer in women using digoxin: tumor characteristics and relapse risk. Breast Cancer Res 15:R13. doi: 10.1186/bcr3386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karasneh RA, Murray LJ, Cardwell CR (2017) Cardiac glycosides and breast cancer risk: A systematic review and meta-analysis of observational studies. Int J Cancer 140:1035–1041. doi: 10.1002/ijc.30520 [DOI] [PubMed] [Google Scholar]
- 42.Ahern TP, Tamimi RM, Rosner BA, Hankinson SE (2014) Digoxin use and risk of invasive breast cancer: evidence from the Nurses’ Health Study and meta-analysis. Breast Cancer Res Treat 144:427–435. doi: 10.1007/s10549-014-2886-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Biggar RJ, Wohlfahrt J, Oudin A, et al. (2011) Digoxin use and the risk of breast cancer in women. J Clin Oncol 29:2165–2170. doi: 10.1200/JCO.2010.32.8146 [DOI] [PubMed] [Google Scholar]
- 44.Curtis C, Shah SP, Chin S-F, et al. (2012) The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature 486:346–352. doi: 10.1038/nature10983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gao J, Aksoy BA, Dogrusoz U, et al. (2013) Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal 6:pl1. doi: 10.1126/scisignal.2004088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cerami E, Gao J, Dogrusoz U, et al. (2012) The cBio Cancer Genomics Portal: An Open Platform for Exploring Multidimensional Cancer Genomics Data: Figure 1. Cancer Discov 2:401–404. doi: 10.1158/2159-8290.CD-12-0095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Azimi I, Milevskiy MJG, Kaemmerer E, et al. (2017) TRPC1 is a differential regulator of hypoxia-mediated events and Akt signalling in PTEN-deficient breast cancer cells. J Cell Sci 130:2292–2305. doi: 10.1242/jcs.196659 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.