Abstract
Genetic knockdown or knockout of the histone methytransferase G9a in the injured dorsal root ganglion (DRG) has been shown to alleviate neuropathic pain development. However, the application of genetic strategy in clinic is highly limited. The present study sought to examine the effect of intrathecal BIX01294, a specific G9a inhibitor, on the development and maintenance of pain hypersensitivities caused by unilateral L5 spinal nerve injury (SNL) or chronic constriction injury (CCI) to the sciatic nerve in rats. We found that intrathecal administration of BIX01294 reduced SNL- or CCI-induced mechanical allodynia, thermal hyperalgesia and cold allodynia not only in the development period but also in the maintenance period. These effects were dose-dependent. Intrathecal administration of BIX01294 also blocked the SNL-induced increase in the level of H3K9me2, a marker of G9a activity, and reversed SNL-induced downregulation of Oprm1 mRNA, Oprk1 mRNA, Oprd1 mRNA, Kcna2 mRNA, and Kcna4 mRNA, the downstream targets of G9a, in the ipsilateral L5 DRG. These findings further implicate that G9a as a potential target in the management of neuropathic pain.
Keywords: G9a, BIX01294, intrathecal injection, neuropathic pain, rats
1. Introduction
Chronic neuropathic pain, resulting from the damages in the peripheral or central nervous system, contributes to prolonged suffering and substantially reduces the quality of life in patients. Treatments strategies for chronic neuropathic pain are highly limited because conventional pharmacologic therapies are often ineffective and/or undesirable due to their side effects [1]. Elucidating the molecular and cellular mechanisms that underlie the development and maintenance of neuropathic pain may provide a novel approach to the treatment of this disorder.
Peripheral nerve injury produces the changes in the expression of pain-associated genes in the dorsal root ganglion (DRG) [2,3]. Recent studies have demonstrated that epigenetic modifications including DNA methylation and histone modifications influence the alterations in gene expression at the transcriptional level in the injured DRGs, contributing to the development of neuropathic pain [4–10]. Histone methytransferase G9a dimethylates histone H3 on lysine residue 9 (H3K9m2) and then condenses chromatin to repress the transcription of gene expression [11,12]. Peripheral nerve injury has been shown to increase the expression of G9a protein and Ehmt2 mRNA, which encodes G9a, in the injured DRG [4,6]. Furthermore, these increases have been implicated in the nerve injury-induced downregulation of opioid receptor-encoding genes and several potassium channel-encoding genes in the injured DRG [4–6,9]. Genetic knockdown or knockout of Ehmt2 in the injured DRG relieved spinal nerve ligation (SNL)-induced pain hypersensitivities, enhanced morphine analgesia and prevented the development of morphine or loperamide-induced analgesic tolerance under neuropathic pain conditions [4–6,9]. Taken together, these findings indicate that G9a in the injured DRG is required for neuropathic pain genesis.
Since the application of genetic strategy in clinic is highly limited, the present study examined the effect of intrathecal BIX01294, a specific G9a inhibitor [13,14], on SNL- or chronic constriction injury of sciatic nerve (CCI)-induced pain hypersensitivities during the development and maintenance periods. We also investigated the effect of intrathecal BIX01294 on the SNL-induced increase in the level of H3K9me2, a marker of G9a activity, in the ipsilateral L5 DRG. Finally, we examined whether intrathecal BIX01294 reversed the expression of downstream targets of G9a, Oprm1, Oprk1, Oprd1, Kcna2, and Kcna4 mRNAs in the injured DRG.
2. Methods
2.1. Animals
Male Sprague-Dawley rats weighing 250 g – 300 g were used in this study. All experimental procedures were approved by the Animal Care and Use Committee at the Rutgers New Jersey Medical School and all experiments were conducted in accordance with the ethical guidelines of the US National Institutes of Health and the International Association for the Study of Pain. All efforts were made to minimize animal suffering and to reduce the number of animals used. All of the experimenters were blind to treatment condition.
2.2. Animal models
SNL or CCI-induced neuropathic pain model was carried out as described previously [15,16]. Before surgery, general anesthesia was introduced in the rats. For the SNL model, the unilateral lumbar 5 (L5) spinal nerve was tightly ligated with 4–0 silk thread and transected distal to the ligature and isolated from the adjacent nerves. For the CCI model, the unilateral sciatic nerve was exposed and loosely ligated with 4–0 silk thread at four sites with an interval of about 1.0 mm proximal to trifurcation of the sciatic nerve. The sham-operated group underwent identical procedures but without ligation and/or transection of the respective nerve.
2.3. Intrathecal catheter implantation and drug administration
Intrathecal catheter implantation was carried out as described previously [17,18]. With the rats under isoflurane anesthesia, about 1 cm of polyethylene-10 (PE-10) catheter was inserted into the subarachnoid space from the intervertebral foramen between the L4 and L5 vertebrae. The residual catheter was anchored to the muscles and tunneled under the skin to the neck area. The outer part of the catheter was exposed, carefully plugged, and fixed onto the skin for drug administration. Any animals with postoperative neurologic deficits (e.g., paralysis) or poor grooming habits after surgery were excluded from this study. After 7 days of recovery, SNL, CCI, or sham surgery was carried out. Saline or BIX01294 (Cayman Chemical, Ann Arbor, MI) was intrathecally injected 30 min before surgery and once daily for 6 days post-surgery starting on post-operative day 1, or once daily for 5 days staring on post-operative day 7.
2.4. Mechanical test
Paw withdrawal thresholds (PWTs) in response to mechanical stimuli (calibrated von Frey filaments) were measured with the up-down testing paradigm described previously [16,17]. Each rat was placed in a Plexiglas chamber on an elevated mesh screen. After acclimation in the environment, the calibrated von Frey filaments in log increments of force (0.69, 1.20, 2.04, 3.63, 5.50, 8.51, 15.14, and 26.00 g) were applied onto the plantar surface of the rats’ hind paws. The 2.04-g von Frey filament was the first to be applied. If a positive response occurred, the next lower force von Frey filament was used; if a negative response was observed, the next higher force one was used. The test was terminated when either one of below conditions occurred: (i) a negative response was obtained with the 26.00-g hair or (ii) three stimuli were applied after the first positive response. PWT was calculated by converting the pattern of positive and negative responses to the von Frey filament stimulation to a 50% threshold value with a formula provided by Dixon and Chaplan [19,20].
2.5. Thermal test
Paw withdrawal latencies (PWLs) to noxious heat were measured with a Model 336 Analgesia Meter (IITC Inc. Life Science Instruments. Woodland Hills, CA) as described previously [15–17]. Before testing, rats were placed in a Plexiglas chamber on a glass for 1 h each day for 3 days to acclimate for the environment. A beam of light that provided radiant heat was aimed at the middle of the plantar surface of each hind paw through the glass plate. When the animal lifted its foot, the light beam was turned off automatically. PWL was defined as the time (s) between the start of the light beam and the foot response. The cutoff time is 20 s to avoid tissue damage. Each trial was repeated five times at 5-min interval for each side.
2.6. Cold plate test
Paw withdrawal latencies (PWLs) to noxious cold (0 °C) were measured with a cold aluminum plate, which was monitored continuously by a thermometer [16,17]. The time started once the rat was placed on the cold aluminum plate in the Plexiglas chamber and stopped when the first paw flinching response was observed. Only one hind paw on the ipsilateral side was recorded in each rat. A cutoff time of 60 s was used to avoid tissue damage if no paw flinching was observed. The length of time that rat stayed on the plate was defined as PWL. Three trials were tested at 15-min interval.
2.7. Locomotor functions
The following locomotor function tests were performed: (1) placing reflex: the rat was held with the hind limbs slightly lower than the forelimbs, and the dorsal surfaces of the hind paws were brought into contact with the edge of a table. The experimenter recorded whether the hind paws were placed on the table surface reflexively; (2) grasping reflex: the rat was placed on a wire grid, and the experimenter recorded whether the hind paws grasped the wire on contact; (3) righting reflex: The rat was placed on its back on a flat surface, and the experimenter recorded whether it immediately assumed the normal upright position. Scores for placing, grasping, and righting reflexes were recorded based on counts of each normal reflex exhibited in five trials.
2.8. Western blotting
Western blot analysis was performed as described previously [5,6,16]. Briefly, to achieve enough proteins, two ipsilateral L5 DRGs from 2 rats were pooled together. DRGs were homogenized in chilled lysis buffer (10 mM Tris, 1 mM phenylmethylsulfonyl fluoride, 5 mM MgCl2, 5 mM EGTA, 1 mM EDTA, 1 mM DTT, 40 μM leupeptin, 250 mM sucrose). After centrifugation at 4 °C for 15 min at 1,000 g, the pellet was collected for nuclear proteins and sonicated in lysis buffer with 1% sodium dodecyl sulfate (SDS) and 0.1% TritonX-100 by ultrasonic. The protein concentration of each sample was measured using the Bio-Rad protein assay (Bio-Rad Laboratories, Hercules, CA). The samples were heated at 99 °C for 5 min before loading onto a 4–15% stacking/7.5% separating SDS-polyacrylamide gel (Bio-Rad). The proteins were then electrophoretically transferred onto a polyvinylidene difluoride membrane (Bio-Rad). After the membranes were blocked with 3% nonfat milk in Tris-buffered saline containing 0.1% Tween-20 for 1 h, rabbit anti-H3K9me2 (1:500, EMD Millipore, Darmstadt, Germany), or rabbit anti-histone H3 (1:1,000, Cell Signaling, Danvers, MA) were incubated at 4 °C overnight. The proteins were detected by horseradish peroxidase-conjugated anti-rabbit secondary antibody (1:3,000, Jackson ImmunoResearch, West Grove, PA), developed by western peroxide reagent and luminol/enhancer reagent (Clarity Western ECL Substrate, Bio-Rad) and visualized by ChemiDoc XRS and System with Image Lab software (Bio-Rad). The intensity of blots was quantified with densitometry using Image Lab software (Bio-Rad). The bands labelled by H3K9me2 were normalized to their corresponding histone H3.
2.9. Quantitative real-time RT-PCR
Two ipsilateral L5 DRGs from 2 rats were pooled together to achieve enough RNA. Total RNA was extracted by the Trizol method (Invitrogen/ThermoFisher Scientific), treated with DNase I (New England Biolabs, Ipswich, MA), and reverse-transcribed using the ThermoScript reverse transcriptase by oligo (dT) primer (Invitrogen/ThermoFisher Scientific, Grand Island, NY). Template (1 µl) was amplified by real-time PCR by using the primers for Oprm1, Oprk1, Oprd1, Kcna2, Kcna4 and Gapdh mRNAs (Integrated DNA Technologies, Coralville, IA). All primes’ sequences are listed in Table 1. Each sample was run in triplicate in a 20 μL reaction with 250 nM forward and reverse primers, 10 µl of SsoAdvanced Universal SYBR Green Supermix (Bio-Rad) and 20 ng of cDNA. Reactions were performed in a BIO-RAD CFX96 real-time PCR system. Ratios of ipsilateral-side mRNA levels to contralateral-side mRNA levels were calculated using the ΔCt method (2-ΔCt). Glyceraldehyde-3-phosphate dehydrogenase (Gapdh) was used as an internal control for normalization. All data were normalized to Gapdh, which has been demonstrated to be stable, even after peripheral nerve injury insult [15,16].
Table 1:
Primers used.
| Genes | Forward primer sequences (5’–3’) | Reverse primer sequences (5’–3’) |
|---|---|---|
| Oprm1 | TTCCTGGTCATGTATGTGATTGT | GGGCAGTGTACTGGTCGCTAA |
| Oprk1 | TTTGTGGTGGGCTTAGTGGG | CTCTGGAAGGGCATAGTGGT |
| Oprd1 | GGGTCTTGGCTTCAGGTGTT | ACGGTGATGATGAGAATGGG |
| Kcna2 | CCCATCTGCAAGGGCAACGT | CACAGCCTCCTTTGGCTGGC |
| Kcna4 | CCCATACCTACCTTCTAATTTGC | TGTTTTTATCTGTCTCGCTGTCA |
| Gapdh | TCGGTGTGAACGGATTTGGC | CCTTCAGGTGAGCCCCAGC |
2.10. Statistical analysis
The rats were randomly distributed into various treatment groups. All of the results were given as means ± SEM. The data were statistically analyzed with two-way Repeated Measure (RM) Analysis of Variance (ANOVA) in behavior tests or one-way ANOVA in Western blotting analysis and quantitative RT-PCR experiments. When ANOVA showed a significant difference, pairwise comparisons between means were tested by the post hoc Turkey method (SigmaPlot 12.5, San Jose, CA). Significance was set at P < 0.05.
3. Results
3.1. Intrathecal BIX01294 attenuates pain hypersensitivities in the development of neuropathic pain
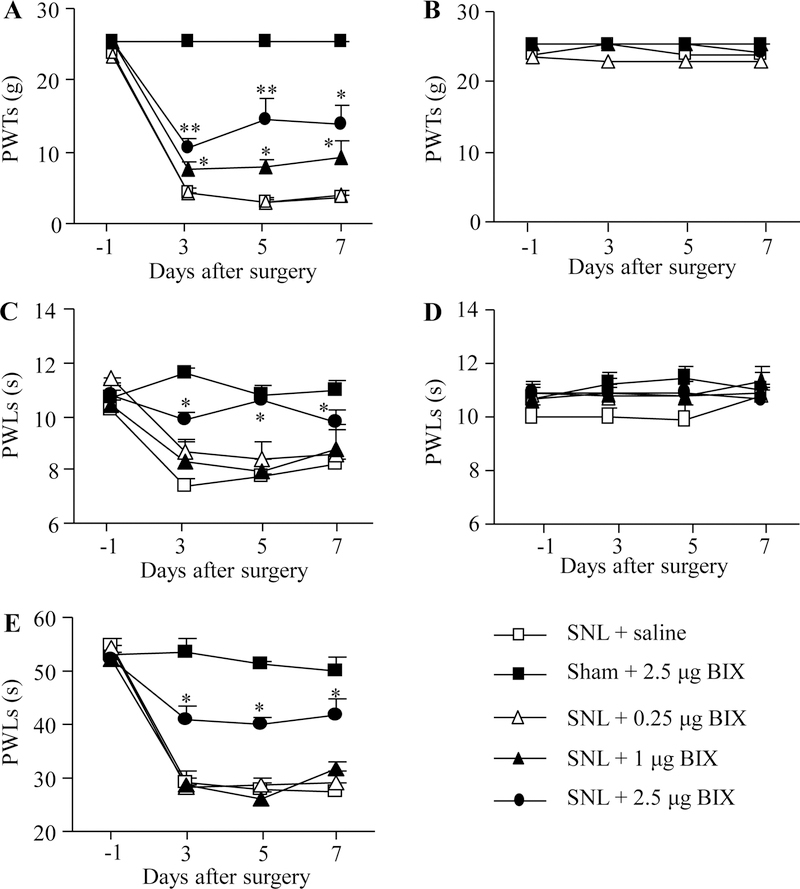
Firstly, we examined the effect of intrathecal BIX01294 on the SNL-induced pain hypersensitivities in the development of neuropathic pain. BIX01294 or saline was intrathecally administrated 30 min prior to surgery on the first day and then once daily for 6 days post-surgery. In concordance with previous data [15,16], SNL led to mechanical allodynia, thermal hyperalgesia and cold allodynia on the ipsilateral (but not contralateral) side of the saline-treated group on days 3, 5 and 7 post-SNL (Fig. 1A–E). These pain hypersensitivities were attenuated by pre-intrathecal injection of BIX01294, in a dose-dependent fashion (Fig. 1A, C, E). Paw withdrawal thresholds (PWTs) to mechanical stimulation and paw withdrawal latencies (PWLs) to thermal and cold stimuli were significantly higher in the 2.5 μg BIX01294-treated group than those in the saline-treated group on the ipsilateral side from days 3 to 7 post-SNL (Fig. 1A, C, E). BIX01294 at 1 μg only markedly reversed the SNL-induced decrease in the PWTs to mechanical stimuli (Fig. 1A), but had no effect on the SNL-induced reductions in the PWLs to thermal or cold stimuli on the ipsilateral side (Fig. 1C, E). BIX01294 at 0.25 μg did not affect the SNL-induced reductions in both PWTs and PWLs on the ipsilateral side. BIX01294 at the highest dose (2.5 μg) did not affect locomotor function (Table 2) or basal paw withdrawal responses on the contralateral side in SNL rats and on both ipsilateral and contralateral sides in sham rats (Fig. 1A–E).
Figure 1.
Effect of intrathecal pre-administration of BIX01294 (BIX) on the SNL-induced pain hypersensitivities during the development period. BIX01294 at three doses (0.25, 1, and 2.5 μg) or saline was administered intrathecally starting 30 min before SNL or sham surgery and once daily for 6 days after surgery. Behavioral tests were performed one day before sham or SNL surgery and on days 3, 5, and 7 after surgery. (A, B) Effect of BIX01294 or saline on paw withdrawal thresholds (PWTs) to mechanical stimulation on the ipsilateral (A) and contralateral (B) sides. (C, D) Effect of BIX01294 or saline on paw withdrawal latencies (PWLs) to thermal stimulation on the ipsilateral (C) and contralateral (D) sides. (E) Effect of BIX01294 or saline on paw withdrawal latencies (PWLs) to cold stimulation on the ipsilateral side. N = 8 rats/group. *P < 0.05 or **P < 0.01 vs. the saline-treated SNL rats at the corresponding time points by two-way RM ANOVA followed by post hoc Tukey test.
Table 2:
Locomotor function tests.
| Groups | Placing | Grasping | Righting |
|---|---|---|---|
| Sham + Saline | 5(0) | 5(0) | 5(0) |
| Sham + 2.5 μg BIX | 5(0) | 5(0) | 5(0) |
| SNL + Saline | 5(0) | 5(0) | 5(0) |
| SNL + 2.5 μg BIX | 5(0) | 5(0) | 5(0) |
N = 8 rats/group; 5 trials. SNL: spinal nerve ligation; BIX: BIX01294.
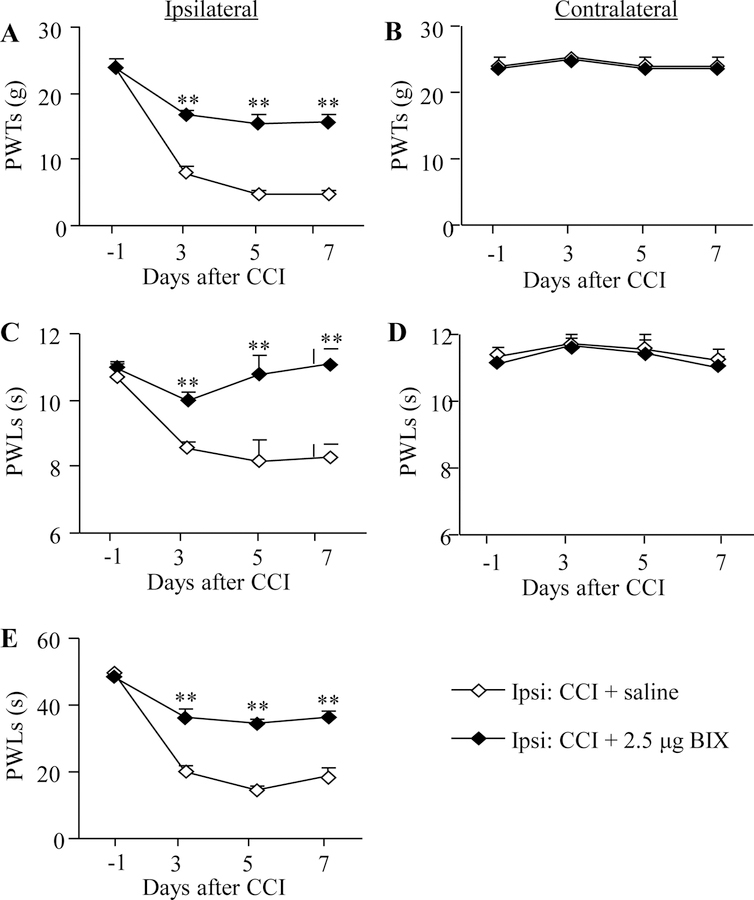
A similar anti-nociceptive effect of BIX01294 at the highest dose (2.5 μg) was also seen in the development of CCI-induced neuropathic pain (Fig. 2A, C, E). Like SNL, CCI produced significant decreases in PWTs to mechanical stimulation and PWLs to thermal or cold stimuli on the ipsilateral (but not contralateral) side (Fig. 2A–E). The PWTs to mechanical stimulation and PWLs to thermal or cold stimuli were significantly higher in the BIX01294-treated group than those in the saline-treated group on the ipsilateral side on days 3, 5 and 7 post-CCI (Fig. 2A, C, E). BIX01294 at the dose used had no effect on basal paw withdrawal responses on the contralateral side in the CCI rats (Fig. 2B, D).
Figure 2.
Effect of intrathecal pre-administration of BIX01294 (BIX) on the CCI-induced pain hypersensitivities during the development period. BIX01294 at the dose of 2.5 μg or saline was administered intrathecally starting 30 min before CCI surgery and once daily for 6 days after CCI surgery. Behavioral tests were carried out one day before CCI surgery and on days 3, 5, and 7 post-CCI. (A, B) Effect of BIX01294 or saline on paw withdrawal thresholds (PWTs) to mechanical stimulation on the ipsilateral (A) and contralateral (B) sides. (C, D) Effect of BIX01294 or saline on paw withdrawal latencies (PWLs) to thermal stimulation on the ipsilateral (C) and contralateral (D) sides. (E) Effect of BIX01294 or saline on paw withdrawal latencies (PWLs) to cold stimulation on the ipsilateral side. N = 8 rats/group. **P < 0.01 vs. the saline-treated CCI rats at the corresponding time points by two-way RM ANOVA followed by post hoc Tukey test.
3.2. Intrathecal BIX01294 attenuates pain hypersensitivities in the maintenance of neuropathic pain
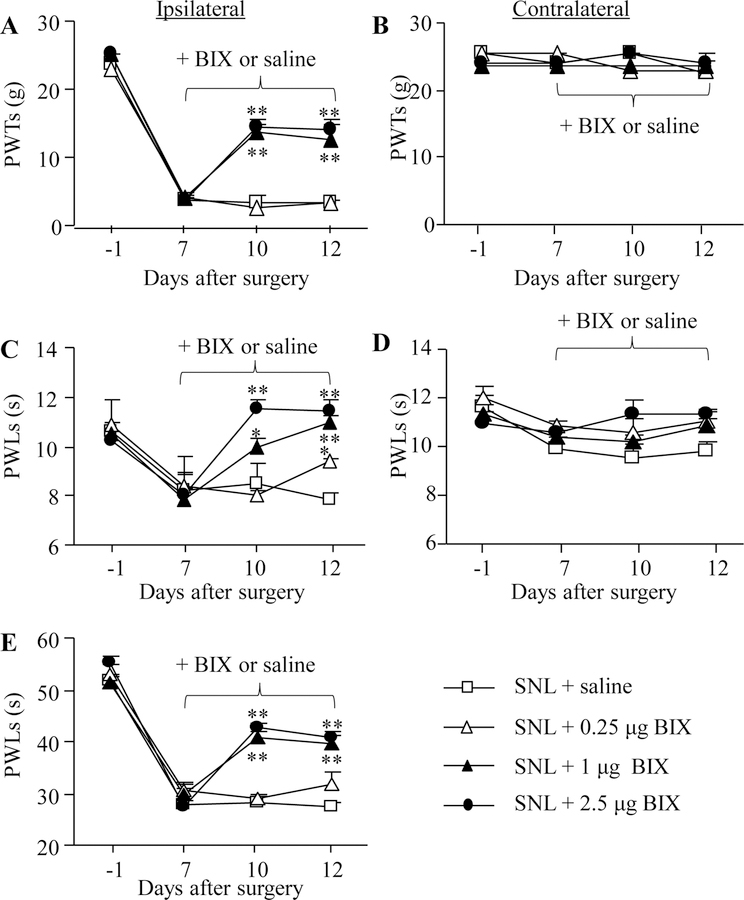
To test the effect of intrathecal BIX01294 on neuropathic pain maintenance, we intrathecally administered BIX01294 for 5 days starting on day 7 post-surgery. Behavioral tests were carried out one day before surgery, on day 7 before BIX01294 or saline administration and on days 10 and 12 after surgery. Mechanical allodynia, thermal hyperalgesia and cold allodynia were dose-dependently diminished by intrathecal BIX01294 on days 10 and 12 post-SNL (Fig. 3A, C, E). The PWTs to mechanical stimulation and PWLs to thermal or cold stimuli were significantly higher in the 2.5 μg and 1 μg BIX01294-treated groups than those in the saline-treated group on the ipsilateral side on days 10 and 12 post-SNL (Fig. 3A, C, E). BIX01294 at 0.25 μg only significantly reversed the SNL-induced decrease in PWLs to thermal stimulation on day 12 post-SNL. BIX01294 at any doses used did not affect basal paw withdrawal responses on the contralateral side (Fig. 3B, D).
Figure 3.
Effect of intrathecal pre-administration of BIX01294 (BIX) on the SNL-induced pain hypersensitivities during the maintenance period. BIX01294 at three doses (0.25, 1, and 2.5 μg) or saline was administered intrathecally once daily for 5 days starting on day 7 post-SNL. Behavioral tests were performed one day before SNL surgery, prior to BIX01294 or saline injection on day 7 post-SNL and on days 10 and 12 post-SNL. (A, B) Effect of BIX01294 or saline on paw withdrawal thresholds (PWTs) to mechanical stimulation on the ipsilateral (A) and contralateral (B) sides. (C, D) Effect of BIX01294 or saline on paw withdrawal latencies (PWLs) to thermal stimulation on the ipsilateral (C) and contralateral (D) sides. (E) Effect of BIX01294 or saline on paw withdrawal latencies (PWLs) to cold stimulation on the ipsilateral side. N = 8 rats/group. *P < 0.05 or **P < 0.01 vs. the saline-treated SNL rats at the corresponding time points by two-way RM ANOVA followed by post hoc Tukey test.
3.3. Intrathecal BIX01294 inhibits G9a activity and reverses some pain-related gene expression in the injured DRG of SNL rats
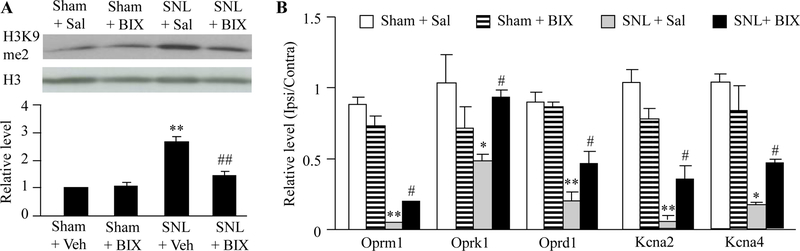
The evidence from our studies confirms observations from previous literature and demonstrated that peripheral nerve injury increased the level of H3K9me2, a G9a substrate that reflects G9a activity, and produced G9a-controlled downregulation of opioid receptors and potassium channels in the injured DRG [4–6,9]. We finally determined whether the anti-nociceptive effect caused by intrathecal BIX01294 was attributed to the inhibition of nerve injury-induced increases in G9a activity and the rescue of G9a-controled downregulation of opioid receptor genes (Oprm1 mRNA, Oprk1 mRNA and Oprd1 mRNA) and potassium channels (Kcna2 mRNA and Kcna4 mRNA) in the injured DRG. As expected, intrathecal pretreatment with BIX01294 at 2.5 μg significantly blocked the SNL-induced increase in the amounts of H3K9me2 in the ipsilateral L5 DRG on day 7 post-SNL, although it had no effect on basal level of H3K9me2 in ipsilateral L5 DRG on day 7 post-sham surgery (Fig. 4A). Consistent with the previous studies [4–6,9], the amounts of Oprm1, Oprk1, Oprd1, Kcna2 and Kcna4 mRNAs were dramatically reduced in the ipsilateral L5 DRG of sham rats on day 7 post-SNL compared with saline-treated sham rats (Fig. 4B). These reductions were significantly reversed by intrathecal pre-treatment of BIX01294 (Fig. 4B).
Figure 4.
Effect of intrathecal pre-administration of BIX01294 (BIX) on SNL-induced increase in H3K9me2 expression and decreases in the levels of opioid receptor genes and some potassium channel genes in the ipsilateral lumbar 5 (L5) DRGs. BIX01294 at the dose of 2.5 μg or saline was administered intrathecally starting 30 min before SNL or sham surgery and once daily for 6 days after surgery. (A) Effect of BIX01294 or saline on H3K9me2 expression in the ipsilateral L5 DRG on day 7 post-SNL or sham surgery. (B) Effect of BIX01294 or saline on the levels of Oprm1, Oprk1, Oprd1, Kcna2, Kcna4 and Kcna1 mRNAs in the ipsilateral L5 DRG on day 7 post-SNL or sham surgery. N = 6 rats/group. *P < 0.05 or **P < 0.01 vs. the saline-treated sham rats. #P < 0.05 or ##P < 0.01 vs. the saline-treated SNL rats. One-way ANOVA followed by post hoc Tukey test.
4. Discussion
In this study, we found that intrathecal BIX01294, a specific inhibitor of G9a [13,14], alleviated SNL- or CCI-induced pain hypersensitivities in the development and maintenance of neuropathic pain. In addition, intrathecal BIX01294 blocked SNL-induced the increase of H3K9me2 and rescued the expression of Oprm1, Oprk1, Oprd1, Kcna2 and Kcna4 mRNAs in the injured DRG. These findings further confirmed the role of DRG G9a in neuropathic pain.
G9a, a histone methyltransferase, methylates histone H3 on lysine residue 9 (H3K9) to produce dimethylation (H3K9me2) and, consequently, results in condensed chromatin and gene transcriptional repression [11,12]. Several studies have provided convincing evidence that G9a in the injured DRG contributes neuropathic pain by epigenetically regulating the pain-related gene expression. G9a knockdown or knockout in the injured DRG attenuated the development of neuropathic pain in rats or mice [4,6]. However, these genetic strategies have limitations in clinical application. Laumet G et al reported that intrathecal G9a inhibitors partially blocked SNL–induced mechanical allodynia starting from 3 weeks post-SNL [21]. However, whether these inhibitors affected heat hyperalgesia and cold allodynia in induction and maintenance of neuropathic pain is unknown. In addition, whether pharmacological G9a inhibition, like genetic knockout/knockdown of DRG G9a, blocks the development of nerve injury-induced mechanical allodynia is unclear. The present study provided further evidence that intrathecal administration of BIX01294 significantly mitigated nerve injury-induced mechanical allodynia, thermal hyperalgesia and cold allodynia during both development and maintenance periods. Although BIX01294 was administered daily for 5 days, it provided full anti-nociceptive effect on day 3 post-SNL or CCI. It appears that a shorter treatment of BIX01294 might produce similar effect. These findings demonstrate that G9a is likely a potential target in neuropathic pain management.
Peripheral nerve injury leads to abnormal ectopic firing in the neuromas at the injured site and DRG neurons. This ectopic firing is considered to participate in neuropathic pain genesis [22,23]. The potassium channels are crucially involved in controlling the membrane potential and excitability of DRG neurons [10,16,24]. Nerve injury-induced downregulation of many potassium channel genes, including Kcna2 mRNA and Kcna4 mRNA, in the injured DRG contributes to neuropathic pain genesis [6,10,16,25,26]. The anti-nociceptive effect of DRG G9a knockdown/knockout/inhibition on neuropathic pain is likely due to the expressional rescue of potassium channels, such as Kcna2, Kcna4, Kcnd2, Kcnq2 and Kcnma1 mRNAs in the injured DRG [4,6]. This conclusion is further supported by our observation that G9a activity inhibition in the injured DRG caused by intrathecal BIX01294 attenuated neuropathic pain development and maintenance possibly through rescuing the expression of Kcna2 and Kcna4 mRNAs in the injured DRG.
In addition, nerve injury-induced downregulation of opioid receptor genes in the injured DRG also participates in neurobiological mechanisms of neuropathic pain genesis and may be responsible, at least in part, for the reduced analgesic effects of opioids in neuropathic pain patients [27–29]. This downregulation is also controlled by nerve injury-induced increase in DRG G9a, as blocking increased G9a in the injured DRG reversed the expression of Oprm1, Oprk1 and Oprd1 mRNAs in the ipsilateral L5 DRG, attenuated opioid receptors-controlled primary afferent neurotransmitter release, enhanced morphine analgesic effect and delayed morphine analgesic tolerance [5,9]. Therefore, the anti-nociceptive effect caused by intrathecal injection of BIX01294 may also be attributed to the rescue of the nerve injury-induced downregulation of Oprm1, Oprk1 and Oprd1 mRNAs in the injured DRG. It should be noted that intrathecal BIX01294 partially, but not fully, attenuated nerve injury-induced pain hypersensitivities. This partial effect may be related to multiple mechanisms by which opioid receptor genes and potassium channel genes are downregulated under neuropathic pain conditions. Besides the role of G9a, DNA methyltransferases (DNMTs), such as DNMT3a and DNMT1, also participated in nerve injury-induced silencing of Kcna2 mRNA, Oprm1 mRNA and Oprd1 mRNA in the injured DRG [7,8,10,15,30]. Nerve injury-induced downregulation of Kcna2 mRNA might also be related to nerve injury-induced upregulation of endogenous long non-coding Kcna2 antisense RNA in the injured DRG [15,16]. Therefore, the combined different strategies that target distinct epigenetic mechanisms underlying neuropathic pain may produce profound effects in management of this disorder.
5. Conclusions
The present study demonstrated the anti-nociceptive effect of the G9a inhibitor BIX01294 on both the development and maintenance of neuropathic pain. Our finding that intrathecal administration of BIX01294 at the study dosage did not affect acute/basal pain responses and locomotor function, suggesting that BIX01294 may have potential application for neuropathic pain treatment in clinic.
Acknowledgements
This work was supported by the National Institutes of Health grants (NS111553, NS094664 and NS094224) to Y.X.T.
Footnotes
Conflict of interest statement
The author(s) declared no compete financial interests.
References
- [1].O’Connor AB. Neuropathic pain: quality-of-life impact, costs and cost effectiveness of therapy. Pharmacoeconomics 2009; 27(2): 95–112. [DOI] [PubMed] [Google Scholar]
- [2].Wu S, Marie LB, Miao X, Liang L, Mo K, Chang YJ et al. Dorsal root ganglion transcriptome analysis following peripheral nerve injury in mice. Mol Pain 2016; 12. [DOI] [PMC free article] [PubMed]
- [3].Xiao HS, Huang QH, Zhang FX, Bao L, Lu YJ, Guo C et al. Identification of gene expression profile of dorsal root ganglion in the rat peripheral axotomy model of neuropathic pain. Proc Natl Acad Sci U S A 2002; 99(12): 8360–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Laumet G, Garriga J, Chen SR, Zhang Y, Li DP, Smith TM et al. G9a is essential for epigenetic silencing of K(+) channel genes in acute-to-chronic pain transition. Nat Neurosci 2015; 18(12): 1746–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Liang L, Zhao JY, Gu X, Wu S, Mo K, Xiong M et al. G9a inhibits CREB-triggered expression of mu opioid receptor in primary sensory neurons following peripheral nerve injury. Mol Pain 2016; 12. [DOI] [PMC free article] [PubMed]
- [6].Liang L, Gu X, Zhao JY, Wu S, Miao X, Xiao J et al. G9a participates in nerve injury-induced Kcna2 downregulation in primary sensory neurons. Sci Rep 2016; 6: 37704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Sun L, Zhao JY, Gu X, Liang L, Wu S, Mo K et al. Nerve injury-induced epigenetic silencing of opioid receptors controlled by DNMT3a in primary afferent neurons. Pain 2017; 158(6): 1153–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Sun L, Gu X, Pan Z, Guo X, Liu J, Atianjoh FE et al. Contribution of DNMT1 to neuropathic pain genesis partially through epigenetically repressing Kcna2 in primary afferent neurons. J Neurosci 2019. [DOI] [PMC free article] [PubMed]
- [9].Zhang Y, Chen SR, Laumet G, Chen H, Pan HL. Nerve Injury Diminishes Opioid Analgesia through Lysine Methyltransferase-mediated Transcriptional Repression of mu-Opioid Receptors in Primary Sensory Neurons. J Biol Chem 2016; 291(16): 8475–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Zhao JY, Liang L, Gu X, Li Z, Wu S, Sun L et al. DNA methyltransferase DNMT3a contributes to neuropathic pain by repressing Kcna2 in primary afferent neurons. Nat Commun 2017. [DOI] [PMC free article] [PubMed]
- [11].Kouzarides T Chromatin modifications and their function. Cell 2007; 128(4): 693–705. [DOI] [PubMed] [Google Scholar]
- [12].Shinkai Y, Tachibana M. H3K9 methyltransferase G9a and the related molecule GLP. Genes Dev 2011; 25(8): 781–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kubicek S, O’Sullivan RJ, August EM, Hickey ER, Zhang Q, Teodoro ML et al. Reversal of H3K9me2 by a small-molecule inhibitor for the G9a histone methyltransferase. Mol Cell 2007; 25(3): 473–81. [DOI] [PubMed] [Google Scholar]
- [14].Park KE, Johnson CM, Cabot RA. IVMBIX-01294, an inhibitor of the histone methyltransferase EHMT2, disrupts histone H3 lysine 9 (H3K9) dimethylation in the cleavage-stage porcine embryo. Reprod Fertil Dev 2012; 24(6): 813–21. [DOI] [PubMed] [Google Scholar]
- [15].Li Z, Gu X, Sun L, Wu S, Liang L, Cao J et al. Dorsal root ganglion myeloid zinc finger protein 1 contributes to neuropathic pain after peripheral nerve trauma. Pain 2015; 156(4): 711–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Zhao X, Tang Z, Zhang H, Atianjoh FE, Zhao JY, Liang L et al. A long noncoding RNA contributes to neuropathic pain by silencing Kcna2 in primary afferent neurons. Nat Neurosci 2013; 16(8): 1024–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Zhang J, Liang L, Miao X, Wu S, Cao J, Tao B et al. Contribution of the Suppressor of Variegation 3–9 Homolog 1 in Dorsal Root Ganglia and Spinal Cord Dorsal Horn to Nerve Injury-induced Nociceptive Hypersensitivity. Anesthesiology 2016; 125(4): 765–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Xu JT, Zhou X, Zhao X, Ligons D, Tiwari V, Lee CY et al. Opioid receptor-triggered spinal mTORC1 activation contributes to morphine tolerance and hyperalgesia. J Clin Invest 2014; 124: 592–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 1994; 53(1): 55–63. [DOI] [PubMed] [Google Scholar]
- [20].Dixon WJ. Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol 1980; 20: 441–62. [DOI] [PubMed] [Google Scholar]
- [21].Laumet G, Garriga J, Chen SR, Zhang Y, Li DP, Smith TM et al. G9a is essential for epigenetic silencing of K(+) channel genes in acute-to-chronic pain transition. Nat Neurosci 2015; 18(12): 1746–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Campbell JN, Meyer RA. Mechanisms of neuropathic pain. Neuron 2006; 52(1): 77–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain 2009; 10(9): 895–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Vydyanathan A, Wu ZZ, Chen SR, Pan HL. A-type voltage-gated K+ currents influence firing properties of isolectin B4-positive but not isolectin B4-negative primary sensory neurons. J Neurophysiol 2005; 93(6): 3401–9. [DOI] [PubMed] [Google Scholar]
- [25].Cao XH, Byun HS, Chen SR, Cai YQ, Pan HL. Reduction in voltage-gated K+ channel activity in primary sensory neurons in painful diabetic neuropathy: role of brain-derived neurotrophic factor. J Neurochem 2010; 114(5): 1460–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Rasband MN, Park EW, Vanderah TW, Lai J, Porreca F, Trimmer JS. Distinct potassium channels on pain-sensing neurons. Proc Natl Acad Sci U S A 2001; 98(23): 13373–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kohno T, Ji RR, Ito N, Allchorne AJ, Befort K, Karchewski LA, Woolf CJ. Peripheral axonal injury results in reduced mu opioid receptor pre- and post-synaptic action in the spinal cord. Pain 2005; 117(1–2): 77–87. [DOI] [PubMed] [Google Scholar]
- [28].Lee CY, Perez FM, Wang W, Guan X, Zhao X, Fisher JL et al. Dynamic temporal and spatial regulation of mu opioid receptor expression in primary afferent neurons following spinal nerve injury. Eur J Pain 2011; 15(7): 669–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Obara I, Parkitna JR, Korostynski M, Makuch W, Kaminska D, Przewlocka B, Przewlocki R. Local peripheral opioid effects and expression of opioid genes in the spinal cord and dorsal root ganglia in neuropathic and inflammatory pain. Pain 2009; 141(3): 283–91. [DOI] [PubMed] [Google Scholar]
- [30].Mo K, Wu S, Gu X, Xiong M, Cai W, Atianjoh FE et al. MBD1 Contributes to the Genesis of Acute Pain and Neuropathic Pain by Epigenetic Silencing of Oprm1 and Kcna2 Genes in Primary Sensory Neurons. J Neurosci 2018; 38(46): 9883–99. [DOI] [PMC free article] [PubMed] [Google Scholar]