Abstract
Individuals of African descent are disproportionately affected by specific complex diseases, such as breast and prostate cancer, which are driven by both biological and non-biological factors. In the case of breast cancer, there is clear evidence that psychosocial factors (environment, socio-economic status, health behaviors, etc.) have a strong influence on racial disparities. However, even after controlling for these factors, overall phenotypic differences in breast cancer pathology remain among groups of individuals who vary by geographic ancestry. There is a growing appreciation that chronic/re-occurring inflammation, primarily driven by mechanisms of innate immunity, contributes to core functions associated with cancer progression. Germline mutations in innate immune genes that have been retained in the human genome offer enhanced protection against environmental pathogens and protective innate immune variants against specific pathogens are enriched among populations whose ancestors were heavily exposed to those pathogens. Consequently, it is predicted that racial/ethnic differences in innate immune programs will translate into ethnic differences in both pro- and antitumor immunity, tumor progression, and prognosis, leading to the current phenomenon of racial/ethnic disparities in cancer. This review explores examples of protective innate immune genetic variants that are 1) distributed disproportionately among racial populations and 2) associated with racial/ethnic disparities of breast and prostate cancer.
Keywords: cancer disparities, Toll-like receptors, single nucleotide polymorphism (SNP), innate immunity, genetic variation, geographical ancestry
Introduction
The Human Genome Project and the discovery of distinctive genetic variations across patient populations associated with geography has shaped our genetic analysis and improved our understanding of disparities in complex diseases among different populations. In particular, the use of geographical ancestry, defined as the flow of genetic information in distinct populations over time and geography, aids in delineating the genetic variations that could explain observed differences in cancer incidence and progression among various populations. We sought to link innate immune variants with racial/ethnic disparities in cancer by describing examples of genetic variants unequally distributed among ethnic populations, which paradoxically protect against infection but impacts cancer incidence and progression.
Inflammation and specific cancers among individuals of African descent
Individuals of African descent, as identified by Ancestry Informative Markers (AIMS) and those that self-identify as African American, suffer disproportionately from specific forms of cancer, cardiovascular disease, inflammatory and autoimmune disease, and neurological dysfunction. Complex diseases are affected by both biological and non-biological factors and, in many cases, the biological (genetic) contributors to disease disparities are less clearly understood than psychosocial factors such as environment, socio-economic status, and health behavior. This article explores evidence that protective innate immune variants contribute to racial disparities in cancer, such as those that occur among individuals of African descent, including colorectal cancer (1) and multiple myeloma (2) in both men and women, breast (3) and uterine (4) cancer in women, and prostate, stomach, and lung cancer in men (5). Except for multiple myeloma, all these tissues have a relatively high exposure to infectious agents that require a strong innate immune defense. The complex association between cancer and inflammation is an increasingly active area of research [reviewed in (6,7)]. More specialized reviews address the relationship between inflammation and/or innate immunity and breast (8), colorectal (9), prostate (10–12), lung (13,14), stomach (15,16), and ovarian (17) cancers. The specific role of innate immunity (and/or members of the toll-like receptor (TLR) family as some of the most common representatives) in tumor progression among these cancers has also received attention (12,18,19). A meta-analysis consisting of 64,591 cancer patients and 74,467 controls of European descent demonstrated that 925 sequence variants in 173 innate immune response markers were significantly associated with lung, ovarian, prostate, breast, and colorectal cancer (20). Unfortunately, few observational studies have tested the hypothesis that genomic aberrations in innate immune response genes are linked to racial/ethnic disparities of cancer.
Inflammation and racial/ethnic disparities in cancer
African American women suffer disproportionately from more aggressive forms of breast cancer (21,22). Both biological and non-biological factors contribute to this disparity, although the relative impact of these factors on breast cancer morbidity and mortality is a matter of debate [reviewed in (3)]. Non-biological factors that contribute to African American disparities in breast cancer include low socio-economic status, limited access to health care, substandard living environments, and nutrient-depleted/high-fat diets [reviewed in (23)]. Nevertheless, several studies indicate that fundamental biological differences are involved in breast cancer health disparities after controlling for differences in socio-economic status, access to healthcare/treatment, and delays in treatment following diagnosis [c.f., (24,25)]. Breast tumors display a high degree of molecular heterogeneity within and between molecular subtypes, which vary by phenotype and prognosis [Cancer Genome Atlas Network, 2012; (26)]. However, the most aggressive breast cancers (i.e., those most commonly found among African American women) are associated with inflammation [reviewed in (27)], and the role of inflammation in breast cancer disparities has become a growing topic of interest [reviewed in (8,28)].
Several lines of evidence are consistent with the idea that variations in innate immune–related genes contribute to breast cancer disparities. First, Elledge and colleagues observed racial disparities in breast cancer survival among black, white, and Hispanic women that existed only when comparing women who had lymph node–positive, locally advanced, and metastatic breast cancers (29). Second, small-sized breast tumors (<2.0 cm) metastasize more extensively among African American women relative to their European counterpart due to what is described as “intrinsic biological differences,” which are indicators of aggressive cancers (i.e., lymph node involvement and distant metastases). Although the source of these differences was not identified, both estrogen receptor status (another marker of breast cancer aggressiveness) and income were ruled out (25). Third, inflammatory breast cancer (IBC) has significantly higher incidence rates and results in shorter lifespans among African Americans compared to European Americans, respectively, based on a meta-analysis of 180,224 breast cancer patients (23). Similarly, African American ancestry, but not Hispanic ancestry (determined by self-identification) or socio-economic status, was identified as an independent predictor of poor prognosis among a cohort of 935 women diagnosed with IBC between 1998 and 2002 (30). Finally, ample evidence exists showing that the frequency distribution of gene sequence variants detected in innate (28) and adaptive (31) immunity differs between breast cancer patients of African and European descent.
Inflammation due to tissue damage or pathogen infection is the result of a coordinated, interdependent protective response that involves both innate and adaptive immunity. Unlike adaptive immunity that requires days to mount a sustained and highly specific inflammatory response, innate immune defense is mobilized immediately. Importantly, the rapid response characteristic of innate immunity can only be achieved by using a pre-determined set of genes that code for products immediately capable of responding to pathogens. The heavy dependence of innate immunity on genetic heritability suggests that it is the contribution of innate, not adaptive, immunity to the mechanisms of inflammation that are inherent in complex disease disparities (32). From the standpoint of population genetics, survival requires genetic adaptation in innate immune defense to counteract the high rate of microbial evolution (33). The need for modifications or genetic variation in innate immune defense is consistent with 1) studies that show these genes are under greater selective pressure than any other class of proteins in the human genome (34,35) and 2) studies that show this selective pressure is pathogen-driven (36,37). Malaria provides a well-characterized example of selective pressure by a pathogen on the development and diversity of innate immune variants, such as sickle-cell hemoglobin (HbS), in the human genome over time [reviewed in (38,39)]. In Africa, the geographic distribution of the HbS variant matches that of malaria (40), and the persistence of HbS in the human genome illustrates a genetic compromise that achieves survival against a deadly infectious agent (Plasmodium) at the cost of introducing another pathology (sickle-cell disease). More relevant to racial disparities in cancer is the example of the Duffy antigen/chemokine receptor (DARC), another non-classical innate immune gene with variants that protect against malaria. Plasmodium vivax binds DARC on erythrocytes to gain entry during infection (41). Genetic variants that reduce DARC expression in combination with the Fy(a–b–) phenotype of the Duffy antigen provide protection against P. vivax but are also associated with pathologies that include increased risk of lymph node and distant metastasis and poor survival in breast cancer (42).
Geographic origin and genomic variation in innate immunity
There are classical innate immune gene variations that display patterns associated with geographic origin. First, Lazarus et al. re-sequenced 16 genes coding for pattern recognition receptors (TLRs, etc.) and related molecules among 93 study participants, including 45 European Americans, 24 African Americans, and 24 Hispanic Americans. These investigators found a total of 705 single-nucleotide polymorphisms (SNPs), with distinct SNP distribution patterns that differed for each of the three ethnic groups (32). Second, Quintana-Murci and collaborators analyzed full genome sequence variations from the 1,000 Genomes Project and found that innate immune genes were under stronger purifying selection than any other protein-coding gene (34). Notably, the diverse functions of these genes included both classical (antigen recognition and response, development, and maintenance of immune cell lineages, etc.) and non-classical innate immunity (structure, motility and adhesion, regulation via kinases, and transcription factors and other modulators of gene expression).
Additional findings provide further insight concerning the unique characteristics of ancestry-specific innate immune gene expression and variation. First, the Kwiatkowski study observed 532 out of the 705 SNP variants in the study (75%) had higher frequencies in African Americans, although only 24 out of 93 individuals in the study (26%) had this ancestry. This suggested that a greater haplotype diversity exists within the African American gene pool (43). Two elegant RNA-sequencing studies used monocyte/macrophage cells from individuals of African and European ancestry to explore ancestry-specific transcriptional responses to activation by pathogens or TLR agonists (35,44). Quintana-Murci and co-workers exposed primary monocytes from 100 Europeans and 100 Africans to TLR agonists LPS (TLR4), Pam3CSK4 (TLR1/2), or R848 (TLR7/8) or to a human seasonal influenza A virus (IAV)(44). In this European study, there was minimal ancestry-related genetic admixture within the two populations. Nevertheless, gene expression in resting monocytes and transcriptional responses to innate immune agonists differed significantly between Europeans and Africans, including 27 innate immune genes that were highly expressed in African but not European monocytes. In a similar study by Barreiro and collaborators, monocyte-derived macrophages from 80 African Americans and 95 European Americans were exposed to Listeria or Salmonella and were evaluated by expression quantitative trait locus (eQTL) analysis (35). Importantly, this study controlled for ancestry-related genetic admixture common among African Americans and reported results according to the degree of African ancestry (45). Results indicated a 9.3% ancestry-related difference in gene expression in response to infection, with those of greatest African ancestry demonstrating the strongest inflammatory response, indicated by higher inflammatory gene expression, enhanced bacterial clearance, and other measures (35).
Among innate immune genes, there is a rapidly expanding body of data concerning the ten human TLRs, their associated molecules (co-receptors, adaptors, regulatory kinases, transcription factors, etc.), and the genetic variants among members of TLR-related pathways. Importantly, TLR pathways have been implicated in cancers that occur disproportionately among individuals of African descent. Although overall TLR function is protective, crosstalk among TLR downstream signaling pathways and other regulatory pathways is complex and nuanced [c.f., (46)]. As a result, the net impact TLRs have on disease risk and progression is likely to involve multiple genes in one or more downstream signaling axes.
Within the TLR family, the subfamily composed of cell surface TLR2, TLR1, TLR6 and TLR10 recognizes the widest range of pathogen-associated molecular patterns (PAMPs), due to the large combination of homo- and heterodimers that can be formed by its members and to the involvement of co-receptors in receptor signaling [reviewed in (47)]. Population genetics analysis shows that among 63, 47, and 48 individuals of African, European, and East Asian ancestry, respectively, the DNA sequence diversity of TLR2 (the most commonly paired member of the subfamily) was equally low in all racial groups and lower than that of TLR1, TLR6, and TLR10 (48). By comparison, TLR1 sequence diversity was widely divergent among the three racial groups, with individuals of African descent exhibiting two times more diversity than those individuals of European and East Asian descent. Similarly, individuals of African descent showed greater nucleotide diversity in TLR6 and TLR10 genes than those of European or East Asian ancestry (36). Intriguingly, the less-well-characterized human TLR10 gene had the largest sequence diversity among all populations, especially among those of African descent (48). The interaction between two polymorphisms in the TLR2 axis, IRAK4 rs4251545 and TLR2 rs1898830, is a significant predictor of prostate cancer risk among African American men (49). In contrast, when tested in a Swedish cohort as one of 99 SNPs (that did not include TLR2 rs1898830) among 20 TLR pathway genes, IRAK4 rs4251545 did not significantly impact prostate cancer mortality (50). IRAK4 rs4251545 alone is also associated with breast cancer risk among a small cohort of African American women (51).
Ribonuclease L (RNASEL) functions in interferon-mediated antiviral responses, in part by degrading viral RNA. Nucleic acid–sensing TLR3, TLR7, TLR8, and TLR9 differ in their ligand specificity, with only TLR7 and TLR8 capable of binding single-stranded RNA fragments generated by RNASEL. An intriguing small study noted that the presence of the RNASEL rs486907 variant on one or both alleles strengthened the association between increased fatty acid consumption and prostate cancer risk among Caucasian men, although no mechanism for this association was proposed (52). In contrast, although the sample size was too small to draw conclusions, the data suggests that fatty acid consumption reduces prostate cancer risk in African Americans, although any association with the RNASEL rs486907 variant in this population could not be addressed. Four studies have evaluated the impact of three RNASEL sequence variants (rs486907, rs56250729, rs627928) in relation to prostate cancer risk. Two independent observational studies and two pooled analyses revealed inheritance of the RNASEL rs486907 1385 G>A (Arg462Gln) variant allele was not significantly related to prostate cancer (53–56). However, upon stratification by racial/ethnic group, Liu and co-workers revealed the RNASEL rs486907 GG+GA genotype was protective for African-Americans in a pooled analysis of 16 studies (56). Notably, this meta-analysis excluded four additional case-control studies from African-Americans, Jamaicans, Caucasian Hispanics, and Caucasian non-Hispanics (53,57,58), which compromised their capacity to generate risk estimates for Caucasian Hispanics and non-Hispanics. This study limitation was resolved in another meta-analysis that demonstrated a marginal increase in prostate cancer risk linked with the RNASEL rs486907 AA genotype among Hispanic Caucasians and African-Americans/Afro-Caribbeans when compared to GG+GA carriers (54). Although the three RNASEL sequence variants (rs486907, rs56250729, rs627928) did not modify PCA risk among Hispanics from Spain, possession of rs486907AA and rs627928 GT/TT genotypes were linked to increased risk for high tumor stage and/or disease progression relative to the referent genotype (55). Although our lab did not establish a link between the RNASEL rs486907 SNP and the risk of developing prostate cancer in a pooled analysis between African-Americans and Jamaicans, we did observe a marginal 2.1-fold increase prostate cancer risk among Jamaicans under the heterozygous genetic model (59). Our findings and those of Alvarez-Cubero and co-workers require confirmation in larger racially diverse studies (55). Overall, mixed genetic findings may be attributed to 1) failure to stratify results by ethnicity or genetic ancestry; 2) differences in the selection of control methods (i.e., population vs. hospital-based) used for allelic discrimination; 3) failure to consider basic confounders (i.e., age, family history, and other prostate cancer risk factors) and effect modifiers (i.e., diet, BMI, physical activity, exposure to environmental/inflammatory insults); 4) studies with small sample sizes that are underpowered to detect true differences; and 5) variations in study designs.
Future directions
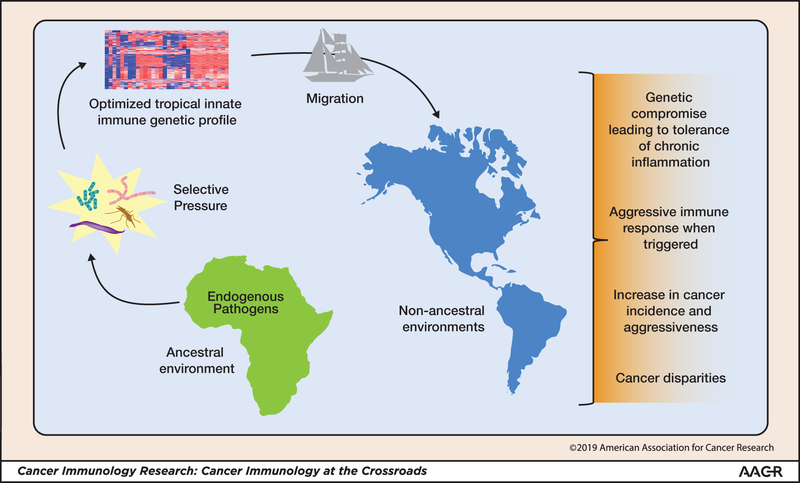
Individuals from environments that include a dense, diverse, and deadly range of pathogens, such as those of African descent, require robust innate immune genetic programs that, from an overarching perspective, might be expected to tolerate a relatively high background of microbes and other environmental insults, but respond rapidly and aggressively to legitimate threats. However, whether the innate immune program is defending against malaria or promoting tumorigenesis, it is becoming increasingly clear that an effective response must be profoundly nuanced, given the capacity for both Plasmodium (60) and cancer (61,62) to subvert immune defense strategies. Gene expression analysis of breast and prostate cancers indicates the existence of distinct immune profiles among groups of individuals who vary by geographical ancestry (Fig. 1)(63–65). Consequently, it is predicted that racial differences in innate immune programs will translate into ethnic differences in both pro- and antitumor immunity, tumor progression, and prognosis (66,67), leading to the current phenomenon that African Americans acquire earlier onset and more aggressive breast (25,68) and prostate cancers (5). It is these genetic variations in the innate immune system that suggest the need for intense scrutiny in identification and targeting of novel immunological therapies and their efficacy in racial/ethnic populations. Such consideration will guarantee that immuno-oncological findings are impactful to all population groups, thus, reducing and eventually eliminating racial/ethnic disparities in cancers.
Figure 1. Geographic origin and effects on racial/ethnic disparities in cancer.
Individuals with innate immune gene profiles optimized for pathogen-rich environments (such as tropical climates) are not optimal in all settings and involve genetic compromises in overall immunity, such as tolerance of low-level chronic inflammation and/or hyper-aggressive immune responsiveness when triggered, that contribute to the disparate incidence and aggressiveness of specific cancers. The migration of individuals with these innate immune variants from a high-pathogen environment to a new environment results in selective pressure that can change innate immune profiles.
Acknowledgements
The authors gratefully acknowledge support from the Clinical Translational Science Pilot Grant (LK); the JGBCC Bucks for Brains “Our Highest Potential” in Cancer Research Endowment (LK); NIH NCMHD grant P20-MD000175 (SK and SY), U54MD012392 (SK) and NIH NCI grant 5R21CA185361 (SK and SY).
Footnotes
Disclosure
The authors declare no conflicts of interest.
References
- 1.Siegel RL, Jemal A. Percentage of colorectal cancer diagnosed in adults aged younger than 50 years. Cancer 2016;122(9):1462–3 doi 10.1002/cncr.29980. [DOI] [PubMed] [Google Scholar]
- 2.Waxman AJ, Mink PJ, Devesa SS, Anderson WF, Weiss BM, Kristinsson SY, et al. Racial disparities in incidence and outcome in multiple myeloma: a population-based study. Blood 2010;116(25):5501–6 doi 10.1182/blood-2010-07-298760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dietze EC, Sistrunk C, Miranda-Carboni G, O’Regan R, Seewaldt VL. Triple-negative breast cancer in African-American women: disparities versus biology. Nat Rev Cancer 2015;15(4):248–54 doi 10.1038/nrc3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Long B, Liu FW, Bristow RE. Disparities in uterine cancer epidemiology, treatment, and survival among African Americans in the United States. Gynecol Oncol 2013;130(3):652–9 doi 10.1016/j.ygyno.2013.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66(1):7–30 doi 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 6.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144(5):646–74 doi 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 7.Pradere JP, Dapito DH, Schwabe RF. The Yin and Yang of Toll-like receptors in cancer. Oncogene 2014;33(27):3485–95 doi 10.1038/onc.2013.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang X, Shapiro DJ. The immune system and inflammation in breast cancer. Mol Cell Endocrinol 2014;382(1):673–82 doi 10.1016/j.mce.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Terzic J, Grivennikov S, Karin E, Karin M. Inflammation and colon cancer. Gastroenterology 2010;138(6):2101–14 e5 doi 10.1053/j.gastro.2010.01.058. [DOI] [PubMed] [Google Scholar]
- 10.Sfanos KS, De Marzo AM. Prostate cancer and inflammation: the evidence. Histopathology 2012;60(1):199–215 doi 10.1111/j.1365-2559.2011.04033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao S, Zhang Y, Zhang Q, Wang F, Zhang D. Toll-like receptors and prostate cancer. Front Immunol 2014;5:352 doi 10.3389/fimmu.2014.00352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weng PH, Huang YL, Page JH, Chen JH, Xu J, Koutros S, et al. Polymorphisms of an innate immune gene, toll-like receptor 4, and aggressive prostate cancer risk: a systematic review and meta-analysis. PLoS One 2014;9(10):e110569 doi 10.1371/journal.pone.0110569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cho WC, Kwan CK, Yau S, So PP, Poon PC, Au JS. The role of inflammation in the pathogenesis of lung cancer. Expert Opin Ther Targets 2011;15(9):1127–37 doi 10.1517/14728222.2011.599801. [DOI] [PubMed] [Google Scholar]
- 14.Gu J, Liu Y, Xie B, Ye P, Huang J, Lu Z. Roles of toll-like receptors: From inflammation to lung cancer progression. Biomed Rep 2018;8(2):126–32 doi 10.3892/br.2017.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang WJ, Du Y, Zhao X, Ma LY, Cao GW. Inflammation-related factors predicting prognosis of gastric cancer. World J Gastroenterol 2014;20(16):4586–96 doi 10.3748/wjg.v20.i16.4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang F, Meng W, Wang B, Qiao L. Helicobacter pylori-induced gastric inflammation and gastric cancer. Cancer Lett 2014;345(2):196–202 doi 10.1016/j.canlet.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 17.Husseinzadeh N, Davenport SM. Role of toll-like receptors in cervical, endometrial and ovarian cancers: a review. Gynecol Oncol 2014;135(2):359–63 doi 10.1016/j.ygyno.2014.08.013. [DOI] [PubMed] [Google Scholar]
- 18.Resler AJ, Malone KE, Johnson LG, Malkki M, Petersdorf EW, McKnight B, et al. Genetic variation in TLR or NFkappaB pathways and the risk of breast cancer: a case-control study. BMC Cancer 2013;13:219 doi 10.1186/1471-2407-13-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu C, Li H, Yin M, Yang T, An L, Yang G. Osteopontin is involved in TLR4 pathway contributing to ovarian cancer cell proliferation and metastasis. Oncotarget 2017;8(58):98394–404 doi 10.18632/oncotarget.21844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hung RJ, Ulrich CM, Goode EL, Brhane Y, Muir K, Chan AT, et al. Cross Cancer Genomic Investigation of Inflammation Pathway for Five Common Cancers: Lung, Ovary, Prostate, Breast, and Colorectal Cancer. J Natl Cancer Inst 2015;107(11) doi 10.1093/jnci/djv246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA 2006;295(21):2492–502 doi 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 22.Huo D, Ikpatt F, Khramtsov A, Dangou JM, Nanda R, Dignam J, et al. Population differences in breast cancer: survey in indigenous African women reveals over-representation of triple-negative breast cancer. J Clin Oncol 2009;27(27):4515–21 doi 10.1200/JCO.2008.19.6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hance KW, Anderson WF, Devesa SS, Young HA, Levine PH. Trends in inflammatory breast carcinoma incidence and survival: the surveillance, epidemiology, and end results program at the National Cancer Institute. J Natl Cancer Inst 2005;97(13):966–75 doi 10.1093/jnci/dji172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Silber JH, Rosenbaum PR, Clark AS, Giantonio BJ, Ross RN, Teng Y, et al. Characteristics associated with differences in survival among black and white women with breast cancer. JAMA 2013;310(4):389–97 doi 10.1001/jama.2013.8272. [DOI] [PubMed] [Google Scholar]
- 25.Iqbal J, Ginsburg O, Rochon PA, Sun P, Narod SA. Differences in breast cancer stage at diagnosis and cancer-specific survival by race and ethnicity in the United States. JAMA 2015;313(2):165–73 doi 10.1001/jama.2014.17322. [DOI] [PubMed] [Google Scholar]
- 26.Gamez-Pozo A, Trilla-Fuertes L, Berges-Soria J, Selevsek N, Lopez-Vacas R, Diaz-Almiron M, et al. Functional proteomics outlines the complexity of breast cancer molecular subtypes. Sci Rep 2017;7(1):10100 doi 10.1038/s41598-017-10493-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schinkel JK, Zahm SH, Jatoi I, McGlynn KA, Gallagher C, Schairer C, et al. Racial/ethnic differences in breast cancer survival by inflammatory status and hormonal receptor status: an analysis of the Surveillance, Epidemiology, and End Results data. Cancer Causes Control 2014;25(8):959–68 doi 10.1007/s10552-014-0395-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kidd LC, Rogers EN, Yeyeodu ST, Jones DZ, Kimbro KS. Contribution of toll-like receptor signaling pathways to breast tumorigenesis and treatment. Breast Cancer (Dove Med Press) 2013;5:43–51 doi 10.2147/BCTT.S29172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elledge RM, Clark GM, Chamness GC, Osborne CK. Tumor biologic factors and breast cancer prognosis among white, Hispanic, and black women in the United States. J Natl Cancer Inst 1994;86(9):705–12. [DOI] [PubMed] [Google Scholar]
- 30.Yang CH, Cristofanilli M. Systemic treatments for inflammatory breast cancer. Breast Dis 2005;22:55–65. [DOI] [PubMed] [Google Scholar]
- 31.Quan L, Gong Z, Yao S, Bandera EV, Zirpoli G, Hwang H, et al. Cytokine and cytokine receptor genes of the adaptive immune response are differentially associated with breast cancer risk in American women of African and European ancestry. Int J Cancer 2014;134(6):1408–21 doi 10.1002/ijc.28458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lazarus R, Vercelli D, Palmer LJ, Klimecki WJ, Silverman EK, Richter B, et al. Single nucleotide polymorphisms in innate immunity genes: abundant variation and potential role in complex human disease. Immunol Rev 2002;190:9–25. [DOI] [PubMed] [Google Scholar]
- 33.Casanova JL, Abel L. Inborn errors of immunity to infection: the rule rather than the exception. J Exp Med 2005;202(2):197–201 doi 10.1084/jem.20050854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deschamps M, Laval G, Fagny M, Itan Y, Abel L, Casanova JL, et al. Genomic Signatures of Selective Pressures and Introgression from Archaic Hominins at Human Innate Immunity Genes. Am J Hum Genet 2016;98(1):5–21 doi 10.1016/j.ajhg.2015.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nedelec Y, Sanz J, Baharian G, Szpiech ZA, Pacis A, Dumaine A, et al. Genetic Ancestry and Natural Selection Drive Population Differences in Immune Responses to Pathogens. Cell 2016;167(3):657–69 e21 doi 10.1016/j.cell.2016.09.025. [DOI] [PubMed] [Google Scholar]
- 36.Barreiro LB, Quintana-Murci L. From evolutionary genetics to human immunology: how selection shapes host defence genes. Nat Rev Genet 2010;11(1):17–30 doi 10.1038/nrg2698. [DOI] [PubMed] [Google Scholar]
- 37.Fumagalli M, Sironi M, Pozzoli U, Ferrer-Admetlla A, Pattini L, Nielsen R. Signatures of environmental genetic adaptation pinpoint pathogens as the main selective pressure through human evolution. PLoS Genet 2011;7(11):e1002355 doi 10.1371/journal.pgen.1002355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gong L, Maiteki-Sebuguzi C, Rosenthal PJ, Hubbard AE, Drakeley CJ, Dorsey G, et al. Evidence for both innate and acquired mechanisms of protection from Plasmodium falciparum in children with sickle cell trait. Blood 2012;119(16):3808–14 doi 10.1182/blood-2011-08-371062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mangano VD, Modiano D. An evolutionary perspective of how infection drives human genome diversity: the case of malaria. Curr Opin Immunol 2014;30:39–47 doi 10.1016/j.coi.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 40.Piel FB, Patil AP, Howes RE, Nyangiri OA, Gething PW, Williams TN, et al. Global distribution of the sickle cell gene and geographical confirmation of the malaria hypothesis. Nat Commun 2010;1:104 doi 10.1038/ncomms1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Horuk R, Chitnis CE, Darbonne WC, Colby TJ, Rybicki A, Hadley TJ, et al. A receptor for the malarial parasite Plasmodium vivax: the erythrocyte chemokine receptor. Science 1993;261(5125):1182–4. [DOI] [PubMed] [Google Scholar]
- 42.Wang J, Ou ZL, Hou YF, Luo JM, Shen ZZ, Ding J, et al. Enhanced expression of Duffy antigen receptor for chemokines by breast cancer cells attenuates growth and metastasis potential. Oncogene 2006;25(54):7201–11 doi 10.1038/sj.onc.1209703. [DOI] [PubMed] [Google Scholar]
- 43.Kwiatkowski DP. The complexity of genetic variation in a simple immune system. Trends Genet 2005;21(4):197–9 doi 10.1016/j.tig.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 44.Quach H, Rotival M, Pothlichet J, Loh YE, Dannemann M, Zidane N, et al. Genetic Adaptation and Neandertal Admixture Shaped the Immune System of Human Populations. Cell 2016;167(3):643–56 e17 doi 10.1016/j.cell.2016.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alexander DH, Novembre J, Lange K. Fast model-based estimation of ancestry in unrelated individuals. Genome Res 2009;19(9):1655–64 doi 10.1101/gr.094052.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaczanowska S, Joseph AM, Davila E. TLR agonists: our best frenemy in cancer immunotherapy. J Leukoc Biol 2013;93(6):847–63 doi 10.1189/jlb.1012501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Bergenhenegouwen J, Plantinga TS, Joosten LA, Netea MG, Folkerts G, Kraneveld AD, et al. TLR2 & Co: a critical analysis of the complex interactions between TLR2 and coreceptors. J Leukoc Biol 2013;94(5):885–902 doi 10.1189/jlb.0113003. [DOI] [PubMed] [Google Scholar]
- 48.Barreiro LB, Ben-Ali M, Quach H, Laval G, Patin E, Pickrell JK, et al. Evolutionary dynamics of human Toll-like receptors and their different contributions to host defense. PLoS Genet 2009;5(7):e1000562 doi 10.1371/journal.pgen.1000562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rogers EN, Jones DZ, Kidd NC, Yeyeodu S, Brock G, Ragin C, et al. Toll-like receptor-associated sequence variants and prostate cancer risk among men of African descent. Genes Immun 2013;14(6):347–55 doi 10.1038/gene.2013.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stark JR, Wiklund F, Gronberg H, Schumacher F, Sinnott JA, Stampfer MJ, et al. Toll-like receptor signaling pathway variants and prostate cancer mortality. Cancer Epidemiol Biomarkers Prev 2009;18(6):1859–63 doi 10.1158/1055-9965.EPI-08-0981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yeyeodu ST, Kidd LR, Oprea-Ilies GM, Burns BG, Vancleave TT, Shim JY, et al. IRAK4 and TLR3 Sequence Variants may Alter Breast Cancer Risk among African-American Women. Front Immunol 2013;4:338 doi 10.3389/fimmu.2013.00338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu X, Schumacher FR, Plummer SJ, Jorgenson E, Casey G, Witte JS. Trans-fatty acid intake and increased risk of advanced prostate cancer: modification by RNASEL R462Q variant. Carcinogenesis 2007;28(6):1232–6 doi 10.1093/carcin/bgm002. [DOI] [PubMed] [Google Scholar]
- 53.Wang MH, Helzlsouer KJ, Smith MW, Hoffman-Bolton JA, Clipp SL, Grinberg V, et al. Association of IL10 and other immune response- and obesity-related genes with prostate cancer in CLUE II. Prostate 2009;69(8):874–85 doi 10.1002/pros.20933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zuo L, Ren KW, Bai Y, Zhang LF, Zou JG, Qin XH, et al. Association of a common genetic variant in RNASEL and prostate cancer susceptibility. Oncotarget 2017;8(43):75141–50 doi 10.18632/oncotarget.20646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alvarez-Cubero MJ, Martinez-Gonzalez LJ, Saiz M, Carmona-Saez P, Alvarez JC, Pascual-Geler M, et al. Prognostic role of genetic biomarkers in clinical progression of prostate cancer. Exp Mol Med 2015;47:e176 doi 10.1038/emm.2015.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu X, Zheng D, Lu G, Yang B. The RNASEL −1385G/A polymorphism is associated with risk of prostate cancer in Africans. Onco Targets Ther 2018;11:97–102 doi 10.2147/OTT.S151398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Winchester DA, Till C, Goodman PJ, Tangen CM, Santella RM, Johnson-Pais TL, et al. Variation in genes involved in the immune response and prostate cancer risk in the placebo arm of the Prostate Cancer Prevention Trial. Prostate 2015;75(13):1403–18 doi 10.1002/pros.23021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Agalliu I, Leanza SM, Smith L, Trent JM, Carpten JD, Bailey-Wilson JE, et al. Contribution of HPC1 (RNASEL) and HPCX variants to prostate cancer in a founder population. Prostate 2010;70(15):1716–27 doi 10.1002/pros.21207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jones DZ, Ragin C, Kidd NC, Flores-Obando RE, Jackson M, McFarlane-Anderson N, et al. The impact of genetic variants in inflammatory-related genes on prostate cancer risk among men of African Descent: a case control study. Hered Cancer Clin Pract 2013;11(1):19 doi 10.1186/1897-4287-11-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stevenson MM, Riley EM. Innate immunity to malaria. Nat Rev Immunol 2004;4(3):169–80 doi 10.1038/nri1311. [DOI] [PubMed] [Google Scholar]
- 61.Vinay DS, Ryan EP, Pawelec G, Talib WH, Stagg J, Elkord E, et al. Immune evasion in cancer: Mechanistic basis and therapeutic strategies. Semin Cancer Biol 2015;35 Suppl:S185–S98 doi 10.1016/j.semcancer.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 62.Muenst S, Laubli H, Soysal SD, Zippelius A, Tzankov A, Hoeller S. The immune system and cancer evasion strategies: therapeutic concepts. J Intern Med 2016;279(6):541–62 doi 10.1111/joim.12470. [DOI] [PubMed] [Google Scholar]
- 63.Hardiman G, Savage SJ, Hazard ES, Wilson RC, Courtney SM, Smith MT, et al. Systems analysis of the prostate transcriptome in African-American men compared with European-American men. Pharmacogenomics 2016;17(10):1129–43 doi 10.2217/pgs-2016-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Reams RR, Agrawal D, Davis MB, Yoder S, Odedina FT, Kumar N, et al. Microarray comparison of prostate tumor gene expression in African-American and Caucasian American males: a pilot project study. Infect Agent Cancer 2009;4 Suppl 1:S3 doi 10.1186/1750-9378-4-S1-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kinseth MA, Jia Z, Rahmatpanah F, Sawyers A, Sutton M, Wang-Rodriguez J, et al. Expression differences between African American and Caucasian prostate cancer tissue reveals that stroma is the site of aggressive changes. Int J Cancer 2014;134(1):81–91 doi 10.1002/ijc.28326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wallace TA, Prueitt RL, Yi M, Howe TM, Gillespie JW, Yfantis HG, et al. Tumor immunobiological differences in prostate cancer between African-American and European-American men. Cancer Res 2008;68(3):927–36 doi 10.1158/0008-5472.CAN-07-2608. [DOI] [PubMed] [Google Scholar]
- 67.Quan B, Qi X, Yu Z, Jiang Y, Liao M, Wang G, et al. Pathway analysis of genome-wide association study and transcriptome data highlights new biological pathways in colorectal cancer. Mol Genet Genomics 2015;290(2):603–10 doi 10.1007/s00438-014-0945-y. [DOI] [PubMed] [Google Scholar]
- 68.Batina NG, Trentham-Dietz A, Gangnon RE, Sprague BL, Rosenberg MA, Stout NK, et al. Variation in tumor natural history contributes to racial disparities in breast cancer stage at diagnosis. Breast Cancer Res Treat 2013;138(2):519–28 doi 10.1007/s10549-013-2435-z. [DOI] [PMC free article] [PubMed] [Google Scholar]