Abstract
Objectives:
Olfaction plays a critical role in health and function in older adults, and impaired sense of smell is a strong predictor of morbidity and mortality. Smoking cigarettes causes olfactory impairment, but the mechanism of damage and ability to recover after cessation are unknown. We investigated the relationship between time since quitting and olfactory dysfunction in order to elucidate the mechanism(s) by which smoking damages the olfactory system and to inform patient counseling.
Methods:
Using longitudinal data from the National Social Life Health and Aging Project (n= 3,528 older adults, including 1,526 former smokers), we analyzed the association between odor identification performance and time since smoking cessation using multivariate ordinal logistic regression, adjusting for cognition and demographic variables. To test whether vascular disease plays a role, we also assessed the relationship between olfactory decline and incidence of heart attack and heart disease.
Results:
Former smokers who quit ≤15 years before testing had significantly impaired olfaction compared to never smokers (p=.04), but those who quit >15 years prior did not. Olfactory decline over 5 years showed modest evidence toward predicting increased incidence of heart attack or heart disease (p=.08).
Conclusions:
Olfactory impairment in smokers persists 15 years after quitting, which is consistent with a vascular mechanism of impairment. Indeed, olfactory decline is a predictor of the development of cardiovascular disease. Taken together, these data suggest that olfactory loss may be a useful sign of underlying vascular pathology. Further investigation of olfactory loss as an early biomarker for cardiovascular disease is warranted.
Keywords: Smell, Tobacco, Smoking cessation, Heart disease, Myocardial infarction, Atherosclerosis, Aging
Introduction
Loss of olfactory function is a common problem among older adults and is associated with a wide array of adverse health effects. Recent studies have shown that patients with impaired olfaction are more likely to suffer from depression and loneliness1, have diminished sleep quality2, develop dementia3, and face increased 5-year mortality4. Despite the profound implications of these well-documented associations, the factors that contribute to olfactory loss and the mechanisms that connect these phenomena are largely unknown.
The olfactory nerve is uniquely susceptible to environmental insult because it is exposed to ambient air in the nose. One of the most common pollutants in inhaled air is tobacco smoke. Several studies have shown that cigarette smokers have worse olfaction than non-smokers5; however, the mechanism by which smoking causes decreased olfaction is unknown. Former smokers perform similarly to never smokers, which suggests that the olfactory damage caused by smoking is reversible, but these studies have treated former smokers as a single cohort without accounting for time since cessation5. There have not been any long-term longitudinal studies of olfaction in former smokers to track their recovery5,6. Therefore, a major clinical question which remains unanswered is how long it takes after quitting smoking for olfactory function to recover to the level of a never smoker. Such information would be useful in counseling patients about what to expect as clinicians encourage them to quit smoking.
Understanding how quitting smoking affects the ability to smell could also provide significant insight into the mechanism by which smoking affects olfactory function. Proposed mechanisms of smoking-mediated olfactory impairment5,7–9 include squamous metaplasia of olfactory mucosa (which might be expected to resolve 6 months after cessation10), inflammatory effects (which may resolve in 5 years11), or vascular effects (which may not return to the level of a never smoker for 15–20 years12,13).
In this study, we examined data from a nationally representative sample of US older adults in which detailed information on smoking behaviors, including time since quitting, was collected. We sought to answer the question of how time since smoking cessation is related to olfactory function, and to investigate whether such information could help elucidate the mechanism of smoking-mediated olfactory loss.
Methods
Subjects
The National Social Health and Aging Project (NSHAP) is a nationally representative, longitudinal study of US older adults. NSHAP was initiated with the goal of examining the interactions between social behaviors, emotional well-being, and physical health of older adults. In 2005, field interviewers from NORC at the University of Chicago collected health information from 3,005 respondents born between 1920 and 1947, including assessments of olfaction and a variety of biomeasures during interviews at respondents’ homes. Interviewers returned five and ten years later in 2010 and 2015 to collect data from the same respondents along with their co-resident romantic partners. Additional details of study design and data collection are available elsewhere14–17. In this study, we used baseline data from all respondents assessed in 2005 (who were born between 1920–1947) and new respondents from the 2010 data collection who were in this age group.
NSHAP received approval from the Institutional Review Board at the University of Chicago and the NORC Institutional Review Board. Written informed consent was obtained from all respondents.
Assessment of Odor Identification
An odor identification test was administered to all respondents in 2005. Two-thirds of respondents were tested in 2010, selected at random by study design. This validated identification method18 measured respondents’ ability to recognize and name five familiar smells via odor impregnated marker pens (Sniffin’ Sticks)19. For each odor presented, respondents were asked to choose from a set of four word/picture options. Briefly, interviewers removed the cap and waved the pen approximately ½ inch below both of the respondents’ nostrils. Respondents were asked to breathe normally for about 2 seconds and then give their answer choice. Refusals to answer were counted as incorrect. An odor identification score was calculated as the number of odors correctly identified (range 0–5). Additional details of the testing are provided elsewhere20.
Assessment of Smoking Behaviors
All respondents were asked if they currently smoked cigarettes and, if they responded yes, the age they started smoking regularly and how many cigarettes they currently smoke per day on average. Respondents who responded no were asked if they had ever regularly smoked cigarettes. Former smokers were then asked the age when they started smoking regularly, the age when they last smoked regularly, and how many cigarettes they used to smoke on average per day.
Duration of smoking was calculated by subtracting age of first smoking from current age for current smokers, and by subtracting age of first smoking from age of smoking cessation for former smokers. Time since quitting was calculated by subtracting age of smoking cessation from current age.
Statistical Analysis
We used multivariate ordinal logistic regression to analyze the effect of smoking status (current vs. former vs. never) on odor identification score. We then investigated how long after smoking cessation olfaction recovers to the level of a never smoker. To do this, we grouped former smokers by number of years since they last smoked regularly: those who stopped smoking greater than 15 years ago and those who stopped 15 or fewer years ago. This time interval,15 years, was chosen a priori based on the time required for the risk of coronary heart disease to return to baseline after smoking cessation13.
We investigated the effect of smoking status and olfactory decline on incidence of heart attack and heart disease. We defined olfactory decline as a decrease in odor identification score of two or more points between baseline and five-year follow-up, as previously reported7. Respondents who were anosmic at baseline (0/5 or 1/5 odors identified correctly) were excluded to account for floor effects. Incident first heart attack or new heart disease was defined as those who reported no history of heart attack or heart disease at baseline but reported a history of heart attack and/or heart disease at 10-year follow-up. In this analysis, we controlled for baseline body mass index (BMI) and self-rated physical health. The goal of this analysis was to measure whether olfactory decline may be a predictor of future cardiovascular morbidity (as it has shown to be for all-cause mortality4).
In all analyses, we controlled for baseline age, gender, race/ethnicity, level of education, and cognition, all of which have been shown to be associated with olfaction21,22. Gender and race/ethnicity were self-reported. Education was defined by the highest certification or degree earned and treated as a continuous variable. In 2005, cognition was measured by the Short Portable Mental Status Questionnaire (SPMSQ)23. In 2010 we used an enhanced measure that better captures a range of function: a modified version of the Montreal Cognitive Assessment (MoCA-survey adapted [SA]), formerly known as the Chicago Cognitive Function Measure (CCFM)24. In order to generate a common cognition variable for use in all respondents, we calculated z-scores for each respondent’s performance on either the SPMSQ or the MoCA-SA, relative to the rest of the respondents given that test. Further details on these measures are available elsewhere25. All analyses were performed using survey weights using Stata 15.0 (StataCorp, College Station, TX).
Results
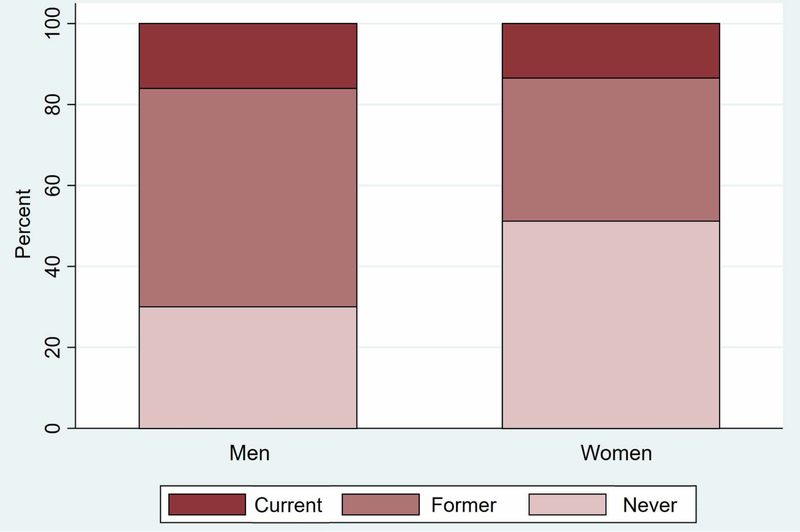
Of 3,528 respondents assessed at baseline, 1,526 (42.5%) reported being former smokers (Table 1). Men were more likely to be former smokers than women and less likely to be never smokers (p<.001) (Figure 1). Former smokers were significantly more likely to be white (p<.001) and have higher cognition scores (p<.04) compared to never smokers. Former smokers and never smokers did not significantly differ in age or education level. Mean duration of smoking was 25.7 years (SD 15.3, range 0–67) among former smokers and mean cigarettes smoked per day was 20.7 (SD 17.2, range 0–100). Mean time since smoking cessation was 25.9 years (SD 14.9, range 0–71). When stratifying former smokers by time since quitting, those who quit 15 or fewer years ago were younger, more likely to be women, and less educated than those who quit more than 15 years ago (p<0.01 for all). The more recent quitters also averaged more years of smoking and more cigarettes smoked per day (p<0.001).
Table 1:
Baseline demographics and smoking behaviors
| Never smokers | Former smokers |
Current smokers | ||
|---|---|---|---|---|
| Quit ≤ 15 years ago | Quit > 15 years ago | |||
| (n=1,500) | (n=413) | (n=1,113) | (n=502) | |
| Gender | ||||
| % Women | 65.8 | 45.5 | 38.0 | 45.2 |
| Race/Ethnicity | ||||
| % White | 69.4 | 74.1 | 77.5 | 65.1 |
| % Black | 15.0 | 15.5 | 12.6 | 24.3 |
| % Hispanic, non-black | 13.0 | 7.8 | 7.7 | 8.4 |
| % Other | 2.6 | 2.7 | 2.1 | 2.2 |
| Education | ||||
| % < High school | 21.6 | 27.1 | 17.6 | 27.5 |
| % High school/equivalent | 24.7 | 29.5 | 24.4 | 31.2 |
| % Vocational/Associates/Some college | 28.3 | 26.1 | 32.1 | 27.3 |
| % Bachelor’s degree or more | 25.3 | 17.1 | 25.9 | 13.9 |
| Age (mean ± SD, range) | 70.3 ± 8.0, 57–91 | 68.2 ± 7.3, 57–88 | 71.0 ± 7.7, 57–90 | 67.2 ± 6.9, 57–85 |
| Years of smoking (mean ± SD, range) | 41.6 ± 11.1, 0–67 | 19.8 ± 12.2, 0–59 | 48.6 ± 9.5, 0–77 | |
| Average cigarettes smoked/day (mean ± SD, range) | 23.5 ± 16.0, 0–80 | 19.7 ± 17.3, 0–100 | 14.9 ± 10.5, 0–60 | |
| Years since smoking cessation (mean ± SD, range) | 7.7 ± 4.8, 0–15 | 32.7 ± 11.2, 16–71 | ||
Demographics of respondents at baseline. Gender, race/ethnic group, education level, and average cigarettes/day were self-reported. Age was calculated from date of birth. Duration of smoking was calculated by subtracting age when respondent began smoking from age when they last smoked regularly. Years since cessation was calculated by subtracting age when respondent last smoked regularly from current age.
Figure 1. Smoking status of respondents at baseline (n=3,528).
Smoking status self-reported by respondents at baseline. Men were more likely to report being former smokers and less likely to report being never smokers (p<.001) than women.
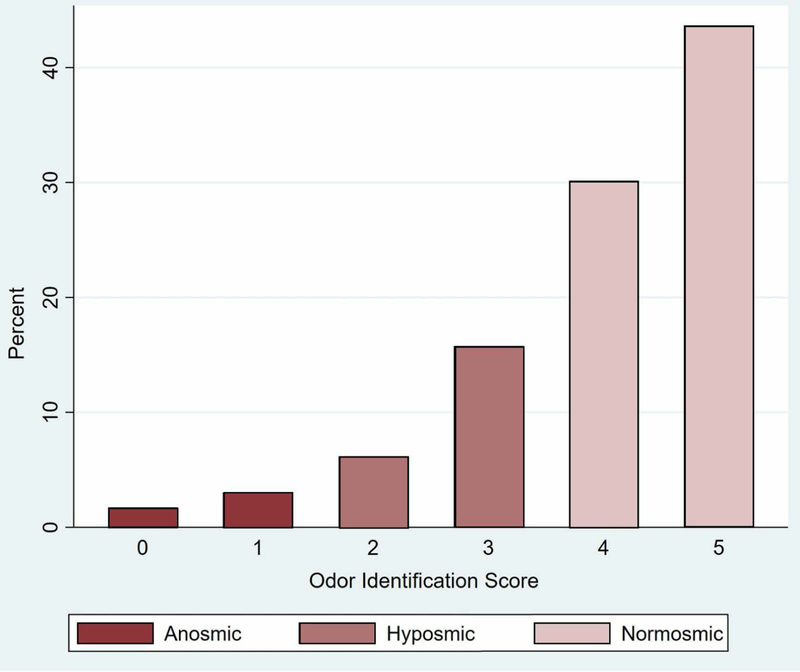
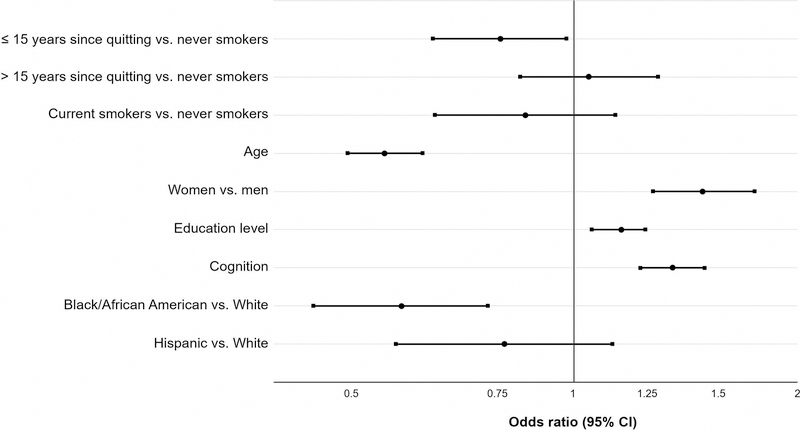
Smoking history did not show an effect on odor identification (Table 2, Model A: Smoking history) in analyses that accounted for age, gender, education level, cognition, and race/ethnicity, consistent with prior studies5. Current smokers also had no statistically significant difference in performance compared to never smokers. Older respondents had worse odor identification scores (OR per decade, 0.57; 95% CI, 0.50–0.64), and Black/African Americans had worse scores compared to Whites (OR, 0.61; 95% CI, 0.46–0.81). Respondents with higher education level (OR, 1.15; 95% CI, 1.06–1.25), and better cognition (OR, 1.36; 95% CI, 1.23–1.51) had better odor identification. Women had better odor identification than men (OR, 1.50; 95% CI, 1.28–1.76). Distribution of odor identification scores at baseline is presented in Figure 2.
Table 2:
Effect of smoking status on odor identification score, controlling for demographic variables
| Covariate | Odds ratio (95% confidence interval) | |
|---|---|---|
| Model A: Smoking history |
Model B: Time since quitting |
|
| Never smokers | ||
| Former smokers | 0.97 (0.82 – 1.15) | |
| Current smokers | 0.87 (0.66 – 1.15) | |
| Never smokers | ||
| Former smokers, by time since quitting | ||
| ≤ 15 years | 0.81 (0.66 – 0.99) * | |
| > 15 years | 1.05 (0.85 – 1.29) | |
| Current smokers | 0.86 (0.65 – 1.14) | |
| Age | 0.57 (0.50 – 0.64) ** | 0.57 (0.50 – 0.64) ** |
| Gender | ||
| Men | ||
| Women | 1.50 (1.28 – 1.76) ** | 1.50 (1.28 – 1.76) ** |
| Education Level | 1.15 (1.06 – 1.25) ** | 1.15 (1.06 – 1.25) ** |
| Cognition | 1.36 (1.23 – 1.51) ** | 1.36 (1.23 – 1.51) ** |
| Race/Ethnicity | ||
| White | ||
| Black | 0.61 (0.46 – 0.81) ** | 0.61 (0.46 – 0.81) ** |
| Hispanic, non-black | 0.83 (0.59 – 1.17) | 0.83 (0.59 – 1.17) |
| Other | 0.77 (0.42 – 1.40) | 0.77 (0.42 – 1.40) |
= p < .05
= p ≤ .001
Former smokers who quit ≤ 15 years ago, but not those who quit >15 years ago, have worse odor identification scores than never smokers. n=3528. Ordinal logistic regression with survey weights. OR for age are per decade. Education treated as a continuous measure with integer scores for education level (higher scores = more education). Cognition measured using z-scores for performance on SPMSQ or MoCA-SA.
Figure 2. Odor identification scores at baseline (n=3,528).
Odor identification score is the number of odors out of 5 that respondent was able to correctly identify. 4–5 correct = normosmic, 2–3 correct = hyposmic, 0–1 correct = anosmic4.
We then stratified former smokers by time since smoking cessation (Table 2, Model B: Time since quitting, and Figure 3). Former smokers who quit over 15 years ago had similar odor identification performance as never smokers (OR, 1.05; 95% CI, 0.85–1.29). However, former smokers who quit 15 or fewer years ago had significantly worse odor identification than never smokers (OR, 0.81; 95% CI, 0.66–0.99). Respondents who quit smoking between baseline and follow-up did not show significant improvement in olfaction compared to those who continued to smoke (data not shown).
Figure 3. Former smokers who quit ≤ 15 years ago, but not those who quit >15 years ago, have worse odor identification scores than never smokers.
n=3528. Ordinal logistic regression with survey weights. Odds ratios for higher score on odor identification test. The effect of age is per decade increase. Education treated as a continuous measure with integer scores for education level (higher scores = more education). Cognition measured using z-scores for performance on SPMSQ or MoCA-SA.
This timepoint of 15 years is consistent with the decline in risk of heart disease after cessation, suggesting a vascular mechanism. Therefore, to evaluate whether olfactory loss might predict vascular disease, we then investigated incidence of heart attack and heart disease at follow-up (Table 3, Model A: Cardiac disease risk). Respondents who identified as former smokers at baseline had significantly increased risk of 10-year incidence of first heart attack or new heart disease (OR, 1.49; 95% CI, 1.05–2.12). Risk was also significantly lower in women than men (OR, 0.52; 95% CI, 0.37–0.72), consistent with epidemiologic studies of cardiovascular disease26,27.
Table 3:
Risk factors for incidence of new heart attack or heart disease at 10-year follow-up
| Covariate | Odds ratio (95% confidence interval) | |
|---|---|---|
| Model A: Cardiac disease risk |
Model B: Cardiac risk accounting for olfactory decline |
|
| Never smokers | ||
| Former smokers | 1.49 (1.05 – 2.12) * | 1.07 (0.67 – 1.71) |
| Current smokers | 2.08 (1.28 – 3.38) * | 2.27 (1.24 – 4.17) * |
| Olfactory decline at 5-year follow-up | 1.75 (0.93 – 3.31) † | |
| Age | 1.13 (0.91 – 1.39) | 1.09 (0.83 – 1.45) |
| Gender | ||
| Men | ||
| Women | 0.52 (0.37 – 0.72) ** | 0.58 (0.38 – 0.88) * |
| Education Level | 0.93 (0.79 – 1.10) | 0.92 (0.74 – 1.14) |
| Cognition | 0.95 (0.78 – 1.15) | 1.09 (0.82 – 1.45) |
| BMI | 1.02 (0.99 – 1.05) | 1.03 (0.99 – 1.07) † |
| Self-rated physical health | 0.91 (0.77 – 1.07) | 0.92 (0.74 – 1.13) |
| Race/Ethnicity | ||
| White | ||
| Black | 0.86 (0.55 – 1.35) | 0.62 (0.33 – 1.17) |
| Hispanic, non-black | 0.65 (0.36 – 1.18) | 0.70 (0.33 – 1.49) |
| Other | 0.43 (0.10 – 1.86) | 0.48 (0.06 – 3.77) |
= p < 0.10
= p < .05
= p ≤ .001
Olfactory decline at 5-year follow-up predicts new cardiac events at 10-year follow-up, independent of smoking status. Model A: n=1,460. Model B: n=935, multivariate logistic regression. Respondents with new cardiac events are those who reported no history of heart attack or heart disease at baseline and history of heart attack and/or heart disease at 10-year follow-up. Olfactory decline at 5-year follow-up is defined by a decrease of 2 or more points in odor identification score. OR for age are per decade. Education treated as a continuous measure with integer scores for education level (higher scores = more education). Cognition measured using z-scores for performance on SPMSQ or MoCA-SA. BMI calculated from direct measurements of height and weight.
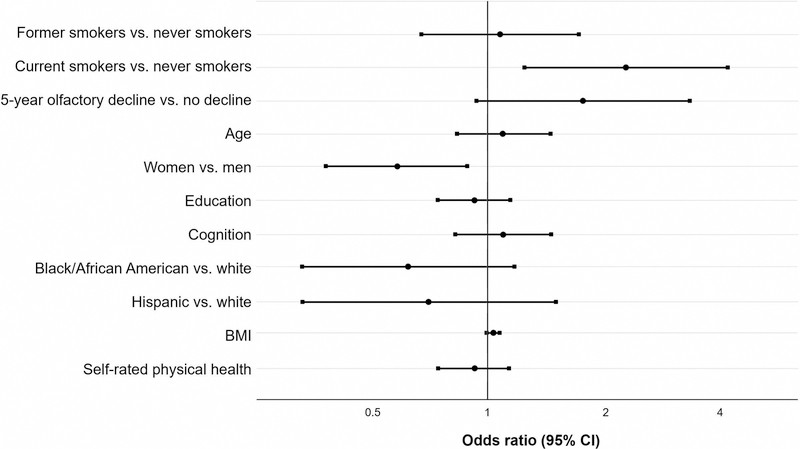
We then added 5-year decline in odor identification to the regression model (Table 3, Model B: Cardiac risk accounting for olfactory decline, and Figure 4). Respondents whose olfaction had declined at 5-year follow-up tended to be more likely to have incidence of first heart attack or new heart disease at 10-year follow-up (OR, 1.75; 95% CI, 0.93–3.31), after accounting for smoking status, BMI, self-rated physical health, and demographic variables.
Figure 4. Olfactory decline at 5-year follow-up predicts new cardiac events at 10-year follow-up, independent of smoking status.
Multivariate logistic regression, n=935. Odds ratios for 10-year incidence of new cardiac events. Reference groups are never smokers, men, and white. Respondents with new cardiac events are those who reported no history of heart attack or heart disease at baseline and history of heart attack and/or heart disease at 10-year follow-up. Olfactory decline at 5-year follow-up is defined by a decrease of 2 or more points in odor identification score. The effect of age is per decade increase. Education treated as a continuous measure with integer scores for education level (higher scores = more education). Cognition measured using z-scores for performance on SPMSQ or MoCA-SA. BMI calculated from direct measurements of height and weight and the effect of BMI is per 1 kg/m2 increase.
Discussion
Here, we report that smoking-mediated olfactory dysfunction is reversible but may persist for 15 years after smoking cessation. Former smokers who had quit within 15 years had significantly impaired olfaction compared to never smokers, but those who quit more than 15 years ago had similar olfaction as never smokers. This novel finding has significant implications for clinical counseling and for advancing understanding of environmental effects of airborne toxicants on the pathophysiology of olfactory loss.
Previous studies on olfaction and smoking have generally only compared current, former, and never smokers as groups5. Most (but not all) have reported significant olfactory deficits in current but not former smokers, suggesting that the damage done by smoking is reversible. However, without stratifying former smokers by time since quitting, the time course of that recovery was unclear. Only one study assessed time since cessation28 and reported a dose-dependent association with olfaction. However, it did not compare former smokers to never smokers and thus was not able to measure how long after cessation olfaction returned to a normal baseline level. In one study which randomized smokers to keep smoking or quit, those who stopped smoking self-reported an improvement in smell after one week6. However, such a self-report may be subject to placebo effect; additionally, subjective reports of olfactory function have been shown to be inaccurate29,30.
There is a wealth of data on how long it takes for various diseases and physiological processes to improve after smoking cessation13, which are central to many public health campaigns to encourage quitting31. Our finding that olfaction can take 15 or more years to recover after smoking cessation extends this to chemosensation. Since olfactory impairment has been linked to depression and decreased quality of life32, we believe that the prospect of eventual recovery can be a significant motivator for smoking cessation.
Additionally, these findings help begin to elucidate the mechanism of smoking-mediated olfactory impairment. The precise mechanism by which smoking impairs the sense of smell has not been established5. Due to the olfactory nerve’s direct exposure to inhaled air, one commonly postulated mechanism is that cigarette smoke causes direct damage to the olfactory epithelium, for example, by induction of squamous metaplasia or olfactory neuron apoptosis5. However, olfactory neurons regenerate every 2–3 months33 and squamous metaplasia in smokers has been shown to resolve 5 months after cessation10, so these mechanisms are not consistent with our finding that smoking-mediated olfactory impairment persists 15 years after cessation. Thus, direct damage to olfactory neurons from inhaled smoke may not be the sole mechanism by which smoking harms the olfactory system.
The 15-year mark matches another commonly-reported interval for reduced risk after smoking cessation. The risk of coronary heart disease returns to that of a never smoker after 15 years 13, and the risk of vascular death approaches that of a never smoker 20 years after cessation12. Thus, our findings here are consistent with a vascular effect on olfaction by smoking. Indeed, the presence of microvascular disease is associated with olfactory dysfunction in diabetes34, and the use of lipid-lowering agents has been shown to decrease risk of olfactory impairment35. Vascular disease was strongly associated with olfactory impairment in the Beaver Dam Offspring Study, in which carotid intima-media thickness and carotid plaques were associated with longitudinal decline in odor identification7. The authors suggested that because such changes are known to be caused by smoking, they may represent a causal vascular mechanism of olfactory dysfunction in smokers. Our data support this hypothesis.
Indeed, olfaction may be impaired due to decreased blood flow to the olfactory system, via relative hypoxia from atherosclerosis of the supplying blood vessels, as hypothesized in the Beaver Dam Offspring Study7. Vascular compromise may also occur at the level of the brain, impairing central olfactory processing. Olfactory impairment has been shown to precede Alzheimer’s Disease (AD)36,37, a neurodegenerative disease that is accelerated by cerebrovascular damage38,39. Thus, olfactory dysfunction may be a signal of cerebrovascular compromise. Indeed, middle cerebral artery occlusion in rats results in olfactory cortex infarcts most consistently of all regions40. Another possible mechanism by which vascular disease could underlie olfactory dysfunction is through damage to the peripheral olfactory apparatus. Smoking may cause microvascular damage to the olfactory nerve and thereby disrupt sensory transduction.
If vascular disease is the cause of smoking-mediated olfactory dysfunction, that may explain why current smokers did not have significantly worse olfaction than never smokers in our study, unlike in most past work. In studies that include smokers of all ages, vascular disease in younger smokers would lead to significantly worse olfaction than age-matched never smokers. However, atherosclerosis increases with age41, so in our sample of older adults, the difference between smokers and non-smokers may be less extreme and lead to a smaller difference in olfaction. A vascular mechanism of smoking on olfaction may also provide insight into the well-documented findings that men have worse olfaction than women and Black/African Americans have worse olfaction than Whites42, considering the increased rates of atherosclerosis and vascular disease in those groups43,44.
Understanding the mechanism of smoking-mediated olfactory impairment is an important goal because loss of olfaction is an early warning sign of future adverse health outcomes. Olfactory decline predicts development of neurodegenerative disease and mortality4,36,37. If smoking-mediated olfactory impairment occurs through atherosclerosis or other vascular disease mechanisms such as endothelial dysfunction or carotid stenosis, then smell impairment may be an early signal of vascular disease more broadly. Indeed, although it did not reach statistical significance, we identified a trend that supports this hypothesis: older adults with olfactory decline at 5-year follow-up tended to be more likely to have new heart attack or heart disease at 10-year follow-up, even when accounting for smoking status, BMI, self-rated physical health, and demographic variables. Given that our analyses did not include people who had died and that we were unable to distinguish respondents who had multiple heart attacks, these results are likely conservative. Therefore, these findings remain suggestive and are an intriguing basis for confirmation in future work. Indeed, further investigation is needed to test the extent to which vascular disease contributes to olfactory impairment and to assess the predictive value of olfactory decline for future heart disease.
This was a retrospective study, with all the attendant limitations of this design. A longitudinal prospective study following current, former, and never smokers of all ages and measuring their olfaction would be ideal for establishing the timeframe for olfactory recovery after smoking cessation. Concurrent measurement of vascular changes such as atherosclerosis and carotid stenosis, including biomarkers and direct test measures, could help establish whether these changes underly olfactory impairment and the precise association. Although measures of salivary cotinine were used to validate current smoking status45, we rely on self-report for duration of smoking and magnitude in former smokers, with the attendant caveats of a retrospective recall. We note that variation among former smokers in terms of smoking histories may affect our results, particularly the fact that the more recent quitters averaged greater durations and magnitudes of smoking. However, neither duration of smoking nor average cigarettes smoked per day were significantly associated with odor identification scores among former smokers. Therefore, we believe that the difference in olfaction can be attributed to recovery after cessation. Finally, by design, we only included older adults. That population is well suited for this study due to the long histories of smoking and cessation and the many risk factors associated with olfactory dysfunction in older adults; however, additional work in younger subjects is needed to generalize these findings.
Conclusions
We report for the first time that olfactory impairment in smokers can persist for 15 years after cessation. This information can be used to counsel patients on what to expect after quitting smoking and supports the concept that smoking may impair olfaction via a vascular mechanism. Olfactory loss has potential to be a sensitive early indicator of risk for not only neurodegenerative disease36,37, but also cardiovascular disease.
Acknowledgements
Members of the Olfactory Research Group and the larger NSHAP team provided intellectual input and useful feedback. Vineet Arora, MD, MAPP, David Meltzer, MD, PhD, and Micah Prochaska, MD provided useful comments. This study was supported by the NIA, the NIEHS, the Department of Surgery at the University of Chicago, and the Pritzker School of Medicine. We thank NSHAP respondents for their generous participation.
Funding sources: NIH NIA (AG030481, AG043538, AG048511, AG000243, AG029795) and NIEHS (ES026718) and the Pritzker School of Medicine
Footnotes
Financial disclosures/conflicts of interest:
None
References
- 1.Sivam A, Wroblewski KE, Alkorta-Aranburu G, et al. Olfactory Dysfunction in Older Adults is Associated with Feelings of Depression and Loneliness. Chem Senses 2016;41(4):293–299. 10.1093/chemse/bjv088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McSorley VE, Pinto J, Schumm LP, et al. Sleep and Olfaction among Older Adults. Neuroepidemiology 2017;48(3–4):147–154. 10.1159/000479066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adams DR, Kern DW, Wroblewski KE, McClintock MK, Dale W, Pinto JM. Olfactory Dysfunction Predicts Subsequent Dementia in Older U.S. Adults. J Am Geriatr Soc 2018;66(1):140–144. 10.1111/jgs.15048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pinto JM, Wroblewski KE, Kern DW, Schumm LP, McClintock MK. Olfactory Dysfunction Predicts 5-Year Mortality in Older Adults. PLOS ONE 2014;9(10):e107541 10.1371/journal.pone.0107541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ajmani GS, Suh HH, Wroblewski KE, Pinto JM. Smoking and olfactory dysfunction: A systematic literature review and meta-analysis. The Laryngoscope 2017;127(8):1753–1761. 10.1002/lary.26558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Etter J-F, Ussher M, Hughes JR. A test of proposed new tobacco withdrawal symptoms. Addiction 2013;108(1):50–59. 10.1111/j.1360-0443.2012.03981.x [DOI] [PubMed] [Google Scholar]
- 7.Schubert CR, Cruickshanks KJ, Fischer ME, et al. Carotid Intima Media Thickness, Atherosclerosis, and 5-Year Decline in Odor Identification: The Beaver Dam Offspring Study. J Gerontol A Biol Sci Med Sci 2015;70(7):879–884. 10.1093/gerona/glu158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yee KK, Pribitkin EA, Cowart BJ, et al. Smoking-associated Squamous Metaplasia in Olfactory Mucosa of Patients with Chronic Rhinosinusitis. Toxicol Pathol 2009;37(5):594–598. 10.1177/0192623309338055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kern RC, Conley DB, Haines GK, Robinson AM. Pathology of the Olfactory Mucosa: Implications for the Treatment of Olfactory Dysfunction. The Laryngoscope 114(2):279–285. 10.1097/00005537-200402000-00018 [DOI] [PubMed] [Google Scholar]
- 10.Lee JS, Lippman SM, Benner SE, et al. Randomized placebo-controlled trial of isotretinoin in chemoprevention of bronchial squamous metaplasia. J Clin Oncol 1994;12(5):937–945. 10.1200/JCO.1994.12.5.937 [DOI] [PubMed] [Google Scholar]
- 11.Tonstad S, Cowan JL. C-reactive protein as a predictor of disease in smokers and former smokers: a review. Int J Clin Pract 2009;63(11):1634–1641. 10.1111/j.1742-1241.2009.02179.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conen D, Everett BM, Kurth T, et al. Smoking, Smoking Cessation and Risk of Symptomatic Peripheral Artery Disease in Women: A Prospective Study. Ann Intern Med 2011;154(11):719–726. 10.1059/0003-4819-154-11-201106070-00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tobacco Control: Reversal of Risk after Quitting Smoking World Health Organization; 2007:235. [Google Scholar]
- 14.O’Muircheartaigh C, Eckman S, Smith S. Statistical Design and Estimation for the National Social Life, Health, and Aging Project. J Gerontol B Psychol Sci Soc Sci 2009;64B(Suppl 1):i12–i19. 10.1093/geronb/gbp045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith S, Jaszczak A, Graber J, et al. Instrument Development, Study Design Implementation, and Survey Conduct for the National Social Life, Health, and Aging Project. J Gerontol B Psychol Sci Soc Sci 2009;64B(Suppl 1):i20–i29. 10.1093/geronb/gbn013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jaszczak A, O’Doherty K, Colicchia M, et al. Continuity and Innovation in the Data Collection Protocols of the Second Wave of the National Social Life, Health, and Aging Project. J Gerontol B Psychol Sci Soc Sci 2014;69(Suppl 2):S4–S14. 10.1093/geronb/gbu031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Waite L. National Social Life, Health and Aging Project (NSHAP): Wave 3. 2017 doi: 10.3886/ICPSR36873.v3. [DOI] [PMC free article] [PubMed]
- 18.Mueller C, Renner B. A new procedure for the short screening of olfactory function using five items from the “Sniffin’’ Sticks” identification test kit.” Am J Rhinol 2006;20(1):113–116. [PubMed] [Google Scholar]
- 19.Kern DW, Wroblewski KE, Schumm LP, Pinto JM, Chen RC, McClintock MK. Olfactory Function in Wave 2 of the National Social Life, Health, and Aging Project. J Gerontol B Psychol Sci Soc Sci 2014;69(Suppl 2):S134–S143. 10.1093/geronb/gbu093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schumm LP, McClintock M, Williams S, et al. Assessment of sensory function in the National Social Life, Health, and Aging Project. J Gerontol B Psychol Sci Soc Sci 2009;64 Suppl 1:i76–85. 10.1093/geronb/gbp048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schubert CR, Cruickshanks KJ, Murphy C, et al. Olfactory Impairment in Adults. Ann N Y Acad Sci 2009;1170(1):531–536. 10.1111/j.1749-6632.2009.04102.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pinto JM, Schumm LP, Wroblewski KE, Kern DW, McClintock MK. Racial Disparities in Olfactory Loss Among Older Adults in the United States. J Gerontol Ser A 2014;69A(3):323–329. 10.1093/gerona/glt063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pfeiffer E A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc 1975;23(10):433–441. [DOI] [PubMed] [Google Scholar]
- 24.Kotwal AA, Schumm LP, Kern DW, et al. Evaluation of a brief survey instrument for assessing subtle differences in cognitive function among older adults. Alzheimer Dis Assoc Disord 2015;29(4):317–324. 10.1097/WAD.0000000000000068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shega JW, Sunkara PD, Kotwal A, et al. Measuring Cognition: The Chicago Cognitive Function Measure in the National Social Life, Health and Aging Project, Wave 2. J Gerontol B Psychol Sci Soc Sci 2014;69(Suppl 2):S166–S176. 10.1093/geronb/gbu106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maas AHEM, Appelman YEA. Gender differences in coronary heart disease. Neth Heart J 2010;18(12):598–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.D’Agostino Ralph B, Vasan Ramachandran S, Pencina Michael J, et al. General Cardiovascular Risk Profile for Use in Primary Care. Circulation 2008;117(6):743–753. 10.1161/CIRCULATIONAHA.107.699579 [DOI] [PubMed] [Google Scholar]
- 28.Frye RE, Schwartz BS, Doty RL. Dose-Related Effects of Cigarette Smoking on Olfactory Function. JAMA 1990;263(9):1233–1236. 10.1001/jama.1990.03440090067028 [DOI] [PubMed] [Google Scholar]
- 29.Nordin S, Monsch AU, Murphy C. Unawareness of Smell Loss in Normal Aging and Alzheimer’s Disease: Discrepancy between Self-Reported and Diagnosed Smell Sensitivity. J Gerontol Ser B 1995;50B(4):P187–P192. 10.1093/geronb/50B.4.P187 [DOI] [PubMed] [Google Scholar]
- 30.Wehling E, Nordin S, Espeseth T, Reinvang I, Lundervold AJ. Unawareness of Olfactory Dysfunction and its Association with Cognitive Functioning in Middle Aged and Old Adults. Arch Clin Neuropsychol 2011;26(3):260–269. 10.1093/arclin/acr019 [DOI] [PubMed] [Google Scholar]
- 31.Encouraging People to Stop Smoking. Department of Mental Health and Substance Abuse. World Health Organization; 2001. http://www.who.int/mental_health/evidence/stop_smoking_whomsdmdp01_4.pdf. Accessed August 7, 2018.
- 32.Gopinath B, Anstey KJ, Sue CM, Kifley A, Mitchell P. Olfactory impairment in older adults is associated with depressive symptoms and poorer quality of life scores. Am J Geriatr Psychiatry Off J Am Assoc Geriatr Psychiatry 2011;19(9):830–834. 10.1097/JGP.0b013e318211c205 [DOI] [PubMed] [Google Scholar]
- 33.Graziadei PP, Karlan MS, Graziadei GA, Bernstein JJ. Neurogenesis of sensory neurons in the primate olfactory system after section of the fila olfactoria. Brain Res 1980;186(2):289–300. [DOI] [PubMed] [Google Scholar]
- 34.Weinstock RS, Wright HN, Smith DU. Olfactory dysfunction in diabetes mellitus. Physiol Behav 1993;53(1):17–21. [DOI] [PubMed] [Google Scholar]
- 35.Gouveri E, Katotomichelakis M, Gouveris H, Danielides V, Maltezos E, Papanas N. Olfactory dysfunction in type 2 diabetes mellitus: an additional manifestation of microvascular disease? Angiology 2014;65(10):869–876. 10.1177/0003319714520956 [DOI] [PubMed] [Google Scholar]
- 36.Stamps JJ, Bartoshuk LM, Heilman KM. A brief olfactory test for Alzheimer’s disease. J Neurol Sci 2013;333(1):19–24. 10.1016/j.jns.2013.06.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilson RS, Arnold SE, Schneider JA, Boyle PA, Buchman AS, Bennett DA. Olfactory impairment in presymptomatic Alzheimer’s disease. Ann N Y Acad Sci 2009;1170(1):730–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Esiri MM, Nagy Z, Smith MZ, Barnetson L, Smith AD. Cerebrovascular disease and threshold for dementia in the early stages of Alzheimer’s disease. The Lancet 1999;354(9182):919–920. 10.1016/S0140-6736(99)02355-7 [DOI] [PubMed] [Google Scholar]
- 39.Jellinger KA. Alzheimer disease and cerebrovascular pathology: an update. J Neural Transm 2002;109(5):813–836. 10.1007/s007020200068 [DOI] [PubMed] [Google Scholar]
- 40.Duverger D, MacKenzie ET. The quantification of cerebral infarction following focal ischemia in the rat: influence of strain, arterial pressure, blood glucose concentration, and age. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab 1988;8(4):449–461. 10.1038/jcbfm.1988.86 [DOI] [PubMed] [Google Scholar]
- 41.Salonen R, Salonen JT. Progression of carotid atherosclerosis and its determinants: a population-based ultrasonography study. Atherosclerosis 1990;81(1):33–40. 10.1016/0021-9150(90)90056-O [DOI] [PubMed] [Google Scholar]
- 42.Pinto JM, Wroblewski KE, Kern DW, Schumm LP, McClintock MK. The Rate of Age-Related Olfactory Decline Among the General Population of Older U.S. Adults. J Gerontol A Biol Sci Med Sci 2015;70(11):1435–1441. 10.1093/gerona/glv072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mosca L, Barrett-Connor E, Wenger NK. Sex/Gender Differences in Cardiovascular Disease Prevention What a Difference a Decade Makes. Circulation 2011;124(19):2145–2154. 10.1161/CIRCULATIONAHA.110.968792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Criqui MH, Vargas V, Denenberg JO, et al. Ethnicity and peripheral arterial disease: the San Diego Population Study. Circulation 2005;112(17):2703–2707. 10.1161/CIRCULATIONAHA.105.546507 [DOI] [PubMed] [Google Scholar]
- 45.Drum ML, Shiovitz-Ezra S, Gaumer E, Lindau ST. Assessment of Smoking Behaviors and Alcohol Use in the National Social Life, Health, and Aging Project. J Gerontol Ser B 2009;64B(suppl_1):i119–i130. 10.1093/geronb/gbn017 [DOI] [PMC free article] [PubMed] [Google Scholar]