Abstract
Background:
A robust predictor of visual outcome in idiopathic intracranial hypertension (IIH) would be useful in management, but there is limited information on this point. The purpose of this study was to ascertain whether visual field mean deviation on standard static perimetry performed at diagnosis in a large patient cohort is a reliable predictor of visual outcome.
Methods:
We retrospectively reviewed the automated visual field mean deviations at diagnosis and at final encounter in 79 patients with IIH examined in the neuro-ophthalmology clinics at a single academic medical center from 1999 to 2015.
Results:
Of the 79 study patients, 66 (84%) entered with visual field mean deviations of −7 dB or better. Of those 66 patients, 59 (89%) had final mean deviations of −4 dB or better and 33 (56%) had final mean deviations of −2 dB or better. The single patient who had an initial mean deviation of −7 dB or better and a poor final mean deviation (−32 dB) was non-adherent to prescribed medication. Of the 13 (21%) patients who entered with mean deviations worse than −7 dB, 11 (85%) ended up with poor visual outcomes, their final mean deviations ranging from −5 dB to −32dB. Over half of those 13 patients had required surgery for IIH, often within 3 weeks of diagnosis, owing to severe papilledema and visual dysfunction at time of diagnosis.
Conclusions:
Based on this retrospective study, patients with IIH who have relatively mild visual dysfunction at diagnosis are likely to have a favorable visual outcome provided they are adherent to recommended treatment. Many of those with poor visual function at diagnosis will have unfavorable visual outcomes despite aggressive treatment.
With an incidence of 1-3 per 100,000 per year in the United States, idiopathic intracranial hypertension (IIH) is a common condition that particularly affects women of child-bearing age (1). Optic neuropathy is the major concern (2). Predicting the risk of lingering optic neuropathy is critical in designing a management program and in providing information regarding visual prognosis. Previous studies have shown that visual outcomes are not related to duration of symptoms, presence of visual obscurations, or recurrence (3). The degree of visual loss at onset (4, 5), systemic hypertension (6,7), male sex (8), African-American race (7), high body mass index (9), absence of headache (8), and thin ganglion cell /internal plexiform layer (5) have all been implicated as being associated with relatively poor visual outcome. A 1982 study of 57 IIH patients who were reexamined 5 to 41 years after diagnosis in the pre-quantitative perimetry era found that nearly 25% had disabling vision loss and that in nearly half of those eyes, the visual loss occurred months to years after diagnosis (4).
Based on our clinical experience that visual outcome could be predicted on the basis of entry visual function, we elected to test the hypothesis that patients with relatively good mean deviations on an automated visual field protocol at diagnosis would have good visual outcomes and those with relatively poor mean deviations on visual fields at diagnosis would have poor visual outcomes despite appropriately aggressive measures to lower intracranial pressure and to protect the optic nerves. We selected visual field mean deviation as a more meaningful psychophysical measure than visual acuity because IIH relatively spares visual acuity. Accordingly, we conducted a retrospective study of patients evaluated and treated at the University of Michigan Neuro-ophthalmology Clinics, using visual field mean deviation as the outcome measure.
METHODS:
After obtaining approval from the University of Michigan’s Institutional Review Board, we used the ICD9 terms “idiopathic intracranial hypertension” and “pseudotumor cerebri” to search the records from 1999 to 2015 for patients examined at the University of Michigan Neuro-ophthalmology Clinics. All patients had been fully evaluated by one or more fellowship-trained neuro-ophthalmologists (JDT, WTC, LBD). They met the modified Dandy criteria for IIH (2) and were above the age of 18 years. Patients were included only if they had a documented minimum of 6 months of inactive disease so that visual outcomes could be reliably assessed. We excluded patients for the following reasons: inadequate follow-up, partially treated elsewhere before reliable visual fields had been performed, refused lumbar puncture, no follow-up visual fields, consumed doxycycline or minocycline within months of symptom onset, surgery for IIH prior to evaluation in our clinics, or organ transplantation.
Data abstracted from the records included age at time of the initial evaluation, sex, disease duration, visual acuity, automated (Humphrey 24-2) visual field mean deviation, and ophthalmoscopic findings at every visit, as well as type of treatment. Information about patient race and initial and follow-up weight measurements were incomplete, so they were not included in the analysis. We defined the date of diagnosis as the date of first encounter in our clinic, which was never more than 1 week from initial identification of papilledema. We defined inactive IIH on follow-up examination as stable visual acuity and visual field, flat or pale optic discs on ophthalmoscopy, and absence of headache, blurred vision, transient obscurations of vision, and pulsatile tinnitus. A recurrence of IIH was defined as resumption of these symptoms or papilledema. Although we initially intended to include ophthalmoscopic data about optic disc appearance in the analysis, we determined that the clinicians’ judgments were too subjective and inconsistent to be useful. Optical coherence tomography and fundus photographs were not available on enough patients to be included. We collected information about symptoms, including headache, but considered it too subjective to be valuable in the analysis. Although the presence of a relative afferent pupil defect was documented on all patients, we did not include it in the analysis because it was so uncommon (present in only 5 patients), given that the optic neuropathy of IIH is generally symmetric between the eyes.
We divided the patients into two groups: those with relatively good visual field at diagnosis (Group 1) and those with relatively poor visual field at diagnosis (Group 2). A cut-off of −7dB mean deviation in the worse eye on Humphrey 24–2 protocol was chosen to separate the two groups based on the Idiopathic Hypertension Treatment Trial (2). We defined a favorable visual outcome as a visual field mean deviation of equal or better than −4 dB in the more affected eye at the last follow-up visit and an unfavorable visual outcome as a mean deviation of worse than −4 dB in the more affected eye at last follow-up visit. We chose a cut-off of −4 dB because our experience has shown that patients are not apt to be disturbed by visual dysfunction at or better than that level of mean deviation. Descriptive statistics were applied to patient demographics, treatment, and clinical course.
RESULTS
Demographics
Of 594 patients identified with a diagnosis of IIH, 79 met entry criteria. The 79 patients included in the data set had a mean age of 32 years (range: 18 to 62 years); 72 (91%) were women. Disease duration from initial diagnosis to prolonged stability ranged from 2.7 months to 13 years (median: 14.9 months); 38/79 (48%) patients had a disease duration of greater than 24 months.
Treatment
Seventy-four patients were treated initially with acetazolamide and advised to lose weight. Among them, 19 (26%) later received a second intracranial pressure-lowering medication (methazolamide, furosemide, or topiramate). Two patients were intolerant of acetazolamide and were treated instead with furosemide; one patient had been started and kept on furosemide for another medical condition. Acetazolamide was always started within 14 days after lumbar puncture at 500-1000mg/day with gradual escalation up to a maximum of 4000mg/day and a slow taper based on evidence of recovery.
Eleven patients (14%) required surgery, including seven ventriculoperitoneal shunts (VPS), one lumboperitoneal shunt (LPS), and three optic nerve sheath fenestrations (ONSF). Four of the 11 surgically-treated patients received their procedure within 20 days of presentation due to the severity of visual dysfunction. The remaining seven surgically-treated patients required the procedure because their visual function continued to worsen despite maximum tolerated medical treatment or because they did not adhere to the medication regimen, owing to intolerance or non-cooperation.
Clinical Course
Group 1.
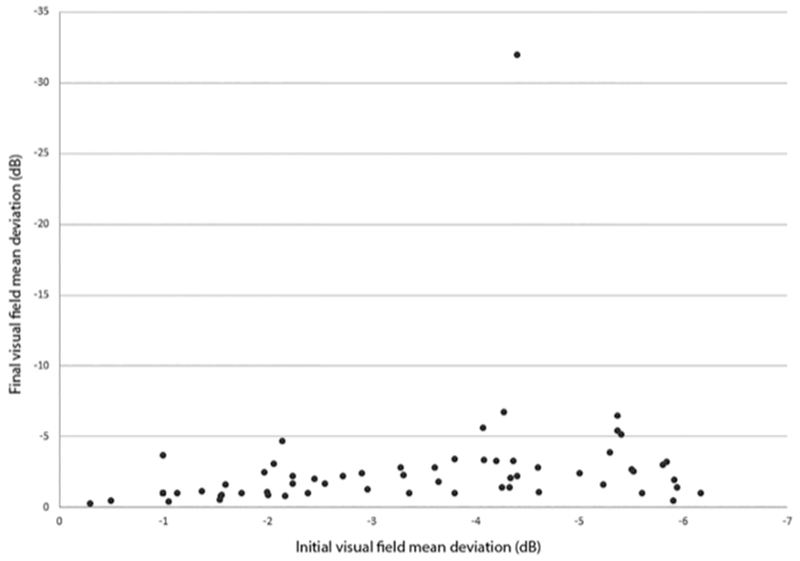
Of the 66 patients in this group, 59 (89%) had a final visual field mean deviation better than −4 dB. Among those 59 patients, 33 (56%) had final mean deviations better than −2 dB. Two of the 59 patients had final mean deviations that were slightly worse than their initial mean deviations, but still better than −4 dB. (Fig 1).
Figure 1:
Initial and final visual field mean deviations in 66 patients with initial mean deviations of −7dB or better (Group 1).
Seven patients (10%) in Group 1 had final mean deviations worse than −4 dB in the more affected eyes. However, six of them had final mean deviations that were only mildly worse than their initial mean deviations. Two of these patients had required surgery after a trial of medication, and 5 had been treated with medication alone. The only patient whose final mean deviation was much worse than the initial mean deviation (−32 dB versus −4.4 dB) had been non-adherent to prescribed acetazolamide owing to inability to afford the cost of this medication. That patient underwent VPS three years after diagnosis because of declining visual function. Forty-seven of the 66 patients in this group were managed with acetazolamide alone; 16 required an additional medication. Six patients eventually required surgical treatment (four VPS, two ONSF) due to medication intolerance, noncompliance, or maximum medical treatment with minimal visual improvement at an interval ranging from four months to nine years after diagnosis.
Group 2.
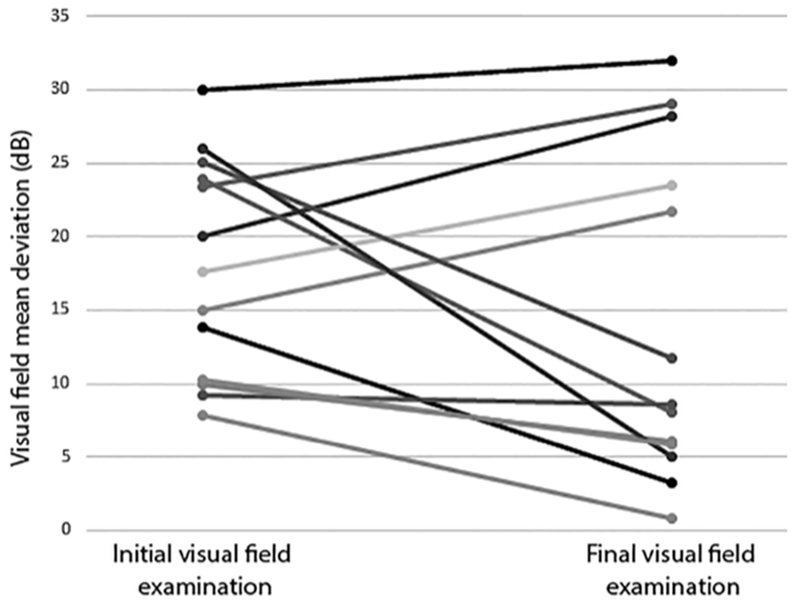
Of the 13 patients in this group, 11 (79%) had a poor visual outcome. Their initial mean deviations were generally much worse than those of patients in Group 1, ranging from −9.3 dB to −30 dB (mean −19 dB) and their final visual mean deviations ranged from −5 dB to −32 dB. Only two patients (21%) in this group had a good visual field outcome, ranging from −0.8 dB to −3.3 dB (mean −2.1 dB); both patients could be managed with acetazolamide alone. (Fig 2).
Figure 2:
Initial and final visual field mean deviations in 13 patients with initial mean deviations worse than −7dB (Group 2).
Among the 11 patients with poor visual outcomes in this group, five (45%) had required surgery (three VPS, one LPS, one ONSF), all occurring within 20 days of the initial visit. Two patients had such severe visual dysfunction at diagnosis that they were taken for VPS immediately. Five patients were initially treated with acetazolamide, but because of minimal improvement in visual function, they were taken to surgery within 20 days of diagnosis. The remaining patient had ONSF on the sixth day after diagnosis, but later required escalating doses of acetazolamide and finally VPS because of lack of visual improvement.
DISCUSSION
In this retrospective cohort of patients with IIH, Humphrey 24–2 visual field mean deviation at time of diagnosis was a strong predictor of final visual field outcome, determined at a time when the disease was considered quiescent for at least six months.
In our study, 84% of the patients had an entry visual field mean deviation of −7dB or better. In that group, 89% had a final visual field mean deviation better than −4 dB. The single patient who had a poor final mean deviation (−32 dB) was non-adherent to medication and required VPS three years after diagnosis. Most of the patients could be managed on acetazolamide alone, with only 10% requiring surgery.
Only 13 (16%) patients entered with mean deviations of worse than −7 dB (Group 2), and 85% of them ended up with relatively poor final visual outcomes, their mean deviations ranging from −5 dB to −32dB. Final mean deviations were worse than −10 dB in over half of this cohort and many had required surgery, often within three weeks of diagnosis, owing to severe papilledema and visual dysfunction at time of diagnosis.
The visual outcomes in our cohort do not necessarily reflect long term outcome, as many patients were not followed for more than 2 years. We acknowledge the findings in a longer term visual outcome study of 57 patients conducted before the advent of quantititative static perimetry (4). In that study, some of the cohort suffered disabling vision loss that did not occur until long after diagnosis. The authors concluded that “visual loss in pseudotumor cerebri may occur either early or late in the disease process” and they counseled that long term vision monitoring is necessary to detect this late-arising vision loss.
Our results conform to those of a more recent smaller study aimed chiefly at assessing the value of OCT GPL thickness as a predictor of visual outcome (5). The patients with retinopathy had better visual outcomes than those with optic neuropathy alone. Our study differs methodologically in that it did not take notice of retinopathy, and did not include OCT or kinetic perimetry.
We believe that our cohort is representative of patients managed for IIH in American tertiary care academic medical centers in terms of latency from symptoms to diagnosis, visual function and degree of papilledema at diagnosis, and treatment. However, we acknowledge several limitations of this retrospective study. First, we did not have sufficient information to examine the other factors reported to be predictive of visual loss in IIH, including systemic hypertension (6,7), African-American race (7), high body mass index (9), absence of headache (8), and OCT (5). Second, patients were not managed in a pre-arranged standard fashion. They were managed by three different neuro-ophthalmologists who may have had non-uniform approaches to care, particularly with regard to indications and timing of surgery. Third, we cannot be certain that visual function remained stable in the long term, as most patients had only brief periods of documented quiescence. Even so, we believe that our results allow the following two inferences:
A large majority of patients managed with IIH in a tertiary care center will have mildly elevated visual field mean deviations at diagnosis; they will tolerate acetazolamide, be adherent to that medication, and ultimately achieve mean deviations at a level that will not disturb their vision; very few patients will require surgery to lower intracranial pressure or to protect the optic nerves; the few patients with poor visual outcomes in this group are those who are non-adherent to medical therapy
A minority of patients managed with IIH in a tertiary care center will have poor visual fields at diagnosis; some will sustain improvement in visual function, but most will not have a satisfactory visual outcome despite aggressive treatment, including surgery
Because lack of complete data in this retrospective study precluded our including patient demographic and optic fundus features in our analysis, we cannot claim that presenting visual field examination is an independent predictor of visual outcome. But with that understanding, we believe that our data show that IIH is generally a manageable condition with minimal visual morbidity in the short term in patients who present with modest visual dysfunction and are adherent to the recommended treatment. For patients with poor visual function at diagnosis, treatment may be effective in preventing a further decline in vision, but it will not usually restore adequate visual function, especially in patients who are non-adherent to medication. Early diagnosis might be the only way to improve the visual prognosis for this unfortunate minority of patients. These results do not alter the need for aggressive treatment to lower intracranial pressure, promote weight loss, and maintain close follow-up.
References
- 1.Bruce BB, Biousse V, Newman NJ Update on idiopathic intracranial hypertension. Am J Ophthalmol 2011;152:163–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wall M, Kupersmith MJ, Kieburtz KD, Corbett JJ, Feldon SE, Friedman DI, Katz DM, Keltner JL, Schron EB, McDermott MP. The idiopathic intracranial hypertension treatment trial: clinical profile at baseline. JAMA Neurol 2014;71:693–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rush JA. Pseudotumor cerebri: clinical profile and visual outcome in 63 patients. Mayo Clinic Proc 1980;55:541–546. [PubMed] [Google Scholar]
- 4.Corbett JJ, Savino PJ, Thompson HS, Kansu T, Schatz NJ, Orr LS, Hopson D. Visual loss in pseudotumor cerebri: Follow-up of 57 patients from 5 to 41 years and a profile of 14 patients with permanent severe visual loss. Arch Neurol 1982;39:461–474. [DOI] [PubMed] [Google Scholar]
- 5.Chen JJ, Thurtell MJ, Longmuir RA, Garvin MK, Jui-Kai W, Wall M, Kardon RH. Causes and prognosis of visual acuity loss at the time of initial presentation in idiopathic intracranial hypertension. Invest Ophthalmol Vis Sci 2015;56:3850–3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wall M George D. Idiopathic intracranial hypertension: A prospective study of 50 patients. Brain. 1991;114155–180. [PubMed] [Google Scholar]
- 7.Bruce BB, Preechawat P, Newman NJ, Lynn MJ, Biousse V. Racial differences in idiopathic intracranial hypertension Neurology. 2008;70:861–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruce BB, Kedar S, Van Stavern GP, Monaghan D, Acierno MD, Braswell RA, Preechawat P, Corbett JJ, Newman J, Biousse V. Idiopathic intracranial hypertension in men. Neurology. 2009;72:304–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Szewka AJ, Bruce BB, Newman NJ, Biousse V. Idiopathic intracranial hypertension: relation between obesity and visual outcomes. J Neuroophthalmol 2013;33:4–8. [DOI] [PMC free article] [PubMed] [Google Scholar]