Abstract
Purpose
The objective of this study is to analyze the potential of immature, denuded, post-GVBD (germinal vesicle breakdown) oocytes (including prometaphase I, metaphase I, and prometaphase II stages) to result live birth after in vitro maturation. Furthermore, we compared two culture media to identify which of them provides better reproductive outcomes when used for in vitro maturation.
Methods
We performed a retrospective cohort study including 4022 IVF-ICSI (in vitro fertilization-intracytoplasmic sperm injection) cycles between 2011 and 2015. A total of 4450 immature post-GVBD oocytes from 1442 cycles were cultured in vitro; of these, 2364 oocytes reached MII (metaphase II) stage (IVMC oocytes, in vitro meiotic completion) and were fertilized. Overall, 3933 embryo transfers were performed: 3579 were embryos derived from MII oocytes (ET-MII); 264 were embryos derived from MII + IVMC oocytes (ET-MIX), and 90 embryos from IVMC oocytes (ET-IVMC). In total, 399 IVMC embryos were transferred.
Results
Maturation rate for immature post-GVBD oocytes was 54.1%. G-2™PLUS (Vitrolife) medium provided significantly higher maturation rate (p < 0.001) than G-IVF™PLUS (Vitrolife) (65.7% vs. 42.5%, p < 0.001). Embryos in ET-IVMC in cleavage stage had an average morphological score of 6.8/10 (7.7 in ET-MII; p < 0.001). Regarding reproductive outcomes, ET-IVMC gave 11.1% biochemical pregnancy rate, 10.0% clinical pregnancy rate, 7.8% ongoing pregnancy rate, and 5.6% live birth rate.
Conclusions
Embryos arising from IVMC oocytes resulted in a live birth rate of 5.6%. We suggest that in vitro maturation of denuded immature post-GVBD oocytes should be performed at the very least when few MII are collected, and likely in all patients, as they provide acceptable maturation and fertilization rates, and a sizeable increase in live birth.
Electronic supplementary material
The online version of this article (10.1007/s10815-019-01540-8) contains supplementary material, which is available to authorized users.
Keywords: IVMC oocyte, In vitro maturation, Live birth, Immature post-GVBD oocyte, Culture medium
Introduction
During each menstrual cycle, a cohort of follicles start a process of growth and maturation during which the oocytes inside the follicles undergo nuclear and cytoplasmic maturation to reach their full developmental competence, i.e., the ability to support early embryonic development. Nuclear maturation requires the progression from prophase I (germinal vesicles (GV)) to metaphase II (MII) of meiosis, signaled by the extrusion of the first polar body (PB). Nevertheless, no visible morphological changes take place when the oocyte completes cytoplasmic maturation, essential for fertilization and early embryonic development [1].
Frequently, a portion of the oocytes is still immature at the time of oocyte pick-up (OPU) [2–4]; about 4% are in metaphase I (MI) and 11% at the GV stage [4], although these rates may differ slightly across studies depending on stimulation protocols and patient demographics. Oocytes might be immature at OPU for several reasons: the follicular stage when the hormonal stimulation was initiated, aspiration of smaller follicles during OPU, patient age, or poor response to stimulation [5]. Within ART patients, the use of in vitro meiotic completion (IVMC), i.e., the culture of immature oocytes for a few hours to allow for the extrusion of the first PB, might be worthwhile [5]. A higher number of mature oocytes could increase the number of oocytes available for fertilization, resulting in a higher number of embryos obtained in each cycle and therefore potentially increasing the chances of pregnancy and live birth [3, 6]. However, immature oocytes at OPU present lower fertilization rates after IVMC [3–6], present higher rates of abnormal fertilization [6, 7], worse embryo development [5–7], diminished implantation capacity [5], and lower pregnancy rates compared with in vivo matured MII oocytes [5, 8].
Culture conditions are crucial for a correct in vitro maturation [1, 9, 10]. Two different aspects should be considered regarding oocyte maturation: the environmental maturation conditions [1] and the incubation time in the media of choice [7]. Regarding the first aspect, a specific medium for the in vitro maturation of denuded immature oocytes at OPU has not been developed so far. Regarding the second aspect, a minimum of 3 h of culture after nuclear maturation and before ICSI has been reported as optimal to reach reasonable fertilization rates in in vitro matured oocytes [7].
The objective of this study is to analyze the potential of immature, denuded, post-GVBD (germinal vesicle breakdown) oocytes to produce a live birth once matured in vitro. Furthermore, we evaluate two culture media to identify which provides better reproductive outcomes when used for in vitro culture.
Materials and methods
Study population and ethical approval
This is a retrospective study including data from 4022 consecutive IVF-ICSI cycles, corresponding to 3339 patients between January 2011 and December 2015. Ethical approval for performing this study was obtained from the institutional Ethics Committee for Clinical Research.
In vitro oocyte maturation and laboratory procedures
Patients underwent controlled ovarian stimulation (COS) with follitropin alfa, follitropin beta, or highly purified urinary hMG (from 150 to 300 IU/day). Pituitary suppression was obtained by either a long protocol of GnRH agonist (triptorelin 0.1 mg, beginning the 21st day of the menstrual cycle previous to the IVF cycle, decreased to 0.05 mg when starting stimulation) or a GnRH antagonist protocol (cetrorelix 0.25 mg/day, beginning when estradiol raised ≥ 400 pg/ml or one follicle reached 14 mm of diameter). When at least one follicle reached 17 mm of diameter, final oocyte maturation was triggered with 250 μg (6500 IU) of choriogonadotropin alfa. Oocyte retrieval was performed strictly 36 h after triggering by ultrasound-guided transvaginal OPU. A strict control of laboratory times was guaranteed by an automated electromagnetic tracking system (RI Witness® System, Origio, Denmark). Cumulus oophorus complexes (COCs) were placed in a dish containing 1.5 ml of G-MOPS™ PLUS (Vitrolife, Sweden) medium covered with 1.5 ml of IRVINE Scientific® oil, where they were kept until the end of the follicular aspiration procedure. COCs were then washed twice in 1.5 ml of G-IVF™PLUS medium (Vitrolife, Sweden) covered with 1.5 ml of OVOIL™ (Vitrolife, Sweden) and incubated for 30 min at 37 °C and 6% of CO2. Cumulus cells were then mechanically removed in hyaluronidase (HYASE 10+™, Vitrolife, Sweden) 1:10 diluted with G-MOPS™PLUS. Oocytes were then classified as MII, immature post-GVBD oocytes (including prometaphase I, metaphase I, and prometaphase II stages), or GV. Immature post-GVBD oocytes were washed in 25 μl of equilibrated G-IVF™PLUS medium and placed for maturation in a droplet of 25 μl of the appropriate medium for further maturation.
Two culture media for final meiotic maturation were used: G-IVF™PLUS and G-2™PLUS (Vitrolife, Sweden). Both are bicarbonate-buffered media containing human serum albumin and gentamicin as an antibacterial agent. G-IVF™PLUS was used from January 2011 to October 2013, after which medium was switched to G-2™PLUS. Usually, ICSI was performed approximately 2–4 h after oocyte pick-up. Before starting ICSI, post-GVDB oocytes were observed to evaluate maturity. If post-GVBD oocytes had extruded the PB, they were injected alongside MII stage oocytes; if not, they were cultured further and checked every 2 h for PB extrusion. Upon PB extrusion, they were injected. Monitoring of the exact incubation time from OPU to ICSI once PB is extruded was carried out for each patient using an automated electromagnetic tracking system (RI Witness® System).
After ICSI, oocytes were washed in a droplet of 15 μl G-1™PLUS (Vitrolife, Sweden) and placed individually in droplets of 15 μl of G-1™PLUS. Oocytes were observed for fertilization results 16–18 h after ICSI. Only embryos with 2PN were kept in culture. Embryo evaluation was performed on the second, third, or fifth day of embryo development (D2, D3, D5), depending on the day of embryo transfer (ET). The score was assessed using a modified scale system initially proposed by Coroleu and colleagues [11], which takes into account number of blastomeres, symmetry of blastomeres, and percentage of fragmentation, giving a maximum score of 10. As no numerical score is assigned to the embryos transferred on D5, the score used for these embryos for statistical analysis corresponds to the numerical score they had on D3. The highest scoring embryos were chosen for ET and the remaining good-quality embryos were cryopreserved. For statistical analysis, only fresh ETs were taken into account. Most ETs were done with embryos originated from MII oocytes. When no embryos from MII oocytes were available, embryos originated from IVMC oocytes were transferred instead. After ET, a protocol for luteal phase support (400 mg of progesterone by vaginal administration for the first 8 weeks of pregnancy) was prescribed to patients.
The procedures described above remained identical during all the study period, limiting potential bias.
Statistical analysis
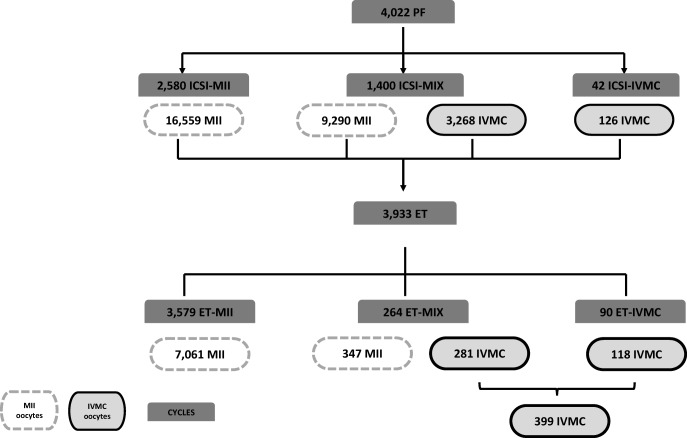
Laboratory outcomes (in vitro maturation rate and fertilization rate) were analyzed according to the database of oocytes available for ICSI (MII oocytes retrieved at OPU and matured IVMC). The 4022 ICSI cycles of this database were classified according to oocyte maturation: the “ICSI-MII” group, comprising cycles with only MII oocytes; the “ICSI-MIX” group, comprising cycles with both MII and IVMC oocytes; and the “ICSI-IVMC” group, comprising cycles with only IVMC oocytes (Fig. 1).
Fig. 1.
Study groups analyzed based on the oocytes available for ICSI and the origin of the oocytes that generated the transferred embryos
Reproductive outcomes (biochemical pregnancy: a positive beta-HCG level in serum 14 days after embryo transfer; clinical pregnancy: one or more intrauterine sacs visible by ultrasound scan at 7 weeks of pregnancy; ongoing pregnancy: pregnancy confirmed at the week 12; and live birth) were analyzed according to the database of embryos available for ET. The 3933 ET cycles of this database were classified according to oocyte maturation in the following: “ET-MII” group, comprising ET cycles of embryos originating only from MII oocytes; the “ET-MIX” group, comprising ET cycles of embryos originating from both MII and IVMC oocytes; and the “ET-IVMC” group, comprising ET cycles of embryos originating only from IVMC oocytes.
Univariable analyses with parametric tests (ANOVA) were performed to evaluate the differences in laboratory outcomes, while non-parametric tests (Pearson’s chi2) were used for reproductive outcomes among the groups defined by oocyte maturation, as well as between culture media. Post hoc pairwise comparisons with Bonferroni’s correction were further applied to identify statistically significant differences compared with the ICSI-MII or the ET-MII groups.
To calculate the pregnancy probability reduction (relative risk) of transferring IVMC and mixed vs. MII embryos adjusted for potential confounders (age, BMI, and number of transferred embryos), a general linear model procedure was performed (Poisson family, robust error variance calculation, log link function), according to the methodology proposed by Zhou et al. [12].
Additionally, the effect of the time elapsed between OPU and ICSI on maturation rate was evaluated by means of locally weighted scatterplot smoothing (LOWESS) regression, with a fit to 50% of the points and an Epanechnikov weight function.
Analyses were performed using SPSS version 22.0 and Stata version 13.0. A p value < 0.05 was set as statistically significant.
Results
Baseline characteristics at times of ICSI and ET
Overall, the mean age of the patients included in the study was 37.7 years old (SD 4.8) and the mean BMI was 23.7 kg/m2 (SD 4.1). Women in the ICSI-IVMC group were significantly older and presented a higher BMI than women in ICSI-MII and ICSI-MIX groups. The mean number of cumulus oocyte complexes (COCs) retrieved was significantly higher for the ICSI-MIX group. The longest mean incubation time for the ICSI-IVMC group was 6.6 h (SD1.7 h). The baseline characteristics at time of ICSI by the study group are presented in Table 1. Of the 4022 ICSI cycles evaluated, 2580 included only MII oocytes (ICSI-MII; n = 16,559 MII oocytes); 1400 included a mixture of MII and IVMC oocytes (ICSI-MIX; n = 9290 MII and 3268 IVMC oocytes); and 42 included only IVMC oocytes (ICSI-IVMC; n = 91 IVMC oocytes) (Fig. 1).
Table 1.
Baseline characteristics of patients at time of ICSI by the study group (ICSI-MII, ICSI-MIX, ICSI-IVMC)
| Overall (n = 4022) | ICSI-MII (n = 2580) | ICSI-MIX (n = 1400) | ICSI-IVMC (n = 42) | p-value1 | |
|---|---|---|---|---|---|
| Age (years); mean (SD) | 37.7 (4.8) | 37.8 (4.8) | 37.2 (4.7)* | 38.8 (5.3) | 0.001 |
| BMI (kg/m2); mean (SD) | 23.6 (4.1) | 23.6 (4.1) | 23.7 (4.1) | 25.9 (5.5)* | 0.001 |
| Oocyte recovered at OPU; mean (SD) | |||||
| COC | 9.2 (6.0) | 8.4 (5.9) | 10.9 (5.8)* | 4.6 (3.8)* | < 0.001 |
| MII | 6.4 (4.4) | 6.4 (4.5) | 6.6 (4.1) | 0* | < 0.001 |
| MI | 1.1 (1.6) | 0.4 (0.9) | 2.3 (1.8)* | 3.0 (2.1)* | < 0.001 |
| GV | 1.1 (1.8) | 0.9 (1.6) | 1.4 (2.0)* | 0.4 (0.9) | < 0.001 |
| IVMC | 0.6 (1.0) | 0 | 1.6 (1.0)* | 2.2 (1.3)* | < 0.001 |
| Timeframe between OPU and ICSI (h); mean (SD) | 4.6 (1.8) | 4.2 (1.5) | 5.3 (2.1)* | 6.6 (1.7)* | < 0.001 |
COC cumulus-oophorus complex, GV germinal vesicle, ICSI = intracytoplasmic injection, IVMC in vitro meiotic completion, MI metaphase I oocytes (immature post-GVBD oocytes), MII metaphase II oocytes (mature oocytes), OPU oocytes pick-up, SD standard deviation
*Statistically siginificant difference after pairwise comparison with Bonferroni’s correction between this group and ICSI-MII
1One-way ANOVA or Pearson’s Chi2 test, as appropriate
Of the 3933 ET performed, 3579 included only embryos developed from MII oocytes (ET-MII; n = 7061 embryos), 264 ET were mixed transfers (ET-MIX; n = 347 MII-embryos and n = 281 IVMC embryos), and 90 ET included only IVMC embryos (ET-IVMC; n = 118 embryos) (Fig. 1). Some of the ET-IVMC embryos came from IVMC oocytes included in the ICSI-MIX group. In total, 399 IVMC embryos were transferred fresh. Double ET was common in ET-MII and ET-MIX (61.0% and 62.1% respectively), whereas single ET was most frequent in the ET-IVMC group (72.2%; p < 0.001). Other ET characteristics are presented in Table 2.
Table 2.
Embryo transfer (ET) characteristics by the study group (ET-MII, ET-MIX, ET-IVMC)
| ET-MII (n = 3579) | ET-MIX (n = 264) | ET-IVMC (n = 90) | p-value1 | |
|---|---|---|---|---|
| Origin of sperm; n (%) | ||||
| Partner | 2002 (55.9) | 133 (50.4) | 60 (66.7) | 0.018 |
| Donor | 1577 (43.7) | 131 (49.6) | 30 (33.3) | |
| Status of sperm; n (%) | ||||
| Fresh | 1347 (37.7) | 90 (34.1) | 35 (38.9) | 0.48 |
| Frozen | 2228 (62.3) | 174 (65.9) | 55 (61.1) | |
| ET day; n (%) | ||||
| D2–4 | 3442 (96.2) | 258 (97.7) | 89 (98.9) | 0.18 |
| D5 | 137 (3.8) | 6 (2.3) | 1 (1.1) | |
| Transferred embryos; n (%) | < 0.001 | |||
| 1 | 747 (20.9) | 0 (0.0)* | 65 (72.2)* | |
| 2 | 2182 (61.0) | 164 (62.1) | 22 (24.4)* | |
| 3 | 650 (18.2) | 100 (37.9)* | 3 (3.3)* | |
| Mean embryo quality at D2–D3 of transferred embryos; mean (SD) | 7.7 (1.3) | 7.6 (1.3) | 6.8 (1.2)* | < 0.001 |
COC cumulus-oophorus complex, GV germinal vesicle, ICSI intracytoplasmic injection, IVMC in vitro meiotic completion, MI metaphase I oocytes (immature post-GVBD oocytes), MII metaphase II oocytes (mature oocytes), n number of cycles, OPU oocyte pick-up, SD standard deviation
*Statistically siginificant difference after pairwise comparison with Bonferroni’s correction between this group and ICSI-MII
1One-way ANOVA or Pearson’s Chi2 test, as appropriate
In vitro maturation, fertilization rates, and embryo quality
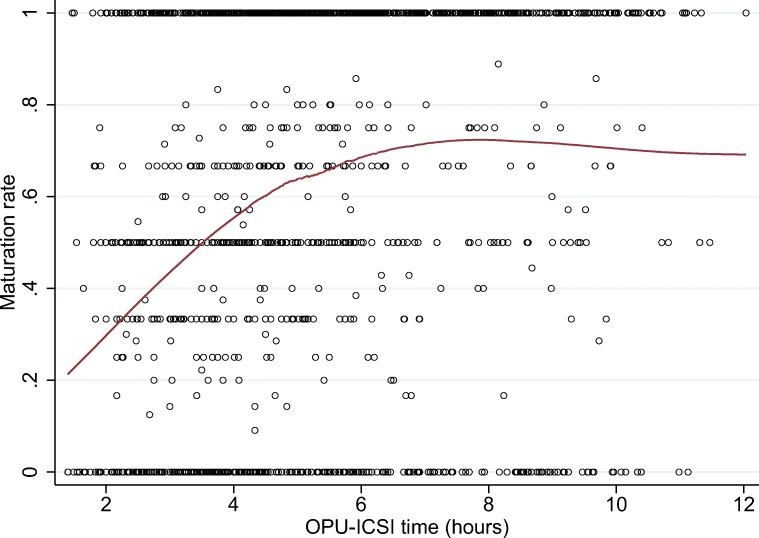
Overall, the in vitro maturation rate was 54.1%. As expected, an increase in in vitro maturation rates was observed as incubation time increased, reaching a plateau after 8 h (Fig. 2).
Fig. 2.
In vitro maturation rate across the time elapsed between the oocyte pick-up (OPU) and ICSI by means of locally weighted scatterplot smoothing (LOWESS) regression
The mean fertilization rates were 68.1% and 47.9% for the ICSI-MII and ICSI-IVMC groups respectively (p < 0.001). The number of non-fertilized oocytes was higher for IVMC oocytes than for MII oocytes: 35.7% vs. 16.1% (p < 0.001). However, we observed similar results for abnormal fertilization (6.4% in the ICSI-MII group vs. 6.8% in the ICSI-IVMC group, p = 0.48), as well as percentage of degenerated oocytes (9.4% in the ICSI-MII group vs. 9.6% in the ICSI-IVMC group, p = 0.79). Embryos in the ET-IVMC group presented lower morphological scores (6.8, SD 1.2) compared with those included in the ET-MII group (7.7, SD 1.3) (p < 0.001).
Reproductive outcomes
Reproductive outcomes by the study group (ET-MII, ET-MIX, ET-IVMC) are presented in Table 3. As expected, all reproductive outcomes were significantly higher in the ET-MII group, although they were also sizeable for the ET-IVMC group: biochemical pregnancy rate was 11.1%, clinical pregnancy rate 10.0%, ongoing pregnancy rate 7.8%, and live birth 5.6%. Supplementary table 1 provides the main characteristics of the five ET-IVMC cycles resulting in live birth.
Table 3.
Reproductive outcomes by the study group (ET-MII, ET-MIX, ET-IVMC)
| ET-MII (n = 3581) | ET-MIX (n = 264) | ET-IVMC (n = 90) | p-value1 | |
|---|---|---|---|---|
| Biochemical pregnancy, n (%) | 1319/3577 (36.9) | 84/264 (31.8) | 10/90 (11.1)* | < 0.001 |
| Implantation rate at 7 weeks, sacs/transferred embryos (%) | 1174/7061 (16.6) | 68/628 (10.8) | 9/118 (7.6)* | < 0.001 |
| Clinical pregnancy, n (%) | 1061/3520 (30.1) | 65/264 (24.6) | 9/90 (10.0)* | < 0.001 |
| Ongoing pregnancy, n (%) | 858/3519 (24.4) | 49/263 (18.7) | 7/90 (7.8)* | < 0.001 |
| Live birth, n (%) | 632/3348 (18.9) | 39/255 (15.3) | 5/89 (5.6)* | 0.003 |
ET embryo transfer, IVMC in vitro meiotic completion, n number of cycles, MII metaphase II
*Statistically siginificant difference after pairwise comparison with Bonferroni’s correction between this group and ICSI-MII
1Pearson’s Chi2 test
Double ET gave an average implantation rate of 13.1% and 20.2% in the ET-MIX and ET-MII groups respectively (p = 0.002). Implantation rates for single ET were significantly lower in the ET-IVMC group compared with the ET-MII group (6.2% vs. 14.2% (p = 0.09)).
Supplementary table 2 shows the pregnancy probability reduction when IVMC-derived embryos or mixed embryos were transferred instead of MII-derived embryos, after adjustment for potential confounders. To note, the probability of live birth was reduced by 53% (95% CI 80% reduction, 11% increase; p = 0.08) and by 19% (95% CI 40% reduction, 9% increase; p = 0.17), for IVMC-derived embryos and mixed embryos, respectively. Nevertheless, the chances of pregnancy with IVMC-derived embryos are not negligible.
Comparison between incubation media
The incubation medium for immature post-GVBD oocytes was either G-IVF™PLUS (n = 2421 oocytes) or G-2™PLUS (n = 2029 oocytes). A significantly higher in vitro maturation rate was registered when using G-2™PLUS vs. G-IVF™PLUS (65.7% vs. 42.5%, p < 0.001). Similarly, higher fertilization rates were observed with G-2™PLUS than with G-IVF™PLUS (54.5% vs. 40.7% (p < 0.001)). Abnormal fertilization rate was, however, comparable between the two media (G-IVF™PLUS, 7.2% vs. G-2™PLUS, 6.7%; p = 0.76), whereas a higher number of non-fertilized oocytes were observed with G-IVF™PLUS (44.5% vs. 27.6%, p < 0.001). The average morphological score for embryos originated from IVMC matured in G-2™PLUS medium (6.3, SD 1.6) was similar to that of embryos matured in G-IVF™PLUS (6.4, SD 1.5) (p = 0.45).
When analyzing reproductive outcomes by maturation media, oocytes in ET-IVMC seemed to give a higher clinical pregnancy rate when matured in G-2™PLUS compared with G-IVF™ PLUS (12.1% vs. 6.3%), although this difference was not statistically significant (p = 0.38). The size of the groups was too small to draw conclusions: 7/58 vs. 2/32 positive clinical pregnancies, respectively (Table 4).
Table 4.
Reproductive outcomes by maturation media
| n = number of cycles | G-IVF™ PLUS (Vitrolife) (n = 32) | G-2™Plus (Vitrolife) (n = 58) | p-value1 |
|---|---|---|---|
| Biochemical, n (%) | 2 (6.3) | 8 (13.8) | 0.28 |
| Clinical, n (%) | 2 (6.3) | 7 (12.1) | 0.38 |
| Ongoing, n (%) | 2 (6.3) | 5 (8.6) | 0.69 |
| Live birth, n (%) | 2 (6.3) | 3 (5.3) | 0.85 |
1Pearson’s Chi2 test
Discussion
The clinical use of immature post-GVBD oocytes might be worthwhile in order to increase the number of transferrable embryos in a patient, hence increasing cycle efficiency and pregnancy rates. However, several studies have shown that IVMC oocytes have a compromised developmental competence [5–7].
Nevertheless, these studies either date back several years ago (i.e., were performed using laboratory techniques and equipment currently superseded in the ART field [4]) or had small sample sizes in terms of ET [3]; in addition, IVMC oocyte developmental competence was not scored. Moreover, since there are no commercial media designed to support the final stages of meiotic maturation, there is no current guidance on the type of medium that might preserve their ability to develop into viable pregnancies. In the present study, a total of 2364 IVMC-fertilized oocytes were analyzed; importantly, 354 ET cycles included at least one embryo derived from IVMC, making it one of the largest studies of IVMC outcomes to date.
Both nuclear and cytoplasmic maturation are required for immature post-GVBD oocytes in order to gain developmental competence [7]. In our study, overall, 54.1% of immature post-GVBD oocytes retrieved at OPU extruded the first PB after in vitro culture. Similar maturation rates had been previously reported: 43.0% when cultured in Sydney IVF Fertilization Medium (Cook, Ltd., Ireland) or Early Cleavage Medium (Irvine Scientific, Belgium) from 2 to 11 h after OPU and 45.1% when cultured in G1.2 (Vitrolife, Sweden) from 1.5 to 4.5 h after OPU [5, 6]. In our study, a significant higher maturation rate was observed when using G-2™PLUS rather than G-IVF™PLUS.
Culture conditions have a significant influence on the ability of oocytes to complete meiosis in vitro [1, 9]. In the absence of a specifically formulated medium for in vitro maturation, G-IVF™PLUS and G-2™PLUS were used in our laboratories for this purpose. Unfortunately, detailed official compositions of these media are not available, although a summary of their components is given by the manufacturer. However, the G-2™PLUS protein content has been analyzed independently [13]. The presence of hyaluronan in G-2™PLUS represents the main difference compared with G-IVF™PLUS. The ability of granulosa cells to synthesize hyaluronan, essential for oocyte maturation and further embryo development, is well known [14]. While culture with cumulus cells did not seem to alter significantly the maturation rate of MI to MII stage oocytes [15], a considerable but not significant improvement in the maturation rates of GV cultured with granulosa cells was reported [16]. Nevertheless, well-designed comparative studies carried out with media specially designed for in vitro maturation in the future would be helpful to identifying a medium ideal for maturation.
In vitro incubation time is another crucial factor [7]. Most immature post-GVBD oocytes extruded the first PB between 2 and 8 h of in vitro culture. The maturation rate did not increase significantly after 8 h, indicating that oocytes with the ability to complete meiosis are already relatively close to doing so when collected from the follicle; in other words, removal of the oocyte from the follicular environment too early after GVBD does not seem to allow for efficient completion of meiosis. Up to 4 h is the most often cited incubation time for maturing oocytes [3, 6], as it seems to balance the time needed for immature post-GVBD oocytes to reach MII stage and post-maturation oocyte aging of the rest of the cohort [6]. The effect of oocyte aging on its developmental competence seems to be mediated by the woman’s age, with little or no clinical effect in younger women [17], and a significant decrease in viability in older women [18]. Time-lapse systems may be optimal to minimize disturbance to oocyte culture during assessment of oocyte maturation in further studies and to record data in a more precise way.
Even though immature post-GVBD oocytes could reach the MII stage, their fertilization rate remains lower compared with MII stage oocytes at OPU (47.9% vs. 68.1%; p < 0.001). Our results are in agreement with those previously reported: 52.0% IVMC vs. 68.0% MII in oocytes cultured in Sydney IVF Fertilization Medium or Early Cleavage Medium [5] and 52.7% IVMC vs. 70.8% MII in oocytes cultured in B2 medium [4]. Currently, oocyte maturity is assessed morphologically by the extrusion of the first PB, i.e., nuclear maturation. An additional time for cytoplasmic maturation after first PB extrusion might be necessary to obtain higher fertilization rates [7].
Embryo developmental competence of IVCM oocytes also appears to be reduced [5–7]; a potentially higher rate of aneuploid blastomeres has been observed in embryos developed from IVCM oocytes [19], as well as abnormalities in embryo development linked to incomplete cytoplasm maturation [20]. Nevertheless, the crucial issue about IVMC oocytes is their capacity to give rise to healthy pregnancies, which is clearly reduced compared with MII oocytes at OPU [4–7]. In our study, a clinical pregnancy rate of 10.0% was measured in IVMC-derived embryos. Our results are in concordance with previously reported results of 7.7% in Sydney IVF Fertilization Medium or Early Cleavage Medium [5]. An overall live birth rate of 5.6% was observed in the ET-IVMC group. We recognize three main limitations to our study. First, the fact that the vast majority of embryos originated from IVMC oocytes were transferred together with one or two embryos developed from a MII oocyte, making it impossible to monitor all pregnancies obtained by an IVMC oocyte. Second, the impossibility to record the exact timing between PB extrusion and ICSI. Third, each culture medium for oocyte maturation was used in a different period of time. Nevertheless, other laboratory and medical procedures remained identical during all the study period, limiting potential bias.
In conclusion, IVMC oocytes should not be discarded, as they offer a sizable improvement in the live birth rate per cycle at no extra cost to the patient. Until a culture medium specifically designed for meiosis completion in absence of cumulus cells is developed, culturing immature post-GVBD oocytes in G-2™PLUS is an acceptable strategy to preserve their limited developmental potential.
Electronic supplementary material
(XLS 26 kb)
(DOC 57 kb)
Acknowledgments
The authors wish to thank Sarai Brazal for her support with the database and Anna Ferrer-Vaquer for critical review of the manuscript.
Authors’ roles
N.M.: involved in study design, data analysis, and manuscript preparation. D.G.: involved in study design, data analysis, statistical analysis, and manuscript preparation. R.V. and A.R.: involved in study design, implementation, supervision, expert knowledge, and manuscript preparation.
Compliance with ethical standards
Ethical approval for performing this study was obtained from the institutional Ethics Committee for Clinical Research.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chian RC, Buckett WM, Tan SL. In-vitro maturation of human oocytes. Reprod BioMed Online. 2004;8(2):148–166. doi: 10.1016/S1472-6483(10)60511-1. [DOI] [PubMed] [Google Scholar]
- 2.Smith SD, Mikkelsen A, Lindenberg S. Development of human oocytes matured in vitro for 28 or 36 hours. Fertil Steril. 2000;73(3):541–544. doi: 10.1016/S0015-0282(99)00574-9. [DOI] [PubMed] [Google Scholar]
- 3.Shin SB, Cho JW, Lee SH, Yang KM, Lim CK, Lee HS. Fertilization and pregnancy potential of immature oocytes from stimulated intracytoplasmic sperm injection cycles. Clin Exp Reprod Med. 2013;40(1):7–11. doi: 10.5653/cerm.2013.40.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Vos A, Van de Velde H, Joris H, Van Steirteghem A. In-vitro matured metaphase-I oocytes have a lower fertilization rate but similar embryo quality as mature metaphase-II oocytes after intracytoplasmic sperm injection. Hum Reprod. 1999;14(7):1859–1863. doi: 10.1093/humrep/14.7.1859. [DOI] [PubMed] [Google Scholar]
- 5.Vanhoutte L, De Sutter P, Van der Elst J, Dhont M. Clinical benefit of metaphase I oocytes. Reprod Biol Endocrinol. 2005;3:71. doi: 10.1186/1477-7827-3-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strassburger D, Friedler S, Raziel A, Kasterstein E, Schachter M, Ron-El R. The outcome of ICSI of immature MI oocytes and rescued in vitro matured MII oocytes. Hum Reprod. 2004;19(7):1587–1590. doi: 10.1093/humrep/deh236. [DOI] [PubMed] [Google Scholar]
- 7.Balakier H, Sojecki A, Motamedi G, Librach C. Time-dependent capability of human oocytes for activation and pronuclear formation during metaphase II arrest. Hum Reprod. 2004;19(4):982–987. doi: 10.1093/humrep/deh158. [DOI] [PubMed] [Google Scholar]
- 8.Ko DS, Lee SH, Park DW, Yang KM, Lim CK. Pregnancy and fertilization potential of immature oocytes retrieved in intracytoplasmic sperm injection cycles. Clin Exp Reprod Med. 2015;42(3):118–125. doi: 10.5653/cerm.2015.42.3.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roberts R, Franks S, Hardy K. Culture environment modulates maturation and metabolism of human oocytes. Hum Reprod. 2002;17(11):2950–2956. doi: 10.1093/humrep/17.11.2950. [DOI] [PubMed] [Google Scholar]
- 10.Chian RC. In-vitro maturation of immature oocytes for infertile women with PCOS. Reprod BioMed Online. 2004;8(5):547–552. doi: 10.1016/S1472-6483(10)61101-7. [DOI] [PubMed] [Google Scholar]
- 11.Coroleu B, Barri PN, Carreras O, Belil I, Buxaderas R, Veiga A, Balasch J. Effect of using an echogenic catheter for ultrasound-guided embryo transfer in an IVF programme: a prospective, randomized, controlled study. Hum Reprod. 2006;21(7):1809–1815. doi: 10.1093/humrep/del045. [DOI] [PubMed] [Google Scholar]
- 12.Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 13.Dyrlund TF, Kirkegaard K, Poulsen ET, Sanggaard KW, Hindkjaer JJ, Kjems J, et al. Unconditioned commercial embryo culture media contain a large variety of non-declared proteins: a comprehensive proteomics analysis. Hum Reprod. 2014;29(11):2421–2430. doi: 10.1093/humrep/deu220. [DOI] [PubMed] [Google Scholar]
- 14.Salustri A, Yanagishita M, Underhill CB, Laurent TC, Hascall VC. Localization and synthesis of hyaluronic acid in the cumulus cells and mural granulosa cells of the preovulatory follicle. Dev Biol. 1992;151(2):541–551. doi: 10.1016/0012-1606(92)90192-J. [DOI] [PubMed] [Google Scholar]
- 15.Johnson JE, Higdon HL, 3rd, Boone WR. Effect of human granulosa cell co-culture using standard culture media on the maturation and fertilization potential of immature human oocytes. Fertil Steril. 2008;90(5):1674–1679. doi: 10.1016/j.fertnstert.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 16.Jahromi BN, Mosallanezhad Z, Matloob N, Davari M, Ghobadifar MA. The potential role of granulosa cells in the maturation rate of immature human oocytes and embryo development: a co-culture study. Clin Exp Reprod Med. 2015;42(3):111–117. doi: 10.5653/cerm.2015.42.3.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barcena P, Rodriguez M, Obradors A, Vernaeve V, Vassena R. Should we worry about the clock? Relationship between time to ICSI and reproductive outcomes in cycles with fresh and vitrified oocytes. Hum Reprod. 2016;31(6):1182–1191. doi: 10.1093/humrep/dew070. [DOI] [PubMed] [Google Scholar]
- 18.Pujol A, Garcia D, Obradors A, Rodriguez A, Vassena R. Is there a relation between the time to ICSI and the reproductive outcomes? Hum Reprod. 2018;33(5):797–806. doi: 10.1093/humrep/dey067. [DOI] [PubMed] [Google Scholar]
- 19.Emery BR, Wilcox AL, Aoki VW, Peterson CM, Carrell DT. In vitro oocyte maturation and subsequent delayed fertilization is associated with increased embryo aneuploidy. Fertil Steril. 2005;84(4):1027–1029. doi: 10.1016/j.fertnstert.2005.04.036. [DOI] [PubMed] [Google Scholar]
- 20.Nogueira D, Staessen C, Van de Velde H, Van Steirteghem A. Nuclear status and cytogenetics of embryos derived from in vitro-matured oocytes. Fertil Steril. 2000;74(2):295–298. doi: 10.1016/S0015-0282(00)00642-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLS 26 kb)
(DOC 57 kb)