Abstract
Purpose
With over 80% of paediatric and adolescent cancer patients surviving into adulthood, quality-of-life issues such as future fertility are increasingly important. However, little is known about regret around decisions to pursue or forgo fertility preservation (FP). We investigated the risk of decision regret in families involved in making a FP decision and explored contributive factors.
Methods
Parents and patients ≥ 15 years were invited to participate. Participants completed a 10-item survey, including a validated Decision Regret Scale. Scores ≥ 30 indicated high regret. Free-text response items allowed participants to provide reasons for satisfaction or regret.
Results
A total of 108 parents and 30 patients participated. Most (81.4%) reported low regret (mean score 13.7). On multivariate analysis, predictors of low regret included having a FP procedure and a fertility discussion pre-treatment. Most participants believed that FP offers hope for future fertility. Some reported dissatisfaction with the process of decision-making.
Conclusion
Overall levels of regret in the study population were low, with factors associated with quality, timely discussion and belief in the success of FP technology being predictors of low regret. However, dissatisfaction with the decision-making process itself revealed that refinements to the programme are required to meet families’ needs.
Electronic supplementary material
The online version of this article (10.1007/s10815-019-01536-4) contains supplementary material, which is available to authorized users.
Keywords: Fertility preservation, Decision regret, Paediatric, Oncofertility, Decision-making
Introduction
The importance of future fertility to oncology patients is well-recognised [1, 2]. International bodies recommend discussing the impact of gonadotoxic therapy on future fertility and fertility preservation (FP) options—strategies implemented in an attempt to retain fertility opportunity when it is threatened by gonadotoxic therapy—at the time of diagnosis [1, 3, 4]. However, time constraints, the anxiety and vulnerability of the patient and the volume of information relayed, as well as potentially limited clinician knowledge regarding FP, can impair the provision of fertility-related information [5–8]. Furthermore, whilst oocyte or sperm salvage have proven efficacy, procedures for pre-pubertal children (ovarian or testicular tissue cryopreservation) are considered experimental [1, 9]—although recent advances may mean this will change rapidly—adding to the complexity of the decision process and the potential for long-term regret around a decision made at diagnosis.
Decision regret (distress or remorse) about healthcare decisions negatively impacts the wellbeing and quality of life of patients [10, 11]. High levels of decision regret have been associated with poorer long-term health outcomes, poorer psychological wellbeing and an overall lower quality of life [10]. Decisions regarding FP have the potential to create high levels of long-term regret. Studies on adult female cancer survivors have demonstrated high regret that can be lessened with pre-treatment counselling [12–18] and thus supports the clinical recommendations for pre-treatment FP discussion in adults.
However, there is minimal published literature evaluating decision regret regarding FP decisions in paediatric and adolescent populations [19]. Paediatric fertility preservation creates a unique situation whereby future cancer survivors and their parents (acting as surrogate decision-makers) could both experience regret. The additional anxiety and responsibility of having to make a decision that is inconsistent with the child’s future preferences may increase their risk [20, 21]. Better understanding of the experiences of regret will support clinical recommendations. We aimed to examine decision regret around FP decisions in families who had a fertility discussion at The Royal Children’s Hospital (RCH) Melbourne, Australia, and to explore contributing factors to the experience of regret.
Materials and methods
A mixed-methods cross-sectional study was undertaken at The RCH, Melbourne, between February 2015 and November 2016, that assessed acceptance of FP decisions. Whilst FP discussions have occurred since 1987, a formalised FP programme was introduced in August 2013 with experimental procedures undertaken as a novel technology with executive approval, clinical ethics governance for individualised cases and research governance for data collection.
Patients and parents who had undergone FP counselling between 1987 and 2016 were invited to complete a 10-item survey. This survey included the validated Decision Regret Scale (DRS) by Brehaut et al [22], two Likert-type questions assessing whether the participant believed that FP procedures were likely to be successful in their lifetime, or within the lifetime of the next generation and two free-text questions allowing for participants to provide reasons as to why they were satisfied with the decision made or regretted it (see Online Resource 1 for questionnaire). Oncofertility data (demographics, diagnosis, type of cancer treatment, FP procedure type, infertility risk and complications) was obtained via an established oncofertility database and the patient’s medical record.
Since the introduction of the formalised programme, the recommended principle of care at The RCH is to discuss the impacts of planned cancer treatments on fertility with all patients and families when the intent of treatment is curative. Clinicians provide individualised fertility recommendations, based on a range of factors. Fertility consultations cover information on the risk to fertility with proposed treatment, the FP options available for the individual patient, current success rates of available procedures and other options if FP was not pursued. Additionally, written resources regarding fertility and FP are provided to patients and their families.
From June 2014, the Fertility Preservation Toolkit has been employed by clinicians to facilitate the communication of up-to-date, consistent information about fertility risk and preservation options to all newly diagnosed paediatric and adolescent oncology patients/families [23]. This practical resource (published in its entirety) [23], includes a clinician instruction booklet, checklist, referral forms, reference information regarding fertility risk of cancer treatments and handouts for patients and families.
Fertility discussion is initiated by the oncologist, and patients are then referred to an oncofertility specialist (either a paediatric gynaecologist or an endocrinologist) for detailed discussion. Referral for an FP procedure is considered for all pubertal patients at any risk of infertility; pre-pubertal patients with a moderate risk (> 30–80%) or high risk (> 80%) of infertility; patients/families who request discussion; and at the discretion of the oncologist. Oncologists can arrange sperm cryopreservation for post-pubertal patients directly without referral. Information sheets about the FP procedures are standardised so that families considering a procedure have access to the same information [23]. In 2016, this was adapted into an electronic resource [24].
For the purposes of this study, eligible participants included parents and their children (if currently aged 15 years or older) who participated in a fertility discussion between January 1987 (when the first FP procedure was performed) and November 2016, who were recruited onto the oncofertility research programme and had consented to being contacted for research purposes. Oncology patients were defined as those with a primary oncology diagnosis (i.e. that of a solid or blood-borne cancer) or those with a non-cancerous disease needing oncological treatment, and therefore under the care of the oncology department. Such diagnoses include but are not limited to immunological, haematological, renal and endocrine conditions.
Both parents were invited to participate, even if they were both present at the same consultation, as it was anticipated that each parent may have unique and independent levels of satisfaction or regret around their decision despite being provided the same information, considering the decision to undergo FP is values-based.
Although paediatric and adolescent (ages 0–17) patients are legally minors, it is recognised ethically and legally that they may have the capacity to give informed consent for some medical procedures. This capacity is relevant to the individual, and judged on a case by case basis. However, capacity or cognitive maturity to weigh one’s values against the advantages and disadvantages of medical procedures begins to emerge from around 11 to 14 years of age. Older teenagers are more engaged with fertility decisions [25]; therefore, it was agreed that minors > 15 years old, who had participated in the FP decision, were able to give informed assent and participate in research with parental consent.
Identification of eligible participants
Families who attended The RCH after August 2013 were recruited onto the oncofertility programme at a hospital visit soon after diagnosis and recorded in the FP database (established 2013). Those participants who consented to future research were invited onto this study.
Prior to August 2013, formalised data collection strategies had not been implemented; therefore, there was no systematic way of identifying patients who had fertility discussions. Patients invited onto the study from 1987- August 2013 were those that had an FP procedure, identified through medical and pathology databases, or were patients seen in survivorship after 2013 and opportunistically recruited onto the oncofertility research programme.
Additionally, families were ineligible to be contacted during the study period if the patient was deceased or receiving palliative treatment, if the treating oncologist determined the patient was too unwell for contact, or if they had just received a diagnosis, (as there was a minimum of 2 months from the time of fertility discussion and recruitment onto the study to minimise burden and distress).
Consent process
Eligible participants were recruited by a researcher (coinciding with a hospital visit), or via mail-out. Survivors who were no longer receiving cancer treatment were invited via mailout or during a follow-up visit. To minimise distress during intensive treatment, in families where the child had recently undergone a FP procedure, parents were invited to participate at least 2 months post-procedure and patients were approached at least 6 months afterwards. In families where the child did not undergo a procedure, parents and patients were contacted at least 6 months after their FP discussion.
Ethical approval
This study was approved by The RCH Human Research Ethics Committee (HREC 34237).
Statistical analysis
Quantitative data analysis was performed using SPSS version 22 (IBM, New York, USA). Descriptive statistics were used to report demographic and clinical characteristics. Decision regret was assessed as a dichotomous variable. Scores from 0—29 were defined as indicative of low regret, and scores ≥ 30 were indicative of high regret [10]. Associations between level of regret and predictor variables were analysed in bivariate analysis using a two-tailed chi-square test. Differences with a two-sided p-value of < 0.05 were considered statistically significant.
To avoid duplication of results from the two-parent families, bivariate analysis was also performed including only the main carer (i.e. the parent who provided consent to fertility preservation or the research programme) in the analysis. Predictor variables with p < 0.05 in this analysis were included in multivariate logistic regression. A progressive, forward elimination modelling strategy was employed until a final model was obtained containing only variables with p < 0.05. Due to their importance, the following a priori factors were assessed for inclusion in the regression model as potential confounders: cancer diagnostic sub-class; time since diagnosis; time since discussion; provider of the fertility discussion; patient’s age at the time of diagnosis, at discussion, at the time of the survey; patient’s treatment status at time of survey; participant ability to recall the discussion. Inductive content analysis was performed on free-text responses using NVivo software.
Results
Background
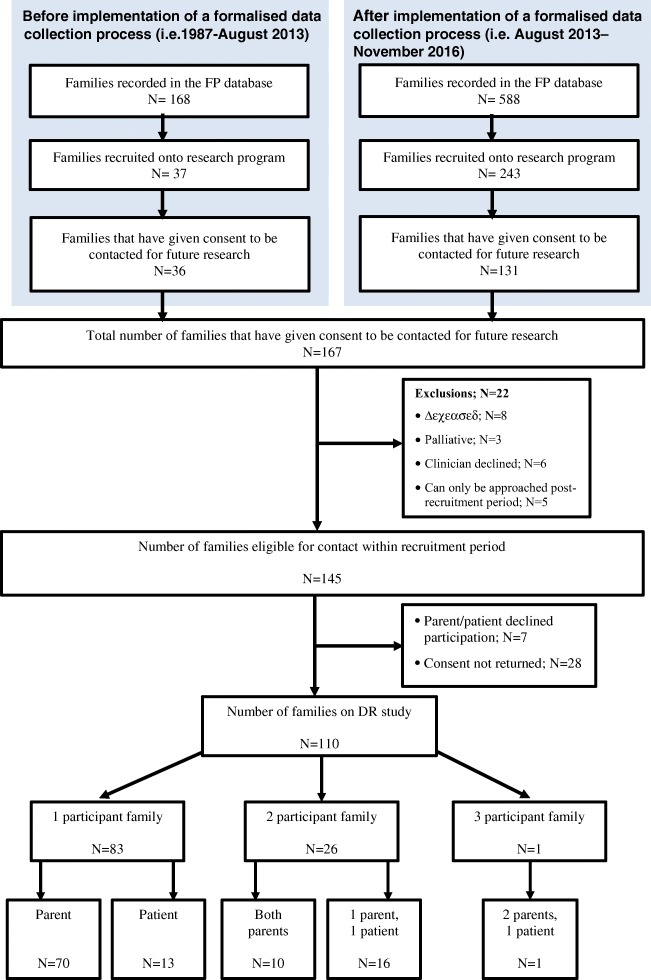
Between 1987 and September 2016, 280 families had given consent for their records to be used for research purposes (and therefore included in our oncofertility research programme); of these, 167 consented to be contacted for future research (36 prior to August 2013, 131 after), such as this study. Twenty-two families were ineligible (eight deceased, three palliative, six clinician declined contact, five could only be approached after the conclusion of the study period) leaving 145 families to be contacted for this study (Fig. 1).
Fig. 1.
Participant recruitment flowchart
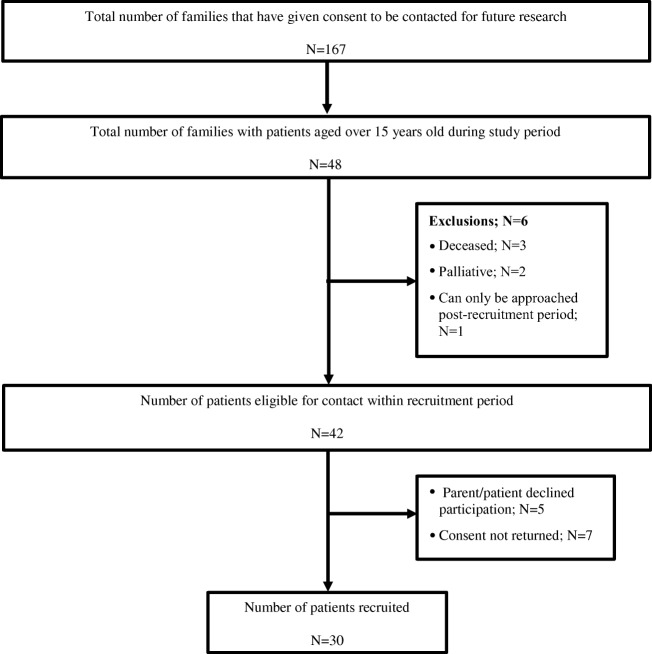
Forty-eight families that had consented to participating in future research had children aged over 15 during the study period. Six patients were excluded: three were deceased, two were palliative at the time of becoming eligible and one patient had consented to future research, but could not yet be approached regarding this study. From 42 eligible patients, five parents did not give consent for their child to participate and seven patients did not return a survey; thus, 30 patients were recruited (Fig. 2).
Fig. 2.
Patient recruitment flowchart
Population characteristics and response rate
Of 145 eligible families, 110 participated (75.9% participation rate) providing a total of 138 respondents made up of 108 parents and 30 patients (Fig. 1). Of these 138, 17 had consultations prior to Aug 2013, 105 had consultations after implementation of the formalised data collection and 16 had no documented consultation date. In 16 families, one parent and the patient took part in the study; in 10 families, both parents participated; and in one family, both parents and the patient participated. Table 1 displays the characteristics of the study population. The mean age of the parents and of the patients that responded was 40.0 ± 6.7 years (range 24.0–64.0) and 20.0 ± 6.3 years (range 14.0–44.0) respectively. The average age of the children of the parents who participated was 8.7 ± 5.5 (1.6–20.7).
Table 1.
Participant demographics and clinical characteristics, and bivariate analysis of their influence on decision regret
| Total number of respondents (n = 138) | Respondents that completed the Decision Regret Scale (n = 129) | |||||||
|---|---|---|---|---|---|---|---|---|
| Parent N = 108 (%) | Patient N = 30 (%) | Total N = 138 (%) | Low regret (DR < 30) N = 105 | High regret (DR ≥ 30) N = 24 | Total N = 129 | X 2 | p value | |
| Participant type—n (%) | 0.0970 | 0.76 | ||||||
| Parent | 108 (100%) | N/A | 108 (78.3%) | 80 (80.8%) | 19 (19.2%) | 99 (100%) | ||
| Patient | N/A | 30 (100%) | 30 (21.7%) | 25 (83.3%) | 5 (16.7%) | 30 (100%) | ||
| Total | 108 (100%) | 30 (100%) | 138 (100%) | |||||
| Gender of participant—n (%) | 1.40 | 0.24 | ||||||
| Male | 19 (17.6%) | 16 (53.3%) | 35 (25.4%) | 23 (74.2%) | 8 (25.8%) | 31 (100%) | ||
| Female | 89 (82.4%) | 14 (46.7%) | 103 (74.6%) | 82 (83.7%) | 16 (16.3%) | 98 (100%) | ||
| Total | 108 (100%) | 30 (100%) | 138 (100%) | |||||
| Participant age at survey (years)—mean ± SD (range) | 40.0 ± 6.7 (24-64) | 20.0 ± 6.3 (14-44) | 35.5 ± 10.6 (14-64) | 35 ± 10.9 (14-64) | 37.3 ± 10.9 (15-51) | 35.5 ± 10.9 (14-64) | ||
| Unknown | 3 | 0 | 3 | 3 | 0 | 3 | ||
| Age of child at survey (years)—mean ± SD (range) | 8.7 ± 5.5 (1.6-20.7) | 20.0 ± 6.3 (14.9-44.3) | 11.3 ± 7.5 (1.6-44.3) | 11.5 ± 7.6 (1.6-44.3) | 12.9 ± 6.8 (2.5-31.4) | 11.8 ± 7.5 (1.6-44.3) | ||
| Country of birth—n (%) | 0.432 | 0.51 | ||||||
| Australia | 86 (79.6%) | 26 (86.7%) | 112 (81.2%) | 86 (80.4%) | 21 (19.6%) | 107 (100%) | ||
| Other | 22 (20.4%) | 4 (13.3%) | 26 (18.8%) | 19 (86.4%) | 3 (13.6%) | 22 (100%) | ||
| Total | 108 (100%) | 30 (100%) | 138 (100%) | |||||
| Gender of patient—n (%) | 0.230 | 0.63 | ||||||
| Male | 42 (38.9%) | 16 (53.3%) | 58 (42.0%) | 45 (83.3%) | 9 (16.7%) | 54 (100%) | ||
| Female | 66 (61.1%) | 14 (46.7%) | 80 (58.0%) | 60 (80.0%) | 15 (20.0%) | 75 (100%) | ||
| Total | 108 (100%) | 30 (100%) | 138 (100%) | |||||
| Patient age at diagnosis (years)—mean ± SD (range) | 7.0 ± 5.6 (0.1-18.6) | 14.7 ± 2.1 (8.6-18.6) | 8.6 ± 6.0 (0.1-18.6) | 8.8 ± 6.1 (0.1-18.6) | 9.8 ± 5.3 (0.3-17.6) | 9.0 ± 5.9 (0.1-18.6) | ||
| Unknown | 0 | 1 | 1 | 1 | 0 | 1 | ||
| Diagnosis—n (%) | 5.70 | 0.34 | ||||||
| Leukaemia | 27 (25.0%) | 7 (23.3%) | 34 (24.6%) | 18 (66.7%) | 9 (33.3%) | 27 (100%) | ||
| Lymphoma | 9 (8.3%) | 11 (36.7%) | 20 (14.5%) | 17 (85.0%) | 3 (15.0%) | 20 (100%) | ||
| CNS | 10 (9.3%) | 0 (0.0%) | 10 (7.2%) | 8 (88.9%) | 1 (11.1%) | 9 (100%) | ||
| Sarcoma | 29 (26.9%) | 8 (26.7%) | 37 (26.8%) | 32 (86.5%) | 5 (13.5%) | 37 (100%) | ||
| Other solid cancer | 10 (9.3%) | 2 (6.7%) | 12 (8.7%) | 10 (90.9%) | 1 (9.1%) | 11 (100%) | ||
| Non-cancerous diseasea | 23 (21.3%) | 2 (6.7%) | 25 (18.1%) | 20 (80.0%) | 5 (20.0%) | 25 (100%) | ||
| Total | 108 (100%) | 30 (100%) | 138 (100%) | |||||
| Diagnosed pre/post programmeb—n (%) | 0.0399 | 0.84 | ||||||
| Pre-programme | 11 (11.1%) | 13 (43.3%) | 25 (18.1%) | 20 (80.0%) | 5 (20.0%) | 25 (100%) | ||
| Post-programme | 96 (88.9%) | 17 (56.7%) | 113 (81.9%) | 85 (81.7%) | 19 (18.3%) | 104 (100%) | ||
| Total | 108 (100%) | 30 (100%) | 138 (100%) | |||||
| Planned treatment infertility risk—n (%) | 0.805 | 0.67 | ||||||
| High (> 80%) | 64 (59.3%) | 15 (50.0%) | 79 (57.2%) | 66 (83.5%) | 13 (16.5%) | 79 (100%) | ||
| Intermediate | 30 (27.8%) | 10 (33.3%) | 40 (29.0%) | 29 (80.6%) | 7 (19.4%) | 36 (100%) | ||
| Low (< 20%) | 14 (13.0%) | 2 (6.7%) | 16 (11.6%) | 8 (72.7%) | 3 (27.3%) | 11 (100%) | ||
| Unknown | 0 (0%) | 3 (10.0%) | 3 (2.2%) | 2 (66%) | 1 (33%) | 3 (100%)ψ | ||
| Total | 108 (100%) | 30 (100%) | 138 (100%) | |||||
| Actual treatment infertility risk—n (%) | 3.88 | 0.14 | ||||||
| High (>80%) | 62 (57.4%) | 16 (53.3%) | 78 (56.5%) | 66 (84.6%) | 12 (15.4%) | 78 (100%) | ||
| Intermediate | 30 (27.8%) | 10 (33.3%) | 40 (29.0%) | 29 (80.6%) | 7 (19.4%) | 36 (100%) | ||
| Low/no riskc (< 20%) | 16 (14.9%) | 2 (6.7%) | 18 (13.0%) | 8 (61.5%) | 5 (38.5%) | 13 (100%) | ||
| Unknown | 0 (0%) | 2 (6.7%) | 2 (1.4%) | 2 (100%) | 0 (0%) | 2 (100%)ψ | ||
| Total | 108 (100%) | 30 (100%) | 138 (100%) | |||||
| Documentation of discussion—n (%) | 2.88 | 0.09 | ||||||
| Yes | 93 (86.1%) | 29 (96.7%) | 122 (88.4%) | 101 (82.8%) | 21 (17.2%) | 122 (100%) | ||
| No | 15 (13.9%) | 1 (3.3%) | 16 (11.6%) | 4 (57.1%) | 3 (42.9%) | 7 (100%) | ||
| Total | 108 (100%) | 30 (100%) | 138 (100%) | |||||
| Recollection of discussion—n (%) | 5.81 | 0.02* | ||||||
| Yes | 96 (88.9%) | 28 (93.3%) | 124 (89.9%) | 102 (82.9%) | 21 (17.1%) | 123 (100%) | ||
| No | 11 (10.2%) | 2 (6.7%) | 13 (9.4%) | 2 (40.0%) | 3 (60.0%) | 5 (100%) | ||
| Unknown | 1 (0.9%) | 0 (0.0%) | 1 (0.7%) | 1 (100%) | 0 (0%) | 1 (100%) | ||
| Total | 108 (100%) | 30 (100%) | 138 (100%) | |||||
| Mean age of patient at discussiond (years)—mean ± SD (range) | 8.1 ± 5.6 (0.9-19.6) | 16.0 ± 3.3 (11.1–29.6) | 10.0 ± 6.1 (0.9-29.6) | 9.6 ± 6.3 (0.9–29.6) | 11.7 ± 5.4 (1.2-18.0) | 10.0 ± 6.1 (0.9-29.6) | ||
| Unknown | 15 | 1 | 16 | 4 | 3 | 7 | ||
| Pubertal status of patient at discussion—n (%) | 0.0704 | 0.79 | ||||||
| Pre-pubertal | 80 (74.1%) | 2 (6.7%) | 82 (59.4%) | 60 (82.2%) | 13 (17.8%) | 73 (100%) | ||
| Post-pubertal | 28 (25.9%) | 28 (93.3%) | 56 (40.6%) | 45 (80.4%) | 11 (19.6%) | 56 (100%) | ||
| Total | 108 (100.0%) | 30 (100%) | 138 (100%) | |||||
| Pubertal status of patient at discussion according to patient gender—n (%) | 0.448 | 0.93 | ||||||
| Pre-pubertal male | 32 (29.6%) | 0 (0%) | 32 (29.6%) | 24 (85.7%) | 4 (14.3%) | 28 (100%) | ||
| Post-pubertal male | 10 (9.3%) | 16 (53.3%) | 26 (18.8%) | 21 (80.8%) | 5 (19.2%) | 26 (100%) | ||
| Pre-pubertal female | 48 (44.4%) | 2 (6.7%) | 50 (36.2%) | 36 (80.0%) | 9 (20.0%) | 45 (100%) | ||
| Post-pubertal female | 18 (16.7%) | 12 (40%) | 30 (21.7%) | 24 (80.0%) | 6 (20.0%) | 30 (100%) | ||
| Total | 108 (100.0%) | 30 (100%) | 138 (100%) | |||||
| Treatment status of patient at discussion—n (%) | 10.0 | <0.01* | ||||||
| Pre-high risk gonadotoxic therapye | 89 (82.4%) | 24 (80.0%) | 113 (81.9%) | 97 (85.8%) | 16 (14.2%) | 113 (100%) | ||
| Post-high risk gonadotoxic therapyf | 4 (3.7%) | 5 (16.7%) | 9 (6.5%) | 4 (44.4%) | 5 (55.6%) | 9 (100%) | ||
| Unknown | 15 (13.9%) | 1 (3.3%) | 16 (11.6%) | 4 (57.1%) | 3 (42.9) | 7 (100%)ψ | ||
| Total | 108 (100%) | 30 (100%) | 138 (100%) | |||||
| Referred for discussion with oncofertility specialistg | 2.59 | 0.11 | ||||||
| Yes | 86 (79.6%) | 20 (66.7%) | 106 (76.8%) | 89 (84.0%) | 17 (16.0%) | 106 (100%) | ||
| No | 22 (20.4%) | 10 (33.3%) | 32 (23.2%) | 16 (69.6%) | 7 (30.4%) | 23 (100%) | ||
| Total | 108 (100%) | 30 (100%) | 138 (100%) | |||||
| Fertility preservation procedure—n (%) | 17.8 | <0.01* | ||||||
| Yes | 71 (65.7%) | 26 (86.7%) | 97 (70.3%) | 87 (89.7%) | 10 (10.3%) | 97 (100%) | ||
| No | 37 (34.3%) | 4 (13.3%) | 41 (29.7%) | 18 (56.3%) | 14 (43.8%) | 32 (100%) | ||
| Total | 108 (100%) | 30 (100%) | 138 (100%) | |||||
| Procedure type—n (%) | 2.72 | 0.84 | ||||||
| Sperm | 5 (7.0%) | 10 (38.5%) | 15 (15.5%) | 12 (80.0%) | 3 (20.0%) | 15 (100%) | ||
| Sperm via TTCP | 4 (5.6%) | 5 (19.2%) | 9 (9.3%) | 8 (88.9%) | 1 (11.1%) | 9 (100%) | ||
| TTCP | 26 (36.6%) | 0 (0%) | 26 (26.8%) | 23 (88.5%) | 3 (11.5%) | 26 (100%) | ||
| OTCP | 33 (46.5%) | 7 (26.9%) | 40 (41.2%) | 37 (92.5%) | 3 (7.5%) | 40 (100%) | ||
| OTCP+ GnRHa | 1 (1.4%) | 2 (7.7%) | 3 (3.1%) | 3 (100%) | 0 (0.0%) | 3 (100%) | ||
| OTCP+ GnRHa+ oocytes | 1 (1.4%) | 1 (3.8%) | 2 (2.1%) | 2 (100%) | 0 (0.0%) | 2 (100%) | ||
| GnRHa | 1 (1.4%) | 1 (3.8%) | 2 (2.1%) | 2 (100%) | 0 (0.0%) | 2 (100%) | ||
| Total | 71 (100%) | 26 (100%) | 97 (100%) | |||||
| Established vs. experimental procedure—n (%) | 1.20 | 0.27 | ||||||
| Established | 6 (8.5%) | 11 (42.3%) | 17 (17.5%) | 14 (82.4%) | 3 (17.6%) | 17 (100%) | ||
| Experimental | 65 (91.5%) | 15 (57.7%) | 80 (82.5%) | 73 (91.3%) | 7 (8.7%) | 80 (100%) | ||
| Total | 71 (100%) | 26 (100%) | 97 (100%) | |||||
| Procedural complications (TTCP & OTCP only)h—n (%) | 0.40 | 0.53 | ||||||
| Yes | 3 (4.6%) | 1 (6.7%) | 4 (5%) | 4 (100.0%) | 0 (0.0%) | 4 (100%) | ||
| No | 62 (95.4%) | 14 (93.3%) | 76 (95%) | 69 (90.8%) | 7 (9.2%) | 76 (100%) | ||
| Total | 65 (100%) | 15 (100%) | 70 (100%) | |||||
| Survey time-point—n (%) | 7.84 | 0.05* | ||||||
| 2 months | 12 (11.1%) | 2 (6.7%) | 14 (10.1%) | 9 (64.3%) | 5 (35.7%) | 14 (100%) | ||
| 6 months | 29 (26.9%) | 1 (3.3%) | 30 (21.7%) | 20 (74.1%) | 7 (25.9%) | 27 (100%) | ||
| 12 months | 24 (22.2%) | 4 (13.3%) | 28 (20.3%) | 27 (96.4%) | 1 (3.6%) | 28 (100%) | ||
| 18 months | 43 (39.8%) | 23 (76.7%) | 66 (47.8%) | 49 (81.7%) | 11 (18.3%) | 60 (100%) | ||
| Total | 108 (100%) | 30 (100%) | 138 (100%) | |||||
| Time since diagnosis (years)—mean ± SD (range) | 1.7 ± 1.7 (0.2-10.8) | 5.0 ± 4.8 (0.2-17.7) | 2.4 ± 3.0 (0.2-17.7) | 2.3 ± 2.7 (0.2-16.5) | 3.1 ± 4.2 (0.2-17.7) | 2.5 ± 3.1 (0.2-17.7) | ||
| Unknown | 0 | 1 | 1 | 1 | 0 | 1 | ||
| Time from discussion to surveyd (years)—mean ± SD (range) | 1.1 ± 0.8 (0.0-4.8) | 3.8 ± 3.5 (0.1-13.4) | 1.7 ± 2.1 (0.0-13.4) | 1.7 ± 1.9 (0.1-10.9) | 2.0 ± 3.0 (0.0-13.4) | 1.7 ± 2.1 (0.0-13.4) | ||
| Unknown | 15 | 1 | 16 | 4 | 3 | 7 | ||
| Treatment status of patient at survey | 8.33 | 0.04* | ||||||
| Active | 29 (26.9%) | 4 (13.3%) | 33 (23.9%) | 26 (86.7%) | 4 (13.3%) | 30 (100%) | ||
| Maintenance | 14 (13.0%) | 2 (6.7%) | 16 (11.6%) | 8 (66.7%) | 4 (33.3%) | 12 (100%) | ||
| Off treatment | 60 (55.6%) | 24 (80.0%) | 84 (60.9%) | 69 (84.1%) | 13 (15.9%) | 82 (100%) | ||
| Otheri | 5 (4.6%) | 0 (0%) | 5 (3.6%) | 2 (40.0%) | 3 (60.0%) | 5 (100%) | ||
| Total | 108 (100%) | 30 (100%) | 138 (100%) | |||||
| DR score—mean ± SD (range) | 13.0 ± 17.2 (0–95) | 16.2 ± 23.1 (0–95) | 13.7 ± 18.7 (0–95) | 6.7 ± 8.2 (0–25) | 44.4 ± 21.0 (30–95) | 13.7 ± 18.7 (0–95) | ||
| Impression of the success of FP in their lifetime—n (%) | 18.5 | < 0.01* | ||||||
| Strongly agree | 30 (27.8%) | 6 (20.0%) | 36 (26.1%) | 30 (83.3%) | 6 (16.7%) | 36 (100%) | ||
| Agree | 50 (46.3%) | 17 (56.7%) | 67 (48.6%) | 59 (90.8%) | 6 (9.2%) | 65 (100%) | ||
| Neither agree or disagree | 16 (14.8%) | 7 (23.3%) | 23 (16.7%) | 14 (60.9%) | 9 (39.1%) | 23 (100%) | ||
| Disagree | 4 (3.7%) | 0 (0.0%) | 4 (2.9%) | 1 (25.0%) | 3 (75.0%) | 4 (100%) | ||
| Unknown | 8 (7.4%) | 0 (0.0%) | 8 (5.8%) | 1 (100%) | 0 (0%) | 1 (100%)ψ | ||
| Total | 108 (100%) | 30 (100%) | 138 (100%) | |||||
CNS, central nervous system; GnRHa, gonadotrophin-releasing hormone analogue; OTCP, ovarian tissue cryopreservation; SD, standard deviation; TTCP, testicular tissue cryopreservation
*Indicates statistically significant factors where p < 0.05 (rounded to two decimal places)
ψUnknown values were not used in bivariate analysis, as the numbers in each regret category were too small for calculation
aNon-cancerous disease includes haematological and immune disorders such as aplastic anaemia, severe combined immunodeficiency and chronic granulomatous disease. These patients underwent or were being considered for bone marrow transplant, and therefore had discussions regarding fertility and fertility preservation prior to receiving chemotherapy or radiotherapy as conditioning treatment
bRefers to whether diagnosis occurred before or after August 2013 when the oncofertility research programme was introduced
cTwo parents have children that did not end up requiring treatment for non-cancerous disease and did not proceed with a fertility preservation procedure
dCalculated based on the date of discussion documented, which was not available for all participants
eIncludes those that have had a discussion prior to any gonadotoxic treatment, and those that had a discussion after commencing low-risk gonadotoxic treatment but before progressing to high-risk gonadotoxic treatment
fIncludes those that have had a discussion after commencing any high-risk gonadotoxic treatment
gAn oncofertility specialist is either a paediatric endocrinologist or a gynaecologist
hProcedural complications are assessed in patients that have undergone OTCP or TTCP, inclusive of patients that have had a secondary procedure (i.e. GnRHa, oocytes or sperm)
iRefers to two patients that did not end up requiring any treatment, and three receiving supportive bone marrow transplant care at the time of the survey but no oncological treatment
All participating families had a discussion regarding fertility. Most (90.5%, 124/137) recalled a discussion, and a discussion was documented in the medical records of 88.4% of the participants (n = 122). The majority (47.8%) of participants completed the survey at least 18 months after a fertility discussion had occurred, with a mean time elapsed of 1.7 years (SD = 2.1; range 0.0–13.4). For 81.9% of participants (n = 113) fertility discussions occurred prior to the initiation of treatment that conferred a high-risk of infertility. This included 16.7% (n = 23) who had a discussion after low-risk gonadotoxic treatment but before starting high-risk treatment. Nine participants (6.5%) had a fertility discussion only after having already received high-risk gonadotoxic treatment.
Seven participants (all parents) indicated that they did not recall a fertility discussion occurring and did not complete the rest of the survey. Another two parents responded speculatively as if a FP procedure had occurred when it had not; therefore, their results were excluded.
Impression of success
One hundred twenty-eight participants responded to the questions relating to impressions of likelihood of success of the fertility preservation procedures. Overall, 80% of parents (78/98) and 77% of patients (23/30) strongly agreed or agreed that FP procedures were likely to be successful in their lifetime, and 80% of parents (78/98) and 70% of patients (21/30) strongly agreed or agreed that FP procedures were likely to be successful in the lifetime of the next generation. A sub-group analysis was performed based on the procedure type undertaken.
Eighty-three percent of parents whose child had an established procedure (sperm or oocyte collection) (5/6) or an experimental procedure (ovarian or testicular tissue preservation or gonadotropin-releasing hormone analogues) (53/64) believed that FP procedures will be successful within their lifetime. In those who had experimental procedures, an additional four believed the technology would be successful in the lifetime of the next generation.
Of the 11 patients who had an established procedure, 90.9% (10/11) believed the procedure would be successful within their lifetime and that of the next generation. Of the 14 patients who had an experimental procedure, 71.4% (10/14) believed success would come in their lifetime and a further 14.3% (2/14) believed the technology would be successful in the lifetime of the next generation.
Decision regret
Responses from 129 participants were analysed for decision regret. The mean total DRS score was 13.7 (SD = 18.7; range 0–95), with 24 (18.6%) participants categorised as having high decision regret.
Overall, respondents felt they had made the right decision (93.0%, 120/129), and would make the same choice again (90.7%, 117/129). Five percent (6/129) of respondents regretted the choice and 6.2% (8/129) felt that the choice caused them harm. Of these eight, two could not recall a discussion occurring prior to high-risk treatment (Table 2).
Table 2.
Characteristics of participants that indicated that their choice caused them harm on item-level analysis of the Decision Regret Scale
| Respondent type | Diagnostic subclass | Treatment status at discussion | Provider of discussion | Pubertal status at discussion | FP Modality | Recalls discussion | DR score | DR category | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Patient | Liquid | Post high-risk treatment | Oncologist + oncofertility specialist | Post-pubertal | None | No | 95 | High |
| 2 | Parent | Liquid | Post high-risk treatment | Oncologist + oncofertility specialist | Post-pubertal | None | No | 95 | High |
| 3 | Parent | Solid | Pre high-risk treatment | Oncologist + oncofertility specialist | Pre-pubertal | TTCP | Yes | 30 | High |
| 4 | Parent | Liquid | Pre high-risk treatment | Oncologist + oncofertility specialist | Pre-pubertal | None | Yes | 30 | High |
| 5 | Parent | Solid | Pre high-risk treatment | Oncologist + oncofertility specialist | Pre-pubertal | OTCP | Yes | 15 | Low |
| 6 | Parent | Not cancer | Pre high-risk treatment | Oncologist + oncofertility specialist | Pre-pubertal | None | Yes | 15 | Low |
| 7 | Parent | Solid | Pre high-risk treatment | Oncologist + oncofertility specialist | Pre-pubertal | OTCP | Yes | 15 | Low |
| 8 | Patient | Solid | Pre high-risk treatment | Oncologist only | Post-pubertal | Sperm | Yes | 20 | Low |
OTCP, ovarian tissue cryopreservation; TTCP, testicular tissue cryopreservation; FP, fertility preservation; DR, decision regret
Bivariate analysis is shown in Table 1. There was no significant difference between parents and patients in terms of regret (Χ2 = 0.097, p = 0.756). Therefore, analysis to determine variables that potentially influenced regret was conducted using the entire study population as a whole. Low decision regret was associated with recalling the fertility discussion, believing FP is likely to be successful in one’s lifetime, having had a FP procedure, and completing the survey at “12 months” post-discussion. Those who had fertility discussions after receiving high-risk gonadotoxic treatment were more likely to have high decision regret. Regret scores were not influenced by the type of fertility preservation procedure undertaken, infertility risk from treatment (low/medium/high), type of cancer diagnosis, and specialty of the clinician who provided the fertility consult.
Twenty-seven families had more than one participant. Table 3 provides an outline of the survey responses from each family unit. There was a discrepancy in the regret category between individuals in 3 of the 11 parent/parent pairs (27.3%) and in 3 of the 17 parent/patient pairs (17.6%).
Table 3.
Survey responses from families with more than one participant. Responses to free-text response items have been summarised in dot-point form. Five families had participants with discrepancy in regret category (2-parent/parent, 2-patient/parent, 1- patient/2 parents)
| Family No. | Procedure Type | Participant | DR Score | Category | Free-text comments | |
|---|---|---|---|---|---|---|
| Reasons for satisfaction | Reasons for regret | |||||
| 2-parent families | ||||||
| 1 | TTCP | Mother | 20 | Low |
• To give child a future opportunity to achieve parenthood • To give the child a choice |
• The discomfort FP caused • Hoping they made the right decision • Dealing with aspects of the clinical care team caused frustration |
| Father | 0 | Low |
• Decision was made for them • To give child a future opportunity to achieve parenthood |
• Did not regret decision • Felt discussion was rushed • Did not get all the information about long term follow-up |
||
| 2 | OTCP | Mother | 10 | Low | • To give the child a possible choice [to have children] later in life | – |
| Father | 5 | Low | • To give the child the best chance at achieving a successful pregnancy. | – | ||
| 3 | OTCP | Mother | 0 | Low | – | – |
| Grandmother | 0 | Low | • To give the child a chance of having a family if unable to fall pregnant | – | ||
| 4 | OTCP | Mother | 5 | Low | • To give the child options | – |
| Father | 5 | Low | – | – | ||
| 5* | TTCP | Mother | 0 | Low | Yes | – |
| Father | 30 | High | – | – | ||
| 6 | TTCP | Mother | 5 | Low | Completely | – |
| Father | 5 | Low | • Chance of a good outcome |
• Did not regret decision • Dissatisfied with process |
||
| 7 | No FP | Mother | 15 | Low |
• Child is currently healthy • Will discuss fertility with child when the time comes |
– |
| Father | 5 | Low | – | – | ||
| 8* | OTCP | Mother | 15 | Low |
• Child is now in remission • Having FP did not cause any harm • There was no delay to starting treatment |
– |
| Father | 30 | High |
• FP caused no harm to child’s health • With a good prognosis, choices are now available to the child later in life because of FP |
– | ||
| 9 | No FP | Mother | 20 | Low |
• FP was not offered given the child’s situation • Grateful for discussion regarding fertility prior to treatment |
– |
| Mother | 25 | Low |
• FP was not an option given child’s situation but fertility was discussed • Would have pursued FP if it was an option |
• No regret- as FP would have been a risk to the child | ||
| 10 | TTCP | Mother | 15 | Low | • Gives the child the best chance of fertility in future, and having their own children | – |
| Father | 0 | Low |
• Gives the child a chance of having their own children • Not proceeding with FP gives an even less of a chance (at fertility) |
|||
| Parent/patient families | ||||||
| 11 | OTCP | Father | 0 | Low |
• Protects daughter’s wellbeing • Gives child a chance at motherhood |
– |
| Patient (F) | 5 | Low | • Chance to become a mother | – | ||
| 12 | None | Mother | 95 | High |
• Not offered any FP options • Daughter is unable to have children now |
|
| Patient (F) | 95 | High | • Not satisfied. Risked ability to have children | • Was not able to make a decision, as FP was not offered | ||
| 13 | None | Father | 30 | High | – | – |
| Patient (F) | 50 | High | – | – | ||
| 14 | Sperm | Mother | 10 | Low | • Gives child every chance of being a parent | – |
| Patient (M) | 0 | Low |
• Had treatment with high risk to fertility • No issues with storage process |
– | ||
| 15 | OTCP | Mother | 0 | Low | • Gives child all chances to conceive | – |
| Patient (F) | 10 | Low |
• Increased chances of fertility • Benefits research in the process |
– | ||
| 16 | TTCP (sperm) | Mother | 0 | Low | • Gives the child a choice in the future of becoming a parent | • No regrets |
| Patient (M) | 15 | Low | – | – | ||
| 17* | Sperm | Mother | 30 | High | • Chance at having own children | – |
| Patient (M) | 10 | Low | • Chance at becoming a parent | – | ||
| 18 | No FP | Mother | 10 | Low | • Child’s health was the main priority- was not fit for FP after undergoing major surgery | – |
| Patient (F) | 15 | Low |
• FP would have caused more harm • Was not fit to have FP |
• Does not regret decision | ||
| 19 | TTCP | Mother | 0 | Low |
• FP was the best opportunity to save fertility • No other choice |
– |
| Patient (M) | 0 | Low |
• Desires children later on • Has choices available for the future |
– | ||
| 20 | OTCP | Mother | 0 | Low |
• Right decision • Gives the patient hope at a normal life after cure |
– |
| Patient (F) | 0 | Low | • FP provides a back-up plan | – | ||
| 21* | No FP | Father | 25 | Low | • Treatment had a low risk to fertility | – |
| Patient (M) | 50 | High | • No current regrets | – | ||
| 22 | OTCP+ GnRHa | Mother | 0 | Low |
• Gives the child choices • FP caused no additional harm |
– |
| Patient (F) | 0 | Low |
• Satisfied with decision • Treatment has impacted fertility • Wants to have children in the future |
– | ||
| 23 | Sperm | Mother | 0 | Low | • Beneficial to the child and their future | – |
| Patient (M) | 0 | Low | • Wants to have children in the future | – | ||
| 24 | TTCP and Sperm | Mother | 10 | Low |
• Treatment may leave child infertile • Child would like to have a family • FP gives some 'insurance' if needed |
– |
| Patient (M) | 25 | Low | • FP may be useful in the future | – | ||
| 25 | OTCP | Mother | 0 | Low |
• Gives daughter the chance to have children • Recurrence may result in oophorectomy or hysterectomy |
– |
| Patient (F) | 0 | Low | • May need stored tissue to have children | – | ||
| 26 | OTCP | Mother | 5 | Low |
• Satisfied as advances in medical research are always occurring • Hopeful that technology would be available [to utilise tissue] when daughter would like to have kids. |
– |
| Patient (F) | 5 | Low |
• FP provides insurance • Gives the best chance of having a family |
– | ||
| 2-parent/1-patient family | ||||||
| 27* | OTCP | Mother | 35 | High | • Best available option | – |
| Father | 15 | Low |
• Risks of treatment explained • Understands that FP is currently experimental • FP allows daughter to have options later in life • Not pursing FP when offered would have played with his conscience |
– | ||
| Patient (F) | 20 | Low | • Best available option | – | ||
BMT, bone marrow transplant; DR, decision regret; FP, fertility preservation; TTCP, testicular tissue cryopreservation; OTCP, ovarian tissue cryopreservation; GnRHa, gonadotrophin-releasing hormone analogue (F): female patient; (M): male patient; -: no response provided
* and italics highlight the families in which there was discordance between family members
A multivariate logistic regression analysis was performed including only the main carer from each two-parent family. The final model is shown in Table 4. Believing that FP will not be successful in one’s lifetime (p = .011, OR = 2.958, CI = 1.289–6.789), not having a FP procedure (p = 0.008, OR = .178, CI = .050–.639), and having a fertility discussion only after high-risk therapy (p = .011, OR = 40.532, CI = 2.352–698.6) were the most significant predictors of high decision regret after adjusting for confounding variables.
Table 4.
Final logistic regression model: Predictors of high regret around the fertility decision. Predictor variables are set to italics; confounders are set to roman. One parent from each two-parent family was excluded from the analysis
| B | SE | Wald | df | p | OR | 95% CI | |
|---|---|---|---|---|---|---|---|
| Impression that FP procedures will not be successful in this lifetime | 1.084 | .424 | 6.543 | 1 | .011* | 2.958 | 1.289–6.789 |
| Having a fertility preservation procedure | − 1.727 | .653 | 7.001 | 1 | .008* | .178 | .050–.639 |
| Having a discussion after high-risk therapy has been commenced | 3.702 | 1.453 | 6.496 | 1 | .011* | 40.532 | 2.352–698.6 |
| Time since diagnosis | − .187 | .197 | .897 | 1 | .344 | .830 | .564–1.221 |
| Age of patient at time of discussion | − .002 | .223 | .000 | 1 | .993 | .998 | .645–1.544 |
| Age of patient at time of survey | 0.045 | .040 | 1.301 | 1 | .254 | 1.046 | .968–1.131 |
B, B coefficient; SE, standard error; Wald, Wald chi-square value; df, degrees of freedom; OR, odds ratio; CI, confidence interval
Analysis of free-text responses
Responses to free-text questions were provided by 115 participants. Most (112, 97.3%) responses related to satisfaction with the FP decision and 22 responses related to regret. Major themes were identified based on the frequency of response (Table 5).
Table 5.
Emergent themes from qualitative analysis
| Future hopes |
“If there is a chance, even slight, that fertility preservation could one day help my son have children...then I’d like to ensure I reserved that opportunity and choice for him” (Parent, TTCP, DR = 20, low regret) “I would like to have children one day, fertility preservation was a necessary and remarkable option to have given the potential dangers to fertility post-treatment from chemotherapy.” (Patient, sperm cryopreservation, DR = 0, low regret) |
| Decision making in behalf of patient | “At the time of diagnosis I was too young and immature to be making my own decisions about fertility preservation… I am happy a decision was made for me by an older individual.” (Patient, TTCP, DR = 0 low regret) |
| Fertility preservation as a back-up plan |
“It acted as insurance in case I was no longer fertile after treatment. I was lucky in that I'm still fertile and no longer need fertility preservation but it made it a lot easier before…[when] I knew that [I had] a backup option.” (Patient, sperm cryopreservation, DR = 0, low regret) “It was …[a] back up option for me however if I didn't do it I know I would have regretted it.” (Patient, OTCP, DR = 10, low regret) |
| Other hopes |
“We decided to take all possible measures for fertility preservation… I believe that I was given the best chance possible.” (Patient, OTCP, GnRHa and oocyte cryopreservation, DR = 0, low regret) “I think giving our son every chance to lead a normal life (including the opportunity to be a father) is very important” (Parent, 44 years, TTCP, DR = 0, low regret) |
| Parental responsibility |
“… as parents we are giving our child every chance and opportunity for her future...” (Parent, OTCP, DR = 5, low regret) “Because we are giving her the best chance we can to have her own children if that is her choice…it wasn't up to us to decide at the age of 2 if she wanted the chance to be a mum...” (Parent, OTCP, DR = 0, low regret) |
| Experimental nature of available techniques |
“It was too much for her to go through at the time and rather experimental for her age” (Parent no FP, DR = 25, low regret) “I didn’t feel that there were any realistic options” (Parent, no FP, DR = 30, high regret) |
| Risk to the individual patient | “Our decision was based more upon the immediate risk to [her] health having just undergone major surgery…” (Parent, no FP, DR = 10, low regret) |
| Current situation | “…As it was ovarian slices, not eggs, my IVF specialist is hesitant to use them as they may contain leukaemic cells- so therefore I will not use them. I wish they had frozen the eggs instead…” (Patient, OTCP, DR = 10, low regret) |
| FP not discussed prior to treatment | “I didn’t get to make [the decision] and I’m extremely lucky to have my baby now.” (Patient, No FP, DR = 95, high regret) |
| Dissatisfaction with the discussion process |
“[I] still think it was the right decision as an option is better than no option in this situation… it was such an intense time I feel more information on pros/cons needs to be provided.” (Parent, TTCP, DR = 15, low regret) “I do not regret the decision.... the [discussion] was extremely rushed…and we perhaps did not get all the information on what follow up appointments etc. would be required....” (Parent, TTCP, DR = 0, low regret) “Discussion was at a very late stage, rushed and without time to adequately address fertility preservation process.” (Parent, sperm cryopreservation, DR = 85, high regret) |
DR, decision regret; FP, fertility preservation; TTCP, testicular tissue cryopreservation; OTCP, ovarian tissue cryopreservation; GnRHa, gonadotrophin-releasing hormone analogue
Responses to “Why are you satisfied with the decision that was made?”
The majority of parents and patients (80%; 92/115) expressed hope around having “options”, “choices” and “chances” of parenthood (n = 27), and in particular, “biological children” (n = 9) in the future. Some described FP as giving them a “back-up plan” or “insurance” measure (n = 5).
The notion of making decisions based on the child’s current (n = 3) and future preferences (n = 5) was also raised by parents. Nineteen patients also reported satisfaction with the decision made, based on their preferences to have children (n = 7) or have options available to them in the future. Parents also expressed satisfaction based on the low risk of the procedure (n = 10) and their desire to upkeep their duty of care and parental responsibility to act in the best interests of their child (n = 5).
For participants who declined FP, satisfaction with their decision was based on the experimental nature of what was available (n = 7) and risks to their child’s health exceeding the expected benefit (n = 4).
Responses to “Why do you regret the decision that was made?”
Despite low regret scores, dissatisfaction stemmed from not having fertility issues raised with them in a timely manner (n = 6) with two parents initiating the discussion themselves and expressing disappointment over the potential for it to have been “missed.” In another situation, despite conceiving naturally, a patient expressed dissatisfaction over a lack of pre-treatment discussion and the potential alternative prospect of infertility.
Five parents who had low decision regret scores after proceeding with FP identified issues with the process, including feeling the consultation process was rushed and that the provision of information was insufficient. The one parent and one patient that regretted the FP decision-making voiced similar concerns.
Suggestions to improve clinical practice
Several participants provided feedback to improve current clinical practice. Fertility discussions with specialists with an interest in fertility; and occurring in a location separate to oncology for clarity and focus were advocated by some. Two patients remember being told that they would never be able to have children and urged for more sensitive language to be adopted during the counselling process, and for consideration of discussion of other measures such as donor sperm. Adequate privacy during sperm collection, early discussion to allow repeat sperm collection for inadequate samples and appropriate follow-up and feedback of results were also flagged as important. Concerns about including children in the fertility consultations and options of having discussions with parents alone, at least initially, were also raised.
Discussion
Among a population of parents and patients who had FP discussions, the majority reported low decision regret. Low regret was associated with having a timely fertility discussion (before the commencement of high-risk gonadotoxic therapy) and having a FP procedure. This is consistent with overall regret scores reported in a wide variety of clinical contexts, including FP decisions in adults, where low regret was associated with high-quality information provision, pre-treatment discussions, and greater involvement in the decision-making process [10, 13].
Our data are similar to other studies examining decision regret in adult cancer patients which have identified significant associations between low regret and having FP counselling and FP procedures [13, 15, 17, 18]. Disentangling the role of the discussion from the procedures has only been investigated by one study, and although both groups were reported as having ‘low’ regret, regret was higher (8.4 on a scale of 5-25) in those that had fertility counselling alone compared to 6.6 in those that then proceeded to preserve fertility [15]. Their method of calculating regret is not directly comparable with our cohort, however, it does suggest that the opportunity to take action to preserve fertility may add to the benefits already gained from counselling.
Among our participants, detailed fertility discussions with an oncofertility specialist (paediatric gynaecologist/endocrinologist), were not associated with higher decision regret, compared to discussions with an oncologist alone. This contrasts with previous studies, where counselling from a fertility specialist in addition to oncology consultations about FP was associated with reduced regret [15, 18]. Our results need to be considered in the context of the fertility service at The RCH. Oncology providers have been partners in the fertility programme, an interdepartmental collaboration, since 2013 and have contributed to principles of care and development of clinical resources and pathways to aid discussion [23, 24]. It is possible that relatively high-quality oncologist-led discussions with families may produce a ceiling effect in the analysis. Furthermore “oncofertility specialist” in this study referred to discussion with a paediatric gynaecologist or endocrinologist, as the vast majority of fertility consultations (apart from those for sperm collection) occur within the paediatric centre. Overall, it is likely that the content, quality and timing of the discussion are more important factors, with respect to post-treatment regret.
As the first to report on the association between late FP discussion and high regret, this study provides evidence to support clinical guideline recommendations around early discussion about FP [1, 3, 4]. The need to start therapy urgently for aggressive malignancies can exclude a patient from being able to pursue FP. However, we recommend a discussion concerning the impact of treatment on fertility and potential reasons why FP cannot be pursued prior to commencing therapy as this may be helpful to minimise fertility-related distress in families.
For participants who had FP, the type of procedure (whether it was experimental or not) did not influence decision regret, consistent with past research demonstrating that lack of efficacy data for tissue cryopreservation is not a deterrent for parents in proceeding with FP [26, 27]. However, expecting that FP would be successful in their lifetime was a predictor of low regret in our study population. This is important in the context of the value expectancy decision-making process. The Health Belief Model theorises that greater satisfaction with a decision is based on a good understanding of risks (or in this case chance of success) and thus greater management of expectations [28–30]. However, regret scores may change in participants who are overly optimistic of future success if fertility does not eventuate. Thus, it is important that parents and patients are provided with accurate information about realistic chances of success and ongoing fertility consultation well into survivorship.
The free-text responses indicated that factors other than the discussion itself can influence regret, such as future hope for biological parenthood, as demonstrated in previous studies [2, 27]. What is unclear from our data and previous studies is whether parental decision-making is also influenced by their desire for future grandchildren and their own experiences with infertility. These unknowns have been raised in the ethical debate surrounding paediatric FP [2, 9]. Parents also wanted their child to know that they “did all [they could]” illustrating the emotional burden of parental decision-making.
In adult populations regardless of the clinical context, high levels of decisional conflict at the time of making a decision [12, 31], lower satisfaction with information provision [12, 17, 18, 32, 33] and less involvement in the decision-making process [34], were associated with higher regret. Similar concerns were raised in our study by parents and patients alike regardless of their level of regret. Namely that the consultation felt “rushed” without time to “adequately address” the FP process, signifying a need for greater information provision and support. From these data, we can conclude that minimising decision regret relies on providing patients with quality written and verbal information support, and allowing shared decision-making, to the extent the family desires. As such, the use of evidence-based decision aids may play a role [35].
Limitations
This was a biased group towards fertility preservation with over 70% having a FP procedure.
All participants in the study consented to be a part of our FP research programme, indicating that some discussion regarding fertility occurred. We were therefore unable to recruit patients and their families who had not had any discussion, for whom regret could be higher due to lack of discussion, or lower if they received low-risk gonadotoxic treatment. In addition, the majority that had discussions had a higher fertility risk profile and thus the sample is not necessarily representative of the general oncology patient population.
Interestingly, eight participants reported harm in their responses to the DRS . “The choice did me a lot of harm” is one item of a 5-point Likert scale that aims to quantify regret. In its current format, this survey does not allow us to explore the experience of harm any further as it has not been directly asked from the participants. The limited amount of qualitative data from participants who selected “strongly agree” or “agree” to this item does not reflect on the harm they experienced from making a decision. In future studies, follow-up questions could be included to allow participants to further elaborate on the harm experienced when making an FP decision as this would be a crucial outcome to evaluate when assessing regret surrounding FP.
In our survey, we asked participants two Likert-type questions ascertaining their impression regarding the likelihood of success of FP procedures in their lifetime and the lifetime of the next generation. We would define success in the context of FP as being a live birth resulting from the FP procedure, however, this was not defined in the survey and therefore success would have been interpreted subjectively by each participant.
This study assessed regret soon after making a decision with the average time to participating in the study from the time of discussion being 1.7 years. Regret may change in the longer term, particularly closer to the time of desiring fertility. Recall bias may impact the results as we were obtaining a self-report of regret surrounding a historical choice. However, any outcome is subject to some level of recall bias, as regret can only be assessed after a decision has been made. To assess the impact of recall bias, time since diagnosis was accounted for in the regression model and was not a confounder. Furthermore, using a DRS score of 30 as an arbitrary cut-off between low and high regret as suggested by Becerra Pérez et al. [10] may not be clinically relevant. Further research is required in all healthcare settings to identify levels of regret that are meaningful to patients and clinicians. Finally, excluding palliative/deceased patients misses an important group who may have potentially different insights into fertility decisions.
Future directions
To shed light on long-term regret, a future longitudinal study of this cohort would be of value, at a time when fertility is desired. Further studies looking at lower risk populations would be useful, to determine if discussions around fertility are being undertaken for this group, to ensure there is no decision regret present in this patient population as well. Financial barriers may have potential to impact regret: our centre funds tissue storage and gonadotrophin-releasing hormone analogue therapy, so that cost does not factor into decision-making. However, this is not the case for all centres and expenses for future in-vitro fertilisation could significantly impact on longer term decisions and regret.
This is the first study to assess decision regret in both parents and patients. We wanted to be as inclusive as possible and felt that although each family member was present at the same consultation, each parent or patient may be satisfied with or regret their decision for different reasons despite being provided the same information. As there are only twenty-seven families for which regret data can be compared between participants, our numbers are insufficient for conclusions to be drawn. However, considering that approximately 20% of these families (5/27) differed in regret category between participants (including in almost a third of parent/parent pairs), there is clearly a need for further investigation in future studies.
Although decisional conflict was not assessed using a validated scale, answers to free-text responses suggest that decisional conflict may have been present at the time of fertility counselling. Previous studies highlight the value of decisional conflict as a predictor variable closely correlated to post-decision regret [2, 12, 21]. Assessment of degree of decisional conflict at the time of decision-making in future studies may help to identify families most at risk of future regret [11, 36]. By doing so, personalised decision-support interventions could be provided to these families, to alleviate their distress, improve the decision-making process and prevent decision regret [30, 37, 38].
Conclusion
This is the first study to use the validated DRS to examine regret in the paediatric FP setting that considers not only regret in the parent as the decision-maker, but also regret in the patients themselves. Most reported low or no regret over their FP decision. The formalised approach to fertility care may be contributing to these results.
It is evident that fertility is an important concern to cancer patients and their parents but there remain many challenges around the best way to convey the information (an unmet information need). Amendments to the decision-making process can ensure parents and patients are better equipped to make quality decisions in a time of great emotional vulnerability, to increase satisfaction in all families. Given the paucity of literature in the field of paediatric oncofertility internationally, this study provides an important contribution towards understanding the needs of this patient population in the immediate years following a cancer diagnosis. Further research and follow-up of children and adolescents with cancer into survivorship are needed to meet their needs in the long term.
Electronic supplementary material
Study questionnaire in table format.pdf (PDF 89 kb)
Acknowledgements
We thank Hannah Clark for her contribution to the oncofertility database, which was sourced for oncofertility data and Matthew Kemertzis for his contribution to this body of work as the past Fertility Preservation Taskforce project manager.
Author contribution
Conception and design: All authors
Data acquisition: SJ and NL
Data analysis: SJ, MP, LG, and interpreted by all authors.
Manuscript: SJ wrote the first draft of the manuscript. All authors critically revised one or more versions of the manuscript and approved the final version.
Funding
The research was supported by the Victorian Cancer Agency (ECSG13027). YJ is funded by the Victorian Cancer Agency and is a National Health and Medical Research Council Translation of Research into Clinical Practice (TRIP) fellow. MP is supported by a NBCF Early Career Fellowship (ECF-0015).
Compliance with ethical standards
Conflict of interest
All authors declare they have no competing interests/conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Oktay K, Harvey BE, Partridge AH, et al. Fertility preservation in patients with cancer: ASCO clinical practice guideline update. J Clin Oncol. 2018;36(19):1994–2001. doi: 10.1200/JCO.2018.78.1914. [DOI] [PubMed] [Google Scholar]
- 2.Schover LR. Patient attitudes toward fertility preservation. Pediatr Blood Cancer. 2009;53(2):281–284. doi: 10.1002/pbc.22001. [DOI] [PubMed] [Google Scholar]
- 3.National Institute for Health and Care Excellence. Fertility problems: assessment and treatment. United Kingdom: NICE2013. Report No. p. CG156. [PubMed]
- 4.AYA cancer fertility preservation guidance working group. Fertility preservation for AYAs diagnosed with cancer: Guidance for health professionals. [Cancer Council Australia Cancer Guidelines Wiki Web site]. October 6, 2017. Available at: https://wiki.cancer.org.au/australia/COSA:AYA_cancer_fertility_preservation. Accessed December 3, 2017.
- 5.Adams E, Hill E, Watson E. Fertility preservation in cancer survivors: a national survey of oncologists' current knowledge, practice and attitudes. Br J Cancer. 2013;108(8):1602–1615. doi: 10.1038/bjc.2013.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McQuillan SK, Malenfant D, Jayasinghe YL, et al. Audit of Current Fertility Preservation Strategies used by individual pediatric oncologists throughout Australia and New Zealand. J Pediatr Oncol. 2013;1:112–118. [Google Scholar]
- 7.Schover LR, Brey K, Lichtin A, et al. Oncologists’ attitudes and practices regarding banking sperm before cancer treatment. J Clin Oncol. 2002;20(7):1890–1897. doi: 10.1200/JCO.2002.07.174. [DOI] [PubMed] [Google Scholar]
- 8.Vadaparampil S, Quinn G, King L. etl al. Barriers to fertility preservation among pediatric oncologists. Patient Educ Couns. 2008;72(3):402–410. doi: 10.1016/j.pec.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 9.Li N, Jayasinghe Y, Kemertzis MA, et al. Fertility preservation in pediatric and adolescent oncology patients: the decision-making process of parents. J Adolesc Young Adult Oncol. 2017;6(2):213–222. doi: 10.1089/jayao.2016.0061. [DOI] [PubMed] [Google Scholar]
- 10.Becerra Perez MM, Menear M, Brehaut JC, et al. Extent and predictors of decision regret about health care decisions: a systematic review. Med Decis Mak. 2016;36(6):777–790. doi: 10.1177/0272989X16636113. [DOI] [PubMed] [Google Scholar]
- 11.O'Connor AM. User manual – Decision Regret Scale [document on the Internet] Ottawa: Ottawa Hospital Research Institute; ©; 1996. [Google Scholar]
- 12.Bastings L, Baysal O, Beerendonk CC, et al. Deciding about fertility preservation after specialist counselling. Hum Reprod. 2014;29(8):1721–1729. doi: 10.1093/humrep/deu136. [DOI] [PubMed] [Google Scholar]
- 13.Benedict C, Thom B, Kelvin JF. Young adult female cancer survivors’ decision regret about fertility preservation. J Adolesc Young Adult Oncol. 2015;4(4):213–218. doi: 10.1089/jayao.2015.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Letourneau JM, Katz PP, Smith JF, et al. The impact of fertility counseling and fertility preservation on long-term psychosocial outcomes in young female cancer survivors. Fertil Steril. 2010;94(4):S65. doi: 10.1016/j.fertnstert.2010.07.253. [DOI] [Google Scholar]
- 15.Letourneau JM, Ebbel EE, Katz PP, et al. Pretreatment fertility counseling and fertility preservation improve quality of life in reproductive age women with cancer. Cancer. 2012;118(6):1710–1717. doi: 10.1002/cncr.26459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mersereau JE, Goodman LR, Deal AM, et al. To preserve or not to preserve: how difficult is the decision about fertility preservation? Cancer. 2013;119(22):4044–4050. doi: 10.1002/cncr.28317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan SW, Niemasik EE, Kao CN, et al. Decisional regret in women diagnosed with cancer who undergo reproductive health counseling (RHC) Fertil Steril. 2014;100(3):S26. doi: 10.1016/j.fertnstert.2013.07.1757. [DOI] [Google Scholar]
- 18.Chan JL, Letourneau J, Salem W, et al. Regret around fertility choices is decreased with pre-treatment counseling in gynecologic cancer patients. J Cancer Surviv. 2017;11(1):58–63. doi: 10.1007/s11764-016-0563-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jadoul P, Guilmain A, Squifflet J, et al. Efficacy of ovarian tissue cryopreservation for fertility preservation: lessons learned from 545 cases. Hum Reprod. 2017;32(5):1046–1054. doi: 10.1093/humrep/dex040. [DOI] [PubMed] [Google Scholar]
- 20.McDougall RJ, Gillam L, Delany C, et al. Ethics of fertility preservation for prepubertal children: should clinicians offer procedures where efficacy is largely unproven? J Med Ethics. 2017;0:1–5. doi: 10.1136/medethics-2016-104042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lorenzo AJ, Pippi Salle JL, Zlateska B, et al. Decisional regret after distal hypospadias repair: single institution prospective analysis of factors associated with subsequent parental remorse or distress. J Urol. 2014;191(5 Suppl):1558–1563. doi: 10.1016/j.juro.2013.10.036. [DOI] [PubMed] [Google Scholar]
- 22.Brehaut JC, O'Connor AM, Wood TJ, Hack TF, Siminoff L, Gordon E, Feldman-Stewart D. Validation of a decision regret scale. Med Decis Mak. 2003;23(4):281–292. doi: 10.1177/0272989X03256005. [DOI] [PubMed] [Google Scholar]
- 23.Kemertzis M, Ranjithakumaran H, Hand M, et al. Fertility Preservation Toolkit: A clinician resource to assist clinical discussion and decision making in pediatric and adolescent oncology. J Pediatr Hematol Oncol. 2018;40(3):e133–e139.22. doi: 10.1097/MPH.0000000000001103. [DOI] [PubMed] [Google Scholar]
- 24.Hand M, Kemertzis M, Peate M, et al. A clinical decision support system to assist pediatric oncofertility: a short report. J Adolesc Young Adult Oncol. 2018;7(4):509–513. doi: 10.1089/jayao.2018.0006. [DOI] [PubMed] [Google Scholar]
- 25.Quinn J, Murphy D, Knapp C, et al. Who Decides? Decision making and fertility preservation in teens with cancer: a review of the literature. J Adolesc Health. 2011;49(4):337–346. doi: 10.1016/j.jadohealth.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wyns C, Collienne C, Shenfield F, et al. Fertility preservation in the male pediatric population: factors influencing the decision of parents and children. Hum Reprod. 2015;30(9):2022–2030. doi: 10.1093/humrep/dev161. [DOI] [PubMed] [Google Scholar]
- 27.Ginsberg JP, Li Y, Carlson CA, et al. Testicular tissue cryopreservation in prepubertal male children: an analysis of parental decision-making. Pediatr Blood Cancer. 2014;61(9):1673–1678. doi: 10.1002/pbc.25078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Janz NK, Becker MH. The Health Belief Model: a decade later. Health Educ Q 1984; 11(1):1-47. [DOI] [PubMed]
- 29.Baysal O, Bastings L, Beerendonk CC, et al. Decision-making in female fertility preservation is balancing the expected burden of fertility preservation treatment and the wish to conceive. Hum Reprod. 2015;30(7):1625–1634. doi: 10.1093/humrep/dev116. [DOI] [PubMed] [Google Scholar]
- 30.Peate M, Meiser B, Hickey M, et al. The fertility-related concerns, needs and preferences of younger women with breast cancer: a systematic review. Breast Cancer Res Treat. 2009;116(2):215–223. doi: 10.1007/s10549-009-0401-6. [DOI] [PubMed] [Google Scholar]
- 31.Peate M, Meiser B, Cheah BC, et al. Making hard choices easier: a prospective, multicentre study to assess the efficacy of a fertility-related decision aid in young women with early-stage breast cancer. Br J Cancer. 2012;106(6):1053–1061. doi: 10.1038/bjc.2012.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harcourt D, Russell C, Hughes J, et al. Patient satisfaction in relation to nipple reconstruction: the importance of information provision. J Plast Reconstr Aesthet Surg. 2011;64(4):494–499. doi: 10.1016/j.bjps.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 33.Zhong T, Hu J, Bagher S, et al. Decision regret following breast reconstruction: the role of self-efficacy and satisfaction with information in the preoperative period. Plast Reconstr Surg. 2013;132(5):724e–734e. doi: 10.1097/PRS.0b013e3182a3bf5d. [DOI] [PubMed] [Google Scholar]
- 34.Lantz PM, Janz NK, Fagerlin A, et al. Satisfaction with surgery outcomes and the decision process in a population-based sample of women with breast cancer. Health Serv Res. 2005;40(3):745–767. doi: 10.1111/j.1475-6773.2005.00383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jayasinghe Y, Kemertzis M, Hand M, et al. Decision support tools in paediatric and adolescent oncofertility for clinicians and parents. Pediatr Blood Cancer. 2017;64:S110–S11134. doi: 10.1002/pbc.26199. [DOI] [Google Scholar]
- 36.Hong P, Gorodzinsky AY, Taylor BA, et al. Parental decision making in pediatric otoplasty: the role of shared decision making in parental decisional conflict and decisional regret. Laryngoscope. 2016;126(Suppl 5):S5–S13. doi: 10.1002/lary.26071. [DOI] [PubMed] [Google Scholar]
- 37.Peate M, Meiser B, Friedlander M, et al. It's now or never: fertility-related knowledge, decision-making preferences, and treatment intentions in young women with breast cancer--an Australian fertility decision aid collaborative group study. J Clin Oncol. 2011;29(13):1670–1677. doi: 10.1200/JCO.2010.31.2462. [DOI] [PubMed] [Google Scholar]
- 38.O'Connor A. Validation of a decisional conflict scale. Med Decis Mak. 1995;15(1):25–30. doi: 10.1177/0272989X9501500105. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Study questionnaire in table format.pdf (PDF 89 kb)