Abstract
Purpose
To identify the disease-causing gene in a family with female infertility and fertilization failure.
Methods
Whole-exome sequencing and Sanger sequencing were used to identify the disease-causing gene in a female with infertility and fertilization failure. Subcellular localization and western blot analysis were used to check the effect of mutations.
Results
We identified novel compound heterozygous mutations c.598C>T (p.Arg200Ter) and c.1319G>C (p.Trp440Ser) in WEE2 gene in a female with infertility and fertilization failure. The p.Arg200Ter mutant WEE2 gene produce truncated protein and mainly located in the nucleus, the same as the wild protein, while the p.Trp440Ser mutant WEE2 proteins are located in the nucleus and cytoplasm and the expression level of p.Trp440Ser mutant WEE2 protein is reduced significantly compared with that of wild-type WEE2.
Conclusions
We discovered novel compound heterozygous mutations c.598C>T (p.Arg200Ter) and c.1319G>C (p.Trp440Ser) in WEE2 gene in a female whose oocytes could not form pronucleus after intracytoplasmic sperm injection (ICSI). Moreover, mutations in WEE2 gene affect the normal function of WEE2 proteins and cause fertilization failure.
Keywords: Female infertility, WEE2, Mutation, Intracytoplasmic sperm injection
Introduction
In general, female infertility is defined as a woman being unable to get pregnant after 1 year of frequent unprotected sexual intercourse [1]. Most cases of female infertility are related to specific disorders, such as environmental factor, ovulatory disorders, endometriosis, and genetic factor [2]. About 5–10% of infertile women are caused by genetic factors such as chromosome aberrations, single or multiple gene mutations, and polymorphisms [3]. For the past few years, several disease genes of female infertility have been reported. From 2014 to the present, investigators found that the mutations of ZP genes cause female infertility with zona pellucida (ZP) defects [4–6]. In 2015, Alazami et al. discovered that TLE6 mutations are a rare cause of human female infertility and human embryonic lethality [7]. In 2016, Feng et al. reported that TUBB8 mutations cause female infertility by disrupting oocyte meiotic and maturation [8]. Xu et al. identified PADI6 mutations can cause female infertility characterized by early embryonic arrest in multiple IVF and ICSI cycles [9]. In 2017, Maddirevula and Chen et al. demonstrated the oocyte-specific translational repressor PATL2 cause female infertility characterized by oocyte maturation arrest [10, 11]. In 2018, Sang et al. identified homozygous mutations in WEE2 that are responsible for human fertilization failure [12]. In 2019, Mu et al. reported that mutations in NLRP2 and NLRP5 cause female infertility characterized by early embryonic arrest [13]. Soon afterwards, Sang et al. described the female infertility characterized by oocyte death and identified heterozygous mutations in PANX1 responsible for this phenotype [14]. Even so, the genetic basis of female fertilization failure has remained largely unknown.
WEE2, also known as Wee1B, is an important oocyte-specific kinase and belongs to the WEE kinase protein family [12]. It is exclusively expressed in oocytes and was conserved among different species [15]. Furthermore, WEE2 can phosphorylate and inhibit Cdc2 (CDK1) and acts as a key regulator of meiosis during prophase I and metaphase II [16, 17]. More and more evidences show that homozygous mutations in WEE2 may lead to female infertility [12, 15, 17–19]. In WEE2 gene, Sang et al. found mutations c.700G>C (p.Asp234His), c.1473dupA (p.Thr493Asnfs*39), and c.1006_1007insTA (p.His337Tyrfs*24) [12]. Zhao et al. discovered mutations c.293_294insCTGAGACACCAGCCCAACC (p.Pro98Pro fsX2), c.1576T>G (p.Tyr526Asp), c.991C>A (p.His331Asn), and so on [15]. Dai et al. showed mutations c.585G>C (p.Lys195Asn), c.1228C>T (p.Arg410Trp), c.1006_1007dup (p.His337Tyrfs*24), and so on [17]. Yang et al. reported mutation c.619C>T (p.R207C) [18]. Zhang et al. displayed mutations such as c.T997C (p.S333P), c.G1221A (p.D408Vfs*1), and c.220_223delAAAG (p.E75Vfs*6) [19].
Here, we identified novel compound heterozygous mutations c.598C>T (p.Arg200Ter) and c.1319G>C (p.Trp440Ser) in WEE2 in a woman with infertility and fertilization failure. The p.Arg200Ter mutant WEE2 gene produce truncated proteins and mostly located in the nucleus like the wild protein; western blot shows that the expression level of p.Trp440Ser mutant WEE2 proteins is reduced significantly compared with that of wild-type WEE2 proteins.
Materials and methods
Clinical samples
There were 17 affected women diagnosed with fertilization failure and recruited from the Reproductive Medicine Center, Tongji Hospital, Huazhong University of Science and Technology. Only the patient in this study showed pronucleus formation failure. Peripheral blood samples were taken for DNA extraction. This study was approved by the ethics committee of Huazhong University of Science and Technology. All participants in the study agreed with informed consent to participate in the investigation.
Genetic studies
Genomic DNA was extracted from blood samples using standard methods and the patient’s genomic DNA was used for whole-exome sequencing. Whole-exome capture (Agilent) and Illumina sequencing were performed following the standard protocols. Basic bioinformatics analysis included mapping the raw FASTQ files to the human reference sequence (NCBI Genome build GRCh37): annotation with the GRCh37, 1000 Genomes, Exome Aggregation Consortium (ExAC, version 0.3.1) databases, and so on. According to the results of whole-exome sequencing, we designed primers around the mutation sites. PCR was performed in reaction system 50 μl, containing 5 μl 10× Taq buffer, 0.2 mM dNTP, 0.5 μM of each primer, 1 unit of Taq DNA polymerase, and 20 ng of human genomic DNA. The amplification program was one cycle for 5 min for denaturation at 94 °C, 35 cycles of 30 s at 94 °C, 30 s at 58 °C, 12 min at 72 °C, and one 10-min extension step at 72 °C. PCR products were purified using the Kangwei Gel Extraction Kit (Kangwei, Beijing, China). DNA sequencing analysis was performed using the BigDye Terminator Cycle Sequencing v3.1 kit and an ABI PRISM 3500 Genetic Analyzer (Applied Biosystems, Foster City, CA).
Expression vector construction and mutagenesis
The wild WEE2 cDNA was cloned into the pEGFP-C1 vector (the cDNA of human WEE2 gene is a gift from Lei Wang’s lab in Fudan University). We used Mut Express II Fast Mutagenesis Kit V2 (Vazyme Biotech Co., Ltd.) to construct the mutant vector. All expression vectors (wild-type and mutant WEE2 expression vector) were confirmed by Sanger sequencing.
Subcellular localization in Hela cells
Hela cells were plated on chamber slide flaskettes (Nalge Nunc, Naperville, IL) and were transiently transfected with expression vectors as described above. Twenty-four hours later, cells were fixed in 4% formaldehyde. The nuclei of cells were stained with DAPI and then visualized by confocal microscopy (× 100 oil immersion lens).
Western blotting
To evaluate the effects of the mutations on WEE2 protein, HEK293T cells were plated onto a 6-well plate and transfected with wild or mutant WEE2 expression vectors using Lipofectamine 2000 (Invitrogen). After being incubated for 48 h, cells were lysed by RIPA lysis buffer (Beyotime Institute of Biotechnology). Total proteins were separated by 10% SDS/PAGE gels and transferred to polyvinylidene difluoride (PVDF) membranes. The primary antibody we used is mouse anti GFP-Tag mAb; it came from ABclonal company and was diluted with 5% bovine serum albumin at a ratio of 1:2000. The second antibody is goat anti-mouse IgG, HRP conjugated (Beijing ComWin Biotech Co., Ltd., 1:20000). The signals were detected with Bio-RAD ChemiDoc XRS+ imaging system. (Bio-RAD, USA). The repetition times were three.
Results
Clinical characteristics of the patient
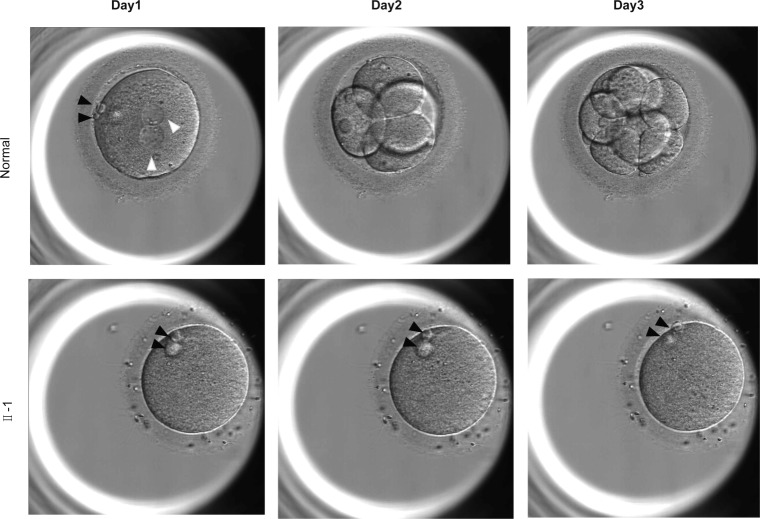
The female-affected individual (Fig. 1a), 27 years old, was diagnosed with primary infertility in Tongji Hospital of unknown cause for 8 years. Her husband has normal seminal parameters (157.32 × 106 spermatozoa per ejaculate, 71.8% sperm motility, and 10% normal sperm morphology). In addition, the sperm of her husband could fertilize with three oocytes which were left by the woman who came to perform IVF and the function was normal. In her first IVF cycle, 15 oocytes were acquired and none of them formed pronucleus. In the second ICSI cycle, we obtained 27 oocytes (22 MII oocytes), 8 MII oocytes were frozen and 14 MII oocytes were performed ICSI. Lamentedly, none of them formed two pronucleus (2PN) and one of them was shown in Fig. 2. The patient’s younger sister had pregnancy history.
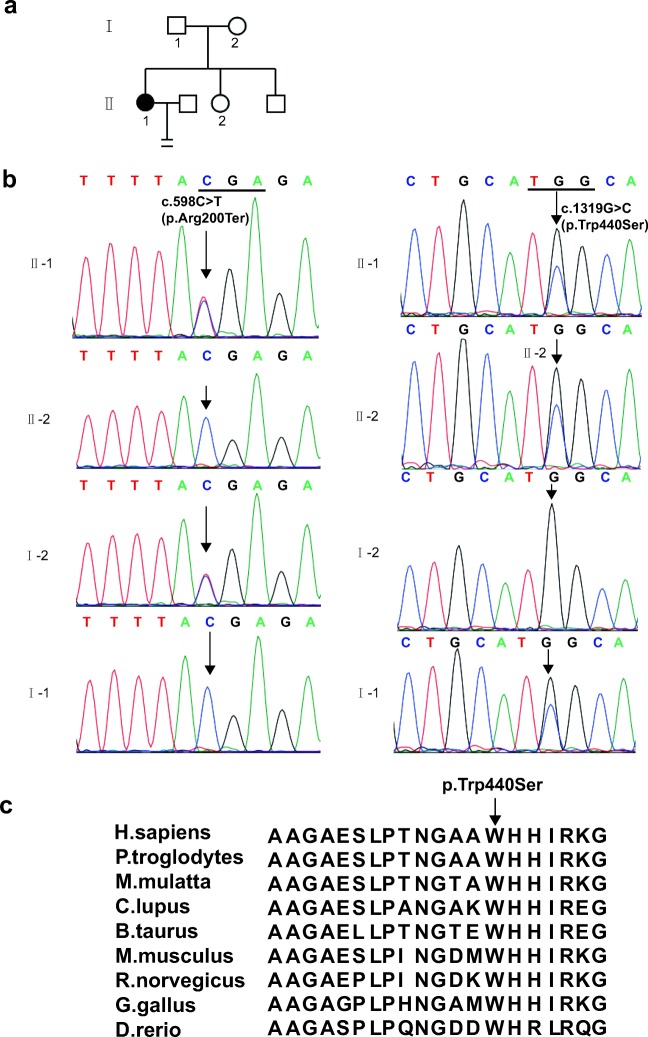
Fig. 1.
Identification of mutations in WEE2. a Pedigree of the family with fertilization failure. The circles filled with black color represent the affected female individual. b Sanger sequencing results for the patient, her younger sister, and her parents. The left shows the heterozygous mutation c.598C>T (p.Arg200Ter) and the right indicates the heterozygous mutation c.1319G>C (p.Trp440Ser). c The conservation of the mutated amino acids is indicated by the alignment of nine vertebrata.
Fig. 2.
Morphology of a normal zygote and affected individual oocytes. Oocytes were captured by optical microscopy after ICSI. The white arrowhead indicates pronuclei, and the black arrowhead indicates the polar body
Identification of a novel compound heterozygous mutations in WEE2
The sequencing depth of WES is 100×; we filtered common SNPs (single nucleotide polymorphisms) and got all the candidate genes which encoded the proteins in the whole genome of the patient. Then, we carefully checked the function of all the genes which carry the mutations; only the mutations in WEE2 were in accordance with recessive inheritance pattern and the function of WEE2 is related to oocyte development. Besides, this gene also has been reported to be related with female infertility and the phenotype of our patient is very similar to the patients who carry the WEE2 mutations. Finally, Sanger sequencing was used to confirm the two mutations in WEE2 in the family. The patient had a nonsense mutation c.598C>T (p.Arg200Ter) which came from her mother and a missense mutation c.1319G>C (p.Trp440Ser) which came from her father (Fig. 1b). Her young sister only had the missense mutation c.1319G>C (p.Trp440Ser) in WEE2. The nonsense mutation c.598C>T (p.Arg200Ter) had been reported in an infertile woman who also had the other frame shift mutation c.220_223delAAAG [18]. The allele frequency of c.1319G>C (p.Trp440Ser) was not found in the ExAC database. The conservation in different species of the missense mutation p.Trp440Ser is shown in Fig. 1c.
Subcellular localization of WEE2 protein in Hela cells
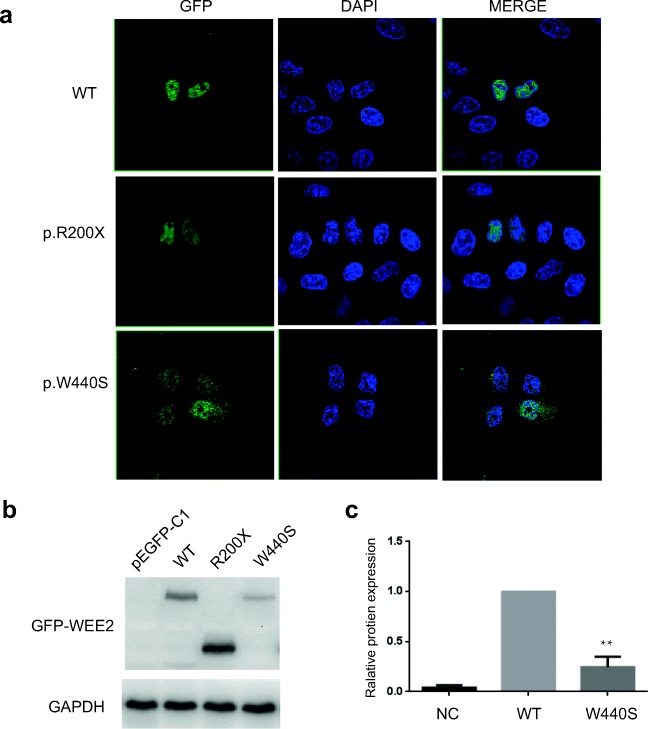
To evaluate the effects of the mutations on protein localization, wild-type and mutant WEE2 expression plasmids were transfected into HeLa cells. As shown in Fig. 3a, the wild-type and the p.Arg200Ter WEE2 are located in the nucleus, while the fluorescence signal of p.Trp440Ser WEE2 is decreased and p.Trp440Ser WEE2 proteins are located both in the nucleus and cytoplasm.
Fig. 3.
Subcellular localization and Western blot analysis of the wild and mutant WEE2 proteins. a PEGFP-C1-WEE2 (WT, Arg200Ter, Trp440Ser) plasmids were transfected in Hela cells. Twenty-four hours post-transfection, cells were fixed in 4% formaldehyde and the nuclei of cells were stained with DAPI (blue color). WEE2 proteins were fused to the green fluorescent protein. b Western blot analysis of wild and mutant WEE2 proteins in HEK293T cells. c Relative total protein expression of wild and p.Trp440Ser WEE2. Results represent the average ± SEM of three independent experiments (**p < 0.01, n = 3)
Expression level of WEE2 protein in the HEK293T cells
To assess the effect of mutations on WEE2 protein expression level in vitro, western blot analysis was performed. HEK293T cells were transfected with wild and mutant WEE2 constructs. As shown in Fig. 3 b and c, the nonsense mutation c.598C>T (p.Arg200Ter) resulted in a truncated protein, while the missense mutation c.1319G>C (p.Trp440Ser) led to decreased expression level of WEE2 protein.
Discussion
In this study, we identified a novel compound heterozygous mutation in WEE2 which led to female infertility and fertilization failure. After intracytoplasmic sperm injection (ICSI), the oocytes from the patient could not form pronucleus. The p.Arg200Ter WEE2 generated a truncated protein but the subcellular localization in Hela cells was unchanged. However, the p.Trp440Ser WEE2 subcellular localization was changed and the protein expression was reduced obviously compared with wild type. The mutation may be located in the nuclear localization sequences (NLS) and nuclear export sequences (NES), which can mediate protein’s shuttle between nucleus and cytoplasm, or the mutation may affect the structure of the WEE2 proteins. Of course, the accurate molecular mechanism why this mutation affected the localization is still unknown; to explain the reason, a long time to deeply study is needed in the future.
Oh et al. showed that WEE2 is essential for metaphase II exit, and WEE2-mediated inhibition of Cdc2 is required for pronuclear formation in mouse oocytes [20]. Sang et al. reported that mutations in WEE2 reduced the protein level in the oocytes and weakened the phosphorylation of WEE2 and Cdc2 in vitro, which blocked oocyte MII exit and led to subsequent fertilization failure [12]. Localization experiments and western blot showed that the compound heterozygous mutations which we identified in this study affected the subcellular localization and protein expression, may lead to decrease of phosphorylation of WEE2 and Cdc2, and then affect the mitosis and cell cycle which eventually leads to fertilization failure and infertility.
Yang et al. reported that ICSI-AOA (artificial oocyte activation) could not rescue fertilization failure in a female patient with a WEE2 mutation, and they considered the genetic diagnosis that female patients who carry WEE2 mutations can avoid repeated treatment with ICSI or ICSI-AOA [18]. By injecting WEE2 cRNA into affected oocytes in vitro, Sang et al. found that the method led to successful fertilization and blastocyst formation [12]. Although injecting WEE2 cRNA into the oocyte has uncertain influence to the embryo, it still provides a potential therapeutic treatment in affected individuals with WEE2 mutations.
In conclusion, we identified a novel compound heterozygous mutation in WEE2 of a woman with infertility and fertilization failure and explained the pathogenesis on molecular level. Our study not only expands on the mutation spectrum of WEE2 but also provides genetic evidence for pronucleus formation failure. In addition, it provides theoretical basis for genetic counseling of female infertility.
Acknowledgments
We thank Lei Wang and Qing Sang in Fudan University for their gift of WEE2 plasmid and the family members for their enthusiastic participation in this study.
Funding information
This work was supported by grants from the National Natural Science Foundation of China (81000079, 81170165, 81870959 to X.Z.) and supported by Program for HUST Academic Frontier Youth Team.
Compliance with ethical standards
This study was approved by the ethics committee of Huazhong University of Science and Technology. All participants in the study agreed with informed consent to participate in the investigation.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xiaopei Zhou, Lixia Zhu, Meiqi Hou and Yanling Wu contributed equally to this work.
Contributor Information
Lei Jin, Email: Ljin@tjh.tjmu.edu.cn.
Xianqin Zhang, Email: xqzhang04@hust.edu.cn.
References
- 1.Usadi RS, Merriam KS. On-label and off-label drug use in the treatment of female infertility. Fertil Steril. 2015;103:583–594. doi: 10.1016/j.fertnstert.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 2.Conforti A, Mascia M, Cioffi G, De Angelis C, Coppola G, De Rosa P, et al. Air pollution and female fertility: a systematic review of literature. Reprod Biol Endocrinol. 2018;16:117. doi: 10.1186/s12958-018-0433-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hanson B, Johnstone E, Dorais J, Silver B, Peterson CM, Hotaling J. Female infertility, infertility-associated diagnoses, and comorbidities: a review. J Assist Reprod Genet. 2017;34:167–177. doi: 10.1007/s10815-016-0836-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang HL, Lv C, Zhao YC, Li W, He XM, Li P, Sha AG, Tian X, Papasian CJ, Deng HW, Lu GX, Xiao HM. Mutant ZP1 in familial infertility. N Engl J Med. 2014;370:1220–1226. doi: 10.1056/NEJMoa1308851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zou Y, Zhou JY, Guo JB, Zhang ZY, Luo Y, Liu FY, Huang H, Wang F, He M, Wang LQ, Huang OP. Mutation analysis of ZP1, ZP2, ZP3 and ZP4 genes in 152 Han Chinese samples with ovarian endometriosis. Mutat Res. 2019;813:46–50. doi: 10.1016/j.mrfmmm.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 6.Zhou Z, Ni C, Wu L, Chen B, Xu Y, Zhang Z, Mu J, Li B, Yan Z, Fu J, Wang W, Zhao L, Dong J, Sun X, Kuang Y, Sang Q, Wang L. Novel mutations in ZP1, ZP2, and ZP3 cause female infertility due to abnormal zona pellucida formation. Hum Genet. 2019;138:327–337. doi: 10.1007/s00439-019-01990-1. [DOI] [PubMed] [Google Scholar]
- 7.Alazami AM, Awad SM, Coskun S, Al-Hassan S, Hijazi H, Abdulwahab FM, et al. TLE6 mutation causes the earliest known human embryonic lethality. Genome Biol. 2015;16:240. doi: 10.1186/s13059-015-0792-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feng R, Sang Q, Kuang Y, Sun X, Yan Z, Zhang S, Shi J, Tian G, Luchniak A, Fukuda Y, Li B, Yu M, Chen J, Xu Y, Guo L, Qu R, Wang X, Sun Z, Liu M, Shi H, Wang H, Feng Y, Shao R, Chai R, Li Q, Xing Q, Zhang R, Nogales E, Jin L, He L, Gupta ML, Jr, Cowan NJ, Wang L. Mutations in TUBB8 and human oocyte meiotic arrest. N Engl J Med. 2016;374:223–232. doi: 10.1056/NEJMoa1510791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu Y, Shi Y, Fu J, Yu M, Feng R, Sang Q, Liang B, Chen B, Qu R, Li B, Yan Z, Mao X, Kuang Y, Jin L, He L, Sun X, Wang L. Mutations in PADI6 cause female infertility characterized by early embryonic arrest. Am J Hum Genet. 2016;99:744–752. doi: 10.1016/j.ajhg.2016.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maddirevula S, Coskun S, Alhassan S, Elnour A, Alsaif HS, Ibrahim N, Abdulwahab F, Arold ST, Alkuraya FS. Female infertility caused by mutations in the oocyte-specific translational repressor PATL2. Am J Hum Genet. 2017;101:603–608. doi: 10.1016/j.ajhg.2017.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen B, Zhang Z, Sun X, Kuang Y, Mao X, Wang X, Yan Z, Li B, Xu Y, Yu M, Fu J, Mu J, Zhou Z, Li Q, Jin L, He L, Sang Q, Wang L. Biallelic mutations in PATL2 cause female infertility characterized by oocyte maturation arrest. Am J Hum Genet. 2017;101:609–615. doi: 10.1016/j.ajhg.2017.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sang Q, Li B, Kuang Y, Wang X, Zhang Z, Chen B, Wu L, Lyu Q, Fu Y, Yan Z, Mao X, Xu Y, Mu J, Li Q, Jin L, He L, Wang L. Homozygous mutations in WEE2 cause fertilization failure and female infertility. Am J Hum Genet. 2018;102:649–657. doi: 10.1016/j.ajhg.2018.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mu J, Wang W, Chen B, Wu L, Li B, Mao X, Zhang Z, Fu J, Kuang Y, Sun X, Li Q, Jin L, He L, Sang Q, Wang L. Mutations in NLRP2 and NLRP5 cause female infertility characterised by early embryonic arrest. J Genet. 2019;56:471–480. doi: 10.1136/jmedgenet-2018-105936. [DOI] [PubMed] [Google Scholar]
- 14.Sang Q, Zhang Z, Shi J, Sun X, Li B, Yan Z, Xue S, Ai A, Lyu Q, Li W, Zhang J, Wu L, Mao X, Chen B, Mu J, Li Q, du J, Sun Q, Jin L, He L, Zhu S, Kuang Y, Wang L. A pannexin 1 channelopathy causes human oocyte death. Sci Transl Med. 2019;11(485):eaav8731. doi: 10.1126/scitranslmed.aav8731. [DOI] [PubMed] [Google Scholar]
- 15.Zhao S, Chen T, Yu M, Bian Y, Cao Y, Ning Y, Su S, Zhang J, Zhao S. Novel WEE2 gene variants identified in patients with fertilization failure and female infertility. Fertil Steril. 2019;111:519–526. doi: 10.1016/j.fertnstert.2018.11.018. [DOI] [PubMed] [Google Scholar]
- 16.Han SJ, Conti M. New pathways from PKA to the Cdc2/cyclin B complex in oocytes: Wee1B as a potential PKA substrate. Cell Cycle. 2006;5:227–231. doi: 10.4161/cc.5.3.2395. [DOI] [PubMed] [Google Scholar]
- 17.Dai J, Zheng W, Dai C, Guo J, Lu C, Gong F, et al. New biallelic mutations in WEE2: expanding the spectrum of mutations that cause fertilization failure or poor fertilization. Fertil Steril. 2019;111:510–518. doi: 10.1016/j.fertnstert.2018.11.013. [DOI] [PubMed] [Google Scholar]
- 18.Yang X, Shu L, Cai L, Sun X, Cui Y, Liu J. Homozygous missense mutation Arg207Cys in the WEE2 gene causes female infertility and fertilization failure. J Assist Reprod Genet. 2019. 10.1007/s10815-019-01418-9. [DOI] [PMC free article] [PubMed]
- 19.Zhang Z, Mu J, Zhao J, Zhou Z, Chen B, Wu L, et al. Novel mutations in WEE2: expanding the spectrum of mutations responsible for human fertilization failure. Clin Genet. 2019;95:520–524. doi: 10.1111/cge.13505. [DOI] [PubMed] [Google Scholar]
- 20.Oh JS, Susor A, Conti M. Protein tyrosine kinase Wee1B is essential for metaphase II exit in mouse oocytes. Science. 2011;332:462–465. doi: 10.1126/science.1199211. [DOI] [PMC free article] [PubMed] [Google Scholar]