Abstract
Eosinophils are the prominent inflammatory cell involved in allergic asthma, atopic dermatitis, eosinophilic esophagitis, and hypereosinophilic syndrome and are found in high numbers in local tissue and/or circulating blood of affected patients. There is recent interest in a family of alarmins, including TSLP, IL-25 and IL-33, that are epithelial-derived and released upon stimulation of epithelial cells. Several genome wide association studies have found SNPs in genes encoding IL-33 to be risk factors for asthma. In two studies examining the direct role of IL-33 in eosinophils, there were differences in eosinophil responses. We sought to further characterize activation of eosinophils with IL-33 compared to activation by other cytokines and chemokines. We assessed IL-33 stimulated adhesion, degranulation, chemotaxis and cell surface protein expression in comparison to IL-3, IL-5, and eotaxin-1 on human eosinophils. Our results demonstrate that IL-33 can produce as potent eosinophil activation as IL-3, IL-5 and eotaxin-1. Thus, when considering specific cytokine targeting strategies, IL-33 will be important to consider for modulating eosinophil function.
Introduction
Eosinophils are the prominent immune cells involved in allergic asthma, atopic dermatitis, eosinophilic esophagitis, and hypereosinophilic syndrome and are found in high numbers in local tissue and/or circulating blood of affected patients [1]. In the tissue, eosinophils can release toxic granule contents including Major Basic Protein (MBP), Eosinophil Derived Neurotoxin (EDN), and Eosinophil Peroxidase (EPX), which may cause intended damage to the target in the case of parasitic infections, but can inadvertently damage surrounding host tissue and trigger remodeling. In severe asthma, this can lead to chronic inflammation of the airway resulting in long-term injury and remodeling. In addition, we and others have demonstrated that eosinophils are pro-inflammatory cells signaling other immune cells through cytokine release especially by driving and propagating the Th2 type immune response [2, 3].
IL-5 is a specific activator of eosinophils and is crucial to their development from bone marrow progenitors. It increases adhesion, survival, and cytokine release as well as inhibits apoptosis. However, treatment of asthmatic patients with anti-IL5 drug (Mepolizumab) fails to completely eliminate tissue eosinophilia, though blood eosinophil numbers and asthma exacerbations are significantly reduced [4, 5]. Thus, it is important to consider alternative eosinophil activators that may contribute to eosinophil-mediated pathology in airway tissue.
There are other well-studied activators of eosinophils such as IL-3, GM-CSF, and TSLP causing varying degrees of activation and involving different signaling kinetics [6]. IL-3 and GM-CSF receptors share the same β chain as IL-5, but IL-3 and GM-CSF have differential effects on eosinophils likely due to the regulation of their specific α chain on eosinophils surface. Eosinophils from bronchial lavage (BAL) have increased expression of IL-3Rα and GM-CSFRα and decreased expression of IL-5Rα compared to circulating blood eosinophils [7]. IL-5 added to eosinophils in vitro leads to up-regulation of IL-3Rα and GM-CSFRα and down-regulation of IL-5Rα [8, 9]. IL-3 more strongly induces eosinophil proteins including CD48, CD13, and Semaphorin 7A than GMCSF and IL-5 [10, 11]. When IL-3 is added along with TNFα, mRNA for MMP-9 and Activin A are more strongly increased by transcription and mRNA stability than with IL-5 and GMCSF stimulation [12, 13].
There is more recent interest in a family of alarmins, including TSLP, IL-25 and IL-33. The alarmins are epithelial-derived, and released in response to a variety of triggers including epithelial trauma, allergic inflammation, protease activity, and rhinovirus infection [14, 15]. These cytokines are involved in the pathophysiology of allergic diseases, including asthma and atopic dermatitis, through Th2 pathway activation [16, 17]. We and others have shown that TSLP can activate eosinophils leading to production of Th2 cytokines, enhanced survival and degranulation [18, 19].
Several genome wide association studies have found SNPs in genes encoding IL-33 or its receptor ST2 (IL1R1) to be risk factors for eosinophilic asthma, early childhood onset asthma, and severe forms of the asthma [20] Airway biopsies of asthmatics have higher IL-33 mRNA expression than healthy subjects[21]. In addition, Rhinovirus infection which is an important risk factor for asthma exacerbations, increases IL-33 levels in nasal lavage of asthmatic patients [22]. In two studies examining the role of IL-33 in eosinophils, there were differences in eosinophil responses. Cherry et al. demonstrated that IL-33 was as potent or more so than IL-5 in inducing adhesion and degranulation measured by EDN release, but did not evaluate chemotaxis [23]. Suzukawa et al. demonstrated that IL-33 was more potent than IL-5 in inducing adhesion, but not EDN release [24]. In addition, CD11b(αM intergrin) was also shown to have increased expression in the presence of IL-33, but no chemotactic effect was observed by Suzukawa et al. We sought to further characterize activation of eosinophils with IL-33 compared to activation by other cytokines and chemokines.
Materials and methods
Eosinophil purification
This study was approved by the University of Wisconsin Health Sciences IRB (UW-HS-IRB-2013-1570). Written informed consent was obtained from all participants. Adult volunteers (18–55 years) with allergic rhinitis or mild atopic asthma provided peripheral blood samples (300 ml) in heparinized syringes. Blood was diluted 1:1 with 1x HBSS (Corning), layered over 1.090g/mL Percoll, and spun at 2000rpm for 20 minutes. The mononuclear cell layer and Percoll layers were removed and the red blood cell and granulocyte pellet was moved to a clean tube. Contaminating erythrocytes were removed through two hypotonic lyses with ddH2O and divided into 200 million cell aliquots. The aliquots were incubated at 4°C for 30 minutes with 200μL HBSS with 4% NCS (Sigma), 200μL of anti-CD16 beads, 15μL of anti-CD14 and anti-CD3 beads, and 30μL of anti-glycophorin-A beads. The diluted cells were passed through an Auto MACS (Miltenyi) to remove all of the CD16, CD14, CD3, and glycophorin-A positive cells. Purified cells were 97% - 99.9% eosinophils with neutrophils and lymphocytes as contaminating cells.
Eosinophil adhesion
Eosinophil adhesion was assessed as previously described by measuring Eosinophil Peroxidase activity (EPX) from adhered cells on 96 well plates (Immulon 4 HBX) stimulated with the respective cytokines for 30 min [25]. Wells were coated with 5ug/ml VCAM1 (RnD Systems) or 5ug/ml Periostin (RnD Systems). Wells were blocked with 0.1% gelatin in HBSS containing Ca2+ and Mg2+ and washed with 3 times with HBSS. Cells, 1x104 eosinophils, were added to each well along with the respective cytokines at a 1ng/ml final concentration. The final concentration of 1ng/mL for IL-33 was based off of prior existing literature [24, 26]. IL-3 and IL-5 were kept at the same concentration as IL-33 for consistency. After 30 min at 37°C, wells were washed 3x with HBSS to remove non adherent cells. 100ul of HBSS was added to sample wells and 100ul with 1x104 eosinophils were added to untreated wells to provide “Total” eosinophils for comparison. Eosinophil peroxidase (EPX) activity was used to provide an index of eosinophil cell numbers as previously performed in our laboratory [27]. EPX substrate (1mN H2O2, 1mM O-phenylenediamine (Sigma), and 0.1% Triton x-100 (Sigma) in Tris buffer, pH8.0) was added to each well for 30 minutes at RT before stopping the reaction using 4M H2SO4. The colorimetric change at 492nm was assessed with the BioTek Synergy HT plate reader and the % adherence was calculated using: sample well OD 492/Total well OD 492 x100%.
Eosinophil EDN release
Eosinophil degranulation was assessed by performing ELISA for Eosinophil Derived Neurotoxin (EDN) on cell free supernatants from eosinophils cultured for 4hrs with N-Formylmethionyl-leucyl-phenylalanine (FMLP) (Calbiochem) or cytokines as previously described in our laboratory [27]. Eosinophils in HBSS with 0.03% gelatin and Ca2+/Mg2+, 2.5 x105 /well, were stimulated with 100nM FMLP as a positive control and IL-5 (RnD Systems), IL-3 (RnD Systems), and IL-33 (RnD Systems) at 1ng/ml final concentration for 4 hours at 37°C. We kept IL-3 and IL-33 at the same final concentration as IL-5 for consistency. A “Total” EDN content well was created by adding 2.5x105 cells directly to 0.1M HCL + 1% Triton x-100 to lyse the cells and their granules. Cell free supernatants were collected and analyzed with EDN ELISA kit per manufacturer’s instructions (MBL).
Eosinophil in vitro chemotaxis
Eosinophil chemotaxis was measured with a 24-well plate and a 6.5mm thick transwell with 5μm diameter pores as previously described in our laboratory [27]. For each condition, 600μL of co-culture media and chemoattractant was added in duplicates to the lower chamber. Eotaxin-1 (Promokine) was used at a concentration of 100ng/mL while IL-3 (RnD Systems), IL-5 (RnD Systems), or IL-33 (RnD Systems) were added at a concentration of 10ng/mL. We and others have used an eotaxin concentration of 100ng/ml for evaluation of eosinophil chemotaxis and have demonstrated that significant changes occur at this concentration [27, 28]. In prior literature, IL-5 at a concentration of 10ng/ml demonstrated significantly increased chemotaxis in eosinophils [29]. We therefore kept concentrations of IL-3 and IL-33 consistent with this. The upper transwell was pre-wet using HBSS with 0.01% gelatin and Ca2+/Mg2+. Purified eosinophils were also diluted in the HBSS with 0.01% gelatin and Ca2+/Mg2+ to a concentration of 3x10^6/mL. Each upper well of the transwell was aliquoted 100μL of the eosinophil solution and the plate incubated at 37°C for one hour. In order to remove any cells stuck to the bottom of the transwell, 250mM EDTA (Boston Bioproducts) was added to the bottom well for 5 minutes at room temperature. The transwell was then removed and 50μL of the solution from the bottom chamber was then diluted 1:1 with trypsin (0.4%, Gibco) and counted.
Flow cytometry
Eosinophils purified through AutoMACS separation were incubated with IL-3 (10ng/mL, RnD Systems), IL-5(10ng/mL, RnD Systems), IL-33(10ng/mL, RnD Systems), Eotaxin-1(100ng/mL, Promokine), in FBS and 1% RPMI for 4 hours at 37°C (0.5x106 cells/tube). These stimulant concentrations are based on prior literature noting changes seen in eosinophil adhesion, degranulation, and chemotaxis as described above. In addition, the publication by Suzukawa et al. demonstrated that with IL-33 at a concentration of 10ng/ml a significant increase in CD11b expression was detectable [24]. Therefore, for IL-3, IL-5, and IL-33, we selected a final concentration of 10ng/ml, while eotaxin-1 was kept at a final concentration of 100ng/mL. An incubation time of 4 hours was selected for consistency with the time periods for adhesion and chemotaxis studies. Two prior studies examining flow cytometry and response to IL-33 performed incubation periods of 30 min and 24 hours for Suzukawa et al. and Cherry et al. respectively [23, 24]. Cells were incubated with antibody-fluorochrome conjugates for 30 minutes at 4°C. Cells were washed, fixed with a paraformaldehyde solution, as described in prior literature [30, 31], except that primary antibody-conjugates were used, and cells were analyzed within 24 hours after fixation. Data were collected using the BD LSR Fortessa or BD LSR II (BD Biosciences). The following antibodies were obtained from BD Biosciences: CD11a BUV395, CD11c Brilliant Violet 421, CD41 Brilliant Violet 510, P-selectin Brilliant Violet 650, CD29 APC, CD14 AlexaFluor 488, CD18 PE-Cy7, CD16 PE-CF594, CD23 Brilliant Violet 421, CD11b Brilliant Violet 510, CD294 BUV395, CD193 BUV737. Additional antibodies used were: CD18 PE-Cy7, CD11b PerCP Cy5.5, CD44 APC, ICAM-1 PE (Biolegend); CD66b PE-Cy7, CD40 PerCP-eFluor 710 (eBioscience); Ghost Dye™ Red 780 (Tonbo); N29 CF568 conjugate was made custom using anti-integrin beta1 antibody (MAB2252, Milipore) and Mix-n-Stain™ CF Dye antibody labeling kit (Biotium). Data was collected using FACSDivaTM Software (BD Biosciences). Data was analyzed with Flow Jo Version 10 (Tree Star Inc.), with 75–150,000 events captured. Experiments were performed with at least 7–9 replicates for each group. Appropriate compensation controls were performed using antibodies and compensation control beads (Invitrogen™ Ebioscience™ UltraComp Ebeads™).
Statistics
EPX activity, EDN release, and in vitro chemotaxis data were analyzed using Kruskal-Wallis test or one-way ANOVA test, based on normality of the data. Following this the Tukey test was used for pair-wise comparisons (Sigmaplot 13.0, Systat Software). Differences were considered significant when p < 0.05.
For flow cytometry studies, MFI values were obtained from flow cytometry analysis software (FlowJo 10.2). MFI fold change values were normalized by performing log transformation of the ratio between cytokine stimulated MFI values and unstimulated MFI values. Ratio-t-tests were performed on the log-transformed data (RStudio Version 1.0.153). Differences were considered significant according to the stated Bonferroni corrected p-value.
Results
Eosinophil adhesion
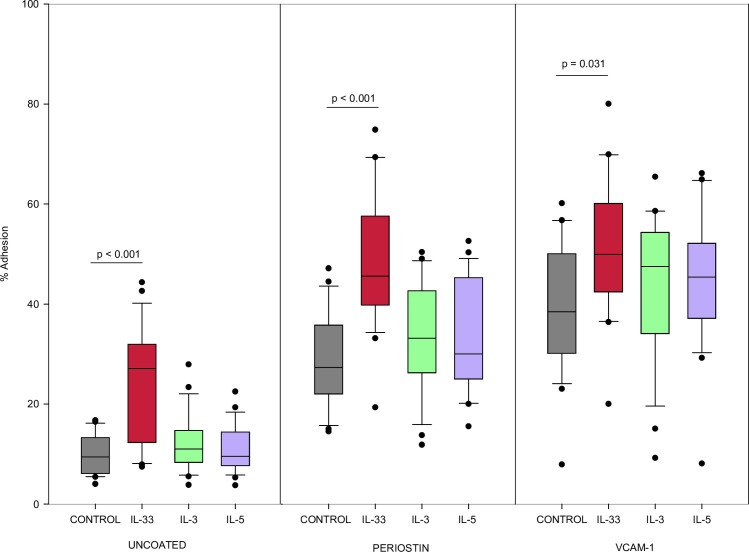
Percent adherence was measured by EPX activity from adhered cells compared to total cells added (N = 22). As shown in Fig 1, eosinophils were more adherent to VCAM-1 coated wells than uncoated wells when stimulated with IL-33 (p < 0.001), IL-3 (p < 0.001) or IL-5 (p < 0.001). Similarly, with periostin as a substrate, eosinophils stimulated with IL-33 (p < 0.001), IL-3 (p <0.001) or IL-5 (p < 0.001) were more adherent compared to uncoated wells.
Fig 1. Eosinophil adhesion with IL-33.
Eosinophil adhesion determined by EPX release from adherent cells cultured for 30min with cytokine stimulation, cytokine concentration 1ng/mL. Values shown are a percentage of optical density (OD) of wells containing 10x10e4 lysed eosinophils (n = 22). Experiments from different blood donors with the exception of 3 donors that were repeated twice in which case, results were averaged from the 2 different experiments using their cells. Lines connect comparison groups with p-value denoting significant difference in pair-wise comparisons.
Eosinophil EDN release
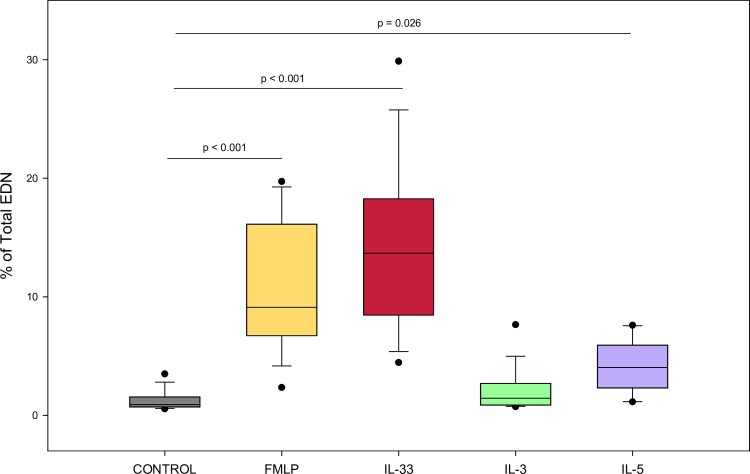
As shown in Fig 2, eosinophils stimulated with FMLP (p < 0.001), IL-33 (p < 0.001), or IL-5 (p = 0.026) for 4 hours induced more percent total EDN release than the negative control (N = 16). However, IL-3 did not demonstrate significantly higher EDN release than the negative control.
Fig 2. Eosinophils stimulated with IL-33 induce more percent total EDN release than negative control.
EDN release determined by ELISA for eosinophils cultured 4 hrs with cytokine stimulation, cytokine concentration 1ng/mL (n = 16). Values are expressed as % of “Total” well containing 0.5x10e6/well of lysed eosinophils. Lines connect comparison groups with p-value denoting significant difference in pair-wise comparisons.
Eosinophil chemotaxis
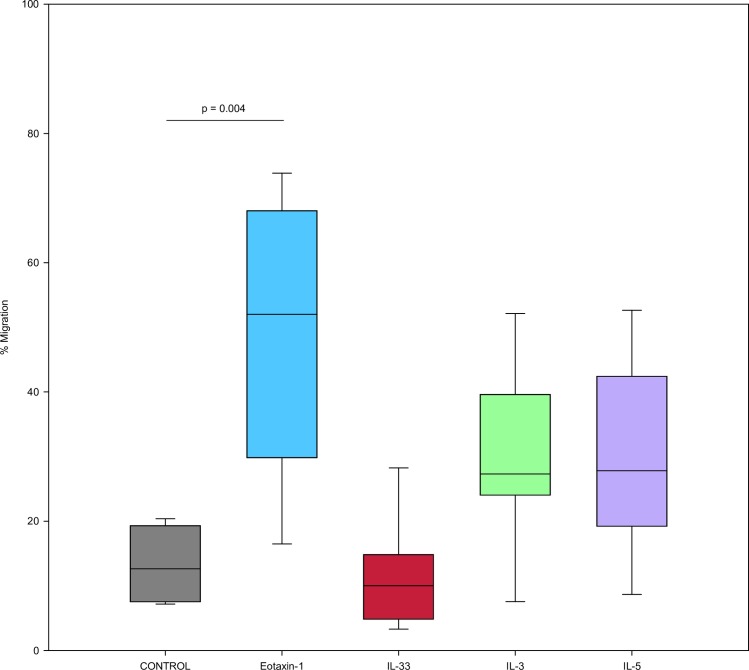
As shown in Fig 3, Percent migration of eosinophils after stimulation using transwell chambers was evaluated (N = 8). Eotaxin-1 demonstrated significant migration of eosinophils across the transwell (p < 0.001). Although IL-3 and IL-5 apparently had increased percent migration when compared to IL-33, there was no significant difference for IL-33, IL-3, and IL-5 compared to control.
Fig 3. IL-33 stimulation does not result in increased eosinophil migration.
Percent migration of eosinophils after stimulation using transwell chambers. Migration assessed after 1 hour after cytokine was placed in lower chamber, eotaxin-1 concentration 100ng/mL, IL-33 and other cytokine concentrations 10ng/mL (n = 8). Values are expressed as % migration of total cells in well containing 0.3x10e6/well of eosinophils. Lines connect comparison groups with p-value denoting significant difference in pair-wise comparisons.
Eosinophil expression of cell surface markers
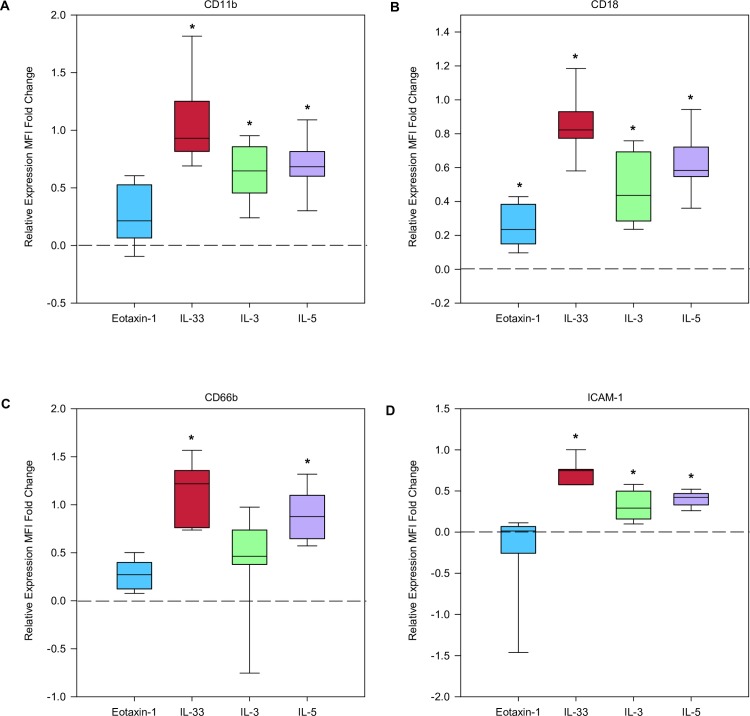
As shown in Fig 4, eosinophil cell surface marker expression was assessed using flow cytometry. We compared the change in cytokine stimulated expression for 4 hours versus baseline expression (N = 7–9). CD18 expression compared to unstimulated control, was significantly increased after incubation with IL-33, IL-3, IL-5, and eotaxin-1 (all p-values < 0.0008). IL-33, IL-3, and IL-5 significantly increased expression of CD11b and ICAM-1 when compared to unstimulated control (all p-values < 0.0008). CD66b expression was significantly increased compared to unstimulated control with only IL-5 and IL-33 (with p-values < 0.0008). The 4hr stimulation experiments did not result in statistically significant change for: N29, CD29, CD11a, CD11c, CD193, CD294, CD23, CD40, CD44, CD41, CD62P (S1 Fig).
Fig 4. IL-33 induces significant changes in cell surface expression.
Box plots of normalized fold change for A) CD11b, B) CD18, C) CD66b, D) ICAM-1 after 4 hour stimulation with either IL-3, IL-5, IL-33, eotaxin-1 compared with unstimulated, represented by dotted line (n = 7–8). The * symbol identifies significant difference when compared to unstimulated control.
Discussion
Our results demonstrate that IL-33 is as potent as IL-3 and IL-5 in inducing eosinophil adhesion and EDN degranulation. In addition, eosinophil stimulation with IL-33 resulted in increased expression of the cell surface markers CD11b, CD18, CD66b, and ICAM-1 in a manner comparable to that of IL-3, IL-5, and eotaxin-1. Our study confirms the lack of chemotaxis from IL-33 stimulation. Few studies have examined the comparative effects of IL-33 vs. IL-3, IL-5 or eotaxin-1 on eosinophils. To our knowledge, increased eosinophil expression of CD18 and CD66b after IL-33 stimulation has not previously been described.
IL-33 is a member of the IL-1 family of cytokines constitutively expressed in the nucleus of endothelial and epithelial cells of mucosal membranes and fibroblasts and can be released in response to cell injury [32]. Characterized as an alarmin, IL-33 warns the immune system of barrier injury when cells undergoing necrosis from infections or physical damage release their contents [33]. In this case, the full- length active form is released and IL-33 signaling can occur. However, if cells undergo programmed death like apoptosis, the full length form is cleaved by caspases 3 and 7 and signaling is abrogated [34]. Proteases from environmental allergens have also been shown to cleave IL-33, which in turn leads to downstream Th2 signaling and allergic inflammation [35]. IL-33 is considered a Th2 type cytokine signaling Th2 lymphocytes and ILC2s to release/produce cytokines such as IL-4, IL-5, IL-6, and IL-13 driving the type 2 immune response. Dendritic cells when stimulated with IL-33, release IL-6 and induce production of IL-5 and IL-13 from naïve CD4+ T cells [36]. Viral infections can also result in production of Th2 cytokines. In addition, viral infections can lead to bronchial epithelial damage and trigger the release of inflammatory mediators. In a study by Han et al., mice infected with rhinovirus were found to have increased lung epithelial TSLP and IL-33 [37]. The production of these two alarmins, in addition to the presence of Th2 cytokines may result in increased eosinophil activity.
The alarmin family also includes TSLP and IL-25. We have previously demonstrated that TSLP promotes eosinophil degranulation, and that its activity may be enhanced by the allergic cytokine milieu [18]. Elevated levels of IL-33 are found in bronchial tissue, and BAL fluid of asthmatics when compared to that of controls [21, 38]. This points to an important role for IL-33 in airway inflammation. The IL-33 and TSLP pathways, may be a point of intersection where viral infections and allergic exposures combine to result in increased eosinophilic inflammation and a heightened risk of exacerbation from the activation of eosinophils.
The CD4+ Th2 cytokines IL-3 and IL-5 are produced during allergic inflammation. It has previously been thought that eosinophil activation was mainly controlled by IL-3, IL-5 and GM-CSF. Eosinophil adhesion is the one of the necessary steps in eosinophil migration to target tissues. We observed that eosinophils were significantly more adherent in uncoated, VCAM-1 or periostin coated wells after 30 minute incubation with IL-33 at a level comparable to to IL-3 and IL-5. This is similar than the findings of Cherry et al. and Suzukawa et al. who both found a significant increase in eosinophil adhesion with IL-33 when compared to IL-5. Our studies used different lengths of time for eosinophil incubation with stimulant than did the previously described studies. In addition, for our adhesion experiments, we used a lower concentration of 1ng/ml for each cytokine, as opposed to the 100ng/mL used in the Cherry et al. study and the 1-100ng/mL used in Suzukawa et al. The concentrations and time intervals for our studies, were selected based on our prior work and the work of others [26–29].
In our studies, IL-33 induced degranulation as measured by EDN release from eosinophils as potently as IL-3 and IL-5. This differs to the results from Suzukawa et al. who also measured EDN after IL-33 incubation. However, our work supports the finding of Cherry et al. that, IL-33 can induce EDN release to a level of at least that of IL-5. One limitation of our experiments is that they were performed at single time points. Previous work has shown that there can be differences in eosinophil activation at different time points. For example, prolonged activation with IL-3 is more potent than IL-5 and GM-CSF in inducing eosinophil expression of specific activation and adhesion molecules [39]. Furthermore, eosinophils are known to exhibit piecemeal degranulation in which some granule proteins may be released while others are not. This poses the question of whether IL-33 demonstrates similar potency when compared to IL-3, IL-5, or eotaxin-1 at time points and with granule proteins not examined in our study.
Our eosinophil chemotaxis studies did not demonstrate increased cell migration after IL-33 incubation. These results are consistent with that of Suzukawa et al. who reported that IL-33 failed to attract eosinophils in their migration study. As demonstrated in our results, IL-33 stimulation led to increased expression of adhesion and migration molecules CD11b, CD18, CD66b and ICAM-1. ICAM-1 is a well known adhesion molecule that binds to Mac-1 and LFA-1 on the surface of cells or endothelium, and has been shown by Suzukawa et al. to increase after IL-33 incubation. Expression of these cell surface molecules was increased with IL-33 to a similar level of IL-3, IL-5. It is possible that IL-33 participates in priming eosinophils to expresses these markers, but does not directly lead to eosinophil migration. Of note, our flow cytometry results revealed changes in expression that are consistent with prior data. For example, others have demonstrated that eosinophils express increased ICAM-1 after incubation with IL-33 [40, 41]. Various studies have demonstrated increased expression of CD11b, CD18, CD66b and ICAM-1 in response to IL-5, IL-3, or GM-CSF, although direct comparisons with IL-33 induced expression were not performed for all of these cell surface markers [39, 42].
CD11b (αM integrin) and CD18 (β2 integrin) form the Mac-1 (macrophage integrin, also known as complement receptor 3, CR3) complex. It has previously been reported that Mac-1 plays a key role in eosinophil degranulation and adhesion [43]. IL-33 has been demonstrated to increase CD11b expression to levels comparable to that of IL-5 [24], and we have extended these observations to demonstrate that the CD18 dimer partner is also upregulated. CD66b (CEACAM8) is GPI anchored glycoprotein, which has been associated with CD11b. Yoon et al. demonstrated that CD66b cross linking by monoclonal antibody, or galectin-3 led to CD11b clustering on the eosinophil cell surface, and promoted degranulation [42]. Therefore, the IL-33 mediated increased CD66b and CD11b may play a role in enhanced eosinophil degranulation.
These eosinophil cell surface molecules have been studied in a number of inflammatory diseases. Sputum eosinophils of asthma patients have been shown to have up-regulated CD66b and CD11b [44]. A study of peripheral eosinophils of asthma patients in response to segmental antigen challenge demonstrated increases in the CD11b and CD18 post-challenge [45]. CD66b has been found to be elevated on the surface of peripheral eosinophils in untreated eosinophilic esophagitis patients when compared to healthy controls. Other inflammatory disorders such as rheumatoid arthritis have demonstrated that CD66b, CD11b, and CD18 are down-regulated by glucocorticoid use. These studies highlight that these cell surface markers likely play an important role in mediating inflammation. Our results indicate that IL-33 increased the expression of CD66b, CD18, and CD11b to a similar degree as IL-3, IL-5 and eotaxin-1.
We recently described relative abundance of proteins of the peripheral blood eosinophil proteome [46]. In comparing protein content of IL-5, IL-3 and IL-33 receptor molecules, IL-5Rα was the highest, with the relative abundance of IL-3Rα and ST2 being 15.6% and 28.1% respectively. This demonstrates that despite less relative abundance of the ST2 receptor when compared to IL-5Rα, IL-33 is still capable of inducing comparable eosinophil adhesion, EDN release and cell surface markers when compared to IL-5.
In this paper, we identify that IL-33 exerts its effects on eosinophils, resulting in as potent adhesion, degranulation of EDN, and cell surface marker expression when compared to IL-3, IL-5, or eotaxin-1. IL-33 as an alarmin has the ability to set off a number of down-stream effects that lead to a skewing toward the Th2 pathway and the development of allergic inflammation. The fact that IL-33 has as potent effects on eosinophils strengthens the argument for use of this molecule as a therapeutic target and broadens our knowledge in regards to how these pathways may be intersecting to produce inflammation. Anti-IL-33 biologics are currently in clinical trials for asthma and other atopic conditions and will be expected to significantly impact eosinophil function in allergic disorders.
Supporting information
Box plots of normalized fold change for A) CD11a, B) CD11c, C) CD23, D) CD29, E) CD40, F) CD41, G) CD44, H) CD193, I) CD294, J) N29, K) CD62P after 4 hour stimulation with either IL-3, IL-5, IL-33, eotaxin-1 compared with unstimulated control, represented by dotted line (n = 7–8). These samples did not demonstrate significant difference when compared to unstimulated control.
(TIF)
Acknowledgments
We thank the nurse coordinators for recruiting patients and providing samples for this study. We also thank the patients who volunteered their time and participated in our study.
Data Availability
All flow cytometry raw data files are available from the Flow Repository database (ID:FR-FCM-Z27L), Link: www.flowrepository.org/id/FR-FCM-Z27L.
Funding Statement
SKM received funding from the following grants: NIH R21 AI122103, NIH P01 HL088594; ELA received funding from the following grant: NIH T32 AI007635, National Institutes of Health, https://www.nih.gov/. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Bochner BS. The eosinophil: For better or worse, in sickness and in health. Annals of allergy, asthma & immunology: official publication of the American College of Allergy, Asthma, & Immunology. 2018;121(2):150–5. Epub 2018/03/03. 10.1016/j.anai.2018.02.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jacobsen EA, Taranova AG, Lee NA, Lee JJ. Eosinophils: Singularly destructive effector cells or purveyors of immunoregulation? Journal of Allergy and Clinical Immunology. 2007;119(6):1313–20. 10.1016/j.jaci.2007.03.043 [DOI] [PubMed] [Google Scholar]
- 3.Liu LY, Mathur SK, Sedgwick JB, Jarjour NN, Busse WW, Kelly EAB. Human airway and peripheral blood eosinophils enhance Th1 and Th2 cytokine secretion. Allergy. 2006;61:589–97. 10.1111/j.1398-9995.2006.01060.x [DOI] [PubMed] [Google Scholar]
- 4.Cabon Y, Molinari N, Marin G, Vachier I, Gamez AS, Chanez P, et al. Comparison of anti-interleukin-5 therapies in patients with severe asthma: global and indirect meta-analyses of randomized placebo-controlled trials. Clinical and experimental allergy: journal of the British Society for Allergy and Clinical Immunology. 2017;47(1):129–38. Epub 2016/11/20. 10.1111/cea.12853 . [DOI] [PubMed] [Google Scholar]
- 5.Kelly EA, Esnault S, Liu LY, Evans MD, Johansson MW, Mathur S, et al. Mepolizumab Attenuates Airway Eosinophil Numbers, but Not Their Functional Phenotype in Asthma. Am J Respir Crit Care Med. 2017. 10.1164/rccm.201611-2234OC . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McBrien CN, Menzies-Gow A. The Biology of Eosinophils and Their Role in Asthma. Frontiers in medicine. 2017;4:93 Epub 2017/07/18. 10.3389/fmed.2017.00093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bates ME, Liu LY, Esnault S, Stout BA, Fonkem E, Kung V, et al. Expression of interleukin-5-and granulocyte macrophage-colony-stimulating factor-responsive genes in blood and airway eosinophils. American Journal of Respiratory Cell and Molecular Biology. 2004;30(5):736–43. 10.1165/rcmb.2003-0234OC [DOI] [PubMed] [Google Scholar]
- 8.Liu LY, Sedgwick JB, Bates ME, Vrtis RF, Gern JE, Kita H, et al. Decreased expression of membrane IL-5 receptor alpha on human eosinophils: II. IL-5 down-modulates its receptor via a proteinase-mediated process. Journal of Immunology. 2002;169(11):6459–66. [DOI] [PubMed] [Google Scholar]
- 9.Gregory B, Kirchem A, Phipps S, Gevaert P, Pridgeon C, Rankin SM, et al. Differential regulation of human eosinophil IL-3, IL-5, and GM-CSF receptor alpha-chain expression by cytokines: IL-3, IL-5, and GM-CSF down-regulate IL-5 receptor alpha expression with loss of IL-5 responsiveness, but up-regulate IL-3 receptor alpha expression. J Immunol. 2003;170(11):5359–66. 10.4049/jimmunol.170.11.5359 [DOI] [PubMed] [Google Scholar]
- 10.Esnault S, Johansson MW, Kelly EA, Koenderman L, Mosher DF, Jarjour NN. IL-3 up-regulates and activates human eosinophil CD32 and alphaMbeta2 integrin causing degranulation. Clinical and experimental allergy: journal of the British Society for Allergy and Clinical Immunology. 2017;47(4):488–98. Epub 2016/12/22. 10.1111/cea.12876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Esnault S, Kelly EA, Johansson MW, Liu LY, Han ST, Akhtar M, et al. Semaphorin 7A is expressed on airway eosinophils and upregulated by IL-5 family cytokines. Clinical immunology (Orlando, Fla). 2014;150(1):90–100. Epub 2013/12/18. 10.1016/j.clim.2013.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelly EA, Esnault S, Johnson SH, Liu LY, Malter JS, Burnham ME, et al. Human eosinophil activin A synthesis and mRNA stabilization are induced by the combination of IL-3 plus TNF. Immunology and cell biology. 2016;94(7):701–8. Epub 2016/03/24. 10.1038/icb.2016.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelly EA, Liu LY, Esnault S, Quinchia Johnson BH, Jarjour NN. Potent synergistic effect of IL-3 and TNF on matrix metalloproteinase 9 generation by human eosinophils. Cytokine. 2012;58(2):199–206. Epub 2012/02/11. 10.1016/j.cyto.2012.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han H, Roan F, Ziegler SF. The atopic march: current insights into skin barrier dysfunction and epithelial cell-derived cytokines. Immunol Rev. 2017;278(1):116–30. Epub 2017/06/29. 10.1111/imr.12546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitchell PD, O'Byrne PM. Epithelial-Derived Cytokines in Asthma. Chest. 2017;151(6):1338–44. Epub 2016/11/08. 10.1016/j.chest.2016.10.042 . [DOI] [PubMed] [Google Scholar]
- 16.Al-Sajee D, Oliveria JP, Sehmi R, Gauvreau GM. Antialarmins for treatment of asthma: future perspectives. Current opinion in pulmonary medicine. 2018;24(1):32–41. Epub 2017/10/31. 10.1097/MCP.0000000000000443 . [DOI] [PubMed] [Google Scholar]
- 17.Ziegler SF, Artis D. Sensing the outside world: TSLP regulates barrier immunity. Nat Immunol. 2010;11(4):289–93. Epub 2010/03/20. 10.1038/ni.1852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cook EB, Stahl JL, Schwantes EA, Koziol-White CJ, Bertics PJ, Graziano FM, et al. Thymic Stromal Lymphopoietin Directly Activates Eosinophil Degranulation. The Journal of Allergy and Clinical Immunology. 2010;125(2):AB133. [Google Scholar]
- 19.Wong CK, Hu S, Cheung PFY, Lam CW. TSLP Induces Chemotactic and Pro-survival Effects in Eosinophils: Implications in Allergic Inflammation. American Journal of Respiratory Cell and Molecular Biology. 2010;43(3):305–15. 10.1165/rcmb.2009-0168OC [DOI] [PubMed] [Google Scholar]
- 20.Jones BL, Rosenwasser LJ. Linkage and Genetic Association in Severe Asthma. Immunol Allergy Clin North Am. 2016;36(3):439–47. Epub 2016/07/13. 10.1016/j.iac.2016.03.002 . [DOI] [PubMed] [Google Scholar]
- 21.Prefontaine D, Nadigel J, Chouiali F, Audusseau S, Semlali A, Chakir J, et al. Increased IL-33 expression by epithelial cells in bronchial asthma. J Allergy Clin Immunol. 2010;125(3):752–4. Epub 2010/02/16. 10.1016/j.jaci.2009.12.935 . [DOI] [PubMed] [Google Scholar]
- 22.Jackson DJ, Makrinioti H, Rana BM, Shamji BW, Trujillo-Torralbo MB, Footitt J, et al. IL-33-dependent type 2 inflammation during rhinovirus-induced asthma exacerbations in vivo. Am J Respir Crit Care Med. 2014;190(12):1373–82. Epub 2014/10/29. 10.1164/rccm.201406-1039OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cherry WB, Yoon J, Bartemes KR, Iijima K, Kita H. A novel IL-1 family cytokine, IL-33, potently activates human eosinophils. J Allergy Clin Immunol. 2008;121(6):1484–90. Epub 2008/06/10. 10.1016/j.jaci.2008.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suzukawa M, Koketsu R, Iikura M, Nakae S, Matsumoto K, Nagase H, et al. Interleukin-33 enhances adhesion, CD11b expression and survival in human eosinophils. Laboratory investigation; a journal of technical methods and pathology. 2008;88(11):1245–53. Epub 2008/09/03. 10.1038/labinvest.2008.82 . [DOI] [PubMed] [Google Scholar]
- 25.Sedgwick JB, Calhoun WJ, Vrtis RF, Bates ME, Mcallister PK, Busse WW. Comparison of Airway and Blood Eosinophil Function After Invivo Antigen Challenge. Journal of Immunology. 1992;149(11):3710–8. [PubMed] [Google Scholar]
- 26.Johansson MW, Annis DS, Mosher DF. alpha(M)beta(2) integrin-mediated adhesion and motility of IL-5-stimulated eosinophils on periostin. Am J Respir Cell Mol Biol. 2013;48(4):503–10. Epub 2013/01/12. 10.1165/rcmb.2012-0150OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mathur SK, Schwantes EA, Jarjour NN, Busse WW. Age-related changes in eosinophil function in human subjects. Chest. 2008;133(2):412–9. Epub 2008/02/07. 10.1378/chest.07-2114 ; PubMed Central PMCID: PMC2919352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Costa GG, Silva RM, Franco-Penteado CF, Antunes E, Ferreira HH. Interactions between eotaxin and interleukin-5 in the chemotaxis of primed and non-primed human eosinophils. Eur J Pharmacol. 2007;566(1–3):200–5. Epub 2007/03/21. 10.1016/j.ejphar.2006.09.075 . [DOI] [PubMed] [Google Scholar]
- 29.Johansson MW, Khanna M, Bortnov V, Annis DS, Nguyen CL, Mosher DF. IL-5-stimulated eosinophils adherent to periostin undergo stereotypic morphological changes and ADAM8-dependent migration. Clin Exp Allergy. 2017;47(10):1263–74. Epub 2017/04/06. 10.1111/cea.12934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johansson MW, Lye MH, Barthel SR, Duffy AK, Annis DS, Mosher DF. Eosinophils adhere to vascular cell adhesion molecule-1 via podosomes. Am J Respir Cell Mol Biol. 2004;31(4):413–22. Epub 2004/06/29. 10.1165/rcmb.2004-0099OC . [DOI] [PubMed] [Google Scholar]
- 31.Johansson MW, Mosher DF. Activation of beta1 integrins on blood eosinophils by P-selectin. Am J Respir Cell Mol Biol. 2011;45(4):889–97. Epub 2011/03/29. 10.1165/rcmb.2010-0402OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moussion C, Ortega N, Girard JP. The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: a novel 'alarmin'? PLoS One. 2008;3(10):e3331 Epub 2008/10/07. 10.1371/journal.pone.0003331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mehraj V, Ponte R, Routy JP. The Dynamic Role of the IL-33/ST2 Axis in Chronic Viral-infections: Alarming and Adjuvanting the Immune Response. EBioMedicine. 2016;9:37–44. Epub 2016/07/12. 10.1016/j.ebiom.2016.06.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luthi AU, Cullen SP, McNeela EA, Duriez PJ, Afonina IS, Sheridan C, et al. Suppression of interleukin-33 bioactivity through proteolysis by apoptotic caspases. Immunity. 2009;31(1):84–98. Epub 2009/06/30. 10.1016/j.immuni.2009.05.007 . [DOI] [PubMed] [Google Scholar]
- 35.Cayrol C, Duval A, Schmitt P, Roga S, Camus M, Stella A, et al. Environmental allergens induce allergic inflammation through proteolytic maturation of IL-33. Nat Immunol. 2018;19(4):375–85. Epub 2018/03/21. 10.1038/s41590-018-0067-5 . [DOI] [PubMed] [Google Scholar]
- 36.Rank MA, Kobayashi T, Kozaki H, Bartemes KR, Squillace DL, Kita H. IL-33-activated dendritic cells induce an atypical TH2-type response. J Allergy Clin Immunol. 2009;123(5):1047–54. Epub 2009/04/14. 10.1016/j.jaci.2009.02.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Han M, Rajput C, Hong JY, Lei J, Hinde JL, Wu Q, et al. The Innate Cytokines IL-25, IL-33, and TSLP Cooperate in the Induction of Type 2 Innate Lymphoid Cell Expansion and Mucous Metaplasia in Rhinovirus-Infected Immature Mice. J Immunol. 2017;199(4):1308–18. Epub 2017/07/14. 10.4049/jimmunol.1700216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Y, Wang W, Lv Z, Chen Y, Huang K, Corrigan CJ, et al. Elevated Expression of IL-33 and TSLP in the Airways of Human Asthmatics In Vivo: A Potential Biomarker of Severe Refractory Disease. J Immunol. 2018;200(7):2253–62. Epub 2018/02/18. 10.4049/jimmunol.1701455 . [DOI] [PubMed] [Google Scholar]
- 39.Esnault S, Kelly EA. Essential Mechanisms of Differential Activation of Eosinophils by IL-3 Compared to GM-CSF and IL-5. Crit Rev Immunol. 2016;36(5):429–44. Epub 2016/01/01. 10.1615/CritRevImmunol.2017020172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chow JYS, Wong CK, Cheung PFY, Lam CWK. Intracellular signaling mechanisms regulating the activation of human eosinophils by the novel Th2 cytokine IL-33: implications for allergic inflammation. Cellular And Molecular Immunology. 2009;7:26 10.1038/cmi.2009.106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wong C-K, Leung KM-L, Qiu H-N, Chow JY-S, Choi AOK, Lam CW-K. Activation of Eosinophils Interacting with Dermal Fibroblasts by Pruritogenic Cytokine IL-31 and Alarmin IL-33: Implications in Atopic Dermatitis. PLOS ONE. 2012;7(1):e29815 10.1371/journal.pone.0029815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yoon J, Terada A, Kita H. CD66b regulates adhesion and activation of human eosinophils. J Immunol. 2007;179(12):8454–62. Epub 2007/12/07. 10.4049/jimmunol.179.12.8454 . [DOI] [PubMed] [Google Scholar]
- 43.Horie S, Kita H. CD11b/CD18 (Mac-1) is required for degranulation of human eosinophils induced by human recombinant granulocyte-macrophage colony-stimulating factor and platelet-activating factor. J Immunol. 1994;152(11):5457–67. Epub 1994/06/01. . [PubMed] [Google Scholar]
- 44.Tak T, Hilvering B, Tesselaar K, Koenderman L. Similar activation state of neutrophils in sputum of asthma patients irrespective of sputum eosinophilia. Clin Exp Immunol. 2015;182(2):204–12. Epub 2015/07/08. 10.1111/cei.12676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johansson MW, Gunderson KA, Kelly EA, Denlinger LC, Jarjour NN, Mosher DF. Anti-IL-5 attenuates activation and surface density of β(2) -integrins on circulating eosinophils after segmental antigen challenge. Clin Exp Allergy. 2013;43(3):292–303. Epub 2013/02/19. 10.1111/j.1365-2222.2012.04065.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wilkerson EM, Johansson MW, Hebert AS, Westphall MS, Mathur SK, Jarjour NN, et al. The Peripheral Blood Eosinophil Proteome. Journal of Proteome Research. 2016;15(5):1524–33. 10.1021/acs.jproteome.6b00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Box plots of normalized fold change for A) CD11a, B) CD11c, C) CD23, D) CD29, E) CD40, F) CD41, G) CD44, H) CD193, I) CD294, J) N29, K) CD62P after 4 hour stimulation with either IL-3, IL-5, IL-33, eotaxin-1 compared with unstimulated control, represented by dotted line (n = 7–8). These samples did not demonstrate significant difference when compared to unstimulated control.
(TIF)
Data Availability Statement
All flow cytometry raw data files are available from the Flow Repository database (ID:FR-FCM-Z27L), Link: www.flowrepository.org/id/FR-FCM-Z27L.