Abstract
Objective
To assess the cephalometric skeletal and soft-tissue of functional appliances in treated versus untreated Class II subjects in the long-term (primarily at the end of growth, secondarily at least 3 years after retention).
Search methods
Unrestricted electronic search of 24 databases and additional manual searches up to March 2018.
Selection criteria
Randomised and non-randomised controlled trials reporting on cephalometric skeletal and soft-tissue measurements of Class II patients (aged 16 years or under) treated with functional appliances, worn alone or in combination with multi-bracket therapy, compared to untreated Class II subjects.
Data collection and analysis
Mean differences (MDs) and 95% confidence intervals (95% CIs) were calculated with the random-effects model. Data were analysed at 2 primary time points (above 18 years of age, at the end of growth according to the Cervical Vertebral Maturation method) and a secondary time point (at least 3 years after retention). The risk of bias and quality of evidence were assessed according to the ROBINS tool and GRADE system, respectively.
Results
Eight non-randomised studies published in 12 papers were included. Functional appliances produced a significant improvement of the maxillo-mandibular relationship, at almost all time points (Wits appraisal at the end of growth, MD -3.52 mm, 95% CI -5.11 to -1.93, P < 0.0001). The greatest increase in mandibular length was recorded in patients aged 18 years and above (Co-Gn, MD 3.20 mm, 95% CI 1.32 to 5.08, P = 0.0009), although the improvement of the mandibular projection was negligible or not significant. The quality of evidence was ‘very low’ for most of the outcomes at both primary time points.
Conclusions
Functional appliances may be effective in correcting skeletal Class II malocclusion in the long-term, however the quality of the evidence was very low and the clinical significance was limited.
Systematic review registration
CRD42018092139
Introduction
Rationale
Class II malocclusion is the most prevalent antero-posterior jaw problem in orthodontics, affecting one third of the population [1, 2]. The majority of Class II patients exhibit mandibular skeletal retrusion [3, 4]. Reduced mandibular size is also a major feature of Class II malocclusion patients [5]. As a result, there has been great interest in the use of ‘functional appliances’, designed primarily to influence the lower dentition and enhance the growth of the mandible [3]. These appliances promote forward posturing of the mandible, although their effects also impact on the upper jaw [6, 7].
The potential that functional appliances could modify skeletal growth is of great importance for patients and orthodontists alike. Improving facial aesthetics is one of the main reasons for seeking orthodontic treatment [8] and it is associated with a high level of patient and parent satisfaction [9]. Mandibular retrusion has a negative impact on perceived attractiveness [10], self-esteem and oral health-related quality of life [11]. The magnitude of the retrusion is also an important factor in treatment decision-making. Small skeletal discrepancies may only need multi-bracket therapy for the correction of malocclusion and refinement of teeth alignment. On the other hand, greater discrepancies may require a surgical treatment to modify the position and length of skeletal structures and to attain better aesthetic results [12].
Post-pubertal growth has been shown to produce dramatic alterations in skeletal and dental relationships [13]. There is no consensus on the age at which growth ends [14–18]. Overall, growth continues up to mid-adulthood, with different patterns in the two genders. Males show an anterior rotation of the mandible, whereas females demonstrate a posterior mandibular rotation [17, 18]. An alternative method to establish when growth comes to an end is through using indicators of the growth phase, such as the hand-and-wrist maturation method [19] or the cervical vertebral maturation method [20].
To fully understand the real effects of functional appliances on the growth of the jaws and profile, it is essential to study these effects at the completion of patient growth, when biases and confounding factors due to natural changes are negligible. The long-term stability of these changes is important too.
To date, most systematic reviews investigating the treatment effects of functional appliances in Class II malocclusion patients have synthesized studies evaluating the skeletal and soft-tissue changes at the end of the orthodontic treatment [6, 7, 21–26]. Only two reviews systematically searched for scientific evidence concerning the long-term stability of treatment results achieved by Class II functional appliance therapy [27, 28]. Another systematic review is ongoing [29]. No previous reviews determined the effects of removable and fixed functional appliances in patients with Class II malocclusion compared to untreated controls at growth completion.
Objective
The objective of this systematic review was therefore to assess the skeletal and soft-tissue effects measured on lateral cephalograms produced by functional appliances in treated versus untreated Class II subjects in the long-term (primarily at the end of growth, secondarily at least 3 years after retention).
Materials and methods
Protocol and registration
The present systematic review was performed according to the guidelines of the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [30], and is reported on the basis of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (S1 table [31]). The protocol was published in the International Prospective Register of Systematic Reviews (PROSPERO) on 03 April 2018 (registration number CRD42018092139).
Information sources
The search strategy covered 11 bibliographic databases, 10 non-bibliographic databases and 3 unpublished studies sources, from their launch to March 2018 [32–35]. Hand-searching of the most common orthodontic journals was performed as well. The Cochrane Master List was consulted to facilitate the identification of these journals [30, 34, 36]. The reference lists of the trials eligible for inclusion and systematic reviews concerning Class II malocclusion treatment were also checked. Information concerning the name of the search source, the date range that were searched, and, for electronic databases, the search platform or provider are presented in S2 table.
Search
Search strategies were developed using medical subject headings (MeSH) and text words related to functional appliances. The search strategies of the preliminarily identified systematic reviews published between 2015 and 2018 were collected [6, 7, 21–26, 28]. As recommended by the Cochrane Collaboration [30], terms related to only three aspects of the review’s question were selected: participants, interventions and timing.
Preliminary searches were conducted to screen the list of queries and define the MEDLINE and Google Scholar search strategies. After the MEDLINE strategy had been finalised, it was adapted to the syntax and subjects headings of the other databases. No restrictions based on language, publication year, or publication status were applied to the search. The search strategy designed for each database is shown in S3 table.
Eligibility criteria
Randomised and non-randomised controlled trials reporting on cephalometric skeletal and soft-tissue measurements of Class II patients (aged 16 years or under) treated with functional appliances, worn alone or in combination with multi-bracket therapy, compared to untreated Class II subjects were included (Table 1). The rationale behind eligibility criteria is provided in S1 Appendix.
Table 1. Eligibility criteria used for the study selection.
| Category | Inclusion | Exclusion |
|---|---|---|
| Study designs | Randomised controlled trials (RCTs), controlled (non-randomised) clinical trials (CCTs), controlled before-after (CBA) studies, and case-control or nested case-control studies | Prospective and retrospective cohort studies, cross-sectional studies, case series, and case reports |
| Participants | Children and adolescents (aged 16 years or under) receiving orthodontic treatment to correct Class II malocclusion | Participants with a cleft lip or palate or both, other craniofacial deformity/syndrome (such as Apert, Crouzon, Hemifacial Microsomia/Goldenhar, Moebius, Pierre Robin, Treacher Collins syndromes or craniosynostosis), syndromes affecting the craniofacial structures or patients with temporo-mandibular joint disorders |
| Active treatment with functional appliances had to be completed by the age of 16 years | ||
| Interventions | Any type of functional appliance, defined as a removable or fixed orthodontic appliance that postures the mandible forward | Association with other Class II devices designed primarily to restrain the maxilla (e.g. headgear) |
| Functional appliances worn alone or in combination with multi-bracket therapy. When functional appliances were worn alone, this therapy could also take place after the functional appliance treatment. | ||
| Functional appliances worn for 6 months or longer | ||
| Comparators | Untreated Class II subjects | |
| Groups with similar ages at the commencement of the observational period (age differences between the treated and untreated groups less than 18 months) | ||
| Outcomes | Cephalometric skeletal measurements evaluating the antero-posterior position of the maxilla and mandible, the total mandibular length or length of its parts (ramus and corpus), the mutual relationship between the two jaws | |
| Soft tissue changes of both lips and chin, measured on lateral cephalograms | ||
| Timing | At the end of growth, defined by age or using indicators of the growth phase | |
| Post-retention period of at least 3 years |
Study selection
Search results from those databases allowing for the export of valid file formats (MEDLINE, EMBASE, CENTRAL, LILACS, Web of Science, Scopus and ProQuest Dissertation & Theses) were uploaded to EndNote software. Results from Google Scholar, TRIP Database, British Library Direct, ISI proceedings, hand-searching, unpublished and ongoing studies were managed manually. A calibration exercise was undertaken to pilot and refine the screening questions, before initiating the formal screening process.
G.C. and A.U. independently screened the titles and abstracts to remove obviously irrelevant reports. After having retrieved full texts of potentially relevant and unclear reports, the reviewers examined if these met the eligibility criteria. Multiple reports of the same study were linked together at the end of the selection process [30]. G.C. sought additional information from study authors when it was deemed necessary to resolve questions about eligibility. Reviewers resolved disagreements by discussion, and an arbitrator (C.S.) adjudicated unresolved disagreements. Primary reasons for excluding trials were recorded.
Data collection process
G.C. and A.U. independently extracted data using a piloted data extraction form. This electronic form originated from those proposed by the Cochrane Collaboration [30] and a previous Cochrane review on Class II malocclusion [26]. To ensure consistency across reviewers, calibration exercises were conducted before starting the review. Disagreements were resolved through discussion.
Data items
Information was extracted from each included study on source and general information, methods, characteristics of participants and interventions, outcomes, data and analysis.
Risk of bias in individual studies
The risk of bias tool for non-randomised studies of interventions (ROBINS-I tool [37]) was used to ascertain the quality of the evidence of included trials.
Summary measures
Data were summarised and considered suitable for pooling only if the same cephalometric measurement was used for the same outcome. To circumvent the issue of the different follow-up periods of included studies, the overall treatment and post-treatment changes were analysed [30]. Mean differences (MDs) and 95% confidence intervals (95% CIs) between these changes were calculated. Whenever necessary, the enlargement of linear measurements due to the radiographic examination was adjusted at 0%. Studies in which the magnification was not reported for linear measurements were excluded from meta-analyses.
Skewed data and non-quantitative data were presented in narrative format.
Synthesis of results
The random-effects model proposed by DerSimonian and Laird [38] was chosen a priori to combine and compare data from included studies. The presence of statistical heterogeneity was assessed by inspecting the overlap of the confidence intervals in the forest plots and by using the chi-squared (Chi2) test, while the impact of between-study heterogeneity on the meta-analysis was tested by calculating the τ2 and the I2 statistics [39].
Since variation applies as much within studies as across them, the choice to treat each independent subgroup as a separate study was preferred to computing a composite effect for each study and using it in the analysis [40].
As there is no consensus on the age at which growth ends, treatment effects were evaluated at 2 primary time points:
-
■
Above 18 years of age. The age threshold of 18 years was chosen to maximise the data available [30];
-
■
At the end of growth documented by the Cervical Vertebral Maturation (CVM) method (cervical vertebral maturation stage 5 or 6 [20]);
A secondary time point was established after a post-retention period of at least 3 years.
Additional analysis
Subgroup and sensitivity analyses were performed in order to explore the source of heterogeneity and test the overall robustness of the data, respectively. All subgroup and sensitivity analyses were pre-specified in the protocol.
For all outcomes, results were divided according to the type of functional appliance.
For the most clinically important outcomes, subgroup analyses were based on the following:
-
■
Patient characteristics (gender);
-
■
Beginning of the functional appliance therapy according to age (early treatments, commencing in children aged between 7 and 11 years; late treatments, beginning in adolescents aged between 12 and 16 years);
-
■
Start of the treatment according to the cervical vertebral maturation method (early treatments, with patients presenting with Cervical Vertebral Maturation Stage [CVMS] 1 or 2 at the first observation; late treatments, with subjects presenting with CVMS 2 or 3);
-
■
Post-retention period duration (3–4, 5–10 years after active treatment with functional appliances);
Sensitivity analysis was performed to examine the impact of the study quality assessment on the overall estimates of effect.
Risk of bias across studies
Outcome reporting bias and publication bias were evaluated. In order to determine whether reporting bias was present, the Clinical Trial Register was screened using the International Clinical Trials Registry Platform of the World Health Organisation (http://apps.who.int/trialssearch). When protocols were identified, discrepancies between the outcomes planned in the protocol and those reported in the final manuscript were assessed. The potential for reporting bias was explored by funnel plots if ≥10 studies were available [40].
The quality of evidence for all outcomes at both primary time points was judged using the Grading of Recommendations Assessment, Development and Evaluation working group methodology [41].
Results
Study selection
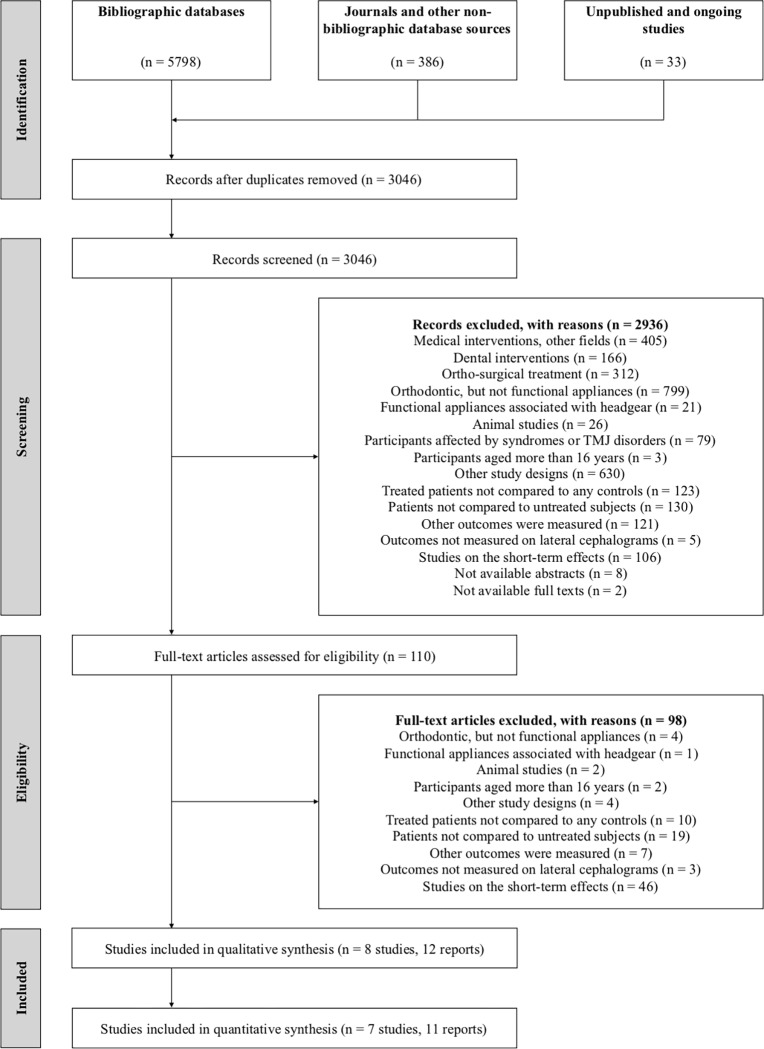
The results of the search are summarised in Fig 1. Among 3046 records, eight non-randomised studies published in 12 papers were identified for inclusion in this review [42–49]. Two authors were contacted to clarify whether duplicate data was used in their trials. Since the study by Pavoni et al. [43] contained partial data of previous studies [50–52] and has the greater sample size and subgroup analysis, it was considered the reference study of the other reports. The thesis by Wigal [47] with complete data of the subsequent published study [53] was included as well. Excluded studies with reasons are listed in supplementary files (S4 Table, S2 Appendix).
Fig 1. PRISMA flow diagram.
Study characteristics
The main characteristics of the 8 included studies are presented in Tables 2–3. All the studies were retrospective controlled clinical trials [42–49]. A wide range of eligibility criteria was found in the included studies. Class II malocclusion was defined by both skeletal and dental parameters. Six trials used historical controls for the comparison with treated patients [43, 45, 46–49].
Table 2. Characteristics of included studies (participants, interventions, outcomes).
| Study | Groups (N) | Participants | Interventions | Outcomes | |||
|---|---|---|---|---|---|---|---|
| T1-T2 | T2-T3 | Mx skeletal | Md skeletal | Mx-Md skeletal | |||
| Wieslander 1979 | TG (23) | ANB > 6 degrees, full Class II molar relationship, mixed dentition | Act | None | A to S perp | Pg to S perp, Co-Gn, Ar-Gn, Co to mand | ANB |
| CG (23) | Matched according to gender, age, ethnicity, and socioeconomic background | None | None | ||||
| Pavoni 2017 | TG (46) | ANB > 4 degrees, full Class II or end-to-end molar relationship, excessive overjet (greater than 5 mm) | Bio / Act | MBA | SNA | SNB, Pg to N perp, Co-Gn, Co-Go | ANB, Wits |
| CG (31) | Matched according to age and skeletal maturation, and starting cephalometric characteristics | None | None | ||||
| Falck 1991 | TG (50) | Class II division 1 malocclusion (no definition) | Fr2 | - | Horiz. A to ORS | Horiz. B or Pg to ORS, Co-Gn | - |
| CG (38) | Matched according to gender and age | None | None | ||||
| Freeman 2009 | TG (30) | Full Class II molar relationship, excessive overjet (no definition) | Fr2 | - | SNA, A to N perp, Co-A | SNB, Pg to N perp, Co-Gn | ANB, Wits, Co-Gn/Co-A diff |
| CG (20) | Matched according to gender, age and skeletal maturation, and starting cephalometric characteristics | None | None | ||||
| Angelieri 2014 | TG (17) | ANB > 2 degrees, full Class II or end-to-end molar relationship, excessive overjet (greater than 5 mm), late mixed dentition | Fr2 | Fr2 / None | SNA, A to N perp, Co-A | SNB, Pg to N perp, Co-Gn | ANB, Wits, Co-Gn/Co-A diff |
| CG (17) | Matched according to gender, age and skeletal maturation | None | None | ||||
| Wigal 2008 | TG (22) | ANB > 4 degrees, mixed dentition | Hb | MBA | SNA, Co-A, Olp-A | SNB, Co-Gn, Olp-Pg, Olp-Co | ANB, Wits, Co-Gn/Co-A diff |
| CG (22) | Matched according to gender, age, and starting cephalometric characteristics | None | None | ||||
| Drosen 2018 | TG (13) | Class II malocclusion (no definition) | Hb +/- MBA | Act / None | SNA | SNB, Ar-Go | ANB, Wits |
| CG (13) | Matched according to gender and age | None | None | ||||
| Alhoraibi 2017 | TG (39) | ANB > 4 degrees, full Class II or end-to-end molar relationship, excessive overjet (greater than 10 mm) | FRD + MBA | None | SNA, A to N perp, Co-A | SNB, Pg to N perp, Co-Gn | ANB, Wits, Co-Gn/Co-A diff |
| CG (39) | Matched according to gender, age and skeletal maturation, and starting cephalometric characteristics | None | None | ||||
N, number of participants; TG, treated group; CG, control group
Act, Activator; Bio, Bionator; Fr2, Frankel-2; Hb, Herbst; FRD, Forsus; MBA, multi-bracket appliances
Mx skeletal, maxillary skeletal outcomes; SNA, SNA angle; A to N perp, A point to N perpendicular distance; A to S perp, A point to S perpendicular distance; Horiz. A to ORS, horizontal distance of A point to occipital reference system; Co-A, Co-A distance; Olp-A, distance of A point to occlusal line perpendicular
Md skeletal, mandibular skeletal outcomes; SNB, SNB angle; Pg to N perp, Pg point to N perpendicular distance; Pg to S perp, Pg point to S perpendicular distance; Horiz. B or Pg to ORS, horizontal distance of B point or Pg point to occipital reference system; Co-Gn, Co-Gn distance; Ar-Gn, Ar-Gn distance; Olp-Pg, distance of Pg point to occlusal line perpendicular; Olp-Co, distance of Co point to occlusal line perpendicular; Co to mand, distance of Co point to mandibular plane; Co-Go, Co-Go distance; Ar-Go, Ar-Go distance
Mx-md skeletal, maxillo-mandibular outcomes; ANB, ANB angle; Wits, Wits appraisal; Co-Gn/Co-A diff, Co-Gn/Co-A difference.
Table 3. Characteristics of included studies (timing).
| Study or subgroup | Groups (N) | Timing | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T1-T2 | T2-T3 | T1-T3 | ||||||||
| Mean | SD | CVSM | Mean | SD | CVSM | Mean | SD | CVSM | |||||
| Wieslander 1979 | TG (23) | ~ 10 | - | - | ~ 13 | - | - | ~ 17 | - | - | 3.0 | 4.0 | 7.0 |
| CG (23) | ~ 10 | - | - | ~ 13 | - | - | ~ 17 | - | - | 3.0 | 4.0 | 7.0 | |
| Pavoni 2017 (early) | TG (23) | 9.5 | 1.2 | 1-2 | 11.4 | 1.2 | 1-3 | 17.9 | 2.3 | 5-6 | 1.9 | 6.5 | 8.4 |
| CG (16) | 9.4 | 0.7 | 1-2 | 11.3 | 0.7 | 1-3 | 17.0 | 1.8 | 5-6 | 1.9 | 5.7 | 7.6 | |
| Pavoni 2017 (late) | TG (23) | 10.2 | 1.3 | 2-3 | 12.5 | 1.2 | 4-5 | 18.5 | 2.1 | 5-6 | 2.3 | 6.0 | 8.3 |
| CG (15) | 10.8 | 1.1 | 2-3 | 12.7 | 1.2 | 4-5 | 18.3 | 1.3 | 5-6 | 1.9 | 5.6 | 7.5 | |
| Falck 1991 (males) | TG (19) | 7.3 | - | - | - | - | - | 17.5 | - | - | - | - | 10.2 |
| CG (18) | 7.0 | - | - | - | - | - | 16.4 | - | - | - | - | 9.4 | |
| Falck 1991 (females) | TG (31) | 7.3 | - | - | - | - | - | 17.2 | - | - | - | - | 9.9 |
| CG (20) | 7.7 | - | - | - | - | - | 17.9 | - | - | - | - | 10.2 | |
| Freeman 2009 | TG (30) | 8.1 | 1.3 | 1-2 | - | - | - | 18.0 | 3.4 | 5-6 | - | - | 9.9 |
| CG (20) | 8.5 | 1.2 | 1-2 | - | - | - | 18.2 | 3.7 | 5-6 | - | - | 9.7 | |
| Angelieri 2014 | TG (17) | 10.8 | 0.6 | 1-3 | 12.5 | 0.6 | 1-4 | 19.7 | 0.7 | 5-6 | 1.7 | 7.2 | 8.9 |
| CG (17) | 11.3 | 0.6 | 1-3 | 12.7 | 0.6 | 2-4 | 18.9 | 2.0 | 5-6 | 1.4 | 6.2 | 7.6 | |
| Wigal 2008 (males) | TG (7) | 8.7 | 1.3 | - | 9.6 | 1.2 | - | 15.2 | 1.5 | - | 0.9 | 5.6 | 6.5 |
| CG (7) | 8.7 | 1.1 | - | 9.6 | 1.1 | - | 15.2 | 1.9 | - | 0.9 | 5.6 | 6.5 | |
| Wigal 2008 (females) | TG (15) | 8.3 | 0.9 | - | 9.1 | 0.4 | - | 14.3 | 1.3 | - | 0.8 | 5.2 | 6.0 |
| CG (15) | 8.3 | 1.1 | - | 9.2 | 0.3 | - | 14.4 | 1.3 | - | 0.9 | 5.2 | 6.1 | |
| Drosen 2018 (males) | TG (13) | 12.4 | 0.9 | - | 14.2 | 1.2 | - | 20.2 | 1.0 | - | 1.8 | 6.0 | 7.8 |
| CG (13) | 12.1 | 0.5 | - | 14.2 | 0.6 | - | 19.8 | 2.3 | - | 2.1 | 5.6 | 7.7 | |
| Alhoraibi 2017 (early) | TG (18) | 11.5 | 0.8 | 1 | 13.1 | 0.8 | - | 16.4 | 1.1 | - | 1.6 | 3.3 | 4.9 |
| CG (18) | 11.8 | 0.9 | 1 | 13.9 | 1.5 | - | 17.1 | 1.3 | - | 2.1 | 3.2 | 5.3 | |
| Alhoraibi 2017 (late) | TG (21) | 13.3 | 0.6 | 2-3 | 15.3 | 0.8 | - | 18.4 | 1.0 | - | 2.0 | 3.1 | 5.1 |
| CG (21) | 13.5 | 0.8 | 2-3 | 15.1 | 0.6 | - | 18.2 | 0.7 | - | 1.6 | 3.1 | 4.7 |
N, number of participants; TG, treated group; CG, control group
T1, at the start of the active phase of functional appliance therapy; T2, at the end of the active phase of functional appliance therapy; T3, long-term follow-up
SD, standard deviation; CVMS, cervical vertebral maturation stage.
Five studies evaluated the treatment effects of three removable functional appliances as follows:
-
■
Activator only [42];
-
■
A mixed group of patients treated either with the Bionator or Activator [43];
- ■
Two trials evaluated respectively the effects of early treatment (mean age at start = 8.4 years [47]) and late treatment (mean age at start = 12.4 years [48]) of a fixed rigid appliance, the Herbst appliance. One study tested a fixed flexible appliance, the Forsus appliance [49]. Multi-bracket therapy was worn concurrently with functional appliance treatment in one study [49], and after functional appliance therapy in 3 trials [43, 47, 48]. A variety of appliances and retention protocols were used in the post-treatment period. All the studies compared Class II malocclusion patients treated with functional appliances to untreated Class II subjects [42–49].
Only cephalometric skeletal measurements were recorded from the 8 studies included in this review [42–49]. Soft tissue changes of both lips and chin measured on lateral cephalograms were investigated only by a report [51] of an included study [43]. Cephalometric magnifications were set at 0% [47, 48], 8% [43, 45, 49], 10% adjusted to 0% [46]. In the rest of the studies, information was not provided [42, 44]. Outcomes were assessed above 18 years in age in 5 trials (5 subgroups [43, 45, 46, 48, 49]) and at the end of growth using the cervical vertebral maturation method in 3 trials (4 subgroups [43, 45, 46]). All the studies had a post-retention period of at least 3 years (Table 3 [42–49]).
Risk of bias within studies
The overall risk of bias ranged from moderate to critical in the included studies (Table 4). Most studies suffered bias in selection of participants and due to deviations from intended interventions [42–49]. The estimated effect can be predicted to be greater than the true effect estimate in studies with the observed selection bias [42, 43, 49]. Multi-bracket therapy, as well as retention appliances, could enhance the treatment effects of functional jaw orthopaedics or control their relapse [43, 47–49].
Table 4. Risk of bias for multiple outcomes within included studies, according to the risk of bias tool for non-randomised studies of interventions (ROBINS-I tool).
| Bias domain | Signalling question | Wieslander and Lagerström, 1979 | Pavoni et al., 2017 | Falck, 1991 | Freeman et al., 2009 | Angelieri et al., 2014 | Wigal, 2008 | Drosen et al., 2018 | Alhoraibi, 2017 |
|---|---|---|---|---|---|---|---|---|---|
| 1. Bias due to confounding | 1.1 | Y | Y | Y | Y | Y | Y | Y | Y |
| 1.2 | N | N | N | N | N | N | N | N | |
| 1.3 | - | - | - | - | - | - | - | - | |
| 1.4 | PY | PY | PY | PY | PY | PY | PY | PY | |
| 1.5 | PY | PY | PY | PY | PY | PY | PY | PY | |
| 1.6 | PN | PN | PN | PN | PN | PN | PN | PN | |
| 1.7 | PY | Y | PY | PY | PY | PY | PY | PY | |
| 1.8 | PY | PY | PY | PY | PY | PY | PY | PY | |
| Risk of bias judgement | Low | Low | Low | Low | Low | Low | Low | Low | |
| 2. Bias in selection of participants into the study | 2.1 | Y | PY | NI | NI | PY | NI | NI | PN |
| 2.2 | Y | Y | - | - | Y | - | - | - | |
| 2.3 | Y | Y | - | - | Y | - | - | - | |
| 2.4 | Y | Y | Y | Y | Y | Y | Y | Y | |
| 2.5 | N | N | - | - | N | - | - | - | |
| Risk of bias judgement | Crit | Ser | Low | Low | Ser | Low | Low | Low | |
| 3. Bias in classification of interventions | 3.1 | Y | Y | Y | Y | Y | Y | Y | Y |
| 3.2 | Y | Y | Y | Y | Y | Y | Y | Y | |
| 3.3 | N | N | N | N | N | N | N | N | |
| Risk of bias judgement | Low | Low | Low | Low | Low | Low | Low | Low | |
| 4. Bias due to deviations from intended interventions | 4.1 | PN | PN | N | N | N | PN | Y | PN |
| 4.2 | - | - | - | - | - | - | Y | - | |
| 4.3 | NI | PN | Y | Y | Y | PN | PN | PN | |
| 4.4 | PY | PY | PY | PY | PY | PY | PY | PY | |
| 4.5 | PY | PY | PY | PY | PY | PY | PY | PY | |
| 4.6 | - | - | - | - | - | - | - | - | |
| Risk of bias judgement | Low | Mod | Low | Low | Low | Mod | Ser | Mod | |
| 5. Bias due to missing data | 5.1 | N | Y | Y | Y | Y | N | Y | Y |
| 5.2 | PN | PN | PN | PN | PN | PN | PN | PN | |
| 5.3 | Y | PN | PN | PN | PN | PN | PN | PN | |
| 5.4 | Y | - | - | - | - | Y | - | - | |
| 5.5 | PN | - | - | - | - | PN | - | - | |
| Risk of bias judgement | Ser | Low | Low | Low | Low | Mod | Low | Low | |
| 6. Bias in measurement of outcomes | 6.1 | NI | NI | NI | NI | NI | NI | NI | NI |
| 6.2 | NI | NI | NI | NI | NI | NI | NI | NI | |
| 6.3 | Y | Y | Y | Y | Y | Y | Y | Y | |
| 6.4 | PN | PN | PN | PN | PN | PN | PN | PN | |
| Risk of bias judgement | Mod | Mod | Mod | Mod | Mod | Mod | Mod | Mod | |
| 7. Bias in selection of the reported result | 7.1 | PN | PN | PN | PN | PN | PN | PN | PN |
| 7.2 | PY | PY | PY | PY | PY | PY | PY | PY | |
| 7.3 | N | N | N | N | N | N | N | N | |
| Risk of bias judgement | Mod | Mod | Mod | Mod | Mod | Mod | Mod | Mod | |
| Overall risk of bias | Crit | Ser | Mod | Mod | Ser | Mod | Ser | Mod | |
Y, yes; PY, probably yes; N, no; PN, probably no; NI, no information.
"-", not applicable or nothing to note
Mod, moderate; Ser, serious; Crit, critical.
Results of individual studies
The main results of the included studies are reported in S5–S6 Tables.
Only one report [51] found that Bionator therapy was able to significantly alter the sagittal position of both the maxillary and mandibular soft tissue profile components. During the overall observation period, functional jaw orthopaedics with the Bionator, followed by multi-bracket appliances produced a restraining effect on the soft tissue A point (-1.8 mm, CI not reported) and a protrusive effect on the soft tissue Pg point (+2.6 mm, CI not reported).
Synthesis of results
Seven studies (10 subgroups [42, 43, 45–49]) were included in the meta-analyses of 9 outcomes at 3 time points (Table 5). Subgroup analyses according to the type of functional appliance are presented together with their overall effects (Tables 6–7). The forest plots concerning the most clinically relevant results are reported in the main text. Other findings are set out in S3 Appendix.
Table 5. Details of the performed meta-analyses with tests on heterogeneity.
| Outcome | Time point | Overall effect | Heterogeneity | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N_s | MD | 95% CI | P | Tau2 | Chi2 | P | I2 | |||
| Mx skeletal | ||||||||||
| SNA (degrees) | Age 18 + | 5 | -0.31 | -0.83, 0.21 | 0.24 | 0.05 | 4.62 | 0.33 | 13% | |
| CVMS 5-6 | 4 | -0.73 | -1.31, -0.15 | 0.01 | 0.00 | 0.02 | 1.00 | 0% | ||
| 3-years + | 9 | -1.03 | -1.88, -0.18 | 0.02 | 1.28 | 50.87 | 0.00 | 84% | ||
| A to N perp (mm) | Age 18 + | 3 | -2.41 | -6.45, 1.62 | 0.24 | 12.54 | 140.47 | 0.00 | 99% | |
| CVMS 5-6 | 2 | -0.48 | -2.74, 1.77 | 0.67 | 2.41 | 11.49 | 0.00 | 91% | ||
| 3-years + | 4 | -2.24 | -4.79, 0.30 | 0.08 | 6.57 | 164.00 | 0.00 | 98% | ||
| Co-A (mm) | Age 18 + | 3 | 0.53 | 0.00, 1.05 | 0.05 | 0.00 | 0.65 | 0.72 | 0% | |
| CVMS 5-6 | 2 | 0.15 | -1.16, 1.46 | 0.82 | 0.00 | 0.27 | 0.60 | 0% | ||
| 3-years + | 6 | -0.96 | -2.32, 0.40 | 0.17 | 2.04 | 39.60 | 0.00 | 87% | ||
| Md skeletal | ||||||||||
| SNB (degrees) | Age 18 + | 5 | 0.66 | 0.03, 1.29 | 0.04 | 0.22 | 7.05 | 0.13 | 43% | |
| CVMS 5-6 | 4 | 0.65 | -0.45, 1.74 | 0.25 | 0.89 | 10.25 | 0.02 | 71% | ||
| 3-years + | 9 | 0.14 | -0.48, 0.76 | 0.67 | 0.52 | 21.67 | 0.01 | 63% | ||
| Pg to N perp (mm) | Age 18 + | 4 | 1.42 | 0.01, 2.84 | 0.05 | 1.39 | 10.02 | 0.02 | 70% | |
| CVMS 5-6 | 4 | 1.54 | -0.25, 3.32 | 0.09 | 2.22 | 9.30 | 0.03 | 68% | ||
| 3-years + | 6 | 0.86 | -0.41, 2.13 | 0.18 | 1.80 | 23.00 | 0.00 | 78% | ||
| Co-Gn (mm) | Age 18 + | 4 | 3.20 | 1.32, 5.08 | 0.00 | 2.61 | 11.89 | 0.01 | 75% | |
| CVMS 5-6 | 4 | 2.87 | 0.47, 5.26 | 0.02 | 4.38 | 11.57 | 0.01 | 74% | ||
| 3-years + | 8 | 1.79 | -0.05, 3.64 | 0.06 | 5.73 | 57.49 | 0.00 | 88% | ||
| Mx-md skeletal | ||||||||||
| ANB (degrees) | Age 18 + | 5 | -1.00 | -2.15, 0.16 | 0.09 | 1.52 | 35.86 | 0.00 | 89% | |
| CVMS 5-6 | 4 | -1.31 | -2.37, -0.24 | 0.02 | 0.97 | 17.21 | 0.00 | 83% | ||
| 3-years + | 10 | -1.11 | -1.82, -0.40 | 0.00 | 1.07 | 57.36 | 0.00 | 84% | ||
| Wits (mm) | Age 18 + | 5 | -3.40 | -4.45, -2.35 | 0.00 | 0.87 | 11.10 | 0.03 | 64% | |
| CVMS 5-6 | 4 | -3.52 | -5.11, -1.93 | 0.00 | 1.85 | 10.71 | 0.01 | 72% | ||
| 3-years + | 9 | -2.89 | -3.64, -2.14 | 0.00 | 0.78 | 23.26 | 0.00 | 66% | ||
| Co-Gn/Co-A diff (mm) | Age 18 + | 3 | 2.07 | 0.79, 3.35 | 0.00 | 0.64 | 3.99 | 0.14 | 50% | |
| CVMS 5-6 | 2 | 2.69 | 1.51, 3.86 | 0.00 | 0.00 | 0.49 | 0.48 | 0% | ||
| 3-years + | 6 | 2.56 | 1.07, 4.05 | 0.00 | 2.64 | 24.57 | 0.00 | 80% | ||
Mx skeletal, maxillary skeletal outcomes; SNA, SNA angle; A to N perp, A point to N perpendicular distance; Co-A, Co-A distance
Md skeletal, mandibular skeletal outcomes; SNB, SNB angle; Pg to N perp, Pg point to N perpendicular distance; Co-Gn, Co-Gn distance
Mx-md skeletal, maxillo-mandibular outcomes; ANB, ANB angle; Wits, Wits appraisal; Co-Gn/Co-A diff, Co-Gn/Co-A difference
Age 18 +, above 18 years of age; CVMS 5–6, at the end of growth according to the cervical vertebral maturation method; 3-years +, after a post-retention period of at least 3 years
N_s, number of studies or subgroups; MD, mean differences; 95% CI, 95% confidence intervals; P, P value.
Table 6. Details of the performed subgroup analysis according to the type of functional appliance (Bionator/Activator and multi-bracket appliances, Frankel-2 appliance).
| Outcome | Time point | Bionator/Activator + multibracket appliances | Frankel-2 appliance | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N_s | MD | 95% CI | P | N_s | MD | 95% CI | P | ||
| Mx skeletal | |||||||||
| SNA (degrees) | Age 18 + | 1 | -0.70 | -2.20, 0.80 | 0.36 | 2 | -0.70 | -1.46, 0.06 | 0.07 |
| CVMS 5-6 | 2 | -0.76 | -1.67, 0.14 | 0.10 | 2 | -0.70 | -1.46, 0.06 | 0.07 | |
| 3-years + | 2 | -0.76 | -1.67, 0.14 | 0.10 | 2 | -0.70 | -1.46, 0.06 | 0.07 | |
| A to N perp (mm) | Age 18 + | - | - | - | - | 2 | -0.48 | -2.74, 1.77 | 0.67 |
| CVMS 5-6 | - | - | - | - | 2 | -0.48 | -2.74, 1.77 | 0.67 | |
| 3-years + | - | - | - | - | 2 | -0.48 | -2.74, 1.77 | 0.67 | |
| Co-A (mm) | Age 18 + | - | - | - | - | 2 | 0.15 | -1.16, 1.46 | 0.82 |
| CVMS 5-6 | - | - | - | - | 2 | 0.15 | -1.16, 1.46 | 0.82 | |
| 3-years + | - | - | - | - | 2 | 0.15 | -1.16, 1.46 | 0.82 | |
| Md skeletal | |||||||||
| SNB (degrees) | Age 18 + | 1 | 1.10 | -0.19, 2.39 | 0.09 | 2 | 1.19 | 0.11, 2.26 | 0.03 |
| CVMS 5-6 | 2 | 0.12 | -1.74, 1.99 | 0.90 | 2 | 1.19 | 0.11, 2.26 | 0.03 | |
| 3-years + | 2 | 0.12 | -1.74, 1.99 | 0.90 | 2 | 1.19 | 0.11, 2.26 | 0.03 | |
| Pg to N perp (mm) | Age 18 + | 1 | 2.90 | 1.11, 4.69 | 0.00 | 2 | 1.16 | -2.26, 4.59 | 0.51 |
| CVMS 5-6 | 2 | 2.05 | 0.11, 3.99 | 0.04 | 2 | 1.16 | -2.26, 4.59 | 0.51 | |
| 3-years + | 2 | 2.05 | 0.11, 3.99 | 0.04 | 2 | 1.16 | -2.26, 4.59 | 0.51 | |
| Co-Gn (mm) | Age 18 + | 1 | 5.10 | 3.29, 6.91 | 0.00 | 2 | 3.18 | 1.31, 5.04 | 0.00 |
| CVMS 5-6 | 2 | 2.35 | -3.23, 7.93 | 0.41 | 2 | 3.18 | 1.31, 5.04 | 0.00 | |
| 3-years + | 2 | 2.35 | -3.23, 7.93 | 0.41 | 2 | 3.18 | 1.31, 5.04 | 0.00 | |
| Mx-md skeletal | |||||||||
| ANB (degrees) | Age 18 + | 1 | -1.80 | -2.74, -0.86 | 0.00 | 2 | -1.82 | -2.69, -0.94 | 0.00 |
| CVMS 5-6 | 2 | -0.87 | -2.64, 0.89 | 0.33 | 2 | -1.82 | -2.69, -0.94 | 0.00 | |
| 3-years + | 3 | -1.19 | -2.41, 0.04 | 0.06 | 2 | -1.82 | -2.69, -0.94 | 0.00 | |
| Wits (mm) | Age 18 + | 1 | -5.40 | -7.66, -3.14 | 0.00 | 2 | -3.64 | -5.59, -1.68 | 0.00 |
| CVMS 5-6 | 2 | -3.45 | -7.17, 0.27 | 0.07 | 2 | -3.64 | -5.59, -1.68 | 0.00 | |
| 3-years + | 2 | -3.45 | -7.17, 0.27 | 0.07 | 2 | -3.64 | -5.59, -1.68 | 0.00 | |
| Co-Gn/Co-A diff (mm) | Age 18 + | - | - | - | - | 2 | 2.69 | 1.51, 3.86 | 0.00 |
| CVMS 5-6 | - | - | - | - | 2 | 2.69 | 1.51, 3.86 | 0.00 | |
| 3-years + | - | - | - | - | 2 | 2.69 | 1.51, 3.86 | 0.00 | |
Mx skeletal, maxillary skeletal outcomes; SNA, SNA angle; A point to N perp, A to N perpendicular distance; Co-A, Co-A distance
Md skeletal, mandibular skeletal outcomes; SNB, SNB angle; Pg point to N perp, Pg to N perpendicular distance; Co-Gn, Co-Gn distance
Mx-md skeletal, maxillo-mandibular outcomes; ANB, ANB angle; Wits, Wits appraisal; Co-Gn/Co-A diff, Co-Gn/Co-A difference
Age 18 +, above 18 years of age; CVMS 5–6, at the end of growth according to the cervical vertebral maturation method; 3-years +, after a post-retention period of at least 3 years
N_s, number of studies or subgroups; MD, mean differences; 95% CI, 95% confidence intervals; P, P value
P_s, test for subgroup differences.
Table 7. Details of the performed subgroup analysis according to the type of functional appliance (Herbst, Forsus and multi-bracket appliances).
| Outcome | Time point | Herbst +/- multibracket appliances | Forsus + multibracket appliances | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N_s | MD | 95% CI | P | N_s | MD | 95% CI | P | P_s | ||
| Mx skeletal | ||||||||||
| SNA (degrees) | Age 18 + | 1 | -0.60 | -1.91, 0.71 | 0.37 | 1 | 0.40 | -0.38, 1.18 | 0.32 | 0.20 |
| CVMS 5-6 | - | - | - | - | - | - | - | - | 0.92 | |
| 3-years + | 3 | -1.62 | -3.17, -0.07 | 0.04 | 2 | -0.92 | -3.47, 1.62 | 0.48 | 0.77 | |
| A to N perp (mm) | Age 18 + | - | - | - | - | 1 | -6.30 | -7.01, -5.59 | 0.00 | 0.00 |
| CVMS 5-6 | - | - | - | - | - | - | - | - | NA | |
| 3-years + | - | - | - | - | 2 | -3.99 | -8.50, 0.52 | 0.08 | 0.17 | |
| Co-A (mm) | Age 18 + | - | - | - | - | 1 | 0.60 | 0.03, 1.17 | 0.04 | 0.54 |
| CVMS 5-6 | - | - | - | - | - | - | - | - | NA | |
| 3-years + | 2 | -4.08 | -6.03, -2.12 | 0.00 | 2 | -0.40 | -2.36, 1.56 | 0.69 | 0.00 | |
| Md skeletal | ||||||||||
| SNB (degrees) | Age 18 + | 1 | -0.30 | -1.69, 1.09 | 0.67 | 1 | 0.30 | -0.27, 0.87 | 0.31 | 0.25 |
| CVMS 5-6 | - | - | - | - | - | - | - | - | 0.33 | |
| 3-years + | 3 | -0.41 | -1.35, 0.54 | 0.40 | 2 | -0.21 | -1.29, 0.87 | 0.70 | 0.15 | |
| Pg to N perp (mm) | Age 18 + | - | - | - | - | 1 | 0.90 | 0.17, 1.63 | 0.02 | 0.13 |
| CVMS 5-6 | - | - | - | - | - | - | - | - | 0.66 | |
| 3-years + | - | - | - | - | 2 | -0.06 | -2.02, 1.89 | 0.95 | 0.32 | |
| Co-Gn (mm) | Age 18 + | - | - | - | - | 1 | 1.60 | 0.62, 2.58 | 0.00 | 0.00 |
| CVMS 5-6 | - | - | - | - | - | - | - | - | 0.78 | |
| 3-years + | 2 | -1.44 | -6.09, 3.22 | 0.55 | 2 | 2.59 | 0.63, 4.55 | 0.01 | 0.35 | |
| Mx-md skeletal | ||||||||||
| ANB (degrees) | Age 18 + | 1 | -0.40 | -1.32, 0.52 | 0.40 | 1 | 0.60 | -0.01, 1.21 | 0.05 | 0.00 |
| CVMS 5-6 | - | - | - | - | - | - | - | - | 0.35 | |
| 3-years + | 3 | -1.48 | -2.72, -0.25 | 0.02 | 2 | 0.17 | -0.80, 1.14 | 0.73 | 0.02 | |
| Wits (mm) | Age 18 + | 1 | -2.40 | -4.11, -0.69 | 0.01 | 1 | -2.70 | -3.53, -1.87 | 0.00 | 0.13 |
| CVMS 5-6 | - | - | - | - | - | - | - | - | 0.93 | |
| 3-years + | 3 | -1.74 | -2.66, -0.81 | 0.00 | 2 | -3.10 | -3.78, -2.42 | 0.00 | 0.09 | |
| Co-Gn/Co-A diff (mm) | Age 18 + | - | - | - | - | 1 | 1.00 | -0.32, 2.32 | 0.14 | 0.06 |
| CVMS 5-6 | - | - | - | - | - | - | - | - | NA | |
| 3-years + | 2 | 1.63 | -0.09, 3.34 | 0.06 | 2 | 2.97 | -0.85, 6.79 | 0.13 | 0.58 | |
Mx skeletal, maxillary skeletal outcomes; SNA, SNA angle; A point to N perp, A to N perpendicular distance; Co-A, Co-A distance
Md skeletal, mandibular skeletal outcomes; SNB, SNB angle; Pg point to N perp, Pg to N perpendicular distance; Co-Gn, Co-Gn distance
Mx-md skeletal, maxillo-mandibular outcomes; ANB, ANB angle; Wits, Wits appraisal; Co-Gn/Co-A diff, Co-Gn/Co-A difference
Age 18 +, above 18 years of age; CVMS 5–6, at the end of growth according to the cervical vertebral maturation method; 3-years +, after a post-retention period of at least 3 years
N_s, number of studies or subgroups; MD, mean differences; 95% CI, 95% confidence intervals; P, P value
P_s, test for subgroup differences.
Maxillary/Upper jaw changes
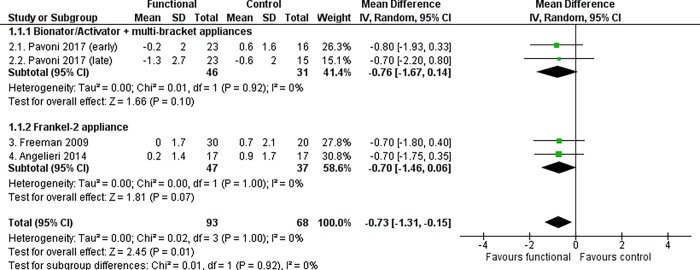
It was found that functional appliances produced a statistically significant reduction in the angular position of the maxilla (SNA angle) at the end of growth according to the CVM method (MD -0.73°, 95% CI -1.31 to -0.15, P = 0.01, I2 = 0%, 4 studies [Fig 2]) and after a post-retention period of at least 3 years (MD -1.03°, 95% CI -1.88 to -0.18, P = 0.02, I2 = 84%, 9 studies [Table 5]).
Fig 2. Meta-analysis; Outcome: SNA angle; Time point: End of growth according to the CVM method.
The most clinically relevant maxillary effects were produced by fixed functional appliances: the Herbst appliance (Co-A distance at least 3 years after retention, MD -4.08 mm, 95% CI -6.03 to -2.12, P < 0.0001, I2 = 0%, 2 studies [Table 7]) and the Forsus device, in combination with multi-bracket therapy (A to N perpendicular distance above 18 years of age, MD -6.30 mm, 95% CI -7.01 to -5.59, P < 0.00001, I2 = Not applicable, 1 study [Table 7]).
Mandibular/Lower jaw changes
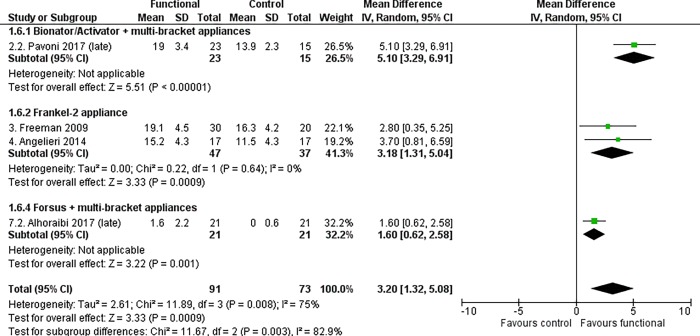
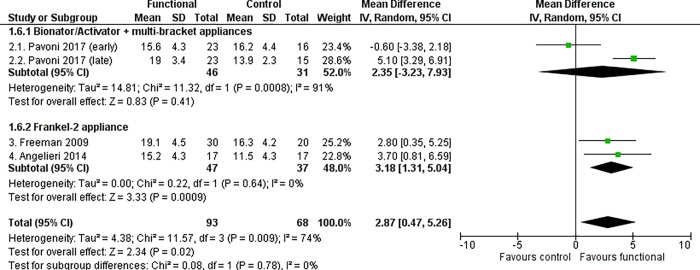
Treated patients showed a statistically significant increase in the mandibular length (Co-Gn distance) compared to untreated subjects, at both primary time points. The increase in the mandibular growth was 3.20 mm in patients aged 18 years and above (95% CI 1.32 to 5.08, P = 0.0009, I2 = 75%, 4 studies [Fig 3]) and 2.87 mm at the end of growth according to the CVM method (95% CI 0.47 to 5.26, P = 0.02, I2 = 74%, 4 studies [Fig 4]).
Fig 3. Meta-analysis; Outcome: Co-Gn distance; Time point: Above 18 years of age.
Fig 4. Meta-analysis; Outcome: Co-Gn distance; Time point: End of growth according to the CVM method.
The angular improvement of the mandibular projection was significant above 18 years of age (SNB angle, MD 0.66°, 95% CI 0.03 to 1.29, P = 0.04, I2 = 43%, 5 studies [Table 5]), however the linear improvement of the same outcome was not significant at any time point (Pg to N perpendicular distance above 18 years of age, MD 1.42 mm, 95% CI 0.01 to 2.84, P = 0.05, I2 = 70%, 4 studies [Table 5]).
Removable functional appliances produced greater treatment effects than fixed devices. The greatest significant increase in the mandibular growth (Co-Gn distance) above 18 years of age was observed in a single study [43], in which a mixed subgroup of patients was treated either with the Bionator or Activator during puberty (MD 5.10 mm, 95% CI 3.29 to 6.91, P < 0.00001, I2 = Not applicable, 1 study [Table 6]). This group also showed a statistically significant improvement of the sagittal projection of the mandible (Pg to N perpendicular distance, MD 2.90 mm, 95% CI 1.11 to 4.69, P = 0.001, I2 = Not applicable, 1 study [Table 6]), although the test for subgroup differences was not significant (P = 0.13, I2 = 51.5%).
Maxillo-mandibular changes
Functional appliance therapy produced a statistically significant improvement of the mutual relationship between the maxilla and mandible, at almost all time points. The most clinically relevant maxillo-mandibular changes were recorded at the end of growth according to the CVM method, when treated patients exhibited an improvement in both angular and linear measurements relative to the controls (ANB angle, MD -1.31°, 95% CI -2.37 to -0.24, P = 0.02, I2 = 83%, 4 studies [Fig 5]; Wits appraisal, MD -3.52 mm, 95% CI -5.11 to -1.93, P < 0.0001, I2 = 72%, 4 studies [Fig 6]; Co-Gn/Co-A difference, MD 2.69 mm, 95% CI 1.51, 3.86, P < 0.0001, I2 = 0%, 2 studies [Fig 7]).
Fig 5. Meta-analysis; Outcome: ANB angle; Time point: End of growth according to the CVM method.
Fig 6. Meta-analysis; Outcome: Wits appraisal; Time point: End of growth according to the CVM method.
Fig 7. Meta-analysis; Outcome: Co-Gn/Co-A difference; Time point: End of growth according to the CVM method.
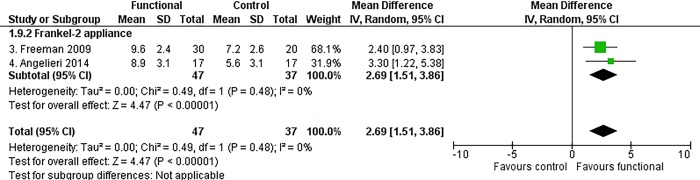
The Frankel-2 appliance worn alone improved all skeletal maxillo-mandibular outcomes regardless of the time point chosen. The statistically significant improvement of the ANB angle, Wits appraisal and Co-Gn/Co-A difference were respectively -1.82° (95% CI -2.69 to -0.94, P < 0.0001, I2 = 38%, 2 studies [Fig 5]), -3.64 mm (95% CI -5.59 to -1.68, P = 0.0003, I2 = 75%, 2 studies [Fig 6), and 2.69 mm (95% CI 1.51 to 3.86, P < 0.00001, I2 = 0%, 2 studies [Fig 7]).
Additional analysis
Few statistically significant differences were found among the subgroups analysed (Tables 8–9, S3 Appendix). Early treatment with functional appliances (commencing in children aged between 7 and 11 years) produced a greater improvement of the angular antero-posterior position of the maxilla (SNA angle) and the relationship between the two jaws (ANB angle) than late treatment (beginning in adolescents aged between 12 and 16 years).
Table 8. Details of the performed subgroup analyses, according to gender, beginning of the functional appliance therapy and post-retention period duration.
| Outcome | Subgroups | Overall effect | Heterogeneity | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N_s | MD | 95% CI | P | Tau2 | Chi2 | P | I2 | ||
| Males Vs females | |||||||||
| SNA (degrees) | Males | 2 | -0.85 | -1.96, 0.27 | 0.14 | 0.00 | 0.50 | 0.48 | 0% |
| Females | 1 | -3.20 | -5.25, -1.15 | 0.00 | NA | ||||
| Total (95% CI) | 3 | -1.62 | -3.17, -0.07 | 0.04 | 1.02 | 4.42 | 0.11 | 55% | |
| Subgroup differences: | 3.92 | 0.05 | 75% | ||||||
| Co-Gn (mm) | Males | 1 | 1.30 | -2.71, 5.31 | 0.52 | NA | |||
| Females | 1 | -3.50 | -5.41, -1.59 | 0.00 | NA | ||||
| Total (95% CI) | 2 | -1.44 | -6.09, 3.22 | 0.55 | 8.95 | 4.49 | 0.03 | 78% | |
| Subgroup differences: | 4.49 | 0.03 | 78% | ||||||
| ANB (degrees) | Males | 2 | -1.26 | -3.11, 0.60 | 0.18 | 1.41 | 4.55 | 0.03 | 78% |
| Females | 1 | -2.00 | -3.11, -0.89 | 0.00 | NA | ||||
| Total (95% CI) | 3 | -1.48 | -2.72, -0.25 | 0.02 | 0.84 | 6.92 | 0.03 | 71% | |
| Subgroup differences: | 0.45 | 0.50 | 0% | ||||||
| Early Vs late treatments according to age | |||||||||
| SNA (degrees) | 7 < age < 11 | 7 | -1.34 | -2.11, -0.57 | 0.00 | 0.66 | 20.39 | 0.00 | 71% |
| 12 < age < 16 | 2 | 0.04 | -0.90, 0.98 | 0.93 | 0.20 | 1.66 | 0.20 | 40% | |
| Total (95% CI) | 9 | -1.03 | -1.88, -0.18 | 0.02 | 1.28 | 50.87 | 0.00 | 84% | |
| Subgroup differences: | 4.99 | 0.03 | 80% | ||||||
| Co-Gn (mm) | 7 < age < 11 | 7 | 1.81 | -0.61, 4.23 | 0.14 | 9.08 | 55.68 | 0.00 | 89% |
| 12 < age < 16 | 1 | 1.60 | 0.62, 2.58 | 0.00 | NA | ||||
| Total (95% CI) | 8 | 1.79 | -0.05, 3.64 | 0.06 | 5.73 | 57.49 | 0.00 | 88% | |
| Subgroup differences: | 0.02 | 0.88 | 0% | ||||||
| ANB (degrees) | 7 < age < 11 | 8 | -1.43 | -2.07, -0.79 | 0.00 | 0.61 | 26.11 | 0.00 | 73% |
| 12 < age < 16 | 2 | 0.16 | -0.81, 1.13 | 0.74 | 0.34 | 3.13 | 0.08 | 68% | |
| Total (95% CI) | 10 | -1.11 | -1.82, -0.40 | 0.00 | 1.07 | 57.36 | 0.00 | 84% | |
| Subgroup differences: | 7.15 | 0.01 | 86% | ||||||
| Early Vs late treatments according to the cervical vertebral maturation method | |||||||||
| SNA (degrees) | CVSM 1-2 | 2 | -1.61 | -2.96, -0.25 | 0.02 | 0.80 | 5.40 | 0.02 | 81% |
| CVSM 2-3 | 2 | 0.04 | -0.97, 1.05 | 0.93 | 0.23 | 1.63 | 0.20 | 39% | |
| Total (95% CI) | 4 | -0.85 | -2.35, 0.64 | 0.26 | 2.06 | 40.60 | 0.00 | 93% | |
| Subgroup differences: | 3.67 | 0.06 | 73% | ||||||
| Co-Gn (mm) | CVSM 1-2 | 2 | 1.71 | -2.39, 5.80 | 0.41 | 7.67 | 7.66 | 0.01 | 87% |
| CVSM 2-3 | 2 | 3.26 | -0.16, 6.69 | 0.06 | 5.57 | 11.11 | 0.00 | 91% | |
| Total (95% CI) | 4 | 2.61 | 0.76, 4.47 | 0.01 | 2.85 | 19.83 | 0.00 | 85% | |
| Subgroup differences: | 0.33 | 0.57 | 0% | ||||||
| ANB (degrees) | CVSM 1-2 | 2 | -0.15 | -0.73, 0.43 | 0.62 | 0.00 | 0.43 | 0.51 | 0% |
| CVSM 2-3 | 2 | -0.57 | -2.92, 1.78 | 0.63 | 2.72 | 17.66 | 0.00 | 94% | |
| Total (95% CI) | 4 | -0.36 | -1.33, 0.61 | 0.47 | 0.81 | 18.10 | 0.00 | 83% | |
| Subgroup differences: | 0.12 | 0.73 | 0% | ||||||
| 3-4 Vs 5-10 years after active functional appliance therapy | |||||||||
| SNA (degrees) | 3-4 years | 2 | -0.92 | -3.47, 1.62 | 0.48 | 3.29 | 36.06 | 0.00 | 97% |
| 5-10 years | 7 | -0.90 | -1.40, -0.40 | 0.00 | 0.00 | 5.72 | 0.46 | 0% | |
| Total (95% CI) | 9 | -1.03 | -1.88, -0.18 | 0.02 | 1.28 | 50.87 | 0.00 | 84% | |
| Subgroup differences: | 0.00 | 0.98 | 0% | ||||||
| Co-Gn (mm) | 3-4 years | 2 | 2.59 | 0.63, 4.55 | 0.01 | 1.73 | 7.46 | 0.01 | 87% |
| 5-10 years | 6 | 1.46 | -1.63, 4.55 | 0.35 | 13.01 | 46.89 | 0.00 | 89% | |
| Total (95% CI) | 8 | 1.79 | -0.05, 3.64 | 0.06 | 5.73 | 57.49 | 0.00 | 88% | |
| Subgroup differences: | 0.37 | 0.55 | 0% | ||||||
| ANB (degrees) | 3-4 years | 3 | -0.53 | -2.06, 1.00 | 0.50 | 1.67 | 25.46 | 0.00 | 92% |
| 5-10 years | 7 | -1.37 | -2.11, -0.63 | 0.00 | 0.74 | 24.20 | 0.00 | 75% | |
| Total (95% CI) | 10 | -1.11 | -1.82, -0.40 | 0.00 | 1.07 | 57.36 | 0.00 | 84% | |
| Subgroup differences: | 0.94 | 0.33 | 0% | ||||||
SNA, SNA angle; Co-Gn, Co-Gn distance; ANB, ANB angle
7 < age < 11; early treatments, commencing in children aged between 7 and 11 years; 12 < age < 16; late treatments, beginning in adolescents aged between 12 and 16 years
CVSM 1–2; early treatments, with patients presenting with Cervical Vertebral Maturation Stage (CVMS) 1 or 2 at the first observation; CVSM 2–3, late treatments, with subjects presenting with CVMS 2 or 3
N_s, number of studies or subgroups; MD, mean differences; 95% CI, 95% confidence intervals; P, P value.
Table 9. Details of the performed sensitivity analyses according to study quality assessment.
| Outcome | Subgroups | Overall effect | Heterogeneity | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N_s | MD | 95% CI | P | Tau2 | Chi2 | P | I2 | ||
| SNA (degrees) | Low-mod | 5 | -1.34 | -2.72, 0.05 | 0.06 | 2.03 | 41.62 | 0.00 | 90% |
| Crit-ser | 4 | -0.71 | -1.31, -0.10 | 0.02 | 0.00 | 0.05 | 1.00 | 0% | |
| Co-Gn (mm) | Low-mod | 5 | 1.19 | -1.17, 3.54 | 0.32 | 5.99 | 41.55 | 0.00 | 90% |
| Crit-ser | 3 | 2.83 | -0.57, 6.23 | 0.10 | 7.39 | 11.36 | 0.00 | 82% | |
| ANB (degrees) | Low-mod | 5 | -1.20 | -2.51, 0.11 | 0.07 | 1.96 | 39.09 | 0.00 | 90% |
| Crit-ser | 5 | -1.05 | -1.84, -0.26 | 0.01 | 0.61 | 16.90 | 0.00 | 76% | |
SNA, SNA angle; Co-Gn, Co-Gn distance; ANB, ANB angle
Mod, moderate; Ser, serious; Crit, critical.
N_s, number of studies or subgroups; MD, mean differences; 95% CI, 95% confidence intervals; P, P value.
Sensitivity analyses revealed that, if only studies with low and moderate risk of bias were considered, differences in the most clinically important outcomes (SNA angle, Co-Gn distance, ANB angle) were not statistically significant (Table 9).
Risk of bias across studies
The protocol of the included studies was not retrieved in the Clinical Trial Register, thus outcome reporting bias could not be assessed. Due to the limited number of included studies, an evaluation for the existence of reporting bias (including publication bias) was not possible [40].
The GRADE assessment for all the outcomes at primary time points were rated as being ‘very low’ (Table 10), except for the Co-A distance when patients were 18 or older (‘low’), and Co-Gn/Co-A difference above the age of 18 (‘low’) and at the end of growth (‘moderate’). Since the included studies were observational, evidence supporting estimates of the intervention effects started to be rated as low-quality. The evidence was down rated for most of the outcomes, as a direct result of the risk of bias and inconsistency of included trials [41].
Table 10. Details for the GRADE assessment of the primary outcomes.
| Outcome | RB | IC | IN | IM | Overall certainty of evidence | No. part. (studies) | Anticipated absolute effects | ||
|---|---|---|---|---|---|---|---|---|---|
| N_C | N_T | Risk with No treatment | Risk with Functional appliances | ||||||
| Above 18 years of age | |||||||||
| SNA | S | NS | NS | S | ⨁◯◯◯ | 190 (5) | The mean ranged from -0.6 to 0.9 degrees | MD 0.31 degrees lower (0.83 lower to 0.21 higher) | |
| VERY LOW | 86 | 104 | |||||||
| A to N perp | NS | S | NS | S | ⨁◯◯◯ | 126 (3) | The mean ranged from 0.1 to 0.9 mm | MD 2.41 mm lower (6.45 lower to 1.62 higher) | |
| VERY LOW | 58 | 68 | |||||||
| Co-A | NS | NS | NS | NS | ⨁⨁◯◯ | 126 (3) | The mean ranged from 0.6 to 9.6 mm | MD 0.53 mm higher (0.00 higher to 1.05 higher) | |
| LOW | 58 | 68 | |||||||
| SNB | S | NS | NS | NS | ⨁◯◯◯ | 190 (5) | The mean ranged from 1.0 to 2.2 degrees | MD 0.66 degrees higher (0.03 higher to 1.29 higher) | |
| VERY LOW | 86 | 104 | |||||||
| Pg to N perp | S | S | NS | NS | ⨁◯◯◯ | 164 (4) | The mean ranged from 0.9 to 3.6 mm | MD 1.42 mm higher (0.01 higher to 2.84 higher) | |
| VERY LOW | 73 | 91 | |||||||
| Co-Gn | S | S | NS | NS | ⨁◯◯◯ | 164 (4) | The mean ranged from 0.0 to 16.3 mm | MD 3.20 mm higher (1.32 higher to 5.08 higher) | |
| VERY LOW | 73 | 91 | |||||||
| ANB | S | S | NS | S | ⨁◯◯◯ | 190 (5) | The mean ranged from -1.6 to -0.8 degrees | MD 1 degrees lower (2.15 lower to 0.16 higher) | |
| VERY LOW | 86 | 104 | |||||||
| Wits | S | S | NS | NS | ⨁◯◯◯ | 190 (5) | The mean ranged from 0.4 to 1.7 mm | MD 3.40 mm lower (4.45 lower to 2.35 lower) | |
| VERY LOW | 86 | 104 | |||||||
| Co-Gn/Co-A diff | NS | NS | NS | NS | ⨁⨁⨁◯ | 126 (3) | The mean ranged from -0.6 to 7.2 mm | MD 2.07 mm higher (0.79 higher to 3.35 higher) | |
| MODERATE | 58 | 68 | |||||||
| At the end of growth according to the cervical vertebral maturation method | |||||||||
| SNA | S | NS | NS | NS | ⨁◯◯◯ | 161 (4) | The mean ranged from -0.6 to 0.9 degrees | MD 0.73 degrees lower (1.31 lower to 0.15 lower) | |
| VERY LOW | 68 | 93 | |||||||
| A to N perp | S | S | NS | S | ⨁◯◯◯ | 84 (2) | The mean ranged from 0.1 to 0.9 mm | MD 0.48 mm lower (2.74 lower to 1.77 higher) | |
| VERY LOW | 37 | 47 | |||||||
| Co-A | S | NS | NS | S | ⨁◯◯◯ | 84 (2) | The mean ranged from 5.7 to 9.6 mm | MD 0.15 mm higher (1.16 lower to 1.46 higher) | |
| VERY LOW | 37 | 47 | |||||||
| SNB | S | S | NS | S | ⨁◯◯◯ | 161 (4) | The mean ranged from 1.0 to 2.2 degrees | MD 0.65 degrees higher (0.45 lower to 1.74 higher) | |
| VERY LOW | 68 | 93 | |||||||
| Pg to N perp | S | S | NS | S | ⨁◯◯◯ | 161 (4) | The mean ranged from 2.8 to 3.6 mm | MD 1.54 mm higher (0.25 lower to 3.32 higher) | |
| VERY LOW | 68 | 93 | |||||||
| Co-Gn | S | S | NS | NS | ⨁◯◯◯ | 161 (4) | The mean ranged from 11.5 to 16.3 mm | MD 2.87 mm higher (0.47 higher to 5.26 higher) | |
| VERY LOW | 68 | 93 | |||||||
| ANB | S | S | NS | NS | ⨁◯◯◯ | 161 (4) | The mean ranged from -1.6 to -0.8 degrees | MD 1.31 degrees lower (2.37 lower to 0.24 lower) | |
| VERY LOW | 68 | 93 | |||||||
| Wits | S | S | NS | NS | ⨁◯◯◯ | 161 (4) | The mean ranged from 0.4 to 1.7 mm | MD 3.52 mm lower (5.11 lower to 1.93 lower) | |
| VERY LOW | 68 | 93 | |||||||
| Co-Gn/Co-A diff | S | NS | NS | NS | ⨁⨁◯◯ | 84 (2) | The mean ranged from 5.6 to 7.2 mm | MD 2.69 mm higher (1.51 higher to 3.86 higher) | |
| LOW | 37 | 47 | |||||||
SNA, SNA angle; A to N perp, A point to N perpendicular distance; Co-A, Co-A distance
SNB, SNB angle; Pg to N perp, Pg point to N perpendicular distance; Co-Gn, Co-Gn distance
ANB, ANB angle; Wits, Wits appraisal; Co-Gn/Co-A diff, Co-Gn/Co-A difference
RB, risk of bias; IC, inconsistency; IN, indirectness; IM, imprecision
No. part., number of participants; N_C, number of not treated subjects; N_T, number of treated patients.
S, serious; NS, not serious
All studies were observational studies.
Discussion
Summary of evidence
The results demonstrated that functional appliances, worn alone or in combination with multi-bracket therapy, produced an improvement of the maxillo-mandibular relationship at almost all time points. The improvement was around -1 degree for the angular measurement (ANB angle) and between -3.5 and 2.5 mm for the linear outcomes (Wits appraisal, Co-Gn/Co-A difference). The decrease in the ANB angle and Wits appraisal was consistent with that reported in previous systematic reviews on the effects of functional appliances in the short- [6, 21, 22, 24, 26, 28] and long-term [28].
In agreement with previous reviews [7, 21, 24], a restraint of maxillary growth (SNA angle, -1 degree) was observed in included studies. Above 18 years of age or at the end of growth according to the cervical vertebral maturation method [20], the increase in the mandibular length (Co-Gn distance) was approximately 3 mm greater in the treated patients compared to that in untreated subjects. Similar results were found in the subgroups of adolescents studied by Perinetti et al. [6, 22]. However, the improvement of the position of the mandible was negligible or not significant, as inferred from results of its measurements (SNB angle, Pg to N perpendicular). During growth, the mandible is translated downward and forward, while at the same time it increases in size by growing upward and backward [12, 14]. Vertical growth can reduce the effects of the increase in mandibular length on its projection.
According to the GRADE Working Group, the quality of evidence was ‘very low’ for most of the outcomes at both primary time points. Most of the studies received a very low rating, because of their risk of bias and inconsistency [41].
Overall, the clinical significance of these findings was limited. Several approaches were described to establish if the ‘statistically significant’ differences were also ‘clinically important’. The small or minimal clinical important, moderate and large effects were conventionally defined as half, one, and two standard deviations of the normal values, respectively [54]. According to these thresholds, functional appliances produced only small clinically significant changes in the linear maxillo-mandibular measurements (Wits appraisal, Co-Gn/Co-A difference) and in the mandibular length (Co-Gn distance).
Strengths and limitations
Strengths of the present systematic review were in the efforts made to respect rigorous standards for quality and reduce risk of bias: original research question; unrestricted electronic search of 24 databases and additional manual searches; pre-defined and unambiguous eligibility criteria with rationale; adjustment for magnified linear measurements; 3 time points evaluated with rationale; pre-defined and broad additional analyses.
However, limitations occurred at some levels. Although both randomised and non-randomised controlled studies were sought, only retrospective controlled clinical trials were retrieved with negative consequences on the quality of evidence of the effect estimates. It needs to be noted that only long-term studies were considered eligible. The whole observational periods of included trials ranged from 4.7 to 10.2 years.
Participants were eligible regardless of their baseline disease severity. The antero-posterior relationship between the two arches or jaws affects the amount of advancement produced by functional appliances, therefore this could influence the treatment effects. The greater the space created between the upper and lower front teeth is, the more protruded position of the mandible can be achieved. Different classifications of malocclusion also bring into question the applicability of results.
Any type of functional appliance, worn alone or in combination with multi-bracket therapy, was included. As anticipated, multi-bracket therapy, as well as retention appliances, could enhance the treatment effects of functional jaw orthopaedics or control their relapse. Moreover, trials with historical untreated controls from growth studies showed larger treatment effects compared to trials with untreated controls from clinical archives [55].
Other limitations concerned the evaluated outcomes. The present systematic review mainly assessed cephalometric skeletal measurements which can be considered as ‘clinically important outcomes’. The effects of functional appliances on the soft-tissue facial structures were searched, but few results were found. Multiple related outcomes were also analysed. In fact, the ANB angle is defined as the difference between the SNA and SNB angles, whilst the Co-Gn/Co-A difference is defined as the total mandibular length (Co-Gn) minus Co-A distance. The greater the number of outcomes, the higher the chance of finding a false positive result [56]. Cephalometric magnification was not reported or retrieved in 2 studies [42, 44]. Linear measurements of these studies were excluded from meta-analyses. The impact of dental movements on the skeletal measurements cannot be examined further, as the objective of this systematic review was to assess the skeletal effects produced by functional appliances in the long-term.
With regards to time points, two alternative methods were used to define the completion of growth. Each of these methods is affected by some limitations. The age threshold of 18 years, as reported in one included trial [48], was chosen to maximise the data available. In studies of long duration with several periods of follow-up, the Cochrane Collaboration recommends to select a single time point and analyse only data at this time [30]. Some investigations reported that growth continues up to 21 years of age [15] or more [16–18]. However, above 18 years of age, most changes in the mandibular growth (Co-Gn distance) appear to be as non-clinically significant (mean change = 0.1 mm per year [17, 18]). None of the included trials evaluated the treatment effects of functional appliances in patients aged at least 21 years old. The cervical vertebral maturation method was also employed. The accuracy of this method is questionable. No skeletal maturity indicator may be considered to have a full diagnostic reliability in the identification of the phases of mandibular growth [57]. All the studies had a post-retention period of at least 3 years, so that a sufficient post-retention period after the functional appliance therapy could be guaranteed [42–49].
Implications for practice
Based on results of this review, weak recommendations can be provided on the long-term effects of functional appliances in treated versus untreated Class II subjects. There is a very low quality evidence that functional appliance therapy produced an improvement of skeletal Class II malocclusion at the end of growth and at least 3 years after retention. Treated patients exhibited an increase in the mandibular length compared to untreated subjects, although with marginal clinical significance.
Implications for research
Further high quality primary studies are needed to confirm or reject the findings of this review. Randomised controlled trials comparing treated patients to untreated subjects (no historical controls) should be carried out. A consensus should be formed on the clinically important measurements to be used for the inclusion in the study and assessment of the effects. Few linear measurements for the position of the maxilla and mandible, the relationship between these jaws, seem to be more appropriate because of their influence on the soft tissue measurements. Patient important outcomes, such as perceived attractiveness, self-esteem and oral health-related quality of life, should be assessed as well.
Conclusions
Functional appliances, worn alone or in combination with multi-bracket therapy, may be effective in correcting skeletal Class II malocclusion in the long-term. The increase in the mandibular length may contribute to the improvement of the maxillo-mandibular relationship, although it brought about a negligible or non-significant improvement of the mandibular projection. The quality of evidence was ‘very low’ for most of the outcomes at both primary time points; the clinical significance of these findings was limited. Further randomised controlled trials evaluating clinically and patient important outcomes are needed to confirm or reject the findings of this review.
Differences between protocol and review
The data extracted were not preliminarily annualised to minimize heterogeneity related to the observation period variability. Annualised changes (mean differences divided by the duration of the whole observational period) seemed to be inappropriate to evaluate the treatment effects in the long-term. If an appliance produced a certain amount of improvement in a given period (reported as degrees/year or mm/year), it does not mean that the device could cause the established improvement for each year of treatment.
An adjustment for magnified linear measurements was introduced to avoid distorted analyses.
Supporting information
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Kelly JE, Sanchez M, Van Kirk LE. An Assessment of the Occlusion of the Teeth of Children 6-11Years, United States. Vital Health Stat 11. 1973;(130):1–60. [PubMed] [Google Scholar]
- 2.Kelly JE, Harvey CR. An assessment of the occlusion of the teeth of youths 12–17 years. Vital Health Stat 11. 1977;(162):1–65. [PubMed] [Google Scholar]
- 3.McNamara JA Jr. Components of class II malocclusion in children 8–10 years of age. Angle Orthod. 1981;51(3):177–202. [DOI] [PubMed] [Google Scholar]
- 4.Pancherz H, Zieber K, Hoyer B. Cephalometric characteristics of Class II division 1 and Class II division 2 malocclusions: a comparative study in children. Angle Orthod. 1997;67(2):111–20. [DOI] [PubMed] [Google Scholar]
- 5.Stahl F, Baccetti T, Franchi L, McNamara JA Jr. Longitudinal growth changes in untreated subjects with Class II Division 1 malocclusion. Am J Orthod Dentofacial Orthop. 2008;134(1):125–37. 10.1016/j.ajodo.2006.06.028 [DOI] [PubMed] [Google Scholar]
- 6.Perinetti G, Primožič J, Furlani G, Franchi L, Contardo L. Treatment effects of fixed functional appliances alone or in combination with multibracket appliances: A systematic review and meta-analysis. Angle Orthod. 2015;85(3):480–92. 10.2319/102813-790.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nucera R, Lo Giudice A, Rustico L, Matarese G, Papadopoulos MA, Cordasco G. Effectiveness of orthodontic treatment with functional appliances on maxillary growth in the short term: A systematic review and meta-analysis. Am J Orthod Dentofacial Orthop. 2016;149(5):600–611.e3. 10.1016/j.ajodo.2015.09.030 [DOI] [PubMed] [Google Scholar]
- 8.Wedrychowska-Szulc B1, Syryńska M. Patient and parent motivation for orthodontic treatment–a questionnaire study. Eur J Orthod. 2010;32(4):447–52. 10.1093/ejo/cjp131 [DOI] [PubMed] [Google Scholar]
- 9.Van Wezel NA, Bos A, Prahl C. Expectations of treatment and satisfaction with dentofacial appearance in patients applying for orthodontic treatment. Am J Orthod Dentofacial Orthop. 2015;147(6):698–703. 10.1016/j.ajodo.2015.01.024 [DOI] [PubMed] [Google Scholar]
- 10.Naini FB, Donaldson AN, Cobourne MT, McDonald F. Assessing the influence of mandibular prominence on perceived attractiveness in the orthognathic patient, clinician, and layperson. Eur J Orthod. 2012;34(6):738–46. 10.1093/ejo/cjr098 [DOI] [PubMed] [Google Scholar]
- 11.Seehra J, Fleming PS, Newton T, DiBiase AT. Bullying in orthodontic patients and its relationship to malocclusion,self-esteem and oral health-related quality of life. J Orthod. 2011;38(4):247–56. 10.1179/14653121141641 [DOI] [PubMed] [Google Scholar]
- 12.Proffit WR, Fields HW. Contemporary orthodontics. St. Louis: Mosby; 2000. [Google Scholar]
- 13.Singer J. Posttreatment change: a reality. Am J Orthod. 1975;67(3):277–89. [DOI] [PubMed] [Google Scholar]
- 14.Bjork A. Variations in the growth pattern of the human mandible: longitudinal radiographic study by the implant method. J Dent Res. 1963;42(1)Pt 2:400–11. [DOI] [PubMed] [Google Scholar]
- 15.Love RJ, Murray JM, Mamandras AH. Facial growth in males 16 to 20 years of age. Am J Orthod Dentofacial Orthop. 1990;97(3):200–6. 10.1016/S0889-5406(05)80052-6 [DOI] [PubMed] [Google Scholar]
- 16.Bishara SE, Treder JE, Jakobsen JR. Facial and dental changes in adulthood. Am J Orthod Dentofacial Orthop. 1994;106(2):175–86. 10.1016/S0889-5406(94)70036-2 [DOI] [PubMed] [Google Scholar]
- 17.West KS, McNamara JA Jr. Changes in the craniofacial complex from adolescence to midadulthood: a cephalometric study. Am J Orthod Dentofacial Orthop. 1999;115(5):521–32. [DOI] [PubMed] [Google Scholar]
- 18.Pecora NG, Baccetti T, McNamara JA Jr. The aging craniofacial complex: a longitudinal cephalometric study from late adolescence to late adulthood. Am J Orthod Dentofacial Orthop. 2008;134(4):496–505. 10.1016/j.ajodo.2006.11.022 [DOI] [PubMed] [Google Scholar]
- 19.Fishman LS. Radiographic evaluation of skeletal maturation. A clinically oriented method based on hand-wrist films. Angle Orthod. 1982;52(2):88–112. [DOI] [PubMed] [Google Scholar]
- 20.Franchi L, Baccetti T, McNamara JA Jr. Mandibular growth as related to cervical vertebral maturation and body height. Am J Orthod Dentofacial Orthop. 2000;118(3):335–40. 10.1067/mod.2000.107009 [DOI] [PubMed] [Google Scholar]
- 21.Koretsi V, Zymperdikas VF, Papageorgiou SN, Papadopoulos MA. Treatment effects of removable functional appliances in patients with Class II malocclusion: a systematic review and meta-analysis. Eur J Orthod. 2015;37(4):418–34. 10.1093/ejo/cju071 [DOI] [PubMed] [Google Scholar]
- 22.Perinetti G, Primožič J, Franchi L, Contardo L. Treatment Effects of Removable Functional Appliances in Pre-Pubertal and Pubertal Class II Patients: A Systematic Review and Meta-Analysis of Controlled Studies. PLoS One. 2015;10(10):e0141198 10.1371/journal.pone.0141198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pacha MM, Fleming PS, Johal A. A comparison of the efficacy of fixed versus removable functional appliances in children with Class II malocclusion: A systematic review. Eur J Orthod. 2016;38(6):621–630. 10.1093/ejo/cjv086 [DOI] [PubMed] [Google Scholar]
- 24.Zymperdikas VF, Koretsi V, Papageorgiou SN, Papadopoulos MA. Treatment effects of fixed functional appliances in patients with Class II malocclusion: a systematic review and meta-analysis. Eur J Orthod. 2016;38(2):113–26. 10.1093/ejo/cjv034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ishaq RA, AlHammadi MS, Fayed MM, El-Ezz AA, Mostafa Y. Fixed functional appliances with multibracket appliances have no skeletal effect on the mandible: A systematic review and meta-analysis. Am J Orthod Dentofacial Orthop. 2016;149(5):612 10.1016/j.ajodo.2015.11.023 [DOI] [PubMed] [Google Scholar]
- 26.Batista KB, Thiruvenkatachari B, Harrison JE, O'Brien KD. Orthodontic treatment for prominent upper front teeth (Class II malocclusion) in children and adolescents. Cochrane Database Syst Rev. 2018;3:CD003452 10.1002/14651858.CD003452.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bondemark L, Holm AK, Hansen K, Axelsson S, Mohlin B, Brattstrom V, Paulin G, Pietila T. Long-term stability of orthodontic treatment and patient satisfaction. A systematic review. Angle Orthod. 2007;77(1):181–91. 10.2319/011006-16R.1 [DOI] [PubMed] [Google Scholar]
- 28.Bock NC, von Bremen J, Ruf S. Stability of Class II fixed functional appliance therapy–a systematic review and meta-analysis. Eur J Orthod. 2016;38(2):129–39. 10.1093/ejo/cjv009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brindeiro D, Cavalcanti Y, Castanha Henriques JF. Long-term stability of post-treatment Class II correction with fixed and removable functional appliances. PROSPERO. 2017. Available from: http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42017075897. [Google Scholar]
- 30.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions (Version 5.1.0). The Cochrane Collaboration; 2011. Available from: http://www.cochrane-handbook.org. [Google Scholar]
- 31.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mallett S, Clarke M. The typical Cochrane review. How many trials? How many participants? Int J Technol Assess Health Care. 2002;18(4):820–3. [DOI] [PubMed] [Google Scholar]
- 33.Greenhalgh T, Peacock R. Effectiveness and efficiency of search methods in systematic reviews of complex evidence: audit of primary sources. BMJ. 2005;331(7524):1064–5. 10.1136/bmj.38636.593461.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hopewell S, Clarke M, Lefebvre C, Scherer R. Handsearching versus electronic searching to identify reports of randomized trials. Cochrane Database Syst Rev. 2007;(2):MR000001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scherer RW, Langenberg P, von Elm E. Full publication of results initially presented in abstracts. Cochrane Database Syst Rev. 2007;(2):MR000005. [DOI] [PubMed] [Google Scholar]
- 36.Dickersin K, Scherer R, Lefebvre C. Identifying relevant studies for systematic reviews. BMJ. 1994;309(6964):1286–91. 10.1136/bmj.309.6964.1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, Carpenter JR, Chan AW, Churchill R, Deeks JJ, Hróbjartsson A, Kirkham J, Jüni P, Loke YK, Pigott TD, Ramsay CR, Regidor D, Rothstein HR, Sandhu L, Santaguida PL, Schünemann HJ, Shea B, Shrier I, Tugwell P, Turner L, Valentine JC, Waddington H, Waters E, Wells GA, Whiting PF, Higgins JP. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919 10.1136/bmj.i4919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.DerSimonian R, Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials. 2015;45(Pt A):139–45. 10.1016/j.cct.2015.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to meta-analysis. Chichester: Wiley; 2009. [Google Scholar]
- 41.Guyatt G, Oxman AD, Sultan S, Brozek J, Glasziou P, Alonso-Coello P, Atkins D, Kunz R, Montori V, Jaeschke R, Rind D, Dahm P, Akl EA, Meerpohl J, Vist G, Berliner E, Norris S, Falck-Ytter Y, Schünemann HJ. GRADE guidelines: 11. Making an overall rating of confidence in effect estimates for a single outcome and for all outcomes. J Clin Epidemiol. 2013;66(2):151–7. 10.1016/j.jclinepi.2012.01.006 [DOI] [PubMed] [Google Scholar]
- 42.Wieslander L, Lagerström L. The effect of activator treatment on class II malocclusions. Am J Orthod. 1979;75(1):20–6. [DOI] [PubMed] [Google Scholar]
- 43.Pavoni C, Cretella Lombardo E, Franchi L, Lione R, Cozza P. Treatment and post-treatment effects of functional therapy on the sagittal pharyngeal dimensions in Class II subjects. Int J Pediatr Otorhinolaryngol. 2017;101:47–50. 10.1016/j.ijporl.2017.07.032 [DOI] [PubMed] [Google Scholar]
- 44.Falck F. Long-term results of treatment of distal occlusion with the function regulator. Fortschr Kieferorthop. 1991;52(5):263–7. [DOI] [PubMed] [Google Scholar]
- 45.Freeman DC, McNamara JA Jr, Baccetti T, Franchi L, Fränkel C. Long-term treatment effects of the FR-2 appliance of Fränkel. Am J Orthod Dentofacial Orthop. 2009;135(5):570e1-6. 10.1016/j.ajodo.2007.11.029 [DOI] [PubMed] [Google Scholar]
- 46.Angelieri F, Franchi L, Cevidanes LH, Scanavini MA, McNamara JA Jr. Long-term treatment effects of the FR-2 appliance: a prospective evalution 7 years post-treatment. Eur J Orthod. 2014;36(2):192–9. 10.1093/ejo/cjt026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wigal TG. Long-term follow-up of patients treated with the edgewise crowned Herbst appliance in the mixed dentition. M.Sc. Thesis, Ann Arbor: West Virginia University. 2008.
- 48.Drosen C, Bock NC, von Bremen J, Pancherz H, Ruf S. Long-term effects of Class II Herbst treatment on the pharyngeal airway width. Eur J Orthod. 2018;40(1):82–89. 10.1093/ejo/cjx032 [DOI] [PubMed] [Google Scholar]
- 49.Alhoraibi L. Long-Term Effects Induced by the Forsus Fatigue Resistant Device in Class II Malocclusion Patients Treated at Pre-Peak, Peak, And Post-Peak Growth Periods. M.Sc. Thesis, Ann Arbor: State University of New York at Buffalo. 2017.
- 50.Faltin KJ, Faltin RM, Baccetti T, Franchi L, Ghiozzi B, McNamara JA Jr. Long-term effectiveness and treatment timing for Bionator therapy. Angle Orthod. 2003;73(3):221–30. [DOI] [PubMed] [Google Scholar]
- 51.Malta LA, Baccetti T, Franchi L, Faltin K Jr, McNamara JA Jr. Long-term dentoskeletal effects and facial profile changes induced by bionator therapy. Angle Orthod. 2010;80(1):10–7. 10.2319/031609-156.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Franchi L, Pavoni C, Faltin K Jr, McNamara JA Jr, Cozza P. Long-term skeletal and dental effects and treatment timing for functional appliances in Class II malocclusion. Angle Orthod. 2013;83(2):334–40. 10.2319/052912-450.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wigal TG, Dischinger T, Martin C, Razmus T, Gunel E, Ngan P. Stability of Class II treatment with an edgewise crowned Herbst appliance in the early mixed dentition: Skeletal and dental changes. Am J Orthod Dentofacial Orthop. 2011;140(2):210–23. 10.1016/j.ajodo.2010.02.036 [DOI] [PubMed] [Google Scholar]
- 54.Sloan J, Symonds T, Vargas-Chanes D, Fridley B. Practical guidelines for assessing the clinical significance of health-related quality of life changes within clinical trials. Drug Inf J. 2003;37(1):23–31. [Google Scholar]
- 55.Papageorgiou SN, Koretsi V, Jäger A. Bias from historical control groups used in orthodontic research: a meta-epidemiological study. Eur J Orthod. 2017;39(1):98–105. 10.1093/ejo/cjw035 [DOI] [PubMed] [Google Scholar]
- 56.Heneghan C, Goldacre B, Mahtani KR. Why clinical trial outcomes fail to translate into benefits for patients. Trials. 2017;18(1):122 10.1186/s13063-017-1870-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Santiago RC, de Miranda Costa LF, Vitral RW, Fraga MR, Bolognese AM, Maia LC. Cervical vertebral maturation as a biologic indicator of skeletal maturity. Angle Orthod. 2012;82(6):1123–31. 10.2319/103111-673.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.