Abstract
Previous studies in rodents have indicated that only a minor fraction of the immunoglobulin heavy chain variable region (IGHV-Cμ) transcripts carry somatic mutations and are considered memory B cells. This is in marked contrast to humans where nearly all marginal zone B (MZ-B) cells are mutated. Here we show in rats that the proportion of mutated IgM+ MZ-B cells varies significantly between the various IGHV genes analyzed, ranging from 27% mutated IGHV5 transcripts to 65% mutated IGHV4 transcripts. The observed data on mutated sequences in clonally-related B cells with a MZ-B cell or follicular B (FO-B) cell phenotype indicates that mutated IgM+ MZ-B and FO-B cells have a common origin. To further investigate the origin of mutated IgM+ MZ-B cells we determined whether mutations occurred in rearranged IGHV-Cμ transcripts using IGHV4 and IGHV5 genes from neonatal rat MZ-B cells and FO-B cells. We were not able to detect mutations in any of the IGHV4 and IGHV5 genes expressed by MZ-B cells or FO-B cells obtained from neonatal rat spleens. Germinal centres (GCs) are absent from neonatal rat spleen in the first few weeks of their life, and no mutations were found in any of the neonatal sequences, not even in the IGHV4 gene family which accumulates the highest number of mutated sequences (66%) in the adult rat. Therefore, these data do not support the notion that MZ-B cells in rats mutate their IGHV genes as part of their developmental program, but are consistent with the notion that mutated rat MZ-B cells require GCs for their generation. Our findings support that the splenic MZ of rats harbors a significant number of memory type IgM+ MZ-B cells with mutated IGHV genes and propose that these memory MZ-B cells are probably generated as a result of an antigen driven immune response in GCs, which still remains to be proven.
Introduction
The splenic marginal zone (MZ) is a distinct anatomical compartment dominated by a unique population of B (MZ-B) lymphocytes, in addition to macrophages, dendritic cells in rodents and in humans also CD4+ T cells [1–3]. This compartment forms an interface between the splenic red and white pulp. This unique localization in combination with the blood flow through this compartment, allows intimate contact between antigens in the blood and cells in the MZ. MZ-B cells have a distinctive phenotype, generally characterized by high levels of IgM and low levels of IgD (IgMhighIgDlow). This contrasts with the dominant population of mature (naïve) follicular B (FO-B) cells located in the follicles of peripheral lymphoid organs, which express low levels of IgM and high levels of IgD (IgMlowIgDhigh). MZ-B cells appear to be in a “pre-activated” state, which is illustrated for example by their high expression of CD80/CD86 and complement receptor 2 (CD21) on their membrane surface in comparison with FO-B cells [4]. MZ-B cells are primarily responsible for T cell-independent (TI) responses to polysaccharide antigens present on the surface of encapsulated bacteria [5, 6]. Another important role of MZ-B cells is facilitation of antigen transport towards the follicles [7]. MZ-B cells constitute a heterogeneous population of cells [8, 9]. The majority of MZ-B cells in rats and mice express unmutated transcripts for IgM heavy chain molecules and are considered to represent naïve B cells. On average their heavy chain complementarity determining region 3 (H-CDR3) is 2–3 amino acids shorter than their FO-B cell counterparts [10]. Autoantigens, rather than exogenous antigens are thought to play a role in the ligand selection of these naïve MZ-B cells [11, 12]. In addition to naïve B cells, a small fraction of the MZ-B cells are either unswitched or class-switched memory B cells as shown by immunization [13–18]. A hallmark of memory B cells is the presence of somatic mutations in the IGV genes [19]. Indeed, approximately 10–20% of rodent IgM+ MZ-B cells carry mutated IgM-encoding IGHV genes [10, 20]. Experimental data by Hendricks et al have revealed in rats the presence of class-switched B cells with a MZ-B cell phenotype, as defined by non-Ig markers, expressing somatically mutated IGHV genes encoding for IgG subclasses [21]. These class-switched memory MZ-B cells exhibited significantly fewer mutations, compared to memory B cells with a FO-B cell phenotype [21]. Their work also provided evidence to suggest that class-switched memory MZ-B cells and FO-B cells originate in a common germinal-center (GC). In contrast to rodents, nearly all MZ-B cells in human spleens express mutated IGHV genes [22, 23]. Phenotypically, these human B cells express CD27, which is an important, but not conclusive, characteristic property of human memory B cells [24]. Human MZ-B cells are therefore defined as IgM+IgD+CD27+ B cells [25]. The reason for the discrepancy between the frequency of mutated MZ-B cells in rodents and humans is not clear. It may result from developmental differences between the species. It has been proposed that, during development, mutations are introduced into the IGHV genes of MZ-B cells in an antigen-independent fashion to diversify the naïve Ig repertoire [26]. Methods of analysis of IGHV genes is another factor that contributes to the discrepancy between adult human and rodent MZ-B cells. Both in humans [22, 23] and rodents [10, 20] mutational analysis of IGHV genes derived from splenic MZ-B cells was carried out on a restricted set of IGHV genes of certain IGHV gene families. Whether these IGHV genes are representative of the mutation frequencies of IGHV genes of the entire MZ-B cell pool is not known. MZ-B cells are ligand selected and for this reason can result in significant differences in the frequencies of mutated IGHV genes between the individual IGHV genes or IGHV gene families in rodents and humans. This issue was addressed in the work described here by determining the mutation frequencies of individual IGHV genes that belong to several IGHV gene families that vary in size (viz. IGHV3, IGHV4, IGHV5) and are expressed in the rat MZ-B or FO-B cells. Whether the mutated MZ-B cells in humans represent bona fide memory B cells is a matter of debate. On the one hand, these B cells are considered true memory cells, generated in GCs during antigen-driven humoral immune responses [27, 28]. On the other hand, it has been proposed that these cells are generated during TI immune responses [29]. Weller and co-workers argued that the presence of mutations reflects an intrinsic property of MZ-B cells that is exploited by these cells to diversify their primary antibody repertoire [25, 26, 30]. According to Weller and colleagues, the IgM+IgD+CD27+ MZ-B cells are not memory cells, but cells that develop in the absence of antigen, in a TI fashion, outside the GCs, along a pathway that differs from classical memory B cells. This conclusion was initially based on the finding that patients with CD40L or CD40 deficiency harbor mutated IgM+IgD+CD27+ MZ-B cells in the blood [25, 30]. These patients lack the classical cognate T-B cell collaboration that is required for the development of GCs. In support of the hypothesis of Weller, the majority of blood and splenic MZ-B cells in young children under the age of 2 years are mutated with no sign of antigen-driven clonal expansion [25, 26]. Scheeren et al. [31] also observed that a low fraction (~20%) of human fetal splenic IgM+IgD+CD27+ B cells are mutated, and these authors hypothesized that somatic hypermutation (SHM) in this population occurs mainly during foetal development and in very young children. MZ-B cells are already found early during ontogeny in the rat spleen [11, 32]. At that time, GCs are absent from spleen [33, 34]. Whether early in neonatal life the IGHV genes expressed by MZ-B cells in rodents are mutated or not is not known and was studied here. We show that, in contrast to humans, neonatal MZ-B cells in rats are all unmutated, supporting the view that, at least in rodents, a significant number of adult IgM+ mutated MZ-B cells are memory B cells that can be considered to be formed in GCs.
Materials and methods
Animals
Adult male at 4.5 months of age and pregnant BN/SsNOlaHsd rats were obtained from Harlan (Horst, The Netherlands). Rats were housed under clean, conventional conditions at the Central Animal Facility of the University Medical Center Groningen. The adult male rats were housed until the age of 9 months. Two-day-old neonatal rats of both sexes were killed by decapitation. All experiments were approved by the Animal Ethics Committee of the University of Groningen.
Isolation and purification of B cell subsets by Flow cytometry
Rat B-lymphocytes were isolated and purified from splenic tissue as described previously [11, 35]. Briefly, spleen cell suspensions from 2 adult male rats and from 5 pooled spleens of two day old neonatal rats were separately prepared and labeled for flow-cytometry with the following mouse monoclonal antibodies: FITC conjugated anti-rat IgM (HIS40; eBioscience, San Diego, CA, USA), biotinylated anti-rat IgD (MaRD3; AbD Serotec, Oxford, UK), APC conjugated anti-rat CD90/Thy1.1 (HIS51; eBioscience) and PE-conjugated anti-rat TCRαβ (R73; eBioscience); TCRγδ (V65; eBioscience), CD161a/NKRP1a (10/78; BD Pharmingen). Biotinylated mAb were revealed with streptavidin conjugated to the tandem fluorochrome PE-Cy5.5 (Ebioscience). The PE channel was used as a “dump” channel: only PE cells negative (i.e. Dump- and CD90- cells) were sorted. Herewith, we were able to exclude immature B cells (i.e. CD90+ B-cells: [11, 35], T cells and NK cells from our sorts. Cell analysis and cell sortings were performed with a MoFlo flow cytometer (Cytomation, Ft Collins, CO). Dead cell, plasma cell, monocyte/macrophage, and erythrocyte contamination was excluded from sorting by using forward and side scatter profiles. Sorted FO-B cells (CD90-IgDhighIgMlow) and MZ-B cells (CD90-IgMhighIgDlow) were collected in sterile FACS tubes (Greiner Bio-One, Alphen a/d Rijn, The Netherlands) containing 500 μl newborn calf serum (PAA laboratories GmbH, Pasching, Austria). At least one million cells per B cell subset were sorted. B cell subsets were obtained with > 95% purity. FlowJo software (Tree Star, San Carlos, CA) was used for flow cytometry data analysis.
Molecular cloning of IGHV-Cμ transcripts
Total RNA was extracted from sorted cells using the Absolutely RNA Miniprep kit (Stratagene, La Jolla, CA, USA) according to instructions of the manufacturer. Briefly, sorted cells were pelleted by 300xg centrifugation for 10 min at 4°C and then resuspended in a total volume of 350μl lysis buffer containing β-mercaptoethanol (Stratagene). First strand cDNA was synthesized using an oligo-(dT)12-18 primer (Invitrogen, Breda, The Netherlands) and SuperScriptTMII reverse transcriptase (200U/μl; Invitrogen) as described in the manufacturer’s protocol. Rearranged IGHV3-Cμ, IGHV4-Cμ IGHV5-Cμ transcripts were amplified in a 50μl reaction mixture, containing 2μl cDNA of either IGHV3-Cμ, IGHV4-Cμ or IGHV5-Cμ family-specific primer, plus 0.6 pmol/μl universal Cμ constant region primer and 2.5U Taq DNA Polymerase (Invitrogen). The IGHV gene family specific primers were: IGHV3:5'-TGAAACCCTCACAGTCACTC-3', IGHV4:5’-GGTGCARCCTGGAAGATCCT-3' and IGHV5'-CTTAGTGCAGCCTGGAAGGT-3' [10]. Individual IGHV gene family specific primers were used in separate RT-PCR reactions in combination with the constant region Cμ primer 5’-CAACACTGAAGTCATCCAGGG-3’. To assess the amount and quality of the cDNA, PCR was also performed for β-actin, using β-actin-specific primers as described by Stoel et al. [36]. The PCR program for amplification of IGHV-Cμ transcripts and β-actin consisted of 35 cycles of 30 sec at 94°C (2 min in the first cycle), 1 min at 58°C and 1 min at 72°C, respectively. This program was followed by an additional incubation period of 25 min at 72°C to allow extension of all IGHV-Cμ products. The quality and size of the PCR products were evaluated by agarose gel electrophoresis.
Cloning and sequencing
PCR products were cloned into the pJET1/blunt vectors using the GeneJETTM PCR Cloning Kit (Fermentas Life Sciences). TOP10F E. coli competent cells (Invitrogen) were transformed with plasmids containing the PCR product. Plasmid DNA was isolated from randomly picked colonies with the Nucleospin Plasmid QuickPure kit (Bioke, Leiden, The Netherlands). Size of the inserts was determined by digestion of a DNA sample of the plasmids with the appropriate restriction enzymes followed by agarose gel electrophoresis. Plasmids containing an insert of approximately 500 bp were sequenced in both directions by ServiceXS (Leiden, The Netherlands). Sequence processing was performed using EMBL/Genbank Data Libraries and Chromas software (Digital River GmbH, Cologne, Germany). IGHV-Cμ sequences displaying 100% identity and obtained from the same PCR amplification, might be derived from a single B cell and were therefore counted only once in our subsequent analysis. The presence of SHM in IGHV genes are another hallmark of memory B cells and therefore mutated IGHV-Cμ transcripts identified with >2 number of mutations in the VDJ region were considered to be IgM+ memory B cells. Mutational analysis was performed using IMGT/V QUEST database (www.imgt.org/IMGT_vquest/share/textes/). We have calculated the mutations (>2 in the VDJ region) of H-CDR and H-FR regions from IGHV-Cμ transcripts as previously reported by Hendricks et al. [21].
Statistical analysis of IGHVDJ–Cμ transcripts
Statistical analysis of the sequence data was performed using SPSS 16 software (SPSS Inc. Chicago, Ill. USA). IGHV-Cμ sequences displaying 100% identity were considered to be derived from a single B cell and counted only once for statistical analysis. Since Taq DNA polymerase errors might be responsible for 1–2 mutations per sequence, we considered only sequences with more than 2 mutations as truly mutated [10]. The number of mutations was determined by counting the number of nucleotide mismatches in comparison with each IGHV gene sequence to its closest germline counterpart. We used Fisher’s exact test to determine possible differences between groups with regard to the binary response variable indicating mutation or not. Non-parametric tests, Kruskal-Wallis and Mann-Whitney, were used to compare groups with respect to the number of mutations. In all statistical tests, a p-value < 0.05 was considered significant.
Results
Analysis of the adult rat IGHV genes in FO-B cells and MZ-B cells
Recently, we have constructed and annotated the complete genomic repertoire of the IGH locus of the BN rat [37]. The completion of the IGH locus has allowed us to analyze individual IGHV genes among different IGHV gene families by FO-B and MZ-B cells. In (Fig 1), the strategy for sorting of FO-B and MZ-B cells is illustrated. Viable lymphocytes were gated on the basis of forward-sideward scatter profiles, and non-T, non-NK cells (Dump- cells) were further analyzed. FO-B cells and MZ-B cells were subsequently defined as CD90-IgMlowIgDhigh and CD90-IgMhighIgDlow. The post sort purity for FO-B cells and MZ-B cells was >95%.
Fig 1. Four colour cytometry of FO-B cells and MZ-B cells.
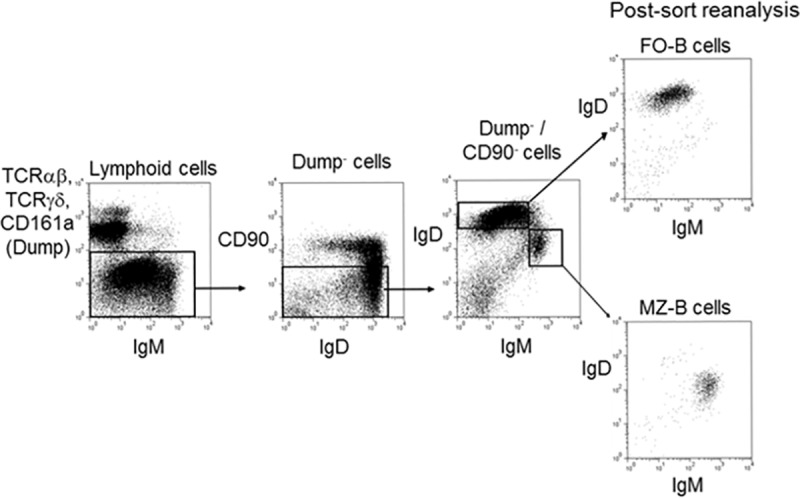
Single cell suspensions of spleen from rats were stained with FITC conjugated anti-rat IgM, biotinylated anti-rat IgD, APC conjugated anti-rat CD90/Thy1.1 and PE-conjugated anti-rat TCRαβ, TCRγδ, CD161a/NKRa. Biotinylated monoclonal antibodies were revealed with streptavidin conjugated to the tandem fluorochrome PE-Cy5.5. Viable lymphocytes were gated by forward scatter and side scatter profiles. Acquisition gates were set to exclude PE positive cells (T cells and NK cells) and CD90 positive (immature) B cells. Mature FO-B cells, defined as CD90-IgDhighIgMlow and MZ-B defined as CD90-IgMhighIgDlowwere sorted. Post sort reanalysis showed that the purity of FO-B cells and MZ-B cells was >95%.
Expressed IGHV3, IGHV4 and IGHV5 genes from adult rats were amplified, cloned and sequenced (Table 1). These IGHV gene families were chosen because of their difference in size, with respectively 4, 2 and 26 potentially functional IGHV genes in the BN rat. We obtained 19 and 17 complete IGHV3-Cμ transcripts from FO-B cells and MZ-B cells, respectively.
Table 1. Sequence analysis of IGHV3,4 and 5-Cμ transcripts expressed by FO-B cells and MZ-B cells from adult BN rat spleen.
| Clonea | IGHV | IGHD | IGHJ | Mutationsb | H-CDR3c | |
| member | member | member | Nf | Amino acids | ||
| Sequences of IGHV3 gene family from FO-Bd cells | ||||||
| A2RFV3-A4 | IGHV3S1 | IGHD1-7 | IGHJ1 | 0 | 12 | ARTTRVYWYFDF |
| A2RFV3-B2 | IGHV3S1 | IGHD4-4 | IGHJ2 | 0 | 9 | ARFVGYFDY |
| A2RFV3-B3 | IGHV3S1 | IGHD4-1 | IGHJ2 | 1 | 11 | AYSPGGYRFDY |
| A2RFV3-C2 | IGHV3S1 | IGHD1-6 | IGHJ2 | 0 | 12 | ARYGATEGIVDY |
| A2RFV3-D1 | IGHV3S1 | IGHD1-7 | IGHJ1 | 0 | 16 | ARFYDGSYYYDWYFDF |
| A2RFV3-D3 | IGHV3S1 | IGHD1-7 | IGHJ3 | 0 | 17 | ARYYGIYYYSSYDWFAY |
| A2RFV3-E1 | IGHV3S1 | IGHD1-8 | IGHJ3 | 0 | 17 | ARAGGRDSYAHVGWFAY |
| A2RFV3-E2 | IGHV3S1 | IGHD1-3 | IGHJ2 | 1 | 11 | ARLYSIAAPYY |
| A2RFV3-E3 | IGHV3S1 | IGHD4-1 | IGHJ3 | 0 | 8 | ARNPGFAY |
| A2RFV3-G3 | IGHV3S1 | IGHD3-3 | IGHJ2 | 2 | 11 | ARSGQKSLFDY |
| A2RFV3-H1 | IGHV3S1 | IGHD1-7 | IGHJ2 | 0 | 16 | ARYGDYYDGSYYAFDY |
| A2RFV3-H3 | IGHV3S1 | IGHD3-1 | IGHJ2 | 0 | 10 | ASPYPGQRWY |
| A2RFV3-A3 | IGHV3S3 | IGHD1-3 | IGHJ2 | 1 | 12 | ARSELQWYYFDY |
| A2RFV3-B4 | IGHV3S3 | IGHD5-1 | IGHJ2 | 0 | 16 | ARRPITGSGGGYYFDY |
| A2RFV3-F2 | IGHV3S3 | IGHD5-1 | IGHJ4 | 0 | 12 | ASGNWDSYVMDA |
| A2RFV3-G1 | IGHV3S3 | IGHD1-7 | IGHJ2 | 0 | 15 | ARSSYYYDGSYSLDY |
| A2RFV3-A1 | IGHV3S5 | IGHD1-5 | IGHJ3 | 0 | 10 | AGNNLDWFAY |
| A2RFV3-F3 | IGHV3S5 | IGHD1-8 | IGHJ4 | 1 | 16 | ASPLDGYYPYYYVMDA |
| Sequences of IGHV3 gene family from MZ-Be cells | ||||||
| A2MZV3-A5 | IGHV3S1 | IGHD1-1 | IGHJ2 | 0 | 9 | ARRTVSFDY |
| A2MZV3-A6 | IGHV3S1 | IGHD1-4 | IGHJ2 | 8 | 12 | ARRDPGITLFDY |
| A2MZV3-B7g | IGHV3S1 | IGHD1-3 | IGHJ2 | 0 | 13 | ARGQQLSEYYFDYC#1 |
| A2MZV3-F5g | IGHV3S1 | IGHD1-3 | IGHJ2 | 1 | 13 | ARGQQLSEYYFDYC#1 |
| A2MZV3-C5 | IGHV3S1 | IGHD1-7 | IGHJ1 | 0 | 14 | ARYDGSYYYWYFDF |
| A2MZV3-C7h | IGHV3S1 | IGHD1-4 | IGHJ2 | 1 | 12 | ARSGGYNYYFDYC#2 |
| A2MZV3-E7h | IGHV3S1 | IGHD1-4 | IGHJ2 | 0 | 12 | ARSGGYNYYFDYC#2 |
| A2MZV3-D4 | IGHV3S1 | IGHD1-6 | IGHJ3 | 5 | 10 | ARYSERGFAY |
| A2MZV3-E4 | IGHV3S1 | IGHD1-5 | IGHJ2 | 0 | 12 | ARGGIYNTYFDY |
| Clonea | IGHV | IGHD | IGHJ | Mutationsb | H-CDR3c | |
| member | member | member | Nf | Amino acids | ||
| A2MZV3-E5 | IGHV3S1 | IGHD4-1 | IGHJ2 | 7 | 13 | ARKGDSNSGLFDY |
| A2MZV3-F6 | IGHV3S1 | IGHD1-1 | IGHJ2 | 0 | 15 | ARGGVYYGLLSSFDY |
| A2MZV3-G6 | IGHV3S1 | IGHD1-7 | IGHJ2 | 0 | 13 | ARSTTVVHYYFDY |
| A2MZV3-G7 | IGHV3S1 | IGHD1-1 | IGHJ2 | 4 | 12 | ARSGYTTDYPDY |
| A2MZV3-H4 | IGHV3S1 | IGHD3-2 | IGHJ2 | 0 | 9 | ARSTDYFDY |
| A2MZV3-E6 | IGHV3S3 | IGHD3-1 | IGHJ2 | 0 | 10 | ARSGSGDFDY |
| A2MZV3-B5 | IGHV3S5 | IGHD1-5 | IGHJ4 | 4 | 12 | ARRTTSDYVMDA |
| Sequences of IGHV4 gene family from FO-Bd cells | ||||||
| A2RFV4-2i | IGHV4S2 | IGHD4-1 | IGHJ2 | 9 | 9 | VREAFGVREC#3 |
| A2RFV4-2.27 | IGHV4S2 | IGHD1-2 | IGHJ1 | 0 | 10 | GGSLYWYFDF |
| A2RFV4-5 | IGHV4S2 | No IGHD | IGHJ3 | 0 | 5 | ASRAY |
| A2RFV4-6 | IGHV4S2 | IGHD1-6 | IGHJ4 | 4 | 11 | TRAGTVLQMDA |
| A3RFV4-12 | IGHV4S2 | IGHD1-8 | IGHJ2 | 0 | 12 | ARASYYDGYGDY |
| A3RFV4-3j | IGHV4S2 | IGHD4-1 | IGHJ2 | 11 | 9 | VREAFGVDYC#4 |
| A3RFV4-3.2 | IGHV4S2 | IGHD1-4 | IGHJ3 | 0 | 13 | ARADGYNFNWFAY |
| A3RFV4-3.4 | IGHV4S2 | IGHD3-3 | IGHJ1 | 0 | 12 | ARLWRRYWYFDF |
| A3RFV4-43 | IGHV4S2 | IGHD1-5 | IGHJ2 | 1 | 12 | ARWNNYDYYFDY |
| A3RFV4-9 | IGHV4S2 | IGHD1-5 | IGHJ2 | 0 | 11 | AREDYNNIGDH |
| A2MZV4-1i | IGHV4S2 | IGHD4-1 | IGHJ2 | 9 | 9 | VREAFGVREC#3 |
| A2MZV4-10 | IGHV4S2 | IGHD1-6 | IGHJ2 | 1 | 9 | AREVGYFDY |
| A2MZV4-11 | IGHV4S2 | IGHD3-4 | IGHJ2 | 1 | 9 | TRARKSVDY |
| A2MZV4-12 | IGHV4S2 | IGHD1-1 | IGHJ3 | 2 | 6 | EGGIIG |
| A2MZV4-13 | IGHV4S2 | IGHD1-6 | IGHJ4 | 11 | 11 | ARASGQRVLDA |
| A2MZV4-14k | IGHV4S2 | IGHD4-1 | IGHJ4 | 5 | 14 | TRREFGPHYYVMDAC#5 |
| A2MZV4-3k | IGHV4S2 | IGHD4-1 | IGHJ4 | 7 | 14 | TRREFGPHYYVMDAC#5 |
| A2MZV4-2.1 | IGHV4S2 | IGHD1-1 | IGHJ3 | 9 | 12 | ARGLYYGFGFAY |
| A2MZV4-2.10 | IGHV4S2 | IGHD4-1 | IGHJ4 | 0 | 13 | ARARNSDYYVMDA |
| A2MZV4-2.11 | IGHV4S2 | IGHD4-1 | IGHJ2 | 0 | 10 | ASHERYTSDY |
| A2MZV4-2.13l | IGHV4S2 | IGHD4-2 | IGHJ2 | 11 | 9 | VREHFGVDFC#6 |
| A2MZV4-2.17l | IGHV4S2 | IGHD4-2 | IGHJ2 | 13 | 9 | VREHFGVDFC#6 |
| A2MZV4-2.14 | IGHV4S2 | IGHD4-1 | IGHJ2 | 8 | 9 | AREAFGVRE |
| A2MZV4-2.18 | IGHV4S2 | IGHD1-6 | IGHJ2 | 10 | 9 | AREEAGIDY |
| A2MZV4-2.20 | IGHV4S2 | IGHD1-6 | IGHJ4 | 5 | 9 | VREALGVNA |
| A2MZV4-2.21 | IGHV4S2 | IGHD2-2 | IGHJ2 | 9 | 9 | VREAYGVDY |
| A2MZV4-2.3 | IGHV4S2 | IGHD1-1 | IGHJ2 | 1 | 25 | AREGVYYYSSYRDVYYGLLPGYFDY |
| A2MZV4-2.4 | IGHV4S2 | IGHD1-7 | IGHJ2 | 8 | 15 | ARGYYYDGSYYHFDY |
| A2MZV4-2.7 | IGHV4S2 | IGHD1-5 | IGHJ1 | 2 | 16 | AREALITTTSYWYFDF |
| A2MZV4-2.8 | IGHV4S2 | IGHD1-6 | IGHJ4 | 16 | 9 | VREALGVDA |
| A2MZV4-7m | IGHV4S2 | IGHD1-1 | IGHJ2 | 4 | 13 | ARARGMSTTDYLYC#7 |
| Clonea | IGHV | IGHD | IGHJ | Mutationsb | H-CDR3c | |
| member | member | member | Nf | Amino acids | ||
| A2MZV4-5 | IGHV4S2 | IGHD4-2 | IGHJ2 | 6 | 9 | VREELGVDY |
| A2MZV4-8 | IGHV4S2 | IGHD5-1 | IGHJ1 | 10 | 13 | GRLSWELYWYFDF |
| A2MZV4-9 | IGHV4S2 | IGHD3-2 | IGHJ2 | 3 | 9 | VRAHSSAGD |
| A3MZV4-1 | IGHV4S2 | IGHD1-4 | IGHJ2 | 2 | 15 | ARGTSYGSSSDYFDY |
| A3MZV4-10 | IGHV4S2 | IGHD1-3 | IGHJ2 | 0 | 15 | ARALDYYSSYIYLDY |
| A3MZV4-35 | IGHV4S2 | IGHD1-6 | IGHJ2 | 13 | 12 | ARGDYYRGDFDY |
| A3MZV4-11n | IGHV4S2 | IGHD1-6 | IGHJ2 | 11 | 9 | VREHLGVDYC#8 |
| A3MZV4-3.1n | IGHV4S2 | IGHD1-6 | IGHJ2 | 11 | 9 | VREHLGVDYC#8 |
| A3MZV4-36 | IGHV4S2 | IGHD1-3 | IGHJ2 | 0 | 11 | AREDYSGDFDY |
| A3MZV4-14 | IGHV4S2 | IGHD1-8 | IGHJ2 | 5 | 10 | SGGLGWIFDY |
| A3MZV4-15 | IGHV4S2 | IGHD1-1 | IGHJ4 | 0 | 17 | ARVLFMYTTDYQGVMDA |
| A3MZV4-16 | IGHV4S2 | IGHD4-1 | IGHJ2 | 19 | 9 | VREDFGVDY |
| A3MZV4-17 | IGHV4S2 | IGHD5-1 | IGHJ2 | 0 | 11 | ARARETGNFDY |
| A3MZV4-18 | IGHV4S2 | IGHD1-2 | IGHJ2 | 9 | 15 | TRGPSYGSDSDFFDY |
| A3MZV4-19 | IGHV4S2 | IGHD1-4 | IGHJ2 | 8 | 15 | ARGTSYGSNSDYFDY |
| A3MZV4-20 | IGHV4S2 | IGHD4-1 | IGHJ2 | 8 | 9 | AREAFGVDY |
| A3MZV4-20Bo | IGHV4S2 | IGHD1-4 | IGHJ2 | 14 | 10 | AKSGPGIIEYC#9 |
| A3MZV4-7o | IGHV4S2 | IGHD1-4 | IGHJ2 | 13 | 10 | AKSGPGIIEYC#9 |
| A3MZV4-22 | IGHV4S2 | IGHD4-1 | IGHJ2 | 9 | 9 | IREAFGVDY |
| A3MZV4-37 | IGHV4S2 | IGHD3-2 | IGHJ1 | 15 | 14 | AGLRSGAPYWYLDF |
| A3MZV4-23 | IGHV4S2 | IGHD1-6 | IGHJ3 | 9 | 12 | ARELSTGEWFAY |
| A3MZV4-29 | IGHV4S2 | IGHD1-7 | IGHJ2 | 2 | 14 | ARSLMVVISHYFDY |
| A3MZV4-3 | IGHV4S2 | IGHD1-6 | IGHJ4 | 0 | 10 | ARRRSDVMDA |
| A3MZV4-3.12 | IGHV4S2 | IGHD1-6 | IGHJ4 | 0 | 14 | ARVGDSSYYYVMDA |
| A3MZV4-3.14 | IGHV4S2 | IGHD1-6 | IGHJ3 | 1 | 11 | VRERSTEGFAY |
| A3MZV4-3.2j | IGHV4S2 | IGHD4-1 | IGHJ2 | 14 | 9 | VREAFGVDYC#4 |
| A3MZV4-3.5 | IGHV4S2 | IGHD4-1 | IGHJ2 | 12 | 9 | VREDLGVDY |
| A3MZV4-30 | IGHV4S2 | IGHD2-2 | IGHJ2 | 14 | 9 | AREIPPVDY |
| A3MZV4-31 | IGHV4S2 | IGHD1-4 | IGHJ4 | 11 | 11 | ARAVISRVLDA |
| A3MZV4-32 | IGHV4S2 | IGHD4-1 | IGHJ2 | 6 | 9 | VREEFGVDY |
| A3MZV4-5N | IGHV4S2 | IGHD1-6 | IGHJ2 | 11 | 9 | VREQRGVDYC15 |
| A3MZV4-5A | IGHV4S2 | IGHD1-2 | IGHJ2 | 8 | 15 | TRGPSYGSDSDYFDY |
| A3MZV4-9 | IGHV4S2 | IGHD1-5 | IGHJ2 | 0 | 11 | ARADNNSGFDY |
| Sequences of IGHV5 genes from FO-Bd cells | ||||||
| A2RFV5-39 | IGHV5S16 | IGHD1-1 | IGHJ3 | 0 | 12 | ARPNYYSGPLAY |
| A3RFV5-11 | IGHV5S13 | IGHD1-2 | IGHJ2 | 0 | 9 | ARRAMGFDY |
| A2RFV5-42p | IGHV5-1 | IGHD1-6 | IGHJ2 | 17 | 9 | TKGVGGPDYC#10 |
| A2RFV5-46 | IGHV5S10 | IGHD5-1 | IGHJ2 | 0 | 9 | ATHLGYFDY |
| A2RFV5-38 | IGHV5S14 | IGHD1-1 | IGHJ2 | 1 | 12 | VRLCGERDYFDY |
| Clonea | IGHV | IGHD | IGHJ | Mutationsb | H-CDR3c | |
| member | member | member | Nf | Amino acids | ||
| A2RFV5-45 | IGHV5S14 | IGHD1-6 | IGHJ1 | 1 | 16 | ARHVPLHYGGHGYFDF |
| A3RFV5-14N | IGHV5S14 | IGHD1-2 | IGHJ2 | 0 | 8 | ARRDDFDY |
| A3RFV5-48 | IGHV5S14 | IGHD1-6 | IGHJ1 | 0 | 17 | ARLPAYYGGYSELPFAY |
| A3RFV5-5 | IGHV5S14 | IGHD1-1 | IGHJ2 | 0 | 18 | ARHLMYTTDYYYPGAFDY |
| A2RFV5-17 | IGHV5S23 | IGHD1-4 | IGHJ3 | 4 | 14 | ARGDYPGITGWFAY |
| A2RFV5-19 | IGHV5S23 | IGHD1-4 | IGHJ2 | 0 | 6 | ARPYSV |
| A3RFV5-12 | IGHV5S23 | IGHD1-8 | IGHJ1 | 1 | 20 | ARPPRWDYDGYYHVGWYFDF |
| A3RFV5-15 | IGHV5S23 | IGHD1-1 | IGHJ4 | 6 | 17 | ARSLMYTTDYYYGVMDA |
| A3RFV5-8 | IGHV5S23 | IGHD1-7 | IGHJ2 | 4 | 12 | ARGDDGSYYFDY |
| A3RFV5-3 | IGHV5S27 | IGHD1-4 | IGHJ2 | 4 | 12 | ARRPPGYNPFDY |
| A3RFV5-45 | IGHV5S27 | IGHD1-7 | IGHJ3 | 0 | 20 | ARHGADGAMMVVITNGWFAY |
| A3RFV5-47 | IGHV5S29 | IGHD2-3 | IGHJ2 | 2 | 10 | TTDRLSTFDY |
| A2RFV5-22 | IGHV5S30 | IGHD1-1 | IGHJ3 | 4 | 17 | ARHMYTTDYYHGDWFAY |
| A2RFV5-23 | IGHV5S30 | IGHD1-4 | IGHJ3 | 2 | 14 | ATRPLPGYNYGFAY |
| A2RFV5-35 | IGHV5S30 | IGHD1-8 | IGHJ3 | 11 | 9 | ARQDQEFAY |
| A2RFV5-37 | IGHV5S30 | IGHD1-7 | IGHJ2 | 4 | 13 | ARLDYYDGSYYDY |
| A2RFV5-42 | IGHV5S30 | IGHD4-1 | IGHJ2 | 0 | 9 | ATVAGYFDY |
| A2RFV5-8 | IGHV5S30 | IGHD1-3 | IGHJ2 | 0 | 8 | ATLLYSGH |
| A3RFV5-13N | IGHV5S30 | IGHD1-1 | IGHJ3 | 0 | 16 | ATDSPTTDYYSNWFAY |
| A3RFV5-2 | IGHV5S30 | IGHD1-6 | IGHJ3 | 0 | 17 | ATDTDYGGYSELGGFAY |
| A3RFV5-4 | IGHV5S43 | IGHD4-2 | IGHJ3 | 1 | 13 | TRDRGYSSHWFAY |
| A3RFV5-46 | IGHV5S43 | IGHD1-3 | IGHJ4 | 0 | 14 | TREPGDYSSYVMDA |
| A3RFV5-13 | IGHV5S43 | IGHD1-7 | IGHJ2 | 2 | 13 | TRVGHYYSSYFDY |
| A2RFV5-18 | IGHV5S45 | IGHD1-1 | IGHJ2 | 2 | 12 | ARRYTTDYWFDY |
| A2RFV5-41 | IGHV5S45 | IGHD1-2 | IGHJ2 | 0 | 10 | ARPPYGAFDY |
| A3RFV5-43 | IGHV5S45 | IGHD1-1 | IGHJ3 | 4 | 24 | TTGAYSSYAVMYTTDYYYAGWFAY |
| A3RFV5-42 | IGHV5S45 | IGHD2-2 | IGHJ1 | 2 | 12 | ARRDTLYWYFDF |
| A2RFV5-47 | IGHV5S57 | IGHD3-3 | IGHJ1 | 1 | 15 | TRASSSYVSDWYFDF |
| A3RFV5-44 | IGHV5S57 | IGHD1-2 | IGHJ2 | 0 | 11 | TRTRVSYYFDY |
| A2RFV5-43 | IGHV5S65 | IGHD1-5 | IGHJ2 | 1 | 13 | AKDQGNNYGYFDY |
| A3RFV5-2 | IGHV5S74 | IGHD5-1 | IGHJ2 | 2 | 11 | ARGHGDYYFDY |
| Sequences of IGHV5 genes from MZ-Be cells | ||||||
| A2MZV5-37p | IGHV5-1 | IGHD1-6 | IGHJ2 | 13 | 9 | TKGVGGPDYC#10 |
| A2MZV5-39 | IGHV5S10 | IGHD4-1 | IGHJ2 | 0 | 11 | ATHPGEYYFDY |
| A3MZV5-4 | IGHV5-1 | IGHD1-6 | IGHJ2 | 16 | 9 | AKGVGGPDY |
| A2MZV5-2.5 | IGHV5S13 | IGHD1-1 | IGHJ2 | 0 | 14 | ARFGLITVAVHFDY |
| A2MZV5-20 | IGHV5S13 | IGHD1-6 | IGHJ1 | 1 | 18 | ARTTGLTTEGIGYWYFDF |
| A2MZV5-38 | IGHV5S13 | IGHD1-1 | IGHJ3 | 4 | 7 | GYYGFAY |
| A3MZV5-16 | IGHV5S13 | IGHD1-6 | IGHJ3 | 2 | 14 | ARHETTVVTGWFAY |
| Clonea | IGHV | IGHD | IGHJ | Mutationsb | H-CDR3c | |
| member | member | member | Nf | Amino acids | ||
| A3MZV5-38 | IGHV5S13 | IGHD1-1 | IGHJ2 | 1 | 11 | ASIITTGYFDY |
| A3MZV5-66 | IGHV5S13 | IGHD1-3 | IGHJ1 | 1 | 12 | ASQSSYNWYFDF |
| A2MZV5-11 | IGHV5S14 | IGHD1-1 | IGHJ2 | 0 | 9 | ARRLLQWDY |
| A2MZV5-33 | IGHV5S14 | IGHD1-4 | IGHJ2 | 1 | 17 | ARGGINNIGTTRGVMDA |
| A3MZV5-3.8 | IGHV5S14 | IGHD1-7 | IGHJ2 | 0 | 12 | ARYYYDGPWGDY |
| A3MZV5-5 | IGHV5S14 | IGHD1-7 | IGHJ2 | 2 | 15 | ARTGFYYYSGDYFDY |
| A3MZV5-8 | IGHV5S14 | IGHD1-7 | IGHJ2 | 1 | 13 | ARHYYDGSYYFDY |
| A2MZV5-14 | IGHV5S16 | IGHD5-1 | IGHJ2 | 4 | 7 | TTDLNNY |
| A2MZV5-19 | IGHV5S16 | IGHD1-8 | IGHJ1 | 0 | 11 | ATCSPYWYFDF |
| A2MZV5-22 | IGHV5S16 | IGHD1-7 | IGHJ4 | 4 | 11 | ATDEGGGVMDA |
| A3MZV5-13 | IGHV5S16 | IGHD1-6 | IGHJ3 | 4 | 12 | TTLYGGPPWFAY |
| A2MZV5-21 | IGHV5S23 | IGHD1-2 | IGHJ1 | 5 | 15 | ARQSTYYEDGWYFDF |
| A3MZV5-3.3 | IGHV5S23 | IGHD1-2 | IGHJ3 | 4 | 14 | ATEGTMGMSDWFAY |
| A2MZV5-23 | IGHV5S27 | IGHD1-4 | IGHJ3 | 0 | 13 | ARPYGYNYRWFAY |
| A3MZV5-61 | IGHV5S29 | IGHD1-6 | IGHJ3 | 0 | 12 | TTDRGNYGWFAY |
| A3MZV5-65 | IGHV5S29 | IGHD1-7 | IGHJ2 | 1 | 13 | TSPLTTVVPYFDY |
| A2MZV5-18 | IGHV5S30 | IGHD1-5 | IGHJ2 | 1 | 13 | ARHDNNYVAYFDY |
| A2MZV5-2.2 | IGHV5S30 | IGHD1-3 | IGHJ2 | 2 | 17 | ATDQYYSSYTLAGYFDY |
| A3MZV5.57 | IGHV5S30 | IGHD1-3 | IGHJ3 | 1 | 15 | ATDRAYRSYIPTFAY |
| A3MZV5-10 | IGHV5S30 | IGHD1-6 | IGHJ1 | 0 | 12 | ATEIDSDWYFDF |
| A3MZV5-14 | IGHV5S30 | IGHD1-8 | IGHJ2 | 0 | 6 | ATLSYY |
| A3MZV5-17 | IGHV5S30 | IGHD1-7 | IGHJ2 | 5 | 15 | AKMWGGSYYYVPFDY |
| A3MZV5-2N | IGHV5S30 | IGHD4-1 | IGHJ2 | 0 | 6 | ATDSSG |
| A3MZV5-24 | IGHV5S30 | IGHD5-1 | IGHJ3 | 0 | 11 | ATDDQLDWFAY |
| A3MZV5-25 | IGHV5S30 | IGHD4-1 | IGHJ3 | 11 | 11 | AHNAGDVWFPY |
| A3MZV5-26 | IGHV5S30 | IGHD1-3 | IGHJ2 | 0 | 13 | ATGVHYSSYIFDY |
| A3MZV5-3.11 | IGHV5S30 | IGHD1-2 | IGHJ2 | 2 | 10 | ATQLGGSFDY |
| A3MZV5-3.4 | IGHV5S30 | IGHD1-8 | IGHJ2 | 1 | 12 | ATGDYYDGYPDY |
| A3MZV5-3.6 | IGHV5S30 | IGHD1-7 | IGHJ2 | 0 | 13 | ATDRSDDGGFFDY |
| A3MZV5-3.7 | IGHV5S30 | IGHD1-1 | IGHJ2 | 0 | 12 | ATDHVYYGLLGA |
| A3MZV5-33 | IGHV5S30 | IGHD1-1 | IGHJ3 | 0 | 14 | ATAGDTTDYSRFAY |
| A3MZV5-39 | IGHV5S30 | IGHD1-6 | IGHJ2 | 0 | 11 | ARGINYGGYAH |
| A3MZV5-6 | IGHV5S30 | IGHD1-1 | IGHJ3 | 2 | 14 | ATEVYYGLSDWFAY |
| A3MZV5-63 | IGHV5S30 | IGHD1-4 | IGHJ2 | 1 | 11 | ATDEAGDTGDY |
| A3MZV5-8 | IGHV5S30 | IGHD1-5 | IGHJ2 | 0 | 12 | ATAFITTTGFDY |
| A2MZV5-4 | IGHV5S30 | IGHD1-7 | IGHJ3 | 0 | 13 | ATDGGYAPRWFAY |
| A2MZV5-25 | IGHV5S45 | IGHD1-4 | IGHJ2 | 2 | 10 | TTGDMGITPY |
| A2MZV5-35 | IGHV5S45 | IGHD1-2 | IGHJ4 | 2 | 11 | ARQGDYGPMDA |
| A2MZV5-9 | IGHV5S45 | IGHD4-2 | IGHJ1 | 1 | 13 | ARRGGSAYWYFDF |
| Clonea | IGHV | IGHD | IGHJ | Mutationsb | H-CDR3c | |
| member | member | member | Nf | Amino acids | ||
| A3MZV5-11 | IGHV5S32 | IGHD1-1 | IGHJ4 | 4 | 17 | ATDGAFTTNYFYDVMAA |
| A3MZV5-12 | IGHV5S32 | IGHD1-6 | IGHJ2 | 1 | 12 | ARQGYGGYPFDY |
| A2MZV5-36 | IGHV5S36 | IGHD1-6 | IGHJ2 | 7 | 11 | TTEVLQWVFDY |
| A2MZV5-40 | IGHV5S36 | IGHD1-3 | IGHJ3 | 1 | 12 | TTGTIAANWFAY |
| A3MZV5-21 | IGHV5S36 | IGHD1-2 | IGHJ2 | 6 | 7 | ATGLGDY |
| A2MZV5-12 | IGHV5S43 | IGHD1-4 | IGHJ2 | 0 | 13 | TREGPYGYNYFDY |
| A3MZV5-3.15 | IGHV5S43 | IGHD1-1 | IGHJ4 | 14 | 10 | TIYSNYVMDA |
| A3MZV5-6 | IGHV5S43 | IGHD1-6 | IGHJ3 | 0 | 9 | TRGTTEAAY |
| A3MZV5-10 | IGHV5S65 | IGHD1-2 | IGHJ2 | 0 | 9 | AKESTMGMG |
| A3MZV5-36 | IGHV5S65 | IGHD1-1 | IGHJ2 | 19 | 7 | AINKYNY |
| A3MZV5-7 | IGHV5S65 | IGHD1-2 | IGHJ2 | 6 | 13 | AKDSYGGYRYFDY |
aCμ (IgM) transcripts from FO-B cells and MZ-B cells
bMutations, nucleotide differences between IMGT germline gene and rearranged Cμ transcript
cH-CDR3, heavy chain complementarity determining region 3
dFO-B cells, recirculating follicular B cells
eMZ-B cells, marginal zone B cells
fLenght of H-CDR3 in amino acids
gThe sequence A2MZV3-B7 and A2MZV3-F5 are from clonally related B cells and designated as clone set C#1
hThe sequence A2MZV3-C7 and A2MZV3-E7 are from clonally related B cells and designated as clone set C#2
iThe sequences A2RFV4-2 and A2MZV4-1 are from clonally related B cells and designated as clone set C#3
jThe sequences A3RFV4-3 and A3MZV4-3.2 are from clonally related B cells and designated as clone set C#4
kThe sequences A2MZV4-14 and A2MZV4-3 are from clonally related B cells and designated as clone set C#5
lThe sequences A2MZV4-2.13 and A2MZV4-2.17 are from clonally related B cells and designated as clone set C#6
mThe sequences A2MZV4-2.9 and A2MZV4-7 are from clonally related B cells and designated as clone set C#7
nThe sequences A3MZV4-11, A3MZV4-3.1 and A3MZV4-9 are from clonally related B cells and designated as clone set C#8
oThe sequences A3MZV4-20B and A3MZV4-7 are from clonally related B cells and designated as clone set C#9
pThe sequences A2RFV5-42 and A2MZV5-37 are from clonally related B cells and designated as clone set C#10
Three of the four IGHV3 germline genes were expressed as productive genes in both B cell subsets, i.e. IGHV3S1, IGHV3S3 and IGHV3S5 (Table 1). From the IGHV4 gene family we were able to successfully amplify only one of the two potentially functional genes (viz. IGHV4S2). In total, we obtained 12 IGHV4-Cμ transcripts from FO-B cells and 59 IGHV4-Cμ transcripts from MZ-B cells (Table 1). From the second largest IGHV gene family in the BN rat, the IGHV5 gene family (also called the PC7183 family), 16 different, out of 26 potentially functional IGHV5 genes were found among 40 and 61 IGHV5-Cμ transcripts (see Table 1) that were amplified from FO-B cells and MZ-B cells, respectively.
MZ-B cells express more mutated IGHV-Cμ transcripts than FO-B cells
We subsequently analyzed the obtained IGHV-Cμ transcripts that were amplified from both B cell subpopulations. The number of mutations within each rearranged IGHV gene was assessed on the basis of the nucleotide identity to the closest corresponding germline of the IGHV gene counterparts. Sequences with only 1 or 2 mutations were considered to be germline because we cannot exclude the possibility that these differences compared to germline IGHV genes were due to PCR artifacts [10]. We first analyzed the proportion of mutated sequences among the combined IGHV3, IGHV4 and IGHV5 sequences. As we show in Fig 2, 18% of FO-B cells and 45% of MZ-B cells expressed mutated IGHV-Cμ transcripts. This percentage of mutated sequences is significantly higher within the MZ-B cell subset compared to the percentage of mutated sequences present within the FO-B cell subset (Fisher’s exact test: P < 0,001).
Fig 2. Percentage of mutated IgM+ FO-B cells and MZ-B cells within different IGHV gene families.
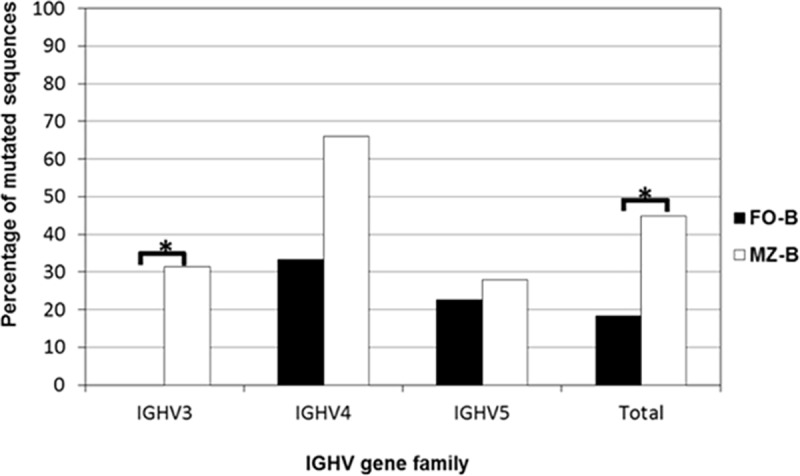
Analysis of the proportion of mutated sequences (>2 mutations compared to the closest germline gene) shows that MZ-B cells express more mutated sequences than FO-B cells, when all sequences from the three IGHV gene families are combined (i.e. “total”) (Fisher’s exact test: P < 0.001). This difference between MZ-B cells and FO-B cells was largely due to a significant difference in the percentage of mutated sequences within the IGHV3 family (Fisher’s exact test: P = 0.016). There are relatively more mutated sequences found in the IGHV4 gene family compared to IGHV3 and IGHV5 both for the MZ-B cells (Fisher’s exact test: P < 0.001) and FO-B cells (Fisher’s exact test: P = 0.023).
The difference in frequency of mutated sequences between MZ-B cells and FO-B cells is largely due to the IGHV3 gene family
We subsequently analyzed whether this difference in percentage of mutated sequences between FO-B cells and MZ-B cells was present in all three IGHV families tested. There was no statistical difference in percentage mutated sequences between the two B cell subsets among sequences of the IGHV4 and IGHV5 gene families, albeit that there was a strong trend with a higher percentage of MZ-B cells expressing mutated IGHV4 genes present compared to the percentage of mutated IGHV4 genes of FO-B cells (Fisher’s exact test: P = 0,051) (Fig 2). In contrast, within the IGHV3 gene family, 31% of the sequences derived from MZ-B cells were mutated, whereas none of IGHV3 sequences derived from FO-B cells were mutated (Fisher’s exact test: P = 0,016) (Fig 2). Thus, the higher frequency of MZ-B cells expressing mutated IGHV sequences is largely due to the contribution of the IGHV3 gene family.
The IGHV4 gene family contains the highest percentage of mutated IGHV-Cμ transcripts, both among MZ-B cells and among FO-B cells
We analyzed whether there was a difference in the percentage of mutated IGHV-Cμ transcripts between the three IGHV gene families (IGHV3, IGHV4, IGHV5) in the two B cell subsets. As we show in Fig 2, within both MZ-B cells and FO-B cells, the IGHV4 gene family contained a significantly higher proportion of mutated IGHV-Cμ transcripts, compared to the two other IGHV gene families (IGHV3 and IGHV5) (Fisher’s exact test: P = 0,023 and P < 0,001, respectively). Of the IGHV4 sequences two-third of the MZ-B cell-derived sequences and one-third of the FO-B cell sequences were mutated. Thus, based upon these findings we conclude that there is a significant difference in the percentage of mutated sequences between the various IGHV gene families, for both MZ-B cells and FO-B cells.
Mutation frequency among the mutated IGHV-Cμ transcripts is higher in MZ-B cells compared to FO-B cells
We next compared the number of mutations among the mutated IGHV-Cμ transcripts (i.e. > 2 mutations per transcript) IGHV genes in FO-B cells and MZ-B cells. When taking all mutated sequences from the three families together, MZ-B cells have a significantly (Mann-Whitney, P = 0.046) higher number of mutations than FO-B cells (Fig 3). In MZ-B cells, the number of mutations is 8.8±4.0 (median 8) and in FO B cells 6.8±3.9 (median 4). Further analysis revealed that the mutation frequency of mutated IGHV-Cμ sequences from MZ-B cells is significantly higher among IGHV4 sequences than among IGHV3 or IGHV5 sequences (Kruskal-Wallis, P = 0.011). These results indicate that the number of mutations in mutated MZ-B cells is higher in comparison to FO-B cells, which appears to be largely due to the higher number of mutations among the IGHV4 sequences (Fig 3).
Fig 3. Mutation frequency of IGHV-Cμ transcripts of different IGHV families within MZ-B cells and FO-B cells.
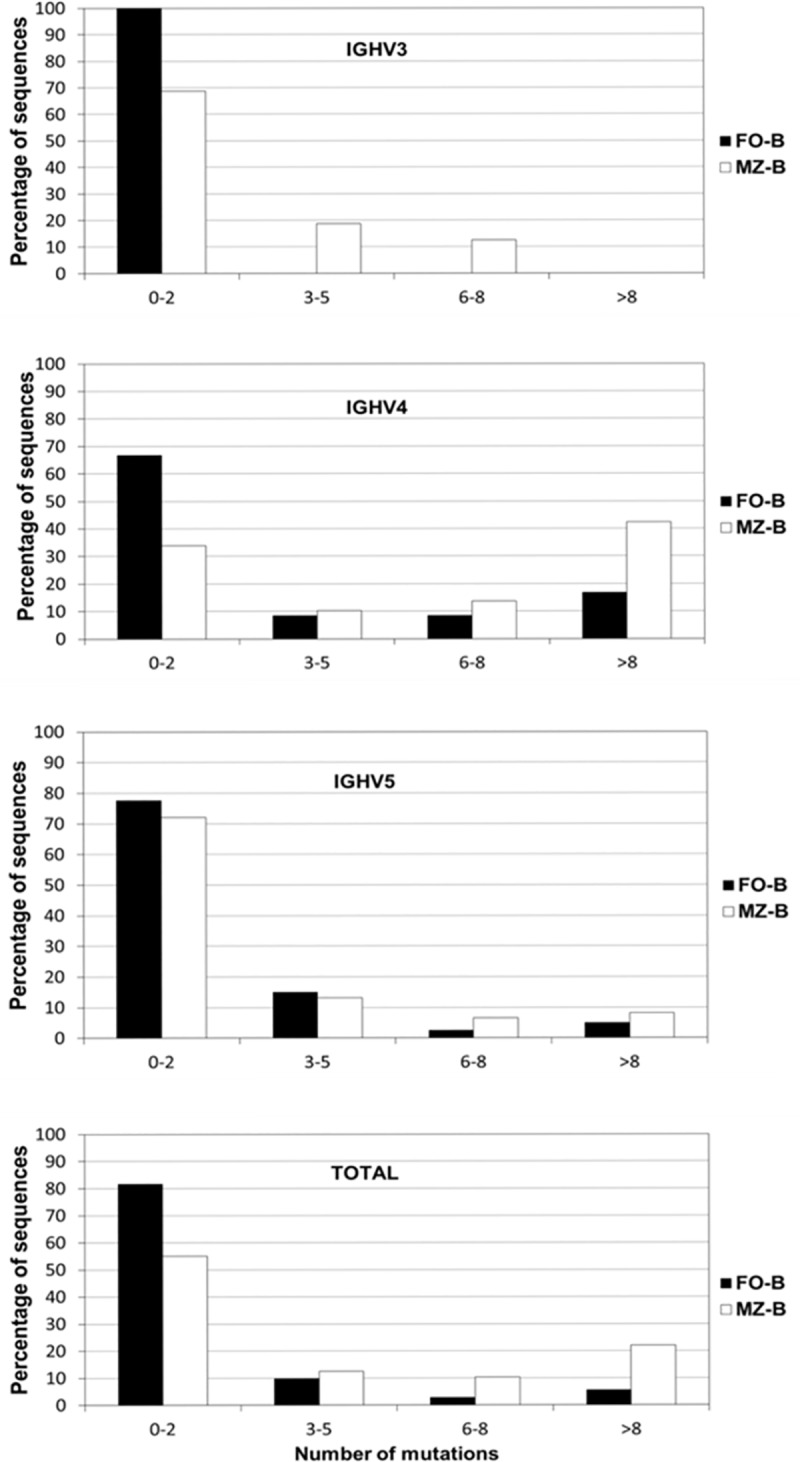
Analysis of the distribution of the number of mutations of all (“total”) IGH-Cμ transcripts shows that MZ-B cells have significantly more mutations per transcript than FO-B cells (Mann-Whitney P = 0.046). Within the MZ-B cell subset, IGHV4 sequences contained more mutations than in IGHV3 or IGHV5 sequences (Kruskal-Wallis, P = 0.011).
Clones of B cells are found within the MZ-B cell subset and some of these clones have members that are also present within the FO-B cell subset
H-CDR3 regions can be used to assess clonal relationships between B cells because the H-CDR3 region is virtually unique for each different IGHV rearrangement. A total of 10 independent clone sets (designated as C#1-C#10) were found among the two B cell subsets. Seven clone sets (2–3 members per clone) had members found exclusively found within the MZ-B cell subset and the remaining three clone sets had shared members between the MZ-B cell subset and the FO-B cell subset (see Table 1). The sequences that belonged to clonally related cells with 2 or 3 members only found in the MZ-B cell subset included clone sets using IGHV3: clone set C#1 (A2MZV3-B7, A2MZV3-F5), clone set C#2 (A2MZV3-C6, A2MZV3-C7, A2MZV3-E7), clone set C#8 (A3MZV4-11, A3MZV4-3.1, A3MZV4-9) and clone set C#9 (A3MZV4-20B, A3MZV4-7) or clone sets using IGHV4: clone set C#5 (IGA2MZV4-14, A2MZV4-3), clone set C#6 (A2MZV4-2.13, A2MZV4-2.17) and clone set C#7 (A2MZV4-2.9, A2MZV4-7). IGHV genes used by members of clone sets C#1 and C#2 had none or only one mutation in their IGHV genes. Members from other clone sets (C#5,6,8 and 9) exhibited more mutations (more than 6 mutations per IGHV sequence); most of these mutations were shared between the members of a clone. Sequences from three clone sets C#3 (A2RFV4-2, A2MZV4-1), C#10 (A2RFV5-42, A2MZV5-37) and C#4 (A3RFV4-3, A3MZV4) have members found in both B cell subsets. Clone sets C#3 and C#10 exhibited an identical mutation pattern, while clone set C#4 showed many shared mutations. Overall, most of these clonally related sequences thus displayed both shared mutations in combination with unique mutations, indicating that members from one clone set were probably derived from the same naive precursor cell.
Analysis of the neonatal IGHV genes in FO-B cells and MZ-B cells
Weller et al and others postulated that mutations in MZ-B cells are not the consequence of an antigen-driven response, but are an intrinsic property of these B cells, introduced during their development [25, 26]. In this study we also addressed this issue, by analysing the occurrence of SHM in IGHV genes, expressed by MZ-B cells present in neonatal rats, i.e. at a time point when antigen-driven humoral immune responses have not been established, as witnessed by the absence of GC in lymphoid organs during the first few weeks of life [33, 34]. MZ-B cells and FO-B cells were sorted from (pooled) neonatal rat spleens, as is illustrated in Fig 4. A large proportion of MZ-B cells in neonatal rats express CD90 and are therefore considered to represent immature MZ-B cells [10]; mature MZ-B cells and FO-B cells are defined as CD90-IgMhighIgDlow cells and CD90-IgMlowIgDhigh respectively. By including CD90 in our staining combination, we excluded immature MZ-B and FO-B cells from our analysis.
Fig 4. Three-colour cytometry was used to analyze FO-B cells and MZ-B cells.
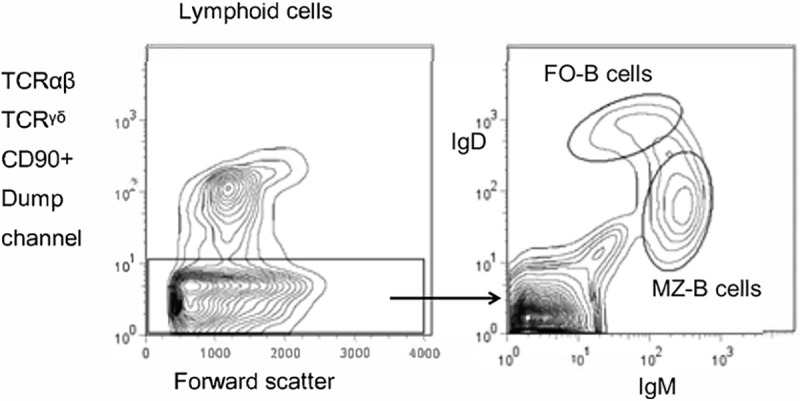
A single neonatal rat splenic cell suspension was stained with FITC conjugated anti-rat IgM (HIS40; eBioscience, San Diego, CA, USA), biotinylated anti-rat IgD (MaRD3; AbD Serotec, Oxford, UK), and APC anti-rat CD90/Thy1.1 (HIS51; eBioscience). Biotinylated mAb were revealed with streptavidin conjugated to the tandem fluorochrome PE-Cy5.5 (Ebioscience). Lymphocytes were sequentially gated by forward scatter and side scatter. Acquisition gates were set to exclude the unwanted immature B cells (CD90+-APC). Gate settings were set appropriately for FO-B cells (CD90negIgDhighIgMlow) and MZ-B (CD90negIgMhighIgDlow) cells.
In the analysis of adult IGHV genes, we showed that the proportion of mutated IgM+ MZ-B cells varied significantly when different IGHV gene families were analyzed. This proportion of mutated MZ-B cell-derived sequences occurred up to 28% for IGHV5 transcripts and up to 66% for IGHV4 transcripts in adult rats. For this reason, we chose to analyze IGHV-Cμ transcripts encoding for IGHV5 and IGHV4 family genes. IGHV-Cμ mRNA transcripts from both neonatal FO-B cells and MZ-B cells were amplified by RT-PCR, cloned, sequenced and analyzed for the presence of SHM. The IGHV5 gene family is the second largest IGHV gene family in the rat and consists of 27 potentially functional IGHV genes [37]. In total, we amplified 16 unique IGHV5-Cμ transcripts from neonatal MZ-B cells and 22 IGHV5-Cμ transcripts from neonatal FO-B cells (Table 2). Various germline genes encoded these transcripts: 10 different IGHV5 germline genes were used by MZ-B cells and 12 by FO-B cells. The IGHV4 gene family is composed of only 2 potentially functional IGHV genes [37], of which only one (IGHV4S2) seemed to be expressed. We obtained 21 unique IGHV4-Cμ sequences derived from neonatal MZ-B cells and nine IGHV4-Cμ sequences from neonatal FO-B cells (Table 2). The IGHV4S2 gene encoded all these transcripts, confirming our previous findings in adult rats, that only this family member is functionally expressed by rat B cells. As shown in (Table 2), the mutational analysis of the IGHV5-Cμ and IGHV4-Cμ transcripts revealed that none of the transcripts from either MZ-B cells or FO-B cells were mutated (i.e. expressed more than two nucleotide differences compared to their germline counterparts). Since GC are absent in the first few weeks of neonatal rats [34, 38], the finding that mutated IgM+ memory B cells are absent in neonatal animals supports the hypothesis that IgM+ memory B cells are generated in the GCs. The absence of mutation profiles of IGHV genes in neonatal rats therefore revealed that mutated IgM MZ-B cells are possibly generated in an antigen GC-dependent process in rats as opposed to the GC-independent process proposed by Weller et al. for humans [25].
Table 2. Neonatal IGHV Cμ mRNA transcripts from FO-B cells and MZ-B cells.
| Clonea | IGHV | IGHD | IGHJ | Mutationsb | H-CDR3c | |
| member | member | member | Nf | Amino acid | ||
| Sequences of IGHV4 gene family from FO-Bd cells | ||||||
| NRFVH4-60 | IGHV4S2 | IGHD5-1 | IGHJ2 | 0 | 7 | ATGSFDY |
| NRFVH4-71g | IGHV4S2 | IGHD1-6 | IGHJ2 | 1 | 9 | ARAPGGYDY C#1 |
| NRFVH4-16 | IGHV4S2 | IGHD1-1 | IGHJ2 | 0 | 14 | ARESYYYYSGDFDY |
| NRFVH4-37g | IGHV4S2 | IGHD1-6 | IGHJ2 | 1 | 9 | ARAPGGYDYC#1 |
| NRFVH4-2.1 | IGHV4S2 | IGHD1-6 | IGHJ2 | 0 | 11 | ARAGGYYYFDY |
| NRFVH4-2.4 | IGHV4S2 | IGHD1-2 | IGHJ2 | 0 | 10 | ARVLWVYFDY |
| Clonea | IGHV | IGHD | IGHJ | Mutationsb | H-CDR3c | |
| member | member | member | Nf | Amino acids | ||
| NRFVH4-2.5 | IGHV4S2 | IGHD1-4 | IGHJ2 | 0 | 12 | ARAYYGYNYFDY |
| NRFVH4-2.6 | IGHV4S2 | IGHD1-4 | IGHJ2 | 0 | 11 | ARYYGYNYFDY |
| NRFVH4-2.7 | IGHV4S2 | IGHD1-4 | IGHJ2 | 0 | 12 | ATYYGYNYYFDY |
| Sequences of IGHV4 gene family from MZ-Be cells | ||||||
| NMZVH4-1 | IGHV4S2 | IGHD1-1 | IGHJ1 | 0 | 15 | AIMYTTDYXYWYFDF |
| NMZVH4-3 | IGHV4S2 | IGHD1-7 | IGHJ2 | 0 | 11 | ARAYYDGSYYY |
| NMZVH4-4 | IGHV4S2 | IGHD1-1 | IGHJ4 | 0 | 16 | ARAHMYTTDYYYVMDA |
| NMZVH4-5 | IGHV4S2 | IGHD1-5 | IGHJ1 | 0 | 10 | AIYNNWYFDF |
| NMZVH4-6 | IGHV4S2 | IGHD1-4 | IGHJ2 | 0 | 10 | ARLPGYNFDY |
| NMZVH4-9 | IGHV4S2 | IGHD2-2 | IGHJ2 | 0 | 9 | ARDTYYFDY |
| NMZVH4-13 | IGHV4S2 | IGHD1-3 | IGHJ1 | 0 | 12 | ARARSSYWYFDF |
| NMZVH4-17 | IGHV4S2 | IGHD1-7 | IGHJ2 | 0 | 12 | ARNYPGMYYFDY |
| NMZVH4-20 | IGHV4S2 | IGHD1-6 | IGHJ2 | 0 | 8 | ARTEGIDY |
| NMZVH4-3.1 | IGHV4S2 | IGHD3-2 | IGHJ2 | 0 | 10 | ARARYNYFDY |
| NMZVH4-3.2h | IGHV4S2 | IGHD1-7 | IGHJ1 | 1 | 16 | ARFYYDGSYYYWYFDFC#2 |
| NMZVH4-3.3 | IGHV4S2 | IGHD1-3 | IGHJ2 | 0 | 10 | ARYSSYYFDY |
| NMZVH4-3.4 | IGHV4S2 | IGHD5-1 | IGHJ2 | 0 | 8 | ATGSYFDY |
| NMZVH4-3.6h | IGHV4S2 | IGHD1-7 | IGHJ1 | 0 | 16 | ARDYYDGSYYYWYFDFC#2 |
| NMZVH4-3.7 | IGHV4S2 | IGHD1-3 | IGHJ2 | 0 | 6 | ARGSYY |
| NMZVH4-3.8 | IGHV4S2 | IGHD4-1 | IGHJ2 | 0 | 9 | ARAQFGVDY |
| NMZVH4-3.9 | IGHV4S2 | IGHD1-5 | IGHJ2 | 0 | 9 | ARIYNNFDY |
| NMZVH4-3.10 | IGHV4S2 | IGHD5-1 | IGHJ2 | 1 | 11 | ARTGYYWSFDF |
| NMZVH4-3.11 | IGHV4S2 | IGHD5-1 | IGHJ2 | 1 | 10 | ARDWELYFDY |
| NMZVH4-3.12 | IGHV4S2 | IGHD5-1 | IGHJ1 | 0 | 12 | ARTGSYYWYFDF |
| NMZVH4-3.13 | IGHV4S2 | IGHD1-4 | IGHJ2 | 0 | 13 | ARRYYGYNYYFDY |
| Sequences of IGHV5 gene family from FO-Bd cells | ||||||
| NRFVH5-50 | IGHV5S30 | IGHD4-1 | IGHJ1 | 1 | 13 | ATDNSGYYWYFDF |
| NRFVH5-72 | IGHV5S30 | IGHD1-4 | IGHJ2 | 0 | 13 | ATIAAISTYYFDY |
| NRFVH5-8 | IGHV5S30 | IGHD5-1 | IGHJ2 | 0 | 8 | ATGSYFDY |
| NRFVH5-18 | IGHV5S30 | IGHD1-2 | IGHJ3 | 1 | 9 | ATGYNWFAY |
| NRFVH5-1 | IGHV5S27 | IGHD1-7 | IGHJ2 | 0 | 12 | ARHYYSGDYFDY |
| NRFVH5-3 | IGHV5S13 | IGHD1-8 | IGHJ2 | 0 | 14 | ARHYYDGYYHYFDY |
| NRFVH5-4 | IGHV5S11 | IGHD4-1 | IGHJ3 | 1 | 12 | ARHNSGYNWFAY |
| NRFVH5-6 | IGHV5-6 | IGHD1-2 | IGHJ2 | 0 | 8 | TTDHYGDY |
| NRFVH5-8 | IGHV5S27 | IGHD1-7 | IGHJ2 | 0 | 14 | ARHYYDGSYYYFDY |
| NRFVH5-9 | IGHV5S10 | IGHD1-5 | IGHJ1 | 2 | 12 | ATHNNYYWYFDF |
| NRFVH5-10 | IGHV5-1 | IGHD1-3 | IGHJ2 | 0 | 11 | ANYYYSSYIDY |
| NRFVH5-11 | IGHV5S74 | IGHD1-1 | IGHJ3 | 1 | 12 | ARMYTTDNWFAY |
| NRFVH5-12 | IGHV5S10 | IGHD1-8 | IGHJ2 | 0 | 10 | ATHYYDGYYY |
| Clonea | IGHV | IGHD | IGHJ | Mutationsb | H-CDR3c | |
| member | member | member | Nf | Amino acids | ||
| NRFVH5-13 | IGHV5-6 | IGHD4-1 | IGHJ2 | 0 | 10 | TTNSGYYFDY |
| NRFVH5-14 | IGHV5S57 | IGHD1-8 | IGHJ2 | 1 | 12 | TNYRDSYAYFDY |
| NRFVH5-15 | IGHV5S45 | IGHD1-6 | IGHJ2 | 1 | 10 | ARQLRRVFDY |
| NRFVH5-16 | IGHV5S10 | IGHD1-6 | IGHJ3 | 0 | 10 | ATYGGYWFAY |
| NRFVH5-17 | IGHV5S57 | no results | IGHJ3 | 0 | 8 | TRGYWFAY |
| NRFVH5-18 | IGHV5S29 | IGHD1-7 | IGHJ2 | 0 | 15 | TTETYYYDGSYYFDY |
| NRFVH5-19 | IGHV5S16 | IGHD5-1 | IGHJ1 | 0 | 9 | ARGSWYFDF |
| NRFVH5-2.3 | IGHV5S30 | IGHD4-1 | IGHJ2 | 0 | 11 | ATDNSGYYFDY |
| Sequences from IGHV5 gene family from MZ-Be cells | ||||||
| NMZVH5-2 | IGHV5S30 | no results | IGHJ2 | 0 | 4 | ATNY |
| NMZVH5-10 | IGHV5S30 | IGHD4-1 | IGHJ2 | 0 | 10 | ATDSGYYFDY |
| NMZVH5-2 | IGHV5-3 | IGHD1-2 | IGHJ4 | 0 | 10 | ARHGYYVMDA |
| NMZVH5-3 | IGHV5-2 | IGHD1-8 | IGHJ2 | 0 | 10 | ARHDGYYFDY |
| NMZVH5-5 | IGHV5S43 | IGHD1-5 | IGHJ2 | 0 | 10 | TRDNNYYFDY |
| NMZVH5-6 | IGHV5S65 | no results | IGHJ2 | 1 | 8 | AKAHYFDY |
| NMZVH5-11 | IGHV5S10 | IGHD1-4 | IGHJ2 | 1 | 11 | ATHYGYNYFDY |
| NMZVH5-12 | IGHV5-6 | IGHD1-7 | IGHJ2 | 1 | 12 | ARHYYDGSYYDY |
| NMZVH5-14 | IGHV5S32 | IGHD1-7 | IGHJ2 | 1 | 14 | ARHYDGSYYYYFDY |
| NMZVH5-15 | IGHV5S16 | IGHD4-1 | IGHJ3 | 0 | 9 | ARHNSGFAY |
| NMZVH5-16 | IGHV5S16 | IGHD1-4 | IGHJ2 | 0 | 6 | ATHNDY |
| NMZVH5-17 | IGHV5S30 | IGHD1-3 | IGHJ4 | 0 | 10 | ATYSSYVMDA |
| NMZVH5-18 | IGHV5S43 | IGHD1-5 | IGHJ2 | 0 | 11 | TRDHNNYYFDY |
| NMZVH5-20 | IGHV5S11 | IGHD1-6 | IGHJ1 | 0 | 16 | ARHNYGGYSDYWYFDF |
| NMZVH5-3.15 | IGHV5S30 | IGHD1-3 | IGHJ2 | 0 | 8 | ATEYWSDY |
| NMZVH5-3.16 | IGHV5S30 | IGHD5-1 | IGHJ2 | 0 | 9 | ATTGSYFDY |
aCμ (IgM) transcripts from FO-B cells and MZ-B cells
b Mutations, nucleotide differences of one or more basis between IMGT germline gene and rearranged Cμ transcript
cH-CDR3, heavy chain complementarity determining region 3
dFO-B cells, recirculating follicular B cells
eMZ-B cells, marginal zone B cells
fLenght of H-CDR3 in amino acids
g The sequence NRFVH4-71 and NRFVH4-37 are from clonally related B cells and designated as clone set C#1
h The sequence NMZVH4-3.2h and NMZVH4-3.6h are from clonally related B cells and designated as clone set C#2
Discussion
Previous studies provided evidence supporting the existence of mutated, IgM+ expressing, memory MZ-B cells in the rat [39]. Dammers et al. [40] demonstrated that less than 20% of the MZ-B cells isolated from spleens of PVG rats carried mutated IGHV genes. These findings were in marked contrast to humans, where >95% of the splenic MZ-B cells are mutated [22, 41, 42]. One possible explanation for this difference could be that only one particular IGHV gene family (viz. the IGHV5 family, the homolog of PC7183 in the mouse) was analyzed in the (PVG) rat and that this IGHV gene family was not representative of other IGHV genes, or IGHV gene families. By establishing the genomic germline IGHV gene repertoire of the BN rat [37] it became possible to accurately analyze other IGHV gene families as well. To avoid possible strain differences we used the BN rat strain, rather than the PVG rat strain that was used previously [39]. Here we report on the frequency of mutated sequences in rearranged IGHV-Cμ transcripts derived from FACS-sorted MZ-B cells (IgMhighIgDlow) in comparison with FO-B cells (IgMlowIgDhigh) obtained from the adult BN rat spleen. The analysis was confined to three different IGHV gene families, which differ in size: IGHV3, IGHV4 and IGHV5. These three IGHV gene families have 4, 2 and 26 functional IGHV genes, respectively. The IGHV3 and IGHV4 gene families were chosen to determine whether there is a difference in mutation frequencies among members of the IGHV gene families that are relatively small and to compare this frequency to the second largest IGHV gene family (IGHV5) in the rat, which had been analyzed previously in the PVG rat [10]. The BN rat strain contains 26 functional IGHV5 (germline) genes compared to the 28 germline genes of the PVG rat. In agreement with previous publications [41, 43, 44], we found that splenic MZ-B cells express a significantly higher percentage of mutated sequences than FO-B cells and all three analyzed IGHV gene families contributed to this difference. In BN rats a slightly higher proportion (27%) of the MZ-B cells expressed mutated IgM molecules encoded by IGHV5 family genes, compared to this proportion in the PVG rat (10–20%) [10]. This difference in mutation frequency can be due to differences that exist between the PVG and BN rat strains, such as for example the fact that BN rats have fewer IGHV genes, or it might also be caused by different environmental conditions (microbial environment; different microbiota) of the two rat strains. Analysis of the IGHV3 gene family showed that a similar proportion (approximately 30%) of mutated IgM encoding sequences can be found among the BN rat-derived MZ-B cells. In marked contrast, to these two IGHV families, a high proportion (66%) of the IGHV4 sequences from purified MZ-B cells was mutated. This family consists of only two potentially functional IGHV genes, although only one of these appeared to be functionally expressed. The presented findings show that the proportion of mutated sequences derived from MZ-B cells varies between the different IGHV gene families in the BN rat. In total a higher proportion (27–66%) of IGHV genes was mutated compared to the 10–20% of mutated sequences found previously for the IGHV5 gene family in PVG rats [10, 20]. Our observation that the highest percentage of mutated frequencies occurred in the single functional member IGHV4 gene family, suggests that there could be more antigen selection pressure on this particular IGHV4 gene in expanding its available repertoire by SHM. Although in total a higher average number of mutated sequences was observed among rat MZ-B cells was observed than previously, the frequency of mutated sequences among human MZ-B cells is still much higher. In humans, nearly all MZ-B cells are mutated [22, 41, 42]. Since the analysis of mutated IGHV genes was restricted to a particular set of IGHV genes in humans, the observed difference in frequency of mutated IGHV genes between the different IGHV families may also contribute to the reported difference in incidence of mutated MZ-B cells in humans and rats. Dunn-Walters et al. [45] analyzed only two particular IGHV genes: the IGHV6 gene and IGHV4.21 gene. It is possible that these IGHV genes are more mutated than other genes. However the analysis of Tangye et al. [42] showed that Ig genes isolated from IgM+ memory B cells among IGHV5 and IGHV6 gene families were all mutated which shows that the high frequency of mutations is not limited due to individual IGHV genes. Further, Colombo et al. [41] found that most of the human IGHV1, IGHV3 and IGHV4 gene families among splenic-derived MZ-B cells (IgMhighCD27+), GC B cells and class-switched B cells were mutated, albeit with a lower average number of mutations than both GC and class-switched B cells. However, the average number of mutations in human MZ-B cells (11.8) [41] was higher than both IgM+ MZ-B cells (8.8) and IgG+ MZ-B cells (7) [21] found in rats. This might be because humans have fewer functional IGHV genes than rats. We postulate that the higher number of germline IGHV genes in rodents is due to rats requiring fewer mutations to diversify their antibody repertoire after immunization than humans, because rats can encode a larger pool of different antibodies from their primary repertoire. In addition, it is possible that differences in life span and environmental conditions also contribute to differences in the average mutation frequency per IGHV gene. During their long lives, humans may encounter a greater variety of antigens than laboratory rats that live in well-controlled laboratory conditions.
Several pathways for the development of IgM and IgG B cells have been proposed. As previously suggested [46], shorter H-CDR3 regions of the IgM molecules expressed by naïve MZ-B cells in rats [40] and mice [8] are associated with polyreactive antibody responses and are ligand selected to bind to TI-antigens such as carbohydrates of micro-organisms [47]. Panda and Ding [48] proposed that splenic MZ-B cells such as B-1 cells might also be involved in the secretion of natural IgM and IgG antibodies. The authors went on to propose that natural antibodies, in particular IgM with diverse immune functions, could link the innate to the adaptive immune system. Findings of earlier experimental studies support these notions, revealing that natural IgM antibodies can recognize foreign antigens such as phosphorylcholine and modify low-density lipoprotein antigens [49]. Indeed IgM antibody production against both TI and TD antigens are induce by neutrophils activating MZ-B cells via BAFF, APRIL and IL-21. In addition to unmutated IgM molecules our data directly showing the presence of mutated IGHV-Cμ transcripts among the pool of purified rat IgMhighIgDlow MZ-B cells.
Memory cells are generally believed to be generated in GCs. It is still controversial, however, whether mutated (memory) IgM+ MZ-B cells are derived from GCs or whether they represent a GC-independent B cell population. Among the mutated sequences, we observed groups of mutated sequences that were derived from clonally related B cells, i.e. these sequences had identical H-CDR3 regions, used the same IGHV gene, expressed shared mutations and were from the same rat. Most (70%) of groups of cells had members that were confined to the MZ-B cell compartment. However, importantly, some clonally related groups of cells had members that were found among both MZ-B cells and FO-B cells. This clonal relationship strongly suggests that mutated IgM+ MZ-B cells and mutated IgM+ FO-B cells have a common origin. This is consistent with our previous finding that clonally related class-switched, mutated B cells co-exist with members in both the MZ-B and FO-B cell compartment [21]. Possibly both unswitched and class-switched B cells are generated in the same fashion. Somatic hypermutations (SHM) are usually introduced in B cells proliferating in the GC environment. Mutated B cells are subsequently subjected to some form of positive selection for B cells expressing immunoglobulins that bind with high affinity to antigen presented by follicular dendritic cells. Genetically engineered mice (such as Bcl6 deficient or CD40 deficient mice) cannot form GCs and lack B cells with mutated IGHV genes, including mutated IgM genes [50, 51]. Thus, at least in mice, GCs appear to be critically involved in SHM of IGHV genes during regular immune responses. This indicates that both mutated IgM+ FO-B cells and MZ-B cells are probably GC derived. In contrast to mice, mutated IgM+ B cells can still be found in humans with CD40 or CD40L deficiency (hyper IgM syndrome patients, HIGM), that lack classical CD40L mediated T cell help and lack GC formation [30, 52–54]. These mutated B cells are IgM+IgD+CD27+ cells, also called natural effector cells, that correspond to splenic MZ-B cells [54]. Other CD27+ B cell populations could not be formed in CD40/CD40L deficient HIGM patients. Berkowska et al. [53] observed that IgM+IgD+CD27+ cells have a relatively low replication history. Furthermore they are already present in very young (< 2 years) children and in human foetuses [26, 31, 54]. These findings suggest that at least a significant proportion of these mutated IgM+IgD+CD27+ MZ-B (-like) cells are not derived from GCs and are generated in the absence of T cell help. Weill and colleagues [54–56] speculated that these MZ-B (-like) cells use SHM in order to diversify their repertoire early during ontogeny outside T-dependent or T-independent humoral immune responses. However, this hypothesis has been challenged in the literature [27, 28]. Our observation that there are clonally related FO-B cells and MZ-B cells expressing mutated IgM molecules indicates that these cells have a common origin and provide evidence that does not support the notion that such a postulated diversification process would then be unique to MZ-B cells. In support of this, the variation in the percentage of mutated sequences in the various IGHV gene families also showed that SHM is used for diversification. In the case of pre-diversification it would be more likely that mutations occur more or less at a similar rate in all IGHV gene families. This variation in mutation frequency between different IGHV gene families is more in favour of a diversification because of antigenic stimulation. In humans, Colombo et al. [41] observed a small number of clonally related sequences that were shared between MZ-B cells and GC B cells, indicating that mutated IgM+ MZ-B cells can be derived from GCs. Recently, Aranburu and co-workers [57] proposed that three different populations of IgM memory B cells exist in humans and that most IgM memory B cells develop independently of the GC. These data suggest that during the first stages of life (i.e. 6–7 years), these cells can enter a GC to become either “remodeled” IgM memory B cells or class-switched memory B cells. In contrast, Weill and colleagues [26, 54, 56] suggested that the mutated IgM+ MZ-B cells are not GC-derived memory B cells. Instead, these authors postulated that the mutations in human MZ-B cells are acquired during their development in order to diversity their primary repertoire, in a GC and T-cell independent fashion. To test this hypothesis in rats we investigated the possible presence of mutated IgM+ MZ-B cells in neonatal rats. Neonatal rats do not develop GCs in the first weeks of life [34, 38]. Thus, when MZ-B cells are unmutated in neonatal rats this would strongly argue against the hypothesis of Weill et al, [25, 26, 56], that SHM is part of the developmental program of MZ-B cells. To this end, we analyzed IGHV-Cμ transcripts using IGHV4 and IGHV5 gene families from both MZ-B cells and FO-B cells. In summary, no mutations were found in any of the neonatal sequences, not even in the IGHV4 gene family genes that have the highest number of mutated sequences (66%) in the adult rat. These results support the notion that at least in rats, the mutated IgM+ MZ-B cells seen in adult animals are bona fide memory cells which are most probably generated under the influence of external antigenic stimuli in the GC.
Acknowledgments
The authors thank Prof Vivienne Russell for her helpful discussions and critical reading of the manuscript.
Abbreviations
- FO-B
follicular B
- GC
germinal centre
- IGHV
immunoglobulin heavy chain variable region
- H-CDR3
immunoglobulin heavy chain complementarity determining region 3
- SHM
somatic hypermutation
- MZ-B
marginal zone B cell
- TI-2
T cell-independent type 2 antigens
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by the Fogarty International Center (FIC), NIH Common Fund, Office of Strategic Coordination, Office of the Director (OD/OSC/CF/NIH), Office of AIDS Research, Office of the Director (OAR/NIH), National Institute of Mental Health (NIMH/NIH) of the National Institutes of Health grant D43TW01013 to JH. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. JH is also recipient of an Ubbo Emmius scholarship.
References
- 1.Martin F, Kearney JF. Marginal-zone B cells. Nature reviews Immunology. 2002;2(5):323–35. 10.1038/nri799 . [DOI] [PubMed] [Google Scholar]
- 2.Mebius RE, Kraal G. Structure and function of the spleen. Nature reviews Immunology. 2005;5(8):606–16. 10.1038/nri1669 . [DOI] [PubMed] [Google Scholar]
- 3.Pillai S, Cariappa A, Moran ST. Marginal zone B cells. Annual review of immunology. 2005;23:161–96. 10.1146/annurev.immunol.23.021704.115728 . [DOI] [PubMed] [Google Scholar]
- 4.Oliver AM, Martin F, Kearney JF. IgMhighCD21high lymphocytes enriched in the splenic marginal zone generate effector cells more rapidly than the bulk of follicular B cells. Journal of immunology. 1999;162(12):7198–207. . [PubMed] [Google Scholar]
- 5.Guinamard R, Okigaki M, Schlessinger J, Ravetch JV. Absence of marginal zone B cells in Pyk-2-deficient mice defines their role in the humoral response. Nature immunology. 2000;1(1):31–6. 10.1038/76882 . [DOI] [PubMed] [Google Scholar]
- 6.Martin F, Oliver AM, Kearney JF. Marginal zone and B1 B cells unite in the early response against T-independent blood-borne particulate antigens. Immunity. 2001;14(5):617–29. . [DOI] [PubMed] [Google Scholar]
- 7.Cinamon G, Zachariah MA, Lam OM, Foss FW Jr., Cyster JG. Follicular shuttling of marginal zone B cells facilitates antigen transport. Nature immunology. 2008;9(1):54–62. 10.1038/ni1542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carey JB, Moffatt-Blue CS, Watson LC, Gavin AL, Feeney AJ. Repertoire-based selection into the marginal zone compartment during B cell development. The Journal of experimental medicine. 2008;205(9):2043–52. Epub 2008/08/20. 10.1084/jem.20080559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schelonka RL, Tanner J, Zhuang Y, Gartland GL, Zemlin M, Schroeder HW Jr. Categorical selection of the antibody repertoire in splenic B cells. European journal of immunology. 2007;37(4):1010–21. 10.1002/eji.200636569 . [DOI] [PubMed] [Google Scholar]
- 10.Dammers PM, Visser A, Popa ER, Nieuwenhuis P, Kroese FG. Most marginal zone B cells in rat express germline encoded Ig VH genes and are ligand selected. Journal of immunology. 2000;165(11):6156–69. 10.4049/jimmunol.165.11.6156 . [DOI] [PubMed] [Google Scholar]
- 11.Dammers PM, Kroese FG. Recruitment and selection of marginal zone B cells is independent of exogenous antigens. European journal of immunology. 2005;35(7):2089–99. Epub 2005/06/09. 10.1002/eji.200526118 . [DOI] [PubMed] [Google Scholar]
- 12.Martin F, Kearney JF. Selection in the mature B cell repertoire. Current topics in microbiology and immunology. 2000;252:97–105. . [DOI] [PubMed] [Google Scholar]
- 13.Gatto D, Bauer M, Martin SW, Bachmann MF. Heterogeneous antibody repertoire of marginal zone B cells specific for virus-like particles. Microbes Infect. 2007;9(3):391–9. 10.1016/j.micinf.2006.12.017 . [DOI] [PubMed] [Google Scholar]
- 14.Gatto D, Ruedl C, Odermatt B, Bachmann MF. Rapid response of marginal zone B cells to viral particles. Journal of immunology. 2004;173(7):4308–16. 10.4049/jimmunol.173.7.4308 . [DOI] [PubMed] [Google Scholar]
- 15.Obukhanych TV, Nussenzweig MC. T-independent type II immune responses generate memory B cells. The Journal of experimental medicine. 2006;203(2):305–10. 10.1084/jem.20052036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pape KA, Kouskoff V, Nemazee D, Tang HL, Cyster JG, Tze LE, et al. Visualization of the genesis and fate of isotype-switched B cells during a primary immune response. The Journal of experimental medicine. 2003;197(12):1677–87. 10.1084/jem.20012065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Phan TG, Gardam S, Basten A, Brink R. Altered migration, recruitment, and somatic hypermutation in the early response of marginal zone B cells to T cell-dependent antigen. Journal of immunology. 2005;174(8):4567–78. 10.4049/jimmunol.174.8.4567 . [DOI] [PubMed] [Google Scholar]
- 18.White HN, Meng QH. Recruitment of a distinct but related set of VH sequences into the murine CD21hi/CD23- marginal zone B cell repertoire to that seen in the class-switched antibody response. Journal of immunology. 2012;188(1):287–93. 10.4049/jimmunol.1101264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tangye SG, Tarlinton DM. Memory B cells: effectors of long-lived immune responses. European journal of immunology. 2009;39(8):2065–75. 10.1002/eji.200939531 . [DOI] [PubMed] [Google Scholar]
- 20.Makowska A, Faizunnessa NN, Anderson P, Midtvedt T, Cardell S. CD1high B cells: a population of mixed origin. European journal of immunology. 1999;29(10):3285–94. . [DOI] [PubMed] [Google Scholar]
- 21.Hendricks J, Visser A, Dammers PM, Burgerhof JG, Bos NA, Kroese FG. Class-switched marginal zone B cells in spleen have relatively low numbers of somatic mutations. Molecular immunology. 2011;48(6–7):874–82. Epub 2011/01/25. 10.1016/j.molimm.2010.12.020 . [DOI] [PubMed] [Google Scholar]
- 22.Dunn-Walters DK, Isaacson PG, Spencer J. Analysis of mutations in immunoglobulin heavy chain variable region genes of microdissected marginal zone (MGZ) B cells suggests that the MGZ of human spleen is a reservoir of memory B cells. The Journal of experimental medicine. 1995;182(2):559–66. 10.1084/jem.182.2.559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tierens A, Delabie J, Michiels L, Vandenberghe P, De Wolf-Peeters C. Marginal-zone B cells in the human lymph node and spleen show somatic hypermutations and display clonal expansion. Blood. 1999;93(1):226–34. . [PubMed] [Google Scholar]
- 24.Klein U, Rajewsky K, Kuppers R. Human immunoglobulin (Ig)M+IgD+ peripheral blood B cells expressing the CD27 cell surface antigen carry somatically mutated variable region genes: CD27 as a general marker for somatically mutated (memory) B cells. The Journal of experimental medicine. 1998;188(9):1679–89. 10.1084/jem.188.9.1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weller S, Braun MC, Tan BK, Rosenwald A, Cordier C, Conley ME, et al. Human blood IgM "memory" B cells are circulating splenic marginal zone B cells harboring a prediversified immunoglobulin repertoire. Blood. 2004;104(12):3647–54. 10.1182/blood-2004-01-0346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weller S, Mamani-Matsuda M, Picard C, Cordier C, Lecoeuche D, Gauthier F, et al. Somatic diversification in the absence of antigen-driven responses is the hallmark of the IgM+ IgD+ CD27+ B cell repertoire in infants. The Journal of experimental medicine. 2008;205(6):1331–42. Epub 2008/06/04. 10.1084/jem.20071555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seifert M, Kuppers R. Molecular footprints of a germinal center derivation of human IgM+(IgD+)CD27+ B cells and the dynamics of memory B cell generation. The Journal of experimental medicine. 2009;206(12):2659–69. Epub 2009/11/18. 10.1084/jem.20091087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tangye SG, Good KL. Human IgM+CD27+ B cells: memory B cells or "memory" B cells? Journal of immunology (Baltimore, Md: 1950). 2007;179(1):13–9. Epub 2007/06/21. 10.4049/jimmunol.179.1.13 . [DOI] [PubMed] [Google Scholar]
- 29.Kruetzmann S, Rosado MM, Weber H, Germing U, Tournilhac O, Peter HH, et al. Human immunoglobulin M memory B cells controlling Streptococcus pneumoniae infections are generated in the spleen. The Journal of experimental medicine. 2003;197(7):939–45. 10.1084/jem.20022020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weller S, Faili A, Garcia C, Braun MC, Le Deist FF, de Saint Basile GG, et al. CD40-CD40L independent Ig gene hypermutation suggests a second B cell diversification pathway in humans. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(3):1166–70. Epub 2001/02/07. 10.1073/pnas.98.3.1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scheeren FA, Nagasawa M, Weijer K, Cupedo T, Kirberg J, Legrand N, et al. T cell-independent development and induction of somatic hypermutation in human IgM+ IgD+ CD27+ B cells. The Journal of experimental medicine. 2008;205(9):2033–42. Epub 2008/08/13. 10.1084/jem.20070447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dammers PM, Lodewijk ME, Zandvoort A, Kroese FG. Marginal zone B cells in neonatal rats express intermediate levels of CD90 (Thy-1). Developmental immunology. 2002;9(4):187–95. 10.1080/10446670310001593488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kroese FG, Wubbena AS, Kuijpers KC, Nieuwenhuis P. The ontogeny of germinal centre forming capacity of neonatal rat spleen. Immunology. 1987;60(4):597–602. Epub 1987/04/01. [PMC free article] [PubMed] [Google Scholar]
- 34.van Rees EP, Dijkstra CD, van Rooijen N. The early postnatal development of the primary immune response in TNP-KLH-stimulated popliteal lymph node in the rat. Cell and tissue research. 1986;246(3):673–7. Epub 1986/01/01. 10.1007/bf00215210 . [DOI] [PubMed] [Google Scholar]
- 35.Dammers PM, de Boer NK, Deenen GJ, Nieuwenhuis P, Kroese FG. The origin of marginal zone B cells in the rat. European journal of immunology. 1999;29(5):1522–31. Epub 1999/06/08. . [DOI] [PubMed] [Google Scholar]
- 36.Stoel M, Jiang HQ, van Diemen CC, Bun JC, Dammers PM, Thurnheer MC, et al. Restricted IgA repertoire in both B-1 and B-2 cell-derived gut plasmablasts. Journal of immunology. 2005;174(2):1046–54. 10.4049/jimmunol.174.2.1046 . [DOI] [PubMed] [Google Scholar]
- 37.Hendricks J, Terpstra P, Dammers PM, Somasundaram R, Visser A, Stoel M, et al. Organization of the variable region of the immunoglobulin heavy-chain gene locus of the rat. Immunogenetics. 2010;62(7):479–86. 10.1007/s00251-010-0448-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kroese FG, Wubbena AS, Seijen HG, Nieuwenhuis P. Germinal centers develop oligoclonally. European journal of immunology. 1987;17(7):1069–72. Epub 1987/07/01. 10.1002/eji.1830170726 . [DOI] [PubMed] [Google Scholar]
- 39.Dammers PM, Visser A, Popa ER, Nieuwenhuis P, Bos NA, Kroese FG. Immunoglobulin VH gene analysis in rat: most marginal zone B cells express germline encoded VH genes and are ligand selected. Current topics in microbiology and immunology. 2000;252:107–17. . [DOI] [PubMed] [Google Scholar]
- 40.Dammers PM, Visser A, Popa ER, Nieuwenhuis P, Kroese FG. Most marginal zone B cells in rat express germline encoded Ig VH genes and are ligand selected. J Immunol. 2000;165(11):6156–69. 10.4049/jimmunol.165.11.6156 [DOI] [PubMed] [Google Scholar]
- 41.Colombo M, Cutrona G, Reverberi D, Bruno S, Ghiotto F, Tenca C, et al. Expression of immunoglobulin receptors with distinctive features indicating antigen selection by marginal zone B cells from human spleen. Molecular medicine (Cambridge, Mass). 2013;19:294–302. Epub 2013/07/24. 10.2119/molmed.2013.00069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tangye SG, Liu YJ, Aversa G, Phillips JH, de Vries JE. Identification of functional human splenic memory B cells by expression of CD148 and CD27. The Journal of experimental medicine. 1998;188(9):1691–703. Epub 1998/11/06. 10.1084/jem.188.9.1691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Makowska A, Faizunnessa NN, Anderson P, Midtvedt T, Cardell S. CD1high B cells: a population of mixed origin. Eur J Immunol. 1999;29(10):3285–94. [DOI] [PubMed] [Google Scholar]
- 44.Spencer J, Perry ME, Dunn-Walters DK. Human marginal-zone B cells. Immunology today. 1998;19(9):421–6. Epub 1998/09/24. . [DOI] [PubMed] [Google Scholar]
- 45.Dunn-Walters DK, Isaacson PG, Spencer J. Analysis of mutations in immunoglobulin heavy chain variable region genes of microdissected marginal zone (MGZ) B cells suggests that the MGZ of human spleen is a reservoir of memory B cells. J Exp Med. 1995;182(2):559–66. 10.1084/jem.182.2.559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hendricks J, Bos NA, Kroese FGM. Heterogeneity of Memory Marginal Zone B Cells. Critical reviews in immunology. 2018;38(2):145–58. Epub 2018/06/12. 10.1615/CritRevImmunol.2018024985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Galson JD, Clutterbuck EA, Truck J, Ramasamy MN, Munz M, Fowler A, et al. BCR repertoire sequencing: different patterns of B-cell activation after two Meningococcal vaccines. Immunology and cell biology. 2015;93(10):885–95. Epub 2015/05/16. 10.1038/icb.2015.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Panda S, Ding JL. Natural antibodies bridge innate and adaptive immunity. Journal of immunology (Baltimore, Md: 1950). 2015;194(1):13–20. Epub 2014/12/21. 10.4049/jimmunol.1400844 . [DOI] [PubMed] [Google Scholar]
- 49.Shaw PX, Horkko S, Chang MK, Curtiss LK, Palinski W, Silverman GJ, et al. Natural antibodies with the T15 idiotype may act in atherosclerosis, apoptotic clearance, and protective immunity. The Journal of clinical investigation. 2000;105(12):1731–40. Epub 2000/06/23. 10.1172/JCI8472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bergqvist P, Stensson A, Lycke NY, Bemark M. T cell-independent IgA class switch recombination is restricted to the GALT and occurs prior to manifest germinal center formation. Journal of immunology (Baltimore, Md: 1950). 2010;184(7):3545–53. Epub 2010/03/09. 10.4049/jimmunol.0901895 . [DOI] [PubMed] [Google Scholar]
- 51.Toyama H, Okada S, Hatano M, Takahashi Y, Takeda N, Ichii H, et al. Memory B cells without somatic hypermutation are generated from Bcl6-deficient B cells. Immunity. 2002;17(3):329–39. Epub 2002/10/02. . [DOI] [PubMed] [Google Scholar]
- 52.Agematsu K, Nagumo H, Shinozaki K, Hokibara S, Yasui K, Terada K, et al. Absence of IgD-CD27(+) memory B cell population in X-linked hyper-IgM syndrome. The Journal of clinical investigation. 1998;102(4):853–60. Epub 1998/08/26. 10.1172/JCI3409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Berkowska MA, Driessen GJ, Bikos V, Grosserichter-Wagener C, Stamatopoulos K, Cerutti A, et al. Human memory B cells originate from three distinct germinal center-dependent and -independent maturation pathways. Blood. 2011;118(8):2150–8. Epub 2011/06/22. 10.1182/blood-2011-04-345579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weller S, Braun MC, Tan BK, Rosenwald A, Cordier C, Conley ME, et al. Human blood IgM "memory" B cells are circulating splenic marginal zone B cells harboring a prediversified immunoglobulin repertoire. Blood. 2004;104(12):3647–54. 10.1182/blood-2004-01-0346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weill JC, Weller S, Reynaud CA. A bird's eye view on human B cells. Seminars in immunology. 2004;16(4):277–81. Epub 2004/11/04. 10.1016/j.smim.2004.08.007 . [DOI] [PubMed] [Google Scholar]
- 56.Weill JC, Weller S, Reynaud CA. Human marginal zone B cells. Annual review of immunology. 2009;27:267–85. Epub 2009/03/24. 10.1146/annurev.immunol.021908.132607 . [DOI] [PubMed] [Google Scholar]
- 57.Aranburu A, Piano Mortari E, Baban A, Giorda E, Cascioli S, Marcellini V, et al. Human B-cell memory is shaped by age- and tissue-specific T-independent and GC-dependent events. European journal of immunology. 2017;47(2):327–44. Epub 2016/11/20. 10.1002/eji.201646642 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.


