Abstract
Background
A severe neurological disorder, Guillain-Barré syndrome (GBS) is the leading cause of acute flaccid paralysis. Enhanced surveillance of GBS in Latin America and the Caribbean (LAC) following the 2015–2016 Zika virus (ZIKV) epidemic presents an opportunity to estimate, for the first time, the regional incidence of GBS.
Methods and findings
For this systematic review and meta-analysis, we searched nine scientific databases and grey literature from January 1, 1980 to October 1, 2018. Sources with primary data on incident GBS cases in LAC within a well-defined population and timeframe, published in English, Spanish, Portuguese, or French, were included. We calculated the annual GBS incidence rates (IRs) and 95% confidence intervals (CIs) for each source based on published data. Following an assessment of heterogeneity, we used random-effects meta-analysis to calculate the pooled annual IR of GBS. The study is registered with PROSPERO, number CRD42018086659. Of the 6568 initial citation hits, 31 were eligible for inclusion. Background annual GBS IRs in Latin America ranged from 0.40 in Brazil to 2.12/100,000 in Chile. The pooled annual IR in the Caribbean was 1.64 (95% CI 1.29–2.12, I2<0.01, p = 0.44). During the ZIKV epidemic, GBS IRs ranged from 0.62 in Mexico to 9.35/100,000 in Martinique. GBS increased 2.6 (95% CI 2.3–2.9) times during ZIKV and 1.9 (95% CI 1.1–3.4) times during chikungunya outbreaks over background rates. A limitation of this review is that the studies included employed different methodologies to find and ascertain cases of GBS, which could contribute to IR heterogeneity. In addition, it is important to consider that data on GBS are lacking for many countries in the region.
Conclusions
Background IRs of GBS appear to peak during arboviral disease outbreaks. The current review contributes to an understanding of the epidemiology of GBS in the LAC region, which can inform healthcare system planning and preparedness, particularly during arboviral epidemics.
Trial registration
Registered with PROSPERO: CRD42018086659.
Author summary
A severe neurological disorder, Guillain-Barré syndrome (GBS) is the leading cause of acute flaccid paralysis. This is the first systematic review on GBS incidence in Latin America and the Caribbean before and during arboviral disease outbreaks. There is a large sub-regional and annual fluctuation in the incidence of GBS. Background annual GBS incidence rates (IRs) in Latin America ranged from 0.40 in Brazil to 2.12/100,000 in Chile. The pooled annual IR in the Caribbean was 1.64 (95% CI 1.29–2.12, I2<0.01, p = 0.44). During the ZIKV epidemic, GBS IRs ranged from 0.62 in Mexico to 9.35/100,000 in Martinique. GBS increased 2.6 times during ZIKV and 1.9 times during chikungunya outbreaks over background rates. GBS is a costly disease, which can result in long-term disability and high mortality rates in resource constrained healthcare settings. Because GBS can be triggered by arboviral infections, baseline incidence of GBS is critical for detecting neglected tropical disease outbreaks. The current review contributes to an understanding of the epidemiology of GBS in the LAC region, which can inform healthcare system planning and preparedness.
Introduction
A rare but severe autoimmune neuropathy, Guillain-Barré syndrome (GBS) is the most common type of acute flaccid paralysis (AFP) [1]. Often preceded by infections such as Campylobacter jejuni, about 25% of patients require mechanical ventilation [2]. Prognosis varies greatly based on GBS type and urgent care availability [2–5]. Many patients report residual deficits, including pain, limited mobility, and fatigue, years after disease onset [4, 6–8]. Mortality rates range from 3−7% [3, 9], although they can be higher in settings with limited access to intensive care [10, 11]. In 2008, the total annual cost of GBS in the U.S. alone was estimated at $1.7 billion (95% CI $1.6 to 1.9 billion) [12].
The median global incidence rate (IR) of GBS was estimated at 1.10 per 100,000 person-years (range, 0.81–1.89) [13]. However, this estimate was based on data from studies conducted in Europe and North America [13]. Worldwide, there are large variations in the incidence of GBS, ranging from 0.38 (95% CI 0.25–0.56) to 2.53 (95% CI 1.87–3.56) per 100,000, with most studies reporting annual IRs between 1.1 and 1.8 per 100,000 [14]. Prior to the 2015–2016 Zika virus (ZIKV) epidemic in Latin America and the Caribbean (LAC), there were few published studies on the incidence of GBS in the region, with an exception among children. As part of polio eradication efforts, AFP in children under 15 years of age has been a notifiable event in all LAC countries since the 1980s [15–17]. Using polio eradication surveillance data, in 2010, Landaverde et al. estimated 0.82 cases of GBS per 100,000 among children under 15 years of age (range, 0.72–0.90) [18].
ZIKV is an enveloped positive-strand RNA member of the Flavivirus genus in the Flaviviridae family. Other flaviviruses include dengue, yellow fever, West Nile virus and Japanese encephalitis virus, many of which are associated with neurological disease. Like these viruses, ZIKV is principally transmitted by a mosquito bite and is thus described as an arthropod-borne virus or ‘arbovirus’. Its primary vector is the Aedes aegypti mosquito, which transmits the virus between humans and is widespread in tropical regions [19].
ZIKV is known to be neurotropic; infection halts proliferation of neural progenitor cells and may induce cell death, leading to ZIKV-related microcephaly [20]. Beyond congenital Zika syndrome, direct viral invasion as well as a parainfective or postinfective autoimmune response may contribute to GBS pathogenesis [21, 22]. An association between GBS and ZIKV was first established in a case-control study in French Polynesia [23]. During the 2015–2016 ZIKV epidemic, many countries in LAC reported increases in GBS cases, particularly in the beginning of 2016 [24, 25]. In 2016, the World Health Organization (WHO) concluded that ZIKV infection was a plausible trigger for GBS [26]. The chikungunya (CHIKV) [27, 28] and dengue (DENV) viruses [29], two other arboviruses that are endemic in parts of the LAC region, have also been investigated as possible GBS antecedent infectious agents.
Enhanced GBS surveillance [30] and increased research present a unique opportunity to assess the background IR of GBS in the LAC region in the aftermath of recent arboviral epidemics. This review aims to assess the background population-wide incidence of GBS in Latin America and the Caribbean through a synthesis of observational studies. For the purposes of this study, “background incidence” refers to the rates reported during time periods with no arboviral epidemic. To our knowledge this is the first systematic review on GBS in this region. A secondary aim of this review is to ascertain the incidence of GBS during arboviral disease outbreaks.
Methods
Search strategy and selection criteria
Our protocol followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses protocols (PRISMA-P) guidelines and the Meta-analyses of Observational Studies in Epidemiology (MOOSE) Checklist (see S1 and S2 Files) [31]. We developed the search word criteria by reviewing PubMed MeSH and Embase Emtree subject headings and keywords. We searched nine electronic databases. Our full search terms are provided in the appendix. We conducted a grey literature search on OpenGrey and OAIster and hand-searched relevant journals and the bulletins of ministries of health cited in bibliographic references of the articles selected for full-text review (see S3 File). We also reviewed the references of all included articles. Inclusion criteria were peer-reviewed and government publications that presented primary data on incident GBS cases in LAC within a well-defined population and timeframe and that were published in English, Spanish, Portuguese, or French between January 1, 1980 to October 1, 2018. The search timeframe was decided upon to update the worldwide review by McGrogan [14] from 1980 to 2008. Review papers were excluded. After removing duplicates, two authors (AC, DLV) independently screened articles by title and abstract based on the inclusion criteria and agreed on those for full-text review. A standard extraction form was developed and tested for reliability. Disagreements were resolved by these two reviewers.
Data analysis
One author (AC) extracted the following items from the included articles onto the extraction form: study design, country, region, data collection year(s), population size, age, sex, GBS type, GBS diagnostic criteria, incident cases, statistical measures, and circulating arboviral diseases (ZIKV, DENV, or CHIKV). Three other authors (DLV, DCO, YT) extracted data from 30% of the records for quality control.
All data were standardized to annual mean IR by dividing the number of GBS cases reported by the number of weeks of data capture and multiplying the result by 52 weeks. The result was entered as annual GBS cases into the software program, and together with the base population, was used to calculate the annual IR per 100,000 persons. When necessary, we attempted to contact the authors to obtain clarifying information. Three authors (AC, DCO, YT) assessed studies for risk of bias using a tool developed for prevalence studies [32]. To reduce publication bias [33], we searched the grey literature, institutional websites, and conference abstracts. The study protocol was registered with PROSPERO (CRD42018086659, available at https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=86659).
We performed the meta-analysis using the metaprop command in Stata version 15 (College Station, Texas, USA). Annualized GBS cases and population sizes were entered for every selected study. Given high heterogeneity, we performed sub-group analyses by: geography (Latin America versus the Caribbean; Southern Cone, Central and North America, and the Caribbean); time (before and during an epidemic outbreak); population (all and under 15 years of age); and case ascertainment (administrative data using ICD codes only and medical record review). Double arcsine transformation and random-effects models were used to calculate pooled IR estimates of GBS [34].
Results
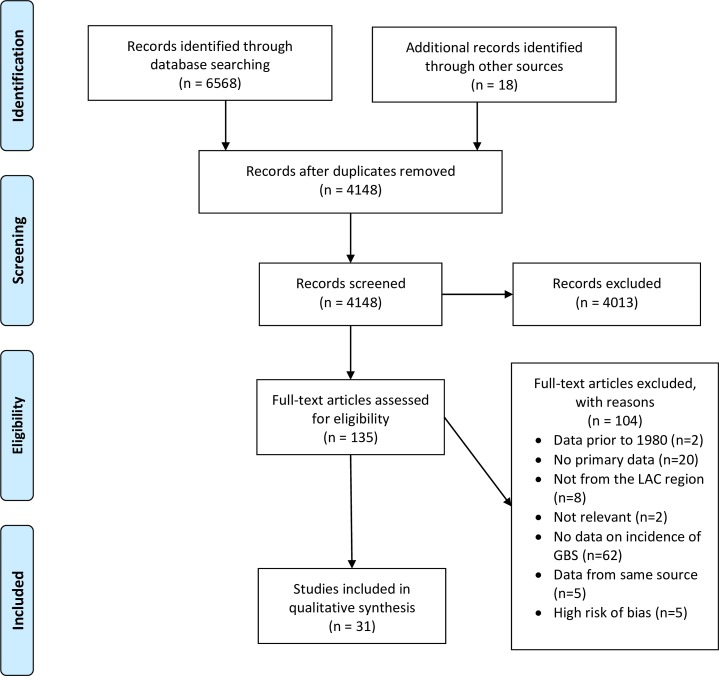
The database search identified 6568 citations. An additional 18 citations were identified through hand searching. After removing duplicates, 4148 citations were screened based on title and abstract. Of these, 4013 did not meet inclusion criteria. We reviewed 135 full-text articles, of which 31 were eligible for inclusion (Fig 1). Of the 31 studies, 28 reported country-specific GBS rates from 13 LAC countries (Tables 1 & 2), 3 regional rates,[18, 35, 36] 15 background rates [18, 35–48], 11 rates during an arboviral outbreak [49–59], and 5 compared rates before and during an outbreak [27, 60–63]. Many studies presented data from Brazil (29%) and Colombia (16%), and the majority (68%) were published in 2015 or later. Of the studies that assessed background IRs, the mean number of years studied was 7.2 (range, 1–13.6 years).
Fig 1. PRISMA flow chart for a systematic review and meta-analysis of Guillain-Barré Syndrome (GBS) in Latin America and the Caribbean before and during the 2015–2016 Zika virus epidemic.
Table 1. Characteristics of the 31 included studies of incidence of Guillain-Barré Syndrome, by location and study period.
| Author/s (Year) | Country | Location | Study Period1 | Case ascertainment | Case definition | Ages |
|---|---|---|---|---|---|---|
| Central America and Mexico | ||||||
| Molinero et al (2003) | Honduras | Nationwide | 1989–1999 | Prospective hospital-based study of acute flaccid paralysis (AFP) cases | Asbury & Cornblath | <15 |
| de la Peña et al (2015) | Mexico | Jalisco (state) | 2005–2009 | Retrospective hospital-based review of medical discharge records | Asbury & Cornblath | ≥18 |
| del Carpio Orantes et al (2018) | Mexico | North Veracruz (delegation) | 2016–2017 | Retrospective hospital-based review of medical discharge records | Brighton Collaboration (1–3) | All |
| South America | ||||||
| Rojas et al (2009) | Argentina | Buenos Aires (city) | 1999–2007 | Retrospective hospital-based review of medical records | ICD-9 357.0 & NINCDS | All |
| Codebó et al (2016) | Argentina | Nationwide | 2007–2013 | Retrospective review of medical discharge records in national database | ICD-10 G61.0 | All |
| Dias-Tosta et al (2002) | Brazil | Nationwide | 1990–1996 | National AFP surveillance system and medical diagnosis | Asbury & Cornblath | <15 |
| Dourado et al (2012) | Brazil | Rio Grande do Norte (state) | 1994–2007 | Prospective hospital-based series | Asbury & Cornblath | All |
| Rocha et al (2004) | Brazil | Sao Paulo (city) | 1995–2002 | Retrospective review of medical discharge records | Asbury & Cornblath | All |
| Barcellos et al (2017) | Brazil | Northeast region | January 2008-May 2015 June-October 2015 | Admission records in national hospital information system | ICD-10 G61.0 | All |
| Souza (2018) | Brazil | Piauí (state) | 2014–2016 | Active hospital-based state surveillance system | Brighton Collaboration (1–3) | All |
| Paploski et al (2016) | Brazil | Salvador (city) | 2015 | Active surveillance and medical records review | Not specified | All |
| Nobrega (2018) | Brazil | Recife (metropolitan) | January-June, 2015 | Retrospective review of medical discharge records | ICD-10 G61.0 & Brighton Collaboration (1–3) | All |
| Styczynski et al (2017) | Brazil | Salvador (metropolitan) | April-July 2015 | Passive state surveillance system and review of medical discharge records | Brighton Collaboration (1–3) | ≥12 |
| Department of Health of Paiuí State (2016) | Brazil | Piauí (state) | November 2015-October 2016 | Active hospital-based state surveillance system | Not specified | All |
| Rivera-Lillo et al (2016) | Chile | Nationwide | 2001–2012 | Passive national surveillance system | ICD-10 G61.0 | All |
| Machado-Alba et al (2016) | Colombia | Nationwide | March 2014-September 2015 October 2015-March 2016 | Private insurance diagnostic database | ICD-10 G61.0 | All |
| Anaya et al (2017) | Colombia | Cúcuta (city) | June 2015-July 2016 | Passive national surveillance system | Brighton Collaboration (1,2) | All |
| Tolosa et al (2017) | Colombia | Nationwide | August 2015-May 2016 | Passive national surveillance system | ICD-10 G61.0 | ≤18 |
| Instituto Nacional de Salud de Colombia (2016) | Colombia | Nationwide | October 2015-March 2016 | Passive national surveillance system | ICD-10 G61.0 | All |
| Salinas et al (2017) | Colombia | Barranquilla (city) | October 2015-April 2016 | Passive national and local surveillance systems and medical records review | ICD-10 G61.0 & Brighton Collaboration (1–3) | All |
| Hart et al (1994) | Paraguay | Nationwide | 1990–1991 | National AFP surveillance and medical diagnosis | Asbury & Cornblath | <15 |
| Caribbean | ||||||
| Suryapranata et al (2016) | Aruba | Nationwide | 2003–2011 | Retrospective review of medical discharge records | ICD-9 357.0 & Asbury & Cornblath | All |
| van Koningsveld et al (2001) | Curaçao | Nationwide | 1987–1999 | Retrospective review of medical discharge records | ICD-9 357.0 & Asbury & Cornblath | All |
| Núnñez R et al (2017) | Dominican Republic | Nationwide | January 2016-October 2016 | Passive national surveillance system | Brighton collaboration (1,2) | All |
| Balavoine et al (2017) | Guadeloupe and Martinique | Nationwide | 2011–2013 2014 (chikungunya) | Retrospective review of medical discharge records | ICD-9 | All |
| Roze et al (2017) | Martinique | Nationwide | 2006–2016 2014 (chikungunya) 2016 (Zika) | Retrospective review of medical discharge records and prospective medical evaluation | ICD-10 & Brighton Collaboration (1,2) | All |
| Salinas et al (2017) | Puerto Rico | Nationwide | 2013 | Retrospective review of medical discharge records and insurance claims | ICD-9357.0 or ICD-10 G61.0 and Brighton Collaboration (1–3) | All |
| Dirlikov (2018) | Puerto Rico | Nationwide | 2017 | National surveillance system followed by medical record review | ICD-10 G61.0 & Brighton Collaboration (1–3) | All |
| Regional | ||||||
| Olivé et al (1997) | 7 countries2 | 1989–1991 | Passive AFP regional surveillance followed by neurologist diagnosis | Asbury & Cornblath | <15 | |
| Silveira et al (1997) | 4 countries3 | 1990–1994 | Passive AFP regional surveillance followed by neurologist diagnosis | PAHO Polio Eradication Field Guide definition | <15 | |
| Landaverde et al (2010) | 19 countries4 | 2000–2008 | Passive AFP regional surveillance followed by neurologist diagnosis | PAHO Polio Eradication Field Guide definition | <15 |
1 When two dates are given, the first is before and the second during the epidemic period
2 El Salvador, Guatemala, Honduras, Paraguay, Peru, Mexico, and Venezuela
3Argentina, Brazil, Chile and Colombia
4 Argentina, Brazil, Chile, Colombia, Cuba, Ecuador, El Salvador, Guatemala, Honduras, Mexico, Nicaragua, Panama, Peru, the Bahamas, Guyana, Jamaica, St Vincent and the Grenadines, Suriname, and Trinidad and Tobago
Table 2. Annual incidence rate of Guillain-Barré Syndrome in 31 selected studies, by location and epidemic status.
| Author/s (Year) | Country | Epidemic arbovirus | Study duration (in years) | Incident cases | Mean annual cases | Population | Annual incidence rate per 100,000 persons (95% CI)1 | |
|---|---|---|---|---|---|---|---|---|
| Central America and Mexico | ||||||||
| Background incidence rate (IR) of GBS | ||||||||
| Molinero et al (2003) | Honduras | 11.0 | 394 | 36 | 2 627 737 | 1.37 (0.96–1.90) | ||
| de la Peña et al (2015) | Mexico | 5.0 | 45 | 9 | 4 513 718 | 0.20 (0.09–0.38) | ||
| IR during epidemic outbreak | ||||||||
| del Carpio Orantes et al (2018) | Mexico | Zika | 2.0 | 34 | 17 | 2 732 286 | 0.62 (0.36–1.00) | |
| South America | ||||||||
| Background IR of GBS | ||||||||
| Rojas et al (2009) | Argentina | 9.0 | 26 | 3 | 145 310 | 2.06 (0.43–6.03) | ||
| Codebó et al (2016) | Argentina | 7.0 | 1 859 | 264 | 41 904 761 | 0.63 (0.56–0.71) | ||
| Dias-Tosta et al (2002) | Brazil | 7.0 | 1 678 | 240 | 52 111 801 | 0.46 (0.40–0.52) | ||
| Dourado et al (2012) | Brazil | 13.6 | 149 | 11 | 2 781 767 | 0.40 (0.20–0.71) | ||
| Rocha et al (2004) | Brazil | 8.0 | 95 | 12 | 3 000 000 | 0.40 (0.21–0.70) | ||
| Barcellos et al (2017) | Brazil | 7.4 | 2 407 | 325 | 54 711 473 | 0.59 (0.53–0.66) | ||
| Rivera-Lillo et al (2016) | Chile | 12.0 | 4 158 | 347 | 16 353 842 | 2.12 (1.90–2.36) | ||
| Machado-Alba et al (2016) | Colombia | 1.6 | 98 | 62 | 6 500 000 | 0.95 (0.73–1.22) | ||
| Hart et al (1994) | Paraguay | 2.0 | 37 | 19 | 1 747 703 | 1.09 (0.65–1.70) | ||
| IR during epidemic outbreak | ||||||||
| Souza (2018) | Brazil | Zika | 3.0 | 73 | 24 | 2 927 711 | 0.82 (0.53–1.22) | |
| Paploski et al (2016) | Brazil | Zika | 1.0 | 51 | 51 | 2 920 300 | 1.75 (1.30–2.30) | |
| Nobrega (2018) | Brazil | Zika | 0.5 | 44 | 88 | 3 890 145 | 2.26 (1.81–2.79) | |
| Styczynski et al (2017) | Brazil | Zika | 0.25 | 48 | 192 | 3 428 571 | 5.60 (4.84–6.45) | |
| Barcellos et al (2017) | Brazil | Zika | 0.42 | 377 | 905 | 56 445 105 | 1.60 (1.50–1.71) | |
| Department of Health of Piauí State (2016) | Brazil | Zika | 1.0 | 23 | 23 | 3 219 257 | 0.71 (0.45–1.07) | |
| Anaya et al (2017) | Colombia | Zika | 1.1 | 29 | 27 | 656 380 | 4.11 (2.71–5.98) | |
| Tolosa et al (2017) | Colombia | Zika | 0.8 | 40 | 51 | 16 236 326 | 0.31 (0.23–0.41) | |
| Machado-Alba et al (2016) | Colombia | Zika | 0.5 | 71 | 142 | 6 500 000 | 2.18 (1.84–2.57) | |
| Instituto Nacional de Salud de Colombia (2016) | Colombia | Zika | 0.5 | 270 | 563 | 49 529 208 | 1.14 (1.04–1.23) | |
| Salinas et al (2017) | Colombia | Zika | 0.5 | 47 | 93 | 1 218 475 | 7.63 (6.16–9.35) | |
| Caribbean | ||||||||
| Background IR of GBS | ||||||||
| Suryapranata et al (2016) | Aruba | 9.0 | 36 | 4 | 100 000 | 4.00 (1.09–10.24) | ||
| van Koningsveld et al (2001) | Curaçao | 12.3 | 49 | 4 | 152 694 | 2.62 (0.71–6.71) | ||
| Balavoine et al (2017) | Guadeloupe and Martinique | 3.0 | 42 | 14 | 792 091 | 1.77 (0.97–2.97) | ||
| Roze et al (2017) | Martinique | 10.0 | 105 | 8 | 378 243 | 2.12 (0.91–4.17) | ||
| Salinas et al (2017) | Puerto Rico | 1.0 | 61 | 61 | 3 595 839 | 1.70 (1.30–2.18) | ||
| IR during epidemic outbreak | ||||||||
| Núñez R et al (2017) | Dominican Republic | Zika | 0.8 | 559 | 671 | 10 075 045 | 6.66 (6.17–7.18) | |
| Balavoine et al (2017) | Guadeloupe and Martinique | Chikungunya | 1.0 | 27 | 27 | 783 336 | 3.45 (2.27–5.01) | |
| Roze et al (2017) | Martinique | Chikungunya | 1.0 | 15 | 15 | 378 243 | 3.97 (2.22–6.54) | |
| Roze et al (2017) | Martinique | Zika | 0.8 | 30 | 36 | 385 103 | 9.35 (6.55–12.94) | |
| Dirlikov (2018) | Puerto Rico | Zika | 1.0 | 123 | 123 | 3 411 307 | 3.61 (3.00–4.30) | |
| Regional | ||||||||
| Background IR of GBS | ||||||||
| Olivé et al (1997) | 7 countries | 3.0 | 1527 | 509 | 55 934 066 | 0.91 (0.83–0.99) | ||
| Silveira et al (1997) | 4 countries | 5.0 | 2 296 | 456 | 73 400 000 | 0.62 (0.57–0.68) | ||
| Landaverde et al (2010) | Regional | 9.0 | 10 486 | 1 165 | 142 086 721 | 0.82 (0.77–0.87) | ||
1 Calculations based on annualized GBS cases and population reported in the paper or from raw data provided by authors.
Background GBS incidence rates
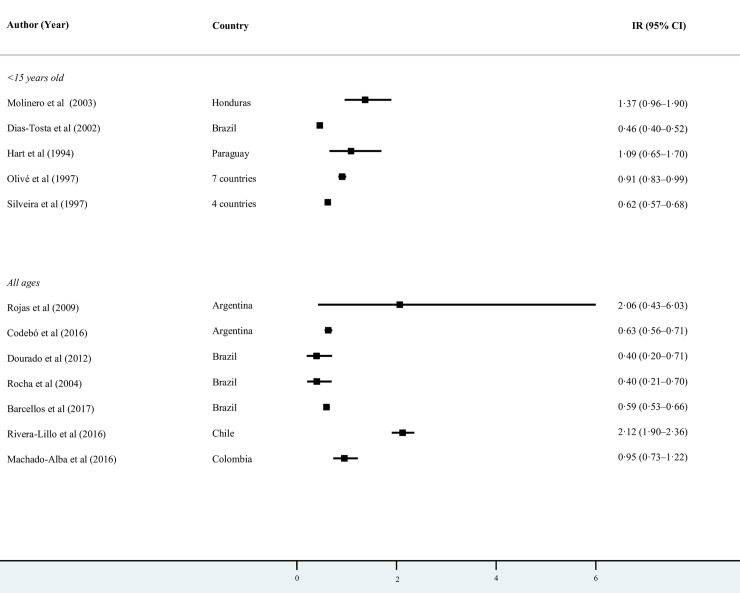
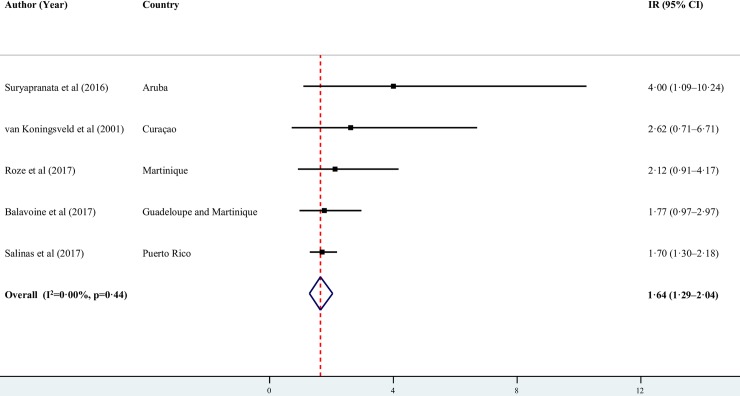
Among the studies in Latin America that reported background GBS IRs in all ages, the highest were reported in Chile (2.12 per 100,000; 95% CI 1.90–2.36)[44] and Argentina (2.06 per 100,000; 95% CI 0.43–6.03) [39], and the lowest in Brazil (0.40 per 100,000; 95% CI 0.20–0.71)[42] (Fig 2). High heterogeneity in Latin America and in other subgroup analyses precluded us from pooling the proportions. The pooled annual IR in the Caribbean was 1.64 per 100,000 (95% CI 1.29–2.12, I2 = 0.00, p = 0.44) (Fig 3).
Fig 2. Annual background incidence rates of GBS by age in Latin America per 100,000 persons.
Fig 3. Annual and pooled background incidence rates of GBS in the Caribbean per 100,000 persons.
There were large annual fluctuations in GBS IRs in any given geographic location. In Chile, over a 12-year period, IRs ranged from 1.61 per 100,000 (n = 250 cases) in 2001 to 2.35 per 100,000 (n = 402 cases) in 2010 [44]. Over a 14-year period, Dourado et al. [42] reported a range of 0.12 to 0.66 per 100,000 in Rio Grande do Norte, Brazil. Another multi-year study from Brazil estimated mean annual IRs of 0.4 per 100,000 (range, 0.3–0.6) [43]. In the Caribbean island of Aruba, over an 11-year period, Suryapranata et al. reported a range of 1 to 11 cases of GBS, the latter occurring during an outbreak of C. jejuni (IRs ranged 1.0–10.37 per 100,000). There was less fluctuation in IRs in Argentina, ranging from 0.56 per 100,000 in 2011 to 0.76 per 100,000 in 2010 over a 7-year period [40]. However, the IR of GBS among members of a private health maintenance organization in Buenos Aires averaged 1.99 per 100,000 over an 8-year period [39].
GBS incidence rates in children
Of the studies reporting background IRs in children under 15, the lowest rates were in Brazil at 0.40 per 100,000 [18], followed by Paraguay at 0.72 per 100,000 [45], and the highest rates in El Salvador at 3.86 per 100,000 [18], Chile at 1.63 per 100,000 [18], and Honduras at 1.37 per 100,000 [37].
The age-specific distribution of GBS in the pediatric population varied widely across countries. Two multi-country studies found the highest GBS IR in the 1−4 age group: Olivé et al. reported that 47% of the GBS cases were in children 1−4 years of age [35] and Silveira et al. reported an average IR of 0.86 per 100,000 (95% CI 0.78–0.89) in this age group as compared to 0.52 per 100,000 (95% CI 0.49–0.53) among 5−14 year-olds [36]. In Paraguay, the IRs ranged from 1.7 per 100,000 among 1−4 year-olds to 0.1 per 100,000 among 10−14 year-olds [45]. In Brazil, a similar trend was reported, with 40% of the cases reported in children under 5 [41]. However, in Chile the IRs were higher among 5−9 year-old children (2.23 per 100,000) than among 0−4 year−olds (2.17 per 100,000) [44].
Distribution of GBS by age in the population as a whole
GBS distribution by age in the general population did not follow a consistent pattern across countries. In Chile, the IR showed a bimodal distribution with a peak in the youngest ages (2.23 per 100,000 in 5−9 year-olds), and increased from 1.22 per 100,000 among 20−29 year-olds to 4.30 per 100,000 among 70−79 years-olds [44]. In São Paulo, Brazil, GBS was most common among 15−40 year-olds (0.15 per 100,000), and the IR was lowest in the over 60 (0.60 per 100,000) and under 15 (0.80 per 100,000) age groups [43]. In Rio Grande do Norte, Brazil, half of the cases were recorded among under 20 years-olds [42]. In Argentina, 37% of the GBS cases were reported in children under 14 years of age [40]. Reporting of higher IRs among children might be due to higher case detection resulting from vaccination safety and polio eradication surveillance efforts.
Sex differences
Studies consistently found a higher burden of GBS among males. In studies of children 15 years of age and younger, the highest male-to-female ratio was documented in El Salvador (1.8:1) [35] and the lowest in Brazil (1.2:1) [41] and Honduras 1.3:1 [37]. Among all ages, the highest male-to-female ratio was documented in Aruba (2.3:1) [46], followed by Argentina (1.6:1) [40], and the lowest in Brazil (1.3:1) [42].
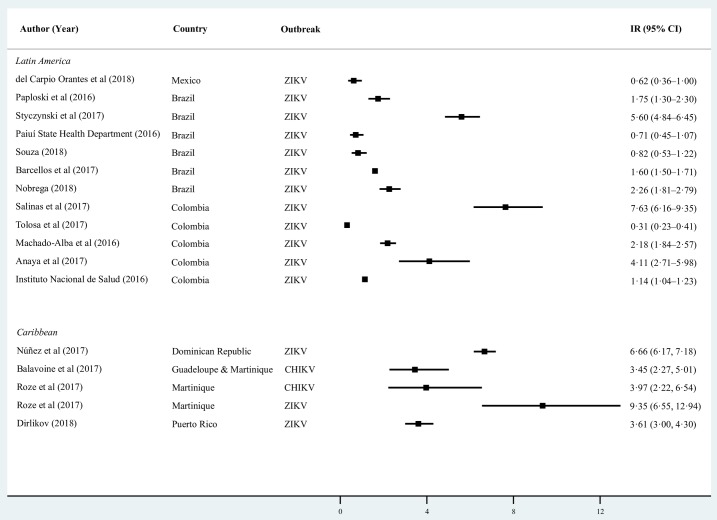
GBS and arboviral infections
We found that 17 studies reported the GBS IR during arboviral disease outbreaks in seven countries; 14 during the 2015–2016 ZIKV epidemic, one during the 2014 CHIKV outbreak in the French West Indies, and 1 during DENV, CHIKV and ZIKV outbreaks in Martinique (See Fig 4). The IR in the French West Indies during the 2014 CHIKV epidemic was 3.45 per 100,000 persons [27], representing a two-fold increase from 1.77 per 100,000 during 2011–2013 (p = 0.006) [27]. The IR in Martinique increased by 4.4 times to 9.35 per 100,000 during the ZIKV epidemic from a mean annual IR of 2.12 per 100,000 during 2006–2015 [Incidence rate ratio (IRR) (2016 vs. 2006–2015) = 4.52; 95% CI 2.80–7.64] [62]. In Puerto Rico, the IR increased by 2.1 times during the ZIKV epidemic from 1.7 to 3.5 per 100,000 [IRR (2016 vs. 2012–2015) = 2.06; 95% CI 1.51–2.85] [48, 63].
Fig 4. Annualized incidence rates of GBS by sub-region during arboviral epidemic outbreaks.
In South America, there was substantial heterogeneity in the GBS IRs during the 2015−2016 ZIKV epidemic for the population as a whole, ranging from 7.63 per 100,000 in Barranquilla, Colombia [58], to 0.71 per 100,000 in the Brazilian state of Piauí [54]. In a multi-year study, Barcellos et al. reported a 2.7 (IRR (2015–2016 vs. 2008–2015) = 2.7; 95% CI 2.38–3.07) increase in the rate of GBS hospitalizations in Brazil’s Northeast region during the peak of the ZIKV epidemic as compared to the mean rate in the eight years preceding the epidemic [60]. In Colombia, Machado et al. reported over a two-fold increase in GBS diagnoses during the peak of the ZIKV epidemic as compared to baseline rates (IRR (2016 vs. 2014–2015) = 2.29; 95% CI 1.69–3.14) [61].
In Veracruz, Mexico, del Carpio Orantes reported a GBS IR of 0.62 per 100,000 during the ZIKV epidemic in 2016 [49].
We performed sub-group analyses by case-ascertainment (i.e., administrative data based on ICD codes only versus both administrative data and medical record review) to assess if the IR heterogeneity was due to this factor. We found significant within-group heterogeneity but not between-group heterogeneity, indicating that case ascertainment did not significantly bias the IRs. This held true when we performed a region-wide analysis, and when we analyzed Latin American and the Caribbean sub-regions separately (See S1 Fig).
Discussion
The pooled background IR of GBS calculated in the Caribbean of 1.64 per 100,000 (95% CI 1.29–2.12, I2 = 0.00, p = 0.44) was higher than the mean IR in North America and Europe but within the estimated range for those regions (0.80−1.90 per 100,000) [13]. In LAC, background IRs of GBS ranged from 0.40 per 100,000 in Rio Grande do Norte [42] and São Paulo [43] in Brazil to 2.12 per 100,000 in Chile [44]. These background IRs exceeded the upper and lower ranges calculated by the aforementioned meta-analysis [13], but were comparable to the range reported in a 2009 GBS literature review from 0.38/100,000/year in Finland to 2.53/100,000/year in Curaçao [14].
Sejvar et al. found that the incidence of GBS increased with age [13]. However, we did not observe similar trends in the LAC population, with the GBS IR exhibiting different age-specific patterns across settings. The higher burden of GBS among males reported in this review was consistent with Sejvar et al., with an estimated relative risk for males of 1.78 (95% CI 1.36–2.33) [13].
This review synthesizes GBS incidence data from CHIKV and ZIKV epidemic outbreaks. However, data on CHIKV infection and GBS are limited to the French West Indies. The association of DENV infection with GBS is not well established. One multi-year study in Aruba found a positive correlation between number of GBS cases and laboratory-confirmed DENV infections (p = 0.004, Kendall’s tau-b) [46]. However, a study from Brazil reported no association between DENV outbreaks and GBS [64]. More data are needed on incidence of GBS during CHIKV and DENV outbreaks in the region. In terms of ZIKV, most studies showed temporal associations of ZIKV infections with GBS rates. However, fewer studies reported GBS rates at baseline and during an outbreak, in part because of sparse historical data on GBS. Dos Santos et al. reported increases in IRs during the weeks of ZIKV transmission ranging from 100% in El Salvador (95% CI 55.7–156.9) to 877% in Venezuela (95% CI 664.1–1149.6) as compared to estimated pre-ZIKV baselines [25]. Consistent with Dos Santos et al., studies that reported rates of GBS at baseline and during epidemic weeks reported increases of 106% in Puerto Rico (IRR (2016 vs. 2012–2015) = 2.06; 95% CI 1.51–2.85), 352% in Martinique (IRR (2016 vs. 2006–2015) = 4.52; 95% CI 2.80–7.64), 171% in Brazil’s Northeastern region (IRR (2015–2016 vs. 2008–2015) = 2.7; 95% CI 2.38–3.07), and 129% in Colombia (IRR (2016 vs. 2014–2015) = 2.29; 95% CI 1.69–3.14). In summary, all studies that reported rates of GBS before and during the ZIKV outbreak showed significant temporal increases in IRs during the outbreak. However, this could be due to publication bias. Furthermore, heterogeneity coupled with limited data precludes us from drawing region-wide comparisons of these differences.
ZIKV emerged in an immunologically naive population and spread rapidly throughout the Americas, with the consequent impact on human health [65]. In the upcoming years it is unlikely that the region will experience similar peaks of GBS due to ZIKV infection given the build-up of population immunity against this arbovirus [66]. However, other emerging pathogens, including different arboviruses and influenza strains [67], may trigger increases in the incidence of GBS. In the future, in addition to background GBS-triggering foodborne infections, we expect GBS incidence to be cyclical as attack rates of arboviral infections fluctuate seasonally as well as in response to population immunity. Foodborne infections are often cited as the most common GBS trigger, responsible for between 25% and 50% of GBS cases worldwide [1]. Of eight studies included in this review that examined C. jejuni as an antecedent infection to GBS, only two found a positive association. On the island of Curaçao, authors found a positive temporal association between C. jejuni infections and GBS in pre-ZIKV years (1987–1999) [47]. In Veracruz, Mexico, 75% of GBS patients tested positive for C. jejuni infection in 2017 (after the peak of ZIKV) [49]. Studies in Aruba, Brazil, and Colombia–all except the one from Aruba were conducted during the ZIKV epidemic–found no association between C. jejuni infection and GBS [43, 46, 50, 52, 55, 58]. A study in Brazil found respiratory infections to be present in 27% of GBS patients compared to 7% with gastrointestinal infections in pre-ZIKV years (1995–2002) [43]. Although examining the role of foodborne infections was outside the scope of this research, this limited evidence suggests that temporal increases in GBS incidence during ZIKV in LAC are not associated with C. jejuni infection. With more robust GBS surveillance and increased awareness, the region would be better prepared to monitor fluctuations in GBS incidence in the future. Although this review focused on GBS in endemic populations, incidence of neurological conditions in other regions might be impacted by international travelers returning from areas with ZIKV and other arboviral disease transmission, or with a sexual partner who returned from such areas [68–76].
Strengths and limitations
Data on GBS, both historical and current, are lacking for many countries in the region. With the exception of studies using AFP data, most are recent and from ZIKV-affected countries, and baseline data are often lacking. Publication bias in favor of significant results may have limited the availability of studies that found no association between arboviral epidemics and GBS. On the other hand, ZIKV’s high public visibility most likely led to increased scientific research and publications on GBS as well as surveillance (i.e., detection) bias, particularly in countries affected by the emerging arbovirus. Few studies reported data from Central America and Mexico. Therefore, estimates for this sub-region have a high risk of bias. All but two studies reported effect sizes for the outcome of interest. However, we addressed this limitation by calculating 95% confidence intervals for all studies.
Methodological differences in case finding limit comparability across studies. The specificity and sensitivity of administrative data varies by setting [48, 77]. Rates of GBS based on administrative records or passive surveillance systems, without medical record review, may be prone to over-reporting [48] and under-reporting [35, 78], respectively. We opted to include surveillance and administrative data because of the paucity of data available. The high cost of capture-recapture methods to compare sensitivity of case identification and ascertainment methods of a rare syndrome limits the feasibility of such studies to assess GBS incidence nationwide. Several researchers have accepted the use of administrative data as a viable low-cost option to assess background GBS IRs [40, 44].
A limitation of this review is that studies employed different methodologies to ascertain GBS cases. Diagnosing GBS is complex and based on clinical observation and electrophysiological studies. Specialists who can properly diagnose GBS may be unavailable in resource-limited settings. Natural annual variations in incidence may be masked or exacerbated by imperfect case identification.
A strength of this research is that all included studies are population based. In addition, many of the studies are multi-year in duration, with a mean of 7.2 years for studies that analyzed background GBS IRs. This gives us a robust estimate of GBS incidence that balances out natural fluctuations in annual IRs. Another strength is the application of a thorough search methodology and inclusion of most languages spoken in LAC. Inclusion of ministry of health bulletins data served to balance publication bias.
Some of the included studies were not carried out with the sole purpose of measuring GBS IRs. While the studies that focused on IR calculations tended to adjust the rates based on population age structures, others did not. This introduces a source of variability. Some studies reported incidence by person-years and others as annual incidence. We addressed this issue by calculating all IRs.
Since GBS is triggered by a variety of antecedent infections, baseline incidence of GBS is critical for detecting and monitoring infectious disease outbreaks. The LAC region has been a pioneer in monitoring of GBS in children over the last 30 years [16]. Countries such as Colombia and Brazil have monitored GBS as part of DENV eradication programs [57, 79]. The ZIKV epidemic and the reported increases in GBS in the Americas have made GBS a notifiable condition in many countries. As the ZIKV epidemic has spread beyond the Americas [80–82], it is important that those countries are particularly prepared for GBS surveillance and management. Enhanced surveillance and increased research have provided us with new data to assess GBS incidence in this region. Because of its severity and lethality in the absence of adequate care, investments are needed to provide information on GBS to populations at-risk and to build healthcare providers’ capacity to diagnose GBS and follow appropriate care protocols [83, 84]. GBS poses an additional burden to health care systems, particularly in resource-limited settings.
Supporting information
(DOC)
(PDF)
(DOCX)
Sub-group analysis by case ascertainment: administrative data and medical record review versus ICD code only.
(PDF)
Risk of bias in GBS observational studies.
(PDF)
Acknowledgments
The authors wish to thank Christovam Barcellos, PhD (Oswaldo Cruz Foundation, Rio de Janeiro, Brazil), Amilton Antunes Barreira, MD, PhD (Universidade de São Paulo, Sao Paulo, Brazil), Luis del Carpio Orantes, MD (Instituto Mexicano del Seguro Social, Veracruz, Mexico), Mario E. T. Dourado Jr., MD, MSc (Hospital Universitario Onofre Lopes/Universidade Federal do Rio Grande do Norte, Natal, Brazil), Jorge E. Machado-Alba, MD, PhD (Universidad Tecnológica de Pereira-Audifarma S.A., Pereira, Colombia), Salim Máttar, PhD (Universidad de Córdoba, Montería, Colombia), Franciska S. T. Suryapranata, MS (VU University Medical Centre, Amsterdam, The Netherlands), and Arnold R. Thompson Cerna, MD (Hospital Nacional Dr. Mario Catarino Rivas, San Pedro Sula, Honduras) for providing additional information about their studies.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
AWS's participation in this study was partially funded by the European Union's Horizon 2020 research and innovation program ZikaPLAN, under Grant Agreement No. 734584. URL:https://ec.europa.eu/programmes/horizon2020/en. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Willison HJ, Jacobs BC, van Doorn PA. Guillain-Barre syndrome. Lancet. 2016;388(10045):717–27. 10.1016/S0140-6736(16)00339-1 [DOI] [PubMed] [Google Scholar]
- 2.Van Den Berg B, Walgaard C, Drenthen J, Fokke C, Jacobs BC, Van Doorn PA. Guillain-Barre syndrome: Pathogenesis, diagnosis, treatment and prognosis. Nature Reviews Neurology. 2014;10(8):469–82. 10.1038/nrneurol.2014.121 [DOI] [PubMed] [Google Scholar]
- 3.Wong AHY, Umapathi T, Shahrizaila N, Chan YC, Kokubun N, Fong MK, et al. The value of comparing mortality of Guillain-Barre syndrome across different regions. J Neurol Sci. 2014;344(1–2):60–2. 10.1016/j.jns.2014.06.021 [DOI] [PubMed] [Google Scholar]
- 4.Darweesh SK, Polinder S, Mulder MJ, Baena CP, van Leeuwen N, Franco OH, et al. Health-related quality of life in Guillain-Barre syndrome patients: a systematic review. J Peripher Nerv Syst. 2014;19(1):24–35. 10.1111/jns5.12051 [DOI] [PubMed] [Google Scholar]
- 5.Jacobs BC, Van Den Berg B, Verboon C, Chavada G, Cornblath DR, Gorson KC, et al. International Guillain-Barré Syndrome Outcome Study: protocol of a prospective observational cohort study on clinical and biological predictors of disease course and outcome in Guillain-Barré syndrome. J Peripher Nerv Syst. 2017;22(2):68–76. 10.1111/jns.12209 [DOI] [PubMed] [Google Scholar]
- 6.Kogos SC Jr, Richards JS, Banos J, Schmitt MM, Brunner RC, Meythaler JM, et al. A descriptive study of pain and quality of life following Guillain-Barre syndrome: One year later. J Clin Psychol Med Settings. 2005;12(2):111–6. [Google Scholar]
- 7.Bersano A, Carpo M, Allaria S, Franciotta D, Citterio A, Nobile-Orazio E. Long term disability and social status change after Guillain-Barre syndrome. J Neurol. 2006;253(2):214–8. 10.1007/s00415-005-0958-x [DOI] [PubMed] [Google Scholar]
- 8.Ruts L, Drenthen J, Jongen JLM, Hop WCJ, Visser GH, Jacobs BC, et al. Pain in Guillain-Barre syndrome A long-term follow-up study. Neurology. 2010;75(16):1439–47. 10.1212/WNL.0b013e3181f88345 [DOI] [PubMed] [Google Scholar]
- 9.Van Den Berg B, Bunschoten C, Van Doorn PA, Jacobs BC. Mortality in Guillain-Barre syndrome. Neurology. 2013;80(18):1650–4. 10.1212/WNL.0b013e3182904fcc [DOI] [PubMed] [Google Scholar]
- 10.Ishaque T, Islam MB, Ara G, Endtz HP, Mohammad QD, Jacobs BC, et al. High mortality from Guillain-Barré syndrome in Bangladesh. J Peripher Nerv Syst. 2017;22(2):121–6. 10.1111/jns.12215 [DOI] [PubMed] [Google Scholar]
- 11.Benamer HTS, Bredan A. Guillain-Barre syndrome in Arab countries: A systematic review. J Neurol Sci. 2014;343(1–2):221–3. 10.1016/j.jns.2014.05.065 [DOI] [PubMed] [Google Scholar]
- 12.Frenzen PD. Economic cost of Guillain-Barre syndrome in the United States. Neurology. 2008;71(1):21–7. 10.1212/01.wnl.0000316393.54258.d1 [DOI] [PubMed] [Google Scholar]
- 13.Sejvar JJ, Baughman AL, Wise M, Morgan OW. Population incidence of Guillain-Barre syndrome: A systematic review and meta-analysis. Neuroepidemiology. 2011;36(2):123–33. 10.1159/000324710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGrogan A, Madle GC, Seaman HE, De Vries CS. The epidemiology of Guillain-Barre syndrome worldwide: A systematic literature review. Neuroepidemiology. 2009;32(2):150–63. 10.1159/000184748 [DOI] [PubMed] [Google Scholar]
- 15.Pan American Health Organization. Polio eradication to be reviewed in August. 1994. 1994-June Report No.: 0251–4710 12345536 [Google Scholar]
- 16.Quadros CA, Hersh BS, Olive JA, Andrus JK, Silveira CMd, Carrasco PA. Eradication of Wild Poliovirus from the Americas: Acute Flaccid Paralysis Surveillance, 1988–1995. J Infect Dis. 1997;175((Suppll)):S37–42. [DOI] [PubMed] [Google Scholar]
- 17.Pan American Health Organization. Director announces campaign to eradicate poliomyelitis from the Americas by 1990. Bull Pan Am Health Organ. 1985;19:213–5. [Google Scholar]
- 18.Landaverde JM, Danovaro-Holliday MC, Trumbo SP, Pacis-Tirso CL, Ruiz-Matus C. Guillain-Barré Syndrome in Children Aged <15 Years in Latin America and the Caribbean: Baseline Rates in the Context of the Influenza A (H1N1) Pandemic. J Infect Dis. 2010;201(5):746–50. 10.1086/650530 [DOI] [PubMed] [Google Scholar]
- 19.Wilder-Smith A, Gubler DJ, Weaver SC, Monath TP, Heymann DL, Scott TW. Epidemic arboviral diseases: priorities for research and public health. Lancet Infect Dis. 2017;17(3):e101–e6. 10.1016/S1473-3099(16)30518-7 [DOI] [PubMed] [Google Scholar]
- 20.Tang H, Hammack C, Ogden SC, Wen Z, Qian X, Li Y, et al. Zika Virus Infects Human Cortical Neural Progenitors and Attenuates Their Growth. Cell Stem Cell. 2016;18(5):587–90. 10.1016/j.stem.2016.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mehta R, Soares CN, Medialdea-Carrera R, Ellul M, da Silva MTT, Rosala-Hallas A, et al. The spectrum of neurological disease associated with Zika and chikungunya viruses in adults in Rio de Janeiro, Brazil: A case series. PLoS Negl Trop Dis. 2018;12(2):e0006212 10.1371/journal.pntd.0006212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilder-Smith A, Preet R, Renhorn KE, Ximenes RA, Rodrigues LC, Solomon T, et al. ZikaPLAN: Zika Preparedness Latin American Network. Glob Health Action. 2017;10(1):1398485 10.1080/16549716.2017.1398485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cao-Lormeau VM, Blake A, Mons S, Lastere S, Roche C, Vanhomwegen J, et al. Guillain-Barre Syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet. 2016;387(10027):1531–9. 10.1016/S0140-6736(16)00562-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pan American Health Organization. Regional Zika Epidemiological Update (Americas) August 25, 2017 Available from: https://www.paho.org/hq/index.php?option=com_content&view=article&id=13622:25-august-2017-zika-epidemiological-update&Itemid=42346&lang=en [Google Scholar]
- 25.Dos Santos T, Rodriguez A, Almiron M, Sanhueza A, Ramon P, De Oliveira WK, et al. Zika Virus and the Guillain-Barre syndrome—Case series from seven countries. N Engl J Med. 2016;375(16):1598–601. 10.1056/NEJMc1609015 [DOI] [PubMed] [Google Scholar]
- 26.Krauer F, Riesen M, Reveiz L, Oladapo OT, Martínez-Vega R, Porgo TV, et al. Zika Virus Infection as a Cause of Congenital Brain Abnormalities and Guillain-Barré Syndrome: Systematic Review. PLoS Med. 2017;14(1):1–27. 10.1371/journal.pmed.1002203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Balavoine S, Pircher M, Hoen B, Herrmann-Storck C, Najioullah F, Madeux B, et al. Guillain-Barre syndrome and chikungunya: description of all cases diagnosed during the 2014 outbreak in the French West Indies. Am J Trop Med Hyg. 2017;97(2):356–60. 10.4269/ajtmh.15-0753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pinheiro TJ, Guimaraes LF, Silva MT, Soares CN. Neurological manifestations of Chikungunya and Zika infections. Arq Neuropsiquiatr. 2016;74(11):937–43. 10.1590/0004-282X20160138 [DOI] [PubMed] [Google Scholar]
- 29.Carod-Artal FJ, Wichmann O, Farrar J, Gascon J. Neurological complications of dengue virus infection. Lancet Neurol. 2013;12(9):906–19. 10.1016/S1474-4422(13)70150-9 [DOI] [PubMed] [Google Scholar]
- 30.World Health Organization. Assessment and management of Guillain-Barré syndrome in the context of Zika virus infection. 2016. Available from: https://www.who.int/csr/resources/publications/zika/guillain-barre-syndrome/en/ [Google Scholar]
- 31.Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350:g7647 10.1136/bmj.g7647 [DOI] [PubMed] [Google Scholar]
- 32.Hoy D, Brooks P, Woolf A, Blyth F, March L, Bain C, et al. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol. 2012;65(9):934–9. 10.1016/j.jclinepi.2011.11.014 [DOI] [PubMed] [Google Scholar]
- 33.Institute of Medicine Committee to Review the Adverse Consequences of P, Rubella V. The National Academies Collection: Reports funded by National Institutes of Health In: Howson CP, Howe CJ, Fineberg HV, editors. Adverse Effects of Pertussis and Rubella Vaccines: A Report of the Committee to Review the Adverse Consequences of Pertussis and Rubella Vaccines. Washington, DC: National Academies Press, 1991. [PubMed] [Google Scholar]
- 34.Barendregt JJ, Doi SA, Lee YY, Norman RE, Vos T. Meta-analysis of prevalence. J Epidemiol Community Health. 2013;67(11):974–8. 10.1136/jech-2013-203104 [DOI] [PubMed] [Google Scholar]
- 35.Olive JM, Castillo C, Castro RG, Quadros CAd. Epidemiologic study of Guillain-Barre syndrome in children <15 years of age in Latin America. J Infect Dis. 1997;175(Suppl. 1):S160–S4. [DOI] [PubMed] [Google Scholar]
- 36.Silveira CMd, Salisbury DM, Quadros CAd. Measles vaccination and Guillain-Barre syndrome. Lancet. 1997;349(9044):14–6. 10.1016/s0140-6736(96)07408-9 [DOI] [PubMed] [Google Scholar]
- 37.Molinero MR, Varon D, Holden KR, Sladky JT, Molina IB, Cleaves F. Epidemiology of childhood Guillain-Barre syndrome as a cause of acute flaccid paralysis in Honduras: 1989–1999. J Child Neurol. 2003;18(11):741–7. 10.1177/08830738030180110801 [DOI] [PubMed] [Google Scholar]
- 38.De la Pena O, Robles-Figueroa M, Chavez-Pena Q, Bedolla-Barajas M. [Features of Guillain-Barre syndrome in adults: results of a university hospital]. Rev Med Inst Mex Seguro Soc. 2015;53(6):678–85. [PubMed] [Google Scholar]
- 39.Rojas JI, Giunta D, Patrucco L, Stefani C, Rosso B, Rugiero M, et al. Incidence of Multiple Sclerosis, Myasthenia Gravis and Guillain-Barre Syndrome in Argentina. Neurology. 2009;72(11):A451–A. [Google Scholar]
- 40.Codebó O, Bonanno D, Almeida V, Dorigo A, Gazia V, Poyard E, et al. Guillain-Barré Syndrome in Argentina: Its Public Health Relevance in Light of Zika Virus Emergence. Revista Argentina de Salud Pública. 2016;7(28):38–40. [Google Scholar]
- 41.Dias-Tosta E, Kuckelhaus CS. Guillain Barre syndrome in a population less than 15 years old in Brazil. Arq Neuropsiquiatr. 2002;60(2 B):367–73. [DOI] [PubMed] [Google Scholar]
- 42.Dourado ME, Felix RH, Silva WKAd, Queiroz JW, Jeronimo SMB. Clinical characteristics of Guillain-Barre syndrome in a tropical country: a Brazilian experience. Acta Neurol Scand. 2012;125(1):47–53. 10.1111/j.1600-0404.2011.01503.x [DOI] [PubMed] [Google Scholar]
- 43.Guimaraes Rocha MS, Dozzi Brucki SM, De Siqueira Carvalho AA, Poti Lima UW. Epidemiologic features of Guillain-Barre syndrome in Sao Paulo, Brazil. Arq Neuropsiquiatr. 2004;62(1):33–7. 10.1590/s0004-282x2004000100006 [DOI] [PubMed] [Google Scholar]
- 44.Rivera-Lillo G, Torres-Castro R, Burgos PI, Varas-Diaz G, Vera-Uribe R, Puppo H, et al. Incidence of Guillain-Barre syndrome in Chile: a population-based study. J Peripher Nerv Syst. 2016;21(4):339–44. 10.1111/jns.12182 [DOI] [PubMed] [Google Scholar]
- 45.Hart DE, Rojas LA, Rosario JA, Recalde H, Roman GC. Childhood Guillain-Barre syndrome in Paraguay, 1990 to 1991. Ann Neurol. 1994;36(6):859–63. 10.1002/ana.410360609 [DOI] [PubMed] [Google Scholar]
- 46.Suryapranata FS, Ang CW, Chong LL, Murk JL, Falconi J, Huits RM. Epidemiology of Guillain-Barre Syndrome in Aruba. Am J Trop Med Hyg. 2016;94(6):1380–4. 10.4269/ajtmh.15-0070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Koningsveld R, Rico R, Gerstenbluth I, Schmitz PI, Ang CW, Merkies IS, et al. Gastroenteritis-associated Guillain-Barre syndrome on the Caribbean island Curacao. Neurology. 2001;56(11):1467–72. 10.1212/wnl.56.11.1467 [DOI] [PubMed] [Google Scholar]
- 48.Salinas JL, Major CG, Pastula DM, Dirlikov E, Styczynski A, Luciano CA, et al. Incidence and clinical characteristics of Guillain-Barre syndrome before the introduction of Zika virus in Puerto Rico. J Neurol Sci. 2017;377:102–6. 10.1016/j.jns.2017.04.006 [DOI] [PubMed] [Google Scholar]
- 49.del Carpio-Orantes L, Peniche Moguel KG, Sánchez Díaz JS, Pola-Ramirez MR, Mata Miranda MP, García-Méndez S, et al. Síndrome de Guillain Barré asociado a Zika, análisis de la cohorte delegacional en la región Veracruz norte durante 2016–2017. Neurologia. 2018. 10.33588/rn.6711.2018380 [DOI] [PubMed] [Google Scholar]
- 50.Souza CO, Vieira MACS, Batista FMA, Eulalio KD, Neves JMM, Sa LC, et al. Serological Markers of Recent Campylobacter jejuni Infection in Patients with Guillain-Barre Syndrome in the State of Piaui, Brazil, 2014–2016. Am J Trop Med Hyg. 2018. 10.4269/ajtmh.17-0666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paploski IAD, Prates APPB, Cardoso CW, Kikuti M, Silva MMO, Waller LA, et al. Time Lags between Exanthematous Illness Attributed to Zika Virus, Guillain-Barré Syndrome, and Microcephaly, Salvador, Brazil. Emerg Infect Dis. 2016;22(8):1438–44. 10.3201/eid2208.160496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nobrega M, Araujo ELL, Wada MY, Leite PLE, Dimech GS, Percio J. Outbreak of Guillain-Barre syndrome possibly related to prior Zika virus infection, Metropolitan Region of Recife, Pernambuco, Brazil, 2015. Epidemiologia e servicos de saude: revista do Sistema Unico de Saude do Brasil. 2018;27(2):e2017039 10.5123/S1679-49742018000200016 [DOI] [PubMed] [Google Scholar]
- 53.Styczynski AR, Malta JM, Krow-Lucal ER, Percio J, Nobrega ME, Vargas A, et al. Increased rates of Guillain-Barre syndrome associated with Zika virus outbreak in the Salvador metropolitan area, Brazil. PLoS Negl Trop Dis. 2017;11(8):e0005869 10.1371/journal.pntd.0005869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Piauí. Secretaria de Estado da Saúde. [Arboviruses and neurological syndromes in Piauí] Bol Epidemiol Semanal. 2016 Nov 12; ed esp. Available from: http://www.saude.pi.gov.br/uploads/document/file/129/Boletim_agravos_neurol_gicos_nov_2016_WORD__2_.pdf
- 55.Anaya JM, Rodriguez Y, Monsalve DM, Vega D, Ojeda E, Gonzalez-Bravo D, et al. A comprehensive analysis and immunobiology of autoimmune neurological syndromes during the Zika virus outbreak in Cucuta, Colombia. J Autoimmun. 2017;77:123–38. 10.1016/j.jaut.2016.12.007 [DOI] [PubMed] [Google Scholar]
- 56.Tolosa N, Tinker SC, Pacheco O, Valencia D, Botero DS, Tong VT, et al. Zika Virus Disease in Children in Colombia, August 2015 to May 2016. Paediatr Perinat Epidemiol. 2017;31(6):537–45. 10.1111/ppe.12391 [DOI] [PubMed] [Google Scholar]
- 57.Instituto Nacional de Salud. Boletín Epidemiológico Semanal. Dirección de Vigilancia y Análisis del Riesgo en Salud Pública. Bogotá, Colombia: Instituto Nacional de Salud; 2016. [Google Scholar]
- 58.Salinas JL, Walteros DM, Styczynski A, Garzon F, Quijada H, Bravo E, et al. Zika virus disease-associated Guillain-Barre syndrome-Barranquilla, Colombia 2015–2016. J Neurol Sci. 2017;381:272–7. 10.1016/j.jns.2017.09.001 [DOI] [PubMed] [Google Scholar]
- 59.Núnñez R, Cornelio Y, Pimentel R. Síndrome de Guillain Barré asociado a Zika-República Dominicana enero-octubre 2016. 9th TEPHINET Global Scientific Conference; Chiang Mai, Thailand 2017.
- 60.Barcellos C, Xavier DR, Pavao AL, Boccolini CS, Pina MF, Pedroso M, et al. Increased Hospitalizations for Neuropathies as Indicators of Zika Virus Infection, according to Health Information System Data, Brazil. Emerg Infect Dis. 2016;22(11):1894–9. 10.3201/eid2211.160901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Machado-Alba JE, Machado-Duque ME, Gaviria-Mendoza A, Orozco-Giraldo V. Diagnosis of neurological disorders and the Zika virus epidemic in Colombia 2014–2016. Int J Infect Dis. 2016;51:133–4. 10.1016/j.ijid.2016.09.010 [DOI] [PubMed] [Google Scholar]
- 62.Roze B, Najioullah F, Ferge JL, Dorleans F, Apetse K, Barnay JL, et al. Guillain-Barre Syndrome Associated with Zika Virus Infection in Martinique in 2016: A Prospective Study. Clin Infect Dis. 2017;65(9):1462–8. 10.1093/cid/cix588 [DOI] [PubMed] [Google Scholar]
- 63.Dirlikov E, Major CG, Medina NA, Lugo-Robles R, Matos D, Munoz-Jordan JL, et al. Clinical Features of Guillain-Barre Syndrome With vs Without Zika Virus Infection, Puerto Rico, 2016. JAMA Neurol. 2018;75(9):1089–97. 10.1001/jamaneurol.2018.1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Santos J, Marques W, Fazan VPS, Barreira AA. No association between guillain-barre syndrome and dengue fever. J Peripher Nerv Syst. 2009;(2):132. [Google Scholar]
- 65.Ferguson NM, Cucunuba ZM, Dorigatti I, Nedjati-Gilani GL, Donnelly CA, Basanez MG, et al. Epidemiology. Countering the Zika epidemic in Latin America. Science. 2016;353(6297):353–4. 10.1126/science.aag0219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.O'Reilly KM, Lowe R, Edmunds WJ, Mayaud P, Kucharski A, Eggo RM, et al. Projecting the end of the Zika virus epidemic in Latin America: a modelling analysis. BMC Med. 2018;16(1):180 10.1186/s12916-018-1158-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vellozzi C, Iqbal S, Broder K. Guillain-Barre syndrome, influenza, and influenza vaccination: the epidemiologic evidence. Clin Infect Dis. 2014;58(8):1149–55. 10.1093/cid/ciu005 [DOI] [PubMed] [Google Scholar]
- 68.Pearce JC, Learoyd TP, Langendorf BJ, Logan JG. Japanese encephalitis: the vectors, ecology and potential for expansion. J Travel Med. 2018;25(suppl_1):S16–S26. 10.1093/jtm/tay009 [DOI] [PubMed] [Google Scholar]
- 69.Lindquist L. Recent and historical trends in the epidemiology of Japanese encephalitis and its implication for risk assessment in travellers. J Travel Med. 2018;25(suppl_1):S3–S9. 10.1093/jtm/tay006 [DOI] [PubMed] [Google Scholar]
- 70.Rezza G. Chikungunya is back in Italy: 2007–2017. J Travel Med. 2018;25(1). 10.1093/jtm/tay004 [DOI] [PubMed] [Google Scholar]
- 71.Nakayama E, Tajima S, Kotaki A, Shibasaki KI, Itokawa K, Kato K, et al. A summary of the imported cases of Chikungunya fever in Japan from 2006 to June 2016. J Travel Med. 2018;25(1). 10.1093/jtm/tax072 [DOI] [PubMed] [Google Scholar]
- 72.Masyeni S, Yohan B, Somia IKA, Myint KSA, Sasmono RT. Dengue infection in international travellers visiting Bali, Indonesia. J Travel Med. 2018;25(1). 10.1093/jtm/tay061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Riddell A, Babiker ZO. Imported dengue fever in East London: a 6-year retrospective observational study. J Travel Med. 2017;24(3). 10.1093/jtm/tax015 [DOI] [PubMed] [Google Scholar]
- 74.Jentes ES, Lash RR, Johansson MA, Sharp TM, Henry R, Brady OJ, et al. Evidence-based risk assessment and communication: a new global dengue-risk map for travellers and clinicians. J Travel Med. 2016;23(6). 10.1093/jtm/taw062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ferguson RW, Henderson SJ, Lee EA, Jung P. Dengue in Peace Corps Volunteers, 2000–14. J Travel Med. 2016;23(3). 10.1093/jtm/taw010 [DOI] [PubMed] [Google Scholar]
- 76.Neuberger A, Turgeman A, Lustig Y, Schwartz E. Dengue fever among Israeli expatriates in Delhi, 2015: implications for dengue incidence in Delhi, India. J Travel Med. 2016;23(3). 10.1093/jtm/taw003 [DOI] [PubMed] [Google Scholar]
- 77.Bogliun G, Beghi E, Italian GBS Registry Study Group. Validity of hospital discharge diagnoses for public health surveillance of the Guillain-Barrè syndrome. Neurol Sci. 2002;23(1):113–7. [DOI] [PubMed] [Google Scholar]
- 78.Lee CD, Jones TF. Optimizing a hospital discharge database for passive surveillance of guillain-barre syndrome. Ann Neurol. 2011;(15):S41. [Google Scholar]
- 79.Carmo E, Gemal A, Oliveira S. Vigilancia en Salud en Suramerica: epidemiologica, sanitaria y ambiental Rio de Janeiro: Instituto Suramericano de Gobierno en Salud and UNASUR; 2013. [Google Scholar]
- 80.Hamer DH, Chen LH. Zika in Angola and India. J Travel Med. 2019. 10.1093/jtm/taz012 [DOI] [PubMed] [Google Scholar]
- 81.Watts AG, Huber C, Bogoch II, Brady OJ, Kraemer MUG, Khan K. Potential Zika virus spread within and beyond India. J Travel Med. 2018;25(1). 10.1093/jtm/tay132 [DOI] [PubMed] [Google Scholar]
- 82.Leder K, Grobusch MP, Gautret P, Chen LH, Kuhn S, Lim PL, et al. Zika beyond the Americas: Travelers as sentinels of Zika virus transmission. A GeoSentinel analysis, 2012 to 2016. PLoS One. 2017;12(10):e0185689 10.1371/journal.pone.0185689 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wilder-Smith A, Chang CR, Leong WY. Zika in travellers 1947–2017: a systematic review. J Travel Med. 2018;25(1). 10.1093/jtm/tay044 [DOI] [PubMed] [Google Scholar]
- 84.Leonhard SE, Lant S, Jacobs BC, Wilder-Smith A, Ferreira MLB, Solomon T, et al. Zika virus infection in the returning traveller: what every neurologist should know. Pract Neurol. 2018;18(4):271–7. 10.1136/practneurol-2017-001789 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(PDF)
(DOCX)
Sub-group analysis by case ascertainment: administrative data and medical record review versus ICD code only.
(PDF)
Risk of bias in GBS observational studies.
(PDF)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.