Abstract
Purpose
To determine whether pharmacological administration of recombinant human anti-Mullerian hormone (rAMH) protects the ovarian reserve and preserves fertility without interfering with anti-tumoural cytotoxic action of chemotherapy.
Methods
Intraperitoneal delivery of rAMH and ovarian post-receptor activity were assessed with immunohistochemistry and western blot. Differential follicle counts and reproductive outcomes were assessed after cyclophosphamide (Cy) administration, with/without concurrent administration of rAMH. Interference of rAMH with Cy chemotoxicity was assessed on a human breast cancer cell line and an in vivo mouse model of human leukaemia.
Results
rAMH reached the ovary after intraperitoneal injection and demonstrated post-receptor bioactivity. Cy administration in mice caused primordial follicle activation, as shown by a decrease in primordial follicle population accompanied by an increase in early growing follicles and granulosa cell proliferation. Co-administration of rAMH reduced follicle activation, thereby protecting the primordial follicle reserve, and improving long-term fertility and reproductive outcomes. rAMH co-administration did not interfere with the cytotoxic actions of Cy in vitro on breast cancer cell line or in vivo in a model of human leukaemia.
Conclusion
This study demonstrates that rAMH is bioactive in the ovary for a limited time, and that pharmacological administration of rAMH during chemotherapy treatment reduces follicle activation and primordial follicle loss and significantly improves reproductive outcomes in a mouse model, and does not interfere with the therapeutic actions of the treatment. Further investigation is necessary to determine whether it has similar protective effects in the human ovary.
Keywords: Fertility preservation, Chemotherapy, Anti-Mullerian hormone, Follicle activation
Introduction
The cause of premature ovarian insufficiency (POI) in cancer survivors is chemotherapy-induced loss of the primordial follicle (PMF) reserve [1]. While there are several methods currently available to help preserve fertility in cancer patients (including embryo, oocyte, and ovarian tissue cryopreservation [2–4], these methods are all invasive procedures and are limited by patient age and status or limited by the timeframe necessary before treatment. As such, there has been a focus on developing preventative pharmacological methods for fertility preservation, which will be suitable for all patients, and enable them to retain their natural fertility by preventing the loss of ovarian follicle reserve during chemotherapy [5, 6].
Chemotherapy-induced loss of the ovarian follicle reserve occurs via multiple routes, both extrinsic to the dormant follicle such as stromal fibrosis [7, 8] and intrinsic to the dormant follicle population. We previously demonstrated that alkylating agent cyclophosphamide (Cy) induces PMF loss in mice via dormant follicle activation and ‘burn-out’ [9]. Other studies have since corroborated this ‘burn-out’ effect, both with Cy ([10]—in mice; [11]—in human ovarian tissue) and with another ovotoxic chemotherapy drug, cisplatin [12, 13]. This accelerated follicle activation appears to be caused by dysregulation in pathways that control follicle dormancy including upregulation of the PI3K-Akt signalling pathway. This has been substantiated by evidence in animal models that treatment with PI3K pathway suppressors [9, 14, 15] attenuates the follicle activation and loss caused by chemotherapy and preserves fertility. It has also been suggested that activation is triggered by the death of growing follicles [16], which play a vital role in maintaining PMF suppression via the excretion of suppression factors, primarily anti-Mullerian hormone (AMH) [17]. AMH is a highly specific negative regulator of follicle activation [18] and in vitro studies of mouse, bovine, and human ovarian tissue have demonstrated that the addition of AMH to culture media reduces the activation and growth of primordial follicles [17, 19–21]. The ‘burn-out’ hypothesis suggests that removal or interruption of this suppression, such as occurs when chemotherapy causes death of the growing follicles, causes PMF activation. Recent studies have shown that virally produced human AMH or recombinant mouse AMH can attenuate chemotherapy-induced loss of ovarian reserve in mice [22, 23]. This study examines delivery, presence, and in vivo bioactivity of human C-terminus recombinant AMH (rAMH), and the efficacy of rAMH to protect the follicle reserve and long-term fertility in a mouse model of ovotoxic chemotherapy. In order to further examine the potential of rAMH for use in conjunction with cancer treatments, we also investigated whether rAMH acts by interfering with the cytotoxic activity of the chemotherapy using two models of human cancer: a breast cancer cell line and an in vivo model of leukaemia.
Materials and methods
Ethical approval
All animal experiments were approved by the Institutional Animal Care and Use Ethics Committee.
Mice
All mice (Envigo, Israel) were housed under special pathogen free (SPF) conditions, with a 12-h light/dark cycle and free access to an autoclaved pelleted diet and water. ‘Low-reserve’ mice were a group of mice which were treated at 6 weeks of age with 4 weekly doses of 75 mg/kg Cy [24], in order to reduce their ovarian reserve and used for initial pharmacokinetic studies 6 months later (n = 3 mice per treatment group per timepoint). Ovaries were removed 2, 7, and 17 h after rAMH injection for histological analysis. For the in vivo treatment study, mice (n = 6–8 mice per group per timepoint) were injected with 5 μg/mouse rAMH (recombinant human MIS/AMH, R&D Systems, USA) half an hour prior to Cy injection (150 mg/kg, IP), and then every 6 h for a total of 5 injections over 24 h (timeframe was based on results of pharmacokinetic study). Ovaries were removed 2, 7, or 21 days post initial Cy injection. The mating experiment was based on our previous study [9]; 5-week-old female Balb/C mice were randomly allocated to one of four treatment groups (n = 10 mice per treatment group). One group received two separate IP injections of 150 mg/kg Cy 2 weeks apart. The second group received the same doses of Cy, along with rAMH during each treatment, as above (5 doses of 5 μg/mouse every 6 h). A third group received only rAMH injections, and a fourth group received PBS in equivalent volumes. One month after the second dose of Cy, the mice were mated for 7 days, 2 females per proved fertile male Balb/C mice. They were then separated from the males and monitored for 3 weeks until they gave birth. This cycle was repeated every 7 weeks for a total of 5 mating rounds. Pregnancy incidence was calculated as the number of mice per group which achieved at least one pregnancy. Pregnancy rate was calculated as the number of pregnancies achieved by each mouse divided by the total number of mating rounds.
Biotin labelling
rAMH was biotin-labelled using Lightning-Link Rapid Biotin Conjugation Kit according to the manufacturer’s instructions (Innova Biosciences, UK). Labelled rAMH was immediately injected IP into mice. Ovaries were harvested 6 h following labelled rAMH injection, and processed for immunohistochemistry.
Western blotting for SMAD 1/5/8
Whole ovaries were homogenised in a RIPA buffer (Sigma-Aldrich, Israel) containing phosphatase and protease inhibitors (Roche, Switzerland). Protein content was determined with a bicinchoninic acid reagent, and 25 μg of protein from each sample was loaded onto 12% SDS-polyacrylamide gels. After transferring proteins to nitrocellulose membranes, blots were probed with appropriate antibodies: Phospho-Smad1 (Ser463/465)/Smad5 (Ser463/465)/Smad8 (Ser465/467) (D5B10) rabbit mAb (no. 13820), vinculin (E1E9V) rabbit mAb (no. 13901) from Cell Signaling Technology (USA); peroxidase-conjugated AffiniPure Goat Anti-Rabbit IgG (111-035-003, Jackson ImmumoResearch Laboratories, USA) was used to detect proteins through enzymatic chemiluminescence substrate (Westar Nova 2.0, Cyanagen).
Histology and follicle counts
Ovaries were fixed in 4% paraformaldehyde (PFA), paraffin embedded, serially sectioned (5 μm sections), and stained with haematoxylin and eosin. Differential follicle counts on whole ovaries were conducted at consecutive intervals of 100 μm to avoid double counting of follicles, and a correction factor applied to produce an estimate of the total follicles in the ovary [9, 15, 25]. Follicle stage was classified according to the accepted definitions [26]. A primordial follicle was counted when the nucleus was clearly identified surrounded by a single layer of flattened squamous follicular cells. A primary follicle was defined as an oocyte surrounded by a single layer of cuboidal granulosa cells. A secondary follicle had 2 or more layers of cuboidal granulosa cells, but no antrum, and was counted only if nucleus was visible.
Immunohistochemistry for KI67
Sections were incubated for 1 h with primary antibodies: Ki-67 (rabbit monoclonal, 275R, 1:200, Cell Marque). Slides stained with secondary antibody only served as negative controls. Murine lungs bearing Lewis lung carcinoma (LLC) served as positive control for Ki-67. HRP detection was conducted using HiDef detection polymer (954D, Cell Marque, USA), and peroxidase substrate kit (SK-4100, Vector Labs, USA) was used as a chromogen and haematoxylin as counterstain.
XTT assay
MDA-MB-231 breast cancer cells were cultured in DMEM supplemented with 10% fetal calf serum and 1% penicillin-streptomycin (all from Biological Industries, Israel). For the XTT assay, cells were plated at 3–5 × 103 cells per well into 96-well plates in media with the addition of 200 ng/ml rAMH, 0–80 μg/ml phosphoramide mustard CS (PM), or a combination of the two compounds at the indicated concentrations. XTT assay was performed according to the manufacturer’s instructions (Biological Industries, Israel). Briefly, after 48 h of treatment, cells were cultured for 2–24 h with XTT reagent. Absorbance of the formazan product was measured at 450 nm with a multichannel microplate spectrophotometer (Tecan). Experiments were done in quadruplicates, and repeated 3 times.
In vivo drug interference assay
Seven- to ten-week-old NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG, Jackson Laboratory) female mice (n = 26) were injected with 5 × 106 B cell precursor leukaemia NALM6 cell line [27, 28]. Once xenografts were successfully engrafted in the bone marrow (BM) (5–10% human blasts verified by BM aspiration), mice were randomised to four treatment groups, PBS, rAMH, Cy, and Cy+rAMH. rAMH (5 μg/mouse) was injected IP half an hour prior to Cy injection (150 mg/kg, IP), and then every 6 h for a total of 5 injections over 24 h. Forty-eight hours after Cy treatment, mice were sacrificed and the BM and spleen were harvested. The spleen weight and size were measured. Spleen and BM samples were labelled with the following antibody mix: 7AAD (BD bioscience, USA), ECD-labelled human CD19 (Beckman Coulter, USA), PE-Cy7-labelled human CD10 (Beckman Coulter, USA), Alexa Flour 450–labelled mouse CD45 (ThermoFisher, USA) for 20 min at 4 °C, and measured by flow cytometer (Beckman Coulter, USA). Disease burden was assessed at this end point by measuring the absolute number of bone marrow and spleen blasts (human CD10+/CD19+ cells) using CountBright absolute counting beads (ThermoFisher, USA).
Statistics
Data from treated and untreated groups were compared using the Kruskal-Wallis test (with multiple comparisons using Dunn’s test) or Tukey’s multiple comparison test (for the in vivo model of leukaemia). All statistical tests were two-sided. A p value < 0.05 was considered to be statistically significant.
Results
Injected human C-terminus rAMH reaches the ovary and has bioactivity in mouse ovaries
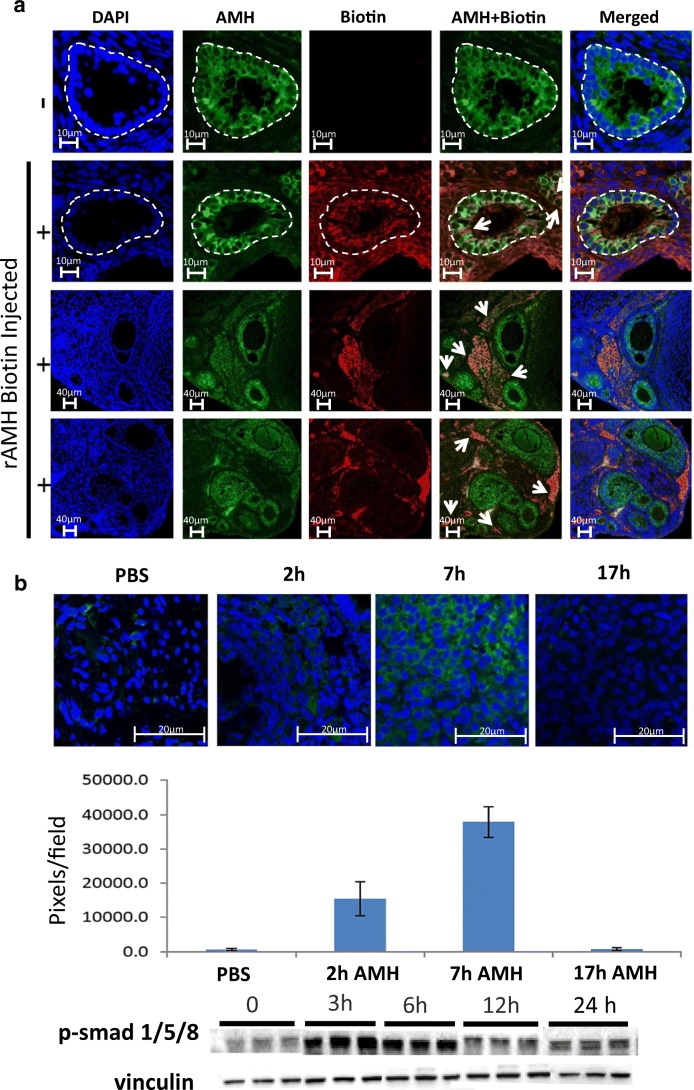
We initially conducted studies to clarify the in vivo kinetics and bioactivity of the rAMH. Biotin-labelled rAMH was delivered via IP injection and was visualised in the ovary 6 h post injection in the cytoplasm of granulosa cells of small growing follicles in the ovarian cortex and in areas of the surrounding stroma (Fig. 1a). The presence of injected rAMH in the ovary was confirmed in a model of low-reserve mice. These mice had been pre-treated with multiple doses of Cy to reduce their ovarian reserve to almost nothing (in previous studies, primordial follicle reserve was reduced by 95% [9], such that their baseline level of AMH was extremely low thereby enabling detection of injected rAMH). In these mice, an increasing presence of rAMH was visible in the ovaries up to 7 h post IP injection, with no rAMH evident by 17 h post injection (Fig. 1b). It was not possible to more specifically localise the rAMH to primordial follicles since as a result of the pre-treatment with Cy, these ovaries contained almost no follicles. Based on this information on the kinetics of rAMH in vivo, in the subsequent in vivo experiments, mice were given IP injections of rAMH at 6 hourly intervals. To investigate the bioactivity and cross-reactivity of the human C-terminus rAMH used in this study, we tested in vivo post-receptor activity in the SMAD pathway in ovaries of mice injected with rAMH (Fig. 1c). AMH has been shown to act via the SMAD molecular pathway, binding to a type I and type II receptor complex which phosphorylates SMAD 1, 5, and 8, triggering in turn the phosphorylation of SMAD 4 [29, 30]. An increase in pSMAD1,5,8 was seen in ovaries between 3 and 6 h after in vivo injection of rAMH (Fig. 1c). These studies demonstrate that IP injection is an efficient method for delivery of rAMH to the ovary, that rAMH is only present in the ovary for a short time, and that rAMH has bioactivity.
Fig. 1.
Bioactivity and IP delivery of rAMH. (a) rAMH was biotin-labelled and injected IP into 6-week-old female mice (10 μg per mouse). Histological analysis was conducted on ovaries removed 6 h post injection (n = 4). PBS-injected (row 1) and biotin-rAMH-injected (rows 2–4) samples were stained with AMH (green) and biotin (red), and DAPI (blue) antibodies. Yellow staining indicates merged AMH and biotin, representing exogenous rAMH. White circles outline the small growing follicles showing the presence of endogenous and exogenous AMH in the cytoplasm of the granulosa cells and in stromal cells in the surrounding tissue. Biotin-labelled exogenous rAMH can be clearly seen in the cytoplasm of granulosa cells of growing follicles (marked by arrows in row 2) and in areas of the ovarian stroma (marked by arrows in rows 3 and 4). Magnification × 63. (b) Mature 6-week-old female mice were treated with 4 weekly doses of 75 mg/kg Cy, a dose which reduced ovarian follicle reserve to below 10% of normal values in our previous studies [9]. Six months later, when almost the entire ovarian reserve was depleted and endogenous levels of AMH were close to non-existent, the mice were injected IP with 10 μg rAMH (unlabelled) (n = 3 mice per timepoint). Histological analysis was conducted on ovaries removed 0, 2, 7, and 17 h post rAMH injection. Samples were stained with AMH (green) antibody and representative images from 4 ovaries from each group were analysed with ImageJ software. Magnification × 63. c Western blot analysis for p-SMAD 1,5,8 and housekeeping protein, vinculin, was conducted on ovaries from 6-week-old mice removed 0, 3, 6, 12, and 24 h post rAMH injection (n = 3 mice per timepoint)
rAMH co-treatment reduces chemotherapy-induced follicle activation and loss in vivo
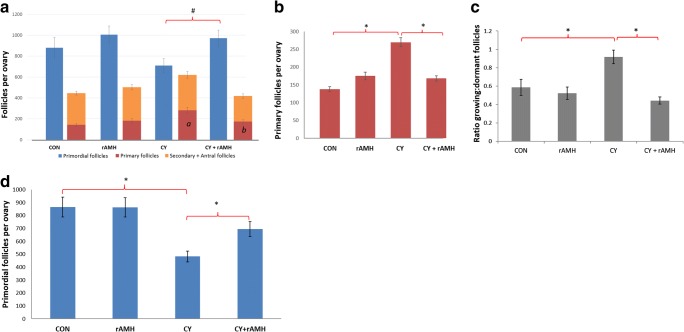
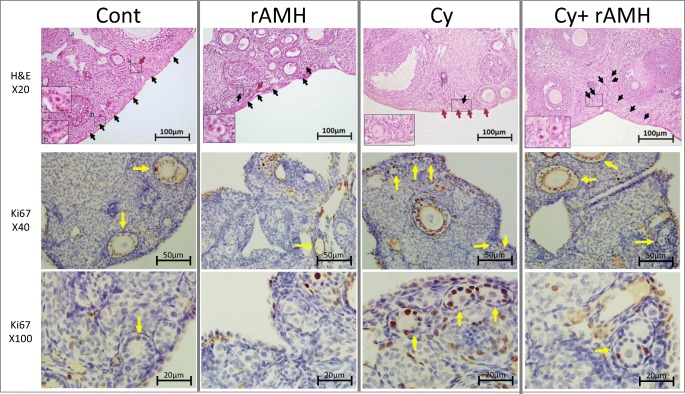
We examined the impact of rAMH co-treatment in vivo on follicle dynamics in a mouse model of Cy-induced ovotoxicity [9, 24]. Differential follicle counts conducted on ovaries removed 7 days after treatment exhibited a reduction in primordial follicles in ovaries from mice treated with Cy alone already at this stage (Fig. 2a), and a significant increase in the number of primary follicles in ovaries from the Cy group compared with controls (270 ± 26 vs. 138 ± 12.9, p < 0.05; Fig. 2b). Co-administration of rAMH, however, significantly reduced the increase in primary follicles that occurred after Cy treatment (168 ± 15 vs. 270 ± 26, p < 0.05; Fig. 2b). The ratio of growing to dormant follicles for mice in the Cy treatment group was significantly increased (0.9 ± 0.07 compared with 0.59 ± 0.09, p < 0.05), and co-administration of rAMH reduced this ratio to levels on par with control groups (0.44 ± 0.04; Fig. 2c). Representative images of ovaries from each treatment group are presented in Fig. 3, showing primordial follicles (black arrows), and primary follicles (red arrows). The changes in follicle dynamics observed immediately after Cy treatment were supported by proliferation staining, which showed increased proliferation (Ki67 staining) in granulosa cells of follicles in Cy-treated mice 2 days after the beginning of the treatment (yellow arrows indicate follicles with positive Ki67 staining, Fig. 3). However, no increase in proliferation was seen in ovaries of mice which were co-treated with rAMH, indicating that rAMH co-administration reduces the activation and growth of the PMFs caused by Cy.
Fig. 2.
rAMH reduces follicle activation and loss in an in vivo mouse model of Cy treatment. Mature 6-week-old female mice were treated with a single dose of 150 mg/kg Cy with or without co-treatment with rAMH (IP injection of 5 μg per mouse every 6 h) for the first 24 h. (a, b, c) Differential follicle counts were conduction on ovaries removed 7 days after treatment. (a) Primordial vs. growing follicle numbers, mean follicle number per ovary ± SEM, n = 6–8 mice per treatment group, *p < 0.05 #p = 0.05). (b) A comparison of the numbers of primary vs. secondary and larger follicles shows the changes within each follicle class incurred by the treatment (7 days after treatment, *p < 0.05). (c) Ratio of growing (primary, secondary, and antral) to dormant (primordial) follicles in ovaries of each treatment group (data represent means of the ratios of each ovary ± SEM, *p < 0.05). (d) PMF counts conducted on ovaries removed 21 days after Cy treatment for assessment of the impact of treatment on primordial follicle reserve (data shown represents mean follicle number per ovary ± SEM, n = 6–8 mice per treatment group, *p < 0.05)
Fig. 3.
Histological evidence of follicle activation and rAMH protection in an in vivo mouse model of Cy treatment. Representative H&E images of ovaries from each of the four treatment groups indicating the changes in primordial follicle (black arrows) and primary follicle (red arrows) populations after treatment. Magnification of boxed areas is shown in the inset pictures. Immunohistochemical staining for Ki67 on ovaries removed 2 days after Cy treatment from mice from each treatment group. Yellow arrows indicate follicles containing granulosa cells which stained positive for Ki67. Sections from a minimum of four animals in each group were stained
Evaluation of ovarian reserve was conducted in a study which examined a longer timepoint of 3 weeks following Cy treatment, once changes in follicle growth would have passed and the final impact of treatment on ovarian reserve could be assessed. Three weeks after Cy treatment, ovaries demonstrated a significantly reduced population of primordial follicles, with a mean of 483 ± 42 PMF per ovary compared with 865 ± 77 in the control group (p < 0.05; Fig. 2d). However, mice which received rAMH co-treatment with Cy had a significantly greater number of PMFs per ovary (695 ± 57), an increase of 34% compared with Cy alone (p < 0.05), demonstrating a protective effect of rAMH on the ovarian reserve.
rAMH co-treatment improves fertility outcomes post chemotherapy in an in vivo mouse model
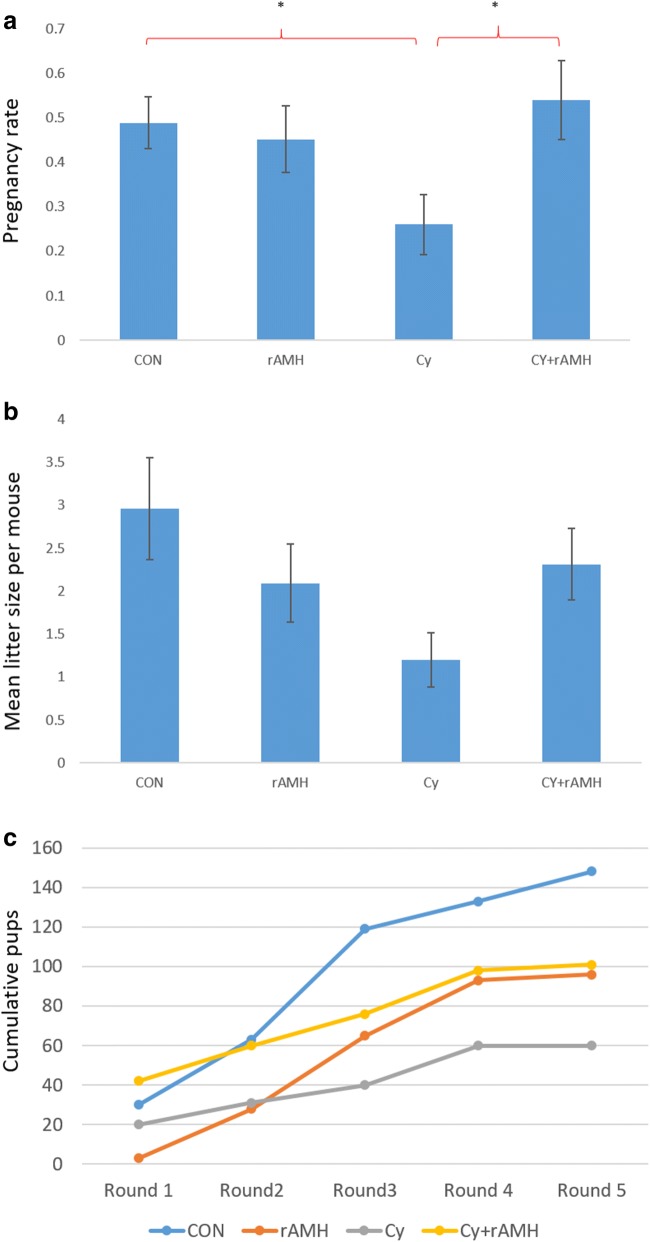
The end point impact of the protection of the ovarian reserve was measured in a mating study of mice treated with two doses of 150 mg/kg Cy at an interval of 2 weeks, either alone or in combination with 5 μg rAMH administered every 6 h for 24 h following each Cy injection. Female mice were mated with proven fertile males 5 weeks after the final treatment, and then again every 7 weeks for a total of five successive mating rounds. Cy treatment alone reduced both pregnancy rate, that is the number of times each mouse became pregnant over the course of the study, (0.26 ± 0.07 vs. 0.5 ± 0.06, p < 0.05; Fig. 4a) and mean litter size per mouse (including all mice in each group) compared with untreated mice (1.2 ± 0.3 vs. 3.0 ± 0.6; Fig. 4b). Co-treatment with rAMH improved fertility outcomes significantly, such that pregnancy rate was similar to that of control mice (0.54 ± 0.09 compared with 0.5 ± 0.06; Fig. 4a), and the overall mean litter size was increased compared with mice that received Cy without rAMH (2.3 ± 0.4 vs. 1.2 ± 0.3; Fig. 4b). Although the dose of Cy administered was not sterilising, pregnancy incidence (the number of mice that achieved pregnancy at least once) among Cy-treated mice was lower than that in the control group (6/10 compared with 9/10), but co-treatment with rAMH resulted in a pregnancy incidence identical to that of the control group (9/10). Assessment of the cumulative number of pups born within each treatment group following each mating round demonstrates that while there is only a small impact on fertility in the Cy-treated mice in the first round of mating, the impact of chemotherapy on fertility becomes more significant with subsequent rounds. The cumulative data from all the mating cycles confirms that rAMH improves fertility outcomes following Cy administration (Fig. 4c).
Fig. 4.
rAMH improves pregnancy rate and reproductive outcomes in Cy-treated mice. Mature 6-week-old female Balb/C mice (n = 10 mice per treatment group) were treated twice with 150 mg/kg Cy at a 2-week interval with or without co-treatment with rAMH (IP injection of 5 μg per mouse every 6 h). One month after the final dose of Cy, the mice were mated for 7 days with proven fertile males. They were then separated and monitored until they gave birth. This cycle was repeated every 7 weeks for a total of 5 mating rounds. (a) Pregnancy rate per mouse (the number of times each mouse became pregnant expressed as a fraction of the number of mating rounds), shown as means ± SEM, *p < 0.05. (b) Mean litter size per mouse per round (includes all mice), over the 5 rounds of mating (± SEM). (C) Cummulative total of pups born per treatment group with each successive mating round
Human rAMH does not diminish Cy chemotoxicity on human cancers
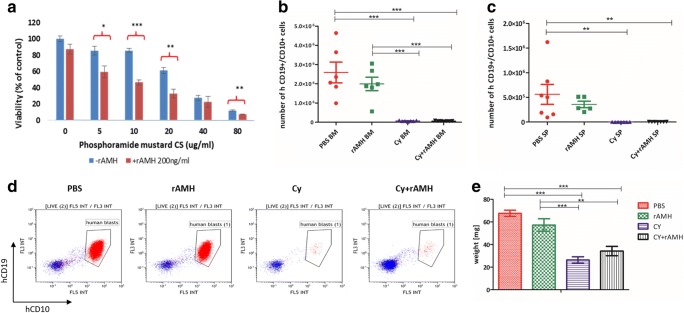
To determine whether rAMH interferes with the anti-tumoural activity of Cy, we tested its effects in vitro on human breast cancer cell line MDA-MB-231, since Cy is commonly used in chemotherapy protocols for breast cancer. In vivo, Cy is metabolised and activated by the liver, resulting in the production of the active metabolite phosphoramide mustard CS (PM), which is generally accepted as the reactive alkylating agent of therapeutic consequence and has been used in similar ex vivo studies [31]. Cells were treated with increasing concentrations of PM with/without rAMH at concentrations of 200 ng/ml, and cell viability was assessed with the XTT assay after 48 h of treatment based on previously described methods [32]. As expected, PM showed a dose-dependent impact on cell viability, and co-treatment with rAMH did not interfere with the chemotoxic activity of PM on the MDA-MB-231 cells, but possibly increased MDA-MB-231 cell sensitivity to PM at specific concentrations (Fig. 5a). In an in vivo model of leukaemia (NALM6 B cell precursor human acute lymphoblastic leukaemia (B-ALL) cells), rAMH did not affect the efficacy of the Cy treatment (Fig.5b–e). All NALM6-engrafted mice treated with Cy exhibited decreased leukaemia burden, as evidence by significant reduction in total blast count in the bone marrow and spleen (from 3.4 ± 0.9 × 106 to 6.5 ± 0.9 × 104, p < 0.001; Fig. 5b–d) and decrease in spleen weight (68 ± 2.8 vs. 26 ± 2.9 mg, p < 0.001; Fig. 5e), and rAMH treatment did not interfere with the Cy treatment (there were no significant differences in any outcome measurements between Cy-treated mice and those which also received rAMH).
Fig. 5.
rAMH does not interfere with chemotoxic activity of Cy in vitro or in vivo. (a) XTT cell viability assay was conducted on MDA-MB-231 human breast cancer cell line. Cells were treated with PM at concentrations of 0, 5, 10, 20, 40, and 80 μg/ml with or without 200 ng/ml rAMH, and XTT assay was performed at 48 h. Viability was calculated relative to the viability of control (culture medium) treated cells. Experiment was repeated 3 times and data represent means ± SEM. (b–e) An in vivo leukaemic mouse model was established using B-ALL NALM6 leukaemic cells injected into NGS female mice. Once leukaemic load was established (2 weeks after initial injection), mice were treated with a single dose of 150 mg/kg Cy with or without co-treatment with rAMH (5 μg per mouse every 6 h) for the first 24 h, and bone marrow (BM) and spleen (SP) were removed 2 days after Cy treatment for assessment of the impact of rAMH on the efficacy of Cy treatment on leukaemic cells in vivo. BM (b) and spleen (c) samples were labelled with antibody mix and the absolute number of human blasts in BM and spleen samples was measured by a flow cytometer, to assess disease burden. Representative FACS dot-plots (d) of gated human blasts in each treated group, in the BM samples. Spleen weights (e) were compared between the different treatment groups. Data represent means ± SEM (n = 5–7 mice per treatment group) and significant differences between each group are indicated where relevant; where *p < 0.05, **p < 0.01, and ***p < 0.001
Discussion
This study demonstrates that pharmacological administration of rAMH in mice reduces chemotherapy-induced follicle activation, attenuating chemotherapy-induced follicle loss, protecting the follicle reservoir, and preserving fertility without interfering with the anti-tumoural actions of chemotherapy. We further showed that recombinant C-terminus human AMH is bioactive, with a relatively short in vivo half-life.
In a mouse model of chemotherapy, a single dose of Cy caused a marked reduction in PMF numbers and a significant increase in early growing follicles shortly after Cy treatment (Fig. 2a, b). As Cy induces massive destruction of the growing follicle population [10, 31, 33], we should see not an increase but rather a vastly reduced number of growing follicles in Cy-treated mice. The existence of an increased population of early growing follicles rather than significantly decreased in the days immediately following Cy treatment (Fig. 2b) provides evidence for activation of the PMFs rather than destruction. This is further substantiated by Ki67 staining showing that 2 days after Cy treatment granulosa cells in these follicles were undergoing proliferation (Fig. 3). This supports the conclusion that Cy destroys large growing follicles in the immediate short term after treatment, which indirectly results in activation of PMFs by reducing negative suppression, generating a replacement population of early growing follicles in the few days after chemotherapy.
Follicle activation and suppression are controlled by both intra-cellular mechanisms, such as AMH secretion by growing follicles, and inter-cellular mechanisms, such as the phosphatidylinositol 3 kinase (PI3K) pathway in the oocyte and granulosa cells [34]. Previous studies have demonstrated that suppression of follicle activation via manipulation of the PI3K pathway responsible for regulating follicle dormancy and activation (using the immunomodulator AS101 [9] or mTOR inhibitors [14, 35]) attenuates the increased follicle activation caused by Cy. However, the PI3K pathway has broad activity, with important cell cycle regulatory roles including direct effects on proliferation, apoptosis, and stem cell differentiation. In contrast, AMH is a molecule that suppresses primordial follicle activation without additional broad activity in rodents [36], thereby presenting a more specific anti-activation agent. AMH is a member of the TGF-β family of proteins, produced by the granulosa cells of small growing follicles as a large homodimeric pro-hormone, before being cleaved into an N-terminus dimer and a C-terminus dimer [37], which then combine to form a stable N-C complex. Receptor binding of the C-terminus triggers phosphorylation of SMAD 1/5/8, which binds to SMAD 4 and enters the nucleus where it regulates gene transcription. In this study, we used a recombinant human C-terminus fragment of the AMH molecule, rAMH, which we demonstrated can be delivered successfully to the ovary where it is present in significant amounts 7 h following a single injection, but no longer evident by 17 h (Fig. 1b). An increase in SMAD1,5,8 phosphorylation beginning 3 h after injection demonstrated that rAMH had cross-reactivity and post-receptor activity in vivo (Fig. 1c).
The timing of the rAMH injections was chosen based on the expected timing of AMH reduction in the ovary after Cy treatment. Cy causes apoptosis in the granulosa cells of the growing follicles which produce AMH, beginning 4–12 h after injection [9]. As we observed that rAMH is biologically active in the ovary 3–6 h after injection (Fig. 1c), injecting the rAMH half an hour prior to the Cy would effectively provide additional AMH in the ovary at the time when the granulosa cells affected by Cy would begin dying and stop producing endogenous AMH.
In this study, mice that received 24 h of co-treatment with rAMH following Cy administration did not show the increased follicle proliferation seen in mice receiving Cy alone (Fig. 3), and as a result, the numbers of growing follicles and proportions of each follicle class 7 days after treatment were almost the same as in untreated mice (Fig. 2a–c). These mice retained significantly more PMFs 3 weeks post treatment—equivalent to control mice (Fig. 2d). The long-term protection generated by rAMH on the PMF reserve was evident in improved fertility and reproductive outcomes in the 9 months post chemotherapy. rAMH administration preserved fertility following Cy treatment with a significant improvement in pregnancy rate and incidence compared with animals which received Cy alone (Fig. 4a). Mice which received rAMH in addition to Cy also had a higher number of cumulative pups over the mating period (Fig. 4c) and mean litter size overall (Fig. 4b). The protection provided by AMH co-treatment seen in this study is supported by previous studies which used recombinant mouse AMH [22] or a virally produced full-length recombinant human AMH protein [23].
While the role of AMH as a PMF suppressor has been established in rodent [18, 38, 39] and bovine [20, 21] studies, the role of AMH in primates is still debated. AMH was shown to inhibit follicle activation in human cortical tissue after 7 days of in vitro culture [19] and in a xenograft model of human ovarian tissue [40], but was also shown to increase follicle activation and growth of primary follicles after 4 weeks of culture [41]. If AMH is to be considered as a potential fertility preservation agent, its role in primate tissue will need to be further elucidated. In addition, while we demonstrate that rAMH can be delivered to the ovary via IP injection and that it has post-receptor bioactivity, we also observed that the molecule has a relatively short half-life and this presents a potential limitation as far as future clinical use is concerned.
There are multiple pathways involved in chemotherapy-induced ovarian PMF loss, especially since different agents have diverse mechanisms of action, and even the same drug can have different effects on different cell types [5, 42]. Apoptosis is a major mechanism of action of many chemotherapy drugs and as such, a number of studies have investigated agents which block the apoptotic pathways in the ovary in order to increase the survival of PMFs [43–45]. However, there is ongoing debate regarding the role of apoptosis in chemotherapy-induced PMF loss. Apoptosis has definitively been shown to occur in granulosa cells of growing follicles as a result of chemotherapy; however, there is lack of in vivo evidence that apoptosis is induced directly in dormant PMFs. Deletion of apoptotic pathway proteins was shown to protect the ovarian reserve from chemotherapy [46], but this could be due to a follow-through effect, whereby protection of growing follicles from apoptosis prevents activation and loss of PMFs. A foremost concern with any potential fertility preservation agent, but particularly with anti-apoptotic agents, is that they could inhibit the anti-cancer actions of the chemotherapy. Given the evidence that AMH, in its various forms, can preserve follicles from chemotherapy-induced activation and death, it was important to investigate whether it achieved this by impeding the cytotoxic actions of the chemotherapy. In this study, we demonstrate that human rAMH does not impede the effectiveness of Cy in vitro on human breast cancer or in vivo in a mouse model of human leukaemia (Fig. 5). This is supported by a study which showed similar results with other chemotherapy agents in vitro [47]. As a potential fertoprotective agent, AMH has additional advantages, in that it is a naturally occurring, highly specific follicle suppressor, unlikely to have systemic effects or toxicity issues [48, 49].
This study demonstrates that pharmacological administration of recombinant C-terminus AMH can preserve the follicle pool and protect fertility in a mouse model of chemotherapy. This highlights the role of follicle suppression, and the importance of AMH in particular, in maintenance of the ovarian follicle reserve. Our results further suggest that rAMH will not interfere with the therapeutic actions of chemotherapy treatment.
Funding information
This work was financially supported by grants from The Israel Innovation Authority (Grant No. 53789), and the Kahn Foundation.
Compliance with ethical standards
All animal experiments were approved by the Institutional Animal Care and Use Ethics Committee.
Conflict of interest
D.M. and H.R. are holders of U.S. Provisional Patent Application No. 62/044,259, titled “Methods for preventing premature follicle activation.”
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jayasinghe YL, Wallace WHB, Anderson RA. Ovarian function, fertility and reproductive lifespan in cancer patients. Expert Rev Endocrinol Metab. 2018;13(3):125–136. doi: 10.1080/17446651.2018.1455498. [DOI] [PubMed] [Google Scholar]
- 2.Shapira M, Raanani H, Cohen Y, Meirow D. Fertility preservation in young females with hematological malignancies. Acta Haematol. 2014;132(3–4):400–413. doi: 10.1159/000360199. [DOI] [PubMed] [Google Scholar]
- 3.Donnez J, Dolmans MM. Fertility preservation in women. N Engl J Med. 2017;377(17):1657–1665. doi: 10.1056/NEJMra1614676. [DOI] [PubMed] [Google Scholar]
- 4.Diaz-Garcia C, Domingo J, Garcia-Velasco JA, Herraiz S, Mirabet V, Iniesta I, Cobo A, Remohí J, Pellicer A. Oocyte vitrification versus ovarian cortex transplantation in fertility preservation for adult women undergoing gonadotoxic treatments: a prospective cohort study. Fertil Steril. 2018;109(3):478–485.e2. doi: 10.1016/j.fertnstert.2017.11.018. [DOI] [PubMed] [Google Scholar]
- 5.Roness H, Kalich-Philosoph L, Meirow D. Prevention of chemotherapy-induced ovarian damage: possible roles for hormonal and non-hormonal attenuating agents. Hum Reprod Update. 2014;20(5):759–774. doi: 10.1093/humupd/dmu019. [DOI] [PubMed] [Google Scholar]
- 6.Lambertini M, Horicks F, del Mastro L, Partridge AH, Demeestere I. Ovarian protection with gonadotropin-releasing hormone agonists during chemotherapy in cancer patients: from biological evidence to clinical application. Cancer Treat Rev. 2019;72:65–77. doi: 10.1016/j.ctrv.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 7.Meirow D, Dor J, Kaufman B, Shrim A, Rabinovici J, Schiff E, Raanani H, Levron J, Fridman E. Cortical fibrosis and blood-vessels damage in human ovaries exposed to chemotherapy. Potential mechanisms of ovarian injury. Hum Reprod. 2007;22(6):1626–1633. doi: 10.1093/humrep/dem027. [DOI] [PubMed] [Google Scholar]
- 8.Himelstein-Braw R, Peters H, Faber M. Morphological study of the ovaries of leukaemic children. Br J Cancer. 1978;38(1):82–87. doi: 10.1038/bjc.1978.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kalich-Philosoph L, Roness H, Carmely A, Fishel-Bartal M, Ligumsky H, Paglin S, Wolf I, Kanety H, Sredni B, Meirow D. Cyclophosphamide triggers follicle activation and “burnout”; AS101 prevents follicle loss and preserves fertility. Sci Transl Med. 2013;5(185):185ra62. doi: 10.1126/scitranslmed.3005402. [DOI] [PubMed] [Google Scholar]
- 10.Chen XY, et al. Follicle loss and apoptosis in cyclophosphamide-treated mice: what’s the matter? Int J Mol Sci. 2016;(6):17. [DOI] [PMC free article] [PubMed]
- 11.Lande Y, Fisch B, Tsur A, Farhi J, Prag-Rosenberg R, Ben-Haroush A, Kessler-Icekson G, Zahalka MA, Ludeman SM, Abir R. Short-term exposure of human ovarian follicles to cyclophosphamide metabolites seems to promote follicular activation in vitro. Reprod BioMed Online. 2017;34(1):104–114. doi: 10.1016/j.rbmo.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 12.Jang H, Lee OH, Lee Y, Yoon H, Chang EM, Park M, Lee JW, Hong K, Kim JO, Kim NK, Ko JJ, Lee DR, Yoon TK, Lee WS, Choi Y. Melatonin prevents cisplatin-induced primordial follicle loss via suppression of PTEN/AKT/FOXO3a pathway activation in the mouse ovary. J Pineal Res. 2016;60(3):336–347. doi: 10.1111/jpi.12316. [DOI] [PubMed] [Google Scholar]
- 13.Chang EM, Lim E, Yoon S, Jeong K, Bae S, Lee DR, Yoon TK, Choi Y, Lee WS. Cisplatin induces overactivation of the dormant primordial follicle through PTEN/AKT/FOXO3a pathway which leads to loss of ovarian reserve in mice. PLoS One. 2015;10(12):e0144245. doi: 10.1371/journal.pone.0144245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou, L., et al., Rapamycin prevents cyclophosphamide-induced over-activation of primordial follicle pool through PI3K/Akt/mTOR signaling pathway in vivo. J Ovarian Res, 2017. 10(1): p. 56. [DOI] [PMC free article] [PubMed]
- 15.Goldman KN, Chenette D, Arju R, Duncan FE, Keefe DL, Grifo JA, Schneider RJ. mTORC1/2 inhibition preserves ovarian function and fertility during genotoxic chemotherapy. Proc Natl Acad Sci U S A. 2017;114(12):3186–3191. doi: 10.1073/pnas.1617233114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roness H, Gavish Z, Cohen Y, Meirow D. Ovarian follicle burnout: a universal phenomenon? Cell Cycle. 2013;12(20):3245–3246. doi: 10.4161/cc.26358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Durlinger, A.L., J.A. Visser, and A.P. Themmen, Regulation of ovarian function: the role of anti-Mullerian hormone. Reproduction, 2002. 124(5): p. 601–9. [DOI] [PubMed]
- 18.Durlinger AL, et al. Anti-Mullerian hormone inhibits initiation of primordial follicle growth in the mouse ovary. Endocrinology. 2002;143(3):1076–1084. doi: 10.1210/endo.143.3.8691. [DOI] [PubMed] [Google Scholar]
- 19.Carlsson IB, Scott JE, Visser JA, Ritvos O, Themmen AP, Hovatta O. Anti-Mullerian hormone inhibits initiation of growth of human primordial ovarian follicles in vitro. Hum Reprod. 2006;21(9):2223–2227. doi: 10.1093/humrep/del165. [DOI] [PubMed] [Google Scholar]
- 20.Yang MY, Cushman RA, Fortune JE. Anti-Mullerian hormone inhibits activation and growth of bovine ovarian follicles in vitro and is localized to growing follicles. Mol Hum Reprod. 2017;23(5):282–291. doi: 10.1093/molehr/gax010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gigli I, Cushman RA, Wahl CM, Fortune JE. Evidence for a role for anti-Mullerian hormone in the suppression of follicle activation in mouse ovaries and bovine ovarian cortex grafted beneath the chick chorioallantoic membrane. Mol Reprod Dev. 2005;71(4):480–488. doi: 10.1002/mrd.20338. [DOI] [PubMed] [Google Scholar]
- 22.Sonigo C, et al. AMH prevents primordial ovarian follicle loss and fertility alteration in cyclophosphamide-treated mice. FASEB J. 2018:p. fj201801089R. [DOI] [PubMed]
- 23.Kano M, Sosulski AE, Zhang LH, Saatcioglu HD, Wang D, Nagykery N, Sabatini ME, Gao G, Donahoe PK, Pépin D. AMH/MIS as a contraceptive that protects the ovarian reserve during chemotherapy. Proc Natl Acad Sci U S A. 2017;114(9):E1688–E1697. doi: 10.1073/pnas.1620729114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meirow D, Lewis H, Nugent D, Epstein M. Subclinical depletion of primordial follicular reserve in mice treated with cyclophosphamide: clinical importance and proposed accurate investigative tool. Hum Reprod. 1999;14(7):1903–7. doi: 10.1093/humrep/14.7.1903. [DOI] [PubMed] [Google Scholar]
- 25.Bucci TJ, Bolon B, Warbritton AR, Chen JJ, Heindel JJ. Influence of sampling on the reproducibility of ovarian follicle counts in mouse toxicity studies. Reprod Toxicol. 1997;11(5):689–696. doi: 10.1016/S0890-6238(97)00034-8. [DOI] [PubMed] [Google Scholar]
- 26.Pedersen T, Peters H. Proposal for a classification of oocytes and follicles in the mouse ovary. J Reprod Fertil. 1968;17(3):555–557. doi: 10.1530/jrf.0.0170555. [DOI] [PubMed] [Google Scholar]
- 27.Jacoby E, Chien CD, Fry TJ. Murine models of acute leukemia: important tools in current pediatric leukemia research. Front Oncol. 2014;4:95. doi: 10.3389/fonc.2014.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Savino AM, Sarno J, Trentin L, Vieri M, Fazio G, Bardini M, Bugarin C, Fossati G, Davis KL, Gaipa G, Izraeli S, Meyer LH, Nolan GP, Biondi A, te Kronnie G, Palmi C, Cazzaniga G. The histone deacetylase inhibitor givinostat (ITF2357) exhibits potent anti-tumor activity against CRLF2-rearranged BCP-ALL. Leukemia. 2017;31(11):2365–2375. doi: 10.1038/leu.2017.93. [DOI] [PubMed] [Google Scholar]
- 29.di Clemente N, Josso N, Gouédard L, Belville C. Components of the anti-Mullerian hormone signaling pathway in gonads. Mol Cell Endocrinol. 2003;211(1–2):9–14. doi: 10.1016/j.mce.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 30.Visser JA. AMH signaling: from receptor to target gene. Mol Cell Endocrinol. 2003;211(1–2):65–73. doi: 10.1016/j.mce.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 31.Desmeules P, Devine PJ. Characterizing the ovotoxicity of cyclophosphamide metabolites on cultured mouse ovaries. Toxicol Sci. 2006;90(2):500–509. doi: 10.1093/toxsci/kfj086. [DOI] [PubMed] [Google Scholar]
- 32.Miller L, Leor J, Rubinsky B. Cancer cells ablation with irreversible electroporation. Technol Cancer Res Treat. 2005;4(6):699–705. doi: 10.1177/153303460500400615. [DOI] [PubMed] [Google Scholar]
- 33.Zhang, Q., et al., Human amniotic epithelial cells inhibit granulosa cell apoptosis induced by chemotherapy and restore the fertility. Stem Cell Res Ther, 2015. 6: p. 152. [DOI] [PMC free article] [PubMed]
- 34.Zhang H, Liu K. Cellular and molecular regulation of the activation of mammalian primordial follicles: somatic cells initiate follicle activation in adulthood. Hum Reprod Update. 2015;21(6):779–786. doi: 10.1093/humupd/dmv037. [DOI] [PubMed] [Google Scholar]
- 35.Goldman KN, Chenette D, Arju R, Duncan FE, Keefe DL, Grifo JA, Schneider RJ. mTORC1/2 inhibition preserves ovarian function and fertility during genotoxic chemotherapy. Proc Natl Acad Sci U S A. 2017;114(12):3186–3191. doi: 10.1073/pnas.1617233114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dewailly D, Andersen CY, Balen A, Broekmans F, Dilaver N, Fanchin R, Griesinger G, Kelsey TW, la Marca A, Lambalk C, Mason H, Nelson SM, Visser JA, Wallace WH, Anderson RA. The physiology and clinical utility of anti-Mullerian hormone in women. Hum Reprod Update. 2014;20(3):370–385. doi: 10.1093/humupd/dmt062. [DOI] [PubMed] [Google Scholar]
- 37.Cate RL, Mattaliano RJ, Hession C, Tizard R, Farber NM, Cheung A, Ninfa EG, Frey AZ, Gash DJ, Chow EP, Fisher RA, Bertonis JM, Torres G, Wallner BP, Ramachandran KL, Ragin RC, Manganaro TF, MacLaughlin DT, Donahoe PK. Isolation of the bovine and human genes for Mullerian inhibiting substance and expression of the human gene in animal cells. Cell. 1986;45(5):685–698. doi: 10.1016/0092-8674(86)90783-X. [DOI] [PubMed] [Google Scholar]
- 38.Nilsson EE, Schindler R, Savenkova MI, Skinner MK. Inhibitory actions of anti-Müllerian hormone (AMH) on ovarian primordial follicle assembly. PLoS One. 2011;6(5):e20087. doi: 10.1371/journal.pone.0020087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hayes E, Kushnir V, Ma X, Biswas A, Prizant H, Gleicher N, Sen A. Intra-cellular mechanism of anti-Mullerian hormone (AMH) in regulation of follicular development. Mol Cell Endocrinol. 2016;433:56–65. doi: 10.1016/j.mce.2016.05.019. [DOI] [PubMed] [Google Scholar]
- 40.Man, L., et al., Engineered endothelium provides angiogenic and paracrine stimulus to grafted human ovarian tissue. Sci Rep, 2017. 7(1): p. 8203. [DOI] [PMC free article] [PubMed]
- 41.Schmidt KL, et al. Anti-Mullerian hormone initiates growth of human primordial follicles in vitro. Mol Cell Endocrinol. 2005;234(1–2):87–93. doi: 10.1016/j.mce.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 42.Morgan S, Anderson RA, Gourley C, Wallace WH, Spears N. How do chemotherapeutic agents damage the ovary? Hum Reprod Update. 2012;18(5):525–535. doi: 10.1093/humupd/dms022. [DOI] [PubMed] [Google Scholar]
- 43.Gonfloni S, di Tella L, Caldarola S, Cannata SM, Klinger FG, di Bartolomeo C, Mattei M, Candi E, de Felici M, Melino G, Cesareni G. Inhibition of the c-Abl-TAp63 pathway protects mouse oocytes from chemotherapy-induced death. Nat Med. 2009;15(10):1179–1185. doi: 10.1038/nm.2033. [DOI] [PubMed] [Google Scholar]
- 44.Hancke K, et al. Sphingosine 1-phosphate protects ovaries from chemotherapy-induced damage in vivo. Fertil Steril. 2007;87(1):172–177. doi: 10.1016/j.fertnstert.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 45.Li F, Turan V, Lierman S, Cuvelier C, de Sutter P, Oktay K. Sphingosine-1-phosphate prevents chemotherapy-induced human primordial follicle death. Hum Reprod. 2014;29(1):107–113. doi: 10.1093/humrep/det391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nguyen QN, Zerafa N, Liew SH, Morgan FH, Strasser A, Scott CL, Findlay JK, Hickey M, Hutt KJ. Loss of PUMA protects the ovarian reserve during DNA-damaging chemotherapy and preserves fertility. Cell Death Dis. 2018;9(6):618. doi: 10.1038/s41419-018-0633-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pieretti-Vanmarcke R, et al. Recombinant human Mullerian inhibiting substance inhibits long-term growth of MIS type II receptor-directed transgenic mouse ovarian cancers in vivo. Clin Cancer Res. 2006;12(5):1593–1598. doi: 10.1158/1078-0432.CCR-05-2108. [DOI] [PubMed] [Google Scholar]
- 48.Woodruff TK. A win-win for women’s reproductive health: a nonsteroidal contraceptive and fertoprotective neoadjuvant. Proc Natl Acad Sci U S A. 2017;114(9):2101–2102. doi: 10.1073/pnas.1700337114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kushnir VA, Seifer DB, Barad DH, Sen A, Gleicher N. Potential therapeutic applications of human anti-Mullerian hormone (AMH) analogues in reproductive medicine. J Assist Reprod Genet. 2017;34(9):1105–1113. doi: 10.1007/s10815-017-0977-4. [DOI] [PMC free article] [PubMed] [Google Scholar]