Abstract
Convolvulaceous species have been reported to contain several bioactive principles thought to be toxic to livestock including the calystegines, swainsonine, ergot alkaloids, and indole diterpene alkaloids. Swainsonine, ergot alkaloids, and indole diterpene alkaloids are produced by seed transmitted fungal symbionts associated with their respective plant host, while the calystegines are produced by the plant. To date, Ipomoea asarifolia and Ipomoea muelleri represent the only Ipomoea species and members of the Convolvulaceae known to contain indole diterpene alkaloids, however several other Convolvulaceous species are reported to contain ergot alkaloids. To further explore the biodiversity of species that may contain indole diterpenes, we analyzed several Convolvulaceous species (n=30) for indole diterpene alkaloids, representing four genera, Argyreia, Ipomoea, Stictocardia, and Turbina, that had been previously reported to contain ergot alkaloids. These species were also verified to contain ergot alkaloids and subsequently analyzed for swainsonine. Ergot alkaloids were detected in 18 species representing all four genera screened, indole diterpenes were detected in two Argyreia species and eight Ipomoea species of the 18 that contained ergot alkaloids, and swainsonine was detected in two Ipomoea species. The data suggest a strong association exists between the relationship of the Periglandula species associated with each host and the occurrence of the ergot alkaloids and/or the indole diterpenes reported here. Likewise there appears to be an association between the occurrence of the respective bioactive principle and the genetic relatedness of the respective host plant species.
Keywords: Convolvulaceous, Ipomoea, ergot alkaloids, indole diterpene, swainsonine
1. Introduction
Ipomoea species, members of the Convolvulaceae plant family, have been reported to contain several bioactive principles thought to be toxic to livestock including the tropane alkaloids (calystegines), indolizidine alkaloids (swainsonine), ergot alkaloids (e.g. ergobalansine), and indole diterpene alkaloids (e.g. paspaline) (Schimming et al. 1998; Schardl et al. 2013; Cook et al. 2014; Panaccione et al. 2014). Swainsonine has also been reported in some genera of the Fabaceae and Malvaceae while the ergot and indole diterpene alkaloids are commonly found in the Poaceae where both or only one may be detected in a given host (Cook et al. 2014; Panaccione et al. 2014). Swainsonine, ergot alkaloids, and indole diterpene alkaloids occur sporadically throughout the convolvulaceous tribe Ipomoeeae and are produced by seed transmitted fungal symbionts associated with some Ipomoea and Turbina species (Schardl et al. 2013; Cook et al. 2014; Panaccione et al. 2014). A symbiont belonging to the Chaetothyriales family has been reported to be associated with the swainsonine-containing species, I. carnea subsp. fistulosa (Cook et al. 2013), while a Clavicipitaceous symbiont, Periglandula species, is associated with ergot and indole diterpene alkaloid-containing Ipomoea species (Kucht et al. 2004; Steiner et al. 2011; Schardl et al. 2013). In contrast, calystegines have been reported to be present in many Ipomoea species and are produced by the plant (Schimming et al. 1998). No Ipomoea species have been reported to contain ergot or indole diterpene alkaloids and swainsonine.
Ipomoea species have been reported to cause a neurologic disease with lesions characteristic of a lysosomal storage disease as well as a tremorgenic syndrome with little or no diagnostic lesions (Everist, 1974; Medeiros et al. 2003; Cook et al. 2014). Swainsonine is the bioactive principle responsible for the lysosomal storage disease while indole diterpenes are likely responsible for the tremorgenic syndrome (Gardner et al. 2018; Welch et al. 2018). Two species, I. asarifolia and I. muelleri are both reported to be associated with a tremorgenic syndrome in livestock (Lee et al. 2017). Both Ipomoea species are host to a Clavicipitaceous symbiont that produces the indole diterpenes and ergot alkaloids (Beaulieu et al. 2015).
Ipomoea asarifolia and I. muelleri represent the only Ipomoea species and members of the Convolvulaceae known to contain indole diterpenes (Lee et al. 2017). In contrast, several Ipomoea species including I. asarifolia and I. muelleri as well as members of other closely related genera (i.e. Argyreia, Stictocardia, and Turbina) of the Convolvulaceae family have been reported to contain ergot alkaloids (Amor-Prats and Harborne, 1993; Eich, 2008, Beaulieu et al. 2015). A summary of most of these species is found in Eich (2008), where some of these are considered unambiguously ergot alkaloid positive while others have contradictory reports on the occurrence of ergot alkaloids. To further explore the biodiversity of species that may contain indole diterpenes, we analyzed several Convolvulaceous species for indole diterpenes representing four genera, Argyreia, Ipomoea, Stictocardia, and Turbina, that had been previously reported to contain ergot alkaloids. These species were specifically targeted since ergot and indole diterpene alkaloids are produced by the fungal symbiont, Periglandula. All species that were surveyed for indole diterpenes were also verified to contain ergot alkaloids and subsequently analyzed for swainsonine.
2. Materials and Methods
2.1. Chemicals and reagents
Terpendole E was purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO). Paxilline was obtained from Tocris Bioscience (Bristol, UK). Terpendole C was obtained from BioVision Inc. (Milpitas, CA). Standards for ergot alkaloids were obtained from Sigma-Aldrich and Alfarma (Prague, Czech Republic) or purified from natural sources (Beaulieu et al. 2013).
2.2. Plant material
All Convolvulaceous species selected for the survey had been previously reported to contain ergot alkaloids as summarized by Eich (2008). Convolvulaceous species (n=30) representing four genera, Argyreia, Ipomoea, Stictocardia, and Turbina, including I. asarifolia and I. muelleri, were surveyed. The number of specimens (n=98) surveyed per species ranged from 1 to 7 and was based upon the number of specimens available from the selected sources. Plant material (seeds and other vegetative parts) was obtained from Missouri Botanical Garden (MO), Western Australian Herbarium, Kensington, Western Australia (PERTH), National Herbarium Netherlands (WAG), GRIN (Germplasm Resource Information Network), and/or in house collections of the authors (Supplemental Table 1). Herbarium specimens have been used to explore the phytochemical diversity of plants (Amor-Prats and Harborne, 1993; Cook et al. 2009; Cook et al. 2017 a, b; Kao et al. 2018). All plant material was ground with a mixer mill for alkaloid extraction (Retsch USA, Newtown, PA, USA).
Ipomoea asarifolia (Desr.) Roem & Schult. seeds were collected near the veterinary hospital of the University of Campina Grande, Campus of Patos in the city of Patos, Paraiba, Brazil (S 7° 04’ 02” W 37° 16’ 53”) (UTC 00260470). Plants derived from the above mentioned seeds were grown in the greenhouse with a 16 hour photoperiod and day/night temperatures of 25 °C/ 20 °C. Leaves from the plant were harvested and separated into 12 aliquots, four replicates of three different drying conditions: (1) frozen at −80 °C and then freeze dried; (2) air dried at room temperature (23 °C); and (3) oven dried at 50 °C.
Plant material was extracted for detection of ergot and indole diterpene alkaloids; in brief, a measured quantity of plant material was weighed into 2 mL screw cap centrifuge tube and extracted with a measured volume of isopropyl alcohol, by mechanical rotation using the Rugged Rotator (Glas Col, LLC, Terre Haute, IN, USA) for 3 hr. The samples were centrifuged (5 min), the isopropyl alcohol removed and filtered. An aliquot was transferred to 300 μL autosample vial for analysis.
2.3. Ergot alkaloid analysis
Ergot alkaloids were analyzed by high performance liquid chromatography (HPLC) with fluorescence detection as described by Panaccione et al. (2012). Briefly, samples were separated on a Prodigy ODS3 column (5-μm particle size; 150 mm by 4.6 mm; Phenomenex, Torrance, CA) with a multilinear gradient from 5% acetonitrile + 95% 50 mM ammonium acetate to 75% acetonitrile + 25% 50 mM ammonium acetate. Analytes were detected in two serially arranged fluorescence detectors; one with excitation and emission wavelengths of 310 nm and 410 nm, respectively, to detect lysergic acid derivatives, and the other at 272 nm/372 nm to detect ergot alkaloids lacking the lysergic acid fluorophore.
2.4. Indole diterpene alkaloid analysis
High performance liquid chromatography – high resolution mass spectrometry (HPLC-HRMS) analysis of plant material was based on published methods (Rasmussen et al. 2012; Lee et al., 2017). Samples were injected (10 μL) onto a Betasil C18 reversed phase column (5 μ; 100 × 2.1 mm i.d.) (Keystone Scientific, Inc. Bellefonte, PA, USA) protected by a guard column of the same phase. The samples were eluted from the column with a gradient flow consisting of 0.1% formic acid and acetonitrile at a flow rate of 0.300 mL/min. The mobile phase program was 0.1% formic acid-acetonitrile, 80:20, v:v for 1 min followed by a linear gradient to a composition of 100% acetonitrile at 40 min. The mobile phase was delivered and samples injected using an Ultimate 3000 HPLC (Thermo Scientific, San Jose, CA, USA) and the column eluent was connected to the heated electrospray source of an Exactive Plus Orbitrap high resolution mass spectrometer (Thermo Scientific) calibrated as per the manufacturer’s instructions and with a scan range 100 – 800 Da, resolution 70000, microscans 1, sheath gas flow 35, auxiliary gas flow 10, spray voltage 4 kV, capillary temperature 320 °C, S lens RF field 55, and auxiliary gas temperature 300 °C. Chromatographic peaks were identified by generating reconstructed HPLC-HRMS chromatograms with the calculated MH+ molecular weight of indole diterpene alkaloids to 5 decimals places and with a mass tolerance of 10 ppm.
2.5. Swainsonine Analysis
Swainsonine was extracted using a modification of the procedure described by Gardner and Cook (2011). A measured quantity of dried plant material was extracted in a measured volume of 2% acetic acid for 18 h with agitation. After extraction, samples were centrifuged and an aliquot from the extraction was diluted into 20 mM ammonium acetate in a 1 mL auto-sampler vial. Samples were analyzed by LC-MS/MS to detect swainsonine as previously described (Gardner et al., 2001). The detection limit of swainsonine was 0.001% of dry weight using this extraction procedure.
Any species that tested positive for swainsonine by the LC-MS/MS method was subsequently verified to contain swainsonine by GC-MS as secondary screen. In brief, a 0.1 mL aliquot of the acetic acid extract from a swainsonine positive species was added to a 8 mL screw cap glass vial and 2 mL of ammoniated methanol (1 to 10 dilution of methanol saturated with NH3) added. The solvent was removed by evaporation under a flow of nitrogen at 60°C. To the vial was then added 0.200 mL of pyridine and 0.050 mL of BSTFA silylation reagent (Supelco, Bellefonte, PA, USA) and the vial capped and heated for 30 min at 60°C. After heating, the samples were diluted with 1.0 mL of chloroform. All samples were analyzed by GC-MS for swainsonine (TMS derivative) using the GC-MS conditions previously described (Gardner et al., 2001).
3. Results and Discussion
Ergot alkaloids were detected in 18 of the 30 species surveyed (Table 1). Among these ergot alkaloid positive species were members of four genera, Argyreia (3 species), Ipomoea (12 species), Stictocardia (2 species), and Turbina (1 species). Seventeen of the 18 species where ergot alkaloids were detected are considered to be unambiguously positive as summarized by Eich (2008). The other species, I. gracilis, where the ergot alkaloids were detected is consistent with a previous report by Beaulieu et al. (2015). For more details regarding the diversity of ergot alkaloids in these species one is referred to (Eich) 2008 and Beaulieu et al. (2015). Not all the accessions within a species contained ergot alkaloids, which may be due to the absence of the seed-transmitted fungal symbiont in some seed collections. Of the 12 species where ergot alkaloids were not detected, six had been considered unambiguously positive as summarized by Eich (2008) while the other six species have contradictory reports in regard to the occurrence of ergot alkaloids (Eich, 2008).
Table 1.
Convolvulaceous species surveyed for ergot alkaloids, indole diterpenes, and swainsonine. Number of specimens analyzed and the number detected for each respective analyte. Details regarding the sourced specimens are found in Supplemental Table 1.
| Specimens Detected | ||||
|---|---|---|---|---|
| Species | Specimens Analyzed | Ergot Alkaloids | Indole Diterpenes | Swainsonine |
| Argyreia acuta Lour.a | 2 | 1 | 1 | 0 |
| Argyreia nervosa (Burm. f.) Bojer a | 4 | 3 | 0 | 0 |
| Argyreia obtusiflia Lour.a | 2 | 2 | 2 | 0 |
| Ipomoea amnicola Morong. a | 5 | 5 | 5 | 0 |
| Ipomoea argillicola R.W. Johnson a | 5 | 4 | 4 | 0 |
| Ipomoea aristolochiifolia G. Don a | 4 | 0 | 0 | 0 |
| Ipomoea asarifolia (Desr). Roem. & Schult. a | 4 | 4 | 4 | 0 |
| Ipomoea carnea subsp. fistulosa (Mart. ex Chosiy) D.F. Austina b | 3 | 0 | 0 | 3 |
| Ipomoea coccinea L. b | 2 | 0 | 0 | 0 |
| Ipomoea costata F. Muell ex Benth. a | 3 | 0 | 0 | 3 |
| Ipomoea dumetorum Roem. & Schult.a | 2 | 0 | 0 | 0 |
| Ipomoea gracilis R. Br. a | 3 | 3 | 3 | 0 |
| Ipomoea hederacea (L.) Jacq. b | 1 | 0 | 0 | 0 |
| Ipomoea hildebrandtii Vatkeba | 1 | 1 | 0 | 0 |
| Ipomoea imperati (Vahl) Griseb. a | 3 | 0 | 0 | 0 |
| Ipomoea leptophylla Torr. a | 5 | 4 | 0 | 0 |
| Ipomoea minutiflora ( M. Martens & Galeotti) House a | 3 | 0 | 0 | 0 |
| Ipomoea muelleri Benth. a | 4 | 4 | 4 | 0 |
| Ipomoea nil (L.) Roth b | 4 | 0 | 0 | 0 |
| Ipomoea parasitica (H.B.K.) G. Don a | 4 | 3 | 0 | 0 |
| Ipomoea pedicellaris Benth. a | 2 | 0 | 0 | 0 |
| Ipomoea pes-caprae (L.) R.Br. a | 7 | 7 | 7 | 0 |
| Ipomoea philomega (Vell.) House a | 3 | 3 | 0 | 0 |
| Ipomoea purpurea (L.) Roth b | 2 | 0 | 0 | 0 |
| Ipomoea quamoclit L. b | 2 | 0 | 0 | 0 |
| Ipomoea setifera Poir. a | 2 | 1 | 1 | 0 |
| Ipomoea tricolor Cav.a | 6 | 4 | 4 | 0 |
| Stictocardia beraviensis (Vatke) Hall. f. a | 4 | 4 | 0 | 0 |
| Stictocardia tiliifolia (Desr.) Hall. f.a | 2 | 1 | 0 | 0 |
| Turbina abutiloides (H.B.K.) O’Donell a | 3 | 1 | 0 | 0 |
Unambiguously ergot alkaloid positive according to Eich (2008)
Contradictory reports regarding ergot alkaloid content according to Eich (2008)
Indole diterpene alkaloids were detected in 10 of the 30 species surveyed (Table 1, Figure 1). These 10 species also contained ergot alkaloids (Table 1). All of the accessions (n=35) among these 10 species that contained indole diterpenes also contained ergot alkaloids. Among these indole diterpene positive species were members of two genera, Argyreia (2 species) and Ipomoea (8 species). The total number of individual indole diterpenes detected among the 10 species ranged from 4 in A. acuta to 39 in I. gracilis (Table 2). In general, the Argyreia species contain fewer individual indole diterpenes than the Ipomoea species. Paspaline was the only indole diterpene detected in all the accessions where indole diterpenes were detected. Seven other indole diterpenes, emindole SB, terpendole E, terpendole H isomer (tR=26.1), terpendole I, terpendole C, 6,7-dehydro-11-hydroxy-12,13-epoxyterpendole A, and terpendole A/M isomer, were detected in greater than 80% of the accessions analyzed from the 10 species that contained indole diterpenes. Thirty three of the 41 indole diterpenes investigated occurred in greater than 50% of the accessions from the 10 species that contained indole diterpenes.
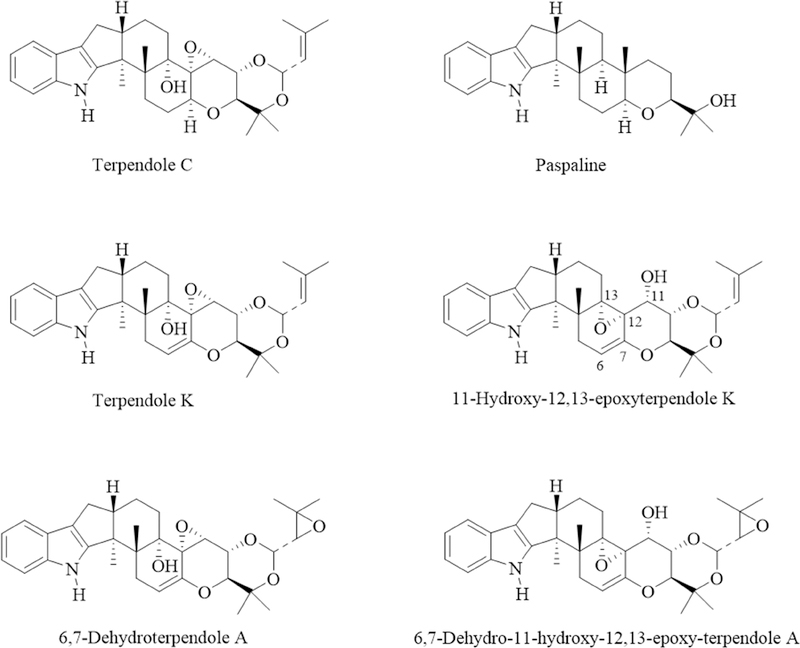
Figure 1.
Structures of representative indole diterpene alkaloids identified in isopropyl alcohol extracts of select Ipomoea and Argyreia species.
Table 2.
Individual indole diterpenes detected in those Convolvulaceous species containing indole diterpene alkaloids. Details regarding the sourced specimens are found in Supplemental Table 1.
| Indole diterpene alkaloidsa | MH+ (m/z) | Published Retention Time b | Argyreia acuta (n=2) | Argyreia obtusifolia (n=2) | Ipomoea amnicola (n=5) | Ipomoea argillicola (n=5) | Ipomoea asarifolia (n=4) | Ipomoea gracilis(n=3) | Ipomoea muelleri (n=4) | Ipomoea pes-caprae (n=7) | Ipomoea setifera (n=2) | Ipomoea tricolor (n=6) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Specimens Detected | ||||||||||||
| Emindole SB | 406.31044 | 35.1 | 0 | 1 | 5 | 4 | 4 | 3 | 4 | 6 | 1 | 0 |
| 13-Desoxypaxilline Isomer | 420.25332 | 22.2 | 0 | 2 | 0 | 4 | 3 | 3 | 4 | 3 | 1 | 0 |
| 13-Desoxypaxilline Isomer | 420.25332 | 26.8 | 0 | 0 | 0 | 4 | 2 | 3 | 4 | 5 | 0 | 0 |
| 13-Desoxypaxilline Isomer | 420.25332 | 33.6 | 0 | 0 | 2 | 4 | 4 | 3 | 4 | 3 | 0 | 2 |
| Terpendole B | 422.26897 | 26.4 | 0 | 0 | 0 | 4 | 4 | 3 | 4 | 4 | 0 | 0 |
| Paspaline Isomer | 422.30535 | 28.0 | 0 | 0 | 3 | 4 | 4 | 3 | 4 | 5 | 0 | 0 |
| Paspaline | 422.30535 | 33.7 | 1 | 2 | 5 | 4 | 4 | 3 | 4 | 7 | 1 | 4 |
| Paxilline Isomer | 436.24823 | 18.9 | 1 | 2 | 0 | 0 | 4 | 1 | 4 | 3 | 1 | 4 |
| Paxilline | 436.24823 | 23.1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Paxilline Isomer | 436.24823 | 29.7 | 0 | 0 | 4 | 1 | 4 | 2 | 4 | 6 | 0 | 4 |
| Paxilline Isomer | 436.24823 | 30.2 | 0 | 0 | 1 | 0 | 4 | 2 | 4 | 0 | 0 | 4 |
| Paxilline Isomer | 436.24823 | 30.9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Paxitriol Isomer | 438.26388 | 22.2 | 0 | 2 | 0 | 4 | 4 | 3 | 4 | 7 | 1 | 1 |
| Paxitriol Isomer | 438.26388 | 33.6 | 0 | 0 | 0 | 4 | 4 | 3 | 4 | 5 | 0 | 2 |
| Terpendole E | 438.30027 | 23.5 | 1 | 0 | 5 | 4 | 4 | 3 | 4 | 7 | 0 | 1 |
| Terpendole E Isomer | 438.30027 | 35.1 | 0 | 0 | 5 | 4 | 4 | 3 | 4 | 4 | 0 | 0 |
| Terpendole H Isomer | 452.24314 | 12.6 | 0 | 0 | 2 | 4 | 3 | 3 | 2 | 3 | 1 | 4 |
| Terpendole H | 452.24314 | 20.4 | 0 | 0 | 2 | 0 | 3 | 3 | 4 | 7 | 1 | 4 |
| Terpendole H Isomer | 452.24314 | 22.1 | 0 | 0 | 3 | 4 | 3 | 3 | 4 | 6 | 0 | 2 |
| Terpendole H Isomer | 452.24314 | 24.9 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 0 | 0 | 0 |
| Terpendole H Isomer | 452.24314 | 26.1 | 0 | 0 | 2 | 3 | 4 | 3 | 4 | 7 | 1 | 4 |
| Terpendole H Isomer | 452.24314 | 26.4 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Terpendole H Isomer | 452.24314 | 29.1 | 0 | 0 | 3 | 4 | 1 | 2 | 3 | 4 | 0 | 0 |
| Terpendole H Isomer | 452.24314 | 30.3 | 0 | 0 | 1 | 4 | 4 | 3 | 4 | 4 | 0 | 4 |
| Terpendole I | 454.25879 | 18.9 | 1 | 2 | 2 | 4 | 4 | 3 | 4 | 6 | 1 | 4 |
| Terpendole I Isomer | 454.25879 | 29.7 | 0 | 0 | 1 | 4 | 4 | 3 | 4 | 4 | 0 | 2 |
| Terpendole I Isomer | 454.25879 | 30.2 | 0 | 0 | 0 | 4 | 4 | 3 | 4 | 2 | 0 | 4 |
| Terpendole D | 506.32648 | 33.6 | 0 | 0 | 0 | 4 | 4 | 3 | 4 | 4 | 1 | 3 |
| Terpendole K Isomer | 518.29009 | 26.1 | 0 | 0 | 2 | 2 | 4 | 2 | 4 | 4 | 1 | 4 |
| 11-Hydroxy,12,13-epoxyterpendole K | 518.29009 | 27.2 | 0 | 0 | 4 | 4 | 4 | 3 | 4 | 7 | 0 | 2 |
| Terpendole K | 518.29009 | 29.1 | 0 | 0 | 4 | 4 | 1 | 3 | 4 | 4 | 0 | 1 |
| Terpendole K Isomer | 518.29009 | 31.4 | 0 | 0 | 2 | 2 | 3 | 2 | 2 | 2 | 0 | 4 |
| Terpendole C | 520.30574 | 29.7 | 0 | 0 | 5 | 4 | 4 | 3 | 4 | 6 | 0 | 4 |
| Terpendole C Isomer | 520.30574 | 30.4 | 0 | 0 | 4 | 4 | 4 | 3 | 4 | 3 | 0 | 4 |
| Terpendole J Isomer | 522.32139 | 26.6 | 0 | 2 | 0 | 0 | 4 | 1 | 0 | 1 | 1 | 3 |
| Terpendole J | 522.32139 | 30.2 | 0 | 0 | 4 | 4 | 4 | 2 | 4 | 3 | 0 | 4 |
| 6,7-Dehydroterpendole A Isomer | 534.28501 | 18.6 | 0 | 0 | 2 | 2 | 3 | 2 | 2 | 3 | 1 | 4 |
| 6,7-Dehydroterpendole A Isomer | 534.28501 | 23.2 | 0 | 0 | 2 | 0 | 3 | 2 | 2 | 3 | 0 | 4 |
| 6,7-Dehydro-11-hydroxy-12,13-epoxyterpendole A | 534.28501 | 24.7 | 0 | 0 | 4 | 4 | 4 | 3 | 4 | 6 | 0 | 4 |
| 6,7-Dehydroterpendole A | 534.28501 | 27.0 | 0 | 0 | 4 | 4 | 4 | 3 | 3 | 4 | 1 | 0 |
| Terpendole A/M Isomer | 536.30066 | 22.1 | 0 | 0 | 5 | 4 | 4 | 3 | 4 | 7 | 1 | 3 |
Indole diterpenes identified according to Lee et al. (2017)
Published retention time (Lee et al. 2017)
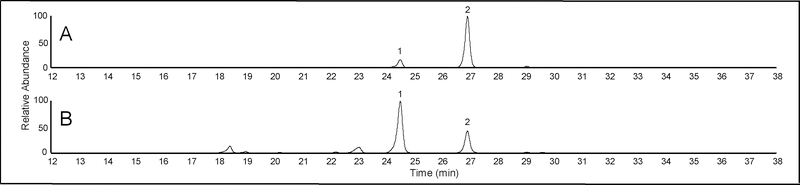
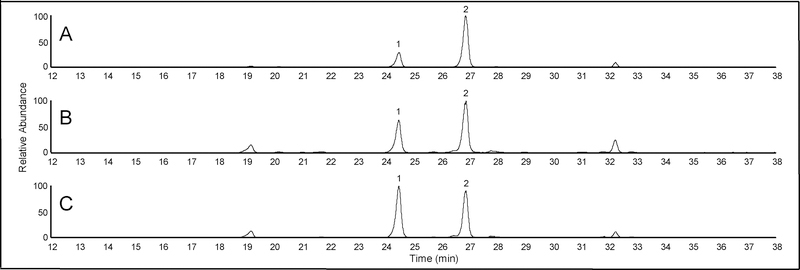
The relative abundance of some indole diterpenes varied among individual accessions of some species. For example, herbarium specimens had varying peak ratios of 6,7-dehydroterpendole A to 6,7-dehydro-11-hydroxy-12,13-epoxyterpendole A (Figure 2A, 2B). We suspected this may be influenced by the age of the specimen. For example, Figure 2A shows an I. muelleri sample collected in 2006 while Figure 2B shows a specimen collected in 1979. In addition to age of the herbarium specimen, the method of drying may also influence the relative abundance of some indole diterpenes. To test this hypothesis, indole diterpene profiles were determined from I. asarifolia collected from greenhouse grown plants that were freeze dried, air-dried, and oven dried. Plants that were snap frozen and freeze dried had a greater peak area of 6,7-dehydroterpendole A compared to 6,7-dehydro-11-hydroxy-12,13-epoxyterpendole A (Figure 3A). In contrast, plants that were oven dried had a greater peak area of the 6,7-dehydro-11-hydroxy-12,13-epoxyterpendole A compared to 6,7-dehydroterpendole A (Figure 3C). Peak area ratios of plants that were air dried were intermediate to the freeze dried and oven dried samples (Figure 3B). Similar trends were observed in regard to relative abundance of terpendole K and 11-hydroxy-12,13-epoxyterpendole K in herbarium specimens as well as in the different drying treatments (data not shown). No other obvious differences were observed in the relative abundance of the other indole diterpenes and or isomers in the different drying treatments (data not shown). These observations are likely explained by observations made by Lee et al. (2017) in the purification procedure of terpendole K and 6,7-dehydroterpendole A, conversion of these compounds was observed to 11-hydroxy-12,13-epoxyterpendole K and 6,7-dehydro-11-hydroxy-12,13-epoxyterpendole A, respectively. Lee et al. (2017) demonstrated the C-12,13 epoxy structure of 11-hydroxy-12,13-epoxyterpendole K was more energetically stable than terpendole K through modeling. The 6,7 double bond appears to facilitate the migration of the epoxide as it occurs in 6,7-dehydroterpendole A and terpendole K but not in terpendole C which lacks the 6,7 double bond. As herbarium specimens are often air dried and have aged, both factors are likely interacting to influence the profile of select indole diterpenes. Due to these results, caution should be taken in comparing indole diterpene profiles among different samples.
Figure 2.
Reconstructed HPLC-HRMS ion chromatograms (m/z 534.28501) from an isopropyl alcohol extract of I. muelleri herbarium specimens: (A) PERTH 7842244 (year collected, 2006) and (B) PERTH 3640787 (year collected, 1979). Peak 1, 6,7-Dehydro-11-hydroxy-12,13-epoxyterpendole A (tR=24.7) and Peak 2, 6,7-Dehydro-terpendole A (tR=27.0).
Figure 3.
Reconstructed HPLC-HRMS ion chromatograms (534.28501) from an isopropyl alcohol extract of I. asarifolia leaves that were (A) freeze dried collected, (B) air dried at room temperature (23 °C), and (C) oven dried at 50 °C. Peak 1, 6,7-Dehydro-11-hydroxy-12,13-epoxyterpendole A (tR=24.7) and Peak 2, 6,7-Dehydro-terpendole A (m/z tR=27.0).
Swainsonine was detected in 2 of the 30 species surveyed, I. carnea subsp. fistulosa and I. costata (Table 1). Ergot and indole diterpene alkaloids were not detected in either species. According to Eich (2008), I. costata was considered unambiguously positive for ergot alkaloids while for I. carnea subsp. fistulosa there were contradictory reports in regard to the occurrence of ergot alkaloids. I. carnea subsp. fistulosa has previously been reported to contain swainsonine (de Balogh et al. 1999; Haraguchi et al. 2003), whereas this is the first report for the occurrence of swainsonine in I. costata. We suspect that the lack of consistent results in regard to the occurrence of ergot alkaloids in I. costata is likely due to improper identification of the plant. The previous report (Amor-Prats and Harborne, 1993) does not reference a voucher specimen which makes it impossible to experimentally verify the previous report.
Ergot alkaloids, indole diterpene alkaloids, or swainsonine were not detected in ten Ipomoea species. Among these Ipomoea species were species that had previously been reported to contain ergot alkaloids, both unambiguously and contradictory (Eich 2008). Differences in our results and previous reports may be due to the fact these compounds are symbiont derived and the accessions analyzed herein were not infected by the symbiont, Periglandula. Alternatively, the accessions analyzed in the previous reports may not have been identified correctly; the genus Ipomoea is the most speciose among the morning glory family and is considered taxonomically difficult.
A recent phylogeny of Periglandula species from several ergot alkaloid containing hosts representing three genera, Ipomoea, Argyreia, and Turbina using the tefA locus reported two distinct clades (Beaulieu et al. 2015). A strong association exists between the two Periglandula clades and the occurrence of ergot and/or indole diterpene alkaloids reported here. One Periglandula clade was associated with six Ipomoea host species, I. amnicola, I. argillicola, I. asarifolia, I gracilis, I. muelleri, and I. pes-caprae, reported to contain ergot and indole diterpene alkaloids while the other clade was associated with four species, A. nervosa, I. leptophylla, I. hildebrandtii, and T. corymbosa, reported to contain only ergot alkaloids. As additional Periglandula species are investigated and the chemical composition of their respective hosts described it will be interesting to see if this association continues.
Distinct indole diterpene profiles are reflective of which functional indole diterpene biosynthetic genes are present, as has been shown with Clavicipitaceous species (Saikia et al. 2012; Schardl et al. 2013; Charlton et al. 2014). Different indole diterpene profiles are reported herein among the Argyreia and some Ipomoea species, which may suggest that the corresponding Periglandula species associated with each host could differ with respect to the presence of functional indole diterpene biosynthetic genes. For example, the Argyreia species contain the simpler indole diterpenes like paspaline and terpendole I that are produced earlier in the pathway while some Ipomoea species contain terpendole C and terpendole K that are later in the pathway (Table 2). Therefore, we propose that the Argyreia species may lack functional idtF and/or idtK, two genes encoding steps in the indole diterpene biosynthetic pathway that are necessary for the production of terpendole C and terpendole K (Saikia et al. 2012; Schardl et al. 2014; Charlton et al. 2014).
Only members of the monophyletic tribe Ipomoeeae, including members of the genera Argyreia, Ipomoea, Stictocardia, and Turbina are reported to contain the ergot alkaloids (Eich, 2008; Eserman et al. 2014). Most of the morning glory species reported by Eich (2008) to contain the ergot alkaloids can be placed within two main clades of the Ipomoeeae, the Argyreiinae and Astripomoeinae (Eserman et al. 2014). Eserman et al. (2014) report that the presence of the ergot alkaloids is ancestral condition in the Ipomoeeae and that the presence of the ergot alkaloids has been lost a minimum of four times. Like the ergot alkaloids the occurrence of the indole diterpenes is likely ancestral and appears to have been lost several more times based upon this limited survey. Many of the species that contain the ergot alkaloids, indole diterpenes, and/or swainsonine are phylogenetically related. For example, all seven species of the Pes-caprae clade (I. amnicola, I. argillicola, I. asarifolia, I gracilis, I. leptophylla, I. muelleri, and I. pes-caprae) which is part of the larger Argyreiinae clade (Miller et al. 1999; Eserman et al. 2014) are reported to contain the ergot alkaloids of which six contain the indole diterpenes as reported here. Similarly, two swainsonine-containing species are reported herein, I. carnea subsp. fistulosa and I. costata, which are part of the Murucoides clade as is another swainsonine-containing species I. polpha. A similar observation has been made between the occurrences of swainsonine, a compound produced by an endophyte in Astragalus species, and the genetic relatedness of the respective host plant species (Cook et al. 2017 a, b).
In summary, ergot alkaloids were detected in 18 of the 30 species evaluated, representing all four genera screened, namely Argyreia, Ipomoea, Stictocardia, and Turbina. Indole diterpene alkaloids were detected in two Argyreia species and eight Ipomoea species, all of which also contained ergot alkaloids. Eight of these 10 species had previously not been known to contain indole diterpenes. Lastly, swainsonine was detected in two Ipomoea species, of which I. costata had previously not been reported to contain swainsonine. The data suggest a strong association exists between the phylogenetic relationship of the Periglandula species associated with each host and the occurrence of ergot and/or indole diterpene alkaloids reported here. Likewise, there appears to be an association between the occurrence of the respective bioactive principle and the genetic relatedness of the respective host plant species.
Supplementary Material
Convolvulaceous species (n=30) were screened for fungal symbiont derived compounds.
Ergot alkaloids were detected in 18 species representing four genera.
Indole diterpenes were detected in two Argyreia species and eight Ipomoea species.
Swainsonine was detected in two Ipomoea species.
Acknowedgements
We thank the Missouri Botanical Garden (MO), Western Australian Herbarium, Kensington, Western Australia (PERTH), National Herbarium Netherlands (WAG) for providing Ipomoea muelleri for analysis. D.G.P. and C.E.L. were supported by NIH grant 2R15GM114774-02. We thank Charles Hailes and Scott Larsen for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Amor-Prats D, Harborne JB, 1993. New sources of ergoline alkaloids within the genus Ipomoea. Biochem. Syst. Ecol 21:455–461. [Google Scholar]
- Beaulieu WT, Panaccione DG, Hazekamp CS, McKee MC, Ryan KL, Clay K, 2013. Differential allocation of seed-borne ergot alkaloids during early ontogeny of morning glories (Convolvulaceae). J. Chem. Ecol 39:919–930. [DOI] [PubMed] [Google Scholar]
- Beaulieu WT, Panaccione DG, Ryan KL, Kaonongbua W, Clay K, 2015. Phylogenetic and chemotypic diversity of Periglandula species in eight new morning glory hosts (Convolvulaceae). Mycologia 107:667–678. [DOI] [PubMed] [Google Scholar]
- Charlton ND, Craven KD, Afkhami ME, Hall BA, Ghimire SR, Young CA, 2014. Interspecific hybridization and bioactive alkaloid variation increases diversity in endophytic Epichloë species of Bromus laevipes. FEMS microbiology ecology, 90:276–289. [DOI] [PubMed] [Google Scholar]
- Cook D, Beaulieu WT, Mott IW, Riet-Correa F, Gardner DR, Grum D, Pfister JA, Clay K, Marcolongo-Pereira C, 2013. Production of the alkaloid swainsonine by a fungal endosymbiont of the Ascomycete order Chaetothyriales in the host Ipomoea carnea. J. Agric. Food Chem 61:3797–3803. [DOI] [PubMed] [Google Scholar]
- Cook D, Lee ST, Gardner DR, Pfister JA, Welch KD, Green BT, Davis TZ, Panter KE, 2009. The alkaloid profiles of Lupinus sulphureus. J. Agric. Food Chem 57:1646–1653. [DOI] [PubMed] [Google Scholar]
- Cook D, Gardner DR, Pfister JA, 2014. Swainsonine-containing plants and their relationship to endophytic fungi. J. Agric. Food Chem 62:7326–7334. [DOI] [PubMed] [Google Scholar]
- Cook D, Gardner DR, Pfister JA, Lee ST, Welch KD, Welsh SL, 2017a. A screen for swainsonine in select North American Astragalus species. Chem. Biodivers 14:e1600364. [DOI] [PubMed] [Google Scholar]
- Cook D, Gardner DR, Martinez A, Robles CA, Pfister JA, 2017b. Screening for swainsonine among South American Astragalus species. Toxicon, 139:54–57. [DOI] [PubMed] [Google Scholar]
- de Balogh KK, Dimande AP, van der Lugt JJ, Molyneux RJ, Naudé TW, Welman WG, 1999. A lysosomal storage disease induced by Ipomoea carnea in goats in Mozambique. J. Vet. Diagn. Invest 11:266–273. [DOI] [PubMed] [Google Scholar]
- Eich E, 2008. Solanaceae and Convolvulaceae: Secondary metabolites: Biosynthesis, chemotaxonomy, biological and economic significance. Springer-Verlag, Berlin, Germany, pp. 213–259. [Google Scholar]
- Eserman LA, Tiley GP, Jarret RL, Leebens-Mack JH, Miller RE, 2014. Phylogenetics and diversification of morning glories (tribe Ipomoeeae, Convolvulaceae) based on whole plastome sequences. Am. J. Bot 101:92–103. [DOI] [PubMed] [Google Scholar]
- Everist SL, 1974. Poisonous plants of Australia Sydney. Angus & Robertson: Sydney, Australia, pp. 59–767. [Google Scholar]
- Gardner DR, Cook D, 2011. A comparison of alternative sample preparation procedures for the analysis of swainsonine using LC-MS/MS. Phytochem. Anal 22:124–127. [DOI] [PubMed] [Google Scholar]
- Gardner DR, Molyneux RJ, Ralphs MH, 2001. Analysis of swainsonine: extraction methods, detection, and measurement in populations of locoweeds (Oxytropis spp.). J. Agric. Food Chem 49:4573–4580. [DOI] [PubMed] [Google Scholar]
- Gardner DR, Welch KD, Lee ST, Cook D, Riet-Correa F, 2018. Tremorgenic indole diterpenes from Ipomoea asarifolia and Ipomoea muelleri and the identification of 6, 7-dehydro-11-hydroxy-12, 13-epoxyterpendole A. J. Nat. Prod 81:1682–1686. [DOI] [PubMed] [Google Scholar]
- Haraguchi M, Gorniak SL, Ikeda K, Minami Y, Kato A, Watson AA, Nash RJ, Molyneux RJ, Asano N, 2003. Alkaloidal components in the poisonous plant, Ipomoea carnea (Convolvulaceae). J. Agric. Food Chem 51:4995–5000. [DOI] [PubMed] [Google Scholar]
- Kao D, Henkin JM, Soejarto DD, Kinghorn AD and Oberlies NH, 2018. Non-destructive chemical analysis of a Garcinia mangostana L.(Mangosteen) herbarium voucher specimen. Phytochem. Lett 28:124–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucht S, Groß J, Hussein Y, Grothe T, Keller U, Basar S, Köning WA, Steiner U, Leistner E, 2004. Elimination of ergoline alkaloids following treatment of Ipomoea asarifolia (Convolvulaceae) with fungicides. Planta 219:619–625. [DOI] [PubMed] [Google Scholar]
- Lee ST, Gardner DR, Cook D, 2017. Identification of indole diterpenes in Ipomoea asarifolia and Ipomoea muelleri, plants tremorgenic to livestock. J. Agric. Food Chem 65:5266–5277. [DOI] [PubMed] [Google Scholar]
- Medeiros RMT, Barbosa RC, Riet-Correa F, Lima EF, Tabosa IM, de Barros SS, Gardner DR, Molyneux RJ, 2003. Tremorgenic syndrome in goats caused by Ipomoea asarifolia in Northeastern Brazil. Toxicon 41:933–935. [DOI] [PubMed] [Google Scholar]
- Miller RE, Rausher MD, Manos PS, 1999. Phylogenetic systematics of Ipomoea (Convolvulaceae) based on ITS and waxy sequences. Syst. Bot 24:209–227. [Google Scholar]
- Panaccione DG, Ryan KL, Schardl CL, Florea S, 2012. Analysis and modification of ergot alkaloid profiles in fungi. Methods Enzymol. 515:267–290. [DOI] [PubMed] [Google Scholar]
- Panaccione DG, Beaulieu WT, Cook D, 2014. Bioactive alkaloids in vertically transmitted fungal endophytes. Funct. Ecol 28:299–314. [Google Scholar]
- Rasmussen S; Lane GA; Mace W, Parsons AJ, Fraser K, Xue H The use of genomic and metabolomics methods to quantify fungal endosymbionts and alkaloids in grasses In Plant Metabolomics: Methods and Protocols, Methods in Molecular Biology, Hardy NW, Hall RD Eds.; Springer: New York, New York, 2012; Vol. 860, pp. 213–226. [DOI] [PubMed] [Google Scholar]
- Saikia S, Takemoto D, Tapper BA, Lane GA, Fraser K, Scott B, 2012. Functional analysis of an indole-diterpene gene cluster for lolitrem B biosynthesis in the grass endosymbiont Epichloë festucae. FEBS letters, 586:2563–2569. [DOI] [PubMed] [Google Scholar]
- Schardl CL, Young CA, Hesse U, Amyotte SG, Andreeva K, Calie. PJ et al. , 2013. Plant-symbiotic fungi as chemical engineers: multi-genome analysis of the Clavicipitaceae reveals dynamics of alkaloid loci. PLoS Genet 9:e1003323. doi: 10.1371/journal.pgen.1003323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimming T, Tofern B, Mann P, Richter A, Jenett-Siems K, Dräger B, Asano N, Gupta MP, Mireya MP, Correa MD, Eich E, 1998. Distribution and taxonomic significance of calystegines in the Convolvulaceae. Phytochemistry 49:1989–1995. [Google Scholar]
- Steiner U, Leibner S, Schardl CL, Leuchtmann A, Leistner. E, 2011. Periglandula, a new fungal genus within the Clavicipitaceae and its association with Convolvulaceae. Mycologia 103:1133–1145. [DOI] [PubMed] [Google Scholar]
- Welch KD, Pfister JA, Cook D, Carriao dos Santos F, Lee ST, 2018. Assessment of endophyte-derived tremorgenic compounds in Ipomoea asarifolia using mouse models. Toxicon 156:52–60. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.