Abstract
Background
Alcohol intake is a leading modifiable cause related to cancer-specific deaths. Various alcohol intake patterns have shown to impact cancer progression differently, however, many studies only evaluated simplified patterns (such as heavy vs. non-heavy drinking) of alcohol intake for cancer survivors. The objective of this study is to provide population-based prevalence of the complex alcohol drinking patterns for cancer survivors, and compare it with that of non-cancer individuals. Additionally, we evaluated the impact of cancer related factors (binary, alcohol-related cancer type, and length of cancer history) to the alcohol intake patterns adjusted for the selected factors.
Methods
A total of 193,197 individuals, including 16,504 cancer survivors, with age ≥18 years old in the 2012–2017 National Health Interview Survey (NHIS) were included in this study. The population-based prevalence of alcohol patterns was estimated. To evaluate cancer related factors associated with the alcohol intake patterns, we applied multinomial logistic models with the appropriate sampling weights and adjusted the selected demographic factors and smoking status.
Results
There were 62.1% of cancer survivors and 66.0% of non-cancer individuals who were current alcohol drinkers in the past year. The prevalence of heavy drinking was identical for 5.2% of cancer and non-cancer individuals. For frequent binge drinking, cancer survivors tended to have less frequent binge than non-cancer individuals (2.8% vs. 4.9%). After adjusting for the selected demographic factors and smoking status, the cancer survivors were less likely to have the intermediate level of alcohol intake (light/moderate or occasional binge drinking) compared with non-cancer individuals, but no difference for the excessive alcohol intake (heavy or frequent binge drinking) was observed for those with and without cancer. As for cancer type, those with non-alcohol related cancer tended to be a current drinker compared with those with alcohol-related cancer. Compared with cancer survivors with a short cancer history (2–4 years), survivors with a cancer history of 5–9 years were more likely to be current drinkers after adjusting for the selected factors. Cancer status, alcohol-related cancer type and length of cancer history had no impact on excessive alcohol intake.
Conclusions
In summary, cancer survivors have similar excessive alcohol drinking patterns but were less likely to have the intermediate level of alcohol intake compared to non-cancer individuals. Alcohol intake may enhance cancer progression, interfere with cancer treatments and increase cancer-related mortality. To improve cancer survivors’ health, custom alcohol interventions and cessation programs should be conducted to minimize alcohol intake for cancer survivors, especially for excessive alcohol drinkers.
Keywords: Alcohol intake, cancer, behavior pattern
Introduction
Alcohol consumption is one of the leading modifiable causes of cancer deaths. The impact of alcohol intake on cancer survivors is gaining more attention with a growing number of cancer survivors. Approximately more than one third of men and women will be diagnosed with cancer during their lifetimes in the United States (1). With improvement of cancer screening, diagnosis, and treatment, the 5-year relative survival rate was more than 60% for all cancers diagnosed during 2008 through 2014 (2). It was estimated that the number of cancer survivors will increase from 15.5 million to 20.3 million from 2016 to 2026 in the United States (1). It is well known that health behaviors such as smoking, physical activity, and alcohol play an important role in cancer progression (3). Heavy alcohol intake is strongly associated with increased deaths from alcohol-related cancers and total mortality (4). In addition, it has been reported that alcohol use contributed to approximately 3.5% of all cancer deaths and approximately 18 years of potential life lost for each alcohol-related cancer death (5). Specifically, 30% of alcohol-related cancer deaths were attributed to heavy drinking (5).
Various alcohol drinking patterns have different impact on cancer prognosis and cancer related deaths. Based on a US national-based study (6), the excessive alcohol intake (heavy or frequent binge drinking) increases risk of cancer-related mortality and overall mortality compared with lifetime infrequent drinkers after adjusting for demographic and lifestyle factors. However, the same study reported no significant or protective impact of the intermediate level of alcohol intake (light/moderate or occasional binge drinking) on cancer-related mortality. The alcohol drinking behaviors of cancer survivors have been widely discussed. However, the majority of studies only tested the dichotomized pattern of alcohol intake (7,8), such as heavy (yes vs. no), binge vs. non-binge (yes vs. no), and risky alcohol intake (yes vs. no). Another study evaluated the combination of moderate and heavy level of drinking (9). Little is known about the pattern of intermediate alcohol intake (light/moderate or occasional binge) for cancer survivors. In order to thoroughly understand alcohol intake for cancer survivors, it is essential to separately evaluate these distinct alcohol intake patterns.
Based on the 2015–2020 U.S. Dietary Guidelines for Americans (10), people who have medical conditions and take medications that can interact with alcohol should stop drinking alcohol. Cancer survivors qualify for the above two conditions of zero alcohol intake. However, it is unrealistic to expect cancer survivors to adhere to the guideline of zero alcohol intake. We can hope cancer survivors will decrease their alcohol intake compared to non-cancer individuals. It has been suggested that cancer diagnosis is a teachable moment for improving health behaviors. It is expected that cancer survivors have a stronger desire to improve health behaviors compared to the general population due to their poor health history (11). Regardless cancer status, cancer survivors also have a higher risk of chronic diseases than the general non-cancer population (12). It has been shown that cancer survivors have more than one comorbid condition and many have died due to chronic diseases (such as cardiovascular disease and diabetes complications) instead of cancer itself (13,14). Thus, we were interested in evaluating whether cancer survivors have better alcohol drinking behaviors compared to non-cancer individuals by testing the detailed alcohol intake patterns. The objective of this study was to provide population-based prevalence of alcohol drinking patterns for cancer and non-cancer individuals, and evaluate the impact of cancer features (binary, alcohol-related cancer type, and length of cancer history) on alcohol intake.
Methods
Study population
A total of 193,197 adults (16,504 cancer survivors and 176,693 non-cancer individuals) in six 1-year survey cycles [2012–2017] performed by the National Health Interview Survey (NHIS) were included in this study. The NHIS is a nationally representative survey designed for monitoring the health of the civilian non-institutionalized population of the United States, conducted by the National Center for Health Statistics of Centers for Disease Control and Prevention. The eligibility criteria of the study population included those with age ≥18 years old and with valid information in alcohol intake and cancer status (yes/no). All alcohol drinking related variables used in this study were based on the status of the past year prior to the interview time. In order to ensure only post-cancer alcohol behaviors were analyzed, we excluded the 268 cancer survivors with a missing value of cancer history and 2,651 recently diagnosed (<2 years) cancer survivors. NHIS was approved by the Research Ethics Review Board of the National Center for Health Statistics and the U.S. Office of Management and Budget. Oral consent was provided for all NHIS respondents.
Measurements
The three cancer related factors of interest are cancer status (yes/no), cancer type (alcohol-related cancer or not), and length of cancer history. The length of cancer history was calculated based on difference between interview age and cancer diagnosis age, which was recorded as integers. In order to address the rounding issue of this cancer history calculation and completely exclude those who had a cancer diagnosis during the reported alcohol intake period (1-year prior interview), we excluded cancer survivors with <2 years of cancer history. The primary cancer type for each cancer survivor was determined using the first diagnosed cancer. If there were multiple cancers diagnosed at the same age, then the most common cancer type was treated as the primary cancer. In this study, the alcohol-related cancer type was defined as breast, colon/rectum, liver, mouth/throat, larynx, and esophagus cancers (15).
The three outcomes of alcohol drinking are general status (never, former and current), heavy drinking (infrequent, light/moderate, and heavy), and binge drinking (no binge, occasional binge and frequent binge). The general status of alcohol intake was classified to never, former and current drinking. “Never drinker”, also called lifetime abstainer, was defined as less than 12 drinks in a lifetime. “Former drinker” was those who drank ≥12 drinks in a lifetime but had no drinks in the past year. “Current drinker” was those who drank ≥12 drinks in a lifetime and at least one drink in the past year. Heavy and binge alcohol intake were only defined for current drinkers. The heavy alcohol intake was based on the average amount of alcohol drinking in the past year. The data were collected using the question “In the PAST YEAR, on those days that you drank alcoholic beverages, on the average, how many drinks did you have?”. “Infrequent drinker” was defined as those who drank 1–11 drinks in the past year. “Light drinkers” were those who drank ≤3 drinks per week in the past year. “Moderate drinkers” were males who drank >3 drinks up to 14 drinks per week or females who drank >3 drinks up to 7 drinks per week. “Heavy drinkers” were defined as males who drank >14 drinks per week or females who drank >7 drinks per week in the past year. This heavy drinking definition is the same as the Centers for Disease Control and Prevention (CDC)’s definition (16).
For binge drinking, or heavy episodic drinking, frequency of binge drinking days was measured based on the question “In the past year, on how many days did you have 5 or more (males)/4 or more drinks (females) of any alcoholic beverage?”. “No binge” was defined as 0 days of binge drinking in the past year. “Occasional binge” was for those with an average of greater than 0 days but less than 4 days of binge drinking per month, and “frequent binge” was defined as an average of ≥4 binge drinking days per month in the past year. Our binge drinking (occasional+ frequent binge) definition is similar to the CDC’s definition, which defines a pattern of drinking with ≥4 drinks for women and ≥5 drinks for men on an occasion in the past 30 days (16). In this study, “excessive” alcohol drinking was defined as heavy drinking or frequent binge alcohol drinking, and the “intermediate” level of alcohol intake was defined as light/moderate or occasional binge.
Statistical analyses
The participants’ demographic, smoking behavior, and cancer characteristics by alcohol intake status were summarized using descriptive statistics. The differences of categorical variables in the sub-groups of alcohol intake were tested using the Rao-Scott chi-square tests; and the differences of continuous variables were tested using the ANOVA test. In order to evaluate cancer related factors (binary cancer status, cancer type and length of cancer history) associated with current alcohol drinking status, a logistic regression model with the appropriate sampling weights was applied. For testing the impact of these cancer related factors on heavy and binge drinking status, the weighted multinomial logistic models were applied. Both univariate and multivariable models adjusted for the selected five demographic factors (age, gender, race, education level, marital status) and smoking status. The sampling weights based on the NHIS guideline (17) were applied for all analyses. A P value of less than 0.05 was considered statistically significance. All statistical analyses were conducted using SAS 9.4 (SAS Institute, Inc., Cary, North Carolina).
Results
We analyzed the complex patterns of alcohol intake of the 16,504 cancer survivors and 176,693 non-cancer individuals in the 2012–2017 NHIS. The demographic characteristics of the eligible adults are shown in Table 1. The mean age for the study samples was 46.82; 51.9% were female, 74.8% were non-Hispanic Caucasian, 62.0% were with a greater than high school degree, 57.3% were married or living with partner, and 16.2% were current smokers.
Table 1. Demographic characteristics and smoking by past-year alcohol intake of the US adults (NHIS 2012–2017)†.
| Characteristic | Total (n=193,197) | Never (n=39,541) | Former (n=29,775) | Current (n=123,881) |
|---|---|---|---|---|
| Age (years), mean ± SE | 46.82±0.09 | 45.32±0.18 | 55.85±0.14 | 45.40±0.10 |
| Age (years) | ||||
| <45 | 82,010 (47.1) | 16,872 (22.3) | 6,643 (7.7) | 58,495 (70.0) |
| 45–64 | 65,170 (34.3) | 10,811 (16.2) | 11,492 (16.4) | 42,867 (67.4) |
| ≥65 | 46,017 (18.6) | 11,858 (24.0) | 11,640 (24.6) | 22,519 (51.4) |
| Gender | ||||
| Female | 106,734 (51.9) | 27,200 (25.4) | 16,133 (13.7) | 63,401 (60.9) |
| Male | 86,463 (48.1) | 12,341 (15.3) | 13,642 (13.9) | 60,480 (70.8) |
| Race | ||||
| Non-Hispanic Caucasian | 141,502 (74.8) | 23,548 (16.8) | 22,107 (14.1) | 95,847 (69.1) |
| Non-Hispanic African-American | 26,492 (12.3) | 7,585 (29.5) | 4,422 (14.5) | 14,485 (56.0) |
| Other Hispanic or others | 25,203 (12.9) | 8,408 (33.8) | 3,246 (11.6) | 13,549 (54.7) |
| Education | ||||
| <High school | 26,197 (12.6) | 8,827 (34.2) | 5,958 (20.7) | 11,412 (45.1) |
| High school | 48,606 (25.4) | 11,470 (24.2) | 9,073 (17.0) | 28,063 (58.8) |
| >High school | 116,834 (62.0) | 18,491 (15.9) | 14,445 (11.1) | 83,898 (73.0) |
| Marital status | ||||
| Married or living with partner | 84,493 (57.3) | 16,201 (18.0) | 12,787 (14.3) | 55,690 (67.8) |
| Never married | 45,133 (24.2) | 10,923 (29.5) | 4,346 (7.9) | 30,077 (62.6) |
| Widowed, divorced, separated | 50,858 (18.5) | 10,954 (20.8) | 11,236 (21.5) | 28,826 (57.7) |
| Smoking | ||||
| Never | 115,622 (61.9) | 33,343 (28.5) | 13,346 (10.3) | 68,933 (61.1) |
| Former | 44,548 (21.9) | 3,381 (6.9) | 10,633 (21.8) | 30,534 (71.3) |
| Current | 32,870 (16.2) | 2,790 (8.4) | 5,754 (16.3) | 24,326 (75.4) |
†, associations between the selected factors and the 3-group alcohol drinking status (and binary current drinking status), all P values <0.001. Data are shown as number (weighted percentage) or mean ± standard error.
Current alcohol drinking
As show in Table 1, prevalence of current drinking was 65.7% and prevalence of former drinking was 13.8%. All of the selected demographic factors and smoking status were significantly associated with current drinking status (P<0.001). Among them, the top two significant demographic factors were education and age. Those with higher education and younger age tended to be a current alcohol drinker. The smoking status also has a strong association with current drinking status. The current smokers were more likely to be a current drinker than never smokers (75.4% vs. 61.1%).
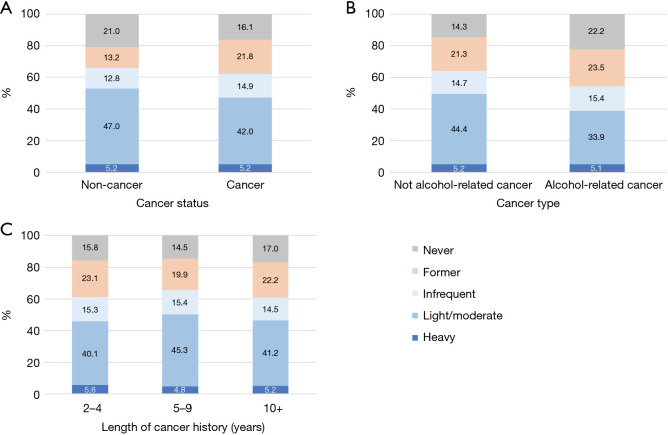
The prevalence of alcohol intake patterns in terms of drinking amount by the cancer related factors is shown in Figure 1. Cancer survivors had lower prevalence of current drinking than non-cancer individuals [62.1% vs. 65.9%, crude odds ratio (OR) =0.85, P<0.001]. After adjusting for the selected factors (Table 2), the impact of cancer diagnosis on current alcohol drinking became insignificant (P=0.781). As for cancer type, alcohol-related cancer survivors had lower prevalence of current drinking compared with non-alcohol-related cancer survivors (Figure 1B, 54.4% vs. 64.3%, crude OR=0.66, P<0.001), and this difference remained significant (P=0.002) after adjusting the selected factors. For length of cancer history, cancer survivors with a cancer history of 2–4 and 10+ years had lower prevalence of current drinking than those with 5–9 years of cancer history (Figure 1C, 61.0%, 65.5% and 60.9% for 2–4, 5–9 and 10+ years of cancer) with and without adjusting for the selected factors (Table 2). As for former drinking status using the two-proportion comparison test, prevalence of former drinkers was higher in cancer survivors than non-cancer individuals (21.8% vs. 13.2%, P<0.001). Alcohol-related cancer survivors were more likely to be former drinkers than non-alcohol-related cancer survivors (23.5% vs. 21.3%, P=0.031). For cancer survivors, prevalence of former drinkers was lower in those with 5–9 years cancer history compared with those with 2–4 and ≥10 years of cancer history (23.1%, 19.9% and 22.2% for those with 2–4, 5–9 and ≥10 years of cancer history, P=0.018).
Figure 1.
Distributions of the heavy alcohol drinking patterns by cancer related factors. (A) By cancer status; (B) by alcohol-related cancer type; and (C) by length of cancer history. Infrequent, light/moderate and heavy drinkers are for current drinkers only. “Never drinker”: <12 drinks in a lifetime; “Former drinker”: ≥12 drinks in a lifetime but with 0 drink in the past year; “Current drinker”: ≥12 drinks in a lifetime and >0 drink in the past year. “Infrequent drinker”: 1–11 drinks in the past year; “Light drinkers”: ≤3 drinks per week in the past year. “Moderate drinkers”: males >3 drinks up to 14 drinks per week or females >3 drinks up to 7 drinks per week; and “Heavy drinkers”: males >14 drinks per week or females >7 drinks per week in the past year.
Table 2. Cancer related factors associated with past-year current alcohol intake.
| Characteristic | Crude OR (95% CI)† | P value | Adjusted OR (95% CI)† | P value |
|---|---|---|---|---|
| Cancer | ||||
| No | 1 | 1 | ||
| Yes | 0.85 (0.81–0.88) | <0.001 | 1.01 (0.96–1.06) | 0.781 |
| Alcohol-related cancer type‡ | ||||
| No | 1 | 1 | ||
| Yes | 0.66 (0.60–0.73) | <0.001 | 0.84 (0.75–0.94) | 0.002 |
| Length of cancer history, year | ||||
| 2–4 | 1 | 1 | ||
| 5–9 | 1.21 (1.07–1.38) | 0.003 | 1.27 (1.11–1.46) | <0.001 |
| 10+ | 0.99 (0.88–1.11) | 0.821 | 1.12 (0.99–1.26) | 0.069 |
†, OR: odds ratio; CI: confidence interval; adjusted OR adjusted for age, gender, race, education, marital status, and smoking. ‡, alcohol related cancer types included breast, colon, rectum, mouth/tongue/lip, larynx-windpipe, esophagus, and liver cancers
Heavy alcohol drinking
For the heavy drinking patterns, the three patterns of interest are infrequent, light/moderate, and heavy drinking. The selected demographic factors and smoking by heavy drinking patterns were summarized in Table S1 and all were significantly associated with binge drinking patterns. Prevalence of heavy drinking was 5.2% and prevalence of light/moderate was 47.4% overall. All of these five demographic factors (age, gender, race, education level, marital status) and smoking status were significantly (all P values <0.001) associated with heavy drinking status among all eligible individuals. Among these selected factors, smoking had a strong association with heavy alcohol drinking. The prevalence of heavy drinking was 11.4% for current smokers, 2.9% for never smokers and 7% for former smokers.
Table S1. Demographic characteristics and smoking by the 5-group heavy alcohol drinking status of the US adults (NHIS 2012-2017)†.
| Characteristic | Never (n=39,541, 20.6%) | Former (n=29,775, 13.8%) | Current | ||
|---|---|---|---|---|---|
| Infrequent (n=24,858, 12.9%) | Light/moderate (n=87,914, 47.4%) | Heavy (n=10,502, 5.2%) | |||
| Age (year) | 45.32±0.18 | 55.85±0.14 | 48.01±0.16 | 44.67±0.11 | 45.87±0.26 |
| Age (years) | |||||
| <45 | 16,872 (22.4) | 6,643 (7.7) | 9,863 (12.1) | 43,554 (52.6) | 4,763 (5.2) |
| 45–64 | 10,811 (16.3) | 11,492 (16.4) | 9,085 (14.1) | 29,630 (47.5) | 3,936 (5.7) |
| ≥65 | 11,858 (24.0) | 11,640 (24.6) | 5,910 (13.0) | 14,730 (34.2) | 1,803 (4.2) |
| Gender | |||||
| Female | 27,200 (25.4) | 16,133 (13.8) | 16,704 (15.9) | 41,074 (39.9) | 5,413 (5.0) |
| Male | 12,341 (15.4) | 13,642 (13.9) | 8,154 (9.7) | 46,840 (55.5) | 5,089 (5.4) |
| Race | |||||
| Non-Hispanic Caucasian | 23,548 (16.8) | 22,107 (14.1) | 17,928 (12.7) | 68,815 (50.5) | 8,716 (5.9) |
| Non-Hispanic African-American | 7,585 (29.7) | 4,422 (14.6) | 3,557 (13.6) | 9,813 (38.6) | 995 (3.6) |
| Other Hispanic or others | 8,408 (33.9) | 3,246 (11.6) | 3,373 (13.6) | 9,286 (38.2) | 791 (2.7) |
| Education | |||||
| <High school | 8,827 (34.4) | 5,958 (20.7) | 3,078 (12.2) | 7,118 (28.7) | 1,069 (3.9) |
| High school | 11,470 (24.3) | 9,073 (17.0) | 6,576 (13.7) | 18,676 (39.6) | 2,620 (5.3) |
| >High school | 18,491 (16.0) | 14,445 (11.1) | 15,076 (12.8) | 61,779 (54.7) | 6,772 (5.4) |
| Marital status | |||||
| Married or living with partner | 16,201 (18.0) | 12,787 (14.3) | 11,373 (13.6) | 40,348 (49.6) | 3,784 (4.6) |
| Never married | 10,923 (29.7) | 4,346 (7.9) | 4,788 (10.5) | 22,047 (46.4) | 3,029 (5.5) |
| Widowed, divorced, separated | 10,954 (20.9) | 11,236 (21.6) | 7,117 (14.2) | 18,873 (38.0) | 2,678 (5.3) |
| Smoking | |||||
| Never | 33,343 (28.6) | 13,346 (10.3) | 14,815 (12.7) | 50,260 (45.4) | 3,608 (2.9) |
| Former | 3,381 (6.9) | 10,633 (21.8) | 5,505 (12.4) | 21,882 (51.8) | 3,023 (7.0) |
| Current | 2,790 (8.4) | 5,754 (16.4) | 4,527 (14.5) | 15,705 (49.3) | 3,864 (11.4) |
†, associations between the selected factors and the 5-group heavy drinking patterns (and 3-group current heavy drinking patterns), all P values <0.001. Data are shown as number (weighted percentage) or mean ± SE.
The prevalence of heavy drinking was the same for those with and without cancer (both 5.2%). For those who currently drank alcohol, we were interested in testing whether the cancer status (yes/no) had an impact on amount of alcohol intake status. After adjusting for the selected factors, cancer survivors were less likely to be light/moderate drinkers versus infrequent drinkers (OR=0.88, P<0.001) compared to non-cancer individuals, but cancer status did not have a significant impact on heavy drinkers versus infrequent drinkers (P=0.089). For alcohol drinking amount by cancer types (Figure 1B), prevalence of heavy drinking was similar among individuals with alcohol-related cancer, and non-alcohol related cancer (5.2% and 5.1%, respectively, P=0.950). The alcohol-related cancer type did not have a significant impact on amounts of alcohol drinking after adjusting for the selected factors (P=0.731 for light/moderate and P=0.132 for heavy vs. infrequent, Table 3). For the impact of length of cancer history, prevalence of heavy drinking was similar regardless of the length of cancer history (5.6%, 4.8% and 5.2% for 2–4, 5–9 and 10+ years, P=0.683, Figure 1C).
Table 3. Cancer related factors associated with past-year heavy and light/moderate alcohol intake among current drinkers.
| Characteristic | Light/moderate vs. infrequent | Heavy vs. infrequent | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Crude OR (95% CI)† | P value | Adjusted OR (95% CI)† | P value | Crude OR (95% CI)† | P value | Adjusted OR (95% CI)† | P value | ||
| Cancer | |||||||||
| No | 1 | 1 | 1 | 1 | |||||
| Yes | 0.75 (0.71–0.80) | <0.001 | 0.88 (0.82–0.94) | <0.001 | 0.86 (0.76–0.98) | 0.024 | 0.89 (0.78–1.02) | 0.089 | |
| Alcohol-related cancer‡ | |||||||||
| No | 1 | 1 | 1 | 1 | |||||
| Yes | 0.73 (0.63–0.84) | <0.001 | 1.03 (0.88–1.20) | 0.731 | 0.92 (0.70–1.20) | 0.537 | 1.22 (0.94–1.59) | 0.132 | |
| Length of cancer history, year | |||||||||
| 2–4 | 1 | 1 | 1 | 1 | |||||
| 5–9 | 1.12 (0.93–1.35) | 0.235 | 1.20 (0.97–1.47) | 0.088 | 0.85 (0.57–1.28) | 0.440 | 0.89 (0.60–1.32) | 0.553 | |
| 10+ | 1.09 (0.92–1.28) | 0.330 | 1.20 (1.01–1.44) | 0.041 | 0.97 (0.66–1.43) | 0.888 | 0.99 (0.70–1.40) | 0.951 | |
†, OR: odds ratio; CI: confidence interval; adjusted OR adjusted for age, gender, race, education, marital status, and smoking. ‡, Alcohol related cancer types included breast, colon, rectum, mouth/tongue/lip, larynx-windpipe, esophagus, and liver cancers.
Binge alcohol drinking
For the binge drinking patterns, we evaluated no binge, occasional binge, and frequent binge among current drinkers. The selected demographic factors and smoking status of the binge drinking patterns were summarized in Table S2 and all were significantly associated with binge drinking patterns. Prevalence of frequent alcohol binge was 4.7% and prevalence of occasional binge was 19.7%. All of these five demographic factors and smoking status were significantly (all P values <0.001) associated with frequent binge. Among these selected factors, smoking and gender were the two factors with the largest impact on frequent binge drinking. The prevalence of frequent binge was 11.8% for current smokers and only 2.8% for never smokers. For gender impact, 7% of males were frequent binge drinkers but only 2.3% of females were frequent binge drinkers.
Table S2. Demographic characteristics and smoking by the 5-group binge alcohol drinking of the US adults (NHIS 2012–2017)†.
| Characteristic | Never: (n=39,541; 20.7%) | Former: (n=29,775; 13.9%) | Current | ||
|---|---|---|---|---|---|
| No Binge: (n=77,426; 41.0%) | Occasional Binge: (n=35,489; 19.7%) | Frequent Binge: (n=9,253; 4.7%) | |||
| Age (year) | 45.32±0.18 | 55.85±0.14 | 49.08±0.11 | 38.98±0.13 | 40.94±0.25 |
| Age (years) | |||||
| <45 | 16,872 (22.6) | 6,643 (7.8) | 29,267 (35.8) | 22,988 (27.8) | 5,353 (6.0) |
| 45–64 | 10,811(16.4) | 11,492 (16.5) | 28,984 (46.3) | 10,195 (16.3) | 3,066 (4.5) |
| ≥65 | 11,858 (24.1) | 11,640 (24.7) | 19,175 (44.1) | 2,306 (5.3) | 834 (1.9) |
| Gender | |||||
| Female | 27,200 (25.5) | 16,133 (13.8) | 44,766 (43.0) | 15,434 (15.4) | 2,569 (2.3) |
| Male | 12,341 (15.5) | 13,642 (14.1) | 32,660 (38.8) | 20,055 (24.3) | 6,684 (7.3) |
| Race | |||||
| Non-Hispanic Caucasian | 23,548 (16.9) | 22,107 (14.2) | 58,667 (42.2) | 28,572 (21.5) | 7,355 (5.1) |
| Non-Hispanic African-American | 7,585 (29.8) | 4,422 (14.6) | 10,267 (39.8) | 3,041 (12.4) | 939 (3.4) |
| Other Hispanic or Others | 8,408 (34.1) | 3,246 (11.7) | 8,492 (35.0) | 3,876 (15.8) | 959 (3.5) |
| Education | |||||
| <High school | 8,827 (34.6) | 5,958 (20.9) | 6,988 (27.0) | 2,867 (12.4) | 1,273 (5.0) |
| High school | 11,470 (24.5) | 9,073 (17.2) | 17,352 (36.1) | 7,657 (16.9) | 2,526 (5.3) |
| >High school | 18,491 (16.0) | 14,445 (11.1) | 52,763 (46.0) | 24,840 (22.4) | 5,413 (4.4) |
| Marital status | |||||
| Married or living with partner | 16,201 (18.1) | 12,787 (14.4) | 37,601 (45.6) | 14,664 (18.3) | 2,854 (3.6) |
| Never married | 10,923 (29.9) | 4,346 (8.0) | 14,941 (31.5) | 11,151 (24.0) | 3,429 (6.5) |
| Widowed, divorced, separated | 10,954 (21.0) | 11,236 (21.7) | 20,304 (40.4) | 6,066 (12.6) | 2,026 (4.2) |
| Smoking | |||||
| Never | 33,343 (28.7) | 13,346 (10.4) | 46,204 (40.9) | 18,773 (17.2) | 3,272 (2.8) |
| Former | 3381 (7.0) | 10,633 (22.0) | 19,500 (44.9) | 8,449 (21.1) | 2,163 (5.0) |
| Current | 2790 (8.5) | 5,754 (16.6) | 11,668 (36.0) | 8,244 (27.2) | 3,815 (11.8) |
†, Associations between the selected factors and the 5-group binge drinking patterns (and 3-group current binge drinking patterns), all P values <0.001. Data are shown as weighted percentage or mean ± SE.
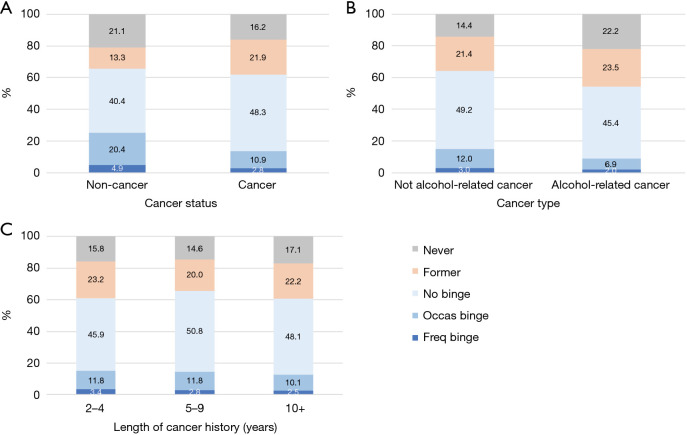
Cancer survivors tended to have fewer binges than non-cancer individuals. The prevalence of frequent binge for cancer survivors was lower than that of non-cancer individuals (Figure 2A, 2.8% vs. 4.9%, P<0.001). In addition, the prevalence of occasional binge was lower in cancer survivors than non-cancer individuals (10.9% vs. 20.4%, P<0.001). We were also interested in testing whether the cancer status had an impact on binge status among current drinkers. After adjusting for other selected factors (Table 4), cancer survivors were less likely to be occasional binge drinkers versus no binge drinkers compared with non-cancer individuals (OR=0.83, P<0.001), but cancer status was not significantly associated with frequent binge versus no binge (P=0.074). By comparing alcohol-related cancer types for frequent binge (Figure 2B), there was a small difference between these two groups (3.0% vs. 2.0%, P=0.013). Prevalence of occasional binge was lower in alcohol-related cancer survivors than in non-alcohol related cancer survivors (6.9% vs. 12.0%, P<0.001). After adjusting for other selected factors (Table 4), alcohol-related cancer types did not have a significant impact on both binge drinking statuses (P=0.143 for occasional binge and P=0.139 for frequent binge vs. no binge). The length of cancer history did not have a significant impact on both alcohol binge drinking behaviors (frequent binge and occasional binge). As shown in Figure 2C, the prevalence of frequent binge for cancer survivors with 2–4, 5–9 and 10+ years of cancer history was 3.4%, 2.8% and 2.5%, respectively (P=0.449).
Figure 2.
Distributions of the binge alcohol drinking patterns by cancer related factors. (A) By cancer status; (B) by alcohol-related cancer type; and (C) by length of cancer history. No binge, occasional binge and frequent binge are for current drinkers only. “Never drinker”: <12 drinks in a lifetime; “Former drinker”: ≥12 drinks in a lifetime but with 0 drink in the past year; “Current drinker”: ≥12 drinks in a lifetime and >0 drink in the past year. “No binge”: 0 days of binge drinking in the past year; “Occasional binge”: an average of >0 days but <4 binge drinking days of binge drinking per month; and “frequent binge”: an average of ≥4 binge drinking days per month in the past year.
Table 4. Cancer related factors associated with past-year frequent and occasional alcohol binge among current drinkers.
| Characteristic | Occasional Binge vs. no Binge | Frequent Binge vs. no Binge | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Crude OR (95% CI)† | P value | Adjusted OR (95% CI)† | P value | Crude OR (95% CI)† | P value | Adjusted OR (95% CI)† | P value | ||
| Cancer | |||||||||
| No | 1 | 1 | 1 | 1 | |||||
| Yes | 0.45 (0.41–0.48) | <0.001 | 0.83 (0.77–0.90) | <0.001 | 0.48 (0.40–0.58) | <0.001 | 0.84 (0.69–1.02) | 0.074 | |
| Alcohol-related cancer‡ | |||||||||
| No | 1 | 1 | 1 | 1 | |||||
| Yes | 0.62 (0.52–0.75) | <0.001 | 0.86 (0.70–1.05) | 0.143 | 0.70 (0.49–1.01) | 0.055 | 1.34 (0.91–1.98) | 0.139 | |
| Length of cancer history, year | |||||||||
| 2–4 | 1 | 1 | 1 | 1 | |||||
| 5–9 | 0.91 (0.75–1.09) | 0.292 | 0.98 (0.80–1.20) | 0.845 | 0.75 (0.41–1.38) | 0.353 | 0.77 (0.44–1.35) | 0.366 | |
| 10+ | 0.82 (0.69–0.97) | 0.020 | 0.99 (0.82–1.19) | 0.883 | 0.71 (0.40–1.25) | 0.237 | 0.78 (0.47–1.28) | 0.320 | |
†, OR: odds ratio; CI: confidence interval; adjusted OR, adjusted for age, gender, race, education, marital status, and smoking. ‡, Alcohol related cancer types included breast, colon, rectum, mouth/tongue/lip, larynx-windpipe, esophagus, and liver cancers.
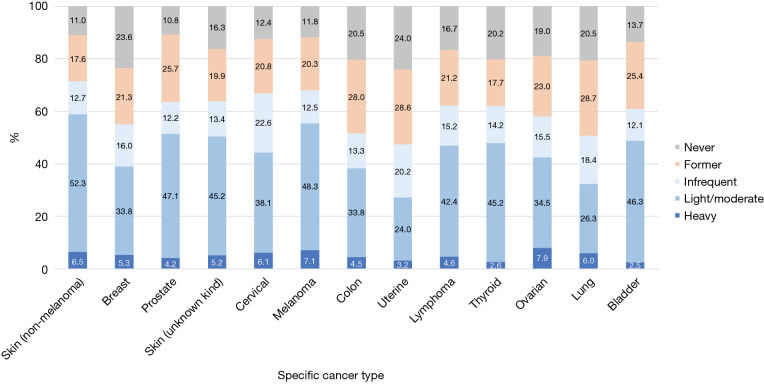
For specific cancer types, the alcohol intake patterns in terms of current, heavy, and binge drinking for the common cancer types are shown in Figures 3,4. The sample sizes of these common cancer types are listed in Table S3. The top three cancer types with the highest prevalence of current alcohol drinking were non-melanoma skin (71.5%), melanoma (67.9) and cervical cancer (66.8%). The top three cancer types with the highest prevalence of heavy drinking were ovarian (7.9%), melanoma (7.1%), and non-melanoma skin cancer (6.5%). For light/moderate alcohol intake, the top three cancer types were non-melanoma skin (52.3%), melanoma (48.3%) and prostate cancer (47.1%). For occasional binge, cervical (16.8%), ovarian (16.1%) and melanoma (14.9%) survivors had a higher prevalence of occasional binge. For frequent binge, cancer survivors with lymphoma (4.6%), skin cancer (unknown type, 3.7%), and colon cancer (3.6%) were likely to be frequent binge drinkers.
Figure 3.
Distributions of the heavy alcohol drinking patterns by common cancer types. Infrequent, light/moderate and heavy drinkers are for current drinkers only. “Never drinker”: <12 drinks in a lifetime; “Former drinker”: ≥12 drinks in a lifetime but with 0 drink in the past year; “Current drinker”: ≥12 drinks in a lifetime and >0 drink in the past year. “Infrequent drinker”: 1–11 drinks in the past year; “Light drinkers”: ≤3 drinks per week in the past year. “Moderate drinkers”: males >3 drinks up to 14 drinks per week or females >3 drinks up to 7 drinks per week; and “Heavy drinkers”: males >14 drinks per week or females >7 drinks per week in the past year.
Figure 4.
Distributions of the binge alcohol drinking patterns by common cancer types. No binge, occasional binge and frequent binge are for current drinkers only. “Never drinker”: <12 drinks in a lifetime; “Former drinker”: ≥12 drinks in a lifetime but with 0 drink in the past year; “Current drinker”: ≥12 drinks in a lifetime and >0 drink in the past year. “No binge”: 0 days of binge drinking in the past year; “Occasional binge”: an average of >0 days but <4 binge drinking days of binge drinking per month; and “frequent binge”: an average of ≥4 binge drinking days per month in the past year.
Table S3. Sample size of common cancer types.
| Cancer type | Sample size |
|---|---|
| Skin (non-melanoma) | 3,035 |
| Breast | 2,930 |
| Prostate | 1,804 |
| Skin (unknown kind) | 1,187 |
| Cervix | 1,058 |
| Melanoma | 1,035 |
| Colon | 854 |
| Uterus | 688 |
| Other | 585 |
| Lymphoma | 434 |
| Thyroid | 408 |
| Ovary | 346 |
| Lung | 325 |
Discussion
Our estimates of past-year alcohol drinking prevalence for non-cancer individuals was similar to other studies or reports. According to our study for non-cancer individuals, the past-year prevalence of current drinking was 65.9%, which is similar to the estimate of 70.1% based on the 2015 National Survey on Drug Use and Health (18). We estimated that prevalence of overall binge (occasional + frequent binge) was for 24.4% for the non-cancer individuals, which is similar to 25.2% based on the National Center for Health Statistics in 2018 (19). For these alcohol use patterns, our study used the ‘past-year’ as the reference timeframe. Comparing with the short-term timeframe (such as past 30 days), the past-year alcohol measure can capture an overall alcohol consumption habit and is especially beneficial for the irregular and infrequent alcohol drinkers (20). It also shown that the past-year alcohol measures provide better prediction on negative drinking consequences and potential alcohol abuse compared with the 2-week alcohol intake (21). Thus, the long-term past-year prevalence for non-cancer individuals, overall cancers and common cancer types (such as breast, colon and prostate cancer) can provide valuable information for physicians and public health policy makers.
Our study showed that prevalence of current drinking, light/moderate drinking, occasional binge and frequent binge was lower in cancer survivors than non-cancer individuals but no difference was observed for heavy drinking. After adjusting for the selected demographic factors and smoking status, cancer diagnosis only had a significant impact on the intermediate alcohol use (light/moderate drinking or occasional binge). Our study shows that smoking and selected demographic factors have a significant impact on alcohol drinking patterns. This is because the distributions of these selected demographic factors and smoking status are different for those with and without cancer. It is necessary to adjust these factors in order to test the real impact of cancer on alcohol intake. There is no direct comparison between our findings and previous studies due to different classification of alcohol use patterns. One similar study based on the NHIS 1998–2001 data found cancer status (yes/no) did not have a significant impact on the combined group of moderate/heavy alcohol intake after adjusting for the demographic and general health status (9).
For excessive alcohol drinking, our tested cancer related factors had no significant impact on heavy alcohol drinking and frequent binge after adjusting the selected factors. The similar prevalence of heavy drinking (4.8–5.6%) was observed regardless of the cancer status, alcohol-related cancer types and length of cancer history. The similar conditions of excessive drinking behaviors between cancer and non-cancer individuals may imply that some heavy-drinking cancer survivors did not reduce their alcohol drinking amount after cancer diagnosis. This reluctant to change condition may be due to many of these excessive drinkers also with alcohol dependence or an alcohol use disorder, which is a chronic relapsing brain disease. It has been shown that binge drinkers have a higher chance to develop alcohol dependence (22). Among past-week heavy drinkers, 41% of them had alcohol dependence and 73% had an alcohol use disorder. Among past-week binge drinkers, 38% of them had alcohol dependence and 65% had an alcohol use disorder (23). It has been shown that alcohol dependence and alcohol use disorders are under-diagnosed because of social exclusion, which may prevent the excessive alcohol drinkers for receiving related medical cares. Thus, we can expect a large portion of excessive drinkers to have addiction problems (24). It is well known that reducing or quitting alcohol intake is difficult for alcohol addicts, so the medical/biological treatments and education interventions are necessary (24,25).
Although our study is focusing on post-cancer alcohol drinking behaviors, it is informative to compare post-and pre-cancer alcohol intake. Using breast cancer as an example, alcohol intake is a confirmed cause of breast cancer incidence (15). The pre-cancer prevalence of heavy and binge drinking based on several breast cancer studies have shown to be higher than the post-cancer prevalence. The estimates of pre-cancer heavy drinking prevalence can be obtained based on several large-scale breast cancer studies. A large-scale prospective breast cancer study followed ~106,000 women for 28 years during 1980–2008 (26), the current drinking prevalence at diagnosis is 62% and heavy drinking rate (with similar definition as this study) is 15%. For the breast cancer survivors with different molecular subtypes [estrogen receptor (ER) +, triple negative (TN) and human epidermal growth factor receptor 2-overexpressing (H2E)], the current drinking prevalence at breast cancer diagnosis was 58–66% and prevalence of ≥4 alcoholic drinks per week was 15–21% (27). Based on another breast cancer prospective cohort that followed ~51,000 women for ~6-year follow-up, the current drinking prevalence at baseline for breast cancer cases was 81%, heavy drinking prevalence (>1 drink/day) was 15%, and prevalence of binge drinking was 15% (28). Based on these large-scale prospective breast cancer studies, the pre-cancer heavy drinking prevalence is ~15% and binge drinking is ~15%. Both of these pre-cancer risky drinking behaviors were much higher than our estimates of the post-cancer prevalence of heavy drinking (5.3%) and binge drinking (7.4%, occasional + frequent binge) for breast cancer survivors (Figures 3,4). For the direct observation of alcohol behavior changes due to cancer diagnosis, one prostate cancer study showed that more than half (52%) of prostate cancer survivors, who were heavy drinkers prior to cancer diagnosis, reduced their alcohol drinking after cancer diagnosis (29). For breast cancer, pre-cancer prevalence of heavy drinking may be higher than the post-cancer prevalence, which was similar to that of the general non-cancer population (7).
Frequent binge drinking (≥4 binge days per month) has been discussed in adolescent studies (22,30,31) but is limited in cancer studies. For binge drinking, the majority of studies evaluated the binary drinking status (yes/no) but did not discuss the frequency of binge drinking days (7). It has been shown that high-frequent alcohol binge increased overall cancer-specific mortality but not for low-frequent binge drinkers (6). In order to thoroughly evaluate the binge drinking frequency patterns, our study classified current drinkers to no binge (0 day of binge drinking), occasional binge (>0 and <4 binge drinking days per month), and frequent binge (≥4 binge drinking days per month) based on alcohol intake during the past year. Our study showed that there were 19% frequent binge drinkers among overall binge drinkers, and cancer survivors were less likely to have the occasional binge habit than non-cancer individuals. However, cancer status did not have a significant impact on those with frequent binge after adjusting the selected factors. Our results were similar compared to the previous study in terms of occasional binge. Another study showed that cancer survivors were less likely to be a binge drinker based on the past 30 days than non-cancer individuals and this significance remains after adjusting for age, race, education and health care coverage (7). This protective effect of cancer on overall binge may be primarily on occasional binge but not frequent binge.
In summary this study showed that cancer survivors have similar excessive alcohol drinking patterns (heavy or frequent binge) but were less likely to have the intermediate level of alcohol intake compared to non-cancer individuals. The strengths of this study include population-based estimates, various alcohol intake patterns (especially for light/moderate, heavy or frequent binge), estimates of habit-like alcohol intake, post-cancer alcohol intake, and consideration of demographic and smoking status. For cancer survivors, only post-cancer alcohol drinking behaviors were evaluated to avoid the mixture of pre- and post-cancer alcohol intake. There are several limitations of this study. First, the alcohol intake data used in this study was based on self-reported questionnaires so recall biases may exist. Second, newly diagnosed cancer survivors (<2 years of cancer history) were excluded, therefore, sample size of cancer survivors was reduced. Regardless these limitations, our study findings provide population-based prevalence of alcohol intake patterns by different cancer characteristics. Our study findings can provide valuable information for physicians and health educators to design custom alcohol interventions or treatments for cancer survivors, especially for those with excessive alcohol drinking habits.
Acknowledgments
We thank our anonymous reviewers for their valuable comments, which have led to many improvements to this article.
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). NHIS was approved by the Research Ethics Review Board of the National Center for Health Statistics and the U.S. Office of Management and Budget. Oral consent was provided for all NHIS respondents.
Footnotes
Provenance and Peer Review: This article was commissioned by the Guest Editors (Hui-Yi Lin, Tung-Sung Tseng) for the series “Population Science in Cancer” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.06.31). The series “Population Science in Cancer” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
References
- 1.Cancer Statistics. Available online: https://www.cancer.gov/about-cancer/understanding/statistics: National Cancer Institute. [updated 2/12/2019].
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7-34. 10.3322/caac.21551 [DOI] [PubMed] [Google Scholar]
- 3.Klein WM, Bloch M, Hesse BW, et al. Behavioral research in cancer prevention and control: a look to the future. Am J Prev Med 2014;46:303-11. 10.1016/j.amepre.2013.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferrari P, Licaj I, Muller DC, et al. Lifetime alcohol use and overall and cause-specific mortality in the European Prospective Investigation into Cancer and nutrition (EPIC) study. BMJ Open 2014;4:e005245. 10.1136/bmjopen-2014-005245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nelson DE, Jarman DW, Rehm J, et al. Alcohol-attributable cancer deaths and years of potential life lost in the United States. Am J Public Health 2013;103:641-8. 10.2105/AJPH.2012.301199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xi B, Veeranki SP, Zhao M, et al. Relationship of Alcohol Consumption to All-Cause, Cardiovascular, and Cancer-Related Mortality in U.S. Adults. J Am Coll Cardiol 2017;70:913-22. 10.1016/j.jacc.2017.06.054 [DOI] [PubMed] [Google Scholar]
- 7.Mowls DS, Brame LS, Martinez SA, et al. Lifestyle behaviors among US cancer survivors. J Cancer Surviv 2016;10:692-8. 10.1007/s11764-016-0515-x [DOI] [PubMed] [Google Scholar]
- 8.Coups EJ, Ostroff JS. A population-based estimate of the prevalence of behavioral risk factors among adult cancer survivors and noncancer controls. Prev Med 2005;40:702-11. 10.1016/j.ypmed.2004.09.011 [DOI] [PubMed] [Google Scholar]
- 9.Bellizzi KM, Rowland JH, Jeffery DD, et al. Health behaviors of cancer survivors: examining opportunities for cancer control intervention. J Clin Oncol 2005;23:8884-93. 10.1200/JCO.2005.02.2343 [DOI] [PubMed] [Google Scholar]
- 10.U.S. Department of Health and Human Services and U.S. Department of Agriculture. 2015 – 2020 Dietary Guidelines for Americans. 8th edition. Washington, D.C.; 2015. [Google Scholar]
- 11.Frazelle ML, Friend PJ. Optimizing the Teachable Moment for Health Promotion for Cancer Survivors and Their Families. J Adv Pract Oncol 2016;7:422-33. [PMC free article] [PubMed] [Google Scholar]
- 12.Berry NM, Miller MD, Woodman RJ, et al. Differences in chronic conditions and lifestyle behaviour between people with a history of cancer and matched controls. Med J Aust 2014;201:96-100. 10.5694/mja13.10701 [DOI] [PubMed] [Google Scholar]
- 13.Ganz PA. The 'three Ps' of cancer survivorship care. BMC Med 2011;9:14. 10.1186/1741-7015-9-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolin KY, Dart H, Colditz GA. Eight ways to stay healthy after cancer: an evidence-based message. Cancer Causes Control 2013;24:827-37. 10.1007/s10552-013-0179-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alcohol and Cancer, Centers of Disease Control and Prevention Centers of Disease Control and Prevention: Centers of Disease Control and Prevention; 2019. Available online: https://www.cdc.gov/cancer/alcohol/index.htm
- 16.Centers for Disease Control and Prevention (CDC) Adult Alcohol Use Information . Available online: https://www.cdc.gov/nchs/nhis/alcohol/alcohol_glossary.htm 2019
- 17.Statistics NCfH. Specifying Weighting Parameters. Available online: http://www.cdc.gov/nchs/tutorials/NHANES/SurveyDesign/Weighting/intro.htm
- 18.Substance Abuse and Mental Health Services Administration (SAMHSA). 2015 National Survey on Drug Use and Health (NSDUH). Table 2.41B—Alcohol Use in Lifetime, Past Year, and Past Month among Persons Aged 12 or Older, by Demographic Characteristics: Percentages, 2014 and 2015. Available online: https://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015.htm#tab2-41b
- 19.Norris T, Clarke TC, Schiller JS. Early release of selected estimates based on data from the National Health Interview Survey. National Center for Health Statistics. Available online: https://public.tableau.com/profile/nhis#!/vizhome/FIGURE9_2/Dashboard9_2. 2018
- 20.Dawson DA. Methodological issues in measuring alcohol use. Alcohol Res Health 2003;27:18-29. [PMC free article] [PubMed] [Google Scholar]
- 21.Cranford JA, McCabe SE, Boyd CJ. A new measure of binge drinking: prevalence and correlates in a probability sample of undergraduates. Alcohol Clin Exp Res 2006;30:1896-905. 10.1111/j.1530-0277.2006.00234.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Courtney KE, Polich J. Binge drinking in young adults: Data, definitions, and determinants. Psychol Bull 2009;135:142-56. 10.1037/a0014414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim SG, Kim JS, Pack HJ, et al. Usefulness of Heavy Drinking and Binge Drinking for the Diagnosis of Alcohol Use Disorder. Korean J Fam Med 2016;37:214-20. 10.4082/kjfm.2016.37.4.214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Substance Abuse and Mental Health Services Administration and National Institute on Alcohol Abuse and Alcoholism, Medication for the Treatment of Alcohol Use Disorder: A Brief Guide. Rockville, MD: Substance Abuse and Mental Health Services Administration. HHS Publication No. (SMA) 15-4907; 2015. [Google Scholar]
- 25.Soyka M, Kranzler HR, Hesselbrock V, et al. Guidelines for biological treatment of substance use and related disorders, part 1: Alcoholism, first revision. World J Biol Psychiatry 2017;18:86-119. 10.1080/15622975.2016.1246752 [DOI] [PubMed] [Google Scholar]
- 26.Chen WY, Rosner B, Hankinson SE, et al. Moderate alcohol consumption during adult life, drinking patterns, and breast cancer risk. JAMA 2011;306:1884-90. 10.1001/jama.2011.1590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baglia ML, Cook LS, Mei-Tzu C, et al. Alcohol, smoking, and risk of Her2-overexpressing and triple-negative breast cancer relative to estrogen receptor-positive breast cancer. Int J Cancer 2018;143:1849-57. 10.1002/ijc.31575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.White AJ, DeRoo LA, Weinberg CR, et al. Lifetime Alcohol Intake, Binge Drinking Behaviors, and Breast Cancer Risk. Am J Epidemiol 2017;186:541-9. 10.1093/aje/kwx118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Farris MS, Courneya KS, Kopciuk KA, et al. Post-diagnosis alcohol intake and prostate cancer survival: A population-based cohort study. Int J Cancer 2018;143:253-62. 10.1002/ijc.31307 [DOI] [PubMed] [Google Scholar]
- 30.Jang JB, Patrick ME, Keyes KM, et al. Frequent Binge Drinking Among US Adolescents, 1991 to 2015. Pediatrics 2017;139. doi: . 10.1542/peds.2016-4023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Powers J, Duffy L, Burns L, et al. Binge drinking and subsequent depressive symptoms in young women in Australia. Drug Alcohol Depend 2016;161:86-94. 10.1016/j.drugalcdep.2016.01.019 [DOI] [PubMed] [Google Scholar]