Abstract
This article provides an overview of hidradenitis suppurativa, lichen planus, lichen sclerosis, calcinosis cuti, pyogenic granuloma, intertrigo, and seborrheic keratosis. This article also focuses on recognition and management of these pleomorphic afflictions of the perianal region.
Keywords: hidradenitis suppurativa, lichen planus, lichen sclerosis, calcinosis cuti, pyogenic granuloma, intertrigo and seborrheic keratosis
Several perianal pathologies fall outside a unifying underlying process—specific etiology. They are commonly important elements within the differential diagnosis of other anorectal diagnoses but with their distinctly individual or unknown pathogenesis. These conditions should be readily recognized and managed by colorectal surgeons. This final section overviews hidradenitis suppurativa, lichen planus (LP), lichen sclerosis (LS), calcinosis cuti (CC), pyogenic granuloma, intertrigo, and seborrheic keratosis.
Hidradenitis Suppurativa
Hidradenitis suppurativa (HS) is also known as acne inversa or Verneuil's disease . It is a chronic, recurrent, painful, inflammatory skin disease that affects apocrine glands. 1 HS usually develops after puberty, commonly seen in early twenties and in the third decade of life. It appears to decline after age 50. Women are more affected than men with a female to male ratio of 3:1—frequently with more genitofemoral, axillary, and mammary lesions. Men tend to have more perianal and perineal involvement. 2 3
Prevalence of HS has been estimated between 0.053 and 4% in US general population. This number probably represents an underestimation because most of the studies are based only from patients that seek medical attention, insurance claims, or complete a questionnaire-based survey. 3 4 5 Vazquez et al reported the incidence of HS in Minnesota, with an annual of 6.0 per 100,000 person-years. They also showed an increase in the incidence from 1970s of 4.3 per 100,000 to more than double in the 2000s of 9.6 per 100,000. 6
Occlusion of the terminal hair follicle is followed by inflammation of the apocrine gland. 7 Typical histology of HS shows infundibular plugging, cyst formation, rupture of the hair follicle, and epidermal psoriasiform hyperplasia followed by abscess formation. Sinus tracts and scarring develop in later stages. 8 9 The inflammatory infiltrate consists of multiple cell types including neutrophils, macrophages, multinucleated giant cells, and B- and T lymphocytes. 9 10 11 Immunological dysregulation may also be involved in the development of HS since perilesional unaffected skin showed perivascular and perifollicular inflammation with significant reduction in sebaceous glands. Combined with friction, hair follicles more readily rupture. 12
Multiple endogenous and exogenous factors are associated with the presence and development of HS. Between 30 and 40% of the patients report another affected family member. HS is associated with an autosomal dominant inheritance pattern with variable penetrance. 13 Several mutations of the γ-secretase complex as PSENEN, PSEN 1, and NCSTN have been identified associated with HS. A small number of cases with severe acne and perifolliculitis capitis have been linked to chromosome 1p21.1–1q25.3 mutations. These are not present in all patients. 14 15 16
Immunological dysregulation in HS is supported since a chronic inflammatory process without the presence of pathogenic bacteria is frequently found. 17 Some studies have shown different inflammatory and anti-inflammatory cytokines elevated in lesions with HS including interleukin-1β(IL-1β), tumor necrosis factor alpha (TNF-α), IL-10, CXCL9 (chemokire 9), monokine induced by interferon-γ, IL-11, B lymphocyte chemoattractant, and IL-17A. 11 18 19 Other immune markers are significantly decreased in HS lesions including TLR 2,3,4,7,9, IL-2, IL-4, IL-5, and B defensing 2. 11 20 More studies are needed to clarify the roll of immunology in HS.
Smoking is a risk factor for the development and severity of HS. Up to 90% of patients are smokers or ex-smokers. Patients who smoke tend to have a more severe disease than nonsmokers. 6 21 Higher concentrations of nicotine are found in intertriginous areas and axillary sweat. Nicotine is associated with stimulation of proinflammatory reactions, chemotaxis of neutrophils, epidermal hyperplasia, and follicular plugging seen in skin of patients with HS. 22 23 Smoking cessation does not improve severity or the disease course of HS. 2
Patients with obesity (body mass index [BMI] > 30) are commonly associated with a more severe disease than those with normal weight. Approximately 50 to 80% of patients with HS are overweight (BMI > 25). 21 This association may be related to the presence of overlapping skin folds, sweat retention, friction, inflamed skin, microtears, and eventually rupture of the hair follicle with superimposed bacterial infection. 12 23
Studies have shown that bacterial infection is a secondary event in the natural course of HS. Bacterial cultures from HS lesions are frequently sterile and superinfection occurs with streptococci, staphylococci, and Escherichia coli that are also commensal skin flora found in cultures. 24 Rifampicin and clindamycin are effective in the treatment of HS. Anti-inflammatory effects and in a lesser extent bactericidal effects may be operant. 25 No studies support the relationship of shaving, chemical substances, deodorants, talcum powder, and use of tight clothing with the appearance of HS. 26
There are other different diseases that share an immunological or genetic base with HS. A group of diseases that present with follicular occlusion are the acne conglobata, pilonidal cyst, dissecting cellulitis of the scalp and HS. The four entities in conjunction are called the follicular occlusion tetrad. Up to 26% of HS patients have a history of severe acne. Up to 30% have a history of pilonidal cyst. These conditions are frequently misdiagnosed in patients with HS. 27 28 Crohn's disease (CD) is associated with HS. The incidence of HS is reported between 17 and 38% in patients with CD. However, it is complicated to delineate if patients with HS are actually cutaneous CD. 29 30 31 Pyoderma gangrenosum is a chronic, inflammatory skin disease with progressive ulcers, affecting the lower extremities or the trunk. Ulcers in the perineal area can be confused with lesions from HS. Misdiagnosis occurs because both pathologies have been associated. 30 32 Other diseases seen with HS in greater frequency include spondyloarthropathy, arthritis (HLA-B27 negative and rheumatoid factor negative), pyogenic arthritis-pyoderma gangrenosum acne syndrome, Down syndrome, and Dowling-Degos disease. 1 24
Squamous-cell carcinoma as Marjolin's ulcers associated with chronic HS lesions ( Fig. 1 ). Association with buccal and hepatocellular cancer is reported. This observation could be confounded by smoking as a risk factor for both these tumors as and HS. 33
Fig. 1.
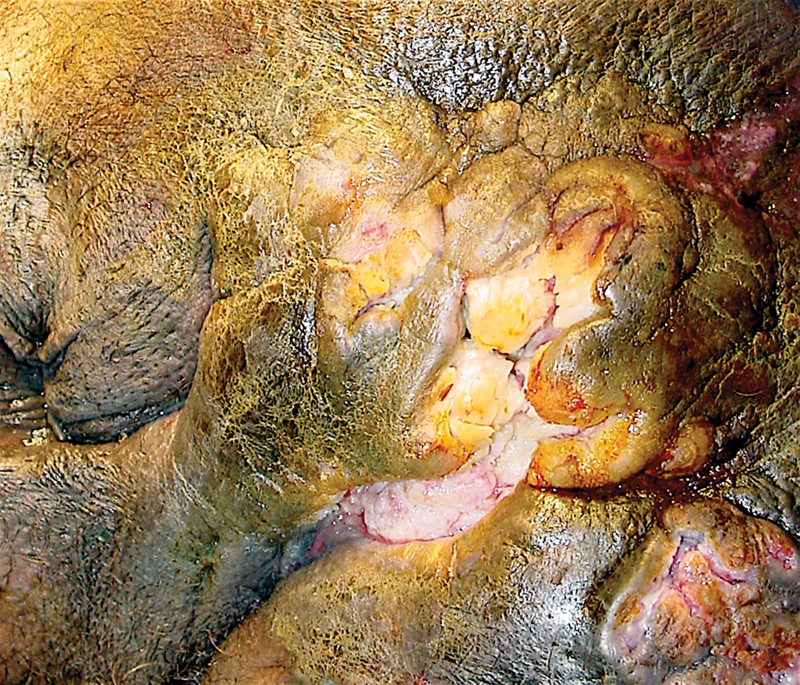
Squamous cell carcinoma transformation in the setting of chronic hidradenitis suppurativa—an example of a Marjolin's ulcer phenomenon (Photo—A. Ortega).
The diagnosis is clinical. Physical examination reveals the characteristic painful, inflammatory and noninflammatory nodules, abscesses, and sinus tract formation. Eventually, scarring and tissue fibrosis are observed. The axillary, inguinal, and anogenital regions are most affected. Secondary lesions as double and tombstone-like comedones are often present. 1 24 A consensus disease definition has been proposed that HS consists of a history of at least five discharging or painful skin lesions at typical sites. 13 Primary lesions appearance are nodules located in the deeper dermis and rounded as purulent boils. Other lesions including pyogenic granulomas in sinus tracts, induration, ropelike scars, and giant multiheaded comedones are dscribed 1 ( Fig. 2 ).
Fig. 2.
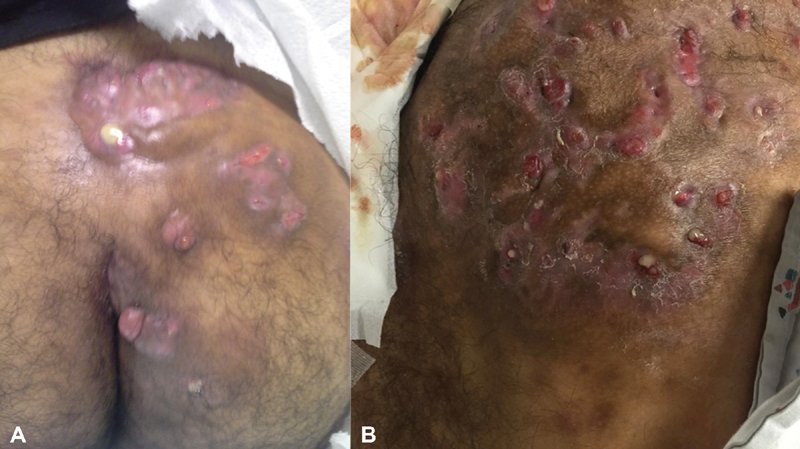
Characteristic features of hidradenitis suppurativa are in evidence including ( A ) diffuse interconnecting tracts with abscesses and pyogenic granuloma; ( B ) demonstrates greater disease extension, scarring, and deformity (Photos—A. Ortega).
Cases refractory to treatment or atypical cases may benefit from biopsies and/or cultures of the lesions. Bacteriologic studies are most frequently negative. Superinfection with Staphylococcus aureus may be associated. 34 These lesions are often treated as ordinary boils with antibiotics that seem to work during the first week, but ultimately result in recurrence and delay in the diagnosis. 1
Hurly described the most commonly used staging system for the classification and treatment of HS. Most patients are diagnosed at stage II—perhaps reflecting a diagnostic delay of stage I disease. 35 Only 1% of patients have progression to stage III disease ( Table 1 ).
Table 1. Hurley stages of lesion in hidradenitis suppurativa.
| Characteristics | |
|---|---|
| Stage I | Localized with formation of single or multiple abscess |
| Stage II | Recurrent abscesses with sinus tract formations and scarring Single or multiple widely separated lesions |
| Stage III | Diffuse or nearly diffuse of the affected region with interconnected tracts and abscesses across the area |
Multiple treatments are reported in the literature for the management of HS. The evidence of most of these interventions is limited to small series and retrospective studies. The variety of therapies reflects a lack of single definitive treatment. Medical versus surgical treatment depends on disease severity. Localized disease (Hurley Stage I) is usually managed with topical therapy. Refractory cases or widespread severe disease (Stage II or III) are candidates for systemic or biological therapy, surgery, or combined treatments. 36 37 Topical clindamycin (10 mg/mL) twice daily during 3 months reduces the number of abscesses, nodules, and pustules in mild disease cases. 38 Resorcinol has less available data in the treatment of localized HS and its irritant effect limits its use to smaller areas. 39
Patients with mild or moderate disease unresponsive to topical treatment are candidates to oral treatment with tetracycline (500 mg twice daily) or doxycycline/minocycline (50–100 mg twice daily). 39 Others use a combination of oral clindamycin (300 mg twice daily) and rifampicin (300 mg twice daily) for 10 to 12 weeks. A prospective study reported limited efficacy showing clinical response in 73% of patients and decreasing to 41% at 1-year follow-up, with a high index of relapse. 40
A small study with minocycline (100 mg daily) combined with colchicine (0.5 mg twice daily) for 6 months and then a maintenance regimen of 0.5 mg of colchicine twice daily for 3 months. Good-to-excellent results were reported in 95% of patients at 9 months. 41 Use of ertapenem, rifampicin, moxifloxacin, and metronidazole combined for 6 weeks has been suggested with modest results. It is an expensive and probably impossible to use in a daily basis scenario in patients with HS. 42
Adalimumab was approved in 2016 by the US Food and Drug Administration. Since then a growing number of high-quality studies have been published to support new monoclonal antibody therapies. Patients with refractory treatment to systemic antibiotics, quick recurrence, or moderate-to-severe disease without secondary bacterial infection are candidates to biological therapy. Adalimumab is a recombinant human immunoglobulin G1 (IgG1) anti-TNF monoclonal antibody. Two phase 3 studies named PIONEER I and PIONEER II by Kimball et al showed that treatment with adalimumab (40 mg weekly) as compared with placebo resulted in significantly higher clinical response rates in both trials at 12 weeks with comparable serious adverse events in the study groups (41.8 vs 26%, p = 0.003 and 58.9 vs 27.6%, p < 0.001 in PIONER I and PIONEER II, respectively). 43 Less evidence supports alternative regimens with adalimumab that start with 160 mg at baseline followed by 80 mg at week 2 and subsequent 40 mg every week from 16 to 24 weeks for maintenance.
Infliximab may be used as a first-line alternative anti-TNF therapy. A dose of 5 mg/kg intravenously at weeks 0, 2, and 6 and then every 8 weeks for maintenance is also recommended in the treatment of severe HS, but with less quality of evidence available. 39 Anakinra, a recombinant IL-1 receptor antagonist and ustekinumab, a human IgG1k monoclonal antibody, were designed to block or inhibit immune response of macrophages, natural killer, and T cells. Recently published small prospective studies showed moderate-to-good results compared with placebo. Further studies are required. 44 45
Patients that required surgery commonly present with scars and tunnels associated with chronic HS. Current evidence is based on retrospective or case series studies. Carbon dioxide laser evaporation or ablation therapy are better suited in patients with mild-to-moderate HS. 46 Abscesses are usually treated only with incision and drainage (I&D) to resolve local sepsis, and pain relief. I&D is not appropriate for solid phlegmatous lesions. Recurrence rates for I&D are near 100%. 47 48 Local excision or tissue-saving methods include unroofing and skin tissue-saving excision with electrosurgical peeling (STEEP). Unroofing implies opening the sinus tract in the same way that a fistulotomy is done over a probe. STEEP entails tangential excisions to progressively resect affected tissue. Both methods reduce scar contractures but recurrence is higher when compared with wide excision procedures. 48 Both excision and unroofing procedures provide better long-term outcomes than I&D because the latter does not remove the contiguous area of intractable disease. 47
Wide excision requires a disease-free tissue margin resection. Wounds generally are left to heal by secondary intention or direct closure. Skin grafts or flaps can be used in larger wounds. Recurrence rates are lower than more conservative methods but morbidity and contracture during healing are higher. A systematic review concluded that wide excision surgery reported the lower recurrence rate compared with local excision or simple unroofing (13% vs 22% vs 27%, respectively). The larger excision also results in a higher probability of cosmetic distortion. 49
Scarce evidence supports intralesional treatment with triamcinolone acetonide (10 mg/mL). Results from a small prospective study suggest improvement in erythema, edema, suppuration, and lesion size after 1 week of injection. The efficacy at long-term is unknown. 50 Neodymium-doped yttrium aluminum garnet laser, intense pulsed light, phototherapy, and botulinum toxin are alternatives reported in small case series. 51
Editorial Comments
It appears that advanced HS has neither an optimal nor definitive treatment. The editors offer the option of serial unroofing procedures based on personal experience covering a quarter of a century practice. Patients with Hurley stage III disease are counseled that their affliction is curable. However, this goal may require up to three operative interventions over several months. It is never a one and done proposition. The first intervention requires aggressive unroofing of all identifiable sinus tracts and abscess. Granulation tissue is debrided by curettage. The wounds are left to heal by secondary intention. The aesthetic aspects of this first intervention are appalling ( Fig. 3 ). However, patients accustomed to sitting upon tissues previously with pus under pressure feel immediate relief and tolerate the intervention remarkably well. The primary wounds are observed until complete or near complete epithelization has taken place. All patients will require a second or third unroofing procedure. Each time, the number of new or persistent sinus tracts diminishes. Eventually, the HS becomes burned out . The approach is admittedly completely anecdotal. It is based on the experience of two generations of surgeons at one of the largest public hospitals in the United States. Advanced perianal HS is a common and pleomorphic affliction in the collective practice of colorectal surgery. It is relatively uncommon in individual practices—even in large teaching hospitals. These factors pose significant obstacles to an evidence-based approach. However, the editors would be remiss in not suggesting serial unroofing procedures as a viable and effective option for the treatment of advanced perianal HS. Biologic treatment appears promising. Multimodal medical treatment and surgery might achieve a lesser recurrence rate than surgery or biological treatment alone. Further study is required. (A. Ortega—guest editor)
Fig. 3.
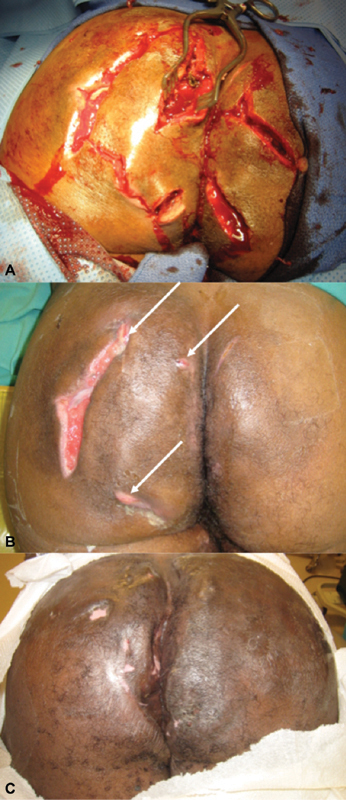
Representative photographs in three stages of advanced hidradenitis treated by serial unroofing procedures: ( A ) An aggressive initial incision and debridement; ( B ) fewer lesions are encountered at second interventions; and ( C ) burned out hidradenitis following multiple unroofing procedures (Photos—A. Ortega).
Perianal lichenification refers to the thickening at the anal verge and surrounding integumentary tissues. Three distinct pleomorphic conditions may be operant: lichen simplex, LP, and LS. Lichen simplex is the manifestation of chronic idiopathic pruritus ani (IPA)—defined as a dermatologic condition characterized as an unpleasant itchy or burning sensation in the perianal region. 52 The etiologic factors that lead to IPA include diet, personal hygiene habits, local irritants, drugs, and psychologic conditions. Secondary pruritus ani is related to infectious, dermatologic, systemic, anorectal as well as other disease-specific conditions discussed previously. 53 LP and LS are two of many specific dermatologic diseases that cause pruritus ani. 54 55 56 57 They present with distinct physical and pathologic findings.
Lichen Planus
This benign dermatosis was first described by Wilson in 1869. 58 It is an idiopathic condition which tends to be chronic or recurrent in nature. It is thought to be caused by an altered, cell-mediated immune response. It presents most commonly during the fifth and sixth decades of life—with a somewhat higher prevalence in women. 59 LP can produce skin eruption and also affects the mucous membranes, genitalia, and nails. Cutaneous LP usually presents as hard reddish-pink or violaceous shiny polygonal papules from 1 to > 1cm in size. These may look semitransparent on the surface and occasionally umbilicated. Additionally, characteristic fine white lines called Wickham's stria are frequently seen on the papules. These lesions can be quite bothersome as itching can occur in up to 80% of cases. 60 61 62 Mucosal LP can occur with or without skin involvement. These lesions are commonly seen on the tongue and buccal mucosa and appear as white to gray streaks on a reticular pattern over a violaceous background. They can be found on the conjunctiva, larynx, tonsils, tympanic membrane, esophagus, stomach, bladder, vulva, vaginal vault, glans penis, throughout the gastrointestinal tract, and around the anus 60 61 ( Fig. 4 ). Occasionally some lesions, especially those that are ulcerated may undergo malignant transformation. Reports of neoplastic transformation of anal LP into squamous-cell carcinoma have been published. 61 63 The diagnosis can be made by macroscopic examination of clinically compatible lesions. Additionally, mineral oil can be applied on to the cutaneous plaques which can serve as an aid to establish the diagnosis as this can reveal Wickham's stria. 53 Usually, LP is a self-limited disease and frequently resolves within an 8 to 12-month period. 64 Less severe cases can be successfully treated with topical steroids. 64 Biopsy may be necessary for the treatment of refractory cases. Anal squamous cell cancer may complicate some cases of LP. Wide local excision is preferred for early lesions without sphincteric involvement. Combined chemoradiotherapy is used for more advanced cases. Persistent or locally recurrent cancers may require an abdominoperineal excision. 53 61
Fig. 4.
Perianal lichen planus confluent at the anal verge associated with peripheral satellite lesions (Photo—A. Ortega).
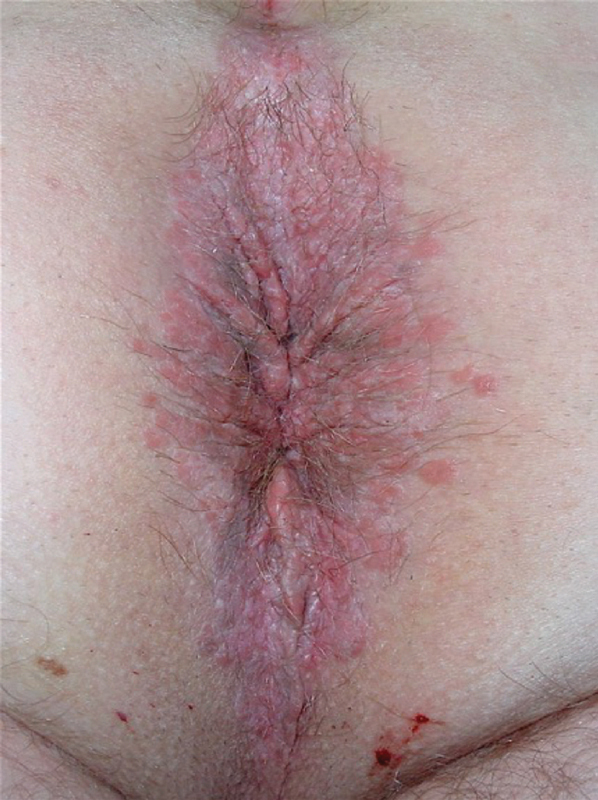
Lichen Sclerosis
LS is also called lichen sclerosis et atrophicus. LS is an idiopathic benign chronic dermatosis. 65 It is thought to be a cell-mediated autoimmune pathology that progresses from erythema and intense itching into thick, indurated, and atrophic skin in affected areas. Sixty percent of LS cases present with a characteristic figure of 8 distribution affecting the vulva and perianal area in females. 66 Involvement of the penis in males is termed balanitis xerotica obliterans. LS prevalence is five to six times greater in females than in males. 65 67 In the first or initial phase of the disease, there are ivory-colored, atrophic papules that tend to break down exposing the underlying raw erythematous tissue that produces intense pruritus and pain. Lesions are replaced by chronic inflammation with sclerosis and atrophy of the affected zone later as this area heals. The typical physical exam findings are white patches around the vulva and anus ( Fig. 5 ). 53 66 67 The diagnosis of LS can be made clinically by the appearance of lesions. Dermoscopy can serve as an aid to confirm diagnosis. 68 69 There is a reported incidence of around 4 to 6% of malignant transformation in LS lesions into squamous cell carcinoma. Patients that do not respond to treatment or those with recurrent sclerosis should have a skin biopsy performed. 70 71 There is evidence supporting that treatment for LS does not reduce this risk. 72 Clobetasol is a powerful topical steroid effective in symptomatic treatment. 65 73 Other options include testosterone creams, retinoids, and tacrolimus ointment. 65 74 75
Fig. 5.
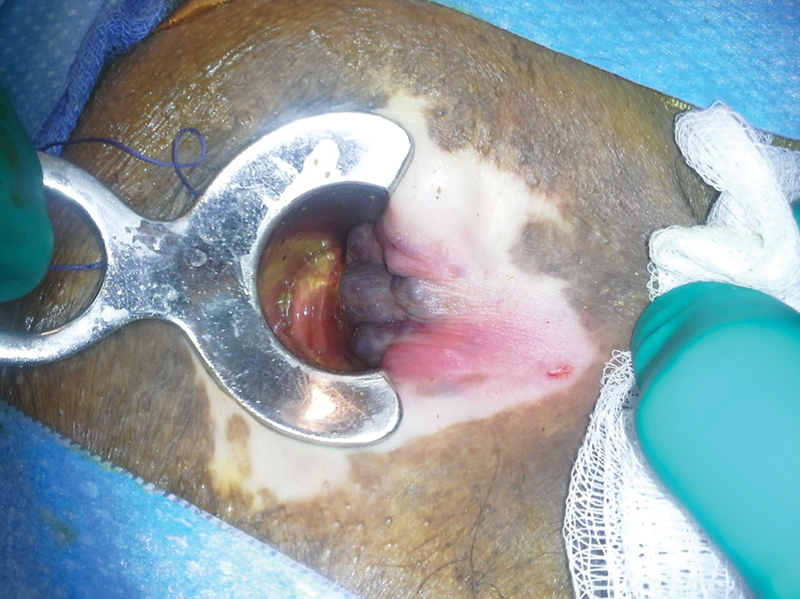
Perianal lichen sclerosis resembles vitiligo (Photo—A. Ortega).
Calcinosis Cutis
CC is a condition in which accumulation of hydroxyapatite crystals and amorphous calcium phosphate occurs in the skin and subcutaneous tissues. 76 77 It appears most frequently in association with autoimmune connective tissue pathologies including systemic sclerosis, dermatomyositis, mixed connective tissue disease, and less frequently in lupus erythematosus 78 79 It is classified into dystrophic, metastatic, iatrogenic, idiopathic, and calciphylaxis. The most frequent affected sites are hands, feet, and limbs in autoimmune diseases. Regions of microtrauma are affected in systemic sclerosis. Limbs and trunk are involved in dermatomyositis. The limbs and buttocks are affected in lupus erythematosus. 80 CC does present in the perianal area in individuals without autoimmune comorbidities ( Fig. 6 ). A previous injury may be operant in this clinical context. Patients can be minimally to very symptomatic. Treatment is based on small case series or isolated cases as there are no controlled clinical trials comparing the different therapeutic options. Small calcium deposits have been treated with ceftriaxone, warfarin, and intravenous immunoglobulins, while larger lesions may respond to other measures including diltiazem, bisphosphonates, probenecid, aluminum hydroxide, and curettage or surgical excision. 80 81 82 83
Fig. 6.
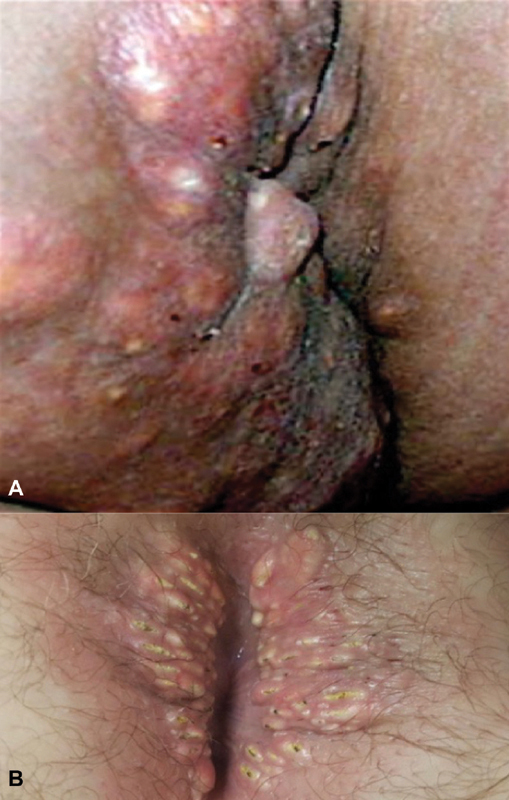
Perianal calcinosis cuti observed in two asymptomatic individuals without identified autoimmune disorders (Photo ( A )—A. Ortega; Photo ( B )—J.M. Parker).
Pyogenic Granuloma
Pyogenic granuloma is a reactive inflammatory process secondary to localized irritation or trauma. It can manifest in any region of the body arising over skin or mucous membranes. It is commonly associated with HS and in cryptoglandular fistula disease. Anal fistula can be associated with an exuberant tissue response at the external opening producing a mass-like appearance. A fistula tract can be associated with a single lobulated mass-like external opening. Multiple fistula tracts can produce more complex growths as demonstrated in Fig. 7 . Like all fistulas, they intermittently discharge purulent and/or bloody matter. It is important not to confuse them with neoplasms. Diagnosis is confirmed as part of the exam under anesthesia demonstrating an associated fistula-in-ano. Treatment is directed to excise the fistula, the external opening(s) and associated hypertrophic tissues.
Fig. 7.
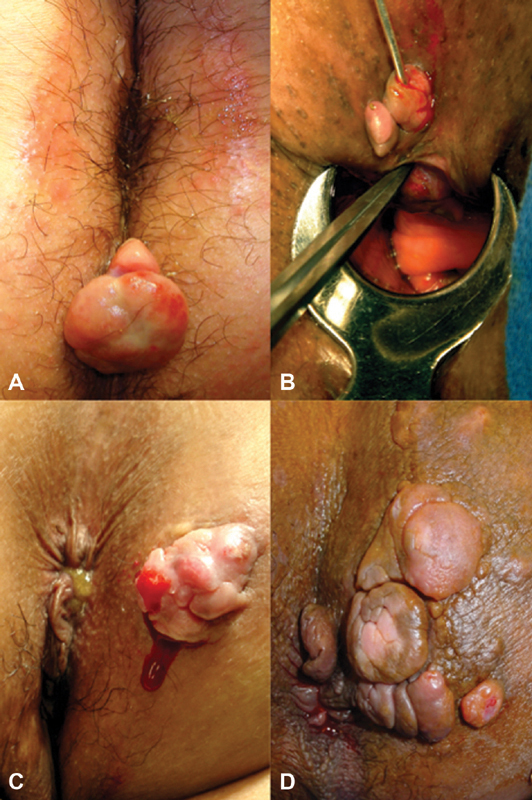
Pyogenic granulomas associated with the external opening(s) of cryptoglandular fistulas: ( A ) solitary, ( B ) double, ( C ) and ( D ) multiple. (Photos—A. Ortega)
Intertrigo
This inflammatory condition affects areas in the body where areas of skin are in close apposition to one another, that is, intertriginous zones. Intertrigo is also known as intertriginous dermatitis. It is induced or aggravated by heat, moisture, maceration, friction, lack of air circulation, and bodily secretions including perspiration, urine, and feces. 84 The moisture inherent in these regions promotes maceration and skin breakdown. These conditions are conducive to bacterial and/or fungal superinfection commonly associated with malodorous qualities. Isolated intertrigo can be relatively asymptomatic. Progression to pruritus, burning, and overt pain may ensue subsequently.
Interrogatories revealing topical application of steroids, antibacterial soaps, and ointments are relevant. A clinical diagnosis of uncomplicated intertrigo may be established on physical examination in this setting. Fungal superinfection is confirmed by potassium hydroxide and/or fungal cultures. The diagnosis can be made clinically by the presence of characteristic appearance associated with papules and pustules ( Fig. 8 ). Wood lamp examination can be useful when it fluoresces green with Pseudomonas or coral-red with Corynebacterium minutissimum . 85
Fig. 8.
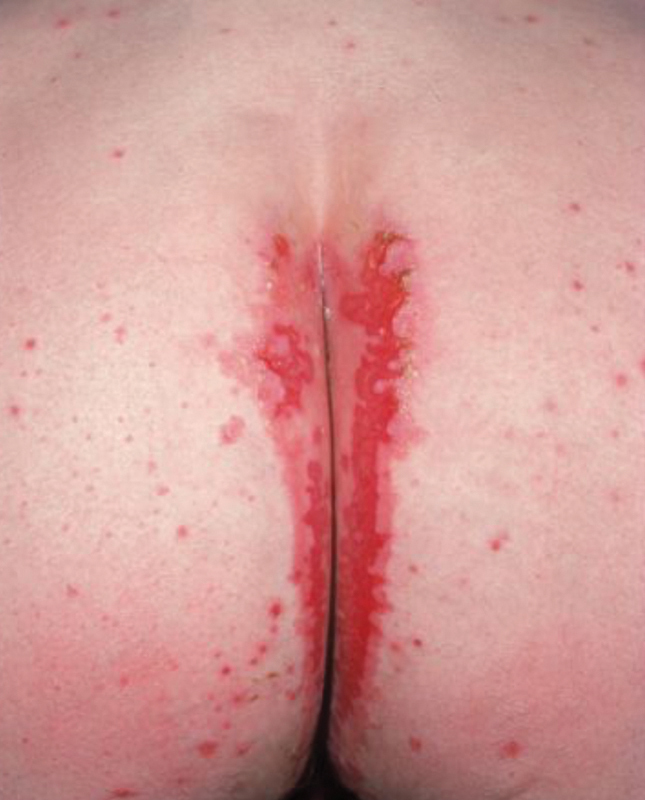
Perianal intertrigo with its characteristic pustules, papules, and superficial ulcerations (Photo—VisualDx.com—with permission).
Therapy in uncomplicated intertrigo is directed to keeping the area dry, clean, cool, and unopposed (e.g., cotton or gauze). Other drying agents include zinc oxide, aluminum sulfate, calcium acetate solution, talcum powder, and corn starch. 86 Intertrigo complicated by fungal or bacterial superinfections requires bacterial and fungal cultures and sensitivities. Topical antifungal treatment may be sufficient in many settings. Nystatin is effective for candida intertrigo. Clotrimazole, ketoconazole, oxiconazole, and econazole are effective for both Candida and dermatophyte superinfections. Fluconazole is reserved for resistant infections. Its hypoglycemic properties require blood sugar monitoring in diabetic patients. 87
Bacterial superinfections encompass a wide spectrum including Staph aureus, group A β-hemolytic strep, Pseudomonas aeruginosa, Proteus mirabilis, Proteus vulgaris, and Corynebacterium minutissimum . Antimicrobial therapies should be tailored according to their respective cultures and sensitivities. 88 89
Seborrheic Keratosis
This noncancerous skin growth is most common in older adults. More than 3 million individuals are diagnosed in the United States annually. Single lesions do occur. Multiple lesions are encountered more commonly over the face, chest, shoulders, and back. Perianal involvement does occur ( Fig. 9 ). The cause is unknown. These growths are described as waxy, wart-like, scaly, and with raised borders. Black, brown, tan, and other colors may be present. Physical diagnosis is generally sufficient. They are harmless and do not require any specific treatment unless associated with a specific skin malignancy (rare). Most are asymptomatic. Patients can find them irritating based on size, location, cosmetic appearance, or interaction with clothing. Excision is reserved for symptomatic lesions. 90
Fig. 9.
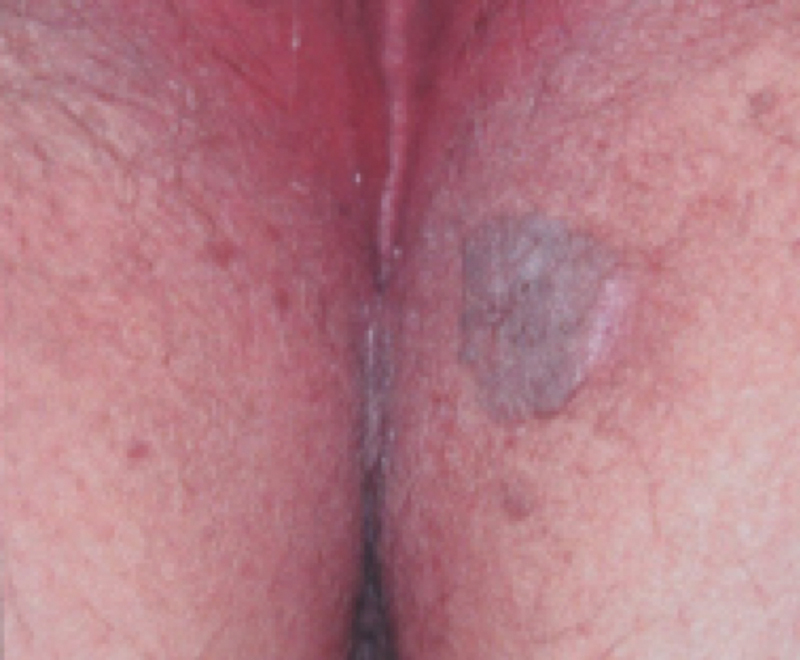
Seborrheic keratosis outside the anal margin in evidence (Photo—Genital & Perianal Diseases—A Color Handbook. Mroczkowski TF, Millikan LE, Parish LC, editors. 2014 © Boca Raton, FL: CRC Press. Reproduced by permission of Taylor & Francis Books UK).
Footnotes
Conflict of Interest None.
References
- 1.Jemec G B. Clinical practice. Hidradenitis suppurativa. N Engl J Med. 2012;366(02):158–164. doi: 10.1056/NEJMcp1014163. [DOI] [PubMed] [Google Scholar]
- 2.Matusiak L, Bieniek A, Szepietowski J C. Hidradenitis suppurativa and associated factors: still unsolved problems. J Am Acad Dermatol. 2009;61(02):362–365. doi: 10.1016/j.jaad.2009.03.043. [DOI] [PubMed] [Google Scholar]
- 3.Revuz J E, Canoui-Poitrine F, Wolkenstein P et al. Prevalence and factors associated with hidradenitis suppurativa: results from two case-control studies. J Am Acad Dermatol. 2008;59(04):596–601. doi: 10.1016/j.jaad.2008.06.020. [DOI] [PubMed] [Google Scholar]
- 4.Jemec G B, Heidenheim M, Nielsen N H.The prevalence of hidradenitis suppurativa and its potential precursor lesions J Am Acad Dermatol 199635(2 Pt 1):191–194. [DOI] [PubMed] [Google Scholar]
- 5.Cosmatos I, Matcho A, Weinstein R, Montgomery M O, Stang P. Analysis of patient claims data to determine the prevalence of hidradenitis suppurativa in the United States. J Am Acad Dermatol. 2013;68(03):412–419. doi: 10.1016/j.jaad.2012.07.027. [DOI] [PubMed] [Google Scholar]
- 6.Vazquez B G, Alikhan A, Weaver A L, Wetter D A, Davis M D. Incidence of hidradenitis suppurativa and associated factors: a population-based study of Olmsted County, Minnesota. J Invest Dermatol. 2013;133(01):97–103. doi: 10.1038/jid.2012.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van der Zee H H, de Ruiter L, Boer J et al. Alterations in leucocyte subsets and histomorphology in normal-appearing perilesional skin and early and chronic hidradenitis suppurativa lesions. Br J Dermatol. 2012;166(01):98–106. doi: 10.1111/j.1365-2133.2011.10643.x. [DOI] [PubMed] [Google Scholar]
- 8.Boer J, Weltevreden E F. Hidradenitis suppurativa or acne inversa. A clinicopathological study of early lesions. Br J Dermatol. 1996;135(05):721–725. [PubMed] [Google Scholar]
- 9.von Laffert M, Helmbold P, Wohlrab J, Fiedler E, Stadie V, Marsch W C. Hidradenitis suppurativa (acne inversa): early inflammatory events at terminal follicles and at interfollicular epidermis. Exp Dermatol. 2010;19(06):533–537. doi: 10.1111/j.1600-0625.2009.00915.x. [DOI] [PubMed] [Google Scholar]
- 10.von Laffert M, Stadie V, Wohlrab J, Marsch W C. Hidradenitis suppurativa/acne inversa: bilocated epithelial hyperplasia with very different sequelae. Br J Dermatol. 2011;164(02):367–371. doi: 10.1111/j.1365-2133.2010.10034.x. [DOI] [PubMed] [Google Scholar]
- 11.van der Zee H H, de Ruiter L, van den Broecke D G, Dik W A, Laman J D, Prens E P. Elevated levels of tumour necrosis factor (TNF)-α, interleukin (IL)-1β and IL-10 in hidradenitis suppurativa skin: a rationale for targeting TNF-α and IL-1β. Br J Dermatol. 2011;164(06):1292–1298. doi: 10.1111/j.1365-2133.2011.10254.x. [DOI] [PubMed] [Google Scholar]
- 12.Kamp S, Fiehn A M, Stenderup K et al. Hidradenitis suppurativa: a disease of the absent sebaceous gland? Sebaceous gland number and volume are significantly reduced in uninvolved hair follicles from patients with hidradenitis suppurativa. Br J Dermatol. 2011;164(05):1017–1022. doi: 10.1111/j.1365-2133.2011.10224.x. [DOI] [PubMed] [Google Scholar]
- 13.Von Der Werth J M, Williams H C, Raeburn J A. The clinical genetics of hidradenitis suppurativa revisited. Br J Dermatol. 2000;142(05):947–953. doi: 10.1046/j.1365-2133.2000.03476.x. [DOI] [PubMed] [Google Scholar]
- 14.Wang B, Yang W, Wen Wet al. Gamma-secretase gene mutations in familial acne inversa Science 2010330(6007):1065. [DOI] [PubMed] [Google Scholar]
- 15.Pink A E, Simpson M A, Desai N, Trembath R C, Barker J NW. γ-Secretase mutations in hidradenitis suppurativa: new insights into disease pathogenesis. J Invest Dermatol. 2013;133(03):601–607. doi: 10.1038/jid.2012.372. [DOI] [PubMed] [Google Scholar]
- 16.Pink A E, Simpson M A, Brice G W et al. PSENEN and NCSTN mutations in familial hidradenitis suppurativa (Acne Inversa) J Invest Dermatol. 2011;131(07):1568–1570. doi: 10.1038/jid.2011.42. [DOI] [PubMed] [Google Scholar]
- 17.Deckers I E, van der Zee H, Prens E P. Epidemiology of hidradenitis suppurativa: Prevalence, pathogenesis, and factors associated with the development of HS. Curr Dermatol Rep. 2014;3(01):54–60. [Google Scholar]
- 18.Nazary M, van der Zee H H, Prens E P, Folkerts G, Boer J.Pathogenesis and pharmacotherapy of Hidradenitis suppurativa Eur J Pharmacol 2011672(1-3):1–8. [DOI] [PubMed] [Google Scholar]
- 19.Schlapbach C, Hänni T, Yawalkar N, Hunger R E. Expression of the IL-23/Th17 pathway in lesions of hidradenitis suppurativa. J Am Acad Dermatol. 2011;65(04):790–798. doi: 10.1016/j.jaad.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 20.Dréno B, Khammari A, Brocard A et al. Hidradenitis suppurativa: the role of deficient cutaneous innate immunity. Arch Dermatol. 2012;148(02):182–186. doi: 10.1001/archdermatol.2011.315. [DOI] [PubMed] [Google Scholar]
- 21.Sartorius K, Emtestam L, Jemec G B, Lapins J. Objective scoring of hidradenitis suppurativa reflecting the role of tobacco smoking and obesity. Br J Dermatol. 2009;161(04):831–839. doi: 10.1111/j.1365-2133.2009.09198.x. [DOI] [PubMed] [Google Scholar]
- 22.Happle R, König A. Smoker's boils. Dermatology. 2011;222(03):282–284. doi: 10.1159/000327923. [DOI] [PubMed] [Google Scholar]
- 23.Kurzen H, Kurokawa I, Jemec G Bet al. What causes hidradenitis suppurativa? Exp Dermatol 20081705455–456., discussion 457–472 [DOI] [PubMed] [Google Scholar]
- 24.Alikhan A, Lynch P J, Eisen D B.Hidradenitis suppurativa: a comprehensive review J Am Acad Dermatol 20096004539–561., quiz 562–563 [DOI] [PubMed] [Google Scholar]
- 25.Bettoli V, Zauli S, Borghi A et al. Oral clindamycin and rifampicin in the treatment of hidradenitis suppurativa-acne inversa: a prospective study on 23 patients. J Eur Acad Dermatol Venereol. 2014;28(01):125–126. doi: 10.1111/jdv.12127. [DOI] [PubMed] [Google Scholar]
- 26.von der Werth J M, Williams H C. The natural history of hidradenitis suppurativa. J Eur Acad Dermatol Venereol. 2000;14(05):389–392. doi: 10.1046/j.1468-3083.2000.00087.x. [DOI] [PubMed] [Google Scholar]
- 27.Canoui-Poitrine F, Le Thuaut A, Revuz J E et al. Identification of three hidradenitis suppurativa phenotypes: latent class analysis of a cross-sectional study. J Invest Dermatol. 2013;133(06):1506–1511. doi: 10.1038/jid.2012.472. [DOI] [PubMed] [Google Scholar]
- 28.Chicarilli Z N. Follicular occlusion triad: hidradenitis suppurativa, acne conglobata, and dissecting cellulitis of the scalp. Ann Plast Surg. 1987;18(03):230–237. doi: 10.1097/00000637-198703000-00009. [DOI] [PubMed] [Google Scholar]
- 29.Church J M, Fazio V W, Lavery I C, Oakley J R, Milsom J W. The differential diagnosis and comorbidity of hidradenitis suppurativa and perianal Crohn's disease. Int J Colorectal Dis. 1993;8(03):117–119. doi: 10.1007/BF00341181. [DOI] [PubMed] [Google Scholar]
- 30.Fimmel S, Zouboulis C C. Comorbidities of hidradenitis suppurativa (acne inversa) Dermatoendocrinol. 2010;2(01):9–16. doi: 10.4161/derm.2.1.12490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van der Zee H H, van der Woude C J, Florencia E F, Prens E P. Hidradenitis suppurativa and inflammatory bowel disease: are they associated? Results of a pilot study. Br J Dermatol. 2010;162(01):195–197. doi: 10.1111/j.1365-2133.2009.09430.x. [DOI] [PubMed] [Google Scholar]
- 32.Hsiao J L, Antaya R J, Berger T, Maurer T, Shinkai K, Leslie K S. Hidradenitis suppurativa and concomitant pyoderma gangrenosum: a case series and literature review. Arch Dermatol. 2010;146(11):1265–1270. doi: 10.1001/archdermatol.2010.328. [DOI] [PubMed] [Google Scholar]
- 33.Lapins J, Ye W, Nyrén O, Emtestam L. Incidence of cancer among patients with hidradenitis suppurativa. Arch Dermatol. 2001;137(06):730–734. [PubMed] [Google Scholar]
- 34.Lapins J, Jarstrand C, Emtestam L. Coagulase-negative staphylococci are the most common bacteria found in cultures from the deep portions of hidradenitis suppurativa lesions, as obtained by carbon dioxide laser surgery. Br J Dermatol. 1999;140(01):90–95. doi: 10.1046/j.1365-2133.1999.02613.x. [DOI] [PubMed] [Google Scholar]
- 35.Hurley H. New York: Marcel Dekker; 1989. Axillary hyperhidrosis, apocrine bromhidrosis, hidradenitis suppurativa, and familial benign pemphigus: surgical approach; pp. 729–739. [Google Scholar]
- 36.Mekkes J R, Bos J D. Long-term efficacy of a single course of infliximab in hidradenitis suppurativa. Br J Dermatol. 2008;158(02):370–374. doi: 10.1111/j.1365-2133.2007.08332.x. [DOI] [PubMed] [Google Scholar]
- 37.Kimball A B, Kerdel F, Adams D et al. Adalimumab for the treatment of moderate to severe Hidradenitis suppurativa: a parallel randomized trial. Ann Intern Med. 2012;157(12):846–855. doi: 10.7326/0003-4819-157-12-201212180-00004. [DOI] [PubMed] [Google Scholar]
- 38.Clemmensen O J. Topical treatment of hidradenitis suppurativa with clindamycin. Int J Dermatol. 1983;22(05):325–328. doi: 10.1111/j.1365-4362.1983.tb02150.x. [DOI] [PubMed] [Google Scholar]
- 39.Saunte D ML, Jemec G BE. Hidradenitis suppurativa: advances in diagnosis and treatment. JAMA. 2017;318(20):2019–2032. doi: 10.1001/jama.2017.16691. [DOI] [PubMed] [Google Scholar]
- 40.Dessinioti C, Zisimou C, Tzanetakou V, Stratigos A, Antoniou C. Oral clindamycin and rifampicin combination therapy for hidradenitis suppurativa: a prospective study and 1-year follow-up. Clin Exp Dermatol. 2016;41(08):852–857. doi: 10.1111/ced.12933. [DOI] [PubMed] [Google Scholar]
- 41.Armyra K, Kouris A, Markantoni V, Katsambas A, Kontochristopoulos G. Hidradenitis suppurativa treated with tetracycline in combination with colchicine: a prospective series of 20 patients. Int J Dermatol. 2017;56(03):346–350. doi: 10.1111/ijd.13428. [DOI] [PubMed] [Google Scholar]
- 42.Join-Lambert O, Coignard-Biehler H, Jais J P et al. Efficacy of ertapenem in severe hidradenitis suppurativa: a pilot study in a cohort of 30 consecutive patients. J Antimicrob Chemother. 2016;71(02):513–520. doi: 10.1093/jac/dkv361. [DOI] [PubMed] [Google Scholar]
- 43.Kimball A B, Okun M M, Williams D A et al. Two phase 3 trials of adalimumab for hidradenitis suppurativa. N Engl J Med. 2016;375(05):422–434. doi: 10.1056/NEJMoa1504370. [DOI] [PubMed] [Google Scholar]
- 44.Blok J L, Li K, Brodmerkel C, Horvátovich P, Jonkman M F, Horváth B. Ustekinumab in hidradenitis suppurativa: clinical results and a search for potential biomarkers in serum. Br J Dermatol. 2016;174(04):839–846. doi: 10.1111/bjd.14338. [DOI] [PubMed] [Google Scholar]
- 45.Tzanetakou V, Kanni T, Giatrakou S et al. Safety and efficacy of anakinra in severe hidradenitis suppurativa: a randomized clinical trial. JAMA Dermatol. 2016;152(01):52–59. doi: 10.1001/jamadermatol.2015.3903. [DOI] [PubMed] [Google Scholar]
- 46.Madan V, Hindle E, Hussain W, August P J. Outcomes of treatment of nine cases of recalcitrant severe hidradenitis suppurativa with carbon dioxide laser. Br J Dermatol. 2008;159(06):1309–1314. doi: 10.1111/j.1365-2133.2008.08932.x. [DOI] [PubMed] [Google Scholar]
- 47.Kohorst J J, Baum C L, Otley C C et al. Surgical management of hidradenitis suppurativa: Outcomes of 590 consecutive patients. Dermatol Surg. 2016;42(09):1030–1040. doi: 10.1097/DSS.0000000000000806. [DOI] [PubMed] [Google Scholar]
- 48.Janse I, Bieniek A, Horváth B, Matusiak Ł. Surgical procedures in hidradenitis suppurativa. Dermatol Clin. 2016;34(01):97–109. doi: 10.1016/j.det.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 49.Mehdizadeh A, Hazen P G, Bechara F G et al. Recurrence of hidradenitis suppurativa after surgical management: a systematic review and meta-analysis. J Am Acad Dermatol. 2015;73(05) 01:S70–S77. doi: 10.1016/j.jaad.2015.07.044. [DOI] [PubMed] [Google Scholar]
- 50.Riis P T, Boer J, Prens E P et al. Intralesional triamcinolone for flares of hidradenitis suppurativa (HS): a case series. J Am Acad Dermatol. 2016;75(06):1151–1155. doi: 10.1016/j.jaad.2016.06.049. [DOI] [PubMed] [Google Scholar]
- 51.Rambhatla P V, Lim H W, Hamzavi I. A systematic review of treatments for hidradenitis suppurativa. Arch Dermatol. 2012;148(04):439–446. doi: 10.1001/archdermatol.2011.1950. [DOI] [PubMed] [Google Scholar]
- 52.Billingham R P, Isler J T, Kimmins M H, Nelson J M, Schweitzer J, Murphy M M. The diagnosis and management of common anorectal disorders. Curr Probl Surg. 2004;41(07):586–645. doi: 10.1016/j.cpsurg.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 53.Gaertner W B. New York: Springer; 2016. Dermatology and pruritus ani; pp. 309–324. [Google Scholar]
- 54.Alexander-Williams J.Pruritus ani Br Med J (Clin Res Ed) 1983287(6386):159–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hanno R, Murphy P. Pruritus ani. Classification and management. Dermatol Clin. 1987;5(04):811–816. [PubMed] [Google Scholar]
- 56.Harrington C I, Lewis F M, McDonagh A J, Gawkrodger D J.Dermatological causes of pruritus ani BMJ 1992305(6859):955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Verbov J. Pruritus ani and its management--a study and reappraisal. Clin Exp Dermatol. 1984;9(01):46–52. doi: 10.1111/j.1365-2230.1984.tb00754.x. [DOI] [PubMed] [Google Scholar]
- 58.Saurat J HLP. Milano: Masson; 1995. Lichen ruber planus e dermatosi lichenoidi; p. 341. [Google Scholar]
- 59.Boyd A S, Neldner K H. Lichen planus. J Am Acad Dermatol. 1991;25(04):593–619. doi: 10.1016/0190-9622(91)70241-s. [DOI] [PubMed] [Google Scholar]
- 60.Lichen planus clinical presentation: History, physical examination, causes.https://emedicine.medscape.com/article/1123213-clinical#b2. Accessed 12/18/2017,2017
- 61.Fundarò S, Spallanzani A, Ricchi E et al. Squamous-cell carcinoma developing within anal lichen planus: report of a case. Dis Colon Rectum. 1998;41(01):111–114. doi: 10.1007/BF02236905. [DOI] [PubMed] [Google Scholar]
- 62.Arndt K A. New York: McGraw Hill; 1993. Lichen planus; p. 1134. [Google Scholar]
- 63.Ruocco V, Satriano R A, De Rosa G, Pettinato G, Gombos F. Malignancy in lichen planus. Int J Dermatol. 1989;28(08):542–544. doi: 10.1111/j.1365-4362.1989.tb04612.x. [DOI] [PubMed] [Google Scholar]
- 64.Lichen planus treatment:https://emedicine.medscape.com/article/1123213-treatment. Updated 12/18/2017
- 65.Nasseri Y Y, Osborne M C. Pruritus ani: diagnosis and treatment. Gastroenterol Clin North Am. 2013;42(04):801–813. doi: 10.1016/j.gtc.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 66.Zuccati G, Lotti T, Mastrolorenzo A, Rapaccini A, Tiradritti L. Pruritus ani. Dermatol Ther. 2005;18(04):355–362. doi: 10.1111/j.1529-8019.2005.00031.x. [DOI] [PubMed] [Google Scholar]
- 67.Smith L E. New York: Informa Healthcare; 2007. Perianal dermatologic disease; pp. 247–260. [Google Scholar]
- 68.Lichen sclerosis; Workup.https://emedicine.medscape.com/article/1123316-workup#c1
- 69.Borghi A, Corazza M, Minghetti S, Bianchini E, Virgili A. Dermoscopic features of vulvar lichen sclerosus in the setting of a prospective cohort of patients: New observations. Dermatology. 2016;232(01):71–77. doi: 10.1159/000439198. [DOI] [PubMed] [Google Scholar]
- 70.Val I, Almeida G. An overview of lichen sclerosus. Clin Obstet Gynecol. 2005;48(04):808–817. doi: 10.1097/01.grf.0000179635.64663.3d. [DOI] [PubMed] [Google Scholar]
- 71.Siddiqi S, Vijay V, Ward M, Mahendran R, Warren S. Pruritus ani. Ann R Coll Surg Engl. 2008;90(06):457–463. doi: 10.1308/003588408X317940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Carli P, Cattaneo A, De Magnis A, Biggeri A, Taddei G, Giannotti B. Squamous cell carcinoma arising in vulval lichen sclerosus: a longitudinal cohort study. Eur J Cancer Prev. 1995;4(06):491–495. doi: 10.1097/00008469-199512000-00008. [DOI] [PubMed] [Google Scholar]
- 73.Neill S M, Tatnall F M, Cox N H; British Association of Dermatologists.Guidelines for the management of lichen sclerosus Br J Dermatol 200214704640–649. [DOI] [PubMed] [Google Scholar]
- 74.Assmann T, Becker-Wegerich P, Grewe M, Megahed M, Ruzicka T. Tacrolimus ointment for the treatment of vulvar lichen sclerosus. J Am Acad Dermatol. 2003;48(06):935–937. doi: 10.1067/mjd.2003.8. [DOI] [PubMed] [Google Scholar]
- 75.Sheth S, Schechtman A D. Itchy perianal erythema. J Fam Pract. 2007;56(12):1025–1027. [PubMed] [Google Scholar]
- 76.Touart D M, Sau P.Cutaneous deposition diseases. Part II J Am Acad Dermatol 199839(4 Pt 1):527–544., quiz 545–546 [DOI] [PubMed] [Google Scholar]
- 77.Balcı D D, Celik E, Sarıkaya G, Yenin J Z, Atik E. The co-existence of vulvar lichen sclerosus, ulcerated calcinosis cutis, and dermatomyositis: coincidence or immunological mechanism? Ann Dermatol. 2011;23 03:S375–S379. doi: 10.5021/ad.2011.23.S3.S375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gutierrez A, Jr, Wetter D A. Calcinosis cutis in autoimmune connective tissue diseases. Dermatol Ther (Heidelb) 2012;25(02):195–206. doi: 10.1111/j.1529-8019.2012.01492.x. [DOI] [PubMed] [Google Scholar]
- 79.Valenzuela A, Chung L. Calcinosis: pathophysiology and management. Curr Opin Rheumatol. 2015;27(06):542–548. doi: 10.1097/BOR.0000000000000220. [DOI] [PubMed] [Google Scholar]
- 80.Jiménez-Gallo D, Ossorio-García L, Linares-Barrios M. Calcinosis cutis and Calciphylaxis. Actas Dermosifiliogr. 2015;106(10):785–794. doi: 10.1016/j.ad.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 81.Dima A, Balanescu P, Baicus C. Pharmacological treatment in calcinosis cutis associated with connective-tissue diseases. Rom J Intern Med. 2014;52(02):55–67. [PubMed] [Google Scholar]
- 82.Reiter N, El-Shabrawi L, Leinweber B, Berghold A, Aberer E.Calcinosis cutis: part II. Treatment options J Am Acad Dermatol 2011650115–22., quiz 23–24 [DOI] [PubMed] [Google Scholar]
- 83.Balin S J, Wetter D A, Andersen L K, Davis M D. Calcinosis cutis occurring in association with autoimmune connective tissue disease: the Mayo Clinic experience with 78 patients, 1996-2009. Arch Dermatol. 2012;148(04):455–462. doi: 10.1001/archdermatol.2011.2052. [DOI] [PubMed] [Google Scholar]
- 84.Kalra M G, Higgins K E, Kinney B S. Intertrigo and secondary skin infections. Am Fam Physician. 2014;89(07):569–573. [PubMed] [Google Scholar]
- 85.Janniger C K, Schwartz R A, Szepietowski J C, Reich A. Intertrigo and common secondary skin infections. Am Fam Physician. 2005;72(05):833–838. [PubMed] [Google Scholar]
- 86.Guitart J, Woodley D T. Intertrigo: a practical approach. Compr Ther. 1994;20(07):402–409. [PubMed] [Google Scholar]
- 87.Del Rosso J Q, Draelos Z D, Jorizzo J L, Joseph W S, Ribotsky B M, Rich P.Modern methods to treat superficial fungal disease Cutis 200779(2, Suppl):6–29. [PubMed] [Google Scholar]
- 88.Holdiness M R. Management of cutaneous erythrasma. Drugs. 2002;62(08):1131–1141. doi: 10.2165/00003495-200262080-00002. [DOI] [PubMed] [Google Scholar]
- 89.Stulberg D L, Penrod M A, Blatny R A. Common bacterial skin infections. Am Fam Physician. 2002;66(01):119–124. [PubMed] [Google Scholar]
- 90.Hafner C, Vogt T. Seborrheic keratosis. J Dtsch Dermatol Ges. 2008;6(08):664–677. doi: 10.1111/j.1610-0387.2008.06788.x. [DOI] [PubMed] [Google Scholar]


