Summary
The diabetes mellitus (DM) drug metformin targets mechanistic/mammalian target of rapamycin and inhibits lymphoma growth in vitro. We investigated whether metformin affected outcomes of newly diagnosed diffuse large B-cell (DLBCL, n = 869) and follicular lymphoma (FL, n = 895) patients enrolled in the Mayo component of the Molecular Epidemiology Resource cohort study between 2002 and 2015. Hazard ratios (HR) and 95% confidence intervals (CI) adjusted for age, sex, body mass index, prognostic index and treatment were used to estimate the association of metformin exposure (No DM/No metformin; DM/No metformin; DM/Metformin) with event-free (EFS), lymphoma-specific (LSS) and overall (OS) survival. Compared to No DM/No metformin DLBCL patients, there was no association of DM/Metformin (n=48; HR=1.05, 95% CI 0.59–1.89) or DM/No metformin(n=54; HR=1.41, 95% CI 0.88–2.26) with EFS; results were similar for LSS and OS. Compared to No DM/No metformin FL patients, there was no association of DM/Metformin (n=37; HR=1.16, 95% CI 0.71–1.89) or DM/No metformin (n=19; HR=1.16, 95% CI 0.66–2.04) with EFS; results were similar for LSS. However, DM/Metformin was associated with inferior OS (HR=2.17; 95% CI 1.19–3.95) compared to No DM/No metformin. In conclusion, we found no evidence that metformin use was associated with improved outcomes in newly diagnosed DLBCL and FL.
Keywords: Metformin, mTOR, Diffuse large B-cell lymphoma, Follicular lymphoma, Diabetes mellitus
Introduction
Metformin (N,N-dimethylbiguanide) is a widely used medication for the treatment of type 2 diabetes mellitus (DM). It exerts its antidiabetic effects by decreasing hepatic glucose production, increasing glucose use by peripheral tissues, and enhancing insulin sensitivity (Ikhlas and Ahmad 2017). The primary molecular targets of metformin include Complex I of the mitochondrial electron transport chain (ETC) and the adenosine monophosphate (AMP)-activated protein kinase (AMPK) (Li, et al 2018a, Mallik and Chowdhury 2018, Vancura, et al 2018). AMPK is a heterotrimeric serine/ threonine protein kinase that plays a central role in metabolism and energy regulation by restricting anabolic processes while promoting catabolic processes (Ikhlas and Ahmad 2017, Vancura, et al 2018).
Type 2 DM is considered as a risk factor for many types of cancer (Cignarelli, et al 2018, Sacerdote and Ricceri 2018, Shlomai, et al 2016). Multiple studies have suggested that metformin use was associated with lower incidences of various solid tumours such as pancreatic, colorectal, gastric, lung and breast cancer (Gandini, et al 2014, Kobiela, et al 2018, Li, et al 2018b, Shlomai, et al 2016, Wang, et al 2014, Zhu, et al 2015). There is also accumulating evidence that supports an association of metformin use with improved outcome of several types of solid tumours (Chu, et al 2018, Hu, et al 2018, Kobiela, et al 2018, Li, et al 2018b, Tang, et al 2018, Xin, et al 2018). There are fewer studies available regarding the role of metformin in haematological malignancies. Metformin was shown to reduce the risk of progression of monoclonal gammopathy of undetermined significance (MGUS) to multiple myeloma (MM) (Chang, et al 2015) and improve the outcome of patients with MM (Wu, et al 2014). However, the role of metformin in lymphoma is unclear. It is unknown whether metformin use reduces the risk of developing lymphoma or improves the treatment outcome in patients with lymphoma.
Activation of AMPK and inhibition of mechanistic/mammalian target of rapamycin complex 1 (mTORC1) are the main mechanisms underlying the potential antineoplastic effects of metformin (Li, et al 2018a, Mallik and Chowdhury 2018, Vancura, et al 2018). The phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K)/mTOR pathway is important for cell proliferation and survival in diffuse large B-cell lymphoma (DLBCL) and follicular lymphoma (FL), two of the most common types of non-Hodgkin lymphoma (NHL) (Majchrzak, et al 2014, Pongas and Cheson 2016). The mTOR inhibitor everolimus has encouraging clinical activity in both relapsed/refractory and untreated DLBCL (Barnes, et al 2013, Johnston, et al 2016, Witzig, et al 2017). The PI3K inhibitors, idelalisib and copanlisib, are efficacious in treating FL and have been approved by the US Food and Drug Administration for this indication (Dreyling, et al 2017, Gopal, et al 2014, Gopal, et al 2017, Salles, et al 2017), and the mTOR inhibitor everolimus was also shown to have activity in FL in early phase clinical trials (Bennani, et al 2017, Tobinai, et al 2010, Witzig, et al 2011). Metformin has been shown to have anti-lymphoma activity in vitro via activation of AMPK and inhibition of mTOR (Shi, et al 2012). However, whether concomitant metformin use in DLBCL and FL patients affects clinical outcomes remains unknown. Two prior small retrospective studies investigated the impact of metformin use on the survival of patients with DLBCL, but the results were inconsistent (Alkhatib, et al 2017, Koo, et al 2011). Whether metformin affects clinical outcome of FL has not been studied before. Here, we investigated the potential impact of metformin use on the clinical outcomes of newly diagnosed DLBCL and FL in a large, prospectively followed cohort.
Patients and methods
Patients
This study was approved by the Mayo Clinic institutional review board. This analysis included all newly diagnosed patients with DLBCL (N = 869) and FL (N = 895) seen at Mayo Clinic from March 2002 to June 2015 who were enrolled in the Molecular Epidemiology Resource (MER) of the University of Iowa/Mayo Clinic Lymphoma Specialized Program of Research Excellence (SPORE). Full details of the MER, a prospective cohort study of lymphoma outcomes, have been previously published (Cerhan, et al 2017). Briefly, consecutive patients with newly diagnosed lymphoma (within 9 months of first diagnosis) who consented participation were enrolled, treated per treating physician choice based on standard of care, and followed prospectively, every 6 months for the first three years and annually thereafter.
Clinical information abstraction
Baseline clinical and pathological characteristics at diagnosis were abstracted from the MER database. Diagnosis of DM was abstracted by MER survey and chart review. Information regarding metformin use was abstracted from electronic medical record review. The following medications containing metformin were included in the search during the initial screening of medical charts: metformin, Glumetza, Glucophage, Glucophage-XR, Fortamet, Riomet, Actoplus Met, Avandamet, Glucovance, Janumet, Metaglip and PrandiMet. The start date, discontinuation date if applicable, and doses of metformin were abstracted from chart review. Patients who were on metformin at the time of diagnosis or within 6 months after lymphoma diagnosis were assigned to the metformin group. Disease progression, relapse, unplanned re-treatment after initial therapy, death and cause of death were verified through medical record review.
Statistical analysis
Event-free survival (EFS) was defined as time from diagnosis to disease progression or relapse, unplanned re-treatment after initial management (rituximab, chemotherapy, radiation or combinations of these; or observation in FL), or death from any cause. Lymphoma-specific survival (LSS) was defined as time from diagnosis to death due to lymphoma, with patients who died from other causes censored at the time of death. Overall survival (OS) was defined as time from diagnosis to death from any cause. Categorical data were analysed using the Chi-square test. Time-to-event data were analysed using the Kaplan-Meier method and Cox regression; from the latter models, we estimated univariate and multivariate adjusted hazard ratios (HR) and 95% confidence intervals (CI). All statistical analyses were done in SPSS (v22, IBM, Armonk, NY).
Results
DLBCL
A total of 869 patients with newly diagnosed DLBCL were included: 767 (88.3%) patients had no DM and did not use metformin (No DM/No metformin), 54 (6.2%) patients had DM but were not using metformin (DM/No metformin) and 48 (5.5%) patients had DM and were using metformin (DM/Metformin). Metformin dose ranged from 500 mg daily to 1000 mg twice daily. The median duration of use was 39 months (range 0.2–165). Baseline characteristics are shown in Table I. DM/No metformin and DM/Metformin patients were older, had higher body mass index (BMI), and had more advanced stage disease. DM/No metformin patients had more extranodal involvement and a higher International Prognostic Index (IPI) score. There were no notable differences regrding sex, Eastern Cooperative Oncology Group performance status (ECOG PS), lactate dehydrogenase (LDH) or DLBCL cell of origin (Hans algorithm) among the three groups. The treatment pattern was similar among the three groups, with the vast majority of patients receiving R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisolone) or R-CHOP-like immunochemotherapy.
Table I.
Baseline characteristics of DLBCL patients
| No DM, No metformin | % | DM, No metformin | % | DM, Metformin | % | P value | |
|---|---|---|---|---|---|---|---|
| Age (years) | <0.01 | ||||||
| ≤60 | 317 | 41.3 | 8 | 14.8 | 13 | 27.1 | |
| >60 | 450 | 58.7 | 46 | 85.2 | 35 | 72.9 | |
| Gender | 0.92 | ||||||
| Male | 440 | 57.4 | 31 | 57.4 | 29 | 60.4 | |
| Female | 327 | 42.6 | 23 | 42.6 | 19 | 39.6 | |
| ECOG PS | 0.14 | ||||||
| <2 | 685 | 89.5 | 44 | 81.5 | 41 | 85.4 | |
| ≥2 | 80 | 10.5 | 10 | 18.5 | 7 | 14.6 | |
| LDH | 0.14 | ||||||
| Normal | 310 | 45.4 | 21 | 43.8 | 14 | 30.4 | |
| Elevated | 373 | 54.6 | 27 | 56.3 | 32 | 69.6 | |
| Extranodal sites | 0.02 | ||||||
| ≤1 | 616 | 80.3 | 36 | 66.7 | 42 | 87.5 | |
| >1 | 151 | 19.7 | 18 | 33.3 | 6 | 12.5 | |
| Ann Arbor stage | 0.05 | ||||||
| I-II | 337 | 44.0 | 16 | 29.6 | 16 | 33.3 | |
| III-IV | 429 | 56.0 | 38 | 70.4 | 32 | 66.7 | |
| IPI score | 0.01 | ||||||
| 0–1 | 294 | 38.3 | 11 | 20.4 | 10 | 20.8 | |
| 2 | 212 | 27.6 | 14 | 25.9 | 18 | 37.5 | |
| 3 | 188 | 24.5 | 18 | 33.3 | 14 | 29.2 | |
| 4–5 | 73 | 9.5 | 11 | 20.4 | 6 | 12.5 | |
| Cell of origin | 0.17 | ||||||
| GCB | 308 | 59.2 | 29 | 74.4 | 19 | 63.3 | |
| Non-GCB | 212 | 40.8 | 10 | 25.6 | 11 | 36.7 | |
| Frontline Therapy | 0.55 | ||||||
| Immunochemotherapy | 712 | 93.4 | 51 | 94.4 | 43 | 89.6 | |
| Other therapy | 50 | 6.6 | 3 | 5.6 | 5 | 10.4 | |
| BMI (kg/m2) | <0.01 | ||||||
| <25 | 221 | 29.4 | 5 | 9.3 | 3 | 3.6 | |
| ≥25 but <30 | 298 | 39.6 | 18 | 33.3 | 12 | 25.0 | |
| ≥30 | 233 | 31.0 | 31 | 57.4 | 33 | 68.8 |
BMI: body mass index; DLBCL: diffuse large B-cell lymphoma; DM: type 2 diabetes mellitus; ECOG PS: Eastern Cooperative Oncology Group performance status; GCB: germinal centre B cell; IPI: International Prognostic Index; LDH: lactate dehydrogenase.
After a median follow-up of 83 months (range 0.2–179), there were 369 events, 183 lymphoma-specific deaths and 294 total deaths. Compared to No DM/No metformin patients, DM/No metformin and DM/Metformin patients had inferior EFS (median 72.5 and 63.8 vs 127.3 months, log rank P = 0.04; Figure 1A). LSS was similar among DM/No metformin, DM/Metformin and No DM/No metformin patients (medians not reached, log rank P = 0.13; Figure 1B). DM/No metformin and DM/Metformin patients also had inferior OS compared to No DM/No metformin patients (median 90.0 and 113.7 vs 165.0 months, log rank P = 0.01; Figure 1C). The results were similar in univariate Cox regression analysis (Table II). After adjusting for sex, age, BMI, IPI score, cell of origin, and immunochemotherapy, compared to No DM/No metformin DLBCL patients, there was no association of DM/Metformin (HR = 1.05; 95% CI 0.59–1.89) or DM/No metformin (HR = 1.41, 95% CI 0.88–2.26) with EFS; results were similar for LSS and OS (Table II). While not a focus of our analysis, the comparison of metformin use in the subset of DLBCL patients with DM also showed no association of metformin use with EFS, LSS or OS (Table SI), acknowledging both small sample size and the probable heterogeneity of the DM/No Metformin group.
Figure 1.
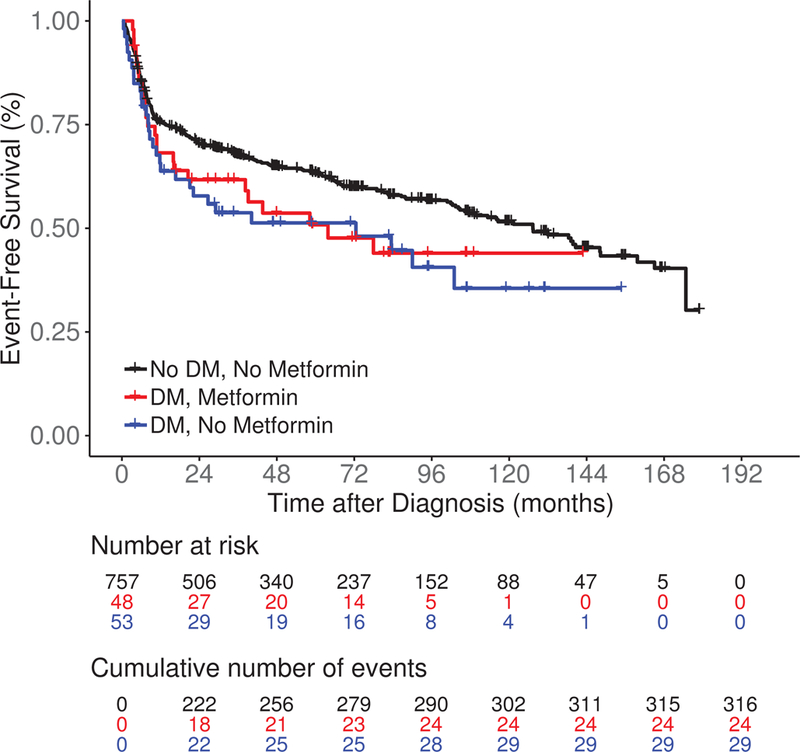
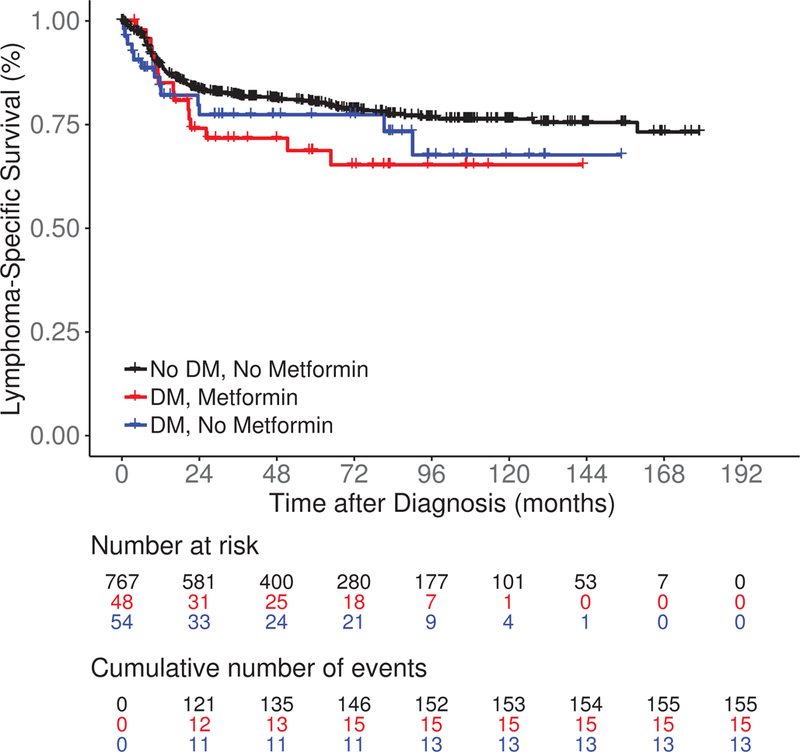
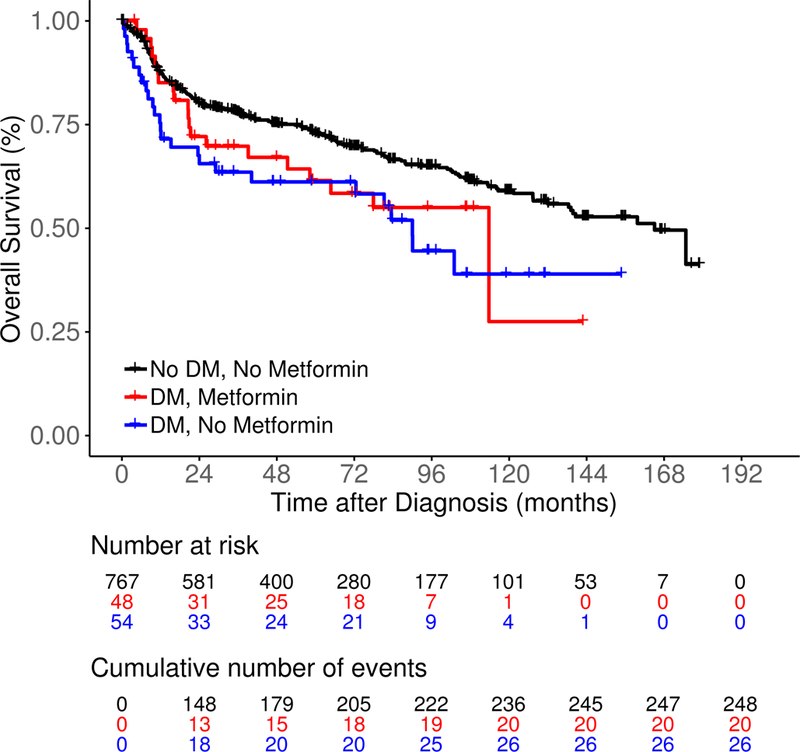
Survival outcomes including (A) event-free survival, (B) lymphoma-specific survival and (C) overall survival in newly diagnosed diffuse large B-cell lymphoma patients by both type 2 diabetes mellitus (DM) and metformin use status.
Table II.
Survival outcomes by DM and metformin status in DLBCL patients
| EFS | Univariate | Multivariate* | ||||||
|---|---|---|---|---|---|---|---|---|
| N | N events | HR | 95% CI | P value | HR | 95% CI | P value | |
| No DM, No metformin | 757 | 316 | 1.00 | reference | 0.04 | 1.00 | reference | 0.35 |
| DM, No metformin | 53 | 29 | 1.52 | 1.04–2.22 | 1.41 | 0.88–2.26 | ||
| DM, Metformin | 48 | 24 | 1.37 | 0.90–2.07 | 1.05 | 0.59–1.89 | ||
| LSS | Univariate | Multivariate* | ||||||
| N | N events | HR | 95% CI | P value | HR | 95% CI | P value | |
| No DM, No metformin | 767 | 155 | 1.00 | reference | 0.14 | 1.00 | reference | 0.19 |
| DM, No metformin | 54 | 13 | 1.39 | 0.79–2.44 | 1.78 | 0.95–3.34 | ||
| DM, Metformin | 48 | 15 | 1.60 | 0.94–2.72 | 1.29 | 0.61–2.75 | ||
| OS | Univariate | Multivariate* | ||||||
| N | N events | HR | 95% CI | P value | HR | 95% CI | P value | |
| No DM, No metformin | 767 | 248 | 1.00 | reference | 0.01 | 1.00 | reference | 0.16 |
| DM, No metformin | 54 | 26 | 1.79 | 1.19–2.68 | 1.63 | 0.99–2.68 | ||
| DM, Metformin | 48 | 20 | 1.44 | 0.91–2.27 | 1.09 | 0.58–2.06 | ||
Adjusted for sex, age (continuous), International Prognostic Index (continuous), cell of origin, frontline therapy (immunochemotherapy vs other therapy) and body mass index (categorical).
CI: confidence interval; DLBCL: diffuse large B-cell lymphoma; DM: type 2 diabetes mellitus; EFS: event-free survival; HR: hazard ratio; LSS: lymphoma-specific survival; OS: overall survival.
FL
A total of 895 patients with newly diagnosed FL were included: 839 (93.7%) patients had no DM and did not use metformin (DM/No metformin), 19 (2.1%) patients had DM but were not using metformin (DM/No metformin) and 37 (4.1%) patients had DM and were using metformin (DM/Metformin). Metformin dose ranged from 500 mg daily to 1000 mg twice daily for the vast majority of patients, with two exceptions - one patient received 2500 mg daily in split doses and another patient treated with 2000 mg twice daily. The median duration of metformin use was 67 months (range 0.4–194). Baseline characteristics are shown in Table III. DM/No metformin and DM/Metformin patients were older and had higher BMI. DM/No metformin patients had worse ECOG PS and more anaemia, although these were based on small numbers. There were no statistical differences in other baseline characteristics among the three groups, including sex, number of nodal sites, stage, LDH and FL International Prognostic Index (FLIPI) score. Initial management strategies (observation vs treatment) were also similar across groups.
Table III.
Baseline characteristics of FL patients
| No DM, No metformin | % | DM, No metformin | % | DM, Metformin | % | P value | |
|---|---|---|---|---|---|---|---|
| Age (years) | <0.01 | ||||||
| ≤60 | 434 | 51.7 | 3 | 15.8 | 12 | 32.4 | |
| >60 | 405 | 48.3 | 16 | 84.2 | 25 | 67.6 | |
| Gender | 0.35 | ||||||
| Male | 438 | 52.2 | 7 | 36.8 | 21 | 56.8 | |
| Female | 401 | 47.8 | 12 | 63.2 | 16 | 43.2 | |
| ECOG PS | <0.01 | ||||||
| <2 | 825 | 98.6 | 15 | 78.9 | 36 | 97.3 | |
| ≥2 | 12 | 1.4 | 4 | 21.1 | 1 | 2.7 | |
| Nodal sites | 0.79 | ||||||
| ≤4 | 554 | 68.7 | 13 | 76.5 | 24 | 68.6 | |
| >4 | 252 | 31.3 | 4 | 23.5 | 11 | 31.4 | |
| Ann Arbor stage | 0.17 | ||||||
| I-II | 275 | 33.2 | 10 | 52.6 | 14 | 38.9 | |
| III-IV | 553 | 66.8 | 9 | 47.4 | 22 | 61.1 | |
| LDH | 0.07 | ||||||
| Normal | 592 | 82.6 | 9 | 60.0 | 27 | 79.4 | |
| Elevated | 125 | 17.4 | 6 | 40.0 | 7 | 20.6 | |
| Haemoglobin (g/l) | <0.01 | ||||||
| ≤120 | 87 | 11.6 | 8 | 47.1 | 8 | 22.9 | |
| >120 | 663 | 88.4 | 9 | 52.9 | 27 | 77.1 | |
| FLIPI score | 0.20 | ||||||
| 0–1 | 371 | 44.2 | 8 | 42.1 | 15 | 40.5 | |
| 2 | 282 | 33.6 | 3 | 15.8 | 11 | 29.7 | |
| ≥3 | 186 | 22.2 | 8 | 42.1 | 11 | 29.7 | |
| Initial management | 0.89 | ||||||
| Observation | 291 | 34.9 | 6 | 31.6 | 14 | 37.8 | |
| Treatment | 543 | 65.1 | 13 | 68.4 | 23 | 62.2 | |
| BMI (kg/m2) | <0.01 | ||||||
| <25 | 269 | 32.4 | 2 | 10.5 | 5 | 13.9 | |
| ≥25 but <30 | 332 | 40.0 | 4 | 21.1 | 13 | 36.1 | |
| ≥30 | 228 | 27.5 | 13 | 68.4 | 18 | 50.0 |
BMI: body mass index; DM: type 2 diabetes mellitus; ECOG PS: Eastern Cooperative Oncology Group performance status; FL: follicular lymphoma; FLIPI: Follicular Lymphoma International Prognostic Index; LDH: lactate dehydrogenase.
After a median follow-up of 85 months (range 0.4–180), there were 421 events, 74 lymphoma-specific deaths and 159 total deaths. There was no significant difference in EFS among DM/No metformin, DM/Metformin and No DM/No metformin patients (median 41.7 vs 118.7 vs 82.8 months, log rank P = 0.27; Figure 2A). LSS was also similar among the three groups (medians not reached, log rank P = 0.10; Figure 2B). DM/No metformin and DM/Metformin patients had inferior OS compared to No DM/No metformin patients (median not reached vs 139.5 months vs not reached, log rank P < 0.01; Figure 2C). The results were similar in univariate Cox regression analysis (Table IV). After adjusting for sex, age, BMI, FLIPI score and initial management strategy, compared to No DM/No metfomin FL patients there was no association of DM/Metformin (HR = 1.16; 95% CI 0.71–1.89) or DM/No metformin (HR = 1.16, 95% CI 0.66–2.04) with EFS; results were similar for LSS (Table IV). However, DM/Metformin was associated with inferior OS (HR = 2.17; 95% CI 1.19–3.95) compared to No DM/No metformin FL patients. When we restricted our analysis to FL patients with DM (Table SII), there was no association of metformin use with EFS, while there was inferior LSS and OS after multivariate adjustment, but the confidence intervals were very wide and the associations were not statistically significant. The same limitations for the analysis of DLBCL also applied to this analysis.
Figure 2.
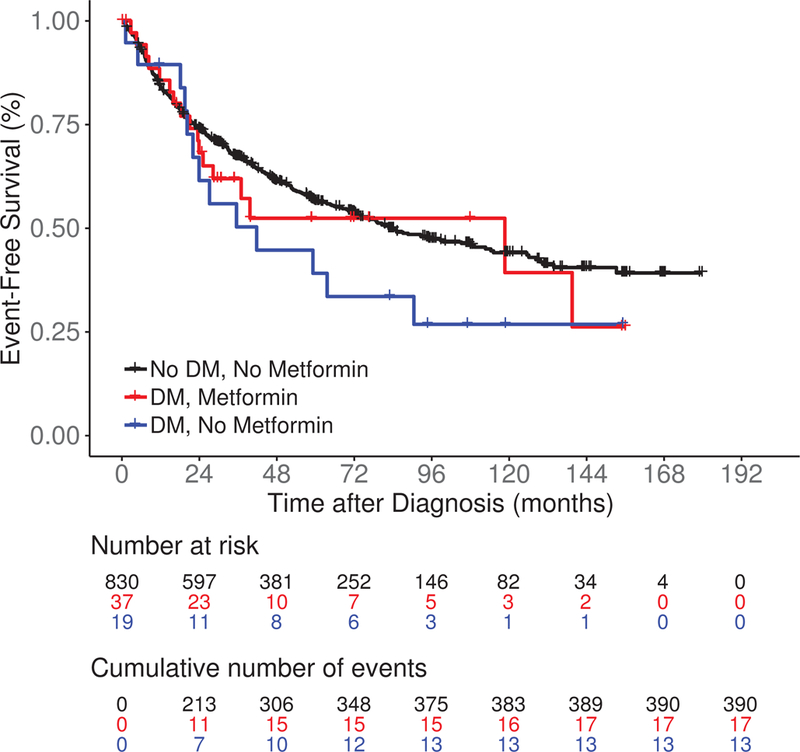
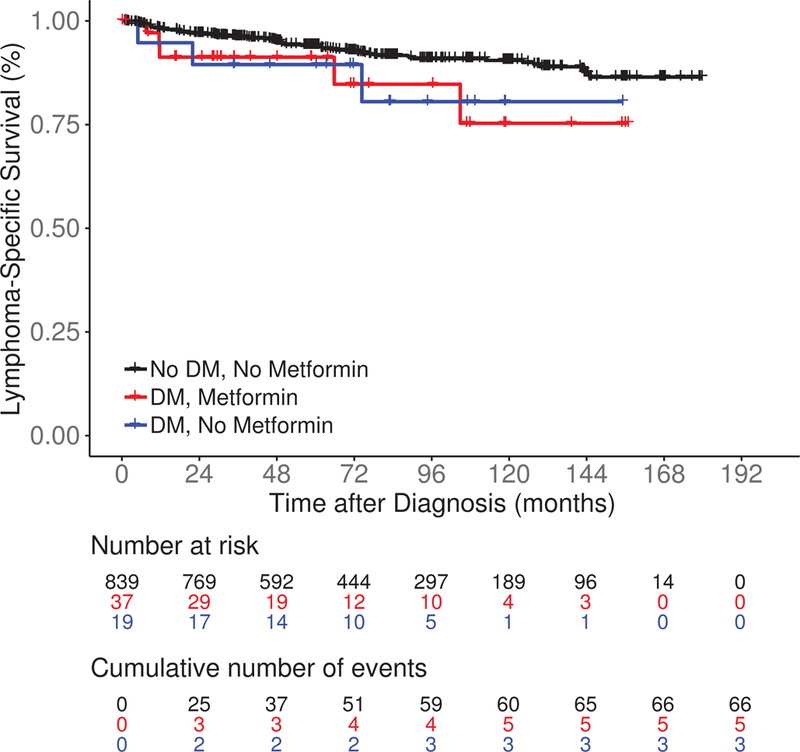
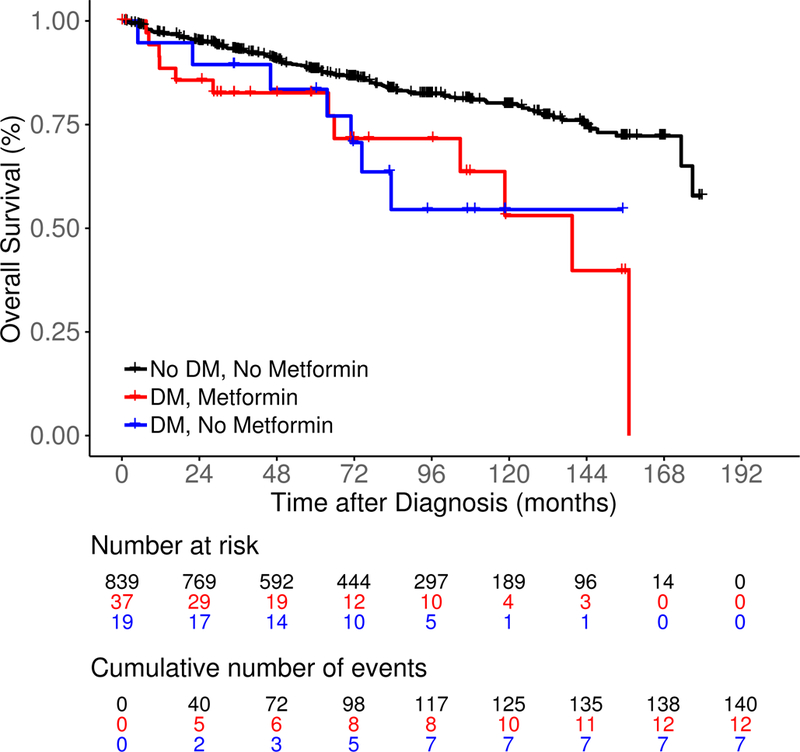
Survival outcomes including (A) event-free survival, (B) lymphoma-specific survival and (C) overall survival in newly diagnosed follicular lymphoma patients by both type 2 diabetes mellitus (DM) and metformin use status.
Table IV.
Survival outcomes by DM and metformin status in FL patients
| EFS | Univariate | Multivariate* | ||||||
|---|---|---|---|---|---|---|---|---|
| N | N events | HR | 95% CI | P value | HR | 95% CI | P value | |
| No DM, No metformin | 830 | 390 | 1.00 | reference | 0.27 | 1.00 | reference | 0.75 |
| DM, No metformin | 19 | 13 | 1.52 | 0.87–2.64 | 1.16 | 0.66–2.04 | ||
| DM, Metformin | 37 | 17 | 1.18 | 0.73–1.92 | 1.16 | 0.71–1.89 | ||
| LSS | Univariate | Multivariate* | ||||||
| N | N events | HR | 95% CI | P value | HR | 95% CI | P value | |
| No DM, No metformin | 839 | 66 | 1.00 | reference | 0.11 | 1.00 | reference | 0.56 |
| DM, No metformin | 19 | 3 | 2.13 | 0.67–6.78 | 1.01 | 0.31–3.31 | ||
| DM, Metformin | 37 | 5 | 2.25 | 0.91–5.59 | 1.67 | 0.66–4.22 | ||
| OS | Univariate | Multivariate* | ||||||
| N | N events | HR | 95% CI | P value | HR | 95% CI | P value | |
| No DM, No metformin | 839 | 140 | 1.00 | reference | <0.01 | 1.00 | reference | 0.03 |
| DM, No metformin | 19 | 7 | 2.43 | 1.14–5.20 | 1.53 | 0.70–3.32 | ||
| DM, Metformin | 37 | 12 | 2.67 | 1.48–4.81 | 2.17 | 1.19–3.95 | ||
Adjusted for sex, age (continuous), Follicular Lymphoma International Prognostic Index (continuous), initial management (observation vs treatment), and body mass index (categorical).
CI: confidence interval; DM: type 2 diabetes mellitus; EFS: event-free survival; FL: follicular lymphoma; HR: hazard ratio; LSS: lymphoma-specific survival; OS: overall survival.
Discussion
This large prospective cohort study found no evidence that metformin use was associated with improved outcomes including EFS, LSS and OS in newly diagnosed DLBCL and FL.
Possible explanations for these results are as follows. First, while the in vitro anti-lymphoma activity was encouraging, the in vivo potency of metformin in blocking mTOR and the clinical anti-lymphoma activity may be limited. In our FL cohort, when we analysed the patients who were initially observed and those who were treated upfront separately, there was no benefit of metformin in either subgroup (data not shown). Metformin did not demonstrate “single agent activity” in FL patients not receiving other active therapy, suggesting that its therapeutic role is probably limited. However, these patients did not require treatment initially and were expected to have an excellent outcome, so any potential benefit of metformin may not be apparent. Whether metformin has single agent clinical activity against lymphoma remains unknown. Second, concurrent DM may lead to a worse prognosis due to possible associations with other comorbidities and complications. In this setting, the benefit of metformin may be limited. Further, a phase II study (NCT02531308) that intended to evaluate the efficacy of metformin when added to R-CHOP in non-diabetic patients with newly diagnosed DLBCL was terminated early due to poor accrual. The role of metformin in non-diabetic lymphoma patients remains unclear. Third, with effective standard-of-care immunochemotherapy for DLBCL and FL, the potential benefit of metformin may be obscured. For example, in DLBCL, 50–70% patients treated with R-CHOP have long term disease-free survival. The addition of several active novel agents to R-CHOP, such as bortezomib and ibrutinib, did not consistently result in improvement of outcomes (Leonard, et al 2017, Younes, et al 2018). Metformin is probably less active compared to these agents, and is probably not expected to provide a significant additional benefit when added to R-CHOP.
Two prior retrospective studies explored the potential clinical activity of metformin in DLBCL. Koo et al (2011) evaluated the effect of concomitant metformin use on rituximab treatment for DLBCL. Metformin users (N = 31) and non-metformin users (N = 182) were compared, and the use of metformin did not affect overall response rate, EFS or OS (Koo, et al 2011). Alkhatib et al ( 2017) performed a study of metformin users (N = 24) and controls (N = 24) matched on clinical characteristics and who did not use metformin. Metformin use was associated with higher complete remission (odds ratio [OR] = 18.6, P = 0.0018) and overall response rates (OR = 9.06, P = 0.0479), improved progression-free survival (P = 0.024) and a trend of better OS (P = 0.22) (Alkhatib, et al 2017). Our study was based on a well-defined prospective cohort study and was able to group patients by both DM status and metformin use. This takes into consideration that concurrent DM may lead to a worse prognosis as mentioned above. Our results were similar to those reported by Koo et al (2011), demonstrating no impact of metformin use on the outcome of DLBCL patients. Our study included similar numbers of metformin users (N = 48) and diabetic non-metformin users (N = 54), who had largely similar clinical characteristics. However, unlike Alkhatib et al (2017), we did not detect a difference in survival outcomes between these two groups. The differences in results may be due to different patient populations, adjustment/matching factors and analysis strategies. Of note, all three studies included a relatively small number of patients who were using metformin, and the results should be interpreted with caution.
Strengths of this study include the prospective cohort study design, central pathology review, relatively large sample size and virtually complete follow-up. Several limitations should be noted. First, there were a relatively small number of DM patients who were using metformin, and the doses and duration varied among patients. It was not feasible to analyse the possible differences between doses and treatment durations. Second, responses to treatment were based on physician assessment and were not systematically evaluated according to a standard protocol as in a clinical trial, so we were not able to assess any potential effect of metformin on treatment response. Third, the role of metformin in non-diabetic patients could not be addressed as this question could only be tested in a clinical trial. Finally, while we postulated that diabetes may be associated with other comorbidities beyond obesity, such data were not analysed in this study. The severity of DM was also not taken into account.
In conclusion, despite promising preclinical rationale, metformin use in patients with newly diagnosed DLBCL or FL was not associated with an improvement of EFS, LSS or OS. While larger, randomized prospective studies could definitely evaluate potential effect of metformin in lymphoma, such studies are unlikely to be conducted considering small if any potential benefit of metformin and competition from other more promising agents targeting similar pathways.
Supplementary Material
Acknowledgements
This study was supported in part by the NIH/NCI awards P50 CA97274 and U01 CA195568, and Predolin Foundation.
Footnotes
Conflict of Interest Disclosures
The authors declare no competing financial interests for this study.
References
- Alkhatib Y, Abdel Rahman Z & Kuriakose P (2017) Clinical impact of metformin in diabetic diffuse large B-cell lymphoma patients: a case-control study. Leuk Lymphoma, 58, 1130–1134. [DOI] [PubMed] [Google Scholar]
- Barnes JA, Jacobsen E, Feng Y, Freedman A, Hochberg EP, LaCasce AS, Armand P, Joyce R, Sohani AR, Rodig SJ, Neuberg D, Fisher DC & Abramson JS (2013) Everolimus in combination with rituximab induces complete responses in heavily pretreated diffuse large B-cell lymphoma. Haematologica, 98, 615–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennani NN, LaPlant BR, Ansell SM, Habermann TM, Inwards DJ, Micallef IN, Johnston PB, Porrata LF, Colgan JP, Markovic SN, Nowakowski GS, Macon WR, Reeder CB, Mikhael JR, Northfelt DW, Ghobrial IM & Witzig TE (2017) Efficacy of the oral mTORC1 inhibitor everolimus in relapsed or refractory indolent lymphoma. Am J Hematol, 92, 448–453. [DOI] [PubMed] [Google Scholar]
- Cerhan JR, Link BK, Habermann TM, Maurer MJ, Feldman AL, Syrbu SI, Thompson CA, Farooq U, Novak AJ, Slager SL, Allmer C, Lunde JJ, Macon WR, Inwards DJ, Johnston PB, Micallef INM, Nowakowski GS, Ansell SM, Kay NE, Weiner GJ & Witzig TE (2017) Cohort Profile: The Lymphoma Specialized Program of Research Excellence (SPORE) Molecular Epidemiology Resource (MER) Cohort Study. Int J Epidemiol, 46, 1753–1754i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SH, Luo S, O’Brian KK, Thomas TS, Colditz GA, Carlsson NP & Carson KR (2015) Association between metformin use and progression of monoclonal gammopathy of undetermined significance to multiple myeloma in US veterans with diabetes mellitus: a population-based retrospective cohort study. Lancet Haematol, 2, e30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu D, Wu J, Wang K, Zhao M, Wang C, Li L & Guo R (2018) Effect of metformin use on the risk and prognosis of endometrial cancer: a systematic review and meta-analysis. BMC Cancer, 18, 438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cignarelli A, Genchi VA, Caruso I, Natalicchio A, Perrini S, Laviola L & Giorgino F (2018) Diabetes and cancer: Pathophysiological fundamentals of a ‘dangerous affair’. Diabetes Research and Clinical Practice, 143, 378–388. [DOI] [PubMed] [Google Scholar]
- Dreyling M, Morschhauser F, Bouabdallah K, Bron D, Cunningham D, Assouline SE, Verhoef G, Linton K, Thieblemont C, Vitolo U, Hiemeyer F, Giurescu M, Garcia-Vargas J, Gorbatchevsky I, Liu L, Koechert K, Pena C, Neves M, Childs BH & Zinzani PL (2017) Phase II study of copanlisib, a PI3K inhibitor, in relapsed or refractory, indolent or aggressive lymphoma. Ann Oncol, 28, 2169–2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandini S, Puntoni M, Heckman-Stoddard BM, Dunn BK, Ford L, DeCensi A & Szabo E (2014) Metformin and cancer risk and mortality: a systematic review and meta-analysis taking into account biases and confounders. Cancer Prev Res (Phila), 7, 867–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopal AK, Kahl BS, de Vos S, Wagner-Johnston ND, Schuster SJ, Jurczak WJ, Flinn IW, Flowers CR, Martin P, Viardot A, Blum KA, Goy AH, Davies AJ, Zinzani PL, Dreyling M, Johnson D, Miller LL, Holes L, Li D, Dansey RD, Godfrey WR & Salles GA (2014) PI3Kdelta inhibition by idelalisib in patients with relapsed indolent lymphoma. N Engl J Med, 370, 1008–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopal AK, Kahl BS, Flowers CR, Martin P, Ansell SM, Abella-Dominicis E, Koh B, Ye W, Barr PM, Salles GA & Friedberg JW (2017) Idelalisib is effective in patients with high-risk follicular lymphoma and early relapse after initial chemoimmunotherapy. Blood, 129, 3037–3039. [DOI] [PubMed] [Google Scholar]
- Hu J, Chen JB, Cui Y, Zhu YW, Ren WB, Zhou X, Liu LF, Chen HQ & Zu XB (2018) Association of metformin intake with bladder cancer risk and oncologic outcomes in type 2 diabetes mellitus patients: A systematic review and meta-analysis. Medicine (Baltimore), 97, e11596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikhlas S & Ahmad M (2017) Metformin: Insights into its anticancer potential with special reference to AMPK dependent and independent pathways. Life Sci, 185, 53–62. [DOI] [PubMed] [Google Scholar]
- Johnston PB, LaPlant B, McPhail E, Habermann TM, Inwards DJ, Micallef IN, Colgan JP, Nowakowski GS, Ansell SM & Witzig TE (2016) Everolimus combined with R-CHOP-21 for new, untreated, diffuse large B-cell lymphoma (NCCTG 1085 [Alliance]): safety and efficacy results of a phase 1 and feasibility trial. Lancet Haematol, 3, e309–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobiela J, Dobrzycka M, Jedrusik P, Kobiela P, Spychalski P, Sledzinski Z & Zdrojewski T (2018) Metformin and Colorectal Cancer - A Systematic Review. Exp Clin Endocrinol Diabetes 2018 Jun 28. doi: 10.1055/a-0621-8830. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Koo YX, Tan DS, Tan IB, Tai DW, Ha T, Ong WS, Quek R, Tao M & Lim ST (2011) Effect of concomitant statin, metformin, or aspirin on rituximab treatment for diffuse large B-cell lymphoma. Leuk Lymphoma, 52, 1509–1516. [DOI] [PubMed] [Google Scholar]
- Leonard JP, Kolibaba KS, Reeves JA, Tulpule A, Flinn IW, Kolevska T, Robles R, Flowers CR, Collins R, DiBella NJ, Papish SW, Venugopal P, Horodner A, Tabatabai A, Hajdenberg J, Park J, Neuwirth R, Mulligan G, Suryanarayan K, Esseltine DL & de Vos S (2017) Randomized Phase II Study of R-CHOP With or Without Bortezomib in Previously Untreated Patients With Non-Germinal Center B-Cell-Like Diffuse Large B-Cell Lymphoma. J Clin Oncol, 35, 3538–3546. [DOI] [PubMed] [Google Scholar]
- Li M, Li X, Zhang H & Lu Y (2018a) Molecular Mechanisms of Metformin for Diabetes and Cancer Treatment. Front Physiol, 9, 1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Zhang C, Gao P, Chen X, Ma B, Yu D, Song Y & Wang Z (2018b) Metformin use and its effect on gastric cancer in patients with type 2 diabetes: A systematic review of observational studies. Oncol Lett, 15, 1191–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majchrzak A, Witkowska M & Smolewski P (2014) Inhibition of the PI3K/Akt/mTOR signaling pathway in diffuse large B-cell lymphoma: current knowledge and clinical significance. Molecules, 19, 14304–14315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallik R & Chowdhury TA (2018) Metformin in cancer. Diabetes Res Clin Pract, 143, 409–419. [DOI] [PubMed] [Google Scholar]
- Pongas G & Cheson BD (2016) PI3K signaling pathway in normal B cells and indolent B-cell malignancies. Semin Oncol, 43, 647–654. [DOI] [PubMed] [Google Scholar]
- Sacerdote C & Ricceri F (2018) Epidemiological dimensions of the association between type 2 diabetes and cancer: A review of observational studies. Diabetes Res Clin Pract, 143, 369–377. [DOI] [PubMed] [Google Scholar]
- Salles G, Schuster SJ, de Vos S, Wagner-Johnston ND, Viardot A, Blum KA, Flowers CR, Jurczak WJ, Flinn IW, Kahl BS, Martin P, Kim Y, Shreay S, Will M, Sorensen B, Breuleux M, Zinzani PL & Gopal AK (2017) Efficacy and safety of idelalisib in patients with relapsed, rituximab- and alkylating agent-refractory follicular lymphoma: a subgroup analysis of a phase 2 study. Haematologica, 102, e156–e159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi WY, Xiao D, Wang L, Dong LH, Yan ZX, Shen ZX, Chen SJ, Chen Y & Zhao WL (2012) Therapeutic metformin/AMPK activation blocked lymphoma cell growth via inhibition of mTOR pathway and induction of autophagy. Cell Death Dis, 3, e275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlomai G, Neel B, LeRoith D & Gallagher EJ (2016) Type 2 Diabetes Mellitus and Cancer: The Role of Pharmacotherapy. J Clin Oncol, 34, 4261–4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang GH, Satkunam M, Pond GR, Steinberg GR, Blandino G, Schunemann HJ & Muti P (2018) Association of Metformin with Breast Cancer Incidence and Mortality in Patients with Type II Diabetes: A GRADE-Assessed Systematic Review and Meta-analysis. Cancer Epidemiol Biomarkers Prev, 27, 627–635. [DOI] [PubMed] [Google Scholar]
- Tobinai K, Ogura M, Maruyama D, Uchida T, Uike N, Choi I, Ishizawa K, Itoh K, Ando K, Taniwaki M, Shimada N & Kobayashi K (2010) Phase I study of the oral mammalian target of rapamycin inhibitor everolimus (RAD001) in Japanese patients with relapsed or refractory non-Hodgkin lymphoma. Int J Hematol, 92, 563–570. [DOI] [PubMed] [Google Scholar]
- Vancura A, Bu P, Bhagwat M, Zeng J & Vancurova I (2018) Metformin as an Anticancer Agent. Trends Pharmacol Sci, 39, 867–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Lai ST, Xie L, Zhao JD, Ma NY, Zhu J, Ren ZG & Jiang GL (2014) Metformin is associated with reduced risk of pancreatic cancer in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes Res Clin Pract, 106, 19–26. [DOI] [PubMed] [Google Scholar]
- Witzig TE, Reeder CB, LaPlant BR, Gupta M, Johnston PB, Micallef IN, Porrata LF, Ansell SM, Colgan JP, Jacobsen ED, Ghobrial IM & Habermann TM (2011) A phase II trial of the oral mTOR inhibitor everolimus in relapsed aggressive lymphoma. Leukemia, 25, 341–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witzig TE, LaPlant B, Habermann TM, McPhail E, Inwards DJ, Micallef IN, Colgan JP, Nowakowski GS, Ansell SM & Johnston PB (2017) High rate of event-free survival at 24 months with everolimus/RCHOP for untreated diffuse large B-cell lymphoma: updated results from NCCTG N1085 (Alliance). Blood Cancer J, 7, e576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, Merriman K, Nabaah A, Seval N, Seval D, Lin H, Wang M, Qazilbash MH, Baladandayuthapani V, Berry D, Orlowski RZ, Lee MH & Yeung SC (2014) The association of diabetes and anti-diabetic medications with clinical outcomes in multiple myeloma. Br J Cancer, 111, 628–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin W, Fang L, Fang Q, Zheng X & Huang P (2018) Effects of metformin on survival outcomes of pancreatic cancer patients with diabetes: A meta-analysis. Mol Clin Oncol, 8, 483–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younes A, Sehn LH, Johnson P, Zinzani PL, Hong X, Zhu J, Samoilova O, Suh C, Matsumura I, Lopez-Hernandez A, Dührsen U, Thieblemont C, Carey J, Liu G, Shreeve SM, Sun S, Vermeulen J, Staudt LM & Wilson WH (2018) A Global, Randomized, Placebo-Controlled, Phase 3 Study of Ibrutinib Plus Rituximab, Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone (RCHOP) in Patients with Previously Untreated Non-Germinal Center B-Cell-like (GCB) Diffuse Large B-Cell Lymphoma (DLBCL). Blood, 132, 784. [Google Scholar]
- Zhu N, Zhang Y, Gong YI, He J & Chen X (2015) Metformin and lung cancer risk of patients with type 2 diabetes mellitus: A meta-analysis. Biomed Rep, 3, 235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


