Abstract
Cortical spreading depolarization (CSD) is the electrophysiological substrate of migraine aura, and a putative trigger of trigeminovascular activation and migraine headache. Many migraineurs report stress or relief after a stress triggers an attack. We tested whether various stress conditions might modulate CSD susceptibility and whether this is dependent on genetic factors. Male and female wild type and familial hemiplegic migraine type1 (FHM1) knock-in mice heterozygous for the S218L missense mutation were subjected to acute or chronic stress, or chronic stress followed by relief (36h). Acute stress was induced by restraint and exposure to bright light and white noise (3h). Chronic stress was induced for 28 days by two cycles of repeated exposure of mice to a rat (7d), physical restraint (3d), and forced swimming (3d). Electrical CSD threshold and KCl-induced (300mM) CSD frequency were determined in occipital cortex in vivo at the end of each protocol. Relief after chronic stress reduced the electrical CSD threshold and increased the frequency of KCl-induced CSDs in FHM1 mutants only. Acute or chronic stress without relief did not affect CSD susceptibility in either strain. Stress status did not affect CSD propagation speed, duration or amplitude. In summary, relief after chronic stress, but not acute or chronic stress alone, augments CSD in genetically susceptible mice. Therefore, enhanced CSD susceptibility may explain why, in certain patients, migraine attacks typically occur during a period of stress relief such as weekends or holidays.
Keywords: Stress Relief, Weekend migraine, FHM1, Cortical spreading depolarization, Acute stress, Chronic stress
INTRODUCTION
Many migraineurs, in particular those with aura or its monogenic subtype familial hemiplegic migraine (FHM), report that stress may provoke attacks (Alstadhaug et al., 2007; Hansen et al., 2011; Hauge et al., 2011; Sauro and Becker, 2009; Torelli et al., 1999). However, clinical evidence for this association is not straightforward. Most studies were retrospective and thus, potentially confounded by recall bias. Moreover, enhanced perceived stress and irritability are common features of the so-called “premonitory symptom complex” (Giffin et al., 2003; Schoonman et al., 2006). These symptoms often precede the aura and headache of migraine attacks by a few hours to days and likely reflect hypothalamic and brainstem changes in the early (preclinical) initiation phase of attacks (Ferrari et al., 2015; Goadsby et al., 2017; Schulte and May, 2017). Therefore, pre-ictal stress may also be a consequence, sub-clinically, of an attack already in progress rather than a trigger. Finally, in a rare prospective study, decline in stress, but not level of stress, was associated with increased migraine onset over the subsequent 6–18 hours (Lipton et al., 2014). This finding is well in line with clinical reports that, in certain patients, attacks typically occur during weekends or holidays, when they relax after a stressful period (Alstadhaug et al., 2007; Nattero et al., 1989; Spierings et al., 1997; Torelli et al., 1999).
Cortical spreading depolarization (CSD) is the electrophysiological substrate of migraine aura, a putative trigger of trigeminovascular activation and migraine headache (Ayata, 2009, 2010; Zhang et al., 2011; Zhang et al., 2010), and a potential surrogate marker of migraine activity in animal studies (Ferrari et al., 2015). It has recently been reported that acute and chronic stress exposure modulates CSD threshold in mice (Yapici-Eser et al., 2018). In a previous study, we showed that exogenously administered corticosterone increases CSD susceptibility in a genetic mouse model of FHM type 1 (FHM1), but not in wild-type (WT) controls (Shyti et al., 2015). FHM1 mice display enhanced susceptibility to CSD, which is linked to abnormal intracellular Ca2+ homeostasis and increased neuronal release of glutamate (Eikermann-Haerter et al., 2015; Eikermann-Haerter et al., 2009b; Ferrari et al., 2015; van den Maagdenberg et al., 2010). Further, FHM1 mutants recapitulate the severe aura phenotype in FHM patients with the same mutation (Eikermann-Haerter et al., 2009b; Kors et al., 2001; Stam et al., 2009; van den Maagdenberg et al., 2010) and display behavioral signs of stress-related head pain (Chanda et al., 2013).
In the present study, we tested the hypothesis that stress or stress relief might trigger migraine attacks by enhancing CSD in genetically susceptible subjects. To this end, we investigated the effects of well-established and widely used experimental paradigms for acute stress, chronic stress, and stress relief after chronic stress, on CSD susceptibility in WT and FHM1 mice.
EXPERIMENTAL PROCEDURES
Ethical approval:
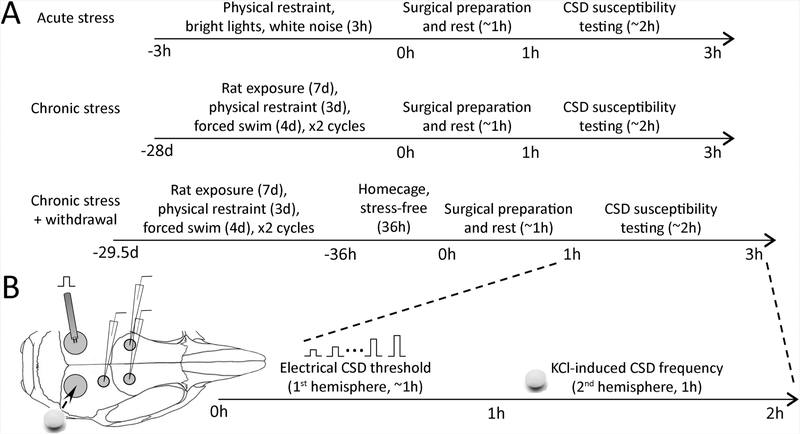
All experiments were carried out in accordance with the ARRIVE guidelines and the Guide for Care and Use of Laboratory Animals (NIH Publication No. 85–23, 1996), and were approved by the institutional review board (MGH Subcommittee on Research Animal Care). We used a total of 98 male and female mice (Table 1). Knock-in mice (RRID:MGI:3836257) heterozygous for the FHM1 S218L missense mutation in Cacnala gene (van den Maagdenberg et al., 2004), their WT littermates, and C57BL/6J mice (Charles River) were used. Only 3 mice were excluded from analyses due to missing data or technical failures (2 naive male WT and 1 naive female S218L FHM1 mutant mouse). All animals were housed with ad libitum access to water. All experiments were carried out with the investigators blinded, and confirmatory genotyping was done after data collection. Experimental protocol and timeline are shown in Figure 1A.
Table 1:
Systemic physiological parameters.
| Experimental groups | N (M/F) | Weight (g) | Age (mo) | pH | pCO2 (mmHg) | pO2 (mmHg) | BP (mmHg) | Adrenal weight (mg/g) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Chronic Stress: | FHM1 | Naïve | 10/4 | 28±6 | 6.4±4.19 | 7.33±0.02 | 36±3 | 107±12 | 93±9 | 0.29±0.09 |
| Stress | 7/6 | 26±5 | 5.7±3.8 | 7.33±0.02 | 35±4 | 119±12# | 89±8 | 0.40±0.11 | ||
| Stress + relief | 6/3 | 28±5 | 7.3±3.4 | 7.35±0.02 | 35±3 | 108±10 | 94±5 | 0.31±0.12 | ||
| WT | Naïve | 9/8 | 29±6 | 6.8±4.3 | 7.35±0.03 | 35±6 | 108±13 | 89±12 | 0.27±0.08 | |
| Stress | 9/7 | 27±5 | 6.6±3.9 | 7.36±0.03 | 35±4 | 108±10 | 95±11 | - | ||
| Stress + relief | 4/3 | 30±3 | 7.0±0.0 | 7.35±0.02 | 34±3 | 113±14 | 80±8† | - | ||
| Acute Stress: | FHM1 | Naïve | 0/5 | 28±5 | 8.6±3.8 | 7.33±0.02 | 36±4 | 104±11 | 79±4 | - |
| Stress | 0/7 | 27±4 | 9.9±3.4 | 7.33±0.02 | 33±4 | 110±10 | 81±9 | - | ||
| WT | Naïve | 0/5 | 29±7 | 8.4±6.5 | 7.34±0.03 | 35±5 | 101±10 | 73±10 | - | |
| Stress | 0/5 | 29±7 | 10.7±5.0 | 7.31±0.02 | 34±2 | 103±10 | 78±5 | - |
Mean ± standard deviation.
p<0.05 vs. FHM1 naïve
p<0.05 vs. WT stress.
Figure 1. Experimental protocols and timeline.
(A) The timeline of acute and chronic stress, and stress withdrawal paradigms. Each cohort had a naive control group maintained in a stress-free environment for the same duration.
(B) Experimental setup to measure the electrical CSD threshold (bipolar stimulation electrode on left occipital cortex) and the frequency of KCl-induced CSDs (topical KCl application via a cotton ball on right occipital cortex) over approximately two hours of CSD susceptibility testing. Microelectrode positions for electrophysiological confirmation of CSDs are also shown.
Stress paradigms:
We simulated acute stress by placing mice in a restrainer tube custom made from 50-cL syringes (internal diameter 30 mm) with six 0.4 cm air holes. Restrained animals were placed inside an empty cage illuminated by two separate light sources (1000 lux on cage floor). Two loudspeakers placed 10 cm away from the sides of the cage exposed the animals to 90-dB (measured inside the cage) white noise. Care was taken not to cause overheating due to the light or respiratory restriction.
Rodents show habituation to repeated stressors of the same type. In addition, response to a particular type of stressor is greatly influenced by individual differences that may render an animal resilient or susceptible (Grissom and Bhatnagar, 2009). To overcome these obstacles, we simulated chronic stress using a previously described protocol of alternating and varying stressors that is shown to increase hypothalamo-pituitary axis (HPA) and sympathetic activity drastically and chronically (Balkaya et al., 2011). Animals were exposed daily to a rat for 7 days, then restrained for 4 days, and then subjected to forced-swimming for 3 days, and the cycle was repeated once more for a total of 28 days of stress. Each daily stress started at the beginning of their active periods at 19:00. For exposure to a rat, mice were placed inside a transparent cylindrical container (radius 8 cm, height 14 cm) fixed to the floor of a rat cage. The container had 0.4 cm fenestrations to ensure sufficient olfactory and visual contact between the animals. After the mouse was introduced to the container and the lid was fixed, a rat was placed inside the cage for 15 hours (19:00 to 10:00). For restraint stress, mice were placed inside the custom-made restrainer tube, as described above, for 2 hours during the dark phase of their cycle and placed inside a new cage (19:00 to 21:00). For forced swimming, mice were placed for 10 minutes inside a container (50 × 40 × 30 cm) filled with water at room temperature (19:00 to 19:10). After acute or chronic stress, animals were examined for signs of a possible spontaneous CSD (e.g. hemiparesis), but none was observed.
Immediately after the completion of the entire stress paradigm (3 hours for acute, 28 days for chronic stress), CSD susceptibility was studied experimentally. In a third cohort, after the completion of chronic stress paradigm, we returned the animals to their home cages for 36 hours (stress relief) and then studied CSD susceptibility. Corticosterone is a primary marker of acute stress response and HPA activity. However, corticosterone measurements at a single time point do not reflect cumulative effects of chronic stress (Ottenweller et al., 1992) and corticosterone responses greatly vary among different stressors, stress durations and sampling times (Gong et al., 2015). It is well documented that adrenal weight increases in response to chronic stress (Ulrich-Lai et al., 2006). These increases are correlated with basal corticosterone levels and have been used to confirm the success of various chronic stress paradigms (Balkaya et al., 2011; Franco et al., 2016). For these reasons, and to avoid potential confounding and stressful effects of repeated blood withdrawal for corticosterone measurements we monitored the success of chronic stress paradigm and its relief by showing increased and normalized adrenal weights, respectively, in a subset of mice, and found a strong trend for both (Table 1).
CSD susceptibility:
CSD susceptibility was assessed under full systemic physiological monitoring (19). Mice were anesthetized (isoflurane 2.5% induction, 1% maintenance, in 70% N2O/30% O2) and the femoral artery was catheterized for blood gas sampling and continuous measurement of blood pressure (PowerLab; ADInstruments, Colorado Springs, MO, USA). Arterial blood gases and pH were measured every 30 minutes in ~25 μL samples. Level of anesthesia was maintained throughout the experiment to eliminate cardiovascular response to tail pinch. Systemic physiological parameters were within normal range for all groups (Table 1). CSD susceptibility was assessed using two independent, but complementary, methods: topical KCl application (300 mM) and electrical stimulation, as described previously (Ayata, 2013; Ayata et al., 2006; Eikermann–Haerter et al., 2009b). Briefly, burr holes were drilled bilaterally under saline cooling at (mm from bregma): (i) posterior 3.5, lateral 2.0 (occipital, 2 mm diameter for KCl application or electrical stimulation); (ii) posterior 1.5, lateral 2.0 (frontoparietal, 0.5 mm diameter for DC electrode 1); (iii) anterior 0.5, lateral 2.0 (frontal, 0.5 mm diameter for DC electrode 2, for KCl frequency, right hemisphere only). The experimental setup is shown in Figure 1B.
We determined the electrical threshold for CSD induction in the left hemisphere by direct cortical stimulation using a bipolar stimulation electrode placed on the pial surface (400 |am tip diameter, 1 mm tip separation; FHC) and a stimulator (Grass Instruments, West Warwick, RI, USA) and constant current unit (WPI, Sarasota, FL, USA). Cathodal square pulses of stepwise doubling intensity (0.5–128 microcoulomb, μC) and alternating polarity were applied at 4-minute intervals by adjusting the current and duration of the stimulus. If CSD was not observed at the highest stimulus, 300 mM KCl was topically applied to the cortical surface to ensure CSD could be induced and recorded in the hemisphere. Thresholds were converted to log scale to ensure data normality and allow parametric testing. We determined the frequency of CSDs evoked upon continuous topical KCl application on the opposite hemisphere by placing a cotton ball (~2 mm diameter) soaked in 300 mM KCl on the occipital dura and replaced every 15 minutes for 60 minutes to maintain constant KCl concentrations for the duration of the experiment. Propagation speed (mm/min) of the first CSD was calculated by dividing the distance between the two recording electrodes by the CSD latency between the two sites. DC shift amplitude and duration at half-maximal amplitude were also measured for each CSD.
Data were analyzed using SAS and GraphPad Prism, and presented as whisker-box plots (whiskers, full range; box, interquartile range; horizontal line median; +, mean) for electrical stimulation threshold and KCl-induced CSD frequency, and mean ± standard deviation for systemic physiology, CSD speed, amplitude and duration. We applied general linear random intercept mixed effects modeling to build a multivariable linear prediction model of mean CSD threshold, frequency and speed as dependent variables, with stress status, genotype and sex as independent variables. Bonferroni correction was applied for multiple comparisons. Two-way ANOVA followed by Sidak’s multiple comparisons test was used to compare systemic physiological parameters and other CSD attributes among groups. P values are two-tailed, p<0.05 was considered statistically significant.
RESULTS
Effects of chronic stress or its withdrawal on CSD susceptibility
The multivariable linear prediction model revealed a significant effect of genotype (F (1, 71) = 36.56; p<0.0001) and chronic stress status (F (2, 71) = 8.24; p=0.0006), but not sex (F (1, 71) = 0.59; p=0.4467), on electrical CSD threshold. The model also showed a significant effect of genotype (F (1, 71) = 80.14; p<0.0001) and a strong trend for stress status (F (2, 71) = 2.93; p=0.0597), but not sex (F (1, 71) = 2.26; p=0.1371), on KCl-induced CSD frequency. Only genotype affected CSD propagation speed in the multivariable model (F (1, 68) = 40.85; p<0.0001), while neither stress status (F (2, 68) = 1.33; p=0.2710) nor sex (F (1, 68) = 2.61; p=0.1108) did.
Post-hoc analyses of the effect of stress status within each genotype showed that chronic stress followed by relief for 36 hours significantly reduced the electrical CSD threshold (F (2, 30) = 5.96; p=0.0066) and increased the frequency of KCl-induced CSDs (F (2, 30) = 6.33; p=0.0051) in FHM1 mutants (Figure 2). The effect was independent of sex for both CSD threshold (F (1, 30) = 0.32; p=0.5780) and CSD frequency (F (1, 30) = 0.44; p=0.5129). In contrast to FHM1 mutants, stress relief in WT mice did not significantly alter the electrical CSD threshold (F (2, 34) = 2.2; p=0.1266) or the frequency of KCl-induced CSDs (F (2, 34) = 1.3; p=0.2903) compared with the naive group. Chronic stress for 28 days without relief did not significantly affect the electrical CSD threshold or the frequency of KCl-induced CSDs in either the WT or the FHM1 mutant mice.
Figure 2. Chronic stress.
Upper panel shows representative intracortical DC potential tracings of electrical CSD threshold (left) and KCl-induced CSD frequency (right) determination in FHM1 mice. Cathodal stimulation was stepwise doubled until a CSD developed. Lower panel shows the CSD threshold (left) and frequency (right) data from wild-type (WT) and FHM1 mutant mice (whiskers, full range; box, interquartile range; line, median, +, mean). Two-way ANOVA (stress status and sex as independent variables) p values, and individual data points are also shown (gray circles male, white circles female). *p<0.05 vs. other groups.
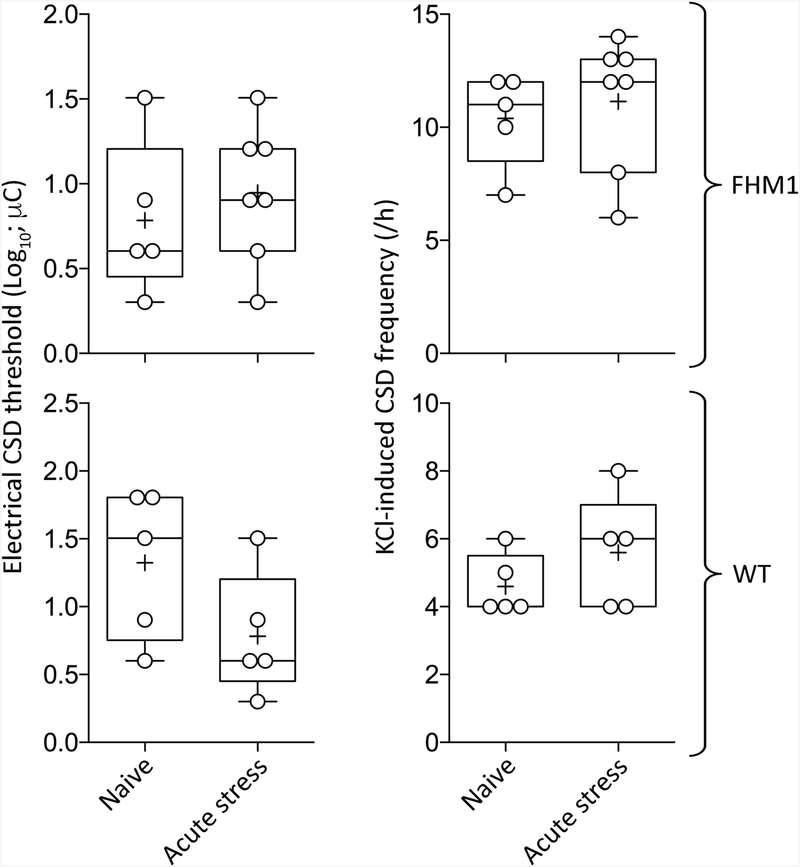
Effects of acute stress on CSD susceptibility
The multivariable linear prediction model revealed that genotype (F (1, 19) = 38.92; p<0.0001), but not acute stress status (F (1, 19) = 0.88; p=0.3603), significantly elevated KCl-induced CSD frequency (Figure 3). Neither genotype (F (1, 19) = 0.60; p=0.4486) nor acute stress (F (1, 19) = 0.60; p=0.4486) affected electrical CSD thresholds. CSD propagation speed was elevated by genotype (F (1, 19) = 15.19; p=0.0010), but not acute stress (F (1, 19) = 1.17; p=0.2925). Post-hoc calculation of achieved power showed 1–β>0.95 for all readouts, suggesting that the acute stress experiment was sufficiently powered.
Figure 3. Acute stress.
CSD threshold (left) and frequency (right) are shown from wild-type (WT) and FHM1 mutant mice (whiskers, full range; box, interquartile range; line, median, +, mean). Individual data points are also shown. All animals were female. There was no statistically significant difference (two-way ANOVA, stress status and genotype as independent variables.
Other CSD attributes and systemic physiological parameters
Stress status, genotype or sex did not consistently affect CSD amplitude or duration, or systemic physiological parameters (Tables 1 and 2).
Table 2:
Electrophysiological SD parameters.
| Genotype | Stress groups | Propagation speed (mm/min) | Amplitude (mV) | Duration (sec) | |
|---|---|---|---|---|---|
| Chronic Stress: | FHM1 | Naïve | 4.4±0.7 | 20±7 | 34±10 |
| Stress | 4.2±0.8 | 21±5 | 40±19 | ||
| Stress + relief | 4.0±0.5 | 23±2 | 40±10 | ||
| WT | Naïve | 3.2±0.4 | 20±7 | 34±10 | |
| Stress | 3.5±0.5 | 17±8 | 30±7 | ||
| Stress + relief | 3.1±1.0 | 23±2 | 54±21* | ||
| Acute Stress: | FHM1 | Naïve | 4.0±0.6 | 19±6 | 50±15 |
| Stress | 4.6±0.9 | 18±5 | 36±12 | ||
| WT | Naïve | 3.0±0.5 | 20±3 | 53±25 | |
| Stress | 3.1±0.9 | 21±2 | 37±13 |
Mean ± standard deviation.
p<0.05 vs. WT naive and stress.
DISCUSSION
In this study, we exposed WT and knock-in mice harboring a human FHM1 mutation to acute stress, chronic stress, or chronic stress followed by relief, and investigated the interaction between stress status and CSD susceptibility. Consistent with clinical observations (Alstadhaug et al., 2007; Lipton et al., 2014; Nattero et al., 1989; Spierings et al., 1997; Torelli et al., 1999), our data show that relief of chronic stress enhances CSD susceptibility in the genetically susceptible FHM1 knock-in mice. Such genetic model-dependent regulation of CSD has previously been observed in relation to gonadal hormones and corticosteroids (Eikermann-Haerter et al., 2009a; Eikermann-Haerter et al., 2009b; Shyti et al., 2015). To our knowledge, this is the first comprehensive study examining the impact of vigorous stress and stress relief paradigms on CSD susceptibility in a genetically susceptible mouse strain.
The prototypical stress response is an adaptive reaction of the body in the face of a challenge and crucial for the survival of the individual. In cases of repetitive or chronic stress however, this adaptive response may become detrimental leading to the so-called allostatic load, where repeated allostatic responses become dysregulated and “interconnected biological systems that overcompensate and eventually collapse leave the organism susceptible to stress-related diseases” (Juster et al., 2010). From that perspective, it has been proposed that migraine and epilepsy (Borsook et al., 2012; McEwen, 2001) are two conditions that are substantially modulated by stress and allostatic load. Epilepsy patients report that stress exacerbates their seizures and monitored case studies suggest increased frequency of seizure episodes under stress (Temkin and Davis, 1984; Thapar et al., 2009). In population studies, seizure frequency and de novo seizure occurrence was increased in environmental stress conditions such as war or natural disasters (Bosnjak et al., 2002; Swinkels et al., 1998). Possible effects of stress relief, however, have not been reported or examined as a pro-convulsant factor.
How stress relief enhances CSD susceptibility in FHM mutants is unknown. Several studies suggest that stress may enhance cerebral glutamatergic excitability (Novakova et al., 2013; Rossi et al., 2009), which is expected to augment susceptibility to CSD. Glucocorticoids have complex actions on excitability through multiple pathways and receptors. Corticosteroids, for example, rapidly trigger glutamate release in hippocampal CA1, and enhance miniature excitatory postsynaptic currents (Aldenhoff et al., 1983; Chameau et al., 2007; Joels, 2009). While rodent stress hormone corticosterone has pro-convulsant effects (Castro et al., 2012; Taher et al., 2005), exposure to acute stress does the contrary (De Lima and Rae, 1991; Joels, 2009; Pericic et al., 2001; Reddy and Rogawski, 2002), possibly through increased GABAergic transmission (Maguire and Mody, 2007; Reddy and Rogawski, 2002). We have recently shown that corticosterone administration increases topical KCl-induced CSD frequency in FHM1 mice via the glucocorticoid receptor, while acute stress was ineffective (Shyti et al., 2015), suggesting that stress is a more complex paradigm that involves multiple mediators.
Other data suggest that chronic stress augments cortical excitability and seizures (Chadda and Devaud, 2004; Jones et al., 2013; Matsumoto et al., 2003; Raudensky and Yamamoto, 2007) and implicates impaired GABAergic inhibition as the mechanism (Dong et al., 2001; Maguire, 2014; Serra et al., 2000). The fact that GABA receptors do not directly modulate CSD susceptibility (Ayata, 2009) may explain why we did not observe a change in CSD susceptibility after acute or chronic stress. Chronic stress or manipulation of glucocorticoid levels also change the expression profiles of a number of ion channels in a time- and brain region-dependent manner (Bali et al., 2013; Nair et al., 1998), including glutamatergic neurotransmission, and voltage-gated Ca2+ channels (Popoli et al., 2011; Shepard and Coutellier, 2017). However, none of these changes were sufficient to impact CSD susceptibility in our chronic stress paradigm.
Our data contradict a recent study reporting a reduction in CSD threshold after acute and chronic stress exposure, and normalization upon acute stress relief, in male wild-type Swiss albino mice (Yapici-Eser et al., 2018). We did not observe a change in CSD susceptibility by either acute or chronic stress in wild-type or FHM1 mutant mice. A third study using different methods and stress paradigms also did not observe a change in CSD susceptibility with exposure to stress alone (i.e. without relief) in male C57Bl6 wild-type mice, the same genetic background strain in our study (Kaufmann and Brennan, 2018). Strain differences in stress response (Bondy et al., 1979; Monteiro et al., 2015; van Bogaert et al., 2006) may have contributed to the discrepant outcomes since C57Bl6 strain is relatively resistant to various stress paradigms (Monteiro et al., 2015). Sex also determines stress response. Female rodents have higher basal corticosterone levels and a stronger and more rapid corticosterone increase in response to stressors (Kant et al., 1983). Molecular responses to stress in relevant brain regions also show sexual dimorphism (Lin et al., 2009). In our study, both male and female FHM1 mutants showed elevated CSD susceptibility upon chronic stress relief, and our multivariate analysis did not reveal sex as an independent predictor of response to stress. The two other studies examined males only (Kaufmann and Brennan, 2018; Yapici-Eser et al., 2018). Therefore, sex is unlikely to explain the discrepant results (Yapici-Eser et al., 2018).
Our finding that stress relief augments CSD susceptibility in FHM mutants only underscores the role of genetic influences in migraine. Although FHM mutations are rare (prevalence ~0.01%), constituting only a small portion of migraine patients (Thomsen et al., 2002), FHM patients display many cardinal features of migraine with similar trigger factors. Indeed, most FHM patients also report migraine attacks following stress (Hansen et al., 2011). The FHM1 mouse model has previously shown selective vulnerability to altered gonadal or stress hormone levels (Eikermann-Haerter et al., 2009a; Eikermann-Haerter et al., 2009b; Shyti et al., 2015), as well as ischemic injury (Eikermann-Haerter et al., 2012), suggesting that the genetic background that confers migraine susceptibility also heightens an individual’s sensitivity to external challenges. Further research with other SD-susceptible genetic mouse strains is needed to test whether increased CSD susceptibility after stress relief is indeed a common phenomenon among migraine prone individuals.
The mechanisms leading to elevated CSD susceptibility upon relief of chronic stress in FHM1 mutant mice are undoubtedly complex and warrant further research. Future work should include a detailed assessment of corticosteroid levels and adrenal weights aimed at understanding the temporal determinants of stress modulation of CSD, dissect the mechanisms, and seek pharmacological targets.
Highlights.
Spreading depolarization is the electrophysiological substrate of migraine aura
Relief after chronic stress enhanced spreading depolarizations in FHM1 mutants only
Acute or chronic stress alone did not alter spreading depolarization susceptibility
Our findings may explain the “weekend migraine” phenomenon
ACKNOWLEDGEMENTS
This work was supported by the following sources: The National Institute of Neurological Disorders and Stroke at the National Institutes of Health (P01NS055104, R01NS102969, R25NS065743), Fondation Leducq, Andrew David Heitman Foundation, Ellison Foundation, Brain Aneurysm Foundation’s Timothy P. Susco and Andrew David Heitman Foundation Chairs of Research, International Headache Society, European Union’s (EU) Seventh Framework programme “EUROHEADPAIN” (nr. 602633) and EU Marie Curie IAPP Program “BRAINPATH” (nr 612360). American Heart Association (10SDG2610275 to K.E.H.); the Massachusetts General Hospital (Claflin Distinguished Award to K.E.H.).
Abbreviations:
- CSD
Cortical spreading depolarization
- SD
spreading depolarization
- FHM1
familial hemiplegic migraine type1
- dB
decibel
- KCl
Potassium Chloride
- ANOVA
analysis of variance
- GABA
γ-aminobutyric acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DECLERATIONS OF INTEREST
None.
REFERENCES
- Aldenhoff JB, Gruol DL, Rivier J, Vale W, and Siggins GR (1983). Corticotropin releasing factor decreases postburst hyperpolarizations and excites hippocampal neurons. Science 221, 875–7. [DOI] [PubMed] [Google Scholar]
- Alstadhaug KB, Salvesen R, and Bekkelund S (2007). Weekend migraine. Cephalalgia 27, 343–6. [DOI] [PubMed] [Google Scholar]
- Ayata C (2009). Spreading depression: from serendipity to targeted therapy in migraine prophylaxis. Cephalalgia 29,1095–114. [DOI] [PubMed] [Google Scholar]
- Ayata C (2010). Cortical spreading depression triggers migraine attack: pro. Headache 50, 725–30. [DOI] [PubMed] [Google Scholar]
- Ayata C (2013). Pearls and pitfalls in experimental models of spreading depression. Cephalalgia 33, 604–13. [DOI] [PubMed] [Google Scholar]
- Ayata C, Jin H, Kudo C, Dalkara T, and Moskowitz MA (2006). Suppression of cortical spreading depression in migraine prophylaxis. Ann Neurol 59, 652–61. [DOI] [PubMed] [Google Scholar]
- Bali A, Gupta S, Singh N, and Jaggi AS (2013). Implicating the role of plasma membrane localized calcium channels and exchangers in stress-induced deleterious effects. Eur J Pharmacol 714, 229–38. [DOI] [PubMed] [Google Scholar]
- Balkaya M, Prinz V, Custodis F, Gertz K, Kronenberg G, Kroeber J, Fink K, Plehm R, Gass P, Laufs U, and Endres M (2011). Stress worsens endothelial function and ischemic stroke via glucocorticoids. Stroke 42, 3258–64. [DOI] [PubMed] [Google Scholar]
- Bondy SC, Tepper JM, and Bettis DB (1979). Seizure proneness and neurotransmitter uptake. Neurochem Res 4, 755–61. [DOI] [PubMed] [Google Scholar]
- Borsook D, Maleki N, Becerra L, and McEwen B (2012). Understanding migraine through the lens of maladaptive stress responses: a model disease of allostatic load. Neuron 73, 219–34. [DOI] [PubMed] [Google Scholar]
- Bosnjak J, Vukovic-Bobic M, and Mejaski-Bosnjak V (2002). Effect of war on the occurrence of epileptic seizures in children. Epilepsy Behav 3, 502–509. [DOI] [PubMed] [Google Scholar]
- Castro OW, Santos VR, Pun RY, McKlveen JM, Batie M, Holland KD, Gardner M, Garcia-Cairasco N, Herman JP, and Danzer SC (2012). Impact of corticosterone treatment on spontaneous seizure frequency and epileptiform activity in mice with chronic epilepsy. PLoS One 7, e46044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadda R, and Devaud LL (2004). Sex differences in effects of mild chronic stress on seizure risk and GABAA receptors in rats. Pharmacol Biochem Behav 78, 495–504. [DOI] [PubMed] [Google Scholar]
- Chameau P, Qin Y, Spijker S, Smit AB, and Joels M (2007). Glucocorticoids specifically enhance L-type calcium current amplitude and affect calcium channel subunit expression in the mouse hippocampus. J Neurophysiol 97, 5–14. [DOI] [PubMed] [Google Scholar]
- Chanda ML, Tuttle AH, Baran I, Atlin C, Guindi D, Hathaway G, Israelian N, Levenstadt J, Low D, Macrae L, O’Shea L, Silver A, Zendegui E, Mariette Lenselink A, Spijker S, Ferrari MD, van den Maagdenberg AM, and Mogil JS (2013). Behavioral evidence for photophobia and stress-related ipsilateral head pain in transgenic Cacna1a mutant mice. Pain 154,1254–62. [DOI] [PubMed] [Google Scholar]
- De Lima TC, and Rae GA (1991). Effects of cold-restraint and swim stress on convulsions induced by pentylenetetrazol and electroshock: influence of naloxone pretreatment. Pharmacol Biochem Behav 40, 297–300. [DOI] [PubMed] [Google Scholar]
- Dong E, Matsumoto K, Uzunova V, Sugaya I, Takahata H, Nomura H, Watanabe H, Costa E, and Guidotti A (2001). Brain 5alpha-dihydroprogesterone and allopregnanolone synthesis in a mouse model of protracted social isolation. Proc Natl Acad Sci U S A 98, 2849–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eikermann-Haerter K, Arbel-Ornath M, Yalcin N, Yu ES, Kuchibhotla KV, Yuzawa I, Hudry E,Willard CR, Climov M, Keles F, Belcher AM, Sengul B, Negro A, Rosen IA, Arreguin A, Ferrari MD, van den Maagdenberg AM, Bacskai BJ, and Ayata C (2015). Abnormal synaptic Ca(2+) homeostasis and morphology in cortical neurons of familial hemiplegic migraine type 1 mutant mice. Ann Neurol 78,193–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eikermann-Haerter K, Baum MJ, Ferrari MD, van den Maagdenberg AM, Moskowitz MA, and Ayata C (2009a). Androgenic suppression of spreading depression in familial hemiplegic migraine type 1 mutant mice. Ann Neurol 66, 564–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eikermann-Haerter K, Dilekoz E, Kudo C, Savitz SI, Waeber C, Baum MJ, Ferrari MD, van den Maagdenberg AM, Moskowitz MA, and Ayata C (2009b). Genetic and hormonal factors modulate spreading depression and transient hemiparesis in mouse models of familial hemiplegic migraine type 1. J Clin Invest 119, 99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eikermann-Haerter K, Lee JH, Yuzawa I, Liu CH, Zhou Z, Shin HK, Zheng Y, Qin T, Kurth T,Waeber C, Ferrari MD, van den Maagdenberg AM, Moskowitz MA, and Ayata C (2012). Migraine mutations increase stroke vulnerability by facilitating ischemic depolarizations. Circulation 125, 335–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari MD, Klever RR, Terwindt GM, Ayata C, and van den Maagdenberg AM (2015).Migraine pathophysiology: lessons from mouse models and human genetics. Lancet Neurol 14, 65–80. [DOI] [PubMed] [Google Scholar]
- Franco AJ, Chen C, Scullen T, Zsombok A, Salahudeen AA, Di S, Herman JP, and Tasker JG (2016). Sensitization of the Hypothalamic-Pituitary-Adrenal Axis in a Male Rat Chronic Stress Model. Endocrinology 157, 2346–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giffin NJ, Ruggiero L, Lipton RB, Silberstein SD, Tvedskov JF, Olesen J, Altman J, Goadsby PJ, and Macrae A (2003). Premonitory symptoms in migraine: an electronic diary study. Neurology 60, 935–40. [DOI] [PubMed] [Google Scholar]
- Goadsby PJ, Holland PR, Martins-Oliveira M, Hoffmann J, Schankin C, and Akerman S (2017). Pathophysiology of Migraine: A Disorder of Sensory Processing. Physiol Rev 97, 553–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong S, Miao YL, Jiao GZ, Sun MJ, Li H, Lin J, Luo MJ, and Tan JH (2015). Dynamics and correlation of serum cortisol and corticosterone under different physiological or stressful conditions in mice. PLoS One 10, e0117503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grissom N, and Bhatnagar S (2009). Habituation to repeated stress: get used to it. Neurobiol Learn Mem 92, 215–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen JM, Hauge AW, Ashina M, and Olesen J (2011). Trigger factors for familial hemiplegic migraine. Cephalalgia 31,1274–81. [DOI] [PubMed] [Google Scholar]
- Hauge AW, Kirchmann M, and Olesen J (2011). Characterization of consistent triggers of migraine with aura. Cephalalgia 31, 416–38. [DOI] [PubMed] [Google Scholar]
- Joels M (2009). Stress, the hippocampus, and epilepsy. Epilepsia 50, 586–97 [DOI] [PubMed] [Google Scholar]
- Jones NC, Lee HE, Yang M, Rees SM, Morris MJ, O’Brien TJ, and Salzberg MR (2013). Repeatedly stressed rats have enhanced vulnerability to amygdala kindling epileptogenesis. Psychoneuroendocrinology 38, 263–70. [DOI] [PubMed] [Google Scholar]
- Juster RP, McEwen BS, and Lupien SJ (2010). Allostatic load biomarkers of chronic stress and impact on health and cognition. Neurosci Biobehav Rev 35, 2–16. [DOI] [PubMed] [Google Scholar]
- Kant GJ, Lenox RH, Bunnell BN, Mougey EH, Pennington LL, and Meyerhoff JL (1983). Comparison of stress response in male and female rats: pituitary cyclic AMP and plasma prolactin, growth hormone and corticosterone. Psychoneuroendocrinology 8, 421–8. [DOI] [PubMed] [Google Scholar]
- Kaufmann D, and Brennan KC (2018). The Effects of Chronic Stress on Migraine Relevant Phenotypes in Male Mice. Front Cell Neurosci 12, 294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kors EE, Terwindt GM, Vermeulen FL, Fitzsimons RB, Jardine PE, Heywood P, Love S, van den Maagdenberg AM, Haan J, Frants RR, and Ferrari MD (2001). Delayed cerebral edema and fatal coma after minor head trauma: role of the CACNA1A calcium channel subunit gene and relationship with familial hemiplegic migraine. Ann Neurol 49, 753–60. [DOI] [PubMed] [Google Scholar]
- Lin Y, Ter Horst GJ, Wichmann R, Bakker P, Liu A, Li X, and Westenbroek C (2009). Sex differences in the effects of acute and chronic stress and recovery after long-term stress on stress-related brain regions of rats. Cereb Cortex 19,1978–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton RB, Buse DC, Hall CB, Tennen H, Defreitas TA, Borkowski TM, Grosberg BM, and Haut SR (2014). Reduction in perceived stress as a migraine trigger: testing the “let-down headache” hypothesis. Neurology 82,1395–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire J (2014). Stress-induced plasticity of GABAergic inhibition. Front Cell Neurosci 8,157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire J, and Mody I (2007). Neurosteroid synthesis-mediated regulation of GABA(A) receptors: relevance to the ovarian cycle and stress. J Neurosci 27, 2155–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K, Nomura H, Murakami Y, Taki K, Takahata H, and Watanabe H (2003). Long-term social isolation enhances picrotoxin seizure susceptibility in mice: up-regulatory role of endogenous brain allopregnanolone in GABAergic systems. Pharmacol Biochem Behav 75, 831–5. [DOI] [PubMed] [Google Scholar]
- McEwen BS (2001). Plasticity of the hippocampus: adaptation to chronic stress and allostatic load. Ann N Y Acad Sci 933, 265–77. [DOI] [PubMed] [Google Scholar]
- Monteiro S, Roque S, de Sa-Calcada D, Sousa N, Correia-Neves M, and Cerqueira JJ (2015). An efficient chronic unpredictable stress protocol to induce stress-related responses in C57BL/6 mice. Front Psychiatry 6, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair SM, Werkman TR, Craig J, Finnell R, Joels M, and Eberwine JH (1998). Corticosteroid regulation of ion channel conductances and mRNA levels in individual hippocampal CA1 neurons. J Neurosci 18, 2685–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nattero G, De Lorenzo C, Biale L, Allais G, Torre E, and Ancona M (1989). Psychological aspects of weekend headache sufferers in comparison with migraine patients. Headache 29, 93–9. [DOI] [PubMed] [Google Scholar]
- Novakova B, Harris PR, Ponnusamy A, and Reuber M (2013). The role of stress as a trigger for epileptic seizures: a narrative review of evidence from human and animal studies. Epilepsia 54, 1866–76. [DOI] [PubMed] [Google Scholar]
- Ottenweller JE, Servatius RJ, Tapp WN, Drastal SD, Bergen MT, and Natelson BH (1992). A chronic stress state in rats: effects of repeated stress on basal corticosterone and behavior. Physiol Behav 51, 689–98. [DOI] [PubMed] [Google Scholar]
- Pericic D, Jazvinscak M, Svob D, and Mirkovic K (2001). Swim stress alters the behavioural response of mice to GABA-related and some GABA-unrelated convulsants. Epilepsy Res 43,145–52. [DOI] [PubMed] [Google Scholar]
- Popoli M, Yan Z, McEwen BS, and Sanacora G (2011). The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nat Rev Neurosci 13, 22–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raudensky J, and Yamamoto BK (2007). Effects of chronic unpredictable stress and ethamphetamine on hippocampal glutamate function. Brain Res 1135,129–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, and Rogawski MA (2002). Stress-induced deoxycorticosterone-derived neurosteroids modulate GABA(A) receptor function and seizure susceptibility. J Neurosci 22, 3795–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi S, De Capua A, Tavanti M, Calossi S, Polizzotto NR, Mantovani A, Falzarano V, Bossini L, Passero S, Bartalini S, and Ulivelli M (2009). Dysfunctions of cortical excitability in drug-naive posttraumatic stress disorder patients. Biol Psychiatry 66, 54–61. [DOI] [PubMed] [Google Scholar]
- Sauro KM, and Becker WJ (2009). The stress and migraine interaction. Headache 49,1378–86. [DOI] [PubMed] [Google Scholar]
- Schoonman GG, Evers DJ, Terwindt GM, van Dijk JG, and Ferrari MD (2006). The prevalence of premonitory symptoms in migraine: a questionnaire study in 461 patients. Cephalalgia 26, 1209–13. [DOI] [PubMed] [Google Scholar]
- Schulte LH, and May A (2017). Of generators, networks and migraine attacks. Curr Opin Neurol 30, 241–245. [DOI] [PubMed] [Google Scholar]
- Serra M, Pisu MG, Littera M, Papi G, Sanna E, Tuveri F, Usala L, Purdy RH, and Biggio G (2000). Social isolation-induced decreases in both the abundance of neuroactive steroids and GABA(A) receptor function in rat brain. J Neurochem 75, 732–40. [DOI] [PubMed] [Google Scholar]
- Shepard R, and Coutellier L (2017). Changes in the Prefrontal Glutamatergic and Parvalbumin Systems of Mice Exposed to Unpredictable Chronic Stress. Mol Neurobiol. [DOI] [PubMed] [Google Scholar]
- Shyti R, Eikermann-Haerter K, van Heiningen SH, Meijer OC, Ayata C, Joels M, Ferrari MD, van den Maagdenberg AM, and Tolner EA (2015). Stress hormone corticosterone enhances susceptibility to cortical spreading depression in familial hemiplegic migraine type 1 mutant mice. Exp Neurol 263, 214–20. [DOI] [PubMed] [Google Scholar]
- Spierings EL, Sorbi M, Maassen GH, and Honkoop PC (1997). Psychophysical precedents of migraine in relation to the time of onset of the headache: the migraine time line. Headache 37, 217–20. [DOI] [PubMed] [Google Scholar]
- Stam AH, Luijckx GJ, Poll-The BT, Ginjaar IB, Frants RR, Haan J, Ferrari MD, Terwindt GM, and van den Maagdenberg AM (2009). Early seizures and cerebral oedema after trivial head trauma associated with the CACNA1A S218L mutation. J Neurol Neurosurg Psychiatry 80, 1125–9. [DOI] [PubMed] [Google Scholar]
- Swinkels WA, Engelsman M, Kasteleijn-Nolst Trenite DG, Baal MG, de Haan GJ, and Oosting J (1998). Influence of an evacuation in February 1995 in The Netherlands on the seizure frequency in patients with epilepsy: a controlled study. Epilepsia 39,1203–7. [DOI] [PubMed] [Google Scholar]
- Taher TR, Salzberg M, Morris MJ, Rees S, and O’Brien TJ (2005). Chronic low-dose corticosterone supplementation enhances acquired epileptogenesis in the rat amygdala kindling model of TLE. Neuropsychopharmacology 30,1610–6. [DOI] [PubMed] [Google Scholar]
- Temkin NR, and Davis GR (1984). Stress as a risk factor for seizures among adults with epilepsy. Epilepsia 25, 450–6. [DOI] [PubMed] [Google Scholar]
- Thapar A, Kerr M, and Harold G (2009). Stress, anxiety, depression, and epilepsy: investigating the relationship between psychological factors and seizures. Epilepsy Behav 14,134–40. [DOI] [PubMed] [Google Scholar]
- Thomsen LL, Eriksen MK, Roemer SF, Andersen I, Olesen J, and Russell MB (2002). A population-based study of familial hemiplegic migraine suggests revised diagnostic criteria. Brain 125,1379–91. [DOI] [PubMed] [Google Scholar]
- Torelli P, Cologno D, and Manzoni GC (1999). Weekend headache: a retrospective study in migraine without aura and episodic tension-type headache. Headache 39,11–20. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Figueiredo HF, Ostrander MM, Choi DC, Engeland WC, and Herman JP (2006). Chronic stress induces adrenal hyperplasia and hypertrophy in a subregion-specific manner. Am J Physiol Endocrinol Metab 291, E965–73. [DOI] [PubMed] [Google Scholar]
- van Bogaert MJ, Groenink L, Oosting RS, Westphal KG, van der Gugten J, and Olivier B (2006). Mouse strain differences in autonomic responses to stress. Genes Brain Behav 5,139–49. [DOI] [PubMed] [Google Scholar]
- van den Maagdenberg AM, Pietrobon D, Pizzorusso T, Kaja S, Broos LA, Cesetti T, van de Ven RC, Tottene A, van der Kaa J, Plomp JJ, Frants RR, and Ferrari MD (2004). A Cacna1a knockin migraine mouse model with increased susceptibility to cortical spreading depression. Neuron 41, 701–10. [DOI] [PubMed] [Google Scholar]
- van den Maagdenberg AM, Pizzorusso T, Kaja S, Terpolilli N, Shapovalova M, Hoebeek FE, Barrett CF, Gherardini L, van de Ven RC, Todorov B, Broos LA, Tottene A, Gao Z, Fodor M, De Zeeuw CI, Frants RR, Plesnila N, Plomp JJ, Pietrobon D, and Ferrari MD (2010). High cortical spreading depression susceptibility and migraine-associated symptoms in Ca(v)2.1 S218L mice. Ann Neurol 67, 85–98. [DOI] [PubMed] [Google Scholar]
- Yapici-Eser H, Donmez-Demir B, Kilic K, Eren-Kocak E, and Dalkara T (2018). Stress modulates cortical excitability via alpha-2 adrenergic and glucocorticoid receptors: As assessed by spreading depression. Exp Neurol 307, 45–51. [DOI] [PubMed] [Google Scholar]
- Zhang X, Levy D, Kainz V, Noseda R, Jakubowski M, and Burstein R (2011). Activation of central trigeminovascular neurons by cortical spreading depression. Ann Neurol 69, 855–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Levy D, Noseda R, Kainz V, Jakubowski M, and Burstein R (2010). Activation of meningeal nociceptors by cortical spreading depression: implications for migraine with aura. J Neurosci 30, 8807–14. [DOI] [PMC free article] [PubMed] [Google Scholar]