Abstract
Background and Purpose:
The purpose of this study was to assess the consistency of a novel MR safe lower extremity motor control neuroimaging paradigm to elicit reliable sensorimotor region brain activity.
Method:
Participants completed multiple sets of unilateral leg presses combining ankle, knee, and hip extension and flexion movements against resistance at a pace of 1.2 Hz while lying supine in a 3T MRI scanner. Regions of Interest (ROI) consisted of regions primarily involved in lower extremity motor control (right and left primary motor cortex, primary somatosensory cortex, pre-motor cortex, secondary somatosensory cortex, basal ganglia, and the cerebellum).
Results:
The group analysis based on mixed effects paired samples t-test revealed no differences for brain activity between sessions (p>0.05). Intraclass correlation coefficients in the sensorimotor regions were good to excellent for average percent signal change (0.621 to 0.918) and Z-score (0.697 to 0.883), with the exception of the left secondary somatosensory cortex percent signal change (0.165).
Conclusions:
These results indicate that a loaded lower extremity force production and attenuation task that simulates the range of motion of squatting, stepping, and landing from a jump is reliable for longitudinal neuroimaging applications and support the use of this paradigm in further studies examining therapeutic interventions and changes in dynamic lower extremity motor function.
Keywords: Reliability, Neuroimaging, Knee, Leg press
Introduction
Coordinated locomotor activities such as jumping, climbing stairs, and running are complex motor skills that require effective coordination between the central and peripheral nervous system. While there is extensive knowledge pertaining to the physiologic,1–3 biomechanical,4–7 and neuromuscular8,9 components of lower extremity dynamic human movement, less is understood regarding the underlying neural activity driving lower extremity complex action. Recent innovations with electroencephalography (EEG) have allowed neural recording during dynamic multi-joint lower extremity coordinated movements.10–13 However, EEG only measures surface cortical activity and lower extremity motor control has an extensive sub-cortical and cerebellar component. Other modalities do allow quantification of cortical function during dynamic tasks such as single-photon-emission-computed-tomography (SPECT),14,15 positron emission tomography (PET)16 and functional near-infrared spectroscopy (fNIRS).17–19 On the other hand, these modalities are limited in their spatial resolution and/or only allow for measuring a minimal degree of lower extremity movement.
Advances in neuroimaging have made it possible to obtain highly accurate spatial images of neural functioning using fMRI.20–25 However, fMRI requires participants to stay in the supine position with their head still for prolonged periods of time. This can be technically challenging in acquiring accurate neural readings, simultaneous with lower limb multi-joint movements.26 As many of the primary hip and knee joint muscles originate from the pelvis, such as hip flexors, gluteals, hamstrings and quadriceps, any lower limb movement proximal to the ankle are predisposed to induce pelvic and trunk translation and head motion artifacts. This has resulted in considerable concern pertaining to the impact of head motion as a confounding factor during motor task fMRI data acquisition5,27 with subsequent image-processing schemes developed to minimize head motion artifacts.28–31 Nonetheless, the image re-alignment processes cannot fully eliminate excessive head motion, nor account for task-correlated motion.32 As a result, researchers have opted towards a solution that includes constraints and supports of the lower limbs and pelvis, allowing for freedom of movement while keeping the head stable.26
A number of studies have developed novel MRI-safe devices permitting participants to execute locomotive-like functions while in the MRI machine.33,34 An early attempt utilized ankle dorsiflexion in order to examine cortical adaptations for walking therapies35 as ankle dorsiflexion is a foundational component for normal gait. Expansions on this work have utilized an isometric leg press device allowing for isometric (muscle contraction without movement) ankle, knee, and hip extension.26 Increased activation in the sensorimotor cortex, the dorsolateral premotor cortex, the supplementary motor area, and in the secondary somatosensory cortex was observed on two separate testing sessions with medium to high reliability demonstrating the efficacy of such apparatus for measuring cortical function associated with ankle and knee extension contractions.26 However, the actions were isometric (no movement) and at very low force levels. A few studies have evaluated more functional knee flexion and extension movements within an MRI scanner,36–38 and while these studies allowed for knee movement, they were not against external resistance nor allowed hip motion. More complex pedaling devices have been used to simulate the rhythmic alterations of lower limb flexion and extension during walking,34 including an MRI safe stepping device termed the Magnetic Resonance Compatible Stepper (MARCOS) which simulates participants’ movements and physical forces as if walking.39 MARCOS was successfully used with fMRI to determine differences in neural activation between active versus passive lower-limb, bilateral, multi-joint movements,24 with active tasks exhibiting greater sensorimotor region activation relative to passive tasks. Greater sensorimotor activation for active tasks were consistent with prior reports demonstrating increased activation during locomotion,34,40 and have provided valuable insight into the neural mechanisms underlying human movement.
While prior studies have helped further scientific understanding of lower limb multi-joint motor movements, there is still a need for devices that simulate other locomotive processes beyond simple single joint movements or simulated gait. For instance, the biomechanics employed during jumping,41–43 squatting,25,44 and running45,46 elicit hip flexion and extension when an individual’s lower extremity is in contact with the ground (i.e., closed-kinetic chain [CKC] exercise) across the entire stretch-shortening cycle. CKC movements are also typically ‘loaded’ in which the knee and hip extension movements are against resistance (typically bodyweight). Functional loaded movements are common behavioral outcome measures of exercise,47,48 injury prevention,49,50 and motor learning interventions,51,52 and are also vital for sport or recreational activities that require proper mechanics to avoid injury.53,54 A neuroimaging paradigm that simulates functional loaded movement could guide novel treatment for musculoskeletal disorders that exhibit both motor control and neural activity alterations (e.g., anterior cruciate ligament [ACL] injury).36,55 However, no reliable fMRI paradigm has been developed that specifically targets these loaded multi-joint movements. The purpose of the present study was to present a novel method to assay brain activation during coordinated lower extremity movement under load and to evaluate the consistency of the neuroimaging paradigm to induce sensorimotor region activity.
Methods
Participants
Thirteen healthy female participants (mean age = 16.23 +/− 0.72 yrs; mean height = 163.85 +/− 4.67 cm; mean weight = 59.56 +/− 8.70 kg) volunteered for this study. Participants were all of similar fitness and participation levels, were members of the same high school soccer team, and did not engage in any specific or novel training beyond school sponsored athletics. All participants (and parent or legal guardian if under 18) signed an informed consent/assent and completed an MRI screening form. Volunteers participated in the full protocol on two separate testing sessions separated by 7.10 (+/− 0.98) weeks. The study was approved by the institutional review board at Cincinnati Children’s Hospital Medical center.
Instrumentation
The leg-press testing apparatus was custom built and comprised of two pedals that ran on tracks. This design permitted the participant to complete repetitive combined ankle, knee and hip extension and flexion movements that mimic the loading pattern for landing. The participant laid on the MR scanner table in the supine position with earplugs for auditory protection. The participants’ head was positioned in the head coil such that the top of the headphones abutted the top of the coil (reducing potential inferior to superior head translation during imaging). Small foam padding was put on the sides of the headphones in order to fill any gaps between the participants’ head and the inner head coil to further secure the head in place. The feet of the participant were strapped into the pedals with their legs in full extension. The pedals moved with flexion and extension of the hip and knee. Rubber resistance bands were put around the pedals and connected to the unit to provide resistance when the participant extended their lower extremity.
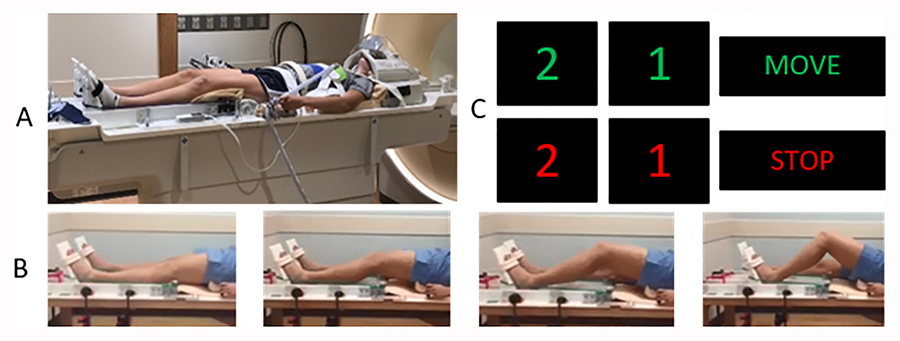
In order to minimize head movement from forces exerted at the knee and hip during the unilateral simulated landing movement, multiple strategies were engaged. Fluidized positioners (Sundance Solutions, White Plains, NY) were placed underneath the participants’ lumbar regions, inferior to the occiput, and in the superior posterior aspect of the head. Velcro straps were fastened to the MRI table and used to secure the participant’s upper torso throughout the scan. Specifically, two straps were fastened bilaterally and stretched over the participant’s acromion process and sternum to the contralateral side of the abdomen, forming an X shape across the chest. Straps were also placed transversely over the sternum and over the anterior superior iliac spines. Our custom apparatus was also constructed with adjustable handlebars for participants to grip while in the MRI scanner. These handlebars were adjustable to be positioned for minimal elbow flexion. A Velcro head strap was fitted to the posterior aspect of the head coil and wrapped over the forehead to further limit head motion. A mirror was also positioned directly on top of the head coil to permit the participant full view of a projector screen for task-related instructions (Figure 1A).
Figure 1.
A: Participant set-up in the MRI, with body straps to reduce accessory movement and demonstrating the leg press set-up. B: Participant completing the leg press motion. C: The visual prompts with a 2, 1 countdown to start moving and stop moving. The 2, 1 was provided to ensure participants were prepared to start moving and to stop.
The unilateral simulated landing task required the participants to flex their dominant (in this case all right) lower extremity to ~ 60-degree knee flexion along the track and then extending their lower extremity to ~ 0-degree knee flexion against resistance set at ~25% of body weight (0.25 × participant body mass = resistance of the band at full extension). The leg press apparatus slid superiorly or inferiorly to standardize the starting placement for all participants relative to individual anthropometrics. Pilot testing revealed that this resistance level was found to not be so high as to induce accessory motion artifact. The participant’s lower extremity movement resulted in the foot moving proximally along the track against the set resistance. The simulated landing device has two tracks, one for each leg, allowing independent unilateral movement. Following the 30s of rest with a blank screen in which the participant began fully extended, a visual countdown stimulus of “2”, “1” would appear on the screen followed by ‘move right’ for right leg or “move left” for left leg (all data reported herein are for the right dominate leg movement only) (Figure 1). Congruent with the onset of the ‘move right’ stimulus, a metronome (1.2 Hz) began which was audible to the participant through the headphones. The visual prompt of the countdown of “2” and “1” was designed to assure a smooth initiation and end of the move period in the fMRI paradigm. The participant was instructed to prepare to begin a slow and controlled movement upon viewing the ‘2’ stimulus and to start the motion in conjunction with the metronome starting when the move command was displayed. Near the end of the 30s move block, the participant would see “2”, “1”, indicating to prepare to stop moving followed by the stop command and participants would relax during the rest period (Figure 1). Akin to the move blocks, participants were instructed to slowly return to full extension when the ‘2’ stimulus appeared preceding the rest blocks to ensure a gradual, non-abrupt motion end to minimize head movement. To further minimize the potential for accessory head motion a complete familiarization session with examiner feedback on head motion was completed on a mock scanner prior to the actual MRI experiment. This familiarization session involved participants first watching a video of the task being completed, then completing the task in a mock scanner that simulated the same physical characteristics and noise of the actual MRI with the same auditory prompts and motor task. A researcher provided feedback during the familiarization session to minimize head motion and ensure task accuracy. All participants completed the same amount of familiarization (completed the entire movement paradigm once in the mock scanner) before actual scanning.
MRI data acquisition and analyses
All scans were performed on a 3T Philips Ingenia MRI scanner (Amsterdam, Netherlands) using a 32-channel head coil. An MPRAGE sequence was used to acquire high-resolution 3D T1-weighted images (sagittal): TR = 8.1 ms; TE = 3.7 ms; field of view = 256 × 256 mm; matrix = 256 × 256; in-plane resolution = 1×1 mm; slice thickness = 1 mm; number of slices = 180. The functional acquisition run consisted of four blocks of 30s cued contractions interleaved with five blocks of 30s rest periods (4:30 minute total scan time) which included 135 whole-brain gradient-echo echoplanar scans: TR = 2000 ms; TE = 35 ms; field of view = 240 × 240 mm; slice thickness = 5 mm; voxel size = 3.75mm × 3.75mm.
fMRI analyses were performed using the FSL software package (The Oxford Centre for Functional MRI of the Brain, Nuffield Department of Clinical Neurosciences, University of Oxford, Oxford, United Kingdom56 with standard processing. This included temporal high-pass filtering (90s), 4D mean intensity normalization, spatial smoothing at 6 mm FWHM, FILM prewhitening, slice timing correction, brain extraction and MCFLIRT motion correction and registration to participant anatomical and standard space via FNIRT.30 First-level subject contrasts (knee movement – baseline [rest]) were completed with z threshold of 2.3 and p <.05 Gaussian random field cluster corrected.
Whole Brain Session Differences
To determine if our paradigm produced similar activation during both testing sessions, second-level between session contrasts (session 1 – session 2) were calculated with a z threshold of 3.5 and p <.05 Gaussian random field cluster corrected.57,58 This higher-level session analysis was completed with FMRIB’s Local Analysis of Mixed Effects (FLAME) stage 1 and stage 259 using paired samples t-tests to contrast both sessions’ whole brain activation (session one > session two & session two > session one).
Region of Interest Selection
In addition to examination of any differences in whole brain activity between the two sessions, a more in-depth analysis of key sensorimotor regions of interest (ROI) were also completed. ROIs were defined in a similar manner as previously described60 and consisted of regions primarily involved in lower extremity motor control (right and left primary motor cortex, primary somatosensory cortex, pre-motor cortex, secondary somatosensory cortex, basal ganglia, and the cerebellum). The cerebellar ROI was created with the cerebellar atlas in MNI152 space after non-linear normalization and included all regions. The basal ganglia was created from the MNI structural atlas by combining the caudate and putamen. The Juelich Histological atlas was used to generate the other 8 cortical ROIs. The respective right and left primary motor cortex consisted of Brodmann area (BA) 4a (anterior) and BA 4p (posterior), primary somatosensory cortex consisted of BA1, 2, 3a and 3b, the secondary somatosensory cortex included BA OP (Operculum)1–4 in the parietal operculum and the pre-motor cortex entailed BA6. Second-level between session contrasts (session 1 – session 2) were calculated with a z threshold of 3.5 and p <.05 Gaussian random field cluster corrected as with the whole brain analysis, but masked for the 10 sensorimotor ROIs.57,58 This higher-level session analysis was completed with FMRIB’s Local Analysis of Mixed Effects (FLAME) stage 1 and stage 259 using paired samples t-tests to contrast both sessions’ whole brain activation (session one > session two & session two > session one). A group by session average masked for the 10 sensorimotor ROIs was also completed to provide descriptive brain activity for the task.
Assessment of test-retest reliability
To estimate the reliability of brain activity within ROIs across sessions, we calculated the intraclass correlation (ICC) for the mean percent signal change and z-score for the anatomical ROI masked regions and computed a two-way mixed model ICC for absolute agreement. A paired samples t-test was also completed on each ROI’s mean percent signal change and z-score in addition to the statistical parametric mapping described above to determine between session differences. ICC values were interpreted as excellent >0.75, good between 0.59 and 0.75, fair between 0.40 and 0.58, and poor if below 0.40.61
Results
All participants completed the leg press during sessions 1 and 2. The time between sessions ranged from 39 to 62 days with a mean of 49.69 (+/− 6.88) days equivalent to 7.10 (+/− 0.98) weeks. 5 of the 13 participants were excluded from the analysis for excessive mean head motion > 0.35 mm, while lower than the 0.5 mm previously reported,26,60,62 was selected to ensure minimal influence of head motion on the brain activation profile (Table 1, head motion for all participants).
Table 1.
Absolute and relative head motion for all participants.
| Participant | Session 1 Absolute Head Motion (mm) | Session 1 Relative Head Motion (mm) | Session 2 Absolute Head Motion (mm) | Session 2 Relative Head Motion (mm) |
|---|---|---|---|---|
| Participant_01* | 0.6 | 0.1 | 0.26 | 0.06 |
| Participant_02 | 0.24 | 0.06 | 0.22 | 0.05 |
| Participant_03 | 0.14 | 0.04 | 0.21 | 0.05 |
| Participant_04 | 0.22 | 0.09 | 0.26 | 0.09 |
| Participant_05 | 0.21 | 0.07 | 0.21 | 0.06 |
| Participant_06* | 0.46 | 0.09 | 0.16 | 0.05 |
| Participant_07 | 0.29 | 0.08 | 0.28 | 0.06 |
| Participant_08 | 0.28 | 0.09 | 0.25 | 0.1 |
| Participant_09 | 0.24 | 0.09 | 0.22 | 0.06 |
| Participant_10* | 0.8 | 0.15 | 0.4 | 0.11 |
| Participant_11* | 0.39 | 0.08 | 0.27 | 0.07 |
| Participant_12* | 0.52 | 0.12 | 0.24 | 0.07 |
| Participant_13 | 0.31 | 0.11 | 0.23 | 0.08 |
| Average ± Standard Deviation for entire group | 0.34±0.19 | 0.09±0.03 | 0.25±0.06 | 0.07±0.02 |
| Average ± Standard Deviation for those analyzed | 0.24±0.05 | 0.08 ± 0.02 | 0.24±0.03 | 0.07±0.02 |
dropped for excessive head motion beyond a priori established 0.35 mm
Changes in Brain Activity between Sessions
At the whole-brain level there was no difference in the pair-wise statistical parametric mapping analysis between session 1 and session 2 nor were there differences when masked for the 10 sensorimotor ROIs. Regarding data extracted from each ROI, no differences in mean percent signal change were found for any ROI between session 1 and 2. Only the left cerebellum showed significantly decreased mean region z-score from session 1 to 2, with no other z-score differences.
Paradigm Brain Activation
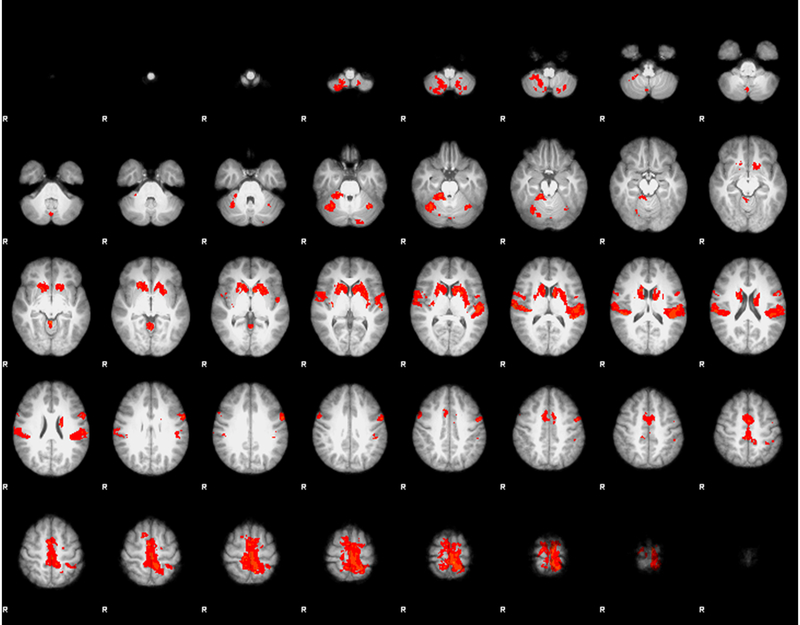
Our leg press task was successful in inducing activation in all ten sensorimotor ROIs investigated including the cerebellum, basal ganglia, the right and left motor cortex, the pre-motor region, and the primary somatosensory and secondary somatosensory cortices (Figure 2, Table 2). Voxel total is the number of voxels in the anatomical region mask, active voxels are from the one sample t-test and represent the group-wise average number of voxels active during the task within the respective anatomical mask. The X, Y, Z location is for the group-wise peak voxel within the respective region.
Figure 2.
Z-statistic activation map for the average combined session 1 and session 2 for the unilateral right leg press within the 10 a priori selected sensorimotor regions of interest. No differences in brain activity were found between session 1 and 2.
Table 2.
Sensorimotor region activity metrics during the right leg press.
| Region of interest | Voxels Total | Active Voxels | Mean % signal change (standard deviation) | Peak % Signal Change | Mean Z-score (standard deviation) | Peak Z-score | MNI Standard Space | ||
|---|---|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||||
| Right Motor Cortex | 5506 | 1030 | 0.25 (0.70) | 3.65 | 0.61 (2.63) | 6.06 | 2 | −18 | 76 |
| Left Motor Cortex | 6297 | 2212 | 0.77 (1.31) | 7.01 | 1.50 (2.75) | 9.78 | −6 | −46 | 80 |
| Right Pre−Motor Cortex | 8732 | 1586 | 0.35 (0.70) | 4.56 | 1.21 (2.36) | 6.06 | 2 | −8 | 74 |
| Left Pre−Motor Cortex | 8592 | 2420 | 0.74 (1.15) | 8.65 | 1.96 (2.13) | 9.78 | −8 | −12 | 80 |
| Right Primary Somatosensory Cortex | 6997 | 641 | 0.12 (0.53) | 3.10 | 0.27(2.30) | 6.05 | 8 | −50 | 80 |
| Left Primary Somatosensory Cortex | 8103 | 1985 | 0.45 (0.98) | 7.01 | 1.28 (2.55) | 9.78 | −6 | −46 | 80 |
| Right Secondary Somatosensory Cortex | 3555 | 1276 | 0.54 (0.55) | 2.98 | 2.26 (2.04) | 7.42 | 62 | 8 | 2 |
| Left Secondary Somatosensory Cortex | 3661 | 1638 | 0.64 (0.59) | 2.95 | 2.73 (1.92) | 6.13 | −66 | −20 | 16 |
| Right Cerebellum | 10278 | 1540 | 0.52 (0.58) | 4.34 | 1.67 (1.57) | 7.75 | 32 | −50 | −58 |
| Left Cerebellum | 8790 | 415 | 0.29 (0.41) | 2.85 | 0.99 (1.30) | 6.81 | −12 | −60 | −62 |
| Right Basal Ganglia | 2656 | 1147 | 0.37 (0.25) | 1.44 | 2.88 (1.44) | 5.92 | 12 | 22 | −10 |
| Left Basal Ganglia | 2680 | 1485 | 0.44 (0.23) | 1.50 | 3.26 (1.36) | 5.80 | −10 | −4 | 16 |
Data presented below is the combined session group average as no differences were found between sessions.
MNI – Montreal Neurological Institute
Reliability of Brain Activity
The between-session ICCs for mean percent signal change in the ROI analysis were in the range between good to excellent (.621-.918) except for the left secondary somatosensory area which was poor (.165; Table 3). The ICC analyses for mean region z-score were all good to excellent (all > 0.697; Table 4).
Table 3:
Intraclass Correlation Coefficient for % Signal Change between sessions for each sensorimotor region of interest
| Region of Interest | Mean (standard deviation) Session 1 | Mean (standard deviation) Session 2 |
p-value for difference | Intraclass Correlation Coefficient | Intraclass Correlation Coefficient p-value |
|---|---|---|---|---|---|
| Right Motor Cortex | 0.25 (0.52) | 0.23 (0.43) | .858 | .834 | .020 |
| Left Motor Cortex | 0.76 (0.48) | 0.76 (0.41) | .989 | .900 | .005 |
| Right Pre-Motor Cortex | 0.41 (0.43) | 0.43 (0.40) | .891 | .811 | .028 |
| Left Pre-Motor Cortex | 0.87 (0.56) | 0.84 (0.36) | .820 | .900 | .005 |
| Right Primary Somatosensory Cortex | 0.18 (0.34) | 0.09 (0.30) | .800 | .879 | .008 |
| Left Primary Somatosensory Cortex | 0.46 (0.36) | 0.39 (0.30) | .412 | .878 | .007 |
| Right Secondary Somatosensory Cortex | 0.66 (0.31) | 0.52 (0.18) | .156 | .621 | .088 |
| Left Secondary Somatosensory Cortex | 0.70 (0.34) | 0.68 (0.17) | .896 | .165 | .419 |
| Right Cerebellum | 0.76 (0.57) | 0.44 (0.50) | .114 | 0.772 | .033 |
| Left Cerebellum | 0.81 (0.72) | 0.32 (0.78) | .083 | 0.662 | .052 |
| Right Basal Ganglia | 0.36 (0.30) | 0.43 (0.22) | .167 | .918 | .001 |
| Left Basal Ganglia | 0.41 (0.39) | 0.51 (0.24) | .315 | .772 | .033 |
Table 4:
Intraclass Correlation Coefficient for Z-stat between sessions of each region of interest
| Region of Interest | Mean (standard deviation) Session 1 | Mean (standard deviation) Session 2 |
p-value for difference | Intraclass Correlation Coefficient | Intraclass Correlation Coefficient p-value |
|---|---|---|---|---|---|
| Right Motor Cortex | 0.31 (0.80) | 0.42 (1.01) | .676 | .831 | .020 |
| Left Motor Cortex | 1.25 (0.76) | 1.47 (1.08) | .381 | .856 | .011 |
| Right Pre-Motor Cortex | 0.60 (0.79) | 0.94 (0.95) | .239 | .775 | .029 |
| Left Pre-Motor Cortex | 1.34 (0.80) | 1.70 (0.89) | .085 | .867 | .004 |
| Right Primary Somatosensory Cortex | 0.04 (0.60) | 0.14 (0.77) | .590 | .871 | .009 |
| Left Primary Somatosensory Cortex | 0.82 (0.62) | 0.94 (0.88) | .547 | .883 | .007 |
| Right Secondary Somatosensory Cortex | 1.35 (0.71) | 1.15 (0.53) | .343 | .776 | .033 |
| Left Secondary Somatosensory Cortex | 1.46 (0.70) | 1.70(0.69) | .302 | .774 | .032 |
| Right Cerebellum | 0.91 (0.78) | 0.53 (0.60) | .138 | .697 | .049 |
| Left Cerebellum | 0.81 (0.93) | 0.33 (0.76) | .016 | .864 | .001 |
| Right Basal Ganglia | 0.94 (0.70) | 1.02 (0.57) | .619 | .870 | .010 |
| Left Basal Ganglia | 1.00 (0.79) | 1.27 (0.66) | .203 | .810 | .017 |
Discussion
The purpose of this study was to present a novel method to assay brain activation during multi-joint lower extremity force production and attenuation while assessing the reliability of a loaded leg press across two-time points using fMRI. Our task was successful in inducing sensorimotor region activity to a similar degree as prior reports with lower extremity motor tasks.60,62 The whole brain paired contrast revealed no differences in activation between sessions, and our ICCs indicated good to excellent reliability for the majority of investigated ROIs. Collectively, these results indicate our task is a reliable fMRI assay of neural function for lower extremity motor control.
Our reliability findings are in line with previous reports for similar motor paradigms of the lower extremity.26,60 A previous isometric set-up achieved ICCs ranging from 0.29 to 0.74 for the contralateral sensorimotor cortex and 0.38 to 0.83 for the contralateral pre-motor cortex for low force knee extension contractions.26 Our results were comparable with a 0.900 ICC for percent signal change in the contralateral motor and pre-motor cortices and 0.856 for motor cortex mean z-score and .867 for pre-motor region z-score. We attribute the improved ICC for our unilateral leg press to the participant familiarization session and differences in patient restraint and apparatus differences. Prior works using an isometric force generation task may have induced accessory head translation as the foot was fixed, but the force being generated may translate the body slightly, whereas in our task the foot moved on tracks and thus force production by the lower extremity musculature translated into hip and knee motion.26 A comparable reliability study examining a stepping-like action machine found comparable ICCs with the present findings across the cerebellum, motor cortex, sensory cortex, and motor planning regions (0.70–0.85).60 Our results differed regarding the secondary somatosensory region, specifically the left side for percent signal change having low reliability, whereas the previous investigation reported an ICC of .85 in the secondary somatosensory area between sessions.60 One key difference is our paradigm was completed unilaterally compared to the bilateral active stepping motor paradigm engaging both legs reported by Jaeger et al.24,60 The bilateral task may induce more reliable secondary sensory region activity bilaterally and facilitate a more uniform sensory experience between legs relative to the unilateral movement reported herein. Also, our participants completed task familiarization before each session, thus the overall novelty and attention during the actual scanning session may influence brain activity compared to prior studies that did not have familiarization sessions.63
Our results indicating high reliability for measuring neural activation for a loaded leg press have important implications for a variety of clinical populations. Leg press related exercises have been shown to improve healing after knee ligament injury and reconstruction surgery,64 reduce pain and increase quadriceps strength in osteoarthritis,65 and reduce pain in patellofemoral pain syndrome.66 Thus, a reliable neuroimaging leg press paradigm could provide a neural assay to examine the neural mechanisms contributing to improved motor control or identify barriers in nonresponses for clinical populations with lower extremity musculoskeletal disorders. Further, such a paradigm can identify possible neural activity contributing to dysfunctional movement in those with injury.67 As prior investigations have reported altered motor cortex excitability with transcranial magnetic stimulation and brain activation patterns for engaging the quadriceps muscle after knee joint injury,68 this multi-joint leg press motion may provide further insights into the neuroplasticity for motor control related to joint injuries. Also, the good to high reliability provides a foundation for studying changes in neural function related to neuromuscular training and rehabilitation, providing neurophysiologic therapeutic targets in addition to the current standard of muscle or functional targets. For instance, decreased motor cortex activity during a loaded leg press exercise was correlated with improved landing mechanics measured during a virtual reality soccer task following real-time biofeedback training.69 The reported changes in motor cortex activity can be safely attributed to the training, as opposed to limitations related to task reliability, especially as the contralateral motor cortex, in this case, had an excellent ICC of .9 for signal change and .86 for z-score between sessions.
While there were no condition differences in the group-wise statistical parametric mapping analysis, the left cerebellum approached significance for percent signal change and was significant for mean z-score in the ROI analysis. We attribute this difference to the nature of the cerebellar ROI analysis encompassing the entire left cerebellum and potentially being contralateral to the movement and thus not the primary cerebellar structure involved as the right cerebellum was less variable, having no pairwise difference. Prior work also found the cerebellum to be less consistent across sessions,60 which found cerebellar activity to be the least reliable with ICCS ranging from 0.53–0.77, with our ICCs comparable or better despite the pair-wise difference. Thus, due to the varied structural and functional nature of the cerebellum, a sub-region may have higher reliability. To that end we ran a pairwise difference analysis between session 1 and session 2 on only the cerebellar regions found to be active during session 1 as opposed to the region as a whole and found no difference in z-score between sessions: session 1: 0.61 (.63); session 2: 0.55 (.41), p = 0.70. Therefore it is possible that the difference found above is due to including the entire left cerebellum, rather than more specific cerebellar ROIs.
The amount of head movement during the leg press task was measured to be 0.24 mm of absolute motion and 0.07 mm of relative motion, indicating that the majority of the brain stayed within the original voxel space throughout the task. In addition, 5 subjects (38%) had to be excluded from the study, which is lower than the 50% exclusion rate found when assessing the reliability of a fMRI passive stepping task.60 This is likely due to the methods utilized to prevent head motion, including using physical restraints (e.g. straps), making the subject comfortable (e.g. lumbar support), and having familiarization sessions prior to the fMRI where the researcher strongly emphasized the importance of keeping the head stationary for the duration of the study in addition to task familiarization. Importantly, the amount of head motion elicited during our gross motor multi-joint movement is comparable to the amount of head motion observed during fine motor single joint movements in which physical restraints, physical comfort, and familiarization sessions are either not used, or less emphasized.
Beyond the novelty of the methods, a strength of this investigation was the repeated measurements being separated by a 7–8 week control period, providing implications for longer-term intervention measurement reliability. The ICCs evaluated in the proposed fMRI metrics were high and associated with consistent ROIs neural activity corresponding to this functional lower extremity movement. The stability of the measured sensorimotor outcomes over an extended period further enhances the clinical validity of the measurements beyond analytical validity commonly evaluated in reliability studies (e.g. within session or day to day reliability). Cumulatively the current data indicate that the proposed methods and metrics are viable outcomes to assess in longitudinal studies of injury risk and the potential effects of therapeutic interventions that aim to optimize movement efficiency and safety.
A limitation of this study was that the leg muscle activation and movement kinematics were not quantified simultaneously. While it would have been beneficial to correlate the brain activation pattern with muscle activation or temporal-spatial leg position, the task was auditory paced and set in tracks with blocks to ensure equivalent timing and range of motion across participants, thereby standardizing kinematic performance. However, these methods providing reliable assessment over time creates the opportunity to merge our MRI-safe leg press task with measurements of leg muscular activity and or a motion capture system.70 A second limitation was that we completed this study utilizing a homogeneous population of female adolescent athletes, which limits our generalizability to other populations but does minimize confounding factors of gender and age. This decision to examine female adolescents specifically was done as knee joint injuries such as ACL rupture occur at a much greater rate in females71–73 and is potentially due to unique neural activity related to knee motor control,74 supporting the need to understand knee motor related neural activity in females. Nevertheless, testing the reproducibility of this task in other populations is warranted. It is also possible that task-related hand movements may have contributed to the reported neural activity, as participants were instructed to keep their hands on the handles for stability. Our ROI analysis did include the medial and lateral primary and secondary sensory and motor cortices, and as the ICCs were still high any participant-level variability in any task-related hand motion did not seem to impact the neural activity reliability. Despite these limitations, this study demonstrates the feasibility in measuring brain activation patterns during a loaded leg press exercise with mostly good to excellent reliability, indicating the utility of the task as a neuroimaging assay for lower extremity motor control. Future investigations should consider refinements to this paradigm with event-related designs and kinematic or kinetic recordings during scanning.
In conclusion, a loaded multi-joint unilateral leg press action that simulates the joint ranges of motion for squatting, stepping, stair climbing and landing from a jump is a reliable motor neuroimaging paradigm over a 7–8 week period.
Acknowledgements & Disclosures
This work was funded in part by the National Institute of Arthritis, Musculoskeletal & Skin NIAMS 1U01AR067997
Authors have no other disclosures
The authors would like to thank the following from Seton High School: Ron Quinn, Lisa Larosa, Holly Laiveling, and the entire soccer coaching staff as well as the Seton administration and athletic director Wendy Smith; from Madeira High School soccer head coach Dan Brady, athletic director Joe Kimling, and principal David Kennedy for their support and assistance to conduct this study. Thank you to the soccer parents and players for participating and support the efforts to complete the project. We appreciate their patience with the testing, scheduling, and follow-up testing. Their enthusiastic support made this study possible. Special acknowledgement goes to the Athletic Trainers at Seton High School, Cindy Busse and Madeira High School, Glenna Knapp. Without their time, commitment, and passion for the health and well-being of their student athletes, this study would not have been possible.
References
- 1.Garg A, Xu D, Laurin A, Blaber AP. Physiological interdependence of the cardiovascular and postural control systems under orthostatic stress. Am J Physiol Heart Circ Physiol 2014;307:H259–H64. [DOI] [PubMed] [Google Scholar]
- 2.Abrahams VC. The physiology of neck muscles; their role in head movement and maintenance of posture. Can J Physiol Pharmacol 1977;55:332–8. [DOI] [PubMed] [Google Scholar]
- 3.Judge JO, Ounpuu S, Davis RB 3rd. Effects of age on the biomechanics and physiology of gait. Clin Geriatr Med 1996;12:659–78. [PubMed] [Google Scholar]
- 4.Slocum DB, James SL. Biomechanics of running. JAMA 1968;205:721–8. [PubMed] [Google Scholar]
- 5.Snyder KR, Earl JE, O’Connor KM, Ebersole KT. Resistance training is accompanied by increases in hip strength and changes in lower extremity biomechanics during running. Clin Biomech (Bristol, Avon) 2009;24:26–34. [DOI] [PubMed] [Google Scholar]
- 6.Winter DA. Human balance and posture control during standing and walking. Gait Posture 1995;3:193–214. [Google Scholar]
- 7.Winter DA. Biomechanics and motor control of human movement. Hoboken, NJ: John Wiley & Sons;2009:1–370. [Google Scholar]
- 8.Hof A Muscle mechanics and neuromuscular control. J Biomech 2003;36:1031–8. [DOI] [PubMed] [Google Scholar]
- 9.Shemmell J, Tresilian JR, Riek S, Barry BK, Carson RG. Neuromuscular adaptation during skill acquisition on a two degree-of-freedom target-acquisition task: dynamic movement. J Neurophysiol 2005;94:3058–68. [DOI] [PubMed] [Google Scholar]
- 10.Oliveira AS, Schlink BR, Hairston WD, Konig P, Ferris DP. A channel rejection method for attenuating motion-related artifacts in EEG recordings during walking. Front Neurosci 2017;11:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oliveira AS, Schlink BR, Hairston WD, Konig P, Ferris DP. Proposing metrics for benchmarking novel EEG technologies towards real-world measurements. Front Hum Neurosci 2016;10:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gwin JT, Ferris DP. An EEG-based study of discrete isometric and isotonic human lower limb muscle contractions. J Neuroeng Rehabil 2012;9:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gwin JT, Ferris D. High-density EEG and independent component analysis mixture models distinguish knee contractions from ankle contractions. Conf Proc IEEE Eng Med Biol Soc 2011;2011:4195–8. [DOI] [PubMed] [Google Scholar]
- 14.Fukuyama H, Ouchi Y, Matsuzaki S, et al. Brain functional activity during gait in normal subjects: a SPECT study. Neurosci Lett 1997;228:183–6. [DOI] [PubMed] [Google Scholar]
- 15.Greenstein J, Gastineau E, Siegel B, Macsata R, Conklin J, Maurer A. Cerebral hemisphere activation during human bipedal locomotion. Hum Brain Mapp 1995;3:320. [Google Scholar]
- 16.Christensen LO, Johannsen P, Sinkjær T, Petersen N, Pyndt H, Nielsen JB. Cerebral activation during bicycle movements in man. Exp Brain Res 2000;135:66–72. [DOI] [PubMed] [Google Scholar]
- 17.Beurskens R, Helmich I, Rein R, Bock O. Age-related changes in prefrontal activity during walking in dual-task situations: a fNIRS study. Int J Psychophysiol 2014;92:122–8. [DOI] [PubMed] [Google Scholar]
- 18.Holtzer R, Mahoney JR, Izzetoglu M, Izzetoglu K, Onaral B, Verghese J. fNIRS study of walking and walking while talking in young and old individuals. J Gerontol A Biol Sci Med Sci 2011;66:879–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Makizako H, Shimada H, Park H, Tsutsumimoto K, Uemura K, Suzuki T. Brain activation during dual-task walking and executive function among older adults with mild cognitive impairment: a fNIRS study. Aging Clin Exp Res 2013;25:539–44. [DOI] [PubMed] [Google Scholar]
- 20.Iranpour J, Morrot G, Claise B, Jean B, Bonny JM. Using high spatial resolution to improve BOLD fMRI detection at 3T. PLoS One 2015;10:e0141358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stelzer J, Buschmann T, Lohmann G, Margulies DS, Trampel R, Turner R. Prioritizing spatial accuracy in high-resolution fMRI data using multivariate feature weight mapping. Front Neurosci 2014;8:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fellner C, Doenitz C, Finkenzeller T, Jung E, Rennert J, Schlaier J. Improving the spatial accuracy in functional magnetic resonance imaging (fMRI) based on the blood oxygenation level dependent (BOLD) effect: benefits from parallel imaging and a 32-channel head array coil at 1.5 tesla. Clin Hemorheol Microcirc 2009;43:71–82. [DOI] [PubMed] [Google Scholar]
- 23.Glover GH. Overview of functional magnetic resonance imaging. Neurosurgery Clinics 2011;22:133–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jaeger L, Marchal-Crespo L, Wolf P, Riener R, Michels L, Kollias S. Brain activation associated with active and passive lower limb stepping. Front Hum Neurosci 2014;8:828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miletello WM, Beam JR, Cooper ZC. A biomechanical analysis of the squat between competitive collegiate, competitive high school, and novice powerlifters. J Strength Cond Res 2009;23:1611–7. [DOI] [PubMed] [Google Scholar]
- 26.Newton JM, Dong Y, Hidler J, et al. Reliable assessment of lower limb motor representations with fMRI: use of a novel MR compatible device for real-time monitoring of ankle, knee and hip torques. Neuroimage 2008;43:136–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hajnal JV, Myers R, Oatridge A, Schwieso JE, Young IR, Bydder GM. Artifacts due to stimulus correlated motion in functional imaging of the brain. Magn Reson Med 1994;31:283–91. [DOI] [PubMed] [Google Scholar]
- 28.Freire L, Roche A, Mangin J-F. What is the best similarity measure for motion correction in fMRI time series? IEEE Trans Med Imaging 2002;21:470–84. [DOI] [PubMed] [Google Scholar]
- 29.Friston KJ, Williams S, Howard R, Frackowiak RSJ, Turner R. Movement-related effects in fMRI time-series. Magn Reson Med 1996;35:346–55. [DOI] [PubMed] [Google Scholar]
- 30.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 2002;17:825–41. [DOI] [PubMed] [Google Scholar]
- 31.Pruim RH, Mennes M, Buitelaar JK, Beckmann CF. valuation of ICA-AROMA and alternative strategies for motion artifact removal in resting state fMRI. Neuroimage 2015;112:278–87. [DOI] [PubMed] [Google Scholar]
- 32.Bullmore E, Brammer M, Rabe-Hesketh S, et al. Methods for diagnosis and treatment of stimulus-correlated motion in generic brain activation studies using fMRI. Hum Brain Mapp 1999;7:38–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fontes EB, Okano AH, De Guio F, et al. Brain activity and perceived exertion during cycling exercise: an fMRI study. Br J Sports Med 2015;49:556–60 [DOI] [PubMed] [Google Scholar]
- 34.Mehta JP, Verber MD, Wieser JA, Schmit BD, Schindler-Ivens SM. The effect of movement rate and complexity on functional magnetic resonance signal change during pedaling. Motor Control 2012;16:158–75. [DOI] [PubMed] [Google Scholar]
- 35.Dobkin BH. Rehabilitation and functional neuroimaging dose-response trajectories for clinical trials. Neurorehabil Neural Repair 2005;19:276–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grooms DR, Page SJ, Nichols-Larsen DS, Chaudhari AM, White SE, Onate JA. Neuroplasticity associated with anterior cruciate ligament reconstruction. J Orthop Sports Phys Ther 2017;47:180–9. [DOI] [PubMed] [Google Scholar]
- 37.Grooms DR, Page SJ, Onate JA. Brain activation for knee movement measured days before second anterior cruciate ligament injury: Neuroimaging in musculoskeletal medicine. J Athl Train 2015;50:1005–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kapreli E, Athanasopoulos S, Papathanasiou M, et al. Lower limb sensorimotor network: issues of somatotopy and overlap. Cortex 2007;43:219–32. [DOI] [PubMed] [Google Scholar]
- 39.Hollnagel C, Brügger M, Vallery H, et al. Brain activity during stepping: a novel MRI-compatible device. J Neurosci Methods 2011;201:124–30. [DOI] [PubMed] [Google Scholar]
- 40.Mehta JP, Verber MD, Wieser JA, Schmit BD, Schindler-Ivens SM. A novel technique for examining human brain activity associated with pedaling using fMRI. J Neurosci Methods 2009;179:230–9. [DOI] [PubMed] [Google Scholar]
- 41.Imwalle LE, Myer GD, Ford KR, Hewett TE. Relationship between hip and knee kinematics in athletic women during cutting maneuvers: a possible link to noncontact anterior cruciate ligament injury and prevention. J Strength Cond Res 2009;23:2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schmitz RJ, Cone JC, Tritsch AJ, et al. Changes in drop-jump landing biomechanics during prolonged intermittent exercise. Sports health 2014;6:128–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shultz SJ, Nguyen A-D, Leonard MD, Schmitz RJ. Thigh strength and activation as predictors of knee biomechanics during a drop jump task. Med Sci Sports Exerc 2009;41:857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Escamilla RF. Knee biomechanics of the dynamic squat exercise. Med Sci Sports Exerc 2001;33127–41. [DOI] [PubMed] [Google Scholar]
- 45.Swanson SC, Caldwell GE. An integrated biomechanical analysis of high speed incline and level treadmill running. Med Sci Sports Exerc 2000;32:1146–55. [DOI] [PubMed] [Google Scholar]
- 46.Mann RA, Hagy J. Biomechanics of walking, running, and sprinting. Am J Sports Med 1980;8:345–50. [DOI] [PubMed] [Google Scholar]
- 47.Paoli A, Gentil P, Moro T, Marcolin G, Bianco A. Resistance training with single vs. multi-joint exercises at equal total load volume: Effects on body composition, cardiorespiratory fitness, and muscle strength. Front Physiol 2017;8:1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wirth K, Hartmann H, Sander A, Mickel C, Szilvas E, Keiner M. The impact of back squat and leg-press exercises on maximal strength and speed-strength parameters. The J Strength Cond Res 2016;30:1205–12. [DOI] [PubMed] [Google Scholar]
- 49.Myer GD, Ford KR, Palumbo OP, Hewett TE. Neuromuscular training improves performance and lower-extremity biomechanics in female athletes. J Strength Cond Res 2005;19:51–60. [DOI] [PubMed] [Google Scholar]
- 50.Myer GD, Ford KR, Brent JL, Hewett TE. Differential neuromuscular training effects on ACL injury risk factors in”high-risk” versus “low-risk” athletes. BMC Musculoskelet Disord 2007;8:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nagata A, Doma K, Yamashita D, Hasegawa H, Mori S. The effect of augmented feedback type and frequency on velocity-based training-induced adaptation and retention. J Strength Cond Res 2018. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 52.Welling W, Benjaminse A, Gokeler A, Otten B. Enhanced retention of drop vertical jump landing technique: A randomized controlled trial. Hum Mov Sci 2016;45:84–95. [DOI] [PubMed] [Google Scholar]
- 53.Kushner AM, Brent JL, Schoenfeld BJ, et al. The back squat part 2: Targeted training techniques to correct functional deficits and technical factors that limit performance. Strength Cond J 2015;37:13–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Myer GD, Kushner AM, Brent JL, et al. The back squat: A proposed assessment of functional deficits and technical factors that limit performance. Strength Cond J 2014;36:4–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Delahunt E, Sweeney L, Chawke M, et al. Lower limb kinematic alterations during drop vertical jumps in female athletes who have undergone anterior cruciate ligament reconstruction. J Orthop Res 2012;30:72–8. [DOI] [PubMed] [Google Scholar]
- 56.Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004;23:S208–S19. [DOI] [PubMed] [Google Scholar]
- 57.Beckmann CF, Jenkinson M, Smith SM. General multilevel linear modeling for group analysis in FMRI. Neuroimage 2003;20:1052–63. [DOI] [PubMed] [Google Scholar]
- 58.Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage 2001;14:1370–86. [DOI] [PubMed] [Google Scholar]
- 59.Woolrich M Robust group analysis using outlier inference. Neuroimage 2008;41:286–301. [DOI] [PubMed] [Google Scholar]
- 60.Jaeger L, Marchal-Crespo L, Wolf P, Riener R, Kollias S, Michels L. Test-retest reliability of fMRI experiments during robot-assisted active and passive stepping. J Neuroeng Rehabil 2015;12:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cicchetti DV. The precision of reliability and validity estimates re-visited: distinguishing between clinical and statistical significance of sample size requirements. J Clin Exp Neuropsychol 2001;23:695–700. [DOI] [PubMed] [Google Scholar]
- 62.Luft AR, Smith GV, Forrester L, et al. Comparing brain activation associated with isolated upper and lower limb movement across corresponding joints. Human Brain Mapp 2002;17:131–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Loubinoux I, Carel C, Alary F, et al. Within-session and between-session reproducibility of cerebral sensorimotor activation: a test--retest effect evidenced with functional magnetic resonance imaging. J Cereb Blood Flow Metab 2001;21:592–607. [DOI] [PubMed] [Google Scholar]
- 64.Jewiss D, Ostman C, Smart N. Open versus closed kinetic chain exercises following an anterior cruciate ligament reconstruction: A systematic review and meta-analysis. J Sports Med (Hindawi Publ Corp) 2017;2017:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Olagbegi OM, Adegoke BO, Odole AC. Effectiveness of three modes of kinetic-chain exercises on quadriceps muscle strength and thigh girth among individuals with knee osteoarthritis. Arch Physiother 2017;7:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Witvrouw E, Lysens R, Bellemans J, Peers K, Vanderstraeten G. Open versus closed kinetic chain exercises for patellofemoral pain. A prospective, randomized study. Am J Sports Med 2000;28:687–94. [DOI] [PubMed] [Google Scholar]
- 67.Silfies SP, Vendemia JMC, Beattie PF, Stewart JC, Jordon M. Changes in brain structure and activation may augment abnormal movement patterns: An emerging challenge in musculoskeletal rehabilitation. Pain Med 2017;18:2051–4. [DOI] [PubMed] [Google Scholar]
- 68.Lepley AS, Grooms DR, Burland JP, Davi SM, Kinsella-Shaw JM, Lepley LK. Quadriceps muscle function following anterior cruciate ligament reconstruction: systemic differences in neural and morphological characteristics. Exp Brain Res 2019:1–12. [DOI] [PubMed] [Google Scholar]
- 69.Grooms DR, Kiefer AW, Riley MA, et al. Brain-behavior mechanisms for the transfer of neuromuscular training adaptions to simulated sport: Initial findings from the train the brain project. J Sport Rehabil 2018;27:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Casellato C, Ferrante S, Gandolla M, et al. Simultaneous measurements of kinematics and fMRI: compatibility assessment and case report on recovery evaluation of one stroke patient. J Neuroeng Rehabil 2010;7:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Agel J, Arendt EA, Bershadsky B. Anterior cruciate ligament injury in national collegiate athletic association basketball and soccer. Am J Sports Med 2005;33:524–31. [DOI] [PubMed] [Google Scholar]
- 72.Joseph AM, Collins CL, Henke NM, Yard EE, Fields SK, Comstock RD. A multisport epidemiologic comparison of anterior cruciate ligament injuries in high school athletics. J Athl Train 2013;48:810–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Malone T, Hardaker W. Garrett WE, Feagin JA, Bassett FH. Relationship of gender to anterior cruciate ligament injuries in intercollegiate basketball players. J Southern Orthop Assoc 1993;2:36–9. [Google Scholar]
- 74.Diekfuss JA, Grooms DR, Yuan W, et al. Does brain functional connectivity contribute to musculoskeletal injury? A preliminary prospective analysis of a neural biomarker of ACL injury risk. J Sci Med Sport 2019;22:169–74. [DOI] [PMC free article] [PubMed] [Google Scholar]