Abstract
The striatum mediates habit formation and reward association. The striatum can be divided into the patch and matrix compartment, which are two distinct regions that sub-serve different aspects of behavior. The patch compartment may mediate reward-related behaviors, while the matrix compartment may mediate adaptive motor functions. Previous studies indicate that enhanced relative activation of the patch versus matrix compartment is associated with inflexible behaviors, such as stereotypy. Habitual behaviors are also inflexible in nature, but whether enhanced activation of the patch compartment contributes to habitual behavior is not known. The goal of the current study was to examine the role of patch compartment in the development of habit formation. We used dermorphin-saporin to ablate neurons of the patch compartment in the dorsolateral striatum prior to training animals to self-administer sucrose on a random interval schedule of reinforcement. Our data showed that patch compartment lesions in the dorsolateral striatum reduced the reinstatement of sucrose self-administration after sucrose devaluation, indicating that destruction of this region prevented the development of habitual behavior. Additionally, in animals with patch compartment lesions in the DLS that did not develop habitual behavior, activation of the dorsolateral striatum and sensorimotor cortex was diminished, while activity in the dorsomedial striatum and prefrontal cortex was increased, suggesting less engagement of regions that mediate habitual behaviors and heightened engagement of regions that mediate goal-directed behaviors occurs with reduced habit formation. These data indicate that the dorsolateral patch compartment may mediate habit formation by altering information flow through basal ganglia circuits.
Keywords: Basal ganglia, cortex, immediate early gene, self-administration, sucrose, striosome
Introduction
An organism’s ability to exert control over their environment is dependent upon instrumental conditioning, where a relationship is detected between specific actions and consequential events (Belin et al., 2009). Experimentally, an instrumental conditioning paradigm involves the arrangement of a contingency between the animal’s behavior and a consequent outcome, such that the animal learns that the occurrence of a reinforcer is controlled by a specific instrumental behavior (Belin et al., 2009). Extensive research has revealed that instrumental behaviors are controlled by two distinct systems of learning that are engaged in different settings: the action-outcome (A-O) system and the stimulus-response (S-R) system. When learning is mediated by the A-O system, performance is goal-directed and dependent upon the association between the action and the consequent outcome (Balleine and Dickinson, 1998; Yin et al., 2005). Since A-O behaviors are controlled by the anticipation of the outcome, this type of instrumental learning is sensitive to the devaluation of that outcome (Balleine and Dickinson, 1998; Yin and Knowlton, 2006; Yin et al., 2005). On the other hand, learning that is mediated by the S-R system relies upon an association between a stimulus and a subsequent response, whereby the behavior occurs automatically in response to sensory inputs with which the action has become associated (Dickinson, 1985; Faure et al., 2005; Yin and Knowlton, 2006; Yin et al., 2004, 2006). Because S-R actions are not driven by the anticipation of the outcome and are automatic in nature, instrumental behaviors that are the result of S-R learning are insensitive to outcome devaluation, and are considered to be habitual in nature (Balleine et al., 2009; Dickinson, 1985; Yin and Knowlton, 2006; Yin et al., 2004, 2006). Thus, once S-R learning is established, the behavior will be elicited by the stimulus, regardless of the value of the outcome and the flexibility of response selection will be kept at a minimum (Balleine et al., 2009; Dickinson, 1985; Yin and Knowlton, 2006; Yin et al., 2004, 2006).
The dorsal striatum plays a significant role in the expression of instrumental behaviors (Balleine et al., 2009; Grahn et al., 2009; Yin and Knowlton, 2006). Instrumental behaviors are mediated by circuits that originate in the cortex and traverse the striatum before returning back to the cortex, with specific instrumental behaviors being controlled by distinct systems. A-O behaviors are mediated by circuits involving the medial prefrontal cortex (mPFC) and the dorsomedial striatum (DMS), and S-R behaviors involving networks through the sensorimotor cortex (SMC) and the dorsolateral striatum (DLS; (Yin and Knowlton, 2006; Yin et al., 2004, 2006; Yin et al., 2005). It is thought that during the initial stages of instrumental learning, behaviors are goal-directed and are mediated by circuits involving the DMS and mPFC, but during the later stages of instrumental learning, circuits through the DLS and SMC dominate, and the behavior becomes habitual in nature (Yin and Knowlton, 2006; Yin et al., 2004, 2006).
It is thought that habitual behaviors may also be related to the enhanced activation of the patch (or striosome) compartment relative to the matrix compartment of the striatum, although this hypothesis has never been explored (Canales, 2005; Crittenden and Graybiel, 2011). The patch and matrix compartments are two functionally and anatomically distinct regions of the striatum that are superimposed upon the DMS and DLS. The neurons of the patch compartment, which contain a high density of mu opioid receptors, process information related to motivation and reward and receive dense inputs from the prelimbic cortex and amygdala (Bolam et al., 1988; Canales and Graybiel, 2000; Gerfen, 1989; McDonald, 1992; Pert et al., 1976). The neurons of the matrix compartment, which do not express mu opioid receptors, mediate adaptive behavioral responses to the environment and receive inputs from sensorimotor cortex (Bolam et al., 1988; Canales and Graybiel, 2000; Gerfen, 1984; Gerfen, 1989). Previous work shows that enhanced activation of the patch compartment relative to the matrix compartment specifically within the DLS is associated with repetitive and inflexible behaviors, such as psychostimulant-induced stereotypy (Canales and Graybiel, 2000; Horner et al., 2012; Horner et al., 2010). For example, destruction of the patch compartment in the DLS (which will also be referred to as the dorsolateral patch compartment) reduced both methamphetamine- and cocaine-induced stereotypy, by increasing the ability of animal to engage in other, non-repetitive behaviors, such as open-field exploration (Murray et al., 2014; Murray et al., 2015). Since stereotypy and habitual behaviors are both inflexible in nature, it is possible that the patch-based circuits within the DLS not only contribute to stereotypy, but also influence the development of habitual behaviors. Specifically, reward-related limbic information contained in the circuits of the patch compartment of the striatum could influence the sensorimotor networks in the DLS that are responsible for S-R learning.
The goal of the current study was to examine the role of the dorsolateral patch compartment in the formation of habitual behaviors by using a toxin that specifically targets mu-opioid receptor containing neurons (Lawhorn et al., 2009; Murray et al., 2014; Murray et al., 2015; Tokuno et al., 2002), to ablate the neurons of the patch compartment in the DLS prior to training rats to self-administer sucrose on random interval schedule of reinforcement, which has been shown to promote S-R behavior (Dickinson, 1985; Son et al., 2011; Yin et al., 2004, 2006). In order to determine whether removal of patch-based circuits in the DLS altered the flow of information through the basal ganglia and associated regions in the context of habit formation, we also examined changes in neuronal activity within S-R and A-O circuits.
Experimental Procedures
Drugs and chemicals
Ketamine hydrochloride and xylazine hydrochloride were obtained from Sigma Aldrich (St. Louis, MO, USA) and dilutions were based on the weight of the salt. All drugs were dissolved saline and delivered in a volume of 1 ml/kg body weight. Demorphin-saporin (DERM-SAP) and unconjugated saporin (SAP) were obtained from Advanced Targeting Systems (San Diego, CA, USA) and dissolved in buffered artificial cerebrospinal fluid (aCSF; 144 mM NaCl, 2.68 mM KCl, 1.6 mM CaCl2, 2.6 mM MgCl2, and 0.4 mM KH2PO4; pH, 7.2). Dermorphin is a potent mu opioid receptor agonist that induces internalization of mu opioid receptors upon binding (Giagnoni et al., 1984), while saporin is a ribosome-inactivating cytotoxin (Wiley and Kline, 2000). Thus, internalization of the DERM–SAP-mu opioid receptor complex will result in the destruction of the mu opioid receptor-containing neurons. The patch compartment of striatum is defined by the presence of mu opioid receptors and these neurons show dense signals for mu opioid receptor mRNA and protein, while the neurons of the matrix compartment do not (Arvidsson et al., 1995; Delfs et al., 1994; Herkenham and Pert, 1981; Mansour et al., 1995; Mansour et al., 1994; Minami et al., 1994). Therefore, treatment with DERM-SAP will selectively ablate the neurons of the patch compartment, while leaving the neurons of the matrix compartment intact.
Animals and intrastriatal infusions
Male Sprague-Dawley rats (Charles River Laboratories, Raleigh, NC, USA), weighing 250–350 g were used in all experiments. Rats were housed in plastic cages in a temperature-controlled room on a 14:10 h light/dark cycle with free access to food and water. All animal care and experimental manipulations were approved by the Institutional Animal Care and Use Committee of Mercer University School of Medicine and were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The minimum possible number of animals (based on power analyses) was used for our experiments and measures were taken to minimize any suffering that might occur during our procedures.
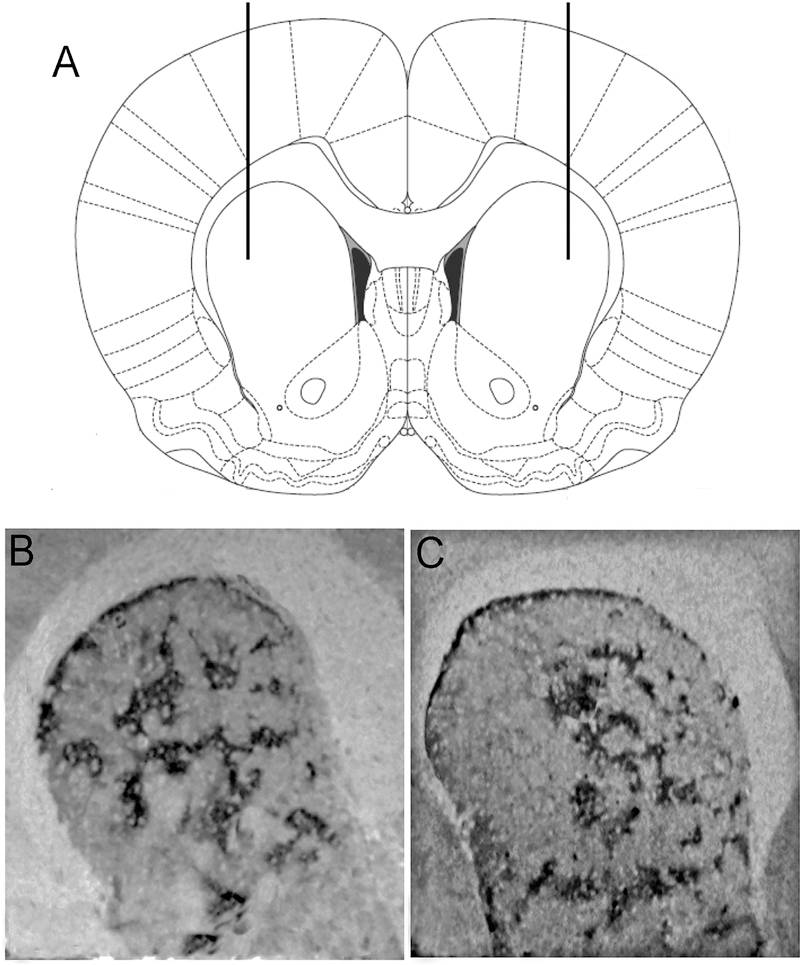
For intrastriatal infusions, animals (n=30) were fixed on a stereotaxic frame (Stoelting Company, Wood Dale, IL, USA) after being anesthetized with ketamine (90 mg/kg, i.p.) and xylazine (9 mg/kg, i.p.). A volume of 2 μl of DERM-SAP (17 ng/μl (Tokuno et al., 2002) or an equivalent amount of unconjugated SAP (as a control) was infused bilaterally, at a rate of 0.5 μl/min by inserting a 31-gauge needle into the DLS, at coordinates based on bregma (Paxinos and Watson, 2005): between +1.7 mm anterior, 3.0 mm lateral, −5.0 mm ventral through a burr hole drilled into the exposed skull (Figure 1A). After infusions were complete, the needles were left in place for one minute before being slowly removed to minimize fluid backflow. Animals were only included in subsequent analyses if during sectioning, their infusions were found to be located in the DLS. Treatment with DERM-SAP resulted in a loss of mu opioid receptor staining in the DLS (Figure 1B and 1C). Following the surgeries, animals were allowed to recuperate for 24–48 hours before being placed in individual home cages, where the animals then started eight days of food restriction (10–15 g chow per day) in order to reach 80–85% of their free-feeding weight.
Figure 1.
Representative images showing of striatum showing the effects of DERM-SAP infusion on mu opioid receptor immunoreactivity in the striatum. Rats were infused with the neurotoxin DERM-SAP (17 ng/ml) in the DLS (+1.7 mm AP, ±3.0 mm ML, −5.0 mm DV; A). Vehicle-infused rats maintained mu opioid receptor staining in the DLS (B), while DERM-SAP infused rats showed a decrease in mu opioid receptor staining in the DLS (C), indicating a loss of patch compartment neurons in this region.
Lever press training
Operant training and testing took place in standard operant conditioning chambers within light- and sound-attenuating boxes (Med Associates, Fairfax, VT). Each chamber had a retractable lever on the right side of a recessed magazine. Illumination was provided by a 3-W/24-V house light, which was mounted on the wall opposite the magazine. Upon lever pressing, a liquid dripper delivered 0.08 ml of a 20% sucrose solution into the magazine. A computer equipped with Med Apps software (Med Associates, Fairfax, VT) controlled the equipment and recorded lever presses.
Rats (SAP infused, n=14; DERM-SAP infused, n=14) first underwent two days of magazine training (twice a day for 30 minutes), where the sucrose solution was delivered on a random time, 60-sec schedule with no levers present, followed by two days of lever training (twice a day for 30 minutes), where the sucrose was delivered on a continuous reinforcement schedule (CRF).
The rats then began self-administration of the sucrose solution using a random interval (RI) training paradigm, which has been shown to promote habitual behavior (Dickinson, 1985; Yin et al., 2004). Sucrose was delivered on a RI-15 second, RI-30 second, and then a RI-60 second schedule of reinforcement, as previously described (Son et al., 2011; Yin et al., 2004). Each rat completed 2 days of training on each schedule, with 2 30-min training sessions per day.
Outcome devaluation and extinction test
One day after the last RI training session, sucrose was devalued with lithium chloride (LiCl) conditioned taste aversion (CTA). For 3 daily sessions, rats were allowed to consume the sucrose solution from a bottle in their home cage for 30 min, a followed by an injection of lithium chloride (LiCl, 0.15 M) or saline, as control. As a result, there were four different treatment groups (n=7, for each group): SAP/valued (saline), SAP/devalued (LiCl), DERM-SAP/valued (saline), DERM-SAP/devalued (LiCl). One day after the final CTA session, all rats were returned to the operant chambers for a 10-min extinction test, where lever pressing had no consequence (Yin et al., 2004).
c-Fos immunohistochemistry
Immediately following the completion of the extinction test, rats were killed by exposure to CO2 for 1 min followed by decapitation. The brains were rapidly harvested, flash-frozen in isopentane and stored at −80°C until they were cut into 12-μm sections through the frontal cortex (at + 4.2 mm anterior to bregma) and striatum at the level of the infusion (approximately + 1.5 mm anterior to bregma (Paxinos and Watson, 2005) on a cryostat (Minotome Plus, Triangle Biomedical Sciences, Durham, NC, USA).
Sections were post-fixed in 4% paraformaldehyde, pH 7.4 and then rinsed three times in phosphate-buffered saline (PBS). Slides were then blocked with 4% normal goat serum (NGS)/0.3% Triton X-100 (TX) for 1 h followed by overnight incubation at 4oC with a polyclonal antibody for c-Fos (Abcam, Cambridge, MA, USA), diluted in 1:1,000 in 0.3 % TX/0.1 M PBS. The slides were then washed several times in PBS and incubated for 2 h at room temperature in biotinylated horse anti-rabbit IgG antiserum (Vector Laboratories, Burlingame, CA, USA) diluted 1:200 in 0.1 M PBS/1% NGS. Slides were then washed three times in PBS, incubated 1 h in ABC solution (Elite ABC Kit, Vector Laboratories) and washed three more times in PBS. Bound antibody was detected using a 3,3’-diaminobenzidine/Ni+ solution (Vector Laboratories). Slides were washed with deionized H2O, dehydrated in a series of alcohols and coverslipped out of xylene.
Mu opioid receptor immunohistochemistry
In order to confirm that treatment with DERM-SAP resulted in a loss of mu opioid-receptor containing neurons in the patch compartment, immunohistochemistry for mu opioid receptors was performed on 12 μm sections through the DLS at the level of infusion (Figure 1). Sections were post-fixed in 4% paraformaldehyde, pH 7.4 and then rinsed three times in phosphate-buffered saline (PBS). Slides were then blocked with 10% bovine serum albumin (BSA)/0.3% Triton X-100 (TX) for 2 h followed by overnight incubation at 4oC with a polyclonal antibody for the mu opioid receptor (Immunostar, Hudson, WI, USA), diluted in 1:1,000 in 5% BSA/0.3 % TX/0.1 M PBS. The slides were then washed several times in PBS and incubated for 2 h at room temperature in biotinylated horse anti-rabbit IgG antiserum (Vector Laboratories, Burlingame, CA, USA) diluted 1:200 in 5% BSA/0.1 M PBS. Slides were then washed three times in PBS, incubated 1 h in ABC solution (Elite ABC Kit, Vector Laboratories) and washed three more times in PBS. Bound antibody was detected using a 3,3’-diaminobenzidine/Ni+ solution (Vector Laboratories). Slides were washed with deionized H2O, dehydrated in a series of alcohols and coverslipped out of xylene.
Image analysis
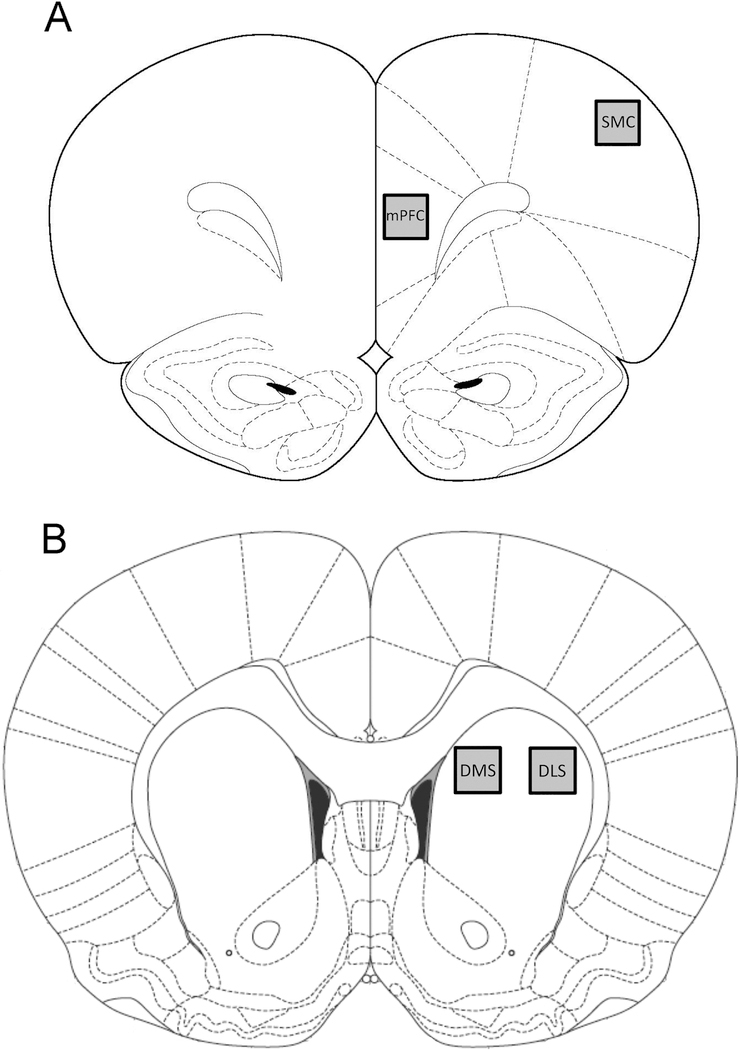
c-Fos-labeled sections were captured by a VistaVision microscope (VWR, Radnor, PA, USA) with a video camera (CCD Moticam 2300, Motic, Richmond, BC, Canada) using a 4× objective. The number c-Fos-labeled particles that exceeded the threshold density in the mPFC (400 × 400 pixel area at 4.2 mm anterior to bregma, in the prelimbic subregion), SMC (400 × 400 pixel area at 4.2 mm anterior to bregma), DLS (400 × 400 pixel area at 1.7 mm anterior to bregma) and DMS (400 × 400 pixel area at 1.7 mm anterior to bregma; Figure 2) were determined using the particle analysis option in Image J. Prior to analysis, the pixel range for particle size was determined by outlining approximately 15–20 positively labeled cells from 10 to 15 randomly selected sections and determining the average size of the labeled cells in terms of pixel area. The lower limit for a “labeled cell” on the particle analysis setting was then set to the smallest number of pixels measured for any cell, whereas the upper limit was set at the maximal particle size on the particle analysis option on Image J. The threshold density was adjusted such that background staining was eliminated and the number of immunoreactive pixels per the selected area in each region of interest was measured above this threshold. Both the left and right hemispheres from one section at each level (+4.2 mm AP and +1.7 mm AP) for each region of interest was analyzed for each animal and the number of c-Fos-labeled particles/area averaged.
Figure 2.
Schematic diagram of the regions of rat brain used for analysis of c-Fos immunoreactivity. A 400 x 400 pixel area was analyzed in the mPFC (within the prelimbic subregion) and SMC (at +4.2 mm from bregma; A) and the DMS and DLS (at +1.7 mm from bregma; B).
Statistical analysis
The effects of DERM-SAP pretreatment on CRF and RI training, as well as the effects of DERM-SAP pretreatment and LiCl treatment on sucrose consumption during the CTA analyzed were using a repeated measure analysis of variance. Extinction responding and changes in c-Fos immunoreactive particles in each region of interest were analyzed using a two-way analysis of variance. All sampled data exhibited a normal distribution, as determined by the D’Agostino and Pearson omnibus normality test (p≥0.05), and equal variances, as determined by Bartlett’s test for equal variances (p≥0.05). Post-hoc analysis of significant effects was accomplished using Tukey multi-comparison tests, which corrects for Type II errors. The alpha level for all analyses was set at 0.05.
Results
Effects of patch compartment lesions in the DLS on S-R-associated learning
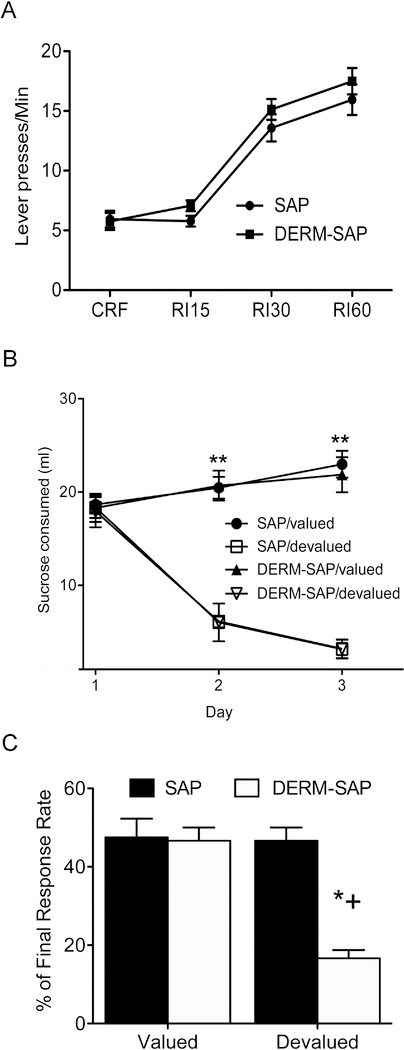
To determine the effects of lesioning the neurons of the dorsolateral patch compartment on the expression of S-R learning, both SAP and DERM-SAP pretreated animals were exposed to a RI schedule of reinforcement, which has been previously shown to promote the development of S-R learning (Dickinson, 1985). A repeated measure ANOVA (pretreatment × training) revealed a main effect of training (F3,90=85.1, p<0.0001), but no main effect of pretreatment (F1, 90=0.92, p=0.35) and no significant pretreatment × training interaction (F1, 90=0.98, p=0.41; Figure 3A), indicating that both SAP and DERM-SAP pretreated rats developed instrumental learning during the RI schedule of reinforcement.
Figure 3.
Effects of patch compartment lesions in the DLS on stimulus-response-associated learning. A.) Response rates (lever presses/min) of SAP- and DERM-SAP-pretreated animals over eight days of continuous reinforcement (CRF) training and random interval schedule of reinforcement training. The data are expressed as the mean lever presses/minute (±SEM) from the average lever presses for each animal across the two sessions per day for two days of CRF, RI15, RI30 and RI60 schedules of reinforcement during instrumental training. B.) Development of lithium chloride (LiCl)-induced conditioned taste aversion over a three-day period following CRF and RI training. Data are expressed as the mean (±SEM) milliliters of sucrose consumed in the home cage during a 30 min access period, after which time animals were injected with either LiCl (0.15 M) or saline. **significantly different from sucrose consumption in LiCl-treated animals, p<0.05. C.) Effect of outcome devaluation due to LiCl-induced CTA on extinction responding in SAP- and DERM-SAP-pretreated animals trained on RI schedules of reinforcement. The data is expressed as the response rate during the 10-min extinction test after outcome devaluation as a percentage of the response rate on the last day of RI60 training (±SEM). *significantly different from all other groups, p<0.05.
To determine whether the observed instrumental behavior was the result of a S-R-associated learning, half of the rats in both the SAP- and DERM-SAP pretreated groups were exposed to CTA with LiCl, in order to devalue the sucrose reinforcer. Rats in both SAP- and DERM-SAP pretreated groups that were given LiCl following sucrose consumption in the home cage decreased their consumption of sucrose of the three-day period (Figure 3B). A repeated-measure ANOVA (pretreatment × devaluation × day) revealed significant main effects of devaluation (F1, 43=148.1, p<0.0001) and day (F1, 43=33.0, p<0.0001), and a significant interaction between the devaluation and day (F1, 43=104.0, p<0.0001). There was not a significant main effect of pretreatment (F1, 43=1.04, p=0.315), nor were there significant interactions between pretreatment and devaluation (F1, 43=0.315, p=0.577), pretreatment and day (F1, 43=0.167, p=0.685) or pretreatment, devaluation and day (F1, 43=0.711, p=0.404). Therefore, CTA resulted in a similar level of devaluation of the sucrose reinforcer in both SAP- and DERM-SAP pretreated rats.
Twenty-four hours after the final day of CTA, rats were re-introduced to the operant chambers in order to determine whether extinction of the operant behavior had occurred as a result of devaluation of the outcome (Figure 3C). A two-way analysis of variance (pretreatment × devaluation) of response rates revealed significant main effects of pretreatment (F1,25=6.1, p=0.02) and devaluation (F1,25=6.1, p=0.02), as well as an interaction between the pretreatment and devaluation of the reinforcer (F1,25=5.8, p=0.02). Post-hoc analyses of significant main effects indicated that there was no significant difference in response rates between SAPpretreated/saline-treated and SAP-pretreated/LiCl-treated rats (p=0.97), nor was there a significant difference in response rates between DERM-SAP-pretreated/saline-treated and DERM-SAP-pretreated/LiCl-treated rats (p=0.97). However, there were significant differences in response rates between SAP-pretreated/LiCl-treated rats and DERM-SAP-pretreated/LiCl treated rats (p=0.002), and between DERM-SAP-pretreated/saline-treated rats and DERM-SAP-pretreated/LiCl-treated rats (p=0.02). These data suggest that in DERM-SAP-pretreated rats, but not SAP-pretreated rats, responding during the extinction test was reduced following devaluation of the sucrose reinforcer.
Effects of patch compartment lesions in the DLS on A-O-associated circuits
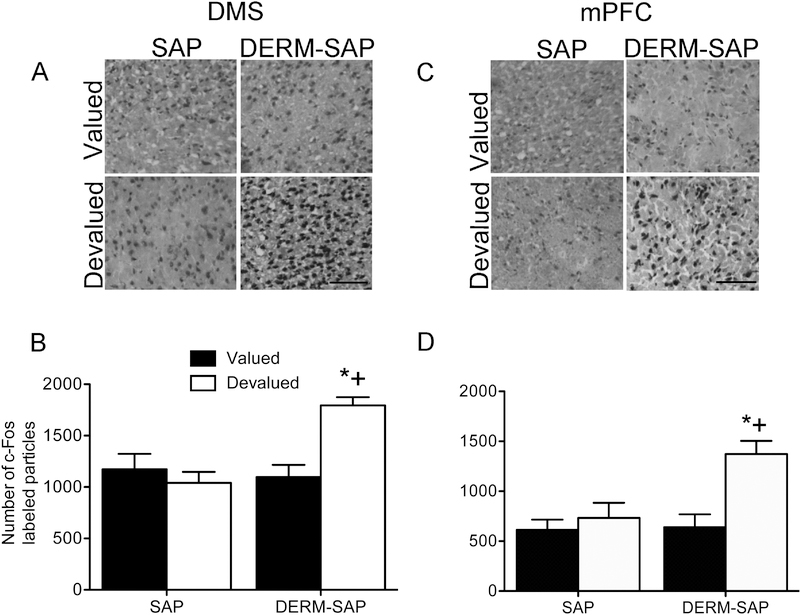
Analysis of c-Fos immunoreactivity within a 400 × 400 pixel region in the DMS revealed significant main effects of reinforcer devaluation (F1,17=10.53, p=0.005) and pretreatment (F1,17=6.44, p=0.02), as well as a significant interaction between reinforcer devaluation and pretreatment (F1,17=5.45, p=0.032). Post-hoc analysis indicated that there was a significant difference in the number of c-Fos-labeled particles in the DMS between SAP-pretreated/LiCltreated and DERM-SAP-pretreated/LiCl-treated rats (p=0.012), as well as a significant difference between DERM-SAP-pretreated/saline-treated and DERM-SAP-pretreated/LiCl-treated rats (p=0.009). There was not a significant difference in the number of c-Fos-labeled particles in the DMS between SAP-pretreated/saline-treated rats and SAP-pretreated/LiCl-treated rats (p=0.85) or SAP-pretreated/saline-treated and DERM-SAP-pretreated/LiCl-treated rats (p=0.64). These data suggest that there was increased activation of the DMS of rats with dorsolateral patch compartment lesions that underwent conditioned taste aversion, as compared to intact animals or lesioned animals that did not undergo conditioned taste aversion (Figure 4A and 4B).
Figure 4.
Effects of patch compartment lesions in the DLS on c-Fos immunoreactivity in the DMS and mPFC (within the prelimbic subregion) following reinforcer devaluation and extinction testing. Photomicrographs showing c-Fos immunoreactivity in the DMS (A) and mPFC (C). Scale bar=100 μM. Quantitative analysis of c-Fos immunoreactivity in the DMS (B) and mPFC (D) in rats infused with SAP or DERM-SAP (17 ng/μl) in the DLS, prior to RI training, CTA and extinction testing. Data are presented as the mean (±SEM) number of c-Fos immunoreactive particles per area. *significantly different from DERM-SAP-pretreated, saline-treated rats, p<0.05; +significantly different from SAP-pretreated, LiCl-treated rats, p<0.05.
Analysis of c-Fos immunoreactivity within a 400 × 400 pixel region in the mPFC (within the prelimbic subregion) revealed significant main effects of reinforcer devaluation (F1,18=6.33, p=0.02) and pretreatment (F1,18=9.13, p=0.007), as well as a significant interaction between reinforcer devaluation and pretreatment (F1,18=13.55, p=0.002). Post-hoc analysis indicated that there was a significant difference in the number of c-Fos-labeled particles in the mPFC between SAP-pretreated/LiCl-treated and DERM-SAP-pretreated/LiCl-treated rats (p=0.0002), as well as a significant difference between DERM-SAP-pretreated/saline-treated and DERM-SAP-pretreated/LiCl-treated rats (p=0.0003). There was not a significant difference in the number of c-Fos-labeled particles in the mPFC between SAP-pretreated/saline-treated rats and SAP-pretreated/LiCl-treated rats (p=0.22) or SAP-pretreated/saline-treated and DERM-SAP-pretreated/LiCl-treated rats (p=0.38). These data suggest that there was increased activation of the mPFC of rats with dorsolateral patch compartment lesions that underwent devaluation of the reinforcer, as compared to intact animals or lesioned animals that did not undergo reinforcer devaluation (Figure 4C and 4D).
Effects of patch compartment lesions in the DLS on S-R-associated circuits
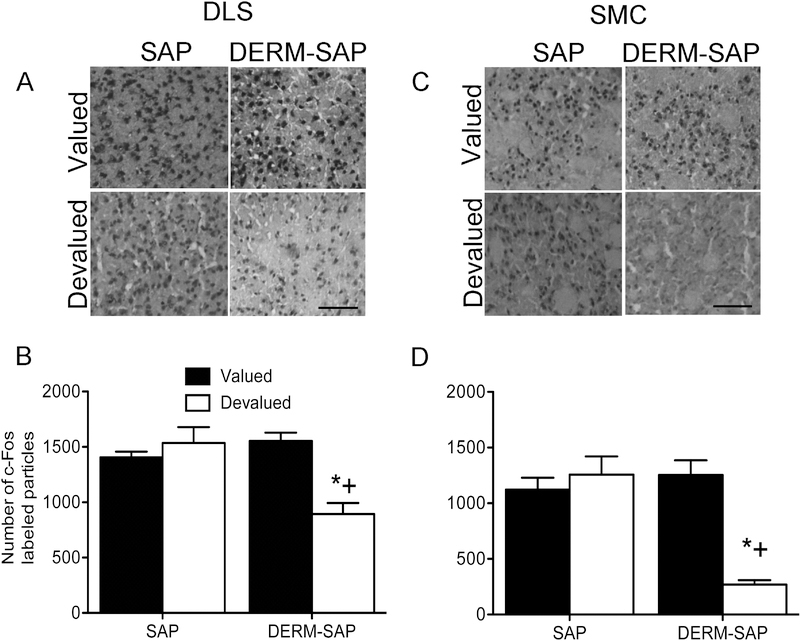
Analysis of c-Fos immunoreactivity within a 400 × 400 pixel region in the DLS revealed significant main effects of reinforcer devaluation (F1,16=12.59, p=0.003) and pretreatment (F1,16=12.82, p=0.003), as well as a significant interaction between reinforcer devaluation and pretreatment (F1,16=21.83, p=0.0003). Post-hoc analysis indicated that there was a significant difference in the number of c-Fos-labeled particles in the DLS between SAP-pretreated/LiCl-treated and DERM-SAP-pretreated/LiCl-treated rats (p=0.0012), as well as a significant difference between DERM-SAP-pretreated/saline-treated and DERM-SAP-pretreated/LiCl-treated rats (p=0.0007). There was not a significant difference in the number of c-Fos-labeled particles in the DLS between SAP-pretreated/saline-treated rats and SAP-pretreated/LiCl-treated rats (p=0.53) or SAP-pretreated/saline-treated and DERM-SAP-pretreated/LiCl-treated rats (p=0.35). These data suggest that there was decreased activation of the DLS of rats with dorsolateral patch compartment lesions that underwent conditioned taste aversion, as compared to intact animals or lesioned animals that did not undergo conditioned taste aversion (Figure 5A and 5B).
Figure 5.
Effects of patch compartment lesions in the DLS on c-Fos immunoreactivity in the DLS and SMC following reinforcer devaluation and extinction testing. Photomicrographs showing c-Fos immunoreactivity in the DLS (A) and SMC (C). Scale bar=100 μM. Quantitative analysis of c-Fos immunoreactivity in the DLS (B) and SMC (D) in rats infused in the DLS with SAP or DERM-SAP (17 ng/μl), prior to RI training, CTA and extinction testing. Data are presented as the mean (±SEM) number of c-Fos immunoreactive particles per area. *significantly different from DERM-SAP-pretreated, saline-treated rats, p<0.05; +significantly different from SAP-pretreated, LiCl-treated rats, p<0.05.
Analysis of c-Fos immunoreactivity within a 400 × 400 pixel region in the SMC revealed significant main effects of reinforcer devaluation (F1,19=7.37, p=0.013) and pretreatment (F1,19=6.41, p=0.02), as well as a significant interaction between reinforcer devaluation and pretreatment (F1,19=16.54, p=0.0007). Post-hoc analysis indicated that there was a significant difference in the number of c-Fos-labeled particles in the SMC between SAP-pretreated/LiCltreated and DERM-SAP-pretreated/LiCl-treated rats (p=0.003), as well as a significant difference between DERM-SAP-pretreated/saline-treated and DERM-SAP-pretreated/LiCl-treated rats (p=0.006). There was not a significant difference in the number of c-Fos-labeled particles in the SMC between SAP-pretreated/saline-treated rats and SAP-pretreated/LiCl-treated rats (p=0.13) or SAP-pretreated/saline-treated and DERM-SAP-pretreated/LiCl-treated rats (p=0.37). These data suggest that there was decreased activation of the SMC of rats with dorsolateral patch compartment lesions that underwent conditioned taste aversion, as compared to intact animals or lesioned animals that did not undergo conditioned taste aversion (Figure 5C and 5D).
Discussion
The goal of the current study was to determine whether destruction of neurons in patch compartment of the DLS altered S-R learning and modified activity in the basal ganglia circuits that mediate instrumental behaviors. Reinstatement of sucrose self-administration following sucrose devaluation was reduced in animals with lesions of the patch compartment in the DLS, whereas animals with an intact patch compartment in the DLS continued to self-administer sucrose following sucrose devaluation, indicating that the expression of S-R behavior was interrupted when the patch compartment of the DLS was compromised. Animals that continued to self-administer sucrose following sucrose devaluation had relatively higher c-Fos levels in the DLS-SMC circuit as compared to animals with lesions of the patch compartment in the DLS that did not continue to self-administer sucrose following sucrose devaluation. However, dorsolateral patch-lesioned animals that were sensitive to devaluation of the sucrose reinforcer exhibited relatively higher levels of c-Fos in the DMS-mPFC circuit, as compared to intact animals that continued to self-administer sucrose after devaluation of the sucrose reward. These data suggest that in the absence of an intact dorsolateral patch compartment, the behavior had become more flexible and goal-directed, as reflected by the relatively greater engagement of A-O-associated circuits in these animals, while animals that had an intact dorsolateral patch compartment that exhibited more inflexible behaviors had relatively greater engagement of S-R-associated circuits. These data are among the first to demonstrate a role for the patch compartment of the DLS in the expression of S-R instrumental behaviors, and suggest that patch-based limbic circuits that traverse the DLS are necessary for the development of habitual behaviors. Furthermore, the current study raises the possibility that the dorsolateral patch compartment functions as a region of contact between the limbic system and the basal ganglia, where information regarding motivation and internal states contained within patch-based circuits influences S-R instrumental learning processes mediated by the DLS.
To date, the majority of studies involving the patch-matrix system have centered on the role that these striatal sub-regions play in psychostimulant-induced stereotypy, which is motor behavior that is repetitive, inflexible and accompanied by an inability to initiate adaptive motor responses to external stimuli (Canales and Graybiel, 2000; Crittenden and Graybiel, 2011). Numerous studies have shown that enhanced activation of the patch compartment is observed during psychostimulant-induced stereotypy, and recent work from our laboratory revealed that an intact patch compartment is necessary for the expression of repetitive behaviors induced by psychostimulants (Canales and Graybiel, 2000; Graybiel et al., 1990; Horner et al., 2012; Horner et al., 2010; Murray et al., 2014; Murray et al., 2015; Saka et al., 2004). Given that both stereotypy and habitual behaviors share aspects of inflexibility and stereotypic behaviors correlate positively with patch-enhanced activity specifically within the DLS, it is possible that circuits contained within the dorsolateral patch compartment are necessary for both stereotypy and habitual behaviors, and potentially other behaviors that also contain elements of inflexibility, such as Tourette syndrome or obsessive-compulsive disorder (Canales, 2005; Canales and Graybiel, 2000; Graybiel and Canales, 2000; Graybiel and Rausch, 2000).
In the current study, we assessed the role of the patch compartment of the DLS in habit learning using a post-training devaluation procedure as our behavioral assay of habitual control over instrumental behavior (Dickinson, 1985; Son et al., 2011; Yin et al., 2004, 2006). Ablation of the patch compartment in the DLS did not prevent the acquisition of S-R learning, but patch-ablated animals did exhibit goal-directed behaviors, as these animals were sensitive to manipulation of the value of the outcome. Otherwise, the behavior of animals that had lesions of the patch compartment in the DLS appeared to be similar to intact animals, and any differences were not evident until the behavior was further probed. Indeed, when the outcome (the delivery of sucrose) was paired with a negative stimulus (LiCl), animals that had lesions of the dorsolateral patch compartment did not execute the behavior (self-administration of sucrose upon re-exposure to the operant chamber) the same way as intact animals, and exhibited behavior that was more A-O-driven, as compared to intact animals that continued to exhibit S-R-driven behaviors. These behavioral findings are supported by our c-Fos data where dorsolateral patch-lesioned animals that were sensitive to outcome devaluation showed decreased activity in DLS-SMC circuits that mediate S-R behaviors and increased activity in DMS-PFC circuits that facilitate A-O behaviors. These data indicate that instrumental performance in dorsolateral patch-lesioned animals is controlled by goal expectancy and that loss of the patch compartment increases the sensitivity of the performance to outcome devaluation. It is possible that in the absence of a fully functioning patch compartment within the DLS, the limbic information concerning internal motivational states that might compete with the processing of negative information regarding the environment is removed, allowing for increased saliency of the negative stimulus that has been associated with the outcome.
Previous work has demonstrated that patch-based circuits may also mediate reward processes. For example, animals will continuously self-stimulate if an electrode is placed in or near the patch compartment (White and Hiroi, 1998). In addition, drug-mediated reward is associated with enhanced activation of the patch compartment, and lesions of the patch compartment reduce drug-mediated reward (Horner et al., 2017). The patch compartment also receives inputs from the anterior cingulate and orbitofrontal cortices, which participate in cognitive processes that are relevant to motivation, drive, the learning of stimulus–reinforcement associations, and decision-making (Canales, 2005; Gehring and Willoughby, 2002; Rolls, 2000; Tucker et al., 1995). Furthermore, ascending recurrent interconnected loops exist between the nucleus accumbens, which encodes information related to reward and reinforcement, and the DLS via midbrain dopamine neurons (Haber et al., 2000). These ascending loops may serve as an anatomical pathway for the transfer of limbic information to regions that mediate habitual learning. The patch compartment of the DLS may eventually accrue synthesized limbic information, resulting in a gradual transfer of behavioral control from accumbal circuits to patch-based circuits (Canales, 2005). Thus, learning processes involving reward-seeking behaviors may initially rely on the nucleus accumbens, but as the behavior becomes more rigid and fixed, reward-seeking behaviors become more reliant on the DLS and the patch compartment neurons contained within this region (Berke and Hyman, 2000; Canales, 2005; Hyman and Malenka, 2001; Natarajan and Yamamoto, 2011; Robbins and Everitt, 2002; Voorn et al., 2004). When dorsolateral patch-based circuits are preferentially activated in the sensorimotor region of the striatum that mediates S-R learning, the focus of the behavior may become narrower, leading to behavior that is inflexible in nature and insensitive to devaluation of the outcome. However, it is important to note that the orbitofrontal cortex (which as noted above, provides inputs to the patch compartment) may also be involved in A-O learning (Gremel and Costa, 2013). This suggests that the patch compartment could also play a role in the execution of goal-directed behaviors. Previous work has shown that during goal-directed behaviors, the DMS and OFC together show increased activation, while the DLS is less engaged (Gremel and Costa, 2013). Therefore, it is possible that the development of A-O occurs when the activity of the patch compartment in the DMS is enhanced as a result of increased stimulatory inputs from the OFC. Additional studies are needed in to determine the possible contribution of the patch compartment of the DMS to A-O learning.
Patch compartment neurons are the only striatal spiny neurons that innervate the substantia nigra pars compacta (SNpc), which is the source of dopaminergic inputs to the striatum (Bolam and Smith, 1990; Fujiyama et al., 2011; Gerfen, 1984; Jimenez-Castellanos and Graybiel, 1989; Tokuno et al., 2002). Thus, patch compartment neurons are in a prime position to modulate dopamine release in the striatum, with potential effects on instrumental behaviors. It has been suggested that patch-based inhibition of nigrostriatal dopamine neurons serves as a mechanism by which a behavior that is well-learned becomes less reliant upon external reinforcers for its maintenance, which is supported by computational models that show timed inhibitory projections from patch compartment neurons prevents burst firing of dopamine neurons in response to an expected reinforcer (Brown et al., 1999; Canales, 2005). It is possible that instrumental behaviors, as they become S-R in nature, could eventually be driven, at least in part by internal reinforcers, due to the accumulation of limbic information in the dorsolateral patch compartment, the neurons of which may also modulate nigrostriatal dopamine release. If the dorsolateral patch compartment has been compromised, there could be an attenuation of this effect, allowing for the behavior to once again rely more heavily upon an external reinforcer, rather than internal stimuli. In the current study, instrumental behaviors in lesioned versus non-lesioned animals might appear to be the same, regardless of whether the behavior was driven by an internal or external reinforcer, until the behavior is further probed by devaluation of the outcome. In intact animals, the devaluation of the outcome would be less significant because the behavior would be more dependent upon internal states than external stimuli. However, in animals with dorsolateral patch compartment lesions, if the behavior has become more reliant upon an external reinforcer, then once the reinforcer has been devalued, the behavior would be more likely to be extinguished.
Our data indicate that in lesioned animals that were sensitive to reinforcer devaluation, there was relatively greater activation of regions that participate in A-O behaviors, as compared to animals that were insensitive to devaluation, while there was relatively greater activation of regions that participate in S-R behaviors in animals that were insensitive to reinforcer devaluation, versus those animals that were lesioned and sensitive to reinforcer devaluation. Together, these data suggest that there is an imbalance in activity between the two circuits associated with habitual behaviors and an intact dorsolateral patch compartment, whereby DLS-SMC circuits show relatively greater levels of engagement, while DMS-mPFC circuits show relatively higher levels of engagement when the behavior is no longer habitual and the dorsolateral patch compartment is compromised. These data are in line with previous work where an imbalance in activity between DLS-SMC and DMS-PFC circuits is observed during the expression of other behaviors that are also inflexible in nature, such as psychostimulant-induced stereotypy (Aliane et al., 2009; Murray et al., 2014). It should be noted that the lack of an increase in c-Fos activity in the DLS of animals that had patch compartment lesions in this region could simply be the result of a loss of medium spiny neurons in this region. However, this scenario is unlikely as the patch compartment comprises only 15% of the striatum as a whole, with roughly 4% of the patch compartment present in the DLS (Johnston et al., 1990). Thus, the loss of less than 5% of the neurons in the DLS cannot account for the approximately 50% difference in the number of c-Fos-labeled neurons in lesioned animals that no longer exhibited habitual behavior versus non-lesioned animals that continued to self-administer sucrose. Nevertheless, these findings suggest that the patch compartment may influence the activity of DLS-SMC circuits during habitual and inflexible behaviors, and elimination of these neurons can result in preferential engagement of DMS-PFC circuits, allowing for the emergence of goal-directed behaviors.
The specific mechanisms by which the patch compartment influences the circuits that mediate instrumental learning are unclear. One possibility is that cross-talk between the patch and matrix compartments underlies the regulation of the circuits that underlie instrumental behaviors. Striatal cholinergic interneurons are preferentially located on the borders of the patch and matrix compartments, where substance P is enriched (Miura et al., 2008). These cholinergic neurons have dendritic fields that extend into the both the patch and matrix compartment, although their axons are largely restricted to the matrix compartment (Kawaguchi, 1992; Tepper and Bolam, 2004). Repetitive activation of patch compartment neurons could lead to increased substance P release and activation of NK-1 receptor-expressing cholinergic interneurons, resulting in increased activation of matrix compartment neurons, which give rise to the majority of striatal efferents (Abudukeyoumu et al., 2019; Crittenden and Graybiel, 2011). In the absence of an intact patch compartment in the DLS, there may be less relative activity of striatal outputs from this area, as compared to the intact DMS. However, recent work indicates that there may be a lack of synaptic connectivity between the patch and matrix compartments, in which case changes in the patch compartment may have little influence on outputs from the matrix compartment (Lopez-Huerta et al., 2016). On the other hand, while the patch compartment is thought to contain the only striatal neurons that innervate the SNpc, there are medium spiny neurons in the patch compartment that project to direct and indirect pathway nuclei (Crittenden and Graybiel, 2011). Therefore, it is possible that a loss of striatal outputs from the patch compartment in the DLS could result in diminished activation of the DLS-SMC circuit relative to the DMS.
In summary, the current study shows that destruction of the neurons of the patch compartment of the DLS prevents the reinstatement of sucrose self-administration after sucrose devaluation, indicating that in the absence of the dorsolateral patch compartment the development of habitual behavior is interrupted. Our data also indicate that in dorsolateral patch-lesioned animals who also exhibited extinction of habitual behaviors following sucrose devaluation with LiCl, c-Fos levels were relatively lower in the DLS and SMC, as compared to other animals that continued to self-administer sucrose, while c-Fos levels were relatively higher in the DMS and PFC in those animals that were sensitive to sucrose devaluation with LiCl following patch compartment lesions versus animals that continued to self-administer sucrose following devaluation. These data suggest that persistent habitual behaviors may be associated with increased engagement of S-R circuits, coupled with relatively less involvement of A-O circuits. On the other hand, in the absence of a fully intact dorsolateral patch compartment, where the outcome is now sensitive to devaluation and the habitual behavior is diminished, there may be less involvement of S-R-associated circuits, coupled with an increased relative engagement of A-O-associated circuits. Together, these data raise the possibility that in the absence of the dorsolateral patch compartment, the flow of information through the basal ganglia is altered such that regions that participate in goal-directed behaviors are preferentially engaged and stimulus-response-associated regions play a relatively smaller role. Over time, the dorsolateral patch compartment may accrue synthesized limbic information, resulting in a gradual transfer of control of behaviors related to reward and reinforcement from accumbal circuits to patch-based circuits and a shift towards patch-enhanced activation in the DLS could represent an enhancement of the rewarding and motivational aspects of behavior. When this happens within the context of the sensorimotor region of the striatum that mediates stimulusresponse learning, and as the imbalance in activity between the patch and matrix compartments in the DLS becomes more pronounced, the focus of the behavior narrows, leading to behavior that is inflexible in nature and insensitive to devaluation of the outcome.
Highlights.
Inflexible, repetitive behaviors are associated with enhanced activity in the patch compartment of striatum
Habits are also inflexible in nature, but it is not known if the patch compartment contributes to their development
Selective destruction of patch compartment neurons reduced habitual sucrose consumption in Sprague-Dawley rats
Acknowledgements
This work was supported by NIH grant DA025303 and a Navicent Foundation research grant (awarded to KAH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Abudukeyoumu N, Hernandez-Flores T, Garcia-Munoz M, Arbuthnott GW, 2019. Cholinergic modulation of striatal microcircuits. Eur J Neurosci 49, 604–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aliane V, Perez S, Nieoullon A, Deniau JM, Kemel ML, 2009. Cocaine-induced stereotypy is linked to an imbalance between the medial prefrontal and sensorimotor circuits of the basal ganglia. Eur J Neurosci 30, 1269–1279. [DOI] [PubMed] [Google Scholar]
- Arvidsson U, Riedl M, Chakrabarti S, Lee JH, Nakano AH, Dado RJ, Loh HH, Law PY, Wessendorf MW, Elde R, 1995. Distribution and targeting of a mu-opioid receptor (MOR1) in brain and spinal cord. J Neurosci 15, 3328–3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine BW, Dickinson A, 1998. Goal-directed instrumental action: contingency and incentive learning and their cortical substrates. Neuropharmacology 37, 407–419. [DOI] [PubMed] [Google Scholar]
- Balleine BW, Liljeholm M, Ostlund SB, 2009. The integrative function of the basal ganglia in instrumental conditioning. Behavioural brain research 199, 43–52. [DOI] [PubMed] [Google Scholar]
- Belin D, Jonkman S, Dickinson A, Robbins TW, Everitt BJ, 2009. Parallel and interactive learning processes within the basal ganglia: relevance for the understanding of addiction. Behavioural brain research 199, 89–102. [DOI] [PubMed] [Google Scholar]
- Berke JD, Hyman SE, 2000. Addiction, dopamine, and the molecular mechanisms of memory. Neuron 25, 515–532. [DOI] [PubMed] [Google Scholar]
- Bolam JP, Izzo PN, Graybiel AM, 1988. Cellular substrate of the histochemically defined striosome and matrix system of the caudate nucleus: a combined golgi and immunohistochemical study. Neuroscience 24, 853–875. [DOI] [PubMed] [Google Scholar]
- Bolam JP, Smith Y, 1990. The GABA and substance P input to dopaminergic neurones in the substantia nigra of the rat. Brain Res 529, 57–78. [DOI] [PubMed] [Google Scholar]
- Brown J, Bullock D, Grossberg S, 1999. How the basal ganglia use parallel excitatory and inhibitory learning pathways to selectively respond to unexpected rewarding cues. J Neurosci 19, 10502–10511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canales JJ, 2005. Stimulant-induced adaptations in neostriatal matrix and striosome systems: transitioning from instrumental responding to habitual behavior in drug addiction. Neurobiol Learn Mem 83, 93–103. [DOI] [PubMed] [Google Scholar]
- Canales JJ, Graybiel AM, 2000. A measure of striatal function predicts motor stereotypy. Nat Neurosci 3, 377–383. [DOI] [PubMed] [Google Scholar]
- Crittenden JR, Graybiel AM, 2011. Basal Ganglia disorders associated with imbalances in the striatal striosome and matrix compartments. Frontiers in neuroanatomy 5, 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfs JM, Kong H, Mestek A, Chen Y, Yu L, Reisine T, Chesselet MF, 1994. Expression of mu opioid receptor mRNA in rat brain: an in situ hybridization study at the single cell level. J Comp Neurol 345, 46–68. [DOI] [PubMed] [Google Scholar]
- Dickinson A, 1985. Actions and habits: the development of behavioral autonomy. Phil Trans R Soc 308, 67–78. [Google Scholar]
- Faure A, Haberland U, Conde F, El Massioui N, 2005. Lesion to the nigrostriatal dopamine system disrupts stimulus-response habit formation. J Neurosci 25, 2771–2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiyama F, Sohn J, Nakano T, Furuta T, Nakamura KC, Matsuda W, Kaneko T, 2011. Exclusive and common targets of neostriatofugal projections of rat striosome neurons: a single neuron-tracing study using a viral vector. Eur J Neurosci 33, 668–677. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Willoughby AR, 2002. The medial frontal cortex and the rapid processing of monetary gains and losses. Science 295, 2279–2282. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, 1984. The neostriatal mosaic: compartmentalization of corticostriatal input and striatonigral output systems. Nature 311, 461–464. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, 1989. The neostriatal mosaic: striatal patch-matrix organization is related to cortical lamination. Science 246, 385–388. [DOI] [PubMed] [Google Scholar]
- Giagnoni G, Parolaro D, Casiraghi L, Crema G, Sala M, Andreis C, Gori E, 1984. Dermorphin interaction with peripheral opioid receptors. Neuropeptides 5, 157–160. [DOI] [PubMed] [Google Scholar]
- Grahn JA, Parkinson JA, Owen AM, 2009. The role of the basal ganglia in learning and memory: neuropsychological studies. Behavioural brain research 199, 53–60. [DOI] [PubMed] [Google Scholar]
- Graybiel AM, Canales JJ, 2000. The neurobiology of repetitive behaviors: clues to the neurobiology of Tourette syndrome. Adv Neurol 85, 123–131. [PubMed] [Google Scholar]
- Graybiel AM, Moratalla R, Robertson HA, 1990. Amphetamine and cocaine induce drugspecific activation of the c-fos gene in striosome-matrix compartments and limbic subdivisions of the striatum. Proc Natl Acad Sci U S A 87, 6912–6916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybiel AM, Rausch SL, 2000. Toward a neurobiology of obsessive-compulsive disorder. Neuron 28, 343–347. [DOI] [PubMed] [Google Scholar]
- Gremel CM, Costa RM, 2013. Orbitofrontal and striatal circuits dynamically encode the shift between goal-directed and habitual actions. Nat Commun 4, 2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Fudge JL, McFarland NR, 2000. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci 20, 2369–2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M, Pert CB, 1981. Mosaic distibution of opiate receptors, parafascilular projections and acetylcholinesterase in rat striatum. Nature 291, 415–418. [DOI] [PubMed] [Google Scholar]
- Horner KA, Hebbard JC, Logan AS, Vanchipurakel GA, Gilbert YE, 2012. Activation of mu opioid receptors in the striatum differentially augments methamphetamine-induced gene expression and enhances stereotypic behavior. J Neurochem 120, 779–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner KA, Logan MC, Fisher TJ, Logue JB, 2017. Blockade of patch-based mu opioid receptors in the striatum attenuates methamphetamine-induced conditioned place preference and reduces activation of the patch compartment. Eur J Pharmacol 796, 207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner KA, Noble ES, Gilbert YE, 2010. Methamphetamine-induced stereotypy correlates negatively with patch-enhanced prodynorphin and arc mRNA expression in the rat caudate putamen: the role of mu opioid receptor activation. Pharmacol Biochem Behav 95, 410–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, 2001. Addiction and the brain: the neurobiology of compulsion and its persistence. Nat Rev Neurosci 2, 695–703. [DOI] [PubMed] [Google Scholar]
- Jimenez-Castellanos J, Graybiel AM, 1989. Compartmental origins of striatal efferent projections in the cat. Neuroscience 32, 297–321. [DOI] [PubMed] [Google Scholar]
- Johnston JG, Gerfen CR, Haber SN, van der Kooy D, 1990. Mechanisms of striatal pattern formation: conservation of mammalian copartmentalization. Dev Brain Res 57, 93–102. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, 1992. Large aspiny cells in the matrix of the rat neostriatum in vitro: physiological identification, relation to the compartments and excitatory postsynaptic currents. J Neurophysiol 67, 1669–1682. [DOI] [PubMed] [Google Scholar]
- Lawhorn C, Smith DM, Brown LL, 2009. Partial ablation of mu-opioid receptor rich striosomes produces deficits on a motor-skill learning task. Neuroscience 163, 109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Huerta VG, Nakano Y, Bausenwein J, Jaidar O, Lazarus M, Cherassse Y, GarciaMunoz M, Arbuthnott G, 2016. The neostriatum: two entities, one structure? Brain structure & function 221, 1737–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour A, Fox CA, Burke S, Akil H, Watson SJ, 1995. Immunohistochemical localization of the cloned mu opioid receptor in the rat CNS. J Chem Neuroanat 8, 283–305. [DOI] [PubMed] [Google Scholar]
- Mansour A, Fox CA, Burke S, Meng F, Thompson RC, Akil H, Watson SJ, 1994. Mu, delta, and kappa opioid receptor mRNA expression in the rat CNS: an in situ hybridization study. J Comp Neurol 350, 412–438. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, 1992. Projection neurons of basolateral amygdala: a correlative golgi and retrograde tract tracing study. Brain Res Bull 28, 179–185. [DOI] [PubMed] [Google Scholar]
- Minami M, Onogi T, Toya T, Katao Y, Hosoi Y, Maekawa K, Katsumata S, Yabuuchi K, Satoh M, 1994. Molecular cloning and in situ hybridization histochemistry for rat mu-opioid receptor. Neurosci Res 18, 315–322. [DOI] [PubMed] [Google Scholar]
- Miura M, Masuda M, Aosaki T, 2008. Roles of micro-opioid receptors in GABAergic synaptic transmission in the striosome and matrix compartments of the striatum. Mol Neurobiol 37, 104–115. [DOI] [PubMed] [Google Scholar]
- Murray RC, Gilbert YE, Logan AS, Hebbard JC, Horner KA, 2014. Striatal patch compartment lesions alter methamphetamine-induced behavior and immediate early gene expression in the striatum, substantia nigra and frontal cortex. Brain structure & function 219, 1213–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray RC, Logan MC, Horner KA, 2015. Striatal patch compartment lesions reduce stereotypy following repeated cocaine administration. Brain Res 1618, 286–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan R, Yamamoto BK, 2011. The Basal Ganglia as a Substrate for the Multiple Actions of Amphetamines. Basal Ganglia 1, 49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C, 2005. The Rat Brain in Stereotaxic Coordinates. Elsevier Academic Press, San Diego, CA. [Google Scholar]
- Pert CB, Kuhar M, Snyder SH, 1976. Opiate receptor: autoradiographic localization in the rat brain. Proc Natl Acad Sci USA 73, 3729–3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ, 2002. Limbic-striatal memory systems and drug addiction. Neurobiol Learn Mem 78, 625–636. [DOI] [PubMed] [Google Scholar]
- Rolls ET, 2000. Memory systems in the brain. Annu Rev Psychol 51, 599–630. [DOI] [PubMed] [Google Scholar]
- Saka E, Goodrich C, Harlan P, Madras BK, Graybiel AM, 2004. Repetitive behaviors in monkeys are linked to specific striatal activation patterns. J Neurosci 24, 7557–7565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son J-H, Latimer C, Keefe KA, 2011. Impaired formation of stimulus-response, but not action-outcome, associations in rats with methamphetamine-induced neurotoxicity. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 36, 2441–2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper JM, Bolam JP, 2004. Functional diversity and specificity of neostriatal interneurons. Curr Opin Neurobiol 14, 685–692. [DOI] [PubMed] [Google Scholar]
- Tokuno H, Chiken S, Kametani K, Moriizumi T, 2002. Efferent projections from the striatal patch compartment: anterograde degeneration after selective ablation of neurons expressing mu-opioid receptor in rats. Neurosci Lett 332, 5–8. [DOI] [PubMed] [Google Scholar]
- Tucker DM, Luu P, Pribram KH, 1995. Social and emotional self-regulation. Ann N Y Acad Sci 769, 213–239. [DOI] [PubMed] [Google Scholar]
- Voorn P, Vanderschuren LJ, Groenewegen HJ, Robbins TW, Pennartz CM, 2004. Putting a spin on the dorsal-ventral divide of the striatum. Trends Neurosci 27, 468–474. [DOI] [PubMed] [Google Scholar]
- White NM, Hiroi N, 1998. Preferential localization of self-stimulation sites in striosomes/patches in the rat striatum. Proc Natl Acad Sci U S A 95, 6486–6491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley RG, Kline IR, 2000. Neuronal lesioning with axonally transported toxins. J Neurosci Methods 103, 73–82. [DOI] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ, 2006. The role of the basal ganglia in habit formation. Nat Rev Neurosci 7, 464–476. [DOI] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ, Balleine BW, 2004. Lesions of dorsolateral striatum preserve outcome expectancy but disrupt habit formation in instrumental learning. Eur J Neurosci 19, 181–189. [DOI] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ, Balleine BW, 2006. Inactivation of dorsolateral striatum enhances sensitivity to changes in the action-outcome contingency in instrumental conditioning. Behavioural brain research 166, 189–196. [DOI] [PubMed] [Google Scholar]
- Yin HH, Ostlund SB, Knowlton BJ, Balleine BW, 2005. The role of the dorsomedial striatum in instrumental conditioning. Eur J Neurosci 22, 513–523. [DOI] [PubMed] [Google Scholar]