Abstract
Background & Aims:
Although low fiber intake has been considered a risk factor for diverticulitis, prospective evidence is limited in women despite having a disproportionate burden of disease, with little known about variation in the protective effects according to food sources. We assessed the associations of intakes of fiber and major food sources of fiber including fruits and vegetables with risk of diverticulitis in a large cohort of women.
Methods:
We followed 50,019 women in the Nurses’ Health Study (1990–2014) who were aged 43–70 years and free of diverticulitis, cancer, and inflammatory bowel disease at baseline. Incident diverticulitis was identified through self-report with validity confirmed by review of medical records.
Results:
We documented 4,343 incident cases of diverticulitis, encompassing 1,106,402 person-years of follow-up. Compared to participants in the lowest quintile, the multivariable hazard ratio (HR) of diverticulitis in the highest quintile of total fiber intake was 0.86 (95% confidence interval [CI]: 0.78–0.95; P-trend=0.002). Fiber from fruits and cereals, but not vegetables, was associated with a decreased risk of diverticulitis. Furthermore, intake of total whole fruit intake and specific fruits such as apples/pears and prunes were associated with reduced risk of diverticulitis with a multivariable HR for diverticulitis of 0.95 (0.92–0.98; P-trend<0.001) for every serving increase of total whole fruit intake per day.
Conclusions:
Higher intake of dietary fiber and fiber from different food sources, except for vegetable fiber, are associated with a lower risk of diverticulitis in women. A greater intake of whole fruit is also associated with reduced risk.
Keywords: dietary fiber, fruit, vegetable, diverticulitis, diverticular disease, women
INTRODUCTION
Diverticulosis, or the presence of colonic diverticula, is very common in Western countries with prevalence increasing with age, reaching 60% by age 70.(1) The principle complication of diverticulosis is diverticulitis, acute inflammation of diverticula and the surrounding colon, which can lead to significant complications that necessitate colectomy. Diverticulitis is among the leading gastrointestinal indications for hospitalizations and outpatient clinic visits in the U.S.(2) Growing evidence has linked lifestyle and dietary factors to the etiopathogenesis of diverticulitis.(3, 4)
Fiber intake is perhaps the most studied dietary risk factor for diverticular disease, a collective term for both clinically significant and symptomatic diverticulosis and diverticulitis. The observations of striking geographical differences and increase after industrialization in the prevalence of diverticular disease have contributed to the belief that fiber intake plays a significant role in its development.(5) This hypothesis is supported by findings from our group and others in prospective cohort studies.(4, 6–9) Furthermore, the protective effects of dietary fiber may vary according to fiber subtypes and food sources.(6–8) However, a potential drawback from prior studies is the inability to distinguish between diverticulitis, diverticular bleeding, and symptomatic uncomplicated diverticulosis. Diverticular bleeding and diverticulitis have demonstrated distinct clinical risk factors and etiopathogenesis(10), and two recent colonoscopy-based studies have cast doubt on the relationship between a low-fiber diet and diverticulosis(1, 11), highlighting the importance of considering diverticulitis separately from other phenotypes of diverticular disease. Additionally, most prior studies relied on registry-level data and included hospitalized patients who generally represent more severe cases. We recently reported that adherence to a healthy lifestyle including higher fiber intake was associated with a substantial decrease in the risk of diverticulitis that included both less severe forms of the disease treated in the outpatient setting and more severe cases that led to hospitalization.(12) Yet, data on fiber subtypes and diverticulitis are still lacking in women, who experience a disproportionate burden of disease.(13–15)
Finally, as major sources of dietary fiber, individual fruits and vegetables vary in fiber content and composition of other nutrients that may impact their beneficial effects. A recent genome-wide association analysis showed that genes associated with diverticular disease shared a common etiology with fresh fruit intake(16), further suggesting a mechanistic link. However, no prior study has examined what types of intake of fruits and vegetables may influence risk of diverticulitis. Therefore, we conducted a comprehensive, prospective evaluation of dietary fiber and fruit and vegetable intake in relation to risk of diverticulitis in a large cohort of women, the Nurses’ Health Study (NHS).
METHODS
Study Population
The NHS is a cohort of 121,700 U.S. female registered nurses aged 30 to 55 years at enrollment in 1976.(17) Participants have been mailed questionnaires every two years since inception querying demographics, lifestyle factors, medical history, and disease outcomes, with a follow-up rate greater than 90% of available person-time. The study was approved by Institutional Review Boards of Brigham and Women’s Hospital. Return of the questionnaires was considered to imply written informed consent.
We excluded participants who reported a diagnosis of diverticulitis, non-melanoma cancers, or inflammatory bowel disease prior to baseline in 1990, those who had incomplete information for dietary data, and those who reported implausible total energy intake (< 500 or > 3500 kcal/day). After exclusions, a total of 50,019 women were included in the primary analysis (Supplemental Figure 1).
Dietary Assessment
Dietary intake data were assessed through administration of a validated 131-item semi-quantitative food frequency questionnaire (FFQ) every 4 years, in which participants were asked how often they typically consumed each food of a standard portion size during the previous year. We included all whole fruits and all vegetables on the FFQ in our analyses, and fruit juice including apple, orange, grapefruit, and other juice was considered separately as it typically includes added sugar and is associated with an increased risk of adverse health outcomes such as diabetes(18) and greater weight gain(19). Fruits and vegetables with similar culinary usages and nutrient profiles were combined, e.g., apples and pears. We ranked fruits and vegetables by their proportional contributions to total intake of fruit fiber or vegetable fiber based on results from all available FFQs.
Daily intake for each nutrient was calculated by multiplying the reported frequency of each food item by its nutrient content, and then summing across foods. Fiber intake was calculated using the Association of Official Analytical Chemists method (accepted by the US Food and Drug Administration and the Food and Agriculture Organization of the World Health Organization for nutrition labeling purposes).(20) Data on soluble fiber and insoluble fiber were available directly from some manufactures, calculations from ingredients (personal communication from USDA), analysis done by Kellogg’s specifically from the Channing laboratory, as well as other dietary fiber food composition data (Horvath and Robertson 1986, USDA 1993).(7) We adjusted fiber intake for total caloric intake using the nutrient residual method.(21) Fiber intake from major food sources, including cereals, vegetables, and fruits, was also considered separately. FFQs have shown good reproducibility and validity for assessing intake of fiber, fruits, and vegetables.(22, 23) With a correction for the ratio of the within-person to the between-person variation, the correlation coefficient comparing diet assessment from FFQ with multiple 7-day dietary records was 0.68 for dietary fiber and ranged from 0.38 (strawberries) to 0.95 (bananas) for individual fruits (median: 0.76) and 0.25 (kale, mustard greens or chard greens) to 0.73 (lettuce) for individual vegetables (median: 0.46).
Ascertainment of Diverticulitis
Our primary endpoint was diverticulitis requiring antibiotic therapy or hospitalization. As described previously(24, 25), follow-up questionnaires in 2008, 2012, and 2014 ascertained using a series of questions diverticulitis requiring antibiotic therapy or hospitalization; diverticular bleeding; and diverticulosis without diverticulitis or diverticular bleeding. This logic structure minimizes misclassification between these entities. If participants reported they ever had a diagnosis of diverticulitis, they were subsequently asked the year of each episode dating back to 1990. In a validation study, we reviewed 107 medical records from women reporting incident diverticulitis and 88% of cases were confirmed.
Assessment of Covariates
Participants reported height at the time of enrollment. At baseline and updated biennially, information on body weight, smoking status, menopausal status and menopausal hormone use, physical examination, and use of multivitamins, aspirin, other nonsteroidal anti-inflammatory drugs (NSAIDs), or acetaminophen were obtained. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Physical activity was assessed every 2–4 years using validated questionnaires.(26) Hypertension and hypercholesterolemia were self-reported, with their validity previously confirmed.(27) In our analysis, we allowed covariates to be time-varying using the most recent information.
Statistical Analysis
Person-time was calculated from the date of baseline questionnaire until the date of diagnosis of diverticulitis, death, last follow-up questionnaire, or the end of the study period (June 2014), whichever came first. We censored participants who reported a new diagnosis of gastrointestinal cancer or inflammatory bowel disease at the date of diagnosis. We used simple updating or the most recent dietary information available (i.e., the intake reported on the most recent FFQ at the start of each 2-year follow-up interval). Missing values for a given FFQ were carried forward from prior available assessments.
Participants were categorized into quintiles according to their energy-adjusted fiber intake(28, 29). Linear trend was assessed by assigning the median value to each category and modeling this as a continuous variable. Using Cox proportional hazards regression with time-varying dietary intake and covariates, we estimated hazard ratios (HRs) and 95% confidence intervals (CIs). Proportional hazards assumption was evaluated by testing the significance of the interaction term between exposure and age by using the Wald test, and no violation of the proportional hazards assumption was observed (P for interaction > 0.05).
We stratified the analysis jointly by age at the start of follow-up and calendar time of the current questionnaire cycle to control for confounding by age, calendar time, and any possible two-way interactions between these two timescales. In multivariate analysis, we adjusted for BMI, menopausal status and menopausal hormone use, vigorous physical activity (METs ≥ 6, including jogging, running, bicycling, swimming, tennis, squash or racquetball, rowing, and heavy outdoor work), alcohol intake, smoking, use of aspirin, other NSAIDs, or acetaminophen, multivitamin use, recent physical examination as a proxy for healthcare engagement, hypertension, hypercholesterolemia, calorie intake, and red meat intake. We further controlled for total whole fruit intake for our analysis of individual fruits and total vegetable intake when testing individual vegetables for any independent association.
Statistical analyses were conducted using SAS software version 9.4 (SAS Institute Inc., Cary, NC). All p-values are 2-sided and < 0.05 was considered statistically significant.
RESULTS
At baseline, women had a mean age of 55 years and an average total fiber intake of 18.0 g. Participant characteristics according to quintiles of energy-adjusted dietary fiber intake are shown in Table 1. Total fiber intake was positively correlated with age, physical activity, multivitamin use, recent history of physical examination, hypercholesterolemia, and diabetes, and was inversely correlated with BMI, alcohol intake, acetaminophen use, other NSAID use, and current smoking. Participants who had higher fiber intake tended to consume more fruit, vegetables, and whole grains but less red meat, resulting in a higher healthy eating index score.
Table 1.
Baseline age-adjusted characteristics of participants according to energy-adjusted dietary fiber intake in Nurses’ Health Study (1990)
| Quintiles of dietary fiber intake | |||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Age, years* | 53.1 (6.5) | 54.2 (6.7) | 55.3 (6.7) | 56.3 (6.7) | 57.4 (6.7) |
| Body mass index, kg/m2 | 25.8 (4.9) | 25.7 (4.7) | 25.5 (4.5) | 25.3 (4.4) | 24.8 (4.3) |
| Alcohol, g/d | 7.3 (12.5) | 5.1 (8.5) | 4.4 (7.4) | 3.7 (6.6) | 2.9 (5.6) |
| Physical activity, MET-h/week | 12.7 (19.9) | 14.8 (20.0) | 16.7 (22.1) | 18.3 (25.1) | 21.7 (25.2) |
| Past smoker, % | 37 | 39 | 42 | 42 | 43 |
| Current smoker, % | 22 | 14 | 11 | 9 | 7 |
| Premenopausal, % | 27 | 27 | 25 | 25 | 23 |
| Multivitamin use, % | 33 | 36 | 39 | 40 | 46 |
| Aspirin use, % | 23 | 23 | 22 | 22 | 22 |
| Other NSAID use, % | 19 | 18 | 18 | 17 | 17 |
| Acetaminophen use, % | 15 | 15 | 14 | 12 | 12 |
| History of hypertension, % | 26 | 26 | 26 | 26 | 26 |
| History of hypercholesterolemia, % | 32 | 35 | 36 | 38 | 41 |
| History of diabetes, % | 2.4 | 2.7 | 3.1 | 3.6 | 3.9 |
| Physical examination, % | 68 | 71 | 73 | 75 | 75 |
| Total energy intake, kcal/d | 1738(526) | 1777(513) | 1779(502) | 1754(491) | 1707(511) |
| Cereal fiber intake, g/d | 3.5(1.3) | 4.4(1.7) | 5.2(2.1) | 6.1(2.5) | 8.9(6.3) |
| Vegetable fiber intake, g/d | 4.6(1.5) | 6.0(1.7) | 6.9(2.0) | 8.1(2.6) | 10.2(4.3) |
| Fruit fiber intake, g/d | 2.2(1.2) | 3.4(1.5) | 4.2(1.8) | 5.2(2.2) | 6.9(3.6) |
| Total fruit intake, serving/d | 0.9(0.6) | 1.4(0.8) | 1.7(0.9) | 2.1(1.0) | 2.7(1.6) |
| Vegetable intake, serving/d | 2.2(1.1) | 3.0(1.2) | 3.6(1.5) | 4.2(1.8) | 5.3(2.8) |
| Red meat intake, serving/d | 1.1(0.7) | 1.0(0.6) | 0.9(0.5) | 0.7(0.5) | 0.5(0.4) |
| Whole grain intake, serving/d | 9.7(7.9) | 15.9(10.5) | 20.8(12.7) | 26.1(14.7) | 36.5(22.0) |
| Alternate Healthy Eating Index score | 41.1(8.5) | 46.3(8.1) | 50.0(8.0) | 53.9(8.1) | 59.6(8.7) |
| Protein intake, g/d | 74.8(14.8) | 76.2(12.4) | 77.2(12.3) | 77.5(12.3) | 77.2(14.4) |
| Cholesterol intake, g/d | 244(72) | 231(60) | 220(60) | 209(59) | 186(67) |
Values are means (SD) or percentages and are standardized to the age distribution of the study population. The mean values (ranges) for quintiles of dietary fiber intake were 12.5 (1.6–14.8), 16.2 (14.9–17.5), 18.8 (17.6–20.2), 21.8 (20.3–23.8), and 28.5 (23.9–135) g/d.
Value is not adjusted for age.
We documented a total of 4,343 incident cases of diverticulitis over 24 years, encompassing 1,106,402 person-years of follow-up. The incidence of diverticulitis in our cohort is similar to other U.S. and European populations(30) taking into consideration that prior population-based studies included only hospitalized events and that the incidence rate is higher in women(13–15). Compared with women in the lowest quintile of total fiber intake, who had a diverticulitis incidence rate of 387 per 100,000 person-years, women in the highest quintile had an incidence rate of 376 per 100,000 person-years, leading to an unadjusted rate difference of 11 fewer cases of diverticulitis in women consuming the most fiber.
In age-adjusted analyses, higher intake of total fiber and fiber from cereals, fruit and vegetables were each associated with reduced risk of diverticulitis (Table 2). After further adjusting for other risk factors, including red meat intake, the associations between incident diverticulitis and total, cereal, and fruit fiber did not materially change, whereas the association with vegetable fiber was attenuated. Compared to women in the lowest quintile of dietary intake, the multivariable HRs (95% CIs) in the highest quintile were 0.86 (0.78–0.95; P-trend = 0.002) for total fiber, 0.90 (0.81–0.99; P-trend = 0.03) for cereal fiber, 0.83 (0.75–0.92; P-trend < 0.001) for fruit fiber, and 0.92 (0.83–1.01; P-trend = 0.31) for vegetable fiber. Additional adjustment for highest degree received (registered nurse, Bachelors, Masters or Doctorate) as a proxy of socioeconomic status did not change the effect estimates. We then categorized participants according to total fiber intake into 3 groups, <18 g/d, 18–25 g/d, and ≥25 g/d. The lower cut-off point of 18 g/d was chosen to represent the mean intake of total fiber among participants in our study at baseline, and the higher cut-off of 25 g/d was based on recommended intake for total fiber for adult women(31). Compared to women who consumed total fiber less than 18 g/d, those consuming total fiber of 25 g/d or more had a 13% reduced risk of incident diverticulitis (multivariable HR: 0.87; 95% CI: 0.79–0.96).
Table 2.
Energy-adjusted dietary fiber intake and risk of diverticulitis in Nurses’ Health Study (1990–2014)
| Quintiles of dietary intake | ||||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | P for trend | |
| Total fiber | ||||||
| Median (range), g/d | 13.0 (0–14.8) | 16.3 (14.8–17.6) | 18.9 (17.6–20.3) | 21.8 (20.3–23.8) | 27.0 (23.8–135) | |
| Incidence per 100,000 person-years | 387 | 396 | 405 | 398 | 376 | |
| Age | 1 (ref) | 0.96 (0.87, 1.05) | 0.94 (0.86, 1.04) | 0.90 (0.81, 0.98) | 0.82 (0.74, 0.90) | <0.001 |
| Multivariable* | 1 (ref) | 0.96 (0.88, 1.06) | 0.95 (0.86, 1.05) | 0.91 (0.83, 1.01) | 0.86 (0.78, 0.95) | 0.002 |
| Multivariable+red meat | 1 (ref) | 0.96 (0.88, 1.06) | 0.95 (0.86, 1.05) | 0.91 (0.83, 1.01) | 0.86 (0.78, 0.95) | 0.002 |
| Cereal fiber | ||||||
| Median (range), g/d | 2.9 (0–3.7) | 4.3 (3.7–4.9) | 5.6 (4.9–6.2) | 7.0 (6.2–8.0) | 9.8 (8.0–122) | |
| Incidence per 100,000 person-years | 367 | 388 | 410 | 413 | 385 | |
| Age | 1 (ref) | 0.99 (0.90, 1.09) | 0.99 (0.90, 1.09) | 0.96 (0.87, 1.06) | 0.86 (0.78, 0.95) | 0.001 |
| Multivariable* | 1 (ref) | 0.98 (0.89, 1.08) | 0.99 (0.90, 1.09) | 0.98 (0.89, 1.08) | 0.89 (0.81, 0.99) | 0.02 |
| Multivariable+red meat | 1 (ref) | 0.98 (0.89, 1.08) | 0.99 (0.90, 1.09) | 0.98 (0.89, 1.08) | 0.90 (0.81, 0.99) | 0.03 |
| Fruit fiber | ||||||
| Median (range), g/d | 1.4 (0–2.0) | 2.6 (2.0–3.2) | 3.8 (3.2–4.4) | 5.2 (4.4–6.1) | 7.7 (6.1–35.1) | |
| Incidence per 100,000 person-years | 405 | 409 | 410 | 386 | 352 | |
| Age | 1 (ref) | 0.99 (0.90, 1.08) | 0.97 (0.88, 1.06) | 0.89 (0.81, 0.98) | 0.79 (0.71, 0.87) | <0.001 |
| Multivariable* | 1 (ref) | 0.99 (0.90, 1.09) | 0.98 (0.89, 1.08) | 0.92 (0.84, 1.01) | 0.83 (0.75, 0.92) | <0.001 |
| Multivariable+red meat | 1 (ref) | 0.99 (0.90, 1.09) | 0.98 (0.89, 1.08) | 0.92 (0.83, 1.01) | 0.83 (0.75, 0.92) | <0.001 |
| Vegetable fiber | ||||||
| Median (range), g/d | 3.1 (0–3.9) | 4.6 (3.9–5.2) | 5.8 (5.2–6.4) | 7.2 (6.4–8.3) | 10.0 (8.3–49.2) | |
| Incidence per 100,000 person-years | 432 | 381 | 380 | 406 | 365 | |
| Age | 1 (ref) | 0.93 (0.84, 1.02) | 0.94 (0.85, 1.03) | 1.01 (0.92, 1.10) | 0.90 (0.82, 0.99) | 0.14 |
| Multivariable* | 1 (ref) | 0.93 (0.85, 1.02) | 0.94 (0.86, 1.04) | 1.02 (0.92, 1.11) | 0.91 (0.83, 1.01) | 0.27 |
| Multivariable+red meat | 1 (ref) | 0.93 (0.85, 1.02) | 0.94 (0.86, 1.04) | 1.02 (0.93, 1.12) | 0.92 (0.83, 1.01) | 0.31 |
Further adjusted for body mass index (<22.5, 22.5–24.9, 25.0–27.4, 27.5–29.9, 30.0–34.9, ≥35 kg/m2), menopausal status and menopausal hormone use (premenopausal and postmenopausal hormone use (never, past, current), vigorous activity (0, 0.1–3.4, 3.5–10.4, 10.5–28.4, ≥28.5 MET-h/week), alcohol intake (0, 0–4.9, 5.0–9.9, 10.0–14.9, ≥15.0 g/d), smoking (never smoker, past smoker, current smoker (1–14, 15–24, ≥25 cigarettes/d)), aspirin use (yes/no), other nonsteroidal anti-inflammatory drug use (yes/no), multivitamin use (yes/no), acetaminophen use (yes/no), physical examination (yes/no), hypertension (yes/no), hypercholesterolemia (yes/no), and calorie intake (quintile). Results for cereal, fruit, and vegetable fiber were similar if further mutually adjusting for fiber from other sources (i.e., except for the one under examination).
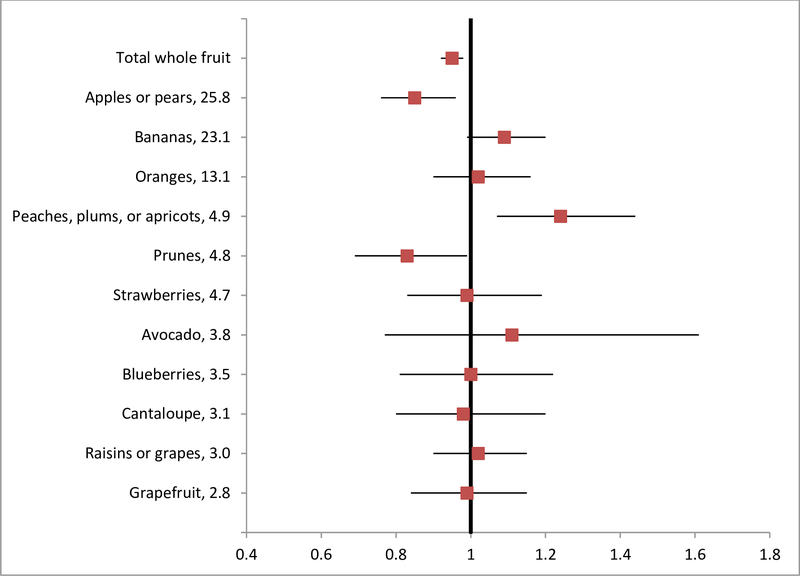
We also found that total whole fruit intake was associated with a reduced risk of diverticulitis (Figure 1). The multivariable HR (95% CI) of diverticulitis was 0.95 (0.92–0.98; P < 0.001) for every serving increase of total whole fruit intake per day. When individual fruits were evaluated, increased intake of apples or pears (HR, 0.85; 95% CI, 0.76–0.96; P = 0.009) and prunes (HR, 0.83; 95% CI, 0.69–0.99; P = 0.04) showed significant associations with lower risk of diverticulitis, independent of total whole fruit intake. In contrast to the inverse association seen for whole fruit, fruit juice was not significantly associated with risk of diverticulitis, with a multivariable HR (95% CI) of 1.04 (0.99–1.08; P-trend = 0.10) for every serving increase of fruit juice per day.
Figure 1.
Total whole and individual fruit intake and risk of diverticulitis in NHS (1990–2014). The values following fruit names represented percentage of contribution to total fruit fiber based on data from food frequency questionnaires in NHS. Total whole fruit does not include fruit juice. Hazard ratios were shown for increase of one serving per day, adjusted for age, body mass index, menopausal status and menopausal hormone use, vigorous activity, alcohol intake, smoking, aspirin use, other nonsteroidal anti-inflammatory drug use, multivitamin use, acetaminophen use, physical examination, hypertension, hypercholesterolemia, calorie intake, and red meat intake. Results for individual fruits were further adjusted for total whole fruit intake.
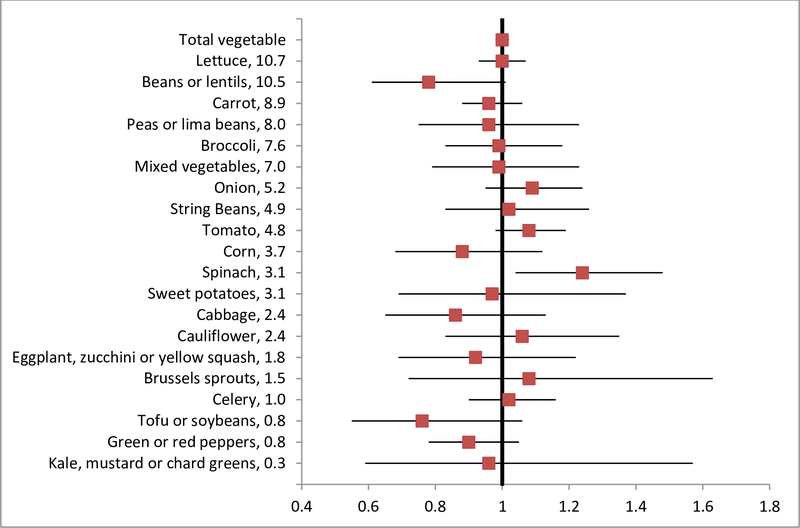
Increased total vegetable intake was not significantly associated with a reduced risk of diverticulitis (HR, 1.00; 95% CI, 0.98–1.02; P = 0.71) (Figure 2). Among individual vegetables, beans or lentils showed a trend towards an inverse association with diverticulitis independent of total vegetable intake, with HR (95% CI) of 0.78 (0.61–1.01; P = 0.06) for every serving increase of intake per day.
Figure 2.
Total and individual vegetable intake and risk of diverticulitis in NHS. The values following vegetable names represented percentage of contribution to total vegetable fiber based on data from food frequency questionnaires in NHS. Hazard ratios were shown for increase of one serving per day, adjusted for age, body mass index, menopausal status and menopausal hormone use, vigorous activity, alcohol intake, smoking, aspirin use, other nonsteroidal anti-inflammatory drug use, multivitamin use, acetaminophen use, physical examination, hypertension, hypercholesterolemia, calorie intake, and red meat intake. Results for individual vegetables were further adjusted for total vegetable intake.
Evaluating soluble and insoluble fiber separately, insoluble fiber showed a stronger association with risk of diverticulitis compared to soluble fiber (Supplemental Table 1). Compared to women in the lowest quintile of dietary intake, the multivariable HRs (95% CIs) in the highest quintile were 0.95 (0.86–1.05; P-trend = 0.57) for soluble fiber and 0.86 (0.78–0.95; P-trend = 0.004) for insoluble fiber.
We also separately examined the relationship between consumption of some other processed vegetable foods (that were not accounted into total vegetable intake), including potatoes (baked or mashed), potato chips, and French fries and diverticulitis. We found that after adjusting for other risk factors including fiber intake, intake of baked or mashed potatoes (HR for every serving increase of intake per day: 1.20; 95% CI: 1.07–1.36; P = 0.003), but not French fries (HR: 0.98; 95% CI: 0.63–1.51; P = 0.91) or potato chips (HR: 1.13; 95% CI: 0.96–1.32; P = 0.13) were associated with a modest increase in the risk of diverticulitis.
DISCUSSION
In a prospective cohort of US women, higher fiber intake was associated with reduced risk of incident diverticulitis. Fiber from fruits and cereals, but not vegetables, was also inversely associated with diverticulitis. Additionally, greater consumption of total whole fruit and specific fruits such as apples/pears and prunes were associated with a lower risk of diverticulitis.
The inverse association between fiber intake and diverticulitis is consistent with findings from previous prospective studies. In an earlier analysis utilizing the parallel male cohort, Health Professionals Follow-Up Study (HPFS), over relatively short-term follow-up (1988 to 1992), Aldoori et al reported that total fiber intake and fiber from fruit and vegetables, but not cereal, were associated with reduced risk of symptomatic diverticular disease in men.(6) The current study extends our prior investigation in men(12, 32) by offering evidence that fiber intake is associated with reduced risk of diverticulitis in women, who experience a disproportionate burden of the disease particularly at older ages(13–15). Crowe et al confirmed the inverse association between fiber intake and risk of diverticular disease requiring hospitalization or as the cause of death among men and women in the UK-based EPIC-Oxford cohort.(4) It was subsequently demonstrated that a higher intake of dietary fiber, specifically fruit fiber and cereal fiber, was associated with a reduction in the risk of diverticular disease among women.(8) However, as noted earlier, these studies are limited by the inability to distinguish between diverticulitis, diverticular bleeding, and symptomatic uncomplicated diverticulosis; additionally, the two aforementioned UK studies were based on registry-level data and included only hospitalized patients; the influence of dietary fiber intake on less severe and more common presentations(33) remains unclear.
In contrast to findings from prospective studies of diverticulitis or diverticular disease requiring hospitalization, low intake of dietary fiber was not associated with increased risk of uncomplicated diverticulosis in cross-sectional, colonoscopy-based studies(1, 11, 34). The divergent associations with diverticulitis and diverticulosis suggest that fiber may not be associated with the development of diverticulosis, but may play a role in preventing the inflammation associated with diverticulitis. Furthermore, data from the colonoscopy-based studies may not be comparable to our findings since diet was assessed after colonoscopy and patients may have modified their diet due to early symptoms or had differential recall according to the awareness of the disease.
The associations of fruits and vegetables with diverticular disease have also been investigated in other observational studies, but results have been less consistent. In a case-control study conducted in Greece, patients with radiologically-confirmed diverticulosis had less frequent consumption of vegetables compared to controls, whereas the frequency of fruit consumption was not significantly different.(35) Another case-control study in Taiwan, however, found consumption frequency of fruit and vegetables was not associated with right-sided asymptomatic diverticulosis.(36) While these studies were limited by the retrospective design and small sample size, it is also likely that diverticulosis, in particular right-sided diverticulosis which is predominant in Asia, has different pathophysiology from left-sided diverticular disease or diverticulitis.(37) An earlier analysis from the HPFS cohort demonstrated energy-adjusted associations of symptomatic diverticular disease with some fruits and vegetables, such as romaine or leafy lettuce, peaches, apricots, or plums, oranges, apples, and blueberries.(6) Due to high correlations between individual and total fruits or vegetables, it is critical to control for total fruit or vegetable intake in the evaluation of any independent relationship between individual fruits or vegetables and diverticulitis. Our findings indicate that overall intake of fruit may be more important compared to individual items in influencing the risk of diverticulitis. Apples or pears did appear to have the strongest individual associations, but this may reflect that these items were by far the most commonly consumed, contributing to 25.8% of total fruit fiber. Meanwhile, differences in contributions to fiber intake did not appear to account for the association of specific fruits and vegetables with disease risk. It is possible that other nutrients in the individual fruits may play a role in conferring the benefits.
The differences in the associations of fiber subtypes with diverticulitis were also consistent with the literature.(8) In parallel with the more pronounced association of fruit fiber and to a lesser extent, cereal fiber, with risk of diverticulitis compared to vegetable fiber, we also observed a stronger association for insoluble vs. soluble fiber. Yet, these results should be carefully interpreted as the distribution of total fiber between soluble and insoluble subtypes is dependent on the method of analysis.(38) It is also worth noting that although we observed a significant linear trend for total, cereal fiber, and fruit fiber, the HRs were only significant by comparing the highest to the lowest quintile, suggesting a potential threshold of fiber intake for achieving the beneficial effects.
The biological mechanism through which dietary fiber may decrease risk of diverticular disease was initially hypothesized to be mediated by its effects on colonic motility and intraluminal pressure.(39, 40) More recent evidence suggests that fiber alleviates obesity-induced chronic inflammation and also has weight-unrelated anti-inflammatory effects, the latter of which is likely mediated through intestinal microbiota.(41, 42) Meanwhile, sex hormones have been shown to alter intestinal permeability(43) and the gut microbiome(44). Prior studies suggested that menopausal hormone therapy was associated with increased risk of diverticular disease in women.(4, 45) Studies also showed that of the three sources of fiber, fruit fiber had the strongest inverse association with circulating concentrations of estradiol, followed by grain fiber; in contrast, vegetable fiber was not associated.(46) Taken together, fiber from different food sources may have divergent interactions with sex hormones and gut microbiome that in turn influence risk of diverticulitis in women. However, future investigations are warranted to verify our hypothesis and better understand the potentially varying roles of fiber subtypes and food sources in the development of the disease.
The strengths of our study include the prospective design, detailed and repeated collection of diet and lifestyle information, long-term follow-up and ascertainment of diverticulitis managed in the inpatient and outpatient setting. Moreover, detailed, contemporaneous information on potential confounders was collected in parallel with fiber, fruit, and vegetable intake.
Several limitations are worth noting. First, the ascertainment of dietary intake and diverticulitis were based on self-report. However, the validity of our dietary instruments has previously been demonstrated(22, 23), and confirmation of self-reported diverticulitis is high. Second, the diagnosis of diverticulitis was based on recall among participants who responded to the 2008, 2012, and 2014 questionnaires, which we believe will not result in bias because our outcome is unlikely to be fatal.(47) Third, information is lacking on the severity of diverticulitis or whether it was associated with hospitalization. Finally, our cohorts included mostly Caucasian health professionals, and future studies in other ethnic groups are needed considering potential ethnic disparities in diverticular disease.
In conclusion, a higher intake of fiber is associated with a lower incidence of diverticulitis. Potential variations exist in the effects of fiber from different food sources. Total whole fruit intake and specific fruits such as apples/pears and prunes are also associated with a reduced risk. Our findings lend support for public health recommendations of maintaining sufficient fiber intake from diet and provide practical dietary guidance for patients in the prevention of diverticulitis.
Supplementary Material
STUDY HIGHLIGHTS.
WHAT IS CURRENT KNOWLEDGE
Diverticulitis is a common gastrointestinal indication for hospitalization and outpatient clinic visits.
The impact of fiber intake on diverticulitis is not well understood in women.
Little is known about the variations in the protective effects of fiber according to food sources.
WHAT IS NEW HERE
Higher fiber intake is associated with a reduced risk of incident diverticulitis in women.
The association may vary according to food sources.
Consumption of total whole fruit and specific fruits are also associated with a reduced risk.
Acknowledgements:
We would like to thank the participants and staff of the Nurses’ Health Study for their valuable contributions.
Funding: This work was supported by grants UM1 CA186107, R01 DK101495 and K24 DK098311 from the National Institutes of Health. The funders had no role in study design, data collection and analysis, interpretation of data, writing of the report, and decision to submit the paper for publication.
Footnotes
Declaration of interests: Andrew T. Chan receives consulting fees from Janssen, Pfizer Inc., and Bayer Pharma AG for work unrelated to the topic of this manuscript. The remaining authors have no conflicts of interest to disclose.
REFERENCES
- 1.Peery AF, Barrett PR, Park D, et al. A high-fiber diet does not protect against asymptomatic diverticulosis. Gastroenterology 2012;142:266–72 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peery AF, Crockett SD, Murphy CC, et al. Burden and Cost of Gastrointestinal, Liver, and Pancreatic Diseases in the United States: Update 2018. Gastroenterology 2019;156:254–272 e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strate LL. Lifestyle factors and the course of diverticular disease. Dig Dis 2012;30:35–45. [DOI] [PubMed] [Google Scholar]
- 4.Crowe FL, Appleby PN, Allen NE, et al. Diet and risk of diverticular disease in Oxford cohort of European Prospective Investigation into Cancer and Nutrition (EPIC): prospective study of British vegetarians and non-vegetarians. BMJ 2011;343:d4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Painter NS, Burkitt DP. Diverticular disease of the colon: a deficiency disease of Western civilization. Br Med J 1971;2:450–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aldoori WH, Giovannucci EL, Rimm EB, et al. A prospective study of diet and the risk of symptomatic diverticular disease in men. Am J Clin Nutr 1994;60:757–64. [DOI] [PubMed] [Google Scholar]
- 7.Aldoori WH, Giovannucci EL, Rockett HR, et al. A prospective study of dietary fiber types and symptomatic diverticular disease in men. J Nutr 1998;128:714–9. [DOI] [PubMed] [Google Scholar]
- 8.Crowe FL, Balkwill A, Cairns BJ, et al. Source of dietary fibre and diverticular disease incidence: a prospective study of UK women. Gut 2014;63:1450–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mahmood MW, Abraham-Nordling M, Hakansson N, et al. High intake of dietary fibre from fruit and vegetables reduces the risk of hospitalisation for diverticular disease. Eur J Nutr 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Urabe M, Nishida T, Shimakoshi H, et al. Distinct Clinical Factors in Hospitalized Patients with Diverticular Bleeding and Diverticulitis. Digestion 2018:1–8. [DOI] [PubMed] [Google Scholar]
- 11.Peery AF, Sandler RS, Ahnen DJ, et al. Constipation and a low-fiber diet are not associated with diverticulosis. Clin Gastroenterol Hepatol 2013;11:1622–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu PH, Cao Y, Keeley BR, et al. Adherence to a Healthy Lifestyle is Associated With a Lower Risk of Diverticulitis among Men. Am J Gastroenterol 2017;112:1868–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wheat CL, Strate LL. Trends in Hospitalization for Diverticulitis and Diverticular Bleeding in the United States From 2000 to 2010. Clin Gastroenterol Hepatol 2016;14:96–103 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nguyen GC, Sam J, Anand N. Epidemiological trends and geographic variation in hospital admissions for diverticulitis in the United States. World J Gastroenterol 2011;17:1600–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kang JY, Hoare J, Tinto A, et al. Diverticular disease of the colon--on the rise: a study of hospital admissions in England between 1989/1990 and 1999/2000. Aliment Pharmacol Ther 2003;17:1189–95. [DOI] [PubMed] [Google Scholar]
- 16.Maguire LH, Handelman SK, Du X, et al. Genome-wide association analyses identify 39 new susceptibility loci for diverticular disease. Nat Genet 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colditz GA, Manson JE, Hankinson SE. The Nurses’ Health Study: 20-year contribution to the understanding of health among women. J Womens Health 1997;6:49–62. [DOI] [PubMed] [Google Scholar]
- 18.Muraki I, Imamura F, Manson JE, et al. Fruit consumption and risk of type 2 diabetes: results from three prospective longitudinal cohort studies. BMJ 2013;347:f5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mozaffarian D, Hao T, Rimm EB, et al. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med 2011;364:2392–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prosky L, Asp NG, Furda I, et al. Determination of total dietary fiber in foods and food products: collaborative study. J Assoc Off Anal Chem 1985;68:677–9. [PubMed] [Google Scholar]
- 21.Willett W, Stampfer MJ. Total energy intake: implications for epidemiologic analyses. Am J Epidemiol 1986;124:17–27. [DOI] [PubMed] [Google Scholar]
- 22.Rimm EB, Giovannucci EL, Stampfer MJ, et al. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol 1992;135:1114–26; discussion 1127–36. [DOI] [PubMed] [Google Scholar]
- 23.Feskanich D, Rimm EB, Giovannucci EL, et al. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc 1993;93:790–6. [DOI] [PubMed] [Google Scholar]
- 24.Ma W, Jovani M, Liu PH, et al. Association Between Obesity and Weight Change and Risk of Diverticulitis in Women. Gastroenterology 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jovani M, Ma W, Joshi AD, et al. Menopausal Hormone Therapy and Risk of Diverticulitis. Am J Gastroenterol 2019;114:315–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolf AM, Hunter DJ, Colditz GA, et al. Reproducibility and validity of a self-administered physical activity questionnaire. Int J Epidemiol 1994;23:991–9. [DOI] [PubMed] [Google Scholar]
- 27.Colditz GA, Martin P, Stampfer MJ, et al. Validation of questionnaire information on risk factors and disease outcomes in a prospective cohort study of women. Am J Epidemiol 1986;123:894–900. [DOI] [PubMed] [Google Scholar]
- 28.Willett W Nutritional Epidemiology Third ed: Oxford University Press; 2012. [Google Scholar]
- 29.Willett WC, Hunter DJ, Stampfer MJ, et al. Dietary fat and fiber in relation to risk of breast cancer. An 8-year follow-up. JAMA 1992;268:2037–44. [PubMed] [Google Scholar]
- 30.Bharucha AE, Parthasarathy G, Ditah I, et al. Temporal Trends in the Incidence and Natural History of Diverticulitis: A Population-Based Study. Am J Gastroenterol 2015;110:1589–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trumbo P, Schlicker S, Yates AA, et al. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. J Am Diet Assoc 2002;102:1621–30. [DOI] [PubMed] [Google Scholar]
- 32.Strate LL, Keeley BR, Cao Y, et al. Western Dietary Pattern Increases, and Prudent Dietary Pattern Decreases, Risk of Incident Diverticulitis in a Prospective Cohort Study. Gastroenterology 2017;152:1023–1030 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schechter S, Mulvey J, Eisenstat TE. Management of uncomplicated acute diverticulitis: results of a survey. Dis Colon Rectum 1999;42:470–5; discussion 475–6. [DOI] [PubMed] [Google Scholar]
- 34.Song JH, Kim YS, Lee JH, et al. Clinical characteristics of colonic diverticulosis in Korea: a prospective study. Korean J Intern Med 2010;25:140–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manousos O, Day NE, Tzonou A, et al. Diet and other factors in the aetiology of diverticulosis: an epidemiological study in Greece. Gut 1985;26:544–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin OS, Soon MS, Wu SS, et al. Dietary habits and right-sided colonic diverticulosis. Dis Colon Rectum 2000;43:1412–8. [DOI] [PubMed] [Google Scholar]
- 37.Matrana MR, Margolin DA. Epidemiology and pathophysiology of diverticular disease. Clin Colon Rectal Surg 2009;22:141–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marlett JA, Chesters JG, Longacre MJ, et al. Recovery of soluble dietary fiber is dependent on the method of analysis. Am J Clin Nutr 1989;50:479–85. [DOI] [PubMed] [Google Scholar]
- 39.Strate LL, Modi R, Cohen E, et al. Diverticular disease as a chronic illness: evolving epidemiologic and clinical insights. Am J Gastroenterol 2012;107:1486–93. [DOI] [PubMed] [Google Scholar]
- 40.Taylor I, Duthie HL. Bran tablets and diverticular disease. Br Med J 1976;1:988–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuo SM. The interplay between fiber and the intestinal microbiome in the inflammatory response. Adv Nutr 2013;4:16–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Llewellyn SR, Britton GJ, Contijoch EJ, et al. Interactions Between Diet and the Intestinal Microbiota Alter Intestinal Permeability and Colitis Severity in Mice. Gastroenterology 2018;154:1037–1046 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Braniste V, Jouault A, Gaultier E, et al. Impact of oral bisphenol A at reference doses on intestinal barrier function and sex differences after perinatal exposure in rats. Proc Natl Acad Sci U S A 2010;107:448–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Markle JG, Frank DN, Mortin-Toth S, et al. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science 2013;339:1084–8. [DOI] [PubMed] [Google Scholar]
- 45.Jovani M, Ma W, Joshi A, et al. Menopausal Hormone Therapy and Risk of Diverticulitis. accepted Am. J. Gastroenterol 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gaskins AJ, Mumford SL, Zhang C, et al. Effect of daily fiber intake on reproductive function: the BioCycle Study. Am J Clin Nutr 2009;90:1061–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liang C, Seeger JD, Dore DD. Implications of immortal person-time when outcomes are nonfatal. Ann Epidemiol 2016;26:212–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.