Summary
We evaluated the prevalence of rapid decline in kidney function, its potential risk factors and influence upon mortality in sickle cell disease (SCD) in a retrospective single-center study. Rapid decline of kidney function was defined as estimated glomerular filtration rate (eGFR) loss of >3.0 ml/min/1.73 m2 per year. A multivariable logistic regression model for rapid eGFR decline was constructed after evaluating individual covariates. We constructed multivariate Cox-regression models for rapid eGFR decline and mortality. Among 331 SCD patients (median age 29 years [intergquartile range, IQR: 20, 41]; 187 [56.5%] female) followed for median 4.01 years (IQR: 1.66, 7.19), rapid eGFR decline was noted in 103 (31.1%). History of stroke (odds ratio [OR]: 2.91, 95% confidenc interval [CI]: 1.25 – 6.77) and use of angiotensin converting enzyme inhibitors/angiotensin receptor blockers (OR: 3.17, 95%CI: 1.28–7.84) were associated with rapid eGFR decline. The rate of eGFR change over time was associated with mortality (hazard ratio [HR]: 0.99, 95%CI: 0.984-0.995, p = 0.0002). In Cox-regression, rapid eGFR decline associated with mortality (HR: 2.07, 95%CI: 1.039-4.138, p = 0.04) adjusting for age, sex and history of stroke. Rapid eGFR decline is common in SCD and associated with increased mortality. Long-term studies are needed to determine whether attenuating loss of kidney function may decrease mortality in SCD.
Keywords: Sickle cell disease, Kidney function, glomerular filtration, chronic kidney disease
INTRODUCTION
Sickle cell disease (SCD), one of the most common monogenic disorders worldwide, results in many structural and functional abnormalities of the kidney (Ataga, et al 2014). Chronic kidney disease (CKD), defined by evidence of kidney damage or decreased kidney function for three or more months (Levey and Coresh 2012), is common in SCD. Albuminuria is the most frequently assessed marker of kidney damage in clinical practice and occurs in up to 68% of adult patients with SCD (Ataga, et al 2014). Although the natural history of CKD in patients with SCD remains inadequately defined, multiple studies suggest that kidney function declines at a more rapid rate in SCD than in healthy individuals (Asnani, et al 2016, Derebail, et al 2019, Xu, et al 2018). We and others have reported on the prevalence of CKD as well as the rate of decline in estimated glomerular filtration rate (eGFR) in adults with SCD. (Asnani, et al 2016, Derebail, et al 2019, Xu, et al 2018) In our earlier publication, the baseline prevalence of CKD in this patient cohort was 19.9%, with an annual rate of decline in eGFR among individuals with HbSS and HbSβ0 thalassaemia of 2.05 ml/min/1.73 m2 per year (Derebail, et al 2019). Prior studies in other populations, including patients with hypertension and diabetes, have demonstrated that those with more rapid decline in kidney function had higher mortality (Rifkin, et al 2008, Shlipak, et al 2009). However, this relationship has never been examined in the sickle cell patient population. In this present study, we examined rapid decline in kidney function in an observational cohort of adult patients with SCD, factors associated with such decline and the association with mortality.
Materials and Methods
Patient Selection and Assessment of Mortality
The study design and participants of this study have been described previously (Derebail, et al 2019). Briefly, patients with SCD aged at least 18 years, followed at a single academic medical centre from 2004 to 2013, were identified via medical record review. We included patients with all common forms of SCD (e.g. homozygous sickle cell disease [HbSS], haemoglobin SC disease [HbSC], sickle-β0-thalassaemia [HbSβ0], sickle-β+-thalassaemia [HbSβ+], haemoglobin SE disease [HbSE], haemoglobin SD disease [HbSD] and sickle cell-hereditary persistence of fetal haemoglobin [SHPFH]). Evaluations were obtained when patients were seen during routine clinic visits in a non-crisis, “steady state” and absent of an acute vaso-occlusive episode at the time of assessment. The baseline visit was defined as the first available serum creatinine measurement during the follow-up period. Only those patients with two or more measures of kidney function over the observation period were included. Evaluation of decline in kidney function was obtained in patients who had no kidney transplant or need for dialysis at baseline. In addition, patients with a history of bone marrow transplantation, systemic lupus erythematosus, human immunodeficiency virus, or hepatitis B or C infection were excluded. Mortality during the observation period was assessed by review of patients’ medical records and by utilizing the US Social Security Death Index. The Institutional Review Board at the University of North Carolina at Chapel Hill approved the study, with a waiver of consent for analysis of deidentified data.
Definitions
We used the creatinine-based Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation to calculate eGFR (Levey, et al 2009). CKD was defined using a modification of Kidney Disease Improving Global Outcomes (KDIGO) guidelines that incorporate eGFR and levels of albuminuria (KDIGO 2013) based on the presence of proteinuria (at least 1+ on dipstick urinalysis) or eGFR < 90 ml/min/1.73 m2 (Becker 2011, Thompson, et al 2007). Hyperfiltration was defined as eGFR > 130 ml/min per 1.73 m2 for women and > 140 ml/min per 1.73 m2 for men (Haymann, et al 2010, Vazquez, et al 2014). Rapid decline of kidney function was defined as eGFR loss of >3.0 ml/min/1.73 m2 per year (Rifkin, et al 2008, Shlipak, et al 2009). Proteinuria was assessed semi-quantitatively by dipstick urinalysis and defined as absent if results were 0 to trace and present if ≥ 1+. Haemoglobinuria was defined as dipstick urinalysis with blood ≥ 1+, but with less than 5 red blood cells per high power field by microscopy. CKD progression was defined as a decline in eGFR to < 90 ml/min and at least a 25% decline in eGFR (KDIGO 2013).
Statistical analyses
All variables of interest were summarized by counts and percentages if categorical, or by median and interquartile ranges (IQR) if continuous. Patients were evaluated together and categorized by presumed disease severity based on the SCD genotype. Individuals with HbSS and HbSβ0 thalassaemia were categorized as “severe genotype” and those with HbSC and HbSβ+ thalassaemia were categorized as “mild genotype.” To evaluate the association of clinical and laboratory variables with rapid decline in eGFR, we first conducted a separate logistic regression analysis for each variable adjusted for baseline age and sex. We then performed a multivariable logistic regression analysis with backward elimination to select the variables significantly associated with rapid eGFR decline. The following variables were included in the initial multivariable model: haemoglobin, reticulocyte count, lactate dehydrogenase, baseline eGFR, history of stroke, hydroxycarbamide therapy, systolic blood pressure, use of angiotensin converting enzyme (ACE) inhibitors/angiotensin receptor blockers (ACE-I/ARB) and history of diabetes. The multivariable analysis was also adjusted for baseline age and sex. As proteinuria measures were missing for many patients, sensitivity analyses of the multivariable analysis were performed using four scenarios: 1) exclude proteinuria in the initial model, 2) include proteinuria in the initial model but use only patients with proteinuria values available, 3) include proteinuria in the initial model and use all patients by assigning no proteinuria to those with missing values, or 4) include proteinuria in the initial model and use all patients by assigning proteinuria to those with missing values.
To evaluate the association of rapid eGFR decline with mortality, we obtained the Kaplan-Meier estimates of survival probabilities for rapid and non-rapid eGFR decline groups, and performed log-rank testing to compare the survival probabilities for the two groups. We performed survival analyses utilizing a Cox regression model in two scenarios: 1) using the rate of eGFR change over time (a continuous variable) as the covariate; 2) using the indicator of rapid eGFR decline (a binary variable) as the covariate. Both analyses were adjusted for baseline age and sex. For the Cox regression model utilizing the binary variable for eGFR decline, we also performed additional multivariable modelling using covariates that were associated with exposure (eGFR decline > 3.0 ml/min/1.73 m2 per year) or with the outcome (death) in the unexposed. All analyses were conducted using SAS software, Version 9 for Windows Copyright © [2014] SAS Institute Inc., Cary, NC, USA.
Results
Patient Demographics and Prevalence of Rapid Decline in Estimated Glomerular Filtration Rate
Of the 427 patients with SCD in the cohort, 331 patients (HbSS = 218, HbSC = 67, HbSβ0 thalassaemia = 18, HbSβ+ thalassaemia= 22, HbSE = 2, HbSD = 2, SHPFH = 2) had at least two assessments of eGFR. Of these 331 patients included in the analysis, 327 (98.8%) self-identified as African-American and 4 (1.2%) were of other race/ethnicity designations. The median age of the patients with at least 2 measurements of eGFR was 29 years (IQR: 20 – 41 years) and 187 (56.5%) were female. The patients had a median follow-up duration of 4.01 years (IQR: 1.66, 7.19). The median number of creatinine measurements was 6 (IQR 3 to 11). Among the severe genotype patients, the median was 7 (IQR 3.75 to 13) and in the mild genotype patients, the median was 5 (IQR 3 to 89). Patients with HbSS/HbSβ0 thalassaemia appeared to have lower median values of systolic blood pressure, diastolic blood pressure and haemoglobin, but higher white blood cell count, total bilirubin and lactate dehydrogenase than those with HbSC/HbSβ+ thalassaemia (Table I). Proteinuria was observed in 16.7% of patients, 19.9% in patients with HbSS/HbSβ0 thalassaemia versus 8.9% in patients with HbSC/HbSβ+ thalassaemia. Rapid decline of kidney function (eGFR decline >3.0 ml/min/1.73 m2 per year) was observed in 103 (31.1%) patients in total, 80 (33.9%) with HbSS/HbSβ0 thalassaemia and 21 (23.6%) with HbSC/HbSβ+ thalassaemia. Of the 103 with rapid eGFR decline, 27 (26.2%) had CKD (8 due to proteinuria alone and 19 due to eGFR < 90 ml/min/1.73 m2). In those without rapid decline (N=228), 35 (15.4%) had CKD (15 due to proteinuria alone and 20 due to eGFR < 90 ml/min/1.73 m2). One hundred and sixty-six (50.2%) patients demonstrated hyperfiltration at baseline, with a greater proportion noted in the severe genotype group than in the mild genotype group (58.5% v. 28.1%). Among the patients with hyperfiltration, 36 (21.7%) had declines in eGFR > 3 ml/min/1.73m2 per year, while among the patients without hyperfiltration, 67 (40.6%) patients had eGFR decline > 3 ml/min/1.73m2 per year. Sixty-six out of 331 patients (19.9%) had CKD progression, with 43 (41.8%) in the > 3ml/min/1.73m2 per year group vs. 23 (10.1%) in the ≤ 3 ml/min/1.73m2 per year group. CKD progression was significantly greater in patients with rapid decline of kidney function than in those without rapid decline of kidney function (difference: 0.32, 95% confidence interval [CI]: 0.21 – 0.43, p < 0.0001).
Table I:
Baseline Demographics, Laboratory and Clinical Data of Patients in the Cohort
| Variable | N | All SCD | N | HbSS/HbSβ0 Thalassaemia | N | HbSC/HBSβ+ Thalasaemia |
|---|---|---|---|---|---|---|
| Age (years) | 331 | 29 (20, 41) | 236 | 28.0 (20.5, 39.5) | 89 | 35 (20, 43) |
| Female sex | 331 | 187 (56.5) | 236 | 133 (56.4) | 89 | 51 (57.3) |
| Weight (kg) | 323 | 67.9 (59.2, 80.1) | 230 | 65.5 (57.1, 75.8) | 87 | 75.6 (65.8, 92.9) |
| Height (cm) | 125 | 170.3 (162.5, 175.7) | 89 | 170.3 (163, 175.9) | 33 | 168 (162, 173) |
| Systolic blood pressure (mm Hg) | 329 | 121 (111, 132) | 236 | 119 (109, 130) | 87 | 128 (118, 134) |
| Diastolic blood pressure (mm Hg) | 329 | 71 (64, 79) | 236 | 70 (62, 76) | 87 | 77 (70, 83) |
| WBC count (109/l) | 330 | 10.2 (7.6, 12.5) | 236 | 10.8 (8.4, 13) | 88 | 8.6 (6.5, 10.5) |
| Haemoglobin (g/l) | 330 | 95 (81, 109) | 236 | 88 (78, 100) | 88 | 120 (105, 130) |
| Reticulocyte count (109/l) | 305 | 189.6 (117.3, 275.9) | 222 | 218.7 (148.5, 303.4) | 79 | 115.6 (83.2, 184.8) |
| Haemoglobin F (%) | 143 | 4.9 (1.9, 9.4) | 124 | 6.5 (3.2, 10.4) | 29 | 0.9 (0.5, 2.6) |
| Blood urea nitrogen (mmol/l) | 311 | 2.86 (2.14, 3.93) | 226 | 2.86 (2.14, 3.57) | 79 | 3.21 (2.50, 3.93) |
| Creatinine (μmol/l) | 331 | 61.88 (53.04, 79.56) | 236 | 61.88 (53.04, 75.14) | 89 | 70.72 (61.88, 88.40) |
| eGFR (ml/min/1.73m2) | 331 | 135 (113.9, 152.1) | 236 | 140.4 (120.1, 155.9) | 89 | 120.7 (105.9, 137.1) |
| Hyperfiltration* | 331 | 166 (52.2) | 236 | 138 (58.5) | 89 | 25 (28.1) |
| Lactate dehydrogenase (u/l) | 267 | 853 (623, 1155) | 189 | 943 (752, 1280) | 73 | 582 (508, 789) |
| Total bilirubin (μmol/l) | 297 | 32.50 (20.52, 56.44) | 213 | 41.04 (25.66, 73.55) | 78 | 17.10 (13.68, 25.66) |
| Indirect bilirubin (μmol/l) | 71 | 34.21 (20.52, 63.28) | 52 | 40.37 (29.08, 68.42) | 18 | 18.81 (13.68, 23.95) |
| Ferritin (μg/l) | 135 | 249 (71, 880) | 95 | 307 (124, 1340) | 37 | 122 (44, 322) |
| Specific gravity | 169 | 1.01 (1.01, 1.01) | 121 | 1.01 (1.01, 1.01) | 44 | 1.01 (1.01, 1.01) |
| Proteinuria (yes) | 180 | 30 (16.7) | 131 | 26 (19.9) | 45 | 4(8.9) |
| Haemoglobinuria (yes) | 167 | 15 (9.0) | 119 | 15 (12.6) | 44 | 0 (0) |
| Hydroxycarbamide use (yes) | 329 | 110 (33.4) | 234 | 98 (41.9) | 89 | 12 (13.5) |
| ACE-I/ARB therapy (yes) | 329 | 36 (10.9) | 235 | 24 (10.2) | 88 | 12 (13.6) |
| Chronic RBC transfusion (yes) | 331 | 13 (3.9) | 236 | 11 (4.7) | 89 | 2 (2.3) |
| History of diabetes (yes) | 331 | 15 (4.5) | 236 | 7 (3.0) | 89 | 8 (9.0) |
| History of acute chest syndrome (yes) | 314 | 254 (80.9) | 226 | 197 (87.2) | 82 | 55 (67.1) |
| History of stroke (yes) | 304 | 41 (13.5) | 216 | 36 (16.7) | 82 | 5 (6.1) |
| History of leg ulcers (yes) | 271 | 39 (14.3) | 197 | 38 (19.3) | 71 | 1 (1.4) |
| History of avascular necrosis (yes) | 233 | 91 (39.1) | 162 | 64 (39.5) | 66 | 25 (37.9) |
| History of priapism** (yes) | 117 | 45 (38.5) | 86 | 37 (43) | 30 | 8 (26.7) |
All SCD groups included 6 patients (SE = 2, SD = 2, SHPFH = 2) not categorized as mild (HbSC/HBSβ+ thalasaemia) or severe genotype (HbSS/HbSβ0 thalassaemia).
Continuous variables presented as median and interquartile range (IQR). Categorial variables presented as count (percentage) - N(%).
Hyperfiltration defined as: as eGFR > 130 ml/min per 1.73 m2 for women and > 140 ml/min per 1.73 m2 for men
Male subjects only.
ACE-I/ARB – angiotensin converting enzyme inhibitors/angiotensin receptor blocker; eGFR – estimated glomerular filtration rate; RBC – red blood cellSCD – sickle cell disease; SD - haemoglobin SD disease; SE - haemoglobin SE disease; SHPFH - sickle cell-hereditary persistence of fetal haemoglobin; WBC – white blood cell
Association of Clinical and Laboratory Variables with Rapid Decline in Kidney Function
We examined baseline laboratory and clinical variables that were associated with rapid decline in eGFR in the cohort. In age- and sex-adjusted analyses, baseline haemoglobin (p = 0.007), ferritin (p = 0.01) and baseline eGFR (p = 0.01) were significantly associated with rapid decline in eGFR in all of the SCD patients (Table II). There was a trend towards an association of proteinuria (p = 0.06) with rapid decline in eGFR. Clinical variables significantly associated with rapid decline in eGFR were history of stroke (p = 0.002) and use of ACE-I/ARB (p = 0.006). Hyperfiltration was less likely to be associated with rapid eGFR decline (p=0.02). In patients with HbSS/HbSβ0 thalassaemia, ferritin (p = 0.03), baseline eGFR (p = 0.0015), history of stroke (p = 0.001) and use of ACE-I/ARB (p = 0.05) were significantly associated with a rapid decline in eGFR (Table II). Presence of proteinuria (p = 0.06) showed a trend towards an association with rapid decline in eGFR, but no association was seen between baseline haemoglobin (p = 0.1) and rapid decline in eGFR in patients with HbSS/HbSβ0 thalassaemia. Similarly, hyperfiltration was less likely to be associated with rapid decline (p=0.01). In patients with HbSC/HbSβ+ thalassaemia, history of diabetes (p = 0.02) and use of ACE-I/ARB (p = 0.04) were significantly associated with a rapid decline in eGFR (Table II). No statistically significant association was noted with hyperfiltration among these patients.
Table II:
Association of Covariates with Rapid Decline in Kidney Function (eGFR > 3 ml/min/1.73m2 per year) Based on Logistic Regression Analyses Adjusted for Age and Sex
| All SCD | HbSS/HbSβ0 | HbSC/HbSβ+ | ||||
|---|---|---|---|---|---|---|
| Variable | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p |
| WBC count | 0.98 (0.92, 1.05) | 0.5 | 0.98 (0.90, 1.06) | 0.6 | 0.91 (0.78, 1.07) | 0.3 |
| Haemoglobin | 0.83 (0.75, 0.95) | 0.007 | 0.87 (0.72, 1.04) | 0.1 | 0.8 (0.56, 1.14) | 0.2 |
| Reticulocyte count | 0.99 (0.99, 1.001) | 0.2 | 0.99 (0.99, 1.0) | 0.07 | 0.99 (0.989, 1.004) | 0.4 |
| Haemoglobin F | 0.99 (0.93, 1.04) | 0.6 | 0.94 (0.87, 1.01) | 0.07 | 1.27 (0.77, 2.1) | 0.4 |
| Ferritin | 1.0 (1.0, 1.001) | 0.01 | 1.0 (1.0, 1.001) | 0.03 | 1.0 (0.99, 1.001) | 0.4 |
| Lactate dehydrogenase | 1.0 (1.0, 1.001) | 0.2 | 1.0 (1.0, 1.001) | 0.5 | 1.001 (0.99, 1.003) | 0.5 |
| Total bilirubin | 0.98 (0.87, 1.10) | 0.7 | 0.95 (0.83, 1.08) | 0.4 | 0.44 (0.14, 1.37) | 0.2 |
| Indirect bilirubin | 0.78 (0.56, 1.09) | 0.1 | 0.80 (0.56, 1.14) | 0.2 | 0.08 (0.003, 2.11) | 0.1 |
| Baseline eGFR | 0.987 (0.98, 1.00) | 0.01 | 0.98 (0.97, 0.99) | 0.002 | 1.004 (0.98, 1.03) | 0.8 |
| Hyperfiltration | 0.48 (0.26, 0.89) | 0.02 | 0.39 (0.18, 0.83) | 0.01 | 0.43 (0.09, 1.96) | 0.3 |
| Baseline proteinuria | 2.22 (0.97, 5.05) | 0.06 | 2.41 (0.97, 5.96) | 0.06 | 1.22 (0.095, 15.8) | 0.9 |
| Baseline haemhaemoglobinuria* | 1.5 (0.5, 4.55) | 0.5 | 1.41 (0.46, 4.35) | 0.6 | - | - |
| Urine specific gravity | <0.001 (<0.001, >999.9) | 0.9 | >999.9 (<0.001, >999.9) | 0.6 | <0.001 (<0.001, >999.9) | 0.5 |
| Systolic blood pressure | 1.02 (0.99, 1.03) | 0.1 | 1.02 (0.99, 1.03) | 0.1 | 1.02 (0.99, 1.05) | 0.3 |
| Diastolic blood pressure | 1.01 (0.99, 1.02) | 0.5 | 1.01 (0.99, 1.03) | 0.3 | 0.99 (0.96, 1.04) | 0.8 |
| Weight | 1.004 (0.99, 1.02) | 0.6 | 1.01 (0.99, 1.03) | 0.4 | 1.01 (0.98, 1.03) | 0.6 |
| History of diabetes | 2.28 (0.76, 6.84) | 0.1 | 1.42 (0.28, 7.09) | 0.7 | 7.45 (1.33, 41.6) | 0.02 |
| History of stroke | 3.0 (1.5, 6.0) | 0.002 | 3.51 (1.62, 7.57) | 0.001 | 0.79 (0.08, 7.84) | 0.8 |
| History of acute chest syndrome | 1.29 (0.68, 2.43) | 0.4 | 1.31 (0.54, 3.17) | 0.6 | 0.93 (0.31, 2.74) | 0.9 |
| History of avascular necrosis | 0.92 (0.50, 1.69) | 0.8 | 0.99 (0.49, 2.02) | 1.0 | 0.87 (0.25, 3.04) | 0.8 |
| History of leg ulcers | 1.04 (0.49, 2.2) | 0.9 | 0.75 (0.33, 1.73) | 0.5 | <0.001 (<0.001, >999.9) | 1.0 |
| ACE-I/ARB therapy | 2.9 (1.35, 6.19) | 0.006 | 2.55 (1.0, 6.51) | 0.05 | 4.51 (1.08, 18.9) | 0.04 |
| Hydroxycarbamide therapy | 1.4 (0.85, 2.3) | 0.2 | 1.18 (0.67, 2.08) | 0.6 | 1.37 (0.31, 5.99) | 0.7 |
| Chronic RBC transfusion | 1.56 (0.48, 5.03) | 0.5 | 2.3 (0.65, 8.1) | 0.2 | <0.001 (<0.001, >999.9) | 1.0 |
Haemoglobinuria not present in individuals with HbSC/HbSβ+ thalassaemia.
ACE-I/ARB – angiotensin converting enzyme inhibitors/angiotensin receptor blocker; CI – confidence interval; eGFR – estimated glomerular filtration rate; OR – odds ratio; RBC – red blood cell; SCD – sickle cell disease; WBC – white blood cell
Proteinuria measures were missing for 151 (45.6%) of the total 331 patients, 104/228 (45.6%) of patients with eGFR decline ≤ 3ml/min/1.73m2 per year and 47/103 (45.6%) of patients with eGFR decline > 3ml/min/1.73m2 per year. We performed sensitivity analyses for the multivariable analyses using four scenarios: in the backward elimination procedure for variable selection, 1) exclude proteinuria in the initial model, 2) include proteinuria in the initial model but use only patients with proteinuria values available, 3) include proteinuria in the initial model and use all patients by assigning no proteinuria to those with missing values, or 4) include proteinuria in the initial model and use all patients by assigning proteinuria to those with missing values. Hyperfiltration was not included in the primary backward elimination models because we included baseline eGFR in the model assessment. We performed subsequent additional analyses adding hyperfiltation to the final selected models to evaluate its potential association with rapid eGFR decline. In all SCD patients, for scenarios (1)(3) and (4), history of stroke (odds ratio [OR]: 2.91, 95% CI: 1.25 – 6.77) and use of ACE-I/ARB (OR: 3.17, 95% CI: 1.28 – 7.84) were retained in the final model after backward elimination and associated with rapid eGFR decline (Table III). In scenarios (3) and (4), proteinuria was not retained in the model after backwards elimination. For scenario (2), only history of stroke (OR: 7.38, 95% CI: 1.72 – 31.65) was associated with rapid eGFR decline. In patients with HbSS/HbSβ0 thalassaemia, for scenarios (1)(3) and (4), history of stroke (OR: 3.41, 95% CI: 1.30 – 8.92) and baseline eGFR (OR: 0.98, 95% CI: 0.97 – 0.998) were associated with rapid eGFR decline; for scenario (2), only history of stroke (OR: 10.85, 95% CI: 1.83 – 64.33) was associated with rapid decline in eGFR. In patients with HbSC/HbSβ+ thalassaemia, for scenarios (1)(3) and (4), only use of ACE-I/ARB (OR: 10.85, 95% CI: 1.83 – 64.33) was associated with rapid eGFR decline. For scenario (2), no variables were associated with rapid decline in eGFR, although the number of evaluated patients was limited (N = 30). In subsequent models in which we evaluated hyperfiltration, among all patients, hyperfiltration was associated with a lower odds of rapid eGFR decline (OR: 0.31, 95% CI: 0.14 – 0.71). Hyperfiltration was not included in this model for severe genotype patients due to its collinearity with baseline eGFR. Among mild genotype patients, hyperfiltration was retained in the model but did not have a statistically significant association with rapid eGFR decline.
Table III:
Multivariable Analysis of Covariates Associated with Rapid Decline in Kidney Function*
| All SCD | HbSS/HbSβ0 | HbSC/HbSβ+ | ||||
|---|---|---|---|---|---|---|
| Variable | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p |
| History of stroke | 2.91 (1.25, 6.77) | 0.01 | 3.41 (1.30, 8.92) | 0.01 | - | - |
| Use of ACE-I/ARB | 3.17 (1.28, 7.84) | 0.01 | - | - | 10.85 (1.83, 64.33) | 0.009 |
| Baseline eGFR | - | - | 0.98 (0.97, 0.998) | 0.02 | - | - |
Results when proteinuria was excluded from the model or when proteinuria status was assigned to those with missing values (proteinuria present or proteinuria absent)
ACE-I/ARB – angiotensin converting enzyme inhibitors/angiotensin receptor blocker; CI – confidence interval; eGFR – estimated glomerular filtration rate; OR – odds ratio; SCD – sickle cell disease
Association of Rapid Decline in Estimated Glomerular Filtration Rate with Mortality
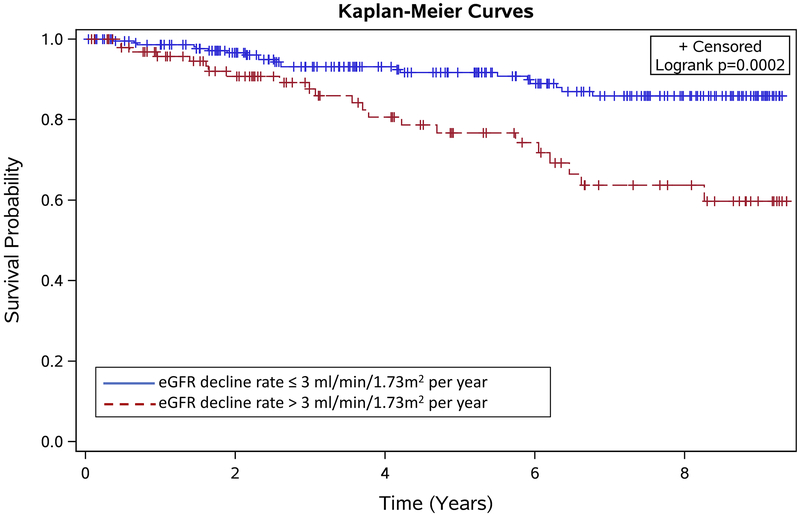
We evaluated the association of eGFR decline with mortality, adjusting for age and sex. There were 43 deaths over the observation period – 34 (79%) in the severe genotype group (21 [61.7%] with eGFR decline > 3 ml/min/1.73 m2 per year) and 8 (18.6%) in the mild genotype group (1 [12.5%] with eGFR decline > 3 ml/min/1.73 m2 per year). One death occurred in a patient with HbSE disease and was not analysed with the mild or severe genotypes. In all patients with SCD, there was an association of the rate of eGFR change over time with mortality (hazard ratio [HR]: 0.99, 95% CI: 0.984 - 0.995, p = 0.0002). This means that mortality is 10% less likely with every 10 ml/min per 1.73 m2 per year increase in the rate of eGFR change. In assessing the association of rapid eGFR decline and mortality, patients with eGFR ≤ 3 ml/min/1.73 m2 per year served as the reference population. Rapid decline in eGFR > 3 ml/min/1.73 m2 per year was significantly associated with increased mortality (HR: 2.40, 95% CI: 1.31 - 4.42, p = 0.005). Kaplan-Meier estimates also showed significantly lower survival probabilities for patients with rapid eGFR decline (log-rank test; p = 0.0002) (Figure 1). In subjects with HbSS/HbSβ0 thalassaemia, the rate of eGFR change over time was significantly associated with mortality (HR: 0.99, 95% CI: 0.98 - 0.99, p< 0.0001). Rapid decline in eGFR was also significantly associated with increased mortality in HbSS/HbSβ0 thalassaemia (HR: 2.96, 95% CI: 1.46 - 5.98, p = 0.003). Due to the low number of deaths (N=8) observed in the HbSC/HbSβ+ thalassaemia group, we did not complete this modelling in this group alone.
Figure 1:
Kaplan-Meier Estimates of the Survival Probabilities for Rapid and Non-Rapid Estimated Glomerular Filtration Rate (eGFR) Decline Groups.
In multivariable Cox regression models, in addition to rapid eGFR decline, we evaluated the association of baseline eGFR, ACE-I/ARB use, history of stroke, baseline haemoglobin, and baseline hydroxycarbamide use with mortality. Only history of stroke was retained in the model, and rapid eGFR decline had a similar association with mortality among all patients (HR: 2.07, 95% CI: 1.04 - 4.14, p = 0.04) and among severe genotype patients (HR: 2.89, 95% CI: 1.32 - 6.36, p = 0.008).
DISCUSSION
Glomerular filtration rate progressively declines with age, with an annual rate of decline of kidney function of 1.27 ± 1.97 ml/min/1.73m2 reported in African-American adults (Young, et al 2016). Multiple previous studies suggest that kidney function progressively declines in SCD (Asnani, et al 2016, Derebail, et al 2019, Gosmanova, et al 2014, van Tuijn, et al 2017, Xu, et al 2018). In a 25-year prospective longitudinal demographic and clinical cohort study, 31 out of 725 patients with HbSS (4.2%) and 5 out of 209 patients with HbSC disease (2.4%) developed chronic renal failure (Powars, et al 1991). However, most of the patients in the study were children in whom prevalence of CKD would be relatively low. A retrospective, single centre study of 98 SCD patients found that the prevalence of CKD, defined according to the 2012 KDIGO recommendations, increased from 28.6% to 41.8% after a mean follow-up of 5 years (Gosmanova, et al 2014). In a more recent single-centre, prospective study of 104 patients in the Netherlands, the prevalence of renal failure increased from 6.7% to 23.4% after 7 years of follow-up (van Tuijn, et al 2017). Although these studies assessed the progression of CKD, they did not evaluate rapid decline in kidney function and its association with mortality.
Rapid decline in kidney function is reported to occur in 11.5% of African-Americans following up to 12 years of follow-up (Young, et al 2016). Rapid decline in GFR, defined as an annual GFR loss of >3 ml/min/1.73 m2 (Rifkin, et al 2008, Shlipak, et al 2009), represents a magnitude of change more than two times the rate of decline in African-American adults and has been shown to predict impaired kidney function in patients with diabetes, end-stage renal disease and cardiovascular outcomes (Bjornstad, et al 2015, Groop, et al 2009, Orchard, et al 2010). However, the association between rapid GFR decline and CKD progression as well as mortality have not been previously described in SCD. In this study, we report on the high prevalence of rapid decline in kidney function in adult patients with SCD. As expected, the prevalence is higher in patients with HbSS/HbSβ0 thalassaemia than in those with HbSC/HbSβ+ thalassaemia, probably due to their more severe disease phenotype. These results are similar to recently published data of 193 subjects with 5-year follow-up data in whom rapid eGFR decline of ≥3 ml/min/year over 5 years was observed in approximately 37% of patients (Xu, et al 2018). In addition, consistent with reports in patients with type 1 diabetes (Young, et al 2016), we find that rapid decline in kidney function is associated with CKD progression in patients with SCD.
In multivariable analysis, we found that patients with history of stroke had an increased risk of rapid decline in kidney function. This relationship may be a result of a shared vasculopathic pathophysiology between cerebrovascular disease and kidney disease. Vaso-occlusion with ischaemia-reperfusion as well as endothelial dysfunction due, at least in part, to ongoing haemolysis probably play roles in these phenomena in SCD (Hebbel, et al 2004). We have previously reported that albuminuria is associated with history of stroke in SCD (Ataga, et al 2010). Although we did not find a significant association of proteinuria with rapid decline in eGFR, we observed that patients on treatment with ACE-I/ARBs had an increased risk of rapid decline in eGFR. This finding is not surprising as the majority of patients on ACE-I/ARBs were on these agents for an indication of proteinuria, a clinical marker of glomerular injury. This still suggests that glomerular injury, assessed clinically by proteinuria, may be a risk factor for rapid decline in kidney function in SCD, much like in other patient populations (Koye, et al 2018, Schwandt, et al 2018, Young, et al 2016). Worsening renal function due to these agents, while possible, seems less likely given their demonstrable benefit in attenuating renal function decline in numerous other settings. Indeed, baseline severe albuminuria (>300 mg/g by urine-albumin-to-creatinine ratio) has previously been reported to be associated with the development and progression of CKD in SCD (Gosmanova, et al 2014). Interestingly, patients with hyperfiltration had a lower likelihood of rapid eGFR decline. This may suggest that those who appear to have “normal” eGFR have already dropped from the hyperfiltration state due to loss of renal mass and are therefore more likely to progress, as has been postulated in diabetes (Tonneijck, et al 2017). This same argument could be made in the severe genotype group, in which higher baseline eGFR was associated with lower odds of eGFR decline.
Although multiple studies show an association of CKD with increased mortality in patients with SCD (Elmariah, et al 2014, Platt, et al 1994, Thrower, et al 2019), there are no previously published studies of the association of rapid decline in kidney function with mortality. Consistent with observations in the general population as well as patients with diabetes (Rifkin, et al 2008, Shlipak, et al 2009). we found that rapid decline in eGFR is associated with increased mortality in adult patients with SCD. With a greater than 2-fold increased risk of death with rapid decline in eGFR, early identification of patients with rapid decline in kidney function is important with regard to modifying risk factors and possibly slowing the decline in eGFR. We have recently reported that treatment with renin-angiotensin-aldosterone system blocking agents results in a slower decline in eGFR in adult SCD patients with proteinuria, although no effect on mortality was observed (Thrower, et al 2019).
Our study is limited by its retrospective design and assessment of proteinuria by dipstick analysis. Despite these limitations, the large size of the cohort, the consistency in practice at our single centre and long duration of follow-up allow us to draw several important conclusions.
We observed that rapid decline in eGFR is common and is associated with CKD progression and mortality in adult patients with SCD. Rapid decline in kidney function is associated with history of stroke and use of ACE-I/ARBs. Although we were unable to demonstrate an association between rapid decline in eGFR and proteinuria, the association of ACE-I/ARB use with rapid decline in kidney function suggests that patients with glomerulopathy have an increased risk of rapid decline in kidney function. Early identification of patients with rapid decline in kidney function is important to address potential modifiable risk factors and possibly slow eGFR decline, which, in turn, may reduce mortality. Prospective, multicentre studies that focus on measures to reduce the loss of kidney function in patients with SCD are needed to assess their potential to impact overall mortality.
Acknowledgments
Funding sources: Direct funding for the study was provided by the National Heart, Lung, and Blood Institute, R01HL111659 (KIA, VKD, JC) and R01FD006030 (KIA, VKD, JC). We also acknowledge the assistance of the NC Translational and Clinical Sciences (NC TraCS) Institute, supported by the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health, through Grant Award Number UL1TR001111.
Footnotes
Conflict of interest: The authors declare that there are no conflicts of interest.
REFERENCES
- Asnani M, Serjeant G, Royal-Thomas T & Reid M (2016) Predictors of renal function progression in adults with homozygous sickle cell disease. Br J Haematol, 173, 461–468. [DOI] [PubMed] [Google Scholar]
- Ataga KI, Brittain JE, Moore D, Jones SK, Hulkower B, Strayhorn D, Adam S, Redding-Lallinger R, Nachman P & Orringer EP (2010) Urinary albumin excretion is associated with pulmonary hypertension in sickle cell disease: potential role of soluble fms-like tyrosine kinase-1. Eur J Haematol, 85, 257–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ataga KI, Derebail VK & Archer DR (2014) The glomerulopathy of sickle cell disease. Am J Hematol, 89, 907–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker AM (2011) Sickle cell nephropathy: challenging the conventional wisdom. Pediatr Nephrol, 26, 2099–2109. [DOI] [PubMed] [Google Scholar]
- Bjornstad P, Cherney DZ, Snell-Bergeon JK, Pyle L, Rewers M, Johnson RJ & Maahs DM (2015) Rapid GFR decline is associated with renal hyperfiltration and impaired GFR in adults with Type 1 diabetes. Nephrol Dial Transplant, 30, 1706–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derebail VK, Ciccone EJ, Zhou Q, Kilgore RR, Cai J & Ataga KI (2019) Progressive Decline in Estimated GFR in Patients With Sickle Cell Disease: An Observational Cohort Study. Am J Kidney Dis. 2019. February 20. doi: 10.1053/j.ajkd.2018.12.027. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmariah H, Garrett ME, De Castro LM, Jonassaint JC, Ataga KI, Eckman JR, Ashley-Koch AE & Telen MJ (2014) Factors associated with survival in a contemporary adult sickle cell disease cohort. Am J Hematol, 89, 530–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosmanova EO, Zaidi S, Wan JY & Adams-Graves PE (2014) Prevalence and progression of chronic kidney disease in adult patients with sickle cell disease. J Investig Med, 62, 804–807. [DOI] [PubMed] [Google Scholar]
- Groop PH, Thomas MC, Moran JL, Waden J, Thorn LM, Makinen VP, Rosengard-Barlund M, Saraheimo M, Hietala K, Heikkila O, Forsblom C & FinnDiane Study G (2009) The presence and severity of chronic kidney disease predicts all-cause mortality in type 1 diabetes. Diabetes, 58, 1651–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haymann JP, Stankovic K, Levy P, Avellino V, Tharaux PL, Letavernier E, Grateau G, Baud L, Girot R & Lionnet F (2010) Glomerular hyperfiltration in adult sickle cell anemia: a frequent hemolysis associated feature. Clin J Am Soc Nephrol, 5, 756–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebbel RP, Osarogiagbon R & Kaul D (2004) The endothelial biology of sickle cell disease: inflammation and a chronic vasculopathy. Microcirculation, 11, 129–151. [PubMed] [Google Scholar]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. (2013) KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl, 3, 1–150. [Google Scholar]
- Koye DN, Magliano DJ, Reid CM, Jepson C, Feldman HI, Herman WH & Shaw JE (2018) Risk of Progression of Nonalbuminuric CKD to End-Stage Kidney Disease in People With Diabetes: The CRIC (Chronic Renal Insufficiency Cohort) Study. Am J Kidney Dis, 72, 653–661. [DOI] [PubMed] [Google Scholar]
- Levey AS & Coresh J (2012) Chronic kidney disease. Lancet, 379, 165–180. [DOI] [PubMed] [Google Scholar]
- Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J & Ckd EPI (2009) A new equation to estimate glomerular filtration rate. Ann Intern Med, 150, 604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orchard TJ, Secrest AM, Miller RG & Costacou T (2010) In the absence of renal disease, 20 year mortality risk in type 1 diabetes is comparable to that of the general population: a report from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetologia, 53, 2312–2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt OS, Brambilla DJ, Rosse WF, Milner PF, Castro O, Steinberg MH & Klug PP (1994) Mortality in sickle cell disease. Life expectancy and risk factors for early death. N Engl J Med, 330, 1639–1644. [DOI] [PubMed] [Google Scholar]
- Powars DR, Elliott-Mills DD, Chan L, Niland J, Hiti AL, Opas LM & Johnson C (1991) Chronic renal failure in sickle cell disease: risk factors, clinical course, and mortality. Ann Intern Med, 115, 614–620. [DOI] [PubMed] [Google Scholar]
- Rifkin DE, Shlipak MG, Katz R, Fried LF, Siscovick D, Chonchol M, Newman AB & Sarnak MJ (2008) Rapid kidney function decline and mortality risk in older adults. Arch Intern Med, 168, 2212–2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwandt A, Bergis D, Denkinger M, Gollisch KSC, Sandig D, Stingl H, Zimny S & Holl RW (2018) Risk factors for decline in renal function among young adults with type 1 diabetes. J Diabetes Complications, 32, 940–946. [DOI] [PubMed] [Google Scholar]
- Shlipak MG, Katz R, Kestenbaum B, Siscovick D, Fried L, Newman A, Rifkin D & Sarnak MJ (2009) Rapid decline of kidney function increases cardiovascular risk in the elderly. J Am Soc Nephrol, 20, 2625–2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J, Reid M, Hambleton I & Serjeant GR (2007) Albuminuria and renal function in homozygous sickle cell disease: observations from a cohort study. Arch Intern Med, 167, 701–708. [DOI] [PubMed] [Google Scholar]
- Thrower A, Ciccone EJ, Maitra P, Derebail VK, Cai J & Ataga KI (2019) Effect of renin-angiotensin-aldosterone system blocking agents on progression of glomerulopathy in sickle cell disease. Br J Haematol, 184, 246–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonneijck L, Muskiet MH, Smits MM, van Bommel EJ, Heerspink HJ, van Raalte DH & Joles JA (2017) Glomerular Hyperfiltration in Diabetes: Mechanisms, Clinical Significance, and Treatment. J Am Soc Nephrol, 28, 1023–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Tuijn CFJ, Schimmel M, van Beers EJ, Nur E & Biemond BJ (2017) Prospective evaluation of chronic organ damage in adult sickle cell patients: A seven-year follow-up study. Am J Hematol, 92, E584–E590. [DOI] [PubMed] [Google Scholar]
- Vazquez B, Shah B, Zhang X, Lash JP, Gordeuk VR & Saraf SL (2014) Hyperfiltration is associated with the development of microalbuminuria in patients with sickle cell anemia. Am J Hematol, 89, 1156–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu JZ, Garrett ME, Soldano KL, Chen ST, Clish CB, Ashley-Koch AE & Telen MJ (2018) Clinical and metabolomic risk factors associated with rapid renal function decline in sickle cell disease. Am J Hematol, 93, 1451–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young BA, Katz R, Boulware LE, Kestenbaum B, de Boer IH, Wang W, Fulop T, Bansal N, Robinson-Cohen C, Griswold M, Powe NR, Himmelfarb J & Correa A (2016) Risk Factors for Rapid Kidney Function Decline Among African Americans: The Jackson Heart Study (JHS). Am J Kidney Dis, 68, 229–239. [DOI] [PMC free article] [PubMed] [Google Scholar]