Abstract
Glutamate is the primary excitatory neurotransmitter in the central nervous system (CNS) that initiates a rapid signal transmission in the synapse before its re-uptake into the surrounding glia, specifically astrocytes. The astrocytic glutamate transporters, glutamate-aspartate transporter (GLAST) and glutamate transporter-1 (GLT-1) and their human homologs excitatory amino acid transporter 1 (EAAT1) and 2 (EAAT2), respectively, are the major transporters that take up synaptic glutamate to maintain its optimal extracellular levels, thus preventing its accumulation in the synaptic cleft and the ensuing excitotoxicity. Growing evidence has shown that excitotoxicity is associated with various neurological disorders, including amyotrophic lateral sclerosis (ALS), Alzheimer’s disease (AD), Parkinson’s disease (PD), manganism, ischemia, schizophrenia, epilepsy, and autism. While the mechanisms of neurological disorders are not well understood, the dysregulation of GLAST/GLT-1 may play a significant role in excitotoxicity and associated neuropathogenesis. The expression and function of GLAST/GLT-1 may be dysregulated at the genetic, epigenetic, transcriptional or translational levels, leading to high levels of extracellular glutamate and excitotoxicity. Consequently, understanding the regulatory mechanisms of GLAST/GLT-1 has been an area of interest in developing therapeutics for the treatment of neurological disorders. Pharmacological agents including β-lactam antibiotics, estrogen/selective estrogen receptor modulators (SERMs), growth factors, histone deacetylase inhibitors (HDACi), and translational activators have shown significant efficacy in enhancing the expression and function of GLAST/GLT-1 and glutamate uptake in both in vitro and in vivo settings. This comprehensive review will discuss the regulatory mechanisms of GLAST/GLT-1, their association with neurological disorders, and the pharmacological agents that mediate their expression and function.
1. Introduction
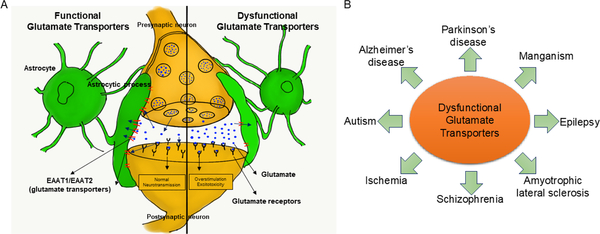
Glutamate is the primary excitatory neurotransmitter in the central nervous system (CNS), where it initiates rapid signal transmission and is involved in learning, memory and synaptic plasticity (Parkin et al., 2018; Willard and Koochekpour, 2013). Following its synaptic release, glutamate is taken up into surrounding astrocytes and the glutamate gradient returns to resting levels (Sulkowski et al., 2014). As high levels of extracellular glutamate are associated with excitotoxic neuronal death, glutamate concentration is optimally maintained via the removal of glutamate from the synapse by astrocytic glutamate transporters after impulse transmission (Figure 1A) (Jia et al., 2015; Karki et al., 2015b). Astrocytic glutamate transporters, also referred to as excitatory amino acid transporters (EAATs) in humans, play a primary role in the rapid termination of glutamate signaling and the maintenance of extracellular glutamate levels (Shigeri et al., 2004).
Fig. 1.
Role of astrocytic glutamate transporters in the central nervous system (CNS) and neurological disorders. (A) The tripartite synapse is comprised of astrocytes, presynaptic and postsynaptic neurons. Astrocytic glutamate transporters, excitatory amino acid transporters 1 (EAAT1) and 2 (EAAT2), uptake glutamate from the synaptic cleft to maintain glutamate homeostasis and prevent excitotoxic neuronal death. (B) Dysfunctional glutamate transporters have been implicated in various neurological disorders such as amyotrophic lateral sclerosis (ALS), Alzheimer’s disease (AD), Parkinson’s disease (PD), schizophrenia, epilepsy, and autism.
Excess levels of synaptic glutamate result in the overstimulation of postsynaptic glutamate receptors, leading to excitotoxic neuronal death (Karki et al., 2018). An increasing body of evidence reveals that excitotoxicity is associated with neurological disorders, including amyotrophic lateral sclerosis (ALS), Alzheimer’s disease (AD), Parkinson’s disease (PD), manganism, ischemia, schizophrenia, epilepsy, and autism (Figure 1B) (Bristot Silvestrin et al., 2013; Garcia-Esparcia et al., 2018; Mironova et al., 2018; Petr et al., 2013a). While the mechanisms of excitotoxicity are not well understood, the dysregulation of EAATs may greatly influence glutamate excitotoxicity and the resulting neuropathology. In particular, EAAT1 and EAAT2, the primary glutamate transporters in the CNS, may significantly impact glutamate excitotoxicity (Karki et al., 2013a). Glutamate-aspartate transporter (GLAST) and glutamate transporter-1 (GLT-1) are homologs (displaying >70% homology) of EAAT1 and EAAT2 in rodents, respectively, and thus can be used interchangeably (Jimenez et al., 2014).
Therefore, understanding transcriptional regulation, as well as the epigenetic and posttranslational modifications (PTMs) of GLAST and GLT-1, may greatly advance the development of therapeutic targets to treat diseases related to the impairment of glutamate transporters. This review will discuss the regulatory mechanisms of GLAST and GLT-1, neurological disorders associated with dysregulated GLAST and GLT-1 and the pharmacological agents modulating GLAST and GLT-1 expression and function.
2. Glutamate transporters
There are five EAAT subtypes identified in humans, referred to as EAAT1–5 (Bridges and Esslinger, 2005). EAAT1 and 2 are predominantly expressed in astrocytes (Karki et al., 2013a), although they are also expressed in other types of glial cells, including microglia and oligodendrocytes (Parkin et al., 2018). Once taken into astrocytes, glutamate is converted to glutamine by glutamine synthase. The newly generated glutamine is subsequently available for transport back to presynaptic neurons, a process referred to as glutamate-glutamine cycling (Shen et al., 2009). EAAT3 is primarily found in neurons, particularly at the post-synaptic terminals (He and Casaccia-Bonnefil, 2008).
Other glutamate transporter subtypes such as EAAT4 and 5 are also expressed in the human CNS (Amara and Fontana, 2002). EAAT4, encoded by the human SLC1A6 gene, is expressed predominantly in the Purkinje cells in the cerebellum, while EAAT5, encoded by human SLC1A7 gene, is expressed in the photoreceptor cells of the retina (Amara and Fontana, 2002). This indicates the important roles of EAAT4 and 5 in glutamate neurotransmission in specific regions of the brain (Amara and Fontana, 2002; Perkins et al., 2018; Yamashita et al., 2006).
2.1. EAAT1 (GLAST)
GLAST (EAAT1) is primarily expressed in the cerebellum and cerebral neocortex (Kim et al., 2011). Studies have shown that GLAST is highly expressed during the developmental stage (Kugler and Schleyer, 2004; Ullensvang et al., 1997). GLAST is a membrane-bound symporter, co-transporting glutamate, three Na+ ions, and one H+ ion—while counter-transporting one K+ ion against the concentration gradient (Ryan et al., 2010). Although GLT-1 is believed to be the major transporter subtype in removing excess glutamate from the synaptic cleft, GLAST also plays a critical role in preventing excitotoxic neuronal injury. Studies have demonstrated that GLAST knockout in mice results in increased susceptibility to traumatic brain injury (TBI) and retinal degeneration (Delyfer et al., 2005; Rao et al., 1998). Inhibition of GLAST also increases extracellular glutamate levels, resulting in excitotoxic neuronal death in mice (Maragakis and Rothstein, 2004; Rothstein et al., 1996).
The SLC1A3 gene, which encodes EAAT1, is located on chromosome 5 (5p13.2) and comprised of 81980 base pairs (Sery et al., 2015). To date, 16 variants of SLC1A3 have been identified, some of which are associated with neurological disorders such as epilepsy and schizophrenia (Bauer et al., 2010; Jen et al., 2005). Posttranslationally, EAAT1 undergoes glycosylation, producing 64 kDa and 70 kDa glycoproteins (Parkin et al., 2018), and these modifications have been linked to changes in its membrane localization and oligomerization, though the pathogenic effects of these modifications are not well understood (Bauer et al., 2010).
2.2. EAAT2 (GLT-1)
GLT-1 (EAAT2) is also a Na+-dependent transmembrane symporter (Kim et al., 2011) and a primary astrocytic glutamate transporter in the adult human brain, accounting for over 90% of synaptic glutamate clearance (Rao et al., 2015a), expressing at levels four to six times higher than GLAST in astrocytes (Lehre and Danbolt, 1998). Although GLAST levels are higher than GLT-1 at early postnatal development, GLT-1 levels overtake GLAST levels between postnatal day P20 and P30 (Kugler and Schleyer, 2004), indicating that the expression of GLT-1 and GLAST are tightly regulated in the developing brains. It has been shown that GLT-1 levels are correlated with glutamate dehydrogenase (GDH) activity in astrocytes, suggesting a critical role of GLT-1 in synaptic glutamate-glutamine cycling (Kugler and Schleyer, 2004). Studies have also reported that astrocytic GLT-1 expression is closely correlated with neuronal activity in an in vitro model of the developing hippocampus (Benediktsson et al., 2012), indicating that the dynamic interaction between neuronal and glial cells is involved in synaptic glutamate homeostasis during brain development.
EAAT2 is encoded by the SLC1A2 gene (Lin et al., 2012). In addition to full-length EAAT2a, two functional splice variants, EAAT2b and EAAT2c, have been identified (O’Donovan et al., 2015). While full-length EAAT2 predominates in the human brain, both splice variants contain unique C-terminal domains whose function is not yet understood (Chen et al., 2002). Interestingly, EAAT2 transporter function is also thought to be regulated via soluble neuronal factors (Gegelashvili et al., 1997).
GLT-1 is a critical mediator of the synaptic glutamate gradient in the adult brain (Karki et al., 2015b). Dysregulation of GLT-1 has been linked to excitotoxicity, neuronal death and neurological disorders (Karki et al., 2013a). GLT-1 knockout mice experienced lethal spontaneous seizures and significant neuronal loss, while functional GLT-1 prevented post-traumatic seizures in a rat TBI model (Tanaka et al., 1997). EAAT2 has also been associated with chronic and acute neurological disorders, including AD, PD, schizophrenia and epilepsy (Karki et al., 2015b; Takahashi et al., 2015; Young et al., 2014).
2.3. EAAT3 (EAAC1)
Although GLAST and GLT-1 are the primary transporters responsible for synaptic glutamate reuptake in the CNS, EAAT3, also known as excitatory amino acid carrier 1 (EAAC1), is ubiquitously expressed in the brain (Bjorn-Yoshimoto and Underhill, 2016) and is primarily found in neurons, particularly at the post-synaptic terminals (He and Casaccia-Bonnefil, 2008). Membrane-associated EAAT3 is associated with post-synaptic neuronal ionotropic receptors (Bjorn-Yoshimoto and Underhill, 2016), suggesting its role in glutamate neurotransmission and synaptic plasticity.
EAAT3 is encoded by the SLC1A1 gene and is highly expressed during the early development of the cortex, as compared to other EAAT subtypes (Bjorn-Yoshimoto and Underhill, 2016). In the adult brain, EAAT3 does not appear to significantly contribute to glutamate clearance (Rothstein et al., 1996), but has been shown to serve as a cysteine transporter (Watts et al., 2014). Despite a lack of involvement in glutamate gradient maintenance, EAAC1 knockdown in rats induced spontaneous seizures and behavioral abnormalities (Sepkuty et al., 2002). Additionally, aberrant SLC1A1 expression has been associated with familial schizophrenia (Myles-Worsley et al., 2013) and in the postmortem brains of patients with idiopathic schizophrenia (Horiuchi et al., 2012). The interplay between the glial EAAT1/2 and neuronal EAAT3 is not yet fully understood. It has been reported that the EAAT1/2 expression was not altered in the spinal cord and cerebral cortex in the absence of EAAT3 in mice (Lee et al., 2010). Moreover, deletion of GLT-1 (EAAT2) did not modulate EAAT3 expression (Petr et al., 2015).
3. Dysregulation of glutamate transporter expression and function
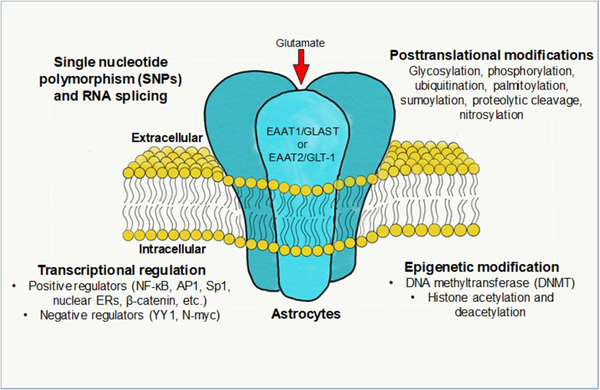
Aberrant glutamate transporter function and expression are associated with various neurological disorders. Therefore, understanding the underlying mechanisms of EAAT1/2 expression may provide therapeutic targets for treating neurological disorders associated with impaired glutamate transporters (Figure 2).
Fig. 2.
Regulatory mechanisms of EAAT1 and EAAT2. Astrocytic glutamate transporters are membrane-bound trimeric proteins which regulate synaptic glutamate levels. The expression, function and trafficking of glutamate transporters are regulated at the transcriptional and translational levels. Single nucleotide polymorphisms (SNPs), aberrant RNA splicing and dysregulated transcription factors modulate the expression of EAAT1/EAAT2. Epigenetic modifications such as DNA methylation and histone acetylation/methylation also modulate their expression. Posttranslational modifications (PTMs) such as glycosylation and phosphorylation regulate function, localization and degradation of EAAT1 and EAAT2.
3.1. Single nucleotide polymorphisms (SNPs)
SNPs are variations in a single nucleotide within the genome that occur naturally in >1% of the population (Zhang et al., 2018). Growing evidence indicates that SNPs are associated with neurological pathogenesis, as well as differences in individual response to treatment (Giacomini et al., 2007). Studies have shown that SNPs in EAAT1 and EAAT2 are associated with neurological disorders such as bipolar disorder (BD), schizophrenia and multiple sclerosis (MS) (Pampliega et al., 2008; Poletti et al., 2014b; Poletti et al., 2018; Spangaro et al., 2014). Since SNPs influence pathogenesis at the transcriptional level and the promoter regions of EAAT1/2 contains consensus binding sequences for transcription factors (Karki et al., 2015a), SNPs in the promoter regions may alter the binding of transcription factors to the promoter, leading to dysregulation of EAAT1/2 expression.
The genetic variants of EAAT1 may contribute to various neurological disorders such as ataxia, seizures, migraines, hemiplegia, BD and schizophrenia (Jen et al., 2005; Poletti et al., 2018; Spangaro et al., 2014). The SNP of EAAT1 replacing C with G at −1047 position (−1047C>G) is associated with ataxia, hemiplegia and seizures (Jen et al., 2005), which is linked to reduced glutamate uptake. The genetic polymorphism of EAAT1, rs2731880, is associated with impairment of cortico-limbic system and emotional dysfunction in BD patients (Poletti et al., 2018), suggesting that EAAT1 polymorphisms may contribute to BD pathology. Studies also revealed that this SNP rs2731880 decreased EAAT1 expression along with declined cognitive function in schizophrenia patients (Spangaro et al., 2014).
Studies show that a genetic variant of EAAT2 substituting the nucleotide A with C in the −181 position (−181A>C) in the promoter region changes the binding site for activator protein-2 (AP-2; a positive regulator) to GC-binding factor 2 (GCF2; a negative regulator) (Mallolas et al., 2006), resulting in reduced EAAT2 expression and glutamate uptake. Accordingly, individuals with this polymorphism show increased plasma glutamate levels and higher susceptibility to stroke (Mallolas et al., 2006). This SNP is also associated with MS (Pampliega et al., 2008), BD and schizophrenia (Dallaspezia et al., 2012; Poletti et al., 2014a; Poletti et al., 2014b). The other SNP identified as rs435668, replacing A with T or G at the −181 position (−181A>T/G) is also associated with a reduced EAAT2 expression in schizophrenia patients (Spangaro et al., 2012). Moreover, the SNPs (−200C>A and −181A>C) increased susceptibility of preterm infants to cerebral palsy and neurodevelopmental disabilities (Rajatileka et al., 2018), indicating that SNPs in the EAAT2 promoter region induce transporter dysfunction, as well as abnormal brain development and increased susceptibility to diseases.
In addition to polymorphisms in the EAAT2 promoter region, SNPs in the EAAT2 coding region may contribute to neurological disorders. A substitution of the amino acid glycine to arginine at residue 603 (G603A variant) in the EAAT2 coding region has been shown to be associated with altered behavior and liver cirrhosis in alcoholics (Foley et al., 2004; Sander et al., 2000b). This EAAT2 variant is believed to reduce EAAT2 functional activity in alcoholics, resulting in elevated glutamate levels in the brain (Sander et al., 2000b). Another study reported that SNPs of the EAAT2 gene did not affect the severity of idiopathic epilepsy (Sander et al., 2000a).
These findings indicate that genetic polymorphisms in EAAT1/2 may contribute to the onset and progression of neurological disorders, potentially by dysregulation of glutamate transporters. Further genome-wide association studies (GWAS) are necessary to identify and understand the role of SNPs in EAAT1/2 across larger populations and various neurological diseases in humans.
3.2. Transcriptional regulation
The promoter regions of EAAT1 (GLAST) and EAAT2 (GLT-1) contain multiple consensus DNA-binding sites for various transcription factors including specificity protein 1 (Sp1), AP-1, nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), nuclear factor of activated T-cells (NFAT), N-myc, cAMP response element-binding protein (CREB) and yin-yang 1 (YY1) (Aguirre et al., 2008; Hagiwara et al., 1996; Karki et al., 2015a; Karki et al., 2014b; Kim et al., 2003a; Su et al., 2003). Studies have shown that various stimulants activated these transcription factors and thus induced their binding to the consensus binding sites of the GLAST/GLT-1 promoters, resulting in altered expression of GLAST/GLT-1.
3.2.1. Positive transcriptional regulation
An increasing body of evidence links the dysregulation of glutamate transporters to excitotoxic neuronal injury (Karki et al., 2013a; Pampliega et al., 2008; Parkin et al., 2018; Wilson et al., 2003). Thus, understanding the transcriptional regulation of GLAST/GLT-1 may provide potential molecular targets to increase GLAST/GLT-1 expression and reverse glutamate excitotoxicity. Several transcription factors including NF-κB, CREB, β-catenin and Sp1 have been shown to increase GLAST/GLT-1 levels (Karki et al., 2015a; Kim et al., 2003b; Lutgen et al., 2016).
The transcription factor NF-κB (primarily found as a p65/p50 dimer) is a critical positive regulator of both GLAST and GLT-1 (Karki et al., 2013b; Karki et al., 2014c; Lee et al., 2012a). NF-ΚBp65 overexpression significantly increased GLAST/GLT-1 expression in astrocyte cultures (Gupta and Prasad, 2014; Sitcheran et al., 2005). The promoter regions of both GLAST and GLT- 1 contain multiple NF-κB binding sites (Karki et al., 2015a; Sitcheran et al., 2005) and mutation of the binding sites in both transporters decreased their expression and abolished the effect of NF-κB overexpression (Karki et al., 2015a; Sitcheran et al., 2005). Pharmacological agents, including arundic acid, 17β-estradiol, tamoxifen, raloxifene, G1 (a selective agonist of G protein-coupled estrogen receptor (GPR30) and transforming growth factor alpha (TGF-α), increased the expression of EAAT1 and EAAT2 via activation of the NF-κB pathway (Figiel et al., 2003; Karki et al., 2018; Lee et al., 2012a; Lee et al., 2009; Unger et al., 2012). Several signaling proteins are known to regulate NF-κB activation to modulate GLAST/GLT-1 expression. Mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) and phosphatidylinositol 3- kinase (PI3K)/Protein Kinase B (Akt) promote NF-κB activation (Karki et al., 2018; Karki et al., 2014c; Lee et al., 2009), resulting in increased GLT-1 levels (Li et al., 2006). The mammalian target of rapamycin (mTOR) activated Akt, resulting in enhanced NF-κB signaling and increased GLAST/GLT-1 expression in rat astrocytes (Abousaab et al., 2016; Han et al., 2016; Ji et al., 2013). These findings indicate that various stimulants targeting these signaling proteins can also modulate NF-κB activation and GLAST/GLT-1 expression.
The transcription factor CREB is known to play an important role in neuronal plasticity and long-term memory formation in the brain (Ortega-Martinez, 2015); and its reduction is consequently implicated in the pathology of AD and other cognitive disorders (Saura and Valero, 2011). CREB binds to cAMP response elements (CRE) in the promoter regions of EAAT1 and EAAT2 and initiates their transcription (Karki et al., 2013b; Kim et al., 2003b). Previous studies have demonstrated that the mutation of CRE sites completely abrogated tamoxifen-induced enhancement of EAAT2 promoter activity in astrocytes (Karki et al., 2013b). Moreover, inhibition of protein kinase A (PKA), an upstream activator of CREB signaling, blocked the enhancing effects of 17ß-estradiol and tamoxifen on EAAT2 expression (Karki et al., 2013b), indicating that CREB serves as a critical positive transcriptional regulator of EAAT1/2 (GLAST/GLT-1). Activation of GPR30) also increased EAAT2 (GLT-1) expression by inducing binding of CREB to the EAAT2 promoter (Lee et al., 2012a).
Selective estrogen receptor modulators (SERMs), which can act as ER agonists or antagonists depending on the tissue type, may also positively regulate EAAT1/2 (Dunn et al., 2001). Some SERMs, such as tamoxifen and raloxifene, can act as ER agonists in the brain and increase expression of GLAST/GLT-1 at the transcriptional level (Colon et al., 2016; Morissette et al., 2008; Pajarillo et al., 2018; Pandey et al., 2016). Indeed, estrogen (17ß-estradiol), tamoxifen and raloxifene enhanced EAAT1/2 expression both in vitro and in vivo (Karki et al., 2014c; Lee et al., 2013; Lee et al., 2009; Pajarillo et al., 2018). Estrogen and SERMs may also activate non-genomic signaling pathways such as PI3K-Akt, ERK, CREB, and NF-κB pathways to increase GLAST/GLT-1 (Karki et al., 2013b; Karki et al., 2014c; Lee et al., 2012a).
3.2.2. Negative transcriptional regulation
Although studies have focused on the positive regulation of glutamate transporters to develop potential therapeutics for neurological disorders related to the reduction of glutamate transporters, some studies have identified the negative regulators of these transporters. The transcription factor N-myc, a member of the Myc proto-oncogene family, is a basic-helix-loop-helix-zipper (bHLHZ) protein, is known to negatively regulate the expression of target genes during neurogenesis. It inhibited the basal and NF-ΚBp65-induced EAAT2 activation in astrocytes (Sitcheran et al., 2005). The inflammatory agent tumor necrosis factor alpha (TNF-α) enhanced the binding of N-myc to its consensus binding sites in the EAAT2 promoter, resulting in repression of EAAT2 (Sitcheran et al., 2005). N-myc is correlated to the down-regulation of GLT-1 expression during postnatal development in mice (Gupta and Prasad, 2014). N-myc levels were increased in the brains of AD and PD patients (Ferrer and Blanco, 2000), suggesting that aberrant N-myc is involved in their pathogenesis. N-myc is clearly involved in the regulation of EAAT2 transcription (Gupta and Prasad, 2014; Sitcheran et al., 2005), but its role in glutamate transporter dysregulation and excitotoxic neuronal damage remains to be elucidated.
The transcription factor YY1 is a potent negative regulator of both GLAST and GLT-1, as it binds to its consensus DNA-binding sites in their promoter regions and leads to decreases in their expression (Aguirre et al., 2008; Karki et al., 2015a; Karki et al., 2014b; Rosas et al., 2007). YY1 can activate or repress transcription of genes, depending on cellular context and co-factor availability (Galvin and Shi, 1997). YY1 plays a critical role in the brain by regulating genes involved in neural development, neuronal function and developmental myelination (He and Casaccia-Bonnefil, 2008; Shiu et al., 2016). YY1 is implicated in neurodegenerative disorders including PD, ALS, AD, Charcot-Marie-Tooth disease and Rett syndrome (Aubry et al., 2015; Bedrosian et al., 2018; Forlani et al., 2010; Nowak et al., 2006; Ratajewski and Pulaski, 2009; Tiwari and Pal, 2017; Yin et al., 2018). Astrocyte-elevated gene-1 (AEG-1) serves as a corepressor of YY1 to inhibit GLT-1 transcription, leading to reduced glutamate uptake in astrocytes (Lee et al., 2011). In addition, YY1 mediates manganese (Mn)- and TNF-α-induced repression of GlAST and GLT-1 (Karki et al., 2015a; Karki et al., 2014b). Activated YY1 recruits the epigenetic modifiers histone deacetylases (HDACs), and the resultant complex binds to its consensus binding sites of GLAST/GLT-1 to repress their expression. Consequently, knockdown of YY1 or mutation of the YY1 binding site reverses its repressive effects on GLAST/GLT-1 promoter activity (Karki et al., 2015a; Karki et al., 2014b).
3.3. RNA splicing
Alternative splicing of astrocytic glutamate transporters contributes to their translational features, posttranslational modifications and functional diversity. Previous studies have reported that an EAAT1 variant lacking exon 9 is expressed in the CNS, both in grey matter and the axonal tracts (Vallejo-Illarramendi et al., 2005). In the absence of exon 9, this EAAT1 variant is non-functional, but it exerts an antagonistic effect on functional EAAT1 (Vallejo-Illarramendi et al., 2005), indicating that exon 9 is critical for plasma membrane localization and function of EAAT1. There are 4 major N- and C-terminal splice variants of EAAT2 which can reach the cell surface for glutamate uptake in astrocytes (Lauriat and McInnes, 2007; Peacey et al., 2009). Although some splice variants are functional, aberrant splicing of EAAT2 induces its rapid degradation and the consequent loss of glutamate uptake (Lin et al., 1998). Aberrant splicing was observed in patients with ALS (Lin et al., 1998; Meyer et al., 1999; Meyer et al., 1998), epilepsy (Hoogland et al., 2004), AD and dementia with Lewy bodies (Scott et al., 2011), which exhibited reduced glutamate uptake in brain regions and consequent neuronal loss (Garcia-Esparcia et al., 2018).
GLT-1b is a splice variant of GLT-1(also known as GLT-1a), which contains a longer 3’-UTR that extends to a stop codon between exons 9 and 10 (Rimmele and Rosenberg, 2016). Treatment with 3-nitropropionic acid (a hypoxic chemical agent) in the APP23 mouse model of AD increased aberrant splice variants of GlT-1 (Munch et al., 2008), suggesting that hypoxia- induced aberrant splicing of GLT-1 is involved in early-onset AD. Since the aberrant GlT-1 transcript is present in only 0.1–0.2% of the major EAAT2/GLT-1 isoforms in the brain (Lauriat and McInnes, 2007), the significance of aberrant splicing of EAAT2/GLT-1 associated with neurological disorders warrants further investigation.
3.4. Epigenetic modulation
Growing evidence indicates that epigenetic modifications, including DNA methylation and histone modification, significantly contribute to neurodegenerative disorders by modulating global gene expression (Gonzalez et al., 2011). Epigenetic dysregulation of EAAT1/2 promoter regions is associated with a decrease in their expression in various neurological disorders, such as BD (Jia et al., 2017), ischemia (Chisholm et al., 2015) and ALS (Yoo and Ko, 2011), as well as in animal models of cocaine abuse (Kim et al., 2018).
3.4.1. DNA methylation
DNA methylation transfers a methyl group from S-adenosyl methionine to CpG island of genes to form 5-methylcytosine (Jin and Liu, 2018). DNA methylation acts primarily to repress gene transcription, and this process is essential for normal development and plays a key role in a number of biological and pathological processes such as aging, carcinogenesis and neurological disorders (Jin and Liu, 2018).
Studies have shown that levels of DNA methylation of CpG islands in the promoter region of GLT-1 are brain region-specific, with high levels of methylation in the cerebellar astrocytes of rats as compared to those in the cortex (Perisic et al., 2012). The pharmacological inhibition of DNA methylation with dexamethasone increased GLT-1 expression in cerebellar glia (Zschocke et al., 2005). Moreover, the EAAT2 promoter displays higher levels of methylation in human glioma cell lines, leading to a decrease in EAAT2 expression and glutamate uptake as compared to normal human brains (Zschocke et al., 2007). Furthermore, self-administration of cocaine and its withdrawal effects were associated with increased EAAT2 DNA methylation, which in turn reduced its expression (Kim et al., 2018). These findings indicate that abnormal DNA hypermethylation is associated with various neurological disorders. Increased methylation of the EAAT2 promoter in BD brains was also noted, but exogenous factors such as nicotine and alcohol addiction may have modified methylation in these patients (Jia et al., 2017), suggesting that exogenous stimulants can contribute to the pathogenicity of global methylation in neurological disorders. Despite the role of CpG methylation in EAAT2, there is no CpG island found in the EAAT1 gene (Sery et al., 2015).
3.4.2. Histone modifications
Histone modifications may also serve as a significant epigenetic contributor to neurodegenerative disease (Berson et al., 2018). Histone modifications are regulated by the covalent modifications of H3 and H4 histone tails, such as acetylation and methylation. Aberrancy of histone acetylation or methylation leads to the dysregulation of global gene expression in various neurological disorders (Landgrave-Gomez et al., 2015). The mechanism of pathogenic histone modifications are beginning to emerge in neurodegenerative diseases related to dysfunctions of glutamate transporters. Aberrant histone methylation following ischemia resulted in dysfunctional GLT-1 and GLAST, but not their expression (Chisholm et al., 2015), suggesting other unknown mechanisms might be involved in histone methylation-induced modulation of glutamate transporters.
Aberrant histone acetylation and deacetylation play a critical role in various neurodegenerative disorders (Bennett et al., 2018; Mai et al., 2009; Park et al., 2016; Selvi et al., 2010). Inhibition of histone deacetylation using HDAC inhibitors (HDACi) such as trichostatin A (TSA), suberoylanilide hydroxamic acid (SAHA), sodium butyrate and valproic acid (VPA), increased GLAST and GLT-1 mRNA and protein levels in vitro and in vivo (Johnson et al., 2018a; Johnson et al., 2018b; Karki et al., 2014b; Lapucci et al., 2017). Moreover, HDACs are potent co-repressors of the negative regulator YY1 on GLAST/GLT-1, and therefore, inhibiting HDACs abolished the negative effects of YY1 on GLAST/GLT-1 (Karki et al., 2015b; Karki et al., 2014b). These findings indicate that histone acetylation/deacetylation plays a critical role in regulation of GLAST/GLT-1 expression and may serve as a molecular target in the development of therapeutics to treat diseases associated with impaired glutamate transporters.
3.5. Posttranslational modifications (PTMs)
In addition to genetic, transcriptional and epigenetic modulations as critical contributors to GLAST/GLT-1 dysregulation, an increasing body of evidence implicates aberrant PTMs in pathogenesis associated with dysfunction of GLAST/GLT-1. Although PTMs play a role in the physiological function and localization of GLAST and GLT-1, aberrant PTMs are implicated in GLAST/GLT-1 dysregulation. Thus, elucidating the mechanisms of PTMs that affect pathogenesis may greatly expand our understanding of neurological disorders associated with the dysfunction of glutamate transporters.
3.5.1. Glycosylation
Glutamate transporters GLAST/GLT-1 require PTMs for their transport and trafficking in the cell. Notably, glycosylation of GLAST/GLT-1 is critical for the localization of transporters on the plasma membrane, as the glycosylation of N-terminal leucine-6 (L6) in GLT-1 is necessary for its export from the endoplasmic reticulum to the plasma membrane (Kalandadze et al., 2004). Interestingly, many splicing variants of GLT-1 are devoid of this motif, which is compensated by a downstream arginine motif (Kalandadze et al., 2004).
Though glycosylation of other residues has been identified (Slotboom et al., 1999), it is still unclear whether aberrant glycosylation contributes to dysfunctional EAATs. Studies have established an association between aberrant glycosylation of GLAST/GLT-1 and schizophrenia (Bauer et al., 2010). Other studies have shown that both glycosylated and non-glycosylated forms of GLT-1 were functional in the plasma membrane, suggesting that glycosylation of GLT-1 may not affect the trafficking or the transport activity of GLT-1 (Raunser et al., 2005). Thus, further investigation is warranted to better understand the role of glycosylation in GLAST/GLT-1 trafficking and functionality.
3.5.2. Phosphorylation
Phosphorylation of GLT-1 has been identified at multiple serine, threonine and tyrosine residues (Casado et al., 1993; Kalandadze et al., 2002). Protein kinase C (PKC)-dependent phosphorylation of serine-113 (S113), serine-486 (S486) and serine-520 (S520) plays a critical role in GLT-1 function and trafficking (Casado et al., 1993; Garcia-Tardon et al., 2012; Kalandadze et al., 2002). Phosphorylation of GLAST and GLT-1 decreased glutamate uptake in HEK293 cells and the ALS-parkinsonism dementia complex (PDC) mouse model, respectively (Conradt and Stoffel, 1997; Wilson et al., 2003), possibly due to rapid intracellular protein sequestration (Anderson and Swanson, 2000). However, other studies have shown that PKC-dependent phosphorylation at serine residues increased with GLT-1 activity (Casado et al., 1993), while others have reported that GLT-1 localization and degradation are independent of its phosphorylation and instead requires ubiquitin ligase Nedd4–2 for ubiquitination (Garcia-Tardon et al., 2012). These contradictory findings may be due to targeting of different serine residues and involvement of PKC-dependent Nedd4–2 ubiquitination associated with GLT-1 internalization and function used in various experimental settings (Garcia-Tardon et al., 2012).
3.5.3. Ubiquitination
Despite the significant role of phosphorylation in GLT-1 function and trafficking, studies have implicated ubiquitination as a necessary PTM for the trafficking and degradation of astrocytic glutamate transporters (Boehmer et al., 2006). Ubiquitin ligase Nedd4–2 facilitates GLAST/GLT-1 ubiquitination (Boehmer et al., 2003; Boehmer et al., 2006), and aberrant Nedd4–2 activity has been shown to disrupt their localization to the astrocytic membrane, resulting in a reduction of membrane-bound GLAST and GLT-1 in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced PD animal models (Zhang et al., 2017).
Ubiquitinated lysine residues in the carboxyl terminus of GLT-1 are required for its endocytosis and degradation (Garcia-Tardon et al., 2012). As GLT-1 prevents excitotoxicity and its loss has been implicated in various pathological conditions, ubiquitination and targeted degradation of GLT-1 may serve as a pathological mechanism for decreasing GLT-1 expression and function in neurodegenerativa conditions (Sheldon et al., 2008). PKC-dependent ubiquitination of GLT-1 has been shown to reduce glutamate uptake in astrocyte cultures (Garcia-Tardon et al., 2012). Activation of PKC increased the ubiquitination of GLT-1, leading to the accumulation of ubiquitinated GLT-1 in the intracellular compartment (Gonzalez-Gonzalez et al., 2008). Excess glutamate increased ubiquitination and internalization of GLT-1, resulting in a reduction of cell surface GLT-1 expression in HEK293 cells (Ibanez et al., 2016). Aberrant ubiquitination of GLT-1 also reduced both its protein levels and glutamate uptake in astrocytes (Munoz-Ballester et al., 2016).
3.5.4. Palmitoylation
Palmitoylation is a PTM that affects the membrane partitioning, trafficking and activity of membrane proteins (Tortosa and Hoogenraad, 2018). The palmitoylation of GLT-1 occurs at cysteine 38 (C38) via a thioester linkage by palmitoyl acyltransferases (Huang et al., 2010). GLT-1 palmitoylation regulates normal glutamate uptake and is greatly reduced in the YAC128 Huntington’s Disease (HD) mouse model (Huang et al., 2010). Further inhibition of palmitoylation by mutation or pharmacological inhibition severely impairs glutamate uptake (Huang et al., 2010). Moreover, studies have shown that reduced palmitoylation of EAAT1 and EAAT2 in glioma patients decreased glutamate re-uptake (Tong et al., 2015). These findings suggest that abnormal palmitoylation of glutamate transporters is associated with various pathological conditions, though the mechanism of pathogenesis remains unclear.
3.5.5. Sumoylation
Sumoylation is a PTM that covalently attaches small ubiquitin-like modifier (SUMO) proteins to other proteins to regulate protein localization and function (Hay, 2005). The localization of GLT-1 in the cells is affected by sumoylation, which is closely associated with proteolytic cleavage (Foran et al., 2011). Caspase-3-mediated cleavage of GLT-1 leads to intracellular accumulation of a sumoylated GLT-1 C-terminus fragment (CTE-SUMO1) at the beginning of disease onset in ALS mouse models (Rosenblum et al., 2017). Moreover, sumoylated proteolytic fragments of GLT-1 were found in the nucleus and endosome in spinal cord astrocytes of ALS mice (Foran et al., 2011; Foran et al., 2014). Nuclear accumulation of GLT-1 fragments in astrocytes also induces neuronal toxicity in co-culture, suggesting that sumoylation of astrocytic GLT-1 contributes to motor neuron degeneration and ALS pathogenesis (Foran et al., 2011). On the other hand, desumoylation facilitates trafficking of GLT-1 to the plasma membrane, resulting in enhanced glutamate uptake in primary astrocytes (Foran et al., 2014). Taken together, these findings warrant further elucidation of the role of sumoylation of GLT-1 in neurodegenerative diseases.
3.5.6. Proteolytic cleavage
Enhanced proteolytic cleavage of GLT-1 by caspase-3 activation is associated with impairment of glutamate uptake in mutant superoxide dismutase (SOD1) ALS mouse models (Boston-Howes et al., 2006). SOD1-mutant mice enhanced caspase-3-mediated cleavage of GLT-1, resulting in the formation of truncated GLT-1 (Boston-Howes et al., 2006). Caspase-3 cleaved GLT-1 at the C-terminal domain, leading to the accumulation of sumoylated GLT-1 C-terminus fragments prior to the onset of ALS. Mutation of this cleavage site did not affect GLT-1 activity, but delayed disease progression and extended lifespan in an ALS mouse model (Rosenblum et al., 2017). Additionally, accumulation of GLT-1 proteolytic fragments in astrocytes increased toxicity, resulting in the impairment of neuronal and axonal growth (Foran et al., 2011). These findings suggest that abnormal GLT-1 cleavage impairs glutamate uptake, leading to excitotoxicity in ALS, providing potential molecular targets for pharmacological interventions.
3.5.7. Nitrosylation
S-Nitrosylation is the covalent attachment of a nitroso group (-NO) to a cysteine thiol to form S-nitrosothiol (SNO), which plays an essential role in nitric oxide (NO) bioactivity (Anand and Stamler, 2012). Recent studies have shown that the nitrosylation of proteins plays a critical role in glutamate neurotransmission by modulating the glutamate/glutamine cycle (Raju et al., 2015). S-nitrosylation of GLT-1 occurs at cysteine-373 (C373) and cysteine-561 (C561), which was abrogated in mice lacking endothelial nitric oxide synthase (Raju et al., 2015). In addition, GLT-1 that was not S-nitrosylated at C373 or C561 showed increased glutamate uptake as compared to nitrosylated GLT-1, suggesting that S-nitrosylation impairs glutamate uptake (Raju et al., 2015). Other studies have shown that chronic Toxoplasma infection and ischemia models showed increased nitric oxide concomitant to decreased GLT-1 levels (David et al., 2016; Yamada et al., 2006), indicating that nitrosylation may contribute to neurological disorders by promoting aberrant glutamate neurotransmission and excitotoxicity.
4. Impairment of glutamate transporters in neurological disorders
4.1. AD
AD is a chronic neurodegenerative disease characterized by progressive memory loss and cognitive decline (Brookmeyer et al., 2018). While several genetic mutations have been linked to AD, most cases are idiopathic in nature and the mechanisms of pathogenesis are not well understood (Tsolaki et al., 2018). Glutamate-mediated excitotoxic neuronal death is implicated in AD pathology (Kornhuber and Wiltfang, 1998), indicating that dysregulation of GLAST and GLT-1 impact AD pathogenesis.
Clinical studies in AD patients and experimental animal studies have shown that a loss of GLT-1 is involved in pathological features of AD (Takahashi et al., 2015). Moreover, AD mouse models with the loss of one GLT-1 allele showed accelerated cognitive decline as compared to wild-type control, whereas overexpression of GLT-1 attenuated AD-associated cognitive deficits in transgenic mice (Mookherjee et al., 2011; Takahashi et al., 2015). Pharmacological restoration of GLT-1 function with LDN 212320, a translational activator, in AD mice also attenuated the cognitive deficits, suggesting that GLT-1 plays a critical role in cognitive function and neuroprotection in AD (Takahashi et al., 2015).
4.2. PD
PD is a chronic, progressive neurodegenerative disorder characterized by the loss of dopaminergic neurons in the substantia nigra, resulting in motor deficits such as hypokinesia, tremors and muscle rigidity (Rees et al., 2018).
Dysregulation of glutamate homeostasis and neurotransmission have been implicated in PD pathogenesis. Glutamate uptake in platelets is reduced by 50% in PD patients as compared to normal human subjects (Ferrarese et al., 2001). PD severity and Parkinsonian symptoms were also correlated with glutamate uptake reduction. Ro 25–6981, an N-methyl-D-aspartate (NMDA) receptor antagonist, attenuated Parkinsonian motor symptoms in the MPTP-induced PD animal model (Loschmann et al., 2004). Further, in a PD rat model, exposure to 6-hydroxydopamine resulted in decreased striatal GLT-1 (Chung et al., 2008). Aberrant ubiquitination of GLT-1 leads to decreases in GLT-1 expression and motor deficits along with DA cell loss in an MPTP-induced PD mouse model (Zhang et al., 2017). Taken together, these findings suggest that GLT-1 is critically involved in the pathogenesis of PD.
4.3. ALS
ALS is a neurological disorder, characterized by progressive degeneration of motor neurons in the brain and spinal cord (Chi et al., 2018). The mechanisms of ALS are not well understood, but growing evidence shows that glutamate-mediated excitotoxicity is closely related to ALS pathogenesis (Lin et al., 2012). Postmortem brain tissue from the ALS patients revealed a 30–95% loss of EAAT2 in the motor cortex and spinal cord, corroborating findings of GLT-1 reduction in transgenic ALS mice (Rothstein et al., 1995). Genetic mutations of SOD1 in the ALS mouse model have also shown a reduction of GLT-1, in addition to the loss of motor neurons (Tortarolo et al., 2004). Moreover, treatment of SOD1 mice with β-lactam antibiotic ceftriaxone attenuated motor symptoms and prolonged survival (Rothstein et al., 2005). These results suggest that GLT-1 dysregulation is closely associated with ALS pathogenesis.
4.4. Manganism
Chronic overexposure to manganese (Mn) results in a neurological disorder known as manganism, which shares pathologic features with sporadic PD (Kwakye et al., 2015). In addition to extrapyramidal motor deficits (Racette et al., 2012), Mn induces dopaminergic neurotoxicity along with dysfunction of GLAST/GLT-1 (Johnson et al., 2018a; Pajarillo et al., 2018). Moreover, Mn neurotoxicity is also known to contribute to the development of other neurodegenerative disorders such as AD, PD, ALS, schizophrenia, and epilepsy (Bowman et al., 2011; Takeda, 2003).
Mn decreases GLAST/GLT-1 expression and function in astrocyte cultures, possibly leading to excitotoxic neuronal injury (Karki et al., 2015b; Karki et al., 2014b). Although the exact mechanisms by which Mn represses these transporters are not completely understood, the transcription factor YY1 appears to mediate the Mn-induced repression of GLAST/GLT-1 (Karki et al., 2013a). This is supported by the findings that Mn increases YY1 expression and enhances YY1 binding to the GLAST/GLT-1 promoters (Karki et al., 2015a; Karki et al., 2015b; Karki et al., 2014b) while mutation of YY1 binding sites in the promoter regions attenuates Mn-induced repression of GLAST/GLT-1 (Karki et al., 2014b).
4.5. Schizophrenia
Schizophrenia is a chronic psychiatric disorder characterized by behavioral disturbances and dissociation from reality (Bartoli et al., 2018). Increasing evidence has shown that dysregulation of glutamate transporters is associated with this disease. It has been reported that EAAT1 expression is decreased in schizophrenia as compared to healthy subjects (Bauer et al., 2008). A genetic variant of EAAT2 (SNP rs4354668) has been correlated with the severity of schizophrenia (Spangaro et al., 2012; Spangaro et al., 2014). At the translational level, N-glycosylation of EAAT2 was reduced in schizophrenic brains (Kalandadze et al., 2004). Together, these findings indicate that EAAT1/2 may be involved in schizophrenic pathogenesis, a phenomenon which warrants further exploration.
4.6. Epilepsy
Epilepsy is a neurological disorder characterized by sensory disturbances, seizures and loss of consciousness, induced by overstimulation of glutamate receptors. There was a significant increase in extracellular glutamate levels in the hippocampus prior to seizure onset in epileptic patients (Cavus et al., 2005), while overexpression of GLT-1 attenuated pilocarpine-induced recurrent seizures and prevented seizure-induced neuronal death in GLT-1 transgenic mice (Kong et al., 2012). Moreover, VPA, an antiepileptic, reversed Mn- or glutamate-reduced GLAST/GLT-1 in both in vitro and in vivo settings (Aguirre et al., 2008; Johnson et al., 2018a). These results suggest that GLAST/GLT-1 might be a therapeutic target in the treatment of epilepsy.
4.7. Cerebral Ischemia
Cerebral ischemia is a condition in which blood flow to the brain is blocked, leading to ischemic stroke (Gulke et al., 2018). A large body of evidence suggests that glutamate-associated excitotoxicity is associated with ischemia (Mayor and Tymianski, 2018). Knockdown of GLT-1 exacerbates neuronal damage in ischemic rat models, indicating that GLT-1-mediated glutamate uptake is critical for neuronal survival (Rao et al., 2001). GLT-1 overexpression was also neuroprotective against ischemia, which was induced by oxygen/glucose deprivation in astrocytes (Weller et al., 2008). Ceftriaxone, a β-lactam antibiotic, also increased GLT-1 expression and decreased cell death in a rat model of ischemia (Chu et al., 2007). Further, overexpression of GLT-1 in a rat model of stroke showed that GLT-1 reduced excess glutamate levels, decreased stroke-associated cell death and improved recovery (Harvey et al., 2011), suggesting that GLT-1 is critically involved in preventing stroke.
4.8. Autism spectrum disorders (ASD)
ASD or autism is characterized by deficits in social interaction and repetitive behavior as well as impairment of language and communication (Kaufmann et al., 2004). Several studies have reported that dysregulated GLT-1 expression and glutamate uptake impairs glutamate clearance from the synaptic cleft, leading to pathogenesis in ASD animal models (Bristot Silvestrin et al., 2013). Moreover, loss of GLT-1 resulted in synaptic over-excitability and pathological repetitive behaviors in mice, suggesting that GLT-1 has an important role in regulating cortical synapses (Aida et al., 2015). Another study reported that the deletion of fragile X mental retardation protein (FMRP) in mice decreased GLT-1 expression and glutamate re-uptake, resulting in abnormal neuronal hyperexcitability, which were reversed by ceftriaxone treatment (Higashimori et al., 2016). The loss of FMRP in the brain produces fragile X syndrome (FXS) phenotypes and is associated with ASD pathogenesis (Kaufmann et al., 2004). These findings indicate a possible link between dysfunctional glutamate transporters and ASD, warranting further exploration.
5. Pharmacological interventions targeting astrocytic glutamate transporters
Several pharmacological agents have been shown to modulate GLAST/GLT-1 expression at the transcriptional and translational levels (Kim et al., 2003b; Kong et al., 2014; Pawlak et al., 2005). Despite the significant efficacy of these compounds, the molecular mechanisms involved in the upregulation of these transporters remain to be elucidated. For example, estrogen (primarily 17ß-estradiol) increased both GLAST and GLT-1 at the transcriptional level and reversed manganese (Mn)-induced reduction of those transporters (Lee et al., 2009; Pajarillo et al., 2018). Various pharmacological agents regulating GLAST/GLT-1 expression and function are compared by their mode of action (Table 1).
Table 1.
List of pharmacological drugs that modulate the expression and function of GLAST/EAAT1 and GLT-1/EAAT2
| Drug | Regulatory Effect | Molecular mechanism/pathways | In vitro/in vivo; disease model | Reference |
|---|---|---|---|---|
| β-lactam antibiotics | ||||
| Ceftriaxone | ↑TEAAT2/GLT-1 | PI3K-Akt, NF-κB | In vitro and in vivo; ALS, ischemia, HD, neuropathic pain, hypoxia, AD, alcohol abuse/dependency, subarachnoid hemorrhage | (Chu et al., 2007; Cudkowicz et al., 2014; Decker et al., 2016; Eljaja et al., 2018; Feng et al., 2014; Jagadapillai et al., 2014; Petr et al., 2013b; Sari et al., 2016) |
| Ampicillin | ↑EAAT1, ↑EAAT2/GLT-1 |
PI3K-Akt | In vivo; alcohol abuse/dependency | (Rao et al., 2015b) |
| Cefazolin | ↑EAAT1, ↑EAAT2/GLT-1 |
PI3K-Akt | In vivo; alcohol abuse/dependency | (Rao et al., 2015b) |
| Cefoperazone | ↑EAAT1, ↑EAAT2/GLT-1 |
PI3K-Akt | In vivo; alcohol abuse/dependency | (Rao et al., 2015b) |
| Estrogen and SERMs | ||||
| 17β-estradiol | ↑EAAT1/GLAST, ↑EAAT2/GLT-1 |
PI3K-Akt, TGF-α, ERK, NF-κB, and nuclear ER binding | In vitro and in vivo; manganism | (Lee et al., 2012a; Lee et al., 2012b; Lee et al., 2009; Pajarillo et al., 2018; Pawlak et al., 2005) |
| Tamoxifen | ↑EAAT1/GLAST, ↑EAAT2/GLT-1 |
PI3K-Akt, TGF-α, ERK, NF-κB, and nuclear ER binding | In vitro and in vivo; manganism | (Lee et al., 2012b; Lee et al., 2009; Pajarillo et al., 2018) |
| Raloxifene | ↑EAAT1/GLAST, ↑EAAT2/GLT-1 |
PI3K-Akt, TGF-α, ERK, NF-κB, and nuclear ER binding | In vitro; manganism | (Karki et al., 2014c) |
| Growth factors | ||||
| EGF | ↑EAAT1/GLAST, ↑EAAT2/GLT-1 |
PI3K-Akt, NF-κB | In vitro | (Figiel et al., 2003; Sitcheran et al., 2005; Zelenaia et al., 2000) |
| TGF-α | ↑EAAT1/GLAST, ↑EAAT2/GLT-1 |
TGF-α, PI3K-Akt | In vitro; manganism | (Figiel et al., 2003; Lee et al., 2012b) |
| TGF-β1 | ↑EAAT1/GLAST, ↑EAAT2/GLT-1 |
TGF-β1 | In vitro and in vivo | (Close et al., 2005; Koeglsperger et al., 2013; Stipursky and Gomes, 2007) |
| BDNF | ↑EAAT1/GLAST, ↑EAAT2/GLT-1 |
BDNF-TrkB, CREB, NF-κB | In vitro and in vivo; depression, AD, hypoxia | (Dai et al., 2012; Figiel et al., 2003; Liu et al., 2016; Rodriguez- Kern et al., 2003) |
| GDNF | ↑EAAT1/GLAST, ↑EAAT2/GLT-1 |
MAPK ERK | In vitro | (Bonde et al., 2003; Delyfer et al., 2005; Figiel et al., 2003; Koeberle and Bahr, 2008) |
| Basic FGF | ↑EAAT1/GLAST, ↑EAAT2/GLT-1 |
MAPK ERK, NF-κB, PI3K-Akt | In vitro | (Figiel et al., 2003) |
| dbcAMP | ↑EAAT1/GLAST, ↑EAAT2/GLT-1 |
PI3K-Akt, NF-κB, cAMP-dependent, PKA | In vitro | (Li et al., 2006; Schluter et al., 2002; Zelenaia et al., 2000) |
| HDACi | ||||
| SAHA | ↑EAAT1, ↑EAAT2 |
Class I and II HDAC inhibition, histone acetylation | In vitro; manganism | (Karki et al., 2014b) |
| VPA | ↑EAAT1/GLAST, ↑EAAT2/GLT-1 |
Class I and HDAC inhibition, histone acetylation | In vitro and in vivo; manganism | (Johnson et al., 2018a; Johnson et al., 2018b; Karki et al., 2014b) |
| Sodium butyrate | ↑EAAT1/GLAST, ↑EAAT2/GLT-1 |
HDAC inhibition, histone acetylation | In vitro and in vivo; manganism | (Johnson et al., 2018b; Karki et al., 2014b) |
| TSA | ↑EAAT1, EAAT2 | Class II HDAC inhibition, histone acetylation | In vitro; manganism | (Karki et al., 2014a) |
| MC1568 | ↑GLT-1 | Class II HDAC inhibition, histone acetylation | In vivo; ALS | (Lapucci et al., 2017) |
| Other compounds | ||||
| Arundic acid | ↑EAAT1 | ERK, NF-κB, Akt | In vitro; manganism | (Karki et al., 2018) |
| LDN 212320 | ↑EAAT2/GLT-1 | PKC, translational activation | In vivo; AD | (Kong et al., 2014) |
| Riluzole | ↑EAAT2 | PKCK1 isoform δ | In vitro and in vivo; ALS, AD, depression, PD | (Carbone et al., 2012a, b; Pereira et al., 2017) |
| Harmine | ↑EAAT2/GLT-1 | - | In vivo, ALS | (Li et al., 2011) |
| Rosiglitazone | ↑EAAT2/GLT-1 | - | In vitro and in vivo; stress, ischemia | (Garcia- Bueno et al., 2007; Romera et al., 2007) |
| Maslinic acid | ↑EAAT1/GLAST, ↑EAAT2/GLT-1 |
NF-κB | In vitro and in vivo; glutamate toxicity, ischemia | (Guan et al., 2011; Qian et al., 2011; Qian et al., 2016) |
| L-threohydroxy aspartate | ↓EAAT1/GLAST, ↓EAAT2/GLT-1 |
Competitive inhibition | In vitro, glutamate toxicity | (Qian et al., 2011) |
| Bromocriptine | ↓EAAT2/GLAST | Competitive inhibition | In vitro | (Shirasaki et al., 2010) |
| Dihydrokainate | ↓EAAT2/GLT-1 | Competitive inhibition | In vitro and in vivo | (Arriza et al., 1994; Kawahara et al., 2002) |
| T0070907 | ↓GLT-1 | Peroxisome proliferator-activated receptor gamma (PPARγ) | In vivo; ischemia | (Romera et al., 2007) |
| dl-threo-beta- benzyloxyaspartic acid (TBOA) | ↓EAAT2 | Competitive inhibition | In vitro | (Shimamoto et al., 1998) |
| Phorbol ester | ↓EAAT1/GLAST, ↓EAAT2/GLT-1 |
PKC | In vitro | (Dunlop et al., 1999; Ganel and Crosson, 1998; Gonzalez et al., 2005; Kalandadze et al., 2002) |
| WAY-855 | ↓EAAT2/GLT-1 | Non-competitive inhibition | In vitro | (Dunlop et al., 2003) |
| WAY-213613 | ↓EAAT2/GLT-1 | Competitive inhibition | In vitro | (Dunlop et al., 2005) |
5.1. β-Lactam antibiotics
Several β-lactam antibiotics have been shown to enhance GLT-1 expression at the transcriptional level in experimental settings (Rothstein et al., 2005). Ceftriaxone increased GLT-1 protein levels as well as glutamate uptake and exerted neuroprotection against ischemia and ALS both in vitro and in vivo (Rao et al., 2015c). At the transcriptional level, ceftriaxone activated the NF-κB signaling pathway to enhance GLT-1 promoter activity and protein levels. Other β-lactam antibiotics, such as ampicillin, cefazolin, and cefoperazone, elicited similar effects in enhancing GLT-1 expression and function (Rao et al., 2015b). These findings indicate that multiple ß-lactam antibiotics upregulate GLT-1 at the transcriptional level and may serve as molecular targets to treat neurological disorders associated with EAAT2 dysregulation.
5.2. Estrogen and SERMs
Growing evidence suggests that estrogen and SERMs exert neuroprotective effects in neurological disorders, including ischemia, AD, PD and Mn-induced toxicity, in several experimental models (Liang et al., 2002; Pajarillo et al., 2018).
Estrogen modulates a broad spectrum of molecular mechanisms to induce neuroprotective effects. One such mechanism is the release of brain-derived neurotrophic factor (BDNF), which promoted neuronal survival and cognitive function in an ischemic rat model (Yang et al., 2010). Estrogen and SERMs, such as tamoxifen and raloxifene, also increase expression of GLT-1 and GLAST at the transcriptional level in vitro and in vivo through genomic pathways (Karki et al., 2014c; Lee et al., 2009; Pajarillo et al., 2018). Additionally, estrogen and tamoxifen enhanced transcription of GLT-1/GLAST via non-genomic pathways, activating MAPK ERK, PI3K-Akt, TGF-α and NF-ΚBsignaling in astrocytes (Lee et al., 2012b; Lee et al., 2009). Raloxifene upregulated GLAST/GLT-1 by the activation of ERK, epidermal growth factor receptor (EGFR), and CREB in astrocytes (Karki et al., 2014c).
Estrogen increased GLAST and GLT-1 expression and attenuated decreases in GLAST/GLT-1 in AD patient-derived astrocytes (Pawlak et al., 2005). Estrogen and tamoxifen also reversed Mn-reduced GLAST and GLT-1 protein levels in mice (Pajarillo et al., 2018). Estrogen receptor (ER)-a has been associated with GLT-1 expression in an ischemic rat model (Cimarosti et al., 2005), indicating that ER-α may serve as the primary receptor involved in GLT-1 expression and neuroprotective actions. These results indicate that estrogen and SERMs promote neuroprotection through a variety of mechanisms, including the regulation of astrocytic glutamate transporter expression and function.
5.3. Growth factors
A variety of growth factors, including TGF-α, TGF-ß1, epidermal growth factor (EGF), insulin-like growth factor I, basic fibroblast growth factor (FGF), glial cell line-derived neurotrophic factor (GDNF) and BDNF, have been shown to enhance the expression and function of GLAST/GLT-1. These growth factors also exert neuroprotective effects in various neurological disorders via attenuation of excitotoxicity (Lee et al., 2012b).
Studies have shown that TGF-ß1-deficient mice exhibited high sensitivity to excitotoxic injury, similar to the tendencies of GLT-1-deficient mice (Koeglsperger et al., 2013). TGF-ß1 induced neuroprotection and attenuated the reduction of GLT-1 in a chronic pain rat model (Chen et al., 2013). EGF and TGF -α increased GLAST/GLT-1 expression in rat primary astrocytes and cell line cultures (Karki et al., 2017; Lee et al., 2012b; Sitcheran et al., 2005; Unger et al., 2012) via activation of the NF-ΚBpathway, likely via PI3K-Akt and MAPK/ERK signaling (Lee et al., 2012b; Sitcheran et al., 2005). TGF-α also mediates the effects of estrogen and SERMs on GLAST/GLT-1 expression via non-genomic signaling in vitro (Lee et al., 2009). Moreover, TGF-α expression is abundant in astrocytes and its expression is increased by estrogen (Ma et al., 1994). Estrogen and tamoxifen increased expression of TGF-α as well as GLT-1/GLAST concomitantly (Pajarillo et al., 2018), indicating that TGF-α at least partially mediates estrogen/SERM-induced neuroprotection by enhancing astrocytic glutamate transporters in mice. These findings suggest that growth factors may be utilized in the development of neurotherapeutics via enhancement of glutamate transporters GLT-1 and GLAST.
5.4. HDACi
HDACs are epigenetic modifiers that remove acetyl groups from histone molecules, resulting in tightening of the DNA around histones and decreased gene transcription (Mai et al., 2009). HDACs are classified into Class I (HDACs 1, 2, 3, 8), Class IIa (HDACs 4, 5, 7, 9), Class IIb (HDACs 6, 10), Class III (sirtuins) and Class IV (HDAC11) (Mai et al., 2009). Studies have shown that HDACi such as MC1568, VPA, and sodium butyrate, increased GLAST/GLT-1 expression and glutamate uptake in in vitro and in vivo models of ALS and Mn-induced neurotoxicity (Johnson et al., 2018a; Johnson et al., 2018b; Karki et al., 2014b; Lapucci et al., 2017). Many HDACi, including sodium butyrate, VPA, SAHA and TSA, increased expression of GLT-1 and GLAST in cell culture models (Karki et al., 2014b). Moreover, VPA and sodium butyrate increased GLAST and GLT-1 expression and protected the loss of dopaminergic neurons against Mn neurotoxicity, resulting in improved motor coordination and activity in mice (Johnson et al., 2018a; Johnson et al., 2018b). Another HDACi, MC1568, has been shown to increase GLT-1 expression and restore spinal cord expression of GLT-1 and glutamate uptake in mouse primary astrocyte cultures and SOD1-mutant mice, an ALS animal model (Lapucci et al., 2017). MC1568 also increased the sumoylation of EAAT2 (Lapucci et al., 2017), suggesting a link between histone acetylation and sumoylation of EAAT2 at the transcriptional and posttranslational levels. The molecular mechanism of HDACi by which GLT-1/GLAST are upregulated remains to be elucidated.
In addition to acting as epigenetic modifiers through the deacetylation of histone molecules, HDACs serve as co-repressors of non-histone protein transcription factor YY1, which binds to its consensus binding sites in the promoter regions of GLT-1 and GLAST, inhibiting their expression (Karki et al., 2015a; Karki et al., 2014b). This indicates that HDACi can target multiple proteins at the cellular levels. HDACi have also been tested clinically in the treatment of various diseases, including cancer (Suraweera et al., 2018) and muscular dystrophy (Bettica et al., 2016), and there is a growing interest in its use in the treatment of neurological disorders.
5.5. Translational activators
The compound LDN 212320, a pyridazine derivative, is known as an activator of GLT-1 translation and has significant neuroprotective effects in vivo (Kong et al., 2014). LDN 212320 protects cultured neurons from glutamate-mediated excitotoxic injury and death via GLT-1 activation (Kong et al., 2014). The compound delayed motor function decline and extended lifespan in an animal model of ALS (Kong et al., 2014). LDN 212320 activated PKC and subsequent Y-box-binding protein 1 (YB-1), which regulates activation of EAAT2 translation (Kong et al., 2014).
LDN 212320 restored GLT-1 protein function with significant improvement of cognitive function, reestablishing synaptic integrity and reducing amyloid plaques in a transgenic AD animal model (Takahashi et al., 2015). These effects lasted a month after compound treatment cessation (Takahashi et al., 2015). These findings indicate that small molecules such as LDN 212320 enhance GLT-1 translation. These small molecules may also contribute to the treatment of neurodegenerative diseases associated with GLT-1 dysfunction.
6. Conclusion
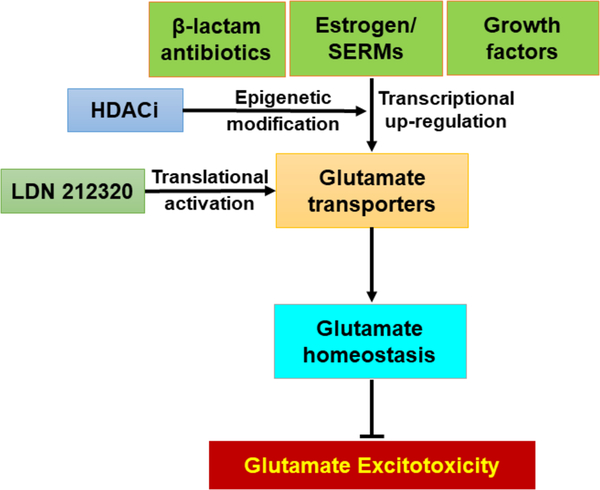
Dysregulation of EAAT1/GLAST and EAAT2/GLT-1 have been strongly linked to the pathogenesis of various neurological disorders such as ALS, AD, PD, manganism, ischemia, schizophrenia, epilepsy, and autism. While epigenetic modifications, transcriptional regulation, RNA splicing and PTMs support the diversity and pleiotropic functions of astrocytic glutamate transporters, aberrancy of these processes contributes to the onset and progression of glutamate excitotoxicity. Accordingly, delineating the molecular mechanisms involved in the genetic, epigenetic, transcriptional and translational regulation of GLAST/GLT-1 expression and function is critical to further our understanding of glutamate excitotoxicity and neuropathogenesis. Likewise, drug targeting of glutamate transporters constitutes an exciting direction for exploration, which would extend our collective comprehension of neurological disorders and aid in the identification of potential therapeutic targets. Pharmacological agents such as ß-lactam antibiotics, estrogen and SERMs, growth factors, HDACi, and translational activators show promising efficacy in increasing GLAST/GLT-1 expression and glutamate uptake in astrocytes, thus preventing excitotoxic neuronal injury (Figure 3).
Fig. 3.
Pharmacological interventions targeting astrocytic glutamate transporters. At the transcriptional level, β-lactam antibiotics, estrogens and growth factors enhance EAAT1 and EAAT2 expression and function. Epigenetic modifiers such as histone deacetylase inhibitors (HDACi) increase transcription of EAAT1/EAAT2. At the translational level, the translational activator of EAAT2 LDN 212320 also increases EAAT2 protein expression and function.
Highlights.
Excitotoxicity plays a critical role in neurological and neurodegenerativa disorders.
Glutamate transporters GLT-1 and GLAST are essential in preventing excitotoxicity.
GLT-1/GLAST expression can be modulated by drugs at multiple levels of gene expression.
Pharmacological agents can target GLT-1/GLAST to treat neurological disorders.
Acknowledgment
The present study was supported in part by National Institutes of Health (NIH) R01 ES024756 (EL), R01 ES10563 (MA), R01 ES07331 (MA) and R01 ES020852 (MA)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abousaab A, Uzcategui NL, Elsir B, Lang F, 2016. Up-Regulation of the Excitatory Amino Acid Transporters EAAT1 and EAAT2 by Mammalian Target of Rapamycin. Cell Physiol Biochem 39, 2492–2500. [DOI] [PubMed] [Google Scholar]
- Aguirre G, Rosas S, Lopez-Bayghen E, Ortega A, 2008. Valproate-dependent transcriptional regulation of GLAST/EAAT1 expression: involvement of Ying-Yang 1. Neurochem Int 52, 1322–1331. [DOI] [PubMed] [Google Scholar]
- Aida T, Yoshida J, Nomura M, Tanimura A, Iino Y, Soma M, Bai N, Ito Y, Cui W, Aizawa H, Yanagisawa M, Nagai T, Takata N, Tanaka KF, Takayanagi R, Kano M, Gotz M, Hirase H, Tanaka K, 2015. Astroglial glutamate transporter deficiency increases synaptic excitability and leads to pathological repetitive behaviors in mice. Neuropsychopharmacology 40, 1569–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amara SG, Fontana AC, 2002. Excitatory amino acid transporters: keeping up with glutamate. Neurochem Int 41, 313–318. [DOI] [PubMed] [Google Scholar]
- Anand P, Stamler JS, 2012. Enzymatic mechanisms regulating protein S-nitrosylation: implications in health and disease. J Mol Med (Berl) 90, 233–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson CM, Swanson RA, 2000. Astrocyte glutamate transport: review of properties, regulation, and physiological functions. Glia 32, 1–14. [PubMed] [Google Scholar]
- Arriza JL, Fairman WA, Wadiche JI, Murdoch GH, Kavanaugh MP, Amara SG, 1994. Functional comparisons of three glutamate transporter subtypes cloned from human motor cortex. J Neurosci 14, 5559–5569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubry S, Shin W, Crary JF, Lefort R, Qureshi YH, Lefebvre C, Califano A, Shelanski ML, 2015. Assembly and interrogation of Alzheimer’s disease genetic networks reveal novel regulators of progression. PLoS One 10, e0120352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoli F, Crocamo C, Di Brita C, Esposito G, Tabacchi TI, Verrengia E, Clerici M, Carra G, 2018. Adjunctive second-generation antipsychotics for specific symptom domains of schizophrenia resistant to clozapine: A meta-analysis. J Psychiatr Res 108, 24–33. [DOI] [PubMed] [Google Scholar]
- Bauer D, Gupta D, Harotunian V, Meador-Woodruff JH, McCullumsmith RE, 2008. Abnormal expression of glutamate transporter and transporter interacting molecules in prefrontal cortex in elderly patients with schizophrenia. Schizophr Res 104, 108–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer D, Haroutunian V, Meador-Woodruff JH, McCullumsmith RE, 2010. Abnormal glycosylation of EAAT1 and EAAT2 in prefrontal cortex of elderly patients with schizophrenia. Schizophr Res 117, 92–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedrosian TA, Quayle C, Novaresi N, Gage FH, 2018. Early life experience drives structural variation of neural genomes in mice. Science 359, 1395–1399. [DOI] [PubMed] [Google Scholar]
- Benediktsson AM, Marrs GS, Tu JC, Worley PF, Rothstein JD, Bergles DE, Dailey ME, 2012. Neuronal activity regulates glutamate transporter dynamics in developing astrocytes. Glia 60, 175–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett SA, Tanaz R, Cobos SN, Torrente MP, 2018. Epigenetics in amyotrophic lateral sclerosis: a role for histone post-translational modifications in neurodegenerative disease. Transl Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berson A, Nativio R, Berger SL, Bonini NM, 2018. Epigenetic Regulation in Neurodegenerative Diseases. Trends Neurosci 41, 587–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettica P, Petrini S, D’Oria V, D’Amico A, Catteruccia M, Pane M, Sivo S, Magri F, Brajkovic S, Messina S, Vita GL, Gatti B, Moggio M, Puri PL, Rocchetti M, De Nicolao G, Vita G, Comi GP, Bertini E, Mercuri E, 2016. Histological effects of givinostat in boys with Duchenne muscular dystrophy. Neuromuscul Disord 26, 643–649. [DOI] [PubMed] [Google Scholar]
- Bjorn-Yoshimoto WE, Underhill SM, 2016. The importance of the excitatory amino acid transporter 3 (EAAT3). Neurochem Int 98, 4–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehmer C, Henke G, Schniepp R, Palmada M, Rothstein JD, Broer S, Lang F, 2003. Regulation of the glutamate transporter EAAT1 by the ubiquitin ligase Nedd4–2 and the serum and glucocorticoid- inducible kinase isoforms SGK1/3 and protein kinase B. J Neurochem 86, 1181–1188. [DOI] [PubMed] [Google Scholar]
- Boehmer C, Palmada M, Rajamanickam J, Schniepp R, Amara S, Lang F, 2006. Post-translational regulation of EAAT2 function by co-expressed ubiquitin ligase Nedd4–2 is impacted by SGK kinases. J Neurochem 97, 911–921. [DOI] [PubMed] [Google Scholar]
- Bonde C, Sarup A, Schousboe A, Gegelashvili G, Noraberg J, Zimmer J, 2003. GDNF pre-treatment aggravates neuronal cell loss in oxygen-glucose deprived hippocampal slice cultures: a possible effect of glutamate transporter up-regulation. Neurochem Int 43, 381–388. [DOI] [PubMed] [Google Scholar]
- Boston-Howes W, Gibb SL, Williams EO, Pasinelli P, Brown RH Jr., Trotti D, 2006. Caspase-3 cleaves and inactivates the glutamate transporter EAAT2. J Biol Chem 281, 14076–14084. [DOI] [PubMed] [Google Scholar]
- Bowman AB, Kwakye GF, Herrero Hernandez E, Aschner M, 2011. Role of manganese in neurodegenerative diseases. J Trace Elem Med Biol 25, 191–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges RJ, Esslinger CS, 2005. The excitatory amino acid transporters: pharmacological insights on substrate and inhibitor specificity of the EAAT subtypes. Pharmacol Ther 107, 271–285. [DOI] [PubMed] [Google Scholar]
- Bristot Silvestrin R, Bambini-Junior V, Galland F, Daniele Bobermim L, Quincozes-Santos A, Torres Abib R, Zanotto C, Batassini C, Brolese G, Goncalves CA, Riesgo R, Gottfried C, 2013. Animal model of autism induced by prenatal exposure to valproate: altered glutamate metabolism in the hippocampus. Brain Res 1495, 52–60. [DOI] [PubMed] [Google Scholar]
- Brookmeyer R, Abdalla N, Kawas CH, Corrada MM, 2018. Forecasting the prevalence of preclinical and clinical Alzheimer’s disease in the United States. Alzheimers Dement 14, 121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone M, Duty S, Rattray M, 2012a. Riluzole elevates GLT-1 activity and levels in striatal astrocytes. Neurochem Int 60, 31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone M, Duty S, Rattray M, 2012b. Riluzole neuroprotection in a Parkinson’s disease model involves suppression of reactive astrocytosis but not GLT-1 regulation. BMC Neurosci 13, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casado M, Bendahan A, Zafra F, Danbolt NC, Aragon C, Gimenez C, Kanner BI, 1993. Phosphorylation and modulation of brain glutamate transporters by protein kinase C. J Biol Chem 268, 27313–27317. [PubMed] [Google Scholar]
- Cavus I, Kasoff WS, Cassaday MP, Jacob R, Gueorguieva R, Sherwin RS, Krystal JH, Spencer DD, Abi-Saab WM, 2005. Extracellular metabolites in the cortex and hippocampus of epileptic patients. Ann Neurol 57, 226–235. [DOI] [PubMed] [Google Scholar]
- Chen NF, Huang SY, Chen WF, Chen CH, Lu CH, Chen CL, Yang SN, Wang HM, Wen ZH, 2013. TGF-beta1 attenuates spinal neuroinflammation and the excitatory amino acid system in rats with neuropathic pain. J Pain 14, 1671–1685. [DOI] [PubMed] [Google Scholar]
- Chen W, Aoki C, Mahadomrongkul V, Gruber CE, Wang GJ, Blitzblau R, Irwin N, Rosenberg PA, 2002. Expression of a variant form of the glutamate transporter GLT1 in neuronal cultures and in neurons and astrocytes in the rat brain. J Neurosci 22, 2142–2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi B, O’Connell JD, Iocolano AD, Coady JA, Yu Y, Gangopadhyay J, Gygi SP, Reed R, 2018. The neurodegenerative diseases ALS and SMA are linked at the molecular level via the ASC-1 complex. Nucleic Acids Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisholm NC, Henderson ML, Selvamani A, Park MJ, Dindot S, Miranda RC, Sohrabji F, 2015. Histone methylation patterns in astrocytes are influenced by age following ischemia. Epigenetics 10, 142–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu K, Lee ST, Sinn DI, Ko SY, Kim EH, Kim JM, Kim SJ, Park DK, Jung KH, Song EC, Lee SK, Kim M, Roh JK, 2007. Pharmacological Induction of Ischemic Tolerance by Glutamate Transporter-1 (EAAT2) Upregulation. Stroke 38, 177–182. [DOI] [PubMed] [Google Scholar]
- Chung EK, Chen LW, Chan YS, Yung KK, 2008. Downregulation of glial glutamate transporters after dopamine denervation in the striatum of 6-hydroxydopamine-lesioned rats. J Comp Neurol 511, 421–437. [DOI] [PubMed] [Google Scholar]
- Cimarosti H, Jones NM, O’Shea RD, Pow DV, Salbego C, Beart PM, 2005. Hypoxic preconditioning in neonatal rat brain involves regulation of excitatory amino acid transporter 2 and estrogen receptor alpha. Neurosci Lett 385, 52–57. [DOI] [PubMed] [Google Scholar]
- Close JL, Gumuscu B, Reh TA, 2005. Retinal neurons regulate proliferation of postnatal progenitors and Muller glia in the rat retina via TGF beta signaling. Development 132, 3015–3026. [DOI] [PubMed] [Google Scholar]
- Colon JM, Torrado AI, Cajigas A, Santiago JM, Salgado IK, Arroyo Y, Miranda JD, 2016. Tamoxifen Administration Immediately or 24 Hours after Spinal Cord Injury Improves Locomotor Recovery and Reduces Secondary Damage in Female Rats. J Neurotrauma 33, 1696–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conradt M, Stoffel W, 1997. Inhibition of the high-affinity brain glutamate transporter GLAST-1 via direct phosphorylation. J Neurochem 68, 1244–1251. [DOI] [PubMed] [Google Scholar]
- Cudkowicz ME, Titus S, Kearney M, Yu H, Sherman A, Schoenfeld D, Hayden D, Shui A, Brooks B, Conwit R, Felsenstein D, Greenblatt DJ, Keroack M, Kissel JT, Miller R, Rosenfeld J, Rothstein JD, Simpson E, Tolkoff-Rubin N, Zinman L, Shefner JM, Ceftriaxone Study I, 2014. Safety and efficacy of ceftriaxone for amyotrophic lateral sclerosis: a multi-stage, randomised, double-blind, placebocontrolled trial. Lancet Neurol 13, 1083–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai M, Xia XB, Xiong SQ, 2012. BDNF regulates GLAST and glutamine synthetase in mouse retinal Muller cells. J Cell Physiol 227, 596–603. [DOI] [PubMed] [Google Scholar]
- Dallaspezia S, Poletti S, Lorenzi C, Pirovano A, Colombo C, Benedetti F, 2012. Influence of an interaction between lithium salts and a functional polymorphism in SLC1A2 on the history of illness in bipolar disorder. Mol Diagn Ther 16, 303–309. [DOI] [PubMed] [Google Scholar]
- David CN, Frias ES, Szu JI, Vieira PA, Hubbard JA, Lovelace J, Michael M, Worth D, McGovern KE, Ethell IM, Stanley BG, Korzus E, Fiacco TA, Binder DK, Wilson EH, 2016. GLT-1-Dependent Disruption of CNS Glutamate Homeostasis and Neuronal Function by the Protozoan Parasite Toxoplasma gondii. PLoS Pathog 12, e1005643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker JM, Kruger L, Sydow A, Dennissen FJ, Siskova Z, Mandelkow E, Mandelkow EM, 2016. The Tau/A152T mutation, a risk factor for frontotemporal-spectrum disorders, leads to NR2B receptor- mediated excitotoxicity. EMBO Rep 17, 552–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delyfer MN, Simonutti M, Neveux N, Leveillard T, Sahel JA, 2005. Does GDNF exert its neuroprotective effects on photoreceptors in the rd1 retina through the glial glutamate transporter GLAST? Mol Vis 11, 677–687. [PubMed] [Google Scholar]
- Dunlop J, Eliasof S, Stack G, McIlvain HB, Greenfield A, Kowal D, Petroski R, Carrick T, 2003. WAY-855 (3-amino-tricyclo[2.2.1.02.6]heptane-1,3-dicarboxylic acid): a novel, EAAT2-preferring, nonsubstrate inhibitor of high-affinity glutamate uptake. Br J Pharmacol 140, 839–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop J, Lou Z, McIlvain HB, 1999. Properties of excitatory amino acid transport in the human U373 astrocytoma cell line. Brain Res 839, 235–242. [DOI] [PubMed] [Google Scholar]
- Dunlop J, McIlvain HB, Carrick TA, Jow B, Lu Q, Kowal D, Lin S, Greenfield A, Grosanu C, Fan K, Petroski R, Williams J, Foster A, Butera J, 2005. Characterization of novel aryl-ether, biaryl, and fluorene aspartic acid and diaminopropionic acid analogs as potent inhibitors of the high-affinity glutamate transporter EAAT2. Mol Pharmacol 68, 974–982. [DOI] [PubMed] [Google Scholar]
- Dunn BK, Anthony M, Sherman S, Costantino JP, 2001. Conclusions: Considerations regarding SERMs. Ann N Y Acad Sci 949, 352–365. [PubMed] [Google Scholar]
- Eljaja L, Bjerrum OJ, Honore PH, Abrahamsen B, 2018. Effects of the excitatory amino acid transporter subtype 2 (EAAT-2) inducer ceftriaxone on different pain modalities in rat. Scand J Pain 2, 132–136. [DOI] [PubMed] [Google Scholar]
- Feng D, Wang W, Dong Y, Wu L, Huang J, Ma Y, Zhang Z, Wu S, Gao G, Qin H, 2014. Ceftriaxone alleviates early brain injury after subarachnoid hemorrhage by increasing excitatory amino acid transporter 2 expression via the PI3K/Akt/NF-kappaB signaling pathway. Neuroscience 268, 21–32. [DOI] [PubMed] [Google Scholar]
- Ferrarese C, Tremolizzo L, Rigoldi M, Sala G, Begni B, Brighina L, Ricci G, Albizzati MG, Piolti R, Crosti F, Dalpra L, Frattola L, 2001. Decreased platelet glutamate uptake and genetic risk factors in patients with Parkinson’s disease. Neurol Sci 22, 65–66. [DOI] [PubMed] [Google Scholar]
- Ferrer I, Blanco R, 2000. N-myc and c-myc expression in Alzheimer disease, Huntington disease and Parkinson disease. Brain Res Mol Brain Res 77, 270–276. [DOI] [PubMed] [Google Scholar]
- Figiel M, Maucher T, Rozyczka J, Bayatti N, Engele J, 2003. Regulation of glial glutamate transporter expression by growth factors. Exp Neurol 183, 124–135. [DOI] [PubMed] [Google Scholar]
- Foley PF, Loh EW, Innes DJ, Williams SM, Tannenberg AE, Harper CG, Dodd PR, 2004. Association studies of neurotransmitter gene polymorphisms in alcoholic Caucasians. Ann N Y Acad Sci 1025, 39–46. [DOI] [PubMed] [Google Scholar]
- Foran E, Bogush A, Goffredo M, Roncaglia P, Gustincich S, Pasinelli P, Trotti D, 2011. Motor neuron impairment mediated by a sumoylated fragment of the glial glutamate transporter EAAT2. Glia 59, 1719–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foran E, Rosenblum L, Bogush A, Pasinelli P, Trotti D, 2014. Sumoylation of the astroglial glutamate transporter EAAT2 governs its intracellular compartmentalization. Glia 62, 1241–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forlani G, Giarda E, Ala U, Di Cunto F, Salani M, Tupler R, Kilstrup-Nielsen C, Landsberger N, 2010. The MeCP2/YY1 interaction regulates ANT1 expression at 4q35: novel hints for Rett syndrome pathogenesis. Hum Mol Genet 19, 3114–3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvin KM, Shi Y, 1997. Multiple mechanisms of transcriptional repression by YY1. Mol Cell Biol 17, 3723–3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganel R, Crosson CE, 1998. Modulation of human glutamate transporter activity by phorbol ester. J Neurochem 70, 993–1000. [DOI] [PubMed] [Google Scholar]
- Garcia-Bueno B, Caso JR, Perez-Nievas BG, Lorenzo P, Leza JC, 2007. Effects of peroxisome proliferator-activated receptor gamma agonists on brain glucose and glutamate transporters after stress in rats. Neuropsychopharmacology 32, 1251–1260. [DOI] [PubMed] [Google Scholar]
- Garcia-Esparcia P, Diaz-Lucena D, Ainciburu M, Torrejon-Escribano B, Carmona M, Llorens F, Ferrer I, 2018. Glutamate Transporter GLT1 Expression in Alzheimer Disease and Dementia With Lewy Bodies. Front Aging Neurosci 10, 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Tardon N, Gonzalez-Gonzalez IM, Martinez-Villarreal J, Fernandez-Sanchez E, Gimenez C, Zafra F, 2012. Protein kinase C (PKC)-promoted endocytosis of glutamate transporter GLT-1 requires ubiquitin ligase Nedd4–2-dependent ubiquitination but not phosphorylation. J Biol Chem 287, 19177–19187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gegelashvili G, Danbolt NC, Schousboe A, 1997. Neuronal soluble factors differentially regulate the expression of the GLT1 and GLAST glutamate transporters in cultured astroglia. J Neurochem 69, 2612–2615. [DOI] [PubMed] [Google Scholar]
- Giacomini KM, Brett CM, Altman RB, Benowitz NL, Dolan ME, Flockhart DA, Johnson JA, Hayes DF, Klein T, Krauss RM, Kroetz DL, McLeod HL, Nguyen AT, Ratain MJ, Relling MV, Reus V, Roden DM, Schaefer CA, Shuldiner AR, Skaar T, Tantisira K, Tyndale RF, Wang L, Weinshilboum RM, Weiss ST, Zineh I, Pharmacogenetics Research N, 2007. The pharmacogenetics research network: from SNP discovery to clinical drug response. Clin Pharmacol Ther 81, 328–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Gonzalez IM, Garcia-Tardon N, Gimenez C, Zafra F, 2008. PKC-dependent endocytosis of the GLT1 glutamate transporter depends on ubiquitylation of lysines located in a C-terminal cluster. Glia 56, 963–974. [DOI] [PubMed] [Google Scholar]
- Gonzalez MI, Susarla BT, Robinson MB, 2005. Evidence that protein kinase Calpha interacts with and regulates the glial glutamate transporter GLT-1. J Neurochem 94, 1180–1188. [DOI] [PubMed] [Google Scholar]
- Gonzalez SJ, Cristiano E, Argibay P, 2011. [Epigenetics and epigenome. A step forward in the etiology and potential treatment of neurological diseases]. Medicina (B Aires) 71, 390–396. [PubMed] [Google Scholar]
- Guan T, Qian Y, Tang X, Huang M, Huang L, Li Y, Sun H, 2011. Maslinic acid, a natural inhibitor of glycogen phosphorylase, reduces cerebral ischemic injury in hyperglycemic rats by GLT-1 up-regulation. J Neurosci Res 89, 1829–1839. [DOI] [PubMed] [Google Scholar]
- Gulke E, Gelderblom M, Magnus T, 2018. Danger signals in stroke and their role on microglia activation after ischemia. Ther Adv Neurol Disord 11, 1756286418774254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta RK, Prasad S, 2014. Differential regulation of GLT-1/EAAT2 gene expression by NF-kappaB and N-myc in male mouse brain during postnatal development. Neurochem Res 39, 150–160. [DOI] [PubMed] [Google Scholar]
- Hagiwara T, Tanaka K, Takai S, Maeno-Hikichi Y, Mukainaka Y, Wada K, 1996. Genomic organization, promoter analysis, and chromosomal localization of the gene for the mouse glial high- affinity glutamate transporter Slc1a3. Genomics 33, 508–515. [DOI] [PubMed] [Google Scholar]
- Han X, Yang L, Du H, Sun Q, Wang X, Cong L, Liu X, Yin L, Li S, Du Y, 2016. Insulin Attenuates Beta-Amyloid-Associated Insulin/Akt/EAAT Signaling Perturbations in Human Astrocytes. Cell Mol Neurobiol 36, 851–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey BK, Airavaara M, Hinzman J, Wires EM, Chiocco MJ, Howard DB, Shen H, Gerhardt G, Hoffer BJ, Wang Y, 2011. Targeted over-expression of glutamate transporter 1 (GLT-1) reduces ischemic brain injury in a rat model of stroke. PLoS One 6, e22135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay RT, 2005. SUMO: a history of modification. Mol Cell 18, 1–12. [DOI] [PubMed] [Google Scholar]
- He Y, Casaccia-Bonnefil P, 2008. The Yin and Yang of YY1 in the nervous system. J Neurochem 106, 1493–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashimori H, Schin CS, Chiang MS, Morel L, Shoneye TA, Nelson DL, Yang Y, 2016. Selective Deletion of Astroglial FMRP Dysregulates Glutamate Transporter GLT1 and Contributes to Fragile X Syndrome Phenotypes In Vivo. J Neurosci 36, 7079–7094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogland G, van Oort RJ, Proper EA, Jansen GH, van Rijen PC, van Veelen CW, van Nieuwenhuizen O, Troost D, de Graan PN, 2004. Alternative splicing of glutamate transporter EAAT2 RNA in neocortex and hippocampus of temporal lobe epilepsy patients. Epilepsy Res 59, 75–82. [DOI] [PubMed] [Google Scholar]
- Horiuchi Y, Iida S, Koga M, Ishiguro H, Iijima Y, Inada T, Watanabe Y, Someya T, Ujike H, Iwata N, Ozaki N, Kunugi H, Tochigi M, Itokawa M, Arai M, Niizato K, Iritani S, Kakita A, Takahashi H, Nawa H, Arinami T, 2012. Association of SNPs linked to increased expression of SLC1A1 with schizophrenia. Am J Med Genet B Neuropsychiatr Genet 159B, 30–37. [DOI] [PubMed] [Google Scholar]
- Huang K, Kang MH, Askew C, Kang R, Sanders SS, Wan J, Davis NG, Hayden MR, 2010. Palmitoylation and function of glial glutamate transporter-1 is reduced in the YAC128 mouse model of Huntington disease. Neurobiol Dis 40, 207–215. [DOI] [PubMed] [Google Scholar]
- Ibanez I, Diez-Guerra FJ, Gimenez C, Zafra F, 2016. Activity dependent internalization of the glutamate transporter GLT-1 mediated by beta-arrestin 1 and ubiquitination. Neuropharmacology 107, 376–386. [DOI] [PubMed] [Google Scholar]
- Jagadapillai R, Mellen NM, Sachleben LR Jr., Gozal E, 2014. Ceftriaxone preserves glutamate transporters and prevents intermittent hypoxia-induced vulnerability to brain excitotoxic injury. PLoS One 9, e100230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jen JC, Wan J, Palos TP, Howard BD, Baloh RW, 2005. Mutation in the glutamate transporter EAAT1 causes episodic ataxia, hemiplegia, and seizures. Neurology 65, 529–534. [DOI] [PubMed] [Google Scholar]
- Ji YF, Zhou L, Xie YJ, Xu SM, Zhu J, Teng P, Shao CY, Wang Y, Luo JH, Shen Y, 2013. Upregulation of glutamate transporter GLT-1 by mTOR-Akt-NF-small ka, CyrillicB cascade in astrocytic oxygen-glucose deprivation. Glia 61, 1959–1975. [DOI] [PubMed] [Google Scholar]
- Jia M, Njapo SA, Rastogi V, Hedna VS, 2015. Taming glutamate excitotoxicity: strategic pathway modulation for neuroprotection. CNS Drugs 29, 153–162. [DOI] [PubMed] [Google Scholar]
- Jia YF, Choi Y, Ayers-Ringler JR, Biernacka JM, Geske JR, Lindberg DR, McElroy SL, Frye MA, Choi DS, Veldic M, 2017. Differential SLC1A2 Promoter Methylation in Bipolar Disorder With or Without Addiction. Front Cell Neurosci 11, 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez E, Nunez E, Ibanez I, Draffin JE, Zafra F, Gimenez C, 2014. Differential regulation of the glutamate transporters GLT-1 and GLAST by GSK3beta. Neurochem Int 79, 33–43. [DOI] [PubMed] [Google Scholar]
- Jin Z, Liu Y, 2018. DNA methylation in human diseases. Genes Dis 5, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J Jr., Pajarillo E, Karki P, Kim J, Son DS, Aschner M, Lee E, 2018a. Valproic acid attenuates manganese-induced reduction in expression of GLT-1 and GLAST with concomitant changes in murine dopaminergic neurotoxicity. Neurotoxicology 67, 112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J Jr., Pajarillo EAB, Taka E, Reams R, Son DS, Aschner M, Lee E, 2018b. Valproate and sodium butyrate attenuate manganese-decreased locomotor activity and astrocytic glutamate transporters expression in mice. Neurotoxicology 64, 230–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalandadze A, Wu Y, Fournier K, Robinson MB, 2004. Identification of motifs involved in endoplasmic reticulum retention-forward trafficking of the GLT-1 subtype of glutamate transporter. J Neurosci 24, 5183–5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalandadze A, Wu Y, Robinson MB, 2002. Protein kinase C activation decreases cell surface expression of the GLT-1 subtype of glutamate transporter. Requirement of a carboxyl-terminal domain and partial dependence on serine 486. J Biol Chem 277, 45741–45750. [DOI] [PubMed] [Google Scholar]
- Karki P, Hong P, Johnson J Jr., Pajarillo E, Son DS, Aschner M, Lee EY, 2018. Arundic Acid Increases Expression and Function of Astrocytic Glutamate Transporter EAAT1 Via the ERK, Akt, and NF-kappaB Pathways. Mol Neurobiol 55, 5031–5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karki P, Johnson J Jr., Son DS, Aschner M, Lee E, 2017. Transcriptional Regulation of Human Transforming Growth Factor-alpha in Astrocytes. Mol Neurobiol 54, 964–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karki P, Kim C, Smith K, Son DS, Aschner M, Lee E, 2015a. Transcriptional Regulation of the Astrocytic Excitatory Amino Acid Transporter 1 (EAAT1) via NF-kappaB and Yin Yang 1 (YY1). J Biol Chem 290, 23725–23737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karki P, Lee E, Aschner M, 2013a. Manganese neurotoxicity: a focus on glutamate transporters. Ann Occup Environ Med 25, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karki P, Smith K, Johnson J Jr., Aschner M, Lee E, 2015b. Role of transcription factor yin yang 1 in manganese-induced reduction of astrocytic glutamate transporters: Putative mechanism for manganese- induced neurotoxicity. Neurochem Int 88, 53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karki P, Smith K, Johnson J Jr., Lee E, 2014a. Astrocyte-derived growth factors and estrogen neuroprotection: role of transforming growth factor-alpha in estrogen-induced upregulation of glutamate transporters in astrocytes. Mol Cell Endocrinol 389, 58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karki P, Webb A, Smith K, Johnson J Jr., Lee K, Son DS, Aschner M, Lee E, 2014b. Yin Yang 1 is a repressor of glutamate transporter EAAT2, and it mediates manganese-induced decrease of EAAT2 expression in astrocytes. Mol Cell Biol 34, 1280–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karki P, Webb A, Smith K, Lee K, Son DS, Aschner M, Lee E, 2013b. cAMP response element-binding protein (CREB) and nuclear factor kappaB mediate the tamoxifen-induced up-regulation of glutamate transporter 1 (GLT-1) in rat astrocytes. J Biol Chem 288, 28975–28986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karki P, Webb A, Zerguine A, Choi J, Son DS, Lee E, 2014c. Mechanism of raloxifene-induced upregulation of glutamate transporters in rat primary astrocytes. Glia 62, 1270–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann WE, Cortell R, Kau AS, Bukelis I, Tierney E, Gray RM, Cox C, Capone GT, Stanard P, 2004. Autism spectrum disorder in fragile X syndrome: communication, social interaction, and specific behaviors. Am J Med Genet A 129A, 225–234. [DOI] [PubMed] [Google Scholar]
- Kawahara K, Hosoya R, Sato H, Tanaka M, Nakajima T, Iwabuchi S, 2002. Selective blockade of astrocytic glutamate transporter GLT-1 with dihydrokainate prevents neuronal death during ouabain treatment of astrocyte/neuron cocultures. Glia 40, 337–349. [DOI] [PubMed] [Google Scholar]
- Kim K, Lee SG, Kegelman TP, Su ZZ, Das SK, Dash R, Dasgupta S, Barral PM, Hedvat M, Diaz P, Reed JC, Stebbins JL, Pellecchia M, Sarkar D, Fisher PB, 2011. Role of excitatory amino acid transporter-2 (EAAT2) and glutamate in neurodegeneration: opportunities for developing novel therapeutics. J Cell Physiol 226, 2484–2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim R, Sepulveda-Orengo MT, Healey KL, Williams EA, Reissner KJ, 2018. Regulation of glutamate transporter 1 (GLT-1) gene expression by cocaine self-administration and withdrawal. Neuropharmacology 128, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Chao W, Choi SY, Volsky DJ, 2003a. Cloning and characterization of the 3’-untranslated region of the human excitatory amino acid transporter 2 transcript. J Neurochem 86, 1458–1467. [DOI] [PubMed] [Google Scholar]
- Kim SY, Choi SY, Chao W, Volsky DJ, 2003b. Transcriptional regulation of human excitatory amino acid transporter 1 (EAAT1): cloning of the EAAT1 promoter and characterization of its basal and inducible activity in human astrocytes. J Neurochem 87, 1485–1498. [DOI] [PubMed] [Google Scholar]
- Koeberle PD, Bahr M, 2008. The upregulation of GLAST-1 is an indirect antiapoptotic mechanism of GDNF and neurturin in the adult CNS. Cell Death Differ 15, 471–483. [DOI] [PubMed] [Google Scholar]
- Koeglsperger T, Li S, Brenneis C, Saulnier JL, Mayo L, Carrier Y, Selkoe DJ, Weiner HL, 2013. Impaired glutamate recycling and GluN2B-mediated neuronal calcium overload in mice lacking TGF-beta1 in the CNS. Glia 61, 985–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong Q, Chang LC, Takahashi K, Liu Q, Schulte DA, Lai L, Ibabao B, Lin Y, Stouffer N, Das Mukhopadhyay C, Xing X, Seyb KI, Cuny GD, Glicksman MA, Lin CL, 2014. Small-molecule activator of glutamate transporter EAAT2 translation provides neuroprotection. J Clin Invest 124, 1255–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong Q, Takahashi K, Schulte D, Stouffer N, Lin Y, Lin CL, 2012. Increased glial glutamate transporter EAAT2 expression reduces epileptogenic processes following pilocarpine-induced status epilepticus. Neurobiol Dis 47, 145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornhuber J, Wiltfang J, 1998. The role of glutamate in dementia. J Neural Transm Suppl 53, 277–287. [DOI] [PubMed] [Google Scholar]
- Kugler P, Schleyer V, 2004. Developmental expression of glutamate transporters and glutamate dehydrogenase in astrocytes of the postnatal rat hippocampus. Hippocampus 14, 975–985. [DOI] [PubMed] [Google Scholar]
- Kwakye GF, Paoliello MM, Mukhopadhyay S, Bowman AB, Aschner M, 2015. Manganese-Induced Parkinsonism and Parkinson’s Disease: Shared and Distinguishable Features. Int J Environ Res Public Health 12, 7519–7540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgrave-Gomez J, Mercado-Gomez O, Guevara-Guzman R, 2015. Epigenetic mechanisms in neurological and neurodegenerative diseases. Front Cell Neurosci 9, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapucci A, Cavone L, Buonvicino D, Felici R, Gerace E, Zwergel C, Valente S, Mai A, Chiarugi A, 2017. Effect of Class II HDAC inhibition on glutamate transporter expression and survival in SOD1-ALS mice. Neurosci Lett 656, 120–125. [DOI] [PubMed] [Google Scholar]
- Lauriat TL, McInnes LA, 2007. EAAT2 regulation and splicing: relevance to psychiatric and neurological disorders. Mol Psychiatry 12, 1065–1078. [DOI] [PubMed] [Google Scholar]
- Lee E, Sidoryk-Wegrzynowicz M, Farina M, Rocha JB, Aschner M, 2013. Estrogen attenuates manganese-induced glutamate transporter impairment in rat primary astrocytes. Neurotox Res 23, 124–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E, Sidoryk-Wegrzynowicz M, Wang N, Webb A, Son DS, Lee K, Aschner M, 2012a. GPR30 regulates glutamate transporter GLT-1 expression in rat primary astrocytes. J Biol Chem 287, 26817–26828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E, Sidoryk-Wegrzynowicz M, Yin Z, Webb A, Son DS, Aschner M, 2012b. Transforming growth factor-alpha mediates estrogen-induced upregulation of glutamate transporter GLT-1 in rat primary astrocytes. Glia 60, 1024–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee ES, Sidoryk M, Jiang H, Yin Z, Aschner M, 2009. Estrogen and tamoxifen reverse manganese- induced glutamate transporter impairment in astrocytes. J Neurochem 110, 530–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SG, Kim K, Kegelman TP, Dash R, Das SK, Choi JK, Emdad L, Howlett EL, Jeon HY, Su ZZ, Yoo BK, Sarkar D, Kim SH, Kang DC, Fisher PB, 2011. Oncogene AEG-1 promotes glioma- induced neurodegeneration by increasing glutamate excitotoxicity. Cancer Res 71, 6514–6523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SN, Li L, Zuo Z, 2010. Glutamate transporter type 3 knockout mice have a decreased isoflurane requirement to induce loss of righting reflex. Neuroscience 171, 788–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehre KP, Danbolt NC, 1998. The number of glutamate transporter subtype molecules at glutamatergic synapses: chemical and stereological quantification in young adult rat brain. J Neurosci 18, 8751–8757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li LB, Toan SV, Zelenaia O, Watson DJ, Wolfe JH, Rothstein JD, Robinson MB, 2006. Regulation of astrocytic glutamate transporter expression by Akt: evidence for a selective transcriptional effect on the GLT-1/EAAT2 subtype. J Neurochem 97, 759–771. [DOI] [PubMed] [Google Scholar]
- Li Y, Sattler R, Yang EJ, Nunes A, Ayukawa Y, Akhtar S, Ji G, Zhang PW, Rothstein JD, 2011. Harmine, a natural beta-carboline alkaloid, upregulates astroglial glutamate transporter expression. Neuropharmacology 60, 1168–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Z, Valla J, Sefidvash-Hockley S, Rogers J, Li R, 2002. Effects of estrogen treatment on glutamate uptake in cultured human astrocytes derived from cortex of Alzheimer’s disease patients. J Neurochem 80, 807–814. [DOI] [PubMed] [Google Scholar]
- Lin CL, Bristol LA, Jin L, Dykes-Hoberg M, Crawford T, Clawson L, Rothstein JD, 1998. Aberrant RNA processing in a neurodegenerative disease: the cause for absent EAAT2, a glutamate transporter, in amyotrophic lateral sclerosis. Neuron 20, 589–602. [DOI] [PubMed] [Google Scholar]
- Lin CL, Kong Q, Cuny GD, Glicksman MA, 2012. Glutamate transporter EAAT2: a new target for the treatment of neurodegenerative diseases. Future Med Chem 4, 1689–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu WX, Wang J, Xie ZM, Xu N, Zhang GF, Jia M, Zhou ZQ, Hashimoto K, Yang JJ, 2016. Regulation of glutamate transporter 1 via BDNF-TrkB signaling plays a role in the anti-apoptotic and antidepressant effects of ketamine in chronic unpredictable stress model of depression. Psychopharmacology (Berl) 233, 405–415. [DOI] [PubMed] [Google Scholar]
- Loschmann PA, De Groote C, Smith L, Wullner U, Fischer G, Kemp JA, Jenner P, Klockgether T, 2004. Antiparkinsonian activity of Ro 25–6981, a NR2B subunit specific NMDA receptor antagonist, in animal models of Parkinson’s disease. Exp Neurol 187, 86–93. [DOI] [PubMed] [Google Scholar]
- Lutgen V, Narasipura SD, Sharma A, Min S, Al-Harthi L, 2016. beta-Catenin signaling positively regulates glutamate uptake and metabolism in astrocytes. J Neuroinflammation 13, 242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma YJ, Hill DF, Junier MP, Costa ME, Felder SE, Ojeda SR, 1994. Expression of epidermal growth factor receptor changes in the hypothalamus during the onset of female puberty. Mol Cell Neurosci 5, 246–262. [DOI] [PubMed] [Google Scholar]
- Mai A, Rotili D, Valente S, Kazantsev AG, 2009. Histone deacetylase inhibitors and neurodegenerative disorders: holding the promise. Curr Pharm Des 15, 3940–3957. [DOI] [PubMed] [Google Scholar]
- Mallolas J, Hurtado O, Castellanos M, Blanco M, Sobrino T, Serena J, Vivancos J, Castillo J, Lizasoain I, Moro MA, Davalos A, 2006. A polymorphism in the EAAT2 promoter is associated with higher glutamate concentrations and higher frequency of progressing stroke. J Exp Med 203, 711–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maragakis NJ, Rothstein JD, 2004. Glutamate transporters: animal models to neurologic disease. Neurobiol Dis 15, 461–473. [DOI] [PubMed] [Google Scholar]
- Mayor D, Tymianski M, 2018. Neurotransmitters in the mediation of cerebral ischemic injury. Neuropharmacology 134, 178–188. [DOI] [PubMed] [Google Scholar]
- Meyer T, Fromm A, Munch C, Schwalenstocker B, Fray AE, Ince PG, Stamm S, Gron G, Ludolph AC, Shaw PJ, 1999. The RNA of the glutamate transporter EAAT2 is variably spliced in amyotrophic lateral sclerosis and normal individuals. J Neurol Sci 170, 45–50. [DOI] [PubMed] [Google Scholar]
- Meyer T, Munch C, Liebau S, Fromm A, Schwalenstocker B, Volkel H, Ludolph AC, 1998. Splicing of the glutamate transporter EAAT2: a candidate gene of amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry 65, 954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mironova YS, Zhukova IA, Zhukova NG, Alifirova VM, Izhboldina OP, Latypova AV, 2018. [Parkinson’s disease and glutamate excitotoxicity]. Zh Nevrol Psikhiatr Im S S Korsakova 118, 50–54. [DOI] [PubMed] [Google Scholar]
- Mookherjee P, Green PS, Watson GS, Marques MA, Tanaka K, Meeker KD, Meabon JS, Li N, Zhu P, Olson VG, Cook DG, 2011. GLT-1 loss accelerates cognitive deficit onset in an Alzheimer’s disease animal model. J Alzheimers Dis 26, 447–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morissette M, Al Sweidi S, Callier S, Di Paolo T, 2008. Estrogen and SERM neuroprotection in animal models of Parkinson’s disease. Mol Cell Endocrinol 290, 60–69. [DOI] [PubMed] [Google Scholar]
- Munch C, Zhu BG, Mink A, Seefried U, Riepe MW, Ludolph AC, Meyer T, 2008. Chemical hypoxia facilitates alternative splicing of EAAT2 in presymptomatic APP23 transgenic mice. Neurochem Res 33, 1005–1010. [DOI] [PubMed] [Google Scholar]
- Munoz-Ballester C, Berthier A, Viana R, Sanz P, 2016. Homeostasis of the astrocytic glutamate transporter GLT-1 is altered in mouse models of Lafora disease. Biochim Biophys Acta 1862, 1074–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myles-Worsley M, Tiobech J, Browning SR, Korn J, Goodman S, Gentile K, Melhem N, Byerley W, Faraone SV, Middleton FA, 2013. Deletion at the SLC1A1 glutamate transporter gene co-segregates with schizophrenia and bipolar schizoaffective disorder in a 5-generation family. Am J Med Genet B Neuropsychiatr Genet 162B, 87–95. [DOI] [PubMed] [Google Scholar]
- Nowak K, Lange-Dohna C, Zeitschel U, Gunther A, Luscher B, Robitzki A, Perez-Polo R, Rossner S, 2006. The transcription factor Yin Yang 1 is an activator of BACE1 expression. J Neurochem 96, 1696–1707. [DOI] [PubMed] [Google Scholar]
- O’Donovan SM, Hasselfeld K, Bauer D, Simmons M, Roussos P, Haroutunian V, Meador-Woodruff JH, McCullumsmith RE, 2015. Glutamate transporter splice variant expression in an enriched pyramidal cell population in schizophrenia. Transl Psychiatry 5, e579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega-Martinez S, 2015. A new perspective on the role of the CREB family of transcription factors in memory consolidation via adult hippocampal neurogenesis. Front Mol Neurosci 8, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajarillo E, Johnson J Jr., Kim J, Karki P, Son DS, Aschner M, Lee E, 2018. 17beta-estradiol and tamoxifen protect mice from manganese-induced dopaminergic neurotoxicity. Neurotoxicology 65, 280–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pampliega O, Domercq M, Villoslada P, Sepulcre J, Rodriguez-Antiguedad A, Matute C, 2008. Association of an EAAT2 polymorphism with higher glutamate concentration in relapsing multiple sclerosis. J Neuroimmunol 195, 194–198. [DOI] [PubMed] [Google Scholar]
- Pandey D, Banerjee S, Basu M, Mishra N, 2016. Memory enhancement by Tamoxifen on amyloidosis mouse model. Horm Behav 79, 70–73. [DOI] [PubMed] [Google Scholar]
- Park G, Tan J, Garcia G, Kang Y, Salvesen G, Zhang Z, 2016. Regulation of Histone Acetylation by Autophagy in Parkinson Disease. J Biol Chem 291, 3531–3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkin GM, Udawela M, Gibbons A, Dean B, 2018. Glutamate transporters, EAAT1 and EAAT2, are potentially important in the pathophysiology and treatment of schizophrenia and affective disorders. World J Psychiatry 8, 51–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlak J, Brito V, Kuppers E, Beyer C, 2005. Regulation of glutamate transporter GLAST and GLT-1 expression in astrocytes by estrogen. Brain Res Mol Brain Res 138, 1–7. [DOI] [PubMed] [Google Scholar]
- Peacey E, Miller CC, Dunlop J, Rattray M, 2009. The four major N- and C-terminal splice variants of the excitatory amino acid transporter GLT-1 form cell surface homomeric and heteromeric assemblies. Mol Pharmacol 75, 1062–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira AC, Gray JD, Kogan JF, Davidson RL, Rubin TG, Okamoto M, Morrison JH, McEwen BS, 2017. Age and Alzheimer’s disease gene expression profiles reversed by the glutamate modulator riluzole. Mol Psychiatry 22, 296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perisic T, Holsboer F, Rein T, Zschocke J, 2012. The CpG island shore of the GLT-1 gene acts as a methylation-sensitive enhancer. Glia 60, 1345–1355. [DOI] [PubMed] [Google Scholar]
- Perkins EM, Clarkson YL, Suminaite D, Lyndon AR, Tanaka K, Rothstein JD, Skehel P, Wyllie DJA, Jackson M, 2018. Loss of cerebellar glutamate transporters EAAT4 and GLAST differentially affects the spontaneous firing pattern and survival of Purkinje cells. Hum Mol Genet. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petr GT, Bakradze E, Frederick NM, Wang J, Armsen W, Aizenman E, Rosenberg PA, 2013a. Glutamate transporter expression and function in a striatal neuronal model of Huntington’s disease. Neurochem Int 62, 973–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petr GT, Schultheis LA, Hussey KC, Sun Y, Dubinsky JM, Aoki C, Rosenberg PA, 2013b. Decreased expression of GLT-1 in the R6/2 model of Huntington’s disease does not worsen disease progression. Eur J Neurosci 38, 2477–2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petr GT, Sun Y, Frederick NM, Zhou Y, Dhamne SC, Hameed MQ, Miranda C, Bedoya EA, Fischer KD, Armsen W, Wang J, Danbolt NC, Rotenberg A, Aoki CJ, Rosenberg PA, 2015. Conditional deletion of the glutamate transporter GLT-1 reveals that astrocytic GLT-1 protects against fatal epilepsy while neuronal GLT-1 contributes significantly to glutamate uptake into synaptosomes. J Neurosci 35, 5187–5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poletti S, Locatelli C, Radaelli D, Lorenzi C, Smeraldi E, Colombo C, Benedetti F, 2014a. Effect of early stress on hippocampal gray matter is influenced by a functional polymorphism in EAAT2 in bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry 51, 146–152. [DOI] [PubMed] [Google Scholar]
- Poletti S, Radaelli D, Bosia M, Buonocore M, Pirovano A, Lorenzi C, Cavallaro R, Smeraldi E, Benedetti F, 2014b. Effect of glutamate transporter EAAT2 gene variants and gray matter deficits on working memory in schizophrenia. Eur Psychiatry 29, 219–225. [DOI] [PubMed] [Google Scholar]
- Poletti S, Riberto M, Vai B, Ghiglino D, Lorenzi C, Vitali A, Brioschi S, Locatelli C, Serretti A, Colombo C, Benedetti F, 2018. A Glutamate Transporter EAAT1 Gene Variant Influences Amygdala Functional Connectivity in Bipolar Disorder. J Mol Neurosci 65, 536–545. [DOI] [PubMed] [Google Scholar]
- Qian Y, Guan T, Tang X, Huang L, Huang M, Li Y, Sun H, Yu R, Zhang F, 2011. Astrocytic glutamate transporter-dependent neuroprotection against glutamate toxicity: an in vitro study of maslinic acid. Eur J Pharmacol 651, 59–65. [DOI] [PubMed] [Google Scholar]
- Qian Y, Tang X, Guan T, Li Y, Sun H, 2016. Neuroprotection by Combined Administration with Maslinic Acid, a Natural Product from Olea europaea, and MK-801 in the Cerebral Ischemia Model. Molecules 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racette BA, Aschner M, Guilarte TR, Dydak U, Criswell SR, Zheng W, 2012. Pathophysiology of manganese-associated neurotoxicity. Neurotoxicology 33, 881–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajatileka S, Odd D, Robinson MT, Spittle AC, Dwomoh L, Williams M, Harding D, Wagstaff M, Owen M, Crosby C, Ching J, Molnar E, Luyt K, Varadi A, 2018. Variants of the EAAT2 Glutamate Transporter Gene Promoter Are Associated with Cerebral Palsy in Preterm Infants. Mol Neurobiol 55, 2013–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju K, Doulias PT, Evans P, Krizman EN, Jackson JG, Horyn O, Daikhin Y, Nissim I, Yudkoff M, Nissim I, Sharp KA, Robinson MB, Ischiropoulos H, 2015. Regulation of brain glutamate metabolism by nitric oxide and S-nitrosylation. Sci Signal 8, ra68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao P, Yallapu MM, Sari Y, Fisher PB, Kumar S, 2015a. Designing Novel Nanoformulations Targeting Glutamate Transporter Excitatory Amino Acid Transporter 2: Implications in Treating Drug Addiction. J Pers Nanomed 1, 3–9. [PMC free article] [PubMed] [Google Scholar]
- Rao PS, Goodwani S, Bell RL, Wei Y, Boddu SH, Sari Y, 2015b. Effects of ampicillin, cefazolin and cefoperazone treatments on GLT-1 expressions in the mesocorticolimbic system and ethanol intake in alcohol-preferring rats. Neuroscience 295, 164–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao PS, Saternos H, Goodwani S, Sari Y, 2015c. Effects of ceftriaxone on GLT1 isoforms, xCT and associated signaling pathways in P rats exposed to ethanol. Psychopharmacology (Berl) 232, 2333–2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao VL, Baskaya MK, Dogan A, Rothstein JD, Dempsey RJ, 1998. Traumatic brain injury down- regulates glial glutamate transporter (GLT-1 and GLAST) proteins in rat brain. J Neurochem 70, 2020–2027. [DOI] [PubMed] [Google Scholar]
- Rao VL, Dogan A, Todd KG, Bowen KK, Kim BT, Rothstein JD, Dempsey RJ, 2001. Antisense knockdown of the glial glutamate transporter GLT-1, but not the neuronal glutamate transporter EAAC1, exacerbates transient focal cerebral ischemia-induced neuronal damage in rat brain. J Neurosci 21, 18761883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratajewski M, Pulaski L, 2009. YY1-dependent transcriptional regulation of the human GDAP1 gene. Genomics 94, 407–413. [DOI] [PubMed] [Google Scholar]
- Raunser S, Haase W, Bostina M, Parcej DN, Kuhlbrandt W, 2005. High-yield expression, reconstitution and structure of the recombinant, fully functional glutamate transporter GLT-1 from Rattus norvegicus. J Mol Biol 351, 598–613. [DOI] [PubMed] [Google Scholar]
- Rees RN, Noyce AJ, Schrag A, 2018. The Prodromes of Parkinson’s disease. Eur J Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimmele TS, Rosenberg PA, 2016. GLT-1: The elusive presynaptic glutamate transporter. Neurochem Int 98, 19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Kern A, Gegelashvili M, Schousboe A, Zhang J, Sung L, Gegelashvili G, 2003. Betaamyloid and brain-derived neurotrophic factor, BDNF, up-regulate the expression of glutamate transporter GLT-1/EAAT2 via different signaling pathways utilizing transcription factor NF-kappaB. Neurochem Int 43, 363–370. [DOI] [PubMed] [Google Scholar]
- Romera C, Hurtado O, Mallolas J, Pereira MP, Morales JR, Romera A, Serena J, Vivancos J, Nombela F, Lorenzo P, Lizasoain I, Moro MA, 2007. Ischemic preconditioning reveals that GLT1/EAAT2 glutamate transporter is a novel PPARgamma target gene involved in neuroprotection. J Cereb Blood Flow Metab 27, 1327–1338. [DOI] [PubMed] [Google Scholar]
- Rosas S, Vargas MA, Lopez-Bayghen E, Ortega A, 2007. Glutamate-dependent transcriptional regulation of GLAST/EAAT1: a role for YY1. J Neurochem 101, 1134–1144. [DOI] [PubMed] [Google Scholar]
- Rosenblum LT, Shamamandri-Markandaiah S, Ghosh B, Foran E, Lepore AC, Pasinelli P, Trotti D, 2017. Mutation of the caspase-3 cleavage site in the astroglial glutamate transporter EAAT2 delays disease progression and extends lifespan in the SOD1-G93A mouse model of ALS. Exp Neurol 292, 145–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein JD, Dykes-Hoberg M, Pardo CA, Bristol LA, Jin L, Kuncl RW, Kanai Y, Hediger MA, Wang Y, Schielke JP, Welty DF, 1996. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron 16, 675–686. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Patel S, Regan MR, Haenggeli C, Huang YH, Bergles DE, Jin L, Dykes Hoberg M, Vidensky S, Chung DS, Toan SV, Bruijn LI, Su ZZ, Gupta P, Fisher PB, 2005. Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature 433, 73–77. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Van Kammen M, Levey AI, Martin LJ, Kuncl RW, 1995. Selective loss of glial glutamate transporter GLT-1 in amyotrophic lateral sclerosis. Ann Neurol 38, 73–84. [DOI] [PubMed] [Google Scholar]
- Ryan RM, Kortt NC, Sirivanta T, Vandenberg RJ, 2010. The position of an arginine residue influences substrate affinity and K+ coupling in the human glutamate transporter, EAAT1. J Neurochem 114, 565–575. [DOI] [PubMed] [Google Scholar]
- Sander T, Berlin W, Ostapowicz A, Samochowiec J, Gscheidel N, Hoehe MR, 2000a. Variation of the genes encoding the human glutamate EAAT2, serotonin and dopamine transporters and Susceptibility to idiopathic generalized epilepsy. Epilepsy Res 41, 75–81. [DOI] [PubMed] [Google Scholar]
- Sander T, Ostapowicz A, Samochowiec J, Smolka M, Winterer G, Schmidt LG, 2000b. Genetic variation of the glutamate transporter EAAT2 gene and vulnerability to alcohol dependence. Psychiatr Genet 10, 103–107. [DOI] [PubMed] [Google Scholar]
- Sari Y, Toalston JE, Rao PSS, Bell RL, 2016. Effects of ceftriaxone on ethanol, nicotine or sucrose intake by alcohol-preferring (P) rats and its association with GLT-1 expression. Neuroscience 326, 117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saura CA, Valero J, 2011. The role of CREB signaling in Alzheimer’s disease and other cognitive disorders. Rev Neurosci 22, 153–169. [DOI] [PubMed] [Google Scholar]
- Schluter K, Figiel M, Rozyczka J, Engele J, 2002. CNS region-specific regulation of glial glutamate transporter expression. Eur J Neurosci 16, 836–842. [DOI] [PubMed] [Google Scholar]
- Scott HA, Gebhardt FM, Mitrovic AD, Vandenberg RJ, Dodd PR, 2011. Glutamate transporter variants reduce glutamate uptake in Alzheimer’s disease. Neurobiol Aging 32, 553 e551–511. [DOI] [PubMed] [Google Scholar]
- Selvi BR, Cassel JC, Kundu TK, Boutillier AL, 2010. Tuning acetylation levels with HAT activators: therapeutic strategy in neurodegenerative diseases. Biochim Biophys Acta 1799, 840–853. [DOI] [PubMed] [Google Scholar]
- Sepkuty JP, Cohen AS, Eccles C, Rafiq A, Behar K, Ganel R, Coulter DA, Rothstein JD, 2002. A neuronal glutamate transporter contributes to neurotransmitter GABA synthesis and epilepsy. J Neurosci 22, 6372–6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sery O, Sultana N, Kashem MA, Pow DV, Balcar VJ, 2015. GLAST But Not Least-Distribution, Function, Genetics and Epigenetics of L-Glutamate Transport in Brain--Focus on GLAST/EAAT1. Neurochem Res 40, 2461–2472. [DOI] [PubMed] [Google Scholar]
- Sheldon AL, Gonzalez MI, Krizman-Genda EN, Susarla BT, Robinson MB, 2008. Ubiquitination-mediated internalization and degradation of the astroglial glutamate transporter, GLT-1. Neurochem Int 53, 296–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Rothman DL, Behar KL, Xu S, 2009. Determination of the glutamate-glutamine cycling flux using two-compartment dynamic metabolic modeling is sensitive to astroglial dilution. J Cereb Blood Flow Metab 29, 108–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigeri Y, Seal RP, Shimamoto K, 2004. Molecular pharmacology of glutamate transporters, EAATs and VGLUTs. Brain Res Brain Res Rev 45, 250–265. [DOI] [PubMed] [Google Scholar]
- Shimamoto K, Lebrun B, Yasuda-Kamatani Y, Sakaitani M, Shigeri Y, Yumoto N, Nakajima T, 1998. DL-threo-beta-benzyloxyaspartate, a potent blocker of excitatory amino acid transporters. Mol Pharmacol 53, 195–201. [DOI] [PubMed] [Google Scholar]
- Shirasaki Y, Sugimura M, Sato T, 2010. Bromocriptine, an ergot alkaloid, inhibits excitatory amino acid release mediated by glutamate transporter reversal. Eur J Pharmacol 643, 48–57. [DOI] [PubMed] [Google Scholar]
- Shiu WL, Huang KR, Hung JC, Wu JL, Hong JR, 2016. Knockdown of zebrafish YY1a can downregulate the phosphatidylserine (PS) receptor expression, leading to induce the abnormal brain and heart development. J Biomed Sci 23, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitcheran R, Gupta P, Fisher PB, Baldwin AS, 2005. Positive and negative regulation of EAAT2 by NF-kappaB: a role for N-myc in TNFalpha-controlled repression. EMBO J 24, 510–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotboom DJ, Konings WN, Lolkema JS, 1999. Structural features of the glutamate transporter family. Microbiol Mol Biol Rev 63, 293–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangaro M, Bosia M, Zanoletti A, Bechi M, Cocchi F, Pirovano A, Lorenzi C, Bramanti P, Benedetti F, Smeraldi E, Cavallaro R, 2012. Cognitive dysfunction and glutamate reuptake: effect of EAAT2 polymorphism in schizophrenia. Neurosci Lett 522, 151–155. [DOI] [PubMed] [Google Scholar]
- Spangaro M, Bosia M, Zanoletti A, Bechi M, Mariachiara B, Pirovano A, Lorenzi C, Bramanti P, Smeraldi E, Cavallaro R, 2014. Exploring effects of EAAT polymorphisms on cognitive functions in schizophrenia. Pharmacogenomics 15, 925–932. [DOI] [PubMed] [Google Scholar]
- Stipursky J, Gomes FC, 2007. TGF-beta1/SMAD signaling induces astrocyte fate commitment in vitro: implications for radial glia development. Glia 55, 1023–1033. [DOI] [PubMed] [Google Scholar]
- Su ZZ, Leszczyniecka M, Kang DC, Sarkar D, Chao W, Volsky DJ, Fisher PB, 2003. Insights into glutamate transport regulation in human astrocytes: cloning of the promoter for excitatory amino acid transporter 2 (EAAT2). Proc Natl Acad Sci U S A 100, 1955–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulkowski G, Dabrowska-Bouta B, Salinska E, Struzynska L, 2014. Modulation of glutamate transport and receptor binding by glutamate receptor antagonists in EAE rat brain. PLoS One 9, e113954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suraweera A, O’Byrne KJ, Richard DJ, 2018. Combination Therapy With Histone Deacetylase Inhibitors (HDACi) for the Treatment of Cancer: Achieving the Full Therapeutic Potential of HDACi. Front Oncol 8, 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Kong Q, Lin Y, Stouffer N, Schulte DA, Lai L, Liu Q, Chang LC, Dominguez S, Xing X, Cuny GD, Hodgetts KJ, Glicksman MA, Lin CL, 2015. Restored glial glutamate transporter EAAT2 function as a potential therapeutic approach for Alzheimer’s disease. J Exp Med 212, 319–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda A, 2003. Manganese action in brain function. Brain Res Brain Res Rev 41, 79–87. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Watase K, Manabe T, Yamada K, Watanabe M, Takahashi K, Iwama H, Nishikawa T, Ichihara N, Kikuchi T, Okuyama S, Kawashima N, Hori S, Takimoto M, Wada K, 1997. Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science 276, 1699–1702. [DOI] [PubMed] [Google Scholar]
- Tiwari PC, Pal R, 2017. The potential role of neuroinflammation and transcription factors in Parkinson disease. Dialogues Clin Neurosci 19, 71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong H, Yu X, Lu X, Wang P, 2015. Downregulation of solute carriers of glutamate in gliosomes and synaptosomes may explain local brain metastasis in anaplastic glioblastoma. IUBMB Life 67, 306–311. [DOI] [PubMed] [Google Scholar]
- Tortarolo M, Crossthwaite AJ, Conforti L, Spencer JP, Williams RJ, Bendotti C, Rattray M, 2004. Expression of SOD1 G93A or wild-type SOD1 in primary cultures of astrocytes down-regulates the glutamate transporter GLT-1: lack of involvement of oxidative stress. J Neurochem 88, 481–493. [DOI] [PubMed] [Google Scholar]
- Tortosa E, Hoogenraad CC, 2018. Polarized trafficking: the palmitoylation cycle distributes cytoplasmic proteins to distinct neuronal compartments. Curr Opin Cell Biol 50, 64–71. [DOI] [PubMed] [Google Scholar]
- Tsolaki AC, Gatzima O, Daniilidou M, Lazarou E, Bamidis PD, Verykouki E, Iakovidou-Kritsi Z, Tsolaki M, 2018. Prevalence of Apolipoprotein E Polymorphisms in Alzheimer’s Disease, Mild Cognitive Impairment, and Healthy Elderly: A Northern Greece Study. Neurodegener Dis 18, 216–224. [DOI] [PubMed] [Google Scholar]
- Ullensvang K, Lehre KP, Storm-Mathisen J, Danbolt NC, 1997. Differential developmental expression of the two rat brain glutamate transporter proteins GLAST and GLT. Eur J Neurosci 9, 1646–1655. [DOI] [PubMed] [Google Scholar]
- Unger T, Lakowa N, Bette S, Engele J, 2012. Transcriptional regulation of the GLAST/EAAT-1 gene in rat and man. Cell Mol Neurobiol 32, 539–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallejo-Illarramendi A, Domercq M, Matute C, 2005. A novel alternative splicing form of excitatory amino acid transporter 1 is a negative regulator of glutamate uptake. J Neurochem 95, 341–348. [DOI] [PubMed] [Google Scholar]
- Watts SD, Torres-Salazar D, Divito CB, Amara SG, 2014. Cysteine transport through excitatory amino acid transporter 3 (EAAT3). PLoS One 9, e109245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller ML, Stone IM, Goss A, Rau T, Rova C, Poulsen DJ, 2008. Selective overexpression of excitatory amino acid transporter 2 (EAAT2) in astrocytes enhances neuroprotection from moderate but not severe hypoxia-ischemia. Neuroscience 155, 1204–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willard SS, Koochekpour S, 2013. Glutamate, glutamate receptors, and downstream signaling pathways. Int J Biol Sci 9, 948–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JM, Khabazian I, Pow DV, Craig UK, Shaw CA, 2003. Decrease in glial glutamate transporter variants and excitatory amino acid receptor down-regulation in a murine model of ALS-PDC. Neuromolecular Med 3, 105–118. [DOI] [PubMed] [Google Scholar]
- Yamada T, Kawahara K, Kosugi T, Tanaka M, 2006. Nitric oxide produced during sublethal ischemia is crucial for the preconditioning-induced down-regulation of glutamate transporter GLT-1 in neuron/astrocyte co-cultures. Neurochem Res 31, 49–56. [DOI] [PubMed] [Google Scholar]
- Yamashita A, Makita K, Kuroiwa T, Tanaka K, 2006. Glutamate transporters GLAST and EAAT4 regulate postischemic Purkinje cell death: an in vivo study using a cardiac arrest model in mice lacking GLAST or EAAT4. Neurosci Res 55, 264–270. [DOI] [PubMed] [Google Scholar]
- Yang LC, Zhang QG, Zhou CF, Yang F, Zhang YD, Wang RM, Brann DW, 2010. Extranuclear estrogen receptors mediate the neuroprotective effects of estrogen in the rat hippocampus. PLoS One 5, e9851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin X, Wang S, Qi Y, Wang X, Jiang H, Wang T, Yang Y, Wang Y, Zhang C, Feng H, 2018. Astrocyte elevated gene-1 is a novel regulator of astrogliosis and excitatory amino acid transporter-2 via interplaying with nuclear factor-kappaB signaling in astrocytes from amyotrophic lateral sclerosis mouse model with hSOD1(G93A) mutation. Mol Cell Neurosci 90, 1–11. [DOI] [PubMed] [Google Scholar]
- Yoo YE, Ko CP, 2011. Treatment with trichostatin A initiated after disease onset delays disease progression and increases survival in a mouse model of amyotrophic lateral sclerosis. Exp Neurol 231, 147–159. [DOI] [PubMed] [Google Scholar]
- Young D, Fong DM, Lawlor PA, Wu A, Mouravlev A, McRae M, Glass M, Dragunow M, During MJ, 2014. Adenosine kinase, glutamine synthetase and EAAT2 as gene therapy targets for temporal lobe epilepsy. Gene Ther 21, 1029–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelenaia O, Schlag BD, Gochenauer GE, Ganel R, Song W, Beesley JS, Grinspan JB, Rothstein JD, Robinson MB, 2000. Epidermal growth factor receptor agonists increase expression of glutamate transporter GLT-1 in astrocytes through pathways dependent on phosphatidylinositol 3-kinase and transcription factor NF-kappaB. Mol Pharmacol 57, 667–678. [DOI] [PubMed] [Google Scholar]
- Zhang X, Wu Y, Cai F, Liu S, Bromley-Brits K, Xia K, Song W, 2018. A Novel Alzheimer-Associated SNP in Tmp21 Increases Amyloidogenesis. Mol Neurobiol 55, 1862–1870. [DOI] [PubMed] [Google Scholar]
- Zhang Y, He X, Meng X, Wu X, Tong H, Zhang X, Qu S, 2017. Regulation of glutamate transporter trafficking by Nedd4–2 in a Parkinson’s disease model. Cell Death Dis 8, e2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zschocke J, Allritz C, Engele J, Rein T, 2007. DNA methylation dependent silencing of the human glutamate transporter EAAT2 gene in glial cells. Glia 55, 663–674. [DOI] [PubMed] [Google Scholar]
- Zschocke J, Bayatti N, Clement AM, Witan H, Figiel M, Engele J, Behl C, 2005. Differential promotion of glutamate transporter expression and function by glucocorticoids in astrocytes from various brain regions. J Biol Chem 280, 34924–34932. [DOI] [PubMed] [Google Scholar]