Abstract
Purpose:
The purpose of this study was to evaluate the role of a 12-month exercise intervention on endocrine-related quality of life (QOL) and overall QOL among breast cancer survivors with aromatase inhibitor (AI)-induced arthralgia in the Hormones and Physical Exercise (HOPE) Study.
Methods:
We conducted a randomized controlled trial of 121 breast cancer survivors who were currently taking AIs and experiencing at least mild arthralgia. QOL was assessed using the Functional Assessment of Cancer Therapy (FACT) questionnaires and the 36-Item Short Form Survey (SF-36) at baseline, 6-, and 12-months. Participants were randomized to either a one-year gym-based, supervised exercise intervention group (150 minutes of aerobic exercise and two strength-training sessions each week) or usual care. Effects of the intervention on QOL were assessed using mixed-model repeated measures analysis.
Results:
At 12 months, the exercise group had greater improvement in the overall QOL measures, as well as the breast cancer-specific (2.2 vs. 0.7, P = 0.02), endocrine-specific (5.6 vs. 1.6, P <0.001), and fatigue-specific (5.8 vs 0.5, P <0.001) subscales compared with the usual care group. Our results show a stronger effect at 12 months compared to 6 months of the intervention.
Conclusion:
In this study, combined aerobic and resistance exercise, such as treadmill walking and strength training, improved endocrine-related and overall QOL among breast cancer survivors experiencing adverse side effects from AIs. Since adverse side effects associated with AI use are quite common and this is the main reason for treatment discontinuation, this non-pharmacologic intervention could benefit many breast cancer survivors and increase successful adherence to AIs in breast cancer treatment.
Keywords: breast cancer, aromatase inhibitors, exercise, quality of life, randomized controlled trial
Presis:
In this study, combined aerobic exercise, such as treadmill walking and strength training, improved endocrine-related and overall QOL among breast cancer survivors experiencing adverse side effects from AIs.
Introduction
Breast cancer is the leading cancer diagnosis in women in the United States. Of those diagnosed, approximately 70% present with estrogen receptor-positive tumors.1 Aromatase inhibitors (AIs) have been shown to be the most effective adjuvant endocrine therapy for postmenopausal women with estrogen receptor-positive breast cancer, and are therefore considered standard of care.1 Because of their physiological mechanism, AIs cause lowered levels of estrogen which are associated with AI-induced arthralgias (i.e., joint pain) and menopausal symptoms, such as hot flashes and night sweats which in turn may impair quality of life.2 Given long-term treatment (up to 10 years) with AIs is effective in reducing both risk of recurrence and breast cancer death, and therefore the strong recommendation by clinicians for their patients to take AIs, understanding how to reduce the severity of AI-related adverse side effects is necessary to increase AI adherence and improve quality of life.
Previous studies have shown that exercise significantly improves quality of life (QOL),3–7 fatigue,7–10 depression,11 and anxiety7, 11 in women diagnosed with breast cancer.12–15 A randomized trial in 62 obese breast cancer survivors observed a positive effect of a 6-week walking intervention on AI-associated side effects (i.e. joint pain) but saw no effect on QOL.16 We conducted a randomized exercise trial in 121 breast cancer survivors randomized to either an exercise intervention or usual-care. We have previously reported that exercise led to a significant 30% improvement in AI-associated arthralgias and improved body composition among previously inactive breast cancer survivors.17, 18 We have also reported that long term exercise adherence is feasible among breast cancer patients experiencing pain due to AI-associated arthralgia.18 To our knowledge, the impact of exercise on endocrine-related QOL in women taking an AI for early stage breast cancer and experiencing arthralgia have not yet been reported. We hypothesized that exercise would have a positive effect on endocrine-related QOL among these women. The purpose of this study was to examine the effect of an exercise intervention vs. usual care within the setting of a randomized trial on endocrine-related QOL and overall QOL among 121 postmenopausal breast cancer survivors who had been taking an AI for at least 6 months and reported at least mild arthralgias in the Hormones and Physical Exercise (HOPE) Study.
Methods
Study Population
The HOPE Study, which has been previously described,17, 19 was a randomized controlled trial of breast cancer survivors with AI-induced arthralgia. Briefly, postmenopausal women diagnosed with hormone-receptor positive stage I-III breast cancer were eligible for the study. Participants had been taking an AI for at least 6 months and were experiencing side effects of the medication (i.e., at least mild arthralgia, defined as ≥ 3 on the Brief Pain Inventory (BPI) Short Form Questionnaire20) for at least 2 months at the time of enrollment. To observe a maximal effect from the exercise intervention, only women reporting less than 90 minutes/week of moderate-to-vigorous intensity aerobic exercise and no strength training in the previous year.
We used the Rapid Case Ascertainment (RCA) Shared Resource Service of the Yale Cancer Center to obtain names of women diagnosed with hormone receptor-positive breast cancer between June 1, 2010 and December 30, 2012 and treated at one of five hospitals in Connecticut. Among those who were screened and eligible, 34% were randomized into this study. Approval for all procedures and written informed consent was obtained from the Yale School of Medicine Human Investigation Committee and Connecticut Department of Public Health Human Investigation Committee.
Data Collection
Clinic visits were completed at baseline, 6-, and 12-months. Participants completed a QOL questionnaire, a 7-day daily activity log, a physical activity questionnaire, and attended a clinic visit for physical measurements at all visits.
Measures
Demographics and medical history.
Self-administered questionnaires were completed by the participants at the baseline visit. Medical history and treatment-related information was obtained from self-report, electronic medical records, and via a physician verification of treatment form.
QOL measures.
QOL was measured by self-report at the baseline, 6-, and 12-month clinic visits using the Functional Assessment of Cancer Therapy (FACT) questionnaires and the 36-Item Short Form Survey (SF-36).21, 22 The FACT-General (FACT-G) is a 27-item questionnaire assessing physical well-being (PWB), social/family well-being (SWB), emotional well-being (EWB), and functional well-being (FWB). The FACT-B (for breast cancer patients) includes the FACT-G as well as 10 additional concerns more specific to women with breast cancer. The endocrine subscale (ES) comprises 19 items (e.g., hot flashes, night sweats, weight gain, and joint pain). The Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-Fatigue) is a 13-item subscale to assess fatigue-related concerns. Participants indicated how true a statement had been for them over the past 7 days using a 5-point scale ranging from 0 (none at all) to 4 (very much). All items received equal weighting for the analysis. The SF-36 uses 36 items to measure eight health concepts, including physical functioning, bodily pain, role limitations due to physical health problems, role limitations due to personal or emotional problems, emotional well-being, social functioning, energy/fatigue, and general health perceptions. Scores were calculated for the eight subscales as well as for the physical component summary score and the mental component summary score. A clinically important difference in physical function and cancer-related fatigue is three points.23–25
Randomization
Participants were randomized to either the exercise group or usual care with equal probability, stratified by whether taking a bisphosphonate and whether joint pain started after initiating the AI. Blocked randomization with random block sizes was used to generate lists by the trial statistician and sealed envelopes were prepared according to the list to allocate participants. Those women randomized to the exercise group were scheduled for their first supervised exercise training session at a local health club immediately. Women randomized to the usual care group were contacted by a trained health professional on a monthly basis to discuss relevant health topics to maintain study compliance.
Exercise Intervention
The exercise intervention group received social and behavioral support and contact time with the exercise trainer to encourage them to increase their exercise level to include twice-weekly strength-training sessions and 150 min of aerobic exercise per week (e.g., three 50-min aerobic exercise sessions or five 30-min sessions) over 12 months. Trainers and participants met twice weekly at a local gym designated by the study during designated times. Gym membership was provided for the duration of the study free of charge. Further details on the exercise intervention were described previously.17
Usual Care
After randomization, participants in the usual care group were told to continue their usual activities and were not given exercise instruction until the end of the study. Each month, women randomized to usual care were contacted monthly to determine AI adherence and discuss health education topics relevant to breast cancer survivors.
Statistical Analysis
Participants were analyzed according to the intention-to-treat procedure in which all participants were grouped according to their intervention assignment at randomization regardless of adherence. We used the Student’s t-test and the Chi-square test to evaluate group differences at baseline. Due to a funding cut, 25 women were only enrolled for a 6-month study rather than 12 months and were therefore not included in the 12-month analyses. At 6 months, 49 controls (82%) and 58 (95%) exercisers completed the QOL assessment. At 12 months, 38 (80%) controls and 45 (94%) exercisers completed the QOL assessment. The primary QOL outcome of interest was endocrine-related QOL as measured by the FACT-B-ES. We performed a mixed model repeated measures analysis with maximum likelihood estimation to examine intervention effects by assessing differences in mean change in QOL measures at the 6-month and 12-month follow-up visits between exercise and usual care groups. This approach is as effective as multiple imputation method to handle missing data with assumption that the data is missing at random.26 Additional sensitivity analyses were performed using multiple imputation under the missing not at random (MNAR) assumption by creating 10 imputed dataset based on observed data in control group only. This method was to show the robustness of results by assuming those who were lost to follow-up in exercise group would have same outcomes as observed in usual care groups.27 Since baseline characteristics did not differ between the two groups, we only adjusted for age and baseline scores for the corresponding outcome measure. Moreover, the exercise group was stratified by weekly exercise time (≥ 150 mins vs. < 150 mins) at the 12-month visit, as well as attendance to training sessions (80% and above vs. < 80%). Effects of baseline outcome measure, age, time, group and group by time interaction were included in the mixed model analysis. We used SAS software (version 9.4; SAS Institute, Cary, NC) in all analyses. All statistical tests were two-tailed, and a P-value of less than 0.05 was considered statistically significant.
Results
Baseline Characteristics
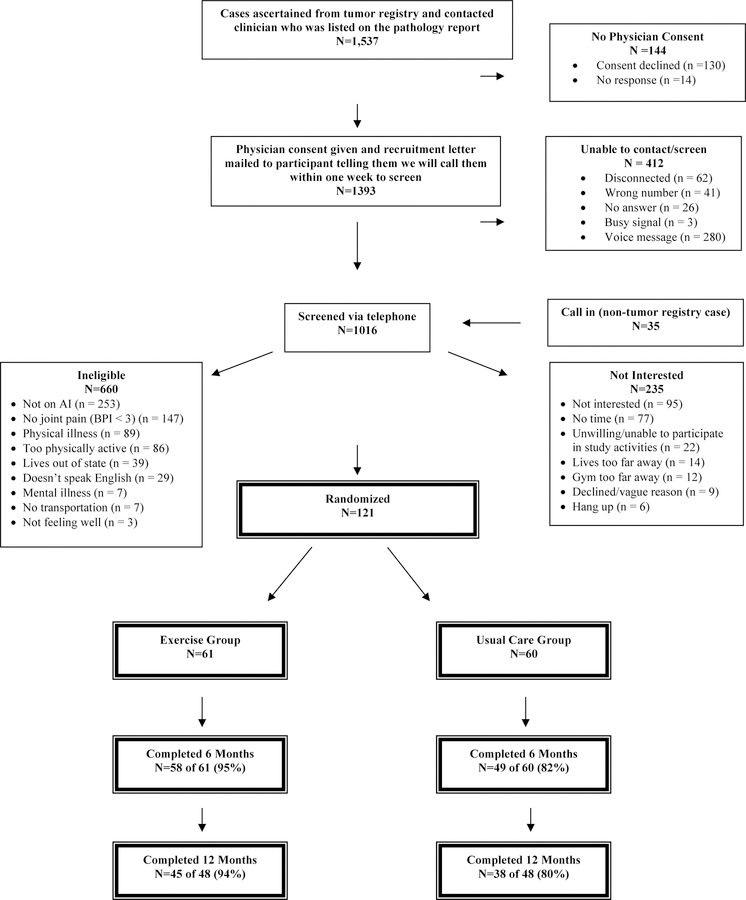
The CONSORT diagram of participants’ screening and enrollment status, as well as baseline demographic and clinical characteristics have been reported previously (Figure 1).17 Briefly, 61 breast cancer survivors were randomized into the exercise arm, while 60 survivors were assigned into the usual care control arm. The average age of study participants in the HOPE study was 61.2 ± 7.0 years old (Table 1). The majority of patients were white (87.6%) and diagnosed with stage I breast cancer (54.5%). The frequencies of race/ethnicity, education, disease stage, radiation therapy, chemotherapy, time on endocrine therapy, and BMI at baseline were similar between the exercise intervention group and the usual care group. Age was slightly higher in the exercise intervention group (62.0 ± 7.0 years) compared to the usual care group (60.5 ± 7.0 years) (P = 0.25).
Figure 1.
Flow of participants through the HOPE Study
Table 1.
Baseline Characteristics among Randomly Assigned Participants in the HOPE Study
| Randomization Group |
|||
|---|---|---|---|
| Usual Care (%) (N = 60) Mean (SD) | Exercise (%) (N = 61) Mean (SD) | P Value | |
| Age, Years | |||
| Mean (SD) | 60.5 (7.0) | 62.0 (7.0) | 0.25 |
| Race/Ethnicity | |||
| Non-Hispanic White | 84 | 85 | 0.85 |
| Hispanic | 5 | 2 | |
| African American | 7 | 10 | |
| Asian/Pacific Islander | 2 | 2 | |
| American Indian | 2 | 0 | |
| Education | |||
| High school graduate | 15 | 10 | 0.25 |
| Some school after high school | 42 | 33 | |
| College graduate | 43 | 57 | |
|
Time since diagnosis, years | |||
| 3.3 (3.9) | 2.7 (3.1) | 0.30 | |
|
Time since initiating AI therapy, years | |||
| 1.8 (1.3) | 1.9 (2.9) | 0.89 | |
| Disease Stage | |||
| 0 | 0 | 1 | 0.70 |
| I | 62 | 59 | |
| II | 32 | 30 | |
| III | 7 | 10 | |
| Chemotherapy | |||
| Yes | 43 | 54 | 0.22 |
| No | 57 | 46 | |
| Radiation Therapy | |||
| Yes | 75 | 82 | 0.65 |
| No | 25 | 18 | |
| BMI, kg/m2 | |||
| 28.7 (5.5) | 30.0 (6.8) | 0.27 | |
| Taking pain medication | |||
| 42 | 52 | ||
| Physician-diagnosed arthritis | |||
| 32 | 49 | ||
| Current glucosamine and chondroitin use | |||
| 18 | 13 | ||
Intervention Adherence
As previously reported, the participants in this study randomized to the exercise group increased their physical activity by 159 minutes per week while the usual-care group increased their physical activity by 49 minutes per week (P < .001).17, 19 The exercise group completed an average of 70% of strength training sessions and reported an average of 119 minutes per week of aerobic exercise. The usual-care group attended an average of 53% of monthly telephone calls.
QOL and Fatigue
Table 2 shows the comparison of mean changes in FACT-B, FACT-ES, FACT-G measures and fatigue scores at 6 months and 12 months by randomly assigned group. At baseline, FACT measure scores did not differ between the two groups. At 12 months, the exercise group had greater improvement in breast cancer subscale (BCS) (2.2 vs. 0.7, P = 0.03), endocrine subscale (ESS) (5.5 vs. 1.7, P < 0.01), FACT-B (10.2 vs. 2.0, P = 0.001), FACT-B-ES (15.6 vs. 3.7, P < 0.001) compared with the usual care group. Additionally, the exercise group had greater improvement in FACT-G (8.0 vs. 1.2, P < 0.01), and Fact-F (5.7 vs. 0.5, P < 0.001) compared with the usual care group. Figure 2 shows the change in total FACT-B-ES score by randomization group at baseline, and after 6 and 12 months of study intervention.
Table 2.
Effect of Exercise Versus Usual Care on FACT Measures at Baseline and Changes at 6 months and 12 months.
| Outcome | Usual Care | Exercise | Exercise Effect | P value | |
|---|---|---|---|---|---|
| FACT-G | |||||
| Physical well-being | Baseline | 21.0 (4.5) | 21.8 (4.2) | 0.31 | |
| 6-month change | 0.6(−0.4, 1.5) | 1.7( 0.8, 2.5) | 1.1(−0.0, 2.2) | 0.05 | |
| 12-month change | −0.1(−1.2, 1.0) | 2.4( 1.4, 3.5) | 2.5( 1.0, 4.0) | 0.001 | |
| Social/Family well-being | Baseline | 21.4 (5.0) | 21.5 (5.4) | 0.89 | |
| 6-month change | −0.7(−1.8, 0.3) | 0.5(−0.5, 1.5) | 1.2(−0.2, 2.7) | 0.09 | |
| 12-month change | 0.4(−0.7, 1.6) | 1.4( 0.3, 2.5) | 1.0(−0.6, 2.5) | 0.23 | |
| Emotional well-being | Baseline | 19.2 (4.4) | 19.3 (4.0) | 0.90 | |
| 6-month change | 0.9( 0.1, 1.7) | 1.1( 0.3, 1.8) | 0.2(−0.8, 1.1) | 0.69 | |
| 12-month change | 0.7(−0.1, 1.6) | 1.7( 0.9, 2.4) | 0.9(−0.1, 2.0) | 0.08 | |
| Functional well-being | Baseline | 20.4 (4.9) | 20.3 (5.2) | 0.92 | |
| 6-month change | 0.9(−0.3, 2.1) | 1.1( 0.0, 2.2) | 0.2(−1.3, 1.8) | 0.77 | |
| 12-month change | 0.1(−1.1, 1.3) | 2.6( 1.5, 3.7) | 2.5( 0.9, 4.1) | 0.002 | |
| FACT-G | Baseline | 82.0 (14.8) | 82.9 (15.1) | 0.73 | |
| 6-month change | 1.5(−1.4, 4.5) | 4.3( 1.6, 7.0) | 2.7(−1.0, 6.5) | 0.153 | |
| 12-month change | 1.2(−2.0, 4.4) | 8.0( 5.1, 11.0) | 6.8( 2.6, 11.0) | 0.002 | |
| FACT-B | |||||
| Breast cancer subscale | Baseline | 18.3 (4.8) | 19.2 (4.7) | 0.26 | |
| 6-month change | 0.7(−0.2, 1.5) | 1.1( 0.3, 2.0) | 0.5(−0.6, 1.6) | 0.39 | |
| 12-month change | 0.7(−0.2, 1.7) | 2.2( 1.3, 3.1) | 1.4( 0.1, 2.7) | 0.03 | |
| FACT-B | Baseline | 100.2 (18.0) | 102.2 (18.7) | 0.57 | |
| 6-month change | 2.2(−1.2, 5.6) | 5.4( 2.3, 8.5) | 3.2(−1.1, 7.6) | 0.14 | |
| 12-month change | 2.0(−1.8, 5.7) | 10.2( 6.7, 13.6) | 8.2( 3.3, 13.2) | 0.001 | |
| FACT-ES | |||||
| Endocrine subscale | Baseline | 54.9 (9.8) | 56.5 (10.1) | 0.38 | |
| 6-month change | 1.3(−0.6, 3.3) | 2.0( 0.2, 3.8) | 0.6(−1.9, 3.2) | 0.62 | |
| 12-month change | 1.7(−0.2, 3.6) | 5.5( 3.7, 7.2) | 3.8( 1.3, 6.2) | 0.003 | |
| FACT-B-ES | |||||
| FACT-B-ES | Baseline | 155.2 (25.5) | 158.7 (26.8) | 0.46 | |
| 6-month change | 3.6(−1.1, 8.2) | 7.4( 3.2, 11.7) | 3.9(−1.9, 9.7) | 0.19 | |
| 12-month change | 3.7(−1.3, 8.7) | 15.6(11.0, 20.2) | 11.9( 5.3, 18.5) | 0.0005 | |
| FACIT-FATIGUE | |||||
| Fatigue subscale | Baseline | 36.2 (10.8) | 37.9 (10.6) | 0.39 | |
| 6-month change | 0.5(−1.7, 2.8) | 3.8( 1.7, 5.9) | 3.3( 0.4, 6.1) | 0.03 | |
| 12-month change | 0.5(−1.6, 2.6) | 5.7( 3.8, 7.7) | 5.2( 2.5, 8.0) | 0.0003 | |
Baseline scores are presented as means (standard deviation). 6-month or 12-month change are presented as least square means and 95% confidence interval from mixed model analysis adjusting for baseline score and age.
At 6 months, 47 controls (78.3%) and 58 (95.1%) exercisers completed the QOL assessment. At 12 months, 38 (79.2%) controls and 45 (93.8%) exercisers completed the QOL assessment. Due to a funding cut, 25 women were only enrolled for a 6-month study rather than 12 months and were therefore not included in the 12-month analyses.
Figure 2.
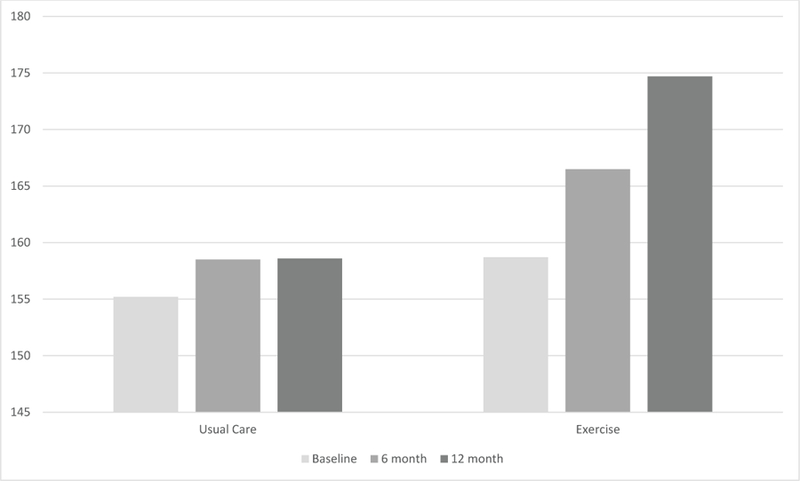
Change in total FACT-B-ES score by randomization group at baseline, and after 6 and 12 months of study intervention.
The exercise intervention group had significantly higher baseline SF-36 vitality subscores compared to the usual care group, but no other differences were seen at baseline (Table 3). In terms of SF-36 eight subscale scores, the exercise group had greater improvement of physical functioning (6.3 vs. −1.1, P < 0.0001), role functioning/physical (7.7 vs. 1.5, P < 0.01), bodily pain (8.1 vs. 0.2, P < 0.0001), general health perceptions (3.0 vs. −0.5, P = 0.01), vitality (6.0 vs. 0.9, P < 0.0001), social role functioning (6.2 vs. 1.4, P = 0.001), and mental functioning (4.0 vs. 0.7, P = 0.01) (Table 3). For the summary scores, the physical component score (7.0 vs. −0.8, P < 0.0001) improved to a greater degree in the intervention compared with the usual care group at 12 months (Table 3). When sensitivity analyses were run under the missing not at random assumption, similar results were obtained (Supplemental Tables 1 and 2).
Table 3.
Effect on Exercise Versus Usual Care on SF-36 at Baseline and Changes at 6 months and 12 months.
| Outcome | Usual Care | Exercise | Exercise Effect | P value | |
|---|---|---|---|---|---|
| SF-36 SCORES | |||||
| Subscales | |||||
| Physical functioning | Baseline | 42.1 (8.7) | 42.8 (8.8) | 0.67 | |
| 6-month change | 0.8 (−1.1, 2.8) | 5.8 (4.0, 7.6) | 4.9 (2.4, 7.4) | 0.0002 | |
| 12-month change | −1.1 (−3.2, 1.1) | 6.3 (4.3, 8.3) | 7.4 (4.5, 10.3) | <.0001 | |
| Roles: physical | Baseline | 42.1 (11.4) | 42.0 (11.7) | 0.97 | |
| 6-month change | 2.6 (−0.4, 5.7) | 4.5 (1.8, 7.3) | 1.9 (−1.9, 5.7) | 0.32 | |
| 12-month change | 1.5 (−1.6, 4.6) | 7.7 (4.8, 10.6) | 6.2 (2.3, 10.1) | 0.002 | |
| Bodily pain | Baseline | 42.5 (7.8) | 42.2 (9.0) | 0.86 | |
| 6-month change | 2.6 (0.1, 5.0) | 4.7 (2.4, 7.0) | 2.1 (−1.1, 5.3) | 0.19 | |
| 12-month change | 0.2 (−2.2, 2.7) | 8.1 (5.9, 10.4) | 7.9 (4.7, 11.1) | <.0001 | |
| General health perceptions | Baseline | 48.1 (9.0) | 49.3 (8.5) | 0.46 | |
| 6-month change | 0.4 (−1.4, 2.3) | 2.2 (0.5, 4.0) | 1.8 (−0.7, 4.2) | 0.15 | |
| 12-month change | −0.5 (−2.5, 1.6) | 3.0 (1.0, 4.9) | 3.4 (0.7, 6.2) | 0.01 | |
| Vitality | Baseline | 44.6 (9.3) | 48.2 (8.7) | 0.03 | |
| 6-month change | 0.8 (−1.2, 2.8) | 4.4 (2.6, 6.2) | 3.6 (1.0, 6.2) | 0.006 | |
| 12-month change | 0.9 (−1.0, 2.8) | 6.0 (4.3, 7.8) | 5.1 (2.6, 7.6) | <.0001 | |
| Social role functioning | Baseline | 46.9 (9.3) | 47.1 (10.4) | 0.92 | |
| 6-month change | 1.6 (−0.9, 4.1) | 2.8 (0.4, 5.2) | 1.2 (−1.9, 4.3) | 0.45 | |
| 12-month change | 1.4 (−1.0, 3.8) | 6.2 (3.9, 8.5) | 4.8 (1.9, 7.7) | 0.001 | |
| Role: emotion | Baseline | 45.3 (12.8) | 44.6 (12.3) | 0.76 | |
| 6-month change | 0.2 (−3.1, 3.5) | 2.9 (−0.1, 6.0) | 2.7 (−1.3, 6.7) | 0.18 | |
| 12-month change | 3.2 (−0.3, 6.6) | 5.1 (1.9, 8.3) | 2.0 (−2.3, 6.2) | 0.36 | |
| Mental functioning | Baseline | 47.2 (11.0) | 48.0 (9.1) | 0.69 | |
| 6-month change | 1.5 (−0.9, 3.8) | 2.0 (−0.2, 4.2) | 0.5 (−2.5, 3.6) | 0.73 | |
| 12-month change | 0.7 (−1.4, 2.8) | 4.0 (2.1, 6.0) | 3.3 (0.7, 5.8) | 0.01 | |
| Component Scores | |||||
| Physical component score | Baseline | 42.4 (8.8) | 42.9 (9.3) | 0.74 | |
| 6-month change | 1.9 (−0.2, 4.1) | 5.2 (3.3, 7.2) | 3.3 (0.6, 6.1) | 0.02 | |
| 12-month change | −0.8 (−3.3, 1.7) | 7.0 (4.7, 9.3) | 7.8 (4.6, 11.0) | <.0001 | |
| Mental component score | Baseline | 48.1 (11.8) | 48.9 (9.9) | 0.68 | |
| 6-month change | 0.5 (−2.2, 3.2) | 1.7 (−0.8, 4.2) | 1.2 (−2.3, 4.6) | 0.50 | |
| 12-month change | 2.4 (−0.2, 5.0) | 4.1 (1.7, 6.6) | 1.7 (−1.5, 5.0) | 0.30 | |
Baseline scores are presented as means (standard deviation). 6-month or 12-month change are presented as least square means and 95% confidence interval from mixed model analysis adjusting for baseline score and age.
Discussion
In this randomized trial, combined aerobic and resistance exercise, such as moderate-intensity walking and twice-weekly supervised strength training, improved endocrine-related QOL and overall QOL by approximately 10% among breast cancer patients experiencing AI-related arthralgias. We noted similar standardized effect sizes for FACT-B, FACT-ES, and FACT-G improvement in the exercise group (0.46, 0.39, and 0.46, respectively). These results are encouraging for postmenopausal breast cancer survivors who are recommended to take AIs to improve their cancer prognosis.
This is the first study to examine the effect of an exercise intervention on endocrine-related and overall QOL in breast cancer patients taking AIs and experiencing AI-related side effects. Our findings were consistent with other physical activity trials among breast cancer patients not taking AIs that showed improved QOL.3–8, 10 Similar to the 10% improvement of FACT-B scores among exercisers in our study, Courneya et al found an improvement of 8.2% in FACT-B scores in their trial of exercise among breast cancer survivors.8 This study did not limit their study to breast cancer survivors taking AIs and experiencing AI-associated side effects but showed similar baseline FACT-B scores and similar improvements in QOL to our study. Additionally, a recent meta-analysis among patients with multiple types of cancer found a favorable effect of exercise on QOL with a standardized mean difference summary estimate of 5.55 (95% CI: 3.19 – 7.90; p<0.001).28 Adverse side effects associated with taking AIs are the main reason for AI treatment discontinuation or poor adherence, thereby reducing treatment effectiveness and increasing mortality.29–33 Adverse side effects of AIs, such as joint pain, are associated with decreased physical activity among breast cancer survivors.34 Previous studies have shown that interventions among breast cancer patients to increase physical activity levels are feasible and effective35 and may improve QOL.36 The breast cancer survivors in our study were all experiencing arthralgia or joint pain associated with taking an AI for cancer treatment. Our intervention further was effective in getting these breast cancer survivors to increase their exercise levels indicating that even survivors experiencing moderate to severe adverse treatment-associated symptoms can increase their exercise levels and improve their QOL. Furthermore, this increase in physical activity can alleviate arthralgia associated with AI use.17
A significant effect of exercise on various QOL measures, both on the FACT and SF-36, was observed in our study, particularly measures of physical QOL. Our participants had lower (i.e. worse) baseline scores across all FACT measures compared to other studies of women who were taking AIs.37, 38 However, the women in these other studies were not required to have joint pain associated with AI use, which may explain lower baseline scores in our study. As we had the FACT subscales, we could examine different aspects of QOL. In this study, we observed significant increases in the physical well-being, functional well-being, breast cancer, endocrine, and fatigue subscales. It is well established that exercise is associated with more beneficial physical and mental health outcomes, including better general and health-related QOL.39 This study further shows that exercise can lead to better QOL among breast cancer patients with AI-associated arthralgias.
Strengths of this study include the randomized design, population-based recruitment, high adherence to the intervention, and 12-month study duration. This is the first study to examine the effect of exercise specifically on endocrine-related QOL (FACT-B-ES) among breast cancer patients taking AIs and experiencing AI-induced arthralgia. Other studies have used various approaches to improve endocrine symptoms in breast cancer survivors, including mindfulness-based stress reduction,40 acupuncture,31 physical therapy, and targeted heat.29 Pharmacological therapies, such as use of non-steroidal anti-inflammatory drugs (NSAIDS), cyclooxygenase-2 (COX-2) inhibitors, glucosamine, and narcotic analgesics, have also been studied to alleviate pain from AI-induced arthralgias, but may be contraindicated or ineffective.29 A randomized trial of duloxetine in 299 breast cancer patients experiencing AI-associated side effects showed an improvement with 12 weeks of treatment, although low-grade toxicities were more frequent in the treatment group.41 This study also has practical implications: 1) the aerobic exercise in this study primarily consisted of brisk walking outside or on a treadmill which can be done by most individuals assuming they have a good pair of walking shoes and a safe place to walk and 2) the Livestrong at the YMCA offers free exercise training for cancer survivors that has been found to be safe and effective in increasing physical activity levels.42
Limitations of our study include participants were not blinded, potential reporting bias due to patient-reported outcomes, and missing data across time points. The exercise group had significantly greater completion rate, indicating a differential dropout rate between the two groups. However, since exercisers showed improvement in joint pain compared to controls, the potential bias due to greater drop-out in control group is likely towards the null. Additionally, we used mixed model analysis that is robust to remedy such bias from missing data. The sensitivity analysis using multiple imputation under MNAR assumption also reached consistent results. Lastly, as our study specifically aimed to treat AI-associated side effects, rather than prevent AI-associated side effects, thus we were unable to assess the impact of our intervention on AI adherence as all women enrolled were taking AIs despite side effects and were planning on staying on AIs for the duration of the study.
In this study, we observed significant and clinically important improvements in endocrine-related QOL symptoms and fatigue among breast cancer patients randomized to the combined aerobic and resistance exercise intervention. The endocrine-related QOL improvement of approximately 12 points observed with exercise exceeds the clinically-defined improvement in QOL of 3 points. Since adverse side effects associated with AI use are quite common among the breast cancer survivors and this is the main reason for treatment discontinuation, this innovative non-pharmacologic intervention could benefit many breast cancer survivors and increase successful implementation of AIs in breast cancer treatment. The effect of combined aerobic and resistance exercise on these overall and symptom-specific QOL measures is promising for breast cancer survivors whose physicians’ have recommended AIs for treatment.
Supplementary Material
Acknowledgements:
We thank Mia Sorkin, Dan Root, Willie Moore, Liz Fraser, Adrienne Viola, Yanchang Zhang, Bridget Winterhalter, Norbert Hootsmans, Celeste Wong, and Meghan Hughes for their assistance. We thank Rajni Mehta and the Rapid Case Ascertainment of Yale Cancer Center, Smilow Cancer Hospital at Yale–New Haven, St Raphael’s Hospital, St Vincent’s Medical Center, Bridgeport Hospital, and Greenwich Hospital and all the clinicians who consented or referred their patients to our study. Most importantly, we thank the participants for their dedication to and time spent on the HOPE (Hormones and Physical Exercise) study.
Supported by National Cancer Institute Grant No. R01 CA132931 and in part by a grant from the Breast Cancer Research Foundation (M.L.I), Yale Cancer Center Support Grant No. P30 CA016359, and Clinical and Translational Science Award Grant No. UL1 TR000142 from the National Center for Advancing Translational Science, a component of the National Institutes of Health. Certain data used in this study were obtained from the Connecticut Tumor Registry, located in the Connecticut Department of Public Health. The authors assume full responsibility for analyses and interpretation of these data.
Footnotes
The authors have no conflicts of interest to disclose.
References
- 1.Systemic treatment of early breast cancer by hormonal, cytotoxic, or immune therapy. 133 randomised trials involving 31,000 recurrences and 24,000 deaths among 75,000 women. Early Breast Cancer Trialists’ Collaborative Group. Lancet 1992;339: 1–15. [PubMed] [Google Scholar]
- 2.Oliveria SA, Felson DT, Cirillo PA, Reed JI, Walker AM. Body weight, body mass index, and incident symptomatic osteoarthritis of the hand, hip, and knee. Epidemiology 1999;10: 161–166. [PubMed] [Google Scholar]
- 3.Mutrie N, Campbell AM, Whyte F, et al. Benefits of supervised group exercise programme for women being treated for early stage breast cancer: pragmatic randomised controlled trial. BMJ 2007;334: 517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sandel SL, Judge JO, Landry N, Faria L, Ouellette R, Majczak M. Dance and movement program improves quality-of-life measures in breast cancer survivors. Cancer Nursing 2005;28: 301–309. [DOI] [PubMed] [Google Scholar]
- 5.Cho OH, Yoo YS, Kim NC. Efficacy of comprehensive group rehabilitation for women with early breast cancer in South Korea. Nursing & Health Sciences 2006;8: 140–146. [DOI] [PubMed] [Google Scholar]
- 6.Basen-Engquist K, Taylor CL, Rosenblum C, et al. Randomized pilot test of a lifestyle physical activity intervention for breast cancer survivors. Patient Education and Counseling 2006;64: 225–234. [DOI] [PubMed] [Google Scholar]
- 7.Milne HM, Wallman KE, Gordon S, Courneya KS. Effects of a combined aerobic and resistance exercise program in breast cancer survivors: a randomized controlled trial. Breast Cancer Res Treat 2008;108: 279–288. [DOI] [PubMed] [Google Scholar]
- 8.Courneya KS, Mackey JR, Bell GJ, Jones LW, Field CJ, Fairey AS. Randomized controlled trial of exercise training in postmenopausal breast cancer survivors: cardiopulmonary and quality of life outcomes. Journal of Clinical Oncology 2003;21: 1660–1668. [DOI] [PubMed] [Google Scholar]
- 9.Pinto BM, Frierson GM, Rabin C, Trunzo JJ, Marcus BH. Home-based physical activity intervention for breast cancer patients. Journal of Clinical Oncology 2005;23: 3577–3587. [DOI] [PubMed] [Google Scholar]
- 10.Campbell A, Mutrie N, White F, McGuire F, Kearney N. A pilot study of a supervised group exercise programme as a rehabilitation treatment for women with breast cancer receiving adjuvant treatment. European Journal of Oncology Nursing 2005;9: 56–63. [DOI] [PubMed] [Google Scholar]
- 11.Badger T, Segrin C, Dorros SM, Meek P, Lopez AM. Depression and anxiety in women with breast cancer and their partners. Nursing Research 2007;56: 44–53. [DOI] [PubMed] [Google Scholar]
- 12.Duijts SF, Faber MM, Oldenburg HS, van Beurden M, Aaronson NK. Effectiveness of behavioral techniques and physical exercise on psychosocial functioning and health-related quality of life in breast cancer patients and survivors--a meta-analysis. Psychooncology 2011;20: 115–126. [DOI] [PubMed] [Google Scholar]
- 13.Schmitz KH, Courneya KS, Matthews C, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc 2010;42: 1409–1426. [DOI] [PubMed] [Google Scholar]
- 14.Bicego D, Brown K, Ruddick M, Storey D, Wong C, Harris SR. Effects of exercise on quality of life in women living with breast cancer: a systematic review. Breast J 2009;15: 45–51. [DOI] [PubMed] [Google Scholar]
- 15.McNeely ML, Campbell KL, Rowe BH, Klassen TP, Mackey JR, Courneya KS. Effects of exercise on breast cancer patients and survivors: a systematic review and meta-analysis. CMAJ: Canadian Medical Association Journal 2006;175: 34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nyrop KA, Callahan LF, Cleveland RJ, Arbeeva LL, Hackney BS, Muss HB. Randomized Controlled Trial of a Home-Based Walking Program to Reduce Moderate to Severe Aromatase Inhibitor-Associated Arthralgia in Breast Cancer Survivors. Oncologist 2017;22: 1238–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Irwin ML, Cartmel B, Gross CP, et al. Randomized exercise trial of aromatase inhibitor-induced arthralgia in breast cancer survivors. J Clin Oncol 2015;33: 1104–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomas GA, Cartmel B, Harrigan M, et al. The effect of exercise on body composition and bone mineral density in breast cancer survivors taking aromatase inhibitors. Obesity (Silver Spring) 2017;25: 346–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arem H, Sorkin M, Cartmel B, et al. Exercise adherence in a randomized trial of exercise on aromatase inhibitor arthralgias in breast cancer survivors: the Hormones and Physical Exercise (HOPE) study. J Cancer Surviv 2016;10: 654–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wolff I, van Croonenborg JJ, Kemper HC, Kostense PJ, Twisk JW. The effect of exercise training programs on bone mass: a meta-analysis of published controlled trials in pre- and postmenopausal women. Osteoporos Int 1999;9: 1–12. [DOI] [PubMed] [Google Scholar]
- 21.Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. Journal of Clinical Oncology 1993;11: 570–579. [DOI] [PubMed] [Google Scholar]
- 22.Ware JE Jr., Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Medical Care 1992;30: 473–483. [PubMed] [Google Scholar]
- 23.Cella D, Hahn EA, Dineen K. Meaningful change in cancer-specific quality of life scores: differences between improvement and worsening. Quality of Life Research 2002;11: 207–221. [DOI] [PubMed] [Google Scholar]
- 24.Cella D, Eton DT, Lai JS, Peterman AH, Merkel DE. Combining anchor and distribution-based methods to derive minimal clinically important differences on the Functional Assessment of Cancer Therapy (FACT) anemia and fatigue scales. Journal of Pain and Symptom Management 2002;24: 547–561. [DOI] [PubMed] [Google Scholar]
- 25.Webster K, Cella D, Yost K. The Functional Assessment of Chronic Illness Therapy (FACIT) Measurement System: properties, applications, and interpretation. Health Qual Life Outcomes 2003;1: 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Allison P Handling missing data by maximum likelihood. SAS Global Forum, 2012.
- 27.Yuan Y Sensitivity analysis in multiple imputation for missing data. SAS Institute 2014.
- 28.Gerritsen JK, Vincent AJ. Exercise improves quality of life in patients with cancer: a systematic review and meta-analysis of randomised controlled trials. Br J Sports Med 2016;50: 796–803. [DOI] [PubMed] [Google Scholar]
- 29.Cella D, Fallowfield LJ. Recognition and management of treatment-related side effects for breast cancer patients receiving adjuvant endocrine therapy. Breast Cancer Res Treat 2008;107: 167–180. [DOI] [PubMed] [Google Scholar]
- 30.Hershman DL, Shao T, Kushi LH, et al. Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Cancer Res Treat 2011;126: 529–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crew KD, Capodice JL, Greenlee H, et al. Pilot study of acupuncture for the treatment of joint symptoms related to adjuvant aromatase inhibitor therapy in postmenopausal breast cancer patients. J Cancer Surviv 2007;1: 283–291. [DOI] [PubMed] [Google Scholar]
- 32.Murphy CC, Bartholomew LK, Carpentier MY, Bluethmann SM, Vernon SW. Adherence to adjuvant hormonal therapy among breast cancer survivors in clinical practice: a systematic review. Breast Cancer Res Treat 2012;134: 459–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sestak I, Cuzick J, Sapunar F, et al. Risk factors for joint symptoms in patients enrolled in the ATAC trial: a retrospective, exploratory analysis. Lancet Oncology 2008;9: 866–872. [DOI] [PubMed] [Google Scholar]
- 34.Brown JC, Mao JJ, Stricker C, Hwang WT, Tan KS, Schmitz KH. Aromatase inhibitor associated musculoskeletal symptoms are associated with reduced physical activity among breast cancer survivors. Breast J 2014;20: 22–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rogers LQ, Hopkins-Price P, Vicari S, et al. A randomized trial to increase physical activity in breast cancer survivors. Medicine and Science in Sports and Exercise 2009;41: 935–946. [DOI] [PubMed] [Google Scholar]
- 36.DeNysschen CA, Burton H, Ademuyiwa F, Levine E, Tetewsky S, O’Connor T. Exercise intervention in breast cancer patients with aromatase inhibitor-associated arthralgia: a pilot study. European Journal of Cancer Care (English Language Edition) 2014;23: 493–501. [DOI] [PubMed] [Google Scholar]
- 37.Lustberg MB, Orchard TS, Reinbolt R, et al. Randomized placebo-controlled pilot trial of omega 3 fatty acids for prevention of aromatase inhibitor-induced musculoskeletal pain. Breast Cancer Res Treat 2017. [DOI] [PMC free article] [PubMed]
- 38.Yagata H, Ohtsu H, Komoike Y, et al. Joint symptoms and health-related quality of life in postmenopausal women with breast cancer who completed 5 years of anastrozole. Supportive Care in Cancer 2016;24: 683–689. [DOI] [PubMed] [Google Scholar]
- 39.Penedo FJ, Dahn JR. Exercise and well-being: a review of mental and physical health benefits associated with physical activity. Curr Opin Psychiatry 2005;18: 189–193. [DOI] [PubMed] [Google Scholar]
- 40.Carlson LE, Speca M, Patel KD, Goodey E. Mindfulness-based stress reduction in relation to quality of life, mood, symptoms of stress, and immune parameters in breast and prostate cancer outpatients. Psychosom Med 2003;65: 571–581. [DOI] [PubMed] [Google Scholar]
- 41.Henry NL, Unger JM, Schott AF, et al. Randomized, Multicenter, Placebo-Controlled Clinical Trial of Duloxetine Versus Placebo for Aromatase Inhibitor-Associated Arthralgias in Early-Stage Breast Cancer: SWOG S1202. Journal of Clinical Oncology 2018;36: 326–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Irwin ML, Cartmel B, Harrigan M, et al. Effect of the LIVESTRONG at the YMCA exercise program on physical activity, fitness, quality of life, and fatigue in cancer survivors. Cancer 2017;123: 1249–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.