Abstract
Glioma, the most common primary malignancy in the brain, has high recurrence and lethality rates, and thus, elucidation of the molecular mechanisms of this incurable disease is urgently needed. Poly-pyrimidine tract binding protein (PTBP1, also known as hnRNP I), an RNA-binding protein, has various mechanisms to promote gliomagenesis. However, the mechanisms regulating PTBP1 expression are unclear. Herein, we report a novel natural antisense noncoding RNA, PTB-AS, whose expression correlated positively with PTBP1 mRNA. We found that PTB-AS significantly promoted the proliferation and migration in vivo and in vitro of glioma cells. PTB-AS substantially increased the PTBP1 level by directly binding to its 3′ UTR and stabilizing the mRNA. Furthermore, staphylococcal nuclease domain-containing 1 (SND1) dramatically increased the binding capacity between PTB-AS and PTBP1 mRNA. Mechanistically, PTB-AS could mask the binding site of miR-9 in the PTBP1-3′ UTR; miR-9 negatively regulates PTBP1. To summarize, we revealed that PTB-AS, which maintains the PTBP1 level through extended base pairing to the PTBP1 3′ UTR with the assistance of SND1, could significantly promote gliomagenesis.
Keywords: glioma, NAT, PTBP1, miR-9, SND1
Zhu et al. show that the upregulated PTB-AS promotes gliomagenesis through elevating PTBP1 expression. Mechanistically, PTB-AS masks the binding site of miR-9, which negatively regulates PTBP1, in the PTBP1-3′ UTR, and finally enhances PTBP1 mRNA stability through extended base pairing to the PTBP1 3′ UTR with the assistance of SND1.
Introduction
Glioma is the most common type of malignant primary brain tumor, with high recurrence and lethality rates.1 The treatment and prognosis of severely ill patients with glioma have shown no significant improvements despite advances in surgery, radiation therapy, and chemotherapy.2 Thus far, the mechanisms of gliomagenesis include the alternative expression of core genes (signal transducer and activator of transcription 3 [STAT3]; positively).3 RNA-binding proteins (RBPs), which can bind to single- or double-stranded RNAs, also participate in regulating gliomagenesis.4 RBPs, which are important and functional protein-coding genes, could influence pre-mRNA processing, as well as the transport, localization, translation, and stability of mRNAs.5 Our research has focused on the regulation of RBPs in glioma, and we previously performed a systematic functional study of poly(RC) binding protein 2 (PCBP2) and adenosine deaminase RNA specific 1 (ADAR1).6, 7 which play important roles in gliomagenesis. In this study, we further explored the regulatory mechanism of another important RBP, poly-pyrimidine tract binding protein (PTBP1), which was dramatically overexpressed in glioma.
PTBP1, also known as hnRNP I, is an RBP with various molecular functions. Upregulating PTBP1 in differentiated cells contributes to gliomagenesis through aberrantly modulating the alternative splicing of genes involved in cell proliferation and migration, including fibroblast growth factor receptor-1 (FGFR-1), pyruvate kinase M (PKM), and ubiquitin-specific peptidase 5 (USP5).8 In addition to its role in splicing, PTBP1 is involved in other aspects of mRNA metabolism, such as 3′ end processing, transport, stability, and internal ribosome entry site (IRES)-mediated translation.9 Although PTBP1 has been extensively investigated, given its crucial role in neural development and gliomagenesis,9 the mechanisms regulating its own expression have been poorly explored. Thus far, the neuron-specific microRNA (miRNA) miR-124 has been shown to directly target PTBP1 mRNA and reduce PTBP1 protein levels, leading to the accumulation of correctly spliced PTBP2 mRNA and a dramatic increase in PTBP2 protein.10 In addition, a previous reports showed that the transcription factor c-Myc directly controls the expression of hnRNPA1/A2 and PTBP1 and then regulates PKM alternative splicing in cancer.11 However, whether long noncoding RNAs (lncRNAs) could influence PTBP1 expression is largely unknown.
Natural antisense transcripts (NATs) are a class of RNA molecules that are complementary to their paired RNA transcripts.12 These molecules can be transcribed in cis from the opposing DNA strands at the same genomic locus or in trans at a separate genomic locus.13 NATs may regulate sense-strand mRNAs in a positive (concordant) or negative (discordant) manner at the transcriptional or post-transcriptional level, to carry out a wide range of biological and cellular functions.14, 15 NATs have been found to function at several levels to regulate gene expression, including the pre-transcriptional, transcriptional, and post-transcriptional levels, through DNA-RNA, RNA-RNA, or protein-RNA interactions in the nucleus or cytoplasm.16 The abnormal expression of NATs has been implicated in the pathogenesis of various diseases, such as cancer or neurological disease.17 Furthermore, NATs were reported to have a close relationship with gliomagenesis. For example, HOXA11-AS was involved in cell cycle progression,18 ZEB1-AS1 was an important regulator of migration and invasion via activating epithelial-to-mesenchymal transition (EMT) in the metastatic progression of glioma,19 downregulation of HIF1A-AS2 led to delayed growth of mesenchymal glioblastoma (GBM) stem-like cell (GSC) tumors,20 TP73-AS1 promotes brain glioma growth and invasion through acting as a competing endogenous RNA (ceRNA) to promote high mobility group box 1 (HMGB1) expression by sponging miR-142,21 and PRKAG2-AS1 is indispensable for early diagnosis or prognosis of glioma.22
In the present study, we discovered that PTB-AS, a novel NAT transcribed from the reverse strand of the PTBP1 gene, partially overlaps with the 3′ UTR of the PTBP1 mRNA and plays an essential role in upregulating PTBP1 gene expression. Knockdown of PTB-AS significantly inhibited glioma proliferation (in vitro and in vivo) and migration. Mechanistically, PTB-AS directly bound to the 3′ UTR of PTBP1 mRNA, protect PTBP1 from being targeted by miR-9, and stabilize PTBP1-mRNA with the help of staphylococcal nuclease domain containing 1 (SND1). These findings elucidated a novel mechanism by which PTBP1 is upregulated in gliomagenesis and identified a novel NAT that could be a potential target for glioma therapy.
Results
Identification of a Novel Antisense lncRNA, PTB-AS, at the PTBP1 Gene Locus
We searched an RBPs database and identified 380 putative RBPs (Table S1) spatially expressed in postmitotic or proliferating regions of the embryonic brain. To analyze the roles of NATs in glioma, we used the NATs Database (NATsDB) (http://natsdb.cbi.pku.edu.cn/)12 to predict whether these RBPs have corresponding NATs in their genomic locus. We found that 199 RBPs (Table S2) had associated NATs (including 117 full overlapping NATs, 40 head-to-head NATs, and 42 tail-to-tail NATs), and 181 other RBPs were predicted to have no NATs in their genomic loci (Figure S1A). We screened the expression of genes among the 199 RBPs in glioma with the NCBI UniGene expressed sequence tab (EST) profile database and found that 23 genes were significantly highly expressed in glioma (data not shown). We found a NAT in the genomic locus of the PTBP1-3′ UTR (Figure S1B; Table S3); then, we identified it as a novel lncRNA and explored its function and mechanism.
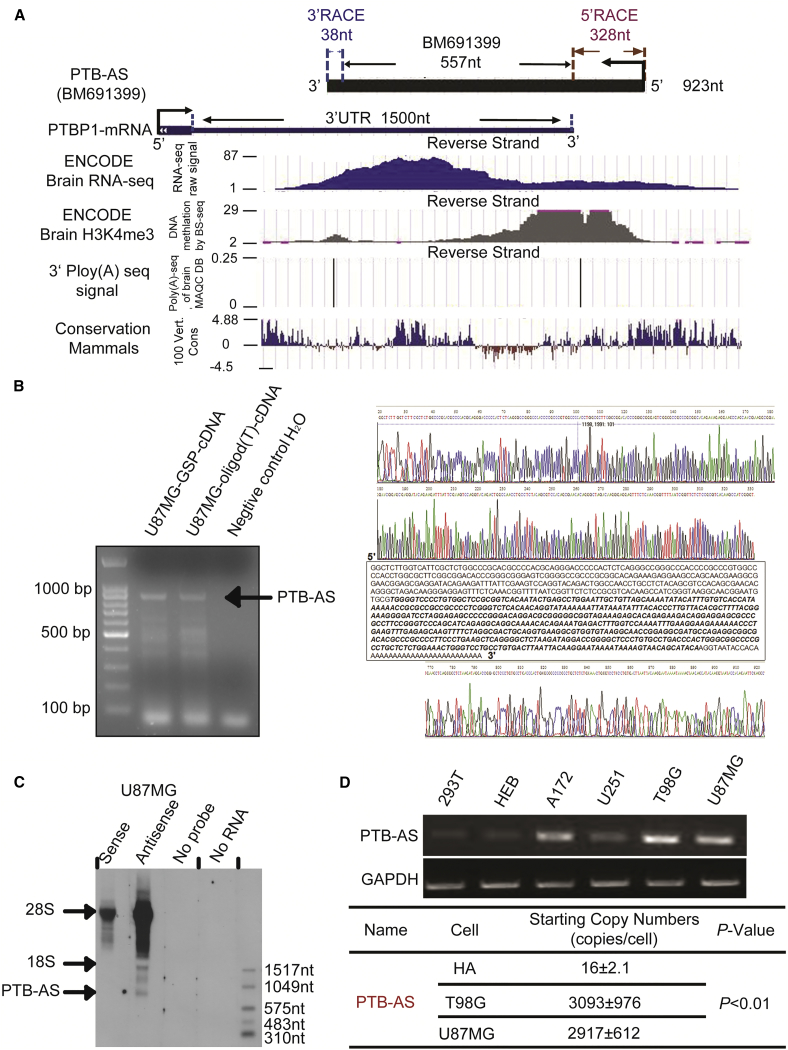
The NAT of PTBP1, BM691399, is a transcript with a length of 557 nt that is completely located in the PTBP1-3′ UTR genomic locus. We analyzed the chromatin marks (H3K4me3) and RNA sequencing data (Encode RNA-seq signals and Poly(A)-seq] and identified the conservation quality among mammals. The results showed that the active transcription signal H3K4me3 exists upstream of BM691399 and that the whole genomic locus has RNA-seq signals in human brain tissue (Figure 1A). To verify the existence of this NAT, we detected its presence and relative abundance in glioma cells compared with normal cells. We identified a robust Poly(A) signal in the 3′ end of BM691399. To clone the full length of this NAT, we performed 5′ and 3′ rapid amplification of cDNA ends (RACE) and observed a distinct band near the 900 nt marker of 900 nt, regardless of the presence of 5′ gene-specific primer 1 (GSP1)-RT-cDNA or 3′ oligo(dT)-RT-cDNA template. We found that the full length of PTBP1 NAT was 923 nt after the strict sequencing step (Figures 1B and S1C). Northern blot analysis was employed to determine the full length of this NAT, and we observed a distinct band near the 1,049 nt RNA marker only, in the probe hybridization treatment group (Figure 1C). Furthermore, the PTB-AS signal was weaker in the knockdown groups than the control group (Figure S1D). We named this NAT PTB-AS, because it originates from the complementary strand of the PTBP1 gene. We found that PTB-AS was upregulated in several glioma cell lines and the starting copy number of PTB-AS, or that PTBP1 was higher in glioma than in normal astrocyte cells (Figures 1D and S1E). To demonstrate the non-coding potential of PTB-AS, we used the classical prediction web server Coding Potential Calculator (CPC) and experimental identification. As a result, PTB-AS was found to be without coding capacity as a lncRNA (Figures S1F and S1G).
Figure 1.
Characterization of the Novel Antisense lncRNA PTB-AS at the PTBP1 Locus
(A) Genome-wide discovery of PTB-AS. PTB-AS (923 nt), derived from the transcript BM691399 (557 nt), was amplified by RACE and overlapped with the PTBP1-3′ UTR. RNA sequencing (RNA-seq) can directly define the transcribed regions of the genome, and the raw signal here shows the primary structure of PTB-AS. Chromatin marks of transcription initiation (histone H3 lysine 4 trimethylation [H3K4me3]) defined the beginning of PTB-AS, and sequencing of polyadenylation ends (3′ Poly(A)-seq) defined the precise ends of these transcripts. The 100 Vert. Cons scores describe the conservation of PTB-AS in mammals. All of these raw data were analyzed using the UCSC genome browser. (B) Identification of the full length of PTB-AS by strand-specific RT-PCR. The total RNA of U87MG cells was extracted. The first lane indicates products that used the GSP for RT as the template. The second lane indicates products that used the oligo (dT) primer for RT as the template. The third lane indicates products that used H2O for RT as the template. The arrow shows the direction of the bands of PTB-AS. The raw sequencing spectrogram of RACE for PTB-AS is shown: the upper one is for 5′ RACE, and the lower one is for 3′ RACE. (C) Northern blot results indicate the full length of PTB-AS. An antisense probe hybridized with PTB-AS RNA, whereas the sense or no-probe and no-total RNA served as negative controls. The single band indicated by the arrow is PTB-AS. (D) Two normal human cell lines and four glioma cell lines were used to extract total RNA, which was reverse transcribed. Semiquantitative PCR was performed to identify the relative expression of the PTBP1 NAT. GAPDH served as the internal parameter reference. The copy number was detected and calculated to quantify the expression of PTB-AS in HA, T98G, or U87MG cell lines, using absolute qPCR.
PTB-AS Is Upregulated in Glioma and Positively Regulates the PTBP1 Level
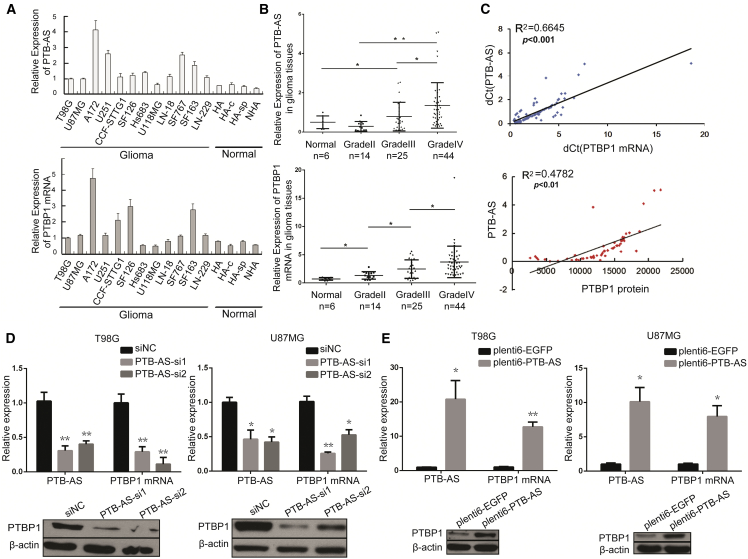
PTBP1 is overexpressed in glioma cell lines and tumor tissues and is essential for gliomagenesis.2, 23 To address the potential coregulation between PTB-AS and PTBP1, we primarily examined the expression of PTB-AS and PTBP1 mRNA. Our results showed that high expression of PTB-AS and PTBP1 mRNA was detected in 12 glioma cell lines compared with the normal astrocyte cell line (Figure 2A). Concordantly, PTB-AS and PTBP1 mRNA levels were upregulated in glioma tissue samples (n = 83) compared with control brain tissue samples (n = 6; Figure 2B). In addition, the PTBP1 protein level was significantly increased in the glioma cell lines and tissue samples (Figures S2A and S2B), which is consistent with the augmentation of PTBP1 observed in glioma in previous studies.24 To determine the potential relationship between PTB-AS expression and patient prognosis, we used the systematic database of the expression profiles and clinical data of genes or ncRNAs from the Chinese Glioma Genome Atlas (CGGA) database of Tiantan Hospital and the Oncomine and The Cancer Genome Atlas (TCGA) databases to screen our transcripts. Kaplan-Meier analysis and log-rank tests were used to evaluate the effects of PTB-AS and PTBP1 expression on overall survival (OS). The results indicated that patients with higher PTB-AS or PTBP1 expression had a significantly poorer prognosis than patients with lower expression (p < 0.0001; Figures S2C and S2D). PTB-AS and PTBP1 showed a similar expression pattern, suggesting that functionally related mechanisms may be present between these two transcripts.
Figure 2.
The Positive Correlation between PTB-AS and PTBP1 Expression and PTB-AS Regulates the PTBP1 mRNA and Protein Levels
(A and B) Expression of PTB-AS and PTBP1 mRNA in the (A) glioma cell lines (n = 12) compared to human normal astrocyte lines (n = 4) or (B) glioma tissues (n = 83) compared to normal brain tissues (n = 6) detected by quantitative real-time PCR. Expression data are presented as the mean ± SD, n = 3. *p < 0.05, **p < 0.01 (Student’s t test). (C) Correlation analysis showing that PTB-AS correlated positively with PTBP1 mRNA or protein expression. The mRNA and protein expression levels were measured in the glioma tissue samples. The delta cycle threshold (dCt) values (normalized to GAPDH) were subjected to Pearson correlation analysis (Student’s t test). (D and E) Changes in PTB-AS expression altered PTBP1 mRNA and protein levels. T98G cells and U87MG cells were infected with (D) two different siRNAs (PTB-AS si1 or PTB-AS si2) to knock down (siNC [small interfering negative control]) or (E) PTB-AS-plenti6 plasmid to overexpress PTB-AS (EGFP-plenti6 was the negative control). PTB-AS and PTBP1 mRNA expression were measured by quantitative real-time PCR. PTBP1 protein level was measured by western blot analysis, n = 3. *p < 0.05, **p < 0.01 (Student’s t test).
Next, we used Pearson’s correlation analysis to identify the relationship between PTB-AS and PTBP1 expression and found that PTB-AS was robustly positively correlated with PTBP1 mRNA, with statistical significance established at p < 0.001. PTB-AS transcripts also correlated positively with the PTBP1 protein (p < 0.01, Figure 2C). Given the close genomic proximity of PTB-AS and PTBP1, we hypothesized that PTB-AS could exert biological effects by modulating PTBP1. We used knockdown and overexpression analyses to test the influence of PTB-AS on PTBP1. The target sites of small interfering RNAs (siRNAs) were carefully chosen to avoid the overlap region with PTBP1 mRNA, as shown in Figure S3A. Quantitative real-time PCR and western blot analysis were performed to examine the effects of PTB-AS on PTBP1. We found that knocking down PTB-AS using two different siRNAs in T98G, U87MG, and A172 glioma cell lines effectively downregulated PTBP1 at both the RNA and protein levels (Figures 2D, S3B, and S3C). Overexpressing PTB-AS in these three glioma cell lines resulted in significant upregulation of PTBP1 mRNA and protein (Figures 2E and S3D). To exclude the possibility of nonspecific effects on other PTB family members, we aligned the 3′ UTRs of the four PTB family members and found that the similarity was only 19.3% (Figure S3E). Therefore, we concluded that the expression of PTB-AS correlated positively with PTBP1. Here, we showed that manipulation of PTB-AS expression affected PTBP1 expression at both the RNA and protein levels, indicating that PTB-AS could specifically regulate PTBP1 expression in glioma.
PTB-AS Contributes to Glioma Cell Proliferation and Migration In Vitro and In Vivo
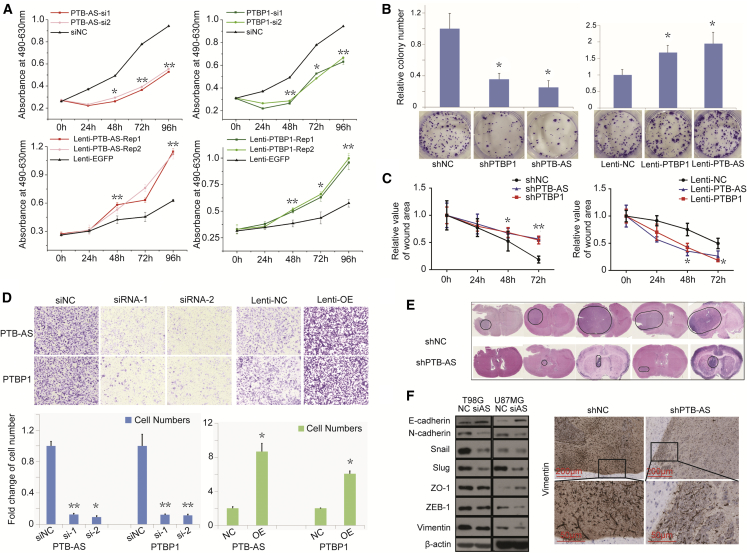
We investigated the biological functions of PTB-AS and PTBP1 by detecting their effects on proliferation and migration of glioma cells. We altered the expression of PTB-AS or PTBP1 by transfecting glioma cells with siRNA or plasmids. RNA or protein expression was detected to confirm transfection efficiency (Figures S4A and S4B). We also overexpressed or reduced PTB-AS or PTBP1 with a stable lentivirus transfection strategy (Figure S4C), and the established stable U87MG cells were used for later functional studies. 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS; Owen’s reagent) and colony-formation assays showed that decreasing the expression of either PTB-AS or PTBP1 significantly inhibited the growth of glioma cells, but overexpressing PTB-AS or PTBP1 partially abrogated this effect (Figures 3A, 3B, and S4D). However, flow cytometry analysis showed that the proportion of apoptotic cells was not obviously different compared with that of the control group (Figure S4E). Next, we studied whether PTB-AS could affect the migration of glioma cells. In the wound-healing assay, PTB-AS or PTBP1 knockdown or overexpressing cells were less motile or more dynamic than lenti-NC-infected cells at closing an artificial wound created over a confluent monolayer (48 and 72 h, **p < 0.01; Figures 3C and S4F). Directional migration was examined by Transwell assay, and we observed that knockdown or overexpression of PTB-AS and PTBP1 dramatically inhibited or promoted the migration of glioma cells, respectively, compared with that of the control cells treated with shRNA or lenti-NC (Figures 3D and S4G). Next, we aimed to address the relevance of these features in vivo. Intracranial orthotropic xenografts were established by implanting approximately 5 × 105 U87MG cells stably expressing either shPTB-AS (lenti-shPTB-AS) or the negative control lentivirus (lenti-shNC). H&E staining revealed that the PTB-AS-silenced glioma cells formed smaller tumors than the control cells (Figure 3E). Moreover, the cells showed obvious increases in epithelial marker level and reductions in mesenchymal markers or in expression of EMT-related transcription factors after knockdown of PTB-AS, followed by the weaker signal of Vimentin when PTB-AS was downregulated in vivo (Figure 3F). These results demonstrated that PTB-AS contributes to glioma proliferation in vitro and in vivo and promotes the migration of glioma cells.
Figure 3.
PTB-AS Could Significantly Promote the Proliferation and Migration of Glioma both In Vitro and In Vivo
(A and B) MTS and colony formation assays showing that PTB-AS, similar to PTBP1, promotes cell viability. (A) The growth curve of T98G cells after knocking down or overexpressing PTB-AS or PTBP1, following individual tests of the absorbance at 490 and 630 nm. (B) Colony formation images and bar charts of the number of colonies. Data are expressed as the mean ± SD, n = 5. *p < 0.05, **p < 0.01 (Student’s t test). (C and D) Wound-healing assays and Transwell migration assays showing that PTB-AS and PTBP1 both promote the migration of glioma cells. (C) The relative area of the remaining open wound calculated in relation to that at time 0 h. (D) The graphs indicate the average number of cells per field of the indicated cell lines in migration assays. Data are the mean ± SD. *p < 0.05, **p < 0.01 (Student’s t test). (E) In vivo assays were performed using shNC and shPTB-AS stable lentivirus infection U87MG cell suspensions (5 × 105 cells/5 μL). The cells were injected intracranially into five nude mice. H&E staining was processed after perfusion and paraffin preparation. The circles in the violet areas indicate the tumor. (F) An immunoblot analysis was performed to show the expression of mesenchymal markers, epithelial markers, and EMT-related transcription factors after knocking down PTB-AS, and representative immunohistochemistry was performed to detect Vimentin in shNC- and shPTB-AS-infected cells in vivo. Scale bars: 200 and 50 μm.
PTB-AS Binds to PTBP1-3′ UTR and Stabilizes PTBP1 mRNA
We performed a rescue experiment and found that overexpressing PTB-AS could upregulate the expression of PTBP1 under PTB-siRNA knockdown conditions. This change also reflected the functional level of glioma (Figures S5A–S5D). Under conditions in which robust PTB-AS elevated PTBP1 expression and influenced glioma cell proliferation and migration, we further investigated the molecular mechanism by which PTB-AS regulates the expression of PTBP1.
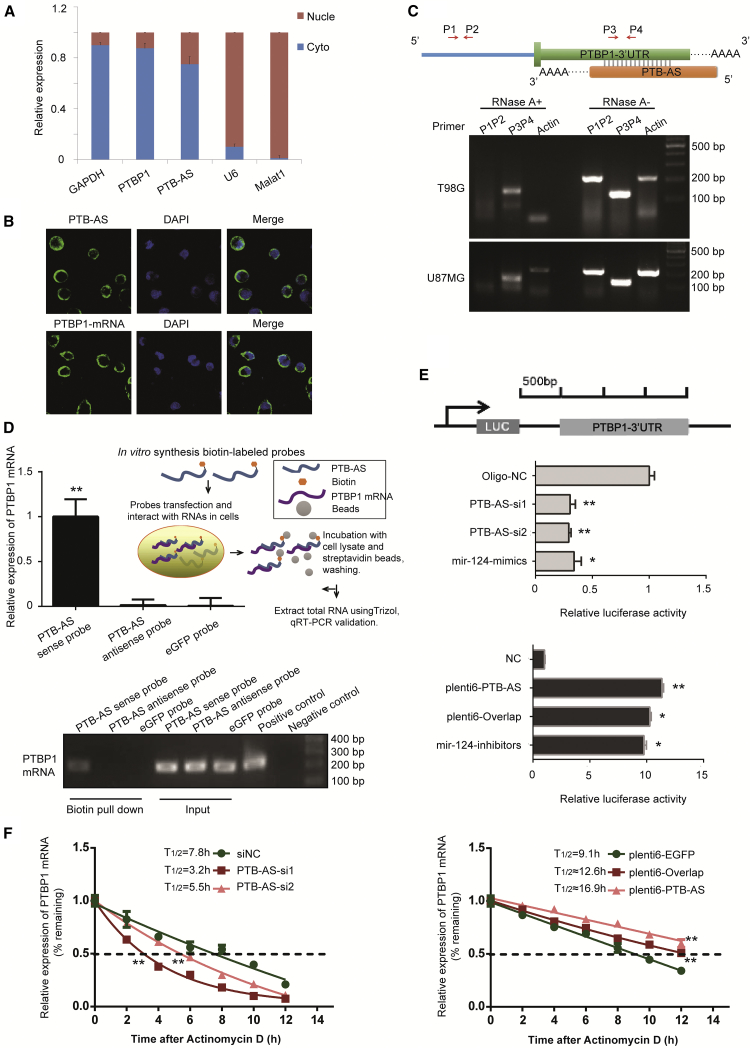
Since the location of lncRNAs generally determines their function, we performed (fluorescence) in situ hybridization ([F]ISH) and RNA fractionation of the nucleus and cytoplasm and showed that PTB-AS is mainly located in the cytoplasm, indicating the potential of post-transcriptional regulation (Figures 4A, 4B, and S5E). The reverse complementary sequence between PTB-AS and PTBP1 indicates that they may have a binding relationship. We then investigated whether PTB-AS could directly bind to the 3′ UTR of PTBP1. To detect the RNA duplex formed by PTB-AS and PTBP1, we performed an RNase protection assay (RPA). The results showed that multiple sites in the overlapping regions were protected from degradation, whereas the non-overlapping regions of PTBP1 and PTB-AS were almost completely degraded by RNase A (Figure 4C). This result indicated that significant proportions of PTB-AS indeed form RNA-RNA duplexes with PTBP1, possibly at the sites of the 3′ UTR genomic locus. The physical interaction between PTB-AS and PTBP1-3′ UTR was further validated by biotin-avidin affinity pull-down assays (brief flowchart shown in Figure 4D). Endogenous PTBP1 mRNA was pulled down by a biotin-labeled PTB-AS probe transcribed in vitro and was detected by quantitative real-time PCR and agarose gel analyses. The biotin-labeled EGFP probe served as the negative control (Figures 4D and S5F). A dual-luciferase assay was used to examine whether PTB-AS could interact with PTBP1-3′ UTR. Our results revealed that knockdown of PTB-AS significantly reduced the luciferase activity, whereas the activity was restored by PTB-AS overexpression (Figure 4E). The above results indicated that PTB-AS could interact with PTBP1-3′ UTR through direct binding.
Figure 4.
PTB-AS Binds to the 3′ UTR of PTBP1 in the Cytoplasm and Promotes PTBP1 mRNA Stability
(A) Relative expression of PTB-AS and PTBP1 in nuclear RNA and cytoplasmic RNA was detected by quantitative real-time PCR in U87MG cells. GAPDH and U6 or MALAT1 were used as the cytoplasmic and nuclear loading controls, respectively. Data are shown as the mean ± SD, n = 3. (B) Fluorescence in situ hybridization for PTB-AS and PTBP1 mRNA on slides through T98G cells. All images show the same magnification (×100, with oil-immersion lens). Scale bar: 100 μm. (C) A ribonuclease protection assay (RPA) was performed to identify the binding relationship between PTB-AS and PTBP1-3′ UTR. The diagram shows the primer position. P1P2 primers were located in the PTBP1-coding sequence region, and P3P4 primers were located in the PTBP1-3′ UTR where it overlapped with PTB-AS. RT-PCR was performed and showed the PCR products after treatment of glioma cells with RNase A. (D) Biotin-based RNA pull-down assays showed that PTB-AS bound to the PTBP1-3′ UTR. A schematic of the biotin pull-down process is shown on the right. U87MG cells were transfected with biotinylated full-length PTB-AS. The antisense probes or biotinylated EGFP acted as a negative control. PTBP1-3′ UTR expression levels were analyzed by quantitative real-time PCR, and PCR products were amplified by agarose gel electrophoresis. The positive control indicates the precise position of the target band and PCR product, which was performed with free cDNAs as the negative control. Data are shown as the mean ± SD of three independent experiments. *p < 0.05; **p < 0.01 (Student’s t test). (E) Luciferase assays revealed that PTB-AS could interact with the 3′ UTR of PTBP1. U87MG cells were separately infected with PTB-AS-siRNAs or the plenti6-PTB-AS vector, accompanied with the luciferase constructs of PTBP1-3′ UTR-pcDNA3.1-Luc or pRL-TK plasmids. The miR-124 group acted as a positive control; this molecule was reported to bind to the PTBP1-3′ UTR. The relative luciferase activity was analyzed, and the data are shown as the mean ± SD of three independent experiments. *p < 005, **p < 0.01 (Student’s t test). (F) The half-life experiments showed that PTB-AS stabilized PTBP1 mRNA. U87MG cells expressing control siRNA, PTB-AS siRNA or plasmids were treated with actinomycin D (5 μg/mL) for the indicated periods of time. Total RNA was purified and then analyzed by quantitative real-time PCR to examine the mRNA half-life of PTBP1. Half-life was calculated by using one-phase decay, and significant differences were calculated using one-way ANOVA. Data shown are the mean ± SD. n = 3. *p < 0.05; **p < 0.01 (two-tailed t test), and the analyses used nonlinear regression (one-phase decay curve fit) to determine the half-life.
Next, we investigated the ability of PTB-AS to regulate the stability of PTBP1 mRNA. U87MG cells were treated with actinomycin D (Act D) to block transcription. Knockdown of PTB-AS decreased the half-life of PTBP1 mRNA from 8.7 to 5.3 or 3.4 h, whereas the half-life was substantially prolonged after overexpression of PTB-AS (Figures 4F and S5G), indicating that PTB-AS stabilizes PTBP1 mRNA. Thus, the data indicated that PTB-AS directly binds to the PTBP1-3′ UTR and strongly enhances the PTBP1 mRNA stability.
PTB-AS Masks the Binding Site of miR-9 in the PTBP1 3′ UTR Locus
miRNAs are ncRNAs that negatively regulate the expression of target genes by binding to their 3′ UTRs. In previous work,25, 26, 27 PTBP1 was shown to be regulated by a series of miRNAs. Accordingly, we investigated whether the effect of PTB-AS on the stability of PTBP1 mRNA was also associated with miRNAs. We predicted miRNA binding at the PTBP1-3′ UTR by integrating the prediction results of the TargetScan, PicTar, Segal Lab, and MicroRNA.org webservers and finally obtained eight miRNAs, including miR-124 (as a positive control), which were reported to target PTBP1. The relative binding sites of the miRNAs are shown in Figure S6A.
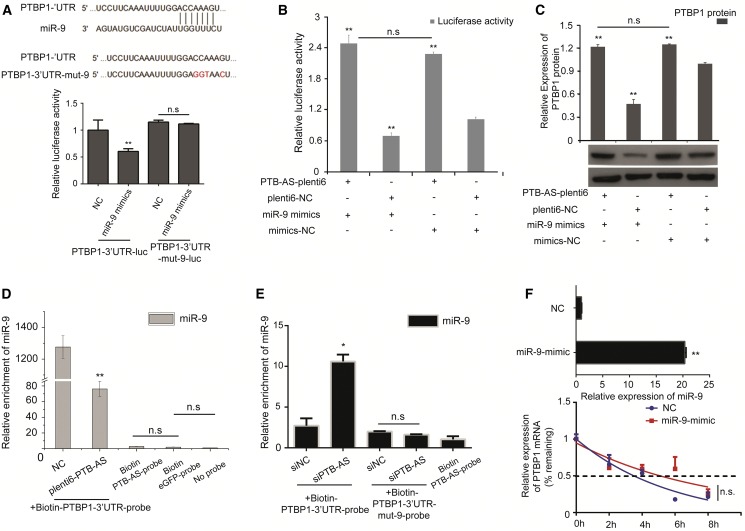
All the results indicate that the relative luciferase activity of PTBP1-3′ UTR was significantly decreased when miR-124, -9, -133, -1, -153, -137, and -429 mimics (which resulted in miRNA overexpression) were transfected, compared with oligo-NC (Figure S6B). Thereafter, we performed western blot analysis to further confirm that the PTBP1 protein level was strongly reduced by overexpressing miR-124, -9, and -133 and slightly reduced by miR-429 (Figure S6C). We concluded that miR-9, -133, and -429 targeted PTBP1 and that inhibition of these molecules could significantly increase the expression of PTBP1 (Figures S6D and S6E). We observed that miR-9 and miR-133 were upregulated in glioma cells compared with normal astrocytes (Figures S6F and S6G), and other miRNAs were downregulated in glioma cells (data not shown). We mutated the binding site of miR-9 at the PTBP1-3′ UTR locus and found that mut-miR-9 could not bind to PTBP1-3′ UTR (Figure 5A). These results suggest that PTBP1 is the target gene of miR-9.
Figure 5.
PTB-AS Contributes to the High Expression of PTBP1 by Masking the Binding Site of miR-9 in the PTBP1-3′ UTR
(A) PTBP1-3′ UTR-mut-9 was constructed to mutate the binding site of miR-9 in the PTBP1-3′ UTR. U87MG cells were separately infected with PTBP1-3′ UTR-luc and PTBP1-3′ UTR-mut-9-luc, all accompanied by transfection of the luciferase constructs of miR-9 mimic/mimic NC and pRL-TK plasmids. A luciferase assay was performed to analyze the relative luciferase activity, and the data are shown as the mean ± SD of three independent experiments. *p < 0.05; **p < 0.01 (Student’s t test); n.s., non-significant. (B) U87MG was cotransfected with miR-9 mimic and plenti6-PTB-AS. Mimic-NC and plenti6-NC were the negative controls. Luciferase reporter assays were performed to validate the failure of miR-9 to target and negatively regulate PTBP1, and the failure was found to be caused by overexpression of PTB-AS. The relative luciferase activity value is shown as the mean ± SD, n = 3. *p < 0.05, **p < 0.01 (Student’s t test). The results were from three biological replicates. (C) The western blot experiments were performed to validate the failure of miR-9 to regulate PTBP1, and the failure was found to be caused by overexpression of PTB-AS. Then, the results of the western blot assays were quantified by densitometry and are shown as the ratios of PTBP1 to β-actin protein levels (values in the right histogram). Relative values are shown as the mean ± SD, n = 3. *p < 0.05, **p < 0.01 (Student’s t test). The results were from three biological replicates. (D and E) U87MG cells were transfected with biotinylated full-length PTBP1-3′ UTR probe or PTBP1-3′ UTR-mut-9 probe accompanied by plenti6-PTB-AS/plenti6-NC (D) or siPTB-AS/siNC (E). The biotinylated PTB-AS was the control. The EGFP probe and no-probe group were the negative controls. Forty-eight hours after transfection, cells were harvested for biotin-based pull-down assays. RNA extraction and RT-PCR were performed. miR-9 expression levels were finally analyzed by quantitative real-time PCR. Data are shown as the mean ± SD of three independent experiments. *p < 0.05, **p < 0.01 (Student’s t test). (F) Half-life assay for PTBP1 mRNA after overexpression of miR-9. The mimic-NC or the miR-9 mimic was transfected into U87MG cells. Transcript decay curves were measured after transfection for 48 h when transcription was blocked by adding actinomycin D (5 μg/mL). Transcripts remaining relative to the control gene were assessed by quantitative real-time PCR. n.s., no significance. The analyses used nonlinear regression (one phase decay curve fit) to determine the half-life. Error bars, SD. The results were from three biological replicates.
To explore the relationship between PTB-AS and miR-9 in regulating PTBP1, co-overexpression of PTB-AS and miR-9 was performed. We found that the expression level, or luciferase activity, of PTBP1 was obviously elevated when co-overexpression of PTB-AS and miR-9 was performed compared with overexpression of miR-9 (Figures 5B and 5C). However, miR-133 did not appear to be a candidate effector of PTBP1 (Figure S6H). We inhibited miR-9 and found that knocking down PTB-AS could significantly rescue the PTBP1 protein expression (Figure S6I). A biotin pull-down assay showed that miR-9 binding to PTBP1-3′ UTR was significantly reduced when PTB-AS was overexpressed. In contrast, enhanced miR-9 enrichment by PTBP1-3′ UTR was observed due to PTB-AS knockdown (Figures 5D and 5E). Unfortunately, changing the expression of miR-9 had no significant effects on the half-life of PTBP1-mRNA in glioma cells (Figures 5F and S6J) and on account of this unexpected phenomenon, we aimed to explore the major mechanism of PTBP1-mRNA stability. The results suggested that miR-9 could not target PTBP1 when PTB-AS was inhibited, because of masking of its binding site in PTBP1-3′ UTR.
SND1 Is Essential for PTB-AS Interaction with PTBP1 mRNA and Improvement of Its Stability
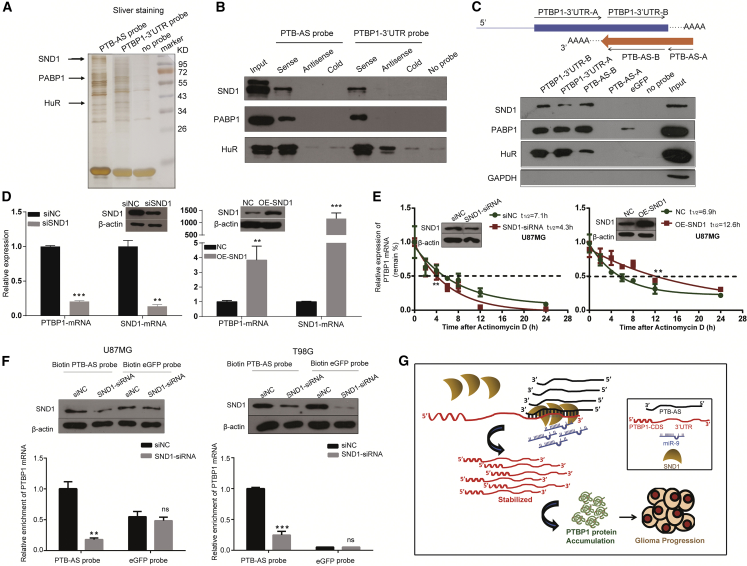
Antisense transcripts usually stabilize their target sense mRNAs through extended base pairing or physical interaction with mRNA-stabilizing proteins. While investigating the mechanism of PTB-AS-mediated stabilization of PTBP1-mRNA, we hypothesized that some RBPs may promote this double-strand RNA binding. We conducted an RNA pull-down assay and performed SDS-PAGE as well as silver staining, followed by mass spectrometry (MS) (Figure 6A). We aimed to identify proteins that bind to both PTB-AS and PTBP1-3′ UTR, and we obtained 87 peptides from 43 proteins (Table S5) from the intersection of the two groups after removing the background noise (Figure S7A).
Figure 6.
SND1, Which Is Necessary for the Interaction of PTBP1 mRNA and PTB-AS, Promotes PTBP1-mRNA Stability
(A) The search for RNA-binding proteins using affinity purification. Biotin-labeled probes corresponding to full-length PTB-AS or the 3′ UTR of PTBP1 were incubated with T98G cell lysates and analyzed by SDS-PAGE, and the proteins were then silver stained. The negative control group used no probe. The pull-down samples were analyzed by mass spectrometry. (B) RNA pull-down assays verified the binding capacity of candidate proteins to PTB-AS and PTBP1-3′ UTR in T98G cells. (C) Identification of specific regions in PTB-AS and PTBP1-3′ UTR bound by candidate proteins. The schematic representation of different segments of transcripts used as probes in affinity purification reactions is shown above. The biotin-labeled EGFP probe as the negative control probe did not bind to any proteins. The probes were incubated with T98G cell lysates. Input protein came from the cell lysates before probes were incubated. GAPDH, as a negative control protein, did not bind to any probes. (D) Knocking down or overexpressing SND1 dramatically changed the expression of PTBP1 mRNA. U87MG cells were infected with siRNA (or siNC) or plasmid (or empty plasmid) targeting SND1, to manipulate the expression of SND1. Western blot analysis demonstrated the SND1 protein level. PTBP1 and SND1 transcripts were measured by quantitative real-time PCR, n = 3. *p < 0.05; **p < 0.01, ***p < 0.001 (Student’s t test). (E) U87MG cells expressing control siRNA or SND1 siRNA- or mock-vector (NC) or pcDNA4.0-SND1 plasmid (OE-SND1) were treated with actinomycin D (5 μg/mL) for the indicated periods of time. Total RNA was analyzed by RT-qPCR to examine the mRNA half-life of PTBP1. Data shown are the mean ± SD, n = 3. *p < 0.05, **p < 0.01 (two-tailed t test). The analyses used nonlinear regression (one-phase decay curve fit) to determine the half-life. (F) Biotin pull-down demonstrates that knockdown of SND1 weakens the binding between PTB-AS and PTBP1-3′ UTR. Cell lysates of U87MG cells and T98G cells expressing control siRNA or SND1-siRNA were incubated with in vitro synthesized biotin-labeled PTB-AS probe or EGFP probe for the biotin pull-down assays, followed by real-time qPCR analysis to examine PTBP1 mRNA levels. Total proteins from cell lysates were subjected to western blot with SND1 antibodies. Data shown are the mean ± SD (n = 3). *p < 0.05, **p < 0.01, ***p < 0.001 (Student’s t test). (G) A schematic illustration of the proposed model depicting the role of PTB-AS in regulating PTBP1 mRNA stability. PTBP1 was significantly upregulated in glioma as a result of the dual-protection of PTB-AS; PTB-AS protected PTBP1 mRNA from inhibition by miR-9 through the masking effect; and SND1 dramatically stabilized PTBP1 mRNA through robustly enhancing the interaction of PTB-AS and PTBP1 mRNA.
Next, we chose the top three proteins that regulate RNA stability, which were SND1, ELAVL1 (Hu-antigen R [HuR]), and PABPC1 (PABP1). To evaluate these candidate proteins, we primarily performed nuclear and cytoplasmic extraction of U87MG cells and found that HuR, SND1, and PABP1 were detected in both the cytoplasm and the nucleus (Figures S7B and S7C). To validate the MS results, we performed an RNA pull-down assay, which showed that the biotin-labeled sense probes of both PTB-AS and PTBP1-3′ UTR could pull down these three proteins (Figure 6B).
We synthesized two-segment constitutive biotin-labeled RNA probes (A and B) of PTB-AS and PTBP1 mRNA, respectively, followed by an RNA pull-down assay to identify the primary specific region binding to the candidate RBPs. Finally, we found that only SND1 tended to bind the overlapping region (segments PTB-AS-B and PTBP1-3′ UTR-B) compared with HuR or PABP1. Glyceraldehyde phosphate dehydrogenase (GAPDH) was used as a negative control, and no GAPDH protein was pulled down by any of the probes (Figure 6C).
SND1, HuR, and PABP1 are well-known stability regulators that usually improve the stability of target genes. We first altered the levels of SND1, HuR, and PABP1 and detected PTBP1 expression. We found that after these three candidate proteins were knocked down, PTBP1 showed various changes. Decreasing SND1 could significantly downregulate the PTBP1 mRNA level, and increasing SND1 strongly upregulated the PTBP1 mRNA level (Figures S7D and S7E). However, PTBP1 mRNA expression was increased when PABP1 was reduced, and no significant differences were observed when HuR was knocked down (Figures S7F and S7G). We further investigated whether HuR, SND1, and PABP1 affect the stability of PTBP1 mRNA in glioma cells. SND1 substantially altered the half-life of PTBP1 mRNA in T98G and U87MG cells (Figures 6D and 6E). Interestingly, the half-life of PTBP1 mRNA did not change significantly after altering the expression of PABP1 or HuR. This result was not consistent with the phenomenon of maintaining mRNA stability (Figures S7H and S7I).
Based on the above results, we hypothesized that SND1 may promote PTB-AS to interact with PTBP1 mRNA and enhance its stability. Previous reports showed that SND1 has a preference for binding proteins or double-stranded RNA28 and can stabilize mRNA.29 Thus, we further explored whether SND1 is essential for the binding of the double-stranded RNA complex. We performed RNA pull-down assays after knocking down SND1 in glioma cells. As shown in Figure 6F, the enrichment of PTBP1 mRNA by the PTB-AS probe was significantly reduced when SND1 was deleted, indicating that SND1 could influence the binding capacity of PTB-AS and PTBP1-3′ UTR. To summarize, we investigated whether SND1 could influence the mRNA level of PTBP1 by altering the binding of the PTB-AS and PTBP1 RNA molecules.
Discussion
PTBP1, a multifunctional component of mRNA metabolism that affects splicing and mRNA stability, has been reported to be the first RBP that affects the invasive and rapid growth of glioma cells.30 However, few detailed mechanisms underlying the upregulated PTBP1 in glioma have been reported. In this study, we identified a novel overexpressed antisense lncRNA, PTB-AS, in glioma cells and elucidated its functional molecular mechanism. In our model, PTB-AS directly binds to target PTBP1 mRNA through extended base pairs on the 3′ UTR and masks the binding sites of miR-9. The masking of the miR-9 binding sites by PTB-AS leads to the derepression of miR-9 on the PTBP1 protein. SND1 acts as a clamp to stabilize the double-stranded RNA complex and is essential for maintaining PTBP1 mRNA stability. In this way, PTBP1 expression is elevated by the combined effects of SND1, PTB-AS, and miR-9. Finally, the entire regulatory pathway substantially contributes to gliomagenesis (Figure 6G).
Several molecules have been shown regulate PTBP1, such as miR-124.31, 32 miR-124 influences tumorigenesis by regulating the PTBP1/PKM1/PKM2 pathway.33 Furthermore, the lncRNA Tcl1 upstream neuron-associated (TUNA) is associated with PTBP1 and participates in recruiting the complex (hnRNP-K, NCL, and PTBP1) as a scaffold.34 Another lncRNA, Pnky, could bind to, but not influence, PTBP1 and regulate the expression and alternative splicing of a core set of transcripts in neural stem cells.35 Here, PTB-AS was shown to be a robust positive regulator of PTBP1 and is the first novel antisense lncRNA originating from the PTBP1 genomic locus to be investigated.
Growing evidence suggests that NATs play a key role in a range of human diseases, especially cancer.36 For example, brain-derived neurotrophic factor (BDNF)-AS and BACE1-AS participate in regulating the neuronal outgrowth and differentiation and development of Alzheimer’s disease through targeting their paired genes in the CNS, respectively.37 Additionally, a NAT can also share the bidirectional regulatory relationship with its paired gene, such as WDR83 and its NAT DHPS, which function in gastric cancer.38 Here, we identified a novel NAT lncRNA named PTB-AS with RACE, and northern blot analysis and revealed that the full-length PTB-AS is 923 nt with a Poly(A) tail structure, which has 595 nt of reverse complementary sequence with the 3′ UTR locus of PTBP1. Unlike TSLC1-AS, which was reported previously in glioma as a tumor suppressor,39 PTB-AS is upregulated in tumor cell lines or tissues and functions as an oncogenic NAT. The high expression of PTB-AS significantly promoted proliferation and migration in glioma. The main cause of upregulated PTB-AS in glioma deserves further research and may be linked to RNA modification. Furthermore, given the increasing expression level of PTB-AS with increased malignancy in glioma, this molecule may serve as a potential biomarker for glioma.
The mechanisms by which NATs regulate gene expression are various and complex and are categorized as those that act in cis or in trans. Generally, NATs can interact with protein complexes as decoys, scaffolds, or tethers or guides; participate in the generation of endogenous siRNAs and miRNAs or modulation of mRNA stability; and mediate the stabilization of long-range chromosomal interactions.40 We discovered that PTB-AS is coexpressed and colocalized with PTBP1 mRNA, and we speculated that it is a putative cis-regulatory factor. To investigate the regulatory mechanism of PTB-AS on PTBP1, we manipulated the expression of PTB-AS, demonstrating that it is closely associated with PTBP1 mRNA stability. Our findings suggested that PTB-AS may stabilize sense PTBP1 mRNA through extended base pairing at the PTBP1 3′ end. Further mechanistic investigation revealed that miR-9 and SND1 are involved in this process.
NATs regulate sense genes mainly through direct antisense-sense RNA interactions, and the most common pattern is RNA masking, in addition to RNA editing, transcriptional or RNA interference and chromatin changes.41 The “masking” of NATs provides a physical barrier against factors that may induce splicing or influence the stability of sense genes. In our study, we found that PTB-AS protects PTBP1 mRNA by masking the miR-9 binding site. Although this overlapping area can be targeted by several miRNAs, miR-9, with a high expression level in glioma cells, seems to be a key miRNA involved in the regulatory network. Evidence has proven that miR-9 is upregulated by CAMP responsive element binding protein (CREB) in glioma cells and negatively regulates the proliferation and migration of glioma.42 In addition, miR-9 could substantially enhance temozolomide resistance and tumor recurrence in GBM through targeting PTCH1, which negatively regulates Sonic Hedgehog (SHH) signaling.43, 44 Considering the inhibitory effect of miR-9 in glioma cells, we conjectured and verified that miR-9 could negatively interfere with PTBP1 and that the binding site of miR-9 in PTBP1-3′ UTR is masked by PTB-AS in glioma. This type of mechanism can be referred to as an “occupation effect,” which has been reported to influence the function of miRNAs.45 This is the first investigation to show that miR-9 regulates the expression of PTBP1 in humans, especially in cancer. However, we cannot explain whether the space-occupying effect of PTB-AS on miR-9 has selectivity yet. The in-depth mechanism of the high expression of miR-9, PTB-AS, and PTBP1 mRNA remains to be further verified.
SND1 is a multifunctional protein that is upregulated in different cancers. SND1 was initially characterized as a transcription coactivator that interacts with several specific transcription factors, including c-Myb,46 STAT6, and STAT5.28 SND1 is also involved in RNA maturation, including but not limited to spliceosome assembly, pre-mRNA splicing,47, 48 and RNA stability.29 In this study, we investigated whether SND1 possesses a new function. SND1 could stabilize the combination of lncRNA PTB-AS and its complementary mRNA.
Structurally, SND1 contains four repeats of staphylococcal nuclease-like domains (SN1–SN4) at the N-terminal followed by a Tudor domain and a fifth truncated SN domain at the C-terminal end.49 This particular structure allows the protein to interact with nucleic acids, individual proteins, and protein complexes in a promiscuous manner. Interestingly, our results showed that compared to HuR or PABP1, SND1 showed more specific binding to the double-stranded RNA structure formed by PTB-AS and PTBP1-3′ UTR in RNA pull-down assays. Thus, we hypothesized that the SN3 and SN4 domains of SND1 may act as a clamp to affect the binding of the duplex structure of PTB-AS and PTBP1-mRNA, which assists PTB-AS in regulating its target mRNA stability. Our results showed that HuR has a regulatory effect on PTBP1 mRNA stability, and thus, HuR cannot be ruled out completely. These results indicate that the regulation of PTBP1 is more complicated than we had anticipated, and the new functions of PTB-AS and RBPs need to be characterized.
Precision medicine is urgently needed. Therapeutic agents that potentially target lncRNAs mostly function via reducing the intracellular transcript levels of lncRNAs or attenuating their activities and molecular functions in cancer cells.50 Furthermore, potential pharmacological principles have suggested the application of NATs in gene therapy for glioma, and several strategies have been designed to use NATs as potential drug targets.51 Historically, lncRNAs can be easily inhibited by oligonucleotide-based drugs (ASO and others), and these molecules seem preferable to undruggable proteins for cancer therapy.52 The presence of lncRNAs (which show specific expression, complicated degradation pathways and little conservation) in biological fluids and their deep involvement in tumorigenesis make them ideal candidates for the development of efficient diagnostic assays;53, 54 therefore, the use of PTB-AS as a diagnostic marker will be easier and more sensitive than PTBP1 protein (antibody-based detection) because glioma tissue samples are generally limited. In addition, PTB-AS could be used for liquid biopsy based on RNA detection in peripheral blood or cerebrospinal fluid. Finally, the secondary structure of PTB-AS may offer clues for designing inhibitor-targeted PTBP1 molecules for glioma therapy applications. To summarize, our findings demonstrate the potential underlying mechanism of PTB-AS in the regulation of PTBP1 expression in glioma and possibly provide a therapeutic application.
Materials and Methods
Cell Culture
The two human normal astrocyte cell lines (HA-c and HA-sp) and glioma cell lines (T98G, U87MG, A172, HS-683, SF-126, SF-763, SF-767, SHG-44, CCF-STGG1, H4, LN-18, LN-229, and U118MG) mentioned in this article were purchased from the American Type Culture Collection (ATCC). The glioma cell line U251 was ordered from the Cell Center of Peking Union Medical College. T98G and U87MG were maintained in modified Eagle’s medium including 1 mM sodium pyruvate, 1% (vol/vol) non-essential amino acids (NEAA), 10% (vol/vol) fetal bovine serum (FBS), 5 mM L-glutamine, 100 U/mL penicillin, and 100 mg/mL streptomycin. The other cell lines were maintained in DMEM supplemented with 10% FBS, 5 mM L-glutamine, and dual antibiotics. The NHA cell line was purchased from the Lonza group and cultured with medium and reagents from Clonetics. The other HA cell line was purchased from ScienCell Research Laboratories and cultured with astrocyte medium (cat. no. 1801; ScienCell).
Tumor Tissues from Clinical Cases
A total of 83 glioma tissue samples and 6 control brain tissues (from the Department of Neurosurgery, Beijing Tiantan Hospital) were included in this research. Malignancy grade (14 samples were grade II, 25 samples were grade III, and 44 samples were grade IV) was defined according to the guidelines of the World Health Organization (WHO). The use of these human materials was in accordance with the policies of the institutional review board at Beijing Tiantan Hospital.
RACE
A172 total RNA was used to generate RACE-ready cDNA by using the commercial kit 5′&3′ RACE System for Rapid Amplification of cDNA Ends (cat. nos. 18374-058 and 18373-019; Invitrogen), according to the manufacturer’s protocol. cDNA ends were amplified with Universal Primer Mix and gene-specific primers. Placental RNA and receptor-specific primers provided by the manufacturer were used as controls. To ensure that we obtained distinct bands for the primary PCR, we performed a “nested PCR” with the nested universal primer and nested gene-specific primers shown in Table S4. Although the sample had a 3′ Poly(A) tail, for regent, we used the Poly(A) polymerase (cat. no. TAK2180; TaKaRa) to add a poly-adenylated tail to the total RNA of 3′ RACE. PCR products were then run in a 1.5% agarose gel, and DNA was extracted, cloned in the pGEM-T plasmid, and sequenced by Invitrogen.
Northern Blot Analysis
Total RNA, extracted using TRIzol reagent from U87MG glioma cells, was subjected to northern blot assays. The PTB-AS-specific DNA sequence (341 nt) was cloned into the pGEM-3zf vector. An RNA probe with a length of 341 nt was generated using digoxin (Roche) and the Riboprobe in vitro transcription labeling system (TaKaRa). The sense and antisense RNA probes were designed according to the principle that minimized nonspecific hybridization against mRNAs after homology searches using BLAST. A total of 3–10 μg of the indicated total RNA was subjected to formaldehyde gel electrophoresis and transferred to a Biodyne Nylon membrane (Pall, NY, USA). A digoxin-labeled PTB-AS sense or antisense probe was prepared. All of the procedures were strictly performed according to the manufacturer’s instructions (cat. no. 12039672910; Roche).
Identification of RNA Copies Using Absolute qPCR
First, the full-length of PTB-AS and PTBP1-CDS were cloned to pGEMT-3zf, then BamHI was used to digest and form the linearized plasmid template A (a serious cascade dilution rate was designed as 1, 1:10, 1:100, and 1:1,000). The standard curve was made according to the starting cycle value (Ct), using a template and aiming at indicating the good linear relationship between the template and the Ct value. Template B was reverse transcribed cDNAs from HA, T98G and U87MG cell lines (∼104 cells), and cDNAs were amplified once to form the double-stranded template in the same way as the linearized plasmid template. Fluorescence qPCR was performed using template A and B together to identify the starting copy numbers of PTB-AS and PTBP1. The calculating formula is as follows (Equation 1):
| (Equation 1) |
RNA Extraction and Quantitative Real-Time PCR
All total RNAs were isolated from corresponding treated cells with TRIzol reagent (Invitrogen) and were reverse transcribed by gene-specific primers (for PTB-AS), oligo(dT) primer (for PTBP1) or stem-loop Reverse-Transcript-PCR primer (for mature miRNAs). The RT process was performed (TransGene) to generate cDNA templates, strictly according to the manufacturer’s instructions. Quantitative real-time PCR was performed with a SYBR Green-containing PCR kit (TaKaRa), and the IQ5 sequence detection system (Applied Biosystems) according to the manufacturer’s instructions. RNA input was normalized to the level of human GAPDH mRNA and U6 snRNA (for miRNA detection) to determine relative gene expression. The primer sequences used for RT-PCR and quantitative real-time PCR are listed in Table S4.
Western Blot Analysis
All of the tumor tissues and treated or untreated glioma cells were harvested using TNTE lysis buffer (pH 7.4), containing 50 mM Tris-HCl, 150 mM NaCl, 0.5% Triton X-100, 1 mM EDTA, 1 mM Na3VO4, 25 mM NaF, and 10 mM Na4P2O7⋅10 H2O and protease inhibitors (5 μg/mL PMSF, 0.5 μg/mL leupeptin, 0.7 μg/mL pepstatin, and 0.5 μg/mL aprotinin). Proteins for immunoblotting were run in 8%–15% SDS-PAGE gels (Invitrogen) and transferred to nitrocellulose membranes. The nitrocellulose membranes were blocked with 5% skim milk in Tris-buffered saline-Tween 20 (TBS-T; 20 mM Tris-HCl [pH 7.4], 150 mM NaCl, 10 mM KCl, and 0.1% Tween 20) for at least 1 h. Antibodies were added in TBS-T containing 5% skim milk, and the blots were washed with TBS-T. Immunoblot signals were developed with an enhanced chemiluminescence reagent (GE Healthcare Bio-Sciences). The antibodies used in this study were as follows: rabbit anti-human PTBP1 (provided by our lab, 1:4,000 dilution) and mouse anti-human β-actin (1:8,000 dilution; cat. no. A5441; Sigma, USA).
Plasmids, shRNA, Lentivirus, siRNAs, and Transfection
The target sequences of siRNAs for PTB-AS and PTBP1 were designed and synthesized by Invitrogen, and the mimics of all miRNAs were synthesized by GenePharma. The cDNA target sequences of short hairpin RNAs (shRNAs) for PTBP1 were purchased from Thermo Fisher Scientific (cat. no. TRCN0000001062), and shRNA-oligos for PTB-AS were designed by us and synthesized by Invitrogen. The full-length PTB-AS and PTBP1-CDS and their overlapping region were cloned into the plenti6 vector. The PTBP1-3′ UTR was cloned into the pcDNA3.1-luc vector. The plasmid used to prepare the probe was segment-pGEM-3zf. All of the plasmids were purified using the EndoFree Plasmid Maxi Kit (QIAGEN) and transfected into T98G, U87MG, A172, or 293T cells using Lipofectamine 2000 (Invitrogen). siRNAs were transfected into glioma cell lines using INTERFERin (PolyPlus) at a final concentration of 100–200 nM.
Growth, Colony Formation, Fluorescence Activated Cell Sorting, Transwell Migration, and Scratch Wound-Healing Assays
T98G, U87MG, and A172 glioma cell line growth was assayed by using the standard MTS method. Twenty-four hours after transfection, the cells were maintained in serum-containing medium, and the number of surviving cells was determined by using the MTS method (G111B; Promega). In colony-formation assays, transfected or infected cells were plated in 12-well plates at 3,000 cells per well. Ten to 14 days later, the cell colonies were stained and counted. For apoptosis analysis, the transfected cells were harvested and assessed for apoptosis using an Annexin V-FITC-PI dual-staining kit (Abchem) by flow cytometry. In the Transwell migration assay, 5 × 104 to 1 × 105 T98G, U87MG, and A172 cells were plated on 8 μm Transwell filters (Corning) after transfection for approximately 24∼48 h. The cells were cultured with serum-free medium for 12 h and induced to migrate toward medium containing 10% FBS for about 24 h in the CO2 incubator. We use a cotton swab to remove non-migrating cells. The cells outside the well were fixed and stained using 0.1% crystal violet. An ix71 inverted microscope (Olympus) was used to count and image migrating cells. We chose four random fields to count and calculate the average number of cells. Scratch wound-healing assays were performed, and after the infected U87MG cells reached a confluence of 90% to 95%, a pipette tip was used to create the scratch wound. The suspended cells were removed by two washes with 1× PBS after scratching, following by addition of fresh medium to the cells. The cells were imaged at 0, 24, 48, and 72 h after scratching, and the migration status was calculated by measuring the wound areas with Nikon imaging software.
Xenograft Model in Nude Mice
The lentivirus-infected U87MG cells (1 × 107) in 100 μL 1× PBS were implanted into 4-week-old BALB/c athymic nu/nu mice (Vital River). Intracranial orthotopic xenografts were established by implanting 5× 105 lenti-U87MG stably transfected cells. The shNC-U87MG and shPTB-AS-U87MG stable transfectants were constructed by our lab. First, the BALB/c athymic nu/nu mice were anaesthetized, and we stereotactically implanted stably infected U87MG cells into the third ventricle (approximately 2 mm lateral and 0.5 mm anterior to the bregma; depth 1.5 mm from the dura). Approximately 1 month later, tumor size was quantified by H&E staining after perfusion. Paraffin-embedded sections of tumors were subjected to standard H&E staining.
ISH and FISH
Following the subcloning protocol, antisense digoxygenin-labeled probes were prepared and hybridized against fixed T98G, U87MG, and A172 cells. Wholemount ISH of glioma cell lines was performed, as previously described, with some modifications. The cells were observed with an optical microscope (ECLIPSE Ni-E; Nikon) and a confocal laser microscope (FV1000; Olympus).
Isolation of Cytoplasmic and Nucleic Cell Protein and RNA
Nuclear and cytoplasmic isolation was performed on U87MG cells using the SurePrep Nuclear or Cytoplasmic RNA Purification Kit (cat. no. BP2805-25; Fisher Bioreagents), according to the manufacturer’s protocols. Then, quantitative real-time PCR was used to identify the RNA levels. In summary, we used an equivalent volume of nuclear and cytoplasmic RNAs to perform RT-PCR to generate the cDNA template. Nuclear and cytoplasmic enrichment ratios were calculated to display the final result.
RNA Pull-Down Assays and Silver Staining
We collected the glioma cell lysate using nuclear and cytoplasmic isolation methods and then combined the cytoplasm and nucleic components. First, streptavidin sepharose was pretreated using RNA binding buffer (50 mmol/L KCl, 1.5 mmol/L MgCl2, 10 mmol/L HEPES [pH 7.5], 0.5% NP40, 2 mmol/L DTT, 1 mmol/L EDTA, 100 U/mL RNase inhibitor [40 U/μL], protease inhibitor, 100 μg/mL tRNA, and 400 μmol/L vanadyl ribonucleoside complexes). Then, we subpackaged 100–300 μL cell lysates for each sample and added 500–700 μL RNA binding buffer and 1–2 μg biotin-labeled probe. The mixture was well blended and formed an RNA-protein complex after incubation at 30°C for 30 min. Then, the RNA-protein mixture was combined with streptavidin sepharose, which had been treated and washed previously, followed by inverting shaking incubation at room temperature for 50 min. Finally, we used 30 μL PBS and 20 μL 6× loading buffer to suspend the streptavidin sepharose after six strict washes with RNA washing buffer (50 mmol/L KCl, 1.5 mmol/L MgCl2, 10 mmol/L HEPES [pH 7.5], and 0.5% NP40) and performed silver staining, MS, or western blot assays. Briefly, the silver staining assay was conducted as follows: an SDS-PAGE gel was run using the protein obtained through RNA pull-down and washed two times for 5 min each with ultrapure water; then, the gel was fixed (50% water:40% methanol:10% acetic acid solution) two times for 15 min each at room temperature or overnight at 4°C; the gel was washed using sensitization solution (30% ethanol, 2.72 g/40 mL sodium acetate, and 1.3 g/40 mL Na2S2O3) two times for 15 min each, and then washed 5 times for 5 min each in ultrapure water; the gel was incubated using stain working solution (0.1 g AgNO3/40 mL) for 30 min in darkness; the gel was washed using Developer Working Solution (1 g/40 mL Na3CO3; 10 μL 37% formaldehyde [40 mL]), and in situ observation was performed; and finally, the gel was washed with EDTA solution (10 mM) and photographed.
Liquid Chromatography-Tandem MS and Data Analysis
The lyophilized samples were redissolved in 0.1% formic acid (buffer A) before MS analysis. The samples were analyzed on a self-packing RP C18 capillary liquid chromatography (LC) column (75 μm × 100 mm, 1.9 μm). The eluted gradient was 5%–30% buffer B (0.1% formic acid, 99.9% ACN; flow rate, 0.3 μL/min) for 40 min. MS data were acquired in TripleTOF MS, using an ion spray voltage of 3 kV, curtain gas of 20 PSI, nebulizer gas of 30 PSI, and an interface heater temperature of 150°C. The precursors were acquired in 500 ms ranging from 350 to 1,250 Da, and the product ion scans were acquired in 50 ms ranging from 250 to 1,800 Da. A rolling collision energy setting was used. A total of 30 product ion scans were collected if they exceeded a threshold of 125 counts per second (counts/s) and with a +2 to +5 charge state for each cycle. The tandem MS (MS/MS) spectra were searched against the UniProt human database from the Uniprot website (https://www.uniprot.org:443/) using Proteinpilot software suite (v4.0, Absciex). Trypsin was chosen for cleavage specificity, with two being the maximum number of allowed missed cleavages. Carbamidomethylation of cysteine was set as fixed modifications. The searches were performed using a peptide tolerance of 0.05 Da and a product ion tolerance of 0.05 Da. A 1% false-positive rate at the protein level was used as a filter, and each protein contained at least two unique peptides.
RPA
T98G, U87MG, and A172 cells were cultured in 10 cm dishes and isolated and purified into nuclear and cytoplasmic components. We performed RNA extraction from the nuclear and cytoplasmic mixture using TRIzol regent and digested the samples with DNase I to remove DNA contamination. The purified RNA was treated with RNase A (Ambion) to digest the single-stranded RNAs in digestion buffer. Following RT-PCR, agarose gel analysis was performed to identify the binding ability and the protective effect of PTB-AS on PTBP1.
Biotin Pull-Down Assay
U87MG cells were transfected with the biotinylated PTB-AS or the PTBP1-3′ UTR probe (50 nM) for 48 h. Then, we harvested the cells using lysis buffer (20 mM Tris [pH 7.5], 200 mM NaCl, 2.5 mM MgCl2, 0.05% Igepal, 60 U/mL, SUPERase-In [TaKaRa], 1 mM DTT, and protease inhibitors [Roche]), and the lysate was placed on ice for 10 min. Fifty microliters of lysates was used as the input control. The remaining lysates were incubated with pre-prepared R10-Flammable streptavidin magnetic beads (GE Healthcare). To prevent nonspecific binding to RNA or protein complexes, we mixed the beads with RNase-free bovine serum albumin (BSA) and yeast tRNA (both from Sigma) when incubated in blocking lysates at 4°C for 3 h to generate probe-coated magnetic beads. Then, the samples were washed twice with ice-cold lysis buffer, three times with low-salt buffer (0.1% SDS, 1% Triton X-100, 2 mM EDTA, 20 mM Tris-HCl [pH 8.0], and 150 mM NaCl), and once with high-salt buffer (0.1% SDS, 1% Triton X-100, 2 mM EDTA, 20 mM Tris-HCl [pH 8.0], and 500 mM NaCl). The bound RNAs were extracted and purified with TRIzol reagent. The RNA complexes bound to the beads were eluted and extracted for quantitative real-time PCR.
Dual-Luciferase Reporter Assays
PTBP1-3′ UTR was amplified and cloned into pcDNA3.1-luc with the restriction endonuclease sites of XhoI and XbaI. U87MG cells (90% confluence) were cultured in 24-well plates (Corning). Then, PTB-AS-siRNAs, siNC, PTB-AS-plenti6, plenti6-EGFP, miRNA mimics or mimic-NC were cotransfected with PTBP1-3′ UTR-luc and pRL-TK (the internal control plasmid constitutively expressing Renilla luciferase) by Lipofectamine 2000-mediated gene transfer. The relative luciferase activity was normalized to Renilla luciferase activity 48 h after transfection. The detailed procedures were performed according to the manufacturer’s instructions. All transfection experiments were performed in triplicate.
Half-Life Assay
U87MG cells and T98G cells were treated with siRNAs targeting PTB-AS or SND1, PABP1, HuR, and control non-targeting siRNA or PTB-AS-plenti6 and pcDNA4.0-SND1 overexpression constructs and plenti6-EGFP vector or mock vector. For analysis of RNA stability, immediately after transfection for 36 h, cells were treated with Act D (5 μg/mL; Sigma-Aldrich), a drug that inhibits RNA polymerase to block the synthesis of new RNA. The cells were then harvested for total cellular RNA isolation at 0, 1, 2, 4, 6, 8, 10, 12, and 24 h after the addition of Act D and analyzed to measure the half-life of PTBP1 mRNA by quantitative real-time PCR. We performed three independent experiments for each data point.
Bioinformatics Analysis and miRNA Target Prediction
The coding potential of PTB-AS was predicted using the CPC and Coding Potential Assessment Tool (CPAT). The University of California, Santa Cruz (UCSC), gene browser was used to analyze the relative gene locus, transcription activity, histone modification, and other information on PTB-AS and PTBP1. Four target prediction databases (TargetScan, PicTar, Segal Lab, and MicroRNA.org) were used to analyze the potential target miRNAs of PTBP1.
Statistical Analysis
All experiments were performed at least in triplicate, and the data are expressed as the mean ± SD. All statistical analyses were performed with SPSS 13.0 for Windows (SPSS Inc., Chicago, IL, USA), and the data were analyzed with Student’s t test or by one-way ANOVA. The results were considered statistically significant at p ≤ 0.05. The Wilcoxon test was used to evaluate the statistical significance of the difference in the expression of PTB-AS or PTBP1 mRNA and protein. The chi-square test was used to determine correlations between PTB-AS and PTBP1 expression.
Author Contributions
L.Z., Q.W., W.H., and X.P. conceived and designed the experiments; L.Z., Q.W., Y.Q., X.R., F.W., L.L., and J.Z. performed the experiments; L.Z. and Q.W. mainly analyzed the data; B.Y., W.L., J.Z., T.J., B.Q., and J.Y. contributed reagents, materials, and analysis tools; and L.Z. and Q.W. wrote the manuscript.
Conflicts of Interest
The authors declare no competing interests.
Acknowledgments
This work was supported by grants from the National Key Research and Development Program of China (2016YFC0902500, 2016YFC0902502, and 2016YFA0100702), and National Sciences Foundation of China (31671316 and 31670789), CAMS Innovation Fund for Medical Sciences (CIFMS; 2016-I2M-1-001, 2016-I2M-2-001, 2016-I2M-1-004, 2017-I2M-2-004, 2017-I2M-3-010, and 2017-I2M-1-004). We thank members of the National Laboratory of Medical Molecular Biology (China) for their valuable input and support throughout this study. We thank Xiaochao Tan from MD Anderson Cancer Center and Xue Yuanchao from the Institute of Biophysics, Chinese Academy of Sciences, for reviewing and editing the manuscript.
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.ymthe.2019.05.023.
Contributor Information
Wei Han, Email: hanwei2012@ibms.pumc.edu.cn.
Xiaozhong Peng, Email: pengxiaozhong@pumc.edu.cn.
Supplemental Information
Document S2. Article plus Supplemental Information
References
- 1.Perdomo-Pantoja A., Mejía-Pérez S.I., Gómez-Flores-Ramos L., Lara-Velazquez M., Orillac C., Gómez-Amador J.L., Wegman-Ostrosky T. Renin angiotensin system and its role in biomarkers and treatment in gliomas. J. Neurooncol. 2018;138:1–15. doi: 10.1007/s11060-018-2789-5. [DOI] [PubMed] [Google Scholar]
- 2.Omuro A., DeAngelis L.M. Glioblastoma and other malignant gliomas: a clinical review. JAMA. 2013;310:1842–1850. doi: 10.1001/jama.2013.280319. [DOI] [PubMed] [Google Scholar]
- 3.Ouédraogo Z.G., Biau J., Kemeny J.L., Morel L., Verrelle P., Chautard E. Role of STAT3 in Genesis and Progression of Human Malignant Gliomas. Mol. Neurobiol. 2017;54:5780–5797. doi: 10.1007/s12035-016-0103-0. [DOI] [PubMed] [Google Scholar]
- 4.Uren P.J., Vo D.T., de Araujo P.R., Pötschke R., Burns S.C., Bahrami-Samani E., Qiao M., de Sousa Abreu R., Nakaya H.I., Correa B.R. RNA-Binding Protein Musashi1 Is a Central Regulator of Adhesion Pathways in Glioblastoma. Mol. Cell. Biol. 2015;35:2965–2978. doi: 10.1128/MCB.00410-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dreyfuss G., Kim V.N., Kataoka N. Messenger-RNA-binding proteins and the messages they carry. Nat. Rev. Mol. Cell Biol. 2002;3:195–205. doi: 10.1038/nrm760. [DOI] [PubMed] [Google Scholar]
- 6.Yang B., Hu P., Lin X., Han W., Zhu L., Tan X., Ye F., Wang G., Wu F., Yin B. PTBP1 induces ADAR1 p110 isoform expression through IRES-like dependent translation control and influences cell proliferation in gliomas. Cell. Mol. Life Sci. 2015;72:4383–4397. doi: 10.1007/s00018-015-1938-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han W., Xin Z., Zhao Z., Bao W., Lin X., Yin B., Zhao J., Yuan J., Qiang B., Peng X. RNA-binding protein PCBP2 modulates glioma growth by regulating FHL3. J. Clin. Invest. 2013;123:2103–2118. doi: 10.1172/JCI61820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Izaguirre D.I., Zhu W., Hai T., Cheung H.C., Krahe R., Cote G.J. PTBP1-dependent regulation of USP5 alternative RNA splicing plays a role in glioblastoma tumorigenesis. Mol. Carcinog. 2012;51:895–906. doi: 10.1002/mc.20859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sawicka K., Bushell M., Spriggs K.A., Willis A.E. Polypyrimidine-tract-binding protein: a multifunctional RNA-binding protein. Biochem. Soc. Trans. 2008;36:641–647. doi: 10.1042/BST0360641. [DOI] [PubMed] [Google Scholar]
- 10.Makeyev E.V., Zhang J., Carrasco M.A., Maniatis T. The MicroRNA miR-124 promotes neuronal differentiation by triggering brain-specific alternative pre-mRNA splicing. Mol. Cell. 2007;27:435–448. doi: 10.1016/j.molcel.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen M., Zhang J., Manley J.L. Turning on a fuel switch of cancer: hnRNP proteins regulate alternative splicing of pyruvate kinase mRNA. Cancer Res. 2010;70:8977–8980. doi: 10.1158/0008-5472.CAN-10-2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y., Li J., Kong L., Gao G., Liu Q.R., Wei L. NATsDB: Natural Antisense Transcripts DataBase. Nucleic Acids Res. 2007;35:D156–D161. doi: 10.1093/nar/gkl782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lapidot M., Pilpel Y. Genome-wide natural antisense transcription: coupling its regulation to its different regulatory mechanisms. EMBO Rep. 2006;7:1216–1222. doi: 10.1038/sj.embor.7400857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katayama S., Tomaru Y., Kasukawa T., Waki K., Nakanishi M., Nakamura M., Nishida H., Yap C.C., Suzuki M., Kawai J., RIKEN Genome Exploration Research Group. Genome Science Group (Genome Network Project Core Group) FANTOM Consortium Antisense transcription in the mammalian transcriptome. Science. 2005;309:1564–1566. doi: 10.1126/science.1112009. [DOI] [PubMed] [Google Scholar]
- 15.Khorkova O., Myers A.J., Hsiao J., Wahlestedt C. Natural antisense transcripts. Hum. Mol. Genet. 2014;23(Suppl R1):R54–R63. doi: 10.1093/hmg/ddu207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Villegas V.E., Zaphiropoulos P.G. Neighboring gene regulation by antisense long non-coding RNAs. Int. J. Mol. Sci. 2015;16:3251–3266. doi: 10.3390/ijms16023251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malhotra S., Freeberg M.A., Winans S.J., Taylor J., Beemon K.L. A Novel Long Non-Coding RNA in the hTERT Promoter Region Regulates hTERT Expression. Noncoding RNA. 2017;4:1. doi: 10.3390/ncrna4010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Q., Zhang J., Liu Y., Zhang W., Zhou J., Duan R., Pu P., Kang C., Han L. A novel cell cycle-associated lncRNA, HOXA11-AS, is transcribed from the 5-prime end of the HOXA transcript and is a biomarker of progression in glioma. Cancer Lett. 2016;373:251–259. doi: 10.1016/j.canlet.2016.01.039. [DOI] [PubMed] [Google Scholar]
- 19.Lv Q.L., Hu L., Chen S.H., Sun B., Fu M.L., Qin C.Z., Qu Q., Wang G.H., He C.J., Zhou H.H. A Long Noncoding RNA ZEB1-AS1 Promotes Tumorigenesis and Predicts Poor Prognosis in Glioma. Int. J. Mol. Sci. 2016;17:14. doi: 10.3390/ijms17091431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mineo M., Ricklefs F., Rooj A.K., Lyons S.M., Ivanov P., Ansari K.I., Nakano I., Chiocca E.A., Godlewski J., Bronisz A. The Long Non-coding RNA HIF1A-AS2 Facilitates the Maintenance of Mesenchymal Glioblastoma Stem-like Cells in Hypoxic Niches. Cell Rep. 2016;15:2500–2509. doi: 10.1016/j.celrep.2016.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang R., Jin H., Lou F. The Long Non-Coding RNA TP73-AS1 Interacted With miR-142 to Modulate Brain Glioma Growth Through HMGB1/RAGE Pathway. J. Cell. Biochem. 2018;119:3007–3016. doi: 10.1002/jcb.26021. [DOI] [PubMed] [Google Scholar]
- 22.Xu, G., Liu, C., Li, G., Yu, Z., Wu, M. New exosomal long intergenic non-coding RNA (lncRNA) PRKAG2 antisense RNA 1 (PRKAG2-AS1), used for early diagnosis or prognosis of glioma. Patent CN103966337 A, filed May 26, 2014, and granted August 6, 2014.
- 23.Van Meir E.G., Hadjipanayis C.G., Norden A.D., Shu H.K., Wen P.Y., Olson J.J. Exciting new advances in neuro-oncology: the avenue to a cure for malignant glioma. CA Cancer J. Clin. 2010;60:166–193. doi: 10.3322/caac.20069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fontana L., Rovina D., Novielli C., Maffioli E., Tedeschi G., Magnani I., Larizza L. Suggestive evidence on the involvement of polypyrimidine-tract binding protein in regulating alternative splicing of MAP/microtubule affinity-regulating kinase 4 in glioma. Cancer Lett. 2015;359:87–96. doi: 10.1016/j.canlet.2014.12.049. [DOI] [PubMed] [Google Scholar]
- 25.Xue Y., Ouyang K., Huang J., Zhou Y., Ouyang H., Li H., Wang G., Wu Q., Wei C., Bi Y. Direct conversion of fibroblasts to neurons by reprogramming PTB-regulated microRNA circuits. Cell. 2013;152:82–96. doi: 10.1016/j.cell.2012.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lages E., Guttin A., El Atifi M., Ramus C., Ipas H., Dupré I., Rolland D., Salon C., Godfraind C., deFraipont F. MicroRNA and target protein patterns reveal physiopathological features of glioma subtypes. PLoS ONE. 2011;6:e20600. doi: 10.1371/journal.pone.0020600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Engels B., Jannot G., Remenyi J., Simard M.J., Hutvagner G. Polypyrimidine tract binding protein (hnRNP I) is possibly a conserved modulator of miRNA-mediated gene regulation. PLoS ONE. 2012;7:e33144. doi: 10.1371/journal.pone.0033144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li C.L., Yang W.Z., Chen Y.P., Yuan H.S. Structural and functional insights into human Tudor-SN, a key component linking RNA interference and editing. Nucleic Acids Res. 2008;36:3579–3589. doi: 10.1093/nar/gkn236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paukku K., Kalkkinen N., Silvennoinen O., Kontula K.K., Lehtonen J.Y. p100 increases AT1R expression through interaction with AT1R 3′-UTR. Nucleic Acids Res. 2008;36:4474–4487. doi: 10.1093/nar/gkn411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheung H.C., Hai T., Zhu W., Baggerly K.A., Tsavachidis S., Krahe R., Cote G.J. Splicing factors PTBP1 and PTBP2 promote proliferation and migration of glioma cell lines. Brain. 2009;132:2277–2288. doi: 10.1093/brain/awp153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coutinho-Mansfield G.C., Xue Y., Zhang Y., Fu X.D. PTB/nPTB switch: a post-transcriptional mechanism for programming neuronal differentiation. Genes Dev. 2007;21:1573–1577. doi: 10.1101/gad.1575607. [DOI] [PubMed] [Google Scholar]
- 32.Hermansen S.K., Kristensen B.W. MicroRNA biomarkers in glioblastoma. J. Neurooncol. 2013;114:13–23. doi: 10.1007/s11060-013-1155-x. [DOI] [PubMed] [Google Scholar]
- 33.Taniguchi K., Sugito N., Kumazaki M., Shinohara H., Yamada N., Nakagawa Y., Ito Y., Otsuki Y., Uno B., Uchiyama K., Akao Y. MicroRNA-124 inhibits cancer cell growth through PTB1/PKM1/PKM2 feedback cascade in colorectal cancer. Cancer Lett. 2015;363:17–27. doi: 10.1016/j.canlet.2015.03.026. [DOI] [PubMed] [Google Scholar]
- 34.Lin N., Chang K.Y., Li Z., Gates K., Rana Z.A., Dang J., Zhang D., Han T., Yang C.S., Cunningham T.J. An evolutionarily conserved long noncoding RNA TUNA controls pluripotency and neural lineage commitment. Mol. Cell. 2014;53:1005–1019. doi: 10.1016/j.molcel.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramos A.D., Andersen R.E., Liu S.J., Nowakowski T.J., Hong S.J., Gertz C., Salinas R.D., Zarabi H., Kriegstein A.R., Lim D.A. The long noncoding RNA Pnky regulates neuronal differentiation of embryonic and postnatal neural stem cells. Cell Stem Cell. 2015;16:439–447. doi: 10.1016/j.stem.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Span P.N., Rao J.U., Oude Ophuis S.B., Lenders J.W., Sweep F.C., Wesseling P., Kusters B., van Nederveen F.H., de Krijger R.R., Hermus A.R., Timmers H.J. Overexpression of the natural antisense hypoxia-inducible factor-1alpha transcript is associated with malignant pheochromocytoma/paraganglioma. Endocr. Relat. Cancer. 2011;18:323–331. doi: 10.1530/ERC-10-0184. [DOI] [PubMed] [Google Scholar]
- 37.Faghihi M.A., Modarresi F., Khalil A.M., Wood D.E., Sahagan B.G., Morgan T.E., Finch C.E., St Laurent G., III, Kenny P.J., Wahlestedt C. Expression of a noncoding RNA is elevated in Alzheimer’s disease and drives rapid feed-forward regulation of bold beta-secretase. Nat. Med. 2008;14:723–730. doi: 10.1038/nm1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Su W.Y., Li J.T., Cui Y., Hong J., Du W., Wang Y.C., Lin Y.W., Xiong H., Wang J.L., Kong X. Bidirectional regulation between WDR83 and its natural antisense transcript DHPS in gastric cancer. Cell Res. 2012;22:1374–1389. doi: 10.1038/cr.2012.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qin X., Yao J., Geng P., Fu X., Xue J., Zhang Z. LncRNA TSLC1-AS1 is a novel tumor suppressor in glioma. Int. J. Clin. Exp. Pathol. 2014;7:3065–3072. [PMC free article] [PubMed] [Google Scholar]
- 40.Carninci P., Kasukawa T., Katayama S., Gough J., Frith M.C., Maeda N., Oyama R., Ravasi T., Lenhard B., Wells C., FANTOM Consortium. RIKEN Genome Exploration Research Group and Genome Science Group (Genome Network Project Core Group) The transcriptional landscape of the mammalian genome. Science. 2005;309:1559–1563. doi: 10.1126/science.1112014. [DOI] [PubMed] [Google Scholar]
- 41.Wight M., Werner A. The functions of natural antisense transcripts. Essays Biochem. 2013;54:91–101. doi: 10.1042/bse0540091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tan X., Wang S., Yang B., Zhu L., Yin B., Chao T., Zhao J., Yuan J., Qiang B., Peng X. The CREB-miR-9 negative feedback minicircuitry coordinates the migration and proliferation of glioma cells. PLoS ONE. 2012;7:e49570. doi: 10.1371/journal.pone.0049570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Munoz J.L., Rodriguez-Cruz V., Rameshwar P. High expression of miR-9 in CD133+ glioblastoma cells in chemoresistance to temozolomide. J. Cancer Stem Cell Res. 2015;3:e1003. doi: 10.14343/JCSCR.2015.3e1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Munoz J.L., Rodriguez-Cruz V., Walker N.D., Greco S.J., Rameshwar P. Temozolomide resistance and tumor recurrence: Halting the Hedgehog. Cancer Cell Microenviron. 2015;2:e747. doi: 10.14800/ccm.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Faghihi M.A., Wahlestedt C. Regulatory roles of natural antisense transcripts. Nat. Rev. Mol. Cell Biol. 2009;10:637–643. doi: 10.1038/nrm2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leverson J.D., Koskinen P.J., Orrico F.C., Rainio E.M., Jalkanen K.J., Dash A.B., Eisenman R.N., Ness S.A. Pim-1 kinase and p100 cooperate to enhance c-Myb activity. Mol. Cell. 1998;2:417–425. doi: 10.1016/s1097-2765(00)80141-0. [DOI] [PubMed] [Google Scholar]
- 47.Shaw N., Zhao M., Cheng C., Xu H., Saarikettu J., Li Y., Da Y., Yao Z., Silvennoinen O., Yang J. The multifunctional human p100 protein ‘hooks’ methylated ligands. Nat. Struct. Mol. Biol. 2007;14:779–784. doi: 10.1038/nsmb1269. [DOI] [PubMed] [Google Scholar]
- 48.Cappellari M., Bielli P., Paronetto M.P., Ciccosanti F., Fimia G.M., Saarikettu J., Silvennoinen O., Sette C. The transcriptional co-activator SND1 is a novel regulator of alternative splicing in prostate cancer cells. Oncogene. 2014;33:3794–3802. doi: 10.1038/onc.2013.360. [DOI] [PubMed] [Google Scholar]
- 49.Ponting C.P. P100, a transcriptional coactivator, is a human homologue of staphylococcal nuclease. Protein Sci. 1997;6:459–463. doi: 10.1002/pro.5560060224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li C.H., Chen Y. Targeting long non-coding RNAs in cancers: progress and prospects. Int. J. Biochem. Cell Biol. 2013;45:1895–1910. doi: 10.1016/j.biocel.2013.05.030. [DOI] [PubMed] [Google Scholar]
- 51.Wahlestedt C. Natural antisense and noncoding RNA transcripts as potential drug targets. Drug Discov. Today. 2006;11:503–508. doi: 10.1016/j.drudis.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 52.Khorkova O., Hsiao J., Wahlestedt C. Basic biology and therapeutic implications of lncRNA. Adv. Drug Deliv. Rev. 2015;87:15–24. doi: 10.1016/j.addr.2015.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Quinn J.J., Chang H.Y. Unique features of long non-coding RNA biogenesis and function. Nat. Rev. Genet. 2016;17:47–62. doi: 10.1038/nrg.2015.10. [DOI] [PubMed] [Google Scholar]
- 54.Huarte M. The emerging role of lncRNAs in cancer. Nat. Med. 2015;21:1253–1261. doi: 10.1038/nm.3981. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Document S2. Article plus Supplemental Information