Abstract
Glioblastoma (GBM) are lethal primary brain tumours whose pathogenesis is aided, at least partly, via a pro-tumorigenic microenvironment. This study investigated whether microglia, a cell component of the GBM microenvironment, mediates pro-tumorigenic properties via the action of cyclophilin A (CypA), a potent secretable chemokine and cytoprotectant that signals via the cell surface receptor, CD147. To this end, intracellular and secreted CypA expression was assessed in human primary microglia and BV2 microglial cells treated with the endotoxin, lipopolysaccharide (LPS) and the oxidative stress inducer, LY83583. We report that human primary microglia and BV2 microglia both express CypA and CD147, and that BV2 microglial cells secrete CypA in response to pro-inflammatory and oxidative stimuli. We also demonstrate for the first time that recombinant CypA (rCypA; 1nM–1000nM) dose-dependently increased wound healing and reduced basal cell death in BV2 microglial cells. To determine the cell–signalling pathways involved, we probed microglial cell lysates for changes in ERK1/2 and AKT phosphorylation, IκB degradation, and IL-6 secretion using Western blot and ELISA analysis. In summary, BV2 microglial cells secrete CypA in response to inflammatory and oxidative stress, and that rCypA increases cell viability and chemotaxis. Our findings suggest that rCypA is a pro-survival chemokine for microglia that may influence the GBM tumour microenvironment.
Keywords: Neuroscience, Biochemistry, Immunology, Neurology, Proteins, Microglia, Cyclophilin A, CD147, Glioblastoma, Inflammation, Oxidative stress
1. Introduction
Glioblastoma (GBM; WHO: grade IV astrocytoma) is the most common, aggressive, and lethal form of astrocytoma. This tumour is typified by progressive and diffuse cancer cell infiltrative properties within the brain, and hence the inability of complete surgical resection of the tumour. Although concomitant radiotherapy and chemotherapy can prolong patient survival, the tumour will invariably recur, hence the need to identify new therapeutic targets critical for GBM tumour progression. To this end, GBM are multicellular in nature due to the infiltration of microglia and macrophages, which comprise up to 30% of the tumour mass (Graeber et al., 2002; Watters et al., 2005), and are thought to play a role in tumour invasiveness (Wu and Watabe, 2017). Glioma cells secrete a variety of growth factors, cytokines, and matrix proteases, which facilitate the recruitment of microglia, as well as influence their role within the tumour environment (Graeber et al., 2002; Hoelzinger et al., 2007; Watters et al., 2005). One potentially pro-tumorigenic factor that is over-expressed in GBM is cyclophilin A (CypA); a multifunctional protein whose elevated intracellular levels are associated with adverse outcomes in many cancers including GBM (Han et al., 2010; Handschumacher et al., 1984; Ryffel et al., 1991; Willenbrink et al., 1995). Moreover, we recently reported that Kaplan-Meier analysis of two large GBM datasets demonstrated that high CypA expression leads to a relative reduction in patient survival of approximately 3 months (Matthews et al., 2018).
CypA is an abundant cellular protein that was first discovered as the main target for the immunosuppressive drug, cyclosporine A (Handschumacher et al., 1984; Ryffel et al., 1991; Willenbrink et al., 1995). Among its functions, CypA contributes to protein folding and assembly and intracellular trafficking, and the regulation of cytokine gene expression (Handschumacher et al., 1984; Ivery, 2000; Kofron et al., 1991). In response to some pathological conditions, such as inflammation, hypoxia, and oxidative stress (Jin et al., 2000; Obchoei et al., 2015; Seko et al., 2004; Sherry et al., 1992; Suzuki et al., 2006), CypA is secreted into the extracellular environment. Extracellular CypA is a potent chemokine that induces chemotaxis in monocytes, neutrophils, eosinophils, and T cells (H. Kim et al., 2005; L. Wang et al., 2010). Interestingly cyclosporine A reportedly inhibits GBM cell proliferation in organotypic rat brain slices and mouse brains (Sliwa et al., 2007). Furthermore, cyclosporine A has been shown to enhance the chemotherapeutic effects of cisplatin and to induce apoptosis in glioma cells (Han et al., 2010; Zupanska et al., 2005).
Extracellular CypA can stimulate the ERK1/2, p38/MAPK, JNK, AKT/PKB, and NFκB signalling pathways via CD147, the main receptor for CypA (H. Kim et al., 2005; Kim et al., 2009). CD147 is frequently over-expressed in cancerous tissues, with up to 86% of tumours testing positive for CD147 (Riethdorf et al., 2006). Likewise, CD147 expression is elevated in human glioblastomas, with higher CD147 expression being associated with poorer patient outcomes (Riethdorf et al., 2006; Tian et al., 2013; M. Yang et al., 2013). CypA-induced CD147 receptor signalling can result in biological effects such as increased Bcl-2 expression, production of matrix metalloproteases (MMPs), cell migration, cell proliferation and cell differentiation (Jin et al., 2000; H. Kim et al., 2005; Seko et al., 2004; Sherry et al., 1992). Moreover, exogenous CypA has been shown to promote cell proliferation through ERK1/2 and p38 MAPK signalling in lung cancer, pancreatic cancer cell lines (Howard et al., 2005; Li et al., 2006; H. Yang et al., 2007) and via CD147 in cholangiocarcinoma cells (Obchoei et al., 2015).
The aims of this study were to examine if extracellular CypA plays a role in promoting microglial cell viability and chemotaxis, and whether microglia secrete CypA. We therefore examined the expression of CypA and CD147 proteins in human and murine microglial cell lines, pro-cell survival signalling events (ERK1/2 and AKT), and cellular effects (migration/chemotaxis and proliferation/viability) of exogenous recombinant CypA (rCypA) in the BV2 murine microglial cell line. We also investigated whether CypA is secreted by BV2 microglial cells and human primary microglia in response to lipopolysaccharide (LPS) and oxidative stress.
2. Materials and methods
2.1. Generation of purified recombinant CypA protein (rCypA)
A pET28a plasmid expression vector encoding the rat CypA sequence 6xHis-tag was transformed into E. coli KRX cells (Promega, USA), with protein induction as previously described (Kanyenda et al., 2014). Briefly, cells were homogenised and rCypA protein was purified by Immobilized Metal Affinity Chromatography (IMAC) using Ni-NTA Superflow Cartridges (Qiagen, Germany) and dialysed in dialysis buffer using Slide-A-Lyser cassettes (Thermo Scientific, USA). Endotoxin was removed by filtration using a Mustang-E Membrane (Pall Corporation, USA) and protein purity verified by coomassie staining of SDS-PAGE gels. Cyclophilin isomerase activity was confirmed by the Kofron method (Kofron et al., 1991). Endotoxin testing by the limulus amebocyte lysate (LAL-Pyrotell T) method confirmed levels were routinely below 0.5 EU/ml.
2.2. Primary human microglia, cell culture, stimulations and signalling
Primary microglia were purified from 14 to 19-week-old aborted human foetuses collected after therapeutic termination and purified as previously described (Guillemin et al., 2005). Consent was obtained prior to collection of tissue, with experimentation approved by the Macquarie University Human Research and Ethics Committee (Approval number: REF 5201300330).
BV2 microglia cells were cultured in DMEM (Life Technologies, Australia) supplemented with 5% foetal bovine serum (FBS), penicillin (0.05 mg/mL), streptomycin (0.1 mg/mL) and maintained in a CO2 (5%) incubator at 37 °C. For cell signalling studies, BV2 cells were seeded at 1.6 × 105 cells per well (24 well plate) and treated with LPS (1 μg/mL, Sigma, USA), ROS generator 6-anilinoquinoline-5,8-quinone (LY83583, Sigma), or rCypA protein. For CypA secretion studies, culture supernatants were centrifuged, transferred to fresh tubes and stored at -80 °C prior to use. Cells were lysed using cytosolic extraction buffer (10 mM HEPES; 3 mM MgCl2; 14 mM KCl; 5% glycerol; 0.2% IPEGAL) containing phosphatase and protease inhibitors (Roche, USA). Cell lysates were cleared, protein concentration calculated using protein assay solution (Bio-Rad, Australia), and the supernatants stored at -80 °C prior to use.
2.3. Western blotting
Western blot analysis was completed as previously described (MacDougall et al., 2017). Briefly, total cell proteins were separated by SDS-PAGE, transferred to PVDF membranes and analysed using the Gel Doc EZ imager (Bio-Rad). Membranes were blocked in TBS-Tween containing ovalbumin (0.1%), followed by incubation with primary antibodies (pERK, Total-ERK, and CD147 Santa Cruz, USA; pAKT, total AKT and IκB Cell Signalling Technology, USA; β-tubulin, Abcam, United Kingdom; CypA, Biomol, Germany; all antibodies at 1:5000 dilution) overnight at 4 °C with gentle rocking. Membranes were washed and probed with anti-mouse or anti-rabbit IgG horseradish peroxidase conjugated secondary antibodies (GE Lifesciences, USA). Immunoreactive bands were visualized using ECL Plus (Amersham) chemiluminescent detection reagent and quantified by densitometry. Total loaded protein levels in gel lanes were also determined by visualising the loaded proteins on TGX stain free gels (Bio-Rad) using the Gel Doc EZ imager (Bio-Rad).
2.4. IL-6 cytokine expression
BV2 cells were incubated overnight in DMEM without serum, then treated with rCypA (100 nM) for a period of 24 h. Cytokine secretion of IL-6 was determined by ELISA (BD OptEIATM; BD Biosciences, USA) and compared to cells treated with vehicle.
2.5. Cell proliferation assays
BV2 microglial cells were seeded at 5 × 103 cells/well (24 well plate format) and incubated at 37 °C (5% CO2) for 24 h. Following incubation, cells were treated with vehicle (PBS) or rCypA (final concentration 1 nM, 10 nM or 100 nM), and incubated at 37 °C (5% CO2) for the required time points (24, 48 and 72 h). Cells were trypsinised with 100 μL of TrypLE express (Life Technologies) and combined with an equal mixture of trypan blue. Viable (trypan blue exclusion) and dead cells (trypan blue positive) were counted using a haemocytometer.
2.6. Scratch wound healing assays
BV2 microglial cells were seeded in 24 well plates, grown to 90% confluency, and washed twice with serum-free DMEM prior to experimentation. The cell monolayer was lesioned 3 times in the same location using a sterile P20 pipette tip to induce an artificial wound. The remaining cells were washed with serum-free DMEM before adding 1 mL of DMEM (2% FBS) containing rCypA protein (10, 100, or 1000 nM) or vehicle (PBS) to each well. Cultured cells were incubated at 37 °C (5% CO2) for 0, 6 and 24 h post injury. Wound closures were analysed using the Scratch Edge Detection Macro feature of Image-J (NIH) software and expressed as percentage area reduction.
2.7. Statistics
Data are presented as mean ± SEM of triplicate wells (experiments were repeated independently three times). Statistical analysis was performed by ANOVA, followed by Tukey multiple comparison or Dunnet post-hoc tests. A value of p < 0.05 was considered to be statistically significant.
3. Results
3.1. CypA and CD147 proteins are expressed by cultured primary human and BV2 microglial cells
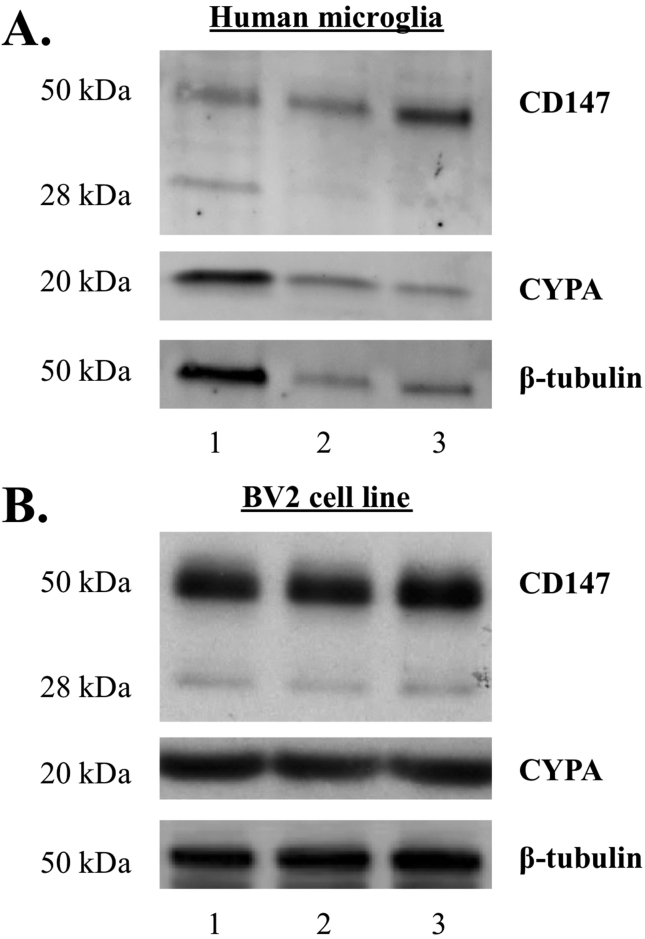
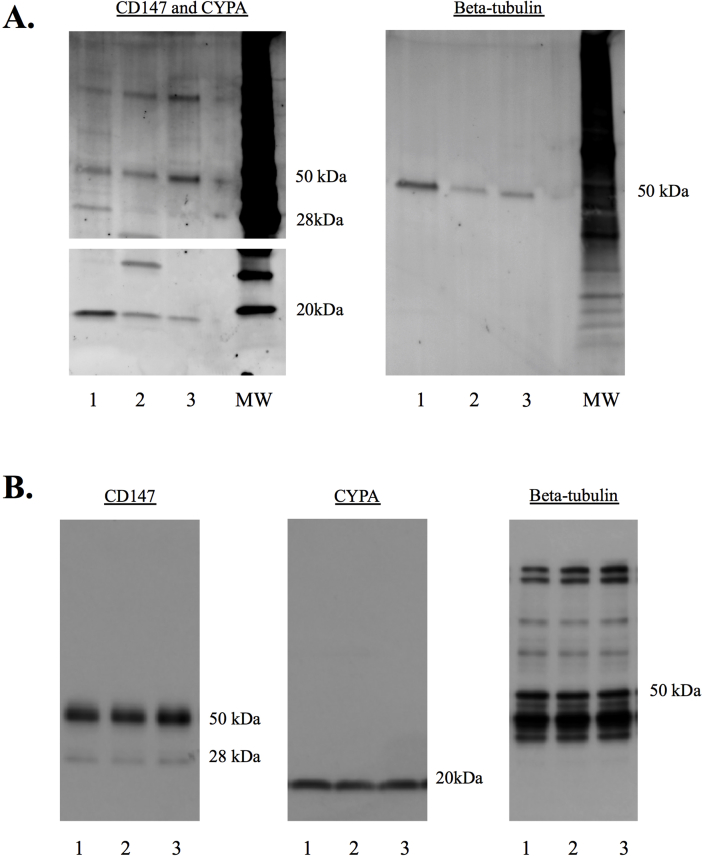
Western blot analysis of three human microglial cell lines and BV2 cell lysates revealed a ∼18kDa protein consistent with the predicted molecular weight of CypA (Figure 1A, B). In addition, the three human microglial cell lines and BV2 cell lysates revealed a ∼30kDa and a ∼50kDa protein for CD147. The CD147 protein is 27kDa in molecular weight and the higher molecular weight protein is likely to represent a glycosylated form of CD147 (Figure 1A, B).
Fig. 1.
Western blot analysis of primary microglia and BV2 microglia cell lysates (A) Intracellular CypA (∼18kDa) and glycosylated CD147 (28–50kDa) expression was detected in human primary microglial and (B) BV2 cell lysates, with loading controlled for using β-tubulin protein expression. N = 3. Original uncropped images of blots are shown in Fig. 1 are presented in Supplementary Fig. 1.
3.2. CypA secretion and ERK1/2 activation in normal BV2 microglia cultures and in cultures subjected to inflammatory (LPS) and oxidative stress (LY83583)
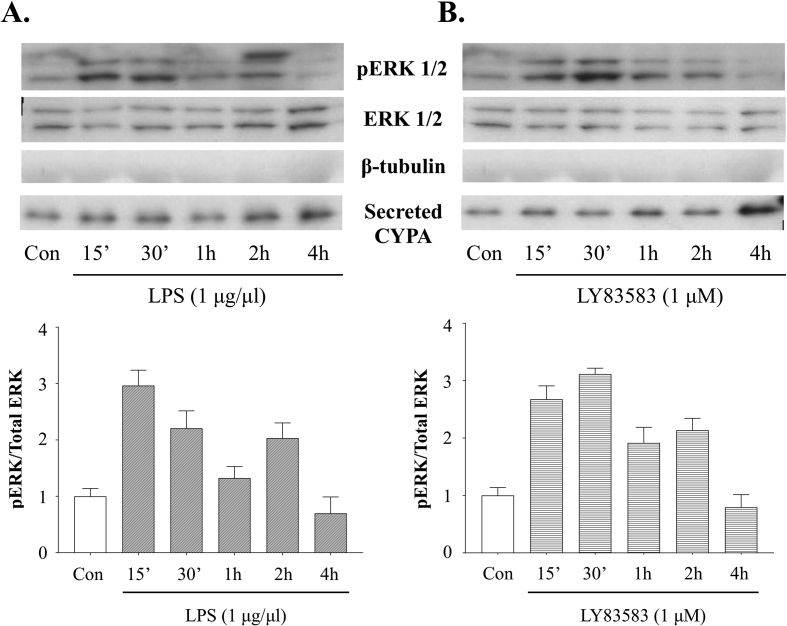
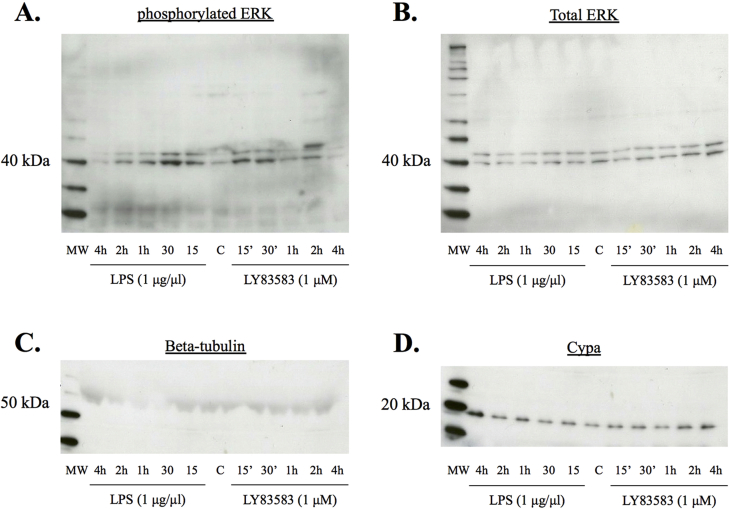
Western analysis of BV2 culture supernatants revealed that the cells constitutively secrete CypA, and that LPS treatment increases CypA secretion 2 and 4 h after exposure (Fig. 2). Likewise, LY83583 treatment increases CypA secretion 4 h after exposure (Fig. 2). Importantly, we did not detect the intracellular cytoskeletal protein β-tubulin in the culture supernatant, thereby confirming that CypA was actively secreted and not the result of cell lysis (Fig. 2). In addition, exposure of BV2 microglia cell cultures to LPS markedly increased ERK1/2 phosphorylation at 15 min, 30 min and 2 h post-exposure, while LY83583 exposure particularly increased ERK1/2 phosphorylation at 15- and 30-minutes post-exposure.
Fig. 2.
Western blot and densitometric analysis of BV2 cell lysates and conditioned media following treatment with (A) LPS or (B) LY83583 for 0–4 h. Blots and densitometry show an increase in pERK1/2 signal at 15min following LPS treatment, when controlled against total ERK1/2. Secreted CypA was detected in conditioned media peaking at 2h and 4h, following LPS treatment. When treated with LY83583, BV2 cell lysates showed a pERK1/2 signal peaking at 30min, when controlled against total ERK1/2 levels. Following LY83583 treatment, an increase in CypA secretion was detected at 4h. The absence of the cytoplasmic protein β-tubulin confirmed conditioned media was free of cell lysate and that the CypA was actively secreted. Con = untreated control. Values are presented as mean +/-SE; N = 3. Original uncropped images of blots are shown in Fig. 2 are presented in Supplementary Fig. 2.
3.3. Recombinant CypA increases the viability but not the rate of cell proliferation of BV2 microglial cells under basal conditions
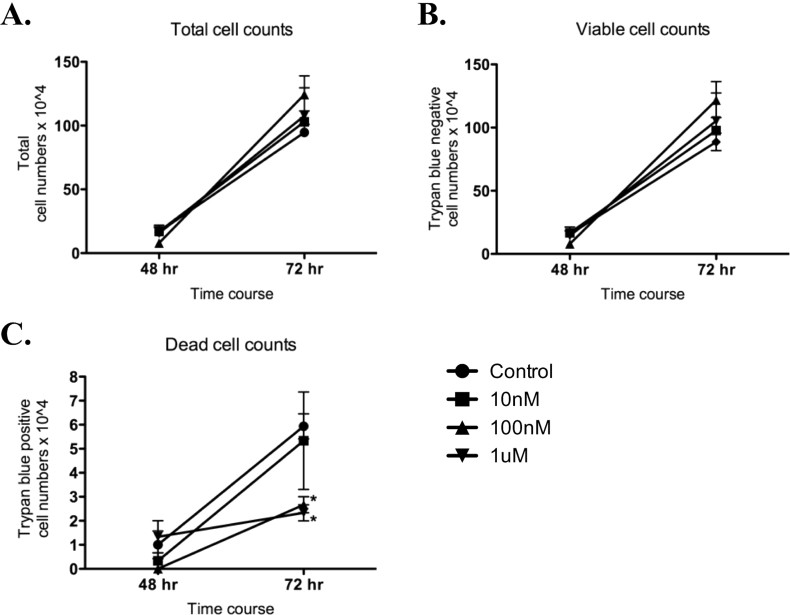
rCypA at 100 and 1000 nM, but not 10 nM significantly reduced BV2 microglia cell death (trypan blue positive cells) at 72 h post-treatment (p < 0.05) when compared to the vehicle treated control (Fig. 3). However, total and viable BV2 cell numbers was not significantly different following exposure to rCypA at the 10, 100 and 1 μM concentrations, suggesting the rate of proliferation was not altered.
Fig. 3.
Recombinant CypA protein decreases basal cell death but does not affect cell proliferation in BV2 microglial cells. Total cell counts of BV2 cell cultures after treatment with vehicle or rCypA protein (A) and viable cell counts (by trypan blue discrimination) of BV2 cell cultures after treatment with vehicle or rCypA protein (B). Total cell numbers (viable and dead cells) increased across all treatments, and rCypA protein addition did not significantly increase cell proliferation. There was no significant increase in viable cells at 48 and 72h post rCypA protein addition compared to vehicle treated cultures (C) Trypan blue positive dead cell counts at 48h and 72h post treatment. Addition of rCypA protein (10 nM and 100 nM) decreased basal cell death at 72h post treatment. Values are presented as mean +/-SE; N = 3, *p < 0.05.
3.4. Recombinant CypA increases BV2 microglial cell migration in a scratch wound healing assay
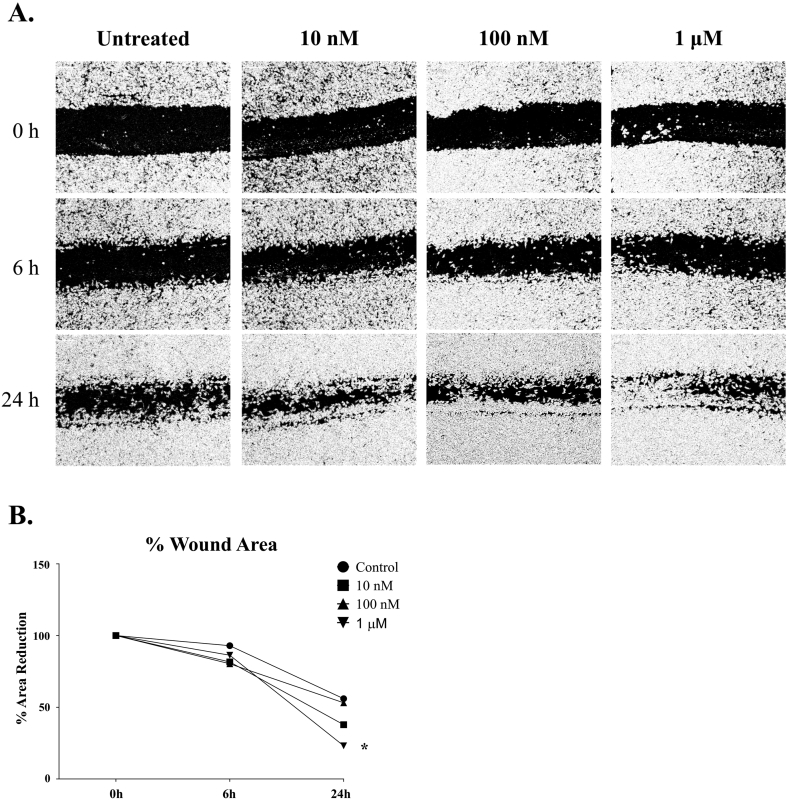
Due to the chemotactic effects of CypA, a scratch wound healing assay was used to determine if CypA could induce the chemotaxis of microglia. In the wound-healing assay, treatment of BV2 microglia with rCypA appeared to increase the rate of wound-gap closure at 6- and 24-hours post-treatment, however only treatment at the 1 μM concentration reached statistical significance compared to the untreated control (p < 0.05; Fig. 4).
Fig. 4.
Recombinant CypA protein enhances BV2 cell migration in a wound-healing assay. A sub-confluent layer of BV2 cells was inflicted with a wound and the resulting gap was imaged at the time of the wound and after 6h and 24h post treatment (A) Images are representative of a typical experiment repeated 3 independent times. The percentage of wound-gap was analysed by the Image J software, graphed and statistically analysed (B). At 24h, cultures treated with rCypA at the 1 μM showed enhanced gap closure compared to the vehicle treated cultures. post treatment (p < 0.05). Values are presented as mean +/-SE; N = 3, *p < 0.05.
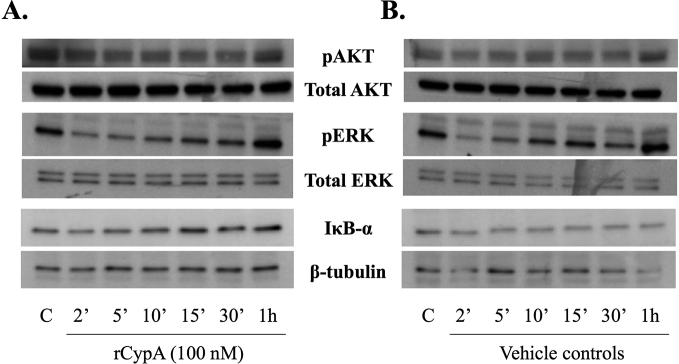
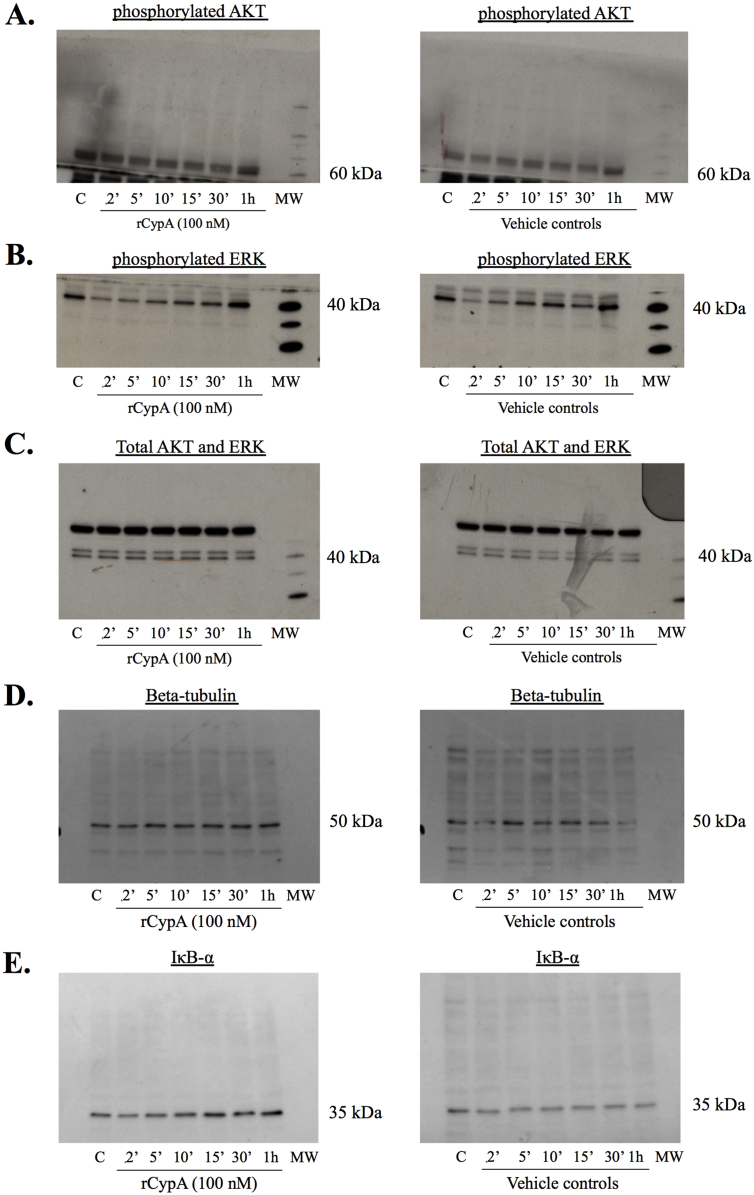
3.5. Recombinant CypA did not activate the AKT, ERK 1/2 or NF-κB pathway in BV2 microglial cells
Following the exposure of cell cultures to rCypA (100 nM), Western blot analysis of BV2 microglia cells lysates revealed no increases in the level of phosphorylated ERK1/2 or AKT compared to vehicle control. Similarly, rCypA treatment did not appear to cause IκB degradation in BV2 cells (Fig. 5).
Fig. 5.
Representative Western blots of rCypA treated BV2 cell lysates with showing no activation of the ERK 1/2, AKT and NFκB signalling pathways (A) Recombinant CypA protein (100 nM) did not activate ERK 1/2 or AKT phosphorylation in the BV2 cells when compared to the time point controls. No IκB degradation was observed following rCypA treatment (100 nM), indicating no activation of the NFκB signalling pathway. The PBS vehicle was used for time point controls in these cell-signalling experiments. C = vehicle control. N = 3. Original uncropped images of blots are shown in Fig. 5 are presented in Supplementary Fig. 3.
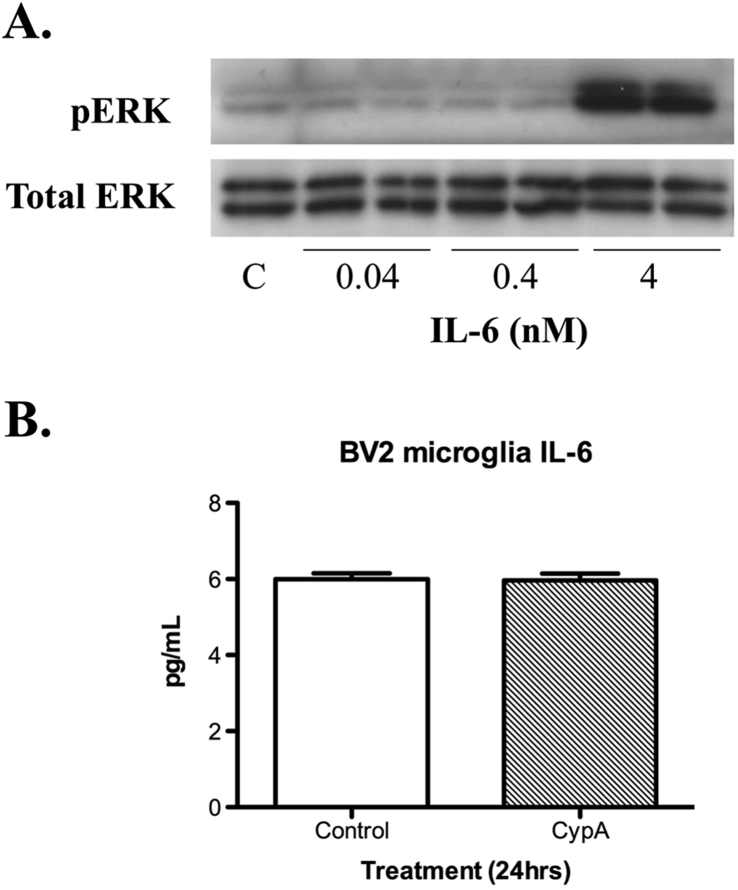
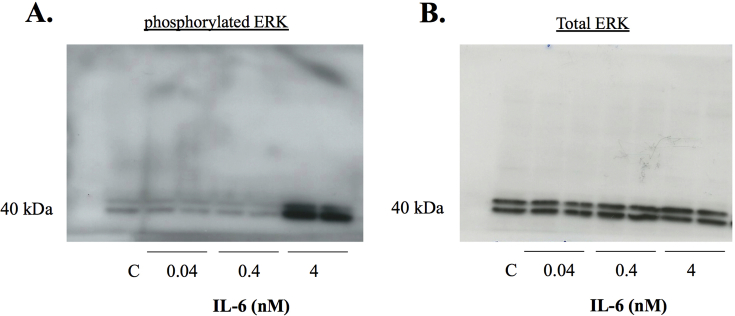
3.6. IL-6 induced ERK1/2 signalling in BV2 microglia cells, but rCypA did not induce the secretion of IL-6
IL-6 is associated with tumour cell invasion, angiogenesis, tumour cell proliferation and poorer prognosis in GBM (Chang et al., 2005; Kudo et al., 2009). Therefore, we tested if IL-6 can activate the ERK 1/2 pathway in BV2 microglia cells. Western blot analysis of BV2 microglia cell lysates following exposure of cell cultures to IL-6 for 24 h at 4 nM, but not 0.04 or 0.4 nM, revealed an increase in the levels of phosphorylated ERK1/2 as compared to the vehicle control (Fig. 6A). Treatment of BV2 microglial cell cultures with rCypA (100 nM) and analysis of culture supernatant 24 h post-treatment by ELISA did not detect an increase in IL-6 levels when compared to the vehicle control (Fig. 6B).
Fig. 6.
Treatment of BV2 cells with IL-6 (24 h) and recombinant CypA (A) IL-6 induces pERK1/2 expression at a 4 nM dose, but not at lower treatment doses (B) Treatment of BV2 cells with rCypA (100 nM) does not enhance the secretion of IL-6 in culture medium. C = vehicle control. Values are presented as mean +/-SE; N = 3. Original uncropped images of blots are shown in Fig. 6 are presented in Supplementary Fig. 4.
4. Discussion
GBM tissues often contain non-malignant cells, in particular, tumour-associated microglia. Microglia are an essential component of the tumour microenvironment and are estimated to make up approximately 30% of GBM (Kostianovsky et al., 2008). Studies have reported that CypA, a potent chemokine linked to cancer proliferation and metastasis, is overexpressed in GBM tissue (Matthews et al., 2018; Sun et al., 2011). Given the known effects of CypA in the promotion of chemotaxis, the present study sought to investigate the role of microglia as a potential source and target of extracellular CypA. In doing so, we demonstrate for the first time that human primary microglial cells and BV2 microglial cells express CypA, and that BV2 microglia secrete CypA under basal conditions, and in response to inflammatory and oxidative stimuli. In conditions of hypoxia, as occurs in the tumour microenvironment, CypA expression has been shown to promote glioma-initiating cell proliferation and radiotherapy resistance (G. Wang et al., 2017). The expression of the main CypA receptor, CD147, positively correlates with GBM severity and patient outcome (Matthews et al., 2018). The present study supports the hypothesis that microglia can contribute to the tumour microenvironment via the actions of CypA. Similar to previous studies on primary microglia and RA2 microglial cells (Inoue et al., 1999), we detected strong CD147 expression in human primary microglial and BV2 microglial cells. The CD147 protein appeared glycosylated, which is a necessary requirement for CypA induced CD147 signalling.
Given that the GBM microenvironment harbours areas of hypoxia and oxidative stress, we also investigated CypA secretion and signalling following inflammatory and oxidative stress stimulation. We observed increased levels of CypA secretion in BV2 microglia cell culture supernatants and increased ERK1/2 activation in BV2 cells following inflammatory and oxidative stress stimulation. The secretion of CypA in response to the pro-inflammatory molecule LPS is in line with the ability of macrophages to secrete CypA in response to inflammatory stressors (Sherry et al., 1992). These finding are consistent with previous studies in showing that CypA is secreted by vascular smooth muscle cells in response to oxidative stress (Jin et al., 2000).
Our observation that BV2 microglia can secrete CypA provides evidence that extracellular CypA may play a role within the GBM micro-environment. While, extracellular rCypA did not induce the proliferation of BV2 cells, it did enhance BV2 cell survival. The cell survival promoting effects of CypA may be mediated by CD147-mediated ERK1/2 anti-apoptotic signalling (Choi et al., 2007). Further, as CypA can act as a potent chemokine for immune cells, we demonstrated that rCypA enhances BV2 microglia migration in a scratch wound-gap healing.
In the context of GBM, targeting of CypA with cyclosporine A resulted in the inhibition of microglial mediated GBM invasion (Sliwa et al., 2007). In addition, CypA-induced cell-migration and invasion has been shown to be mediated by the CD147 receptor (Guo et al., 2015; C.-h. Wang et al., 2014), which as we demonstrate is expressed by BV2 microglial cells. However, further work is needed to confirm that CypA mediates these actions via the CD147 receptor.
CypA is known to promote tumour growth and correlates with poor clinical outcomes in various cancers (Nakano et al., 2017), however the exact mechanism of this activity remains unclear. In an effort to identify the mechanisms responsible for the CypA-induced cell survival and migration of BV2 cells, key signal pathways were studied following recombinant CypA treatment. The results from this study showed rCypA treatment did not induce significant changes in the expression of phosphorylated AKT, ERK1/2 or NFκB pathways. These results were somewhat surprising as we have previously shown that CD147-mediated activation with rCypA mediates ERK1/2-dependent survival pathways in neurons during in vitro oxidative and ischemic injury (Boulos et al., 2007). In the present study, while we demonstrated activation of ERK1/2 and AKT pathways following LPS and oxidative stress, subsequent experiments showed rCypA induced cell migration occurred independent of any induced stress. However, it is possible that incubation in serum-free media for a 72-hour time point may have provided conditions of stress to allow for CypA-mediated survival pathway activation, which was not present in western blot experiments. However, the lack of such downstream effects observed following rCypA treatment in the present study is surprising, and may therefore be a product of the BV2 cell line.
To further investigate the signalling properties of BV2 microglia, IL-6 was studied as a cytokine known to be secreted from microglia (W.-Y. Wang, Tan, Yu and Tan, 2015), and which can activate pro-survival signalling in cancer cells. We demonstrated that IL-6 could induce ERK1/2 activation in BV2 microglia cells and confirms that while we did not detect ERK1/2 activation in BV2 cells exposed to rCypA, such a pathway is able to be activated following cytokine treatment. Neurons, astrocytes, microglia and endothelial cells are all essential sources of IL-6 in the CNS, and upon stimulation (such as injury) are capable of actively secreting IL-6 (Erta et al., 2012). In addition, activated microglia are the primary source of inflammatory cytokines in non-malignant neuronal diseases (e.g. Alzheimer's disease) (Bachstetter and Van Eldik, 2010). Therefore, the inability of rCYPA to induce IL-6 secretion in microglia was surprising, however, while the morphological appearance of microglia located in or around GBM tumours suggests microglia activation, such cells do not release the prototypical inflammatory cytokines such as TNF-α or IL-6 (Gabrusiewicz et al., 2011; Sliwa et al., 2007). Nevertheless, the results in the present study may also be a result of the BV2 cell line, and requires further confirmation in primary microglia.
Microglia and astrocytes play a protective and curative function in the CNS against pathogens by producing cytokines and chemokines that promote the recruitment of circulating immune cells. Peripheral bacterial infections are recognised by toll like receptors (TLR) which activate innate immune cells. The activation of TLRs can lead to the upregulation of NFKB which can increase the transcription of genes encoding the IL-1 family cytokines and TNF with downstream effects of cytokine secretion that act on the innate immune system and fight infections. In our study, we showed that LPS induces the secretion of CypA and LPS is known to trigger TLRs to induce its effects.
The finding that microglia can secrete CypA in response to oxidative stress and pro-inflammatory stimulation has broader implications in neuroinflammation. Stress and inflammation are pathogenic processes, known to induce the onset and progression of many diseases. Chronic stress due to physical and environment stimuli, such as those present in the GBM microenvironment, can induce several unwanted changes within the CNS, which includes secretion of high levels of proinflammatory cytokines and chemokines, increased oxidative stress and neuroinflammation (Kempuraj et al., 2019). Stress induced neuroinflammation can involve mast cell activation, the subsequent generation of proinflammatory cytokines (Caraffa et al., 2018), which in turn can activate glial cells. A recent report indicates targeting inflammation through the administration of natural flavonoid compounds could inhibit neuroinflammation and the severity of neurodegenerative diseases (Theoharides and Kavalioti, 2018; Theoharides and Tsilioni, 2018). These reports also suggest that the interaction of mast cells and microglia in the hypothalamus could induce stress mediated neuroinflammation.
In summary, we have demonstrated that the BV2 microglial cell line secretes CypA, and that rCypA can promote cell viability and chemotaxis in these cells. In addition, we have shown that inflammatory and oxidative stress stimulates the secretion of CypA by BV2 microglial cells. Definitive elucidation of the cell-signalling pathways by which CypA mediates its cell-supporting role in microglia will probably require the use of organotypic brain slice cultures, in which quiescent primary microglial cells are more likely to be found. Finally, our findings are important because they indicate that extracellular CypA is likely to be secreted within the GBM micro-environment wherein its pro-tumorigenic actions maybe mediated, not just though microglia, but via the high levels of CD147 receptor expression found on GBM cells. Finally, the secretion of CypA by microglia has broader implications in regards to stress induced neuroinflammation, which is associated with other diseases of the CNS.
Declarations
Author contribution statement
Gurkiran Kaur Flora: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data.
Ryan S. Anderton: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Bruno P. Meloni: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Gilles J. Guillemin: Performed the experiments.
Neville W. Knuckey: Conceived and designed the experiments.
Gabriella MacDougall: Performed the experiments; Analyzed and interpreted the data.
Vance Matthews, Sherif Boulos: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors would like to acknowledge Dr Benjamin Heng for assistance with microglial lysate collection, Mr. Vince Clark for his assistance with programming the software used to analyse the scratch wound-healing assay, and Mr. Marty Firth for his assistance with statistical analysis.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
Supplementary Figure 1.
Supplementary Figure 2.
Supplementary Figure 3.
Supplementary Figure 4.
References
- Bachstetter A.D., Van Eldik L.J. The p38 MAP kinase family as regulators of proinflammatory cytokine production in degenerative diseases of the CNS. Aging Dis. 2010;1(3):199–211. [PMC free article] [PubMed] [Google Scholar]
- Boulos S., Meloni B.P., Arthur P.G., Majda B., Bojarski C., Knuckey N.W. Evidence that intracellular cyclophilin A and cyclophilin A/CD147 receptor-mediated ERK1/2 signalling can protect neurons against in vitro oxidative and ischemic injury. Neurobiol. Dis. 2007;25(1):54–64. doi: 10.1016/j.nbd.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Caraffa A., Conti C., O C.D., Gallenga C.E., Tettamanti L., Mastrangelo F.…Conti P. New concepts in neuroinflammation: mast cells pro-inflammatory and anti-inflammatory cytokine mediators. J. Biol. Regul. Homeost. Agents. 2018;32(3):449–454. [PubMed] [Google Scholar]
- Chang C.Y., Li M.C., Liao S.L., Huang Y.L., Shen C.C., Pan H.C. Prognostic and clinical implication of IL-6 expression in glioblastoma multiforme. J. Clin. Neurosci. 2005;12(8):930–933. doi: 10.1016/j.jocn.2004.11.017. [DOI] [PubMed] [Google Scholar]
- Choi K.J., Piao Y.J., Lim M.J., Kim J.H., Ha J., Choe W., Kim S.S. Overexpressed cyclophilin a in cancer cells renders resistance to hypoxia- and cisplatin-induced cell death. Cancer Res. 2007;67(8):3654. doi: 10.1158/0008-5472.CAN-06-1759. [DOI] [PubMed] [Google Scholar]
- Erta M., Quintana A., Hidalgo J. Interleukin-6, a major cytokine in the central nervous system. Int. J. Biol. Sci. 2012;8(9):1254–1266. doi: 10.7150/ijbs.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrusiewicz K., Ellert-Miklaszewska A., Lipko M., Sielska M., Frankowska M., Kaminska B. Characteristics of the alternative phenotype of microglia/macrophages and its modulation in experimental gliomas. PLoS One. 2011;6(8) doi: 10.1371/journal.pone.0023902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graeber M.B., Scheithauer B.W., Kreutzberg G.W. Microglia in brain tumors. Glia. 2002;40:252–259. doi: 10.1002/glia.10147. [DOI] [PubMed] [Google Scholar]
- Guillemin G.J., Smythe G., Takikawa O., Brew B.J. Expression of indoleamine 2,3-dioxygenase and production of quinolinic acid by human microglia, astrocytes, and neurons. Glia. 2005;49(1):15–23. doi: 10.1002/glia.20090. [DOI] [PubMed] [Google Scholar]
- Guo N., Zhang K., Lv M., Miao J., Chen Z., Zhu P. CD147 and CD98 complex-mediated homotypic aggregation attenuates the CypA-induced chemotactic effect on Jurkat T cells. Mol. Immunol. 2015;63(2):253–263. doi: 10.1016/j.molimm.2014.07.005. [DOI] [PubMed] [Google Scholar]
- Han X., Yoon S.H., Ding Y., Choi T.G., Choi W.J., Kim Y.H.…Kim S.S. Cyclosporin A and sanglifehrin A enhance chemotherapeutic effect of cisplatin in C6 glioma cells. Oncol. Rep. 2010;23:1053–1062. doi: 10.3892/or_00000732. [DOI] [PubMed] [Google Scholar]
- Handschumacher R.E., Harding M.W., Rice J., Drugge R.J., Speicher D.W. Cyclophilin: a specific cytosolic binding protein for cyclosporin A. Science. 1984;226:544–547. doi: 10.1126/science.6238408. [DOI] [PubMed] [Google Scholar]
- Hoelzinger D.B., Demuth T., Berens M.E. Autocrine factors that sustain glioma invasion and paracrine biology in the brain microenvironment. J. Natl. Cancer Inst. 2007;99:1583–1593. doi: 10.1093/jnci/djm187. [DOI] [PubMed] [Google Scholar]
- Howard B.A., Furumai R., Campa M.J., Rabbani Z.N., Vujaskovic Z., Wang X.F., Patz E.F., Jr. Stable RNA interference-mediated suppression of cyclophilin A diminishes non-small-cell lung tumor growth in vivo. Cancer Res. 2005;65:8853–8860. doi: 10.1158/0008-5472.CAN-05-1219. [DOI] [PubMed] [Google Scholar]
- Inoue H., Sawada M., Ryo A., Tanahashi H., Wakatsuki T., Hada A.…Tabira T. Serial analysis of gene expression in a microglial cell line. Glia. 1999;28(3):265–271. doi: 10.1002/(sici)1098-1136(199912)28:3<265::aid-glia10>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Ivery M.T. Immunophilins: switched on protein binding domains? Med. Res. Rev. 2000;20:452–484. doi: 10.1002/1098-1128(200011)20:6<452::aid-med2>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Jin Z.G., Melaragno M.G., Liao D.F., Yan C., Haendeler J., Suh Y.A.…Berk B.C. Cyclophilin A is a secreted growth factor induced by oxidative stress. Circ. Res. 2000;87:789–796. doi: 10.1161/01.res.87.9.789. [DOI] [PubMed] [Google Scholar]
- Kanyenda L.J., Verdile G., Martins R., Meloni B.P., Chieng J., Mastaglia F.…Boulos S. Is cholesterol and amyloid-beta stress induced CD147 expression a protective response? Evidence that extracellular cyclophilin a mediated neuroprotection is reliant on CD147. J. Alzheimer's Dis. 2014;39(3):545–556. doi: 10.3233/JAD-131442. [DOI] [PubMed] [Google Scholar]
- Kempuraj D., Thangavel R., Selvakumar G.P., Ahmed M.E., Zaheer S., Raikwar S.P.…Zaheer A. Mast cell proteases activate astrocytes and glia-neurons and release interleukin-33 by activating p38 and ERK1/2 MAPKs and NF-κB. Mol. Neurobiol. 2019;56(3):1681–1693. doi: 10.1007/s12035-018-1177-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H., Kim W.-J., Jeon S.-T., Koh E.-M., Cha H.-S., Ahn K.-S., Lee W.-H. Cyclophilin A may contribute to the inflammatory processes in rheumatoid arthritis through induction of matrix degrading enzymes and inflammatory cytokines from macrophages. Clin. Immunol. 2005;116(3):217–224. doi: 10.1016/j.clim.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Kim J.Y., Kim W.J., Kim H., Suk K., Lee W.H. The stimulation of CD147 induces MMP-9 expression through ERK and NF-kappaB in macrophages: implication for atherosclerosis. Immune Netw. 2009;9:90–97. doi: 10.4110/in.2009.9.3.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofron J.L., Kuzmic P., Kishore V., Colon-Bonilla E., Rich D.H. Determination of kinetic constants for peptidyl prolyl cis-trans isomerases by an improved spectrophotometric assay. Biochemistry. 1991;30:6127–6134. doi: 10.1021/bi00239a007. [DOI] [PubMed] [Google Scholar]
- Kostianovsky A.M., Maier L.M., Anderson R.C., Bruce J.N., Anderson D.E. Astrocytic regulation of human monocytic/microglial activation. J. Immunol. 2008;181(8):5425. doi: 10.4049/jimmunol.181.8.5425. [DOI] [PubMed] [Google Scholar]
- Kudo M., Jono H., Shinriki S., Yano S., Nakamura H., Makino K.…Kuratsu J. Antitumor effect of humanized anti-interleukin-6 receptor antibody (tocilizumab) on glioma cell proliferation. Laboratory investigation. J. Neurosurg. 2009;111(2):219–225. doi: 10.3171/2008.12.JNS081284. [DOI] [PubMed] [Google Scholar]
- Li M., Zhai Q., Bharadwaj U., Wang H., Li F., Fisher W.E.…Yao Q. Cyclophilin A is overexpressed in human pancreatic cancer cells and stimulates cell proliferation through CD147. Cancer. 2006;106:2284–2294. doi: 10.1002/cncr.21862. [DOI] [PubMed] [Google Scholar]
- MacDougall G., Anderton R.S., Edwards A.B., Knuckey N.W., Meloni B.P. The neuroprotective peptide poly-arginine-12 (R12) reduces cell surface levels of NMDA NR2B receptor subunit in cortical neurons; investigation into the involvement of endocytic mechanisms. J. Mol. Neurosci. 2017;61(2):235–246. doi: 10.1007/s12031-016-0861-1. [DOI] [PubMed] [Google Scholar]
- Matthews V., Candy P., West K., Knuckey N., Robbins P., Jeffcote B., Boulos S. CYPA and CD147 expression analysis in human glioblastoma: evidence for a correlation with reduced survival. J. Transl. Sci. 2018;4(5):1–7. [Google Scholar]
- Nakano N., Sakashita S., Matsuoka R., Murata Y., Shiba-Ishii A., Kobayashi N.…Noguchi M. Cyclophilin A expression and its prognostic significance in lung adenocarcinoma. Pathol. Int. 2017;67(11):555–563. doi: 10.1111/pin.12593. [DOI] [PubMed] [Google Scholar]
- Obchoei S., Sawanyawisuth K., Wongkham C., Kasinrerk W., Yao Q., Chen C., Wongkham S. Secreted cyclophilin A mediates G1/S phase transition of cholangiocarcinoma cells via CD147/ERK1/2 pathway. Tumour Biol. 2015;36(2):849–859. doi: 10.1007/s13277-014-2691-5. [DOI] [PubMed] [Google Scholar]
- Riethdorf S., Reimers N., Assmann V., Kornfeld J.W., Terracciano L., Sauter G., Pantel K. High incidence of EMMPRIN expression in human tumors. Int. J. Cancer. 2006;119:1800–1810. doi: 10.1002/ijc.22062. [DOI] [PubMed] [Google Scholar]
- Ryffel B., Woerly G., Greiner B., Haendler B., Mihatsch M.J., Foxwell B.M. Distribution of the cyclosporine binding protein cyclophilin in human tissues. Immunology. 1991;72:399–404. [PMC free article] [PubMed] [Google Scholar]
- Seko Y., Fujimura T., Taka H., Mineki R., Murayama K., Nagai R. Hypoxia followed by reoxygenation induces secretion of cyclophilin A from cultured rat cardiac myocytes. Biochem. Biophys. Res. Commun. 2004;317:162–168. doi: 10.1016/j.bbrc.2004.03.021. [DOI] [PubMed] [Google Scholar]
- Sherry B., Yarlett N., Strupp A., Cerami A. Identification of cyclophilin as a proinflammatory secretory product of lipopolysaccharide-activated macrophages. Proc. Nat. Acadamy Sci. 1992;89:3511–3515. doi: 10.1073/pnas.89.8.3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sliwa M., Markovic D., Gabrusiewicz K., Synowitz M., Glass R., Zawadzka M.…Kaminska B. The invasion promoting effect of microglia on glioblastoma cells is inhibited by cyclosporin A. Brain. 2007;130(2):476–489. doi: 10.1093/brain/awl263. [DOI] [PubMed] [Google Scholar]
- Sun S., Wang Q., Giang A., Cheng C., Soo C., Wang C.-Y.…Chiu R. Knockdown of CypA inhibits interleukin-8 (IL-8) and IL-8-mediated proliferation and tumor growth of glioblastoma cells through down-regulated NF-κB. J. Neuro Oncol. 2011;101(1):1–14. doi: 10.1007/s11060-010-0220-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki J., Jin Z.-G., Meoli D.F., Matoba T., Berk B.C. Cyclophilin a is secreted by a vesicular pathway in vascular smooth muscle cells. Circ. Res. 2006;98(6):811. doi: 10.1161/01.RES.0000216405.85080.a6. [DOI] [PubMed] [Google Scholar]
- Theoharides T.C., Kavalioti M. Stress, inflammation and natural treatments. J. Biol. Regul. Homeost. Agents. 2018;32(6):1345–1347. [PubMed] [Google Scholar]
- Theoharides T.C., Tsilioni I. Tetramethoxyluteolin for the treatment of neurodegenerative diseases. Curr. Top. Med. Chem. 2018;18(21):1872–1882. doi: 10.2174/1568026617666181119154247. [DOI] [PubMed] [Google Scholar]
- Tian L., Zhang Y., Chen Y., Cai M., Dong H., Xiong L. EMMPRIN is an independent negative prognostic factor for patients with astrocytic glioma. PLoS One. 2013;8(3) doi: 10.1371/journal.pone.0058069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C.-h., Rong M.-y., Wang L., Ren Z., Chen L.-n., Jia J.-f.…Zhu P. CD147 up-regulates calcium-induced chemotaxis, adhesion ability and invasiveness of human neutrophils via a TRPM-7-mediated mechanism. Rheumatology. 2014;53(12):2288–2296. doi: 10.1093/rheumatology/keu260. [DOI] [PubMed] [Google Scholar]
- Wang G., Shen J., Sun J., Jiang Z., Fan J., Wang H.…Guo M. Cyclophilin a maintains glioma-initiating cell stemness by regulating Wnt/β-catenin signaling. Clin. Cancer Res. 2017;23(21):6640. doi: 10.1158/1078-0432.CCR-17-0774. [DOI] [PubMed] [Google Scholar]
- Wang L., Wang C.-h., Jia J.-f., Ma X.-k., Li Y., Zhu H.-b.…Zhu P. Contribution of cyclophilin a to the regulation of inflammatory processes in rheumatoid arthritis. J. Clin. Immunol. 2010;30(1):24–33. doi: 10.1007/s10875-009-9329-1. [DOI] [PubMed] [Google Scholar]
- Wang W.-Y., Tan M.-S., Yu J.-T., Tan L. Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Ann. Transl. Med. 2015;3(10) doi: 10.3978/j.issn.2305-5839.2015.03.49. (June 2015): Annals of Translational Medicine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watters J.J., Schartner J.M., Badie B. Microglia function in brain tumors. J. Neurosci. Res. 2005;81:447–455. doi: 10.1002/jnr.20485. [DOI] [PubMed] [Google Scholar]
- Willenbrink W., Halaschek J., Schuffenhauer S., Kunz J., Steinkasserer A. Cyclophilin A, the major intracellular receptor for the immunosuppressant cyclosporin A, maps to chromosome 7p11.2-p13: four pseudogenes map to chromosomes 3, 10, 14, and 18. Genomics. 1995;28:101–104. doi: 10.1006/geno.1995.1112. [DOI] [PubMed] [Google Scholar]
- Wu S.-Y., Watabe K. The roles of microglia/macrophages in tumor progression of brain cancer and metastatic disease. Front. Biosci. 2017;22:1805–1829. doi: 10.2741/4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Chen J., Yang J., Qiao S., Zhao S., Yu L. Cyclophilin A is upregulated in small cell lung cancer and activates ERK1/2 signal. Biochem. Biophys. Res. Commun. 2007;361:763–767. doi: 10.1016/j.bbrc.2007.07.085. [DOI] [PubMed] [Google Scholar]
- Yang M., Yuan Y., Zhang H., Yan M., Wang S., Feng F.…Wang L. Prognostic significance of CD147 in patients with glioblastoma. J. Neuro Oncol. 2013;115(1):19–26. doi: 10.1007/s11060-013-1207-2. [DOI] [PubMed] [Google Scholar]
- Zupanska A., Dziembowska M., Ellert-Miklaszewska A., Gaweda-Walerych K., Kaminska B. Cyclosporine a induces growth arrest or programmed cell death of human glioma cells. Neurochem. Int. 2005;47(6):430–441. doi: 10.1016/j.neuint.2005.05.010. [DOI] [PubMed] [Google Scholar]