Abstract
Amniotic membrane (AM) is used to treat a range of ophthalmic indications but must be presented in a non-contaminated state. AM from elective caesarean sections contains natural microbial contamination, requiring removal during processing protocols. The aim of this study was to assess the ability of antibiotic decontamination of AM, during processing by innovative low-temperature vacuum-drying. Bioburden of caesarean section AM was assessed, and found to be present in low levels. Subsequently, the process for producing vacuum-dried AM (VDAM) was assessed for decontamination ability, by artificially loading with Staphylococcus epidermidis at different stages of processing. The protocol was highly efficient at removing bioburden introduced at any stage of processing, with antibiotic treatment and drying the most efficacious steps. The antibacterial activity of non-antibiotic treated AM compared to VDAM was evaluated using minimum inhibitory/biocidal concentrations (MIC/MBC), and disc diffusion assays against Meticillin-resistant Staphylococcus aureus, Meticillin-resistant S. epidermidis, Escherichia coli, Pseudomonas aeruginosa and Enterococcus faecalis. Antibacterial activity without antibiotic was low, confirmed by high MIC/MBC, and a no inhibition on agar lawns. However, VDAM with antibiotic demonstrated effective antibacterial capacity against all bacteria. Therefore, antibiotic decontamination is a reliable method for sterilisation of AM and the resultant antibiotic reservoir is effective against gram-positive and –negative bacteria.
Subject terms: Antimicrobials, Corneal diseases, Translational research
Introduction
Treatment of ocular surface disease, ulcers and infections remains a challenge. Severe bacterial ocular infections are commonly treated with corticosteroids and an intensive course of antibiotics1,2. However, the toxic side-effects of antibiotics can be harmful to epithelial cells of the cornea2,3.
Human amniotic membrane (AM), processed and preserved for therapeutic application is reported to possess anti-inflammatory, anti-scarring, anti-angiogenic and anti-microbial properties, which are valued for their role in wound healing and prevention of post-surgery complications4–7. Application of AM in the treatment of corneal disease has been reported to reduce infection-causing microorganisms and accelerate healing of infected corneas8, having been shown to be superior to antibiotic treatment alone7,9.
AM intervention is an option that mitigates the prolonged use of topical, broad-spectrum antibiotics, preventing steroid-related complications. Studies supporting the use of AM for the treatment of corneal ulcers show significantly enhanced epithelial healing and a reduction in inflammation, corneal scarring, neovascularization, and infection complications5,7,9. Although the mechanism of action of AM is not well explained, it is often attributed to bioactive substances such as growth factors, pro-inflammatory cytokines and anti-inflammatory cytokines, that enhance the function of structural constituents, working as a substrate for local cell migration and adhesion10.
Freshly collected AM is rarely used for clinical treatment due to post-transplant complications11, increased probability of disease transmission, and short shelf-life. Therefore, most clinically used AM is subject to processing and preservation steps prior to use10. Cryopreservation12, heat-drying13 and freeze-drying14 are the most common methods for AM preservation. Although associated with low post-transplantation infection complications15, cryopreservation has been shown to damage the amnion16 which impairs the viability and proliferative capacity of epithelial cells17, decrease levels of many bioactive substances including epidermal growth factors (EGF) and transforming growth factors (TGF), and is constrained by distribution difficulties18. Dried AM followed by radiation sterilisation has been shown to be degraded with reduced growth factor content14. This effect on the stability and function of biological molecules such as growth factors, cytokines and structural proteins, affects the quality of the AM as a biological treatment for eye injury, and justifies the development of an appropriate approach for AM preservation.
Due to potential degradation by terminal sterilisation techniques19, AM is often aseptically prepared in order to ensure no contamination is introduced during processing and to limit the spread of any natural bioburden. In vivo during pregnancy, AM is considered sterile, however, potential microbial contamination originates from skin and vaginal flora during delivery of the placenta during birth, with the retrieving staff being an additional source of contamination20. Therefore, any manufacturing process must involve steps to effectively decontaminate such bioburden and allow for long-term preservation of AM.
Fresh AM processing generally involves a series of washing steps in physiological solutions to remove raw contamination and blood. Subsequent preservation via heat, freeze-drying, or deep freezing at below −60 °C are applied to suppress water activity to maintain tissue stability, and prevent microbial growth. However, to address bioburden contamination, antibiotics are typically added to the collection medium or storage medium for frozen AM20,21. For dried AM products, vacuum-packing followed by radiation sterilisation is often applied to ensure complete microbial inactivity22.
The sterility and integrity of final manufactured AM is necessary for safe and direct application. However, maintaining AM sterility, quality and functional properties during processing or storage is a challenge. Manufacturing AM in the presence of selected antibiotic/antifungal agents can have several advantageous features, including avoiding deleterious effects on the matrix and biomolecules caused by terminal sterilisation, providing sterility, and inadvertently working as a potential antibiotic reservoir that offers a prophylactic treatment following transplantation.
The aim of this study was to validate the microbial decontamination potential of a novel vacuum-drying, aseptic manufacturing technique, and to then investigate further the lasting anti-microbial effects of antibiotics added during processing over long-term storage. We identify the level of microbiological contamination, or bioburden, of elective caesarean section (ECS) AM, and then go on to assess the robustness of decontamination during our optimised, innovative protocol for preserving AM, using vacuum-drying and a broad spectrum antibiotic/antifungal cocktail18. This is done by artificially loading AM with high levels of Staphylococcus epidermidis, a skin floral species likely to contaminate amnion23, and investigating the decontamination efficacy of the washing, antibiotic treatment and vacuum-drying steps by counting the surviving colony forming units (CFU) using a spread plate method. The implications of biological and physical antimicrobial activity of fresh AM and the inadvertent antibiotic reservoir effect of vacuum-dried AM (VDAM) against strains of gram-positive and gram-negative bacteria are then studied using minimum inhibitory and biocidal concentrations (MIC/MBC) and disc-diffusion assays.
Results
Natural bioburden of fresh ECS AM
Results demonstrating the normal (unloaded) floral contamination of fresh ECS AM can be seen in Table 1. Results showed that 40% of the fresh AM obtained from caesarean section placentae were not contaminated with detectable bioburden. Of the remaining 60%, any contamination was predominantly identified as Neisseria cinerea or Micrococcus spp. Enhanced anaerobic incubation for seven days showed increased growth of Cutibacterium acnes bacteria in samples that were negative at aerobic conditions.
Table 1.
Bioburden assessment and identification of natural bioburden of 10 freshly collected ECS AM (NG – no growth).
| AM | Bacterial Identification | |||
|---|---|---|---|---|
| Blood Agar Culture Plates | Basal Broth Culture | |||
| Aerobic (48 h) | Anaerobic (7 days) | Aerobic(48 h) | Anaerobic (7 days) | |
| 1 | Neisseria cinerea | NG | NG | NG |
| 2 | Micrococcus spp. | Cutibacterium acnes | Micrococcus spp. | P. acnes |
| 3 | NG | 30 CFU/mL | NG | Cutibacterium acnes P. acnes Staph. schleiferi |
| 4 | NG | NG | NG | NG |
| 5 | NG | NG | Micrococcus spp. | NG |
| 6 | NG | NG | NG | NG |
| 7 | NG | NG | Micrococcus spp. | NG |
| 8 | NG | NG | NG | Micrococcus spp. |
| 9 | NG | NG | NG | NG |
| 10 | NG | NG | NG | NG |
Validation of vacuum-drying protocol with artificially loaded bioburden
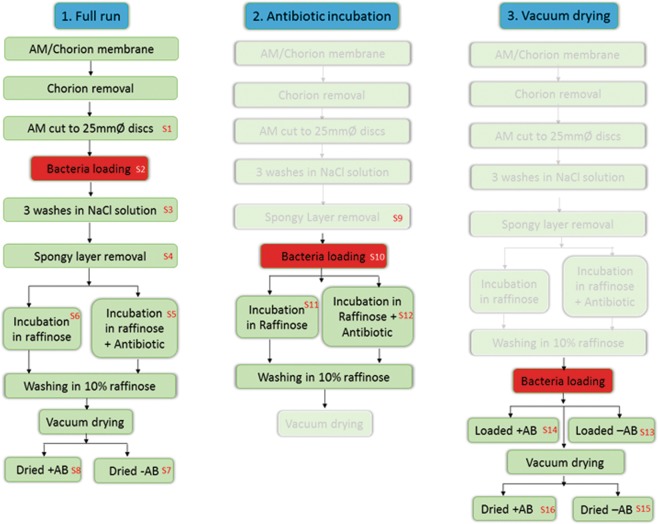
Due to low and inconsistent levels of natural bioburden on ECS AM, we artificially loaded 5 donated amnion with high bioburden (106 CFU/mL), at different stages of processing, to effectively test the decontamination efficacy of the VDAM process. Figure 1, shows a schematic of the bacterial loading and sampling procedure performed. Unloaded samples of each donor (n = 3 per donor) were tested before washing in NaCl and no detectable contamination was seen.
Figure 1.
Schematic diagram showing sampling procedures for the S. epidermidis loaded bioburden experiment. Discs of 25 mm diameter were collected at each point numbered in red (S1–S15). Experiment was performed on 5 separate donors.
Decontamination efficacy of full manufacturing run
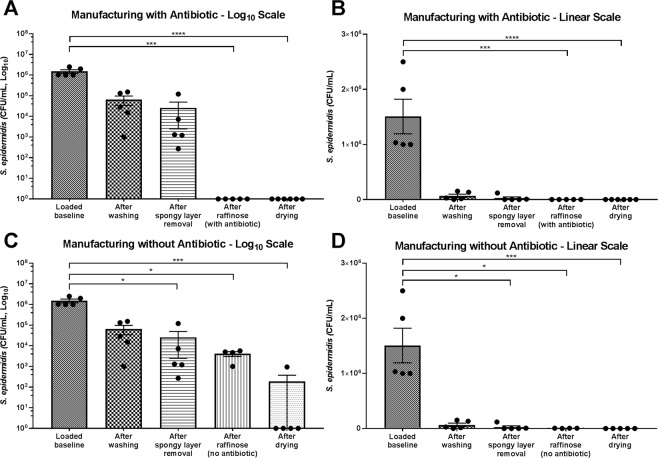
The efficacy of the entire processing procedure to detach and devitalise bacterial contamination loaded at the start was examined by counting CFU/mL after each processing step (Fig. 2, graphs shown on log10 and linear scales). AM samples loaded with S. epidermidis showed significant successive reduction from the original load of 106 CFU/mL as processing progressed. Washing three times in NaCl reduced the bacterial load by almost one log cycle (post wash, 95.65%) and the removal of the spongy layer contributed to further lowering the remaining microbial load by 60.53%. Incubating the AM in raffinose solution containing a broad spectrum antibiotic cocktail (components listed in Table 2), caused the bioburden to be essentially eliminated, with no growth detected after raffinose/antibiotic incubation, or after the combined processing of raffinose/antibiotic incubation and vacuum drying (Fig. 2A,B). Incubating in raffinose without antibiotic (Fig. 2C,D) resulted in a further reduction of the remaining bacteria by 87.29% and continuing to the drying step without antibiotic treatment excluded more CFU by 94.26%.
Figure 2.
Effect of VDAM processing on reduction of artificially loaded S. epidermidis bacterial counts. Loading of 100 μL of 106 CFU/mL S. epidermidis was performed before washing procedures in 0.9% NaCl. AM samples were taken throughout processing and assessed for CFU/mL. Manufacturing was performed with antibiotic included in the raffinose step (A) graph shown with log10 scale, (B) graph shown with linear scale. Manufacturing with antibiotic included (C) graph shown with log10 scale, (D) graph shown with linear scale. Data shows individual experiments performed with 5 different donors and is represented as mean ± SEM. Data compared by one-way ANOVA, Kruskal-Wallis test followed by multiple comparison uncorrected Dunn’s test. Statistical significance vs. baseline represented by *p ≤ 0.05, ***p ≤ 0.001, ****p ≤ 0.0001.
Table 2.
Concentrations and activity of the antibiotic/antifungal cocktail used in this study.
| Antibiotic | Concentration | % w/v | Activity |
|---|---|---|---|
| Gentamicin | 200 mg/mL | 15–20 | Gram-positive, gram-negative |
| Imipenem | 10 mg/mL | 5–10 | Gram-positive, gram-negative |
| Polymyxin B | 10 mg/mL | 5–10 | Gram-negative |
| Vancomycin | 2.5 mg/mL | <0.5 | Gram-positive |
| Nystatin | 125,000 U/mL | <0.05 | Antifungal |
Efficacy of raffinose/antibiotic incubation step
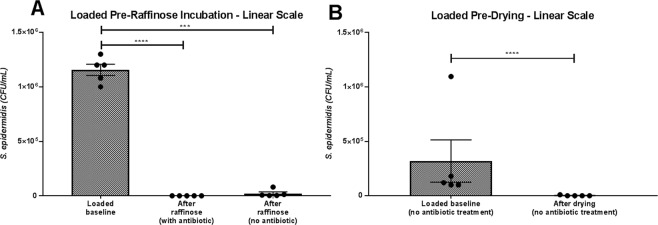
To test the efficacy of the raffinose incubation step with and without antibiotics, loading the AM was performed after the spongy layer removal step (Fig. 3A). This removes any effect that previous manufacturing steps had on the bacterial reduction. Incubation in raffinose containing antibiotic effectively eliminated the loaded bacteria. Raffinose incubation without antibiotic removed the majority of loaded bacteria resulting in a 98.17% reduction, similar to that seen during the full run experiment.
Figure 3.
Efficiency of antibiotic incubation and drying steps in reducing artificially loaded S. epidermidis bacterial counts. (A) Loading of 100 μL of 106 S. epidermidis was performed before raffinose incubation with/without antibiotic. AM samples were taken after loading and after incubation and assessed for CFU/mL. (B) Loading of 100 μL of 106 CFU/mL S. epidermidis was performed after raffinose incubation with/without antibiotic and before drying. AM samples were taken after loading and after drying and assessed for CFU/mL. Only samples processed without antibiotic are shown, as no colonies were seen either in the loaded baseline or after drying. Data represents experiments performed on 5 different donors and is represented as mean ± SEM. Data compared by one-way ANOVA, Kruskal-Wallis test followed by multiple comparison uncorrected Dunn’s test. Statistical significance vs. baseline represented by ***p ≤ 0.001, ****p ≤ 0.0001.
Efficacy of vacuum-drying step
To test the efficacy of the vacuum-drying step, samples were loaded with S. epidermidis after the raffinose incubation and was performed with and without antibiotic (Fig. 3B). When antibiotic had been included in the process prior to loading, no CFU were found either in the loaded baseline or after drying (not shown on graph). When processed without antibiotics, the loaded baseline was lower than seen in previous experiments, indicating an inefficiency of loading at this point of processing. Subsequent drying then resulted in a 99.41% reduction of loaded bioburden.
Antibacterial efficacy of AM
MIC/MBC test
MIC tests showed that washed AM extract without antibiotic treatment did not exert any consistent antimicrobial activity. Bacterial growth was observed across the 96-well plates (Table 3); therefore, the MIC could not be determined. When the AM was incubated with antibiotic, the MIC results for each donor ranged from 0.78 µg/mL to 12.5 µg/mL, and the MBC results ranged from 6.25 µg/mL to 25 µg/mL. These results indicate that the AM itself has very low natural antimicrobial properties but inclusion of the antibiotics in the process, even in small concentrations, can cause bacterial elimination.
Table 3.
MIC (Minimum Inhibitory Concentration) and MBC (Minimum Bactericidal Concentration) of serially diluted AM extracts treated with raffinose, with and without antibiotic treatment. Results are shown as average values from three separate experiments.
| Processed without antibiotics | ||||||
|---|---|---|---|---|---|---|
| Bacterial Strain | Sample 1 | Sample 2 | Sample 3 | |||
| MIC (µg/mL) | MBC (µg/mL) | MIC (µg/mL) | MBC (µg/mL) | MIC (µg/mL) | MBC (µg/mL) | |
| S. aureus | 100 | 100 | 100 | 100 | 25 | 25 |
| S. epidermidis | 50 | 100 | 100 | 100 | 100 | 100 |
| C. diphtheria | 100 | 100 | 100 | 100 | 100 | 100 |
| Moraxella | 100 | 100 | 100 | 100 | 50 | 100 |
| S. aureus | 6.25 | 6.25 | 3.13 | 6.25 | 6.25 | 12.5 |
| S. epidermidis | 6.25 | 12.5 | 0.78 | 3.13 | 6.25 | 12.5 |
| C. diphtheria | 12.5 | 12.5 | 12.5 | 12.5 | 3.13 | 6.25 |
| Moraxella | 3.13 | 6.25 | 6.25 | 6.25 | 12.5 | 25 |
Disc diffusion assay
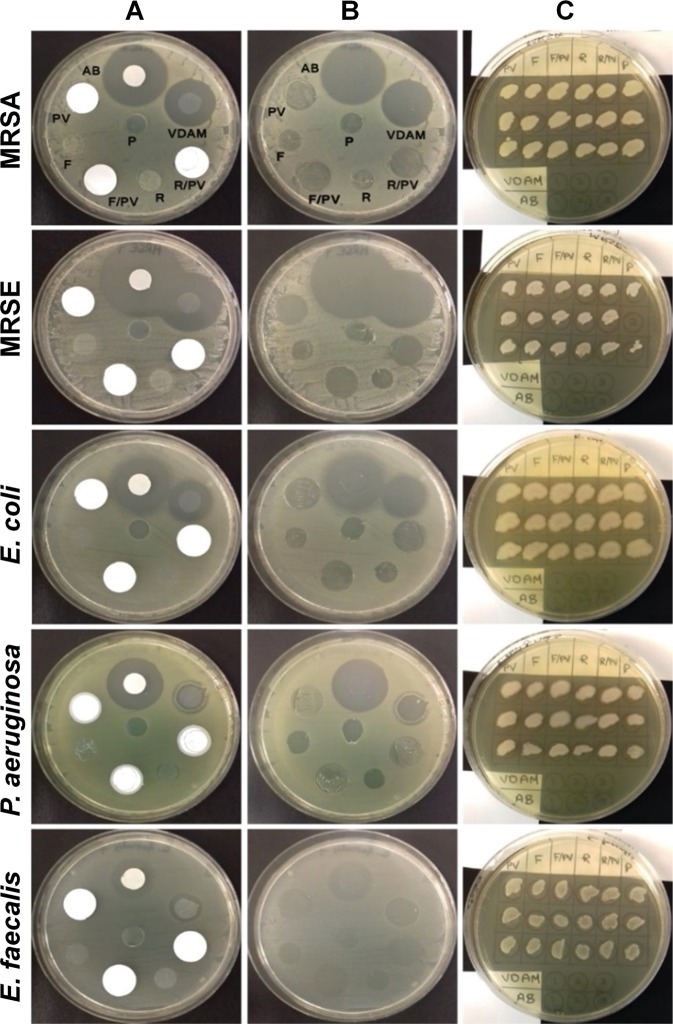
Results from the disc diffusion assay can be seen in Fig. 4. The only samples to form a zone of inhibition (ZOI) against all 5 bacteria studied were VDAM and the positive control (AB). Effects from VDAM on MRSA (ZOI: 10.74 mm) and MRSE (ZOI: 15.87 mm) were higher than on those of E. coli (ZOI: 8.82 mm), P. aeruginosa (ZOI: 2.48 mm) and E. faecalis plates (ZOI: 1.96 mm). All samples of VDAM processed without antibiotic (R) VDAM, processed without raffinose or antibiotic (F), and the PVDF sample discs were not bactericidal to the five strains and did not produce ZOI. However, once the discs were removed there was reduced bacterial growth found below the sample (Fig. 4B). To test the number of bacteria that remained once the discs had been removed, swabs were taken and performed on a new plate (Fig. 4C), before visual assessment. VDAM and AB controls had eliminated all bacteria found directly below them. All other samples showed that viable bacteria remained below where the disc had been placed.
Figure 4.
Antibacterial activity of various amniotic membrane preparations. Discs of 10 mm diameter were prepared of VDAM; non-raffinose and non-antibiotic treated AM (F), non-antibiotic treated AM (R) and antibiotic soaked Whatman no. 3 filter paper (AB) as a positive control. Polyvinylidene fluoride (PVDF) transfer membrane discs (PV) were used as a control to the following disc samples: F over PVDF (F/PV) and R over PVDF (R/PV). The transparent plastic material (P) was used as a carrier to transfer VDAM onto new for a further incubation period. All discs were laid on lawns of MRSA, MRSE, E. coli, P. aeruginosa and E. faecalis. Images show representative MHA plates after overnight incubation at 35 °C. (A) Bacterial lawns with discs still present. (B) Same plates after removal of samples. (C) Streaks from areas underneath discs, shown in B, onto lawns of corresponding bacteria on MHA plates after 35 °C overnight incubation. Experimental error caused MRSE sample P2 not to be streaked. Experiment conducted on three different donors.
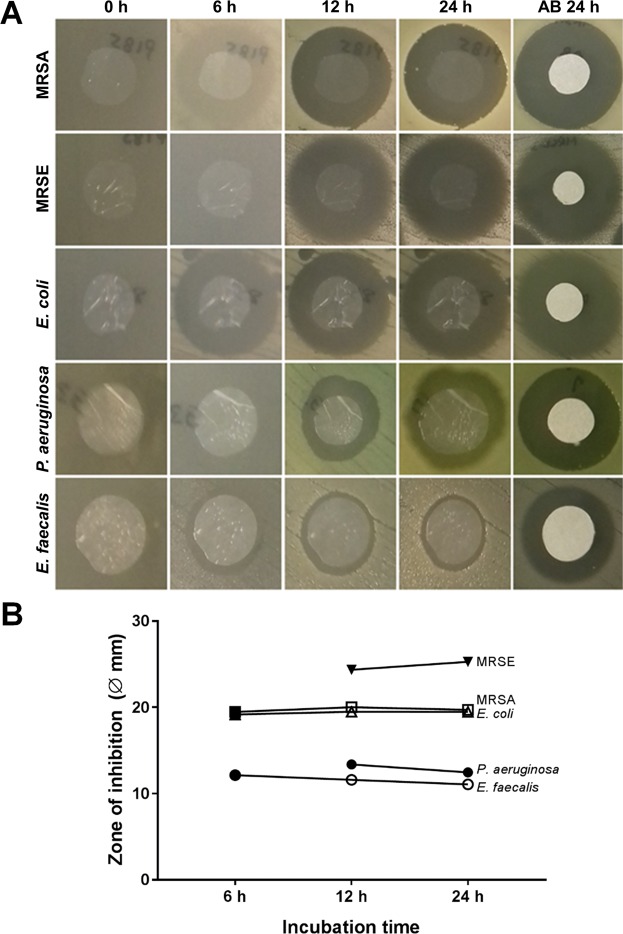
Diffusion assays performed over time (Fig. 5) showed that the antibacterial effects of the antibiotic release from VDAM could only be seen in full effect after certain time points. At 6 hours incubation, ZOI were measured from MRSA, E. coli and E. faecalis, but not from MRSE and P. aeruginosa. Notably, the antibiotic content of VDAM is released rapidly in the medium before the bacteria show a distinct growth in the first 6 hours.
Figure 5.
Antibiotic release from VDAM over time. (A) Antibiotic release from 10 mm diameter discs of VDAM placed on bacterial lawns. Representative images show zones of inhibition of the same disc after 6, 12 and 24 hours of incubation at 35 °C. Filter paper discs impregnated in antibiotic were used as control in every plate. Experiment performed on triplicate samples from 3 different donors and repeated 3 times for all bacteria. (B) Quantification of the zone of inhibition (including disc diameter) at the 6, 12 and 24 hour time points.
Sterility test of VDAM held in long-term storage
VDAM samples that had been stored, vacuum-packed, at 4–6 °C for over two years were placed into TSB or thioglycolate broth medium to test for contaminant growth. Of the 21 samples of 25 mm diameter that were tested, only one sample reported any growth, and only of aerobic bacteria. Two others from the same donor did not show any contamination. Of the 30 samples of 10 mm diameter, 1 sample showed contaminant growth of both aerobic and anaerobic bacteria. Once again, two other samples from the same donor, processed identically, showed no growth. These samples were judged to have been contaminated during test handling.
Discussion
The natural floral contamination of fresh AM obtained by ECS in the Queens’s Medical Centre, Nottingham University Hospitals NHS trust, was shown to be considerably lower than literature reported from normal vaginal deliveries20,24,25. 40% of the tested AM showed no signs of bioburden contamination, similar to results previously reported (39%)20. Of those that demonstrated contamination, only Micrococcus spp, Propionibacterium acnes, S. schleiferi and N. cinerea were identified, most likely from skin contact of the placenta during delivery. Previous reports have identified up to 12 species in their surveys including the ones named in our study20,24,26. Staphylococcus spp have been identified as the most prevalent contamination of AM15,20,26. This study indicates that the risk of major contamination from AM obtained through ECS is low, reducing the risk of contaminated AM being transplanted to the eye. Additional results from PBS samples collected immediately from raw AM before washing (incubated overnight at 35 °C on BA) contained no bioburden (n = 15 from 5 donors), demonstrating that fresh AM could be bacteriostatic before processing.
VDAM was developed to be stored dry at room temperature in a stable state until use, avoiding the cold chain storage and distribution challenges associated with fresh and frozen AM. A bioburden-free VDAM final product was confirmed during investigation into our protocol, and long-term sterility test results confirm sterility of end-product samples, even when artificially high levels of S. epidermidis were loaded during different stages of processing. S. epidermidis is an ocular pathogen and was selected as a model of staphylococcus genera which is one of the most prevalent causes of bacterial keratitis27. It is considered to be one of the skin floral species most likely to contaminate AM, and has also been shown to adhere well to fresh AM23.
Our results revealed that three successive washes in 0.9% NaCl reduced bioburden by 95.65%, in agreement with Germain et al., (2010) who reported that frequent washing can reduce bioburden in processed heart valves28. Most AM preparation protocols use repeated washing in physiological saline to remove blood and immunogens, as well as remains of amniotic fluid. However, we have found that to retain the integrity of the epithelial surface and matrix biomolecules appropriate washing times have to be optimised18. As an added safety precaution for preserved AM, different antibiotic/antimycotic combinations can be used to cover a wide range of gram-positive, gram-negative bacteria and fungi, including penicillin, streptomycin, neomycin, amphotericin B, gentamycin, imipenem, nystatin, polymyxin and vancomycin29,30. We used a combination of the latter five agents, a cocktail widely used in the UK for tissue allograft banking31. Reports show that antibiotic solutions used for tissue allograft preservation can be stored for up to one-year storage at −20 and −80 without effect on potency. Dried forms of these antibiotics can withstand storage at 37 °C for up to 12 months32.
The microbicide activity of antibiotic treatment was found to be more effective at 37 °C than at 4 °C or 22 °C28. Long antibiotic incubation, such as 12 hours at 37 °C, has been shown to be more efficient, but could potentially compromise the cell viability of tissue, including AM28,33,34. The current study showed that a two-hour incubation at 37 °C resulted in full eradication of bacteria loaded just before the antibiotic treatment step and that longer incubation times are not required. The antibiotic cocktail was capable of completely eradicating a high load of bacteria.
AM prepared in antibiotics can serve as a reservoir during transplantation, particularly effective against microbial keratitis frequently caused by different bacteria strains including the conjunctival normal flora S. epidermidis27,35,36. AM can absorb and release antibiotics after rehydration on a wet surface such as agar medium or the corneal surface. Our results showed that the pattern of VDAM release of antibiotic was consistent with the antibiotic control discs, with rapid release at the beginning of application (6 hours) without considerable change in ZOI after 12 and 24 hours of incubation on bacterial lawns. AM has been shown to have slow antibiotic release over seven hours, dependent on the time of AM soaking in antibiotic37, however the release profile may be different for different antibiotics38.
Dehydration of AM by air, heat or freeze-drying (lyophilisation) can be applied after washing procedures to decrease the water content of AM, hindering the spread of contamination during storage, and extending the shelf-life. Drying is applied as a final step before packaging, and has been found to maintain AM structural and biological molecules39. AM desiccated to lower or equal to 5% water content prevents microbial growth, thus there is less chance for product contamination. To confirm this, we showed that vacuum-drying alone, without antibiotic treatment, was efficient in removing 94.26% of bacteria, when samples were loaded after the raffinose washing step. Drying in our protocol is primarily applied to AM as a method of preservation and not as a decontamination step, however, so as it was not efficient in reducing all of the bacterial load it would only ever be used as an adjunct to antibiotic treatment for decontamination.
Previous studies have shown that AM antibacterial function after washing or prolonged rehydration was significantly reduced40. No ZOI was observed around washed AM against S. epidermidis38. Tehrani et al. (2013), showed a decrease in antibacterial activity against S. aureus, P. aeruginosa and E. coli of freeze dried AM after 2 hours rehydration, and after 3 times rinsing in PBS41. Our MIC/MBC in vitro study of AM antibacterial activity showed that AM processed without antibiotics has little effect on S. aureus, S. epidermidis, C. diphtheria and Moraxella, indicating that AM has little intrinsic antimicrobial action without the inclusion of antibiotics during the manufacture process. To then investigate the role of the included antibiotics, we then decided to use bacterial strains that may show more resistance to the included antibiotic cocktail in disc diffusion assays. The results of these assays showed antibiotic processed VDAM has a variant ZOI on MRSA (10.74 mm) and MRSE (15.87 mm). Reduced activity was observed on E. coli (8.82 mm), P. aeruginosa (2.48 mm) and E. faecalis (1.96 mm) plates, but was still present and in a consistent manner. AM processed without antibiotic and/or raffinose were ineffective against MRSA, MRSE, E. coli, P. aeruginosa and E. faecalis strains with no ZOI, further indicating that washed AM untreated with antibiotic is not bactericidal.
Moreover, visual assessment of confluence of growth underlying the discs showed that bacteria did grow slightly underneath the control discs of plastic, PVDF and untreated AM, but not under VDAM and antibiotic-soaked filter paper. AM antibacterial action by adhesion was higher against gram-positive bacteria compared to gram-negative bacteria. This could be explained by the differences in defence mechanisms between both groups of bacteria42, and implies that fresh AM may have only a static antimicrobial action by adherence rather than a bactericidal effect, in agreement with Elian et al, 199140. Placing fresh AM on PVDF membrane, to evaluate the biological antibacterial activity by anti-microbial peptide stripping, demonstrated that samples of AM without antibiotics have no such effects against any tested bacteria. These findings disagree with that of N. Kjaergaard et al., 2001, who showed that saline washed AM placed over a nitrocellulose paper was inhibitive to underlying bacterial growth of group B streptococcus, Staphylococcus saprophyticus, P. aeruginosa and S. aureus43.
The current study was limited to investigating whether the antibacterial action of the dried amnion was due to the intrinsic properties of amniotic membrane or due to the antibiotics added during processing. This study could have been further extended by investigating the individual activity of the antibiotics within the cocktail and by considering the effect of long-term storage on each of the individual antibiotics. The study could also be extended by investigating the anti-fungal properties of both amniotic membrane and the antibiotic/antimycotic cocktail added during processing.
AM processed under approved guidelines and as a licensed protocol results in better wound healing results and less post-transplant infections15. This effect is most likely due to antibiotic use, providing an antibiotic reservoir, substituting for the fresh AM’s lost antibacterial property and effective in prohibiting bacterial growth on wounds44. AM also functions as a physical barrier against infective pathogens40,45, with elastic, pliable properties allowing it to adhere completely to wounds23, preventing external infection recurrence.
The risk of major bioburden contamination of caesarean section collected AM is low. Nevertheless, the combined AM processing procedure used in this paper is a robust method to remove any bioburden, sub-clinical infection, and manufacturing-related contamination. Non-antibiotic processed AM may be bacteriostatic but has no bactericidal effect. The specific VDAM processing steps of washing in NaCl, spongy layer removal, raffinose incubation and drying, all contribute to significantly reduce the bacterial load, with drying and antibiotic treatment being considered a sterilisation step. The antibiotic reservoir of VDAM can eliminate natural and acquired contamination of gram-negative and gram-positive bacteria, such that VDAM is a reliably sterile product suitable for clinical application. Combined with vacuum packing, VDAM can be confidently and stably stored long-term without the risk of infection-related degradation.
Methods
Human amniotic membrane procurement and preparation
This study was carried out with the approval of the Nottingham Research Ethics Committee and the study (OY110101) complied with the tenets of the Declaration of Helsinki. Human placental samples were collected from consenting donors undergoing elective caesarean sections. Patients not complying with the inclusion criteria for tissue donation or having a history of antenatal problems were excluded. Written informed consent from the donor was obtained for sample use and the placenta membranes were prepared according to previously published methodology46.
Identification of the natural flora of amniotic membrane natural flora
AM samples (separated from the chorion membrane) from 10 different donors were studied for the natural bacterial flora contamination. Unwashed AM samples were collected in 5 mL PBS (Gibco®, ThermoFisherScientific, UK) before sonication for 5 minutes. 200 µL of each sample was plated onto blood agar plates (Blood Agar Base No. 2 with sheep blood, Oxoid, UK) and incubated aerobically for 48 hours and anaerobically for 7 days at 37 °C, before colony forming units (CFU)/mL were counted. 100 µL volumes of sample were inoculated into Anaerobic Basal Broth (Oxoid), for 48 hours and 7 days at 37 °C in aerobic conditions to enhance bacterial proliferation. Bacterial growth was identified by turbidity and API® Staph Rapid 32 A microbial identification kit (BioMerieux, UK) was used to identify microorganisms.
Vacuum-dried amniotic membrane (VDAM) processing
AM was separated from the chorion membrane by finger blunt dissection and triple washed in 250 mL 0.9% sodium chloride (NaCl) solution (Baxter, UK) for 15 minutes with rocking agitation to wash out blood. The spongy layer was removed by scalpel blade size 22 attached to a custom-made tool. The AM were soaked in 100 mM raffinose pentahydrate (Acros Organics, UK) solution in DPBS containing 5 mL of a broad-spectrum antibiotic/antifungal combination (Source Bioscience, UK, see Table 2) and incubated at 37 °C for 2 hours. To prepare for vacuum-drying, the AM was spread out epithelial side down over a sheet of parafilm and placed in a freeze dryer (Alpha 1–4 LSC, Advanced Freeze Dryer Christ® Osterode, Germany). Prior to sample drying the condenser was cooled to −45 °C to allow for vapour removal. Drying cycles consisted of a main dry phase of 30 minutes with a shelf temperature of 15 °C and vacuum pressure of 1.03 mbar followed by a final drying phase with a shelf temperature of 15 °C and vacuum pressure of 0.001 mbar for 15 minutes. After drying, AM was cut to the desired size and individually vacuum-packed in plastic pouches (Orved Multiple 315 Vacuum Sealer, Italy).
Validation of VDAM decontamination process
Bacterial culture
A pure culture of a clinical isolate of S. epidermidis was transferred from a BA plate into 20 mL universal tubes containing Tryptone Soya Broth (TSB) (Casein Soya Bean Digest Medium, CM 0129, Oxoid) and incubated overnight at 37 °C. The next day absorbance at 490 nm was measured using a Jenway 6705 UV/Vis Spectrophotometer (Fisher Scientific, Leicestershire, UK) and the bacterial suspension adjusted to 108 CFU/mL.
Bacterial loading of AM
Artificial loading of S. epidermidis was performed individually at three different stages of the VDAM process to assess the efficiency of the decontamination process at different steps (Fig. 1). To assess the entire process, the bacteria were loaded immediately after chorion removal and the samples taken and assessed for CFU at each stage. To assess the raffinose/antibiotic cocktail wash, the bacteria were loaded after spongy layer removal. To assess the drying stage bacteria were loaded after the incubation in raffinose (with/without antibiotic) and before vacuum drying. Sampling of unloaded and loaded tissues was performed throughout processing. Discs of 25 mm diameter were cut after collection of the AM. Bacteria inoculation was applied before each of the examined processing steps. To load the discs with S. epidermidis, a 2 mL microcentrifuge tube containing the AM sample in 1 mL PBS was inoculated with 100 µL of 108 CFU/mL S. epidermidis and agitated by rolling for 1 hour at 4 °C.
CFU counting
Loaded and fresh AM samples in 1 mL PBS were twice diluted to give 10−1 and 10−2 dilutions of each sample, then 200 µL of each dilution was spread onto each of three BA plates. The plates were incubated for 48 hours aerobically (37 °C). The average of viable colonies was then counted and expressed as CFU/mL.
Minimum inhibitory concentration/minimum bactericidal concentration
Dry AM discs from 3 donors were prepared with and without antibiotics. Proteins were then extracted from the tissue by grinding with pestle and mortar in a liquid nitrogen bath. AM extracts were then tested against 106 CFU/mL of gram-positive; Staphylococcus aureus (S. aureus), S. epidermidis, Corynebacterium diphtheriae, and gram-negative Moraxella lacunata bacterial strains. 50 µL/well of lysogeny broth (LB) medium (Sigma-Aldrich) was added to appropriate wells of a 96-well plate, followed by 50 µL of AM extract at concentrations of 100, 50, 25, 12.5, 6.25, 3.13, 1.56, 0.78, 0.39, 0.20, and 0.10 µg/mL loaded in triplicate across the plate. Culture medium only and medium with 100 mg/L gentamicin were used as negative and positive controls respectively. The bacterial strains were grown up in LB medium until optical density = 0.1 at 600 nm. 50 µL/well of the bacterial cell suspension added to each well of the plate. Plates were incubated overnight at 200 rpm on a rocker at 37 °C. Results were assessed visually with the minimum inhibitory concentration (MIC) equivalent to the last clear well, where bacterial growth is completely inhibited, and the minimum bacterial concentration (MBC) equivalent to the lowest concentration of test article required to kill 99.9% of the initial bacterial inoculum.
Disc diffusion assay
The following AM samples were prepared for disc diffusion assays; VDAM processed with raffinose and antibiotic (VDAM), VDAM processed in raffinose without antibiotic (R) and VDAM without raffinose or antibiotic (F). All samples were cut into discs of 10 mm diameter and kept in vials at 4 °C for a maximum of 4 days before the experiment was performed. Cultures of multidrug resistant S. aureus (MRSA), multidrug resistant S. epidermidis (MRSE), Enterococcus faecalis, Escherichia coli, and Pseudomonas aeruginosa bacteria were prepared from fresh pure cultures in TSB. The viable bacterial count of each was adjusted to 108 CFU/mL using 0.5 McFarland standard. Each of the five bacterial cultures were seeded onto three Mueller-Hinton agar (MHA) (Oxoid) plates in 90 mm Petri dishes using a sterile cotton swab and subsequently the AM discs were aseptically placed on the even lawns of the bacterial strains. An antibiotic-soaked disc of Whatman no.3 filter paper (AB) was performed as a positive control and a disc of transparent laminating plastic (P) was used as a negative control. Three additional discs were also assessed, using hydrophobic highly stripping polyvinylidene fluoride (PVDF) transfer membrane discs, pore size 0.45 µm (Hybond™-P, Amersham Biosciences, UK), one of which was used alone as a control (PV). The PVDF was then used to separate the MHA surface and VDAM-R (R/PV) and VDAM-F (F/PV) to assess biological antibacterial activity, rather than physical blocking. MHA plates were incubated at 35 °C for 18–24 hours and zone of inhibition (ZOI) were measured in mm using a digital calliper (Mitutoyo Absolute Digimatic Caliper, UK). To confirm whether there was any bacterial growth beneath the samples, sterile disposable loops were used to touch areas under the discs of the same bacterial plates and streak on another Tryptone Soya Agar (TSA, Casein soya bean digest agar, CM0131, Oxoid) plate before overnight incubation at 35 °C.
To specifically investigate time line of antibiotic release from VDAM samples, ZOI were measured at 0, 6, 12 and 24 hours.
Sterility test of VDAM held in long-term storage
Triplicate samples of 25 mm diameter (average storage time 757 days, 7 donors each with 3 samples) and 10 mm diameter (average storage time 862 days, 10 donors each with 3 samples) VDAM discs were tested for sterility. Discs were either aseptically immersed in falcon tubes containing 40 mL TSB for aerobic bacteria, facultative bacteria and fungus, or placed in thioglycolate broth medium U.S.P. (CM0173, Oxoid) for anaerobic and facultative bacteria. TSB tubes were incubated at 35 °C with shaking (80 rpm), for 21 days along with negative and positive control tubes. Thioglycolate medium tubes were incubated for 21 days at 35 °C without shaking. Turbidity was used as an indicator of non-sterility and was checked daily. Streaking of 10 µL TSB from each tube was performed weekly on TSA plates, which were subsequently incubated for 5 days at 37 °C and observed for bacterial growth.
Statistical analysis
Statistical analysis was performed using GraphPad Prism version 7.01.
Acknowledgements
This study was funded by The Authority for Research, Science and Technology of the Ministry of Higher Education and Scientific Research-Libya (Scholarship 404/2013), an Innovate UK Biomedical Catalyst Feasibility Award (132044) and the Defence Science & Technology Laboratory, UK.
Author Contributions
The study was conceived and coordinated by A.H., C.A., L.S. and R.B. Experiments were conducted by N.M., C.A. and W.A., with support on experimental design by C.A., L.S., E.B. and O.M. The data was analysed by N.M., C.A. and L.S. Figures were created by N.M. and L.S. and the manuscript written by N.M., L.S., O.M. and E.B. All authors reviewed the manuscript and accepted the final version.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing Interests
Dr. Hopkinson and Dr. Allen own shares in NuVision Biotherapies. Dr. Hopkinson, Dr. Britchford and Mr. McIntosh were partially employed by NuVision Biotherapies whilst this study took place. Dr. Sidney, Mr. Ashraf and Prof. Bayston declare no potential conflict of interest.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Nagi M. Marsit and Laura E. Sidney contributed equally.
References
- 1.Azuara-Blanco A, Pillai C, Dua HS. Amniotic membrane transplantation for ocular surface reconstruction. Br. J. Ophthalmol. 1999;83:399–402. doi: 10.1136/bjo.83.4.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gicquel JJ, Bejjani RA, Ellies P, Mercie M, Dighiero P. Amniotic membrane transplantation in severe bacterial keratitis. Cornea. 2007;26:27–33. doi: 10.1097/ICO.0b013e31802b28df. [DOI] [PubMed] [Google Scholar]
- 3.Liu J, Sheha H, Fu Y, Liang L, Tseng SCG. Update on amniotic membrane transplantation. Expert Rev. Ophthalmol. 2010;5:645–661. doi: 10.1586/eop.10.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim JS, Kim JC, Hahn TW, Park WC. Amniotic membrane transplantation in infectious corneal ulcer. Cornea. 2001;20:720–726. doi: 10.1097/00003226-200110000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Bouchard CS, John T. Amniotic membrane transplantation in the management of severe ocular surface disease: Indications and outcomes. Ocul. Surf. 2004;2:201–211. doi: 10.1016/S1542-0124(12)70062-9. [DOI] [PubMed] [Google Scholar]
- 6.Tseng SC. Amniotic membrane transplantation for ocular surface reconstruction. Biosci. Rep. 2001;21:481–489. doi: 10.1023/A:1017995810755. [DOI] [PubMed] [Google Scholar]
- 7.Chen H-C, et al. Amniotic membrane transplantation for persistent corneal ulcers and perforations in acute fungal keratitis. Cornea. 2006;25:564–572. doi: 10.1097/01.ico.0000227885.19124.6f. [DOI] [PubMed] [Google Scholar]
- 8.Dua HS. Amniotic membrane transplantation. Br. J. Ophthalmol. 1999;83:748–752. doi: 10.1136/bjo.83.6.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tabatabaei SA, et al. A randomized clinical trial to evaluate the usefulness of amniotic membrane transplantation in bacterial keratitis healing. Ocul. Surf. 2017;15:218–226. doi: 10.1016/j.jtos.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 10.Dua HS, Rahman I, Miri A. Variations in amniotic membrane: Relevance for clinical applications. Br. J. Ophthalmol. 2010;94:963–964. doi: 10.1136/bjo.2009.157941. [DOI] [PubMed] [Google Scholar]
- 11.Khokhar SMD, Sharma NMD, Kumar HMD, Soni AMD. Infection after use of nonpreserved human amniotic membrane for the reconstruction of the ocular surface. Cornea. 2001;20:773–774. doi: 10.1097/00003226-200110000-00023. [DOI] [PubMed] [Google Scholar]
- 12.Cheng AM, et al. Accelerated restoration of ocular surface health in dry eye disease by self-retained cryopreserved amniotic membrane. Ocul. Surf. 2016;14:56–63. doi: 10.1016/j.jtos.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koob TJ, et al. Biological properties of dehydrated human amnion/chorion composite graft: Implications for chronic wound healing. Int. Wound J. 2013;10:493–500. doi: 10.1111/iwj.12140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paolin A, et al. Cytokine expression and ultrastructural alterations in fresh-frozen, freeze-dried and gamma-irradiated human amniotic membranes. Cell Tissue Bank. 2016;17:399–406. doi: 10.1007/s10561-016-9553-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marangon FB, et al. Incidence of microbial infection after amniotic membrane transplantation. Cornea. 2004;23:264–269. doi: 10.1097/00003226-200404000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Hopkinson A, McIntosh RS, Tighe PJ, James DK, Dua HS. Amniotic membrane for ocular surface reconstruction: Donor variations and the effect of handling on tgf-beta content. Invest. Ophthalmol. Vis. Sci. 2006;47:4316–4322. doi: 10.1167/iovs.05-1415. [DOI] [PubMed] [Google Scholar]
- 17.Kruse F, et al. Cryopreserved human amniotic membrane for ocular surface reconstruction. Graefes Arch. Clin. Exp. Ophthalmol. 2000;238:68–75. doi: 10.1007/s004170050012. [DOI] [PubMed] [Google Scholar]
- 18.Allen CL, et al. Augmented dried versus cryopreserved amniotic membrane as an ocular surface dressing. PloS one. 2013;8:e78441. doi: 10.1371/journal.pone.0078441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marsit NM, Sidney LE, Branch MJ, Wilson SL, Hopkinson A. Terminal sterilization: Conventional methods versus emerging cold atmospheric pressure plasma technology for non-viable biological tissues. Plasma Process. Polym. 2017;14:e1600134. doi: 10.1002/ppap.201600134. [DOI] [Google Scholar]
- 20.Aghayan HR, et al. Bacterial contamination of amniotic membrane in a tissue bank from iran. Cell Tissue Bank. 2013;14:401–406. doi: 10.1007/s10561-012-9345-x. [DOI] [PubMed] [Google Scholar]
- 21.Marsit N, et al. Safety and efficacy of human amniotic membrane in primary pterygium surgery. Cell Tissue Bank. 2016;17:407–412. doi: 10.1007/s10561-016-9554-9. [DOI] [PubMed] [Google Scholar]
- 22.Singh R, et al. Radiation resistance of the microflora associated with amniotic membranes. World J. Microbiol. Biotech. 2006;22:23–27. doi: 10.1007/s11274-005-2890-8. [DOI] [Google Scholar]
- 23.John T, Allen S, John AG, Lai CI, Carey RB. Staphylococcus epidermidis adherence to human amniotic membrane and to human, rabbit, and cat conjunctiva. J. Cataract Refract. Surg. 2003;29:1211–1218. doi: 10.1016/S0886-3350(02)01980-6. [DOI] [PubMed] [Google Scholar]
- 24.Adds PJ, Hunt C, Hartley S. Bacterial contamination of amniotic membrane. Br. J. Ophthalmol. 2001;85:228–230. doi: 10.1136/bjo.85.2.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Binte Atique F, Ahmed KT, Asaduzzaman SM, Hasan KN. Effects of gamma irradiation on bacterial microflora associated with human amniotic membrane. BioMed research international. 2013;2013:586561. doi: 10.1155/2013/586561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Souza CEB, et al. Evaluation of microbiological contamination of amniotic membrane and amniotic fluid. Arq. Bras. Oftalmol. 2004;67:709–712. doi: 10.1590/S0004-27492004000500003. [DOI] [Google Scholar]
- 27.Sotozono CMDPD, et al. Methicillin-resistant staphylococcus aureus and methicillin-resistant staphylococcus epidermidis infections in the cornea. Cornea. 2002;21:S94–S101. doi: 10.1097/01.ico.0000263127.84015.3f. [DOI] [PubMed] [Google Scholar]
- 28.Germain M, Thibault L, Jacques A, Tremblay J, Bourgeois R. Heart valve allograft decontamination with antibiotics: Impact of the temperature of incubation on efficacy. Cell Tissue Bank. 2010;11:197–204. doi: 10.1007/s10561-009-9155-y. [DOI] [PubMed] [Google Scholar]
- 29.Dekaris I, Gabric N. Preparation and preservation of amniotic membrane. Dev. Ophthalmol. 2009;43:97–104. doi: 10.1159/000223842. [DOI] [PubMed] [Google Scholar]
- 30.Agraval U, Rundle P, Rennie IG, Salvi S. Fresh frozen amniotic membrane for conjunctival reconstruction after excision of neoplastic and presumed neoplastic conjunctival lesions. Eye. 2017;31:884–889. doi: 10.1038/eye.2016.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parker, R. In Essentials of tissue banking Vol. 1 (ed. George Galea) Ch. 5, 69–80 (Springer Netherlands, 2010).
- 32.Mathijssen NM, et al. Impregnation of bone chips with antibiotics and storage of antibiotics at different temperatures: An in vitro study. BMC Musculoskelet. Disord. 2010;11:96. doi: 10.1186/1471-2474-11-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aykut V, Celik U, Celik B. The destructive effects of antibiotics on the amniotic membrane ultrastructure. Int. Ophthalmol. 2015;35:381–385. doi: 10.1007/s10792-014-9959-z. [DOI] [PubMed] [Google Scholar]
- 34.Gall KL, Smith SE, Willmette CA, O’Brien MF. Allograft heart valve viability and valve-processing variables. Ann. Thorac. Surg. 1998;65:1032–1038. doi: 10.1016/S0003-4975(98)00085-X. [DOI] [PubMed] [Google Scholar]
- 35.Jensen O, Gluud B. Bacterial growth in the conjunctival sac and the local defense of the outer eye. Acta Ophthalmol. 1985;63:80–82. doi: 10.1111/j.1755-3768.1985.tb06849.x. [DOI] [PubMed] [Google Scholar]
- 36.Goodman DF, Gottsch JD. Methicillin-resistant staphylococcus epidermidis keratitis treated with vancomycin. Arch. Ophthalmol. 1988;106:1570–1571. doi: 10.1001/archopht.1988.01060140738046. [DOI] [PubMed] [Google Scholar]
- 37.Resch MD, et al. Drug reservoir function of human amniotic membrane. J. Ocul. Pharmacol. Ther. 2011;27:323–326. doi: 10.1089/jop.2011.0007. [DOI] [PubMed] [Google Scholar]
- 38.Mencucci R, Menchini U, Dei R. Antimicrobial activity of antibiotic-treated amniotic membrane: An in vitro study. Cornea. 2006;25:428–431. doi: 10.1097/01.ico.0000214207.06952.23. [DOI] [PubMed] [Google Scholar]
- 39.Paolin A, et al. Cytokine expression and ultrastructural alterations in fresh-frozen, freeze-dried and γ-irradiated human amniotic membranes. Cell and tissue banking. 2016;17:399–406. doi: 10.1007/s10561-016-9553-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Inge E, Talmi YP, Sigler L, Finkelstein Y, Zohar Y. Antibacterial properties of human amniotic membranes. Placenta. 1991;12:285–288. doi: 10.1016/0143-4004(91)90010-D. [DOI] [PubMed] [Google Scholar]
- 41.Tehrani FA, Ahmadiani A, Niknejad H. The effects of preservation procedures on antibacterial property of amniotic membrane. Cryobiology. 2013;67:293–298. doi: 10.1016/j.cryobiol.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 42.Malanovic N, Lohner K. Gram-positive bacterial cell envelopes: The impact on the activity of antimicrobial peptides. Biochim. Biophys. Acta. 2016;1858:936–946. doi: 10.1016/j.bbamem.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 43.Kjaergaard N, et al. Antibacterial properties of human amnion and chorion in vitro. Eur. J. Obstet. Gynecol. Reprod. Biol. 2001;94:224–229. doi: 10.1016/S0301-2115(00)00345-6. [DOI] [PubMed] [Google Scholar]
- 44.Malhotra C, Jain AK. Human amniotic membrane transplantation: Different modalities of its use in ophthalmology. World J. Transplant. 2014;4:111–121. doi: 10.5500/wjt.v4.i2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Singh R, Gupta P, Kumar P, Kumar A, Chacharkar M. Properties of air dried radiation processed amniotic membranes under different storage conditions. Cell Tissue Bank. 2003;4:95–100. doi: 10.1023/B:CATB.0000007030.72031.12. [DOI] [PubMed] [Google Scholar]
- 46.Hopkinson A, et al. Optimization of amniotic membrane (am) denuding for tissue engineering. Tissue Eng. Part C Methods. 2008;14:371–381. doi: 10.1089/ten.tec.2008.0315. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.