Graphical abstract
Keywords: Cloudy apple juice, Cold plasma, Spark discharge, PPO inactivation, Quality parameters, Shelf life study
Highlights
-
•
Cold plasma spark discharge was effective for PPO inactivation in cloudy apple juice.
-
•
Enzyme inactivation was dependent on treatment time.
-
•
No reactivation of PPO was found during storage in any plasma treated sample.
-
•
Cold plasma improved color of the juice after longest treatments.
-
•
Antioxidant capacity of the juice was increased after cold plasma treatments.
Abstract
Direct cold plasma treatment has been investigated as an alternative non-thermal technology as a means of maintaining and improving quality of fresh cloudy apple juice. Process variables studied included type of plasma discharge, input voltage and treatment time on polyphenol oxidase (PPO) inactivation. Spark discharge plasma at 10.5 kV for 5 min was the best treatment, with near total inactivation of PPO achieved, although good PPO inactivation was also recorded using shorter treatment times. Residual activity (RA) of PPO was 16 and 27.6% after 5 and 4 min of treatment respectively. This PPO inactivation was maintained throughout the storage trials, but decreased with samples treated for a shorter time. Plasma treatment improved key quality parameters of Golden delicious cloudy apple juice, with retention of critical quality parameters during extended storage trials. Color was the most noticeable change, which was enhanced with retention of a greener color. An increase of 69 and 64% was obtained in the total phenolic content after 4 and 5 min of treatment, respectively. Therefore, cold plasma was demonstrated to be a good alternative to traditional heat treatments for enhanced quality retention of fresh cloudy apple juice and over its storage.
1. Introduction
Fruit juices are rich in bioactive compounds and meet consumer demand for healthy, tasty and practical foods, which encourages consumption (Faria et al., 2017). It has been shown that consuming cloudy apple juice can be more beneficial to health than clarified juice, since cloudy apple juice is a good source of bioactive and prebiotic compounds such as ascorbic acid, polyphenols, and pectin (Fonteles and Rodrigues, 2018, Oszmianski et al., 2007). For cloudy apple juice, the most important factor responsible for quality degradation is enzymatic browning, which is catalyzed by polyphenol oxidase (PPO) through the oxidation of naturally present polyphenols. The production of juices with fresh-like characteristics and stable quality during processing and storage remains a challenge for the beverage industry (Rodríguez, Gomes, Rodrigues, & Fernandes, 2017). In recent decades, the development of non-thermal technologies such as cold plasma, pressurized fluid processes, ultrasonication and high-pressure applications has been an important aim of food technology research as alternatives to thermal treatments for foods quality retention. The effect of High Pressure Carbon Dioxide (HPCD) on PPO and other enzymes have been extensively studied on different fruit juices by Briongos et al., 2016, Illera et al., 2018, Illera et al., 2018, Zhou et al., 2009 among others. Thermosonication has also emerged as an alternative for enzymatic inactivation (Abid et al., 2015, Illera et al., 2018, as well as Pulsed Electric Fields (Aguiló-aguayo et al., 2009, Yang et al., 2004). Cold plasma is a promising non-thermal technology for foods that is already recognized as an industrial surface functionalization and decontamination method. Plasma is known as the fourth state of matter, consisting of an ionized gas composed of electrons, ions, radicals and atoms which are both in ground and excited states (Tappi et al., 2014). Plasma can be generated in any neutral gas by providing enough energy for its ionization (Pankaj & Keener, 2017). Cold plasma is generated at 30–60 °C and it does not present thermodynamic equilibrium (Coutinho et al., 2018). Some advantages of this technology are short treatment times and reduced thermal effects, which makes it suitable for treating heat sensitive food (Mandal, Singh, & Singh, 2018). When air is used as working gas the formation of reactive oxygen species (ROS) and reactive nitrogen species (RNS) occurs, and they can interact with different compounds present in the food, altering their properties (Ramazzina et al., 2016). Cold plasma has been applied to a wide range of foods including dairy products (Coutinho et al., 2019, Silveira et al., 2019, Silveira et al., 2019), meat (Lee et al., 2018) and bakery products (Bahrami et al., 2016) among others. However, few studies have evaluated or understood the effect of cold plasma on enzymatic inactivation. Furthermore, existing studies were mainly focused on solid fruits; tomato (Ma et al., 2015, Misra et al., 2014), kiwifruit (Ramazzina et al., 2015) or melon (Tappi et al., 2016). The non-thermal treatment and impact on the quality enhancement of fruit juices is an increasingly important area of study for high economic and nutritional value beverages, including apple juice (Liao et al., 2018, Xiang et al., 2018), blueberry juice (Hou et al., 2019) or pomegranate juice (Putnik et al., 2016, Putnik et al., 2019). Thus, we addressed the gap identified in relation to quality retention of high value fresh cloudy apple juice and optimized a bench scale cold plasma process using air as the inducer gas. A custom built system that allows control of key plasma reactive species through changing the mode of discharge was employed (Lu et al., 2017, Lu et al., 2017). Direct treatment to liquid was used, with two discharge modes initially compared for controlled effects on PPO inactivation; corona glow discharge and spark discharge. The effect of cold plasma enzyme inactivation depends on gas composition, electrical input, exposure mode, treatment time and food composition (Coutinho et al., 2018, Liao et al., 2017). In the present study, these variables were studied for PPO inactivation in cloudy apple juice along with other critical quality parameter responses of color, cloud stability, polyphenol content and antioxidant activity evaluated after spark discharge plasma treatments and during 28 days of storage at 4 °C, which have not been examined to date.
2. Materials and methods
2.1. Sample preparation
2.1.1. Cloudy apple juice
Golden delicious apples were peeled and cubed. To avoid enzymatic browning during processing, apple cubes were submerged in a 0.3% of l-ascorbic acid solution for 5 min and then they were wiped and immediately squeezed with a juicer (Breville JE3). The juice was filtered and analyzed immediately after preparation, which was considered as untreated sample (control). Cloudy apple juice was stored frozen (−18 °C) in 25 mL tubes until required.
2.1.2. Pure PPO solution
In order to compare PPO inactivation between a pure enzymatic solution and in apple juice, a pure Polyphenol oxidase solution was prepared. The enzyme used was tyrosinase from mushroom (Sigma-Aldrich®). Following the manufactureŕs recommendation the enzyme was diluted in a 0.1 M sodium phosphate buffer pH = 6.5 (enzyme optimum pH = 6–7). Final enzyme concentration was 0.04 mg/mL. Activity of PPO was measured right after preparation and considered as untreated sample. Enzyme solution was frozen (−18 °C) until further use.
2.2. Cold plasma treatments
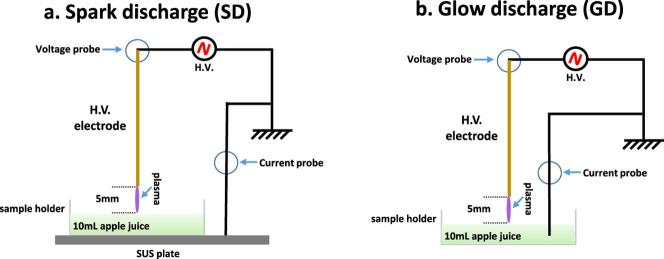
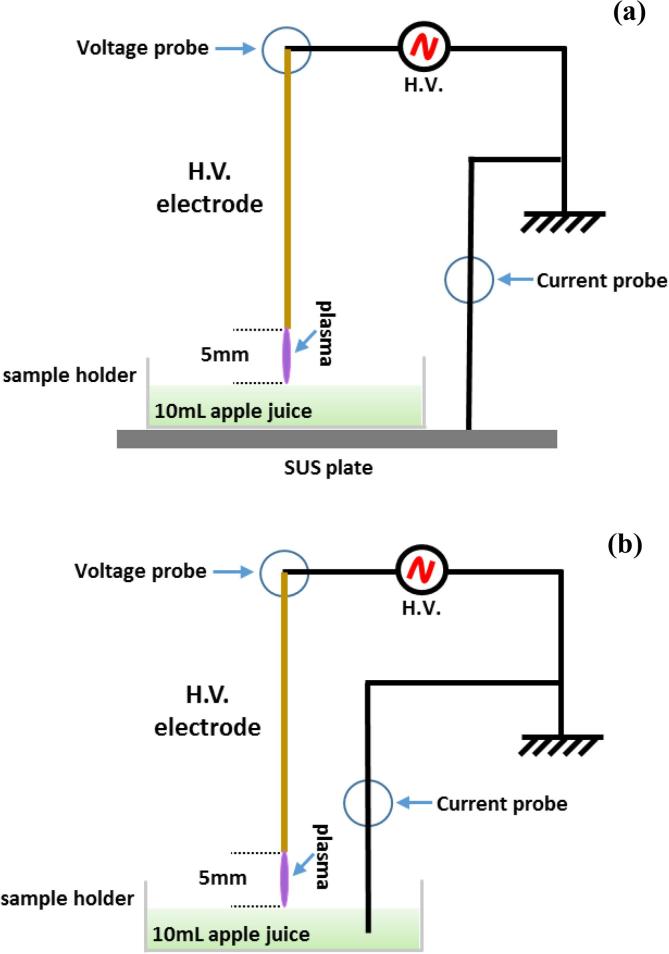
Two types of electrical discharge configurations were employed (Fig. 1); namely spark or glow discharge to liquid, with system characteristics and diagnostics previously reported in detail (Lu et al., 2017, Lu et al., 2017). Briefly, in both setups, a stainless-steel needle served as the high voltage (HV) electrode and was fixed perpendicular to the juice surface. The distance between the HV needle tip and the juice surface was fixed to 5 mm in all experiments. For each treatment of the juice or model enzyme solution, 10 mL of the liquid sample was accurately added into a plastic petri dish (55 mm inner dimeter) serving as a sample holder, which corresponded to a liquid layer of about 4.2 mm depth. To realize two different discharge modes, the ground electrode connection was adjusted in two setups. In Fig. 1(a), the plastic petri dish was placed on a stainless-steel plate which was connected to the ground; in Fig. 1(b) a thin ground electrode rod was submerged into the juice sample contained in the petri dish. The two electrical discharge configurations were investigated in order to compare their effect on PPO inactivation on cloudy apple juice as they can generate different plasma reactive species profiles (Lu et al., 2017, Lu et al., 2017). Both treatments are plasma discharge to liquid sample, with both discharges operated in open air. The power supply used for driving plasma discharges was a HV half bridge resonant inverter circuit (PVM500, Information unlimited). Its maximum output voltage was 20 kV with a variable frequency of 20–65 kHz depending on the plasma load capacitance. Applied voltage and discharge current were monitored by a Tektronix P6015A HV probe and an ELDITEST CP6990 current probe, respectively. The HV probe and the current probe were connected to the high-impedance inputs of an InfiniVision DSO-2014A oscilloscope (Agilent Technologies, 100-MHz bandwidth and 2-G Samples/s sampling rate).
Fig. 1.
Schematic of plasma discharge on juice, (a) spark discharge, (b) glow discharge. Adapted from Lu et al., 2017, Lu et al., 2017.
The effect of input power in spark discharge was evaluated in apple juice using a voltage range from 7.9375 to 10.875 kV to determine the optimum voltage. Then, using the optimum voltage level, the effect of treatment time was studied from 1 to 5 min in both cloudy apple juice and in pure enzyme solution. Measurement of PPO activity and physical-chemical analysis of samples were carried out immediately after each treatment.
2.3. Physico-chemical analysis
2.3.1. Determination of polyphenol oxidase (PPO) activity
The activity of PPO was determined spectro-photometrically. It was analyzed by mixing 100 µL of sample with 2.9 mL of substrate solution, which was a 0.05 M catechol (Sigma Aldrich®) solution prepared in a 0.1 M phosphate buffer (pH 6.5), which was kept at 30 °C in a water bath. Oxidation of catechol was determined immediately by the increase of absorbance at 420 nm during 120 s by using a spectrophotometer (DR6000 Hach Lange). The solution of catechol was used as blank. PPO activity was taken as the very first linear part of the reaction curve (Illera, Sanz, Beltran, & Solaesa, 2018).
Relative residual activity of PPO was expressed as:
| (1) |
2.3.2. Determination of pH, color and non-enzymatic browning
pH of cloudy apple juice was determined with a pH-meter (Eutech™ pH 700) at room temperature (21 ± 1 °C).
Color was measured at room temperature (21 ± 1 °C) using an UltraScan PRO colorimeter (Hunterlab, Inc.). The observation angle was 10°, equal to the perception of a human observer, and illuminant D65 was used (daylight source), following the CIE recommendations. For calibration, a white reference standard was used before the measurements. An appropriate cell was filled with 20 mL of cloudy apple juice and color was measured. Color parameters obtained were L* (lightness), a* (redness) and b* (yellowness) and the CIE L*, a*, b* parameters were used to report the total change in color (ΔE) (Eq. (2)).
| (2) |
Depending on the value of ΔE, differences in perceivable color can be analytically classified as very distinct (ΔE > 3), distinct (1.5 < ΔE < 3) and small difference (ΔE < 1.5) (Misra et al., 2014).
Non-enzymatic browning (NEB) was determined spectrophotometrically. For its determination, the method described by Queiroz et al. (2011) was applied with slight modifications; 0.7 mL of apple juice was mixed with 0.7 mL of ethanol in a 1.5 mL Eppendorf tube. The mixture was centrifuged at 12,000g for 10 min (Legend Micro 21R, Thermo Scientific™). Supernatant was recovered and 1 mL was mixed with 1 mL of a trichloroacetic acid solution (734 mM) and 1 mL of a thiobarbituric acid solution (25 mM). Samples were incubated at 40 °C during 50 min, and then, absorbance was measured at 443 nm at room temperature (21 ± 1 °C). A blank was also prepared with distilled water instead of juice. Non enzymatic browning of juice was expressed as the absorbance value at 443 nm.
2.3.3. Determination of cloud value, cloud stability and particle size distribution (PSD)
Cloud value was measured according to the method described by Versteeg, Rombouts, Spaansen, and Pilnik (1980) with slight modifications. 4 mL of juice were centrifuged at 760g during 10 min in a thermostatic centrifuge at 4 °C, then the supernatant was collected and its absorbance was measured at 660 nm.
Cloud stability was determined using the method of Baslar and Ertugay (2014). 4 mL of juice were centrifuged at 4200g for 15 min, and the absorbance of the supernatant was measured at 625 nm. Cloud stability was calculated according to Eq. (3):
| (3) |
where Ac and A0 are the absorbance after and before centrifugation respectively. In both determinations, a spectrophotometer (DR6000 Hach Lange) was used for the measurements and distilled water was used as blank.
Particle size distribution (PSD) was determined by laser diffraction with a Mastersizer Hydro-2000S (Malvern® Inst., MA) following the method used by Illera, Sanz, Benito-Román et al. (2018). Apple juice was added to a stirred vessel containing distilled water, until an obscuration level of more than 10% was achieved. The system uses a laser light to size particles from 0.02 to 2000 µm by light diffraction. Particle size distribution was calculated by the Fraunhofer model. Size distributions (volume fractions against particle size) were calculated and the weight-average sizes expressed as:
-
•
The equivalent surface area mean diameter:
| (4) |
-
•
The equivalent volume mean diameter:
| (5) |
where dlc is the diameter of the particle and nc is the percentage of particles.
The Span value was also evaluated:
| (6) |
where dv,0.9, dv,0.1 and dv0.5 are the particle size bellow which, 90%, 50% and 10% of the particles lies, and it describes distribution width.
2.3.4. Determination of total phenolic content and antioxidant capacity
Total phenolic content (TPC) was determined by using the Folin-Ciocalteau method described by Illera, Sanz, Benito-Román et al. (2018). First, 100 µL of juice were mixed with 2.8 mL of water and subsequently with 100 µL of the Folin-Ciocalteau reagent (VWR), in that order. After that, 2 mL of sodium carbonate 7.5% (w/v) were added to the mixture and the reaction immediately started. Color was measured spectrophotometrically at 750 nm after 60 min of reaction at room temperature (21 ± 1 °C). A blank was also prepared using water instead of juice. Total phenolic content retention of juice was expressed as:
| (7) |
Antioxidant capacity was determined by the DPPH assay described by Illera, Sanz, Beltran, and Solaesa (2018) with modifications. This method is based on the discoloration of the radical 2,2-diphenil-1-picrylhydrazyl, which occurs in presence of an oxidant agent. DPPH was prepared in advance, consisting of 50.7 μM DPPH in methanol and kept in the dark for a minimum of 4 h before use. For the antioxidant test, juice samples were diluted 1:10 with deionized water. The reaction took place when 300 μL of diluted cloudy apple juice were mixed with 3 mL of DPPH solution. Absorbance was measured at 517 nm at room temperature after 60 min of reaction in the dark. Results were expressed as inhibition percentage of the radical.
2.4. Shelf life study
Physico-chemical parameters and PPO activity were analyzed in apple juice directly after cold plasma treatments. Then, apple juice samples were stored at 4 °C and periodically analyzed every 7 days up to 28 days to determine key parameters evolution. PPO activity of pure enzyme solution was studied immediately after the treatments and 24 h later (4 °C).
2.5. PPO inactivation kinetics
Different kinetic models were tested to fit the evolution of the residual enzymatic activity as a function of time:
2.5.1. Weibull model
The non-linear Weilbull model can be written in the power-law form as (Van Boekel, 2002):
| (8) |
where A0 is the initial activity of the enzyme, A is the residual activity at different treatment times (t), b is the non-linear rate parameter and n is the shape factor.
2.5.2. Two-fraction kinetic model
This model takes into account the existence of several isoenzymes of PPO in apple juice, grouped into two fractions, a labile and a stable fraction. Both enzymes were considered to be inactivated according to first-order kinetics, but independently of each other (Briongos et al., 2016):
| (9) |
where AL and AS (AS = 1-AL) are the activity of the labile and stable fractions respectively and kL and kS (min−1) the inactivation rate constants of both the labile and stable fractions respectively.
2.6. Statistical analysis
All analyses were conducted using software Statgraphics X64. The results are presented as a mean ± standard deviation of at least three replicates. The significance of the differences was determined based on an analysis of the variance with the Tukey's honestly significant difference (HSD) method at p-value ≤ 0.05.
To estimate the kinetic parameters for the different models tested in this work, non-linear regression was performed by using the Marquardt algorithm (Statgraphics X64). The mean relative deviation (MRD) between experimental and calculated residual activities was also evaluated:
| (10) |
3. Results and discussion
3.1. Effect of plasma treatment on PPO activity
After preliminary studies using PPO inactivation as the indicator, spark discharge mode was found to be more effective for PPO inactivation of cloudy apple juice than glow discharge configuration. No significant difference was found after 1 min of treatment, since RA was 85.9 ± 1.4 and 82.9 ± 3.7 after glow and spark treatments respectively, but after a treatment of 5 min the difference was much higher, being in this case RA 55.5 ± 2.5 and 16.6 ± 2.2. Lu et al., 2017, Lu et al., 2017 explained that in a spark discharge, a higher concentration of H2O2 and NO3− is produced than in glow discharge, which may produce a higher enzyme inactivation due to the inactivation effect of these species (Illera, Sanz, Benito-Román et al., 2018). Therefore, further experiments were performed using the spark discharge mode.
3.1.1. Effect of input power
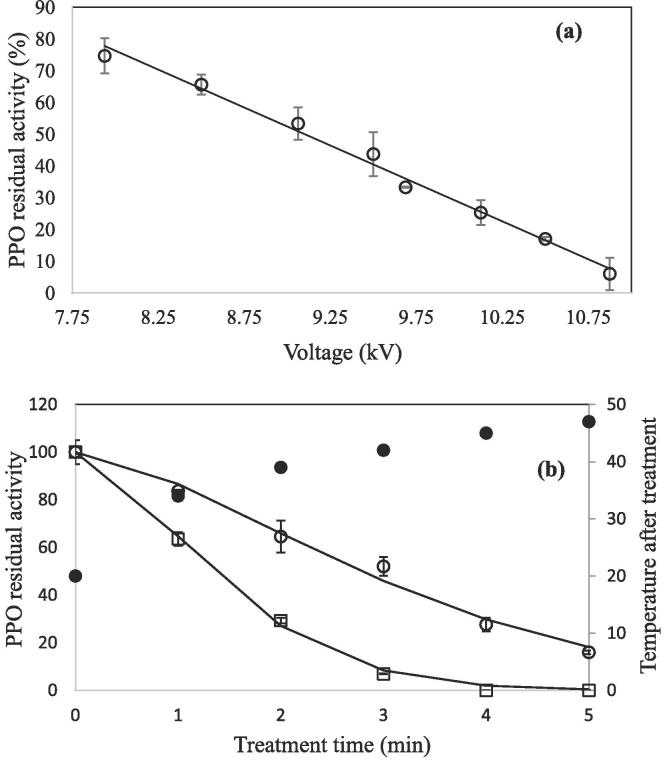
The effect of input power (voltage and frequency) was the first variable evaluated. Eight different spark plasma treatments using voltage values from 7.9375 to 10.875 kV were carried out, with treatment times of 5 min. PPO residual activity for each treatment is presented in Fig. 2a. Higher input voltage is linearly related to greater inactivation efficacy of Cold Plasma on PPO (PPO Residual activity = −23.871·Voltage +267.15; R2 = 0.9905). Although the highest voltage applied (10.875 kV) produced the best inactivation results, reaching a final residual activity of 6 ± 5%, it was not significantly different to the one obtained at 10.5 kV (16 ± 0.8%) at p-value ≤ 0.05. Temperatures of the juices after the treatments were 41 and 50 °C for 10.5 and 10.875 kV respectively, therefore, 10.5 kV was chosen as the best input voltage for the following experiments.
Fig. 2.
Effect of (a) input voltage. Continuous line represents linear adjustment (b) treatment time on PPO inactivation of (○) cloudy apple juice and (□) PPO solution (pH = 6.5) after plasma treatment. Continuous line represents Weibull model (Apple juice R2 = 98.9; PPO solution R2 = 99.8). Filled spots (•) represent final temperature after plasma treatments.
3.1.2. Effect of treatment time
As can be observed in Fig. 2b, increasing treatment time increases PPO inactivation. Best results were found after longer treatment times, where final PPO activity in cloudy apple juice was 27.6 ± 2.9 and 16 ± 0.8% after 4 and 5 min respectively, although this difference is significant. When treatment time increased, an increase in the final temperature of the juice was also observed (Fig. 2b). In order to know if the higher inactivation at longer treatment times was attributable to this increase in temperature, independent heating treatments were also performed on apple juice. No inactivation was observed after heat treatments in the time range from 1 to 5 min; however, some enzyme activation was observed, so it can be concluded that inactivation of the enzyme was due to the effects of plasma treatment. Tappi et al. (2014) treated fresh-cut apples using Dielectric Barrier Discharge (DBD) plasma, and observed that increasing treatment time significantly decreased PPO activity, where after 10 min treatment, residual activity of PPO was still 88%, which decreased to 42% after 30 min treatment. In comparison, in the present study, higher PPO inactivation and in significantly less time was achieved by the use of spark plasma. Pankaj, Misra, and Cullen (2013) studied the kinetics of isolated tomato peroxidase inactivation by in package DBD plasma, where they obtained low inactivation after 1 min of treatment (30%) and nearly total inactivation after 3 min. In the present study, different treatment times were also applied on pure enzyme solution (pH = 6.5), and the same trend was observed (Fig. 2b), but in this case, total inactivation was reached after 4 min plasma treatments, and higher inactivation rates were observed, showing that inactivation is easier and faster when enzyme was isolated than when it was present in a complex food matrix. For both cloudy apple juice and the pure enzyme solution data, the Weibull model fitted very well (for apple juice: b = 3.5 ± 0.1, n = 1.5 ± 0.2, R2 = 98.9; for pure PPO solution: b = 1.7 ± 0.1, n = 1.6 ± 0.1, R2 = 99.8). It can be seen that the shape value, n, is very similar in both samples, but b value of pure PPO solution is the half of the value for apple juice, due to higher inactivation rates. Surowsky, Fischer, Schlueter, and Knorr (2013) also treated polyphenol oxidase (PPO) from mushroom and they observed that its inactivation depended greatly on the plasma exposure time. Inactivation kinetics were very similar to the ones of the present study, after 4 min of treatment, final residual activity was around 90%, although they employed argon as working gas. Employing air as a working gas provides ease of operation and cost effectiveness to the process.
3.1.3. PPO activity during storage
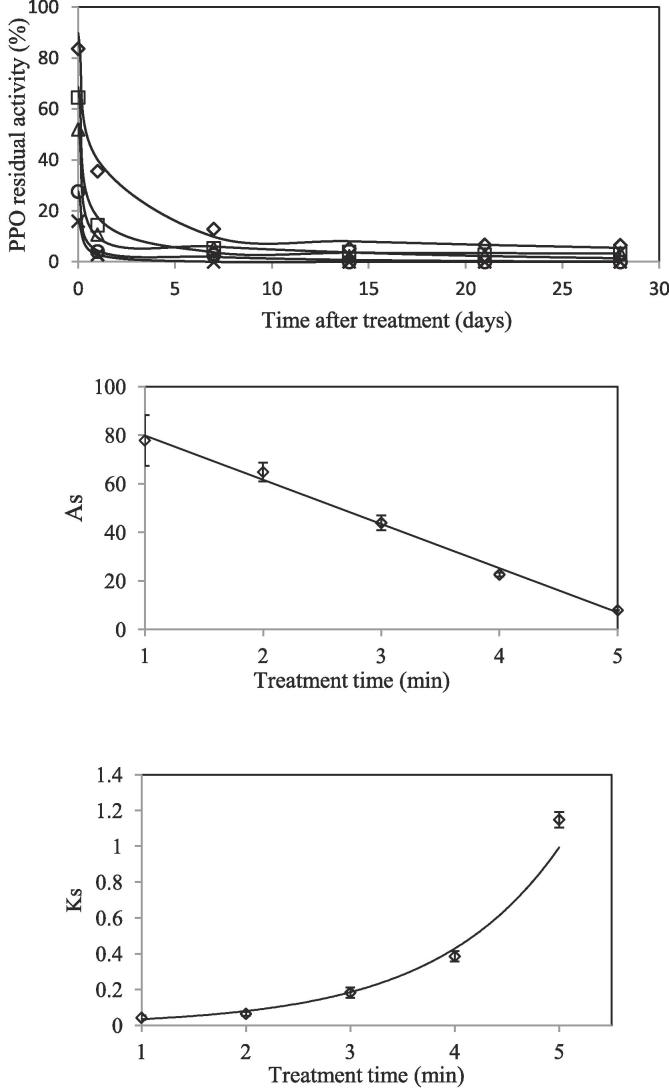
Treated apple juices were stored during 28 days at 4 °C, and PPO activity was measured just after the treatment and 24 h later, and then weekly for up to four weeks. During the first day of storage, a significant decrease in PPO activity was observed. Juice that had been treated for just one minute experienced a decrease from 83.7 ± 0.5 to 35.6 ± 0.8% of residual PPO activity after only 24 h storage. Juice that had been treated for 4 or 5 min had minimal PPO activity 24 h post treatment, and no residual activity was found after 7 days up to the end of the storage study (Fig. 3). No reactivation was observed in any of the treated juices over the storage trial as well as in the pure PPO solution, although this one was only measured until 24 h after treatment. After 24 h post treatment, there was still residual activity in the juices that had been treated for 1, 2 and 3 min, but this was very low, e.g. residual activity changed from 63.5 ± 2.8 just after the treatment to 25.6 ± 0.6 after 24 h of storage. Many studies propose that enzymatic inactivation by plasma treatments is produced by ROS (Reactive Oxygen Species) and RNS (Reactive Nitrogen Species) formed and their effect on conformational changes and structure changes of the enzyme (Misra et al., 2016, Surowsky et al., 2013, Takai et al., 2012). Although formation of these species has not been studied during storage time, according to Surowsky, Fröhling, Gottschalk, Schlüter, and Knorr (2014), H2O2 formed during plasma treatment and during in container storage time has a longer half-life than other reactive species, and could remain active in the treated juice post plasma treatment, which could cause a further inactivation of PPO in the juice during prolonged storage. PPO residual inactivation kinetics during storage were fitted to the Weibull model and to the two fractional kinetic model (Table supplementary 1). Inactivation data during storage fitted quite well to both models, although the two fractional kinetic model fitted better, especially for short plasma treatments. At the one minute plasma treatment time, inactivation kinetic data fitting during storage was R2 = 95.6 for the Weibull model and R2 = 98.6 for the two fraction one (Table S1). The activity (AS) and the inactivation rate constant (KS) during storage of the stable fraction of the enzyme showed an increase and decrease, respectively, when increasing treatment time, and are plotted in Fig. 3b and 2c. As can be observed, the activity of the stable fraction of PPO decreased linearly (AS = −18.2·treatment time +98; R2 = 0.9934) when treatment time was increased, decreasing from 77.8 ± 10.4 (1 min treatment) to 7.9 ± 0.1% (5 min treatment). In the same way, the constant that refers to the inactivation rate of the enzyme increased after longer treatments, showing a noticeable increase after 5 min of treatment (Fig. 3c).
Fig. 3.
(a) PPO residual activity in cloudy apple juice just after different plasma treatments of (◊) 1 min, (□) 2 min, (Δ) 3 min, (○) 4 min and (×) 5 min and during storage at 4 °C. Solid symbols represent the experimental inactivation values obtained, and continuous lines represent the two fraction kinetic model data. (b) As values from the two fraction kinetic model after different treatment times. Continuous line represents linear adjustment (c) Ks values from the two fraction kinetic model after different treatment times. Continuous line represents exponential adjustment.
3.2. Effect of spark plasma on physical-chemical properties of the juice
3.2.1. pH, color and non-enzymatic browning
A significant decrease in the pH value of all the plasma treated samples was observed, and this decreasing trend was aligned to treatment time. After 4 and 5 min of treatment the decrease in pH changed from 3.73 to 3.58 and 3.59 respectively (Table 1). After 28 days of storage, the pH value of the juice was similar to that measured immediately after the treatment. After a DBD treatment of just 2 min, Xiang, Liu, Li, Liu, and Zhang (2018) found that pH of commercial apple juice decreased from 3.96 ± 0.01 to 3.34 ± 0.01. Liao et al. (2018) also observed a pH decrease after a 30 kV DBD treatment, where pH of commercial apple juice changed from approximately 3.71 to 3.66 after just 40 s of treatment. Thirumdas, Kothakota, Annapure, and Siliveru (2018) explained that this effect is produced due to the formation of chemical species by the action of plasma, such as hydrogen peroxides, that contribute to the acidity of liquid samples.
Table 1.
Non enzymatic browning (NEB), cloud value, cloud stability (%), pH, total polyphenolic content retention (%) and DPPH (% inhibition) values of apple juice after plasma treatment (AT) at 10.5 kV during 5 min and during storage at 4 °C.
| Sample | Time (days) | pH | NEB | Cloud value | Cloud stability | TPC | DPPH |
|---|---|---|---|---|---|---|---|
| AT | 3.73 ± 0.00b | 0.12 ± 0.01a | 0.44 ± 0.02a | 8.57 ± 0.10a | 100 ± 7a | 79.4 ± 0.8a | |
| 7 | 3.76 ± 0.01a | 0.09 ± 0.01b | 0.38 ± 0.01b | 8.33 ± 0.19a | 88 ± 5b | 57.2 ± 0.3b | |
| Untreated juice | 14 | 3.76 ± 0.01a | 0.10 ± 0.01ab | 0.31 ± 0.01c | 5.72 ± 0.05b | 96 ± 1ab | 58.5 ± 0.5b |
| 21 | 3.69 ± 0.01c | 0.10 ± 0.01ab | 0.32 ± 0.01c | 5.60 ± 0.04b | 90 ± 3ab | 61.6 ± 3.5b | |
| 28 | 3.70 ± 0.01c | – | 0.30 ± 0.01c | 3.25 ± 0.12c | 86 ± 3b | – | |
| AT | 3.69 ± 0.00c | 0.20 ± 0.01a | 0.37 ± 0.01a | 8.83 ± 0.15a | 111 ± 10a | 78.4 ± 0.3a | |
| 7 | 3.74 ± 0.01a | 0.14 ± 0.01b | 0.37 ± 0.01a | 7.46 ± 0.06b | 101 ± 3a | 60.0 ± 0.1b | |
| 1 min treated | 14 | 3.71 ± 0.01b | 0.13 ± 0.01b | 0.33 ± 0.01b | 5.18 ± 0.10c | 114 ± 2a | 61.6 ± 0.1b |
| 21 | 3.66 ± 0.01d | 0.12 ± 0.01b | 0.07 ± 0.01c | 2.19 ± 0.05d | 101 ± 4a | 60.7 ± 0.2b | |
| 28 | 3.67 ± 0.01d | – | 0.06 ± 0.01c | 1.88 ± 0.03d | 101 ± 5a | – | |
| AT | 3.65 ± 0.00b | 0.23 ± 0.01a | 0.31 ± 0.01c | 8.93 ± 0.06a | 136 ± 2b | 85.0 ± 0.2a | |
| 7 | 3.70 ± 0.01a | 0.13 ± 0.01c | 0.37 ± 0.01a | 7.22 ± 0.21b | 134 ± 5b | 68.8 ± 0.8b | |
| 2 min treated | 14 | 3.68 ± 0.01a | 0.16 ± 0.01b | 0.34 ± 0.01b | 5.47 ± 0.17c | 147 ± 2a | 68.9 ± 1.1b |
| 21 | 3.63 ± 0.01c | 0.13 ± 0.01c | 0.09 ± 0.01d | 2.04 ± 0.04d | 123 ± 5c | 68.1 ± 0.1b | |
| 28 | 3.63 ± 0.01c | – | 0.06 ± 0.01e | 1.16 ± 0.03e | 124 ± 1c | – | |
| AT | 3.63 ± 0.00c | 0.23 ± 0.01a | 0.39 ± 0.01c | 8.12 ± 0.05a | 146 ± 1a | 92.9 ± 0.3a | |
| 7 | 3.70 ± 0.01a | 0.14 ± 0.01c | 0.44 ± 0.01a | 8.22 ± 0.08a | 117 ± 1c | 74.2 ± 0.9b | |
| 3 min treated | 14 | 3.66 ± 0.01b | 0.18 ± 0.01b | 0.42 ± 0.01b | 6.98 ± 0.20b | 128 ± 6b | 74.5 ± 1.2b |
| 21 | 3.61 ± 0.01d | 0.15 ± 0.01c | 0.09 ± 0.01d | 2.26 ± 0.05c | 136 ± 3b | 72.0 ± 0.4c | |
| 28 | 3.62 ± 0.01c | – | 0.05 ± 0.01e | 1.22 ± 0.06d | 135 ± 4b | – | |
| AT | 3.59 ± 0.00c | 0.22 ± 0.01a | 0.42 ± 0.01a | 7.66 ± 0.14a | 169 ± 1a | 93.4 ± 1.5a | |
| 7 | 3.66 ± 0.01a | 0.14 ± 0.01c | 0.43 ± 0.01a | 7.39 ± 0.01ab | 143 ± 6b | 75.9 ± 0.5c | |
| 4 min treated | 14 | 3.64 ± 0.01b | 0.18 ± 0.01b | 0.38 ± 0.01b | 7.06 ± 0.25b | 151 ± 12b | 78.3 ± 0.3b |
| 21 | 3.58 ± 0.01c | 0.14 ± 0.01c | 0.22 ± 0.01c | 3.81 ± 0.08c | 145 ± 1b | 74.4 ± 0.6c | |
| 28 | 3.59 ± 0.01c | – | 0.05 ± 0.01d | 1.05 ± 0.02d | 145 ± 1b | – | |
| AT | 3.58 ± 0.00b | 0.22 ± 0.01a | 0.45 ± 0.01a | 9.41 ± 0.23a | 164 ± 1a | 92.8 ± 0.2a | |
| 7 | 3.63 ± 0.01a | 0.14 ± 0.01c | 0.43 ± 0.01ab | 8.75 ± 0.01b | 166 ± 1a | 76.4 ± 0.4c | |
| 5 min treated | 14 | 3.62 ± 0.01a | 0.13 ± 0.01b | 0.42 ± 0.01b | 7.80 ± 0.23c | 159 ± 2b | 78.2 ± 0.5b |
| 21 | 3.57 ± 0.01b | 0.17 ± 0.01c | 0.35 ± 0.02c | 7.18 ± 0.14d | 158 ± 2b | 73.3 ± 1.0d | |
| 28 | 3.58 ± 0.01b | – | 0.18 ± 0.01d | 1.03 ± 0.06e | 143 ± 2c | – | |
Values with different letters in each column, and each treatment time (a,b) are significantly different when applying the Tukeýs honest significant difference (HSD) method at p-value ≤ 0.05.
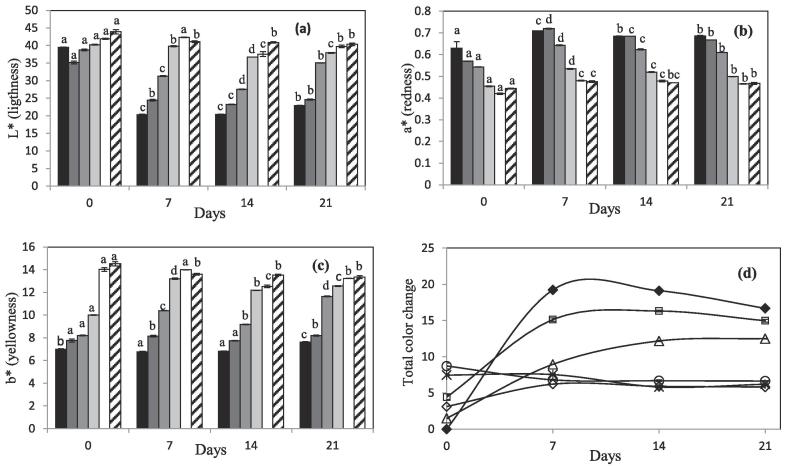
Color parameters of different samples are plotted in Fig. 4. Total change in color (ΔE) has been calculated (Eq. (2)) and is also represented. This value is the total change in color between the treated samples during storage compared to the untreated juice. After plasma treatment, significant changes in color parameters can be observed depending on the duration of the treatment applied to each juice. Lightness (L) of the juice only decreased after 1 min of plasma treatment, and after longer treatments, this value was maintained and even increased in juice treated for 4–5 min. The optimal value was obtained after the 5 min treatment, where L value significantly increased from 39.5 ± 0.1 (untreated sample) to 43.9 ± 0.6, and, although this value was not maintained over storage, it was still higher than the value in the untreated juice, at 40.3 ± 0.4 after 21 days. Similar values can be seen for the 4 min treated sample. In untreated and 1 min treated samples lightness of the juice significantly decreased during storage time, pointing that juice had suffered severe browning, which could also be probed visually. Another important value related to browning in the juice is redness (a* value), and usually, an increase in this value is related to browning. In Fig. 4b, the difference of this value between different treated samples is evident, the longer the plasma treatment, the lower the value, which means that the juice has gone to a greener color and has not browned. During storage this value increased in all the samples, but at the highest treatment times it was more or less constant over storage. This value is correlated with the b* values obtained, indicating that the longer the plasma treatment, the greener the juice, and the values are generally maintained during storage (Fig. 4c). With the changes observed in color parameters, it can be said that plasma treatment effect on color is treatment time dependent, and the best results for color quality were obtained at 4 and 5 min treatment were juice is more green and yellow, and lightness increased. In Fig. 4d, total color change of the samples is represented, and it can be clearly seen that juices with longer treatments suffered a bigger change after the treatment due to lightening, but stability was maintained during the storage. In all cases, ΔE value during storage was higher than 3, so the change in color was very distinct (Misra et al., 2014), and clearly appreciable visually. No studies of the effect of plasma on color of fresh juice have been found in the literature, but there are some studies of color changes on commercial apple juices after plasma treatments (Dasan and Boyaci, 2018, Liao et al., 2018). In these studies, apple juice was treated during very short times (2 min or lower) with a plasma jet device and DBD plasma respectively. After both treatments, juice was brown due to an increase in a* and b* values and a decrease in lightness. Also total color change was greater than that obtained in this work. It can be observed that spark plasma discharge using air, and the associated chemistry has a better effect on the color properties of the juice than other plasma devices.
Fig. 4.
Color parameters (a) L* (b) a* and (c) b* of (black) untreated samples and plasma treated samples after (dark grey) 1 min, (grey) 2 min, (light grey) 3 min, (white) 4 min and (striped) 5 min and during storage at 4 °C. Values with different letters in each figure are significantly different for each treatment time when applying the Tukeýs honestly significant difference (HSD) method at p-value ≤ 0.05. (d) Total color change during storage of (♦) untreated juice and after plasma treatment of (□) 1 min, (Δ) 2 min, (◊) 3 min, (×) 4 min and (○) 5 min.
Browning in juice is not only produced by the action of the enzymes, but non-enzymatic browning (NEB) also occurs by different reactions in food during processing and storage, and it can be used as an index of the freshness and quality of juices. As it can be seen in Table 1, just after treatment non-enzymatic browning increased in all the samples, from Abs443nm = 0.12 ± 0.01 to approximately 0.22. During storage, those values decreased, which is an unusual trend, since non enzymatic browning usually increases during storage. Pankaj, Wan, Colonna, and Keener (2017) studied non-enzymatic browning in white grape juice, and after 4 min treatment the value of the absorbance increased from 0.09 ± 0.00 to 0.23 ± 0.01, which is similar to that observed in this work. There is no literature regarding changes in this property during liquid food storage post plasma treatments.
3.2.2. Cloud value, cloud stability and particle size distribution
Both cloud value and cloud stability are two parameters of great interest in fresh juices and cloudy apple juices. Initial cloud value of untreated apple juice was 0.44 ± 0.02 (Table 1). Although cloud value did not change to a great extent post plasma treatments, the worst results were found after 2 min of treatment, where cloud value was 0.31 ± 0.01. After 4 and 5 min cloud value was maintained post treatment. During storage, all samples showed a decrease in the cloud value, with a faster decline in the case of samples treated for a short time. The optimal values were obtained in the 5 min treated juice, where cloud value was 0.18 ± 0.01 after 28 days of storage, although it was lower than in the untreated sample after the same storage time (0.30 ± 0.01). Cloud stability results are in accordance with cloud value results, where after 5 min of plasma treatment, cloud stability of the juice increased from 8.57 ± 0.10 to 9.41 ± 0.23 (Table 1), although, as it happened in cloud values, the stability significantly decreased during storage. All cloud stability values after 28 days of storage were similar and lower than the cloud value of untreated samples.
The main components responsible for the desirable cloud appearance of apple juice are proteins, pectin and polyphenols (Zhao, Zong, & An, 2008), and the different interactions that can occur between them during and after plasma treatments will determine its stability. Cloud stability of the juice is usually related to the diameters of the particles present in the juice, homogenization occurs when particle size decreases, and therefore, cloud stability increases. According to Beveridge and Beveridge (2010), particle sizes above 0.5 μm to 0.65 μm are unstable and settle out, while the particles below this range are held in suspension and do not settle.
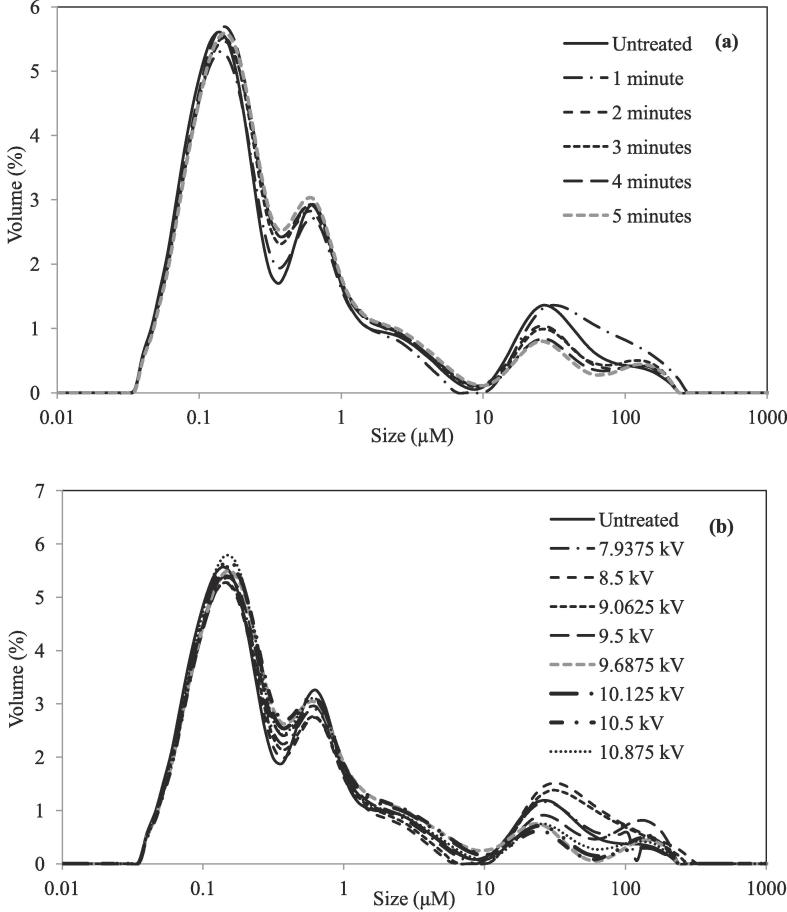
Particle size distribution results are shown in Table 2 and plotted in Fig. 5a. Particle size was not measured during the storage study; results correspond to the juices after different treatment times. As it can be observed in D[4,3] values, particle size did not change much, although a significant decrease was observed for 4 and 5 min treatments, where it changed from 8.53 ± 0.05 to 7.00 ± 0.02 and 6.85 ± 0.02 respectively. In contrast, after the one minute treatment, the D[4,3] value significantly increased to 13.34 ± 0.06. Obtained values for d(0.1) and d(0.5) parameters were also practically unchanged after plasma treatment, but a bigger change was seen in d(0.9) values. This parameter indicates the size where 90% of the particles are below it. As seen in Table 2, after a treatment of one minute, particle size in the juice increased, since d(0.9) increased from 28.2 ± 1.2 to 45.5 ± 2, which may have been caused by coagulation of particles. After longer treatments, d(0.9) value decreased, therefore increasing treatment time decreased the particle size. After 5 min treatment, d(0.9) was 7.8 ± 0.1, which is lower than the value for the untreated juice, but it is still high enough for a stable juice. This can be seen in Fig. 5a, where in the particle size range between 0.1 and 1 μm, there is an increase in the volume (%) of the treated samples compared to the untreated one, as well as a decrease in the particles between 10 and 100 μm (except for 1 min treated). This change is clearly visible in Span values, which also increased after 1 min, but decreased thereafter with longer treatments. As expected, the lowest Span value was after 5 min treatment, decreasing from 106 ± 7 in the untreated sample to 28 ± 2 in the treated one. Span value represents the distribution width, so this decrease indicates a homogenization of the juice, which should contribute to its cloud stability. Although particles are much higher than 0.5 μm and cloud stability was low, no phase separation was observed in the juices that had been treated during 4 and 5 min during the shelf life study. One possible explanation of the cloud maintenance in these juices is the inactivation of another enzyme that is usually present in apple juice and which activity has not been measured in this study, pectinmethylesterase (PME). This enzyme causes pectin demethylation that can precipitate with calcium ions present in the juice, producing clarification and loss of turbidity (Illera, Sanz, Beltran, and Solaesa (2018)). Based on the good results obtained in PPO inactivation after these treatments of 4 and 5 min, total or partial inactivation of this other enzyme could have happened. Zhao et al. (2008) also explained that a factor important for cloud stability could be the electrostatic repulsion by negative charges present in the partly de-methylated pectin, which would avoid aggregation.
Table 2.
PSD values of untreated and plasma treated juices after different treatment times.
| Sample | D [3,2] | D [4,3] | d (0.1) | d (0.5) | d (0.9) | Span* |
|---|---|---|---|---|---|---|
| Untreated | 0.19 ± 0.01a | 8.53 ± 0.05b | 0.084 ± 0.001a | 0.26 ± 0.01a | 28.2 ± 1.2b | 106 ± 7b |
| 1 min | 0.21 ± 0.02c | 13.34 ± 0.06a | 0.087 ± 0.001b | 0.30 ± 0.02c | 45.5 ± 2a | 152 ± 8a |
| 2 min | 0.20 ± 0.01b | 7.53 ± 0.05d | 0.088 ± 0.001b | 0.28 ± 0.01b | 21.1 ± 0.8c | 75 ± 5c |
| 3 min | 0.20 ± 0.01b | 8.23 ± 0.03c | 0.088 ± 0.001b | 0.28 ± 0.01b | 22.5 ± 0.6c | 81 ± 7c |
| 4 min | 0.20 ± 0.01b | 7.00 ± 0.02e | 0.087 ± 0.001b | 0.26 ± 0.01a | 13.9 ± 0.1d | 53 ± 5d |
| 5 min | 0.20 ± 0.01b | 6.85 ± 0.02f | 0.089 ± 0.001b | 0.27 ± 0.01b | 7.8 ± 0.1e | 28 ± 2e |
Values with different letters in each column, and each treatment (a,b) are significantly different when applying the Tukeýs honestly significant difference (HSD) method at p-value ≤ 0.05.
Span has no units.
Fig. 5.
Particle size distribution of (a) Untreated and treated cloudy apple juice at different treatment times (b) Untreated and treated juices at different voltages input.
Particle size distribution of the juices treated at different voltages is plotted in Fig. 5b. The distribution did not change to a great extent, but some differences can be found especially in the bigger size range, where lower voltage treatments produced an increase in the volume of big particles sizes.
3.2.3. Total phenolic content and antioxidant capacity
Table 1 shows the evolution of total polyphenolic compounds retention after plasma treatments and during the shelf life study at 4 °C. Total phenolic compounds (TPC), expressed as percentage of the initial value, increased after all plasma treatments, and it was significantly higher when treatment time increased, reaching the highest values in the 4 and 5 min treated juices, where TPC increased by 69% and 64% respectively. During storage, all samples suffered a decrease in TPC content, but after 28 days, these values were still higher than in the untreated juice. Rodríguez et al. (2017) observed the same trend, the TPC showed a significantly higher increase with increasing the treatment time. They observed an increment of 114% in TPC of cashew apple juice after 5 min of plasma treatment. The same increase was obtained by Dasan and Boyaci (2018) after a DBD treatment of 2 min on commercial apple juice. Both studies explained that the increase in phenolic compounds is probably caused by an increase in cell membrane breakdown, where these compounds are located. The energy applied by the plasma treatment and also the degradation of the cell membrane by the action of plasma reactive species could produce this breakdown and enhance the phenolic extraction. Ramazzina et al. (2015) studied TPC in processed kiwifruit and they also obtained a decrease of around 5% after storage of 4 days.
The determination of phenolic content is related to antioxidant capacity of the sample (Muhammad et al., 2018), as it can be observed in DPPH inhibition results (Table 1). A very similar trend to the changes observed in TPC can be seen, with an increase in antioxidant capacity with treatment time, although in this case, the 3 min treatment was as effective as 4 and 5 min. In these samples DPPH % inhibition increased from 79.4 ± 0.8% to approximately 93%. As happened with TPC, despite this initial increase, antioxidant capacity decreased during storage, although from day seven onwards, antioxidant capacity remained constant until the end of the shelf life study. This overall decrease of antioxidant capacity can be explained by the ability of antioxidant compounds present in the juice to scavenge the free radicals generated with plasma, which would decrease their concentration in the juice (Dasan & Boyaci, 2018). This explanation also includes phenolic compounds, and explains their decrease during storage, although according to (Muhammad et al., 2018), further work is needed to clarify the reaction chemistry between plasma and antioxidants in food.
4. Conclusions
Cold plasma treatment using spark discharge to liquid was an effective alternative for the treatment and quality preservation of cloudy apple juice. The optimal conditions of treatment were a power input of 10.5 kV and a treatment time of 5 min. Enzyme inactivation and physico-chemical properties were dependent on treatment time. Significant PPO inactivation was achieved after 5 min of treatment, and after 24 h it was totally inactivated. No reactivation of PPO was found in any sample. pH significantly decreased after plasma treatments due to the formation of reactive species. Color of juice was improved after long treatments, being lighter than the untreated sample right after treatment, and it was maintained during storage. Antioxidant capacity of the sample was also increased, as well as polyphenols content, and, although cloud stability didńt show high values and particle size distribution didńt significantly change, the juice quality was stable during storage in samples of long treatments. The study of the properties of juice during extended storage following cold plasma spark discharge treatment, contributes to the development of the implementation strategy for cold plasma technology in juice processing. Further studies are needed for this purpose, with an important aspect of scale up to large volume processing required with a focus on retaining air as the working gas as a renewable resource. Prior to scale up, a detailed study of the sensorial acceptance of the product as well as nutritional impact of consumption with appropriate models are needed to determine the viability and other potential advantages of treating cloudy apple juice by cold plasma technology at an industrial scale.
Funding
This work was supported by the Spanish Government (MINECO) and the European Regional Development Fund (ERDF) [Project CTQ2015-64396-R] and in part by a research grant from Science Foundation Ireland (SFI) under the Grant Number SFI/16/BBSRC/3391 and the BBSRC under the Grant Reference BB/P008496/1. A.E. Illera mobility to Technological University Dublin was funded by a mobility grant of the University of Burgos, Spain.
Declaration of Competing Interest
The authors declared that there is no conflict of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2019.100049.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- Abid M., Jabbar S., Wu T.A.O., Hashim M.M., Hu B., Saeeduddin M., Zeng X. Qualitative assessment of sonicated apple juice during storage. Journal of Food Processing and Preservation. 2015;39:1299–1308. [Google Scholar]
- Aguiló-aguayo I., Soliva-fortuny R., Martín-belloso O. Avoiding non-enzymatic browning by high-intensity pulsed electric fields in strawberry, tomato and watermelon juices. Journal of Food Engineering. 2009;92(1):37–43. [Google Scholar]
- Bahrami N., Bayliss D., Chope G., Penson S., Perehinec T., Fisk I.D. Cold plasma: A new technology to modify wheat flour functionality. Food Chemistry. 2016;202:247–253. doi: 10.1016/j.foodchem.2016.01.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baslar M., Ertugay M.F. The effect of ultrasonic treatments on cloudy quality-related quality parameters in apple juice. Innovative Food. 2014;26:226–231. [Google Scholar]
- Beveridge T., Beveridge T. Opalescent and cloudy fruit juices : Formation and particle stability. Opalescent and Cloudy Fruit Juices. 2010 doi: 10.1080/10408690290825556. [DOI] [PubMed] [Google Scholar]
- Briongos H., Illera A.E., Sanz M.T., Melgosa R., Beltran S., Solaesa A.G. Effect of high pressure carbon dioxide processing on pectin methylesterase activity and other orange juice properties. LWT - Food Science and Technology. 2016;74:411–419. [Google Scholar]
- Coutinho N.M., Silveira M.R., Fernandes L.M., Moraes J., Pimentel T.C., Freitas M.Q., Rodrigues S. Processing chocolate milk drink by low-pressure cold plasma technology. Food Chemistry. 2019;278:276–283. doi: 10.1016/j.foodchem.2018.11.061. [DOI] [PubMed] [Google Scholar]
- Coutinho N.M., Silveira M.R., Rocha R.S., Moraes J., Vinicius M., Ferreira S., Cruz A.G. Cold plasma processing of milk and dairy products. Trends in Food Science & Technology. 2018;74:56–68. [Google Scholar]
- Dasan B.G., Boyaci I.H. Effect of cold atmospheric plasma on inactivation of escherichia coli and physicochemical properties of apple, orange, tomato juices, and sour cherry nectar. Food Bioprocess Technology. 2018;11:334–343. [Google Scholar]
- Faria W., Kumar B., Rodriguez Ó., Sousa E., Brito D., André F., Rodrigues S. Effect of ultrasound followed by high pressure processing on prebiotic cranberry juice. Food Chemistry. 2017;218:261–268. doi: 10.1016/j.foodchem.2016.08.132. [DOI] [PubMed] [Google Scholar]
- Fonteles T.V., Rodrigues S. Prebiotic in fruit juice : Processing challenges, advances, and perspectives. Current Opinion in Food Science. 2018;22:55–61. [Google Scholar]
- Hou Y., Wang R., Gan Z., Shao T., Zhang X., He M., Sun A. Effect of cold plasma on blueberry juice quality. Food Chemistry. 2019;290:79–86. doi: 10.1016/j.foodchem.2019.03.123. [DOI] [PubMed] [Google Scholar]
- Illera A.E., Sanz M.T., Beltran S., Solaesa A.G. Evaluation of HPCD batch treatments on enzyme inactivation kinetics and selected quality characteristics of cloudy juice from Golden delicious apples. Journal of Food Engineering. 2018;221 [Google Scholar]
- Illera A.E., Sanz M.T., Benito-Román O., Varona S., Beltrán S., Melgosa R., Solaesa A.G. Effect of thermosonication batch treatment on enzyme inactivation kinetics and other quality parameters of cloudy apple juice. Innovative Food Science and Emerging Technologies. 2018;47:71–80. [Google Scholar]
- Lee J., Jo K., Lim Y., Joon H., Ho J., Jo C. The use of atmospheric pressure plasma as a curing process for canned ground ham. Food Chemistry. 2018;240:430–436. doi: 10.1016/j.foodchem.2017.07.148. [DOI] [PubMed] [Google Scholar]
- Liao X., Li J., Muhammad A.I., Suo Y., Chen S., Ye X.…Ding T. Application of a dielectric barrier discharge atmospheric cold plasma (Dbd-Acp) for eshcerichia coli inactivation in apple juice. Journal of Food Science. 2018;83(2):401–408. doi: 10.1111/1750-3841.14045. [DOI] [PubMed] [Google Scholar]
- Liao X., Liu D., Xiang Q., Ahn J., Chen S., Ye X. Inactivation mechanisms of non-thermal plasma on microbes: A review. Food Control. 2017;75:83–91. [Google Scholar]
- Lu P., Boehm D., Bourke P., Cullen P.J. Achieving reactive species specificity within plasma-activated water through selective generation using air spark and glow discharges. Plasma Processes and Polymers. 2017;1–9 [Google Scholar]
- Lu P., Boehm D., Cullen P., Bourke P. Controlled cytotoxicity of plasma treated water formulated by open-air hybrid mode discharge. Applied Physics Letters. 2017;110 [Google Scholar]
- Ma R., Wang G., Tian Y., Wang K., Zhang J., Fang J. Non-thermal plasma-activated water inactivation of food-borne pathogen on fresh produce. Journal of Hazardous Materials. 2015;300:643–651. doi: 10.1016/j.jhazmat.2015.07.061. [DOI] [PubMed] [Google Scholar]
- Mandal R., Singh A., Singh A.P. Recent developments in cold plasma decontamination technology in the food industry. Trends in Food Science & Technology. 2018;80:93–103. [Google Scholar]
- Misra N.N., Keener K.M., Bourke P., Mosnier J., Cullen P.J. In-package atmospheric pressure cold plasma treatment of cherry tomatoes. Journal of Bioscience and Bioengineering. 2014;118(2):177–182. doi: 10.1016/j.jbiosc.2014.02.005. [DOI] [PubMed] [Google Scholar]
- Misra N.N., Pankaj S.K., Segat A., Ishikawa K. Cold plasma interactions with enzymes in foods and model systems. Trends in Food Science & Technology. 2016;55:39–47. [Google Scholar]
- Muhammad A.I., Liao X., Cullen P.J., Liu D., Xiang Q., Wang J., Ye X. Effects of nonthermal plasma technology on functional food components. Comprehensive Reviews in Food Science and Food Safety. 2018;17:1379–1394. doi: 10.1111/1541-4337.12379. [DOI] [PubMed] [Google Scholar]
- Oszmianski J., Wolniak M., Wojdylo A., Wawer I. Comparative study of polyphenolic content and antiradical activity of cloudy and clear apple juices. Journal of the Science of Food and Agriculture. 2007;87:573–579. [Google Scholar]
- Pankaj S.K., Keener K.M. Cold plasma: Background, applications and current trends. Current Opinion in Food Science. 2017;16:49–52. [Google Scholar]
- Pankaj S.K., Misra N.N., Cullen P.J. Kinetics of tomato peroxidase inactivation by atmospheric pressure cold plasma based on dielectric barrier discharge. Innovative Food Science and Emerging Technologies. 2013;19:153–157. [Google Scholar]
- Pankaj S.K., Wan Z., Colonna W., Keener K. Effect of high voltage atmospheric cold plasma on white grape juice quality. Journal of Science and Food Agriculture. 2017;97:4016–4021. doi: 10.1002/jsfa.8268. [DOI] [PubMed] [Google Scholar]
- Putnik P., Dragovic V., Pedisic S., Rez A. Effects of cold atmospheric gas phase plasma on anthocyanins and color in pomegranate juice. Food Chemistry. 2016;190:317–323. doi: 10.1016/j.foodchem.2015.05.099. [DOI] [PubMed] [Google Scholar]
- Putnik P., Kresoja Ž., Bosiljkov T., Režek A., Barba F.J., Lorenzo J.M., Žuntar I. Comparing the effects of thermal and non-thermal technologies on pomegranate juice quality: A review. Food Chemistry. 2019;279:150–161. doi: 10.1016/j.foodchem.2018.11.131. [DOI] [PubMed] [Google Scholar]
- Queiroz C., Jorge A., Lúcia M., Lopes M., Fialho E., Valente-mesquita V.L. Polyphenol oxidase activity, phenolic acid composition and browning in cashew apple (Anacardium occidentale, L.) after processing. Food Chemistry. 2011;125:128–132. [Google Scholar]
- Ramazzina I., Berardinelli A., Rizzi F., Tappi S., Ragni L., Sacchetti G., Rocculi P. Postharvest Biology and Technology Effect of cold plasma treatment on physico-chemical parameters and antioxidant activity of minimally processed kiwifruit. Postharvest Biology and Technology. 2015;107:55–65. [Google Scholar]
- Ramazzina I., Tappi S., Rocculi P., Sacchetti G., Berardinelli A., Marseglia A., Rizzi F. Effect of cold plasma treatment on the functional properties of fresh-cut apples. Journal of Agricultural and Food Chemistry. 2016;64:8010–8018. doi: 10.1021/acs.jafc.6b02730. [DOI] [PubMed] [Google Scholar]
- Rodríguez Ó., Gomes W.F., Rodrigues S., Fernandes F.A.N. Effect of indirect cold plasma treatment on cashew apple juice (Anacardium occidentale L.) LWT - Food Science and Technology. 2017;84:457–463. [Google Scholar]
- Silveira M.R., Coutinho N.M., Esmerino E.A., Moraes J., Fernandes L.M., Pimentel T.C., Nazzaro F. Guava- flavored whey beverage processed by cold plasma technology: Bioactive compounds, fatty acid profile and volatile compounds. Food Chemistry. 2019;279:120–127. doi: 10.1016/j.foodchem.2018.11.128. [DOI] [PubMed] [Google Scholar]
- Silveira M.R., Esmerino E.A., Tatiana C., Raices R.S.L., Senaka C., Neto R.P.C., Fonteles T.V. Guava flavored whey-beverage processed by cold plasma: Physical characteristics, thermal behavior and microstructure. Food Research International. 2019;119:564–570. doi: 10.1016/j.foodres.2018.10.033. [DOI] [PubMed] [Google Scholar]
- Surowsky B., Fischer A., Schlueter O., Knorr D. Cold plasma effects on enzyme activity in a model food system. Innovative Food Science and Emerging Technologies. 2013;19:146–152. [Google Scholar]
- Surowsky B., Fröhling A., Gottschalk N., Schlüter O., Knorr D. International journal of food microbiology impact of cold plasma on citrobacter freundii in apple juice : Inactivation kinetics and mechanisms. International Journal of Food Microbiology. 2014;174:63–71. doi: 10.1016/j.ijfoodmicro.2013.12.031. [DOI] [PubMed] [Google Scholar]
- Takai E., Kitano K., Kuwabara J., Shiraki K. Protein inactivation by low-temperature atmospheric pressure plasma in aqueous solution. Plasma Processes and Polymers. 2012;9(1):77–82. [Google Scholar]
- Tappi S., Berardinelli A., Ragni L., Dalla M., Guarnieri A., Rocculi P. Atmospheric gas plasma treatment of fresh-cut apples. Innovative Food Science and Emerging Technologies. 2014;21:114–122. [Google Scholar]
- Tappi S., Gozzi G., Vannini L., Berardinelli A., Romani S., Ragni L., Rocculi P. Cold plasma treatment for fresh-cut melon stabilization. Innovative Food Science and Emerging Technologies. 2016;33:225–233. [Google Scholar]
- Thirumdas R., Kothakota A., Annapure U., Siliveru K. Plasma activated water (PAW): Chemistry, physico-chemical properties, applications in food and agriculture. Trends in Food Science & Technology. 2018;77:21–31. [Google Scholar]
- Van Boekel M.A.J.S. On the use of the Weibull model to describe thermal inactivation of microbial vegetative cells. International Journal of Food Microbiology. 2002;74:139–159. doi: 10.1016/s0168-1605(01)00742-5. [DOI] [PubMed] [Google Scholar]
- Versteeg C., Rombouts F.M., Spaansen C.H., Pilnik W. Thermostability and orange juice cloud destabilizing properties of multiple pectinesterases from orange. Journal of Food Science. 1980;45(4):969–971. [Google Scholar]
- Xiang Q., Liu X., Li J., Liu S., Zhang H. Effects of dielectric barrier discharge plasma on the inactivation of Zygosaccharomyces rouxii and quality of apple juice. Food Chemistry. 2018;254(136):201–207. doi: 10.1016/j.foodchem.2018.02.008. [DOI] [PubMed] [Google Scholar]
- Yang R.J., Li S.Q., Zhang Q.H. Effects of pulsed electric fields on the activity of enzymes in aqueous solution. Food Chemistry and Toxicology. 2004;69(4):241–248. [Google Scholar]
- Zhao G.Y., Zong W., An G.J. Effect of storage on cloud stability of cloudy apple juice. Food Science and Technology International. 2008;14(1):105–113. [Google Scholar]
- Zhou L., Wang Y., Hu X., Wu J., Liao X. Effect of high pressure carbon dioxide on the quality of carrot juice. Innovative Food Science and Emerging Technologies. 2009;10(3):321–327. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.