Abstract
The lessons are: (a) Human cancers certainly respond to immunological manipulations. Efforts at human cancer immunotherapy are therefore worthwhile. (b) Prophylaxis is very different from therapy of pre-existing disease, and hence much enthusiasm should not be derived from successful prophylaxis studies. Even in case of infectious agents against which robust prophylaxis is routinely achieved, therapy is nearly impossible once the disease has established. (c) Studies with appropriate cancer models of mice and rats are useful. The notion that it is easy to cure cancers in mice is generally advanced the most confidently by those who have never cured a mouse of cancer by immunotherapy. (d) With a nod to James Carville, it is the antigen(s), stupid! We still do not know the identity of protective tumor antigens. If any lesson can be drawn at all, it may well be that cancer immunotherapy must move away from the one-shoe-fits-all therapeutic models of chemotherapy and must move to individualized approaches. (e) All targets are equal, but some are more equal than others. The key is specificity for cancer. That does not necessarily mean specificity for cancer cells. (f) Vaccitherapy must be attempted preferably in the minimal residual disease setting, even though this is certain to be time-taking and expensive. In the setting of bulky disease, vaccitherapy must be combined with blockade of inhibitory signals, or depletion of down-regulatory T cells. Inhibition of effector level suppression of immune response is a key. Vaccitherapy alone or immuno-modulation alone is unlikely to succeed in therapy of bulky metastatic disease.
Keywords: cancer vaccines, personalized treatment, heat shock proteins, melanoma, renal cell carcinoma, unique antigens, shared antigens
Introduction
Although immunotherapy of human cancer has a record going back well over a century, its modern era may be said to have begun in the 1980s when new insights into antibody diversity and into the bases of MHC-restriction and T cell functions began to emerge. Since it took about another decade before these insights could actually begin to be tested in patients, we have now had over a decade of experience with treating cancer patients immunologically. Clear cut clinical successes have been rare as hen’s teeth; however, some lessons have emerged, and if they are acted upon, there is reason to be optimistic about the next decade or two.
Lessons
(a) There is absolutely no doubt that human cancers respond to immunological manipulations. Efforts at human cancer immunotherapy are therefore worthwhile. A small number of clinical studies have demonstrated un-ambiguously that immunological manipulations affect the natural course of disease favorably. The anti-idiotype therapy of B cell lymphoma was in the forefront of such studies (1), and the wide spread use of the anti-CD20 antibody for treatment of non-Hodgkin’s lymphoma (2) is a clear example. The necessity of use of mis-matched transplants for eradication of chronic myelogenous leukemia (3) remains a solid clinical demonstration of the power of cancer immunotherapy. Among solid tumors, the durable complete responses elicited by high dose IL2 in patients with metastatic melanoma (4) and metastatic renal cell carcinoma (5) are two of the early instances. The enhanced relapse-free and overall survival seen in melanoma patients who received interferon α, and who developed autoantibodies (6), is another case in point. The dramatic tumor regressions in melanoma and ovarian cancer patients who were immunized with autologous vaccines and then received blocking antibody to CTLA4 are powerful demonstrations of the power of the host immune system to modulate the course of cancer. The regressions seen upon withdrawal of immunosuppressive therapy in patients with post transplant lymphoproliferative disorders are yet another example. Collectively, these examples span nearly the entire gamut of immunological therapies from administration of antibodies to cytokines to inhibition of negative co-stimulatory effects on T cells. Interestingly, efforts at vaccitherapy cannot yet be included in this list (discussed later in d).
Each of the examples cited above has its detractors; mainly, that the benefits are seen only in small proportion of patients, or that the adverse reactions are severe. These are serious limitations indeed. The point here however, is that these examples, howsoever limited, teach us un-ambiguously that immunological intervention have the potential to alter the natural course of human malignant disease. That it has been shown to do so in such limited contexts, only indicates that our understanding of the immune system and the interplay of the cancers and the immune system, is quite limited. These examples tell us unmistakably that we are moving steadily towards successful immunotherapy of human cancers, and that the journey is not an asymptote.
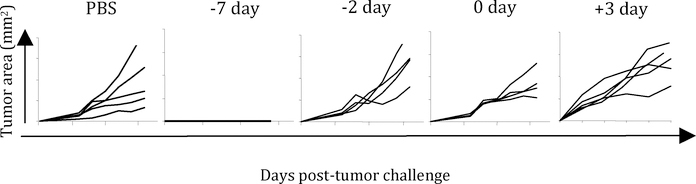
(b) Prophylaxis is very different from therapy of pre-existing disease, and hence much enthusiasm should not be derived from successful prophylaxis studies. The impressive record of successful prophylactic vaccinations against many infectious agents gave many a false sense of ease with which we shall be able to treat human cancers immunologically. Lost in translation was the fact that the impressive successes in infectious diseases have been seen purely in prophylaxis; therapy is nearly impossible once the disease has established. Prophylaxis and therapy are, immunologically speaking, apples and oranges, and not too much comfort should be derived from studies in prophylaxis. Fig. 1 shows the results of a very simple experiment where groups of mice were injected once with irradiated tumor cells at various time points prior to or after receiving a live tumor challenge. The data clearly define the distinct window of time during which prophylactic treatment (days −7 or earlier) protects animals from tumor challenge; immunizing mice after this time does not elicit tumor.
Figure 1.
Groups of BALB/c mice were immunized once at the indicated time point with an intradermal injection of 106 irradiated Meth A tumor cells. On day 0, all groups received live tumor challenge and were monitored for tumor growth on subsequent days. Data are presented as tumor area for individual mice in each group (5 mice per group). The lack of any lines in the panel corresponding to “−7 day” denotes complete lack of tumor growth in 5/5 mice.
Only a very small number of immunological approaches have been (even modestly) successful in therapy of pre-existing tumor (7–10). The reasons for the profound difference between prophylaxis and therapy are multi-factorial (tolerance, suppression, micro-environmental factors) and beyond the scope of this article.
(c) Studies with appropriate cancer models of mice and rats are useful. The notion that it is easy to cure cancers in mice is generally advanced the most confidently by those who have never cured a mouse of cancer by immunotherapy. The failures of several advanced human clinical trials, which were apparently based on solid data from mouse tumors, has lead to a common “wisdom” that tumors of mice are easy to cure and that human tumors represent an entirely different beast, to which the lessons from mice do not apply. This “wisdom” is largely misguided. True, human tumors are different from mouse tumors in at least one significant way: human tumors have lived in their hosts for long periods of time, in fact longer than the entire life of a mouse, and hence are more likely to have developed potent immune evasive mechanisms than most mouse tumors (11). However, the mouse or rat tumor models which are the bases of human clinical trials, are generally unrepresentative of the human disease in other mundane ways. The animal studies often show activity in prophylaxis, and are not tested in appropriate models of therapy (see lesson #b); animal studies are often done in non-metastatic models, while human disease may be metastatic. It is important to recognize the information that can, and cannot, be obtained from animal models. The more sophisticated models of prostate (12), breast (13,14), lymphoma (15) and other cancers (16,17), have been largely under-utilized in pre-clinical studies.
(d) It is the antigen(s), stupid! This is the central lesson in this article. The ability to identify T cell defined antigens lead to a great effort to identify the MHC I and II - restricted antigens on human cancers (see 18 for review). This was followed by a reasonably extensive effort at treating human cancers (in Phase 1 and Phase 2 trials) by immunizing against these antigens. Although no randomized Phase 3 trial has been conducted with such antigens, the early results suggest, at a minimum, that the notion that one could affect an anti-cancer response by simply expanding the numbers of CD8 T cells against normal self antigens, which also happen to be expressed on cancers, was a bit simple minded. Immunization with defined differentiation antigen(s) presented by normal and cancer cells may or may not elicit a measurable T cell response; however, there is no evidence that a T cell response, when elicited, translates into a demonstrable anti-cancer effect. (The jury is still out on the cancer testes antigens, which appear the more promising because of the specificity of their expression on cancers, and germ line tissues, but not normal adult tissues. See 19 for review) Regardless of the lack of correlation between measurable T cell response and clinical activity, extensive monitoring of such responses must remain an integral part of clinical studies (see 20 as an example). Indeed, the immunological monitoring of clinical trials needs to be broadened in scope (to CD4 responses, NK activity, antibody response etc) if we are ever to understand the immunological correlates to protective tumor immunity (21).
Where are we then with respect to the identity of the protective tumor antigens of human cancers (see 22)? We do not really know, and it is unlikely that we will know anything with certainty for some years to come. In contrast to the epitopes shared between normal tissues and cancers discussed above, another category of truly tumor-specific epitopes has been defined during the past several years. These epitopes are truly tumor-specific because they are created by a mutation in a normal gene; these mutations are almost always unique to the specific tumor in which they are identified [23-35]. Interestingly, in almost every instance where immune response can be correlated to tumor rejection, the immune response is directed to these true tumor-specific mutations [23,26,28,29,30-32,35]. Such individually unique tumor-specific epitopes have been suggested to be created by random mutations inherent in any cell division (36, N. Srivastava and P.K. Srivastava, personal communication), and the rates of their occurrence may well be much higher in tumors than in normal tissues.
These individually tumor-specific mutation-derived epitopes are reminiscent of the individually unique protective antigens of experimentally induced mouse and rat tumors (37,38). Are these the true protective antigens of human tumors? The question is difficult to resolve because of the obvious and necessary constraints on what can be tested clinically. Regardless, should these individually unique antigens turn out to be the protective antigens of human cancers, how can that knowledge be translated into a generally applicable clinical effort? Three avenues come to mind.
Heat shock proteins of the hsp70 and hsp90 family have been shown extensively to chaperone the antigenic epitopes generated in the cells from which the HSPs are purified; the HSPs chaperone the epitope precursors from the point of their generation in the cytosol to the transporters associated with antigen processing in the endoplasmic reticulum for loading on the MHC I molecules (39–43). The HSP-peptide complexes isolated from cancers or cells infected with intracellular organisms such as viruses or intracellular bacteria have also been shown to elicit potent and protective immunity against the cognate cancers and infectious agents (see 44 for review). Immunization with such cancer-derived HSP-peptide complexes carries the distinct advantage that one does not require prior knowledge of the identity of the antigenic epitopes in order to vaccinate with them. One simply needs to isolate the HSP-peptide complexes from each patient’s cancer. After a series of Phase 1 and Phase 2 trials, two randomized Phase 3 trials, one each in stage IV melanoma (45) and adjuvant renal cell carcinoma (46), have been completed using this approach.
In the melanoma trial (45), 322 patients were randomly assigned 2:1 to receive HSP gp96-peptide complexes derived from each patient’s autologous tumor or physician’s choice of a treatment containing one or more of the following: dacarbazine, temozolomide, interleukin-2, or complete tumor resection. Patients assigned to the HSP arm received variable number of injections (range, 0 to 87; median, 6) in part because of the autologous nature of such therapy. Intention-to-treat analysis showed that overall survival in the HSP arm is statistically indistinguishable from that in the control arm. Exploratory landmark analyses show that patients in the M1a and M1b sub-stages receiving a larger number of gp96 immunizations survived longer than those receiving fewer such treatments. Such difference was not detected for sub-stage M1c patients, who have the worst prognosis. The results suggest patients with M1a and M1b disease who are able to receive 10 or more doses of the HSP vaccine as the candidate population for a confirmatory study.
In the adjuvant renal cell carcinoma trial (46), patients were randomized 1:1 to receive the autologous tumor-derived gp96-peptide complexes or observation postnephrectomy in an open-label trial. Recurrence free survival and overall survival were similar between arms in the intent-to-treat analysis (n = 728). There was a strong positive trend associated with the gp96 vaccine in earlier-stage (stage I and II) patients with no baseline disease (n = 240) as well as in earlier-stage patients with prognostic factors indicating intermediate risk for recurrence (stage Ib/II highgrade, III T1/2/3a low-grade; n= 362). Given the lack of adjuvant treatments for renal cell carcinoma and the excellent safety profile of the gp96 vaccine, the positive results from this trial warrant further exploration of the autologous tumorderived gp96 vaccine as an adjuvant treatment for this group of patients. These results formed the basis of approval of the gp96-based vaccine for treatment of intermediate stage adjuvant renal cell carcinoma, and an application for approval is pending in the European Union.
These results are consistent with the immunologic mechanism of action of HSP-peptide complexes, indicating delayed onset of clinical activity after exposure to the vaccine. However, based as they are on retrospective sub-set analyses, they require confirmation in a prospectively defined patient population, in order for them to achieve the requisite standard of evidence.
Immunization with RNA isolated from individual tumors is another possible way to treat patients with antigens corresponding to their individual antigenic repertoires. Transfection of DCs with mRNA has been reported to be superior to other antigenloading techniques. The ability to amplify RNA from microscopic amounts of tumor tissue potentially extends the use of DC vaccination to virtually every cancer patient (47). In phase I clinical trials, patients with prostate and renal cell cancers have been administered cognate mRNA-transfected DCs and a majority of patients exhibited a vaccine-induced T-cell response. Similar observations have been made in melanoma patients (48).
The most extreme form of individualization of immunotherapy would of course consist of sequencing of the entire genome of each patient’s tumor, followed by listing of the unique tumor-specific epitopes and immunization against a panel of such epitopes. With the rapid and continuing decline in the cost of sequencing, such approaches are not beyond the bounds of possibility in the near future.
To return to the primary theme of this lesson, and with a nod to James Carville, it is the antigen(s), stupid! There is little hope that we can manipulate the immune response to advantage, unless we can harness the protective antigens. At this point, we have little real knowledge of what these antigens are in human tumors. The protective antigens of all the mouse tumors where this information is available, are unique mutational epitopes (26,28,31,32); the non-mutated antigens of mouse tumors do not elicit protective immunity (49,50). There are good reasons, and good evidence, to believe that the human tumor antigens will follow the same pattern. Some of these have been discussed in the beginning of this section.
The antigens are central in their own right, but also in terms of how they affect the manipulability of the immune response by other factors. The use of the blocking antibody to CTLA4 is a good example. In a remarkable study (51), Hodi et al. infused a number of pre-vaccinated patients with a blocking antibody to CTLA4. The infusion “stimulated extensive tumor necrosis with lymphocyte and granulocyte infiltrates in three of three metastatic melanoma patients and the reduction or stabilization of CA-125 levels in two of two metastatic ovarian carcinoma patients previously vaccinated with irradiated, autologous granulocyte-macrophage colony-stimulating factor-secreting tumor cells. MDX-CTLA4 did not elicit tumor necrosis in four of four metastatic melanoma patients previously immunized with defined melanosomal antigens.” Although the patient numbers here were small, the complete concordance between prior vaccitherapy with autologous tumors (or shared antigens) and clinical response was striking. A recent study essentially confirmed those results, and further showed that in patients immunized with autologous vaccines, the anti-tumor effects appeared to be dissectable from the pathological autoimmunity generated by infusion with blocking antibody to CTLA4 (52). These results highlight the centrality of the antigens chosen for vaccitherapy.
In spite of the considerable evidence suggesting, indeed indicating, that the protective antigens of human tumors are individually specific, that is not a widely shared view. The overwhelming volume of papers and reviews take it as a matter of fact that the “tumor antigens” are normal self antigens and that the only way to immunize cancer patients successfully, is to find ways to break tolerance. There is no rational basis for this divergence between the data and belief. There are really no serious experimental or theoretical grounds opposed to the concept of antigenic individuality of human tumors. One could make the questionable case that we are culturally uncomfortable with the idea of a vast, practically un-definable antigenic complexity and antigenic individuality that arises out of randomness! An unwitting but real contributor to opposition to the idea of antigenic individuality of human tumors is the pharmaceutical industry, which is arguably too comfortable with mass production and mass marketing of one-shoe-fits-all, “well defined” drugs. As the biomedical community has, with the best intentions, almost entirely surrendered the choices and modalities of drug development to the pharmaceutical industry, the space for curiosity-driven, hypothesis-driven clinical research in drug development has been steadily shrinking. Regardless of these constraints, we hope that whether through hypothesis-driven clinical research, or through market-driven trials and errors, we will come to harness the protective antigens of human tumors.
(e) All targets are equal, but some are more equal than others. The key is specificity for cancer. That does not necessarily mean specificity for cancer cells. A plethora of cancer “targets” have been identified over the years, and most of them have gone the way of the parchment paper. The “targets” include oncogene products like p53, and ras, telomerase, and as classes, molecules involved in signal transduction, apoptosis, protein turnover, DNA repair, and other normal physiological processes. The oncogene products have drawn the attention of immunologists, while the other targets have been targeted by developers of small molecule inhibitors, and antibodies. The lesson regarding the oncogene products as targets of vaccination is as follows. These are attractive targets because they are cancer-specific. They are unattractive targets because cancers have many many ways to keep the malignant phenotype, even if one or two specific targets such as p53 and ras mutations are eliminated. And the possibility of vaccination against all the pathways that cells can utilize to remain malignant, is incompatible with life. Summary: paradoxical as it may seem, targets that appear essential for cancer cells to retain the malignant phenotype, are not good targets for vaccination.
The lesson regarding the non-cancer-specific targets (such as signal transducers, DNA repairers etc) is less straight forward. At the face of it, they are not cancer-specific targets; however, we have learnt that toxicities can be acceptable and lack of specificity alone is not a valid case. It is reasonable to believe that these targets shall follow the traditional course of drug development, and shall become drugs or not, depending on the merits of each target and each inhibitor tested.
Specificity however has another face, and that is, specificity for the cancer, as distinct from specificity for cancer cells. The cancer mass contains the stroma, the blood supply, infiltrating cells, in addition to the cancer cells. Much attention has focused on cancer cells, and the infiltrating cells and the blood supply, howsoever unsatisfactorily. The stroma is only now gaining its due, with the recognition of markers that are relatively specific for the cancer stroma (53), and that destruction of stroma can eliminate even large bulky tumor masses (54,55).
(f) Vaccitherapy must be attempted in minimal residual disease setting. In the setting of bulky disease, vaccitherapy must be combined with immune modulation. This lesson may be too self-evident to be written about, and we do so only because what is self-evident may be in the eye of the beholder. Successes in vaccitherapy of pre-existing mouse tumors have been few and far between, as previously discussed, and these have been more convincing in the setting of micrometastatic disease rather than bulky and disseminated disease (7). One could argue that one should attempt vaccitherapy in human clinical trials in the corresponding setting of minimal residual disease, with the end points of time to progression or overall survival. The problem with this approach is that no real indicator of efficacy in the minimal residual disease setting can be achieved in Phase 1 and Phase 2 trials and the Phase 3 trials take a very long time. The requirement of that length of time is incompatible with the temperament and the needs of drug industry that largely controls such trials. That fact does not augur well for trials in the adjuvant or minimal residual disease setting, precisely the setting where vaccitherapy has the best activity.
Conversely, immuno-modulators, be they inhibitors of CTLA4 or PD1 blockade, or depletors of T regulatory cells (56) have shown the potential to mediate regression of large tumor masses. These agents and others of their ilk, with and without vaccitherapy, are proceeding ahead in clinical development. There is not so much of a lesson here as a statement of fact, and the bias that immuno-modulators have a better chance to succeed with than without vaccitherapy.
Conclusions
The challenges in human cancer immunotherapy today, are less conceptual and more logistical. Several worthy approaches, with strong scientific and clinical rationales and track records, are on the table. It is nearly certain that a judicious application of some of these shall have a powerful impact on cancer as a clinical entity. However, the motivation to do the necessary studies, the ownership of intellectual property and materials, the knowhow to conduct the studies, and the resources necessary, are all highly fragmented. This might appear at first blush, to be a rather gloomy scenario but in fact it is not. As some initial successes, howsoever small but real, are obtained, the current fragmented picture will begin to congeal into a more coherent scene, where more combinations will be testable, more resources will be available to test them, and there will be sufficient patience to do what has to be done
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Meeker TC, Lowder J, Maloney DG, Miller RA, Thielemans K, Warnke R and Levy R A clinical trial of anti-idiotype therapy for B cell malignancy Blood 1985. 65: 1349–1363 [PubMed] [Google Scholar]
- 2.McLaughlin P, Grillo-Lopez AJ, Link BK, Levy R, Czuczman MS, Williams ME, Heyman MR, Bence-Bruckler I, White CA, Cabanillas F, Jain V, Ho AD, Lister J, Wey K, Shen D, Dallaire BK. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J Clin Oncol. 1998. August;16(8):2825–33 [DOI] [PubMed] [Google Scholar]
- 3.Horowitz MM, et al. Blood. 1990. February 1;75(3):555–62. Graft-versus-leukemia reactions after bone marrow transplantation. [PubMed] [Google Scholar]
- 4.Atkins MB, Lotze MT, Dutcher JP, Fisher RI, Weiss G, Margolin K, Abrams J, Sznol M, Parkinson D, Hawkins M, Paradise C, Kunkel L, Rosenberg SA. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999. July;17(7):2105–16 [DOI] [PubMed] [Google Scholar]
- 5.McDermott DF, Regan MM, Clark JI, Flaherty LE, Weiss GR, Logan TF, Kirkwood JM, Gordon MS, Sosman JA, Ernstoff MS, Tretter CP, Urba WJ, Smith JW, Margolin KA, Mier JW, Gollob JA, Dutcher JP, Atkins MB. Randomized phase III trial of high-dose interleukin-2 versus subcutaneous interleukin-2 and interferon in patients with metastatic renal cell carcinoma. J Clin Oncol. 2005. January 1;23(1):133–41. Erratum in: J Clin Oncol. 2005 Apr 20;23(12):2877 [DOI] [PubMed] [Google Scholar]
- 6.Gogas H, Ioannovich J, Dafni U, Stavropoulou-Giokas C, Frangia K, Tsoutsos D, Panagiotou P, Polyzos A, Papadopoulos O, Stratigos A, Markopoulos C, Bafaloukos D, Pectasides D, Fountzilas G, Kirkwood JM. Prognostic significance of autoimmunity during treatment of melanoma with interferon. N Engl J Med. 2006. February 16;354(7):709–18 [DOI] [PubMed] [Google Scholar]
- 7.Tamura Y, Peng P, Liu K, Daou M, Srivastava PK. Immunotherapy of tumors with autologous tumor-derived heat shock protein preparations. Science. 1997. October 3;278(5335):117–20. Erratum in: Science 1999 Feb 19;283(5405):preceding 1119. [DOI] [PubMed] [Google Scholar]
- 8.Finkelstein SE, Heimann DM, Klebanoff CA, Antony PA, Gattinoni L, Hinrichs CS, Hwang LN, Palmer DC, Spiess PJ, Surman DR, Wrzesiniski C, Yu Z, Rosenberg SA, Restifo NP. Bedside to bench and back again: how animal models are guiding the development of new immunotherapies for cancer. J Leukoc Biol. 2004. August;76(2):333–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kovalchin JT, Murthy AS, Horattas MC, Guyton DP, Chandawarkar RY. Determinants of efficacy of immunotherapy with tumor-derived heat shock protein gp96. Cancer Immun. 2001. April 27;1:7. [PubMed] [Google Scholar]
- 10.Heckman KL, Schenk EL, Radhakrishnan S, Pavelko KD, Hansen MJ, Pease LR. Fast-tracked CTL: rapid induction of potent anti-tumor killer T cells in situ. Eur J Immunol. 2007. July;37(7):1827–35 [DOI] [PubMed] [Google Scholar]
- 11.Parsa AT, Waldron JS, Panner A, Crane CA, Parney IF, Barry JJ, Cachola KE, Murray JC, Tihan T, Jensen MC, Mischel PS, Stokoe D, Pieper RO. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat Med. 2007. January;13(1):84–8 [DOI] [PubMed] [Google Scholar]
- 12.Gingrich JR, Barrios RJ, Morton RA, Boyce BF, DeMayo FJ, Finegold MJ, Angelopoulou R, Rosen JM, Greenberg NM. Metastatic prostate cancer in a transgenic mouse. Cancer Res. 1996. September 15;56(18):4096–102 [PubMed] [Google Scholar]
- 13.Cardiff RD, Moghanaki D, Jensen RA. Genetically engineered mouse models of mammary intraepithelial neoplasia. J Mammary Gland Biol Neoplasia. 2000. October;5(4):421–37 [DOI] [PubMed] [Google Scholar]
- 14.Vargo-Gogola T, Rosen JM. Modelling breast cancer: one size does not fit all. Nat Rev Cancer. 2007. September;7(9):659–72 [DOI] [PubMed] [Google Scholar]
- 15.Scaglione BJ, Salerno E, Balan M, Coffman F, Landgraf P, Abbasi F, Kotenko S, Marti GE, Raveche ES. Murine models of chronic lymphocytic leukaemia: role of microRNA-16 in the New Zealand Black mouse model. Br J Haematol. 2007. December;139(5):645–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanahan D, Wagner EF, Palmiter RD. The origins of oncomice: a history of the first transgenic mice genetically engineered to develop cancer. Genes Dev. 2007. September 15;21(18):2258–70 [DOI] [PubMed] [Google Scholar]
- 17.Frese KK, Tuveson DA. Maximizing mouse cancer models. Nat Rev Cancer. 2007. September;7(9):645–58 [DOI] [PubMed] [Google Scholar]
- 18.Database of human tumor antigens recognized by T cells. Istituto Nazionale per lo Studio e la Cura dei Tumori (INT) - Milan, Italy: http://www.istitutotumori.mi.it/INT/AreaProfessionale/Human_Tumor/default.asp?LinkAttivo=17B [Google Scholar]
- 19.Cancer/testis (CT) Gene Database, Ludwig Institute for Cancer Research, Office of Information Technology - Lausanne, Switzerland: http://www.cancerimmunity.org/CTdatabase/ [Google Scholar]
- 20.Rosenberg SA, Sherry RM, Morton KE, Scharfman WJ, Yang JC, Topalian SL, Royal RE, Kammula U, Restifo NP, Hughes MS, Schwartzentruber D, Berman DM, Schwarz SL, Ngo LT, Mavroukakis SA, White DE, Steinberg SM. Tumor progression can occur despite the induction of very high levels of self/tumor antigen-specific CD8+ T cells in patients with melanoma. J Immunol. 2005. November 1;175(9):6169–76 [DOI] [PubMed] [Google Scholar]
- 21.Srivastava PK. Immunotherapy of human cancer: lessons from mice. Nature Immunol. 2000. November;1(5):363–6. [DOI] [PubMed] [Google Scholar]
- 22.Srivastava PK. Do human cancers express shared protective antigens? or the necessity of remembrance of things past. Semin Immunol. 1996. October;8(5):295–302 [DOI] [PubMed] [Google Scholar]
- 23.Baurain JF, Colau D, van Baren N, Landry C, Martelange V, Vikkula M, Boon T, Coulie P-G (2000) High Frequency of Autologous Anti- Melanoma CTL Directed Against an Antigen Generated by a Point Mutation in a New Helicase Gene. J Immunol 164: 6057–6066 [DOI] [PubMed] [Google Scholar]
- 24.Chiari R, Foury F, De Plaen E, Baurain J-F, Thonnard J, Coulie P-G (1999) Two Antigens Recognized by Autologous Cytolytic T Lymphocytes on a Melanoma Result from a Single Point Mutation in an Essential Housekeeping Gene Cancer Res 59: 5785–5792 [PubMed] [Google Scholar]
- 25.Coulie PG, Lehmann F, Lethe B, Herman J,Lurquin C,Andrawiss M, Boon T (1995) A Mutated Intron Sequence Codes for an Antigenic Peptide Recognized by Cytolytic T Lymphocytes on a Human Melanoma. Proceedings of the National Academy of Sciences 92: 7976–7980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dubey P, Hendrickson RC, Meredith SC, Siegel CT,Shabanowitz J, Skipper JCA, Engelhard VH, Hunt DF, Schreiber H (1997) The Immunodominant Antigen of an Ultraviolet-induced Regressor Tumor Is Generated by a Somatic Point Mutation in the DEAD Box Helicase p68. J. Exp. Med 185: 695–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Echchakir H, Mami-Chouaib F, Vergnon I, Baurain J-F, Karanikas V, Chouaib S, Coulie P-G (2001) A Point Mutation in the alpha-Actinin-4 Gene Generates an Antigenic Peptide Recognized by Autologous Cytolytic T Lymphocytes on a Human Lung Carcinoma Cancer Res 2001 61: 4078–4083 [PubMed] [Google Scholar]
- 28.Ikeda H, Ohta N, Furukawa K, Miyazaki H, Wang L, Furukawa K, Kuribayashi K, Old LJ, Shiku H (1997) Mutated mitogen-activated protein kinase: A tumor rejection antigen of mouse sarcoma. Proceedings of the National Academy of Sciences 94: 6375–6379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karanikas V, Colau D, Baurain J-F, Chiari R, Thonnard J, Gutierrez-Roelens I, Goffinet C, van Schaftingen E, Weynants P, Boon T, Coulie PG (2001) High Frequency of Cytolytic T Lymphocytes Directed against a Tumorspecific Mutated Antigen Detectable with HLA Tetramers in the Blood of a Lung Carcinoma Patient with Long Survival. Cancer Res 2001 61: 3718–3724 [PubMed] [Google Scholar]
- 30.Lennerz V, Fatho M, Gentilini C, Frye RA, Lifke A, Ferel D, Wolfel C, Huber C, Wlfel T (2005) The response of autologous T cells to a human melanoma is dominated by mutated neoantigens. Proceedings of the National Academy of Sciences 102: 16013–16018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsutake T, Srivastava PK (2001) The immunoprotective MHC II epitope of a chemically induced tumor harbors a unique mutation in a ribosomal protein. Proceedings of the National Academy of Sciences 98:3992–3997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Monach PA, Meredith SC, Siegel CT, Schreiber H (1995) A unique tumor antigen produced by a single amino acid substitution. Immunity 2:45–59 [DOI] [PubMed] [Google Scholar]
- 33.Sensi M, Nicolini G, Zanon M, Colombo C, Molla A, Bersani I, Lupetti R, Parmiani G, Anichini A (2005) Immunogenicity without Immunoselection: A Mutant but Functional Antioxidant Enzyme Retained in a Human Metastatic Melanoma and Targeted by CD8+ T Cells with a Memory Phenotype.Cancer Res 2005 65: 632–64024 [PubMed] [Google Scholar]
- 34.Takenoyama M, Baurain J-F, Yasuda M, So T, Sugaya M, Hanagiri T, Sugio K, Yasumoto K, Boon T, Coulie PG (2006) A point mutation in the NFYC gene generates an antigenic peptide recognized by autologous cytolytic T lymphocytes on a human squamous cell lung carcinoma. Int. J. Cancer 118:1992–1997 [DOI] [PubMed] [Google Scholar]
- 35.Zorn E and Hercend T (1999) A natural cytotoxic T cell response in a spontaneously regressing human melanoma targets a neoantigen resulting from a somatic point mutation. Eur. J. Immunol 29:592–601 [DOI] [PubMed] [Google Scholar]
- 36.Srivastava PK. Peptide-binding heat shock proteins in the endoplasmic reticulum: role in immune response to cancer and in antigen presentation. Adv Cancer Res. 1993;62:153–77 [DOI] [PubMed] [Google Scholar]
- 37.Srivastava PK, Old LJ. Individually distinct transplantation antigens of chemically induced mouse tumors. Immunol Today. 1988. March;9(3):78–83. [DOI] [PubMed] [Google Scholar]
- 38.Mumberg D, Wick M, Schreiber H. Unique tumor antigens redefined as mutant tumor-specific antigens. Semin Immunol. 1996. October;8(5):289–93 [DOI] [PubMed] [Google Scholar]
- 39.Srivastava PK, Udono H, Blachere NE, Li Z. Heat shock proteins transfer peptides during antigen processing and CTL priming. Immunogenetics. 1994;39(2):93–8 [DOI] [PubMed] [Google Scholar]
- 40.Binder RJ, Blachere NE, Srivastava PK. Heat shock protein-chaperoned peptides but not free peptides introduced into the cytosol are presented efficiently by major histocompatibility complex I molecules. J Biol Chem. 2001. May 18;276(20):17163–71 [DOI] [PubMed] [Google Scholar]
- 41.Kunisawa J, Shastri N. The group II chaperonin TRiC protects proteolytic intermediates from degradation in the MHC class I antigen processing pathway. Mol Cell. 2003. September;12(3):565–76 [DOI] [PubMed] [Google Scholar]
- 42.Kunisawa J, Shastri N. Hsp90alpha chaperones large C-terminally extended proteolytic intermediates in the MHC class I antigen processing pathway. Immunity. 2006. May;24(5):523–34 [DOI] [PubMed] [Google Scholar]
- 43.Callahan MK, Garg M, Srivastava PK. Heat-shock protein 90 associates with N-terminal extended peptides and is required for direct and indirect antigen presentation. Proc Natl Acad Sci U S A. 2008. February 5;105(5):1662–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Srivastava P Interaction of heat shock proteins with peptides and antigen presenting cells: chaperoning of the innate and adaptive immune responses. Annu Rev Immunol. 2002;20:395–425. [DOI] [PubMed] [Google Scholar]
- 45.Testori A, Richards J, Whitman E, Mann GB, Lutzky J, Camacho L, Parmiani G, Tosti G, Kirkwood JM, Hoos A, Yuh L, Gupta R, Srivastava PK. Phase III comparison of vitespen, an autologous tumor-derived heat shock protein gp96 peptide complex vaccine, with physician’s choice of treatment for stage IV melanoma. J Clin Oncol. 2008. February 20;26(6):955–62. [DOI] [PubMed] [Google Scholar]
- 46.Wood CG, Srivastava PK, Lacombe L, Wentworth K, Yuh L, Gupta R, Figlin R, Flanigan R, Escudier B. A randomized Phase 3 trial comparing adjuvant therapy with autologous tumor-derived heat shock protein gp96 (vitespen) vs. observation in patients at high risk of recurrence of renal cell carcinoma. Lancet, in press (2008). [Google Scholar]
- 47.Gilboa E, Vieweg J. Cancer immunotherapy with mRNA-transfected dendritic cells. Immunol Rev. 2004. June;199:251–63 [DOI] [PubMed] [Google Scholar]
- 48.Kyte JA, Gaudernack G. Immuno-gene therapy of cancer with tumourmRNA transfected dendritic cells. Cancer Immunol Immunother. 2006. November;55(11):1432–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ramarathinam L, Sarma S, Maric M, Zhao M, Yang G, Chen L, Liu Y. Multiple lineages of tumors express a common tumor antigen, P1A, but they are not cross-protected. J Immunol. 1995. December 1;155(11):5323–9 [PubMed] [Google Scholar]
- 50.Sarma S, Bai XF, Liu JQ, May KF Jr, Zheng P, Liu Y. On the role of unmutated antigens in tumor rejection in mice with unperturbed T-cell repertoires. Cancer Res. 2003. September 15;63(18):6051–5. [PubMed] [Google Scholar]
- 51.Hodi FS, Mihm MC, Soiffer RJ, Haluska FG, Butler M, Seiden MV, Davis T, Henry Spires R, MacRae S, Willman A, Padera R, Jaklitsch MT, Shankar S, Chen TC, Korman A, Allison JP, Dranoff G. Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc Natl Acad Sci U S A. 2003. April 15;100(8):4712–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hodi FS, Butler M, Oble DA, Seiden MV, Haluska FG, Kruse A, Macrae S, Nelson M, Canning C, Lowy I, Korman A, Lautz D, Russell S, Jaklitsch MT, Ramaiya N, Chen TC, Neuberg D, Allison JP, Mihm MC, Dranoff G. Immunologic and clinical effects of antibody blockade of cytotoxic T lymphocyte-associated antigen 4 in previously vaccinated cancer patients. Proc Natl Acad Sci U S A. 2008. February 26;105(8):3005–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Garin-Chesa P, Old LJ, Rettig WJ. Cell surface glycoprotein of reactive stromal fibroblasts as a potential antibody target in human epithelial cancers. Proc Natl Acad Sci U S A. 1990. September;87(18):7235–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Singh S, Ross SR, Acena M, Rowley DA, Schreiber H. Stroma is critical for preventing or permitting immunological destruction of antigenic cancer cells. J Exp Med. 1992. January 1;175(1):139–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yu P, Rowley DA, Fu YX, Schreiber The role of stroma in immune recognition and destruction of well-established solid tumors. Curr Opin Immunol. 2006. April;18(2):226–31 [DOI] [PubMed] [Google Scholar]
- 56.Waldmann TA. Effective cancer therapy through immunomodulation. Annu Rev Med. 2006;57:65–81 [DOI] [PubMed] [Google Scholar]