Abstract
Microtubule (MT)-associated proteins perform diverse functions in cells. These functions are dependent on their interactions with MTs. Dynactin, a cofactor of dynein motor, assists the binding of dynein to various organelles and is crucial to the long-distance processivity of dynein-based complexes. The largest subunit of dynactin, the p150Glued, contains an N-terminus segment that is responsible for the MT-binding interactions and long-range processivity of dynactin. We employed solution and magic angle spinning NMR spectroscopy to characterize the structure and dynamics of the p150Glued N-terminal region, both free and in complex with polymerized MTs. This 191-residue region encompasses the cytoskeleton-associated protein glycine-rich domain, the basic domain, and serine/proline-rich (SP-rich) domain. We demonstrate that the basic and SP-rich domains are intrinsically disordered in solution and significantly enhance the binding affinity to MTs as these regions contain the second MT-binding site on the p150Glued subunit. The majority of the basic and SP-rich domains are predicted to be random coil, whereas the segments S111–I116, A124–R132, and K144–T146 in the basic domain contain short α-helical or β-sheet structures. These three segments possibly encompass the MT-binding site. Surprisingly, the protein retains a high degree of flexibility upon binding to MTs except for the regions that are directly involved in the binding interactions with MTs. This conformational flexibility may be essential for the biological functions of the p150Glued subunit.
Significance
Microtubule (MT)-associated proteins perform diverse functions in cells. Many of them comprises intrinsically disordered regions whose structural flexibility are central to MT-based cellular functions of MT-associated proteins. We employed solution and magic angle spinning NMR spectroscopy to characterize the structure and dynamics of the p150Glued N-terminal region encompassing the cytoskeleton-associated protein glycine-rich domain, the basic domain, and the serine/proline-rich domain, both free and in complex with polymerized MTs. The results reveal that the basic and serine/proline-rich domains are largely unstructured and retain a high degree of flexibility upon binding to MTs, except for the regions that are possibly involved in the binding interactions with MTs. This approach is informative for dynamic studies of intrinsically disordered MT-associated proteins and other disordered proteins in large biological assemblies.
Introduction
Microtubules (MTs) play fundamental roles in intracellular transport, cell motility, cytoskeletal organization, maintenance of cell shape, and separation of chromosomes during mitosis (1, 2). MT-associated proteins are involved in these cellular activities by interacting with MTs and/or regulating MT dynamics (3, 4). The dynein motor mediates the retrograde organelle transport along MTs (5, 6). Dynactin, a cofactor of dynein, assists the binding of dynein to various organelles, especially messenger RNA, chromosomes, viruses, and endomembrane vesicles, and is crucial for the long-distance processivity of dynein-based complexes (7, 8).
Most of the MT-associated proteins have specific MT-binding domains that mediate their interactions with MTs. The 1.2 MDa dynactin complex contains 20 subunits (9); the cytoskeleton-associated protein glycine-rich (CAP-Gly) domain at the N-terminus of the largest subunit p150Glued is the MT-binding domain of dynactin. Mutations in the CAP-Gly domain lead to severe neurodegenerative diseases, such as Perry syndrome and distal spinal bulbar muscular atrophy (10). Multiple investigations have been carried out into the structure of the CAP-Gly domain to understand the mechanism of its interaction with MTs (11, 12, 13, 14, 15).
Other domains of p150Glued subunit are also essential for the dynactin’s function. As reported previously, the basic domain of p150Glued (residues 115–145) has the ability to bind with MTs in the absence of CAP-Gly domain and to independently skate along MTs in the absence of dynein (16). In addition, this domain enhances dynein processivity by fourfold and is responsible for the long-range motility of dynein and its long-time interactions with MTs (16). More specifically, the K-rich domain (132–152) antagonizes the inhibitory effect of the coiled-coil 1 domain (CC1) (residues 214–547) in the MT-binding affinity of p150Glued (17). In the N-terminal segment of p150Glued (residues 1–144), the N-terminal tail (residues 1–25) and the basic patch (residues 106–144) greatly increase the binding affinity of the segment to MTs, and these patches affect the lateral associations of tubulins by interacting with the tubulin’s E-hook (13). Despite these studies, atomic-resolution information concerning the structure and dynamics of the p150Glued subunit bound to the MTs is still lacking, limiting our understanding of dynactin’s function.
Here, we focus on the N-terminal segment of p150Glued subunit, which includes the CAP-Gly domain, the basic domain, and the serine/proline-rich (SP-rich) domain. This functional region of p150Glued comprises the N-terminal residues 1–191 of p150Glued and is dubbed here p150Glued(1–191). We performed a structure and dynamics investigation of p150Glued(1–191) free and in complex with MTs by magic angle spinning (MAS) NMR and solution NMR spectroscopy. On the basis of these experiments, we determined the likely MT-binding site and functional domains in the extended region. Our results inform on the conformation and mobility of the basic and the SP-rich domains of p150Glued(1–191) free and bound to MTs at atomic resolution. The structural and dynamics information of the p150Glued(1–191) subunit bound to MTs gained in this study is beneficial for understanding the structure-function relationships of p150Glued subunit. Our work lays the foundation for the determination of the structure of the dimeric p150Glued subunit bound to polymerized MTs.
Materials and Methods
Materials
Common chemicals were purchased from Thermo Fisher Scientific (Waltham, MA) or Sigma-Aldrich (St. Louis, MO). Isotopically labeled chemicals, including 15NH4Cl and U-13C6-glucose, were purchased from Cambridge Isotope Laboratories (Tewksbury, MA). Chromatography columns were purchased from GE Healthcare (Chicago, IL). Bovine brain tubulin, GTP, and paclitaxel (Taxol) were purchased from Cytoskeleton (Denver, CO). 400-mesh copper grids coated with Formval and stabilized with evaporated carbon films were purchased from Electron Microscopy Sciences (Hatfield, PA). The SMT3 fusion vector (pET28b-His6-SMT3) and His6-Ulp1 protease expression system were generous gifts from Dr. Christopher Lima (Weill Medical College, Cornell University, Ithaca, NY).
Protein expression and purification
The N-terminal region of p150Glued subunit of Rattus norvegicus dynactin that encompasses residues 1–191 was subcloned into the pET28b-His6-SMT3 vector using BamHI and XhoI restriction sites. The construct was transformed into Escherichia coli BL21 (DE3) competent cells for expression and purification. A cysteine residue was added to the C-terminal end of p150Glued(1–191) for cysteine-based cross-linking. This p150Glued(1–191) construct was expressed as His6-SMT3-tagged recombinant protein using the above construct. The natural abundance p150Glued(1–191) protein was overexpressed in Luria-Bertani (LB) media. The E. coli cells were grown at 37°C in LB media after antibiotic selection on kanamycin LB/agar plates. Bacterial cells were induced with 0.4 mM isopropyl-β-1-thiogalactopyranoside at 37°C for 4–5 h after the OD600 value reached ∼0.6–∼0.7 and expressed at 37°C for 4–5 h and were harvested with centrifugation at 4000 × g for 30 min.
Overexpression of U-15N-labeled and U-13C,15N-labeled p150Glued(1–191) was carried out in minimal media as follows. Cells were grown in 2 L of unlabeled LB media at 37°C and harvested when the OD600 reached ∼1.4. The cell pellets were prewashed with M9 minimal media depleted of nitrogen and carbon sources and collected after centrifugation at 4000 × g for 30 min. The pelleted cells were resuspended in 1 L of M9 media that contained 15NH4Cl, 2 g U-13C6 glucose, basal vitamins, 0.1 mM CaCl2, and 2 mM MgSO4 at cell concentrations fourfold greater than LB media. 15NH4Cl and 4 g of natural abundance glucose were used for the preparation of U-15N-labeled p150Glued(1–191). After a recovery in labeled M9 media for 1 h, the cells were induced with 0.8 mM isopropyl-β-1-thiogalactopyranoside at 37°C, and expression proceeded for 4–5 h. Cells were harvested with centrifugation at 4000 × g for 40 min. The pelleted cells were resuspended in phosphate-buffered saline and stored at −80°C.
The p150Glued(1–191) protein was purified using Ni-affinity chromatography and gel-filtration chromatography. The cells were lysed after the addition of DNase, protease inhibitor, and 1 mM dithiothreitol (DTT). The supernatant was collected from cell lysis with centrifugation at 15,000 RPM for 30 min and ultracentrifugation at 69,700 × g for 40 min. The protein solution was loaded onto a His-Trap Ni-affinity column (GE Healthcare), and most impurities were removed by a slow gradient to 140 mM of imidazole in 1× PBS (pH 7.4), 1 mM DTT. p150Glued(1–191) fused with His6-SMT3-tag was eluted at 300–350 mM of imidazole. The His6-SMT3-p150Glued(1–191) was digested with ∼1 mg SUMO protease (Ulp1) at 4°C overnight after the collected fractions were dialyzed against a buffer solution containing 50 mM Tris, 100 mM NaCl, 1mM DTT, and 1mM EDTA to an imidazole concentration of lower than 20 mM. The purified p150Glued(1–191) was concentrated to the final volume of 2.0 mL (protein concentration of 0.5 mM for U-13C,15N-p150Glued(1–191)) and loaded onto the gel-filtration column (Superdex 75 pg; GE Healthcare). If needed, cation exchange chromatography can be pursued as a third-step purification using HiTrap SP HP column (GE Healthcare). After purification, the extended p150Glued(1–191) protein solution was exchanged into the phosphate buffer and concentrated to a final concentration of 0.4 mM for the preparation of complexes with MTs. The typical yield of pure p150Glued(1–191) was 25 mg per 1 L of LB for unlabeled protein, 20 mg per 1 L M9 media for U-15N-p150Glued(1–191), and 13 mg per 1 L M9 for U-13C,15N- p150Glued(1–191).
Preparation of p150Glued(1–191)/MT complexes
Paclitaxel-stabilized MTs were prepared by the polymerization of lyophilized bovine tubulin (Cytoskeleton). Precleared tubulin was dissolved in 80 mM piperazine-N,N′-bis(2-ethanesulfonic acid) (PIPES), 1 mM MgCl2, and 1 mM EGTA (pH 6.8) (BRB 80). Tubulin solution was typically incubated at 37°C for 30 min with 20 μM paclitaxel, 1 mM GTP, and 10% dimethyl sulfoxide in BRB 80 buffer. Polymerized MTs were pelleted by ultracentrifugation at 108,900 × g (TLA-120, fixed angle, 50,000 RPM) for 20 min and resuspended in 20 mM phosphate buffer containing 50 mM NaCl and 1 mM DTT (pH 6.0) for co-sedimentation assays and transmission electron microscopy (TEM) characterization.
To measure the binding affinity of p150Glued(1–191) to MT, the co-sedimentation assay was performed at molar ratios of p150Glued(1–191) to tubulin dimer ranging from 1:1 to 8:1. The concentration of MTs was typically 20 μM, and concentrations of p150Glued(1–191) were varied from 0 μM (control) to 160 μM. After the mixture was incubated at 25°C for 1 h, the p150Glued(1–191)/MT complex was pelleted with ultracentrifugation at 108,900 × g for 20 min. The contents of supernatant and pellets were characterized by sodium dodecyl sulfate polyacrylamide gel electrophoresis analysis using 15% acrylamide gel.
For solution NMR experiments, the p150Glued(1–191) sample with a final protein concentration of 250 μM was prepared in 500 μL phosphate buffer (20 mM phosphate, 50 mM NaCl, 1 mM DTT, and 10% D2O (pH 6.0)).
For solid-state NMR samples of p150Glued(1–191)/MT assemblies, polymerized MTs were prepared from 6 mg of lyophilized tubulin. The resulting solution was directly resuspended in the p150Glued(1–191) solution at a 1:2 molar ratio of tubulin dimer to p150Glued(1–191) and containing 10% sucrose cushion. After 1 h incubation at 25°C, the assemblies were pelleted by ultracentrifugation at 108,900 × g and transferred into a MAS rotor. 9.6 mg hydrated p150Glued (1–191)/MT complex was packed in a 1.6-mm Varian rotor.
For 31P MAS NMR experiments, 8.5 mg polymerized MTs and 6.3 mg CAP-Gly19−107/MT complex containing ∼1 mg U-13C,15N-CAP-Gly19−107 were packed in two 1.6-mm Varian rotors. Both samples were hydrated. To avoid any background 31P signals from buffers (31P is a 100% natural abundant nucleus), the CAP-Gly19−107 solution was exchanged into BRB15 buffer (15 mM PIPES, 1 mM MgCl2, and 1 mM EGTA (pH 6.8)), and the CAP-Gly19−107/MT complex was prepared in BRB15 buffer instead of phosphate buffer. Sodium phosphate dibasic heptahydrate (Na2HPO4⋅7H2O) was packed in a 1.6-mm Varian rotor as a standard compound.
TEM
The morphologies of polymerized MTs and their complexes with p150Glued(1–191) and CAP-Gly19−107 were characterized by negatively stained TEM with a final concentration of MTs of 5–10 μM. The MT samples were stained with uranyl acetate (5% w/v) and deposited onto 400 mesh copper grids coated with Formval and carbon films. The TEM images were acquired by a Zeiss Libra 120 transmission electron microscope operating at 120 kV.
Solution NMR spectroscopy
All solution NMR spectra were acquired on a 14.1 T (1H Larmor frequency of 600.1 MHz) Bruker Avance spectrometer using a triple-resonance (HCN) CryoProbe at 298 K. The two-dimensional (2D) heteronuclear single quantum coherence (HSQC) spectra were recorded with U-15N-p150Glued(1–191), and the three-dimensional (3D) spectra were acquired with U-13C,15N-p150Glued(1–191). For the H-N HSQC experiment, 256 complex points were recorded in the 15N dimension; States-time proportional phase incrementation (TPPI) was used for phase sensitive detection. The 3D HNCA and HN(CO)CA spectra were acquired in the States-TPPI mode (18) in both indirect dimensions, and 15N decoupling was applied during the acquisition. For the HNCA experiment, 104 complex points were recorded in indirect 13C and 15N dimensions. For the HN(CO)CA experiment, 112 points and 100 points were recorded in the 13C and 15N dimension, respectively. A recycle delay of 1.0 s was used, and the experimental time was 2 days for both 3D spectra. The WATERGATE sequence (19) was applied to suppress the 1H signals arising from water. Chemical shifts in all spectra were referenced to 4,4-dimethyl-4-silapentane-1-sulfonic acid. All NMR spectra were processed using NMRPipe (20) and analyzed in CcpNmr (21).
MAS NMR spectroscopy
31P- and 13C-detected MAS NMR spectra were collected at 14.1 T on a narrow bore Varian InfinityPlus instrument using a 1.6 mm triple-resonance HXY probe. The Larmor frequencies were 599.5 MHz for 1H, 242.8 MHz for 31P, 150.7 MHz for 13C, and 60.7 MHz for 15N. The MAS frequency was 14 kHz controlled at ±10 Hz for all experiments using the Varian MAS controller. The sample temperature was calibrated using KBr as the temperature sensor (22).
For 31P experiments, the sample temperature was ∼5.6°C for paclitaxel-stabilized MTs, 7.1°C for CAP-Gly19−107/MT complex, and 26.0°C for Na2HPO4⋅7H2O, maintained to within ±0.2°C by a Varian temperature controller. The typical 90° pulse lengths were 2.5 μs for 31P and 2.4 μs for 1H. The Na2HPO4⋅7H2O was used as the standard to calibrate the pulse lengths as well as to optimize the 1H-31P cross polarization (CP) Hartmann-Hahn matching conditions. The 31P direct-excitation experiments were performed with recycle delays ranging from 10 to 15 s to assure the equilibrium magnetization recovery. For 1H-31P CPMAS experiments, the CP contact time was 2.5 ms. TPPM (23) with radio frequency (RF) field strength of 100–110 kHz was used for 1H decoupling during all experiments. The 31P chemical shift was referenced to H3PO4 (85% H3PO4 in H2O) and used as an external referencing standard (24).
In 13C-detected MAS NMR experiments on p150Glued(1–191)/MT assemblies, a series of one-dimensional (1D) 1H-13C CPMAS spectra were acquired at the sample temperatures of −27.0, −25.8, −24.5, −17, −10, and 4°C. The typical 90° pulse lengths were 2.5 μs for 1H, 3.5 μs for 13C, and 3.0 μs for 15N. During the 1H-13C CP transfer, a linear amplitude ramp of 80–100% was applied on the 1H channel. The RF field strength was ∼100 kHz for 1H TPPM decoupling during the acquisition period. The 2D 13C-13C correlation spectra were acquired at several sample temperatures ranging from −27 to 4°C. TPPI (25) acquisition mode was used for phase-sensitive detection in the indirect dimension of all 2D spectra. In the CORD (26) experiments, the 1H RF fields during the 13C-13C mixing were matched to the spinning frequency (14 kHz) or half of the spinning frequency (7 kHz), and the mixing time was 50 ms. All 2D CORD spectra were processed with either 90° or 60° shifted sine-bell function in both dimensions.
The 2D heteronuclear correlation NCA and NCO spectra were acquired at a sample temperature of −24.5°C. The 1H-15N CP was performed with the RF fields of 97 kHz on 1H and 83 kHz on 15N, respectively; a linear amplitude ramp of 80–100% was applied on 1H. For SPECIFIC CP, the Hartmann-Hahn condition was matched at a 13C RF field strength of 35 kHz and a 15N RF field strength of 63 kHz using a tangent amplitude ramp. The carrier frequencies in the 13C dimension were set to 55 and 170 ppm in the NCA and NCO experiments, respectively.
Results and Discussion
Biochemical characterization of p150Glued(1–191) binding with polymerized MTs
The p150Glued(1–191) was isolated as a monomer in a two-step purification procedure, as shown in Fig. 1 B and Document S1. Supporting Materials and Methods, Figs. S1–S3, and Tables S1–S3, Document S2. Article plus Supporting Material. The binding affinity of monomeric p150Glued(1–191) to polymerized MTs was assessed by co-sedimentation assay (Fig. 1 C). More than 98% of p150Glued(1–191) binds to MTs when equivalent molar concentrations of the protein and the tubulin dimer were used (Fig. 1 C). In comparison, as we and others reported previously, under the identical conditions, less than half of the CAP-Gly domain protein encompassing residues 19–107 (CAP-Gly19–107) binds to MTs (15). Thus, the binding affinity of p150Glued(1–191) to MTs is significantly higher than that of CAP-Gly19–107. This result validates that p150Glued(1–191) contains a second MT-binding domain other than CAP-Gly, and the extended region comprised of the basic domain and SP-rich domain assists the interactions between the p150Glued and MTs (16). Furthermore, the amount of p150Glued(1–191) bound to MTs increases as the molar ratio of p150Glued(1–191) to tubulin dimer becomes higher, indicating that the binding interaction between p150Glued(1–191) and MTs is promoted by the higher molar concentration of the protein. The fraction of MT-bound p150Glued(1–191) decreases when the molecular ratio of p150Glued(1–191) to tubulin dimer is higher than 2:1. Therefore, the p150Glued(1–191)/MT assemblies were prepared with the 2:1 p150Glued(1–191)/tubulin dimer molar ratio to ensure the stoichiometric formation of the assembly.
Figure 1.
(A) (Top) Domain structure of p150Glued subunit of dynactin. (Bottom) Primary sequence of p150Glued(1–191) domain is shown. The p150Glued(1–191) consists of the CAP-Gly domain (19–107), basic domain (115–145), and SP-rich domain (146–191). The basic and SP-rich domains are located in the linker connecting the CAP-Gly and coiled-coil 1 domains (CC1). (B) Sodium dodecyl sulfate polyacrylamide gel electrophoresis gel of p150Glued(1–191) after purification is shown. (C). Co-sedimentation assay of p150Glued(1–191) with MTs is shown. “S” and “P” denote supernatant and pellets after ultracentrifugation of the p150Glued(1–191)/MT solution, respectively. (D) Shown are negatively stained TEM images of MT alone (left), p150Glued(1–191)/MT assemblies (middle), and CAP-Gly/MT assemblies (right). To see this figure in color, go online.
The p150Glued(1–191) was also isolated as a cross-linked dimer by dialysis against a buffer solution containing no DTT (see Fig. S1 B). The dimer appears to bind to polymerized MTs with higher binding affinity than the monomer (Fig. S1 C). Although in this work we have not pursued structural characterization of the dimeric p150Glued(1–191) complex with MTs, such studies will be conducted in the future.
To assess the morphology of the assembly of p150Glued(1–191) bound with polymerized MTs, we used negatively stained TEM. As shown in Fig. 1 D, p150Glued(1–191) bound to polymerized MTs forms straight tubules of the same dimensions (within the resolution of the images) and morphology as the pure MT preparations as well as the assembly of CAP-Gly(19–107) with polymerized MTs.
31P MAS NMR spectroscopy of MTs and CAP-Gly19–107/MT assembly
We have recorded 31P MAS NMR spectra to characterize the nucleotide states in MTs and assess the conformational homogeneity of the MTs in the samples under investigation. As shown in Fig. 2 C, the spectra of MTs are well resolved, and signals from the GTP and guanosine diphosphate (GDP) are present and well resolved. Specifically, the direct excitation and/or CPMAS spectra contain three resonances corresponding to GTP, two corresponding to GDP, and two corresponding to the inorganic phosphate PO4− (Pi). The signal assignments are shown in Fig. 2 C and Table S1. The intensities of the Pα and Pβ resonances of GTP are higher than those of the Pα′ and Pβ′ resonances of GDP. This is consistent with the fact that there are more GTP than GDP molecules bound. In polymerized MTs, the α-tubulin binds to GTP and β-tubulin binds to either GTP or GDP with a “GTP cap” at the plus-end of MTs that contains the GTP-bound β-tubulins. The two resonances of Pi indicate the potential existence of stabilized GDP-Pi-capped MTs in the sample of free MTs (27, 28, 29). Important to note is that the 31P peaks in all spectra are narrow (the line widths are 0.4–0.8 ppm), indicating that the conformational homogeneity of the MT in our preparations is high, both in the free and CAP-Gly19−107 bound states.
Figure 2.
(A) The structure of a tubulin heterodimer, showing the positions of GTP, GDP, and paclitaxel (Protein Data Bank [PDB]: 1JFF (34)). (B) Shown are the chemical structures of GTP and GDP, indicating the phosphorus atoms in each molecule. (C) 31P MAS NMR spectra of guanosine nucleotides in MTs and CAP-Gly/MT complex are shown. The direct excitation and CPMAS spectra contain three resonances corresponding to GTP, two corresponding to GDP, and two corresponding to the inorganic phosphate PO4− (Pi). Each peak was assigned to corresponding phosphorus resonance of GTP and GDP. The 31P MAS NMR spectra were acquired with (from top to bottom) 20,000 scans, 4864 scans, 8360 scans, and 7756 scans. To see this figure in color, go online.
Solution NMR spectroscopy of p150Glued(1–191)
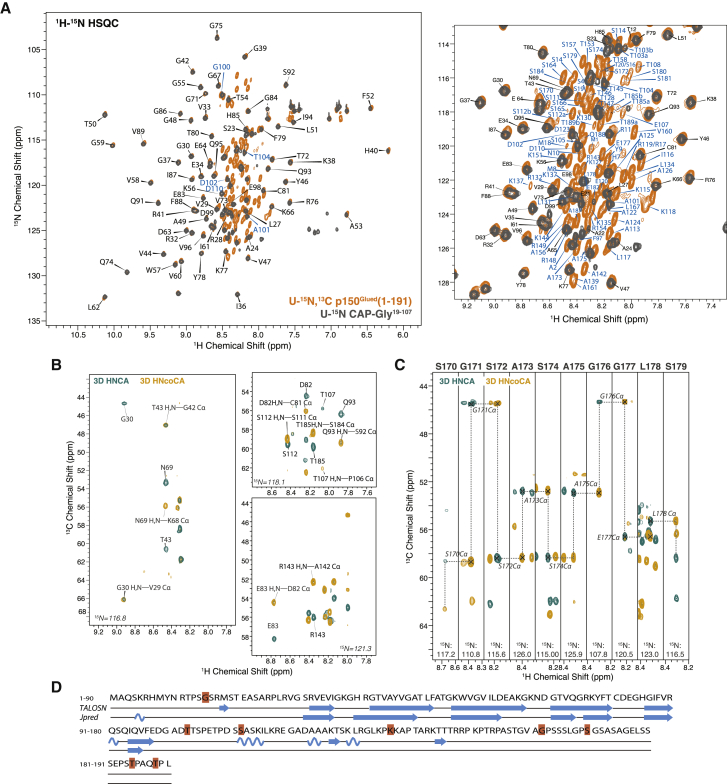
The 2D 1H-15N HSQC and 3D HNCA and HN(CO)CA spectra of U-15N-CAP-Gly19−107 and U-13C,15N-p150Glued(1–191) are shown in Fig. 3. The 1H and 15N chemical shift perturbations (CSPs) with respect to the free CAP-Gly domain are summarized in Table S3. In the 2D HSQC spectra, the signals that correspond to residues in the CAP-Gly domain of p150Glued(1–191) are well dispersed and generally overlay well with those in the spectra of CAP-Gly19–107 reported by us previously (15) (see Fig. 3 A). The residues with large 15N CSPs are S19, E21, and A22 (CSPs greater than 0.4 ppm) as well as T20, S23, A24, Q95-F97, T104, and S105 (CSPs greater than 0.2 ppm but smaller than 0.4 ppm) (Fig. 4 A). All of these residues are located at the N- and C-termini of CAP-Gly domain (Fig. 4 C), so these changes are not surprising. These residues are more rigid and structured in the context of the p150Glued(1–191) protein compared to the CAP-Gly domain alone. In addition to these perturbations, several residues in the β-sheet core region of CAP-Gly exhibit small chemical shift changes commensurate with minor structural rearrangements; these are R25, R28, R41, N69, H85, and R90 (see Fig. 4 C), which are mostly positively charged residues.
Figure 3.
Solution NMR spectra of p150Glued(1–191). (A) (Left) Shown is the superposition of 2D 1H-15N HSQC spectra of U-13C,15N-p150Glued(1–191) (orange, light trace) and U-13C,15N CAP-Gly19–107 (gray, darker trace). (Right) An expanded view of 2D HSQC spectra shows the resonance assignments of the poorly dispersed regions, including the basic and SP-rich domains. Residues T103, S112, T185, and T189 show double peaks. The assignments of crosspeaks corresponding to residues in different domains are designated as follows: CAP-Gly domain, black, darker letters, and extended regions including basic domain and SP-rich domain, blue, lighter letters. (B) Shown are the representative 2D 1H-15N planes of 3D HNCA (cyan, darker contours) and HN(CO)CA (yellow, lighter contours) solution NMR spectra. (C) Shown is the sequential backbone walk for residues S170–S179 using the 3D solution NMR spectra. (D) Shown is the secondary structure of p150Glued(1–191) predicted by TALOS-N based on solution NMR chemical shifts compared to that predicted by Jpred from the primary sequence. To see this figure in color, go online.
Figure 4.
(A) CSPs of the CAP-Gly domain plotted against residue number in p150Glued(1–191) versus CAP-Gly19–-107. (B) Shown are glycine regions of 2D HSQC and 2D slices of 3D spectra showing peak doubling for representative residues in p150Glued(1–191). Residues that exhibit two resonances include G15, T103, S112, K137, G162, S170, T185, and T189. (C) Mapping of residues exhibits two resonances (left) and 15N CSPs (right) onto 3D CAP-Gly structure (PDB: 2COY (35)). The residues are color coded as follows: residues with 15N chemical shifts perturbation (Δ15N) larger than 0.4 ppm, yellow; residues with Δ15N larger than 0.2 ppm, orange; residues with Δ15N larger than 0.15 ppm, dark blue; and residues with Δ15N larger than 0.1 ppm, light purple. To see this figure in color, go online.
In contrast to the CAP-Gly domain, the signals of other domains in the protein are poorly dispersed. This is an indication that the extended region of p150Glued(1–191) comprising the basic and SP-rich domains is much less structured than the CAP-Gly domain and may have a substantial proportion of random coil. We have assigned the resonances on the basis of the 3D HNCA and HN(CO)CA spectra and the assignments have been validated by the complete and unique backbone walk. Both intraresidue and sequential correlations are revealed in the HNCA spectra. As shown in Fig. 3, B and C, the spectral resolution is excellent, permitting full assignments, despite the presence of many Pro residues and sequence repeats. For the unstructured proline-rich regions that have a lower sequence complexity, the inter-residue backbone connectivities were established based on the unique sequence pattern and specific neighboring amino acids with characteristic chemical shifts (Ala, Val, Thr, Gly, Ile, Ser, etc.). As shown in Fig. S2, the assignments of different proline-containing domains are unambiguous and distinguishable as validated by the averaged backbone chemical shifts of each amino acid. The solution chemical shifts are summarized in Table S2.
We have carried out the secondary structure prediction on the basis of the chemical shifts, using TALOS-N (30) and Jpred (31) programs. As shown in Fig. 3 D, the predictions for the core regions in the CAP-Gly domain are consistent with the secondary structure of isolated CAP-Gly19−107, which is comprised of loop regions and a core region formed by four β-sheets. The SP-rich domain and the majority of the basic domains are random coil. The exceptions are three separate segments in the basic domain, S111–I116, A124–R132, and K144–T146, which are predicted to contain short α-helical or β-sheet stretches.
It is worth noting that several residues exhibit peak doubling in solution NMR spectra (Fig. 4 B and right panel of Fig. 3 A), indicating the existence of conformational isoforms for the segments that contain these residues. These residues include G15, T103, S112, K137, G162, S170, T185, and T189, which are mostly in the random coil or loop regions.
Our results thus indicate that despite the presence of some secondary structures in these segments, the basic and SP-rich domains are intrinsically disordered and unfolded in free state in solution.
MAS NMR spectroscopy of p150Glued(1–191) in complex with MTs
As shown in Fig. 5 A, 1D 1H-13C CPMAS spectra of U-13C,15N-p150Glued(1–191) assembled with polymerized MTs show a strong temperature dependence, indicating the presence of dynamics. The spectral intensity and resolution depend on whether a particular temperature was reached by heating or cooling the sample in the range of 4 to −27°C, which is consistent with our previous observations in CAP-Gly19–107 free and assembled with MTs as well as LC8 (11, 12, 14, 32). Different spectra were observed upon temperature cycling, as shown in Fig. 5 A. Specifically, at the starting temperature of 4°C, spectral resolution is excellent but deteriorates progressively accompanied by an increase in sensitivity as the experimental temperature is decreased. At −27°C, the signal-to-noise ratio of the aliphatic and carbonyl 13C signals increases by four and eightfold, respectively, compared to that at 4°C. When the temperature is raised from −25.8 to −10°C after the sample was cooled down to −27°C, the resolution and sensitivity of the 1H-13C CPMAS spectra do not change dramatically. The increase in resolution is only observed at −25.8°C when warming up the sample from −27°C.
Figure 5.
(A) Temperature dependence of 1D 1H-13C CPMAS spectra of U-13C,15N-p150Glued(1–191)/MT complex. (left) Spectral resolution is excellent at 4°C but deteriorates progressively accompanied by an increase in sensitivity as the experimental temperature is decreased to −27°C. The number of transients is 512 for +4°C and 128 for −27°C. (right) The spectral resolution and sensitivity does not change dramatically when the temperature is raised from −25.8 to −10°C after the sample has been cooled down to −27°C. The number of transients is 128 for −24.5°C and 256 for −25.8, −17, and −10°C. (B and C) Shown are 2D 13C-13C correlation MAS NMR spectra of U-13C,15N-p150Glued(1–191)/MTs (B) and U-13C,15N-CAP-Gly19–107/MT (C) at different temperatures. At near or above 0°C, a finite number of correlations are detected for both p150Glued(1–191)/MT and CAP-Gly19–107/MT due to motions of flexible regions. For p150Glued(1–191)/MT complex, a large number of correlations only appear at −27°C because motions of residues in flexible regions are restricted. The contour levels were set to 3× noise level. All 2D 13C-13C spectra were processed with 60° shifted sine-bell function. To see this figure in color, go online.
Interestingly, the temperature profile of the p150Glued(1–191)/MT complex is different from that for the CAP-Gly19−107/MT complex, indicating different dynamic behaviors. Specifically, the CPMAS spectra of the CAP-Gly19−107/MT complex at temperatures close to −10°C experience significant loss of sensitivity and resolution, whereas the sensitivity and resolution in the CPMAS spectra of extended p150Glued(1–191)/MT complex at −10°C are the same as at −25.8°C. This result suggests overall reduced dynamics in the p150Glued(1–191) in complex with MTs compared to that in the CAP-Gly19−107/MT complex on timescales of microseconds to milliseconds throughout the entire protein molecule.
To further probe the relative flexibility of different segments in p150Glued(1–191) assembled with MTs, 2D 13C-13C CORD spectra were acquired at −27, −10, and 4°C (Fig. 5 B). Although the spectrum acquired at 4°C exhibits good resolution, only a finite number of correlations are present because of motions of flexible regions occurring at temperatures above 0°C. This behavior is similar to that of CAP-Gly19−107 and indicates that the limited number of residues detected at 4°C belong to rigid regions of p150Glued(1–191) assembled on MTs. At −27°C, numerous intense crosspeaks are present in the spectra, consistent with efficient dipolar-based transfers and spin diffusion, which indicates that the motions are restricted under these conditions. The majority of these correlations are only detected at −27°C and disappear in the 13C-13C spectra at −10°C, providing evidence that these are associated with residues in the flexible segments of the protein.
We next pursued resonance assignments of the p150Glued(1–191)/MT complex on the basis of the 2D homonuclear and heteronuclear correlation spectra (shown in Fig. 6). For this, we used our previously reported solution NMR chemical shifts of p150Glued(1–191) and solid-state NMR assignments of CAP-Gly19−107 assembled on MTs as guides (33). We first assigned the unique signals in 2D heteronuclear NCA spectra, which comprise ∼37% of residues, of which half are located at CAP-Gly domain and half in other regions. Next, we analyzed 2D 13C-13C spectra on the basis of which we made assignments for additional 68 residues. The 2D CORD spectra of p150Glued(1–191)/MT were processed with different resolution enhancements and were employed to assist site-specific resonance assignments of an extended region (residues 108–191) (Fig. S3). Most of the signals in the CORD spectra acquired at 4°C were unambiguously assigned, except for several signals of high intensity, which possibly contain multiple peaks. The comparison of CORD spectra acquired at −27 and −10°C was very useful for distinguishing residues in the flexible segments of the protein, as shown in Fig. 6 C. Specifically, the correlations detected only at −27°C correspond to the flexible regions of the protein (Fig. 6 E), whereas those at −10°C correspond to more rigid residues. Correlations only present at 4°C correspond to residues in the most rigid segments (Fig. 6 B). As shown in Fig. 6 E, the flexibilities of different regions in p150Glued(1–191) are found to be generally consistent with the predicted secondary structure by TALOS-N. In the basic domain, three segments are predicted to have α-helical or β-sheet structures, S112–R119, A126–R312, and T141–T146. Most of these residues are present in the spectra at 4°C. At the same time, the SP-rich domain is comprised of flexible segments, both according to the secondary structure predictions and the presence/absence of crosspeaks in the CORD spectra acquired at different temperatures.
Figure 6.
MAS NMR spectra of U-13C,15N-p150Glued(1–191)/MTs. (A) (left) 2D 13C-13C CORD spectrum of U-13C,15N-p150Glued(1–191)/MTs acquired at 4°C (blue, darker contours) were superimposed with the CORD spectrum acquired at −27°C (orange, lighter contours). (right) 2D CORD spectrum acquired at −10°C (purple, darker contours) were superimposed with the CORD spectrum acquired at −27°C (lighter contours). The assignments of crosspeaks corresponding to residues in different domains are color coded as follows: CAP-Gly domain, black (dark trace), and extended regions including basic domain and SP-rich domain, red (light trace). (B) Shown is an expanded view of the 2D 13C-13C CORD spectrum acquired at 4°C. (C) 2D 13C-13C CORD spectrum acquired at −27°C shows resonance assignments. The 2D CORD spectrum recorded at −10°C (darker contours) was superimposed to show the correlations that are present at −10°C. (D) Shown is the 2D 15N-13C NCA correlation MAS NMR spectra of U-13C,15N-p150Glued(1–191)/MT (orange, lighter contours) and U-13C,15N-CAP-Gly19−107/MT (gray, darker contours). (E) Primary sequence of p150Glued(1–191) shows the relative flexibilities of the different regions of the protein. The correlations detected only at −27°C correspond to the flexible segments. Resonances detected only at −10°C are associated with moderately flexible residues, and those present at 4°C indicate rigid residues. The color coding of the individual residues is as follows: residues appearing at 4°C, cyan; residues appearing only at −27°C, green; and residues appearing at both −10 and −27°C, pink. The color coding of the segments of secondary structure is as follows: rigid, purple; flexible, light red; and moderately flexible, yellow. The contour levels were set to 3.5× noise level. All 2D 13C-13C spectra were processed with 90° shifted sine-bell function. To see this figure in color, go online.
The comparison of rigid residues in CAP-Gly19−107 with residues at the MT-binding interface reveals that the rigid residues in CAP-Gly domain contain the majority of binding sites for MTs (Fig. 7 B). This observation is very informative and indicates that the rigid residues detectable at nonfreezing conditions are actually the ones in close contact with MTs. Therefore, we hypothesize that many rigid residues detected at 4°C in the extended region 108–191 are possibly involved in binding with MTs. The rigid residues in CAP-Gly19−107 and p150Glued(1–191) assembled on MTs revealed by 2D CORD MAS NMR spectra indicate that multiple segments in CAP-Gly domain become more rigid in p150Glued(1–191) than in CAP-Gly19–107 (Fig. 7 A). These results support the notion that the extended region in p150Glued(1–191) assists the overall binding interactions with MTs. For the CORD spectra of extended CAP-Gly/MTs acquired at 4°C and CORD spectra of CAP-Gly19−107/MTs acquired at 0°C, we are able to perform the unambiguous resonance assignments of residues in CAP-Gly domain.
Figure 7.
Rigid residues in CAP-Gly19–107 and p150Glued(1–191) assembled on MTs revealed by 2D CORD MAS NMR spectra. (A) Shown are CORD spectra of p150Glued(1–191)/MTs acquired at 4°C (blue, darker contours) and CORD spectra of CAP-Gly19–107/MTs acquired at 0°C (yellow, lighter contours), showing unambiguous resonance assignments of residues in CAP-Gly domain. (B) Shown is a comparison of rigid residues in CAP-Gly19–107 with residues at MT-binding interface. Rigid residues in CAP-Gly19–107 contain the majority of binding residues for MTs. Multiple segments in CAP-Gly domain become more rigid in p150Glued(1–191) than in CAP-Gly19–107. (C) Shown is a schematic representation of p150Glued(1–191) and regions of its basic domain possibly involved in binding to MTs. The segments comprised of residues S112–R119, A124–R132, and T141–R146 are structured and remain rigid upon binding with MTs, which likely contain many MT-binding residues. R11 is the only residue that is detected at 4°C in the N-terminal segment 1–25. To see this figure in color, go online.
Our results thus indicate that the rigid segments in the basic domain of p150Glued(1–191), S112–R119, A124–R312, and T141–T146, likely comprise most of the residues at the intermolecular interface with MTs (Fig. 7 C). Together with the CAP-Gly domain, they appear to be responsible for the MT-binding interactions of the p150Glued subunit. This finding is consistent with previous studies demonstrating that the basic domain is the second MT-binding segment of p150Glued (16). Furthermore, the K-rich segment R132–P152 represses the inhibitory effect of the CC1 domain on the MT binding, whereas the SP domain and CC1 do not bind to MTs (17). We also speculate that the N-terminal tail (1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25) of p150Glued(1–191) may contain an anchoring point at residue R11 for interacting with the tubulin’s C-terminal tail (Fig. 7). In a previous study, the N-terminal segment 1–25 has been proposed to secure the binding of CAP-Gly to MTs by wrapping around tubulin’s E-hook (13). According to our results, R11 is the only residue that is detected at 4°C and exhibits strong peak intensity in the segment 1–25, indicating that R11 is possibly involved in binding interactions with tubulin.
Conclusions
Our results demonstrate that the p150Glued(1–191) has a stronger binding affinity with polymerized MTs than the isolated CAP-Gly domain. Residues 108–191 of p150Glued(1–191) enhance the binding affinity and contain a second MT-binding region, albeit it is largely unstructured in solution and dynamic upon binding to MTs. Three short and rigid segments in the basic domain, S111–I116, A124–R132, and K144–T146, are predicted to form α-helical and β-sheet structures and are likely to encompass the MT-binding site.
We conclude p150Glued(1–191) remains flexible upon binding to MTs except for regions that are directly involved in the MT-binding interactions. This conformational flexibility may be important for the p150Glued(1–191) interactions with MTs. Our results lay the foundation for the atomic-resolution structure characterization of dynactin’s p150Glued subunit bound to polymerized MTs.
Author Contributions
T.P. and J. C. W. conceived and directed the project. C.G. performed experiments and analyzed data. C.G and T.P. took the lead in writing the manuscript. All authors discussed the results.
Acknowledgments
We thank Dr. Si Yan for valuable discussions and initial work on the p150Glued(1–191) construct. We are grateful to Dr. Shi Bai for help in using solution NMR instruments and Shannon Modla at Bioimaging Center at the Delaware Institute of Technology for assistance with acquiring TEM images.
We acknowledge the support of the National Institutes of Health grant P30GM110758 for the support to the core instrumentation infrastructure at the University of Delaware.
Footnotes
Editor: David Eliezer.
Supporting Material can be found online at https://doi.org/10.1016/j.bpj.2019.07.036.
Supporting Material
References
- 1.Fletcher D.A., Mullins R.D. Cell mechanics and the cytoskeleton. Nature. 2010;463:485–492. doi: 10.1038/nature08908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Conde C., Cáceres A. Microtubule assembly, organization and dynamics in axons and dendrites. Nat. Rev. Neurosci. 2009;10:319–332. doi: 10.1038/nrn2631. [DOI] [PubMed] [Google Scholar]
- 3.Olmsted J.B. Microtubule-associated proteins. Annu. Rev. Cell Biol. 1986;2:421–457. doi: 10.1146/annurev.cb.02.110186.002225. [DOI] [PubMed] [Google Scholar]
- 4.Mallik R., Rai A.K., Kunwar A. Teamwork in microtubule motors. Trends Cell Biol. 2013;23:575–582. doi: 10.1016/j.tcb.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 5.Hancock W.O. Bidirectional cargo transport: moving beyond tug of war. Nat. Rev. Mol. Cell Biol. 2014;15:615–628. doi: 10.1038/nrm3853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roberts A.J., Kon T., Burgess S.A. Functions and mechanics of dynein motor proteins. Nat. Rev. Mol. Cell Biol. 2013;14:713–726. doi: 10.1038/nrm3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karki S., Holzbaur E.L. Cytoplasmic dynein and dynactin in cell division and intracellular transport. Curr. Opin. Cell Biol. 1999;11:45–53. doi: 10.1016/s0955-0674(99)80006-4. [DOI] [PubMed] [Google Scholar]
- 8.Moore J.K., Li J., Cooper J.A. Dynactin function in mitotic spindle positioning. Traffic. 2008;9:510–527. doi: 10.1111/j.1600-0854.2008.00710.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Urnavicius L., Zhang K., Carter A.P. The structure of the dynactin complex and its interaction with dynein. Science. 2015;347:1441–1446. doi: 10.1126/science.aaa4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cronin M.A., Schwarz T.L. The CAP-Gly of p150: one domain, two diseases, and a function at the end. Neuron. 2012;74:211–213. doi: 10.1016/j.neuron.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yan S., Zhang H., Polenova T. Internal dynamics of dynactin CAP-Gly is regulated by microtubules and plus end tracking protein EB1. J. Biol. Chem. 2015;290:1607–1622. doi: 10.1074/jbc.M114.603118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yan S., Guo C., Polenova T. Atomic-resolution structure of the CAP-Gly domain of dynactin on polymeric microtubules determined by magic angle spinning NMR spectroscopy. Proc. Natl. Acad. Sci. USA. 2015;112:14611–14616. doi: 10.1073/pnas.1509852112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Q., Crevenna A.H., Mizuno N. Structural basis for the extended CAP-Gly domains of p150(glued) binding to microtubules and the implication for tubulin dynamics. Proc. Natl. Acad. Sci. USA. 2014;111:11347–11352. doi: 10.1073/pnas.1403135111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yan S., Hou G., Polenova T. Three-dimensional structure of CAP-gly domain of mammalian dynactin determined by magic angle spinning NMR spectroscopy: conformational plasticity and interactions with end-binding protein EB1. J. Mol. Biol. 2013;425:4249–4266. doi: 10.1016/j.jmb.2013.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun S., Siglin A., Polenova T. Solid-state and solution NMR studies of the CAP-Gly domain of mammalian dynactin and its interaction with microtubules. J. Am. Chem. Soc. 2009;131:10113–10126. doi: 10.1021/ja902003u. [DOI] [PubMed] [Google Scholar]
- 16.Culver-Hanlon T.L., Lex S.A., King S.J. A microtubule-binding domain in dynactin increases dynein processivity by skating along microtubules. Nat. Cell Biol. 2006;8:264–270. doi: 10.1038/ncb1370. [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi T., Miyashita T., Toyoshima Y.Y. Dynactin has two antagonistic regulatory domains and exerts opposing effects on dynein motility. PLoS One. 2017;12:e0183672. doi: 10.1371/journal.pone.0183672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marion D., Ikura M., Bax A. Rapid recording of 2D NMR spectra without phase cycling. Application to the study of hydrogen exchange in proteins. J. Magn. Reson. 1989;85:393–399. [Google Scholar]
- 19.Piotto M., Saudek V., Sklenár V. Gradient-tailored excitation for single-quantum NMR spectroscopy of aqueous solutions. J. Biomol. NMR. 1992;2:661–665. doi: 10.1007/BF02192855. [DOI] [PubMed] [Google Scholar]
- 20.Delaglio F., Grzesiek S., Bax A. NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J. Biomol. NMR. 1995;6:277–293. doi: 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]
- 21.Stevens T.J., Fogh R.H., Laue E.D. A software framework for analysing solid-state MAS NMR data. J. Biomol. NMR. 2011;51:437–447. doi: 10.1007/s10858-011-9569-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thurber K.R., Tycko R. Measurement of sample temperatures under magic-angle spinning from the chemical shift and spin-lattice relaxation rate of 79Br in KBr powder. J. Magn. Reson. 2009;196:84–87. doi: 10.1016/j.jmr.2008.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ernst M. Heteronuclear spin decoupling in solid-state NMR under magic-angle sample spinning. J. Magn. Reson. 2003;162:1–34. doi: 10.1016/s1090-7807(03)00074-0. [DOI] [PubMed] [Google Scholar]
- 24.Jameson C.J., Dedios A., Jameson A.K. Absolute shielding scale for 31P from gas-phase NMR studies. Chem. Phys. Lett. 1990;167:575–582. [Google Scholar]
- 25.Marion D., Wüthrich K. Application of phase sensitive two-dimensional correlated spectroscopy (COSY) for measurements of 1H-1H spin-spin coupling constants in proteins. Biochem. Biophys. Res. Commun. 1983;113:967–974. doi: 10.1016/0006-291x(83)91093-8. [DOI] [PubMed] [Google Scholar]
- 26.Hou G., Yan S., Polenova T. Broadband homonuclear correlation spectroscopy driven by combined R2(n)(v) sequences under fast magic angle spinning for NMR structural analysis of organic and biological solids. J. Magn. Reson. 2013;232:18–30. doi: 10.1016/j.jmr.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brouhard G., Sept D. Microtubules: sizing up the GTP cap. Curr. Biol. 2012;22:R802–R803. doi: 10.1016/j.cub.2012.07.050. [DOI] [PubMed] [Google Scholar]
- 28.Panda D., Jordan M.A., Wilson L. Differential effects of vinblastine on polymerization and dynamics at opposite microtubule ends. J. Biol. Chem. 1996;271:29807–29812. doi: 10.1074/jbc.271.47.29807. [DOI] [PubMed] [Google Scholar]
- 29.Melki R., Carlier M.F., Pantaloni D. Direct evidence for GTP and GDP-Pi intermediates in microtubule assembly. Biochemistry. 1990;29:8921–8932. doi: 10.1021/bi00490a007. [DOI] [PubMed] [Google Scholar]
- 30.Shen Y., Bax A. Protein backbone and sidechain torsion angles predicted from NMR chemical shifts using artificial neural networks. J. Biomol. NMR. 2013;56:227–241. doi: 10.1007/s10858-013-9741-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Drozdetskiy A., Cole C., Barton G.J. JPred4: a protein secondary structure prediction server. Nucleic Acids Res. 2015;43:W389–W394. doi: 10.1093/nar/gkv332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun S. PhD thesis (University of Delaware); 2011. Structural investigations of microtubule-associated motor proteins by magic angle spinning solid-state NMR spectroscopy. [Google Scholar]
- 33.Fritz M., Quinn C.M., Gronenborn A.M. Toward closing the gap: quantum mechanical calculations and experimentally measured chemical shifts of a microcrystalline lectin. J. Phys. Chem. B. 2017;121:3574–3585. doi: 10.1021/acs.jpcb.6b09479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nogales E., Wolf S.G., Downing K.H. Structure of the alpha beta tubulin dimer by electron crystallography. Nature. 1998;391:199–203. doi: 10.1038/34465. [DOI] [PubMed] [Google Scholar]
- 35.Saito K., Koshiba S., …, RIKEN Structural Genomics/Proteomics Initiative. 2005. 2COY: Solution structure of the CAP-Gly domain in human dynactin 1. Protein Data Bank, https://www.rcsb.org/structure/2COY.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.