Abstract
Taste loss is one of the debilitating complications in radiation-induced oral mucositis (RIOM), as occurs in head and neck cancer patients undergoing radiotherapy. We report here a radio-mitigation effect of Sirtuin 1 (SIRT1) inhibitors in taste bud organoids and a mouse model of radiation-induced taste bud injury. The organoids, developed from circumvallate (CV) papilla, were irradiated with single dose of X-rays and inhibitors of SIRT1 or SIRT2 were added 24 h later. The survival was evaluated by measuring the number and size of regenerated organoids after irradiation (IR). Oral mucositis (OM) was induced by IR of the oral region of Lgr5-lacZ transgenic mice. The surviving Lgr5+ taste bud stem cells were identified after lacZ-staining and the mucosal ulceration on tongue dorsal surface was determined by histological methods. Results showed that SIRT1 inhibitors (nicotinamide, EX527, salermide and sirtinol), but not SIRT2 inhibitors, significantly improve taste bud organoid survival after IR. Remarkably, administration of nicotinamide (NAM), a recognized inhibitor of SIRT1 to mice 24 h after IR promotes the survival of Lgr5+ taste bud stem cells, resulting in alleviated tongue mucositis. In conclusion, SIRT1 inhibitors promote Lgr5+ taste bud stem cell survival and mitigate RIOM in mice. These observations have important implications for efforts to develop therapeutic strategies against taste dysfunction and mucosal ulceration in RIOM.
Keywords: Taste bud stem cell, radiation, SIRT1, organoid, oral mucositis, Lgr5
Introduction
Taste bud injury is one of the major complications in radiation-induced oral mucositis (RIOM), as occurs in radiotherapy of head and neck cancers [1-4]. Taste loss or dysfunction, and severe mucosal lesions, disturbs oral intake after irradiation (IR) due to anorexia and swallowing pain [5]. These, combined with other oral dysfunctions (xerostomia, mucositis) may lead to more serious problems due to interruption of cancer therapy, and septic complication [6]. Despite its frequency, severity and symptomatic and economic impact, there is no effective intervention for oral mucositis (OM) induced by chemotherapeutic or radiotherapy [6]. At present, several possible therapies that are based on mechanism-defined strategies are undergoing evaluation as potential interventions, such as amifostine, N-acetylcysteine and melatonin, in prevention and/or management of RIOM [6,7]. The only approved treatment for mucositis thus far is palifermin (Kepivance), and its application is limited to mucositis in patients undergoing conditioning regimens prior to hematopoietic stem cell transplant [8].
The taste buds are located in the epithelial structures known as lingual papillae found mostly on the dorsal surface of tongue. Three types of taste papillae are present among the most numerous non-taste filiform papillae, of which fungiform papillae (FFP) sit in the anterior two-thirds part, foliate taste papillae (FolP) and circumvallate papillae (CVP) sit in the posterior region [9]. Cells in taste bud are modified epithelial cells and continually renewed from lingual progenitors or stem cells [10-13]. A stem cell population marked by Lgr5 (leucine-rich-repeat containing G-protein coupled receptor 5) is identified in mice, which give rise to the taste cell types and non-taste epithelial cells in the posterior region of dorsal surface including circumvallate papillae [14,15]. While Lgr6+ stem cells supply epithelial cells in the anterior part including FFP [16]. Based on advances in culture technology of gastrointestinal stem cells, lingual organoids have been developed in vitro from isolated Lgr5+ or Lgr6+ lingual stem cells [16,17].
The protein product of the gene SIRT1, human homolog of the S.cerevisiae Sir2 protein, and mainly act as a NAD-dependent deacetylase [18]. Through deacetylation, SIRT1 modifies the activities of a variety of target proteins that contribute to oxidative response, cell cycle control. Accumulating evidence shows that SIRT1 has been involved in the response to DNA damage, binds and deacetylates the p53 protein, modification of which has been implicated in the activation of p53 after IR [19,20]. These data led us to speculate that SIRT1 inhibitors might also be able to mitigate the radiation-induced oral mucosal toxicity.
Adult tissue stem cells are responsible for maintenance of tissue homeostasis and play a vital role in tissue regeneration after injury. Following continued apoptosis of dying cells, the temporary or permanently disruption in the supply of new cells from reduced or impaired taste bud stem cells leads to taste loss and mucosal ulceration in the irradiated epithelia [21]. Taste bud organoid culture system allows us to study these cells long-term in vitro. In order to explore the effect of SIRT1 inhibition on the survival of Lgr5+ taste bud stem cell after IR, the Lgr5-lacZ transgenic mice were used. The role of SIRT1 inhibition on regeneration capability of taste bud stem cells and healing of oral mucositis were evaluated.
Materials and methods
Mice
Lgr5-lacZ mice, a kind gift from Dr. Hans Clevers (Hubrecht Institute, Utrecht, Netherlands) which carrying lacZ gene integrated into the last exon of the Lgr5 gene, were genotyped and used as described [22]. C57BL/6J mice were provided by Shanghai Model Organisms Center, Inc. All mice used in this study were 8-10 weeks of age, and of both sexes. Mice were maintained in a specific pathogen-free (SPF) facility and exposed to a 12 h/12 h light/dark cycle. All animal protocols were approved by the Shanghai Medical College Fudan University Animal Care and Use Committee.
Organoid culture, X-ray exposure and treatment
Circumvallate (CV) papillae tissues were isolated from mouse tongue using a recommended protocol as previously described [23]. Briefly, the CV tissue in posterior tongue was isolated under an anatomic microscope and minced into tiny fragments in the sterile dish on ice. Then tissue fragments were subjected to enzymatic digestion in 8 mL digestion medium containing with 500 U/mL collagenase IV (C9407, Sigma-aldrich), 1.5 mg/mL collagenase II (C81150, Solarbio), 20 μg/mL hyaluronidase (h8030, Solarbio) and 0.1 mg/mL dispase typeII (D4693, Sigma-aldrich) for 30-40 min at 37°C. After vortexing, suspension was passed through 70-mm cell strainer (352350, BD biosciences) and then centrifuged at 200 g for 5 minutes. The pellets of cell were suspended and embedded in Matrigel (BD biosciences) and incubated in advanced DMEM/F12 medium supplemented with 1 × GlutaMax (35050061, Gibco™), 10 mM HEPES (15630080, Gibco™), penicillin/streptomycin (15140122, Gibco™), 1 × N2 (17502, Invitrogen), 1 × B27 (17504, Invitrogen), 1 × n-Acetylcysteine (A9165, Sigma-aldrich), 500 ng/mL R-spondin-conditioned media (11083-HNAS, Sino Biological Inc), 50 ng/mL EGF (50482-MNCH, Sino Biological Inc) and 100 ng/mL Noggin (50688-M02H, Sino Biological Inc). Culture medium was refreshed every 3 days. The developed organoids were monitored and imaged under microscopy (Vert.A1, Zeiss).
The organoids cultured for 3-5 days were irradiated with single dose of 6 Gy or 8 Gy X-ray at a dose rate of 246 cGy/min (produced by X-Rad 320 with 0.25 mm Cu filtration). SIRT1 inhibitors: 10 mM Nicotinamide (NAM) (N0636, Sigma-aldrich), 10 μM EX527 (S1541, Selleck), 50 μM Sirtinol (S2804, Selleck) and 50 μM Salermide (S8460, Selleck) or SIRT2 inhibitors: 50 μM AGK2 (S7577, Selleck) and 50 μM SirReal2 (S7845, Selleck) were used respectively 24 h later. Images of surviving organoid were obtained under microscopy (Vert.A1, Zeiss) at day 10 after IR. The size of alive organoids was measured using Image-Pro Plus (Version 6.0, Media Cybernetics, Inc.).
5-ethynyl-2’-deoxyuridine (EdU) incorporation assay
Cell proliferation in organoids was determined using 5-ethynyl-2’-deoxyuridine (EdU) incorporation into DNA as described [24]. The organoids were incubated in 10 μM EdU solution in culture medium for 30 min at 37°C. EdU was detected with the Click-iT® EdU cell proliferation kit (Thermos, C10337) according to manufacturer’s instruction.
Radiation-induced oral mucositis
The oral region of mouse were irradiated by 250 kVp X-rays at a dose rate of 246 cGy/min (produced by X-Rad 320 with 0.25 mm Cu filtration) under anesthesia in a tray according previous reports [25]. During IR, the remaining body of the animal (below neck) was protected by a 5 mm-thick lead shield. 24 h later, the mice were injected intraperitoneally with a reference dose 1000 mg/kg NAM [26] or same volume of phosphate buffer saline (PBS).
Toluidine blue (TB) staining and histological study
Mice were euthanized 10 days after IR, and the whole tongue were dissected completely and immediately fixed for 12 h in ice-cold 4% paraformaldehyde in PBS at 4°C. After washed with PBS, the samples were then stained for 1-2 min in 1% toluidine blue (TB) in 10% acetic acid according to previous reports [25,27]. After vigorous washing until no more dye could be removed from the tissue, the mucosal lesions with ulcer (TB stained area) at the dorsal surface of tongue were photographed and qualified by Image-Pro Plus.
Tissue was then dissected longitudinally in the median plane to divide the ulcer into identical halves for paraffin embedding. 5 μm sections were made and hematoxylin-eosin (H&E) staining was performed. The injury of epithelia was evaluated by epithelium height based on the microscopic images (Imager M2, Zeiss).
β-galactosidase (LacZ) staining
For tracing the stem cells in circumvallate papillae of tongue, Lgr5-lacZ mice were used and β--galactosidase (LacZ) staining was performed with modified method as described [28]. Briefly, the tongue tissue was immediately fixed for 2 h in a 20-fold volume of ice-cold fixative (1% formaldehyde, 0.2% gluteraldehyde and 0.02% NP40 in PBS) at 4°C. Fixative was removed and samples were washed twice in PBS for 20 min at room temperature. X-gal solution (6 mM potassium ferrocyanide, 6 mM potassium ferricyanide, 2 mM MgCl2, 0.02% NP40, 0.01% sodium deoxycholate, and 1 mg/ml X-gal in PBS) was then added and tissues incubated in the dark overnight at room temperature. Tissues were then washed with PBS and fixed overnight in a 20-fold volume of 4% paraformaldehyde in PBS at 4°C in the dark for paraffin process. 5 μm sections were made and counter staining with nuclear fast red was performed.
Statistical analysis
The data are presented as the mean ± standard deviation (SD). GraphPad Prism software (version 5.0) was used for statistical analysis. Statistical significance was determined using unpaired Student’s T-test (in Figures 3, 5, 6 and 7), or one-way ANOVA with Dunnett’s multiple comparison post-hoc test (in Figures 2 and 4). P<0.05 was considered significant.
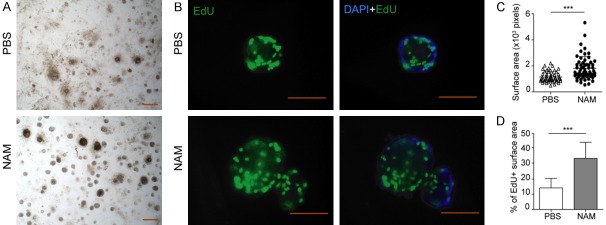
Figure 3.
SIRT1 inhibition stimulates the regeneration of taste bud organoid after irradiation. A. Bright field images of taste bud organoids treated with or without NAM at day 10 after IR. B. Representative images of taste bud organoid stained with DAPI and EdU; Scale bar 100 μm. C. Statistical analysis of the organoid size (surface area in pixels). D. Percentage of EdU positive area in taste bud organoid treated with or without NAM after IR. Data presented as the mean ± SD, n=55 from three independent tests, ***P<0.001.
Figure 5.
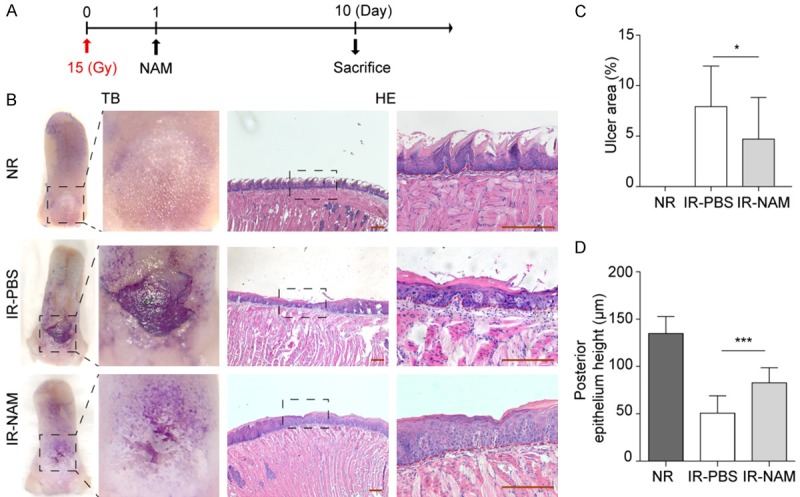
NAM suppresses the histological signs of oral mucositis induced by single dose irradiation. A. Single dose radiation and drug treatment scheme for mice. A single dose of Nicotinamide (1000 mg/kg, NAM) or PBS administered 24 h after 15 Gy IR. B. Representative pictures of tongues stained with TB and its histological analysis (right panels, HE stained), from control mice (non-irradiated, NR), and irradiated mice on day 10 post IR, after they received vehicle (PBS) or NAM. Scale bar 200 μm. C. Mean ulcer size percentage to the total epithelialized upper surface of the tongue. D. Quantification of the posterior epithelium height. Data presented as the mean ± SD (n=8), *P<0.05, ***P<0.001.
Figure 6.
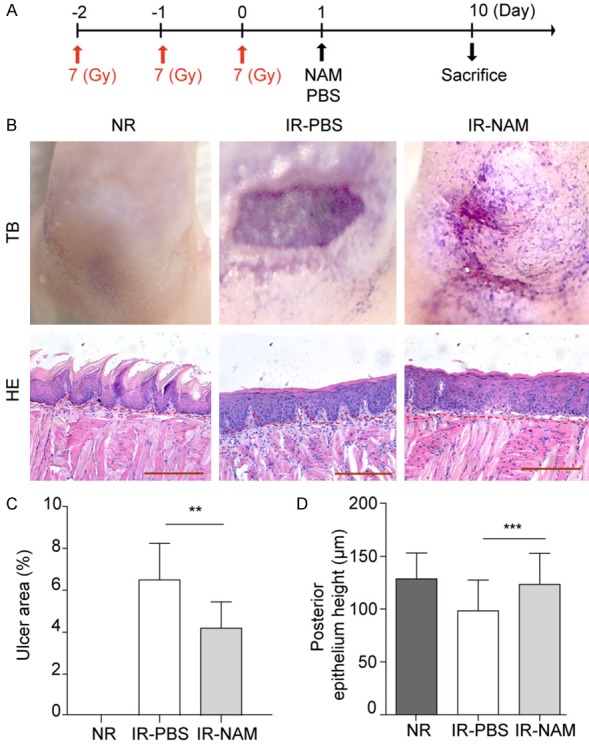
NAM suppresses the histological signs of oral mucositis induced by fractionated dose irradiation. A. Fractioned radiation and drug treatment scheme for mice. 7 Gy/day/mouse for 3 days of X-ray IR, NAM (1000 mg/kg) was given 24 h after last dose of IR. B. Representative pictures of tongues stained with TB and its histological analysis (HE stained), from control mice (non-irradiated, NR), and irradiated mice on day 10 after last dose IR, after they received vehicle (PBS) or NAM treatment; Scale bar 200 μm. C. Mean ulcer size percentage to the total epithelialized upper surface of the tongue. D. Quantification of the posterior epithelium height. Data presented as the mean ± SD (n=6). **P<0.01, ***P<0.001.
Figure 7.
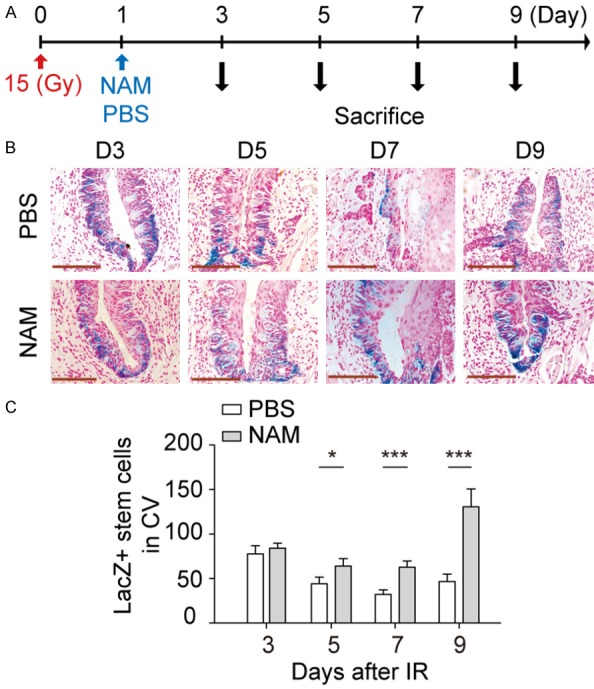
SIRT1 inhibitors promote the survival of Lgr5+ taste bud stem cells in mice. A. Radiation and drug treatment scheme for mice. Tissue collected on day 3, 5, 7 and 9 from groups with or without NAM injection. B. Representative images of stem cells after IR, Scale bar 100 μm. C. Quantitative comparison of Lgr5+ taste bud stem cell depletion kinetics after 15 Gy IR in CV papilla tissue. Data presented as the mean ± SD (n=4). *P<0.05, ***P<0.001.
Figure 2.
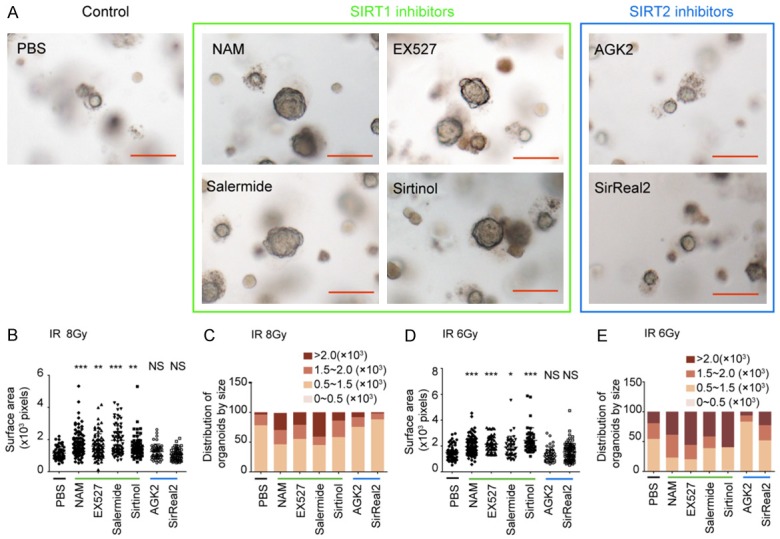
Sirt1 inhibitors mitigate radiation toxicity in taste bud organoid. A. Representative images of taste bud organoid treated with vehicle or SIRT1 inhibitors (NAM, EX527, salermide and sirtinol) or SIRT2 inhibitors (AGK2 and sirReal2) at day 10 after 8 Gy IR, Scale bar 100 μm. B. Statistical analysis of the average sizes (surface area in pixels, mean ± SD) of taste bud organoids after 8 Gy IR. C. Distribution of organoids by size after 8 Gy IR. D. Statistical analysis of the average sizes (surface area in pixels, mean ± SD) of taste bud organoids after 6 Gy IR. E. Distribution of organoids by size after 6 Gy IR. n=68 from three independent tests. *P<0.05, **P<0.01, ***P<0.001 vs. Ctrl (PBS), NS indicates no significance.
Figure 4.
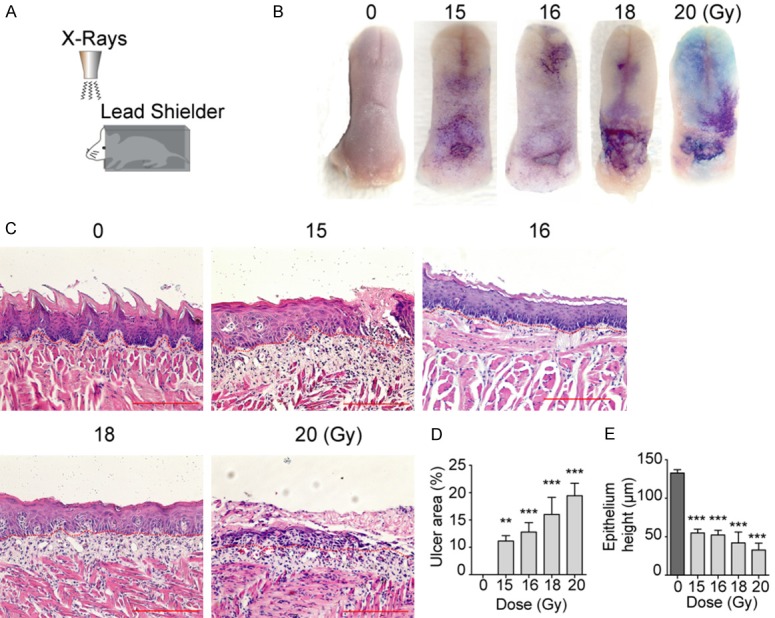
Irradiation induces RIOM in Lgr5-lacZ transgenic mice. A. Schematic illustration of setup of head-only radiation for mice. B. Representative pictures of tongues stained with toluidine blue (TB) after various dose of IR. C. Histological analysis of the tongues collected from mice at day 10 after IR, Scale bar 200 μm. D. Mean ulcer size percentage to the total epithelialized upper surface of the tongue. E. Quantification of the posterior epithelium height. Data presented as the mean ± SD (n=6). **P<0.01, ***P<0.001 vs. 0 Gy group.
Results
Establishment of a taste bud organoid model of radiation-induced lingual epithelial cell damage
Lgr5 expressing cells have recently been shown to reside in taste tissue of the posterior tongue and give rise to new mature taste bud epithelial cells during the normal cycles of growth. However, the role of Lgr5+ stem cells during the regeneration of taste buds after injury remains poorly elucidated. In order to verify the Lgr5 expression in CV papilla tissue, we used Lgr5-lacZ transgenic mice, commonly used to mark Lgr5+ cells, because lacZ gene is integrated into the last exon of the Lgr5 allele. The fidelity of the Lgr5-lacZ knock-in reporter has been validated in multiple tissues [29]. As shown in Figure 1A and 1B, LacZ positive cells were detected at the trench area and the base of the taste buds in CV papilla, and Lgr5 expression is most pronounced within the basal epithelium surrounding taste buds, which is consistent with previously published data [15]. No LacZ staining was detected in the fungiform papillae, foliate papillae or filiform papillae, therefore, we focused on the Lgr5 expressing cells in CV papilla during radiation induced tongue ulceration.
Figure 1.
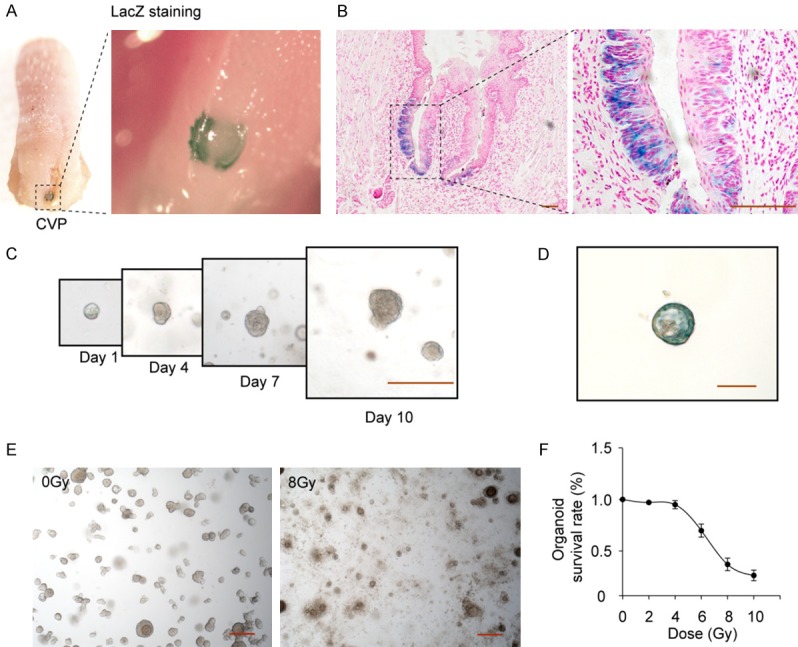
Establishment of a taste bud organoid model of radiation-induced lingual epithelial cell damage. (A) The macroscopic images of β-galactosidase-stained area (blue) in CV papilla of posterior tongue, and (B) the microscopic images of β-galactosidase stained stem cells. LacZ+ stem cells are visible at the base of the taste buds in CV papilla tissue. (C) Development of taste bud organoids in 3D cultures on days 1, 4, 7 and 10, and (D) LacZ staining. (E) radiation-induced damage of taste bud organoids, and (F) the dose curve. Dose range from 2 Gy to 10 Gy, Values are means ± SD (n=4). Scale bar 100 μm.
Recent development of taste bud organoid culture system allows us to visualize these cells long-term in vitro, and define their functional and molecular phenotypes after IR. CV papillae tissues isolated from mice were seeded in 3-dimensional (3D) Matrigel to develop in vitro taste bud organoids with Lgr5 expression (Figure 1C and 1D). Initial studies examined the dose response of taste bud organoid to IR (3-5 days after plating) and the survival rate was 70% by 6 Gy and 37% by 8 Gy on day 10 respectively (Figure 1E). Radiation survival curve, performed over a wide range of doses (2-10 Gy; Figure 1F), showed the taste bud organoid model could be used to monitor the radiation effect on lingual epithelium.
SIRT1 inhibitors mitigate radiation toxicity in taste bud organoids
To investigate the effect of SIRT1 inhibition after IR on the survival of lingual epithelial cells, taste bud organoids were treated with SIRT1 inhibitors, including NAM, EX527, sirtinol and salemide, at 24 h after IR. Treatment with SIRT1 inhibitors at 24 h after 8 Gy IR significantly improved the number and size of surviving taste bud organoids compared with untreated group (Figure 2A-C). Radio-mitigation effect of SIRT1 inhibition was also confirmed at 6 Gy IR (Figure 2D and 2E). In contrast, treatment with SIRT2 inhibitors (SirReal2 and AGK2) after 6 and 8 Gy IR did not significantly affect the organoid survival (Figure 2). Thus, SIRT1 inhibitors, not SIRT2 inhibitors, could serve as effective mitigators against radiation-induced taste bud injury.
SIRT1 inhibition stimulates the proliferation of lingual epithelial cells in taste bud organoid after IR
To test whether the SIRT1 inhibition also could affect the proliferation of lingual epithelial cells after IR, EdU incorporation assay was applied in this study. Taste bud organoids were treated with NAM, a recognized inhibitor of SIRT1, at 24 h after IR. Treatment with 10 mM NAM at 24 h after 8 Gy IR significantly improved the size of taste bud organoids compared with untreated group (Figure 3A and 3C). EdU labeled proliferating cells increased by NAM treatment after IR (Figure 3B and 3D, IR vs. IR + NAM: 14.19%±6.291 vs. 33.59%±10.58, P<0.001).
Radiation induces RIOM in Lgr5-lacZ transgenic mice
To examine the mitigative effect of SIRT1 inhibition on RIOM in vivo, we first defined the phenotypic response of Lgr5-lacZ mice to oral region IR with respect to induction of RIOM (illustrated in Figure 4A). RIOM was macroscopically evaluated using TB staining (Figure 4B). The mucosal ulceration and epithelium damage at the upper posterior surface of tongue were determined 10 days after IR (Figure 4B and 4C). Consistent with previous data [25], we found that a single dose of 15 Gy was the minimal dose needed to induce oral mucositis, and oral epithelia in Lgr5-lacZ mice typically showed radiation dose-dependent damage (Figure 4B and 4C). We observed oral mucosa ulcer and thinning epithelium after 15 Gy IR and more damaged epithelial layer after 18 Gy and 20 Gy IR (Figure 4D and 4E). These studies also reveal that loss of a single Lgr5 allele has no significant impact on the initiation of RIOM. Thus, the dose of 15 Gy was choose to induce RIOM in the following studies in vivo.
Administration of NAM suppresses RIOM in mice
Lgr5-lacZ transgenic mice received a single dose IR (15 Gy), 24 h after IR, mice were injected with vehicle (PBS) or NAM with a reference dose 1000 mg/kg [26]. Mice were euthanized at day 10 after IR (Figure 5A). Tongues were removed and processed for analysis to examine the mucosal damage of IR. SIRT1 inhibition by NAM significantly suppresses the oral mucositis/ulcers in irradiated mice (Figure 5B). Histological analysis of the tongues revealed the preservation of the epithelial layer in irradiated NAM-treated mice, although radiation associated tissue changes were observed (Figure 5B and 5C). The area of ulcer, relative to the total dorsal surface, was markedly decreased in NAM treated group (Figure 5C, NAM vs. PBS: 4.701% vs. 7.926%, P<0.05), along with significant increase in the thickness of epithelia layer (Figure 5D, NAM vs. PBS, 82.7 μm vs. 50.7 μm, P<0.001).
Considering the remarkable effects of SIRT1 inhibition in single dose radiation model, we next asked if NAM could rescue the deleterious effects of fractioned dose IR in oral epithelia. For these experiments, Lgr5-lacZ mice received fractionated IR of a 7 Gy dose/day/mouse for 3 days (Figure 6A), and NAM were given after last dose of IR. Aligned with our single dose results, the mitigation effect of NAM on RIOM was also observed in fractioned radiation model (Figure 6B-D).
SIRT1 inhibitors promote the survival of Lgr5+ taste bud stem cells in mice
Adult tissue stem cells are responsible for tissue regeneration after injury [21]. To determine the underlined mechanisms, the surviving Lgr5+ stem cells in CV papillae were kinetically quantified by LacZ staining at indicated times after IR (Figure 7A). The LacZ+ stem cells in taste bud were gradually depleted from day 3 to day 7 after 15 Gy IR (Figure 7B), and about 40% (P<0.001) of stem cells were lost during 7 days after IR. Further, when comparing impact of 15 Gy at 7 days with 9 days, it is apparent that damage to Lgr5+ taste bud stem cells is reversed upon transition to 9 days (Figure 7B and 7C). A single dose of NAM administration significantly increases the number of surviving of Lgr5+ stem cells in CV. Our stem cell data show a mitigation effect of NAM with 46.8±8.4 lacZ+ stem cells detected in untreated Lgr5-lacZ mice increased to 130.5±20.1 lacZ+ stem cells in NAM treated group at day 9 after 15 Gy IR (Figure 7C). These results indicated that SIRT1 inhibition promotes the survival of taste bud stem cells after IR in vivo, resulting in alleviated tongue ulceration.
Discussion
The tongue is gustatory tissues containing numerous taste buds to detect taste stimuli. The complexity of oral mucositis as a biological process remain elusive.
The taste bud epithelium is rapidly renewed, with a complete turnover every 10-14 days, making them particularly vulnerable to chemical or radiation insults compromising the repopulating capacity of the epithelial stem cell compartment [10,11]. The self-renewal is driven by taste bud stem cells, which are marked by Lgr5 and localized at the base of the taste buds in CV papilla tissue. In mice, the number of taste progenitors or stem cells decreased within 1-3 days after IR, mostly due to cell cycle arrest and apoptosis [21]. Following continued apoptosis of dying cell, the temporary or permanent disruption in the supply of new cells lead to taste loss and mucosal ulceration in the irradiated epithelia [21]. A better understanding of the taste bud stem cell responses to radiation, will help to develop new approaches to identify new therapeutic targets. In this study, we established the taste bud organoid based assay to investigate the radio-mitigation effect of the SIRT1 inhibitors in vitro. The organoid data indicates that treatment with SIRT1 inhibitors at 24 h post IR promotes the regeneration of lingual organoid and improves organoid survival against radiation. Aligned with in vitro data, NAM treatment correlated with increased number of surviving Lgr5 taste bud stem cells in vivo, thereby enhancing their repopulating capacity and preventing the appearance of ulcers. These observations have important implications for efforts to develop medical countermeasures against oral toxicity induced by radiation.
SIRT1 belongs to sirtuin family proteins and has dual activities as a NAD+-dependent deacetylase and as a mono-ADP ribosyltransferase [18]. SIRT1 known to be involved in cell aging and in the response to DNA damage, binds and deacetylates the p53 protein, modification of which has been implicated in the activation of p53 [19]. Through deacetylation, SIRT1 modifies the activities of histones and non-histone proteins that contribute to many processes that affect cell survival, senescence or life span such as oxidative stress, DNA damage repair, cell cycle control, inflammation, and energy and oxygen metabolism [30]. SIRT1 was reported positively affects stem cells function or renewal in variety of adult tissues [31-36]. It has also been reported that SIRT1 inhibition protect against oxidative stress induced apoptosis in neurons [37,38]. In fact, the beneficial effect of SIRT1 inhibition has also been observed in inflammatory injury of lung [39], liver [40,41] and in septic rats [42]. In this study, we found that SIRT1 inhibition, not SIRT2, could promote the survival of taste bud stem cells and mitigate the radiation induced oral toxicity. Yet the potential mechanisms underlying these observations remain unknown and will require further studies for elucidation.
Conclusion
Altogether, our results indicate that pharmacological inhibition of SIRT1 promote Lgr5+ taste bud stem cell survival and mitigates radiation-induced oral mucositis in mice. The taste bud organoid system is reliable model to mimic the stem cell radiation response in vivo. These findings have important implications for efforts to develop medical countermeasures against taste dysfunction and mucosal ulceration in RIOM.
Acknowledgements
This work was supported by grants from the National Science Foundation of China (No. 31470826, and No. 31670858 to G.H). We would like to thank Shungao Tong for guidance on the use of X-Rad 320 and assistance in animal caring. All animal protocols were approved by the Institutional Animal Care and Use Committee of Fudan University.
Disclosure of conflict of interest
None.
Abbreviations
- RIOM
Radiation-induced oral mucositis
- SIRT1
Sirtuin 1
- SIRT2
Sirtuin 2
- IR
Irradiation
- NAM
Nicotinamide
- CVP
Circumvallate papillae
- Lgr5
Leucine-rich repeat-containing G-protein-coupled receptor 5
- LacZ
Beta-galactosidase
- Gy
Gray
- EdU
5-ethynyl-2’-deoxyuridine
References
- 1.Mossman KL. Gustatory tissue injury in man: radiation dose response relationships and mechanisms of taste loss. Br J Cancer Suppl. 1986; 7:9–11. [PMC free article] [PubMed] [Google Scholar]
- 2.Epstein JB, Barasch A. Taste disorders in cancer patients: pathogenesis, and approach to assessment and management. Oral Oncol. 2010;46:77–81. doi: 10.1016/j.oraloncology.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 3.Yamashita H, Nakagawa K, Hosoi Y, Kurokawa A, Fukuda Y, Matsumoto I, Misaka T, Abe K. Umami taste dysfunction in patients receiving radiotherapy for head and neck cancer. Oral Oncol. 2009;45:e19–23. doi: 10.1016/j.oraloncology.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Irune E, Dwivedi RC, Nutting CM, Harrington KJ. Treatment-related dysgeusia in head and neck cancer patients. Cancer Treat Rev. 2014;40:1106–1117. doi: 10.1016/j.ctrv.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 5.Sciubba JJ, Goldenberg D. Oral complications of radiotherapy. Lancet Oncol. 2006;7:175–183. doi: 10.1016/S1470-2045(06)70580-0. [DOI] [PubMed] [Google Scholar]
- 6.Maria OM, Eliopoulos N, Muanza T. Radiation-induced oral mucositis. Front Oncol. 2017;7:89. doi: 10.3389/fonc.2017.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abdel Moneim AE, Guerra-Librero A, Florido J, Shen YQ, Fernandez-Gil B, Acuna-Castroviejo D, Escames G. Oral mucositis: melatonin gel an effective new treatment. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18051003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sonis ST. Efficacy of palifermin (keratinocyte growth factor-1) in the amelioration of oral mucositis. Core Evid. 2010;4:199–205. doi: 10.2147/ce.s5995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reiner DJ, Jan TA, Boughter JD Jr, Li CX, Lu L, Williams RW, Waters RS. Genetic analysis of tongue size and taste papillae number and size in recombinant inbred strains of mice. Chem Senses. 2008;33:693–707. doi: 10.1093/chemse/bjn025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farbman AI. Renewal of taste bud cells in rat circumvallate papillae. Cell Tissue Kinet. 1980;13:349–357. doi: 10.1111/j.1365-2184.1980.tb00474.x. [DOI] [PubMed] [Google Scholar]
- 11.Beidler LM, Smallman RL. Renewal of cells within taste buds. J Cell Biol. 1965;27:263–272. doi: 10.1083/jcb.27.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barlow LA, Klein OD. Developing and regenerating a sense of taste. Curr Top Dev Biol. 2015;111:401–419. doi: 10.1016/bs.ctdb.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng P, Huang L, Wang H. Taste bud homeostasis in health, disease, and aging. Chem Senses. 2014;39:3–16. doi: 10.1093/chemse/bjt059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takeda N, Jain R, Li D, Li L, Lu MM, Epstein JA. Lgr5 identifies progenitor cells capable of taste bud regeneration after injury. PLoS One. 2013;8:e66314. doi: 10.1371/journal.pone.0066314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yee KK, Li Y, Redding KM, Iwatsuki K, Margolskee RF, Jiang P. Lgr5-EGFP marks taste bud stem/progenitor cells in posterior tongue. Stem Cells. 2013;31:992–1000. doi: 10.1002/stem.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ren W, Lewandowski BC, Watson J, Aihara E, Iwatsuki K, Bachmanov AA, Margolskee RF, Jiang P. Single Lgr5- or Lgr6-expressing taste stem/progenitor cells generate taste bud cells ex vivo. Proc Natl Acad Sci U S A. 2014;111:16401–16406. doi: 10.1073/pnas.1409064111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schuijers J, Clevers H. Adult mammalian stem cells: the role of Wnt, Lgr5 and R-spondins. EMBO J. 2012;31:2685–2696. doi: 10.1038/emboj.2012.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen HY, Miller C, Bitterman KJ, Wall NR, Hekking B, Kessler B, Howitz KT, Gorospe M, de Cabo R, Sinclair DA. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science. 2004;305:390–392. doi: 10.1126/science.1099196. [DOI] [PubMed] [Google Scholar]
- 19.Vaziri H, Dessain SK, Ng Eaton E, Imai SI, Frye RA, Pandita TK, Guarente L, Weinberg RA. hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell. 2001;107:149–159. doi: 10.1016/s0092-8674(01)00527-x. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Q, Zeng SX, Zhang Y, Zhang Y, Ding D, Ye Q, Meroueh SO, Lu H. A small molecule Inauhzin inhibits SIRT1 activity and suppresses tumour growth through activation of p53. EMBO Mol Med. 2012;4:298–312. doi: 10.1002/emmm.201100211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nguyen HM, Reyland ME, Barlow LA. Mechanisms of taste bud cell loss after head and neck irradiation. J Neurosci. 2012;32:3474–3484. doi: 10.1523/JNEUROSCI.4167-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 23.Aihara E, Mahe MM, Schumacher MA, Matthis AL, Feng R, Ren W, Noah TK, Matsu-ura T, Moore SR, Hong CI, Zavros Y, Herness S, Shroyer NF, Iwatsuki K, Jiang P, Helmrath MA, Montrose MH. Characterization of stem/progenitor cell cycle using murine circumvallate papilla taste bud organoid. Sci Rep. 2015;5:17185. doi: 10.1038/srep17185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salic A, Mitchison TJ. A chemical method for fast and sensitive detection of DNA synthesis in vivo. Proc Natl Acad Sci U S A. 2008;105:2415–2420. doi: 10.1073/pnas.0712168105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maria OM, Syme A, Eliopoulos N, Muanza T. Single-dose radiation-induced oral mucositis mouse model. Front Oncol. 2016;6:154. doi: 10.3389/fonc.2016.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horsman MR, Chaplin DJ, Brown JM. Radiosensitization by nicotinamide in vivo: a greater enhancement of tumor damage compared to that of normal tissues. Radiat Res. 1987;109:479–489. [PubMed] [Google Scholar]
- 27.Muanza TM, Cotrim AP, McAuliffe M, Sowers AL, Baum BJ, Cook JA, Feldchtein F, Amazeen P, Coleman CN, Mitchell JB. Evaluation of radiation-induced oral mucositis by optical coherence tomography. Clin Cancer Res. 2005;11:5121–5127. doi: 10.1158/1078-0432.CCR-05-0403. [DOI] [PubMed] [Google Scholar]
- 28.Burn SF. Detection of beta-galactosidase activity: X-gal staining. Methods Mol Biol. 2012;886:241–250. doi: 10.1007/978-1-61779-851-1_21. [DOI] [PubMed] [Google Scholar]
- 29.Huch M, Dorrell C, Boj SF, van Es JH, Li VS, van de Wetering M, Sato T, Hamer K, Sasaki N, Finegold MJ, Haft A, Vries RG, Grompe M, Clevers H. In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature. 2013;494:247–250. doi: 10.1038/nature11826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nogueiras R, Habegger KM, Chaudhary N, Finan B, Banks AS, Dietrich MO, Horvath TL, Sinclair DA, Pfluger PT, Tschop MH. Sirtuin 1 and sirtuin 3: physiological modulators of metabolism. Physiol Rev. 2012;92:1479–1514. doi: 10.1152/physrev.00022.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang T, Zhou Y, Li L, Wang HH, Ma XS, Qian WP, Shen W, Schatten H, Sun QY. SIRT1, 2, 3 protect mouse oocytes from postovulatory aging. Aging (Albany NY) 2016;8:685–696. doi: 10.18632/aging.100911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Imperatore F, Maurizio J, Vargas Aguilar S, Busch CJ, Favret J, Kowenz-Leutz E, Cathou W, Gentek R, Perrin P, Leutz A, Berruyer C, Sieweke MH. SIRT1 regulates macrophage self-renewal. EMBO J. 2017;36:2353–2372. doi: 10.15252/embj.201695737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rathbone CR, Booth FW, Lees SJ. Sirt1 increases skeletal muscle precursor cell proliferation. Eur J Cell Biol. 2009;88:35–44. doi: 10.1016/j.ejcb.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singh SK, Williams CA, Klarmann K, Burkett SS, Keller JR, Oberdoerffer P. Sirt1 ablation promotes stress-induced loss of epigenetic and genomic hematopoietic stem and progenitor cell maintenance. J Exp Med. 2013;210:987–1001. doi: 10.1084/jem.20121608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rimmele P, Bigarella CL, Liang R, Izac B, Dieguez-Gonzalez R, Barbet G, Donovan M, Brugnara C, Blander JM, Sinclair DA, Ghaffari S. Aging-like phenotype and defective lineage specification in SIRT1-deleted hematopoietic stem and progenitor cells. Stem Cell Rep. 2014;3:44–59. doi: 10.1016/j.stemcr.2014.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Niu B, Wu J, Mu H, Li B, Wu C, He X, Bai C, Li G, Hua J. miR-204 Regulates the proliferation of dairy goat spermatogonial stem cells via targeting to Sirt1. Rejuvenation Res. 2016;19:120–130. doi: 10.1089/rej.2015.1719. [DOI] [PubMed] [Google Scholar]
- 37.Li Y, Xu W, McBurney MW, Longo VD. SirT1 inhibition reduces IGF-I/IRS-2/Ras/ERK1/2 signaling and protects neurons. Cell Metab. 2008;8:38–48. doi: 10.1016/j.cmet.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sansone L, Reali V, Pellegrini L, Villanova L, Aventaggiato M, Marfe G, Rosa R, Nebbioso M, Tafani M, Fini M, Russo MA, Pucci B. SIRT1 silencing confers neuroprotection through IGF-1 pathway activation. J Cell Physiol. 2013;228:1754–1761. doi: 10.1002/jcp.24334. [DOI] [PubMed] [Google Scholar]
- 39.Huang J, Tian R, Yang Y, Jiang R, Dai J, Tang L, Zhang L. The SIRT1 inhibitor EX-527 suppresses mTOR activation and alleviates acute lung injury in mice with endotoxiemia. Innate Immun. 2017;23:678–686. doi: 10.1177/1753425917733531. [DOI] [PubMed] [Google Scholar]
- 40.Ponugoti B, Kim DH, Xiao Z, Smith Z, Miao J, Zang M, Wu SY, Chiang CM, Veenstra TD, Kemper JK. SIRT1 deacetylates and inhibits SREBP-1C activity in regulation of hepatic lipid metabolism. J Biol Chem. 2010;285:33959–33970. doi: 10.1074/jbc.M110.122978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cui X, Chen Q, Dong Z, Xu L, Lu T, Li D, Zhang J, Zhang M, Xia Q. Inactivation of Sirt1 in mouse livers protects against endotoxemic liver injury by acetylating and activating NF-kappaB. Cell Death Dis. 2016;7:e2403. doi: 10.1038/cddis.2016.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao T, Li Y, Liu B, Bronson RT, Halaweish I, Alam HB. Histone deacetylase III as a potential therapeutic target for the treatment of lethal sepsis. J Trauma Acute Care Surg. 2014;77:913–919. doi: 10.1097/TA.0000000000000347. [DOI] [PMC free article] [PubMed] [Google Scholar]