Abstract
To investigate the effect of pantoprazole on acute lung and kidney injury with sepsis and its possible mechanism. Rats were randomly divided into six groups, the status and lung wet/dry weight ratio were determined at various time points. Hematoxylin Eosin staining (HE) for pathological changes in the lungs and kidneys of rats with sepsis. Western blot (WB) and immunohistochemistry were used to detect the pulmonary surfactant protein A(SPA) and D(SPD). The levels of markers for kidney damage in serum and urine various time points were measured by ELISA. The apoptosis in lung and kidney tissue of rats were detected using TUNEL assay. Subsequently, the cell apoptosis of LPS-induced BEAS-2B and HK-2 cells after pantoprazole treatment were detected using flow cytometry. The levels of RHOA/ROCK signaling pathway proteins in the lung and kidney tissues and cells were detected using WB. Our results indicated that Pantoprazole could suppress the expression of inflammatory factors in the blood and alleviate pathological damage of lung and kidney tissues in rats with sepsis. Pantoprazole treatment could reduce apoptosis in lung and kidney tissues and inhibit cell apoptosis induced by LPS. In addition, pantoprazole can inhibit RHOA/ROCK signaling pathway proteins and the levels of inflammatory factors in LPS-induced BEAS-2B and HK-2 cells. Pantoprazole can improve the symptoms of acute lung and kidney injury in septic rats, which suggested that pantoprazole might be used to guide the treatment of sepsis.
Keywords: Pantoprazole, sepsis, acute lung and kidney injury, apoptosis, RHOA
Introduction
Sepsis as a systemic inflammatory response syndrome caused by various factors, can affect multiple systems and organs. It is an important factor leading to the death of patients [1]. In the case of sepsis, various inflammatory factors and inflammatory mediators such as tumor necrosis factor alpha (TNF-α), interleukin (IL)-6, IL-8 are released and activated in large quantities, which can cause proinflammatory and anti-inflammatory processes, as well as the immune function of the body is damaged. Among them, acute lung and kidney injury is a common serious complication in patients with sepsis, the incidence rate is as high as 40% to 70% [2], and the mortality rate is more than 70% [3], serious threat to the safety of patients.
Pantoprazole is a proton pump inhibitor and an effective antagonist of gastric acid secretion for the treatment of many gastroesophageal diseases, including dyspepsia, gastroesophageal reflux disease (GERD) and peptic ulcer disease [4,5]. Beyond its role on the suppression of acid secretion, it has been reported that PPI exerts anti-inflammatory effects as well [6]. It has been suggested that PPI attenuates inflammation via different mechanisms such as inhibition of NF-κB activation in endothelial and gastric parietal cells, modulating the calcium concentration in polymorphonuclear neutrophils, impairing the neutrophils migration and prevention of pro-inflammatory cytokines [6,7]. In addition, PPI plays significant anti-oxidative and anti-apoptotic properties through direct scavenging of reactive oxygen species (ROS) [7-9]. Recent studies have shown that pantoprazole reduces vasodilation in vitro and in vitro, and interferes with blood coagulation in animal models, as well as anti-inflammatory, anti-oxidant, anti-cancer and chemotherapy sensitization effects [10,11]. Currently, there is no data available to investigate the effect of pantoprazole on acute lung and kidney injury caused by sepsis.
Therefore, the purpose of this study was to investigate the effects of pantoprazole on lung and kidney tissues of the animal model in vivo, and the LPS-induced BEAS-2B and HK-2 cells models in vitro, demonstrating that pantoprazole inhibits inflammatory factors in lung epithelial cells and human renal cortical proximal tubular epithelial cells by RHOA/ROCK signaling. The aim of the present study is to investigate the mechanism of action of pantoprazole sodium combined with monitoring on acute lung and kidney injury caused by sepsis.
Materials and methods
Animal
Male Sprague Dawley (SD) rats weighing 200~210 g were housed under controlled temperature (20-25°C), humidity (20-30%), constant light cycle (12 h light/dark) and were allowed access to food and water ad libitum during the study. All mice received humane care and experimental procedures were carried out in strict accordance with the health and care guidelines for experimental animals. All animals received humane care and experimental procedures were carried out in strict accordance with the health and care guidelines for experimental animals. All experimental operations performed on all rats were approved by the Animal Experiment Ethics Committee of The Second Xiangya Hospital of Central South University.
The method of CLP model preparation
An animal model of sepsis was established using an internationally recognized cecal ligation and puncture (CLP) sepsis model. The standard steps mainly refer to the report of Rittirsch et al. [12], Anesthetize with intraperitoneal injection of 1% pentobarbital sodium injection 50 mg/kg. After anesthesia, cut off the abdominal body hair of the rats, routinely disinfect and spread the sterile towel, make an incision about 1 cm along the midline of the abdomen, find the cecum and carefully dissociate it. The vascular injury was avoided at the distal end. The cecum was ligated with the No. 4 suture on the distal side of the ileocecal valve. The ligation range was about 1/3 of the entire cecum, avoiding ligation of the ileum and cecal mesenteric vessels. The 18th needle was used to penetrate the cecum twice, and a small amount of intestinal contents was squeezed out. After that, the cecum was also placed in the abdominal cavity, and the abdominal wall incision was sutured layer by layer.
Experimental protocol
The rats were randomly divided into 6 groups, including control group (normal rats), model group (rats with CLP and without any treatment), sham group (except for surgery without CLP, other operations are the same as model group), lower dose pantoprazole-treated group (LG group, rats with CLP and 26 mg/kg pantoprazole-treated), medium-dose pantoprazole-treated group (MG group, rats with CLP and 52 mg/kg pantoprazole-treated), higher dose pantoprazole-treated group (HG group, rats with CLP and 104 mg/kg pantoprazole-treated). The control, model and sham groups of rats were housed with free access to food and no drug treatment, whereas the rats in the LG, MG and HG groups were treated with different dose of pantoprazole via intragastric gavage. According to the experimental requirements, all groups were set experiment time points at 0, 6, 12, and 24 h after the modeling. The blood and the urine samples were collected and the lung and kidney tissue were taken after sacrificed. The mouse hair, mental state, body weight, eating condition and spontaneous activity were determined everyday [13].
Histopathological examination
The lung and kidney were taken from the rats which had been executed after 24 h of CLP respectively and fixed in 10% buffered formalin and the tissues were embedded in paraffin, sectioned at 5 μm and stained with hematoxylin and eosin (HE). The H&E stained slides were observed and evaluated under light microscopy for histological examination.
Dry/wet ratio of lung
The lung specimen was weighed and placed in an oven at 80°C for 72 h to constant weight to calculate the lung wet/dry weight ratio (W/D).
Immunohistochemistry (IHC) staining
The samples were incubated with the indicated primary antibody (1:100) at 4°C overnight and then rinsed with PBS for 5 min three times and incubated with biotinylated secondary antibody (1:100) at room temperature for 30 min. After PBS washing, DAB was then added for color development. The reaction was controlled under a microscope. When the color was fully developed, the sample was rinsed with PBS thoroughly to quench the reaction. The sample was then patched, dehydrated, cleared, and mounted with neutral gum. Image analysis was performed using Biosens Digital Imaging System.
Tunel assay
Cell apoptosis was detected using a terminal deoxynucleotidyl transferase 2’-deoxyuridine-5’-triphosphate nick-end labelling (TUNEL) assay (Millipore; Merck KGaA, Darmstadt, Germany). Cells were washed with PBS and cells were fixed with 1% paraformaldehyde. TUNEL reagents were used to stain the apoptotic cells. Optical microscopy (Olympus Corp., Tokyo, Japan) was used to analyze samples (magnification, ×200).
Cell culture and transfection
BEAS-2B and HK-2 cells were obtained from Cell Bank of the Chinese Academy of Sciences, and cultured in Dulbecco’s modified Eagle’s media (DMEM, Gibco) supplemented with 10% fetal bovine serum (Gibco) and 100 μ/ml penicillin and 100 mg/ml streptomycin (Gibco) and streptomycin at 37°C with 5% CO2.
Construction of RhOA overexpression plasmid, the BEAS-2B and HK-2 cells were plated in six-well plates, respectively. Transfection used Lipofectamine 3000 (Invitrogen, Thermo Fisher Scientific, Inc., USA), Opti-MEM and RMPI DMEM (serum-free) according to the manufacturer’s instruction. The cells were cultured for 24-72 h at 37°C after transfection.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
The total RNA was extracted using Trizol reagent (TaKaRa, Dalian, Liaoning, China) and the cDNA was synthesized with RNA Transcription Kit (TaKaRa). Then, RT-qPCR was performed using the SYBR Green RT-qPCR system. The primer sequences: β-Actin, Forward: CTGAGAGGGAAATCGTGCGT; Reverse: CCACAGGATTCCATACCCAAGA; RhoA, Forward: AGCCTGTGGAAAGACATGCTT; Reverse: TCAAACACTGTGGGCACATAC. Conditions for RT-qPCR was set as the following: 95°C for 10 min, 40 cycles of 95°C for 30 s, 60°C for 30 s, 72°C for 30 s and a final extension of 10 min at 72°C.
Flow cytometry apoptosis detection assay
According to the instructions of the kit, cells were seeded in six-well plates for a whole night and washed in 4°C PBS, pelleted, and resuspended in 0.5 ml of hypotonic fluorochrome solution containing 50 μg/ml propidium iodide (PI), 0.1% sodium citrate, and 0.1% Triton X-100 (Sigma) to quantitate the cellular DNA content under the permeabilized condition. Phosphatidyl-serine (PS) exposure due to flipping of the plasma membrane, a concomitant feature during apoptosis, was evaluated by annexin V-FITC staining. Five microliters of annexin V/FITC and 5 μl PI were added to the cells and then incubated in the dark. Apoptosis rate was assessed by flow cytometric analysis.
Western blots
Total protein from the cells and tissues was extracted using RIPA lysis buffer and protein concentrations were determined using a BCA protein kit. Protein samples (30 μg) were separated by 10% SDS-PAGE, then the proteins were transferred to PVDF membranes. Membranes were blocked with TBS containing 5% nonfat milk and 0.1% Tween-20 for 1 h at room temperature. The primary antibodies overnight at 4°C. After three washes with TBST buffer, the membranes were incubated with secondary antibody at 4°C for 3 h and visualized by enhanced chemiluminescence (ECL) reaction reagents. The antibodies used were as follows: A(SPA) (1:1,000, Santa Cruz), D(SPD) (1:1,000, Santa Cruz), RHOA (1:1,000, Santa Cruz), ROCK1 (1:1,000, Santa Cruz), ROCK2 (1:1,000, Millipore), LIMK1 (1:1,000, Abcam), LIMK2 (1:1,000, Abcam), mouse anti-rabbit secondary antibody (1:10000, Santa Cruz -2077), rabbit anti-mouse secondary antibody (1:10000, Santa Cruz -2061).
ELISA
The levels of KIM-1, IL-18, Scr, BUN and ScysC in serum, the levels of α-glutathione S transferase (α-GST) and albumin in urine, and the levels of IL-1β, TNF-α and MCP-1 in serum as well as in BEAS-2B and HK-2 cells, were determined using ELISA assay kits (Andihuatai Technology Co. Ltd., Beijing, China) according to the manufacturer’s instructions.
Statistical analysis
All data are expressed as the mean ± standard deviation (SD). Statistical differences between the groups were assessed by one-way ANOVA statistical analysis followed by a Tukey’s test if necessary. All experiments were repeated at least three times, and P < 0.05 was considered significant.
Results
Pantoprazole improves the status of septic rats
There was no significant difference in the initial body weight in each group. After surgery, the Sham group had faster waking after surgery and flexible activities. They were free to drink water, eat, and had little or no secretions. In the model group, the rats had delayed recovery, mental dysfunction, slow activity, and shortness of breath. After 24 hours, the degree was reduced, but it lasted for 48-72 hours, and gradually returned to normal after 7 days.
We treated mice in the model group with different concentrations of pantoprazole sodium by intragastric administration. After treatment, it was observed that the hair became obedient. The mental state has improved, not getting together, the activity has improved, and the diet has gradually returned to normal.
Effect of pantoprazole on the lung water content of CLP-induced spesis rat
As shown in Figure 1, the lung water content in model group was significantly increased (P < 0.05) at every time point compared with control group and sham group, indicating the severe pulmonary edema in model group. When it comes with the pantoprazole treatment groups, at 6, 12 and 24 h post-CLP, lung water content of LG, MG and HG groups was significantly lower (P < 0.001) than model group. The results indicated that pantoprazole can release changes in pulmonary edema.
Figure 1.

Pantoprazole reduced lung W/D ratio in CLP-induced spesis rats. *P < 0.05, **P < 0.01, ***P < 0.001 vs. Control group; #P < 0.05, ##P < 0.01, ###P < 0.001 vs. model group and ΔP < 0.05, ΔΔP < 0.01, ΔΔΔP < 0.001 vs. sham group.
Pantoprazole attenuates pathological damage of lung and kidney tissues in rats with sepsis
Histopathological examination (Figure 2A) showed that the lung tissue of the sham-operated group was similar to that of the control group. But compared with the sham groups, severe damage on the pulmonary alveoli structure was observed for all of the model group with the appearance of histopathological changes such as complete destruction of pulmonary alveoli structure and inflammation cell infiltration. And the harm was more and more serious over time. Pantoprazole treated groups (LG, MG and HG groups), gradually alleviated the pulmonary alveoli damage by increase in dosage administration.
Figure 2.
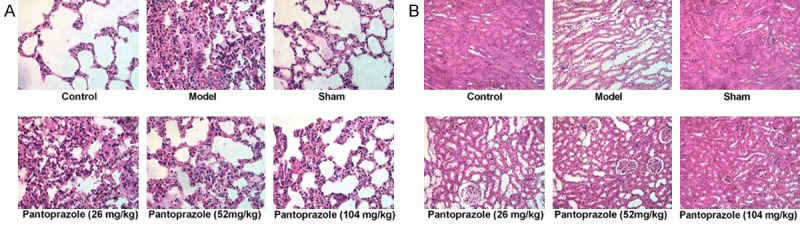
Pantoprazole ameliorated histopathological changes in lung and kidney tissues of CLP-induced spesis rats. (A) Representative images of hematoxylin and eosin (H&E) staining from each experimental group of lung tissues at 6 h, 12 h and 24 h after CLP. (Original magnification) (B) Representative images of hematoxylin and eosin (H&E) staining from each experimental group of kidney tissues at 6 h, 12 h and 24 h after CLP. (Original magnification).
Meanwhile, the results of HE staining (Figure 2B) showed that the cells in the kidney of the sham operation group were arranged regularly, the structure was intact, the nucleus was in the middle, the nucleolus was clear, the cytoplasm was uniformly stained, no degeneration and necrosis occurred. In model and pantoprazole treated groups (LG, MG and HG groups), the tubular epithelial cells showed swelling, watery and vacuolar degeneration, tubular dilatation, exfoliated cells, renal interstitial congestion. Compared with the model group, the rats in the pantoprazole group had reduced renal tissue damage, decreased cell abnormalities.
Pantoprazole reduces the expression of markers in lung injury
Compared with the sham group, the expression levels of A(SPA) and D(SPD) in the lung tissue of the model group and pantoprazole treatment groups were increased, and the differences were statistically significant (P < 0.05). Compared with the model group and control group, the relative expressions of A(SPA) and D(SPD) protein in the lung tissue of pantoprazole treatment group were decreased, the difference was statistically significant (P < 0.05) (Figure 3A). The immunohistochemistry results showed that the pantoprazole treatment groups gradually reduced the expression of A(SPA) (Figure 3B) and D(SPD) (Figure 3C) in the lung tissue by increasing the dose administered compared with the sham group and the control group at 24 hours. The immunohistochemistry results are similar with the western blot results (Figure 3B).
Figure 3.

Pantoprazole reduces the expression of A(SPA) and D(SPD) in lung injury. (A) Western blot to show the protein levels of A(SPA) and D(SPD) in control, model, sham and Pantoprazole-treatment groups. Quantification assay of the A(SPA) and D(SPD) band’s intensity. Error bars indicate ± SD. **P < 0.01 vs. Control group. **P < 0.01, ***P < 0.001 vs. Control group; ###P < 0.001 vs. model group and ΔΔP < 0.01, ΔΔΔP < 0.001 vs. sham group. The expression of A(SPA) (B) and D(SPD) (C) in lung injury tissues were assessed by IHC analysis (Original magnification).
Pantoprazole reduces markers of kidney damage in serum and urine
We examined the levels of serum inflammatory factors. The ELISA assay indicated that the levels of serum Scr, BUN and Scys C were significantly up-regulated in the model group and pantoprazole-treated groups compared with the sham group and control group (Figure 4A). As shown in Figure 4B, the levels of Serum kidney injury markers KIM-1, IL-18 of model group rats at each time point increased significantly (P < 0.05) compared with the sham group and gradually increased with time, eventually reaching a peak of 24 h. The KIM-1 and IL-18 level in pantoprazole-treated groups were significantly decreased (P < 0.05) at all of the three time points. Then we detected the changes of α-GST and albumin in urine at 0 h, 6 h, 12 h and 24 h, the results indicated that the expression of α-GST and albumin in model group is much higher than control group, and in pantoprazole-treated groups (LG, MG, and HG groups), the expression of α-GST and albumin are decreased as the metering increases (Figure 4C).
Figure 4.
Pantoprazole reduces markers of kidney damage in serum and urine. A. The levels of serum Scr, BUN and ScysC; B. The levels of Serum kidney injury markers KIM-1, IL-18; C. The changes of α-GST and albumin were detected by ELISA. ***P < 0.001 vs. Control group; #P < 0.05, ##P < 0.01, ###P < 0.001 vs. model group and ΔP < 0.05, ΔΔP < 0.01, ΔΔΔP < 0.001 vs. sham group.
Pantoprazole reduces the expression of inflammatory factors in the blood
Detection of proinflammatory cytokines TNFα, MCP-1, IL-1β in blood by ELISA. TNFα, MCP-1, IL-1β were significantly up-regulated in the model group compared with the sham group. Additionally, the levels of serum TNFα, MCP-1, IL-1β were significantly higher in all of the pantoprazole-treated groups (LG, MG, and HG groups) than the sham groups in a dose-dependent manner (Figure 5).
Figure 5.
Pantoprazole inhibited TNFα, MCP-1 and IL-1β expression in the blood of CLP-induced spesis rats. ***P < 0.001 vs. Control group; ##P < 0.01, ###P < 0.001 vs. model group and ΔΔP < 0.01, ΔΔΔP < 0.001 vs. sham group.
Pantoprazole reduces apoptosis in lung and kidney tissues
To confirm apoptotic in lung and kidney tissues, TUNEL staining was performed. TUNEL assay showed high FITC positivity in Pantoprazole-treated cells compared to the vehicle-treated cells confer apoptotic cell death. Compared with the model group, the apoptosis rate of pantoprazole-treated groups decreased, the difference was statistically significant (P < 0.05) (Figure 6).
Figure 6.

Observation of apoptosis of lung (A) and kidney (B) tissues in 24 h by TUNEL staining. Presented are the representative photographs of TUNEL staining. Nuclei are stained with blue and TUNEL-positive cells green (arrow).
Pantoprazole inhibits RHOA/ROCK signaling pathway proteins
Western blot was used to detect the expression of RHOA, ROCK1, ROCK2, LIMK1 and LIMK2 signaling proteins in lung and kidney tissues after 24 h sacrifice. As shown in Figure 7, the expression of RHOA, ROCK1, ROCK2, LIMK1 and LIMK2 in model group was significantly increased (P < 0.05) at every time point compared with control group and sham group. Compared with the sham group, the expression levels RHOA, ROCK1, ROCK2, LIMK1 and LIMK2 in the lung and kidney tissue of the model group and pantoprazole treatment groups were increased, and the differences were statistically significant (P < 0.05). Pantoprazole treatment significantly inhibited the levels of RHOA/ROCK in three pantoprazole-treated groups in a dose-dependent manner in the LG, MG, and HG groups, indicating that a higher concentration of pantoprazole has a stronger inhibitory effect on the lung and kidney damage (Figure 7).
Figure 7.
Pantoprazole inhibits RHOA/ROCK signaling pathway proteins. Pantoprazole reduces the expression of RHOA, ROCK1, ROCK2, LIMK1 and LIMK2 in lung (A) and kidney (B) tissues after 24 h sacrifice. Quantification assay of the RHOA/ROCK signaling pathway proteins band’s intensity. Error bars indicate ± SD. *P < 0.05, **P < 0.01, ***P < 0.001 vs. Control group; #P < 0.05, ##P < 0.01, ###P < 0.001 vs. model group and ΔP < 0.05, ΔΔP < 0.01, ΔΔΔP < 0.001 vs. sham group.
Flow cytometry to detect apoptosis
The BEAS-2B and HK-2 cells were cultured, and the RhOA overexpression plasmid was constructed and transfected into BEAS-2B and HK-2 cells respectively. The results of western blot showed that the expression of overexpression-RHOA group is much higher than overexpression-NC and control group (Figure 8A, 8C). RT-qPCR assay showed the similar results to those of western blot (Figure 8B, 8D).
Figure 8.

Western blot to show the protein levels of RHOA in BEAS-2B (A) and HK-2 (C) cell after transfect overexpression-RHOA. RHOA mRNA in BEAS-2B (B) and HK-2 (D) was examined by RT-qPCR. Quantification assay of the RHOA proteins band’s intensity. Error bars indicate ± SD. ***P < 0.001 vs. Control group; ###P < 0.001 vs. model group and ΔΔΔP < 0.001 vs. sham group.
Detect cell apoptosis by Flow cytometry, the results showed that significant increase in cell apoptosis after LPS induction. As the dose of Pantoprazole increases, the level of apoptosis in cells gradually decreases. Compared with the LPS (10 ug/ml) + P (100 μmol/l) + overexpression-NC (72 h) group and control group, the apoptosis rate of LPS (10 ug/ml) + P (100 μmol/l) + overexpression-RhOA (72 h) group were increased and the difference were statistically significant. The results indicated that pantoprazole can attenuate the apoptosis of BEAS-2B and HK-2 cells and has a certain protective effect (Figure 9).
Figure 9.
Detect apoptosis rate of BEAS-2B (A, B) and HK-2 (C, D) cells after overexpression-RHOA by flow cytometry. ***P < 0.001 vs. Control group; #P < 0.05, ###P < 0.001 vs. LPS group.
Pantoprazole attenuates LPS-induced expression of inflammatory factors TNF-α, IL-1β, MCP-1 in lung epithelial cells and mesangial cells by inhibiting RHOA/ROCK signaling
Detection of inflammatory factors TNF-α, IL-1β and MCP-1 in cells by ELISA. The levels of TNFα, MCP-1, IL-1β in lung epithelial cells and mesangial cells were significantly higher in the pantoprazole-treated groups (LG and MG group) than the control groups. And HG group are similar to control group. Compared with the LPS (10 ug/ml) + P (100 μmol/l) + overexpression-NC group and control group, the expression of TNFα, MCP-1 and L-1β in LPS (10 ug/ml) + P (100 μmol/l) + overexpression-RhOA group were increased and the difference was statistically significant (Figure 10).
Figure 10.

Pantoprazole attenuates LPS-induced expression of inflammatory factors TNF-α, IL-1β and MCP-1 in BEAS-2B (A) and HK-2 (B) cells by inhibiting RHOA/ROCK signaling. Protein levels of TNF-α, IL-1β and MCP-1 proteins in BEAS-2B (A) and HK-2 (B) cells were determined by Western blotting, respectively. Data were presented as the mean ± SD of three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001 vs. Control group; ##P < 0.01, ###P < 0.001 vs. LPS group.
Expression of signal proteins in lung epithelial cells and mesangial cells
Western blot was used to detect the expression of RHOA, ROCK1, ROCK2, LIMK1 and LIMK2 signaling proteins in lung epithelial cells and mesangial cells. The results showed that significant increase in the expression of signaling proteins after LPS induction. Pantoprazole treatment significantly inhibited the levels of RHOA/ROCK in three pantoprazole-treated groups in a dose-dependent manner in the LG, MC, and HG groups, indicating that a higher concentration of pantoprazole has a stronger inhibitory effect on the lung epithelial cells and mesangial cells. Compared with the LPS (10 ug/ml) + P (100 μmol/l) + overexpression-NC group and control group, the expression of T RHOA, ROCK1, ROCK2, LIMK1 and LIMK2 signaling proteins in LPS (10 ug/ml) + P (100 μmol/l) + overexpression-RhOA group were significant increased (Figure 11).
Figure 11.
Pantoprazole reduces the expression of RHOA, ROCK1, ROCK2, LIMK1 and LIMK2 in BEAS-2B (A) and HK-2 (B) cells. Quantification assay of the RHOA/ROCK signaling pathway proteins band’s intensity. Error bars indicate ± SD. ***P < 0.001 vs. Control group; ##P < 0.01, ###P < 0.001 vs. LPS group.
Discussion
As the leading fatal disease, sepsis has a high incidence and is increasing year by year [14], which seriously threatens human life and health. At present, the mechanism of sepsis is not fully understood, and multiple organ damage can be caused during the pathogenesis. Among them, acute lung and kidney injury is a common complication, and the condition is critical, the clinical treatment effect is not good, and the mortality rate is high [15]. Pantoprazole is an irreversible proton pump inhibitor that reduces gastric acid secretion [9]. Recent studies have shown that pantoprazole also has anti-inflammatory, anti-oxidant, anti-cancer and chemotherapy sensitization effects. Pantoprazole has anti-cancer effects on glioma cells, Pantoprazole mediates apoptosis and inhibits NF-κB signaling to inhibit glioma cell growth and induce cell death [16,17].
In this study, a rat model of sepsis was established using the Rittirsch [12] method. The rats’ hairs are mess and loss of appetite. The results of HE staining showed that the tubular epithelial cells in the kidney tissue of the model group showed swelling, watery degeneration, vacuolar degeneration, tubular dilatation and pulmonary edema. Exfoliated cells were observed, renal interstitial congestion and edema, and glomerulonephritis appeared. At the same time, the Scr and BUN levels in the serum of the model group rose, indicating that AKI occurred in the septic rats, and the model was successfully constructed.
The pathogenesis of AKI caused by sepsis is complicated. With the in-depth study of the pathogenesis of AKI caused by sepsis, it is believed that the apoptosis of renal tubular epithelial cells may play a key role in AKI caused by sepsis [18,19]. The results of this experiment show that the apoptosis of renal tissue in AKI group caused by sepsis was significantly higher than that in sham operation group and the renal tissue apoptosis rate was positively correlated with renal injury markers IL-18 and KIM-1, indicating sepsis. The results showed that compared with the model group and the saline group, the expression levels of pulmonary surfactant protein A(SPA) and D(SPD) were increased in the pantoprazole group, and the levels of Scr, BUN and ScysC in serum were decreased. The level of serum damage markers KIM-1, IL-18 is decreased. The results showed that the renal tissue damage of the pantoprazole group was alleviated, the cell abnormality was reduced, and the interstitial congestion and edema was alleviated, indicating that the acute lung and kidney injury of sepsis can be effectively alleviated by the intervention of pantoprazole. When the apoptosis of renal tissue increases, the kidney damage is aggravated. The reason for considering the increase of renal cell apoptosis may lead to renal dysfunction. For example, apoptosis of renal tubular epithelial cells may lead to the return of primary urine, tubular formation, and kidney. Apoptosis of vascular endothelial cells leads to changes in the renal vascular bed, promoting inflammatory cell infiltration and microthrombosis [20].
IL-1β and TNF-α are inflammatory factors produced by mononuclear macrophages, which are closely related to the level of the inflammatory response in the body and are indicators of the degree of inflammation in the body [21,22]. The results of this study showed that the levels of IL-1β and TNF-α in serum and kidney tissues of the model group, control water group and pantoprazole group were higher than those of the sham operation group, indicating that the AKI rats in sepsis Oxidative stress and inflammatory response occurred in the body and kidney tissues. At the same time, the results showed that IL-1β and TNF-α levels in serum and kidney tissues of pantoprazole group compared with model group and saline group. Decreased, indicating that after the administration of pantoprazole, the levels of oxidative stress and inflammatory reaction in the body and kidney tissues of septic AKI rats decreased, suggesting that pantoprazole may exert lung and kidney protection by inhibiting oxidative stress and inflammatory response. The results showed that compared with the sham operation group, the apoptosis rate of the model group, the control group and the pantoprazole group increased, and the apoptosis rate of the pantoprazole group decreased compared with the model group and the control group. It indicated that exogenous administration of pantoprazole can effectively reduce epithelial cell apoptosis in renal tissue of septic AKI rats. Bcl-2 and Bax are important members of the Bcl-2 family. Bcl-2 plays an important role in inhibiting apoptosis, while Bax promotes apoptosis. Bcl-2/Bax balance can lead to a series of levels [23]. The Caspase-3 molecule, which ultimately leads to the activation of apoptosis, initiates apoptosis and induces apoptosis in cells [24]. This study shows that the anti-apoptotic protein Bcl-2 in the kidney tissue of sepsis AKI rats. The expression is down-regulated, and the proapoptotic protein Bax is up-regulated, which activates Caspase-3 to cause apoptosis. After exogenous administration of pantoprazole, this process is reversed, thereby reducing the apoptosis of renal tubular epithelial cells. In summary, pantoprazole can effectively improve renal function in rats with sepsis AKI and play a protective role in the kidney. The mechanism may be related to the reduction of oxidative stress, inflammatory response, inhibition of apoptosis-related gene activation, thereby reducing renal tissue. Studies have shown that the RohA/ROCK pathway regulates downstream LIM kinase phosphorylation cleaved proteins, which phosphorylate F-actin filaments, while non-phosphorylated shredded proteins enable F-actin Protein filaments depolymerize.
The Rho family protein is a small G protein with GTP enzymatic activity that is widely present in all eukaryotic tissues and plays an important role in cell proliferation, apoptosis, and gene expression [25,26]. LIMK is a class of kinases involved in the regulation of the actin skeleton, which phosphorylates Cofilin, an actin-depolymerizing protein, which is inhibited by phosphorylation of LIMK. Phosphorylation of LIMK by ROCK enhances its inhibition of Cofilin-mediated actin skeleton depolymerization and increases the number of actin skeletons. ROCK also activates and regulates other downstream substrates, such as addiction, ERM protein complex, GFAP, and NF-L [27], which play different roles in cells. The result of this study showed that pantoprazole treatment significantly inhibited the levels of RHOA/ROCK in three pantoprazole-treated groups in a dose-dependent manner in the LG, MC, and HG groups, indicating that a higher concentration of pantoprazole has a stronger inhibitory effect on the lung and kidney damage.
Conclusion
To conclude, it is the first report to investigate the effect of pantoprazole on acute lung and kidney injury with sepsis. Pantoprazole can improve the symptoms of acute lung and kidney injury in septic rats, which suggested that pantoprazole might be used to guide the treatment of sepsis.
Disclosure of conflict of interest
None.
References
- 1.Seckel MA, Ahrens T. Challenges in sepsis care: new sepsis definitions and fluid resuscitation beyond the central venous pressure. Crit Care Nurs Clin North Am. 2016;28:513–532. doi: 10.1016/j.cnc.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 2.Olvera L, Dutra D. Early recognition and management of maternal sepsis. Nurs Womens Health. 2016;20:182–195. doi: 10.1016/j.nwh.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Han IM, Yoon CY, Shin DH, Kee YK, Han SG, Kwon YE, Park KS, Lee MJ, Oh HJ, Park JT, Han SH, Kang SW, Yoo TH. Delta neutrophil index is an independent predictor of mortality in septic acute kidney injury patients treated with continuous renal replacement therapy. BMC Nephrol. 2017;18:94. doi: 10.1186/s12882-017-0507-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shih CJ, Chen YT, Ou SM, Li SY, Chen TJ, Wang SJ. Proton pump inhibitor use represents an independent risk factor for myocardial infarction. Int J Cardiol. 2014;177:292–297. doi: 10.1016/j.ijcard.2014.09.036. [DOI] [PubMed] [Google Scholar]
- 5.Wedemeyer RS, Blume H. Pharmacokinetic drug interaction profiles of proton pump inhibitors: an update. Drug Saf. 2014;37:201–211. doi: 10.1007/s40264-014-0144-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zeng X, Liu L, Zheng M, Sun H, Xiao J, Lu T, Huang G, Chen P, Zhang J, Zhu F, Li H, Duan Q. Pantoprazole, an FDA-approved proton-pump inhibitor, suppresses colorectal cancer growth by targeting T-cell-originated protein kinase. Oncotarget. 2016;7:22460–22473. doi: 10.18632/oncotarget.7984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Udelnow A, Kreyes A, Ellinger S, Landfester K, Walther P, Klapperstueck T, Wohlrab J, Henne-Bruns D, Knippschild U, Wurl P. Omeprazole inhibits proliferation and modulates autophagy in pancreatic cancer cells. PLoS One. 2011;6:e20143. doi: 10.1371/journal.pone.0020143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geeviman K, Babu D, Prakash Babu P. Pantoprazole induces mitochondrial apoptosis and attenuates nf-kappab signaling in glioma cells. Cell Mol Neurobiol. 2018;38:1491–1504. doi: 10.1007/s10571-018-0623-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen M, Huang SL, Zhang XQ, Zhang B, Zhu H, Yang VW, Zou XP. Reversal effects of pantoprazole on multidrug resistance in human gastric adenocarcinoma cells by down-regulating the V-ATPases/mTOR/HIF-1alpha/P-gp and MRP1 signaling pathway in vitro and in vivo. J Cell Biochem. 2012;113:2474–2487. doi: 10.1002/jcb.24122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kimura H. Hydrogen sulfide and polysulfides as signaling molecules. Proc Jpn Acad Ser B Phys Biol Sci. 2015;91:131–159. doi: 10.2183/pjab.91.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feliers D, Lee HJ, Kasinath BS. Hydrogen sulfide in renal physiology and disease. Antioxid Redox Signal. 2016;25:720–731. doi: 10.1089/ars.2015.6596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rittirsch D, Huber-Lang MS, Flierl MA, Ward PA. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat Protoc. 2009;4:31–36. doi: 10.1038/nprot.2008.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang C, Guo D, Wang Q, You S, Qiao Z, Liu Y, Dai H, Tang H. Aliskiren targets multiple systems to alleviate cancer cachexia. Oncol Rep. 2016;36:3014–3022. doi: 10.3892/or.2016.5118. [DOI] [PubMed] [Google Scholar]
- 14.Henriksen DP, Laursen CB, Jensen TG, Hallas J, Pedersen C, Lassen AT. Incidence rate of community-acquired sepsis among hospitalized acute medical patients-a population-based survey. Crit Care Med. 2015;43:13–21. doi: 10.1097/CCM.0000000000000611. [DOI] [PubMed] [Google Scholar]
- 15.Dupuis C, Sonneville R, Adrie C, Gros A, Darmon M, Bouadma L, Timsit JF. Impact of transfusion on patients with sepsis admitted in intensive care unit: a systematic review and meta-analysis. Ann Intensive Care. 2017;7:5. doi: 10.1186/s13613-016-0226-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang YF, Chen YT, Luo JC, Chen TJ, Wu JC, Wang SJ. Proton-pump inhibitor use and the risk of first-time ischemic stroke in the general population: a nationwide population-based study. Am J Gastroenterol. 2017;112:1084–1093. doi: 10.1038/ajg.2017.101. [DOI] [PubMed] [Google Scholar]
- 17.Wang WJ, Sun AN, Guo F. Noncanonical NF-kappaB pathway and hematological malignancies. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2010;18:1069–1073. [PubMed] [Google Scholar]
- 18.Bagshaw SM, George C, Bellomo R ANZICS Database Management Committee. Early acute kidney injury and sepsis: a multicentre evaluation. Crit Care. 2008;12:R47. doi: 10.1186/cc6863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ostermann M, Chang RW. Acute kidney injury in the intensive care unit according to RIFLE. Crit Care Med. 2007;35:1837–1843. doi: 10.1097/01.CCM.0000277041.13090.0A. [DOI] [PubMed] [Google Scholar]
- 20.Lerolle N, Nochy D, Guerot E, Bruneval P, Fagon JY, Diehl JL, Hill G. Histopathology of septic shock induced acute kidney injury: apoptosis and leukocytic infiltration. Intensive Care Med. 2010;36:471–478. doi: 10.1007/s00134-009-1723-x. [DOI] [PubMed] [Google Scholar]
- 21.Figueras M, Busquets S, Carbo N, Almendro V, Argiles JM, Lopez-Soriano FJ. Cancer cachexia results in an increase in TNF-alpha receptor gene expression in both skeletal muscle and adipose tissue. Int J Oncol. 2005;27:855–860. [PubMed] [Google Scholar]
- 22.Himmelfarb J, McMonagle E, Freedman S, Klenzak J, McMenamin E, Le P, Pupim LB, Ikizler TA, The PG. Oxidative stress is increased in critically ill patients with acute renal failure. J Am Soc Nephrol. 2004;15:2449–2456. doi: 10.1097/01.ASN.0000138232.68452.3B. [DOI] [PubMed] [Google Scholar]
- 23.Sutton TA. Alteration of microvascular permeability in acute kidney injury. Microvasc Res. 2009;77:4–7. doi: 10.1016/j.mvr.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mahadevaiah S, Robinson KG, Kharkar PM, Kiick KL, Akins RE. Decreasing matrix modulus of PEG hydrogels induces a vascular phenotype in human cord blood stem cells. Biomaterials. 2015;62:24–34. doi: 10.1016/j.biomaterials.2015.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hasan Z, Palani K, Zhang S, Lepsenyi M, Hwaiz R, Rahman M, Syk I, Jeppsson B, Thorlacius H. Rho kinase regulates induction of T-cell immune dysfunction in abdominal sepsis. Infect Immun. 2013;81:2499–2506. doi: 10.1128/IAI.00126-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loirand G, Sauzeau V, Pacaud P. Small G proteins in the cardiovascular system: physiological and pathological aspects. Physiol Rev. 2013;93:1659–1720. doi: 10.1152/physrev.00021.2012. [DOI] [PubMed] [Google Scholar]
- 27.Nunes KP, Rigsby CS, Webb RC. RhoA/Rho-kinase and vascular diseases: what is the link? Cell Mol Life Sci. 2010;67:3823–3836. doi: 10.1007/s00018-010-0460-1. [DOI] [PMC free article] [PubMed] [Google Scholar]







