Abstract
Stroke is a common cause of physical disability. Biomarkers have been used to predict prognosis in ischemic stroke, but studies linking biomarkers to physical recovery from ischemic stroke have not been systematically evaluated since 2011. The purpose of this paper is to report the findings of a systematic review of the intervening literature to identify potential predictive biomarkers for recovery of physical function following ischemic stroke. The PubMed, Embase, and CINAHL databases were searched for studies reported between January 1, 2011, and September 18, 2018. Search criteria were adult ischemic stroke patients, blood sample collection within 24 ± 6 hrs of stroke onset, and outcome measures, including physical function. Identified from 18 studies and representing four biological classifications, 34 biomarkers were significantly associated with physical recovery after ischemic stroke: (1) immune response (15, 44%); (2) lipids/metabolism (4, 12%); (3) neuronal function (4, 12%); and (4) blood vessel/circulation (11, 32%). Of the predictive biomarkers associated with 1-month recovery, 60% (6 of 10) was classified into blood vessel/circulation; 54% (14 of 26) of the biomarkers associated with 3-6 month physical recovery involved the immune response. Blood biomarkers might provide useful information to improve the prediction of physical outcome after ischemic stroke. The data suggest that biomarkers from four biological classifications may predict physical recovery in patients after ischemic stroke.
Keywords: Biomarker, ischemic stroke, recovery of function
Introduction
Stroke is the 5th leading cause of death in the U.S. [1], with an annual incidence of approximately 795,000 cases. Ischemic stroke accounts for 87% of all strokes [1]. In addition, stroke is the most common neurological disease in the adult population worldwide, and the third leading cause of chronic disability [2]. Up to 74% of stroke survivors are dependent in activities of daily living [3].
Motor impairment, sensory dysfunction, and dysphasia are common manifestations of stroke. Up to 30% of stroke survivors in the U.S. suffer permanent disability, and 20% of survivors require inpatient rehabilitation within 3 months after stroke [4]. Improvements in acute stroke care have reduced stroke-related mortality over the past two decades; however, the increased survival rate leaves many survivors with severe disability, placing a tremendous burden on the healthcare system and caregivers [5].
Prior studies indicated that up to 91% of physical recovery occurs within the first 3 months after stroke [6]. Physical recovery includes motor function, sensation, language, and swallowing ability [7]. Because these physical functions often are interdependent, all are included in the assessment of recovery after stroke, and no single measure fully describes disability or functional outcome from stroke.
The most widely used scales for stroke outcomes are the National Institutes of Health Stroke Scale (NIHSS), the modified Rankin Scale (mRS), and the modified Barthel Index (mBI) [8]. The NIHSS is used to quantify stroke severity based on language, motor function, sensory loss, consciousness, visual fields, extraocular movements, coordination, neglect, and speech [9]. Global disability, with a focus on mobility, is assessed with the mRS [10]. Functional outcomes and daily life activities are measured with the mBI [11]. A statistically significant inverse correlation has been demonstrated between the mRS and the mBI in the post-stroke population [12]; the more disability on the mRS, the less independent the patient scores on the mBI. Furthermore, admission NIHSS score has been found to be positively correlated with mRS score over time [13].
A biomarker is a molecule measured in blood, urine, cerebrospinal fluid, or tissue, or an imaging test, such as magnetic resonance imaging or computed tomography. Blood biomarkers have been commonly used to provide prognostic information following ischemic stroke. For example, levels of brain natriuretic peptide (BNP), D-Dimer, matrix metallopeptidase-9 (MMP-9), and S100B in blood were positively correlated with mortality at 4 months post-stroke [14]. Cardiac markers, such as BNP and troponin, have been shown to have a consistent association with poor outcome after ischemic stroke [15]. Moreover, the most recent systematic review of blood biomarkers for acute stroke found that levels of glucose, glutamate, and fibrinogen at stroke onset were associated with poor prognosis in ischemic stroke patients [16].
Emerging biomarkers from new discoveries in the field of stroke research may help predict stroke outcome and recovery. As the Hasan et al. [16] publication is the most recent and relevant systematic review, the purpose of this review was to synthesize the literature published after 2011 on the relationship between biomarkers and physical recovery from ischemic stroke. Ultimately, biomarkers may provide prognostic information to guide clinicians with patient-centered rehabilitation efforts.
Methods
Search strategy
PubMed, Embase, and CINAHL databases were searched for studies that examined blood biomarkers and functional outcome in patients with ischemic stroke. The hierarchical search strategies and keywords were (1) biomarker, (2) ischemic stroke, (3) physical recovery, and (4) adult (Supplemental Table 1). Duplications were removed from the list, and titles and abstracts of articles retrieved were reviewed for eligibility. All relevant articles from reference lists of each reviewed paper were identified. After screening the abstracts, final eligibility was determined based on the full content of the articles.
Inclusion and exclusion criteria
Inclusion criteria for the selection of articles to include in the review were: (1) primary source of quantitative study in a peer-reviewed journal published in English between January 2011 and September 2018; (2) patients ≥ age 18 years diagnosed with ischemic stroke; (3) biomarkers measured from blood samples within 24 ± 6 hrs of stroke onset; (4) measure(s) of physical outcome (e.g., NIHSS, mRS and/or mBI); and (5) reported the association with physical outcome for each biomarker. Note that the short window, within 24 ± 6 hrs for blood sampling relative to stroke onset, maximizes the predictive potential of the biomarkers to provide time-sensitive prognostic information in the early clinical evaluation of ischemic stroke. Unpublished theses, dissertations, and conference proceedings were excluded.
Study quality appraisal
A code sheet was used to extract information from each article. The information included author(s), year of publication, subjects, time frame of blood draw, biomarker(s), measurement of physical outcomes, and findings [17]. Where more than one cohort was examined within a study, the results for each cohort were extracted separately. Quality of the study was assessed using the modified questionnaire implemented by Whiteley et al. [15] for the stroke population (Supplemental Table 2). These quality components correspond to the sections on study design and assay methods sections of the REporting recommendations for tumor MARKers prognostic studies (REMARK) [18].
Results
Based on the search keywords, 333 studies were identified from PubMed, Embase, and CINAHL. Three more studies were found through Google Scholar and reference lists of relevant articles. The search procedure (Figure 1) followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram [19]. After screening the title and abstract of each article, 98 full-text studies were assessed for eligibility; 18 studies met the eligibility criteria and were included in the systematic review.
Figure 1.
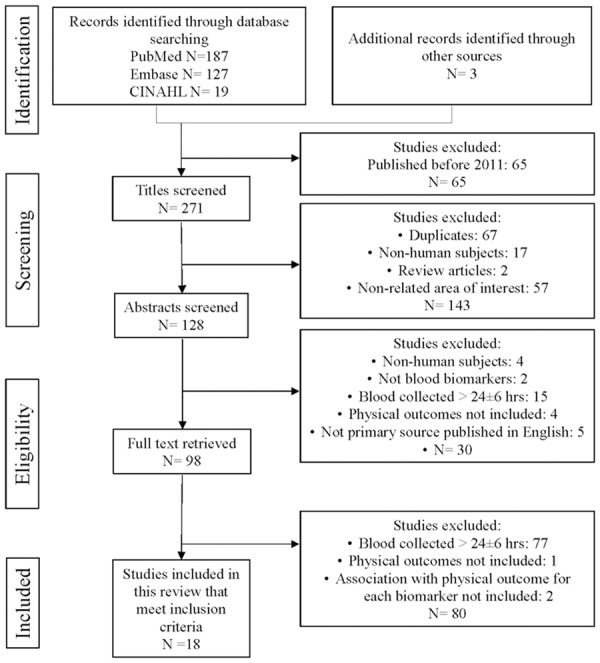
Flow diagram of the literature search. Adapted from “The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration” by Liberati, A. and Altman, D. G., 2009, British Medical Journal, 339, b2700.
Characteristics of the studies
The articles are listed in ascending chronological order in Supplemental Table 3. Studies in this review were published between 2011 and 2018. Sample sizes varied from 50 to 783 patients. The proportion of male patients was higher than females (54.3% male vs. 45.7% female). The overall means of patient ages varied from 59 to 73 years. All studies enrolled patients with ischemic stroke, and 5 (28%) included healthy controls as clinical comparisons. Only 2 (11%) of the studies selected patients with first-episode ischemic stroke; the others did not explicitly address history of stroke. Studies were conducted in Spain (7, 39%), Germany (3, 17%), Japan (2, 11%), and 1 (6%) each in the Netherlands, Turkey, the United Kingdom, Poland, the United States, and Italy. Of 73 molecules tested in the 18 studies, 34 (47%) were significantly associated with physical recovery after ischemic stroke.
Methodological assessment
The percentages of reports reviewed that met the modified REMARK criteria for methodological quality are shown in Supplemental Table 4 and Figure 2. All study authors defined clinical outcome and provided the characteristics of the study population; 94% used a prospective design; 94% developed a logistic regression model and reported such adjustment variables as age and stroke severity; 72% defined enrollment period; and 67% provided information on the measurement of biomarkers. Few authors reported sample size calculation (6%), and blinded biomarker measurement (33%).
Figure 2.
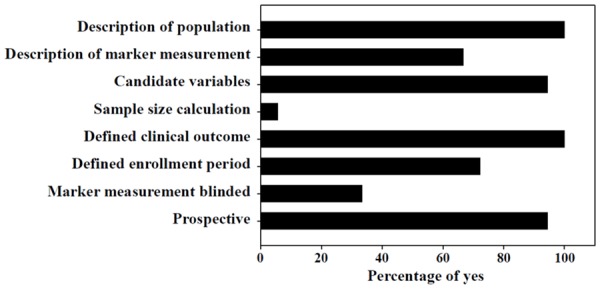
Study quality of the 18 articles in the systematic review assessed by the modified REMARK questionnaire (Supplemental Table 4).
Physical function was defined as an individual’s capacity to perform daily personal living tasks like eating, walking, and bathing [20]. A number of outcome measures following ischemic stroke were reported (Supplemental Table 3). Various instruments (NIHSS, mRS, or mBI) were used and outcomes were measured at different time points after stroke (admission, 1, 3, or 6 months). All studies presented NIHSS stroke severity at admission, but only one study reported the 3-month NIHSS score. Therefore, in this review, NIHSS score was not used to define physical recovery from ischemic stroke. In 15 studies (83%), physical outcomes at 3 months were evaluated by mRS or mBI; 2 (11%) and 1 (6%) of the studies reported physical outcomes at 1 and 6 months after stroke, respectively. Poor outcome was defined according to definitions used in the reviewed articles: mRS ≥ 3 in 13 (72%) studies, mRS ≥ 2 in 2 (11%) studies and mBI < 15 in 1 (6%) study; two studies did not provide cut-off scores for the mRS. Likewise, for this review, we defined physical recovery as used by the authors of the reviewed articles.
Biomarker findings
Biomarkers related to biological functions
The 34 biomarkers that were significantly associated with physical outcome after ischemic stroke were divided into four categories based on biomarker biological function (Table 1). In terms of immune response, patients with lower levels of C-C motif chemokine 11 (CCL11), interleukin (IL)-1β and IL-8, and monocyte chemoattractant protein 1 (MCP1) and higher levels of adiponectin, IL-1Ra, IL-6, IL-10, IL-12, copeptin, C-reactive protein (CRP), growth differentiation factor-15 (GDF-15), osteopontin, tumor necrosis factor-alpha (TNFα), and white blood cell (WBC) count at stroke onset showed worse physical recovery (poor outcomes at 1, 3, or 6 months post-stroke). Notably, patients with poor outcome exhibited more than twofold higher levels of copeptin, IL-6, and TNFα compared to those with good outcome.
Table 1.
Categories of biomarkers significantly associated with physical outcome after ischemic stroke
| Category | Biomarker | Article Reference Numbers |
|---|---|---|
| Immune response | Adiponectin, CCL11, Copeptin, CRP, GDF-15, IL-1β, IL-1Ra, IL-10, IL-12, IL-6, IL-8, MCP1, Osteopontin, TNFα, WBC | [28-31,37,49,54-57] |
| Lipids/Metabolism | Cholesterol, Glucose, HDL-C, LDL-C | [30,31,37-39,49,58] |
| Neuronal function | BDNF, Glutamate, GOT, GPT | [38,59] |
| Blood vessel/Circulation | CrCl, D-Dimer, eGFR, Endostatin, Fibrinogen, Fibronectin, Hematocrit, Hemoglobin, MR-proANP, proMMP-10, Troponin | [29-31,38,49,60] |
BDNF = Brain-derived neurotrophic factor; CCL11 = C-C motif chemokine 11; CrCl = Creatinine Clearance; CRP = C-reactive protein; eGFR = Estimated glomerular filtration rate; GDF-15 = Growth Differentiation Factor-15; GOT = Glutamic oxaloacetic transaminase; GPT = Glutamic pyruvic transaminase; HDL-C = High-density lipoprotein cholesterol; IL = Interleukin; LDL-C = Low-density lipoprotein cholesterol; MCP1 = Monocyte chemoattractant protein 1; MMP = Matrix Metalloproteinase; MR-proANP = Midregional proatrial natriuretic peptide; TNFα = Tumor necrosis factor-alpha; WBC = White blood cell.
For lipids/metabolism biomarkers, decreased high-density lipoprotein cholesterol (HDL-C) and increased cholesterol, low-density lipoprotein cholesterol (LDL-C), and glucose at stroke onset were significantly associated with worse functional outcome at 1, 3, or 6 months post-stroke. With respect to biomarkers of neuronal function, patients with ischemic stroke who had higher levels of glutamate and lower levels of glutamic oxaloacetic transaminase (GOT), glutamic pyruvic transaminase (GPT), and brain-derived neurotrophic factor (BDNF) tended to have worse outcomes at 1, 3, or 6 months after stroke. Patients with poor outcome at 1, 3, or 6 months post-stroke showed increased levels of the blood vessel/circulation biomarkers D-Dimer, endostatin, fibronectin, pro-matrix metalloproteinase-10 (proMMP-10), troponin, and midregional proatrial natriuretic peptide (MR-proANP) and decreased levels of estimated glomerular filtration rate (eGFR), creatinine clearance (CrCl), hematocrit, and hemoglobin at stroke onset. Furthermore, elevated levels of fibrinogen at 1 and 3 months after stroke were associated with poor outcome.
Biomarkers related to physical recovery
Among 34 blood biomarkers that were significantly associated with physical outcome after ischemic stroke (Table 2), 10, 26, and 2 biomarkers, respectively, showed a significant association with physical outcome at 1, 3, or 6 months following ischemic stroke: 60% (6 of 10) of biomarkers predictive of physical outcome at 1 month were classified in the blood vessel/circulation category; 54% (14 of 26) of biomarkers that predicted recovery between 3 and 6 months post-stroke were related to the immune response. Elevated levels of CRP and fibrinogen were found to be significant predictors of poor prognosis at 1 and 3 months post-stroke.
Table 2.
Biomarkers significantly associated with physical outcome after ischemic stroke by time after stroke
| Time after stroke | Biomarker associated with physical outcome | Article Reference Numbers | |||
|---|---|---|---|---|---|
|
| |||||
| Immune response | Lipids/Metabolism | Neuronal function | Blood vessel/Circulation | ||
| 1 month | CRP, WBC | Cholesterol, LDL-C | CrCl, D-Dimer, Fibrinogen, Hematocrit, Hemoglobin, Troponin | [49,61] | |
| 3 months | Adiponectin, CCL11, Copeptin, CRP, GDF-15, IL-1β, IL-1Ra, IL-6, IL-8, IL-10, IL-12, MCP1, Osteopontin, TNFα | Glucose, HDL-C | BDNF, Glutamate, GOT, GPT | Endostatin, eGFR, Fibrinogen, Fibronectin, MR-proANP, proMMP-10 | [28-31,37,38,54-60] |
| 6 months | CCL11 | Glucose | [39,56] | ||
BDNF = Brain-derived neurotrophic factor; CCL11 = C-C motif chemokine 11; CrCl = Creatinine Clearance; CRP = C-reactive protein; eGFR = Estimated glomerular filtration rate; GDF-15 = Growth Differentiation Factor-15; GOT = Glutamic oxaloacetic transaminase; GPT = Glutamic pyruvic transaminase; HDL-C = High-density lipoprotein cholesterol; IL = Interleukin; LDL-C = Low-density lipoprotein cholesterol; MCP1 = Monocyte chemoattractant protein 1; MMP = Matrix Metalloproteinase; MR-proANP = Midregional proatrial natriuretic peptide; TNFα = Tumor necrosis factor-alpha; WBC = White blood cell.
Low level of CCL11 and high level of blood glucose were significantly associated with poor outcome at 3 and 6 months after ischemic stroke. Nine (50%) of the reviewed studies reported biomarkers and physical outcomes in patients who had received tissue plasminogen activator (tPA) intravenously, the gold standard treatment of acute ischemic stroke [21]. In this review, patients who received tPA treatment with poor physical outcomes compared to those with good outcomes had higher levels of IL-6, TNFα, osteopontin, fibronectin, endostatin, proMMP-10, CRP, IL-1Ra, IL-10, and IL-12; and lower levels of HDL, BDNF, IL-1β, IL-8, and MCP1.
Discussion
Of 73 putative biomarkers tested, 34 were found in this review to be statistically significantly associated with physical recovery after ischemic stroke. The biomarkers showed an association with poor outcome by different instruments (mRS or mBI) and measurement time points (admission, 1, 3, or 6 months after stroke). To provide a functional view of the biomarkers, we divided the 34 into four categories based on biological function of the individual biomarker: immune response, lipids/metabolism, neuronal function, and blood vessel/circulation.
Immune response
Brain injury from ischemia is exacerbated by the inflammatory response to cell injury and necrosis [22,23]. To repair tissue damage, inflammatory cytokines and chemokines attract immune cells from the circulation into the brain [24,25], and over-activated immune cells adversely augment brain damage leading to an unfavorable recovery [26,27]. We found that 54% of the 26 biomarkers that predicted long-term (3-6 month) stroke recovery are related to immune response. Consistently, IL-6 [28,29], TNFα [28,29], and CRP [28,30,31] were found to be robust predictors of long-term functional outcome in ischemic stroke. Therefore, higher levels of immune-related biomarkers after ischemic stroke may reflect worse physical recovery.
Lipids/metabolism
We identified four lipids/metabolism biomarkers (high glucose, cholesterol, HDL-C, and low LDL-C) that were significantly associated with poor outcome after ischemic stroke. High blood glucose, cholesterol, and LDL-C and low HDL-C levels have been associated with increased risk for atherosclerosis and stroke [32-34]. LDL-C and hemoglobin A1c (HbA1c) levels guide recommendations in the American Heart Association/American Stroke Association stroke prevention guidelines [35]. A prior systematic review indicated that low HDL-C level is associated with worse physical outcome after ischemic stroke [36]. Five of the articles [30,31,37-39] reviewed in this paper indicated that hyperglycemia was associated with poor outcome in patients with ischemic stroke, which is consistent with previous studies [16,40,41]. Hasan et al. [16] reported that admission glucose level might predict poor outcome following tPA treatment; however, the majority of biomarkers we found associated with physical recovery after treatment with tPA were related to immune response and blood vessel/circulation, not to glucose or other biomarkers in the lipids/metabolism category.
Neuronal function
Although different brain regions have different thresholds for ischemic cell damage, neurons are the most sensitive to hypoxia [42]. Glutamate is the major excitatory neurotransmitter in the brain that mediates the signal of neuronal degeneration following ischemic stroke [43,44]. Elevated levels of glutamate, with decreased GOT and GPT (enzymes to decrease the level of glutamate in peripheral blood), may induce neuronal apoptosis [22,45]. Higher levels of glutamate, and lower levels of GOT, GPT, and BDNF were associated with less favorable physical recovery after ischemic stroke in this review. Hasan et al. [16] reported similar findings that elevated glutamate may indicate progressive stroke.
Blood vessel/circulation
Ischemia disrupts the mitochondrial membrane potential, which generates excessive reactive oxygen species (ROS) in endothelial cells of cerebral blood vessels [46]. ROS damages mitochondrial DNA, activates the inflammatory response, induces secretion of MMPs, and leads to endothelial cell swelling and death [47]. This process triggers breakdown of the blood-brain barrier and thus increases the risk of hemorrhagic transformation (i.e., bleeding into an area of ischemic brain when cerebral blood flow is restored to damaged vasculature), which adversely affects stroke outcome.
Fibrinogen, an acute phase protein, is involved in platelet activation, coagulation, and hemostasis [48]. Our results support earlier findings that elevated fibrinogen level is associated with poor functional outcome at 1 [49] and 3 months [38] after ischemic stroke. Interestingly, a prior study indicated that an early reduction in fibrinogen increases the risk of intracerebral hemorrhage after tPA treatment in ischemic stroke patients [50]. Therefore, the relationship between fibrinogen and physical outcome among ischemic stroke patients, with or without tPA intervention, merits further research.
Strengths and weaknesses of the research
A strength of this systematic review is the criterion for timing of blood collection for biomarker determination. The short window-within 24 ± 6 hrs - for blood sampling relative to stroke onset maximizes the predictive potential of the biomarkers to provide time-sensitive prognostic information in the early clinical evaluation of ischemic stroke. Moreover, because stroke recovery is a chronic process, this review addressed potential prognostic prediction of physical recovery at 1, 3, and 6 months after ischemic stroke. Categorization of predictive biomarkers into four broad categories based on biological function may further inform our understanding of the clinicopathology of stroke. On the other hand, the short window for blood collection precluded biomarkers that are expressed in the later phases of ischemic stroke, which may have prognostic importance for physical recovery. Congruent with the prior systematic review [16], high levels of glucose, glutamate, and fibrinogen within 24 ± 6 hrs of stroke onset were repeatedly found to be associated with poor outcome after ischemic stroke.
The review is limited by the information that could not be accounted for because it was either not controlled or not reported in the primary source articles. Biomarker expression typically follows a circadian rhythm. For example, expression of TNFα, IL-1β, and IL-6 is known to peak in the evening [51], whereas copeptin level remains unchanged throughout the 24-hour day [52]. Unknown biomarker expression pattern and/or inconsistency in timing of blood collection within and across studies could have adversely affected the findings. Such issues should be addressed in future prospective studies on biomarker expression after ischemic stroke. Another limitation is the small sample size in some of the eligible studies, which inherently compromises the statistical power of the relationships between blood biomarkers and physical recovery from ischemic stroke.
Implications for clinical practice and future research
The biomarkers we found in the acute period may provide useful insights into prognosis and mechanism(s) of action. These findings can facilitate biomarker-based care in patients with ischemic stroke, and thus match patients with appropriate intervention. A robust number and types of biomarkers have been and continue to be investigated in patients with ischemic stroke. Biomarkers show promise, especially in the prognosis of functional recovery and evaluation of therapeutic response, but the current application of their usefulness in practice is elusive without further research evidence from controlled studies. Future research is expected to align timing of putative predictive biomarker levels with phases of physical recovery; the timing may well correlate with the biological classification of the biomarker and suggest different pathways that are mechanistically different for acute and chronic stroke recovery. For instance, troponin, a blood vessel/circulation marker, indicates myocardial damage in acute coronary syndromes; patients with higher levels of troponin on admission or a peak of troponin measured within 48 hours of a myocardial infarction are likely to have a more difficult and prolonged recovery than patients with lower troponin levels [53].
Prior studies have shown a circadian rhythm influence on cytokine expression [51,52]. Because 44% of the identified biomarkers in this review are related to immune response; future studies should control the time of day the blood is collected for biomarker determination. Finally, the time of the blood sample collection was constrained to within 24 ± 6 hrs of stroke onset in this review. These early markers may be used in a predictive way to identify subjects at high risk for poor recovery beyond the discharge NIHSS and mRS, so they could be targeted for more aggressive interventions, Additionally, future investigations may extend the time of blood collection to 3 days, 7 days, or even 3 months after stroke to help understand how molecules released in the later response phase correlate with physical outcome after ischemic stroke.
Summary/Conclusions
A systematic review of biomarkers for prediction of stroke recovery has not been updated since 2011. In the present review, 34 blood biomarkers were significantly associated with physical outcome after ischemic stroke. These biomarkers fall into the biological classifications of immune response, lipids/metabolism, neuronal function, and blood vessel/circulation. The majority of biomarkers appears to predict physical recovery at 3 months following ischemic stroke, with fewer biomarkers predictive of recovery at 1 and 6 months.
Acknowledgements
The authors express gratitude to Marcus Spann and Amy Sisson, the Texas Medical Center Library liaisons to the UTHealth Cizik School of Nursing, for their help with database searches. YJL was funded by the American Heart Association (Summer 2018 Predoctoral Fellowship, 18PRE34060017).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, de Ferranti SD, Ferguson JF, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Lutsey PL, Mackey JS, Matchar DB, Matsushita K, Mussolino ME, Nasir K, O’Flaherty M, Palaniappan LP, Pandey A, Pandey DK, Reeves MJ, Ritchey MD, Rodriguez CJ, Roth GA, Rosamond WD, Sampson UKA, Satou GM, Shah SH, Spartano NL, Tirschwell DL, Tsao CW, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. American heart association council on epidemiology, prevention statistics committee and stroke statistics subcommittee. Heart disease and stroke statistics-2018 update: a report from the American heart association. Circulation. 2018;137:e67–e492. doi: 10.1161/CIR.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 2.Feigin VL, Norrving B, Mensah GA. Global burden of stroke. Circ Res. 2017;120:439–448. doi: 10.1161/CIRCRESAHA.116.308413. [DOI] [PubMed] [Google Scholar]
- 3.Miller EL, Murray L, Richards L, Zorowitz RD, Bakas T, Clark P, Billinger SA American Heart Association Council on Cardiovascular Nursing and the StrokeCouncil. Comprehensive overview of nursing and interdisciplinary rehabilitation care of the stroke patient: a scientific statement from the American heart association. Stroke. 2010;41:2402–2448. doi: 10.1161/STR.0b013e3181e7512b. [DOI] [PubMed] [Google Scholar]
- 4.Creutzfeldt CJ, Holloway RG, Walker M. Symptomatic and palliative care for stroke survivors. J Gen Intern Med. 2012;27:853–860. doi: 10.1007/s11606-011-1966-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mu F, Hurley D, Betts KA, Messali AJ, Paschoalin M, Kelley C, Wu EQ. Real-world costs of ischemic stroke by discharge status. Curr Med Res Opin. 2017;33:371–378. doi: 10.1080/03007995.2016.1257979. [DOI] [PubMed] [Google Scholar]
- 6.Lee KB, Lim SH, Kim KH, Kim KJ, Kim YR, Chang WN, Yeom JW, Kim YD, Hwang BY. Six-month functional recovery of stroke patients: a multi-time-point study. Int J Rehabil Res. 2015;38:173–80. doi: 10.1097/MRR.0000000000000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harvey RL. Predictors of functional outcome following stroke. Clin Interv Aging. 2016;11:985–95. doi: 10.2147/CIA.S111637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harrison JK, McArthur KS, Quinn TJ. Assessment scales in stroke: clinimetric and clinical considerations. Clin Interv Aging. 2013;8:201–11. doi: 10.2147/CIA.S32405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adams HP Jr, Davis PH, Leira EC, Chang KC, Bendixen BH, Clarke WR, Woolson RF, Hansen MD. Baseline NIH Stroke Scale score strongly predicts outcome after stroke: a report of the trial of Org 10172 in acute stroke treatment (TOAST) Neurology. 1999;53:126–131. doi: 10.1212/wnl.53.1.126. [DOI] [PubMed] [Google Scholar]
- 10.Banks JL, Marotta CA. Outcomes validity and reliability of the modified rankin scale: implications for stroke clinical trials: a literature review and synthesis. Stroke. 2007;38:1091–1096. doi: 10.1161/01.STR.0000258355.23810.c6. [DOI] [PubMed] [Google Scholar]
- 11.Ohura T, Hase K, Nakajima Y, Nakayama T. Validity and reliability of a performance evaluation tool based on the modified barthel index for stroke patients. BMC Med Res Methodol. 2017;17:131. doi: 10.1186/s12874-017-0409-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Memis D, Kozanoglu E, Kelle B, Goncu MK. Assessment of demographic and clinical characteristics on functional status and disability of patients with stroke. Neurosciences (Riyadh) 2016;21:352–357. doi: 10.17712/nsj.2016.4.20160212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saver JL, Altman H. Relationship between neurologic deficit severity and final functional outcome shifts and strengthens during first hours after onset. Stroke. 2012;43:1537–1541. doi: 10.1161/STROKEAHA.111.636928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iemolo F, Sanzaro E, Duro G, Giordano A, Paciaroni M. The prognostic value of biomarkers in stroke. Immun Ageing. 2016;13:19. doi: 10.1186/s12979-016-0074-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whiteley W, Chong WL, Sengupta A, Sandercock P. Blood markers for the prognosis of ischemic stroke: a systematic review. Stroke. 2009;40:e380–389. doi: 10.1161/STROKEAHA.108.528752. [DOI] [PubMed] [Google Scholar]
- 16.Hasan N, McColgan P, Bentley P, Edwards RJ, Sharma P. Towards the identification of blood biomarkers for acute stroke in humans: a comprehensive systematic review. Br J Clin Pharmacol. 2012;74:230–40. doi: 10.1111/j.1365-2125.2012.04212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cooper HM. Research synthesis and meta-analysis: a step-by-step approach. Los Angeles: SAGE; 2017. [Google Scholar]
- 18.McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM Statistics Subcommittee of the NCI-EORTC Working Group on Cancer Diagnostics. Reporting recommendations for tumor marker prognostic studies (REMARK) J Natl Cancer Inst. 2005;97:1180–1184. doi: 10.1093/jnci/dji237. [DOI] [PubMed] [Google Scholar]
- 19.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ilunga Tshiswaka D, Seals SR, Raghavan P. Correlates of physical function among stroke survivors: an examination of the 2015 BRFSS. Public Health. 2018;155:17–22. doi: 10.1016/j.puhe.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 21.Adeoye O, Hornung R, Khatri P, Kleindorfer D. Recombinant tissue-type plasminogen activator use for ischemic stroke in the united states: a doubling of treatment rates over the course of 5 years. Stroke. 2011;42:1952–1955. doi: 10.1161/STROKEAHA.110.612358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nat Med. 2011;17:796–808. doi: 10.1038/nm.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Q, Tang XN, Yenari MA. The inflammatory response in stroke. J Neuroimmunol. 2007;184:53–68. doi: 10.1016/j.jneuroim.2006.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lakhan SE, Kirchgessner A, Hofer M. Inflammatory mechanisms in ischemic stroke: therapeutic approaches. J Transl Med. 2009;7:97. doi: 10.1186/1479-5876-7-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simats A, Garcia-Berrocoso T, Montaner J. Neuroinflammatory biomarkers: from stroke diagnosis and prognosis to therapy. Biochim Biophys Acta. 2016;1862:411–424. doi: 10.1016/j.bbadis.2015.10.025. [DOI] [PubMed] [Google Scholar]
- 26.Dziedzic T. Systemic inflammation as a therapeutic target in acute ischemic stroke. Expert Rev Neurother. 2015;15:523–531. doi: 10.1586/14737175.2015.1035712. [DOI] [PubMed] [Google Scholar]
- 27.Kim JY, Kawabori M, Yenari MA. Innate inflammatory responses in stroke: mechanisms and potential therapeutic targets. Curr Med Chem. 2014;21:2076–2097. doi: 10.2174/0929867321666131228205146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gori AM, Giusti B, Piccardi B, Nencini P, Palumbo V, Nesi M, Nucera A, Pracucci G, Tonelli P, Innocenti E, Sereni A, Sticchi E, Toni D, Bovi P, Guidotti M, Tola MR, Consoli D, Micieli G, Tassi R, Orlandi G, Sessa M, Perini F, Delodovici ML, Zedde ML, Massaro F, Abbate R, Inzitari D. Inflammatory and metalloproteinases profiles predict three-month poor outcomes in ischemic stroke treated with thrombolysis. J Cereb Blood Flow Metab. 2017;37:3253–3261. doi: 10.1177/0271678X17695572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodriguez JA, Sobrino T, Orbe J, Purroy A, Martinez-Vila E, Castillo J, Paramo JA. proMetalloproteinase-10 is associated with brain damage and clinical outcome in acute ischemic stroke. J Thromb Haemost. 2013;11:1464–1473. doi: 10.1111/jth.12312. [DOI] [PubMed] [Google Scholar]
- 30.De Marchis GM, Schneider J, Weck A, Fluri F, Fladt J, Foerch C, Mueller B, Luft A, Christ-Crain M, Arnold M, Katan M. Midregional proatrial natriuretic peptide improves risk stratification after ischemic stroke. Neurology. 2018;90:e455–e465. doi: 10.1212/WNL.0000000000004922. [DOI] [PubMed] [Google Scholar]
- 31.De Marchis GM, Katan M, Weck A, Fluri F, Foerch C, Findling O, Schuetz P, Buhl D, El-Koussy M, Gensicke H, Seiler M, Morgenthaler N, Mattle HP, Mueller B, Christ-Crain M, Arnold M. Copeptin adds prognostic information after ischemic stroke: results from the CoRisk study. Neurology. 2013;80:1278–1286. doi: 10.1212/WNL.0b013e3182887944. [DOI] [PubMed] [Google Scholar]
- 32.Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107:1058–1070. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brites F, Martin M, Guillas I, Kontush A. Antioxidative activity of high-density lipoprotein (HDL): mechanistic insights into potential clinical benefit. BBA Clin. 2017;8:66–77. doi: 10.1016/j.bbacli.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao W, An Z, Hong Y, Zhou G, Guo J, Zhang Y, Yang Y, Ning X, Wang J. Low total cholesterol level is the independent predictor of poor outcomes in patients with acute ischemic stroke: a hospital-based prospective study. BMC Neurol. 2016;16:36. doi: 10.1186/s12883-016-0561-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kernan WN, Ovbiagele B, Black HR, Bravata DM, Chimowitz MI, Ezekowitz MD, Fang MC, Fisher M, Furie KL, Heck DV, Johnston SC, Kasner SE, Kittner SJ, Mitchell PH, Rich MW, Richardson D, Schwamm LH, Wilson JA American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, and Council on Peripheral Vascular Disease. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke. 2014;45:2160–2236. doi: 10.1161/STR.0000000000000024. [DOI] [PubMed] [Google Scholar]
- 36.Amarenco P, Labreuche J, Touboul PJ. High-density lipoprotein-cholesterol and risk of stroke and carotid atherosclerosis: a systematic review. Atherosclerosis. 2008;196:489–496. doi: 10.1016/j.atherosclerosis.2007.07.033. [DOI] [PubMed] [Google Scholar]
- 37.Brea D, Sobrino T, Rodriguez-Yanez M, Ramos-Cabrer P, Agulla J, Rodriguez-Gonzalez R, Campos F, Blanco M, Castillo J. Toll-like receptors 7 and 8 expression is associated with poor outcome and greater inflammatory response in acute ischemic stroke. Clin Immunol. 2011;139:193–198. doi: 10.1016/j.clim.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 38.Campos F, Rodriguez-Yanez M, Castellanos M, Arias S, Perez-Mato M, Sobrino T, Blanco M, Serena J, Castillo J. Blood levels of glutamate oxaloacetate transaminase are more strongly associated with good outcome in acute ischaemic stroke than glutamate pyruvate transaminase levels. Clin Sci (Lond) 2011;121:11–17. doi: 10.1042/CS20100427. [DOI] [PubMed] [Google Scholar]
- 39.Luitse MJ, van Seeters T, Horsch AD, Kool HA, Velthuis BK, Kappelle LJ, Biessels GJ. Admission hyperglycaemia and cerebral perfusion deficits in acute ischaemic stroke. Cerebrovasc Dis. 2013;35:163–167. doi: 10.1159/000346588. [DOI] [PubMed] [Google Scholar]
- 40.Nair SS, Sylaja PN, Sreedharan SE, Sarma S. Maintenance of normoglycemia may improve outcome in acute ischemic stroke. Ann Indian Acad Neurol. 2017;20:122–126. doi: 10.4103/0972-2327.194301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Putaala J, Sairanen T, Meretoja A, Lindsberg PJ, Tiainen M, Liebkind R, Strbian D, Atula S, Artto V, Rantanen K, Silvonen P, Piironen K, Curtze S, Happola O, Mustanoja S, Pitkaniemi J, Salonen O, Silvennoinen H, Soinne L, Kuisma M, Tatlisumak T, Kaste M. Post-thrombolytic hyperglycemia and 3-month outcome in acute ischemic stroke. Cerebrovasc Dis. 2011;31:83–92. doi: 10.1159/000321332. [DOI] [PubMed] [Google Scholar]
- 42.Woodruff TM, Thundyil J, Tang SC, Sobey CG, Taylor SM, Arumugam TV. Pathophysiology, treatment, and animal and cellular models of human ischemic stroke. Mol Neurodegener. 2011;6:11. doi: 10.1186/1750-1326-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lai TW, Zhang S, Wang YT. Excitotoxicity and stroke: identifying novel targets for neuroprotection. Prog Neurobiol. 2014;115:157–88. doi: 10.1016/j.pneurobio.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 44.Bano D, Nicotera P. Ca2+ signals and neuronal death in brain ischemia. Stroke. 2007;38:674–676. doi: 10.1161/01.STR.0000256294.46009.29. [DOI] [PubMed] [Google Scholar]
- 45.Teichberg VI, Cohen-Kashi-Malina K, Cooper I, Zlotnik A. Homeostasis of glutamate in brain fluids: an accelerated brain-to-blood efflux of excess glutamate is produced by blood glutamate scavenging and offers protection from neuropathologies. Neuroscience. 2009;158:301–308. doi: 10.1016/j.neuroscience.2008.02.075. [DOI] [PubMed] [Google Scholar]
- 46.Kalogeris T, Bao Y, Korthuis RJ. Mitochondrial reactive oxygen species: a double edged sword in ischemia/reperfusion vs preconditioning. Redox Biol. 2014;2:702–714. doi: 10.1016/j.redox.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hu H, Doll DN, Sun J, Lewis SE, Wimsatt JH, Kessler MJ, Simpkins JW, Ren X. Mitochondrial impairment in cerebrovascular endothelial cells is involved in the correlation between body temperature and stroke severity. Aging Dis. 2016;7:14–27. doi: 10.14336/AD.2015.0906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koenig W. Fibrin(ogen) in cardiovascular disease: an update. Thromb Haemost. 2003;89:601–9. [PubMed] [Google Scholar]
- 49.Potpara TS, Polovina MM, Djikic D, Marinkovic JM, Kocev N, Lip GY. The association of CHA2DS2-VASc score and blood biomarkers with ischemic stroke outcomes: the belgrade stroke study. PLoS One. 2014;9:e106439. doi: 10.1371/journal.pone.0106439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vandelli L, Marietta M, Gambini M, Cavazzuti M, Trenti T, Cenci MA, Casoni F, Bigliardi G, Pentore R, Nichelli P, Zini A. Fibrinogen decrease after intravenous thrombolysis in ischemic stroke patients is a risk factor for intracerebral hemorrhage. J Stroke Cerebrovasc Dis. 2015;24:394–400. doi: 10.1016/j.jstrokecerebrovasdis.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 51.Scheiermann C, Kunisaki Y, Frenette PS. Circadian control of the immune system. Nature Reviews Immunology. 2013;13:190–198. doi: 10.1038/nri3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Beglinger S, Drewe J, Christ-Crain M. The circadian rhythm of copeptin, the c-terminal portion of arginine vasopressin. J Biomark. 2017;2017:4737082. doi: 10.1155/2017/4737082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wettersten N, Maisel A. Role of cardiac troponin levels in acute heart failure. Card Fail Rev. 2015;1:102–106. doi: 10.15420/cfr.2015.1.2.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Groschel K, Schnaudigel S, Edelmann F, Niehaus CF, Weber-Kruger M, Haase B, Lahno R, Seegers J, Wasser K, Wohlfahrt J, Vollmann D, Stahrenberg R, Wachter R. Growth-differentiation factor-15 and functional outcome after acute ischemic stroke. J Neurol. 2012;259:1574–9. doi: 10.1007/s00415-011-6379-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mendioroz M, Fernández-Cadenas I, Rosell A, Delgado P, Domingues-Montanari S, Ribó M, Penalba A, Quintana M, Álvarez-Sabín J, Montaner J. Osteopontin predicts long-term functional outcome among ischemic stroke patients. J Neurol. 2011;258:486–93. doi: 10.1007/s00415-010-5785-z. [DOI] [PubMed] [Google Scholar]
- 56.Roy-O’Reilly M, Ritzel RM, Conway SE, Staff I, Fortunato G, McCullough LD. CCL11 (Eotaxin-1) levels ppredict long-term functional outcomes in patients following ischemic stroke. Transl Stroke Res. 2017;8:578–584. doi: 10.1007/s12975-017-0545-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kuwashiro T, Ago T, Kamouchi M, Matsuo R, Hata J, Kuroda J, Fukuda K, Sugimori H, Fukuhara M, Awano H, Isomura T, Suzuki K, Yasaka M, Okada Y, Kiyohara Y, Kitazono T. Significance of plasma adiponectin for diagnosis, neurological severity and functional outcome in ischemic stroke-research for biomarkers in ischemic stroke (REBIOS) Metabolism. 2014;63:1093–1103. doi: 10.1016/j.metabol.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 58.Makihara N, Okada Y, Koga M, Shiokawa Y, Nakagawara J, Furui E, Kimura K, Yamagami H, Hasegawa Y, Kario K, Okuda S, Naganuma M, Toyoda K. Effect of serum lipid levels on stroke outcome after rt-PA therapy: SAMURAI rt-PA registry. Cerebrovasc Dis. 2012;33:240–7. doi: 10.1159/000334664. [DOI] [PubMed] [Google Scholar]
- 59.Lasek-Bal A, Jedrzejowska-Szypulka H, Rozycka J, Bal W, Holecki M, Dulawa J, Lewin-Kowalik J. Low concentration of BDNF in the acute phase of ischemic stroke as a factor in poor prognosis in terms of functional status of patients. Med Sci Monit. 2015;21:3900–5. doi: 10.12659/MSM.895358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Navarro-Sobrino M, Rosell A, Hernandez-Guillamon M, Penalba A, Boada C, Domingues-Montanari S, Ribo M, Alvarez-Sabin J, Montaner J. A large screening of angiogenesis biomarkers and their association with neurological outcome after ischemic stroke. Atherosclerosis. 2011;216:205–211. doi: 10.1016/j.atherosclerosis.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 61.Selçuk Ö, Yayla V, Çabalar M, Güzel V, Uysal S, Gedikbaşi A. The relationship of serum s100b levels with infarction size and clinical outcome in acute ischemic stroke patients. Noro Psikiyatr Ars. 2014;51:395–400. doi: 10.5152/npa.2014.7213. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


